Abstract
RNF144A is a DNA damage-induced E3 ubiquitin ligase that targets proteins involved in genome instability for degradation, e.g., DNA-PKcs and BMI1. RNF144A is frequently mutated or epigenetically silenced in cancer, providing the rationale to evaluate RNF144A loss of function in tumorigenesis. Here we report that RNF144A-deficient mice are more prone to the development of bladder tumors upon carcinogen exposure. In addition to DNA-PKcs and BMI1, we identify the immune checkpoint protein PD-L1 as a novel degradation target of RNF144A, since these proteins are expressed at higher levels in Rnf144a KO tumors. RNF144A interacts with PD-L1 in the plasma membrane and intracellular vesicles and promotes poly-ubiquitination and degradation of PD-L1. Therefore, Rnf144a KO stabilizes PD-L1 and leads to a reduction of tumor-infiltrating CD8+ T cell populations in the BBN-induced bladder tumors. The bladder tumors developed in WT and Rnf144a KO mice primarily express CK5 and CK14, markers of basal cancer subtype, as expected in BBN-induced bladder tumors. Intriguingly, the Rnf144a KO tumors also express GATA3, a marker for the luminal subtype, suggesting that RNF144A loss of function promotes features of cellular differentiation. Such differentiation features in Rnf144a KO tumors likely result from a decrease of EGFR expression, consistent with the reported role of RNF144A in maintaining EGFR expression. In summary, for the first time our study demonstrates the in vivo tumor suppressor activity of RNF144A upon carcinogenic insult. Loss of RNF144A promotes the expression of DNA-PKcs, BMI1 and PD-L1, likely contributing to the carcinogen-induced bladder tumorigenesis.
Keywords: RNF144A, Bladder cancer, BMI1, DNA-PKcs, PD-L1
Introduction
RBR (RING-Between-RING) E3 ubiquitin ligase is an emerging subgroup of the ring finger proteins (RNF) [1-3]. It contains two conserved zinc-binding domains surrounding a novel zinc-binding motif and may catalyze the transfer of ubiquitin (Ub) by a RING/HECT hybrid-like mechanism [4]. Typically, the first RING domain binds to the Ub-loaded E2, and then Ub is transferred to the second RING domain, and the received Ub then conjugates to the substrate protein via the second RING domain. Many studies have revealed that E3 ligases, including RBR E3 ligases, are involved in tumorigenesis by regulating cell proliferation, apoptosis, cell cycle, inflammation, and other biological processes [5]. For example, Parkin knockout mice develop hepatocellular carcinomas [6], and PARK2 mutations are involved in lung cancer [7], glioma and neuroblastoma tumorigenesis [8]. However, the roles of many RBR E3 ligases, including RNF144A, in human diseases and tumorigenesis are yet to be determined.
RNF144A and RNF144B belong to the RNF144 subfamily, which only contains an RBR domain at the N-terminus and a transmembrane (TM) domain at the C-terminus. The TM domain is essential for RNF144A E3 ligase activity and subcellular localization [9]. RNF144A also uses this TM domain to bind to EGFR and further maintains EGFR protein stability and signaling [10]. On the other hand, epigenetic silencing of RNF144A has been found in various human cancer types, such as breast cancer [11] and glioblastoma (GBM) [12], suggesting that RNF144A may act as a tumor suppressor. Various DNA damaging agents dramatically induce p53-dependent up-regulation of RNF144A and further promote apoptosis of cancer cells [13-15]. RNF144A inhibits DNA-PKcs-dependent cell survival signaling by directly ubiquitinating and degrading DNA-PKcs [13, 16]. In addition, RNF144A binds to and degrades proto-oncoprotein BMI1 [12]. BMI1 enhances the repair of double-strand breaks and promotes cell survival upon DNA damage [17]. BMI1 is overexpressed in many human cancers [18], including bladder cancer [19]. Knockdown of BMI1 inhibits proliferation and invasion of bladder cancer cells [20]. In contrast, BMI1-dependent oncogenic pathways are upregulated in tumor-initiating cells, as shown in clinical bladder cancer specimens [21]. Bladder cancer is the sixth common cancer in the United States, with roughly 70,000 new cases each year (PDQ Cancer Information Summaries; https://www.ncbi.nlm.nih.gov/books/NBK65962/). Although RNF144A can degrade BMI1 via the ubiquitin-proteasome pathway, the effect of RNF144A deficiency on bladder tumorigenesis remains unknown.
Certain subtypes of bladder cancer are more immunogenic [22]. In particular, the basal-squamous subtype of human bladder tumors expresses relatively low levels of RNF144A and high levels of immune checkpoint protein programmed cell death ligand-1 (PD-L1) [23]. PD-L1 binds to its receptor, programmed cell death protein-1 (PD-1), which can hijack the activation state of CD28/MHC, and help evade immune surveillance by inhibiting T lymphocyte proliferation, cytokine production and cytolytic activity [24, 25]. Blocking of PD-L1/PD-1 inhibitory pathway can reactivate T cell activity and has been used in clinical trials for various cancers, including bladder cancer [26, 27]. Both RNF144A and PD-L1 are localized on the plasma membrane and in the intracellular vesicles and can be induced by DNA damage [13, 28]. Although RNF144A has been shown to regulate the stability of several plasma membrane proteins, the relationship between RNF144A and PD-L1 has not yet been determined.
In the current study, we for the first time show that RNF144A interacts with the glycosylated PD-L1 protein, mediating its polyubiquitination and proteasomal degradation. We generated two different RNF144A-deficient (knockout-first and whole body Cre/lox deletion KO) C57BL/6 mice for in vivo studies. Both RNF144A-deficient mutant mice are viable and fertile. Intriguingly, RNF144A deficiency promotes bladder cancer development upon exposure to the carcinogen N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN). These tumors express high levels of the RNF144A degradation targets, i.e., DNA-PKcs, BMI1, and PD-L1 proteins, which likely contribute to the development of bladder tumorigenesis in Rnf144a KO mice upon BBN carcinogen exposure.
Materials and Methods
Animal care
All mouse experiments were carried out under the approval of the Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine (BCM) (Protocol AN-5529). Mice were housed in the BCM Transgenic Mouse Facility under the care of the Center for Comparative Medicine. Free food and water are supplied for all mice. Body weight was measured under free-feeding weekly from 9 to 19 weeks of age.
Rnf144a knockout-first mice (Rnf144aβgeo) generation and breeding
Rnf144a knockout-first mouse was generated as previously described [10]. Briefly, the Rnf144a targeting vector construct (HTGR6005_A_5_G08) was purchased from the KOMP repository (https://www.komp.org). Targeting vector DNA was electroporated into the C57BL/6N embryonic stem cell line JM8A3 [29] by the BCM Mouse Embryonic Stem Cell Core. Correctly targeted clones were microinjected into C57BL/6-Tyrc-Brd (albino) blastocysts and transplanted into pseudopregnant foster mothers by the BCM Genetically Engineered Mouse Core. Chimeric male offspring were crossed with C57BL/6N females to test for germline transmission of the targeted Rnf144a allele, referred to as Rnf144aβgeo. Rnf144a knockout-first mouse was crossed with new C57BL/6N breeders for more than ten generations. Genotyping of mouse genomic DNA was performed by PCR amplification using different oligos: LoxP-F: 5’-ACT GAT GGC GAG CTC AGA CC- 3’, LoxP-R: 5’-GCC TTT GAA TCC TAC AAC AG-3’, LoxP-F-genotyping: 5’-CTG CAA GGA GTA CCC AGC TT-3’.
Generation of Rnf144aflox and Rnf144ako mice
Mice with conditional (Rnf144aflox) allele were made by removing the entire FRT-flanked lacZ-neomycin (βgeo) gene-trap cassette from the Rnf144aβgeo allele. In brief, the conditional-ready Rnf144aβgeo female mice were crossed with the Flp recombinase germline deleter (FLPeR; B6.129S4-Gt(ROSA)26Sortm1(FLP1)Dym/RainJ, The Jackson Laboratory) male mice [30]. Mice harboring Rnf144aflox allele were confirmed by genomic DNA PCR analysis using different oligos: 5-ARM-F: 5’-ttc ctc cct cca ttt tcc tt-3’; EN2-R: 5’-cca caa cgg gtt ctt ctg tt-3’; Neo-F: 5’-atc gcc ttc tat cgc ctt ct-3’; FRT2-R: 5’-agt aac cgc cta ctg cga ct-3’. Mice heterozygous for the Rnf144a floxed allele (Rnf144aflox/+) were crossbred to generate mice homozygous for the floxed Rnf144a allele (Rnf144aflox/flox). The Rnf144a floxed allele was designed to delete exon 5 and exon 6 through Cre-mediated recombination. Deletion of exon 5 and exon 6 eliminates the coding sequences for the partial RING1 and IBR domains (a.a. 46-100), resulting in a frameshift and nonsense-mediated mRNA decay. To generate mice with an Rnf144ako allele, Rnf144aflox/flox female mice were crossed with EIIaCre deleter (B6.FVB-TgN(EIIa-Cre)C5379Lmgd) mice (purchased from the Jackson Laboratories), which are homozygous for the transgenic cre gene with an adenovirus EIIa promoter [31, 32]. PCR analysis of tail genomic DNA was performed to identify the Cre-mediated recombination in the Rnf144aflox/−/EIIa-cre mice. Rnf144aflox/−/EIIa-cre mice were further crossed with Rnf144aflox/flox mice to generate Rnf144aflox/− mice. Mice heterozygous for the Rnf144a floxed-out allele (Rnf144aflox/−) were crossbred to generate mice homozygous for the floxed-out Rnf144a allele (Rnf144a−/−, also named as Rnf144ako in this study). Rnf144aflox/flox (WT) offspring were used as controls in this study.
Carcinogen BBN treatment of mice
Mice (4-5 weeks old; 10 WT males, 10 WT females, 10 Rnf144a KO males, and 5 Rnf144a KO females) were treated with N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN; TCI America) as previously described [33]. In brief, a standard dose (0.05%) BBN was supplied ad libitum in drinking water for 12 weeks, followed by regular drinking water for four weeks. No difference in water consumption was observed among different groups. Mouse body weight was measured under free-feeding weekly from each week under BBN water consumption. Mice were euthanized at 16 weeks after the first BBN treatment (endpoint). After euthanasia, bladders were excised and washed in phosphate-buffered saline. Mouse bladders were fixed in 10% formalin, embedded in paraffin, and then stored at room temperature until sectioning for histological analysis.
Mouse bladder echography
Mouse bladder echography was performed to evaluated potential bladder tumors at week 11 of BBN treatment. The mouse was anesthetized, the abdomen was shaved, and an ultrasound examination was performed to identify tumors. The chi-square statistic is performed, and the P-value < 0.05 consider as significant.
