Abstract
Due to their improved structural and functional properties as well as biocompatibility, biodegradability, and nontoxicity, chitosan and its nanoparticles are currently grasping the interest of researchers. Although numerous attempts have been made to apply chitosan and its derivatives to biological applications, few have reported in achieving its pharmacological and drug delivery. The goal of the current work is to provide a summary of the chitosan biopolymer's physical, chemical, and biological properties as well as its synthesis of nanoparticles and characterization of its modified nanocomposites. The drug delivery method and pharmaceutical applications of chitosan biopolymer and its modified nanocomposites are examined in further detail in this research. We will introduce also about the most current publications in this field of study as well as its recent expansion.
Keywords: Chitosan, Biopolymer, Nanocomposite, Pharmaceutical, Drug delivery
ChitosanBiopolymerNanocompositePharmaceuticalDrug delivery
1. Introduction
Due to their great biocompatibility and biodegradability, biopolymers are crucial in biomedical applications [1, 2]. Biopolymer-based nanoparticles are the most promising nano carriers for delivering various therapeutic drugs to tumor cells such ovarian cancer cell lines because they have good biodegradation and biodistribution in biological systems [3, 4]. Additionally, biopolymers are utilized in a number of biomedical applications, including genes delivery [1], tissue engineering, and drug delivery [5, 6]. Due to their usage and limitations, biopolymers are a major chemistry and biology interface [7, 8]. There is a need to create awareness and create novel ways for biomedical and agricultural applications given the variety of biopolymer applications [1, 9]. The research community is currently very interested in biopolymers such chitosan, alginate, pectin, cellulose, agarose, and gelatin [10, 11].
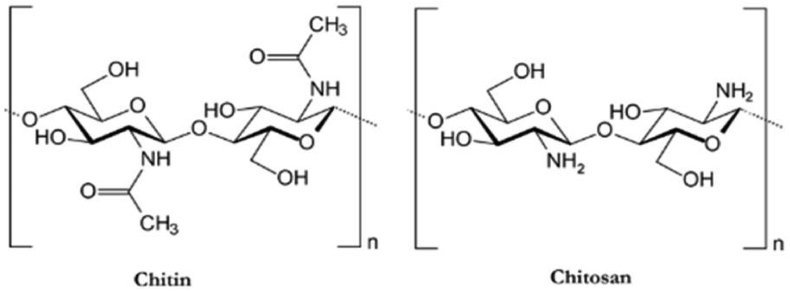
Chitosan (CS), a biopolymer, has drawn a lot of interest due to its adaptability, accessibility, and special qualities in medical applications [12, 13]. It is made up of 2-acetamido-2-deoxy-D-glucopyranose and 2-amino-2-deoxy-D-glucopyranose units and is the second most prevalent copolymer after cellulose [14, 15, 16]. It is often created from chitin by partial deacetylation in an alkaline environment, as shown structurally in Figure 1 [17-20]. The polymer is digested by human enzymes and helps with wound healing by promoting hemostasis and accelerating tissue regeneration [21, 22, 23]. Additionally, chitosan is made from renewable resources, which are currently extending the range of applications [24]. It has been coupled with a range of polymeric biomaterials and inorganic bioactive chemicals for possible use in orthopedics as bone graft substitutes, intervertebral discs, and bone and cartilage tissue engineering [25].
Figure 1.
Chemical structure of chitin and chitosan [26].
Due to its antibacterial action, chitosan and its nanoparticles (NPs) are beneficial for a range of biological applications, including food preservation [27]. They also affect fish and crustaceans in an immunomodulatory manner, which directly benefits the aquaculture and fish farming industries [28]. Additionally, CS NPs are now being used to treat illnesses in fish and other animals [29]. Chitosan NPs are attractive candidates for a variety of uses in fish medicine due to their many beneficial biological features, including safety, biocompatibility, biodegradability, and antibacterial capacity [30].
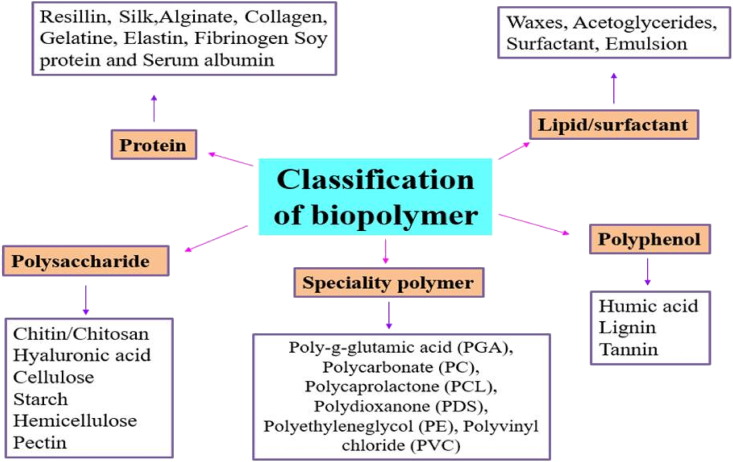
Due to its polyelectrolytic nature and its capacity to chelate substances because of the presence of amino groups, chitosan is used for the majority of applications [31, 32]. As a result of its beneficial physicochemical features, which enable the creation of reactive surfaces, chitosan and its derivatives are the materials that are most heavily investigated [33, 34]. Chitosan has numerous uses as a bio-pesticide in agriculture, a packaging material for the food and pharmaceutical sectors, and a membrane filtration system for wastewater treatment [28, 35, 36]. Chitosan is amenable to modification because of the presence of functional groups like amino (NH2+) and hydroxyl (OH-) [37, 38, 39]. Furthermore, chitosan is distinguished by its substantial biological and chemical characteristics and safety [40]. Figure 2 illustrates how distinct groups of biopolymers can be categorized according to the presence and covalent bonding of monomers.
Figure 2.
Classification of biopolymers.
2. Physicochemical and biological properties of chitosan biopolymer
Chitosan has a wide range of physicochemical and biological properties, which are listed in Table 1. It can be used in its natural state or in a modified state produced through physical or chemical methods to produce novel qualities and functionalities.
Table 1.
| Physical properties | Chemical properties | Biological properties |
|---|---|---|
| • High molecular weight (1.2 × 105 g mol−1) • White yellow in color • Weak base (powerful nucleophile, pKa 6.3) • Flakes, bead or powder • Intermolecular hydrogen bonding • Optical clarity. • Amorphous solid • Density 0.18–0.33 g/cm3 • Soluble in diluted aqueous acid solution • Insoluble in water • Conductivity |
• Rigid D-glucosamine structure • Degree of acetylation range 70–95% • Cationic polyamine • High charge density at pH < 6.5 • Forms gels with Poly-anions Polyelectrolyte • Adheres to negatively charged surfaces • Amiable to chemical modification • Additive in paper industry • Filmogenic properties • Linear polyamine • Numerous reactive groups (amino and hydroxyl) • Linear amino-polysaccharide with high nitrogen content |
• Biocompatibility • Bacteriostatic • Wound management • Anticancerogen • Accelerates bone formation • Accelerates the formation of osteoblast • Antioxidant • Biodegradable • Homeostatic • Natural polymer • Bone formation • Safe and non-toxic |
3. Synthesis of chitosan nanoparticles
Due to their low prices, eco-friendly, and non-toxic natures, metal oxide nanoparticles (NPs) have been created utilizing green chemical technologies in recent decades, and they are good therapeutics for animals and people [43, 44]. Chemically produced metal oxide NPs including hazardous chemical reducing agents such as hydrazinium hydroxide, sodium hypophosphite, and sodium borohydride, on the other hand, have had an environmental impact. The precursors would cling to the broad surfaces of NPs, increasing their toxicity and negatively impacting the environment and biological applications [17, 44, 45]. Chitosan nanoparticles (CNPs) are nontoxic, biocompatible, biodegradable, and functionalized nanostructures derived primarily from by-products of the seafood industry. CNPs have shown potential as green fillers in biodegradable composite reinforcement for food packaging and biomedical applications [44, 46]. In the following section, the majority of the common methods for synthesizing chitosan nanoparticles are thoroughly explained, with their benefits and drawbacks.
