Abstract
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide with an increasing trend of its incidence. Alcohol consumption, smoking, and viral infections, such as the mucosal high-risk (HR) human papillomaviruses (HPVs) are major risk factors for HNSCC development. In particular, HR HPVs are mainly associated with a subset of oropharyngeal squamous cell carcinoma (OPSCC), while other head and neck sites are marginally affected by HPV infection. HPV16 is the most frequently HR HPV type associated with HNSCC. In contrast to the cervix, no screening programs or identifiable pre-malignant lesions have been characterized for HPV-related HNSCC. Therefore, identification of general diagnostic algorithms and HPV biomarkers that could facilitate the early diagnosis, disease evolution and recurrence for HPV-driven HNSCCs are urgently needed. We herein review the role of HPV in HNSCC with a focus on epidemiology, biology, applied diagnostic algorithms and available biomarkers in body fluids as early diagnostic tools in HPV-driven HNSCCs.
Keywords: HPV, HNC, HNSCC, Biomarkers, Liquid biopsy, Cancer of the oropharynx, Early diagnosis, Diagnostic algorithm
Abbreviations: HNSCC, Head and neck squamous cell carcinoma; HNC, head and neck cancer; HR, high-risk; OPSCC, oropharyngeal squamous cell carcinoma; HPV, human papillomavirus; cfDNA, circulating free DNA; ISH, in situ hybridization; IHC, immunohistochemistry; CC, cervical cancer; FFPE, Formalin Fixed Paraffin Embedded
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) includes several malignancies which may arise in the oral cavity, oropharynx, larynx, and hypopharynx. HNSCCs have been associated with well-established risk factors like tobacco smoking, alcohol use [1,2] and viral infections, such as human papillomaviruses (HPVs), referred to as mucosal high-risk (HR) HPV types. This HR HPV group include 12 different types, namely 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 [3] that have been clearly associated with the anogenital cancers [3]. However, it is now well demonstrated that they are also responsible for a subset of oropharyngeal squamous cell carcinoma (OPSCC) [4,5] as well as for a low proportion of oral and laryngeal tumors [6]. Among the mucosal HR HPV types, HPV16 appears to be the most oncogenic type, being present in approximately 50% of cervical cancers worldwide [7]. Interestingly, HPV16 is responsible for more than 90% of virus-driven HNSCC, while the other mucosal HR HPVs are seldom associated with this pathological condition [8]. Many studies have clearly shown that HPV-driven HNSCC incidence is increasing [9,10] in Europe and North America [8,11,12], while the incidence of mucosal HR HPV-positive HNSCCs in low-income countries is still low. This because tobacco and alcohol consumption remain the principal risk factors in these countries [8,13]. Thus, HNSCCs can be stratified in HPV-related and -unrelated cancers, of which those HPV-related show different biological characteristics and a better clinical outcome in comparison to the HPV-negative counterpart [14,15]. Differently from cervical cancer, no clear pre-cancerous lesions for HNSCC have been identified yet. In addition, no screening protocols for early-stage detection of these malignancies have been fully validated [16].
Many studies have provided important insights on the mechanism of HPV-driven HNSCC (reviewed in Ref. [17]) and several biomarkers have been identified and proposed as possible novel diagnostic and prognostic tools [[18], [19], [20]]. Finally, a few findings highlighted the efficacy of the HPV prophylactic vaccine in preventing oral infections, providing indirect evidence for a positive impact of the vaccine on HPV-driven carcinogenesis in head and neck [[21], [22], [23]]. In this review, we summarize the HPV biology and epidemiology of HPV-positive HNSCC, together with novel prophylactic and diagnostic strategies.
2. HNSCC risk factors and epidemiology
2.1. Risk factors
As with many types of cancer, the risk of developing HNSCC is higher for patients exposed to certain environmental factors or who have a history with specific lifestyle behaviors.
2.1.1. Tobacco and alcohol
Two main substances, tobacco and alcohol, greatly increase the risk of developing HNSCC [24,25].
Tobacco use includes smoking cigarettes, cigars, or pipes; chewing tobacco; and using snuff. It is the single largest risk factor for HNSCC [1], and prognosis may be influenced by the intensity of tobacco use and the duration [24,25]. Smoking tobacco increases the risk of developing many types of cancer that affect the head and neck area. In UK, smoking is associated with 64% of laryngeal cancer, 37% pharyngeal cancer, 25% nasopharyngeal cancer and 17% oral cavity cancers [26]. In addition, passive smoking may also increase the risk of HNSCC development [1].
Frequent and heavy alcohol consumption strongly impact on the HNSCC establishment, specifically in the oral cavity, pharynx, larynx, as well as in the upper degistive tract, suh as eophagus [27].
Studies have shown that people who use tobacco and alcohol together have a substantially greater risk of oral cancer than people who only smoke or drink. At least 75% of head and neck cancers are caused by the combined use of tobacco and alcohol [24].
2.1.2. Human papillomaviruses
In recent years, tumors that arise in the oropharynx, especially cancers at the back of the tongue and in the tonsils, are linked to prior infection with mucosal HR HPVs, primarily HPV16, and, to a lesser extent, HPV18 and other HR HPV types [28,29]. Specifically, in OPSCCs it was reported a 93% prevalence of HPV16, with a lowest prevalence of other high-risk types belonging to alpha-9 species, such as HPV33 (1.4%), HPV35 (0.5%) or alpha-7 species, like HPV18 (1.3%). Thus, differently from cervical cancer, HPV-positive HNSCCs are mainly attributable to HPV16, with an underrepresentation of HPV18 and other HR-HPVs commonly seen in cervical cancer.
HPV-related HNSCCs tend to occur in younger age groups with little exposure to tobacco and alcohol and differ from HPV-unrelated HNSCCs with respect to the molecular mechanisms underlying their oncogenic processes [9,30]. HPV-related OPSCC are reported to have a better response to treatment as compared with HPV-unrelated OPSCC [[31], [32], [33], [34]]. Therefore, it became more important to accurately discriminate between HPV-related and HPV-unrelated OPSCCs.
2.1.3. Age, sex, gender, genetic background, oral hygiene
Other factors that can impact on HPV-positive HNSCC development are age, sex, gender, genetic background, and poor oral and dental hygiene [35].
An increase in the median age was observed in HPV-positive OPSCC patients classified by p16INK4a positivity and diagnosed between 1995 and 2000 or 2001–2013, changing from 53 to 58 years, respectively [36].