Immunohistochemistry and Immunofluorescence
Serial 3-μm or 5-μm transverse sections were cut and collected on slides. Mouse bladder sections were stained with Hematoxylin and Eosin, or subjected to the immunohistochemistry analysis following standard protocols. In brief, the paraffin-embedded sections were deparaffinized and hydrated. Antigen unmasking was performed according to the recommendations on the commercial antibody data sheet. Sections were washed by dH2O and incubated in 3% hydrogen peroxide for 10 min. Sections were blocked by blocking solution for 1 h at room temperature followed by the primary antibody incubation overnight at 4°C. The sections were further incubated with biotinylated secondary antibodies, and combined with streptavidin-HRP in the ABC (Avidin-Biotin Complex) method, followed a DAB (3,3’-Diaminobenzidine) staining and a counterstain in hematoxylin. For CD8/granzyme B double staining, CD8 was stained with DAB (brown) and granzyme B with AP/Red (red) to produce a red-brown color combination. Primary antibodies used are listed as follows, CK5 (Covance, 905501, 1:1000), CK14 (Covance, 905301, 1:1000), GATA3 (Santa Cruz, sc-268, 1:200), DNA-PKcs (NeoMarkers, MS-423-P1, 1:100), EGFR (Santa Cruz, sc-373746, 1:250), p63 (Santa Cruz, sc-8431, 1:50), BMI1 (Cell signaling, #6964, 1:200), PD-L1 (Cell signaling, #13684 and #15165, 1:500), active Caspase-3 (Cell signaling, #9661, 1:200), Ki-67 (Abcam, ab16667, 1:100), granzyme B (R&D systems, AF1865, 1:200), and CD8 (Cell signaling, #98941, 1:200). Immunofluorescence analysis was performed as described previously [13]. Cells were fixed with 4% paraformaldehyde for 10 min, followed by permeabilization with 0.5% Triton-X 100 in 1X PBS for 10 min. The fixed cells were then subjected to blocking with 2% BSA in 1X PBS for two hours, followed by incubation with indicated primary antibodies overnight and the Texas Red X- or FITC-conjugated secondary antibody for an hour. The nuclei were stained with Hoechst 33258. Images were captured on a Zeiss fluorescence microscope (Axio Observer Inverted Microscope) equipped with ApoTome 2 (Zeiss).
Data mining
The genetic alternations of the RNF144A gene in bladder cancer were analyzed using the cBioPortal for Cancer Genomics [34, 35]. To evaluate the correlation between RNF144A mRNA (RNA Seq V2 RSEM) and DNA methylation (HM450), a total of three studies were selected (Bladder, TCGA 2014; BLCA, TCGA 2017; and TCGA, Firehose Legacy 2016), and the plot was generated according to the cBioPortal’s online instructions. RNA-seq data of RNF144A and CD274 in 29 subsets of immune cells were extracted [36] for analysis.
Cell culture and plasmid transfection
Cell culture and plasmid transfection were performed as described previously [9, 10, 13]. In brief, cells were maintained in media supplemented with 10% Fetal Bovine Serum (FBS), penicillin (50 IU/ml) and streptomycin (50 μg/ml), and were grown in a humidified incubator at 37 °C with 5% CO2 and 95% air. Specifically, Lenti-X™ 293T, T24, HT-1197, 639V, 647V and MDA-MB-468 cells were maintained in DMEM medium. U2OS cells were grown in McCOY’s 5A medium. The plasmids expressing FLAG-tagged WT or mutant RNF144A were constructed as described previously [9, 13]. RNF144A hairpin shRNA constructs (V2LHS_ 254611, and V2LHS_72643) were purchased from Open Biosystem. RNF144A was knocked out by CRISPR/Cas9 in U2OS cells as described [10]. The human PD-L1-HA plasmid was purchased from Sino Biological. The human pEGFP-N1/PD-L1 was a gift from Dr. Mien-Chie Hung (Addgene plasmid #121478 [37]). A standard Lipofectamine 2000 (Life Technologies) or Polyethylenimine (PEI; Sigma-Aldrich) [38] methods were used for transfection of all cells.
Immunoprecipitation and Western Blot Analysis
To perform co-immunoprecipitation, transfected or untransfected cells were harvested in TNN buffer as described previously [13]. Equal amounts of cell lysates were subjected to immunoprecipitation using indicated antibodies and were resolved by 10% or 12% (vol/vol) gradient SDS-PAGE (Bio-Rad). Co-immunoprecipitated proteins were detected by Western blotting using specific antibodies. An aliquot of cell lysates were also subjected to Western blot analysis to serve as input controls. Alternatively, cells were lysed in SDS lysis buffer and subjected to Western blot analysis. The anti-FLAG Rabbit or mouse antibody (F7245 or F3165), actin antibody (A2066), anti-HA mouse monoclonal antibody conjugated agarose (A2095), and anti-FLAG® M2 mouse monoclonal antibody-conjugated affinity gel (A2220) were purchased from Sigma-Aldrich. The anti-GAPDH (sc-47724) and anti-HA (sc-805) antibodies were purchased from Santa Cruz Biotechnology. The anti-RNF144A (LS-C162648) antibody was purchased from LifeSpan BioSciences. The anti-c-Myc rabbit (A-14, sc-789) and mouse (9E10, sc-40) antibodies were from Santa Cruz Biotechnology. The anti-Vinculin (V9131) antibody was from Sigma-Aldrich. Antibodies for DNA-PKcs, EGFR, BMI and PD-L1 were described in the IF/IHC sections.
Cellular Ubiquitination Assay
The cellular ubiquitination assay was performed as described previously [13]. Briefly, HEK293T cells were transfected with Myc-tagged ubiquitin, HA-tagged PD-L1 and FLAG-tagged RNF144A (WT or mutants) for 24 h as indicated. Cells were treated with 20 μM MG-132 for 3 h before collection. Cells were then lysed in radioimmunoprecipitation assay (RIPA) buffer or SDS lysis buffer (1% SDS and 60 mM Tris (pH 6.8)), followed by boiling for 5 min at 95 °C. The denatured cell lysates were reconstituted to 0.1% SDS by 1:10 dilution in TNN buffer supplemented with a mixture of protease inhibitors. The lysates were then sonicated and clarified by centrifugation at 12,000 rpm for 10 min in a microfuge. Equal amounts of lysates were incubated overnight with anti-HA antibody-conjugated agarose at 4°C. Beads were washed five times with RIPA buffer and subjected to SDS-PAGE, followed by Western blot analysis using indicated antibodies.
Real-time RT-PCR and PCR
RNA was extracted using TRIzol reagent (Invitrogen) and was reverse transcribed into cDNA as described previously [13]. Real-time PCR was then performed in triplicates on an MX3005P Thermal Cycler (Stratagene) using SYBR Green dye to measure amplification and ROX as a reference dye. Transcript levels were normalized with GAPDH levels, which were analyzed in parallel with test genes. Results were analyzed with MxPro 4.1 Quantitative PCR software (Stratagene). The PCR primer sequences are: Human GAPDH-F, 5’-ATTGGGCGCCTGGTCACCAGGGCTG-3’; Human GAPDH-R, 5’-AAATGAGCCCCAGCCTTCTCCATG-3’; Human PD-L1-F, 5’-TGCCGACTACAAGCGAATTACTG-3’; Human PD-L1-R, 5’-CTGCTTGTCCAGATGACTTCGG-3’; Human RNF144A-qPCR-1F, 5′-CTGTTTGATCCCTGTCGGACT-3′; Human RNF144A-qPCR-1R, 5′-GATGGGCGCGTCATCTTCTT-3′.
Subcellular Fractionation
Lenti-X 293T cells were transiently transfected with FLAG-RNF144A and/or PD-L1-HA for 24 h, and then were harvested in a hypotonic buffer containing 20 mM Tris-HCl, pH 7.4 and 2 mM EDTA on ice for 15 min. The nuclei were first pelleted by centrifugation at 500 x g, and the supernatant was further subjected to centrifugation at 16,000 x g for 60 min to spin down the membrane-enriched insoluble fraction. The enriched nuclei and insoluble membrane fraction were dissolved in TNN lysis buffer. After sonication, the total cell lysates, nuclear extract, insoluble membrane fraction and supernatant (cytosol) were subjected to immunoblotting. The blot was probed with an anti-GAPDH antibody, an anti-p84 and an anti-E-Cadherin antibody, which serve as cytosolic, nuclear and membrane markers, respectively.
Statistics
The age- and weight-matched mice of each genotype were randomly assigned to the untreated group and BBN-treatment group. The sample size for each group was estimated according to our prior experience in the BBN-induced murine bladder cancer model [33]. All histology slides were de-identified and blind to the pathologist. Chi-squared and Student’s two-sample t-test were used to determine if there was a statistical difference between the means of two groups (e.g., WT and KO groups). P-values were two-sided and p-value < 0.05 was considered statistically significant. Quantified data shown represent at least three independent experiments. Data were represented as means ± s.d. (standard deviation). GraphPad software was used in this manuscript.
Results
Generation of Rnf144a knockout-first and exons-deletion mutant mice
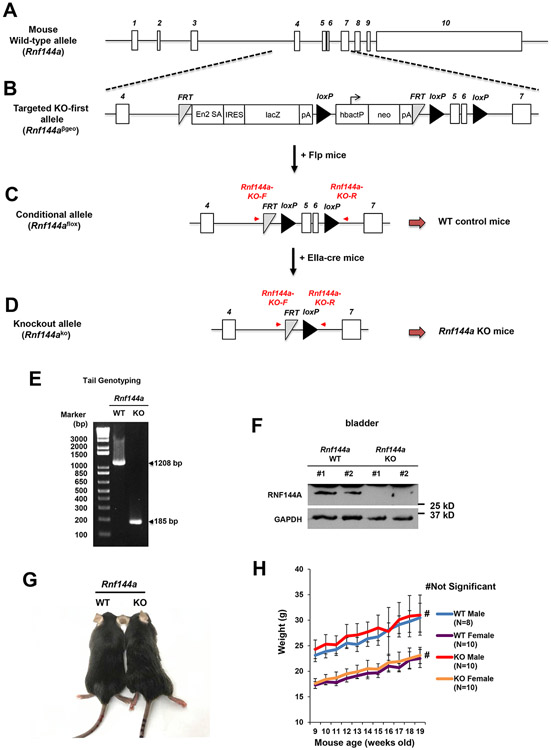
To investigate the physiological role of RNF144A, we generated two mutant mouse strains with RNF144A deficiency (Fig. 1). The mouse Rnf144a gene is located on chromosome 12 and contains ten exons. Its protein sequences are encoded from exon 4 to exon 10 (Fig. 1A and Fig. S1). We took the knockout-first strategy [39, 40] using the Rnf144a knockout-first vector (Fig. 1B) obtained from the KOMP repository (https://www.komp.org). The C57BL/6N mouse ES cells were microinjected with the Rnf144a knockout-first vector, which contains a promoter-driven selection cassette between exon 4 and exon 7 of Rnf144a (Fig. 1B). This resulted in a non-expressing RNF144A transcript, which was then degraded through nonsense-mediated mRNA decay (NMD). Rnf144a knockout-first mice were fertile and viable with normal life span (age > 500 days, N = 14). We did not observe significant developmental defects or gross abnormalities among these Rnf144a knockout-first mice (up to age > 365 days; N = 62). RNF144A mRNA was still detectable, albeit at a much-reduced level, in embryonic fibroblasts derived from Rnf144a knockout-first deficient mice [10]. To disrupt the Rnf144a gene completely, we further generated Rnf144a knockout mice by deleting exons 5 and 6 that encode a.a. 46-100 (spanning parts of RING1 and IBR domains) of RNF144A protein (Fig. 1C-D and Fig. S1). The deletion causes a frameshift and subsequent NMD. To this end, Rnf144a knockout-first allele (Rnf144aβgeo) was converted into a conditional Rnf144a knockout allele (Rnf144aflox) (Fig. 1C) by crossing Rnf144a knockout-first mouse with the Flp recombinase germline deleter mouse [30]. To further generate mice with the Rnf144ako allele (Fig. 1D), Rnf144aflox/flox female mice were crossed with EIIaCre deleter male mice [31, 32]. Mice heterozygous for the Rnf144a floxed-out allele (Rnf144aflox/ko) were crossbred to generate mice homozygous for the floxed-out Rnf144a allele (Rnf144ako/ko; Rnf144a KO). Rnf144aflox/flox (Rnf144a WT) offspring were used as controls in this study. The PCR genotyping of tail genomic DNA confirmed that 1024 base pairs of Rnf144a were deleted in the KO mice (Fig. 1E). The expression of Rnf144a protein was also completely eliminated in Rnf144a KO mice (Fig. 1F). Similar to Rnf144a knockout-first mice, Rnf144a KO mice were viable and fertile with a normal life span (age > 350 days, N = 15). We also did not observe significant differences in growth, body weights, or development between Rnf144a WT and KO mice (Fig. 1G-H).
