Abstract
Background
Eastern Khyber Pakhtunkhwa is home to a vast range of medicinal and edible waterbird species due to its diverse geographical environment. Waterbird species have been used for various ailments and cultural practices since ancient times, while ethno-pharmacological applications and cultural uses of waterbird species in this area have seldom been documented. This study is the first ethnomedicinal and cultural assessment of waterbird species, and the first compilation and listing of all known data on these species in Eastern Khyber Pakhtunkhwa, Pakistan.
Methods
Interviews and questionnaires were used to collect data from native respondents (N = 100). To analyze the data, principal component analysis (PCA), relative frequency of citation (RFC), fidelity level (FL%), relative popularity level (RPL), rank order priority, and similarity index were used.
Results
In total, 64 waterbird species were utilized in cultural practices, of which 40 species are used to cure different infectious and chronic diseases such as cold, cough, flu, fever, respiratory disorders, asthma, TB, gastric ulcers, kidney stones, male impotency, obesity, paralysis, piles, cancer, arthritis, body pain, and weakness. PCA showed significant differences in the use of waterbird species among the local inhabitants of the study area, separated along the axis-2 (p < 0.05). The FL% of waterbird species varied from 12 to 100%. 100% FL was analyzed for four waterbird species, i.e., Charadrius mongolus (cold), Gallicrex cinerea (asthma), Anas platyrhynchos (cancer), and Esacus recurvirostris (body weakness). In this study, Mallard (Anas platyrhynchos) was the most popular species used in the healthcare system of Eastern Khyber Pakhtunkhwa, with high RFC (4.06), FL% (100), and RPL (1.0) values.
Conclusion
We concluded that waterbird species are more used for medicine and food purposes in the study area. However, in vitro/in vivo assessment of biochemical activities of waterbird species with a maximum FL% might be significant to produce novel drugs. Recent research shows important ethno-ornithological information about native people and their links with waterbird species, which might be helpful for the sustainable use of waterbird diversity in the research area.
Keywords: Ethno-ornithology, Waterbirds, Pakistan, Principal component analysis, Chord diagram
Introduction
Ethno-ornithology is a natural scientific approach that explains the relationship between people’s knowledge and the use of birds in their culture [1–5]. It is essential in ethno-ornithological research that a bird's presence, movements, habits, and associated local knowledge be recorded correctly and in a way that all people can access the information [6]. In several human ethnic communities, bird species constitute the major source of protein [7–9] and fats [10]; they are used in medicine, commercial as well as in folklore [11, 12]. In Pakistan, herbivores, granivores, frugivores, and omnivore species (which do not eat dead animals) were edible and used as food [11]. Birds and parts of birds are also known for their healing properties around the world [13]. Bird’s highest percentages of recipes are used to treat respiratory disorders, body weakness, gastrointestinal problems, and skin infections [11]. For example, the insides of a Neotropic Cormorant, Phalacrocorax brasilianus, spread on the chest, were an antidote for a person suffering from asthma [14].
Pakistan currently has a diverse and dense bird population [1, 15–23] with almost 668 known species [24, 25], and a number of waterbird species are utilized by societies [2, 11, 12]. Very old associations have been developed between waterbirds and human societies, and these waterbird species are documented in the thoughts of human societies in various ways [26]. Waterbird species are generally documented in terms of their roles as entertainment, commercial, pets, magic, medicine [1, 2, 5, 8], or sources of food [3, 11], though these birds have other significant symbolic and medicinal relationships with humans [27]. Birds are among the fauna commonly utilized in ethnomedicine in Pakistan [1, 3–5, 11, 19, 28] and other countries on this planet [29, 30]. Anatidae and other waterbirds have cultural significance in many parts of the world [31]. Cultural uses of waterbird species (i.e., food, hunting, medicine, entertainment, religious practice, and trade) may promote beliefs and behaviors that help to conserve these species [32, 33]. However, if they are practiced unsustainably, or affected by commercialization or other political and economic factors, they may negatively affect or even endanger these species [33]. The use of waterbird species in traditional medicine and for cultural purposes by local communities must also be considered in relation to other factors, such as changes in climate and habitat [26, 34]. Because of anthropogenic influences, wetlands are rapidly diminishing biodiversity and risking freshwater supplies for waterbird habitats [35]. Waterbirds have been identified as being extremely sensitive to climate change [36] and even more vulnerable to changes in land cover or human-engineered land use [37]. Studies have also demonstrated that agricultural runoff into wetlands can reduce waterbird populations by contaminating the water with pesticides [38–40].
Waterbirds are major players in the aquatic ecosystem theater, providing a variety of important ecosystem services [33, 41]. Waterbirds can help to preserve the diversity of other species by controlling pests, acting as effective bio-indicators of environmental conditions, and responding as indicator species for potential disease outbreaks [33, 42]. They also provide essential provisioning (eggs, meat, feathers, etc.) and cultural services to both modern and indigenous societies [43, 44]. Various waterbird species are currently used for ethnomedicine, folklore drugs, and nanomedicine. However, there is an urgent need to investigate the ecological, cultural, social, and ethnomedicine aspects of waterbird species’ use for sustainable management and conservation of bio-resources. The main purpose of this study was to (i) document the cultural uses of waterbirds as well as traditional knowledge about the medicinal uses of waterbird species, and (ii) collect data on traditional therapies for a variety of diseases, including parts used, preparation methods, and applications, to preserve the traditional knowledge of medicinal uses against various human ailments.
Materials and methods
Description of the study area
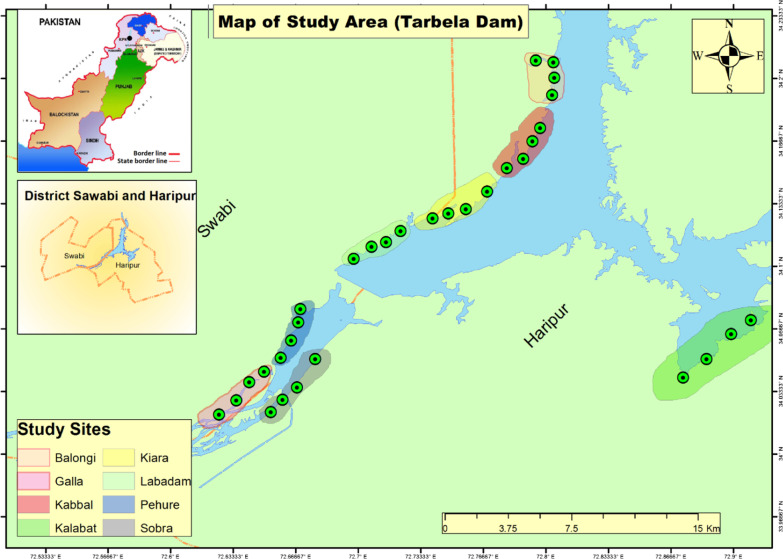
Swabi and Haripur, the most populous districts, are situated between the Indus River and Kabul River in Eastern KPK (Fig. 1). The Tarbela Dam is present on the Indus River (34° 7′ 35″ North, 72° 48′ 37″ East) in Haripur District, KPK, about 50 km northwest of Islamabad. The Indus is the largest flow through the Karakorum and Himalayan Mountains and passes from Tarbela Dam. The flow of water is higher in the period of monsoon compared with other seasons. Most of the area (78.0%) of the district is mountainous, and the rest (21.0%) is plain dry land. The climate of the study area is hot in summer (April to September) with maximum temperatures between 38 and 46 °C. June is the hottest month, and winters are relatively cold with minimum temperatures between 3 and 14 °C. The average rainfall recorded was 1026 mm/annum. The humidity is relatively high throughout the year [45]. Yousafzai are in majority and other tribes are Razar, Utman kheil, Jadoon, Khattak, and Hindkyan (Hindko speakers). Most of the people are directly or indirectly involved in agriculture [46]. According to a literature review, 60 percent of the population in this area derives their income from the forests [47]. A total of 29 mammalian species, 9 species of amphibians, 26 species of reptiles, and 89 species of waterbird including 68 migratory waterbird species have been documented from Tarbela Dam in Eastern Khyber Pakhtunkhwa, Pakistan [45, 48–52]. The unique characteristics of this region, such as significant temperature, altitude, geography, soil type, and moisture variation, make it extremely valuable from a medicinal point of view.
Fig. 1.
Map of the study area showing the sampling sites in Eastern KPK, Pakistan
Ethno-ornithology documentation and identification
During the field survey, the main focus was on quantifying, exploring, and comparing ethno-ornithological knowledge among different rural communities in the study area. The data were collected from the selected sub-areas such as Kalabat town, Kiara, Labadam, Pehur, Sobera, Balongi, Kabbal, and Gala from March 2019 to February 2020 (Fig. 1). Data on ethnomedicinal applications of waterbird species were obtained through semi-structured interviews and discussions using the methods previously described [53, 54]. The study's principal author is a local resident who visited the various places (high and low altitude) in the region with a photographer. Prior to collecting data, verbal consent was obtained from all local respondents after briefing the research objectives. Ethical guidelines of the International Society of Ethno-biology (http://www.ethnobiology.net/) were strictly followed. Questionnaires and semi-structured interviews were conducted with 100 informants, i.e., farmers, teachers, herdsmen, hunters, and traditional health practitioners (THPs). Informants were selected based on their traditional knowledge on the medicinal and cultural importance of bird species. Personal information of informants, local names of waterbird species, cultural importance of waterbird species, and ethno-pharmacological uses of waterbirds were all included in the questionnaires. Books of “Birds of Pakistan” were noted for correct identification of waterbird species of the region [50, 51]. The diversity of waterbirds in the study area was estimated through the linear count survey method, and the direct (i.e., physical count mean direct observation with camera and naked eye and voices) and indirect (i.e., nests and group questionnaire surveys or meetings) methods were utilized [55]. Moreover, the species’ scientific names were checked and corrected by using the Global Biodiversity Information Facility (https://www.gbif.org) and Catalogue of Life (https://www.catalogueoflife.org).
Ethical approval
The proposed research on birds, especially waterbird species, was duly approved by the Institutional Ethical Committee (IEC), PMAS-Arid Agriculture University Rawalpindi, Pakistan (Ref No. PMAS-AAUR/IEC/15), focusing on the ethnomedicinal research and intellectual property rights of informants before the field survey. In addition, the ethical guidelines and rules of the International Society of Ethno-biology (ISE) (http://www.ethnobiology.net/) were strictly followed.
Quantitative analysis
The waterbird data were observed using six various indices: “Principal Component Analysis,” “Relative Frequency of Citation,” “Fidelity Level,” “Relative Popularity Level,” “Rank Order Priority,” and “Similarity Index.”
Frequency of citation (FC)
“FC” presents the number of local participants who cited the ethnomedicinal uses of each waterbird species [11, 56].
Relative frequency of citation (RFC)
“RFC” presents the significance of all species from the region [57, 58]. RFC is calculated by dividing the number of informers citations of a particular waterbird species (FC) by the whole number of respondents in the study area (N) [53, 59]. “RFC” was analyzed by Eq. (1) as follows:
| 1 |
Fidelity level (FL%)
FL is the percentage of informers declaring the uses of an exacting kind of particular number of ethnomedicinal uses of diversity of birds through informer in the region. The FL index was analyzed utilizing Eq. (2) as follows [56, 60]:
| 2 |
where “Np” is the informers’ number for exacting types of ethnomedicinal uses of fauna and “N” is the total informers that noted the fauna for uses. A high “FL” index documented the significance and a high number of uses of fauna for ethno-cultural use by the informers of the region.
Relative popularity level (RPL)
The “RPL” is the proportion of the ethno-cultural use number by notifying fauna and the sum number of informers for sickness. Though waterbird species with similar “FL” values, however, were recognized by various informers, that may be different in their curing capability. A scale was as a result created as follows: All the waterbirds documented were separated into “popular” as well as “unpopular” factions. The “RPL” presumes a value “zero” and “one” with 1.0 being the total popularity of a particular waterbird species for major sickness and “zero” for no sickness cured by a particular species. While all livings are uniformly important for major sicknesses, a “popularity index” would be at a maximum of “one” and reduce toward “zero” as the relative popularity of the waterbird species deviates away from the popular part. For popular waterbirds, the “RPL” value is logically preferred to equivalent “1.0.” For waterbirds within “unpopular group,” the “RPL” value is lower than “one.” The “RPL” value may be resolute for each particular species in accordance with its accurate place on the grid [56, 61].