HPV is sexually acquired, and early sexual debut as well as a high number of sexual partners, including oral sex partners, and previous genital warts, correlate with an increased risk for HPV-positive oropharyngeal squamous cell carcinoma (OPSCC) [37].
In addition, there is a higher prevalence of HPV-positive OPSCC in men compared with women, and white populations compared with black populations and Asians [17]. In general, men are at 2–4-fold higher risk than women for developing HNSCC [38].
2.1.4. Pre-existing diseases, pre-cancerous conditions and genetic syndromes
On top, certain diseases, such as gastroesophageal reflux disease (GERD) [39] or laryngopharyngeal reflux disease (LPRD) [40,41], can increase the risk on head and neck cancer, as well as a weakened immune system [16], and especially a previous history of head and neck cancer.
People with certain syndromes - such as Fanconi Anemia, Dyskeratosis Congenita and Plummer-Vinson syndrome - caused by inherited defects in certain genes, have a very high risk of developing HNSCC (primarily cancers of the oral cavity) at substantially younger ages than the general population [[42], [43], [44], [45]].
2.2. HNSCC epidemiology
According to estimates of the International Agency for Research on Cancer, approximately 504,000 men and 157,000 women developed cancer originating from oral, oropharyngeal or laryngeal sites in 2020 [46]. Approximately 31% of the oropharyngeal cancers and 2% percent of oral cavity or laryngeal cancers can be etiologically attributed to HPV infection [47].
From the HPV attributed fractions and 2020 IARC cancer burden reports, we updated previous estimates [48] of the burden of head and neck cancer for the year 2020. The most frequent HPV-related malignancy was oropharyngeal cancer affecting 79,000 men and 19,000 women. An estimated 7900 patients developed oral cavity cancer (5400 men and 2500 women) whereas 4400 patients developed laryngeal cancer (3800 men and 600 women), all driven by HPV infection (Table 1).
Table 1.
World-wide burden of head and neck cancers attributable to high-risk HPV infection (estimates for 2020).
| New |
Proportion |
New cases |
||
|---|---|---|---|---|
| ICD | Cancer site | casesa | Attributed to HPVb | due to HPV |
| Male | ||||
| C00-06 | Oral cavity cancer | 264,211 | 2.1% | 5400 |
| C09-10 | Oropharyngeal cancer | 79,045 | 30.9% | 24,400 |
| C32 | Laryngeal cancer | 160,265 | 2.4% | 3800 |
| Total | 503,521 | 6.7% | 33,600 | |
| Female | ||||
| C00-06 | Oral cavity cancer | 113,502 | 2.2% | 2500 |
| C09-10 | Oropharyngeal cancer | 19,367 | 31.2% | 6000 |
| C32 | Laryngeal cancer | 24,350 | 2.3% | 600 |
| Total | 157,219 | 5.8% | 9100 | |
| Both Sexes | ||||
| C00-06 | Oral cavity cancer | 377,713 | 2.1% | 7900 |
| C09-10 | Oropharyngeal cancer | 98,412 | 31.0% | 30,400 |
| C32 | Laryngeal cancer | 184,615 | 2.4% | 4400 |
| Total | 660,740 | 6.5% | 42,700 | |
3. Human papillomaviruses biology
Human Papillomaviruses (HPVs) are non-enveloped viruses belong to the Papillomaviridae family with circular double-stranded DNA (dsDNA) genome of approximately 8000 base pairs (bp). Over 200 HPVs have been identified so far and classified into five genera, namely alpha beta, gamma, mu and nu (www.hpvcenter.se) [49]. A large number of biological and epidemiological studies in the last forty years have clearly highlighted the oncogenic nature of the mucosal alpha HR-HPV types. Their genome contains three functional regions, (i) the early region (E) encodes regulatory proteins, namely E1, E2, E4, E5, E6 and E7 [50,51], (ii) the late region (L) encodes the structural viral capsid proteins L1 and L2, and (iii) the long control region (LCR) or upstream regulatory region (URR), contains a regulatory region important for viral replication and transcription. The products of the early E6 and E7 genes are the major oncoproteins of the mucosal HR HPV types, being able to alter the regulation of fundamental cellular events, such as cell cycle, apoptosis, and DNA repair. They induce proliferation of basal and parabasal layers of the epithelium. As a consequence they facilitate the accumulation of DNA damage and the progression toward malignancy. However, establishment of a persistent infection is an essential event for the development of HR HPV-associated malignant lesions. Indeed, if the HPV-infected cells are rapidly eliminated by the immune system, the viral oncoproteins do not have sufficient time to promote accumulation of chromosomal abnormalities and cellular transformation.
HR HPV E6 and E7 exert their oncogenic function by interacting and inactivating several cellular proteins, including the products of several tumor suppressor genes.
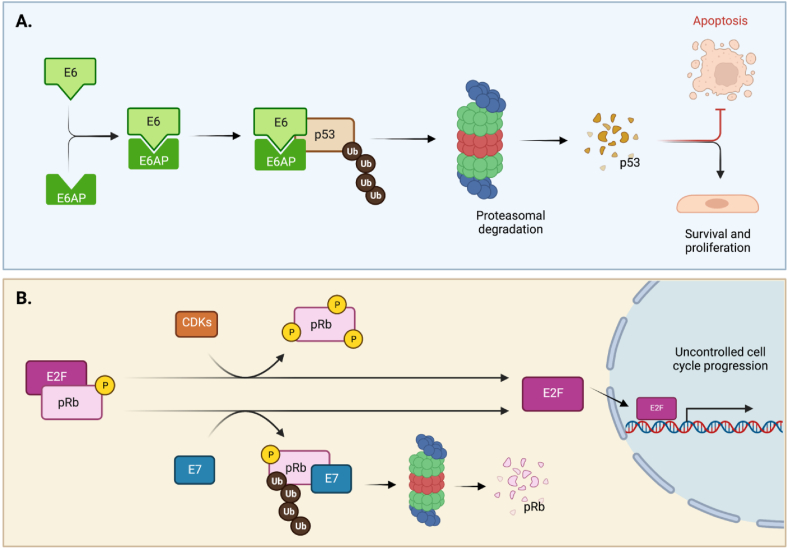
Specifically, E6 induces the degradation of the tumor suppressor p53 that plays a fundamental role in preventing cancer establishment through induction of DNA damage response (DDR), cell cycle arrest and/or apoptosis [52]. In normal cells, DNA damage leads to p53 accumulation and cell cycle arrest before the S phase, allowing DNA repair before DNA duplication [53]. Alternatively, if the damage is too large and unrepairable, p53 activates the apoptotic events, preventing the generation of potentially transformed progeny [53]. In HPV-infected cells, E6-mediated p53 degradation inhibits cell cycle arrest and apoptosis, causing chromosomal instability and leading to carcinogenesis [54,55]. The key event for E6-induced p53 degradation is the formation of a ternary complex containing E6, the ubiquitin E3 ligase E6-associated protein (E6AP) and p53 (Fig. 1A). Importantly, E6AP/p53 interaction can only occur in presence of E6 that acts as a bridge between the two cellular proteins [56]. Upon formation of this ternary complex, p53 is rapidly ubiquitinated by E6AP and degraded by the proteasome pathway (Fig. 1A). In the genus alpha, the mucosal HR HPV types are the only ones able to induce p53 degradation [57,58]. Indeed, the E6 form of the LR HPV types binds p53 without inducing its degradation [59,60], however they are still able to alter some transcriptional p53 functions [61,62].