Figure 1. Generation of Rnf144a knockout-first and exons-deletion mutant mice.
(A-D) Schematic diagrams show the allele of WT Rnf144a (A), the targeted Knockout-first Rnf144aβgeo (B), the conditional Rnf144aflox (C), and the knockout Rnf144ako (D).
(E) PCR genotyping of genomic DNA confirms the deletion of a fragment of the Rnf144a gene in Rnf144ako alleles.
(F) Western blot analysis confirms the absence of RNF144A protein in the bladder tissues of RNF144A-deficient mice.
(G-H) No significant change in body size (G) and body weight (H) between Rnf144a WT and KO mice.
Deficiency of RNF144A promotes BBN-induced bladder tumor progression.
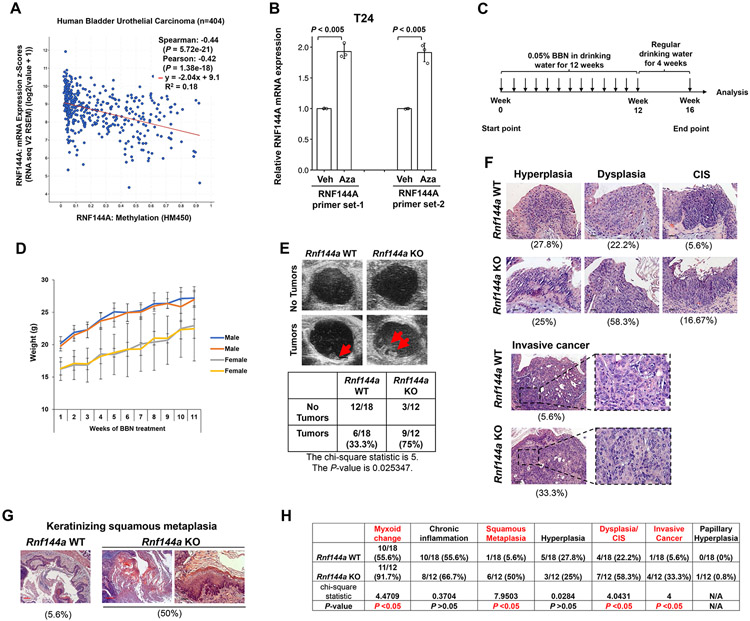
It has been reported that RNF144A can promote DNA damaging reagent-induced cancer cell death [13] and reduce tumor growth in cancer cell xenografts [15]. Here we used Rnf144a KO mutant mice to further determine the effects of RNF144A deficiency on tumorigenesis in vivo. Spontaneous occurrence of tumors from Rnf144a KO or WT mice (age > 300 days; N = 20 per group) was not observed, suggesting that RNF144A deficiency alone is not sufficient to drive tumorigenesis in mice. In human bladder cancer, the levels of RNF144A mRNA are negatively correlated with its promoter methylation levels (Fig. 2A), suggesting RNF144A loss of function in bladder tumorigenesis. Besides, treatment with DNA methylation inhibitor 5-Azacitidine can reactivate the expression of RNF144A in human bladder cancer cells (Fig. 2B). These data suggest epigenetic silencing of RNF144A in some bladder cancers. Given that chemical carcinogen BBN is a well-established chemical carcinogen for inducing murine tumors that recapitulate human muscle-invasive bladder cancer [41, 42] and that RNF144A is endogenously expressed in mouse bladders (Fig. 1F), we investigated the physiological effect of RNF144A deficiency on BBN-induced bladder tumorigenesis (Fig. 2C). There was no significant difference in body weight between WT and KO mice throughout BBN treatment for 11 weeks (Fig. 2D). We first detected potential bladder tumors by sonography at week 11 of BBN treatment. While both Rnf144a WT and KO mice developed bladder tumors, the Rnf144a KO mice showed significantly higher incidence rates of the tumor formation rate (75%) compared to WT mice (33.3%) by week 11 (Fig. 2E). Further, a board-certified GU pathologist evaluated the frequency of Rnf144a KO to WT at different stages of BBN-induced bladder hyperplasia, dysplasia, carcinoma in situ (CIS) and invasive cancer (Fig. 2F), as well as keratinizing squamous metaplasia (Fig. 2G), using hematoxylin and eosin (H&E) analysis at endpoint week 16. The frequency of each pathological change is shown under each panel (Fig. 2F-G). Consistent with sonographic findings, Rnf144a KO mice showed more advanced tumor progression compared to WT mice, i.e., a higher rate of dysplasia/CIS (58.3% in KO mice vs. 22.2% in WT mice) and invasive cancer (33% in KO mice vs. 5.6% in WT mice). BBN treatment can cause squamous metaplasia, a precancerous condition, in the rodent bladder [43]. There is also an increased rate of BBN-induced keratinizing squamous metaplasia in Rnf144a KO mice (50%) vs. WT mice (5.6%) (Fig. 2G-H). Detailed results and statistical analysis are tabulated in Fig. 2H. Noticeably, the BBN challenge also induced papillary hyperplasia in one (0.8%) Rnf144a KO mice. In summary, both ultrasound examination and bladder H&E staining showed that Rnf144a KO mice had a higher incidence rate of BBN-induced bladder tumors. These data suggest that deficiency of RNF144A promotes carcinogen BBN-induced bladder tumorigenesis in mice.
Figure 2. RNF144A deficiency promotes carcinogen-induced bladder cancer development.
(A) TCGA data mining shows a negative correlation between RNF144A mRNA expression and its promoter methylation level in human bladder urothelial carcinoma. (N = 404)
(B) Treatment with 5 μM DNA methylation inhibitor 5-Azacitidine (Aza) for 72 h increases RNF144A mRNA expression in T24 human bladder tumor cells. Veh: vehicle.
(C) A diagram showing the timeline of BBN treatment.
(D) There is no significant difference in body weight between WT and Rnf144a KO mice during the period of BBN treatment. Data shown are the mean mouse body weight ± s.d. for male and female mice of each genotype during BBN treatment (N=5-8 as indicated on the graph).
(E) More RNF144A-deficient mice are found to develop bladder tumors after 11 weeks of BBN treatment as detected by sonography. The chi-square statistic is 5, and the P-value is 0.025347.
(F) Representative H&E stained histopathologic images of bladder tumors in Rnf144a WT and KO mice at the endpoint of BBN treatment. The two bottom right panels show high (40X) magnification of invasive cancers. Black scale bars represent 20 μm. The incidence rate for each pathological finding is listed below each panel.
(G) Rnf144a KO mouse bladders developed more squamous metaplasia after BBN treatment. Pictures shown are the representative H&E stained images of squamous metaplasia in Rnf144a WT and KO mice. Red scale bars represent 100 μm.
(H) RNF144A deficiency predisposes urothelial cells to BBN-induced CIS and invasive cancers. The table shown is the incidence rate of BBN-induced tumorigenesis and pathological changes in each group of mice based on the H&E staining. Chi-square statistic was used to compare the difference in each event between Rnf144a WT and KO mice. P-value < 0.05 was considered significant. N/A: Not Available.
RNF144A regulates urothelial cell survival and proliferation in response to carcinogen treatment.
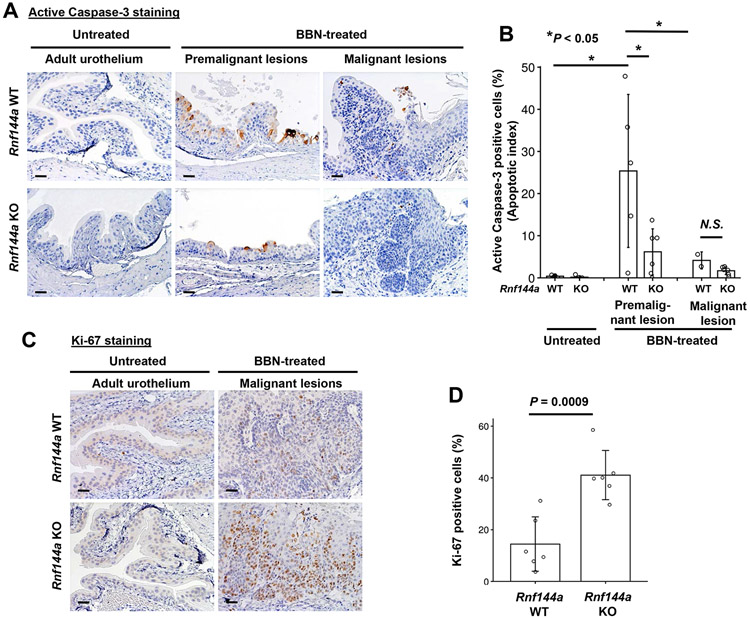
Given that RNF144A deficiency promoted BBN-induced bladder tumor development, we next evaluated the underlying mechanisms by performing immunohistochemical staining of active Caspase-3 and Ki-67, respectively, to determine apoptosis and proliferation in mouse bladder tissues. We previously reported that RNF144A promotes apoptosis in response to DNA damaging agents, so we hypothesized that RNF144A deficiency might confer better survival of urothelial cells in response to BBN treatment, which is a DNA-damaging alkylating carcinogen [44, 45]. The percentage of apoptotic cells in early premalignant lesions was significantly lower in RNF144A-deficient compared to WT mice (Fig. 3A-B). We also observed that carcinogen treatment induced an increased population of active Caspase-3-positive cells, in the premalignant lesions of the superficial urothelium (Fig. 3A) with a significant decrease during the late-stage malignant bladder tissues.
