Abstract
Escherichia coli Nissle 1917 has been used as a probiotic against intestinal disorders for many decades. It is a good colonizer of the human gut and has been reported to be able to express type 1 fimbriae. Type 1 fimbriae are surface organelles which mediate α-d-mannose-sensitive binding to various host cell surfaces. The expression is phase variable, and two tyrosine recombinases, FimB and FimE, mediate the inversion of the fimbrial phase switch. Current evidence suggests that FimB can carry out recombination in both directions, whereas FimE-catalyzed switching is on to off only. We show here that under liquid shaking growth conditions, Nissle 1917 did not express type 1 fimbriae, due to a truncation of the fimB gene by an 1,885-bp insertion element. Despite its fimB null status, Nissle 1917 was still capable of off-to-on switching of the phase switch and expressing type 1 fimbriae when grown under static conditions. This phase switching was not catalyzed by FimE, by truncated FimB, or by information residing within the insertion element. No further copies of fimB seemed to be present on the chromosome of Nissle 1917, suggesting that another tyrosine recombinase in Nissle 1917 is responsible for the low-frequency off-to-on inversion of the phase switch that is strongly favored under static growth conditions. This is the first report documenting the non-FimB- or non-FimE-catalyzed inversion of the fim switch.
Escherichia coli Nissle 1917, of serotype O6:K5:H1, is an excellent colonizer of the human gut (27, 28, 39). This strain was originally isolated during World War I from a soldier who escaped a severe outbreak of diarrhea affecting his detachment. Nissle 1917, alias SK22 or DSM6601 (7, 28), seems to have a beneficial effect on several types of intestinal disorders and has been marketed as a probiotic for several decades (30, 31, 39, 42). The strain has been reported to be able to colonize and establish itself in the human intestine even in the presence of a natural resident bacterial flora. This ability has been variously attributed to the capacity of Nissle 1917 to produce microcins, siderophores, and adhesins. Nissle 1917 has been reported to produce the type 1 fimbrial adhesin (7).
Bacterial adherence is normally a prerequisite for successful colonization of a specific host tissue. The best-characterized group of bacterial adhesins is that of fimbriae (for reviews, see reference 17). The most common and best characterized of the enterobacterial adhesive surface organelles are type 1 fimbriae. Type 1 fimbriae are found on the majority of E. coli strains and are widespread among other members of the Enterobacteriaceae (20).
A type 1 fimbria is a thin, 7-nm-wide and approximately 1-μm-long, rod-shaped surface organelle. It is a heteropolymer consisting of four different subunits. Approximately 1,000 copies of the major building element, FimA, are polymerized into a right-handed helical structure also containing small percentages of the minor components, FimF, FimG, and FimH (9, 18, 22). It has been shown that the receptor-recognizing element of type 1 fimbriae is the 30-kDa FimH protein (21). The FimH protein is located at the tip and also is interspersed along the fimbrial shaft (15, 21). The FimF and FimG components are probably required for integration of the FimH adhesin into the fimbriae (15, 18).
By virtue of the FimH adhesin, type 1-fimbriated bacteria confer α-d-mannose-sensitive agglutination of a number of eucaryotic cell types displaying this molecular motif, such as certain erythrocytes and yeast cells. Interactions between type 1 fimbriae and receptor structures have been shown to play a key role in the colonization by E. coli of various host tissues in a number of studies (4, 44). Recently, the adhesion conferred by type 1 fimbriae in an E. coli strain was shown to be linked to urinary tract pathogenesis (11, 40). In mouse models, immunization with FimH was shown to prevent urogenital mucosal infection by E. coli (24).
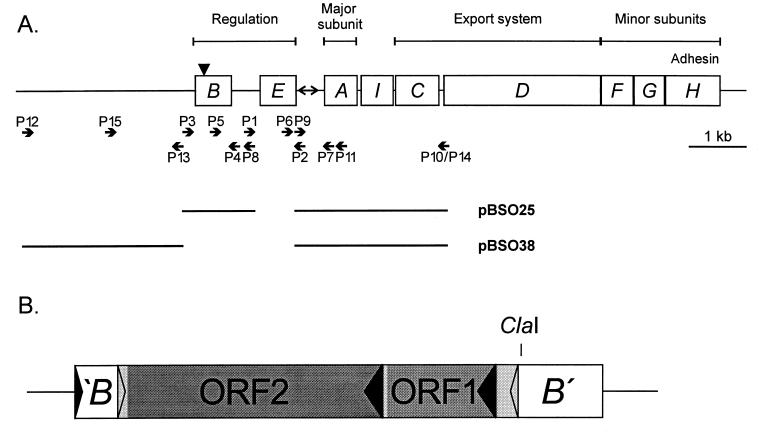
The chromosomally located fim gene cluster (19) encodes the components of the fimbrial organelle. In addition to structural components, this 9.5-kb DNA segment also encodes the fimbrial biosynthesis machinery as well as regulatory elements (Fig. 1). Expression is phase variable, with individual cells switching between fimbriated and nonfimbriated states. This characteristic is due to inversion of a 314-bp DNA segment located immediately upstream of the fimA gene, encoding the major subunit protein (1). A promoter residing in this phase switch drives the expression of the fim genes (33) when the switch is in the on orientation but not when it is in the off orientation. Two recombinases, FimB and FimE (14, 16), mediate the inversion of the phase switch. FimB and FimE are members of the tyrosine recombinase family (12); like other members of this recombinase class, they contain characteristic invariant sequence motifs which contribute to the active site, viz., H-X-LRH and LGH-X5-T-X2-Y, located in the C-terminal parts of the proteins (12). Both FimB and FimE have been shown to bind to half-sites that flank and overlap the left and right inverted repeats of the switch (14). In addition to the recombinases, a number of accessory proteins are involved in wrapping and bending the switch DNA into a recombination-proficient complex (5, 13). FimB and FimE were originally proposed to mediate off-to-on and on-to-off phase switching, respectively (16). A number of in vivo and in vitro studies have since suggested that FimB can carry out recombination in both directions, whereas the current evidence indicates that FimE-catalyzed phase switching is unidirectional, viz., on-to-off only (6, 29). No other recombinases have so far been reported to act on the fim phase switch.
FIG. 1.
(A) fim gene cluster. The positions of oligonucleotide primers used in the study are indicated. The black triangle indicates the location of the insertion element in fimB. The fim segments of plasmids pBSO25 and pBSO38, used to create ΔfimE and ΔfimBE derivatives of Nissle 1917, respectively, are indicated. (B) Localization of the insertion element in the fimB gene of Nissle 1917. Black arrowheads indicate the orientations of the fimB gene and the two ORFs; grey arrowheads indicate the inverted repeats which flank the IS.