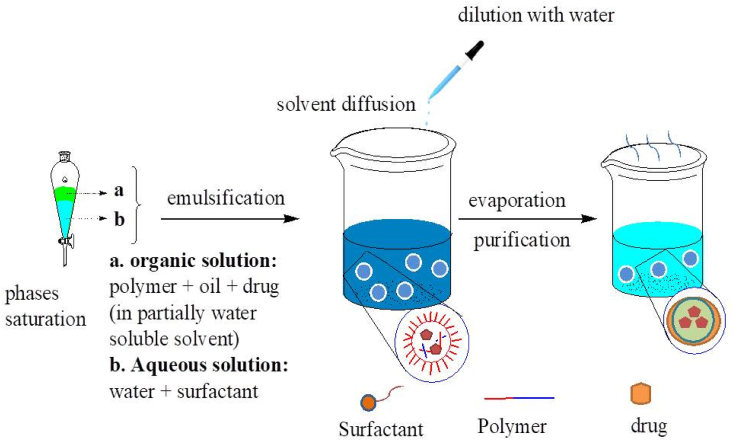
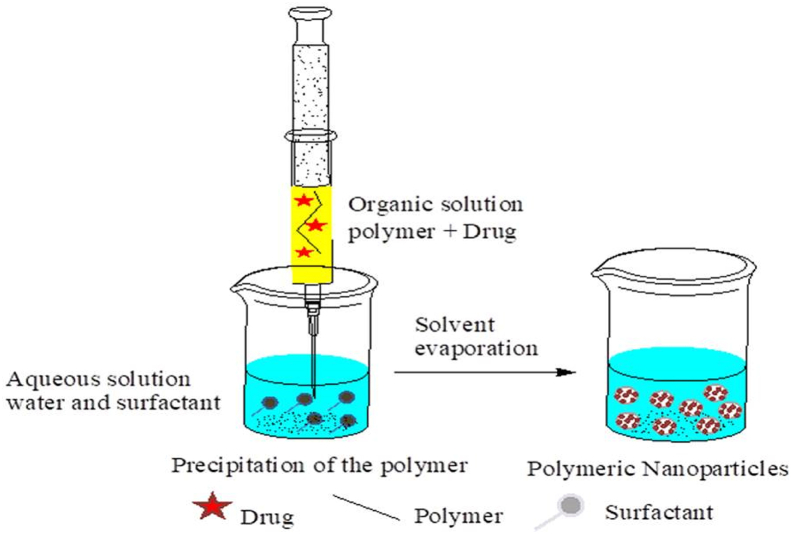
3.1. Emulsification method
As depicted in Figure 3 below, emulsions interior phase is made up of a semi-hydrophobic organic solvent like benzyl alcohol or ethyl acetate. Both phases were pre-saturated with water to ensure that they were in thermodynamic equilibrium at ambient temperature [12, 44]. The approach is based on emulsifying a polymer organic solution into a water phase, then evaporating the organic solvent [44, 47]. Following dilution with a large amount of water, solvent diffusion from the dispersed droplets into the outer phase causes the formation of colloidal particles. Finally, evaporation or filtration can be used to remove the organic solvent depending on their boiling point Figure 4 [39,48]. Emulsification reduces the size of the emulsion droplet by using a high-shear force. Following emulsification, the system evaporates the organic solvent under vacuum, resulting in polymer precipitation and the formation of nanoparticles [49]. Finally, NPs with diameters ranging from 80 to 900 nm can be obtained. Despite the need for a large volume of aqueous phase to be removed from the colloidal dispersion and the risk of hydrophilic drug diffusion into the aqueous phase, this method is frequently used for the production of polymeric NPs [39, 50].
Figure 3.
Schematic representation of the emulsification/solvent diffusion method.
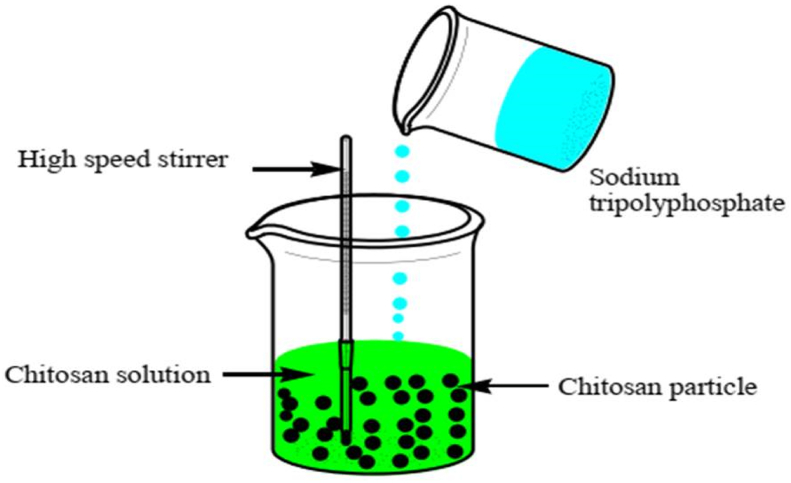
Figure 4.
Preparation of chitosan NPs by ion gelation technology.
3.2. Ionic gelation method
Ionic crosslinking is used to create chitosan NPs (Figure 4). A positively charged amine group and a negatively charged polyanion, such as tripolyphosphate (TPP), make up the ionic compound [51, 52]. Chitosan was made into a cationic solution by dissolving it in diluted acetic acid, while TPP was made into an anionic solution by dissolving it in distilled water. The TPP solution was then added one drop at a time to the cationic chitosan solution [48, 53]. At room temperature, mechanical churning created NPs rapidly. Adjusting the amount of chitosan and crosslinking agent, as well as the pH value of the solution, can affect the physiochemical parameters of the resultant NPs, such as particle size and surface charge [54, 55].
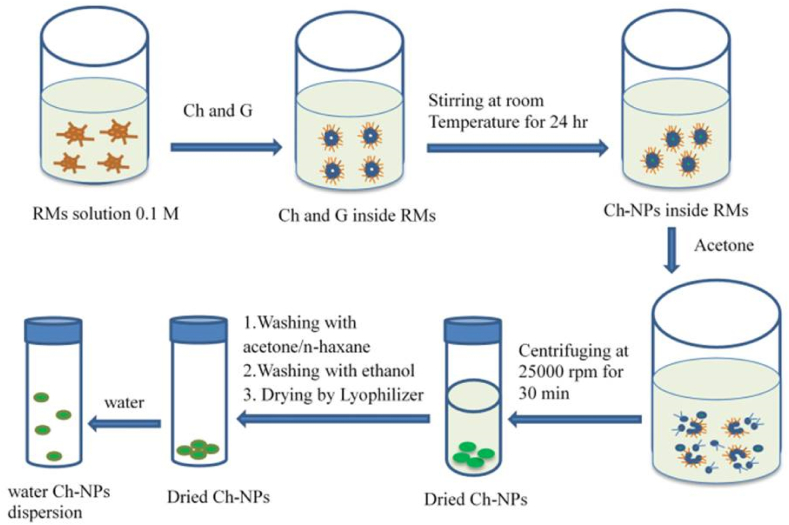
3.3. Reverse micellar method
Making chitosan NPs using the reverse micellar technique entails producing NPs in the aqueous core of reverse micellar droplets and then crosslinking them with glutaraldehyde (Figure 5). A surfactant was dissolved in an organic solvent to form reverse micelles in this manner [56]. To prevent turbidity, an aqueous chitosan solution was introduced while constantly whirling [57]. This transparent solution was given a crosslinking agent while being constantly agitated. To complete the cross-linking process and ensure that the free amine group of chitosan was conjugated with glutaraldehyde, the system was kept stirred overnight [56]. The organic solvent was removed by evaporation under low pressure. The surplus surfactant yields and cross-linked chitosan NP yields were achieved. By precipitating the surplus surfactant with an appropriate salt and centrifuging the precipitate, the excess surfactant was removed. The final NPs suspension was dialyzed before lyophilyzation. This approach yielded chitosan NPs with a size of less than 100 nm and a high degree of mono dispersing [48, 56].
Figure 5.
Preparation of chitosan NPs reverses micellar method.
3.4. Nanoprecipitation method
Fessi's group was the first to develop and apply nanoprecipitation, also known as solvent displacement or interfacial deposition [58, 60]. The nanoparticles are made in a colloidal suspension using the nanoprecipitation method, which entails adding the oil phase to the aqueous phase slowly while stirring moderately (Figure 6). It has the advantage of being rapid and simple to utilize because the production of the NPs is instantaneous and takes only one step. The rate of organic phase injection, the rate of aqueous phase agitation, and the oil phase/aqueous phase ratio are all critical manufacturing parameters that have a significant impact on the nanoprecipitation process [50, 59, 61]. Particles with an incredibly narrow dispersion can be created because there is no shearing tension. Entrapment of hydrophobic and hydrophilic medicines is a common application of this method [50, 62]. The polymer and medication are dissolved in a water miscible organic solvent such as acetone or methanol. The solution is then dropped into an aqueous solution containing surfactant one drop at a time. Due to rapid solvent diffusion, the NPs are formed quickly. Following that, the solvents are extracted at a reduced pressure [61, 63].
Figure 6.