Figure 3. RNF144A regulates BBN-induced apoptosis and urothelial cell proliferation.
(A) Images shown are immunohistochemical staining of active caspase-3 in the adult urothelium and BBN-induced premalignant and malignant lesions, respectively.
(B) The graph shown is the apoptotic index of untreated adult urothelium and BBN-induced premalignant and malignant lesions as indicated. Data shown are the means ± s.d. (N=5-6 mice per group in premalignant lesions; N=2 WT and 6 KO mice in malignant lesions; more than 400 urothelial cells were scored for each mouse). *P < 0.05; N.S.: Not significant.
(C) Images shown are the immunohistochemical staining of Ki-67 in the adult urothelium and BBN-induced malignant lesions.
(D) The graph shown is the Ki-67 proliferation index (% of Ki-67 positive cells) of the BBN-induced malignant lesions in Rnf144a WT and KO mice, respectively. Data shown are the means ± s.d. (N=6). All scale bars represent 20 μm.
We next determined the effect of RNF144A deficiency on BBN-induced bladder tumor proliferation by immunohistochemical staining of the proliferation marker, Ki-67. We observed an increase of Ki-67-positive cell population in BBN-induced malignant lesions compared to untreated adult urothelium (Fig. 3C). In contrast to the active Caspase 3-positive population in premalignant lesions, the Ki-67-positive cell population is significantly higher in the malignant lesions in Rnf144a KO mice compared to WT mice (Fig. 3D). Together, these data are consistent with a pro-apoptotic role of RNF144A in response to DNA damage [13] (in this case BBN treatment) and suggest that RNF144A deficiency promotes BBN-induced tumor proliferation (Fig. 3D).
RNF144A regulates its target proteins in human bladder cancer cells.
Prior studies identified DNA-PKcs and BMI1 as target proteins of RNF144A in various cell lines [10, 12, 13]. In addition to targeting DNA-PKcs for degradation, RNF144A has been shown to negatively regulate the stability of BMI1 proto-oncoprotein [12, 46], a protein that promotes EMT and tumor-initiating capability in head and neck cancer patients [47]. To investigate whether these regulations also occur in bladder cancer, we first examined the effect of RNF144A depletion on the expression of these proteins in a number of bladder cancer cell lines. Indeed, RNF144A knockdown increased the levels of DNA-PKcs and BMI1, but substantially decreased EGFR protein levels in 639V, 647V, and HT-1197 bladder cancer cells (Fig. 4A-C). More importantly, colony formation assay demonstrated that knockdown of RNF144A in HT-1197 bladder cancer cells promoted cell survival after cisplatin treatment (Fig. 4D), supporting the pro-apoptotic role of RNF144A during DNA damage [13] in bladder cancer.
Figure 4. RNF144A deficiency increases the expression of DNA-PKcs and BMI1 in BBN-induced bladder tumors.
(A-C) Western blot analysis shows that knockdown of RNF144A decreases EGFR protein expression but increases the levels of DNA-PKcs and BMI1 in 639V (A), 647V (B), or HT-1197 bladder cancer cells (C).
(D) Colony formation assay shows that Knockdown of RNF144A in HT-1197 cells promotes cell survival after treatment with 1 μM cisplatin (Cis) for 14 days. Veh: vehicle control. P values were calculated from a two-tailed t test (N=3).
(E) Immunohistochemical analysis shows increased DNA-PKcs expression in the urothelium of untreated and BBN-treated Rnf144a KO mice. The boxed images in the top right corner indicate the magnified images of DNA-PKcs staining. Data shown are representative images (N=6 per group).
(F) Immunohistochemical staining shows increased BMI1 expression in the premalignant lesions of BBN-treated Rnf144a KO mice. The red arrows show spotty BMI1-weak positive cells in the untreated urothelium. Data shown are representative images (N=6 per group).
(G) Immunohistochemical staining shows increased BMI1 expression in invasive bladder tumors of BBN-treated Rnf144a KO mice. The right panels show magnified images of the boxed area in the left panels. Data shown are representative images (N=6 per group). Black scale bars represent 20 μm. Red scale bars represent 100 μm.
RNF144A deficiency increases DNA-PKcs expression and further promotes BBN-induced up-regulation of DNA-PKcs and BMI1.
To further validate these cellular results and characterize BBN-induced bladder tumors in Rnf144a KO mice, we next performed immunohistochemical staining of DNA-PKcs. DNA-PKcs could be detected in the adult urothelium of Rnf144a WT and KO mice; however, RNF144A deficiency increased its levels (Fig. 4E, left panel). BBN treatment increased DNA-PKcs expression in the premalignant lesions, and to a lesser degree, in the malignant lesions, which could be further enhanced by knockout of Rnf144a (Fig. 4E, middle and right panels), confirming that DNA-PKcs is indeed a target of RNF144A in vivo.
The BMI1-dependent oncogenic pathways are upregulated in tumor-initiating cells, as found in clinical bladder cancer specimens [21], and the expression of BMI1 may function as a prognostic marker in bladder cancer [19]. Therefore, to confirm the effect of RNF144A in the regulation of BMI1 expression in vivo, we next examined the expression of BMI1 in BBN-induced bladder tumors in WT and Rnf144a KO mice (Fig. 4F-G). Similar to the previous report regarding BMI1 expression in non-cancerous human bladder tissues [19], we found that BMI1 protein was weakly present in a few adult urothelial cells in both WT and Rnf144a KO mice (Fig. 4F, red arrows). We also observed that BBN treatment increased BMI1 expression and the number of BMI1-positive cells (Fig. 4F, right panels). Furthermore, in BBN-induced invasive bladder tumors, Rnf144a KO mice showed higher levels of BMI1 than WT mice (Fig. 4G). Thus, the BMI1-dependent activation of oncogenic pathways may in part contribute to the effect of RNF144A deficiency on promoting BBN-induced bladder tumorigenesis.
Bladder tumors in Rnf144a KO mice express additional features of luminal differentiation.
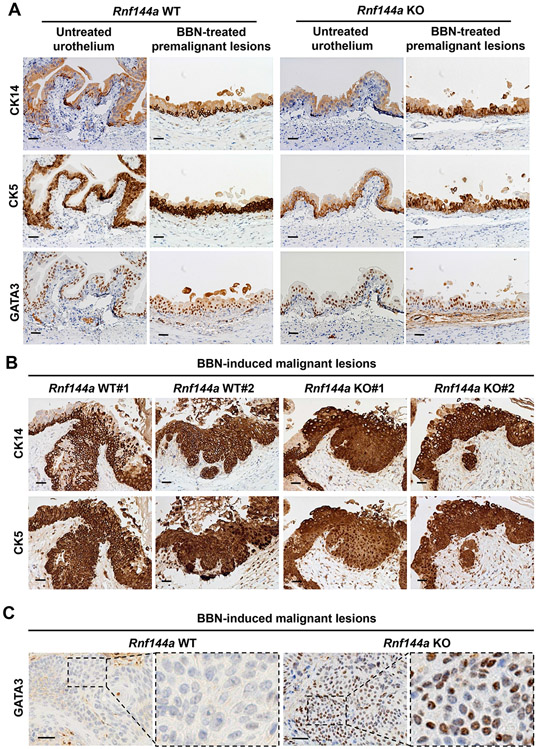
Based on their molecular signatures, muscle-invasive bladder cancers can be classified into two major molecular subtypes, basal and luminal [48, 49]. The basal subtype often manifests squamous differentiation [48] and is also referred as basal-squamous subtype [23]. BBN-induced tumors often show high expression of basal subtype markers [42, 50], such as CK5 and CK14. Indeed, consistent with the previous report [51], the population of CK5+ and CK14+ basal cells, but not GATA3+ luminal cells, was increased in BBN-treated uroepithelium of both WT and Rnf144a KO mice (Fig. 5A). The tumors developed in both Rnf144a WT and KO mice express CK14 and CK5 (Fig. 5B), indicating that they are a basal subtype of bladder tumors. Tumors developed in Rnf144a WT mice were mainly GATA3-negative; intriguingly, tumors developed in Rnf144a KO mice showed a mixed distribution of GATA3-positive and -low/negative cells (Fig. 5C). Those GATA3-mixed lesions in KO tumors also express CK14 (Fig. S2), indicating they are still basal subtype tumors, but also express a luminal marker GATA3. BBN induced a papillary hyperplasia tumor in Rnf144a KO, but not in WT mice (Fig. 2H). These data suggest features of luminal differentiation in Rnf144a KO BBN-induced tumors.
Figure 5. In WT mice, BBN-induced bladder tumors show the molecular markers of basal subtype, whereas those in Rnf144a KO mice show the molecular markers of both basal and luminal subtypes.
(A) Immunohistochemical analysis of the molecular markers of basal (CK5 and CK14) and luminal (GATA3) bladder subtypes in the adult urothelium and BBN-induced premalignant lesions of Rnf144a WT and KO mice (N=6 per group). Shown are representative images at 20X magnification.
(B) Immunohistochemical analysis reveals strong CK5+/CK14+ expression in the malignant lesions of both Rnf144a WT and KO mice. Shown are representative images at 20X magnification (N=6 per group).
(C) Immunohistochemical analysis shows GATA3 expression in the malignant lesions of Rnf144a KO mice, but not those of WT mice. Shown are representative images at 20X magnification (N=6 per group). Panels 2 and 4 represent higher magnification of the boxed area. Black scale bars represent 20 μm.
EGFR expression is decreased in BBN-induced bladder tumors in Rnf144a KO mice.
Our prior study showed that RNF144A can bind to EGFR and maintain EGFR protein expression during EGFR ligand stimulation [10]. Knockdown of RNF144A also reduced the expression of EGFR in human bladder cell lines (Fig. 4A-C). Thus, we next examined EGFR expression in BBN-induced bladder tumors. Consistently, the immunoblotting showed that RNF144A deficiency resulted in decreased expression of EGFR in the bladder tissues (Fig. 6A), and the immunohistochemical study also showed lower expression of EGFR in the urothelium of Rnf144a KO mice (Fig. 6B, leftmost panels). Although BBN treatment increased EGFR expression in the urothelium of both WT and Rnf144a KO mice, BBN-induced premalignant lesions of Rnf144a KO mice showed low or loss of expression of EGFR in the basal layer between the intermediate layer and lamina propria (Fig. 6B, middle panel). Furthermore, the malignant lesions of Rnf144a KO tumors showed a dramatic decrease of EGFR expression (Fig. 6C, bottom panels) compared to Rnf144a WT tumors that heavily expressed EGFR (Fig. 6C, upper panels). Meanwhile, EGFR could be detected in the normal urothelium of Rnf144a KO mice (Fig. 6D, arrows), whereas the adjacent malignant lesions barely expressed EGFR (Fig. 6D, left panel, boxed area), which may suggest a gradual loss of EGFR during tumor progression in the Rnf144a KO mice. To further confirm that the cells with low/loss of EGFR are basal cells, we then performed immunohistochemical staining of p63, a marker for proliferating basal cells of the urothelium [52]. Indeed, those cells with low/loss expression of EGFR in the premalignant lesions expressed p63 (Fig. 6E, 1st and 2nd panels). These cells were also CK14-positive (Fig. 6E, 3rd panel), further confirming that they are basal cells. Likewise, the malignant lesions of Rnf144a KO tumors also showed strong p63 expression (Fig. 6E, rightmost panels). Since both premalignant and malignant lesions of Rnf144a KO mice showed low/loss of EGFR expression, these results suggest that the malignant lesions may be derived from clonal expansion of the basal layer of premalignant lesions during BBN treatment.
