Abstract
Background
Impaired insight poses a challenge in the treatment of patients with schizophrenia because of its potential to jeopardize therapeutic engagement and medication adherence. This study explored how insight impairment, graded from none to extreme, is related to patient-reported mental health status, depression, and neurocognition in schizophrenia.
Methods
In a post hoc analysis of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study (NCT00014001), insight was measured using the Positive and Negative Syndrome Scale (PANSS) Item G12 (lack of insight). Additional assessments for this analysis included the 12-Item Short-Form Health Survey (SF-12) Mental Component Summary (MCS), physician- and patient-reported Clinical Global Impression–Severity (CGI-S), MATRICS Consensus Cognitive Battery, and Calgary Depression Scale for Schizophrenia. Relationships between patient-reported outcomes and PANSS total and Item G12 ratings were evaluated.
Results
Among 1431 CATIE study participants in this analysis, increasingly impaired insight at baseline was significantly associated with better patient-reported quality of life (QoL), lower baseline depression, and greater divergence between physician- and patient-reported illness severity. Patients with more severely impaired insight reported milder illness compared with physician reports, particularly those with moderate-severe to extreme impairment (PANSS Item G12 rating ≥ 5), approximately 10% (138/1431) of CATIE participants. For the 90% of patients with PANSS Item G12 ratings < 5, patient-reported QoL decreased with increasing symptoms. SF-12 MCS scores were linearly related to baseline PANSS total score only in patients with PANSS total score < 90 (moderately ill or better), and better symptom scores were associated with higher QoL. No significant relationship between insight and neurocognition was observed.
Conclusions
In the small subgroup (10%) of CATIE study patients with schizophrenia and PANSS Item G12 ratings ≥5, moderate-severe–severe/extreme insight impairment was associated with significantly more positive perception of QoL and illness severity by the patient versus the treating physician. This was not observed in the remaining 90% of patients with normal to moderately impaired insight, suggesting that poor insight as a threat to the validity of self-report is uncommon.
Keywords: Insight, PANSS, Patient-reported outcome, Neurocognition, Quality of life, Schizophrenia, SF-12, CATIE schizophrenia trial
Introduction
Between 50 and 80% of patients with schizophrenia are reported to have impaired insight into their illness [1–3]. In the context of a life-altering disease such as schizophrenia, retaining insight helps patients make sense of the meaning of life events. In contrast, poor insight could be understood as a failure to construct a coherent account of complex and potentially traumatic life experiences that can result in loss of functioning and hospitalization [4]. Insight is a multidimensional concept that includes awareness of illness, the capacity to re-label psychotic experiences as abnormal, and adherence to treatment, which vary along a continuum [5, 6]. Poor insight is a feature of schizophrenia across different cultures and across all stages of the illness, and it persists even after symptoms have remitted [2, 4]. From a treating clinician’s perspective, impaired insight is one of the most vexing aspects of the illness because of the challenges it poses for therapeutic engagement and medication adherence [7, 8].
The relationship between insight and health is complex. Poor clinical insight in schizophrenia has been associated with poorer medication adherence, which can lead to an increase in positive symptoms and relapse risk [2, 4, 9–12]. Conversely, poor insight also may exert a protective effect. In patients with schizophrenia or other psychotic illnesses, small but positive associations have been reported between insight and depression or suicidal thoughts or actions [9, 13–15]. However, more complex relationships between insight and suicidal thoughts or actions, mediated by other symptoms and changing over the course of illness, have been observed in several analyses [16, 17]. This dual nature of insight in schizophrenia outcomes has been called the insight paradox [4, 14, 18, 19]. Because symptoms of depression affect health-related quality of life (QoL), there may be an interaction between poor insight and better health-related QoL arising from a lower level of depressive symptoms. Negative impacts of depression on health-related QoL in patients with good insight may prevent these individuals from attaining personal goals and increase the risk of suicide [4, 19]. However, such determinations may be limited if insight is viewed by category (i.e., good versus poor insight) rather than as a continuum or assessed using only population means, which is typically how such data have been reported [20–22].
Neurocognition may also play a role in potential interactions between insight and mental health status, depression, and health-related QoL, although the specific connections between these domains and global insight are unclear. For example, one study in patients with psychotic disorders found that improvement in insight over 3 years was significantly associated both with fewer clinical symptoms and with better neurocognitive performance at baseline. Gradual improvement in insight over 3 years was associated with symptom improvement but not with improved neurocognition [23]. Additionally, a meta-analysis identified positive correlations between neurocognition and objective health-related QoL (i.e., observable life conditions) but negative or no association between neurocognition and subjective health-related QoL (e.g., patient-reported satisfaction with life conditions) [24].
To date, one of the major limitations of insight research is that insight generally has been conceptualized and reported as a categorical variable, whereas it seems more appropriate to consider insight as a dimensional phenomenon, lying along a continuum with gradations in the severity of lack of awareness [4]. As a result, it remains unknown whether there is a particular level of impaired insight (e.g., mild versus moderate versus severe) at which a lack of agreement with others’ appraisal of the patient’s well-being, neurocognitive abilities, and depression becomes apparent. The current post hoc analysis used data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) [25] study to explore how insight, assessed both as a categorical variable (more versus less impairment) and based on gradations along a continuum from none to extreme, is related to patient-reported mental health status, depression, and neurocognition in patients with schizophrenia.
Methods
We conducted a post hoc analysis using baseline, 6-month, and 12-month data from phase 1/1A of the National Institutes of Health (NIH)-supported CATIE study (NCT00014001) [25]. CATIE was conducted from January 2001 to December 2004 at 57 centers in the United States.
Data source
The CATIE schizophrenia trial was a multiphase, randomized, controlled trial that compared the effectiveness of first- and second-generation antipsychotic medications in patients with schizophrenia for up to 18 months of treatment [25, 26]. Results from this landmark study are reported in numerous publications; the study design is therefore described here briefly. In phase 1 of CATIE, patients were randomly assigned to receive olanzapine, perphenazine, quetiapine, risperidone, or ziprasidone under double-blind conditions and were followed for up to 18 months or until discontinuation of assigned medication, whichever came first. Patients with tardive dyskinesia at study entry were excluded from the perphenazine arm and were randomly assigned to olanzapine, quetiapine, risperidone, or ziprasidone (phase 1A). Data from phase 1/1A were pooled. Further details on the rationale, design, and study methods of CATIE have been described previously [25, 26].
Outcome measures
Baseline patient demographics and socioeconomic characteristics from the pooled phase 1 and phase 1A CATIE data included antipsychotic medication, age, sex, race, marital status, patient education, employment status, and living arrangements. Baseline insight was assessed using the Positive and Negative Syndrome Scale (PANSS) Item G12 (lack of judgment and insight). This single-item scale is a commonly used measure in published studies of insight [27] and is reported to be sufficiently sensitive to distinguish patient-rated functioning and symptom severity in schizophrenia [22, 28]. Significant correlations have been established between PANSS Item G12 and multidimensional insight scales, including the clinician-rated VAGUS Insight Into Psychosis scale [29], the Schedule for the Assessment of Insight–Expanded [30], and the Insight and Treatment Attitudes Questionnaire [31]. PANSS Item G12 is rated from 1 to 7 (1 = absent, 2 = minimal, 3 = mild, 4 = moderate, 5 = moderate-severe, 6 = severe, and 7 = extreme) [32], where higher scores indicate increasing symptom severity. In scoring PANSS Item G12, clinicians consider several dimensions of clinical insight, including awareness or understanding of the disorder, acknowledgment of common symptoms of schizophrenia, and awareness of the need for psychiatric treatment [22]. For this analysis, the goal was to determine whether there was a level of impairment along the Item G12 rating continuum that presented noticeable and clinically relevant insight challenges.