Preparation of chitosan NPs by nanoprecipitation method.
4. Characterization of chitosan biopolymer and its modified nanocomposites
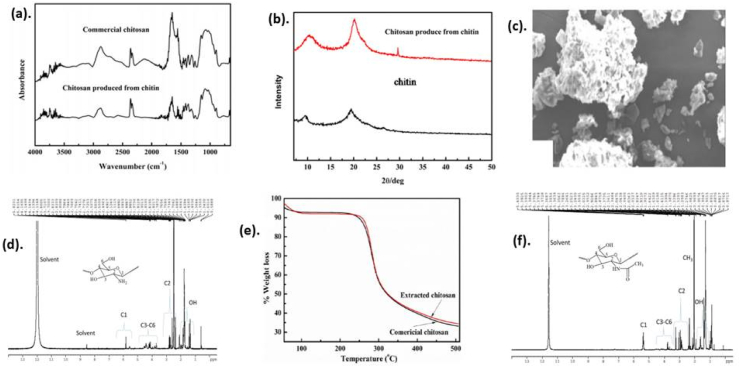
According to the degree of deacetylation (DD), which is assessed by the percentage of D-glucosamine and N-acetyl-D-glucosamine, the biopolymer is classified as either chitin or chitosan [64, 65]. Table 2 shows the most widely used methodologies for characterizing chitosan and its modified nanocomposites. Chromatographic and spectroscopic techniques can be used to analyze chemically modified chitosan derived from chitin, as shown in Figure 7 which is taken from our previous work.
Table 2.
Instruments commonly used for characterization of chitosan and its modified nanocomposites, as well as their applications.
| No | Instruments | Application | Ref |
|---|---|---|---|
| 1 | Thermogravimetric | Thermal stability of chitosan and its nanocomposites | [66] |
| 2 | FT-IR spectroscopy | elucidate the structure of a compound | [67] |
| 3 | Viscometric analysis | Molecular weight determination | [68] |
| 4 | X-ray diffraction | Crystallinity and phase purity | [69] |
| 5 | Scanning electron microscopy | Morphology | [70, 71] |
| 6 | 1H NMR | Characteristic peaks of proton | [71] |
Figure 7.
Characterization of chitin and chitosan. (a). FTIR, (b). XRD, (c). SEM, (d and f). 1HNMR and (e). Thermogravimetric analysis. Reproduced with permission from [68].
5. Pharmaceutical applications of chitosan biopolymer, chitosan derivatives and its modified nanocomposites
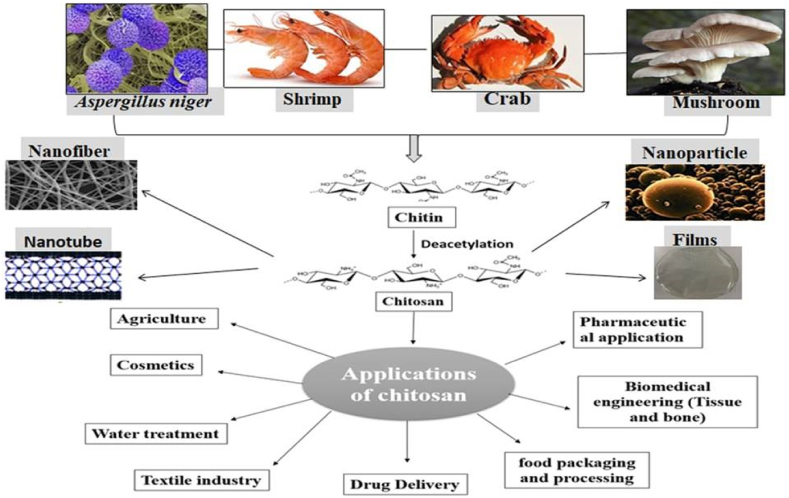
Chitosan is used in a wide range of sectors, from agriculture to advanced biotechnology and nanotechnology professions (Table 3). Figure 8 shows chitosan derivatives and modified composites applications in wastewater treatment, cosmetics, textiles, biomedical, food packaging and processing, and other applications [72, 73]. Bacterial and viral infections can be life-threatening as a result of drug overuse and the emergence of antibiotic-resistant pathogens. Biopolymers are currently considered to be the most promising medicinal materials [74, 75]. Chitosan is a surface-modified polysaccharide that is used in drug delivery systems [76, 77]. It has attracted a lot of attention because of its biocompatibility, low cost, nontoxicity, environmental friendliness, absorbability, biodegradability, recyclability, and superior antibacterial characteristics [72, 78]. Chemical modifications to the chitosan structure have sparked a lot of attention because they improve transfection efficiency and stability [79, 80].
Table 3.
Reports chitosan based nanocomposite and their pharmaceutical applications.
| Chitosan derivatives and chitosan based nanocomposite | Properties | Pharmaceutical applications | Reference |
|---|---|---|---|
| Chitosan/Polyvinyl alcohol and modified thiabendazolium-montmorillonite. | Biodegradable and antimicrobial activities | Show a good antibacterial activities against S. aureus and E. coli) and active packaging applications | [30] |
| Carboxylic acid functionalized carbon nanotubes dispersed in chitosan as a selective layer on the polsulfone membrane (CNTs-COOH/CHIT/PS) Carbon nanotubes dispersed in chitosan as a selective layer on the polsulfone membrane (CNTs/CHIT/PS) |
Eco-friendly adsorbent Eco-friendly adsorbent |
Efficient rejection of heavy metal ions from aqueous solutions. Less efficient than (CNTs-COOH/CHIT/PS) in rejection of heavy metal ions from aqueous solutions. |
[87] |
| Porous nickel molybdate nanosheets/chitosan (NiMoO4/CHIT). | Sensitive, selective, reproducible, biocompatible & biodegradable | As biosensor and practical pharmaceutical analysis (detection of amlodipine drug). | [91] |
| Gold nanoparticles and a chitosan nanocomposite film coated on a screen printed electrode (Au-NPs/CHI/SPE). | Sensitivity, stability, reproducibility, immuno sensor & cancer biomarkers | Exhibited potential in clinical screening of cancer biomarkers. Diagnosis of prostate cancer using prostate-specific antigen. | [92] |
| (Nickel Ferrite cores/bovine serum albumin/chitosan/folic acid) NFs-BSA–CS–FA or BSA-CMC-FA conjugates. | Hydrophilicity, nontoxicity, cancer-specific capability and biocompatible | Green approach for breast cancer MR imaging, treatment, tumor diagnosis and therapy. | [93] |
| Six novel N,N,O tridentate water soluble hydrazide based O-carboxymethyl chitosan Schiff base derivatives | Anti-inflammatory, antioxidant & antidiabetic agent | Could be used for treatment of body pain, as anti-diabetes and cancer. | [94] |
| Gold and silver-based chitosan nocomposites | antimicrobial, antitumor, anti-inflammatory and antioxidant effects | Possess potential applications in nanomedicine. Used as wound dressing and anti-bacterial activities. | [86] |
| N,N,N-Trimethyl ammonium chitosan (TMC) | Water solubility, pH sensitivity antibacterial, anti-inflammatory agents | Widely used in medicine as antibacterial, anti-inflammatory drugs, filler fiber in materials for dressing wounds | [95] |
| Chitosan (CS) Deacetylation Degree(DD) + Alginate (ALG) Chitosan + Lecithin liposomes + L-Arginine |
Bioavailability, mucoadhesion and blood glucose lowering properties. | Used as effective insulin oral delivery for treatment of diabetes | [96] |
| Ag-chitosan nanoparticles | Durable effects and antibacterial activity | Portray encouraging antibacterial reduction of textile materials | [97] |
| N-quaternized chitosan/poly(vinyl alcohol) hydrogels | Biocompatibility, biodegradability, nontoxicity, availability in abundance and antifungal agent | Used as antifungal agent and in wound dressing materials | [98] |
| Chitosan beads & Chitosan stabilized bimetallic Fe/Ni nanoparticles, Grafted chitosan hydrogel with acrylic acid, MgO/Chitosan/Graphene oxide and Chitosan-g-poly(glycidyl methacrylate) | Adsorbents of antibiotic pharmaceuticals | Removal of Amoxicillin, Enrofloxacin, Norfloxacin and Cephalosporin respectively from aquatic environment. Used for waste water treatment by removing antibiotic pharmaceuticals. | [99] |
| 2,6-Diamino chitosan (2,6-DAC) | Biodegradable, biocompatible and synergistic activity | Exhibit broad bactericidal efficacy toward both Gram-positive and Gram-negative bacteria with minimum inhibitory concentrations and has synergistic activity with antibiotics including amikacin, tobramycin, novobiocin, rifampicin, and tazobactam. | [100] |
| Chitosan/polylactic acid/calcium phosphate Chitosan/calcium phosphate nanosheet |
Tough bone-resembling and osteoblast enlargement biodegradability and low cytotoxicity | Bone tissue engineering (bone implants) Vaccine carrier |
[101] |
| Carboxymethyl chitosan (CMCs) + glutathione-glycylsarcosine (G-GS) & carboxymethyl chitosan (CMCs) + glutathione-valyl-valin (G-VV)-LDH hybrid |
Noncytotoxicity and permeability | As topical administration drug delivery to the posterior segment of the eye. | [102] |
| Fucoidan-based chitosan carrier | Non toxicity and biocompatibility | Used test Human breast cancer cell line and Colon cancer Caco-2 cells and treatment. | [103] |
| Chemically modified O-carboxymethyl chitosan Schiff base and their metal complex | Good solubility in water, high viscosity, low toxicity and biocompatibility | Possess better antibacterial, antifungal, anti-inflammatory, antidiabetic and antioxidant | [104] |
| modified cellulose and cross-linked chitosan with covalently bound 8-hydroxyquinoline | Non-digestible, non resorbable, biocompatible | Potential for treatment of Wilson's disease. | [105] |
| Chitosan-g-poly (N-isopropyl acrylamide) | Biodegradable and injectable thermo gel, antioxidant and drug delivery | Suppressing oxidative stress, lowering ocular hypertension, reducing retinal ganglion cell loss and enhancing myelin growth and neuron regeneration. | [106] |
Figure 8.
