Introduction
Paraneoplastic pemphigus (PNP), first described by Anhalt et al1 in 1990, is a rare and often fatal autoimmune bullous dermatosis associated with lymphoproliferative diseases. The variability of clinical and histopathologic features makes diagnosis difficult.
The diagnostic findings are severe erosive mucositis and serologic detection of antiplakin antibodies in patients with underlying malignancy. The pathogenesis of PNP is poorly understood. Humoral and cell-mediated autoimmune responses against desmosomal and hemidesmosomal proteins triggered by the neoplastic process play a pivotal role. Treatment of PNP is based on evidence from a limited number of case reports.2 Here, we describe a case of PNP associated with chronic lymphocytic leukemia (CLL) and its response to obinutuzumab, a novel anti-CD20 antibody.
Case presentation
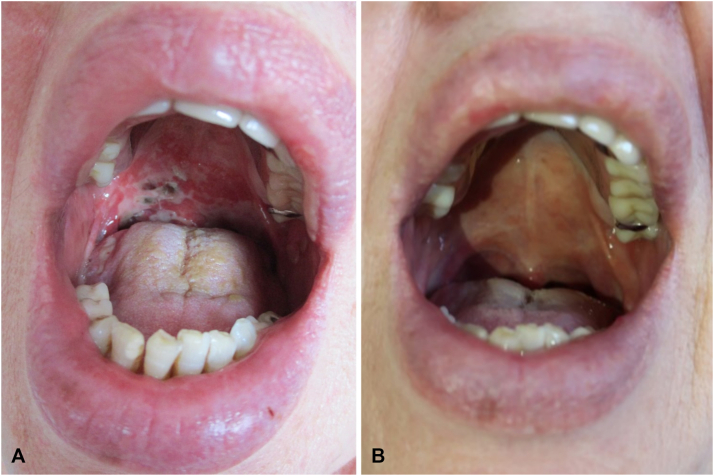
A 70-year-old woman with progressive erosive stomatitis presented to our department. Hoarseness and odynophagia had started the week before. Her medical history was otherwise uneventful. She had not been taking any medication. Her physical examination showed extensive painful erosions on the soft and hard palate, the buccal mucosa, and the tongue. A laryngoscopic examination revealed symmetric swelling of the larynx. Enlarged lymph nodes were prominent on the right side of the neck (Fig 1).
Fig 1.
Clinical presentation. A, Initial presentation: extensive fibrin-coated erosions on the hard and soft palate of the patient, (B) 21 months after disease onset.
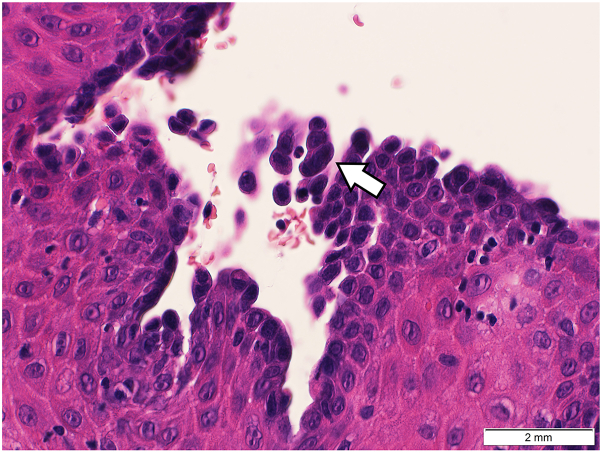
Histopathologic investigation of a 4-mm punch biopsy of the oral mucosa revealed suprabasal acantholysis and a sparse lymphocytic infiltrate in the dermis (Fig 2). Direct immunofluorescence (DIF) test results showed complement component 3, IgG, IgA, and immunoglobulin M on cytoid bodies. Serologic analysis performed via enzyme-linked immunosorbent assay revealed elevated antidesmoglein (Dsg) antibodies-3 (21.16 U/mL; reference value: <7 U/mL, negative; >20 U/mL, positive). Anti–Dsg-1, envoplakin, and bullous pemphigoid antigen antibodies 180 and 230 were negative. Indirect immunofluorescence testing presented a positive antiintercellular substance antibody IgG titer of >1:160 on monkey esophagus epithelia and a positive titer of 1:40 on rat bladder epithelia. Furthermore, immunoblot analysis of a cellular extract of cultured human keratinocytes detected IgG antibodies against periplakin and envoplakin. Microbiologic investigations of herpes simplex virus, bacteria, and fungi were negative.