The primary outcomes of interest were patient-reported evaluation of mental and physical health (at baseline and at 6 and 12 months), as measured by the 12-Item Short-Form Health Survey (SF-12) Mental and Physical Component Summary (MCS and PCS), where lower scores indicate poorer health-related QoL [33], and a patient-reported overall mental/emotional health status score at baseline from a single-item, stand-alone query: “Please rate your current state of mental or emotional health by choosing a number from 1 to 100,” where a score of 1 is the worst possible state and a score of 100 is the best possible state. The association between baseline insight level and patient-reported schizophrenia symptom severity at baseline was also examined by comparing patient-reported severity of illness (assessed using the Patient Global Impression−Severity [PGI-S] scale) [34] versus physician-reported severity of illness (assessed using the Clinical Global Impression−Severity [CGI-S] scale). To better characterize the clinical impact of different levels of insight, we also examined the relationship between baseline scores on neurocognition, depression, and schizophrenia symptom scales and baseline PANSS Item G12 ratings in the CATIE population. Those assessments included PANSS components identified through an analysis of patients at the West Haven Veterans Affairs Medical Center (i.e., negative, positive, cognitive, emotional discomfort, and hostility) [35], neurocognition measured by the Neurocognitive Composite Score (MATRICS Consensus Cognitive Battery [MCCB]) [36], and scores of depression measured by the Calgary Depression Scale for Schizophrenia [37].
Statistical analyses
Patient characteristics and demographics were summarized using means and SDs for continuous variables and frequency and percentages for categorical variables. Associations between baseline PANSS Item G12 rating and baseline scores on patient-reported outcomes, symptoms scales, and neurocognitive scales were explored using analysis of variance. Based on results of those analyses, patient characteristics and demographics were then compared for patients with PANSS Item G12 ratings of 1 to 4 (good or fair insight) versus those with ratings of 5 to 7 (poor insight) using t tests for continuous variables and Chi-square tests for categorical values.
Local regression (LOESS), a nonparametric technique that uses local weighted regression to fit a smooth curve through points of a scatterplot [38], was used to explore the relationship between SF-12 MCS and PANSS total scores using baseline data. To simplify the curve, a piecewise linear model was fit and the impact of poor insight was tested. A mixed model was used to evaluate and quantify the relationship between patient-reported change in SF-12 MCS score and PANSS Item G12 insight level change, as well as dichotomized PANSS Item G12 rating (< 5 versus ≥5), controlling for baseline SF-12 MCS score, baseline PANSS total score, and time point (6 versus 12 months). In all analyses, a 2-sided P < 0.05 was the threshold by which differences were statistically significant. SAS version 9.4 was used to conduct the analyses.
Results
Patient demographics and baseline scores
A total of 1431 patients with schizophrenia from the CATIE phase 1/1A study were included in this post hoc analysis; most patients were male (74.3%) and white (61.1%), and mean age was 40.6 years (Table 1). At baseline, mean (SD) SF-12 MCS score was 40.9 (11.7) and SF-12 PCS score was 48.2 (10.2).
Table 1.
Baseline characteristics and demographics of CATIE study participants by PANSS Item G12 insight rating
| Characteristic | All Patients N = 1431 | PANSS Insight Item Rating 1 to 4 (Absent to Moderate) n = 1293 (90%) | PANSS Insight Item Rating 5 to 7 (Moderate-Severe to Extreme) n = 138 (10%) | P value* |
|---|---|---|---|---|
| Treatment for phase 1/1A, n (%a) | 0.7710 | |||
| Olanzapine | 330 (23.1) | 304 (92.1) | 26 (7.9) | |
| Perphenazine | 256 (17.9) | 228 (89.1) | 28 (10.9) | |
| Quetiapine | 330 (23.1) | 298 (90.3) | 32 (9.7) | |
| Risperidone | 333 (23.3) | 299 (89.8) | 34 (10.2) | |
| Ziprasidone | 182 (12.7) | 164 (90.1) | 18 (9.9) | |
| Age, y, mean (SD) | 40.6 (11.1) | 40.7 (11.0) | 39.6 (12.1) | 0.2815 |
| Age group, n (%a) | 0.0998 | |||
| 18–35 years | 449 (31.4) | 395 (88.0) | 54 (12.0) | |
| 36–45 years | 476 (33.3) | 438 (92.0) | 38 (8.0) | |
| 46+ years | 506 (35.4) | 460 (90.9) | 46 (9.1) | |
| Sex, n (%a) | 0.0820 | |||
| Male | 1063 (74.3) | 952 (89.6) | 111 (10.4) | |
| Female | 368 (25.7) | 341 (92.7) | 27 (7.3) | |
| Race, n (%a) | 0.2376 | |||
| White | 875 (61.1) | 789 (90.2) | 86 (9.8) | |
| Black | 506 (35.4) | 462 (91.3) | 44 (8.7) | |
| Other | 50 (3.5) | 42 (84.0) | 8 (16.0) | |
| Marital status, n (%a) | 0.0478 | |||
| Married | 166 (11.6) | 156 (94.0) | 10 (6.0) | |
| Previously married | 414 (28.9) | 381 (92.0) | 33 (8.0) | |
| Never married | 851 (59.5) | 756 (88.8) | 95 (11.2) | |
| Patient education, n (%a) | 0.2445 | |||
| < 12 years | 365 (25.5) | 336 (92.1) | 29 (7.9) | |
| 12 years | 506 (35.4) | 449 (88.7) | 57 (11.3) | |
| > 12 years | 560 (39.1) | 508 (90.7) | 52 (9.3) | |
| Employed, n (%a) | 0.3161 | |||
| No | 1213 (84.8) | 1092 (90.0) | 121 (10.0) | |
| Yes | 218 (15.2) | 201 (92.2) | 17 (7.8) | |
| Lives alone, n (%a) | 0.2536 | |||
| No | 1084 (75.8) | 974 (89.9) | 110 (10.1) | |
| Yes | 347 (24.2) | 319 (91.9) | 28 (8.1) |
Abbreviations: PANSS Positive and Negative Syndrome Scale, SD standard deviation
*For comparisons between patients with PANSS Item G12 ratings of 1–4 versus 5–7, t tests were performed on continuous variables, and Chi-square tests were performed on categorical values
aFor “All Patients,” percentages indicate proportion of total patients. For PANSS Insight subgroups, percentages indicate proportions of patients with the given characteristic
Table 2 presents baseline scores on health-related QoL, illness severity, depression, and neurocognitive scales by PANSS Item G12 ratings from the 1431 patients who had complete data for physician-rated measures and patient-reported outcomes at baseline. PANSS Item G12 ratings for lack of insight were 1 (absent) in 22% (n = 309), 2 (minimal) in 18% (n = 258), 3 (mild) in 27% (n = 387), 4 (moderate) in 24% (n = 339), 5 (moderate-severe) in 6% (n = 80), and 6 to 7 (severe to extreme) in 4% (n = 58) of patients (patients scoring 6 or 7 were grouped due to small sample size). Patients meeting the predefined criterion for impaired insight (PANSS Item G12 rating ≥ 5) at baseline represented 10% (n = 138) of the total population.