Chitin, chitosan and chitosan nanostructure formation and potential applications.
The -NH2 and -OH groups of chitosan molecules make them a good base for interacting with other monomers, biological molecules, polymers, and nanoparticles [20, 81]. A variety of methods can be used to make quality films, fibers, gels, microspheres-microcapsules, and micro/nanoparticles [82, 83]. Because of their physicochemical properties, chitosan and its derivatives are good materials for biomedical and pharmaceutical applications, and they are also compatible with the human body environment [84, 85]. In biological systems, chitosan-based nanoparticles have good biodegradation and bio distribution, making them one of the most promising nano carriers for delivering various therapeutic medicines to tumor cells, particularly ovarian cancer cell lines [4, 79]. In recent years, metal and chitosan composites have been a hotspot of antibacterial research, with the addition of metals to chitosan increasing its antibacterial activity and potentially having applications in nanomedicine [86, 87]. Antibacterial activity of metal-chitosan nanocomposite films was found to be superior to that of chitosan [88, 89]. Biotechnologists and microbiologists have created various types of chitosan nanocomposites for distinct uses in the biomedical and pharmaceutical industries due to its outstanding physical, chemical, and biological properties [56, 88]. Chitosan biopolymer is a remarkable substance for cosmetics, food, medicine, and pharmacy because of these properties [82]. As a result, a number of researchers in a variety of fields have contributed to the field of chitosan-based nanocomposites, and a variety of chitosan-based materials have been made and evaluated for bioactivity studies [89, 90].
5.1. Chitosan biopolymer and its modified nanocomposite for drug delivery system
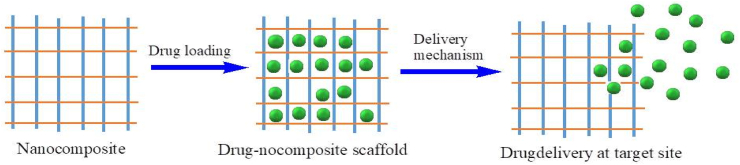
Natural polymers are considered appropriate hosting materials for nanoparticles, particularly for biological applications, due to their sustainability, eco-friendliness, nontoxicity, biodegradability, and biocompatibility [107, 108]. The development of effective drug delivery techniques that allow bioactive molecules to reach their site of action despite avoiding non-target cells, organs, or tissues is becoming a public health research priority [109, 110]. Drug targeting and regulated drug delivery are concepts that are used to increase the therapeutic index of drugs by improving their localization to specific parts of the human body and reducing potentially detrimental side effects under normal circumstances [111, 112]. This method has a number of benefits, including easy drug adjustments after parenteral administration to achieve target disease sight, increased drug treatment efficacy, and less drug side effects [113, 114]. The medicines can be incorporated into the systems without passing through any chemical processes, which is important for preserving drug activity, and the system can be used for a range of administration routes, such as oral, nasal, parenteral, and intraocular [115, 116]. Figure 9 shows the schematic representation of the drug loading and delivery system for biopolymer nanocomposites.
Figure 9.
Schematic diagram of drug loading and delivery mechanism by biopolymer nanocomposites.
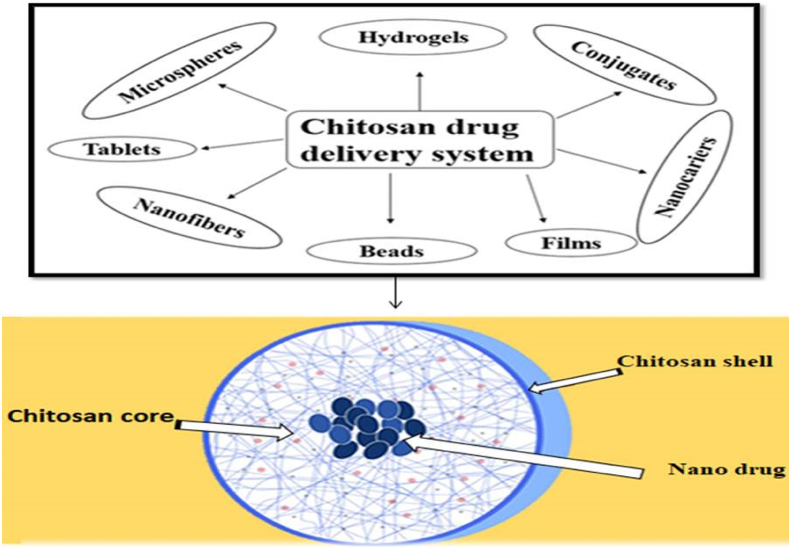
Chitosan nanocomposites, which can be modified for drug delivery system (Figure 10), are frequently employed in the treatment of diseases such as cancer and osteoarthritis [117, 118]. Drug-embedded nanocomposites provide a number of advantages, including improved pharmacokinetics and the capacity to deliver pharmaceuticals to the right location or tumor [119, 120]. A number of recent research have established the capacity to synthesize and describe chitosan biopolymer modified with nano-clay, reduced graphene oxide, zeolites, SiO2, hydroxyapatite, and gold nanoparticle for use as a targeted drug carrier in drug delivery systems (Table 4).
Figure 10.
Processing of chitosan and its modified nanocomposite for drug delivery system.
Table 4.
Chitosan biopolymer based drug delivery system with fabricated materials and loaded drugs.