Fig. 1.
E6 and E7 HPV oncoproteins degrade p53 and pRb, respectively.
A. E6-induced p53 degradation through the formation of a ternary complex. Binding of E6 to p53 induces its ubiquitylation and proteasomal-mediated degradation, via the ubiquitin E3 ligase E6-associated protein (E6AP). B. Degradation of pRb, overexpression of CDKs and inactivation of CDK inhibitors all contribute to E2F overexpression and uncontrolled cell-cycle progression in cancers. Whereas hypo-phosphorylation of pRb prevents effects of E2F and renders cells quiescent, CDK/D-cyclin association causes its phosphorylation and release from E2F. Binding of E7 also causes release of E2F through ubiquitylation and proteasomal degradation of pRb [[163], [164], [165], [166], [167]].
Created withBioRender.com.
In addition to p53 functions, E6 also activates the transcription of the telomerase reverse transcriptase (hTERT), which is a ribonucleoprotein responsible for telomeres elongation. hTERT is usually overexpressed in cancer cells, but not or at low levels in normal somatic cells. In somatic cells, the very low telomerase activity results in telomere shortening after each cellular division to finally reach a critical size, leading to fusion among neighbour chromosomes and replicative senescence [63]. In contrast, in HPV16-infected cells the high level of telomerase activity results in telomere length maintenance and indefinite proliferation. It appears that the viruses have multiple mechanisms to activate hTERT transcription. The expression of hTERT is negatively regulated by the transcriptional repressor NFX1-91. HPV16, via E6/E6AP interaction, induces the degradation of NFX1-91 with consequent activation of hTERT transcription [64]. In addition, HPV16 can activate hTERT expression through the induction of Myc, which is recruited to the hTERT promoter [65].
Other important targets of the HR HPV E6 oncoprotein are the cellular proteins containing the PSD-90/Dlg/ZO-1 homology (PDZ) domain [66]. The HR HPV E6s bind several PDZ proteins via a motif of 4 amino acid located at the C-terminus. An alignment of the HR HPV E6 protein sequences resulted in the identification of a canonical X–S/T-X-ΦCOOH consensus site and highlighted some amino acid sequence variability that can impact on the E6 affinity for binding the different PDZ proteins [67]. The mucosal LR HPV type E6 proteins do not contain the C-terminus PDZ-binding motif, highlighting the importance of this interaction in the transformation processes induced by the HR HPV types. Indeed, many PDZ proteins are involved in the regulation of cell–cell contact and cell polarity events that are heavily altered in cancer cells.
The oncoprotein E7 contains at the N-terminus the LXCXE motif that mediates the binding of the tumour suppressor gene product retinoblastoma (pRb1) and its related proteins p107 and p130. In normal cells, pRb1 acts as negative regulator of the E2F transcription factors (E2F1−3), preventing the expression of pro-proliferation factors, such as cyclins, and maintaining the cell in a quiescent state (reviewed in Ref. [68]). When cells are exposed to mitogenic factors, signaling pathways are activated and pRb1 is rapidly phosphorylated with consequent release of active E2F and cell cycle progression. In HR HPV-infected cells, E7/pRb1 interaction leads to degradation of the tumor suppressor via proteasomal pathways [69,70], with consequent activation of E2F and deregulation of cellular proliferation independently of external stimuli (Fig. 1B).
In HPV-positive lesions, HPV DNA is often integrated into the host genome, or alternatively can be found as episomal DNA or in a mix of both forms [71]. Interestingly in cervical lesions, viral DNA integration increases with the severity of the disease, while in HNSCC this phenomenon appears to be less frequent.
Many studies have shown that HPV DNA integration results in the loss of the expression of several viral genes, including E2 that is intimately involved in the regulation of E6 and E7 expression. Loss of E2 promotes high E6 and E7 expression, further stimulating the progression of infected cells towards malignancy. In addition, other studies reported that HPV16 E6/E7 mRNA stability is increased after integration and specific alterations of host cell gene expression have been detected upon HPV genome integration [72]. The HR HPV E6 and E7 expression increases with neoplasia stage and greatly contributes to malignant progression [73,74]. Cells that express E6/E7 from integrated HR HPV sequences have a selective growth advantage over cells with episomal HPV genomes that still present HPV E2 expression [75]. In cancer cells mainly containing the episomal viral DNA form, a key event in deregulation of E6 and E7 expression can occur by genetic and epigenetic modifications at the early promotor region [76].
4. Diagnostic tools and applied algorithm in HNSCC classification
4.1. HPV diagnostic tools
Several procedures/algorithms have been fully developed and validated for the screening of pre-malignant and malignant cervical lesions. Regarding HPV-positive HNSCC, several assays have been proposed by independent studies, however they remain to be validated in screening programs. Classification of HPV-driven and -non driven HNSCC can be based on the use of direct HPV markers, such as viral DNA detection by PCR or by in situ hybridization (ISH), E6/E7 mRNA detection by RT-PCR or RT-qPCR, and indirect/surrogate HPV markers, like p16INK4a staining. HPV DNA detection by PCR-based assays in cancer tissue has a high sensitivity, but lower specificity, since the test positivity may be also due to a transitory infection in the upper respiratory/digestive tracts [77]. HPV DNA detection by ISH provides information on viral localization within the cells, however it is affected by a lower sensitivity when compared with PCR-based assays [78,79]. The ‘gold standard’ laboratory method to establish the oncogenic role of HPV is the identification of the full length E6/E7 mRNA in cancer tissue, which can also be performed using FPPE material [[80], [81], [82], [83], [84], [85], [86], [87]]. Alternatively to E6/E7 full length mRNA, the spliced version of E6 gene (E6*I mRNA) has been validated in several studies as a good marker for the classification of HR HPV-positive HNSCCs [87,88]. However, a possible limitation for the use of HPV mRNA detection assays in clinical settings is that they require more specialized laboratories.