Rank order priority (ROP)
The “ROP” of the waterbirds is utilized to suitably rank the waterbirds with “FL” values and “RPL” values utilized as correction feature. The “ROP” is derived from “FL” multiplying with “RPL.” The “ROP” value was analyzed by Eq. (3) [56, 61].
| 3 |
Similarity index (SI)
SI is collected by the following formula (4):
| 4 |
Note that Sa = similar documented ailment in the previous and present study; Ta = total documented ailment in the present study.
Statistical analysis
We used multivariate ordination principal component analysis (PCA) to discover patterns of the ethno-cultural and ethnomedicinal uses of waterbird species by using the ethno-data variables. A one-way ANOVA was performed to check the significance of PCA scores. The contribution of different part use and mode of application were displayed in chord diagrams using “circlize package (24)” in R software 3.6.1 [62]. All graphical data analyses were performed using Microsoft Excel 2010 (Microsoft, Redmond, WA, USA), R software 3.6.3, and PAST 3.20 [63].
Results
Demographic features of respondents
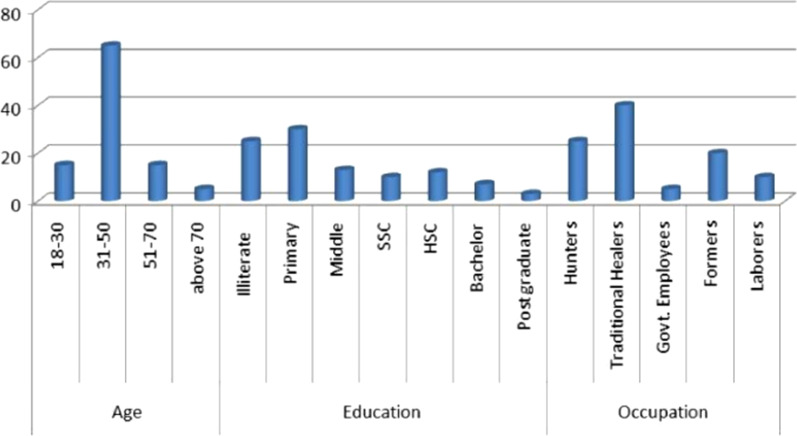
In total, 100 informers were selected from 18 to 75 years of age (Fig. 2). However, the maximum number of respondents (n = 64) was between the ages of 31 and 50 years. Approximately 75 respondents were literate, viz. primary (n = 30), middle (n = 13), secondary school certificate (SSC) (n = 10), higher school certificate (HSC) (n = 35), bachelor (n = 7), and post-graduate (n = 3). 79% of the respondents were from rural areas. The older informers have important traditional ecological knowledge as compared to the younger ones. Selected informants belong to different occupations such as hunters, traditional health practitioners (THPs), government employees, formers, and laborers (Fig. 2).
Fig. 2.
Number of study participants in Eastern KPK, Pakistan. Respondents of different age, occupation, and education were interviewed
Taxonomic classification
In total, 64 waterbird species from 9 orders and 17 families were reported (Table 1 and Fig. 3). Anseriformes was the most dominant order with 18 species, followed by Charadriiformes (16 species), Pelecaniformes (8 species), Passeriformes (7 species), Gruiformes (5 species), Coraciiformes (4 species), Laridae, Podicipediformes, and Suliformes (2 species each) (Table 1).
Table 1.
Ethno-ornithological applications among the local people of study area
| Sr. No. | Scientific name Common name (local name) |
Family (order) | FC | MD | FD | SPS | HN | EX | OR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ceryle rudis (Linnaeus, 1758) Pied Kingfisher (Mahe Khawarak) | Alcedinidae (Coraciiformes) | 16 | 0 | 0 | 2 | 0 | 0 | 15 |
| 2 | Alcedo atthis (Linnaeus, 1758) Common Kingfisher (Shentagh) | Alcedinidae (Coraciiformes) | 15 | 0 | 0 | 2 | 0 | 0 | 15 |
| 3 | Anas strepera Linnaeus, 1758 Gadwall (Khar sari batha/gadwall) | Anatidae (Anseriformes) | 81 | 39 | 55 | 11 | 79 | 6 | 26 |
| 4 | Anas crecca Linnaeus, 1758 Green-winged Teal (Warri choraki) | Anatidae (Anseriformes) | 79 | 42 | 60 | 11 | 79 | 10 | 26 |
| 5 | Anas platyrhynchos Linnaeus, 1758 Mallard (Sheen sari Batha) | Anatidae (Anseriformes) | 85 | 47 | 78 | 26 | 84 | 17 | 30 |
| 6 | Anas acuta Linnaeus, 1758 Northern Pintail (Laki mar Batha) | Anatidae (Anseriformes) | 78 | 39 | 65 | 11 | 78 | 3 | 23 |
| 7 | Anas clypeata Linnaeus, 1758 Northern Shoveler (Shabli) | Anatidae (Anseriformes) | 70 | 36 | 47 | 11 | 68 | 13 | 20 |
| 8 | Netta rufina (Pallas, 1773) Red-crested Pochard (Shabar) | Anatidae (Anseriformes) | 74 | 14 | 32 | 11 | 69 | 0 | 13 |
| 9 | Aythya ferina (Linnaeus, 1758) Common Pochard (Sor-sari Batha) | Anatidae (Anseriformes) | 80 | 27 | 72 | 17 | 76 | 13 | 15 |
| 10 | Aythya fuligula (Linnaeus, 1758) Tufted Duck (Ziar Stargi Batha) | Anatidae (Anseriformes) | 42 | 9 | 39 | 3 | 25 | 0 | 17 |
| 11 | Anas querquedula Linnaeus, 1758 Garganey (Kar kari/Gargany) | Anatidae (Anseriformes) | 67 | 45 | 63 | 0 | 45 | 7 | 12 |
| 12 | Anas Penelope Linnaeus, 1758 Eurasian Wigeon (Seti mar) | Anatidae (Anseriformes) | 70 | 48 | 60 | 5 | 68 | 5 | 11 |
| 13 | Bucephala clangula (Linnaeus, 1758) Common Goldeneye (Ziar Stargi Batha/ Zangli Charga) | Anatidae (Anseriformes) | 55 | 17 | 51 | 0 | 8 | 0 | 0 |
| 14 | Tadorna tadorna (Linnaeus, 1758) Common shelduck (Spena Batha) | Anatidae (Anseriformes) | 64 | 39 | 64 | 3 | 43 | 0 | 15 |
| 15 | Tadorna ferruginea (Pallas, 1764) Ruddy Shelduck (Sorhab) | Anatidae (Anseriformes) | 67 | 36 | 67 | 5 | 43 | 3 | 15 |
| 16 | Aythya nyroca (Güldenstädt, 1770) Ferruginous Duck (Seti mar wari Batha) | Anatidae (Anseriformes) | 38 | 32 | 37 | 3 | 25 | 2 | 12 |
| 17 | Mergellus albellus (Linnaeus, 1758) Smew (Spena Batha) | Anatidae (Anseriformes) | 36 | 26 | 36 | 3 | 15 | 0 | 7 |
| 18 | Anser anser (Linnaeus, 1758) Greylag Goose (Warri margabi) | Anatidae (Anseriformes) | 73 | 9 | 45 | 7 | 73 | 17 | 36 |
| 19 | Anser albifrons (Scopoli, 1769) Great White-fronted Goose (Ghatti margabi) | Anatidae (Anseriformes) | 73 | 9 | 45 | 7 | 73 | 17 | 36 |
| 20 | Mergus merganser Linnaeus, 1758 Common Merganser (Torsari Bathe) | Anatidae (Anseriformes) | 48 | 27 | 47 | 0 | 32 | 3 | 15 |
| 21 | Mesophoyx intermedia (Wagler, 1829) Intermediate Egret (Dermeiani Bagle) | Ardeidae (Pelecaniformes) | 18 | 0 | 0 | 7 | 0 | 0 | 6 |
| 22 | Egretta garzetta (Linnaeus, 1766) Little Egret (Warri-spene Bagle) | Ardeidae (Pelecaniformes) | 8 | 0 | 0 | 7 | 0 | 0 | 5 |
| 23 | Ardea cinerea Linnaeus, 1758 Grey Heron (Khari Bagle) | Ardeidae (Pelecaniformes) | 18 | 0 | 0 | 9 | 0 | 0 | 16 |
| 24 | Nycticorax nycticorax (Linnaeus, 1758) Black-crowned Night Heron (Taj-wala Bagle) | Ardeidae (Pelecaniformes) | 17 | 0 | 0 | 9 | 0 | 0 | 16 |
| 25 | Botaurus stellaris (Linnaeus, 1758) Eurasian Bittern (Eurasian Bagla) | Ardeidae (Pelecaniformes) | 10 | 0 | 0 | 3 | 0 | 0 | 9 |
| 26 | Ardeola grayii (Sykes, 1832) Indian Pond Heron (Mashriqi Bagla) | Ardeidae (Pelecaniformes) | 13 | 0 | 0 | 3 | 0 | 0 | 13 |
| 27 | Ardea alba Linnaeus, 1758 Great Egret (Gaati Bagla) | Ardeidae (Pelecaniformes) | 7 | 0 | 0 | 7 | 0 | 0 | 1 |
| 28 | Bubulcus ibis (Linnaeus, 1758) Cattle Egret (Zenawaro Bagla) | Ardeidae (Pelecaniformes) | 10 | 0 | 0 | 5 | 0 | 0 | 9 |