| Natural polymer | Method of preparation | Modified material | Drug/model drug | Reference |
|---|---|---|---|---|
| Chitosan | Spray-drying | - | Diphenylhydamine and mebeverine | [121] |
| Ionic gelation | Chitosan -β-cyclodextrin grafted N- maleoyl | Cyclodextrin | [122] | |
| Nano/microencapsulation methods | Chitosan and Poly(lactide-co-glycolide) (PLGA). | - | [63] | |
| Carboxymethyl chitosan | Hydrothermal method | Carboxymethyl chitosan - folate/Fe3O4/CdTe nanoparticle | Adriamycin | [85] |
| Chitosan | Precipitated and solvent method | Chitosan -Clay | Ibuprofen | [123] |
| ionic-gelation method | Ionically Cross-Linked Chitosan and sodium tripolyphosphate (STPP) | Docetaxel | [52] | |
| Freeze-drying | Chitosan and Calcium carbonate | Methotrexate | [7] | |
| Esterification reaction | Folate modified chitosan/carboxymethyl | Paclitaxel | [93] | |
| Freeze-drying | Catechol modified-Chitosan -Genipin | Sulfasalazine | [7] | |
| N-maleoyl chitosan | Precipitation | N-maleoyl chitosan -β-cyclodextrin | Ketoprofen | [124] |
| Chitosan | Dissolution | Chitosan and 2-chloro-N,N-diethylethylamine hydrochloride | Quercetin | [19, 125] |
| Freeze-drying | Chitosan/Succinic anhydride, glutaric anhydride | Paclitaxel and docetaxel | [122] | |
| Oxidation | Chitosan/Glycidyltrimethyl ammonium chloride, gelatin | Dopamine | [7] | |
| Freeze-drying | Chitosan/Poly(DL-lactide-co-glycolide) | Donepezil | [122] | |
| - | lauryl succinyl/Chitosan/tripolyphosphate | Insulin | [65] | |
| Crosslinking methods | Chitosan/5-fluorouracil | 5-fluorouracil | [62] | |
| Encapsulation | Chitosan nanoparticles loaded with plasmid DNA encoding Rho1-GTPase protein of Schistosoma mansoni. | - | [126] | |
| Ionotropic gelation | Chitosan–fluorescein isothiocyanate-bovine serum albumin | fluorescein | [25] | |
| Freeze-drying | Chitosan/Gold nanoparticle | Curcumin | [127] | |
| Chitosan,aspartate, glutamate, and hydrochloride | Dispersion | AgSD- incorporated bilayer chitosan wound dressing | silver sulfadiazine (AgSD) | [128] |
| Chitosan | Ionic-gelation method | Chitosan and alginate | Amygdalin | [129] |
| Ionic cross-linking | Chitosan and Graphene | Isosfamide | [18] | |
| Ionic gelation | Chitosan and xanthan gum | Ciprofloxacin | [130] | |
| Complex coacervation | CS/Dz13Scr NPs | Insulin | [131] | |
| Ionic cross-linking | Chitosan cross-linked-6-phosphogluconic Trisodium | - | [132] |
6. Conclusion
Biopolymer nanocomposite have attracted a lot of research due to its special qualities, which include biocompatibility, biodegradability, and nontoxicity as well as better structural and functional features. The most difficult component of this technology is developing bio-based materials with equivalent quality and functions to synthetic materials. Various naturally occurring polymers, such as starch, collagen, alginate, cellulose, and chitin, are appealing candidates because they can reduce reliance on manufactured goods while remaining environmentally beneficial. Chitosan is one of the most exploited biopolymers in biomedical science, and it is the second most abundant next to cellulose, a naturally occurring amino polysaccharide. De-acetylated chitin and its amino-polysaccharide present in nature are used to make chitosan biopolymer. Because of its biocompatible and biodegradable nature, it has inspired a lot of interest in biological applications. Based on various reported study chitosan has been used in a variety of pharmaceutical application including antimicrobial, antioxidant, anti-inflammatory, anticancer and drug delivery systems throughout the last few decades. A range of chitosan sources, modification procedures, and manufacturing methods are also widely discussed. This review stated that chitosan and its nanocomposite have a bright future with improved distinctive qualities of their especial biocompatibility, biodegradability, mechanical and thermal stabilities, barrier, and nontoxicity, suggesting their uniqueness in the biomedical application based on numerous recent publications.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Reference
- 1.Gheorghita R., Anchidin-Norocel L., Filip R., Dimian M., Covasa M. Applications of biopolymers for drugs and probiotics delivery. Polymers. 2021;13(16) doi: 10.3390/polym13162729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao D., Yu S., Sun B., Gao S., Guo S., Zhao K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers. 2018;10(4):462. doi: 10.3390/polym10040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao K.S.V.K., Reddy P.R., Rao K.M., Kumar S.P. A Green Approach to Synthesize Silver Nanoparticles from Natural Polymer for Biomedical Application. Indian Journal of Advances in Chemical Science. 2015;5:340–344. [Google Scholar]
- 4.Ribeiro L.N.M., Alcântara A.C.S., Darder M., Aranda P., Araújo-moreira F.M., Ruiz-hitzky E. Pectin-coated chitosan – LDH bionanocomposite beads as potential systems for colon-targeted drug delivery. Int. J. Pharm. 2014;463(1):1–9. doi: 10.1016/j.ijpharm.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Rebitski E.P., Darder M. Chitosan and pectin core–shell beads encapsulating metformin–clay intercalation compounds for controlled delivery. New J. Chem. 2020;44(24):10102–10110. [Google Scholar]
- 6.Cheikh D., García-villén F., Majdoub H., Zayani M.B. Applied clay science complex of chitosan pectin and clay as diclofenac carrier. Appl. Clay Sci. 2019;172(3):155–164. [Google Scholar]
- 7.de Oliveira Pedro R., Goycoolea F.M., Pereira S., Schmitt C.C., Neumann M.G. Synergistic effect of quercetin and PH-responsive DEAE-chitosan carriers as drug delivery system for breast cancer treatment. Int. J. Biol. Macromol. 2018;106:579–586. doi: 10.1016/j.ijbiomac.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 8.Tomoda B.T., Yassue-Cordeiro P.H., Ernesto J.V., Lopes P.S., Péres L.O., da Silva C.F., de Moraes M.A. Characterization of biopolymer membranes and films: physicochemical, mechanical, barrier, and biological properties. Biopolym. Membr. Film. 2020:67–95. [Google Scholar]
- 9.Jiménez-Gómez C.P., Cecilia J.A. Chitosan: a natural biopolymer with a wide and varied range of applications. Molecules. 2020;25(17) doi: 10.3390/molecules25173981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustine R., Rajendran R., Cvelbar U., Mozetič M., George A. Biopolymers for health, food, and cosmetic applications. Handb. Biopolym. Mater. From Blends Compos. to Gels Complex Networks. 2013:801–849. [Google Scholar]
- 11.Wong T.W. 2011. Alginate Graft Copolymers and Alginate – Co-excipient Physical Physicochemical and Biological; pp. 1497–1512. [DOI] [PubMed] [Google Scholar]
- 12.Sharma K., Porat Z., Gedanken A. Designing natural polymer-based capsules and spheres for biomedical applications—a review. Polymers. 2021;13(24):1–41. doi: 10.3390/polym13244307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan A., Alamry K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: a review. Carbohydr. Res. 2021;506(5):108–368. doi: 10.1016/j.carres.2021.108368. [DOI] [PubMed] [Google Scholar]
- 14.Cheba B. ScienceDirect available ScienceDirect ScienceDirect chitosan : properties , modifications and food nanobiotechnology chitosan : properties , modifications and a , b , food nanobiotechnology. Procedia Manuf. 2020;46:652–658. [Google Scholar]
- 15.Ahmad M., Rahim M.A., Jabar A. Ionically cross-linked chitosan nanoparticles for sustained delivery of docetaxel: fabrication , post-formulation and acute oral toxicity evaluation. Int. J. Nanomed. 2019;14:10035–10046. doi: 10.2147/IJN.S232350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noshirvani N., Ghanbarzadeh B., Gardrat C., Rezaei M.R., Hashemi M., Le Coz C., Coma V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocolloids. 2017;70:36–45. [Google Scholar]
- 17.Soni B., Mahmoud B., Chang S., El-Giar E.M., Hassan E.B. Physicochemical, antimicrobial and antioxidant properties of chitosan/TEMPO biocomposite packaging films. Food Packag. Shelf Life. 2018;17(5):73–79. [Google Scholar]
- 18.Hu Y.L., Qi W., Han F., Shao J.Z., Gao J.Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011;6:3351–3359. doi: 10.2147/IJN.S25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu B., Luo Y. A review on the preparation and characterization of chitosan-clay nanocomposite films and coatings for food packaging applications. Carbohydr. Polym. Technol. Appl. 2021;2:100–102. [Google Scholar]