Table 2.
CATIE population baseline scale scores by PANSS Item G12 insight rating
| Assessments, mean (SD) | PANSS Item G12 Rating | ||||||
|---|---|---|---|---|---|---|---|
| 1 Absent n = 309 |
2 Minimal n = 258 |
3 Mild n = 387 |
4 Moderate n = 339 |
5 Moderate-Severe n = 80 |
6/7 Severe/Extreme n = 58 |
P value* | |
| Patient-reported outcomes | |||||||
| Mental/emotional health item | 58.5 (26.3) | 58.0 (26.1) | 59.1 (26.1) | 59.0 (27.7) | 72.7 (26.7) | 74.1 (28.5) | < 0.0001 |
| SF-12 PCS | 47.7 (10.8) | 47.7 (10.1) | 48.1 (10.6) | 48.3 (9.9) | 50.5 (7.8) | 50.3 (8.1) | < 0.0001 |
| SF-12 MCS | 39.7 (12.0) | 40.2 (11.0) | 40.0 (11.5) | 41.4 (11.2) | 44.4 (11.9) | 49.1 (11.7) | < 0.0001 |
| Schizophrenia severity | |||||||
| CGI-S | 3.7 (1.0) | 3.8 (0.9) | 3.9 (0.9) | 4.2 (0.9) | 4.5 (1.0) | 4.6 (1.0) | < 0.0001 |
| PGI-S | 3.5 (1.5) | 3.6 (1.6) | 3.6 (1.5) | 3.6 (1.6) | 3.0 (1.7) | 2.6 (1.7) | < 0.0001 |
| PANSS components** | |||||||
| Negative | 17.5 (6.4) | 20.1 (5.8) | 21.6 (6.1) | 23.3 (6.9) | 24.1 (7.2) | 27.1 (8.0) | < 0.0001 |
| Positive | 14.2 (5.3) | 15.5 (4.8) | 17.0 (4.8) | 18.7 (5.7) | 19.1 (6.0) | 20.0 (6.2) | < 0.0001 |
| Cognitive | 13.5 (4.2) | 16.5 (4.2) | 18.2 (4.0) | 20.6 (4.4) | 23.2 (4.7) | 24.8 (5.6) | < 0.0001 |
| Emotional discomfort | 10.6 (4.2) | 11.2 (3.6) | 11.4 (3.6) | 11.2 (3.9) | 10.3 (3.6) | 9.1 (3.2) | < 0.0001 |
| Hostility | 6.0 (2.5) | 6.7 (2.5) | 7.5 (2.8) | 7.5 (3.1) | 8.1 (3.2) | 7.8 (3.8) | < 0.0001 |
| Neurocognitive Composite Score (MCCB)*** | 51.3 (9.8) | 51.3 (9.7) | 49.8 (9.3) | 48.7 (11.1) | 46.8 (10.0) | 49.2 (9.7) | 0.1648 |
| Calgary total score | 5.0 (4.8) | 4.9 (4.3) | 4.8 (4.4) | 4.3 (4.3) | 2.9 (3.7) | 2.1 (2.4) | < 0.0001 |
Abbreviations: CGI-S Clinical Global Impression–Severity, MCCB MATRICS Consensus Cognitive Battery, MCS Mental Component Summary, PANSS Positive and Negative Syndrome Scale, PCS Physical Component Summary, PGI-S, Patient Global Impression–Severity, SF-12 12-Item Short-Form Health Survey
*Overall difference
**Based on West Haven Veterans Administration sample [35]
***MCCB was standardized to a baseline cohort from 5 other component scores (mean = 0; standard deviation = 1). The T-score reported was calculated as 50 + 50 + 10 × Neuro_SS, a normative value used for MCCB
At baseline, scores for most outcomes varied significantly with level of insight (PANSS Item G12; P < 0.0001). In general, greater physician-rated symptom severity and impairment in functional outcomes were observed for patients with greater lack of insight at baseline (Table 2). Conversely, patients with greater insight impairment had lower levels of depression (Calgary Depression total score) compared with patients with lower levels of impairment. Neurocognition (based on the MCCB) did not appear to be significantly related to insight (Table 2).
Insight impairment and physician-reported versus patient-reported schizophrenia severity
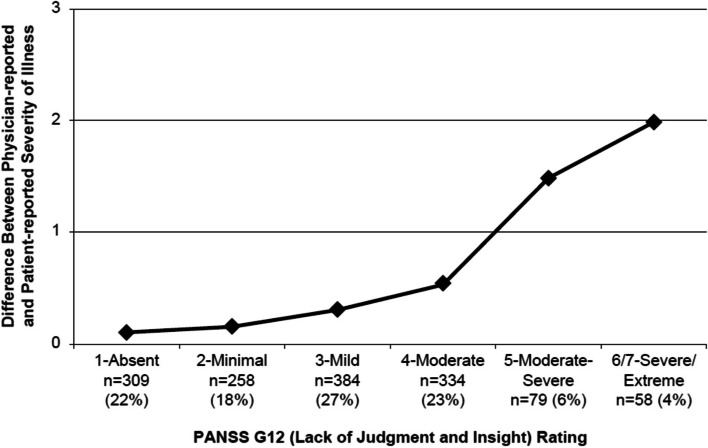
Level of insight at baseline was significantly associated with both physician- and patient-reported illness severity scores at baseline (both P < 0.0001; Table 2). However, whereas physician-reported schizophrenia severity (CGI-S) was higher for patients with the most severe levels of impaired insight, these patients had the lowest patient-reported illness severity (PGI-S) (Table 2). Consequently, the difference between physician- and patient-reported schizophrenia severity was greatest for patients with the greatest impairment in insight (Fig. 1 and Table 2). The divergence between physician and patient ratings appeared to become clinically relevant (1.5 to 2 points in magnitude) for patients with moderate-severe to extreme lack of insight (Item G12 rating ≥ 5; Fig. 1). For the remaining patients (Item G12 rating < 5), mean CGI-S and PGI-S scores were comparable, within approximately 0.5 points of each other at each level of impairment.
Fig. 1.
