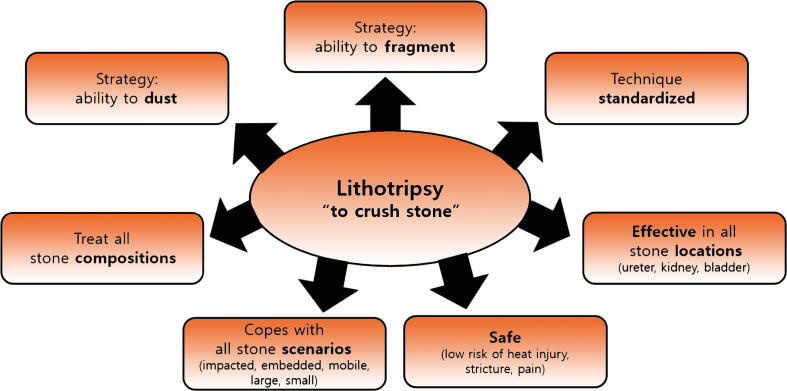
The term tripsy means the intentional crushing of a structure. The ideal laser for lithotripsy must tackle different stone compositions and sizes and be effective in a range of scenarios such as mobile, impacted, embedded, and hard-to-reach stones. Some of these factors cannot be predicted on imaging. The laser in the operating room must provide the surgeon with confidence that it can handle any situation effectively and safely.
Modern strategies for laser lithotripsy consist of fragmenting stones into smaller parts for retrieval or breaking them into fine fragments, often called dust, for spontaneous passage. The former is performed in the ureter while the latter is now popular for retrograde intrarenal surgery (RIRS) because of the availability of high-frequency holmium lasers.
The thulium fiber laser (TFL; wavelength 1940 nm) was launched after in vitro studies demonstrated a 1.5- to three-fold increase in stone ablation compared to holmium laser [1]. The question thus arises as to whether this is the end of the road for holmium. The answer is no, holmium laser is here to stay because of fundamental differences from TFL in the parameter domains of peak power, fluid absorption, and stone carbonization. These factors may lead to variation in TFL performance and impact the efficacy of surgery in certain scenarios.
TFL has low peak power. The pulse duration is very long, even when on a short pulse mode. While this translates to low retropulsion and small particles coming off the stone, the pulse does not provide sufficient forward penetration for fracture of all stones, as we are accustomed to when fragmenting stones with holmium laser. This feature may not be necessary when dusting renal stones but is critical when dealing with impacted or embedded stones. For these reasons, we use holmium laser when treating ureteral stones.
Because of the TFL wavelength, another critical difference is that absorption by fluid is four-fold greater with TFL than with holmium laser [1]. From a technique perspective, TFL is a contact laser for which the fiber tip must be exactly on the stone for effective fragmentation. By contrast, holmium has a greater reach. Fragmentation can occur 1–2 mm away from the tip [2]. We do not realize how helpful this feature is until confronted with a case for which it is challenging to get in contact with the stone. The remedy for the handicaps of peak power and fluid absorption with TFL has been to increase the power so that energy can reach the stone. However, high power settings can be injurious to tissue and have consequences, especially in the ureter.
Stone carbonization (charring) and sparks of light can occur during TFL lithotripsy. There are multiple hypotheses for why this occurs, including the long TFL pulse duration and the chaotic bubble stream of TFL pulses. Pulses with a long duration result in photothermal ablation with carbonization and collateral damage [3]. Our observation is that this happens more frequently with calcium phosphate stones, which may be somewhat resistant to ablation.
How do we reconcile the higher fragmentation observed with TFL in the laboratory? These in vitro experiments are performed in tubes containing phantom Begostones, with the fiber in contact with the stone, and micro-sieves that evacuate small fragments from the field. Laser flashes do not stop these automated experiments. In the clinical realm, the pauses needed to perform lithotripsy, either because of dust residue limiting vision or stone carbonization, may reduce the efficiency of TFL and lead to different results.
Ulvik and colleagues [4] recently published results from a randomized trial comparing ureteroscopy with TFL versus a 30-W holmium laser. The computed tomography (CT)-based complete stone-free rate (cSFR) for renal stones was 66% among 36 patients treated with TFL compared to 33% among 39 patients treated with holmium laser. While this seems promising on first glance, comparison to outcomes with high-frequency holmium laser reveals that it is not. In a recent study of 86 patients undergoing RIRS for 10–20-mm renal stones with a 120-W holmium using a range of dusting settings (0.2–0.5 J, 50–80 Hz), Peretti et al. [5] demonstrated a CT-based cSFR of 69%. The efficacy for dusting may not differ between TFL and high-power holmium lasers, and large comparative clinical trials are warranted.