Fig 2.
Punch biopsy of the oral mucosa, suprabasal acantholysis.
Blood counts revealed leukocytosis (24.3 G/L; reference limits 3.6-10.5 G/L) with 58% lymphocytes (reference limits 20%-44%). Lymphocyte phenotyping of the peripheral blood showed a B-cell clone characteristic of CLL. PNP with underlying CLL (Binet stage B) was diagnosed.
High-dose glucocorticoid therapy (prednisolone 250 mg/d) was initiated. However, the disease progressed rapidly with severe laryngeal involvement requiring intubation and tracheotomy.
A single infusion of cyclophosphamide (250 mg/m2) and weekly rituximab (375 mg/m2) were added to the concomitant glucocorticoid therapy for 5 weeks without clinical improvement. Two months after disease onset, staging investigation with computed tomography showed generalized lymphadenopathy, and ibrutinib (420 mg/d) was started. Although lymphocytosis improved, PNP responded insufficiently. Therefore, monthly intravenous immunoglobulin (IVIG, 2g/kg) and dexamethasone pulse therapy (100 mg/d for 3 days) were added. Mucositis improved and remained stable for 3 months. Despite ongoing treatment, PNP relapsed and obinutuzumab was initiated (1000 mg on days 1, 8, and 15) and then every 4 weeks for 6 months. Ibrutinib had to be stopped after 3 months because of drug-induced atrial fibrillation. Eight weeks after the initiation of obinutuzumab, the mucosal erosions had completely healed and the initially detected disease-related autoantibody had disappeared. CLL was in full remission with no detectable B cells in the peripheral blood. Radiologic imaging displayed no signs of active disease. IVIG and glucocorticoid pulse therapy were continued for a further 3 months and then slowly tapered. After 3 months without any specific therapy, small ulcerations on the tongue and buccal erosions reappeared. With B cells still depleted, we resumed IVIG (2g/kg) and remission was achieved. Monthly IVIG administration was maintained. The patient is still in complete remission 32 months after the disease onset.
Discussion
In 1990, Anhalt et al1 reported 5 patients with underlying malignomas and sudden onset of severe mucosal ulceration and polymorphous cutaneous eruptions, resembling erythema multiforme. The histopathologic findings showed an unspecific pattern. Immunofluorescence testing revealed atypical pemphigus-like autoantibodies that were different from those typically seen in pemphigus vulgaris (Dsg-3) and foliaceus (Dsg-1). In 1998, Borradori et al3 and Kiyokawa et al4 shed some more light on the PNP antigen complex by detecting periplakin (190 kDa) and envoplakin (210 kDa). Svoboda et al5 proposed erosive mucositis with or without cutaneous involvement as a required clinical sign of PNP. Further, major diagnostic criteria are the underlying hematologic neoplasm and the serologic detection of antiplakin autoantibodies. Minor criteria are evidence of acantholysis and/or lichenoid interface dermatitis observed on histopathology and DIF test displaying intercellular and/or basement membrane staining patterns. The diagnosis of PNP is fulfilled if all the 3 major criteria or the 2 major and minor criteria are met.5 Common differential diagnoses of PNP are other autoimmune bullous skin diseases (eg, pemphigus vulgaris), severe drug reaction (eg, Stevens-Johnson syndrome), erythema exsudativum multiforme, and mucocutaneous eruptions associated with mycoplasma infections. Unlike pemphigus vulgaris and pemphigus foliaceus, in which DIF test is the gold standard in diagnosis, negative DIF test results in PNP are not uncommon.6
High-dose systemic glucocorticoids with and without rituximab are often used as a first-line treatment of PNP. In refractory cases, the addition of further immunosuppressive agents (mycophenolate mofetil or azathioprine) or IVIG may be used. The successful treatment of PNP with ibrutinib, an oral inhibitor of Bruton tyrosine kinase, in combination with rituximab was reported by Ito et al.7
Obinutuzumab is a humanized monoclonal antibody causing B-cell depletion by targeting CD20. Because of altered glycosylation, obinutuzumab binds with higher affinity to the CD20 epitope of the immune effector cells, which increases the antibody-dependent cell-mediated cytotoxicity/phagocytosis. Furthermore, obinutuzumab is thought to be more effective in B-cell depletion than previous agents (eg, rituximab) because of a different binding geometry.8,9 Kuriyama et al10 described a case of follicular lymphoma and PNP that was successfully treated with combined therapy of bendamustine and obinutuzumab.