Insight impairment and perceived schizophrenia severity: difference* between physician- and patient-reported severity of illness. Abbreviations: CGI-S, Clinical Global Impression–Severity; PANSS, Positive and Negative Syndrome Scale; PGI-S, Patient Global Impression–Severity. *Calculated as mean CGI-S score – mean PGI-S score
Relationship between insight impairment and patient-reported outcomes
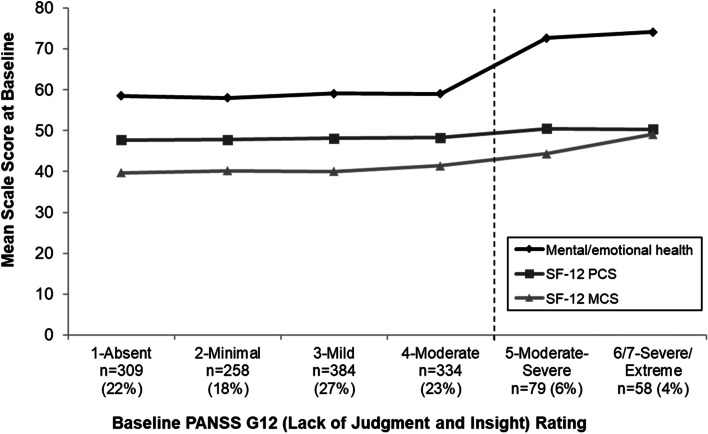
Baseline scores for all patient-reported outcomes varied significantly with level of insight (all P < 0.0001; Table 2). Specifically, patients with poorer insight had higher (better) scores on the mental/emotional health item, SF-12 MCS, and SF-12 PCS, with most apparent differences between patients with PANSS Item G12 ≥ 5 versus < 5 (Fig. 2 and Table 2).
Fig. 2.
Patient-reported mental and physical health by insight score. Abbreviations: MCS, Mental Component Summary; PANSS, Positive and Negative Syndrome Scale; PCS, Physical Component Summary; SF-12, 12-Item Short-Form Health Survey
Based on the observed difference between patients with moderate-severe to extreme insight impairment (PANSS Item G12 rating ≥ 5) and those with absent to moderate impairment on the mental/emotional health item, SF-12 MCS, and CGI-S and PGI-S scores, baseline demographic characteristics were explored by PANSS Item G12 ≥ 5 versus < 5. The two groups were comparable on all baseline characteristics except marital status (Table 1).
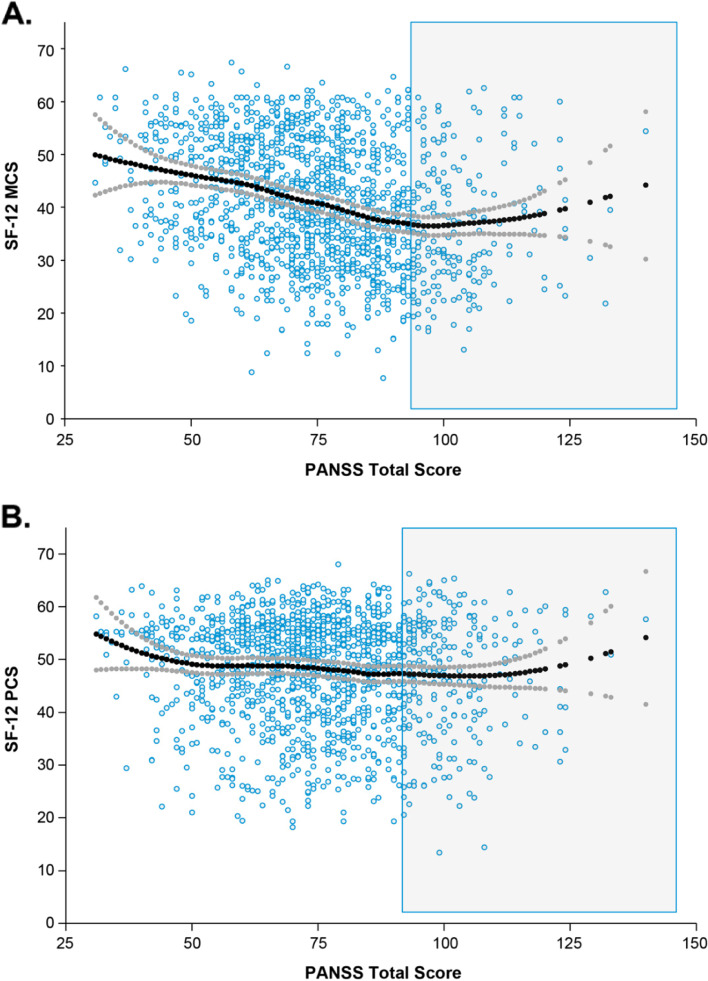
Relationship between patient-reported SF-12 MCS and PCS scores and PANSS total score
LOESS regression analysis suggested a piecewise linear relationship between SF-12 MCS score and PANSS total score, but no piecewise linear relationship between SF-12 PCS score and PANSS total score (Fig. 3); therefore, no further analyses were conducted using SF-12 PCS scores. The relationship between level of insight impairment and MCS score was assessed using a piecewise linear model. In the model with baseline PANSS data, when the baseline PANSS total score was <90, the SF-12 MCS score decreased by 2.6 points for each additional 10-point increase in the baseline PANSS total score. For example, a patient with a baseline PANSS total score of 80 would be expected to have an SF-12 MCS score 2.6 points lower than a patient with a baseline PANSS score of 70 (i.e., worse SF-12 MCS score was associated with more severe disease as measured by PANSS score). When the baseline PANSS score was ≥90, there was no significant relationship between PANSS total score and SF-12 MCS score.
Fig. 3.
LOESS relationships between patient-reported outcomes and PANSS total score. Shown are LOESS relationships between patient-reported outcomes SF-12 MCS (A) and SF-12 PCS (B) and PANSS total score. Abbreviations: LOESS, locally weighted scatterplot smoothing; MCS, Mental Component Summary; PANSS, Positive and Negative Syndrome Scale; PCS, Physical Component Summary; SF-12, 12-Item Short-Form Health Survey
In the model with longitudinal data, the SF-12 MCS score increased by 2.2 points (i.e., improved) for each 10-point decrease in PANSS total score from baseline to 6 months (Table 3). Therefore, if a patient’s PANSS score fell from 80 to 70 from baseline to 6 months, a 2.2-point increase in MCS would be expected.
Table 3.
Models for SF-12 MCS and PANSS total score and insight level
| Parameter | Estimate | SE | P value |
|---|---|---|---|
| Model with baseline data | |||
| Intercept | 35.86 | 0.54 | < 0.0001 |
| PANSS total score | |||
| When score < 90 | −0.26 | 0.02 | < 0.0001 |
| When score ≥ 90 | −0.02 | 0.06 | 0.6958 |
| PANSS Item G12 ≥ 5 | 8.63 | 1.01 | < 0.0001 |
| Model with longitudinal data | |||
| Intercept | 35.23 | 2.18 | < 0.0001 |
| Visit 12: 12 months vs 6 months | 0.24 | 0.47 | 0.6142 |
| Baseline MCS | 0.44 | 0.03 | < 0.0001 |
| Baseline PANSS total score | −0.15 | 0.02 | < 0.0001 |
| Change in PANSS total score from baseline | −0.22 | 0.02 | < 0.0001 |
| Follow-up PANSS Item G12 ≥ 5 | 4.48 | 1.23 | 0.0003 |
Abbreviations: MCS Mental Component Summary, PANSS Positive and Negative Syndrome Scale, SE standard error
Relationship between patient-reported SF-12 MCS score change and insight
The association between level of insight impairment and SF-12 MCS score was examined. Patients with poor insight (PANSS Item G12 rating ≥ 5) reported an 8.63-point higher SF-12 MCS score (P < 0.0001) at baseline and a 4.48-point higher score (P = 0.0003) at follow-up compared with patients with good or fair insight (PANSS Item G12 rating < 5; Table 3).