Figure 6. Rnf144a KO mice show lower EGFR expression in normal urothelium and BBN-induced bladder tumors compared to WT mice.
(A) Western blot analysis demonstrates lower EGFR protein expression in the bladder tissue of Rnf144a KO mice compared to WT mice. #1 and #2 represent different mice of each genotype.
(B) In the BBN-induced premalignant lesions of Rnf144a KO mice, loss of EGFR is found in the basal layer between EGFR-positive intermediate cells and the lamina propria. Immunohistochemical staining was performed to detect EGFR expression in the urothelium of untreated and BBN-treated WT or Rnf144a KO mice. Panel 4 shows higher magnification of the boxed area in panel 3. Data shown are representative images (N=6 per group).
(C) Immunohistochemical staining shows the loss of EGFR in malignant lesions of BBN-treated Rnf144a KO mice. Shown are two representative images of Rnf144a WT or KO mice (N=6 per group).
(D) Immunohistochemical staining of EGFR in Rnf144a KO tumors shows that the loss of EGFR is only found in the cancerous area but not in adjacent normal tissues. Black arrows indicate normal urothelial cells with EGFR expression. The left panel shows 5X magnification, and the right panel shows 20X magnification of the boxed area in the left panel. Data shown are representative images (N=6).
(E) Immunohistochemical staining of EGFR, p63, or CK14 in the urothelium of BBN-treated Rnf144a KO mice (N=6). Data shown are representative images. Black scale bars represent 20 μm. Red scale bars represent 100 μm.
RNF144A mediates ubiquitination and degradation of PD-L1.
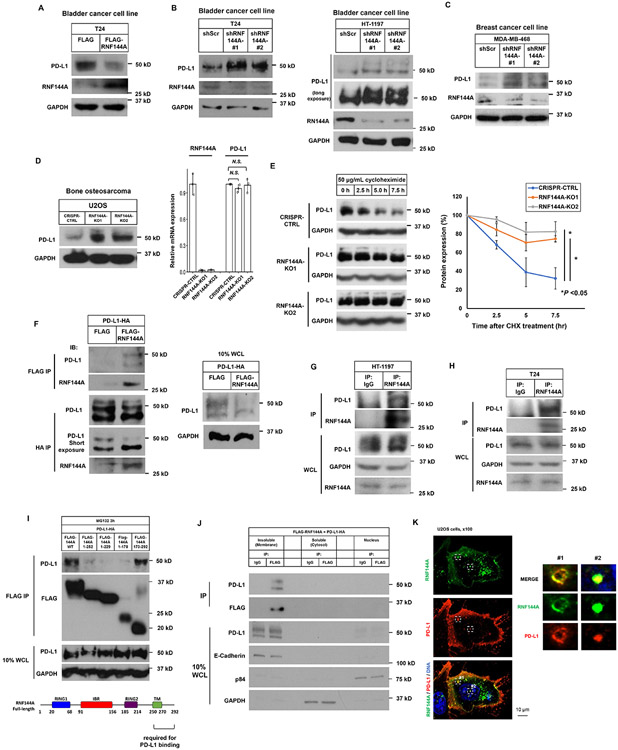
Urothelial carcinoma is a highly immunogenic cancer. The increased PD-L1 levels have been shown to associate with high grade/stage of bladder tumors and poor prognosis. As such, we next examined PD-L1 protein expression in BBN-induced bladder tumors. Although several E3 ligases (e.g. STUB1 [53] and βTrCP [37]) have been reported to regulate PD-L1 protein stability, these E3 ligases are not membrane proteins. Therefore, it is unclear whether these E3 ligases directly target membrane PD-L1 for degradation. Thus, identification of the E3 ligase for functional membrane PD-L1 can provide a better understanding of PD-L1 regulation. Among the 49 RING finger-containing candidate membrane E3 ligases with at least one TM domain, the mRNA expression of RNF144A shows the highest correlation with that of PD-L1 in 29 subsets of immune cells [36] (r=0.77) (Supplementary Fig. 3). In fact, it is the top 29th gene correlated with PD-L1 expression among more than 17,000 genes in that database. This suggests their functional relationship. Therefore, we examined the effect of RNF144A on PD-L1 expression in human bladder cancer cells. Ectopic expression of RNF144A decreased PD-L1 levels in human bladder cancer T24 cells (Fig. 7A); in contrast, RNF144A knockdown elevated its levels in various cancer cell lines, including T24, HT-1197 (Fig. 7B), and MDA-MB-468 (Fig. 7C). Similarly, knockout of RNF144A [10] also increased the levels of PD-L1 protein in U2OS cells without affecting PD-L1 mRNA levels (Fig. 7D), suggesting post-transcriptional regulation of PD-L1 by RNF144A. Indeed, knockout of RNF144A stabilized PD-L1 protein (Fig. 7E). Furthermore, co-immunoprecipitation experiments confirmed the interaction between ectopically expressed FLAG-tagged RNF144A and HA-tagged PD-L1 in Lenti-X 293T cells (Fig. 7F). DNA damage can induce the mRNA expression of both RNF144A and PD-L1 [13, 28]. Their interaction could also be detected after treatment with DNA-damaging agent neocarzinostatin (NCS) (Supplementary Fig. 4). Moreover, under physiological conditions, we detected the interaction between RNF144A and PD-L1 at the endogenous levels in HT-1197 and T24 cells (Fig. 7G-H). Using a series of truncated RNF144A proteins, we further determined that the carboxyl-terminal region (a.a. 250-292) of RNF144A is responsible for its interaction with PD-L1 (Fig. 7I). RNF144A interacted with PD-L1 mainly in the insoluble fraction, which contains the plasma membrane and intracellular vesicles (Fig. 7J). Immunofluorescence studies confirmed that RNF144A colocalized with PD-L1 in the intracellular vesicles or on the plasma membrane (Fig. 7K). The time-lapse imaging further showed that RNF144A partially formed a complex with PD-L1 in the intracellular vesicles, and they shuttled together between the plasma membrane and the cytoplasm (Supplementary movies S1 & S2).
Figure 7. RNF144A interacts with PD-L1 in the plasma membrane and intracellular vesicles and decreases PD-L1 expression.
(A) Overexpression of RNF144A decreases the level of PD-L1 protein in T24 cells. T24 cells transiently transfected with a FLAG empty vector or FLAG-RNF144A expression vector were harvested, and the whole cell lysates were subjected to Western blot analysis to detect the indicated proteins.
(B-D) Knockdown or knockout of RNF144A increases the levels of PD-L1 in T24, HT-1197 (B) or MDA-MB-468 (C) cells stably expressing a scrambled shRNA or an RNF144A shRNA (#1 or #2) [13], or U2OS cells in which RNF144A was knocked out by an RNF144A sgRNA (KO1 or KO2) (D). Cells were harvested and the levels of PD-L1, RNF144A or GAPDH were detected by Western blot analysis. The mRNA levels of RNF144A and PD-L1 in U2OS cells were measured by qRT-PCR (right panel).
(E) Western blot analysis of endogenous PD-L1 to determine its half-life in RNF144A WT or KO cells. Cells were treated with 50 μg/ml cycloheximide (CHX) for the indicated time intervals, and the total cell lysates were subjected to Western blot analysis to determine the protein levels of PD-L1 or GAPDH. The intensity of PD-L1 or GAPDH was quantified using Image J software, and the relative level of PD-L1 was normalized to GAPDH (right panel).
(F) Co-immunoprecipitation experiment shows that transfected FLAG-RNF144A interacts with PD-L1-HA in Lenti-X 293T cells. The right panel shows 10% input of whole cell lysates (WCL) used in the co-immunoprecipitation.
(G-H) Endogenous RNF144A immunoprecipitates with endogenous PD-L1 in HT-1197 (G) or T24 (H) cells.
(I) Deletion analysis shows that the interaction of RNF144A with PD-L1 is mainly mediated by the carboxyl-terminal region (a.a. 250-292) of RNF144A. Truncated FLAG-RNF144A as indicated was transfected with PD-L1-HA in Lenti-X 293T cells. After 10 μM MG-132 treatment for 3 h to prevent protein degradation, co-immunoprecipitation was performed.
(J) Subcellular fractionation analysis shows that RNF144A interacts with PD-L1 mainly in the insoluble fraction, which contains the plasma membrane and intracellular vesicles. Lenti-X 293T cells were co-transfected with FLAG-RNF144A and PD-L1-HA. Cells were harvested in a hypotonic buffer on ice for 15 min, and subcellular fractionation was performed by differential centrifugation to enrich the nuclei, membrane-enriched insoluble fraction or cytosol as described in Materials and Methods.
(K) Immunofluorescence staining shows that RNF144A colocalizes with PD-L1 in the intracellular vesicles and plasma membrane. FLAG-RNF144A and PD-L1-HA were cotransfected to U2OS cells for 24 h. After fixation, immunostaining was performed using anti-FLAG mouse monoclonal antibody and Alexa Fluor 488-conjugated mouse secondary antibody to detect FALG-RNF144A. Subsequently, anti-HA rabbit antibody and Texas red-X-conjugated rabbit secondary antibody were used to stain PD-L1-HA. The nuclei were stained with Hoechst 33258. Right panels are magnified images of boxes #1 and #2.