We have previously used Nissle 1917 for the display of chimeric type 1 fimbriae containing heterologous inserts; in this respect, the Nissle 1917 strain proved to be superior to E. coli K-12 strains (41). In the present study, we have investigated the capacity of Nissle 1917 to produce type 1 fimbriae and have found it to be a natural fimB null mutant, yet surprisingly still capable of off-to-on switching of the phase switch and expressing type 1 fimbriae.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A list of E. coli strains used in this study is provided in Table 1. E. coli Nissle 1917 was purchased as a commercial preparation from a pharmacy in Germany; another isolate (C1433-82) was obtained from the State Serum Institute, Copenhagen, Denmark. No difference was observed between the isolates. In this study, we used an F′ lacI Tn5 variant of the E. coli K-12 strain HB101 (8) as an intermediate host during plasmid construction work. Cells were grown either on solid medium or in liquid Luria-Bertani (LB) broth (37) supplemented with the appropriate antibiotics. Shaken cultures were grown overnight. Static cultures were inoculated with 10 μl of shaken overnight culture and allowed to stand for 48 h.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotypea | Reference or source |
|---|---|---|
| Strains | ||
| Nissle 1917 | fimB | 7 |
| PC31 | gal tonA phx argF rel | 19 |
| HB101/F′ | F′ lacI Tn5 derivative of HB101 | 8 |
| BOD11 | Nissle 1917 ΔfimE | This study |
| BOD25 | Nissle 1917 ΔfimBE | This study |
| Plasmids | ||
| pBSO8 | fimE from Nissle 1917 (B) | This study |
| pBSO10 | fimB from Nissle 1917 (U) | This study |
| pBSO11 | fimB′ and fimE from Nissle 1917 (U) | This study |
| pBSO13 | Phase switch from Nissle 1917 (U) | This study |
| pBSO21 | Insert′ and fimB′ from Nissle 1917 (B) | This study |
| pBSO22 | Insert′ fimB′ phase switch and fimAIC from Nissle 1917 (B) | This study |
| pBSO25 | Insert′ fimB′ phase switch and fimAIC from Nissle 1917 (M) | This study |
| pBSO26 | fimE from Fim-positive Nissle 1917 colony (U) | This study |
| pBSO28 | Phase switch from Fim-positive Nissle 1917 colony (U) | This study |
| pBSO31 | Phase switch from Fim-positive BOD11 colony (U) | This study |
| pBSO36 | Region upstream of fimB from Nissle 1917 (B) | This study |
| pBSO37 | Region upstream of fimB, phase switch, and fimAIC from Nissle 1917 (B) | This study |
| pBSO38 | Region upstream of fimB, phase switch, and fimAIC from Nissle 1917 (M) | This study |
| pMAK700oriT | Ts origin | 9a |
| pPKL4 | All fim genes (B) | 19 |
| pPKL9 | fimB (B) | 16 |
U, pUC19 based; B, pBR322 based; M, pMAK700oriT based.
Plasmids.
The plasmids used in this study are listed in Table 1. The locations of all primers used are indicated in Fig. 1A.
pBSO8 was made by inserting a HindIII/SalI-cut PCR fragment generated from Nissle 1917 with primers P1 and P2 into pBR322 cut with the same enzymes. pBSO10, pBSO11, and pBSO13 were all made by inserting PCR fragments containing different parts of the fim regulatory region from Nissle 1917 into pUC19 cut with HincII and phosphorylated with T4 kinase. The following primers were used: P3 and P4 for pBSO10 (2,611-bp fragment), P5 and P2 for pBSO11 (1,421-bp fragment), and P6 and P7 for pBSO13 (664-bp fragment).
pBSO21, pBSO22, and pBSO25 were made as follows. A PCR fragment containing fimB was generated with primers P3 and P8 (NotI and SalI sites). It was cut with EcoRV and SalI, and the 2,314-bp fragment was joined with the 3,899-bp EcoRV/SalI fragment of pBR322, yielding pBSO21. Another PCR fragment containing the phase switch and fimAIC was generated with primers P9 (NotI site) and P10 (EcoRV and SalI sites). It was cut with NotI and SalI, and the 2,482-bp fragment was inserted into pBSO21 cut with NotI and SalI, yielding pBSO22. The 4,785-bp EcoRV fragment of pBSO22 containing the fim genes with the fimE deletion was inserted in pMAK700oriT cut with EcoRV, yielding pBSO25.
pBSO26 and pBSO28 contain DNA from a Fim-positive Nissle 1917 colony isolated after static growth. pBSP26 was made by inserting a PCR fragment generated with primers P1 and P2 into pUC19 cut with SmaI and HincII and phosphorylated with T4 kinase. pBSO28 was made by inserting a PCR fragment generated with primers P6 and P7 into pUC19 cut with HincII and phosphorylated with T4 kinase. pBSO31 contains DNA from a Fim-positive BOD11 colony isolated after static growth. It was made by inserting a HindIII/XbaI-cut PCR fragment generated with primers P1 and P11 into pUC19 cut with the same enzymes.
pBSO36, pBSO37, and pBSO38 were made as follows. A PCR fragment containing the region upstream of fimB was generated with primers P12 (ClaI site) and P13 (NotI and SalI sites). It was cut with ClaI and SalI, and the 2,466-bp fragment was joined with the 3,734-bp ClaI/SalI fragment of pBR322, yielding pBSO36. Another PCR fragment containing the phase switch and fimAIC was generated with primers P9 (NotI site) and P14 (ClaI and SalI sites). It was cut with NotI and SalI, and the 2,482-bp fragment was inserted into pBSO36 cut with NotI and SalI, yielding pBSO37. The 4,936-bp ClaI fragment of pBSO37 containing the fim region with the fimBE deletion was inserted into pMAK700oriT cut with ClaI, yielding pBSO38. Plasmids pPKL4 and pPKL9 have been described previously (16, 19).
DNA manipulations.
Chromosomal DNA was purified with a GenomicPrep Cell and Tissue DNA Isolation Kit (Amersham Pharmacia Biotech Inc.). Plasmid DNA was isolated with a QIAprep Spin Miniprep Kit (Qiagen). PCR products were purified with a GFX PCR DNA and Gel Band Purification Kit (Amersham Pharmacia Biotech Inc.). Restriction endonucleases were used according to the manufacturer's specifications (New England Biolabs or Pharmacia).
PCR methodology.