Discussion
This post hoc analysis of CATIE study data provides a greater understanding of how the degree of insight may influence responses of individuals with schizophrenia on patient-reported outcomes, including health-related QoL measures. At baseline, statistically significant associations were observed between CATIE participants’ levels of insight, based on PANSS Item G12 rating, and most outcomes assessed, including patient-reported health-related QoL outcomes, illness severity, PANSS component scores, and depression scale scores. Among CATIE participants, insight that was moderately severe or worse (i.e., PANSS Item G12 rating ≥ 5) was associated with better mental/emotional health status and better health-related QoL (based on SF-12 MCS and PCS scores) compared with those with less impairment (PANSS Item G12 rating < 5). Patients with more impaired insight (i.e., PANSS Item G12 ≥ 5) accounted for 10% of CATIE participants and had lower levels of baseline depression compared with patients with less impaired insight (i.e., PANSS Item G12 rating < 5). Also, among the group with poor insight, there was a discrepancy in appraisal of schizophrenia severity, with lower severity reported by patients than by physicians. These results are consistent with the finding that, compared with the overall CATIE population, patients with substantially greater insight impairment were less likely to adhere to their medication [39]. For the 90% of CATIE patients with baseline PANSS Item G12 rating < 5, physician- and patient-reported severity ratings were generally more consistently close in their agreement with one another.
The validity of patient-reported outcomes to predict health outcomes in schizophrenia has been questioned because of possible confounding due to impaired insight, such that patients with poor insight might lack the ability to appraise their own health-related QoL accurately [40, 41]. Some studies have shown that poor insight has a weaker correlation with self- and reviewer-rated health-related QoL measurements than good insight [42, 43]. However, other studies have found that self-reported health-related QoL measures in patients with schizophrenia are reliable [44], although this appears to be most reproducible among those with better insight (and potentially less severe symptoms) [45]. The current results indicate that although severe insight impairment can be associated with significant divergence between physician- and patient-reported ratings of illness, the proportion of patients in which this occurs is likely small. For a large majority of patients with schizophrenia—90% in the current analysis—physician and patient ratings aligned. These findings have important implications for the use of patient-reported outcomes in the clinical trials enrolling patients with schizophrenia. Because successful treatment of schizophrenia requires functional recovery in addition to remission of symptoms [46], assessments of health-related QoL and daily functioning are essential for evaluating treatment efficacy in patients with schizophrenia [47]. Impairment in insight would not be expected to have a substantial effect on measurement of those outcomes at a study population level, as patient-reported outcomes diverge from physician-reported outcomes only with moderate-severe to extreme impairment in insight. However, on the individual patient level, patients with very poor insight may report better mental health status relative to those with fair or good insight, and this should be taken into consideration when interpreting such data.
In the current post hoc analysis, self-reported mental health (SF-12 MCS) status was found to be linearly related to symptom severity (PANSS total score) when PANSS was <90, but no relation was found in patients with greater symptom severity (≥90). Linear regression modeling demonstrated a negative association between self-reported mental health and PANSS total scores, as expected given that higher SF-12 MCS scores indicate better health-related QoL, whereas higher PANSS scores indicate greater symptom severity. For both baseline and longitudinal data, an approximate 2-point change in SF-12 MCS was associated with a 10-point change in PANSS total score. No relationship was observed between self-reported physical health (SF-12 PCS score) and PANSS total score.
In this analysis, we found that depression scores (based on Calgary total score) decreased with increasing impairment in insight. The change across the insight continuum was small but consistent with the findings from a recent meta-analysis, in which a significant association between global clinical insight and depression was observed, with better insight associated with higher levels of depressive symptoms [48]. While the current post hoc analysis (in line with other studies [3, 13, 31]) reports better health-related QoL among patients with schizophrenia with impaired insight relative to those with fair or good insight, other studies have found that patients with impaired insight have decreased health-related QoL [42, 49] or have found no significant association between the two [28, 50–52]. Several factors may explain the discrepancies between these findings, including differences in assessments used to measure insight [28, 31, 52]. In addition, characteristics of the study population, such as treatment history, symptom stability, status of social and living situations, and objective QoL [42, 49] may also be operant. The single measure in this analysis that did not vary significantly with insight was the Neurocognitive Composite Score; however, an association was observed between insight and the PANSS cognitive component score. Significant associations between measures of insight and cognition have been reported in meta-analyses of patients with schizophrenia (11 studies) and in patients with schizophrenia or psychosis (35 studies) [53]. However, the authors observed small and inconsistent correlations in a number of included studies and posited that the relationship between insight and cognitive deficits is nonlinear [53]. Results of published studies suggest that the relation between insight and cognition may vary with age, severity of disease, or number of previous episodes [54, 55].
Treatment of poor insight in schizophrenia has been approached using both pharmacological and psychological therapies. Clozapine and second-generation antipsychotics have been associated with improvements in insight in schizophrenia [20, 56]. However, the association was not observed in an analysis that controlled for other clinical factors, indicating that effects on impaired insight were mediated by overall improvement in symptoms [20]. Several psychological therapies have been assessed for effect on insight in schizophrenia, including cognitive behavioral therapy, psycho-education, adherence therapy, social skills training, and metacognitive training [8, 57]. Overall, meta-analyses have shown small to moderate effect sizes for these interventions on insight, although the approaches vary in effectiveness [8, 57]. Cognitive behavioral therapy for psychosis, which is specifically adapted for individuals with psychosis, has been associated with improvements in insight in several studies [58–61] and is recommended in the American Psychiatric Association practice guideline for the treatment of patients with schizophrenia [62]. Metacognitive training for psychotic illnesses and metacognitive reflection and insight therapy (MERIT) are emerging therapies that address insight in schizophrenia and aim to help patients strengthen their understanding of their distorted mental processes (through metacognitive training) or their self-appraisal abilities (through MERIT) [63–65]. One study assessing MERIT found that patients with first-episode psychosis and poor clinical insight who received 6 months of MERIT had statistically significant improvements in objective measures of insight without any increases in hopelessness or emotional distress relative to those who had standard meetings with therapists [66]. A systematic review in patients with schizophrenia spectrum disorders concluded that metacognitive training improved cognitive insight, illness awareness, and awareness of delusions and hallucinations, while MERIT was found to be less effective [67]. These types of therapy hold promise for patients with poor insight.
Limitations of the current study include the post hoc nature of the analysis and the use of a single-item measurement for insight, PANSS Item G12. In addition, while the goal of the current analysis was to better understand how impairment in insight might affect responses on patient-reported measures of symptom severity and mental health, observed associations between the outcomes assessed do not necessarily indicate a causal relationship. Finally, the results observed here may not generalize to patients with schizophrenia who differ from those who enrolled in the CATIE study. CATIE study participants were predominantly male and white and notably were willing to enroll in a clinical trial for the treatment of schizophrenia; such patients may differ in their level of insight from those who are unwilling to enroll in a clinical study. Our analysis is strengthened by use of a nonparametric regression method (LOESS) to examine the relationship between insight and subjective health-related QoL. By characterizing relatively large groups for each of the levels of insight, this approach revealed a spectrum of insight levels based on PANSS Item G12 ratings.