Finally, the evaluation of p16INK4a over-expression by immunohistochemistry (IHC), which has been widely used in the cervix as HPV-infection surrogate marker, represents the most common clinical marker also applied to the classification of HPV-positive OPSCC. Accordingly, evaluation of HPV-status based on p16INK4a staining has been incorporated into the latest TNM staging guidelines (TNM8). However, lines of evidence indicate that p16INK4a positivity should be confirmed with a HPV-specific test for a more accurate classification [89]. p16INK4a accumulation in HPV-transformed cells is mainly linked to the deregulation of the cell cycle mediated by the E7 oncoprotein. However, several studies have also showed that p16INK4a overexpression can be detected in some HPV-negative HNSCC. Thus, as suggested for HPV DNA tests, p16INK4a staining should be used in combination with other HPV markers to have a precise classification of OPSCC.
Although, p16INK4a detection appears to be a good assay for HPV-positive OPSCC [90], some limitations on its use have been observed for the classification of the other head and neck sites [[91], [92], [93]].
4.2. Applied algorithm in HNSCC classification
It appears that HPV-driven OPSCCs have a better prognosis than HPV-negative OPSCCs [94,95]. Therefore, many studies have focused on the establishment of algorithms that could result in a rapid classification of HNSCC. As already mentioned above, the use of multiple HPV markers considerably increases the specificity of a specific algorithm without affecting the sensitivity [96]. Smeets and colleagues developed an algorithm that is based on the combined use of p16INK4a and HPV DNA [82]. This algorithm resulted in 98–100% sensitivity and specificity in oral cavity and oropharynx FFPE samples [82,83]. Conversely, the combined use of DNA ISH and p16INK4a in HNSCCs reported a p16NK4a sensitivity of 100% and a reduced specificity (85%) [97]. In a meta-analysis by Prigge et al., comprising 24 studies [98], the combined use of p16INK4a and HPV DNA PCR resulted in a 93% sensitivity and 96% specificity for viral mRNA-positive OPSCCs, which was not significantly less sensitive but significantly more specific compared to the two tests separately.
Although the use of p16INK4a staining together with other HPV tests [80,[99], [100], [101]] represent a valid algorithm for HNSCC classification, some precautions should be taken for the correct interpretation of p16INK4a staining. Indeed, p16INK4a expression can be altered by other risk factors in some specific HNSCCs [17,102]. Accordingly, discrepancy in p16INK4a and HPV DNA PCR positivity was reported [103,104].
HPV DNA PCR and p16INK4a have been evaluated together with E6/E7 mRNA in some studies [[105], [106], [107]]. The combined detection of E6 mRNA, HPV DNA PCR and p16INK4a in FFPE specimens, resulted in a lower HPV-attributable fraction than those previously reported [12]. In an Indian study, about 60% of HNSCC specimens which tested HPV DNA/mRNA double positive resulted p16INK4a negative, while about 18% of HPV mRNA negative HNSCC resulted p16INK4a positive, underling the concept that other risk factors may impact on p16INK4a expression in this geographical region [105].
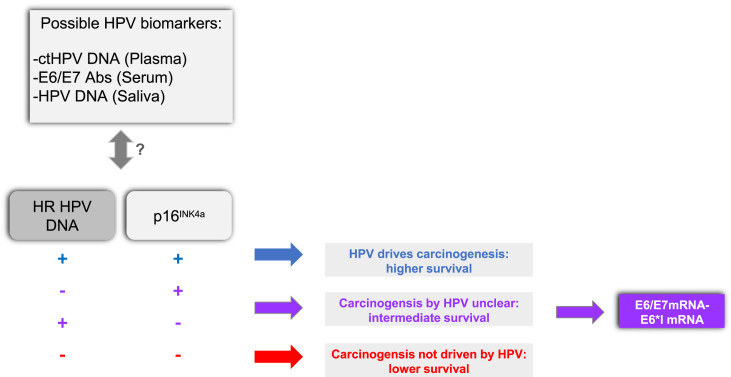
Thus, including triage with direct HPV markers, such as HR HPV DNA detection complemented with mRNA and indirect cellular markers, such as p16INKa (Fig. 2) could improve the stratification of HPV-driven HNSCC. Indeed, HPV DNA test can improve the specificity of p16INKa staining, while the mRNA assay allows the identification of clinically relevant HPV infections, especially in case of discordancy between HPV markers (e.g. HPV DNA−/p16+and vice-versa).
Fig. 2.
Diagnostic assays and viral biomarkers in HPV-related HNSCCs identification
Concepts for possible diagnostic algorithms to identify HPV-driven HNSCC using a combination of direct (HPVDNA + E6*I mRNA/E6-E7 mRNA) and surrogate cellular markers (p16INK4a) of oncogenic HPV infection in fresh and FFPE tissues (lower panel). HPV-biomarkers in body fluids used in combination with diagnostic algorithm for early diagnosis and follow-up of HNSCCs (upper panel).
5. HPV biomarkers in body fluids
In addition to available HPV markers described above, further biomarkers released in body fluids are of increasing interest, since they could be easily implemented into clinical practice (Fig. 2). Cancer cells release several biomarkers, which have been investigated as diagnostic, prognostic, and relapse markers in a large number of studies. Due to the non-invasive methods for their collection, body fluids have become possible cancer surrogate specimens for an easy and rapid diagnostic tool at different anatomical sites [[108], [109], [110], [111]]. Importantly, the use of biomarkers could simplify and synergize with the diagnostic conventional protocols to rapidly obtain key clinical information about the type of cancer, outcome, treatment responses and cancer recurrence, without requiring tumoral tissue specimens. In the context of precision medicine the role of biomarkers, such as viral circulating-tumor DNA (ctDNA), have high value, being indicated as a promising tool for HPV-associated OPSCC [[112], [113], [114]]. The ctDNA represents a fraction of cfDNA (circulating free DNA), which is released by cancer cells under necrosis, apoptosis or through active secretion mechanisms in the bloodstream compartment [115,116]. CfDNA detection in blood-derived samples, such as serum or plasma, could provide a ‘liquid biopsy’ specimen which can be used in early detection and/or cancer monitoring as an alternative to a solid tumor specimen, in the near future. The utility of ctHPV DNA is emerging for early diagnosis, post-treatment surveillance and recurrence, being efficiently increased in consecutively collected specimens of patients with HPV-positive OPSCC [20,117]. In a recent prospective study, it was reported that cfHPV DNA detection in plasma is specific biomarkers with high sensitivity, representing a useful non-invasive diagnostic approach to identify HPV-related HNSCCs [118]. In addition, it was reported in a case-control study that in a subset of patients, cfHPV16 DNA was detected 3 years before the clinical diagnosis of HPV16-related HNSCC [119].