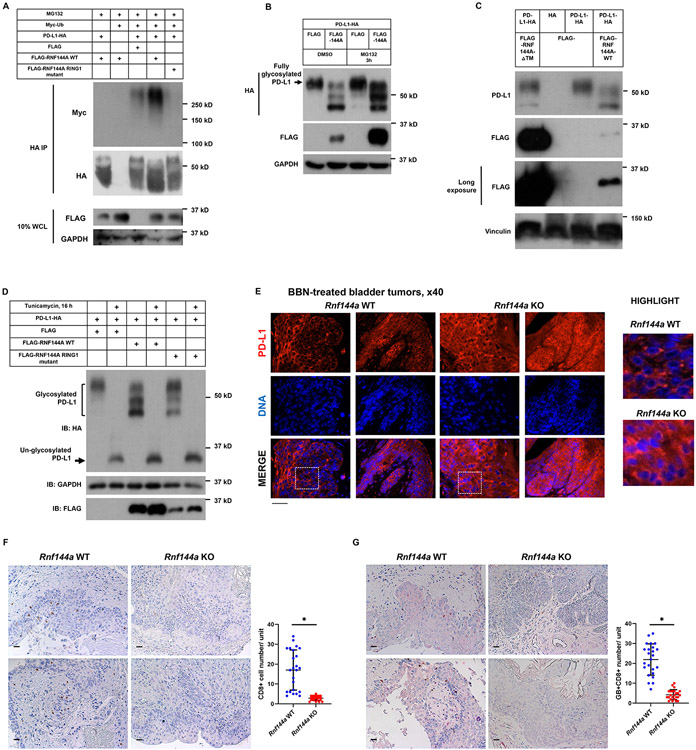
Given that RNF144A is an E3 ubiquitin ligase and the depletion of RNF144A can stabilize PD-L1, we then determined whether RNF144A could mediate PD-L1 ubiquitination. Our data showed that WT RNF144A, but not its ligase-dead mutant, promoted poly-ubiquitination of PD-L1 (Fig. 8A). Moreover, ectopic expression of RNF144A decreased the levels of fully glycosylated PD-L1, which could be rescued by pretreatment with proteasome inhibitor MG-132 (Fig. 8B). The carboxyl terminus of RNF144A, including the transmembrane (TM) domain, is required for PD-L1 binding (Fig. 7H). Thus, we examined whether the TM domain is also required for RNF144A to promote PD-L1 degradation. Indeed, unlike WT RNF144A, RNF144A-△TM (lacking TM domain) failed to promote PD-L1 degradation even though it was expressed at a much higher level than WT RNF144A (Fig. 8C). Together, these data demonstrate that RNF144A binds PD-L1 and promotes its poly-ubiquitination and proteasomal degradation. It should be noted that PD-L1-HA was expressed primarily as a 55 kDa, presumably fully glycosylated, product in Lenti-X 293T cells. However, when co-expressed with RNF144A, two products of PD-L1 with lower molecular weight were also found, in particular the 45 kDa product (Fig. 8B-D). This effect was greatly attenuated when co-expressed with the ligase-dead mutant of RNF144A. To confirm that these PD-L1-HA products are partially glycosylated products, we then treated cells with tunicamycin to inhibit protein glycosylation. Indeed, PD-L1-HA was expressed solely as a 33 kDa un-glycosylated product without or with co-expression of RNF144A in tunicamycin-treated Lenti-X 293T cells (Fig. 8D). Tunicamycin treatment also prevented the effect of RNF144A. These results suggest that RNF144A mainly regulates the degradation of fully glycosylated PD-L1. It also raises a possibility that RNF144A might interfere with full glycosylation or promote de-glycosylation of PD-L1 as well.
Figure 8. RNF144A promotes poly-ubiquitination and degradation of PD-L1.
(A) Overexpression of WT, but not ligase-dead (C20A/C23A), RNF144A promotes ubiquitination of PD-L1. Myc-Ub and PD-L1-HA were co-transfected with either FLAG empty vector or FLAG-RNF144A into Lenti-X 293T cells for 24 h. Cells were treated with MG-132 for 3 h and then were harvested in RIPA buffer. PD-L1-HA was immunoprecipitated with the anti-HA antibody-conjugated agarose bead and was resolved by SDS-PAGE. Western blotting was performed using indicated antibodies.
(B) Overexpression of RNF144A results in the degradation of glycosylated PD-L1-HA, which can be rescued by pretreatment with a proteasome inhibitor MG-132. Lenti-X 293T cells transiently expressing PD-L1-HA and either a FLAG empty vector or FLAG-RNF144A were pretreated with MG-132 for 3 h, followed by Western blot analysis to detect the indicated proteins.
(C) Western blot analysis shows that unlike WT, overexpression of RNF144A-△TM does not promote degradation of glycosylated PD-L1 in Lenti-X 293T cells.
(D) Overexpression of WT RNF144A, but not the ligase-dead mutant of RNF144A, promotes degradation of glycosylated PD-L1 in Lenti-X 293T cells; however, pretreatment with tunicamycin (5 μg/ml) overnight inhibits PD-L1 glycosylation and prevents RNF144A-mediated PD-L1 degradation.
(E) Immunofluorescence staining shows increased PD-L1 levels in BBN-induced bladder tumors of Rnf144a KO mice. Data from two representative Rnf144a WT or KO mice are shown (N=6 per group). The highlighted bottom panels are magnified images of the boxed area. Black scale bars represent 20 μm.
(F-G) Immunohistochemistry of CD8 (brown) alone (F) or CD8 (brown) with granzyme B (red) (G) in the BBN-treated bladder samples of Rnf144a WT or KO mice. Black scale bar, 20 μm. Data represent means ± s.d. (N=24; 4 tissue slides per mouse, 6 mice per group). *P<0.001. Unit = 132,088 μm2. Black scale bars represent 20 μm.
Importantly, we observed a substantial increase of PD-L1 levels in the BBN-induced bladder tumors in Rnf144a KO mice compared to those in WT mice (Fig. 8E). In contrast, the tumor-infiltrating CD8+ T cell populations were significantly decreased in Rnf144a KO mice compared to those in WT mice (Fig. 8F-G). PD-L1 is a critical immune checkpoint molecule exploited by tumor cells to escape immune surveillance. These data suggest that the upregulation of PD-L1 protein expression in RNF144A-deficient bladder cells may inhibit T cell activation and thus contribute to tumorigenesis in the BBN-induced bladder model.
Discussion
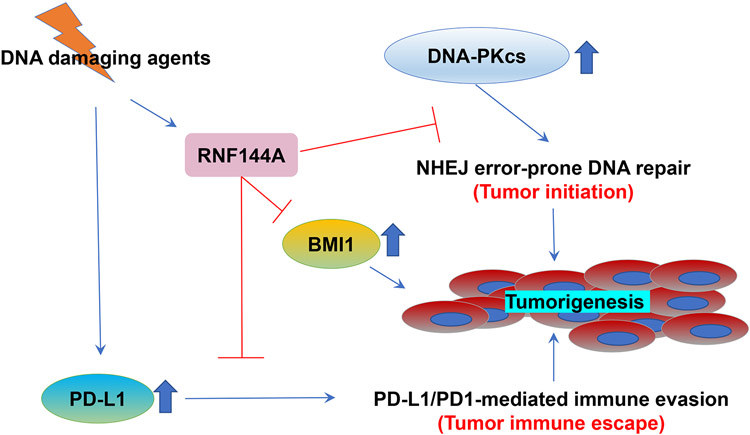
Growing evidence suggests that RNF144A may act as a tumor suppressor in vitro. Although the epigenetic silencing of RNF144A has been shown in a few human cancers, including breast cancer [11] and glioblastoma (GBM) [12], a role for RNF144A in preventing tumorigenesis has not been demonstrated in vivo. The current study provides the first evidence that the deficiency of RNF144A promotes carcinogen-induced tumorigenesis (Fig. 2). Although RNF144A-deficient mice did not develop spontaneous tumors, they developed more invasive bladder cancer after chronic treatment of BBN. Similar to the major carcinogen associated with tobacco smoke for human bladder cancer, the metabolites of BBN cause alkylating DNA damage in the bladder epithelium [54, 55]. RNF144A is induced by p53 upon treatment with various DNA damaging agents and promotes apoptosis in cultured cells [13]. The current study suggests that RNF144A may contribute to tumor suppression through at least three distinct pathways (Fig. 9). Specifically, it can promote apoptosis of tumor-initiating cells at an early stage, inhibit tumor cell proliferation at the later stage, and decrease tumor immune escape.
Figure 9. A proposed model for the role of RNF144A in tumorigenesis after DNA damage insults.
DNA damage induces and stabilizes RNF144A, which can target DNA-PKcs, BMI1, and PD-L1 for ubiquitination and degradation, and therefore prevents DNA-PKcs- and BMI1-dependent tumor initiation as well as PD-L1/PD1-mediated inhibition of T cell response and immune evasion.
Previously our group and others have identified more than 150 RNF144A-interacting proteins [10, 12, 13, 15]. To confirm the physiological role of RNF144A in the regulation of these targets, we examined the effect of RNF144A deficiency on the expression of DNA-PKcs and BMI1, which are known to be targeted by RNF144A for proteasomal degradation. DNA-PKcs is a significant component of error-prone NHEJ DNA repair, which promotes genome instability and cell survival under DNA damage condition. BMI1 proto-oncoprotein is a major component of polycomb repressive complexes, silencing many important tumor suppressors and leading to tumorigenesis in many human cancers, including bladder cancer. Indeed, the deficiency of RNF144A increased the levels of DNA-PKcs and BMI1 (Fig. 4), which provides a mechanism for BBN-induced bladder tumorigenesis in Rnf144a KO mice. The enhanced expression of DNA-PKcs and BMI1 may also be responsible for the reduction of apoptosis and the increase of proliferation index observed in Rnf144a KO tumors (Fig. 3). Additionally, we identify the glycosylated PD-L1 as a novel target of RNF144A (Fig. 7 and 8). RNF144A deficiency increases PD-L1 protein levels and leads to the suppression of CD8+ tumor-infiltrating lymphocyte (TIL) activation (Fig. 8E-G) and subsequent cytotoxic T-lymphocyte (CTL)-mediated cancer cell death. Therefore, the RNF144A-PD-L1 regulatory mechanism may link RNF144A with immune surveillance and anti-tumor immunity. Interestingly, the expression of RNF144A mRNA is relatively high in the T cell population, but is relatively low in the B cell population and other immune cells (Supplementary Fig. 3) [36]. Future research on whether RNF144A is involved in the development and function of immune cells will also bring significant scientific and clinical benefit. In contrast to GSK3β-βTrCP that specifically targets non-glycosylated PD-L1 for degradation [37], RNF144A mainly targets glycosylated PD-L1 for degradation. The upregulation of PD-L1 in RNF144A-deficient tumors may provide an opportunity for immune checkpoint blockade therapy to treat these cancers. It has been reported that glioma stem cells with RNF144A down-regulation are highly sensitive to BMI1 inhibitor [12]. Thus, this study may provide a potential clinical utility for BMI1 inhibitor in treating bladder cancer that expresses low levels of RNF144A but high levels of BMI1.
Both knockout-first and exons-deletion RNF144A-deficient mice were fertile and had a normal life span, indicating that RNF144A is dispensable for embryonic development. Previously, our cellular studies showed that RNF144A can maintain EGFR protein stability during EGFR signaling [10]. Consistently, Rnf144a KO mice also showed reduced EGFR expression in the bladder (Fig. 6A). Although Egfr KO mice have defects in epithelial development in skin, lung, gastrointestinal tract, and other organs [56], we did not observe similar phenotypes in Rnf144a KO mice. It may be because the reduced expression of EGFR is not severe enough to affect gross development. More studies are needed to address this question in the future.