In conclusion, this post hoc analysis assessed the relationship between degrees of impairment in insight and physician- and patient-reported outcomes. Results indicate that the inverse relationships between insight and self-perceived mental health and mood are most robust once moderate-severe levels of impairment of insight are reached. In this analysis, agreement about health status, depression, and neurocognition differed between patients with different degrees of insight impairment, but most notably between the 10% of patients with moderate-severe to extreme lack of insight and the 90% with lower levels of impairment. For most patients with schizophrenia enrolled in the CATIE study, there was little effect of insight impairment on the convergence of ratings for patient- versus physician-reported outcomes. By providing a greater understanding of the relationship between insight impairment and patient-reported outcomes in schizophrenia, the results from this analysis may be informative for clinicians seeking to address impairment in insight to help patients achieve their therapeutic goals.
Acknowledgments
Medical writing and editorial support for the preparation of this article was provided by Susan Bartko-Winters, PhD, and Esther Tazartes, MS, of Global Outcomes Group, and Gina Daniel, PhD and Kathleen Dorries, PhD of Peloton Advantage, LLC (Parsippany, NJ), an OPEN Health company, and funded by Alkermes, Inc.
The data used in the preparation of this manuscript were obtained from the limited-access datasets distributed from the NIH-supported Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study (NCT00014001). This was a multisite, clinical trial of persons with schizophrenia comparing the effectiveness of randomly assigned medication treatment. The study was supported by National Institute of Mental Health (NIMH) Contract #N01MH90001 to the University of North Carolina at Chapel Hill. The CATIE study was conducted by investigators from the University of North Carolina, Duke University, Columbia University, the University of Southern California, the University of Rochester, and Yale University along with program staff of the Division of Interventions and Services Research, NIMH, and investigators from each of the 57 research sites in the United States. This manuscript reflects the views of the authors and may not reflect the opinions or views of the CATIE Study Investigators or the NIMH.
Role of the funding source
This study was sponsored by Alkermes, Inc. (Waltham, MA, USA). The study sponsor was involved in the design, collection, and analysis of the data. Interpretation of the results was by the authors, and the decision to submit the manuscript for publication was made by the authors.
Abbreviations
- CATIE
Clinical Antipsychotic Trials of Intervention Effectiveness
- CGI-S
Clinical Global Impression−Severity
- LOESS
Locally weighted scatterplot smoothing
- MCCB
MATRICS Consensus Cognitive Battery
- MCS
Mental Component Summary
- PANSS
Positive and Negative Syndrome Scale
- PCS
Physical Component Summary
- PGI-S
Patient Global Impression−Severity
- QoL
Quality of life
- SD
Standard deviation
- SE
Standard error
- SF-12
12-Item Short-Form Health Survey
Authors’ contributions
All authors contributed to the conception and design of the study. Xiaowu Sun analyzed the data. All authors were involved in interpreting the findings, drafting and revising the manuscript, and giving final approval for submission. All authors agree to be personally accountable for their contributions and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and the resolution documented.
Funding
Research and editorial support were funded by Alkermes, Inc., Waltham, MA, USA.
Availability of data and materials
The data used in the preparation of this manuscript are proprietary to Alkermes, Inc. Alkermes, Inc. is committed to public sharing of data in accordance with applicable regulations and laws, and requests can be submitted to the corresponding author.
Declarations
Ethics approval and consent to participate
The CATIE study (Lieberman et al., 2005) was approved by the institutional review board at each site, and written informed consent was obtained from the patients or their legal guardians. We confirm that the dataset from the CATIE investigators was all de-identified. We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
Dr. Lysaker has no competing interests. Dr. McEvoy has received consulting fees from Alkermes, Inc., in the past. Dr. O’Sullivan is an employee of Alkermes, Inc., and may own stock in the company. At the time of the study, Drs. Sun and Weiden were employees of Alkermes, Inc., and may own stock in the company.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vohs JL, George S, Leonhardt BL, Lysaker PH. An integrative model of the impairments in insight in schizophrenia: emerging research on causal factors and treatments. Expert Rev Neurother. 2016;16(10):1193–1204. doi: 10.1080/14737175.2016.1199275. [DOI] [PubMed] [Google Scholar]
- 2.Lysaker PH, Vohs J, Hillis JD, Kukla M, Popolo R, Salvatore G, et al. Poor insight into schizophrenia: contributing factors, consequences and emerging treatment approaches. Expert Rev Neurother. 2013;13(7):785–793. doi: 10.1586/14737175.2013.811150. [DOI] [PubMed] [Google Scholar]
- 3.Lysaker PH, Buck KD, Salvatore G, Popolo R, Dimaggio G. Lack of awareness of illness in schizophrenia: conceptualizations, correlates and treatment approaches. Expert Rev Neurother. 2009;9(7):1035–1043. doi: 10.1586/ern.09.55. [DOI] [PubMed] [Google Scholar]
- 4.Lysaker PH, Pattison ML, Leonhardt BL, Phelps S, Vohs JL. Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry. 2018;17(1):12–23. doi: 10.1002/wps.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David AS. Insight and psychosis. Br J Psychiatry. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- 6.Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873–879. doi: 10.1176/ajp.150.6.873. [DOI] [PubMed] [Google Scholar]
- 7.Leonhardt BL, Benson K, George S, Buck KD, Shaie R, Vohs JL. Targeting insight in first episode psychosis: a case study of metacognitive reflection insight therapy (MERIT) J Contemp Psychother. 2016;46:207–216. doi: 10.1007/s10879-016-9332-9. [DOI] [Google Scholar]
- 8.Pijnenborg GH, van Donkersgoed RJ, David AS, Aleman A. Changes in insight during treatment for psychotic disorders: a meta-analysis. Schizophr Res. 2013;144(1–3):109–117. doi: 10.1016/j.schres.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Lincoln TM, Lüllmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull. 2007;33(6):1324–1342. doi: 10.1093/schbul/sbm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed S, Rosenheck R, He H, Yuping N. Insight and attitudes towards medication among inpatients with chronic schizophrenia in the US and China. Soc Psychiatry Psychiatr Epidemiol. 2014;49(7):1063–1070. doi: 10.1007/s00127-014-0824-1. [DOI] [PubMed] [Google Scholar]