| 29 | Esacus recurvirostris (Cuvier, 1829) Great Stone-curlew (Ghatee Kharari) | Burhinidae (Charadriiformes) | 4 | 5 | 0 | 0 | 2 | 0 | 4 |
| 30 | Pluvialis squatarola (Linnaeus, 1758) Grey Plover (Kharari) | Charadriidae (Charadriiformes) | 14 | 5 | 12 | 0 | 1 | 0 | 4 |
| 31 | Charadrius alexandrinus Linnaeus, 1758 Kentish or Snowy Plover (Speni Kharari) | Charadriidae (Charadriiformes) | 10 | 5 | 5 | 0 | 2 | 0 | 3 |
| 32 | Vanellus indicus indicus (Boddaert, 1783) Red-wattled Lapwing (Sor titara) | Charadriidae (Charadriiformes) | 16 | 0 | 0 | 9 | 0 | 0 | 16 |
| 33 | Vanellus malabaricus (Boddaert, 1783) Yellow-wattled Lapwing (Zair titara) | Charadriidae (Charadriiformes) | 18 | 0 | 0 | 9 | 0 | 0 | 16 |
| 34 | Charadrius mongolus Pallas, 1776 Lesser Sand Plover (Warri Kharari) | Charadriidae (Charadriiformes) | 5 | 5 | 0 | 0 | 1 | 0 | 1 |
| 35 | Ciconia nigra (Linnaeus, 1758) Black stork (Tor Zanari) | Ciconiidae (Ciconiiformes) | 23 | 9 | 23 | 5 | 13 | 0 | 13 |
| 36 | Ciconia ciconia Linnaeus, 1758 White stork (Spen Zanari) | Ciconiidae (Ciconiiformes) | 28 | 7 | 28 | 5 | 13 | 0 | 13 |
| 37 | Grus grus (Linnaeus, 1758) Common Crane (Zanrai) | Gruidae (Gruiformes) | 38 | 0 | 23 | 0 | 37 | 11 | 26 |
| 38 | Haematopus ostralegus Linnaeus, 1758 Eurasian Oystercatcher (Mahe Khawarak) | Haematopodidae (Charadriiformes) | 15 | 0 | 0 | 0 | 0 | 0 | 13 |
| 39 | Riparia riparia (Linnaeus, 1758) Sand Martin (Khar Totakarki) | Hirundinidae (Passeriformes) | 21 | 0 | 0 | 21 | 0 | 0 | 3 |
| 40 | Cecropis daurica (Laxmann, 1769) Red-rumped Swallow (Sor Totakarki) | Hirundinidae (Passeriformes) | 5 | 0 | 0 | 5 | 0 | 0 | 2 |
| 41 | Ptyonoprogne obsoleta (Cabanis, 1850) Pale Crag Martin (Beranga Totakarki) | Hirundinidae (Passeriformes) | 22 | 0 | 0 | 21 | 0 | 0 | 2 |
| 42 | Larus ridibundus (Linnaeus, 1766) Common Black-headed Gull (Ghatti Torsari Bagle) | Laridae (Charadriiformes) | 5 | 0 | 0 | 3 | 0 | 0 | 2 |
| 43 | Larus marinus Linnaeus, 1758 Great Black-backed Gull (Ghatti obo Bagle) | Laridae (Charadriiformes) | 5 | 0 | 0 | 3 | 0 | 0 | 3 |
| 44 | Sterna acuticauda (Gray, 1832) Black-bellied tern (Totakarki) | Laridae (Charadriiformes) | 26 | 0 | 0 | 21 | 0 | 0 | 26 |
| 45 | Larus cachinnans Pallas, 1811 Caspian Gull (Obo Bagla) | Laridae (Charadriiformes) | 8 | 0 | 0 | 2 | 0 | 0 | 7 |
| 46 | Larus fuscus Linnaeus, 1758 Lesser Black-backed Gull (Warri-torsari Bagle) | Laridae (Charadriiformes) | 14 | 0 | 0 | 3 | 0 | 0 | 14 |
| 47 | Sterna hirundo Linnaeus, 1758 Common Tern (Kaaz/Babozi) | Laridae (Charadriiformes) | 13 | 1 | 0 | 13 | 7 | 0 | 8 |
| 48 | Motacilla alba Linnaeus, 1758 White Wagtail (Spina Chinchi lakai) | Motacillidae (Passeriformes) | 10 | 0 | 0 | 1 | 0 | 0 | 6 |
| 49 | Motacilla cinerea Tunstall, 1771 Grey Wagtail (Chinchi Lakai/Tan Tanai) | Motacillidae (Passeriformes) | 3 | 0 | 0 | 1 | 0 | 0 | 3 |
| 50 | Pelecanus onocrotalus Linnaeus, 1758 Great White Pelican (Kotanara) | Pelecanidae (Pelecaniformes) | 80 | 79 | 0 | 0 | 13 | 17 | 21 |
| 51 | Microcarbo niger (Vieillot, 1817) Little Cormorant (Warri-tore elli) | Phalacrocoracidae (Suliformes) | 55 | 36 | 54 | 0 | 46 | 0 | 15 |
| 52 | Phalacrocorax carbo (Linnaeus, 1758) Great Cormorant (Ghati-tore elli) | Phalacrocoracidae (Suliformes) | 54 | 36 | 54 | 0 | 46 | 0 | 15 |
| 53 | Podiceps cristatus (Linnaeus, 1758) Great Crested Grebe (Ghati grab) | Podicipedidae (Podicipediformes) | 57 | 21 | 57 | 11 | 35 | 3 | 13 |
| 54 | Tachybaptus ruficollis (Pallas, 1764) Little Grebe (Warri greb) | Podicipedidae (Podicipediformes) | 57 | 21 | 57 | 11 | 35 | 3 | 13 |
| 55 | Porphyrio porphyrio (Linnaeus, 1758) Purple swamphen (Jamani Charga) | Rallidae (Gruiformes) | 15 | 5 | 0 | 0 | 0 | 0 | 0 |
| 56 | Rallus aquaticus Linnaeus, 1758 Water Rail (Khawar chargai) | Rallidae (Gruiformes) | 10 | 3 | 0 | 0 | 0 | 0 | 8 |
| 57 | Fulica atra Linnaeus, 1758 Eurasian Coot (Jal Kokar) | Rallidae (Gruiformes) | 76 | 54 | 76 | 4 | 72 | 3 | 14 |
| 58 | Gallinula chloropus (Linnaeus, 1758) Common Moorhen (Obo Charga) | Rallidae (Gruiformes) | 8 | 2 | 0 | 0 | 0 | 0 | 7 |
| 59 | Gallicrex cinerea (Gmelin, 1789) Watercock (Zanglai Charga) | Rallidae (Gruiformes) | 7 | 7 | 0 | 0 | 0 | 0 | 6 |
| 60 | Himantopus himantopus (Linnaeus, 1758) Black-winged stilt (Tor tetari) | Recurvirostridae (Charadriiformes) | 8 | 3 | 0 | 0 | 0 | 0 | 5 |
| 61 | Recurvirostra avosetta Linnaeus, 1758 Pied avocet (Loi mahoki tetara) | Recurvirostridae (Charadriiformes) | 20 | 2 | 11 | 0 | 9 | 0 | 17 |
| 62 | Calidris temminckii (Leisler, 1812) Temminck’s stint (Saheli teteeri) | Scolopacidae (Charadriiformes) | 18 | 3 | 16 | 0 | 0 | 0 | 7 |
| 63 | Gallinago gallinago (Linnaeus, 1758) Common Snipe (Drum Tel) | Scolopacidae (Charadriiformes) | 10 | 3 | 3 | 0 | 1 | 0 | 8 |
| 64 | Tringa stagnatilis (Bechstein, 1803) Marsh Sandpiper (Drum Tel) | Scolopacidae (Charadriiformes) | 5 | 3 | 3 | 0 | 0 | 0 | 3 |
MD, medicinal; FD, food; SPS, superstitious; HN, hunting; EX, export; OR, ornamental
Fig. 3.
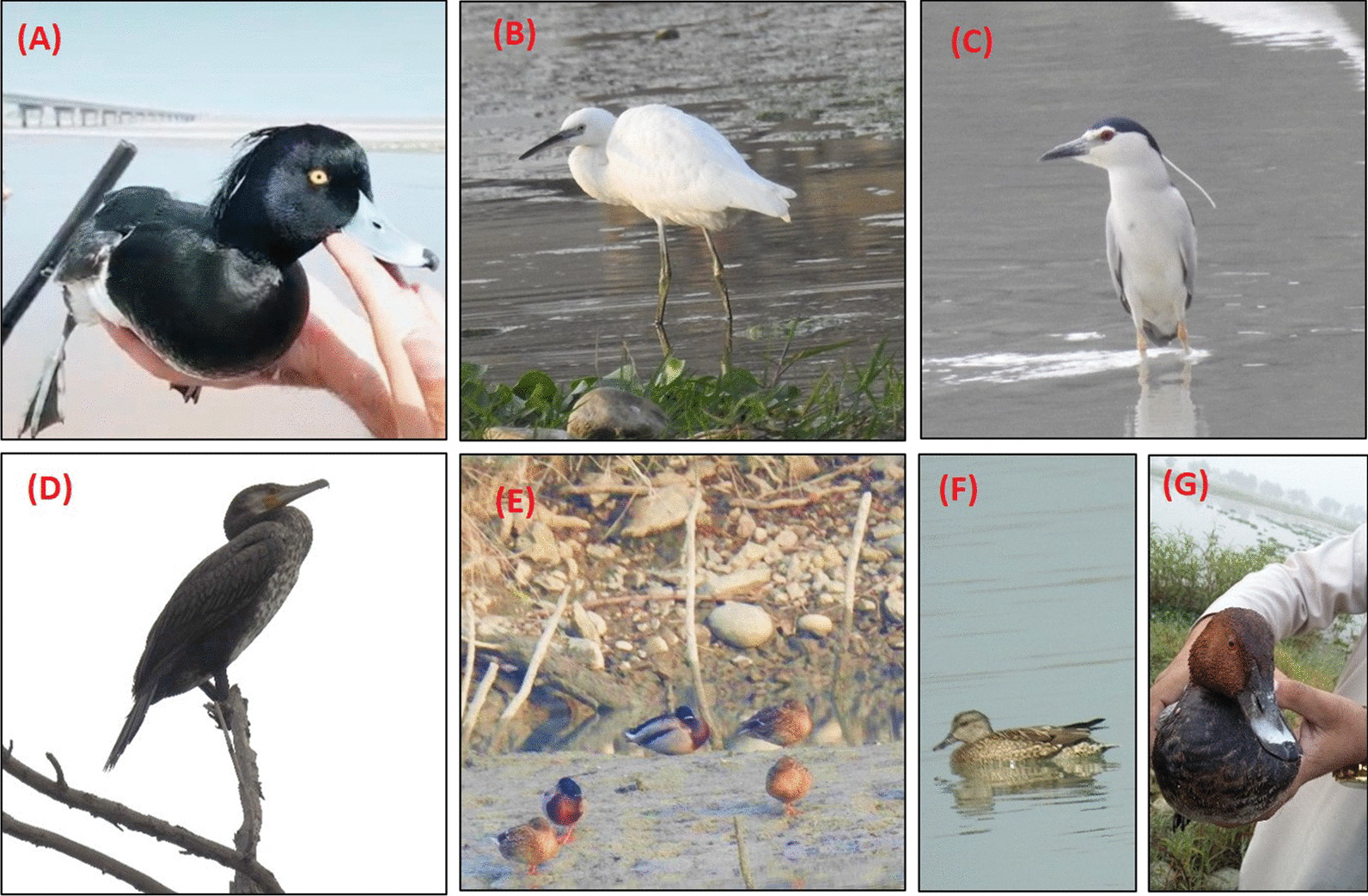
Some important waterbirds of the study area. A Tufted duck, B little white egret, C black-crowned night heron, D great cormorant, E mallard, F gadwall and G common pochard
Significant differences in the use of waterbird species
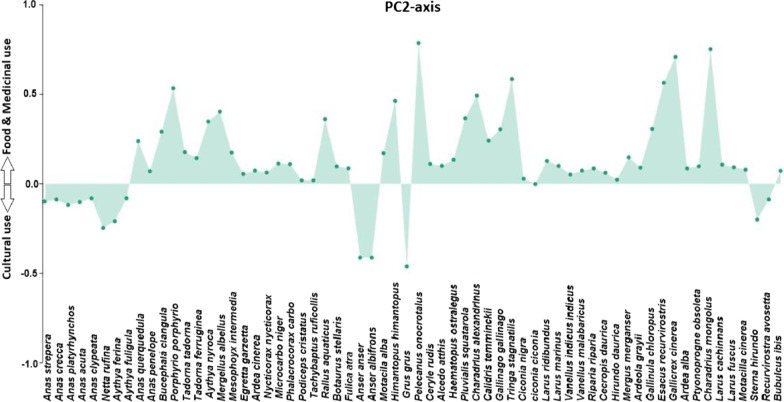
There were significant differences in the use of water bird species for cultural and medicinal purposes separated along the axis-2 (p < 0.05) as shown in Fig. 4. The significance of PCA scores was confirmed by one-way ANOVA, which calculated the analytic differences between cultural and medicinal use of waterbird species. PC1 and PC2 elucidated 86% of the variance in the PCA conducted with MD (medicinal), FD (food), SPS (superstitious), HN (hunting), EX (export), and OR (ornamental). Loadings of variables in PC2 showed that Anas strepera, Anas crecca, Anas platyrhynchos, Anas acuta, Anas clypeata, Netta rufina, Aythya ferina, Aythya fuligula, Anser anser, Anser albifrons, Grus grus, Sterna hirundo, Recurvirostra avosetta, and Bubulcus ibis were negatively correlated with cultural use value (Fig. 5).
Fig. 4.
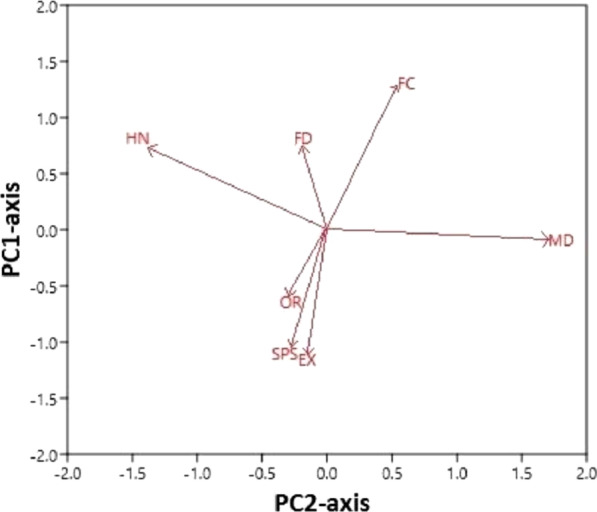
Principal component analysis of folklore data; codes are written in Table 1
Fig. 5.