It is interesting to note that although EGFR expression is modestly reduced in the untreated bladder tissues of Rnf144a KO mice (Fig. 6A and the leftmost panels of 6B), a profound loss of EGFR was observed in the basal layer of premalignant lesions (Fig. 6B, panels 2 and 3) and in the malignant lesions (Fig. 6D-E) after BBN treatment. Given the role of RNF144A in sustaining EGFR stability [10], these results imply that RNF144A plays a more central role in maintaining EGFR expression in cancer cells during BBN treatment. Bladder cancers express variable levels of EGFR, ranging from overexpression to an undetectable level. EGFR protein cannot be detected in about one quarter of bladder cancer (ranging from 24% to 27.8% [57-59]). Loss of EGFR expression has been associated with poor differentiation and invasiveness in the bladder and oral squamous cell carcinomas [60, 61]. Absent or low EGFR expression is quite common in bladder cancer, particularly in invasive tumors. In one study examining EGFR expression by immunohistochemistry, absent EGFR was found in 5/19 (26.3%) non-metastatic bladder tumors, but in 29/51 (57%) primary invasive bladder tumors with metastasis and in 31/51 (60.8%) metastatic tumors, demonstrating the prevalence of loss of EGFR in aggressive bladder cancer [62]. Our data also suggest that EGFR is not a key driver for BBN carcinogen-induced tumorigenesis. In fact, many patients with muscle-invasive bladder cancers are resistant to EGFR inhibitor therapy [63]. Furthermore, EGFR signaling protects some tissues, such as the intestine and liver, against apoptosis when suffering an injury [64, 65]. More intriguingly, mice lacking EGFR in hepatocytes developed more hepatocellular carcinoma due to increasing hepatocyte damage and compensatory proliferation [66]. These data suggest a paradoxical role for EGFR in preventing tumorigenesis in some contexts.
BBN is known to cause the development of keratinizing squamous metaplasia in the rodent bladder [43]. Metaplasia usually is an adaptive response to chronic irritation, such as BBN challenges, and the lesions are sometimes regarded as precancerous [67, 68]. Rnf144a KO mice develop a much higher frequency of keratinizing squamous metaplasia after chronic treatment of BBN (Fig. 2G-H). Thus, RNF144A plays a role in preventing metaplasia upon chronic irritation by BBN. BBN-induced bladder tumors are mainly basal-like subtype [42, 50], but their molecular subtypes and progression can be influenced by the genetic background of mice [69]. In WT mice, BBN-induced bladder tumors only express markers for basal subtype (CK5 and CK14), whereas tumors developed in Rnf144a KO also express a marker for luminal subtype (GATA3) in addition to CK5 and CK14 (Fig. 5C and Fig. S2). It is possible that the low-EGFR condition in Rnf144a KO cells may be conducive to the expression of a luminal marker GATA3, given an inhibitory effect of EGFR on urothelial differentiation [70]. It has been reported that BBN can induce an early expansion of CK14+ urothelial stem cells in premalignant lesions [33]. Thus, the EGFR-/p63+/CK5+/CK14+/GATA3+/− cells in the malignant lesions of Rnf144a KO mice are likely expanded from the EGFR-/p63+/CK5+/CK14+ cells present in the basal layer of premalignant lesions. Our data are consistent with a prior lineage tracing study showing that CK5+ basal cells are progenitors of carcinoma-in-situ, basal-squamous subtype, and squamous cell carcinoma of the bladder [71].
E3 ligase participates in human cancer development by regulating the degradation of tumor promoters or suppressors. RNF144A is an attractive RBR E3 ligase due to its potential tumor suppressor function and epigenetic silencing in certain human cancers. Our study demonstrates that the deficiency of RNF144A promotes carcinogen-induced tumorigenesis, supporting the tumor suppressor role for RNF144A in vivo. The Rnf144a KO mice provides a very useful model for the investigation of the physiological role of RNF144A.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants R01CA100857 (to W.-C.L.) and R01CA203824 and Department of Defense Grants W81XWH-18-1-0329 and W81XWH-19-1-0369 (to W.-C.L and F.-T.L.). S.-R.H. was supported by T32 Fellowship (T32CA174647). We want to thank the members of the Lin Laboratories for the discussion, and BCM Genetically Engineered Mouse Core for the assistance of Rnf144a knockout-first mouse, Pathology & Histology Core and Breast center for IHC and other services. We also want to thank Jocelyn Jea and BCM Mouse Metabolic and Phenotyping Core for their help in sonography service.
Abbreviations:
- RING
Really Interesting New Gene
- RBR
RING-Between-RING
- RNF
Ring finger proteins
- BBN
N-butyl-N-(4-hydroxybutyl)nitrosamine
Footnotes
Supplementary Information
This article contains four supplementary figures and 6 supplementary movies (S1A-C and S2A-C).
Disclosure of Potential Conflicts of Interest
All authors disclose that they have no financial interests that will pose a conflict of interest regarding the submitted article.
References
- [1].Marin I, RBR ubiquitin ligases: Diversification and streamlining in animal lineages, J Mol Evol, 69 (2009) 54–64. [DOI] [PubMed] [Google Scholar]
- [2].Spratt DE, Walden H, Shaw GS, RBR E3 ubiquitin ligases: new structures, new insights, new questions, Biochem J, 458 (2014) 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smit JJ, Sixma TK, RBR E3-ligases at work, EMBO Rep, 15 (2014) 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wenzel DM, Lissounov A, Brzovic PS, Klevit RE, UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids, Nature, 474 (2011) 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang D, Ma L, Wang B, Liu J, Wei W, E3 ubiquitin ligases in cancer and implications for therapies, Cancer Metastasis Rev, 36 (2017) 683–702. [DOI] [PubMed] [Google Scholar]
- [6].Fujiwara M, Marusawa H, Wang HQ, Iwai A, Ikeuchi K, Imai Y, Kataoka A, Nukina N, Takahashi R, Chiba T, Parkin as a tumor suppressor gene for hepatocellular carcinoma, Oncogene, 27 (2008) 6002–6011. [DOI] [PubMed] [Google Scholar]
- [7].Quinsay MN, Lee Y, Rikka S, Sayen MR, Molkentin JD, Gottlieb RA, Gustafsson AB, Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism, J Mol Cell Cardiol, 48 (2010) 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, Carter SL, Cibulskis K, Hanna M, Kiezun A, Kim J, Lawrence MS, Lichenstein L, McKenna A, Pedamallu CS, Ramos AH, Shefler E, Sivachenko A, Sougnez C, Stewart C, Ally A, Birol I, Chiu R, Corbett RD, Hirst M, Jackman SD, Kamoh B, Khodabakshi AH, Krzywinski M, Lo A, Moore RA, Mungall KL, Qian J, Tam A, Thiessen N, Zhao Y, Cole KA, Diamond M, Diskin SJ, Mosse YP, Wood AC, Ji L, Sposto R, Badgett T, London WB, Moyer Y, Gastier-Foster JM, Smith MA, Guidry Auvil JM, Gerhard DS, Hogarty MD, Jones SJ, Lander ES, Gabriel SB, Getz G, Seeger RC, Khan J, Marra MA, Meyerson M, Maris JM, The genetic landscape of high-risk neuroblastoma, Nat Genet, 45 (2013) 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ho SR, Lee YJ, Lin WC, Regulation of RNF144A E3 Ubiquitin Ligase Activity by Self-association through Its Transmembrane Domain, J Biol Chem, 290 (2015) 23026–23038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ho SR, Lin WC, RNF144A sustains EGFR signaling to promote EGF-dependent cell proliferation, J Biol Chem, 293 (2018) 16307–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang Y, Yang YL, Zhang FL, Liao XH, Shao ZM, Li DQ, Epigenetic silencing of RNF144A expression in breast cancer cells through promoter hypermethylation and MBD4, Cancer Med, 7 (2018) 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jin X, Kim LJY, Wu Q, Wallace LC, Prager BC, Sanvoranart T, Gimple RC, Wang X, Mack SC, Miller TE, Huang P, Valentim CL, Zhou QG, Barnholtz-Sloan JS, Bao S, Sloan AE, Rich JN, Targeting glioma stem cells through combined BMI1 and EZH2 inhibition, Nat Med, 23 (2017) 1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ho SR, Mahanic CS, Lee YJ, Lin WC, RNF144A, an E3 ubiquitin ligase for DNA-PKcs, promotes apoptosis during DNA damage, Proc Natl Acad Sci U S A, 111 (2014) E2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang J, Xu LG, Liu T, Zhai Z, Shu HB, The p53-inducible E3 ubiquitin ligase p53RFP induces p53-dependent apoptosis, FEBS Lett, 580 (2006) 940–947. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Liao XH, Xie HY, Shao ZM, Li DQ, RBR-type E3 ubiquitin ligase RNF144A targets PARP1 for ubiquitin-dependent degradation and regulates PARP inhibitor sensitivity in breast cancer cells, Oncotarget, 8 (2017) 94505–94518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu YH, Hong CW, Wang YC, Huang WJ, Yeh YL, Wang BJ, Wang YJ, Chiu HW, A novel histone deacetylase inhibitor TMU-35435 enhances etoposide cytotoxicity through the proteasomal degradation of DNA-PKcs in triple-negative breast cancer, Cancer Lett, 400 (2017) 79–88. [DOI] [PubMed] [Google Scholar]
- [17].Ginjala V, Nacerddine K, Kulkarni A, Oza J, Hill SJ, Yao M, Citterio E, van Lohuizen M, Ganesan S, BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair, Mol Cell Biol, 31 (2011) 1972–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang MC, Li CL, Cui J, Jiao M, Wu T, Jing LI, Nan KJ, BMI-1, a promising therapeutic target for human cancer, Oncol Lett, 10 (2015) 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qin ZK, Yang JA, Ye YL, Zhang X, Xu LH, Zhou FJ, Han H, Liu ZW, Song LB, Zeng MS, Expression of Bmi-1 is a prognostic marker in bladder cancer, BMC Cancer, 9 (2009) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liang W, Zhu D, Cui X, Su J, Liu H, Han J, Zhao F, Xie W, Knockdown BMI1 expression inhibits proliferation and invasion in human bladder cancer T24 cells, Mol Cell Biochem, 382 (2013) 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J Jr., Chang HY, van de Rijn M, Shortliffe L, Weissman IL, Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells, Proc Natl Acad Sci U S A, 106 (2009) 14016–14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li H, Zhang Q, Shuman L, Kaag M, Raman JD, Merrill S, DeGraff DJ, Warrick JI, Chen G, Evaluation of PD-L1 and other immune markers in bladder urothelial carcinoma stratified by histologic variants and molecular subtypes, Sci Rep, 10 (2020) 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, Castro MAA, Gibb EA, Kanchi RS, Gordenin DA, Shukla SA, Sanchez-Vega F, Hansel DE, Czerniak BA, Reuter VE, Su X, de Sa Carvalho B, Chagas VS, Mungall KL, Sadeghi S, Pedamallu CS, Lu Y, Klimczak LJ, Zhang J, Choo C, Ojesina AI, Bullman S, Leraas KM, Lichtenberg TM, Wu CJ, Schultz N, Getz G, Meyerson M, Mills GB, McConkey DJ, Network TR, Weinstein JN, Kwiatkowski DJ, Lerner SP, Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer, Cell, 171 (2017) 540–556 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Spranger S, Bao R, Gajewski TF, Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity, Nature, 523 (2015) 231–235. [DOI] [PubMed] [Google Scholar]