- 11.James AV, Hasson-Ohayon I, Vohs J, Minor KS, Leonhardt BL, Buck KD, et al. Metacognition moderates the relationship between dysfunctional self-appraisal and social functioning in prolonged schizophrenia independent of psychopathology. Compr Psychiatry. 2016;69:62–70. doi: 10.1016/j.comppsych.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed S, Rosenheck R, McEvoy J, Swartz M, Stroup S, Lieberman JA. Cross-sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes in chronic schizophrenia. Schizophr Bull. 2009;35(2):336–346. doi: 10.1093/schbul/sbn067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res. 2003;61(1):75–88. doi: 10.1016/s0920-9964(02)00316-x. [DOI] [PubMed] [Google Scholar]
- 14.Palmer EC, Gilleen J, David AS. The relationship between cognitive insight and depression in psychosis and schizophrenia: a review and meta-analysis. Schizophr Res. 2015;166(1–3):261–268. doi: 10.1016/j.schres.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Villa J, Choi J, Kangas JL, Kaufmann CN, Harvey PD, Depp CA. Associations of suicidality with cognitive ability and cognitive insight in outpatients with schizophrenia. Schizophr Res. 2018;192:340–344. doi: 10.1016/j.schres.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayesa-Arriola R, Terán JMP, Moríñigo JDL, Rivero MC, Setién-Suero E, Al-Halabi S, et al. The dynamic relationship between insight and suicidal behavior in first episode psychosis patients over 3-year follow-up. Eur Neuropsychopharmacol. 2018;28(10):1161–1172. doi: 10.1016/j.euroneuro.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Morinigo JD, Di Forti M, Ajnakina O, Wiffen BD, Morgan K, Doody GA, et al. Insight and risk of suicidal behaviour in two first-episode psychosis cohorts: effects of previous suicide attempts and depression. Schizophr Res. 2019;204:80–89. doi: 10.1016/j.schres.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Lysaker PH, Roe D, Yanos PT. Toward understanding the insight paradox: internalized stigma moderates the association between insight and social functioning, hope, and self-esteem among people with schizophrenia spectrum disorders. Schizophr Bull. 2007;33(1):192–199. doi: 10.1093/schbul/sbl016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belvederi Murri M, Amore M, Calcagno P, Respino M, Marozzi V, Masotti M, et al. The "insight paradox" in schizophrenia: magnitude, moderators and mediators of the association between insight and depression. Schizophr Bull. 2016;42(5):1225–1233. doi: 10.1093/schbul/sbw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattila T, Koeter M, Wohlfarth T, Storosum J, van den Brink W, Derks E, et al. The impact of second generation antipsychotics on insight in schizophrenia: results from 14 randomized, placebo controlled trials. Eur Neuropsychopharmacol. 2017;27(1):82–86. doi: 10.1016/j.euroneuro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Schennach R, Meyer S, Seemüller F, Jäger M, Schmauss M, Laux G, et al. Insight in schizophrenia-course and predictors during the acute treatment phase of patients suffering from a schizophrenia spectrum disorder. Eur Psychiatry. 2012;27(8):625–633. doi: 10.1016/j.eurpsy.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Gharabawi GM, Lasser RA, Bossie CA, Zhu Y, Amador X. Insight and its relationship to clinical outcomes in patients with schizophrenia or schizoaffective disorder receiving long-acting risperidone. Int Clin Psychopharmacol. 2006;21(4):233–240. doi: 10.1097/00004850-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Quee PJ, van der Meer L, Krabbendam L, de Haan L, Cahn W, Wiersma D, et al. Insight change in psychosis: relationship with neurocognition, social cognition, clinical symptoms and phase of illness. Acta Psychiatr Scand. 2014;129(2):126–133. doi: 10.1111/acps.12138. [DOI] [PubMed] [Google Scholar]
- 24.Tolman AW, Kurtz MM. Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: a meta-analytic investigation. Schizophr Bull. 2012;38(2):304–315. doi: 10.1093/schbul/sbq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. 10.1056/NEJMoa051688. [DOI] [PubMed]
- 26.Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, et al. The National Institute of Mental Health clinical antipsychotic trials of intervention effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 2003;29(1):15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- 27.Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86(1–3):54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Gharabawi G, Bossie C, Turkoz I, Kujawa M, Mahmoud R, Simpson G. The impact of insight on functioning in patients with schizophrenia or schizoaffective disorder receiving risperidone long-acting injectable. J Nerv Ment Dis. 2007;195(12):976–982. doi: 10.1097/NMD.0b013e31815c1982. [DOI] [PubMed] [Google Scholar]
- 29.Jeong SH, Chung IW, Jung HY, Hwang SS, Kim SH, Youn T, et al. Comparison of clinician-rated and self-report insight in Korean patients with schizophrenia using VAGUS insight scale. Psychiatry Res. 2017;258:93–100. doi: 10.1016/j.psychres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Larabi DI, Marsman JC, Aleman A, Tijms BM, Opmeer EM, Pijnenborg GHM, et al. Insight does not come at random: individual gray matter networks relate to clinical and cognitive insight in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2021;109:110251. doi: 10.1016/j.pnpbp.2021.110251. [DOI] [PubMed] [Google Scholar]
- 31.Siu CO, Harvey PD, Agid O, Waye M, Brambilla C, Choi WK, et al. Insight and subjective measures of quality of life in chronic schizophrenia. Schizophr Res Cogn. 2015;2(3):127–132. doi: 10.1016/j.scog.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. 10.1093/schbul/13.2.261. [DOI] [PubMed]
- 33.Ware JE, Kosinski M, Keller SD. SF-12: how to score the SF-12 physical and mental health summary scales. 2. Boston: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 34.Guy W. ECDEU assessment manual for psychopharmacology. Revised. Rockville: US Department of Health, Education and Welfare; 1976. [Google Scholar]
- 35.Bell MD, Lysaker PH, Beam-Goulet JL, Milstein RM, Lindenmayer JP. Five-component model of schizophrenia: assessing the factorial invariance of the positive and negative syndrome scale. Psychiatry Res. 1994;52(3):295–303. doi: 10.1016/0165-1781(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 36.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 37.Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6(3):201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- 38.Cleveland WS, Devlin SJ, Grosse E. Regression by local fitting: methods, properties, and computational algorithms. J Econom. 1988;37:87–114. doi: 10.1016/0304-4076(88)90077-2. [DOI] [Google Scholar]
- 39.Kim J, Ozzoude M, Nakajima S, Shah P, Caravaggio F, Iwata Y, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi: 10.1016/j.neuropharm.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Awad AG, Voruganti LN. Measuring quality of life in patients with schizophrenia: an update. Pharmacoeconomics. 2012;30(3):183–195. doi: 10.2165/11594470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Boyer L, Baumstarck K, Boucekine M, Blanc J, Lançon C, Auquier P. Measuring quality of life in patients with schizophrenia:an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):343–349. doi: 10.1586/erp.13.15. [DOI] [PubMed] [Google Scholar]