As additional HPV blood-based markers, previous studies focused also on serum antibodies against early and late HPV proteins, such as E6, E7, E1, E2, and L1 [120,121] The presence of antibodies against the major capsid L1 protein is mainly indicative of HPV exposure [[122], [123], [124]], while those against early regulatory proteins E1 and E2 have been associated with OPSCCs [125,126]. Seropositivity for HPV16 E6 and E7 are specific markers of HPV-related anogenital cancers, such as anal and cervical, however seroconversion does not occur in all cancer cases [124,127,128]. In addition, HPV16 E6 antibodies are more frequently detected than E7 antibodies [129], but their use is rather limited for their low diagnostic sensitivity in CC [129]. By contrast, in OPSCCs antibodies against HPV16 E6 and E7 are more clinically relevant [5], since HPV16 E6 antibodies are detectable even a decade before the appearance of the lesions and clinical diagnosis [125,[130], [131], [132]]. In contrast, they are rarely detected in healthy individuals [133,134]. A recent meta-analysis highlighted that HPV16 E6 antibodies are sensitive and specific biomarkers for the diagnosis of HPV-related OPSCCs [135], while not predictive of recurrence [136].
In addition to HPV biomarkers in blood-derived specimens, some studies have proposed the use of saliva for identification of specific HPV markers in HNC patients [137,138]. Saliva represents an easy-to-collect body fluid in respect to blood. However, its use could be hampered by the low biomarker concentration in comparison with blood. In several independent studies, it has been observed that positivity for any HPV type in the oral cavity could range from 3.5% to about 10% in healthy population [[139], [140], [141]]. While in HPV-related HNSCC patients, high prevalence of HPV DNA positivity in saliva and gargles was detected [5,19,142,143]. In HNSCC patients, oral HPV prevalence and viral load tends to decrease after treatment, while its persistency was associated with recurrence [144]. In addition to HPV DNA, detection of oral HPV mRNA reported a sensitivity ranging from 23% up to 82% in detecting HPV-related OPSCCs [19,145,146]. In some studies, a combined analysis of oral HPV mRNA with ctHPV16 DNA in plasma improved the sensitivity for HPV16-positive OPSCCs detection [19]. In addition, a double-positivity for HPV16 DNA in saliva and plasma resulted in an increased sensitivity and early detection of HPV16-positive OPSCC recurrence [147].
Beside the HPV biomarkers, the characterization of HPV-positive OPSCC could be improved if host deregulated genes could be also considered. A recent study suggests that the host SYCP2 gene is up-regulated in HPV-positive premalignant tissue [148].
Validation of HPV and host biomarkers in body fluids may results in useful diagnostic, prognostic, and progression/recurrence tools, which can be easily applied, in correlation with the available diagnostic methods, in HPV-driven cancers. However, additional biomarkers as well as additional validation studies are required.
6. Preventive strategy by HPV vaccination
In the last decades, a vast number of studies resulted in the generation of prophylactic vaccines against some mucosal HPV types [149]. The rational of the HPV vaccine is based on specific features of the major capsid HPV L1 protein, which retains the ability to acquire the correct tridimensional structure when produced as a recombinant protein in eukaryotic cells, such as yeast or insect cells. The recombinant HPV L1 proteins are able to assemble in penta-capsomers and subsequently in virus like particles (VLPs). Immunization with VLPs leads to the generation of neutralizing antibodies that can prevent the HPV infection. Three prophylactic HPV vaccines have been licensed since 2006: (i) Cervarix® is a bivalent vaccine that targets the HR types, HPV16 and HPV18; (ii) Gardasil® is a quadrivalent HPV vaccine targeting the LR types HPV6 and HPV11 and the HR types HPV16 and HPV18 and (iii) Gardasil 9® is a nonavalent HPV vaccine that targets five additional HR types, namely HPV31, HPV33, HPV45, HPV52, and HPV58, together with the HPV types 6, 11, 16 and HPV18. They are all effective in reducing HPV-associated ano-genital infections and the development of pre-malignant and malignant lesions in women [[150], [151], [152], [153], [154], [155]]. The quadrivalent HPV vaccine is also effective in preventing genital lesions [156] and in reducing anal intraepithelial neoplasia in men [157]. Therefore, in several countries HPV vaccination has recently been recently extended to boys. A recent study performed in England, clearly showed a substantial reduction in cervical cancer and incidence of CIN3 in young women after the implementation of the HPV immunization program in adolescents [155]. Eradication of infections with oncogenic HPV types may also have an impact on other HPV-related cancers, including HPV-associated OPSCC. The available epidemiological data reports efficacy of the HPV vaccine in preventing oral infection [22,23,[158], [159], [160]]. In addition, a decline of oral prevalence of the vaccine types HPV16, 18, 6 and HPV11 from 2009 to 2016 has been observed in a cohort of unvaccinated men and women in parallel with an increase of the vaccination campaign, suggesting a herd protection [21]. Experimental studies conducted in animal models provide additional evidence for the efficacy of PV vaccination in preventing oral infections. Indeed, immunization of dogs with canine oral papillomavirus (COPV) L1 VLPs resulted in a protection against the development of oral lesions [161]. However, additional epidemiological studies are desirable to further determine the impact of HPV vaccination on preventing viral oral infection, considering the type of vaccine and the different vaccination protocols, e.g. different number of doses. HPV vaccines are not yet licensed for OPSCC, in part because of the lack of identified precursor lesions [162] to be used as efficacy end-point for OPSCCs. However, as suggested by IARC-WHO, the use of persistent viral infection as end-point for risk of OPSCC would be a feasible surrogate to be applied (IARC HPV Working Group. Primary End-points for Prophylactic HPV Vaccine Trials. Lyon (FR): International Agency for Research on Cancer; 2014. (IARC Working Group Reports, No. 7.).