- 20.Upadhyaya L., Singh J., Agarwal V., Tewari R.P. The implications of recent advances in carboxymethyl chitosan based targeted drug delivery and tissue engineering applications. J. Contr. Release. 2014:1–34. doi: 10.1016/j.jconrel.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Burkatovskaya M., Castano A.P., Demidova-Rice T.N., Tegos G.P., Hamblin M.R. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16(3):425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathogens F., Granata G., Stracquadanio S., Leonardi M., Napoli E., Malandrino G., Cafiso V., Stefani S., Geraci C. Oregano and thyme essential oils encapsulated in chitosan nanoparticles as effective antimicrobial agents against. Foodborne pathogens. Molecules. 2021;26:40–55. doi: 10.3390/molecules26134055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biocompatibility F.S., Adrian G., Mihai M., Vodnar D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers. 2019;11:1837. doi: 10.3390/polym11111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eliana M., Zapata V., Mina H., Blanca V., San J., Rojo L. Novel bioactive and antibacterial acrylic bone cement nanocomposites modified with graphene oxide and chitosan. Int. J. Mol. Sci. 2019;20:2938. doi: 10.3390/ijms20122938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Salamanca A.E., Diebold Y., Calonge M., García-Vazquez C., Callejo S., Vila A., Alonso M.J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Investig. Ophthalmol. Vis. Sci. 2006;47(4):1416–1425. doi: 10.1167/iovs.05-0495. [DOI] [PubMed] [Google Scholar]
- 26.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13(3):1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Characteristics P., Aguila D. Chitosan nanoparticles : production , physicochemical characteristics and nutraceutical applications. Rev. Virtual Quim. 2017;9(1):3–24. [Google Scholar]
- 28.Ahmed F., Soliman F.M., Adly M.A., Soliman H.A.M., El-Matbouli M., Saleh M. Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: a review. Res. Vet. Sci. 2019;126(2):68–82. doi: 10.1016/j.rvsc.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed F., Soliman F.M., Adly M.A., Soliman H.A.M. Research in veterinary science recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture : a review. Res. Vet. Sci. 2019;126(7):68–82. doi: 10.1016/j.rvsc.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Bourakadi K. El, Merghoub N., Fardioui M., Mekhzoum M.E.M., Kadmiri I.M., Essassi E.M., Qaiss A. el K., Bouhfid R. Chitosan/polyvinyl alcohol/thiabendazoluim-montmorillonite bio-nanocomposite films: mechanical, morphological and antimicrobial properties. Compos. B Eng. 2019;172(1):103–110. [Google Scholar]
- 31.Ahmed S., Ikram S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016;10(1):27–37. [Google Scholar]
- 32.Darder M., Ruiz-hitzky E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003;15:3774–3780. [Google Scholar]
- 33.Ziegler-Borowska M., Chełminiak D., Kaczmarek H., Kaczmarek-Kędziera A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016;124(3):1267–1280. [Google Scholar]
- 34.Hussien N.A., Işıklan N., Türk M. Pectin-conjugated magnetic graphene oxide nanohybrid as a novel drug carrier for paclitaxel delivery. Artif. Cell Nanomed. Biotechnol. 2018;46(1):264–273. doi: 10.1080/21691401.2017.1421211. [DOI] [PubMed] [Google Scholar]
- 35.Hoang N.H., Thanh T. Le, Thepbandit W., Treekoon J., Saengchan C. 1–22. 2022. Efficacy of chitosan nanoparticle loaded-salicylic acid and -silver on management of cassava leaf spot disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris G.A., Kök S.M., Harding S.E., Adams G.G., Morris G.A., Kök S.M., Harding S.E., Adams G.G., Kok S., Gordon A., Harding E. Polysaccharide drug delivery systems based on pectin and chitosan polysaccharide. Biotechnol. Genet. Eng. Rev. 2013;27:257–284. doi: 10.1080/02648725.2010.10648153. [DOI] [PubMed] [Google Scholar]
- 37.Pan Y., Li Y., Zhao H., Zheng J., Xu H. Bioadhesi v e Polysaccharide in Protein Deli v Ery System : chitosan Nanoparticles Impro v e the Intestinal Absorption of Insulin in v i v O. Int J Pharm. 2002;249:139–147. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 38.Hembram K.C., Prabha S., Chandra R., Ahmed B., Nimesh S. Advances in preparation and characterization of chitosan nanoparticles for advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cell Nanomed. Biotechnol. 2014;1:1–10. doi: 10.3109/21691401.2014.948548. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Li P., Tran T.T., Zhang J., Kong L. 2016. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaffari A. Pectin/chitosan/eudragit ® RS mixed-film coating for bimodal drug delivery from theophylline pellets: preparation and evaluation. Acta Pharm. 2006;56:299–310. [PubMed] [Google Scholar]
- 41.Crini G., Morin-Crini N., Fatin-Rouge N., Déon S., Fievet P. Metal removal from aqueous media by polymer-assisted ultrafiltration with chitosan. Arab. J. Chem. 2017;10:3826–3839. [Google Scholar]
- 42.Hosseinnejad M., Jafari S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016;85:467–475. doi: 10.1016/j.ijbiomac.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K., Xu J., Wang K.Y., Cheng L., Wang J., Liu B. Preparation and characterization of chitosan nanocomposites with vermiculite of different modification. Polym. Degrad. Stabil. 2009;94(12):2121–2127. [Google Scholar]
- 44.Sathiyavimal S., Vasantharaj S., Kaliannan T., Pugazhendhi A. Eco-biocompatibility of chitosan coated biosynthesized copper oxide nanocomposite for enhanced industrial (azo) dye removal from aqueous solution and antibacterial properties. Carbohydr. Polym. 2020;241(4) doi: 10.1016/j.carbpol.2020.116243. [DOI] [PubMed] [Google Scholar]
- 45.Garavand F., Cacciotti I., Vahedikia N., Rehman A., Tarhan Ö., Akbari-Alavijeh S., Shaddel R., Rashidinejad A., Nejatian M., Jafarzadeh S., Azizi-Lalabadi M., Khoshnoudi-Nia S., Jafari S.M. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2022;62(5):1383–1416. doi: 10.1080/10408398.2020.1843133. [DOI] [PubMed] [Google Scholar]
- 46.Mesquita P. De, Donnici C.L., Pereira F.V. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules. 2010;11:473–480. doi: 10.1021/bm9011985. [DOI] [PubMed] [Google Scholar]
- 47.Moinard-Chécot D., Chevalier Y., Briançon S., Beney L., Fessi H. Mechanism of nanocapsules formation by the emulsion-diffusion process. J. Colloid Interface Sci. 2008;317(2):458–468. doi: 10.1016/j.jcis.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Li P., Tran T.T.D., Zhang J., Kong L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials. 2016;6(2):1–18. doi: 10.3390/nano6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernández-Giottonini K.Y., Rodríguez-Córdova R.J., Gutiérrez-Valenzuela C.A., Peñuñuri-Miranda O., Zavala-Rivera P., Guerrero-Germán P., Lucero-Acuña A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: effects of formulation parameters. RSC Adv. 2020;10(8):4218–4231. doi: 10.1039/c9ra10857b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zielinska A., Carreiró F., Oliveira A.M., Neves A., Pires B., Nagasamy Venkatesh D., Durazzo A., Lucarini M., Eder P., Silva A.M., Santini A., Souto E.B. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16):3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanat M., Schroën K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021;161(12) [Google Scholar]
- 52.Mahmood M.A., Madni A., Rehman M., Rahim M.A., Jabar A. Ionically cross-linked chitosan nanoparticles for sustained delivery of docetaxel: fabrication, post-formulation and acute oral toxicity evaluation. Int. J. Nanomed. 2019;14:10035–10046. doi: 10.2147/IJN.S232350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao D., Yu S., Sun B., Gao S., Guo S., Zhao K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers. 2018;10(4):1–12. doi: 10.3390/polym10040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahalingam M., Krishnamoorthy K. Selection of a suitable method for the preparation of polymeric nanoparticles: multi-criteria decision making approach. Adv. Pharmaceut. Bull. 2015;5(1):57–67. doi: 10.5681/apb.2015.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang J., Yan H., Puligundla P., Gao X., Zhou Y., Wan X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: a review. Food Hydrocolloids. 2017;69:286–292. [Google Scholar]
- 56.Orellano M.S., Longo G.S., Porporatto C., Correa N.M., Falcone R.D. Role of micellar interface in the synthesis of chitosan nanoparticles formulated by reverse micellar method. Colloids Surfaces A Physicochem. Eng. Asp. 2020;599(2) [Google Scholar]
- 57.Hembram K.C., Prabha S., Chandra R., Ahmed B., Nimesh S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cell Nanomed. Biotechnol. 2016;44(1):305–314. doi: 10.3109/21691401.2014.948548. [DOI] [PubMed] [Google Scholar]