- [25].Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T, Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation, J Exp Med, 192 (2000) 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stenehjem DD, Tran D, Nkrumah MA, Gupta S, PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer, Onco Targets Ther, 11 (2018) 5973–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Eckstein M, Cimadamore A, Hartmann A, Lopez-Beltran A, Cheng L, Scarpelli M, Montironi R, Gevaert T, PD-L1 assessment in urothelial carcinoma: a practical approach, Ann Transl Med, 7 (2019) 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, Yoshimoto Y, Held KD, Suzuki Y, Kono K, Miyagawa K, Nakano T, Shibata A, DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells, Nat Commun, 8 (2017) 1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pettitt SJ, Liang Q, Rairdan XY, Moran JL, Prosser HM, Beier DR, Lloyd KC, Bradley A, Skarnes WC, Agouti C57BL/6N embryonic stem cells for mouse genetic resources, Nat Methods, 6 (2009) 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Farley FW, Soriano P, Steffen LS, Dymecki SM, Widespread recombinase expression using FLPeR (flipper) mice, Genesis, 28 (2000) 106–110. [PubMed] [Google Scholar]
- [31].Holzenberger M, Lenzner C, Leneuve P, Zaoui R, Hamard G, Vaulont S, Bouc YL, Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene, Nucleic Acids Res, 28 (2000) E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Doetschman T, Georgieva T, Li H, Reed TD, Grisham C, Friel J, Estabrook MA, Gard C, Sanford LP, Azhar M, Generation of mice with a conditional allele for the transforming growth factor beta3 gene, Genesis, 50 (2012) 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ho PL, Lay EJ, Jian W, Parra D, Chan KS, Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer, Cancer Res, 72 (2012) 3135–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N, Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal, Sci Signal, 6 (2013) pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N, The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data, Cancer Discov, 2 (2012) 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Monaco G, Lee B, Xu W, Mustafah S, Hwang YY, Carre C, Burdin N, Visan L, Ceccarelli M, Poidinger M, Zippelius A, Pedro de Magalhaes J, Larbi A, RNA-Seq Signatures Normalized by mRNA Abundance Allow Absolute Deconvolution of Human Immune Cell Types, Cell Rep, 26 (2019) 1627–1640 e1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Ding Q, Wang Y, Yao J, Lee CC, Wu HJ, Sahin AA, Allison JP, Yu D, Hortobagyi GN, Hung MC, Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity, Nat Commun, 7 (2016) 12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Longo PA, Kavran JM, Kim MS, Leahy DJ, Transient mammalian cell transfection with polyethylenimine (PEI), Methods Enzymol, 529 (2013) 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A, A conditional knockout resource for the genome-wide study of mouse gene function, Nature, 474 (2011) 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Testa G, Schaft J, van der Hoeven F, Glaser S, Anastassiadis K, Zhang Y, Hermann T, Stremmel W, Stewart AF, A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles, Genesis, 38 (2004) 151–158. [DOI] [PubMed] [Google Scholar]
- [41].Bryan GT, The pathogenesis of experimental bladder cancer, Cancer Res, 37 (1977) 2813–2816. [PubMed] [Google Scholar]
- [42].Fantini D, Glaser AP, Rimar KJ, Wang Y, Schipma M, Varghese N, Rademaker A, Behdad A, Yellapa A, Yu Y, Sze CC, Wang L, Zhao Z, Crawford SE, Hu D, Licht JD, Collings CK, Bartom E, Theodorescu D, Shilatifard A, Meeks JJ, A Carcinogen-induced mouse model recapitulates the molecular alterations of human muscle invasive bladder cancer, Oncogene, 37 (2018) 1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Herman CJ, Vegt PD, Debruyne FM, Vooijs GP, Ramaekers FC, Squamous and transitional elements in rat bladder carcinomas induced by N-butyl-N-4-hydroxybutyl-nitrosamine (BBN). A study of cytokeratin expression, Am J Pathol, 120 (1985) 419–426. [PMC free article] [PubMed] [Google Scholar]
- [44].Hsu JW, Hsu I, Xu D, Miyamoto H, Liang L, Wu XR, Shyr CR, Chang C, Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor, Am J Pathol, 182 (2013) 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Airoldi L, Magagnotti C, Bonfanti M, Chiappetta L, Lolli M, Medana C, De Gregorio G, Fanelli R, Detection of O6-butyl- and O6-(4-hydroxybutyl)guanine in urothelial and hepatic DNA of rats given the bladder carcinogen N-nitrosobutyl(4-hydroxybutyl)amine, Carcinogenesis, 15 (1994) 2297–2301. [DOI] [PubMed] [Google Scholar]
- [46].Chen X, Hu L, Yang H, Ma H, Ye K, Zhao C, Zhao Z, Dai H, Wang H, Fang Z, DHHC protein family targets different subsets of glioma stem cells in specific niches, J Exp Clin Cancer Res, 38 (2019) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, Wu KJ, Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition, Nat Cell Biol, 12 (2010) 982–992. [DOI] [PubMed] [Google Scholar]
- [48].Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, Melquist J, Bondaruk J, Majewski T, Zhang S, Pretzsch S, Baggerly K, Siefker-Radtke A, Czerniak B, Dinney CP, McConkey DJ, Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy, Cancer Cell, 25 (2014) 152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dadhania V, Zhang M, Zhang L, Bondaruk J, Majewski T, Siefker-Radtke A, Guo CC, Dinney C, Cogdell DE, Zhang S, Lee S, Lee JG, Weinstein JN, Baggerly K, McConkey D, Czerniak B, Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use, EBioMedicine, 12 (2016) 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Saito R, Smith CC, Utsumi T, Bixby LM, Kardos J, Wobker SE, Stewart KG, Chai S, Manocha U, Byrd KM, Damrauer JS, Williams SE, Vincent BG, Kim WY, Molecular Subtype-Specific Immunocompetent Models of High-Grade Urothelial Carcinoma Reveal Differential Neoantigen Expression and Response to Immunotherapy, Cancer Res, 78 (2018) 3954–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shin K, Lim A, Odegaard JI, Honeycutt JD, Kawano S, Hsieh MH, Beachy PA, Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma, Nat Cell Biol, 16 (2014) 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F, p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities, Mol Cell, 2 (1998) 305–316. [DOI] [PubMed] [Google Scholar]
- [53].Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, Lam EYN, Henderson MA, Bell CC, Stolzenburg S, Gilan O, Bloor S, Noori T, Morgens DW, Bassik MC, Neeson PJ, Behren A, Darcy PK, Dawson SJ, Voskoboinik I, Trapani JA, Cebon J, Lehner PJ, Dawson MA, CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity, Nature, 549 (2017) 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nagao M, Suzuki E, Yasuo K, Yahagi T, Seino Y, Mutagenicity of N-butyl-N-(4-hydroxybutyl)nitrosamine, a bladder carcinogen, and related compounds, Cancer Res, 37 (1977) 399–407. [PubMed] [Google Scholar]
- [55].Wada K, Yoshida T, Takahashi N, Matsumoto K, Effects of seven chemicals on DNA damage in the rat urinary bladder: a comet assay study, Mutat Res Genet Toxicol Environ Mutagen, 769 (2014) 1–6. [DOI] [PubMed] [Google Scholar]
- [56].Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R, Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor, Nature, 376 (1995) 337–341. [DOI] [PubMed] [Google Scholar]
- [57].Chaux A, Cohen JS, Schultz L, Albadine R, Jadallah S, Murphy KM, Sharma R, Schoenberg MP, Netto GJ, High epidermal growth factor receptor immunohistochemical expression in urothelial carcinoma of the bladder is not associated with EGFR mutations in exons 19 and 21: a study using formalin-fixed, paraffin-embedded archival tissues, Hum Pathol, 43 (2012) 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS, Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder, Clin Cancer Res, 7 (2001) 1957–1962. [PubMed] [Google Scholar]
- [59].Kramer C, Klasmeyer K, Bojar H, Schulz WA, Ackermann R, Grimm MO, Heparin-binding epidermal growth factor-like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome, Cancer, 109 (2007) 2016–2024. [DOI] [PubMed] [Google Scholar]
- [60].Tungekar MF, Linehan J, Patterns of expressions of transforming growth factor and epidermal growth factor receptor in squamous cell lesions of the urinary bladder, J Clin Pathol, 51 (1998) 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kimura I, Kitahara H, Ooi K, Kato K, Noguchi N, Yoshizawa K, Nakamura H, Kawashiri S, Loss of epidermal growth factor receptor expression in oral squamous cell carcinoma is associated with invasiveness and epithelial-mesenchymal transition, Oncol Lett, 11 (2016) 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rotterud R, Nesland JM, Berner A, Fossa SD, Expression of the epidermal growth factor receptor family in normal and malignant urothelium, BJU Int, 95 (2005) 1344–1350. [DOI] [PubMed] [Google Scholar]
- [63].Mooso BA, Vinall RL, Mudryj M, Yap SA, deVere White RW, Ghosh PM, The role of EGFR family inhibitors in muscle invasive bladder cancer: a review of clinical data and molecular evidence, J Urol, 193 (2015) 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Berasain C, Avila MA, The EGFR signalling system in the liver: from hepatoprotection to hepatocarcinogenesis, J Gastroenterol, 49 (2014) 9–23. [DOI] [PubMed] [Google Scholar]
- [65].Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW, Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage, Mol Cell Biol, 24 (2004) 8907–8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic M, Sieghart W, Trauner M, Wang H, Sibilia M, EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation, Nat Cell Biol, 16 (2014) 972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Akdas A, Turkeri L, The impact of squamous metaplasia in transitional cell carcinoma of the bladder, Int Urol Nephrol, 23 (1991) 333–336. [DOI] [PubMed] [Google Scholar]
- [68].Giroux V, Rustgi AK, Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence, Nat Rev Cancer, 17 (2017) 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kobayashi T, Owczarek TB, McKiernan JM, Abate-Shen C, Modelling bladder cancer in mice: opportunities and challenges, Nat Rev Cancer, 15 (2015) 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Varley CL, Stahlschmidt J, Lee WC, Holder J, Diggle C, Selby PJ, Trejdosiewicz LK, Southgate J, Role of PPARgamma and EGFR signalling in the urothelial terminal differentiation programme, J Cell Sci, 117 (2004) 2029–2036. [DOI] [PubMed] [Google Scholar]
- [71].Van Batavia J, Yamany T, Molotkov A, Dan H, Mansukhani M, Batourina E, Schneider K, Oyon D, Dunlop M, Wu XR, Cordon-Cardo C, Mendelsohn C, Bladder cancers arise from distinct urothelial sub-populations, Nat Cell Biol, 16 (2014) 982–991, 981-985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.