PCR for plasmid construction, Southern blotting, probe labeling, and switch orientation assays were performed with a Perkin-Elmer GeneAmp machine. Oligonucleotide primers were purchased from Gibco-BRL. The primers used in this study are listed in Table 2. Amplification was done for 20 to 25 cycles as follows: hot start without polymerase at 94°C for 3 min; primary denaturation at 94°C for 30 s to 1 min; primer annealing at 50°C for 30 s to 1 min; and primer extension at 72°C for 30 s to 3 min. Each reaction was carried out with 50 pmol of each primer and 2 U of polymerase (Taq [Pharmacia] for switch orientation assay; Expand High Fidelity [Boehringer Mannheim Biochemicals] for plasmid construction).
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Nucleotide sequence |
|---|---|
| P1 | 5′-CGGCAAGCTTGGAAGTTAATTCACTGC |
| P2 | 5′-GCGCGTCGACGTTAAATCAAACCTCTTC |
| P3 | 5′-GGCGTCGACTAACCCAGCACAGCTA |
| P4 | 5′-GCGCGGATCCGTAAGAATAATGTAGT |
| P5 | 5′-CTGGTTGAGTATCCGTACTTCG |
| P6 | 5′-GGCGGCCGATCGATCGTTTTGCCGGATTATGGG |
| P7 | 5′-AGTGAACGGTCCCACCATTAACC |
| P8 | 5′-GCTCGTCGACGCGGCCGCTGCAGTGGATTAACTTCC |
| P9 | 5′-GCACGCGGCCGCGAGAAGAGGTTTGATTTAAC |
| P10 | 5′-CCGCGTCGACGATATCCTCCGGCTTAAAATCACC |
| P11 | 5′-TGCTCTAGATTAGCCTGCATCAACTGCGCA |
| P12 | 5′-GCGCATCGATCAGCGTAACTCCATTGC |
| P13 | 5′-GCTCGTCGACGCGGCCGCGTTCAACTAGCCGTCTC |
| P14 | 5′-CCGCGTCGACATCGATCTCCGGCTTAAAATCACC |
| P15 | 5′-CCCGCATAAACAATTAGCA |
Switch orientation assay.
Samples (1 μl) of chromosomal DNA were amplified with primers situated on either site of the phase switch. The following primers were used: P6 and P7 for the E. coli K-12 strains and wild-type Nissle 1917, P1 and P7 for BOD11, and P15 and P7 for BOD25. The products were purified and cut with either HinfI or SnaBI. The digests were separated on a 2% Tris-borate-EDTA gel.
DNA sequencing.
The DNA sequence was determined from double-stranded plasmid templates by dideoxy chain termination (38). Double-stranded templates were denatured, and the sequencing reactions were carried out with T7 DANN polymerase as suggested in the Plasmid FdATP +AutoreadKit from Pharmacia-Amersham (Freiburg, Germany). Sequencing reactions were primed by vector and custom-made oligonucleotide primers labeled at their 5′ ends with the fluorescent carbocyamine dye Cy5 (Pharmacia). The labeled reaction mixtures were separated on 6% Hydrolink Long Ranger gels, and sequences were automatically detected by use of a red helium neon laser (633 nm) and fixed photodiodes in an ALFexpress DNA sequencer.
Deletion of the fimE or fimBE genes in Nissle 1917.
In order to delete the fimE gene in Nissle 1917, part of the fim gene cluster (from the EcoRV site in the insertion sequence to downstream of fimC), excluding fimE, was inserted into integration vector pMAK700oriT (Camr). This plasmid carries a temperature-sensitive origin of replication which permits it to replicate only at temperatures below 30°C. The resulting plasmid (pBSO25) was transformed into Nissle 1917 and allowed to grow overnight at 30°C on plates containing 17 μg of chloramphenicol per ml. Single colonies were streaked on plates containing 17 μl of chloramphenicol per ml and placed at 42°C to select for recA-mediated single-crossover events. Camr colonies were then grown in liquid LB medium containing 17 μg of chloramphenicol per ml at 42°C overnight. Overnight cultures were diluted 1:1,000 in liquid LB medium without antibiotics, grown to an optical density at 600 nm of 0.5, plated on LB medium plates, and incubated at 30°C overnight. Colonies from these plates were replicated on chloramphenicol (17 μg/ml) plates and LB medium plates, and chromosomal DNA from Cams colonies was tested by PCR with primers P5 and P7. A colony which had fimE deleted was selected and named BOD11.
The Nissle 1917 fimBE mutant was made in the same way, except that pBSO38, containing part of the fim gene cluster and upstream region, excluding fimB and fimE, was used. Chromosomal DNA from Cams colonies was tested by PCR with primers P15 and P7. A clone in which fimB, including the insertion sequence, and fimE were deleted was selected and named BOD25.
Agglutination of yeast cells.
The capacity of bacteria to express an α-d-mannose-binding phenotype was assayed by their ability to agglutinate Saccharomyces cerevisiae cells on glass slides. Aliquots of liquid bacterial cultures at an optical density at 550 nm of 10 and 2% (wt/vol) yeast cells were mixed, and the time until agglutination occurred was measured.
Antisera.
Rabbit anti-type 1 fimbrial serum raised against purified type 1 fimbriae has previously been described (22). Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit serum was supplied by Dako, Glostrup, Denmark.
Fixation of bacterial cells and fluorescence labeling.
Cells from shaken overnight cultures were diluted 1:10 and incubated for 1 h with shaking. Statically grown cells were not subjected to this treatment. Cells were harvested and washed gently in phosphate-buffered saline (PBS; 0.145 M NaCl, 0.15 M sodium phosphate). Cells were fixed by mixing 250 μl of cells with 750 μl of a 4% (wt/vol) solution of paraformaldehyde in PBS. This mixture was incubated on ice for 20 min. To remove the fixative, cells were washed twice in PBS. Samples of 15 μl were placed on a poly-l-lysine-coated slide and air dried. After the samples were washed in PBS plus 0.1% (vol/vol) Tween 20, 15 μl of a 1:10 dilution of antifimbria primary antiserum in PBS plus 0.1% (vol/vol) Tween 20 was placed on top of each sample, and the samples were left in a moist incubation chamber for 1 h. The slide was washed three times, and 15 μl of a 1:25 dilution of FITC-conjugated secondary antiserum was added. After 2 h in a dark moist incubation chamber, the slide was washed three times and air dried. A drop of Citiflour was placed on top of each sample before microscopy.
Microscopy and image analysis.
Differential interference contrast microscopy (DICM) and image analysis were carried out as previously described (35).
Southern blotting.
Southern hybridizations were performed at high stringency with a digoxigenin nonradioactive DNA labeling and detection kit (Boehringer). A 389-bp fimB′ probe specific for the 3′ end of fimB downstream of the ClaI site was made by direct labeling by PCR with primers P5 and P4 and pPKL4 as a template.