Another consideration is the variation in laser setting selection when using TFL. Despite the fanfare of very low pulse energies (0.05 J), surgeons are not using this setting because it does not achieve effective fragmentation. In a study of surgeon settings for TFL, there was no consensus on the ideal setting [6]. Since idiosyncratic physician practice patterns are recognized as an indication of uncertainty, this could be concerning for TFL. Training the next generation of urologic surgeons may not be standardized, which could lead to safety concerns.
Belle and co-workers [7] recently compared the heat generated during lithotripsy with TFL against holmium in a ureteroscopic model, when treating an impacted ureteral stone with 35cc/min irrigation. At equivalent power settings of 3.6 W, 10 W, and 30 W, TFL generated significantly higher intra-ureteral temperatures. Importantly, with TFL at 30 W, the temperature exceeded 43 °C, the threshold for tissue damage. These findings are consistent with reports of thermal injury and stricture after ureteroscopy with TFL in the ureter [8]. In clinical practice, 60–70% of upper urinary tract stones treated with ureteroscopy are located in the ureter [9]. For this location, holmium laser offers predictability and safety.
The future with holmium laser is bright. Pulse modulation with Moses technology increases fragmentation by 30% in comparison to short or long pulse modes [2]. Holmium lasers with micro-pulse packets are the next step. A prototype holmium system with these features provided significantly greater stone ablation in comparison to TFL [10].
TFL is a novel surgical technology that will evolve. However, the physics and evidence so far suggest it is a dusting laser. In the correct circumstances, it is effective. However, laser lithotripsy must cope with different strategies and holmium laser provides the correct balance for either fracturing or dusting stones, making it more versatile (Fig. 1). Similar to the second car in a family household, TFL is likely to become the second laser in the operating room used for specific cases requiring dusting. However, not every family can afford a second car. The holmium laser will remain the primary vehicle for lithotripsy.
Fig. 1.
Features for ideal laser lithotripsy.
Conflicts of interest: Khurshid R. Ghani is a consultant for Boston Scientific, Coloplast, Olympus, and Karl Storz, and has received investigator funding from Boston Scientific and Coloplast. Hyung Joon Kim has nothing to disclose.
Associate Editor: Silvia Proietti
References
- 1.Fried N.M., Irby P.B. Advances in laser technology and fibre-optic delivery systems in lithotripsy. Nat Rev Urol. 2018;15:563–573. doi: 10.1038/s41585-018-0035-8. [DOI] [PubMed] [Google Scholar]
- 2.Aldoukhi A.H., Roberts W.W., Hall T.L., Ghani K.R. Watch your distance: the role of laser fiber working distance on fragmentation when altering pulse width or modulation. J Endourol. 2019;33:120–126. doi: 10.1089/end.2018.0572. [DOI] [PubMed] [Google Scholar]
- 3.Chan K.F., Pfefer T.J., Teichman J.M.H., Welch A.J. A perspective on laser lithotripsy: the fragmentation processes. J Endourol. 2001;15:257–273. doi: 10.1089/089277901750161737. [DOI] [PubMed] [Google Scholar]
- 4.Ulvik Ø, Sørstrand Æsøy M, Juliebø-Jones P, Gjengstø P, Beisland C. Thulium fibre laser versus holmium:YAG for ureteroscopic lithotripsy: outcomes from a prospective randomised clinical trial. Eur Urol. In press. 10.1016/j.eururo.2022.02.027. [DOI] [PubMed]
- 5.Peretti D., Dalmasso E., Pecoraro A., et al. Low-energy high-frequency Ho-YAG lithotripsy: is RIRS going forward? A case-control study. Urolithiasis. 2022;50:79–85. doi: 10.1007/s00240-021-01282-2. [DOI] [PubMed] [Google Scholar]
- 6.Sierra A, Corrales M, Pinero A, Traxer O. Thulium fiber laser pre-settings during ureterorenoscopy: Twitter’s experts’ recommendations. World J Urol. In press. 10.1007/s00345-022-03966-9. [DOI] [PubMed]
- 7.Belle JD, Chen R, Srikureja N, Amasyali A, Keheila M, Baldwin DD. Does the novel thulium fiber laser have a higher risk of urothelial thermal injury than the conventional holmium laser in an in vitro study? J Endourol. In press. 10.1089/end.2021.0842. [DOI] [PubMed]
- 8.US Food and Drug Administration. Class 2 device recall Olympus. Silver Spring, MD: FDA; 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=188172.
- 9.Hiller S.C., Daignault-Newton S., Pimentel H., et al. Ureteral stent placement following ureteroscopy increases emergency department visits in a statewide surgical collaborative. J Urol. 2021;205:1710–1717. doi: 10.1097/JU.0000000000001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang B., Parab I., Cancino J., et al. MP07-17 Stone ablation efficacy of a new prototype Holmium:YAG pulse-modulated laser at working distances of up to 3 mm. J Urol. 2021;206:e146. doi: 10.1097/JU.0000000000001980.17. [DOI] [Google Scholar]