Loadings of variables in PC2-axis separating the cultural, food, and medicinal use of waterbird species
Quantitative assessment of medicinal waterbird species
Relative frequency of citation (RFC)
The highest value of “relative frequency of citation” is documented in mallard (Anas platyrhynchos) as 4.06, followed by Gadwall (Anas strepera) (3.87), Common Pochard (Aythya ferina) (3.82), and Great White Pelican (Pelecanus onocrotalus) (3.82) (Table 2).
Table 2.
Ethnomedicinal uses of water bird species in the study area
| Sr. No. | Scientific name | Local name | DIS | Code | BPU | MOA | Ailments | RFC | FAC | FL | RPL | ROP | Reported use | SI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Anas strepera | Khar Sari batha | WV | AGS | Meat | Oral | Paralysis, cold, male impotency | 3.87 | 39 | 48.15 | 1.00 | 48.1 | 0 | |
| 2 | Anas crecca | Warri Chorak | WV | ACCT | Meat | Oral | Cough, cold, male impotency | 3.77 | 42 | 53.16 | 1.00 | 53.2 | 0 | |
| 3 | Anas platyrhynchos | Sheen sar Batha | WV | APM | Meat | Oral | Cancer, cough, cold, male impotency, diabetes, BP piles, arthritis, body sickness during pregnancy, fever, heart problems, cut and wound, eye pain, TB | 4.06 | 85 | 100 | 1.00 | 100 | Fever, weakness, BP, cancer, weight loss, eye [12], paralysis, weakness [11, 64], erectile dysfunction [65], TB [66] | 1 |
| 4 | Anas acuta | Laki mar Batha | WV | ACNT | Meat | Oral | Male impotency, cough, cold, | 3.72 | 39 | 50.00 | 1.00 | 50.0 | 0 | |
| 5 | Anas clypeata | Shabli | WV | ACS | Meat | Oral | Cough, cold, male impotency | 3.34 | 36 | 51.43 | 1.00 | 51.4 | 0 | |
| 6 | Netta rufina | Shabar | WV | NRRP | Meat | Oral | Cough, cold, male impotency | 3.53 | 14 | 18.92 | 1.00 | 18.9 | 0 | |
| 7 | Aythya ferina | Sor-sari Batha | WV | AFCP | Meat | Oral | Paralysis, male impotency | 3.82 | 27 | 33.75 | 1.00 | 33.8 | 0 | |
| 8 | Aythya fuligula | Ziar Stergi Batha | WV | AFTD | Meat | Oral | Cough, cold, male impotency | 2.00 | 9 | 21.43 | 0.99 | 21.2 | 0 | |
| 9 | Anas querquedula | Gergani | WV | AQG | Meat | Oral | Cough, cold, male impotency | 3.20 | 45 | 67.16 | 1.00 | 67.2 | 0 | |
| 10 | Anas penelope | Seti mar Batha | WV | MPW | Meat | Oral | Cough, cold, male impotency | 3.34 | 48 | 68.57 | 1.00 | 68.6 | 0 | |
| 11 | Bucephala clangula | Zangli Charga | WV | BC | Meat | Oral | Cough, cold, male impotency | 2.63 | 17 | 30.91 | 1.00 | 30.9 | 0 | |
| 12 | Porphyrio porphyrio | Jamani Charga | R | PPH | Egg | Oral | Cough, asthma | 0.72 | 5 | 33.33 | 0.35 | 11.8 | 0 | |
| 13 | Tadorna tadorna | Spena Batha | WV | TTS | Meat | Oral | Male impotency, cough, cold, | 3.05 | 39 | 60.94 | 1.00 | 60.9 | 0 | |
| 14 | Tadorna ferruginea | Sorhab | WV | TTRS | Meat | Oral | Cough, cold, male impotency | 3.20 | 36 | 53.73 | 1.00 | 53.7 | 0 | |
| 15 | Aythya nyroca | Seti mar wari Batha | WV | ANFD | Meat | Oral | Cough, cold, male impotency | 1.81 | 32 | 84.21 | 0.89 | 75.3 | 0 | |
| 16 | Mergellus albellus | Spena Bata | WV | MASD | Meat | Oral | Cough, cold, male impotency | 1.72 | 26 | 72.22 | 0.85 | 61.2 | 0 | |
| 17 | Microcarbo niger | Warri tori Heley | R | MNLC | Meat | Oral | Cough, body pain | 2.63 | 36 | 65.45 | 1.00 | 65.5 | 0 | |
| 18 | Phalacrocorax carbo | Gati Tori Heley | R | BSEB | Meat | Oral | Cough, body pain | 2.58 | 36 | 66.67 | 1.00 | 66.7 | 0 | |
| 19 | Podiceps cristatus | Ghat greb | WV | PCCG | Meat | Oral | Cold, cough, male impotency | 2.72 | 21 | 36.84 | 1.00 | 36.8 | 0 | |
| 20 | Tachybaptus ruficollis | Warri Grab | R | TRLB | Meat | Oral | Cough, cold, male impotency | 2.72 | 21 | 36.84 | 1.00 | 36.8 | 0 | |
| 21 | Rallus aquaticus | Khawar cherge | R | RAWR | Egg | Oral | Asthma | 0.48 | 3 | 30.00 | 0.24 | 7.1 | 0 | |
| 22 | Fulica atra | Jal kokar | R | FAC | Meat | Oral | Male impotency, cold | 3.63 | 54 | 71.05 | 1.00 | 71.1 | 0 | |
| 23 | Anser anser | Warri mergabi | WV | AAGG | Meat | Oral | Arthritis, body pain | 3.48 | 9 | 12.00 | 1.00 | 12.3 | 0 | |
| 24 | Anser albifrons | Ghati mergabi | WV | AAG | Meat | Oral | Arthritis, body pain | 3.48 | 9 | 12.00 | 1.00 | 12.3 | 0 | |
| 25 | Himantopus himantopus | Tor Tetare | R | HHBS | Meat | Oral | Kidney stone | 0.38 | 3 | 37.50 | 0.19 | 7.1 | 0 | |
| 26 | Pelecanus onocrotalus | Kotanra | WV | POGP | Fat, skin | Oral | Arthritis, body Pain | 3.82 | 79 | 98.75 | 1.00 | 98.8 | 0 | |
| 27 | Pluvialis squatarola | Kherari | WV | FSGP | Meat | Oral | Cold | 0.67 | 5 | 35.71 | 0.33 | 11.8 | 0 | |
| 28 | Charadrius alexandrinus | Speni Kherari | WV | CASK | Meat | Oral | Cold | 0.48 | 5 | 50.00 | 0.24 | 11.8 | 0 | |
| 29 | Calidris temminckii | Saheli Tetari | WV | CTTS | Bone | Oral | TB, kidney stone | 0.86 | 3 | 16.67 | 0.42 | 7.1 | 0 | |
| 30 | Gallinago gallinago | Drum tel | WV | GGCS | Meat, bone | Oral | Piles | 0.48 | 3 | 30.00 | 0.24 | 7.1 | 0 | |
| 31 | Tringa stagnatilis | Drum tel | WV | TSMS | Meat, bone | Oral | Piles | 0.24 | 3 | 60.00 | 0.12 | 7.1 | 0 | |
| 32 | Ciconia nigra | Tor Zarhi | WV | CNBS | Meat, fat, skin | Oral | Male impotency, arthritis | 1.10 | 9 | 39.13 | 0.54 | 21.2 | 0 | |
| 33 | Ciconia ciconia | Spen Zarhi | WV | CCWS | Meat, fat, skin | Oral | Male impotency, arthritis | 1.34 | 7 | 25.00 | 0.66 | 16.5 | 0 | |
| 34 | Mergus merganser | Tor sar Bata | WV | MMCM | Meat | Oral | Respiratory disorder, body pain, male impotency | 2.29 | 27 | 56.25 | 1.00 | 56.3 | 0 | |
| 35 | Gallinula chloropus | Obo Charga | R | GCCM | Egg | Oral | Asthma | 0.38 | 2 | 25.00 | 0.19 | 4.7 | 0 | |
| 36 | Esacus recurvirostris | Ghatee kharare | R | ERGT | Meat | Oral | Body weakness | 0.19 | 4 | 100 | 0.09 | 11.8 | 0 | |
| 37 | Gallicrex cinerea | Zanglai Charga | R | GCWC | Egg | Oral | Asthma | 0.33 | 7 | 100 | 0.16 | 16.5 | 0 | |
| 38 | Charadrius mongolus | Warri Kharari | R | CMLP | Meat | Oral | Cold | 0.24 | 5 | 100 | 0.12 | 11.8 | 0 | |
| 39 | Sterna hirundo | Kaaz/babozi | S | SHCT | Meat | Oral | Gastric ulcer, obesity | 0.62 | 1 | 7.69 | 0.31 | 2.4 | 0 | |
| 40 | Recurvirostra avosetta | Loi mahoki tetara | S | RAPA | Meat | Oral | Male impotency, cold | 0.95 | 2 | 10.00 | 0.47 | 4.7 | 0 |
DIS, distribution; WV, winter visited; R, resident; S, summer breeder; BPU, body parts used; MOA, mode of application; FC, frequency of citation; FAC, frequency of ailment citation; FC, fidelity level; RPL, relative popularity level; ROP, rank order priority; SI, similarity index
Relative popularity level (RPL)
We documented 40 species that are used in ethno-pharmacological applications. Of total, 20 birds’ species, i.e., Gadwall, green-winged teal, mallard, northern pintail, northern shoveler, red-crested pochard, common pochard, garganey, Eurasian wigeon, common golden eye, common shelduck, ruddy shelduck, little cormorant, great cormorant, great crested grebe, little grebe, Eurasian coot, graylag goose, great white-fronted goose, great white pelican, and common merganser, were found more popular by respondents and have the highest “RPL” value (RPL = 1.00) (Fig. 6).
Fig. 6.
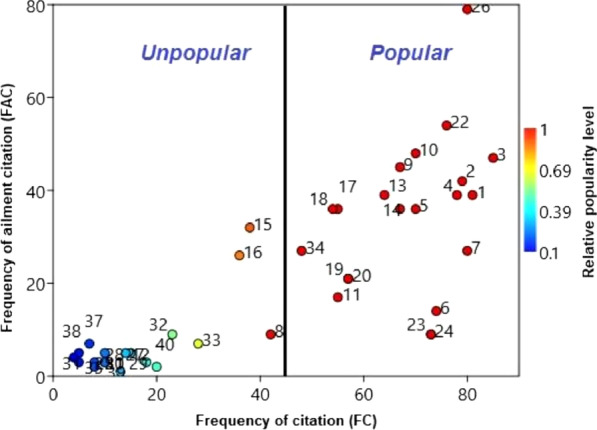
Relationship between numbers of informants (FC) claimed use of certain waterbird species for particular ailment (FAC). The species' relative popularity level (RPL) is determined and classified as popular or unpopular. Numbers represent the species names as they appear in Table 2
Fidelity level (FL%)
“Fidelity level” of waterbird species varied from 12 to 100%. A 100% “fidelity level” was calculated for only four waterbird species, i.e., mallard, great stone-curlew, watercock, and lesser sand plover. A total of 10 bird species showed an “FL%” value greater than 60%, i.e., common shelduck (60.94%), little cormorant (65.45%), great cormorant (66.67%), garganey (67.16%), Eurasian wigeon (68.57%), Eurasian coot (71.05%), smew (72.22%), ferruginous duck (84.21%), and great white pelican (98.75%) (Table 2).
Rank order priority (ROP)
The “rank order priority” is utilized to determine the appropriate position of species of birds with different “fidelity level” values and the “rank order priority” (Table 2). In total, only 7 species attained a value of “rank order priority” above 60. These species are common shelduck (60.9), smew (61.2), little cormorant (65.5), great cormorant (66.7), garganey (67.2), Eurasian wigeon (68.6), Eurasian coot (71.1), ferruginous duck (75.3), and great white pelican (98.8) (Table 2).