RESULTS
A 1.9-kb insert truncates the fimB gene in Nissle 1917.
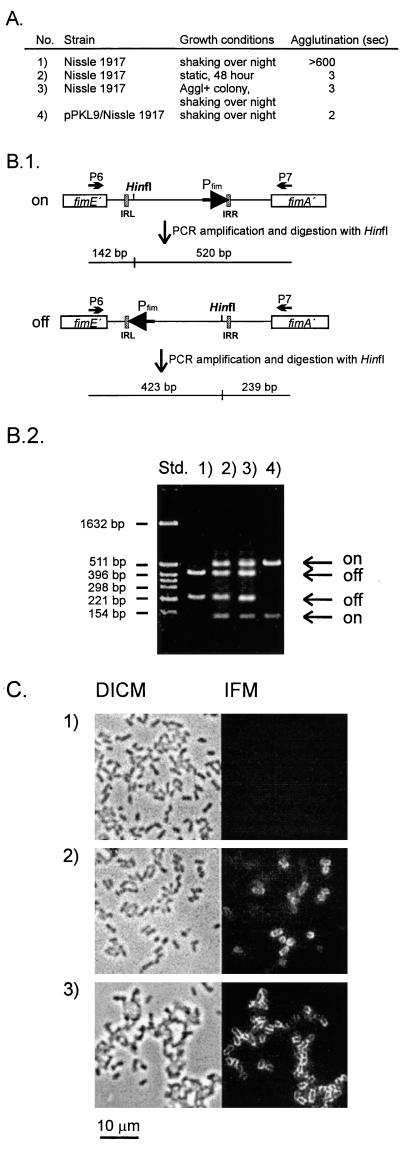
When grown as liquid shaken cultures in LB medium, Nissle 1917 was found to be incapable of expressing type 1 fimbriae, as evidenced from the fact that it was unable to agglutinate yeast cells or react with a specific antiserum against type 1 fimbriae (Fig. 2). Since this observation differed from the reported phenotype of Nissle 1917 (7), we investigated the status of the phase switch and the fimB and fimE genes. In line with the observed Fim-negative phenotype, it was found, by PCR amplification of the phase switch region and analysis of orientation, that the phase switch state was exclusively off (Fig. 2). In E. coli K-12, the fimB and fimE genes are well characterized; they encode proteins of 200 and 198 amino acids, respectively (16). PCR amplification with two primer sets, flanking the fimB and fimE genes, revealed that the fimB gene of Nissle 1917 harbored an insert of about 1.9 kb, whereas the amplified segment containing the fimE gene was of the expected size. The amplified segment containing the fimB gene with an insert was cloned in pUC19, resulting in plasmid pBSO10, and sequenced (GenBank accession no. AF188737). The insert, containing 1,885 bp plus two 4-bp terminal duplications of fim DNA, was located in a position corresponding to codon 67 in the fimB reading frame (Fig. 1B). Since the sectors of the FimB recombinase involved in the catalysis of DNA inversion are located in the C-terminal part of the protein, the truncation of the fimB gene of Nissle 1917 apparently represented a knockout mutation. In accordance with the reported directionality of the FimB and FimE recombinase activities, a knockout insertion in fimB would readily account for the off orientation of the phase switch and the Fim-negative status of the cells.
FIG. 2.
Agglutination phenotypes and orientation of the fimbrial phase switch in Nissle 1917. (A) Agglutination times of Nissle 1917 grown under different conditions:1, with shaking overnight; 2, static growth for 48 h; 3, a single agglutinating (Aggl+) colony, isolated by plating statically grown Nissle 1917, grown with shaking overnight; and 4, Nissle 1917 containing pPKL9 grown with shaking overnight. Yeast was used at 2%. (B.1) The orientation of the phase switch can be determined by combined PCR and restriction analysis, since HinfI cuts asymmetrically. IRL and IRR, left and right inverted repeats, respectively. (B.2) Orientation of the phase switch in Nissle 1917 grown under different conditions, as in panel A. Std., pBR322 cut with HinfI. (C) DICM and corresponding immunofluorescence microscopy (IFM) of Nissle 1917 cells. Bacteria were fixed, reacted with polyclonal anti-type 1 fimbriae primary antibodies, washed, reacted with FITC-labeled secondary antibodies, washed, and subjected to microscopy. Images were recorded with charge-coupled device technology (see Materials and Methods). 1, Nissle 1917 grown with shaking overnight; 2, Nissle 1917 grown statically for 48 h; 3, a single agglutinating colony, isolated by plating statically grown Nissle 1917, grown with shaking overnight. Expression of type 1 fimbriae is seen only in panels 2 and 3.
Characterization of the insertion element in the Nissle 1917 fimB gene.
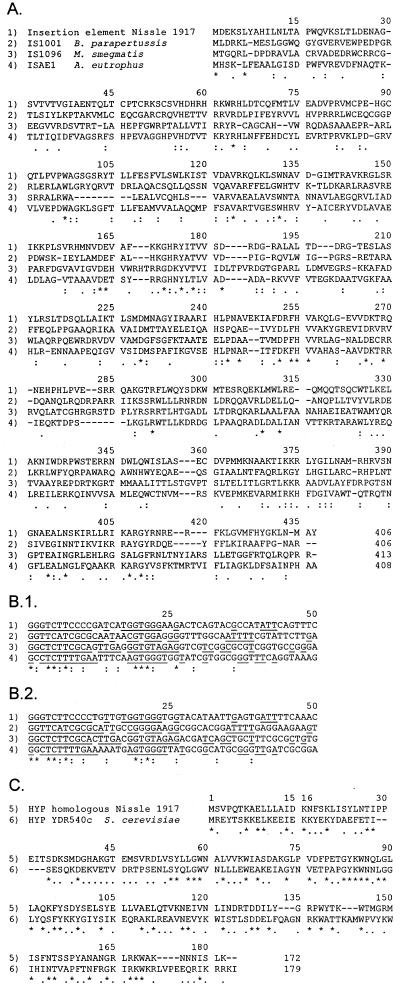
Analysis of the insert sequence revealed the presence of an open reading frame which could encode a protein of 406 amino acids. Also, this reading frame was in the orientation opposite that of fimB. A database search indicated significant homology to several transposases, i.e., TnpA of insertion element IS1096 from Mycobacterium smegmatis (10) and IS1001 from Bordetella parapertussis (43), and to an open reading frame residing in ISAE1 in Alcaligenes eutrophus (23) (Fig. 3). Furthermore, the insert sequence had 26-bp nearly perfect inverted terminal repeats which resembled the inverted repeats of IS1001, IS1096, and ISAE1 (Fig. 3). Taken together, these features suggested that the insert in fimB of Nissle 1917 could be an insertion element similar to IS1001, IS1096, and ISAE1.