7. Conclusions
In the last two decades many studies significantly increased our general knowledge on the role of HPV in HNSCCs, providing important insights for the generation of novel strategies for their prevention and early diagnosis. HPV vaccination and organized screening programs, reduce the incidence of HPV infections and HPV-associated pre-cancer, cervical cancer and probably also other HPV-associated anogenital and oropharyngeal cancers, whereas well organized cervical cancer screening has reduced incidence of and mortality from cervical cancer. Therefore, it is crucial that future efforts will be focused on the increase of HPV vaccination coverage worldwide.
Several studies led to the identification of novel diagnostic markers for early detection of HPV-positive HNSCC. However, these markers need to be validated in large-population studies together with accurate diagnostic algorithms for management of screen-positive subjects.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Author statement
All authors contributed to the preparation of the manuscript.
Declaration of competing interest
MA and CS were supported by the Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the RISCC Network (Grant No. 847845).The other authors declare no competing interests.
Acknowledgements
The authors apologize to those scientists whose important contributions to HPV research could not be cited or adequately discussed due to space limitations. We thank Mrs Nicole Suty for the preparation of this manuscript. The work performed in the groups is partially supported by grants from the European Commission, HPV-AHEAD (FP7-HEALTH-2011-282562), and the Italian Ministry of Health (Ricerca Corrente Reti, RCR-2020-23670066, Alliance Against Cancer (ACC): Head and neck tumors, WP 5).
Contributor Information
Tarik Gheit, Email: gheitt@iarc.fr.
Massimo Tommasino, Email: m.tommasino@oncologico.bari.it.
References
- 1.Tobacco smoke and involuntary smoking. IARC Monogr. Eval. Carcinog. Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 2.Baan R., Straif K., Grosse Y., et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–293. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 3.Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvard V., Baan R., Straif K., et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza G., Kreimer A.R., Viscidi R., et al. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 6.Castellsagué X., Mena M., Alemany L. Epidemiology of HPV-positive tumors in Europe and in the world. Recent Results Cancer Res. Fortschr. Krebsforsch. Progr. Rech. Cancer. 2017;206:27–35. doi: 10.1007/978-3-319-43580-0_2. [DOI] [PubMed] [Google Scholar]
- 7.Clifford G.M., Smith J.S., Plummer M., et al. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Martel C., Plummer M., Vignat J., et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillison M.L., Chaturvedi A.K., Anderson W.F., et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2015;33:3235–3242. doi: 10.1200/jco.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi A.K., Engels E.A., Pfeiffer R.M., et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/jco.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ndiaye C., Mena M., Alemany L., et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/s1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 12.Castellsagué X., Alemany L., Quer M., et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J. Nat. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 13.Hashibe M., Sturgis E.M. Epidemiology of oral-cavity and oropharyngeal carcinomas: controlling a tobacco epidemic while a human papillomavirus epidemic emerges. Otolaryngol. Clin. N. Am. 2013;46:507–520. doi: 10.1016/j.otc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Gillison M.L., Koch W.M., Capone R.B., et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Nat. Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 15.Ang K.K., Harris J., Wheeler R., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson D.E., Burtness B., Leemans C.R., et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatini M.E., Chiocca S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer. 2020;122:306–314. doi: 10.1038/s41416-019-0602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ginkel J.H., Slieker F.J.B., de Bree R., et al. Cell-free nucleic acids in body fluids as biomarkers for the prediction and early detection of recurrent head and neck cancer: a systematic review of the literature. Oral Oncol. 2017;75:8–15. doi: 10.1016/j.oraloncology.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H., Suzuki M., Takemoto N., et al. Performance of oral HPV DNA, oral HPV mRNA and circulating tumor HPV DNA in the detection of HPV-related oropharyngeal cancer and cancer of unknown primary. Int. J. Cancer. 2022;150:174–186. doi: 10.1002/ijc.33798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chera B.S., Kumar S., Shen C., et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2020;38:1050–1058. doi: 10.1200/jco.19.02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi A.K., Graubard B.I., Broutian T., et al. Prevalence of oral HPV infection in unvaccinated men and women in the United States, 2009-2016. JAMA. 2019;322:977–979. doi: 10.1001/jama.2019.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo T., Eisele D.W., Fakhry C. The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer. 2016;122:2313–2323. doi: 10.1002/cncr.29992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beachler D.C., Kreimer A.R., Schiffman M., et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J. Nat. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashibe M., Brennan P., Benhamou S., et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Nat. Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 25.Hashibe M., Brennan P., Chuang S.C., et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. : Publ. Am. Assoc. Cancer Res. 2009;18:541–550. doi: 10.1158/1055-9965.Epi-08-0347. cosponsored by the American Society of Preventive Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown K.F., Rumgay H., Dunlop C., et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer. 2018;118:1130–1141. doi: 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alcohol drinking . vol. 44. IARC Working Group; Lyon: 1988. pp. 1–378. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans). 13-20 October 1987. [PMC free article] [PubMed] [Google Scholar]
- 28.Stein A.P., Saha S., Kraninger J.L., et al. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer J. 2015;21:138–146. doi: 10.1097/ppo.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillison M.L., D'Souza G., Westra W., et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Nat. Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 30.El-Mofty S.K., Lu D.W. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am. J. Surg. Pathol. 2003;27:1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Dayyani F., Etzel C.J., Liu M., et al. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC) Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klussmann J.P., Mooren J.J., Lehnen M., et al. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2009;15:1779–1786. doi: 10.1158/1078-0432.Ccr-08-1463. [DOI] [PubMed] [Google Scholar]