Discussion
Socio-demographic data
Gathering socio-demographic data on participants (gender, age, educational level, occupation, and ethnicity) is particularly beneficial in ethnobiological research, as this element plays a significant role in analyzing and interpreting the responses received [67]. The older respondents, particularly those aged over 30 years, were highly populated in the study area (Fig. 2) and possessed significantly more traditional knowledge compared to younger participants. Community elders are often the holders of the most species information [68]. They are engaged in family responsibilities such as finance, health, and education and do not pass their knowledge to the next generation. As a result, knowledge of medicinal waterbird usage is diminishing. Similar research conducted in Pakistan and other countries showed that older respondents had significant traditional knowledge than younger participants [69–72].
Educated individuals in the study region were found to be less knowledgeable about the use of medicinal waterbirds than illiterate people, due to their higher exposure to modernization. Similar findings were reported in the research studies conducted in southern KPK [73] and central Punjab [11].
Temporal shifts of folk knowledge and local nomenclature
According to traditional health practitioners (THPs), knowledge about the use of medicinal waterbird(s) was derived from either one or more of these sources: (i) medicinal knowledge regarding the use of waterbird(s) was passed from generation to generation within the family, (ii) folk knowledge was gained from teachers, religious scholars, and hakeems, (iii) knowledge was gained from reading published traditional folklore books, (iv) knowledge was obtained by experimentation with waterbird species, which was then applied on humans, (v) traditional knowledge was gained in aspirations, and (vi) a comparable assortment of medicinal waterbird(s) to treat any specific ailment of the human body parts. Transfer of cultural knowledge and traditional information from parents to children, preferably to sons, was found to be the most prevalent, as in other communities across the world [74–79]. Moreover, local taxonomy represents the vernacular names of species which give clues about social associations, myths, morphological differences, and ecology [80].
Folklore and cultural applications of waterbird species
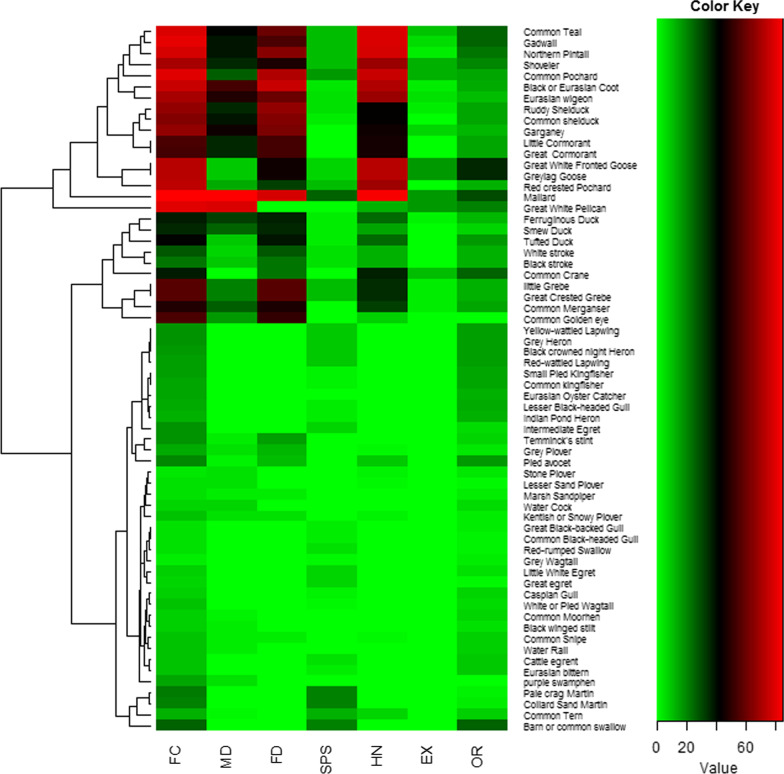
Some waterbird species are more used as food, medicine, and hunting, e.g., mallard, common teal, gadwall, northern pintail, shoveler, common pochard, Eurasian coot, Eurasian wigeon, garganey, great white-fronted goose, graylag goose, little cormorant, great cormorant, and red-crested pochard (Fig. 7). In total, 40 waterbird species were utilized as foodstuffs in the study area (Table 1). A total of 18 species are exported from the study area, while the feathers of 62 waterbird species are utilized in decoration (Table 1 and Fig. 7). Waterbird species are also utilized as food, according to other ornithologists [2, 3, 11].
Fig. 7.
Heatmap of waterbird species usage by informants for MD (medicinal), FD (food), SPS (superstitious), HN (hunting), EX (export), and OR (ornamental) purposes in Eastern KPK, Pakistan. Green and red colors indicate increased and decreased values of informants, respectively
Forty-four species of birds are connected with superstitious beliefs, such as people of the local area believing that ducks (i.e., gadwall, common teal, mallard, northern pintail, northern shoveler, red-crested pochard, common pochard, tufted duck, garganey, European wigeon, common shelduck, ruddy shelduck, ferruginous duck, smew, great crested grebe, Eurasian coot, and little grebe), kingfishers (i.e., pied kingfisher and common kingfisher), and gooses (i.e., graylag goose and great white-fronted goose) are a sign of prosperity. The following are superstitions about egrets (i.e., intermediate egret, little white egret, cattle egret, and great egret) and terns (i.e., black-bellied tern and common tern): If someone harms egrets, it will be bad luck (i.e., gray heron, Indian pond heron, and black-crowned night heron). It is documented that herons are a sign of bad luck if they are present at home. Superstition about storks (black stork and white stork) is that when storks lay down their heads and necks back over their bodies at this time, it means a storm will come. Gulls are also superstitious in the study area, as if three gulls (i.e., common black-headed gull, Caspian gull, lesser black-headed gull, and great black-backed gull) are flying directly over a person; it is a sign of the death of this person. Likewise, it is noted that if red-rumped swallows and martens (i.e., sand martin and pale crag martin) are settled in any house, it is a sign of poverty. Similarly, lapwings (yellow-wattled lapwing and red-wattled lapwing) have superstitions that if these birds cry at your house, it is a sign of a visitor (Table 1). These findings were also documented by other ornithologists [5, 11, 81].
Ethnomedicinal uses of waterbird species
The meat of waterbird species was the most utilized body part in the study area (Fig. 8). Meat of waterbird species was commonly used to treat various human ailments such as respiratory disorders, gastric ulcers, arthritis, obesity, body pain, and piles (Fig. 9). People specifically hunted waterbirds for meat. Cold, cough, fever, flu, bronchitis, breathing problems, infertility, asthma, abscess, anemia, body weakness, body strength, enhanced memory, immune enhancer, epilepsy, menorrhagia, paralysis, puberty in young girls, skin diseases, sexual power, and wound healing are all treated with meat from various waterbird species [1, 3, 7, 17, 28, 82–86]. The inhabitants of the study area also use fat to treat arthritis, body pain, and male impotency (Table 2 and Fig. 8). In fact, the presence of “omega-3 fatty acid” in fat cures inflammation [87]. Moreover, “omega-3 fatty acid” is also useful in atherosclerosis, thrombotic, neurological disorders, and aging effects [10, 88–90].
Fig. 8.
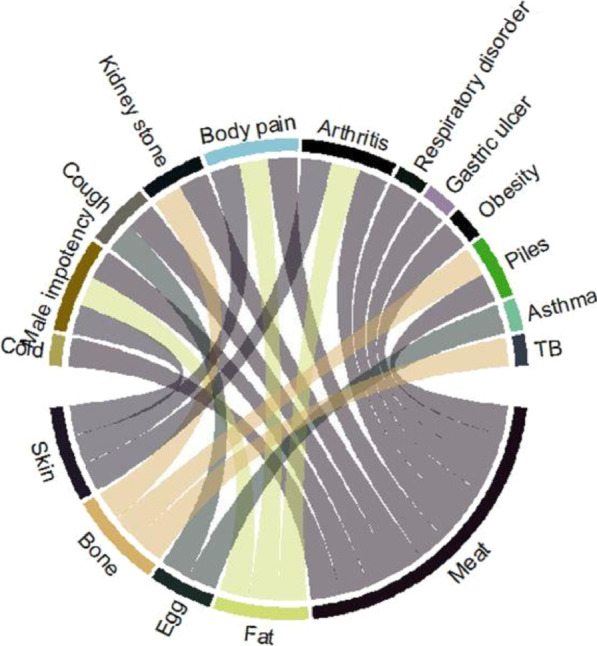
Relationship between body parts and diseases used in the study area
Fig. 9.
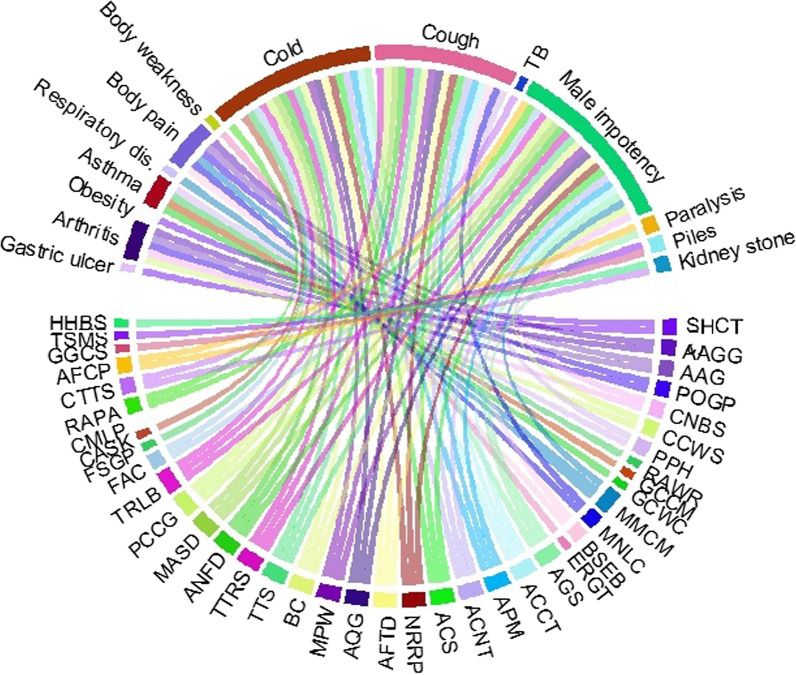
Waterbird species distribution according to the treatment of various ailments in Eastern KPK, Pakistan. Codes represent the species names as they appear in Table 2
It was found that local inhabitants of the study area used various waterbird species to treat different infectious and chronic diseases like cold, cough, flu, fever, respiratory disorders, asthma, TB, gastric ulcers, kidney stones, male impotency, obesity, paralysis, piles, cancer, arthritis, body pain, and weakness (Fig. 9). Other studies also reported that waterbird species were used to treat respiratory disorders (asthma, pneumonia, and cough), cardiovascular disorders, and skin infections [11, 91]. Moreover, waterfowl are a major part of the diet of indigenous people at high latitudes in North America [92]. The main reasons for the higher number of diseases in this remote area might be a lack of exercise, nutritional deficiency, and a polluted environment. However, THPs are more familiar with the use of parts and products of waterbird species for the treatment of various human ailments. Some of the local inhabitants hunted bird species and sold them in local markets or to hakeems, normally at low prices. THPs use the products or parts of waterbird species in suitable seasons or at specific times. Many THPs kept written notes for medicinal preparations but usually did not share such information publicly, so as not to increase the number of practitioners.
The separating line between the popular and unpopular groups falls at the point where the average number of uses per species ceases to increase with a further increase in the number of informants (Fig. 8). Based on the RPL index analysis, we found certain popular species that are utilized to cure a greater number of diseases in the study region, i.e., mallard, gadwall, green-winged teal, garganey, Eurasian wigeon, and Eurasian coot. The high popularity of these plant species might be attributed to their high efficacy which specifies their use as therapeutic medicine. Moreover, 100% FL was noted for four waterbird species, i.e., Charadrius mongolus (cold), Gallicrex cinerea (asthma), Anas platyrhynchos (cancer), and Esacus recurvirostris (body weakness). Mainly, waterbird species with 100% FL are utilized more in the traditional healthcare system of the study area [93, 94]. The high familiarity of waterbird species might be recognized by their wider distribution, diversity, and familiarity with the people of the study area, which specifies their use in ethno-pharmacological applications. These findings are supported by other ethnobiologists [56, 61]. Waterbird species with high RPL and FL values showed the importance of these species and are proposed for further pharmacological evaluation to analyze their therapeutic potential and for screening of unknown bioactive chemicals.