FIG. 3.
(A) Homology between transposases and transposase homologues encoded by the insertion element from Nissle 1917 (ORF2) (1), IS1001 from B. parapertussis (2), IS1096 from M. smegmatis (3), and ISAE1 from A. eutrophus (4). Dashes indicate gaps introduced to increase the number of matches. Asterisks represent identical amino acids in all four sequences. Colons represent identical amino acids in Nissle 1917 and two of the other sequences. Dots represent similar amino acids in Nissle 1917 and at least two of the other sequences. (B) Sequences of the left end (B.1) and of the right end (B.2) of the insertion element from Nissle 1917 (1), IS1001 from B. parapertussis (2), IS1096 from M. smegmatis (3), and ISAE1 from A. eutrophus (4). The sense strands are presented. Asterisks represent identical amino acids in all four sequences. Colons represent identical amino acids in Nissle 1917 and two of the other sequences. The inverted repeats are underlined. (C) Homology between ORF1 of the insertion element in Nissle 1917 (5) and YDR540c (6), which encodes a hypothetical (HYP) yeast protein in S. cerevisiae (GenBank accession no. 2131555). Asterisks represent identical amino acids. Dots represent similar amino acids. Dashes indicate gaps.
Despite its fimB null status, Nissle 1917 is capable of producing type 1 fimbriae.
Strain Nissle 1917 was also routinely checked for type 1 fimbriation after cultivation under static (nonshaking) growth conditions. Surprisingly, we observed that the strain was now capable of expressing type 1 fimbriae. This finding was reflected in good agglutination of yeast cells and a reaction with a specific anti-type 1 fimbria serum (Fig. 2). Furthermore, when the orientation of the fimbrial phase switch was investigated, the pattern indicative of the on orientation of the phase switch was observed (Fig. 2). The introduction of a plasmid, pPKL9, encoding the fimB recombinase of E. coli K-12 strain PC31 into Nissle 1917 resulted in cells that had a fimbriated phenotype when grown as liquid shaken cultures. The phase switch of such cells was predominantly in the on orientation, indicating that the phase switch of Nissle 1917 indeed was receptive to FimB-mediated inversion (Fig. 2).
Since Nissle 1917 is not well characterized at the genetic level, we sought evidence for the presence of potential intact copies of fimB on its genome by Southern hybridization. In line with our PCR results, the only signals that were seen could be accounted for by the fimB version containing the insertion sequence (IS)-like element. It should also be noted that PCR analysis of fimB and Southern blotting with fimB probes of cells grown under nonshaking conditions did not reveal the presence of intact copies of fimB, which could result from a clean excision of the insertion element in fimB. This result would suggest that the IS in fimB is not prone to excision.
Cells in which the fim switch was turned on were capable of switching back.
When cells from static cultures of Nissle 1917 were plated, individual colonies exhibiting agglutination and nonagglutination phenotypes were observed. In order to determine whether the observed Fim-positive variants could have resulted from clonal selection under static growth conditions of switch-locked-on mutant cells from Fim-positive colonies, these cells were investigated for their ability to undergo phase variation. Liquid shaken cultures of such cells were investigated by immunofluorescence microscopy (Fig. 2C); additionally, the relative orientation of the fimbrial phase switch was investigated (Fig. 2B). The data indicated that progeny from Fim-positive colonies gave rise to a mixed population of both switch-locked-on and switch-locked-off cells. It was therefore clear that there was no phase locking of the invertible element in Fim-positive cells.
Investigation of the fimE gene and the phase switch of Nissle 1917.
To further analyze the basis for the ability of Nissle 1917 to perform off-to-on phase switching, we proceeded to investigate the fimE gene and the phase switch region of this strain. Relevant sectors containing these elements were cloned and sequenced (pBSO11 and pBSO13, respectively). The nucleotide sequence of the Nissle 1917 fimE gene turned out to be identical to the K-12 fimE sequence, except for a single base change in a Leu codon, TTG to CTG, resulting in another Leu codon. Accordingly, the phase-switching ability of Nissle 1917 was not compatible with a model based on a FimE variant whose catalytic activity differs from the activity of the K-12 variant. The sequence of the Nissle 1917 phase switch sector differed from that of K-12 in four positions, one inside the phase switch and three outside. All four changes were located at considerable distances from the inverted repeats and the proximal flanking sequences to which the FimB and FimE recombinases have been shown to bind (14). In light of this finding, it did not seem conceivable that the structure of the Nissle 1917 fim switch could account for the observed off-to-on phase switching. Interestingly, the unique change inside the phase switch resulted in a shortening of the distance, by 1 base, between the −35 and −10 consensus sequences of the switch-located promoter.
The phase switch and the fimE gene were also cloned from the Fim-positive Nissle 1917 colonies isolated from static cultures and sequenced (pBSO28 and pBSO26, respectively). No difference was observed between these constructs and the wild type, supporting the notion that phase locking does not occur in such cells due to the accumulation of mutations in the phase switch (data not shown).
Construction and investigation of a Nissle 1917 fimE mutant.