- 58.Detsi A., Kavetsou E., Kostopoulou I., Pitterou I., Rozaria A., Pontillo N., Tzani A., Christodoulou P., Siliachli A., Zoumpoulakis P. Nanosystems for the encapsulation of natural products : the case of chitosan biopolymer as a matrix. Pharmaceutics. 2020;12:669. doi: 10.3390/pharmaceutics12070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roul J., Mohapatra R., Kumar Sahoo S. QR code for mobile users preparation, characterization and drug delivery behavior of novel biopolymer/hydroxyapatite nanocomposite beads. Asian J. Biomed. Pharm. Sci. 2013;3(24):33–38. [Google Scholar]
- 60.Antony V., Samrot T., Chuan S., Teeshalini K., Ummu B., Anita M., Etel F., Amira Abubakar M., Hawwa H., Ali J., Lavanya A., Angalene S., Suresh K. Production, characterization and application of nanocarriers made of polysaccharides, proteins, bio-polyesters and other biopolymers: a review. Int. J. Biol. Macromol. 2020:1–136. doi: 10.1016/j.ijbiomac.2020.10.104. [DOI] [PubMed] [Google Scholar]
- 61.Jelvehgari M., Salatin S., Barar J., Barzegar-Jalali M., Adibkia K., Kiafar F., Jelvehgari M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into eudragit RL nanoparticles. Res. Pharm. Sci. 2017;12(1):1–14. doi: 10.4103/1735-5362.199041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamidi M., Azadi A., Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60(15):1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Essa D., Kondiah P.P.D., Choonara Y.E., Pillay V. The design of poly(lactide-Co-glycolide) nanocarriers for medical applications. Front. Bioeng. Biotechnol. 2020;8(2):1–20. doi: 10.3389/fbioe.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Alvarenga E.S. Characterization and properties of chitosan. Biotechnology of Biopolymers. 2011;6:92–108. [Google Scholar]
- 65.Rekha M.R., Sharma C.P. Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J. Contr. Release. 2009;135(2):144–151. doi: 10.1016/j.jconrel.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Han Y.S., Lee S.H., Choi K.H., Park I. Preparation and characterization of chitosan-clay nanocomposites with antimicrobial activity. J. Phys. Chem. Solid. 2010;71(4):464–467. [Google Scholar]
- 67.Badawy M.E.I., Rabea E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011;2011:1–29. [Google Scholar]
- 68.Tolesa L.D., Gupta B.S., Lee M.J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019;130:818–826. doi: 10.1016/j.ijbiomac.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 69.Alagumuthu G., Kumar T.A. Synthesis and characterization of chitosan/TiO 2 nanocomposites using liquid phase deposition technique. Int. J. Nanosci. Nanotechnol. 2013;4(1):105–111. [Google Scholar]
- 70.Gülmen S.S.T., Güvel E.A., Kızılcan N. Preparation and characterization of chitosan/polypyrrole/sepiolite nanocomposites. Procedia - Soc. Behav. Sci. 2015;195:1623–1632. [Google Scholar]
- 71.Alipour A., Mansour Lakouraj M., Tashakkorian H. Study of the effect of band gap and photoluminescence on biological properties of polyaniline/CdS QD nanocomposites based on natural polymer. Sci. Rep. 2021;11(1):1–15. doi: 10.1038/s41598-020-80038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomes L.P., Paschoalin V.M.F., Del Aguila E.M. Chitosan nanoparticles: production, physicochemical characteristics and nutraceutical applications. Rev. Virtual Quim. 2017;9(1):387–409. [Google Scholar]
- 73.Gough C.R., Callaway K., Spencer E., Leisy K., Jiang G., Yang S., Hu X. Biopolymer-based filtration materials. ACS Omega. 2021;6(18):11804–11812. doi: 10.1021/acsomega.1c00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasirajan K., Balaji M., Nithya P., Sundrarajan M. International journal of biological macromolecules synthesis of biogenic chitosan-functionalized 2D layered MoS 2 hybrid nanocomposite and its performance in pharmaceutical applications : in-vitro antibacterial and anticancer activity. Int. J. Biol. Macromol. 2020;149:1019–1033. doi: 10.1016/j.ijbiomac.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Hu Q., Luo Y. International journal of biological macromolecules chitosan-based nanocarriers for encapsulation and delivery of curcumin : a review. Int. J. Biol. Macromol. 2021;179:125–135. doi: 10.1016/j.ijbiomac.2021.02.216. [DOI] [PubMed] [Google Scholar]
- 76.Haponiuk T., Thomas S., Gopi S., Jacob J. Biopolymer based nanomaterials in drug delivery systems: a review. Mater. Today Chem. 2018;9:43–55. [Google Scholar]
- 77.Venkatesan J., Singh S.K., Anil S., Shim M.S. Applications of biosynthesized silver nanoparticles with chitosan-fucoidan coating. Molecules. 2018;23:1429. doi: 10.3390/molecules23061429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renault F., Sancey B., Badot P.M., Crini G. Chitosan for coagulation/flocculation processes - an eco-friendly approach. Eur. Polym. J. 2009;45(5):1337–1348. [Google Scholar]
- 79.Iravani K., Torkzadeh-mahani M., Mosaddegh E. Materials science & engineering C synthesis and characterization of aminotetrazole-functionalized magnetic chitosan nanocomposite as a novel nanocarrier for targeted gene delivery. Mater. Sci. Eng. C. 2018;89(4):166–174. doi: 10.1016/j.msec.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 80.Ahmed S. Chitosan & its derivatives: a review in recent innovations. IJPSR. 2015;6(1):14–30. [Google Scholar]
- 81.Aizat M.A., Aziz F. Chitosan nanocomposite application in wastewater treatments. Nanotechnology in Water and Wastewater Treatment. 2019:243–265. [Google Scholar]
- 82.Peniche H., Peniche C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers. 2018;6(3):235. doi: 10.3390/polym10030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-naamani L., Dutta J. Nanocomposite zinc oxide-chitosan coatings on polyethylene films for extending storage life of okra (abelmoschus esculentus) Nanomaterials. 2018;8:479. doi: 10.3390/nano8070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmad M., Manzoor K., Ikram S. 2018. Chitosan Based Nanocomposites for Drug, Gene Delivery, and Bioimaging Applications. Applications of Nanocomposite Materials in Drug Delivery; pp. 27–38. [Google Scholar]
- 85.Shen J.M., Tang W.J., Zhang X.L., Chen T., Zhang H.X. A novel carboxymethyl chitosan-based folate/Fe 3O 4/CdTe nanoparticle for targeted drug delivery and cell imaging. Carbohydr. Polym. 2012;88(1):239–249. [Google Scholar]
- 86.Katas H., Moden N.Z., Lim C.S., Celesistinus T., Chan J.Y., Ganasan P., Suleman S., Abdalla I. 2018. Biosynthesis and Potential Applications of Silver and Gold Nanoparticles and Their Chitosan-Based Nanocomposites in Nanomedicine; p. 13. [Google Scholar]
- 87.Refaat A., Ali A., Ali T., Mohamed N. Polysulfone membranes with CNTs/chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020;388(11):124–267. [Google Scholar]
- 88.Saikia C., Gogoi P. Chitosan: a promising biopolymer in drug delivery applications. J. Mol. Genet. Med. 2015;7:2–10. [Google Scholar]
- 89.Boominathan T., Sivaramakrishna A. SpringerInternational Publishing; 2021. Recent Advances in the Synthesis , Properties , and Applications of Modified Chitosan Derivatives : Challenges and Opportunities; p. 379. [DOI] [PubMed] [Google Scholar]
- 90.Phil L., Naveed M., Mohammad I.S., Bo L., Bin D. Chitooligosaccharide: An evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomed. Pharmacother. 2018;102(3):438–451. doi: 10.1016/j.biopha.2018.03.108. [DOI] [PubMed] [Google Scholar]
- 91.Lou B., Rajaji U., Chen S., Chen T. A simple sonochemical assisted synthesis of porous NiMoO4/chitosan nanocomposite for electrochemical sensing of amlodipine in pharmaceutical formulation and human serum. Ultrason. Sonochem. 2019:104–827. doi: 10.1016/j.ultsonch.2019.104827. [DOI] [PubMed] [Google Scholar]
- 92.Suresh L., Brahman P.K., Reddy K.R., Bondili J.S. Development of an Electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzym. Microb. Technol. 2017;112:43–51. doi: 10.1016/j.enzmictec.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Bano S., Afzal M., Mahmood M., Alamgir K., Nazir S. Paclitaxel loaded magnetic nanocomposites with folate modi fi ed chitosan/carboxymethyl surface ; a vehicle for imaging and targeted drug delivery. Int. J. Pharm. 2016;513(1–2):554–563. doi: 10.1016/j.ijpharm.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 94.Manimohan M., Pugalmani S., Aboobucker M. Biologically active water soluble novel biopolymer/hydrazide based O - carboxymethyl chitosan schiff bases : synthesis and characterisation. J. Inorg. Organomet. Polym. Mater. 2020;13:1–18. [Google Scholar]
- 95.Wang W., Meng Q., Li Q., Liu J., Zhou M., Jin Z., Zhao K. Chitosan derivatives and Their application in biomedicine. Int. J. Mol. Sci. 2020;21(2):487. doi: 10.3390/ijms21020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seyam S., Nordin N.A., Alfatama M. Recent progress of chitosan and chitosan derivatives-based nanoparticles: pharmaceutical perspectives of oral insulin delivery. Pharmaceuticals. 2020;14(10):307. doi: 10.3390/ph13100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shahid-ul-Islam, Butola B.S. Elsevier B.V; 2019. International Journal of Biological Macromolecules; pp. 905–912. (Recent advances in chitosan polysaccharide and its derivatives in antimicrobial modification of textile materials). [DOI] [PubMed] [Google Scholar]