- 42.Doyle M, Flanagan S, Browne S, Clarke M, Lydon D, Larkin C, et al. Subjective and external assessments of quality of life in schizophrenia: relationship to insight. Acta Psychiatr Scand. 1999;99(6):466–472. doi: 10.1111/j.1600-0447.1999.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 43.Whitty P, Browne S, Clarke M, McTigue O, Waddington J, Kinsella T, et al. Systematic comparison of subjective and objective measures of quality of life at 4-year follow-up subsequent to a first episode of psychosis. J Nerv Ment Dis. 2004;192(12):805–809. doi: 10.1097/01.nmd.0000146733.26005.bd. [DOI] [PubMed] [Google Scholar]
- 44.Voruganti L, Heslegrave R, Awad AG, Seeman MV. Quality of life measurement in schizophrenia: reconciling the quest for subjectivity with the question of reliability. Psychol Med. 1998;28(1):165–172. doi: 10.1017/s0033291797005874. [DOI] [PubMed] [Google Scholar]
- 45.Karow A, Pajonk FG. Insight and quality of life in schizophrenia: recent findings and treatment implications. Curr Opin Psychiatry. 2006;19(6):637–641. doi: 10.1097/01.yco.0000245754.21621.c9. [DOI] [PubMed] [Google Scholar]
- 46.Karadayi G, Emiroglu B, Ucok A. Relationship of symptomatic remission with quality of life and functionality in patients with schizophrenia. Compr Psychiatry. 2011;52(6):701–707. doi: 10.1016/j.comppsych.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D. Remission in schizophrenia: validity, frequency, predictors, and patients' perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):393–407. 10.31887/DCNS.2010.12.3/mlambert. [DOI] [PMC free article] [PubMed]
- 48.Belvederi Murri M, Respino M, Innamorati M, Cervetti A, Calcagno P, Pompili M, et al. Is good insight associated with depression among patients with schizophrenia? Systematic review and meta-analysis. Schizophr Res. 2015;162(1–3):234–247. doi: 10.1016/j.schres.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Ritsner M, Modai I, Endicott J, Rivkin O, Nechamkin Y, Barak P, et al. Differences in quality of life domains and psychopathologic and psychosocial factors in psychiatric patients. J Clin Psychiatry. 2000;61(11):880–889. doi: 10.4088/jcp.v61n1113. [DOI] [PubMed] [Google Scholar]
- 50.Browne S, Garavan J, Gervin M, Roe M, Larkin C, O'Callaghan E. Quality of life in schizophrenia: insight and subjective response to neuroleptics. J Nerv Ment Dis. 1998;186(2):74–78. doi: 10.1097/00005053-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Hofer A, Rettenbacher MA, Widschwendter CG, Kemmler G, Hummer M, Fleischhacker WW. Correlates of subjective and functional outcomes in outpatient clinic attendees with schizophrenia and schizoaffective disorder. Eur Arch Psychiatry Clin Neurosci. 2006;256(4):246–255. doi: 10.1007/s00406-005-0633-3. [DOI] [PubMed] [Google Scholar]
- 52.Williams CC, Collins A. Factors associated with insight among outpatients with serious mental illness. Psychiatr Serv. 2002;53(1):96–98. doi: 10.1176/appi.ps.53.1.96. [DOI] [PubMed] [Google Scholar]
- 53.Aleman A, Agrawal N, Morgan KD, David AS. Insight in psychosis and neuropsychological function: meta-analysis. Br J Psychiatry. 2006;189:204–212. doi: 10.1192/bjp.189.3.204. [DOI] [PubMed] [Google Scholar]
- 54.Bhat PS, Raj J, Chatterjee K, Srivastava K. Cognitive dysfunction in first-episode schizophrenia and its correlation with negative symptoms and insight. Ind Psychiatry J. 2021;30(2):310–315. doi: 10.4103/ipj.ipj_107_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerretsen P, Voineskos AN, Graff-Guerrero A, Menon M, Pollock BG, Mamo DC, et al. Insight into illness and cognition in schizophrenia in earlier and later life. J Clin Psychiatry. 2017;78(4):e390–e3e7. doi: 10.4088/JCP.16m10741. [DOI] [PubMed] [Google Scholar]
- 56.Hou CL, Cai MY, Ma XR, Zang Y, Jia FJ, Lin YQ, et al. Clozapine prescription and quality of life in Chinese patients with schizophrenia treated in primary care. Pharmacopsychiatry. 2015;48(6):200–204. doi: 10.1055/s-0035-1555939. [DOI] [PubMed] [Google Scholar]
- 57.Sauvé G, Lavigne KM, Pochiet G, Brodeur MB, Lepage M. Efficacy of psychological interventions targeting cognitive biases in schizophrenia: a systematic review and meta-analysis. Clin Psychol Rev. 2020;78:101854. doi: 10.1016/j.cpr.2020.101854. [DOI] [PubMed] [Google Scholar]
- 58.Habib N, Dawood S, Kingdon D, Naeem F. Preliminary evaluation of culturally adapted CBT for psychosis (CA-CBTp): findings from developing culturally-sensitive CBT project (DCCP) Behav Cogn Psychother. 2015;43(2):200–208. doi: 10.1017/s1352465813000829. [DOI] [PubMed] [Google Scholar]
- 59.Li ZJ, Guo ZH, Wang N, Xu ZY, Qu Y, Wang XQ, et al. Cognitive-behavioural therapy for patients with schizophrenia: a multicentre randomized controlled trial in Beijing, China. Psychol Med. 2015;45(9):1893–1905. doi: 10.1017/s0033291714002992. [DOI] [PubMed] [Google Scholar]
- 60.Naeem F, Saeed S, Irfan M, Kiran T, Mehmood N, Gul M, et al. Brief culturally adapted CBT for psychosis (CaCBTp): a randomized controlled trial from a low income country. Schizophr Res. 2015;164(1–3):143–148. doi: 10.1016/j.schres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Guo ZH, Li ZJ, Ma Y, Sun J, Guo JH, Li WX, et al. Brief cognitive-behavioural therapy for patients in the community with schizophrenia: randomised controlled trial in Beijing, China. Br J Psychiatry. 2017;210(3):223–229. doi: 10.1192/bjp.bp.116.183285. [DOI] [PubMed] [Google Scholar]
- 62.American Psychiatric Association . The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Washington, DC: American Psychiatric Association; 2021. [Google Scholar]
- 63.Lysaker PH, Buck KD, Carcione A, Procacci M, Salvatore G, Nicolò G, et al. Addressing metacognitive capacity for self reflection in the psychotherapy for schizophrenia: a conceptual model of the key tasks and processes. Psychol Psychother. 2011;84(1):58–69. doi: 10.1348/147608310x520436. [DOI] [PubMed] [Google Scholar]
- 64.Moritz S, Andreou C, Schneider BC, Wittekind CE, Menon M, Balzan RP, et al. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev. 2014;34(4):358–366. doi: 10.1016/j.cpr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Moritz S, Klein JP, Lysaker PH, Mehl S. Metacognitive and cognitive-behavioral interventions for psychosis: new developments. Dialogues Clin Neurosci. 2019;21(3):309–317. doi: 10.31887/DCNS.2019.21.3/smoritz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vohs JL, Leonhardt BL, James AV, Francis MM, Breier A, Mehdiyoun N, et al. Metacognitive reflection and insight therapy for early psychosis: a preliminary study of a novel integrative psychotherapy. Schizophr Res. 2018;195:428–433. doi: 10.1016/j.schres.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 67.Lopez-Morinigo JD, Ajnakina O, Martínez AS, Escobedo-Aedo PJ, Ruiz-Ruano VG, Sánchez-Alonso S, et al. Can metacognitive interventions improve insight in schizophrenia spectrum disorders? A systematic review and meta-analysis. Psychol Med. 2020;50(14):2289–2301. doi: 10.1017/s0033291720003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the preparation of this manuscript are proprietary to Alkermes, Inc. Alkermes, Inc. is committed to public sharing of data in accordance with applicable regulations and laws, and requests can be submitted to the corresponding author.