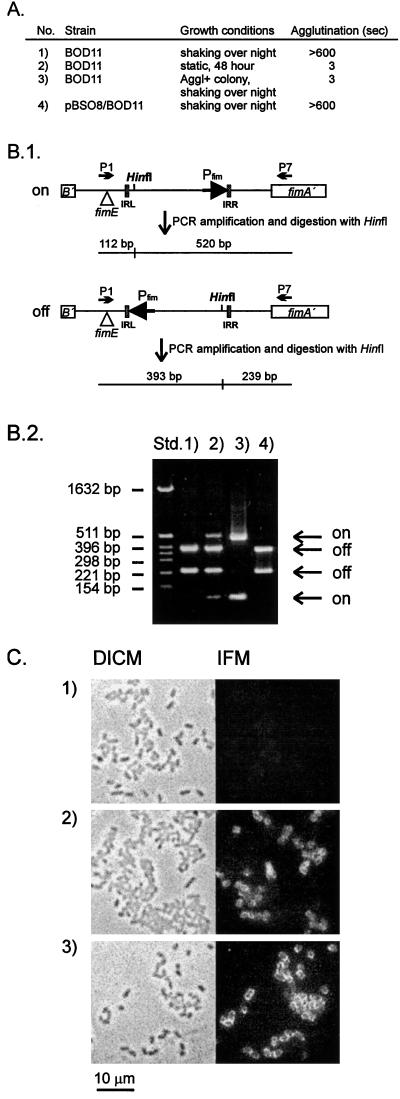
It is conceivable that FimE was responsible for the off-to-on phase switching observed under static growth conditions. To test this hypothesis, a fimE mutant of Nissle 1917, BOD11, was constructed by use of plasmid pBSO25 (see Materials and Methods). After growth under shaking conditions, this strain expressed no type 1 fimbriae. However, when grown under static conditions, BOD11 was still capable of inverting the phase switch from the off to the on orientation. Thus, when the orientation of the fimbrial phase switch was investigated, a clear signal, representing an on orientation of the phase switch, was observed (Fig. 4B). Type 1 fimbriation was also reflected in the ability of statically grown BOD11 to agglutinate yeast cells (Fig. 4A) and to react with a specific anti-type 1 fimbria serum (Fig. 4C). When cells from static cultures of BOD11 were plated, individual colonies exhibiting agglutination and nonagglutination phenotypes were observed, as was the case with wild-type Nissle 1917. However, in contrast to the wild-type strain, Fim-positive colonies could no longer undergo phase variation. That is, the phase switch of such colonies remained in the on orientation after several passages both in shaken LB broth and on LB agar plates, growth conditions known to favor the nonfimbriated state (Fig. 4B) (6). When the phase switch of a Fim-positive BOD11 colony was cloned and sequenced (pBSO31), no difference was observed between this construct and wild-type Nissle 1917. In addition, introducing a plasmid, pBSO8, encoding the Nissle 1917 fimE gene into a Fim-positive BOD11 colony resulted in cells with the phase switch uniquely in the off orientation after growth under shaking conditions (Fig. 4B). Therefore, the phase locking seen for Fim-positive BOD11 colonies under static growth conditions was not due to a defective phase switch.
FIG. 4.
Agglutination phenotypes and orientation of the fimbrial phase switch in BOD11, i.e., Nissle 1917 fimE. (A) Agglutination times of BOD11 grown under different conditions: 1, with shaking overnight; 2, static growth for 48 h; 3, a single agglutinating (Aggl+) colony, isolated by plating statically grown BOD11, grown with shaking overnight; and 4, the same colony (from 3) containing pBSO8 grown with shaking overnight. Yeast was used at 2%. (B.1) The orientation of the phase switch can be determined by combined PCR and restriction analysis, since HinfI cuts asymmetrically. Triangles indicate the fimE deletion. IRL and IRR, left and right inverted repeats, respectively. (B.2) Orientation of the phase switch in BOD11 grown under different conditions, as in panel A. Std., pBR322 cut with HinfI. (C) DICM and corresponding immunofluorescence microscopy (IFM) of BOD11 cells. Bacteria were fixed, reacted with polyclonal anti-type 1 fimbria primary antibodies, washed, reacted with FITC-labeled secondary antibodies, washed, and subjected to microscopy. Images were recorded with charge-coupled device technology (see Materials and Methods). 1, BOD11 grown with shaking overnight; 2, BOD11 grown statically for 48 h; 3, a single agglutinating colony, isolated by plating statically grown BOD11, grown with shaking overnight. Expression of type 1 fimbriae is seen only in panels 2 and 3.
Construction and investigation of a Nissle 1917 fimBE double mutant.
To exclude the hypothesis that mRNA splicing of the fimB transcript, which would remove the IS, could be involved in the observed phase switching, a “clean” Nissle 1917 fimBE double mutant, BOD25, was constructed by use of plasmid pBSO38 (see Materials and Methods). This strain behaved in exactly the same way as BOD11; i.e., phase switching from the off to the on orientation was observed after static growth. However, individual Fim-positive BOD25 colonies were incapable of switching back. The phase locking in the on orientation was not due to a defective phase switch, since transformation of Fim-positive BOD25 colonies with plasmid pBSO8 (fimE+) resulted in cells with the phase switch uniquely in the off orientation after growth under shaking conditions (data not shown).
DISCUSSION
Nissle 1917 has been marketed as a probiotic remedy against various intestinal disorders in several European countries since the 1920s (39). During this period of time, it has been ingested by an appreciable number of people and arguably represents the best “field-tested” E. coli strain in the world. We have previously shown Nissle 1917 to be an excellent vehicle for the expression of chimeric type 1 fimbriae in connection with the development of vaccine systems (41). In order to shed light on the efficient colonization properties of Nissle 1917, we initiated an analysis of the regulation of the indigenous fim gene cluster in this strain.
When grown as liquid shaken cultures, Nissle 1917 had a Fim-negative phenotype, and the fim switch was found to be in the off orientation. PCR-based and nucleotide sequence analyses revealed a 1,893-bp insertion in codon 67 of the fimB gene. This element contained an open reading frame encoding a product of potentially 406 amino acids. Interestingly, the sequence of this putative protein exhibited considerable homology, 20 to 26% identity, to those of several transposase-like proteins encoded on IS elements characterized from a number of distantly related microorganisms (Fig. 3). Additionally, the insert in fimB also contained an open reading frame for a hypothetical protein of 172 amino acids which exhibited 31% identity to an uncharacterized open reading frame in the S. cerevisiae genome (Fig. 3). The insert was flanked by nearly perfect inverted repeats, which themselves showed considerable homology to the flanking elements of IS1096, IS1001, and ISAE1 of M. smegmatis, B. parapertussis, and A. eutrophus, respectively. The position in fimB where the insert is located is AT rich and actually in itself represents a dyad symmetry motif. Indeed, such structures of A's and T's have been reported to be preferential insertion sites for IS1001 (43). In addition, the observed terminal duplications of host DNA are often reported in connection with IS transposition. Taken together, these observations suggest that the IS in the Nissle 1917 fimB gene belongs to a group of related IS elements with a considerable host range.
In spite of being a natural fimB null mutant, Nissle 1917 was capable of producing type 1 fimbriae when grown under static conditions. In different experiments, the invertible fim switch was observed to be in the on orientation in 10 to 50% of the cells. Two tyrosine class recombinases, encoded by the fimB and fimE genes, control the orientation of the phase switch. These recombinases have been intensively studied; FimB has been shown to be able to catalyze both off-to-on inversion and on-to-off inversion of the phase switch, whereas several reports indicate that FimE uniquely catalyzes on-to-off inversion (6, 29). Furthermore, FimE-catalyzed inversion seems to be highly efficient and causes on-to-off phase switching at a rate of about 0.3 per cell per generation, whereas FimB-mediated phase switching occurs at a rate of about 0.001 per cell per generation (13). A knockout mutation of fimB would therefore phenotypically result in a Fim-negative state with the phase switch in the off orientation.