Critical analysis of medicinal waterbird species
The ethno-pharmacological data were calculated using PCA, which assigned the six variables for the ordination of designs in terms of MD, FD, SPS, HN, EX, and OR. It is clear from our results that local residents used the waterbird species more for medicinal and food purposes (Fig. 5). Previous results showed that wild birds are used as a source of food in many areas of the world, i.e., India [95, 96], Pakistan [11, 28], Philippines [97], and Brazil [91, 98]. However, statistical analysis is highly valuable in ethnobiological studies because it provides important information for pharmacological and clinical studies.
Waterbird species are used to treat different human ailments, which reflects that the people of Eastern KPK have more information to control the healthcare system and that traditional pharmacological applications have not been eliminated from the culture. The high usage of waterbird species may be due to the abundance and widespread dispersion of these species in the study area. Furthermore, traditional medicine for curing various ailments may also result in high RFC, RPL, and FL [99–101]. In this study, mallard was the most popular species in Eastern KPK with high FL (100%), which show the abundance and wider use of this species' by-products for cancer treatment (Table 2). In their study, Altaf, Umair [12] reported that mallard was used to treat cancer by the local communities of Punjab, Pakistan.
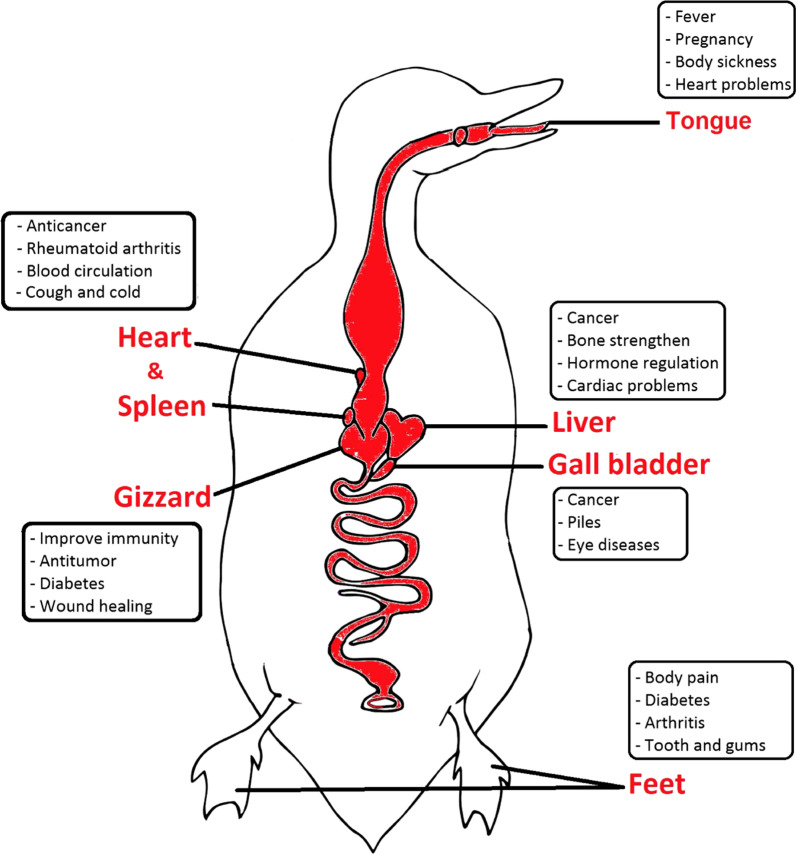
Most wild duck by-products, such as liver, gizzard, heart, and spleen (Fig. 10), are rich sources of essential nutrients and polyunsaturated fatty acids [102]. In comparison with other tissues, El-Sayed, Farag [103] found that the liver and gizzard are the best sources of high-quality protein. A high-protein diet has been demonstrated to boost metabolism, control appetite, and enhance muscle growth and preservation during weight reduction [104, 105]. Despite this, it is also high in minerals and vitamins, including copper, vitamin A, and several essential amino acids [106]. Trace elements are also known as microelements and are essential for bone formation, hormone production, and heart rate regulation [107, 108]. Furthermore, all of the by-products, particularly the liver, had larger quantities of microelements (e.g., Cu, Fe, Mn, and Zn) than muscle tissues [109]. Copper (Cu) is an essential microelement, and the human body requires only a minimal amount [108]. According to Garber [110], copper has higher antioxidant properties and can help to fight cancer.
Fig. 10.
Graphical representation of the medicinal uses of mallard (Anas platyrhynchos) in Eastern KPK, Pakistan
Liver and fat are used to treat swelling wounds and pneumonia [66], influenza, bronchitis, asthma [111], blisters, and skin problems [112, 113]. Duck tongue meat is said to be especially beneficial to people recovering from illness and to alleviate body sickness during pregnancy. In another study, duck bile is used to treat cancer, traumatic hemorrhage, and dyspepsia [114, 115]. Likewise, duck gizzard peptides can provide a plentiful source of natural antioxidants for applications in the food industry [116]. The gizzard is a low-fat, high-protein organ that has great natural levels of iron and zinc [103]. These nutrients support a healthy immune system, promote wound healing, and aid in cell division. The dark-colored large duck hearts are very low in calories, and in terms of their nutritional value, they are as good as the hearts of other animals [117]. Both the heart and spleen are rich in protein and saturated fatty acids, which are helpful in improving blood circulation and curing cancer, cough, cold, and rheumatoid arthritis [118]. Duck feet are a natural source of glucosamine, chondroitin, and collagen [119], which provide joint health by producing joint fluid, reducing the risk of brittle bones, improving mobility, and helping maintain healthy teeth and gums.
Bio-conservation or sustainable use of the reported species
For the design and integration of biodiversity conservation plans, understanding the knowledge of human–animal interaction and the use of natural resources is critical [120]. However, the documentation of indigenous knowledge on animal-based medicines is very helpful in the formulations of strategies for sustainable management and conservation of bio-resources [121]. Ethno-ornithological studies, in addition to integrating biological factors and giving traditional knowledge on medicinal values of species in any region, also cover social, economic, traditional, and cultural values of animal species in human societies and thus make a significant contribution in animal conservation [26].
Use of waterbird species in traditional therapies and for cultural purpose by humans is not the only threat to bird diversity in any region. Factors also include changes in climate and various types of interactions in an ecosystem, i.e., food chain and food webs also contribute significantly to threatening waterbird population and diversity [26, 34]. Given the great need to find solutions to deal with the current crisis of biodiversity loss [122], more specifically that of bird species, it is obligatory to adopt strategies that address the problem in all its complexity. And for this, ethnozoology presents itself as an interdisciplinary tool, approaching the issue in an additional comprehensive method [123].
Novelty of the study
The current study is a collective effort that includes both documenting and cross-cultural comparisons of the reported species in order to better understand the different waterbirds usage traditions. We found a high degree of overlap in the use of specific waterbirds among ethnic groups. Because of their food value, certain species were found to be more prevalent in all cultures. Moreover, the collected data are unique because these waterbird species have no previous records. We found that all waterbird species have a 0.00 “similarity index.” Only 1 species (i.e., mallard) has a 1.00 similarity index and has been reported for ethnomedicine applications previously. In the current study, this species was used to treat cancer, cough, cold, male impotency, diabetes, BP piles, arthritis, body sickness during pregnancy, fever, heart problems, cut, wounds, eye pain, and TB, while in reported use, this species was used to cure fever, weakness, colds, BP, cancer, weight loss, eye pain [12], paralysis, weakness [64], erectile dysfunction [65], and TB [66].
Conclusion
To treat human ailments, the local inhabitants of Eastern KPK used 40 species of waterbirds. The present collected data showed that a lot of medicinal waterbird species are used by confined societies. The native people still rely on traditional medicine in Eastern KPK instead of the presence of other healthcare departments; thus, medicinal waterbird species have significant value in treating a variety of human ailments. Compiled data showed that high RFC, FL, RPL, and ROP values showed that popular waterbird species are the most preferred for specific ailments. These results could be helpful for the sustainable use of waterbird species in the traditional healthcare system. However, the main threats to the diversity of waterbirds in the area are hunting, trading, and cultural use.
Acknowledgements
The authors are thankful for the help of Govt. employees and people in Eastern KPK. They are grateful to local participants for sharing their traditional knowledge.
Author contributions
QR and MU prepared the first draft; QR, MAJ, and MSN were involved in field surveys and data collection; AP, SA, JN, MHH, and TS critically revised the manuscript; QR, MU, MA, and AMA were involved in data analysis, interpolation, and final write up. All authors read and approved the final manuscript.
Funding
We have not received any funding for this study.
Availability of data and materials
All the data are presented in tables and figures in the article or as supplementary material, and further inquiries can be directed to the corresponding authors.
Declarations
Ethics approval and consent to participate
The proposed research on birds (waterbird species) was duly approved by the Institutional Ethical Committee (IEC), PMAS-Arid Agriculture University Rawalpindi, Pakistan (Ref No. PMAS-AAUR/IEC/15), before the field survey concerning ethnomedicinal data collection and intellectual property rights of informants. We declared that all curative properties are simply described as ethnographic folklore with no proven beneficial effect on human health. This study is based on a field survey rather than human or animal trails. However, verbal consent was taken from participants regarding data collection and publication. In addition, the ethical guidelines and rules of the International Society of Ethno-biology (ISE) (http://www.ethnobiology.net/) were strictly followed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Umair, Email: umair.bot@gmail.com, Email: umairm@zjnu.edu.cn.
Muhammad Altaf, Email: altafweco@gmail.com.
References
- 1.Mughal S, Pervaz M, Bashir SM, Shamashad SS. Assessment of diversity and ethnopharmacological uses of birds in Chakar, Hattian Bala district, Azad Jammu and Kashmir—Pakistan. J Wild Ecol. 2020;4:35–44. [Google Scholar]
- 2.Rauf K, Altaf M, Mumtaz B, Altaf M, Haider R, Safeer B, et al. Assessment of behavior, distribution, ecology and interaction study of Cinnamon Tree Sparrow (Passer rutilans) in district Bagh-Pakistan. J Wild Ecol. 2017;1:43–49. [Google Scholar]
- 3.Hakeem F, Altaf M, Manzoor S, Rauf K, Mumtaz B, Bashir M, et al. Assessment of behavioral study, human activities impacts and interaction with Streak laughingthrush (Trochalopteron lineatum) in district Bagh, Azad Jammu and Kashmir-Pakistan. J Wild Ecol. 2017;1:1–7. [Google Scholar]
- 4.Haider R, Altaf M, Rasheed Z, Rauf K, Mumtaz B, Altaf M, et al. Assessment of behavioral study, human activities impacts and interaction with white cheeked bulbul (Pycnonotus leucotis) in district Bagh, Azad Jammu and Kashmir, Pakistan. J Wild Ecol. 2017;1:17–24. [Google Scholar]
- 5.Bashir SM, Rashid Z, Mumtaz B, Altaf M, Rauf K, Haider R, et al. Assessment of behavioral ecology, folklore and medicinal uses of Barn Swallow (Hirundo rustica) in district Bagh-Pakistan. J Wild Ecol. 2018;2:13–21. [Google Scholar]
- 6.Bibby CJ, Jones M, Marsden S. Bird surveys. London: Expedition Advisory Centre; 1998. [Google Scholar]
- 7.Haidar R, Bashir SM. Chemical composition, traditional and modern uses of meat of animals-a review. J Wild Ecol. 2021;5:47–55. [Google Scholar]
- 8.Adil S, Tariq S. Study of traditional and modern applications of feathers-a review. J Wild Ecol. 2020;4:141–150. [Google Scholar]
- 9.Tariq S. Chemical composition and traditional uses of eggs of different avian species—a review. J Wild Ecol. 2020;4:45–50. [Google Scholar]
- 10.Ijaz S, Faiz M. Chemical composition, folk and modern uses of fats and oil—a review. J Wild Ecol. 2021;5:104–110. [Google Scholar]
- 11.Altaf M, Javid A, Umair M, Iqbal KJ, Rasheed Z, Abbasi AM. Ethnomedicinal and cultural practices of mammals and birds in the vicinity of river Chenab, Punjab-Pakistan. J Ethnobiol Ethnomed. 2017;13:41. doi: 10.1186/s13002-017-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altaf M, Umair M, Abbasi AR, Muhammad N, Abbasi AM. Ethnomedicinal applications of animal species by the local communities of Punjab, Pakistan. J Ethnobiol Ethnomed. 2018;14:55. doi: 10.1186/s13002-018-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tidemann S, Chirgwin S, Sinclair JR. Indigenous knowledges, birds that have ‘spoken’ and science. In: Ethno-ornithology. Routledge; 2012. pp. 25–34.