This case report highlights the importance of considering PNP in the differential diagnosis of severe erosive stomatitis. The treatment of PNP is challenging and the prognosis is often poor, so obinutuzumab could be a promising ace in the hole.
Conflicts of interest
None disclosed.
Acknowledgments
The authors would like to acknowledge the patient for agreeing to the publication of her medical history. Further, we would like to show our gratitude to Primary Associate Professor Dr Melitta Kitzwögerer, head of the Department for Clinical Pathology and Molecular Pathology, who contributed significantly to case processing and histopathology. We also would like to thank Dr Carolina Neudorfer, who cared for the patient and was involved in the patient’s documentation.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Supplementary data
References
- 1.Anhalt G.J., Kim S.C., Stanley J.R., et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729–1735. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.H., Kim S.C. Paraneoplastic pemphigus: paraneoplastic autoimmune disease of the skin and mucosa. Front Immunol. 2019;10:1259. doi: 10.3389/fimmu.2019.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borradori L., Trueb R.M., Jaunin F., et al. Autoantibodies from a patient with paraneoplastic pemphigus bind periplakin, a novel member of the plakin family. J Invest Dermatol. 1998;111:338–340. doi: 10.1046/j.1523-1747.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- 4.Kiyokawa C., Ruhrberg C., Nie Z., et al. Envoplakin and periplakin are components of the paraneoplastic pemphigus antigen complex. J Invest Dermatol. 1998;111:1236–1238. doi: 10.1046/j.1523-1747.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- 5.Svoboda S.A., Huang S., Liu X., Hsu S., Motaparthi K. Paraneoplastic pemphigus: revised diagnostic criteria based on literature analysis. J Cutan Pathol. 2021;48:1133–1138. doi: 10.1111/cup.14004. [DOI] [PubMed] [Google Scholar]
- 6.Anhalt G.J. Paraneoplastic pemphigus. J Investig Dermatol Symp Proc. 2004;9:29–33. doi: 10.1111/j.1087-0024.2004.00832.x. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y., Makita S., Maeshima A.M., et al. Paraneoplastic pemphigus associated with B-cell chronic lymphocytic leukemia treated with ibrutinib and rituximab. Intern Med. 2018;57:2395–2398. doi: 10.2169/internalmedicine.0578-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman C.L., Sehn L.H. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182:29–45. doi: 10.1111/bjh.15232. [DOI] [PubMed] [Google Scholar]
- 9.Tobinai K., Klein C., Oya N., Fingerle-Rowson G. A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies. Adv Ther. 2017;34:324–356. doi: 10.1007/s12325-016-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuriyama K., Kitamura Y., Tsuji T., et al. Successful treatment of paraneoplastic pemphigus and bronchiolitis obliterans associated with follicular lymphoma with obinutuzumab and bendamustine. Curr Probl Cancer. 2022;46(2) doi: 10.1016/j.currproblcancer.2021.100813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.