FimB and FimE have both been shown, by footprinting, to interact with the inverted repeats and their immediate flanking sequences (14). FimB and FimE exhibit 48% sequence identity (16). However, the bias in phase switching directionality must be due at least partially to differences in the respective domains of the proteins which interact with the inverted repeat sectors of the fim switch. FimE of E. coli K-12 strain PC31 was reported to confer a low level of off-to-on phase switching when present on a high-copy-number plasmid (34). It was therefore conceivable that alterations in the FimE sequence could influence the directionality bias toward the catalysis of off-to-on switch inversion. However, the amino acid sequences of the products of the fimE genes of Nissle 1917 and E. coli K-12 turned out to be identical, thus ruling out this possibility.
Changes in the nucleotide sequence of the phase switch of Nissle 1917 could be invoked to explain the observed off-to-on phase switching. In certain clinical isolates, changes in the DNA sequence of the fim switch, mainly clustering in and proximal to the right inverted repeat, have been shown to influence the orientation of the phase switch (26). However, DNA sequence analysis of the phase switch region of Nissle 1917 revealed only a few changes, located at considerable distances from the inverted repeats. Thus, it seemed highly improbable that these could account for the ability of Nissle 1917 to perform off-to-on phase switching under static growth conditions.
The behavior of the Nissle 1917 fimE mutant BOD11 indicated that FimE was not the prime suspect for conferring off-to-on phase switching in Nissle 1917. Thus, BOD11, like its parent, was capable of off-to-on phase switching under static growth conditions. Since there was no evidence that the insert sequence in fimB could be excised or that any residual activity could originate from truncated FimB, we hypothesized that the insert sequence in Nissle 1917 fimB could belong to the family of prokaryotic group I introns (2, 3). These introns catalyze their own splicing from the precursor RNA, an ability related to their highly conserved secondary and tertiary structures (36). According to this tenet, intact FimB might be produced from a correctly spliced mRNA. However, when the clean Nissle 1917 fimBE double mutant, BOD25, was examined after growth under static conditions, it was still capable of off-to-on phase switching. In fact, the Nissle 1917 fimBE and fimE mutants behaved identically. Accordingly, we concluded that some other recombinase encoded on the Nissle 1917 chromosome could confer off-to-on inversion of the fimbrial phase switch.
It has long been known that fimbriated bacteria outgrow nonfimbriated bacteria in static liquid medium (32). The mechanism of selection depends on the special ability of fimbriated bacteria to establish themselves rapidly in a pellicle on the surface of the broth, where their growth is promoted by the free supply of oxygen. Thus, although starting as a small minority (e.g., 1 in 106), the number of fimbriated bacteria approaches or even surpasses the number of nonfimbriated bacteria after 48 h. Given the strong selection bias for fimbriated cells under static liquid growth conditions, one can argue that the mechanism which confers off-to-on phase switching of the fim switch in Nissle 1917 would not need to be very efficient compared to the Fim recombinases yet would still give rise to a large fraction of fimbriated cells in the population.
Our results indicate that no other copies of fimB are present on the Nissle 1917 genome. It is conceivable, however, that another tyrosine recombinase is encoded by the yet-uncharacterized genome of Nissle 1917 and that this enzyme, acting with a low frequency on the fim switch, accounts for the reported observations. In this respect, it is interesting to note that the E. coli K-12 genome encodes several tyrosine recombinases, i.e., XerC/XerD, as well as an uncharacterized ORF product (GenBank accession no. U73857), which all exhibit a fair amount of homology to the fim recombinases (12). The genes for such recombinases, as well as others, are probably located on the Nissle 1917 chromosome and might be the target for future research.
Our results represent the first report of non-FimB- or non-FimE-catalyzed inversion of the fim switch. Although Nissle 1917 is indeed capable of expressing type 1 fimbriae under selective pressure, the fimB insertional mutation might result in attenuation of the strain. In accordance with this assumption, it was recently shown that an IS1 element located in the csgB gene, involved in curly fibril formation, reduced the virulence of two E. coli O78:K80 strains in poultry infection (25). Another noteworthy aspect of our study is the fact that two distinct fimbrial phenotypes arose from cultivation of a bacterial strain under different growth conditions, emphasizing the fact that phenotype assessment should be done with caution.
ACKNOWLEDGMENTS
This work was supported by the Danish Medical Research Council and the Danish Natural Sciences Research Council (grants 9503048 and 9601682).
REFERENCES
- 1.Abraham J M, Freitag C S, Clements J R, Eisenstein B I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci USA. 1985;82:5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belfort M, Perlman P S. Mechanisms of intron mobility. J Biol Chem. 1995;270:30237–30240. doi: 10.1074/jbc.270.51.30237. [DOI] [PubMed] [Google Scholar]
- 3.Belfort M, Reaban M E, Coetzee T, Dalgaard J Z. Prokaryotic introns and inteins: a panoply of form and function. J Bacteriol. 1995;177:3897–3903. doi: 10.1128/jb.177.14.3897-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch C A, Stocker B A D, Orndorff P E. A key role for type 1 pili in enterobacterial communicability. Mol Microbiol. 1992;6:697–701. doi: 10.1111/j.1365-2958.1992.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 5.Blomfield I C, Kulasekara D H, Eisenstein B I. Integration host factor stimulates both FimB- and FimE-mediated site-specific DNA inversion that controls phase variation of type 1 fimbriae expression in Escherichia coli. Mol Microbiol. 1997;23:705–717. doi: 10.1046/j.1365-2958.1997.2241615.x. [DOI] [PubMed] [Google Scholar]
- 6.Blomfield I C, McClain M S, Princ J A, Calie P J, Eisenstein B I. Type 1 fimbriation and fimE mutants of Escherichia coli K-12. J Bacteriol. 1991;173:5298–5307. doi: 10.1128/jb.173.17.5298-5307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum G, Marre R, Hacker J. Properties of Escherichia coli strains of serotype O6. Infection. 1995;23:1–3. doi: 10.1007/BF01781204. [DOI] [PubMed] [Google Scholar]
- 8.Boyer H W, Roulland-Dussoix D. A complementary analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 9.Brinton C C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in Gram negative bacteria. Trans N Y Acad Sci. 1965;27:1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- 9a.Chakraborty, T. Unpublished data.