- 98.Yangchao L., Qin W., Yaqiong Z. Biopolymer-based nanotechnology approaches to deliver bioactive compounds for food applications: a perspective on the past, present, and future. J. Agric. Food Chem. 2020;1:1–8. doi: 10.1021/acs.jafc.0c00277. [DOI] [PubMed] [Google Scholar]
- 99.Karimi-Maleh H., Ayati A., Davoodi R., Tanhaei B., Karimi F., Malekmohammadi S., Orooji Y., Fu L., Sillanpää M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: a review. J. Clean. Prod. 2021:291. [Google Scholar]
- 100.Si Z., Hou Z., Vikhe Y.S., Reddy K., Thappeta V., Marimuthu K., De P.P., Ng O.T., Li P., Zhu Y., Pethe K., Chan-park M.B. Antimicrobial e ff ect of a novel chitosan derivative and its synergistic e ff ect with antibiotics. ACS Appl. Mater. Interfaces. 2021;13(2):3237–3245. doi: 10.1021/acsami.0c20881. [DOI] [PubMed] [Google Scholar]
- 101.Salama A. International journal of biological macromolecules recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021;178:240–252. doi: 10.1016/j.ijbiomac.2021.02.143. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y., Zhou L., Fang L., Cao F. Multifunctional carboxymethyl chitosan derivatives-layered double hydroxide hybrid nanocomposites for efficient drug delivery to the posterior segment of the eye. Acta Biomater. 2020;1:104–114. doi: 10.1016/j.actbio.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 103.Etman S.M., Elnaggar Y.S.R., Abdallah O.Y. Fucoidan , a natural biopolymer in cancer combating : from edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2019;15:799–808. doi: 10.1016/j.ijbiomac.2019.11.191. [DOI] [PubMed] [Google Scholar]
- 104.Manimohan M., Pugalmani S., Aboobucker M. Synthesis. Spectral characterisation and biological activities of novel biomaterial/N, N , O donor tridentate Co (II), Ni (II) and Zn (II) complexes of hydrazide based biopolymer schiff base ligand. J. Inorg. Organomet. Polym. Mater. 2020:1–10. [Google Scholar]
- 105.Vetrik M., Mattova J., Mackova H., Kucka J., Pouckova P., Kukackova O., Brus J., Eigner-henke S., Sedlacek O., Sefc L., Stepanek P., Hruby M. Biopolymer strategy for the treatment of Wilson ’ s disease. J. Contr. Release. 2018;273(11):131–138. doi: 10.1016/j.jconrel.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 106.Luo L., Dung D., Lai J. Benzoic Acid Derivative-Modi Fi Ed Chitosan- g -Poly (N -Isopropylacrylamide): methoxylation e Ff Ects and Pharmacological Treatments of Glaucoma-Related Neurodegeneration. J. Contr. Release. 2020;317(11):246–258. doi: 10.1016/j.jconrel.2019.11.038. [DOI] [PubMed] [Google Scholar]
- 107.Reddy M.S.B., Ponnamma D., Choudhary R., Sadasivuni K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers. 2021;13(7) doi: 10.3390/polym13071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muvva A., Chacko I.A., Ghate V., Lewis S.A. Modified pectins for colon-specific drug delivery. IJPER. 2020;54(2) [Google Scholar]
- 109.Han J., Zhao D., Li D., Wang X., Jin Z., Zhao K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers. 2018;10(1):1–14. doi: 10.3390/polym10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zirak S., Kiadeh H., Ghaee A., Farokhi M., Nourmohammadi J., Bahi A., Ko F.K. International journal of biological macromolecules electrospun pectin/modi fi ed copper-based metal – organic framework (MOF) nano fi bers as a drug delivery system. Int. J. Biol. Macromol. 2021;173:351–365. doi: 10.1016/j.ijbiomac.2021.01.058. [DOI] [PubMed] [Google Scholar]
- 111.Tiwari G., Tiwari R., Bannerjee S., Bhati L., Pandey S., Pandey P., Sriwastawa B. Drug delivery systems: an updated review. Int. J. Pharm. Investig. 2012;2(1):2. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hassan M., Dou D. Biopolymers; definition, classification and applications. Egypt. J. Chem. 2019;62(9):1725–1737. [Google Scholar]
- 113.Gursoy M., Sargin I., Mujtaba M., Akyuz B., Ilk S., Akyuz L., Kaya M., Cakmak Y.S., Salaberria A.M., Labidi J., Erdem N. False flax (camelina sativa) seed oil as suitable ingredient for the enhancement of physicochemical and biological properties of chitosan films. Int. J. Biol. Macromol. 2018;114:1224–1232. doi: 10.1016/j.ijbiomac.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 114.Vunain E., Mishra A.K., Mamba B.B. Vol. 1. Elsevier; 2017. pp. 3–30. (Fundamentals of Chitosan for Biomedical Applications). [Google Scholar]
- 115.Wen H., Jung H., Li X. Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. AAPS J. 2015;17(6):1327–1340. doi: 10.1208/s12248-015-9814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zare M. Chitosan-functionalized Fe 3 O 4 nanoparticles as an excellent biocompatible nanocarrier for silymarin delivery. Polymers. 2021;5:1–7. [Google Scholar]
- 117.Gu W., Wu C., Chen J., Xiao Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 2013;8:2305–2317. doi: 10.2147/IJN.S44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katti K.S., Katti D.R., Dash R. Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomed. Mater. 2008;3(3) doi: 10.1088/1748-6041/3/3/034122. [DOI] [PubMed] [Google Scholar]
- 119.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1):1–33. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fahmy H.M., Salah E., Abu S., Elsayed Z.A., Edrees A., Shams-eldin E., Shalan A.E. 2020. RSC Advances Advances in Nanotechnology and Antibacterial Properties of Biodegradable Food Packaging; pp. 20467–20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kamrani M., Akbari A., Yunessnia lehi A. Chitosan-modified acrylic nanofiltration membrane for efficient removal of pharmaceutical compounds. J. Environ. Chem. Eng. 2018;6(1):583–587. [Google Scholar]
- 122.Hou X., Zhang W., He M., Lu Y., Lou K., Gao F. Preparation and characterization of β-cyclodextrin grafted N-maleoyl chitosan nanoparticles for drug delivery. Asian J. Pharm. Sci. 2017;12(6):558–568. doi: 10.1016/j.ajps.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abdeen R., Salahuddin N. Modified chitosan-clay nanocomposite as a drug delivery system intercalation and in vitro release of ibuprofen. J. Chem. 2013;9 [Google Scholar]
- 124.Hashad R.A., Ishak R.A.H., Fahmy S., Mansour S., Geneidi A.S. Chitosan-tripolyphosphate nanoparticles: optimization of formulation parameters for improving process yield at a novel PH using artificial neural networks. Int. J. Biol. Macromol. 2016;86:50–58. doi: 10.1016/j.ijbiomac.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 125.Aranaz I., Paños I., Peniche C., Heras Á., Acosta N. Chitosan spray-dried microparticles for controlled delivery of venlafaxine hydrochloride. Molecules. 2017;22(11):1–13. doi: 10.3390/molecules22111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oliveira C.R., Rezende C.M.F., Silva M.R., Borges O.M., Pêgo A.P., Goes A.M. Oral vaccination based on DNA-chitosan nanoparticles against schistosoma mansoni infection. Sci. World J. 2012;2012:11. doi: 10.1100/2012/938457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takeuchi I., Takeshita T., Suzuki T., Makino K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for positively charged drugs. Colloids Surf. B Biointerfaces. 2017;160:520–526. doi: 10.1016/j.colsurfb.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 128.Hao J.Y., Mi F.L., Shyu S.S., Wu Y.B., Schoung J.Y., Tsai Y.H., Huang Y. Bin. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002;59(3):438–449. doi: 10.1002/jbm.1260. [DOI] [PubMed] [Google Scholar]
- 129.Qi L., Xu Z. In Vivo antitumor activity of chitosan nanoparticles. Bioorg. Med. Chem. Lett. 2006;16(16):4243–4245. doi: 10.1016/j.bmcl.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 130.Hanna D.H., Saad G.R. Encapsulation of ciprofloxacin within modified xanthan gum- chitosan based hydrogel for drug delivery. Bioorg. Chem. 2019;84(11):115–124. doi: 10.1016/j.bioorg.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 131.Wong C.Y., Al-Salami H., Dass C.R. Formulation and characterisation of insulin-loaded chitosan nanoparticles capable of inducing glucose uptake in skeletal muscle cells in vitro. J. Drug Deliv. Sci. Technol. 2020;57(1):101–738. [Google Scholar]
- 132.Martínez-Martínez M., Rodríguez-Berna G., Gonzalez-Alvarez I., Hernández M., Corma A., Bermejo M., Merino V., Gonzalez-Alvarez M. Ionic hydrogel based on chitosan cross-linked with 6-phosphogluconic trisodium salt as a drug delivery system. Biomacromolecules. 2018;19(4):1294–1304. doi: 10.1021/acs.biomac.8b00108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.