- 33.Fakhry C., Westra W.H., Li S., et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Nat. Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 34.O'Rorke M.A., Ellison M.V., Murray L.J., et al. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48:1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Guha N., Boffetta P., Wünsch Filho V., et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am. J. Epidemiol. 2007;166:1159–1173. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 36.Windon M.J., D'Souza G., Rettig E.M., et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer. 2018;124:2993–2999. doi: 10.1002/cncr.31385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syrjänen S., Syrjänen K. HPV in head and neck carcinomas: different HPV profiles in oropharyngeal carcinomas - why? Acta Cytol. 2019;63:124–142. doi: 10.1159/000495727. [DOI] [PubMed] [Google Scholar]
- 38.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 39.Anis M.M., Razavi M.M., Xiao X., et al. Association of gastroesophageal reflux disease and laryngeal cancer. World J. Otorhinolaryngol. Head Neck Surg. 2018;4:278–281. doi: 10.1016/j.wjorl.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tae K., Jin B.J., Ji Y.B., et al. The role of laryngopharyngeal reflux as a risk factor in laryngeal cancer: a preliminary report. Clin. Exp. Otorhinolaryngol. 2011;4:101–104. doi: 10.3342/ceo.2011.4.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo C. Acid reflux increases susceptibility to laryngopharyngeal and oropharyngeal cancers. Arch. Otorhinolaryngol. Head Neck Surg. 2020;1:2. doi: 10.24983/scitemed.aohns.2020.00123. [DOI] [Google Scholar]
- 42.Velleuer E., Dietrich R. Fanconi anemia: young patients at high risk for squamous cell carcinoma. Mol. Cell. Pediatr. 2014;1:9. doi: 10.1186/s40348-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kutler D.I., Singh B., Satagopan J., et al. A 20-year perspective on the international Fanconi anemia registry (IFAR) Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 44.Novacek G. Plummer-Vinson syndrome. Orphanet J. Rare Dis. 2006;1:36. doi: 10.1186/1750-1172-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alter B.P., Giri N., Savage S.H. Head and neck cancer in Fanconi anemia and dyskeratosis congenita. J. Clin. Oncol. 2012;30:5563. doi: 10.1200/jco.2012.30.15_suppl.5563. [DOI] [Google Scholar]
- 46.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 47.de Martel C., Georges D., Bray F., et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Global Health. 2020;8:e180–e190. doi: 10.1016/s2214-109x(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 48.Arbyn M., de Sanjosé S., Saraiya M., et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int. J. Cancer. 2012;131:1969–1982. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bzhalava D., Eklund C., Dillner J. International standardization and classification of human papillomavirus types. Virology. 2015;476:341–344. doi: 10.1016/j.virol.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Z.M., Baker C.C. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front. Biosci. : J. Vis. Literacy. 2006;11:2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doorbar J., Egawa N., Griffin H., et al. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015;25(Suppl 1):2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zilfou J.T., Lowe S.W. Tumor suppressive functions of p53. Cold Spring Harbor Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane D., Levine A. p53 Research: the past thirty years and the next thirty years. Cold Spring Harbor Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas J.T., Laimins L.A. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J. Virol. 1998;72:1131–1137. doi: 10.1128/jvi.72.2.1131-1137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson D.A., Belinsky G., Chang T.H., et al. The human papillomavirus-16 E6 oncoprotein decreases the vigilance of mitotic checkpoints. Oncogene. 1997;15:3025–3035. doi: 10.1038/sj.onc.1201495. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Zapien D., Ruiz F.X., Poirson J., et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature. 2016;529:541–545. doi: 10.1038/nature16481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheffner M., Werness B.A., Huibregtse J.M., et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 58.Scheffner M., Münger K., Huibregtse J.M., et al. Targeted degradation of the retinoblastoma protein by human papillomavirus E7-E6 fusion proteins. EMBO J. 1992;11:2425–2431. doi: 10.1002/j.1460-2075.1992.tb05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crook T., Tidy J.A., Vousden K.H. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- 60.Lechner M.S., Laimins L.A. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J. Virol. 1994;68:4262–4273. doi: 10.1128/jvi.68.7.4262-4273.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lechner M.S., Mack D.H., Finicle A.B., et al. Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. EMBO J. 1992;11:3045–3052. doi: 10.1002/j.1460-2075.1992.tb05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas M., Massimi P., Jenkins J., et al. HPV-18 E6 mediated inhibition of p53 DNA binding activity is independent of E6 induced degradation. Oncogene. 1995;10:261–268. [PubMed] [Google Scholar]
- 63.Shay J.W., Wright W.E. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 64.Gewin L., Myers H., Kiyono T., et al. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004;18:2269–2282. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veldman T., Liu X., Yuan H., et al. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8211–8216. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiyono T., Hiraiwa A., Fujita M., et al. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganti K., Broniarczyk J., Manoubi W., et al. The human papillomavirus E6 PDZ binding motif: from life cycle to malignancy. Viruses. 2015;7:3530–3551. doi: 10.3390/v7072785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kouzarides T. Transcriptional control by the retinoblastoma protein. Semin. Cancer Biol. 1995;6:91–98. doi: 10.1006/scbi.1995.0012. [DOI] [PubMed] [Google Scholar]
- 69.Roman A., Munger K. The papillomavirus E7 proteins. Virology. 2013;445:138–168. doi: 10.1016/j.virol.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dyson N., Howley P.M., Münger K., et al. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science (New York, N.Y.) 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 71.Kristiansen E., Jenkins A., Holm R. Coexistence of episomal and integrated HPV16 DNA in squamous cell carcinoma of the cervix. J. Clin. Pathol. 1994;47:253–256. doi: 10.1136/jcp.47.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Münger K., Baldwin A., Edwards K.M., et al. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004;78:11451–11460. doi: 10.1128/jvi.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Isaacson Wechsler E., Wang Q., Roberts I., et al. Reconstruction of human papillomavirus type 16-mediated early-stage neoplasia implicates E6/E7 deregulation and the loss of contact inhibition in neoplastic progression. J. Virol. 2012;86:6358–6364. doi: 10.1128/jvi.07069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Münger K., Hayakawa H., Nguyen C.L., et al. Viral carcinogenesis and genomic instability. EXS. 2006:179–199. doi: 10.1007/3-7643-7378-4_8. [DOI] [PubMed] [Google Scholar]