- 10.Cirillo J D, Barletta R G, Bloom B R, Jacobs W R., Jr A novel transposon trap for mycobacteria: isolation and characterization of IS1096. J Bacteriol. 1991;173:7772–7780. doi: 10.1128/jb.173.24.7772-7780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell H, Agace W, Klemm P, Schembri M, Mårild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito D, Scocca J J. The integrase family of tyrosine recombinases: evolution of a conserved active site domain. Nucleic Acids Res. 1997;25:3605–3614. doi: 10.1093/nar/25.18.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gally D L, Bogan J A, Eisenstein B I, Blomfield I C. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J Bacteriol. 1993;175:6186–6193. doi: 10.1128/jb.175.19.6186-6193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gally D L, Leathart J, Blomfield I C. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol Microbiol. 1996;21:725–738. doi: 10.1046/j.1365-2958.1996.311388.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones C H, Pinkner J S, Roth R, Heuser J, Nicholes A V, Abraham S, Hultgren S J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986;5:1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemm P, editor. Fimbriae, adhesion, genetics, biogenesis and vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. [Google Scholar]
- 18.Klemm P, Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987;208:439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- 19.Klemm P, Jørgensen B J, van Die I, de Ree H, Bergmans H. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli. Mol Gen Genet. 1985;199:410–414. doi: 10.1007/BF00330751. [DOI] [PubMed] [Google Scholar]
- 20.Klemm P, Krogfelt K A. Type 1 fimbriae of Escherichia coli. In: Klemm P, editor. Fimbriae, adhesion, genetics, biogenesis and vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 9–26. [Google Scholar]
- 21.Krogfelt K A, Bergmans H, Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect Immun. 1990;58:1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krogfelt K A, Klemm P. Investigation of minor components of Escherichia coli type 1 fimbriae: protein chemical and immunological aspects. Microb Pathog. 1988;4:231–238. doi: 10.1016/0882-4010(88)90073-3. [DOI] [PubMed] [Google Scholar]
- 23.Kung S-S, Chen J, Chow W-Y. Molecular and genetic characterization of an Alcaligenes eutrophus insertion element. J Bacteriol. 1992;174:8023–8029. doi: 10.1128/jb.174.24.8023-8029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langermann S, Palaszynsky S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 25.La Ragione R M, Collighan R J, Woodward M J. Non-curliation of Escherichia coli O78:K80 isolates associated with IS1 insertion in csgB and reduced persistence in poultry infection. FEMS Microbiol Lett. 1999;175:247–253. doi: 10.1111/j.1574-6968.1999.tb13627.x. [DOI] [PubMed] [Google Scholar]
- 26.Leathart J B S, Gally D L. Regulation of type 1 fimbrial expression in uropathogenic Escherichia coli: heterogeneity of expression through sequence changes in the fim switch region. Mol Microbiol. 1998;28:371–381. doi: 10.1046/j.1365-2958.1998.00802.x. [DOI] [PubMed] [Google Scholar]
- 27.Lodinova-Zadnikova R, Sonnenborn U. Effect of preventive administration of a nonpathogenic Escherichia coli strain on the colonization of the intestine with microbial pathogens in newborn infants. Biol Neonate. 1997;71:224–232. doi: 10.1159/000244421. [DOI] [PubMed] [Google Scholar]
- 28.Lodinova-Zadnikova R, Tlaskalova-Hogenova H, Sonnenborn U. Local and serum antibody response in full-term and premature infants after artificial colonization of the intestine with E. coli strain Nissle 1917 (Mutaflor) Pediatr Allergy Immunol. 1992;3:43–48. [Google Scholar]
- 29.McClain M, Blomfield I C, Eisenstein B I. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1991;173:5308–5314. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mertz A. Behandlungesversuche bei ernährungsgestörten Säuglingen mit Mutaflor (Aufschwemmung von Kolibakterien mit hohem antagonistischen Index nach Nissle) Monatsschr Kinderheilkd. 1920;18:401–416. [Google Scholar]
- 31.Nissle A. Die Heilung der chronischen Obstipation mit Mutaflor, ihre Grundlagen, ihre Bedeutung. Muench Med Wochenschr. 1929;76:1745–1748. [Google Scholar]
- 32.Old D C, Duguid J P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970;103:447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen P B, Klemm P. Localization of promoters in the fim gene cluster and the effect of H-NS on the transcription of fimB and fimE. FEMS Microbiol Lett. 1994;116:95–100. doi: 10.1111/j.1574-6968.1994.tb06681.x. [DOI] [PubMed] [Google Scholar]
- 34.Pallesen L, Madsen O, Klemm P. Regulation of the phase switch controlling expression of type 1 fimbriae in Escherichia coli. Mol Microbiol. 1989;3:925–935. doi: 10.1111/j.1365-2958.1989.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 35.Pallesen L, Poulsen L K, Christiansen G, Klemm P. Chimeric FimH adhesin of type 1 fimbriae: a bacterial display system for heterologous sequences. Microbiology. 1995;141:2839–2848. doi: 10.1099/13500872-141-11-2839. [DOI] [PubMed] [Google Scholar]
- 36.Saldanha R, Mohr G, Belfort M, Lambowitz A M. Group I and group II introns. FASEB J. 1993;7:15–24. doi: 10.1096/fasebj.7.1.8422962. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulze J, Sonnenborn U. Re.: Oral administration of a certain strain of live Escherichia coli for intestinal disorders? Infection. 1995;23:184–188. doi: 10.1007/BF01793863. [DOI] [PubMed] [Google Scholar]
- 40.Sokurenko E V, Chesnokova V, Doyle R J, Hasty D L. Diversity of the Escherichia coli type 1 fimbrial lectin. Differential binding to mannosides and uroepithelial cells. J Biol Chem. 1997;272:17880–17886. doi: 10.1074/jbc.272.28.17880. [DOI] [PubMed] [Google Scholar]
- 41.Stentebjerg-Olesen B, Pallesen L, Jensen L B, Christiansen G, Klemm P. Authentic display of a cholera toxin epitope by chimeric type 1 fimbriae: effects of insert positions and host background. Microbiology. 1997;143:2027–2038. doi: 10.1099/00221287-143-6-2027. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich P. Klinishe Beobachtungen über Mutaflor bei verschiedenen Erkrankungen. Med Klin. 1926;30:1152–1153. [Google Scholar]
- 43.van der Zee A, Agterberg C, van Agterveld M, Peeters M, Mooi F R. Characterization of IS1001, an insertion sequence element of Bordetella parapertussis. J Bacteriol. 1993;175:141–147. doi: 10.1128/jb.175.1.141-147.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto T, Fujita K, Yokota T. Adherence characteristics to human intestinal mucosa of Escherichia coli isolated from patients with diarrhea or urinary tract infections. J Infect Dis. 1990;162:896–908. doi: 10.1093/infdis/162.4.896. [DOI] [PubMed] [Google Scholar]