- 14.Del Hoyo J, Elliott A, Sargatal J. Handbook of the birds of the world. Barcelona: Lynx Edicions; 1992. [Google Scholar]
- 15.Jadoon A, Bibi S, Rehman A. Birds’ population in district Haripur, Khyber Pakhtunkhwa. Pakistan J Wild Ecol. 2019;3:18–25. [Google Scholar]
- 16.Ashraf S, Kanwal S, Haider MS, Altaf M. Diversity of birds in rural and urban habitats of district Sargodha, Pakistan. J Wild Ecol. 2018;2:26–36. [Google Scholar]
- 17.Ali A, Khan MSH, Altaf M. Winter survey of birds at district of the Badin, Pakistan. J Wild Ecol. 2018;2:11–22. [Google Scholar]
- 18.Altaf M, Javid A, Khan AM, Umair M, Irfan S, Ashraf M, et al. Assessment of water fowl diversity of River Chenab Pakistan. J Anim Plant Sci. 2015;25:382–388. [Google Scholar]
- 19.Altaf M. Assessment of avian and mammalian diversity at selected sites along river Chenab. In: Wildlife and ecology. University of Veterinary and Animal Sciences, Lahore; 2016. p. 197.
- 20.Altaf M, Javid A, Khan AM, Khan M, Umair M, Ali Z. Anthropogenic impact on the distribution of the birds in the tropical thorn forest, Punjab, Pakistan. J Asia Pac Biodivers. 2018;11:229–236. doi: 10.1016/j.japb.2018.03.001. [DOI] [Google Scholar]
- 21.Ali A, Altaf M, Khan MSH, Khan AM, Ashraf S, Chattha SA. Avifauna diversity along the coastline of Banbhore (Gharo creek), district Thatta, Sindh, Pakistan. J Wild Ecol. 2017;1:8–16. [Google Scholar]
- 22.Ashraf S, Riaz A, Muhammad N. Assessments of avian diversity of Uchhali lake. Pakistan J Wild Ecol. 2019;3:8–15. [Google Scholar]
- 23.Ali A, Khan MSH, Altaf M. Analysis of anthropogenic activities on avian diversity along the coastal landscape of Sindh, Pakistan. J Wild Ecol. 2020;4:94–110. [Google Scholar]
- 24.Grimmett R, Inskipp C, Inskip T. Birds of the Indian subcontinent. Christopher Helm an imprint of A and C Black (Publisher) Ltd, London; 1998.
- 25.Mirza ZB, Wasiq H. A field guide to birds of Pakistan. Lahore: Bookland; 2007. [Google Scholar]
- 26.Alves RR. Relationships between fauna and people and the role of ethnozoology in animal conservation. Ethnobiol Conserv. 2012;1:1–69. [Google Scholar]
- 27.Alves RR, Rosa IL, Neto NAL, Voeks R. Animals for the gods: magical and religious faunal use and trade in Brazil. Hum Ecol. 2012;40:751–780. doi: 10.1007/s10745-012-9516-1. [DOI] [Google Scholar]
- 28.Arshad M, Ahmad M, Ahmed E, Saboor A, Abbas A, Sadiq S. An ethnobiological study in Kala Chitta hills of Pothwar region, Pakistan: multinomial logit specification. J Ethnobiol Ethnomed. 2014;10:13. doi: 10.1186/1746-4269-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alves RR, Alves HN. The faunal drugstore: animal-based remedies used in traditional medicines in Latin America. J Ethnobiol Ethnomed. 2011;7:9. doi: 10.1186/1746-4269-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bezerra DMM, de Araujo HFP, Alves ÂGC, Alves RRN. Birds and people in semiarid northeastern Brazil: symbolic and medicinal relationships. J Ethnobiol Ethnomed. 2013;9:3. doi: 10.1186/1746-4269-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kear J. Man and wildfowl. London: Poyser, A&C Black; 2010. [Google Scholar]
- 32.Diamond J. The world until yesterday: What can we learn from traditional societies? Penguin; 2013.
- 33.Green AJ, Elmberg J. Ecosystem services provided by waterbirds. Biol Rev. 2014;89:105–122. doi: 10.1111/brv.12045. [DOI] [PubMed] [Google Scholar]
- 34.Alves RRN, Silva JS, da SilvaChaves L, Albuquerque UP. Ethnozoology and animal conservation. In: Ethnozoology. Elsevier; 2018. pp. 481–496.
- 35.Cantonati M, Poikane S, Pringle CM, Stevens LE, Turak E, Heino J, et al. Characteristics, main impacts, and stewardship of natural and artificial freshwater environments: consequences for biodiversity conservation. Water. 2020;12:260. doi: 10.3390/w12010260. [DOI] [Google Scholar]
- 36.Bateman BL, Wilsey C, Taylor L, Wu J, LeBaron GS, Langham G. North American birds require mitigation and adaptation to reduce vulnerability to climate change. Conser Sci Prac. 2020;2:e242. [Google Scholar]
- 37.Saunders SP, Meehan TD, Michel NL, Bateman BL, DeLuca W, Deppe JL, et al. Unraveling a century of global change impacts on winter bird distributions in the eastern United States. Glob Change Biol. 2022;28:2221–2235. doi: 10.1111/gcb.16063. [DOI] [PubMed] [Google Scholar]
- 38.Ackerman JT, Eagles-Smith CA. Agricultural wetlands as potential hotspots for mercury bioaccumulation: experimental evidence using caged fish. Environ Sci Technol. 2010;44:1451–1457. doi: 10.1021/es9028364. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly JP, King SL, Silverman NL, Collins DP, Carrera-Gonzalez EM, Lafón-Terrazas A, et al. Climate and human water use diminish wetland networks supporting continental waterbird migration. Glob Change Biol. 2020;26:2042–2059. doi: 10.1111/gcb.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Zhang S, Li Z, Lu X, Yang Q. Impacts on wetlands of large-scale land-use changes by agricultural development: the small Sanjiang Plain, China. Ambio. 2004;33:306–310. doi: 10.1579/0044-7447-33.6.306. [DOI] [PubMed] [Google Scholar]
- 41.Wenny DG, Devault TL, Johnson MD, Kelly D, Sekercioglu CH, Tomback DF, et al. The need to quantify ecosystem services provided by birds. Auk. 2011;128:1–14. doi: 10.1525/auk.2011.10248. [DOI] [Google Scholar]
- 42.Whelan CJ, Şekercioğlu ÇH, Wenny DG. Why birds matter: from economic ornithology to ecosystem services. J Ornithol. 2015;156:227–238. doi: 10.1007/s10336-015-1229-y. [DOI] [Google Scholar]
- 43.Mahendiran M, Azeez P. Ecosystem services of birds: a review of market and non-market values. Entomol Ornithol Herpetol. 2018;7:2161-0983. [Google Scholar]
- 44.Dennis JR. The effects of land use change, climate change, and conservation on waterbird habitat in the Upper Klamath Basin. Oregon State University; 2022. [Google Scholar]
- 45.GOP. Environmental and social assessment. Water and Power Development Authority and National Transmission and Despatch Company; 2016. p. 100.
- 46.Khalid M, Bilal M, Hassani D, Zaman S, Huang D. Characterization of ethno-medicinal plant resources of karamar valley Swabi. Pakistan J Radiat Res Appl Sci. 2017;10:152–163. doi: 10.1016/j.jrras.2017.03.005. [DOI] [Google Scholar]
- 47.Rabbi F, Bauer S, Idalinya J. Contribution of forests to rural inequality reduction: present scope and future options for rural development and sustainable use of forests. Int J Sustain Dev World Ecol. 2010;17:4–14. doi: 10.1080/13504500903488271. [DOI] [Google Scholar]
- 48.Bhutia, TK. Tarbela Dam dam, Pakistan. 2018.
- 49.Khan MS. Amphibians and reptiles of Pakistan. Florida: Krieger Publishing Company Malabar; 2006. [Google Scholar]
- 50.Roberts TJ. The birds of Pakistan. Karachi: Oxford University Press; 1992. p. 617. [Google Scholar]
- 51.Roberts TJ. The birds of Pakistan. Karachi Oxford University Press; 1991. p. 598. [Google Scholar]
- 52.Rafique A, Burian S, Hassan D, Bano R. Analysis of operational changes of Tarbela Reservoir to improve the water supply, hydropower generation, and flood control objectives. Sustainability. 2020;12:7822. doi: 10.3390/su12187822. [DOI] [Google Scholar]
- 53.Saleem R, Altaf M, Umair M, Amjad MS, Abbasi AM. Ethnopharmacological applications of the amphibians and reptiles among the people in the vicinity of Margalla Hill National Park, Islamabad, Pakistan. J Wild Ecol. 2021;5:13–25. [Google Scholar]
- 54.Khan A, Mehmood S, Khan RA. Ethnobotanical study of some wild herb medicinal Xerophytes of district Bannu, Khyber Pakhtunkhwa, Pakistan. J Wild Ecol. 2017;1:37–51. [Google Scholar]
- 55.Rahman Q, Nadeem M, Altaf M, Khan S, Saeed A, Naseer J, et al. Assessment of anthropogenic-causing-agents act on waterbirds-diversity in the vicinity of Tarbela Dam, Indus River, Pakistan. Braz J Biol. 2021;84. [DOI] [PubMed]
- 56.Friedman J, Yaniv Z, Dafni A, Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J Ethnopharmacol. 1986;16:275–287. doi: 10.1016/0378-8741(86)90094-2. [DOI] [PubMed] [Google Scholar]
- 57.Ilker U, Suleyman B, Nurettin Y, Yunus D. The investigation and quantitative ethnobotanical evaluation of medicinal plants used around Izmir province, Turkey. J Med Plant Res. 2009;3:345–367. [Google Scholar]
- 58.Vitalini S, Iriti M, Puricelli C, Ciuchi D, Segale A, Fico G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—an alpine ethnobotanical study. J Ethnopharmacol. 2013;145:517–529. doi: 10.1016/j.jep.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Tardío J, Pardo-de-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain) 1. Econ Bot. 2008;62:24–39. doi: 10.1007/s12231-007-9004-5. [DOI] [Google Scholar]
- 60.Alexiades MN, Sheldon JW. Selected guidelines for ethnobotanical research: a field manual. Boranx: The New York Botanical Garden; 1996. [Google Scholar]
- 61.Ali-Shtayeh MS, Yaniv Z, Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J Ethnopharmacol. 2000;73:221–232. doi: 10.1016/S0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 62.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 63.Hammer Ø, Harper D, Ryan P. Paleontological statistics software: package for education and data analysis. Palaeontol Electron. 2001;1:9. [Google Scholar]
- 64.Medeiros Costa Neto E. Traditional use and sale of animals as medicines in Feira de Santana City, Bahia, Brazil. Indigenous Knowledge and Development Monitor (Netherlands); 1999.
- 65.Mootoosamy A, Mahomoodally MF. A quantitative ethnozoological assessment of traditionally used animal-based therapies in the tropical island of Mauritius. J Ethnopharmacol. 2014;154:847–857. doi: 10.1016/j.jep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Kendie FA, Mekuriaw SA, Dagnew MA. Ethnozoological study of traditional medicinal appreciation of animals and their products among the indigenous people of Metema Woreda, North-Western Ethiopia. J Ethnobiol Ethnomed. 2018;14:1–12. doi: 10.1186/s13002-018-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Easthope G. Ethnicity and health. In: Macmillan NJ, Lupton G, editors. Sociology of health and illness: Australian readings. Sydney: Macmillan Education AU; 1995. pp. 143–161. [Google Scholar]
- 68.Nolan JM, Turner NJ. Ethnobotany: the study of people-plant relationships. Ethnobiology. 2011;9:135–141. [Google Scholar]
- 69.Wanjohi BK, Sudoi V, Njenga EW, Kipkore WK. An ethnobotanical study of traditional knowledge and uses of medicinal wild plants among the Marakwet Community in Kenya. Evid Based Complement Alternat Med. 2020;2020. [DOI] [PMC free article] [PubMed]
- 70.Amjad MS, Zahoor U, Bussmann RW, Altaf M, Gardazi SMH, Abbasi AM. Ethnobotanical survey of the medicinal flora of Harighal, Azad Jammu & Kashmir, Pakistan. J Ethnobiol Ethnomed. 2020;16:1–28. doi: 10.1186/s13002-020-00417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Umair M, Altaf M, Abbasi AM. An ethnobotanical survey of indigenous medicinal plants in Hafizabad district, Punjab-Pakistan. PLoS ONE. 2017;12:e0177912. doi: 10.1371/journal.pone.0177912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kayode J, Omotoyinbo MA. Ethnobotanical utilization and conservation of chewing sticks plants species in Ekiti state, Nigeria. Res J Bot. 2009;4:1–9. doi: 10.3923/rjb.2009.1.9. [DOI] [Google Scholar]
- 73.Mussarat S, Ali R, Ali S, Mothana RA, Ullah R, Adnan M. Medicinal animals and plants as alternative and complementary medicines in southern regions of Khyber Pakhtunkhwa, Pakistan. Front Pharmacol. 2021;1764. [DOI] [PMC free article] [PubMed]
- 74.Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20:127–134. [Google Scholar]
- 75.Uniyal SK, Singh K, Jamwal P, Lal B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J Ethnobiol Ethnomed. 2006;2:14. doi: 10.1186/1746-4269-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Upadhyay B, Roy S, Kumar A. Traditional uses of medicinal plants among the rural communities of Churu district in the Thar Desert, India. J Ethnopharmacol. 2007;113:387–399. doi: 10.1016/j.jep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124:69–78. doi: 10.1016/j.jep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Panghal M, Arya V, Yadav S, Kumar S, Yadav JP. Indigenous knowledge of medicinal plants used by Saperas community of Khetawas, Jhajjar District, Haryana, India. J Ethnobiol Ethnomed. 2010;6:4. doi: 10.1186/1746-4269-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Islam MK, Saha S, Mahmud I, Mohamad K, Awang K, Uddin SJ, et al. An ethnobotanical study of medicinal plants used by tribal and native people of Madhupur forest area, Bangladesh. J Ethnopharmacol. 2014;151:921–930. doi: 10.1016/j.jep.2013.11.056. [DOI] [PubMed] [Google Scholar]
- 80.Clément D. The historical foundations of ethnobiology (1860–1899) J Ethnobiol Ethnomed. 1998;18:161–161. [Google Scholar]
- 81.Farooq A, Kayani AK. Prevalence of superstitions and other supernaturals in rural Punjab: a sociological perspective. J South Asia Stud. 2012;5:335–344. [Google Scholar]
- 82.Bagde N, Jain S. Study of traditional man-animal relationship in Chhindwara district of Madhya Pradesh, India. J Glob Biosci. 2015;4:1456–1463. [Google Scholar]
- 83.Vijayakumar S, Yabesh JM, Prabhu S, Ayyanar M, Damodaran R. Ethnozoological study of animals used by traditional healers in Silent Valley of Kerala, India. J Ethnopharmacol. 2015;162:296–305. doi: 10.1016/j.jep.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 84.Chattha SA, Malik MF, Altaf M, Mahmood S, Khan J, Ali A, et al. Human pursuits cause of road killing of wild and domestic animals by accident on National Highway of Punjab, Pakistan. J Wild Ecol. 2017;1:8–16. [Google Scholar]
- 85.Aloufi A, Eid E. Zootherapy: a study from the northwestern region of the kingdom of Saudi Arabia and Hashemite Kingdom of Jordan. Ind J Trad Knowl. 2016;15:561–569. [Google Scholar]
- 86.Vijayakumar S, Prabhu S, Yabesh JM, Prakashraj R. A quantitative ethnozoological study of traditionally used animals in Pachamalai hills of Tamil Nadu, India. J Ethnopharmacol. 2015. [DOI] [PubMed]
- 87.Wilson L. Fats and oils for optimum health. The Center for Development; 2015.
- 88.Breteler MM. Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging. 2000;21:153–160. doi: 10.1016/S0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 89.Kalmijn S. Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies. J Nut Health Aging. 2000;4:202–207. [PubMed] [Google Scholar]
- 90.Haag M. Essential fatty acids and the brain. Can J Psych. 2003;48:195–203. doi: 10.1177/070674370304800308. [DOI] [PubMed] [Google Scholar]
- 91.Alves RRN, Leite RCL, Souto WMS, Bezerra DMM, Loures-Ribeiro A. Ethno-ornithology and conservation of wild birds in the semi-arid Caatinga of northeastern Brazil. J Ethnobiol Ethnomed. 2013;9:14–14. doi: 10.1186/1746-4269-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krcmar E, van Kooten GC, Chan-McLeod A. Waterfowl harvest benefits in northern aboriginal communities and potential climate change impacts. 2010.
- 93.Bibi T, Ahmad M, Tareen RB, Tareen NM, Jabeen R, Rehman S-U, et al. Ethnobotany of medicinal plants in district Mastung of Balochistan province-Pakistan. J Ethnopharmacol. 2014;157:79–89. doi: 10.1016/j.jep.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 94.Srithi K, Balslev H, Wangpakapattanawong P, Srisanga P, Trisonthi C. Medicinal plant knowledge and its erosion among the Mien (Yao) in northern Thailand. J Ethnopharmacol. 2009;123:335–342. doi: 10.1016/j.jep.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 95.Chinlampianga M, Singh R, Shukla AC, Soil C. Ethnozoological diversity of Northeast India: empirical learning with traditional knowledge holders of Mizoram and Arunachal Pradesh. 2013.
- 96.Jaroli DP, Mahawar MM, Vyas NJ. An ethnozoological study in the adjoining areas of Mount Abu wildlife sanctuary, India. J Ethnobiol Ethnomed. 2010;6:6–6. doi: 10.1186/1746-4269-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ploeg J, Weerd Mv. Agta bird names: an ethno-ornithological survey in the Northern Sierra Madre Natural Park, Philippines. Forktail. 2010;26:127–131. [Google Scholar]
- 98.Teixeira PHR, Thel TDN, Ferreira JMR, de Azevedo SM, Júnior WRT, Lyra-Neves RM. Local knowledge and exploitation of the avian fauna by a rural community in the semi-arid zone of northeastern Brazil. J Ethnobiol Ethnomed. 2014;10:1–10. doi: 10.1186/1746-4269-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amjad MS, Qaseem MF, Ahmad I, Khan SU, Chaudhari SK, Zahid Malik N, et al. Correction: Descriptive study of plant resources in the context of the ethnomedicinal relevance of indigenous flora: a case study from Toli Peer National Park, Azad Jammu and Kashmir, Pakistan. PLoS ONE. 2017;12:e0180917. doi: 10.1371/journal.pone.0180917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farooq A, Amjad MS, Ahmad K, Altaf M, Umair M, Abbasi AM. Ethnomedicinal knowledge of the rural communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. J Ethnobiol Ethnomed. 2019;15:1–30. doi: 10.1186/s13002-019-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kayani S, Ahmad M, Zafar M, Sultana S, Khan MPZ, Ashraf MA, et al. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies-Abbottabad, Northern Pakistan. J Ethnopharmacol. 2014;156:47–60. doi: 10.1016/j.jep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Cobos A, Veiga A, Diaz O. Chemical and fatty acid composition of meat and liver of wild ducks (Anas platyrhynchos) Food Chem. 2000;68:77–79. doi: 10.1016/S0308-8146(99)00164-8. [DOI] [Google Scholar]
- 103.El-Sayed SM, Farag SE, El-Sayed SA. Utilization of some chicken edible internal organs and wheat germ in production of sausage. J Food Dairy Sci. 2018;9:353–358. doi: 10.21608/jfds.2018.36027. [DOI] [Google Scholar]
- 104.Baeza E. The meat of duck: production and main characteristics. 1995.
- 105.Adeola O. Review of research in duck nutrient utilization. Int J Poult Sci. 2006;5:201–218. doi: 10.3923/ijps.2006.201.218. [DOI] [Google Scholar]
- 106.Savran E, Pavlova V. Amino acid makeup of the soft by-products of various poultry species. Vopr Pitan. 1980:71–74. [PubMed]
- 107.Nollet LM, Toldrá F. Analysis of edible animal by-products. 2011.
- 108.García-Llatas G, Alegría A, Barberá R, Farré R. 11 minerals and trace elements. Boca Raton: CRC Press; 2011. [Google Scholar]
- 109.Seong PN, Cho SH, Park KM, Kang GH, Park BY, Moon SS, et al. Characterization of chicken by-products by mean of proximate and nutritional compositions. Korean J Food Sci Anim Resour. 2015;35:179. doi: 10.5851/kosfa.2015.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garber K. Targeting copper to treat breast cancer. American Association for the Advancement of Science; 2015. [DOI] [PubMed]
- 111.Costa-Neto EM, Motta PC. Animal species traded as ethnomedicinal resources in the Federal District, Central West Region of Brazil. Open Complement Med J. 2010;2:24–30. doi: 10.2174/1876391X01002020024. [DOI] [Google Scholar]
- 112.Kim H, Song M-J. Analysis of ethnomedicinal practices for treating skin diseases in communities on Jeju Island (Korea) Ind J Trad Knowl. 2014;13:673–680. [Google Scholar]
- 113.de Queiroz Dias D, Sales DL, Andrade JC, da Silva ARP, Tintino SR, de Morais Oliveira-Tintino CD, et al. Body fat modulated activity of Gallus gallus domesticus Linnaeus (1758) and Meleagris gallopavo Linnaeus (1758) in association with antibiotics against bacteria of veterinary interest. Microb Pathog. 2018;124:163–169. doi: 10.1016/j.micpath.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 114.Bi Y, Yi L, Zhang L, Sun Y, Li M. Ethnomedicine investigation of the medicinal plants and animals in Daur, Inner Mongolia, China. J Ethnobiol Ethnomed. 2020.
- 115.Wang DQ, Carey MC. Therapeutic uses of animal biles in traditional Chinese medicine: an ethnopharmacological, biophysical chemical and medicinal review. World J Gastroenterol. 2014;20:9952. doi: 10.3748/wjg.v20.i29.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Su W, Tang S, Xie C, Mu Y, Li Z, Yang X, et al. Antioxidant and DNA damage protection activities of duck gizzard peptides by chemiluminescence method. Int J Food Prop. 2016;19:760–767. doi: 10.1080/10942912.2015.1043605. [DOI] [Google Scholar]
- 117.Wen B, Chen Z, Qu H, Gao J. Growth and fatty acid composition of discus fish Symphysodon haraldi given varying feed ratios of beef heart, duck heart, and shrimp meat. Aquac Fish. 2018;3:84–89. doi: 10.1016/j.aaf.2018.01.002. [DOI] [Google Scholar]
- 118.Weng W, Chen J. The eastern perspective on functional foods based on traditional Chinese medicine. Nutr Rev. 1996;54:S11. doi: 10.1111/j.1753-4887.1996.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 119.Seidavi A, Zaker-Esteghamati H, Scanes C. Chicken processing: impact, co-products and potential. World’s Poult Sci J. 2019;75:55–68. doi: 10.1017/S0043933918000764. [DOI] [Google Scholar]
- 120.Albuquerque UP, de Sousa DCP. Ethnobiology and biodiversity conservation. In: Introduction to ethnobiology. Springer; 2016. pp. 227–232.
- 121.Borah MP, Prasad SB. Ethnozoological study of animals based medicine used by traditional healers and indigenous inhabitants in the adjoining areas of Gibbon Wildlife Sanctuary, Assam, India. J Ethnobiol Ethnomed. 2017;13:1–13. doi: 10.1186/s13002-017-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boivin NL, Zeder MA, Fuller DQ, Crowther A, Larson G, Erlandson JM, et al. Ecological consequences of human niche construction: examining long-term anthropogenic shaping of global species distributions. Proc Natl Acad Sci. 2016;113:6388. doi: 10.1073/pnas.1525200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dickman AJ. Complexities of conflict: the importance of considering social factors for effectively resolving human–wildlife conflict. Anim Conserv. 2010;13:458–466. doi: 10.1111/j.1469-1795.2010.00368.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are presented in tables and figures in the article or as supplementary material, and further inquiries can be directed to the corresponding authors.