- 75.Jeon S., Allen-Hoffmann B.L., Lambert P.F. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 1995;69:2989–2997. doi: 10.1128/jvi.69.5.2989-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McBride A.A., Warburton A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis J.S., Jr. Human papillomavirus testing in head and neck squamous cell carcinoma in 2020: where are we now and where are we going? Head Neck Pathol. 2020;14:321–329. doi: 10.1007/s12105-019-01117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevens T.M., Caughron S.K., Dunn S.T., et al. Detection of high-risk HPV in head and neck squamous cell carcinomas: comparison of chromogenic in situ hybridization and a reverse line blot method. Appl. Immunohistochem. Mol. Morphol. : AIMM. 2011;19:574–578. doi: 10.1097/PAI.0b013e318215248a. [DOI] [PubMed] [Google Scholar]
- 79.Schlecht N.F., Brandwein-Gensler M., Nuovo G.J., et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod. Pathol. : Off. J. United States Can. Acad. Pathol. Inc. 2011;24:1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marur S., D'Souza G., Westra W.H., et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/s1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung A.C., Briolat J., Millon R., et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int. J. Cancer. 2010;126:1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
- 82.Smeets S.J., Hesselink A.T., Speel E.J., et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int. J. Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 83.Rietbergen M.M., Leemans C.R., Bloemena E., et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in The Netherlands as assessed by a validated test algorithm. Int. J. Cancer. 2013;132:1565–1571. doi: 10.1002/ijc.27821. [DOI] [PubMed] [Google Scholar]
- 84.Rietbergen M.M., Snijders P.J., Beekzada D., et al. Molecular characterization of p16-immunopositive but HPV DNA-negative oropharyngeal carcinomas. Int. J. Cancer. 2014;134:2366–2372. doi: 10.1002/ijc.28580. [DOI] [PubMed] [Google Scholar]
- 85.Mes S.W., Heideman D.A.M., Bloemena E., et al. Development and validation of a novel and rapid molecular detection method for high-risk human papillomavirus in formalin-fixed, paraffin-embedded tumor tissue. J. Mol. Diagn. : J. Mod. Dynam. 2020;22:262–271. doi: 10.1016/j.jmoldx.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 86.Schache A.G., Liloglou T., Risk J.M., et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br. J. Cancer. 2013;108:1332–1339. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Halec G., Schmitt M., Dondog B., et al. Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int. J. Cancer. 2013;132:63–71. doi: 10.1002/ijc.27605. [DOI] [PubMed] [Google Scholar]
- 88.von Knebel Doeberitz M. The causal role of human papillomavirus infections in non-anogenital cancers. It's time to ask for the functional evidence. Int. J. Cancer. 2016;139:9–11. doi: 10.1002/ijc.30059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Craig S.G., Anderson L.A., Schache A.G., et al. Recommendations for determining HPV status in patients with oropharyngeal cancers under TNM8 guidelines: a two-tier approach. Br. J. Cancer. 2019;120:827–833. doi: 10.1038/s41416-019-0414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rischin D., Young R.J., Fisher R., et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2010;28:4142–4148. doi: 10.1200/jco.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lechner M., Chakravarthy A.R., Walter V., et al. Frequent HPV-independent p16/INK4A overexpression in head and neck cancer. Oral Oncol. 2018;83:32–37. doi: 10.1016/j.oraloncology.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Bussu F., Sali M., Gallus R., et al. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br. J. Cancer. 2013;108:1157–1162. doi: 10.1038/bjc.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dalianis T., Grün N., Koch J., et al. Human papillomavirus DNA and p16(INK4a) expression in hypopharyngeal cancer and in relation to clinical outcome, in Stockholm, Sweden. Oral Oncol. 2015;51:857–861. doi: 10.1016/j.oraloncology.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Mirghani H., Amen F., Blanchard P., et al. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int. J. Cancer. 2015;136:1494–1503. doi: 10.1002/ijc.28847. [DOI] [PubMed] [Google Scholar]
- 95.Chera B.S., Amdur R.J., Green R., et al. Phase II trial of de-intensified chemoradiotherapy for human papillomavirus-associated oropharyngeal squamous cell carcinoma. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2019;37:2661–2669. doi: 10.1200/jco.19.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng Z., Hasegawa M., Aoki K., et al. A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int. J. Oncol. 2014;45:67–76. doi: 10.3892/ijo.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singhi A.D., Westra W.H. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 98.Prigge E.S., Arbyn M., von Knebel Doeberitz M., et al. Diagnostic accuracy of p16(INK4a) immunohistochemistry in oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis. Int. J. Cancer. 2017;140:1186–1198. doi: 10.1002/ijc.30516. [DOI] [PubMed] [Google Scholar]
- 99.Rietbergen M.M., Brakenhoff R.H., Bloemena E., et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2013;24:2740–2745. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 100.Taberna M., Mena M., Pavón M.A., et al. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2017;28:2386–2398. doi: 10.1093/annonc/mdx304. [DOI] [PubMed] [Google Scholar]
- 101.Holzinger D., Schmitt M., Dyckhoff G., et al. Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res. 2012;72:4993–5003. doi: 10.1158/0008-5472.Can-11-3934. [DOI] [PubMed] [Google Scholar]
- 102.Hoffmann M., Tribius S., Quabius E.S., et al. HPV DNA, E6*I-mRNA expression and p16INK4A immunohistochemistry in head and neck cancer - how valid is p16INK4A as surrogate marker? Cancer Lett. 2012;323:88–96. doi: 10.1016/j.canlet.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 103.Anantharaman D., Abedi-Ardekani B., Beachler D.C., et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer. 2017;140:1968–1975. doi: 10.1002/ijc.30608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perrone F., Gloghini A., Cortelazzi B., et al. Isolating p16-positive/HPV-negative oropharyngeal cancer: an effort worth making. Am. J. Surg. Pathol. 2011;35:774–777. doi: 10.1097/PAS.0b013e3182116a45. author reply 7-8. [DOI] [PubMed] [Google Scholar]
- 105.Gheit T., Anantharaman D., Holzinger D., et al. Role of mucosal high-risk human papillomavirus types in head and neck cancers in central India. Int. J. Cancer. 2017;141:143–151. doi: 10.1002/ijc.30712. [DOI] [PubMed] [Google Scholar]
- 106.Simoens C., Gorbaslieva I., Gheit T., et al. HPV DNA genotyping, HPV E6*I mRNA detection, and p16(INK4a)/Ki-67 staining in Belgian head and neck cancer patient specimens, collected within the HPV-AHEAD study. Cancer Epidemiol. 2021;72 doi: 10.1016/j.canep.2021.101925. [DOI] [PubMed] [Google Scholar]
- 107.Shinn J.R., Davis S.J., Lang-Kuhs K.A., et al. Oropharyngeal squamous cell carcinoma with discordant p16 and HPV mRNA results: incidence and characterization in a large, contemporary United States cohort. Am. J. Surg. Pathol. 2021;45:951–961. doi: 10.1097/pas.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esposito A., Bardelli A., Criscitiello C., et al. Monitoring tumor-derived cell-free DNA in patients with solid tumors: clinical perspectives and research opportunities. Cancer Treat Rev. 2014;40:648–655. doi: 10.1016/j.ctrv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 109.Tie J., Wang Y., Tomasetti C., et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arantes L., De Carvalho A.C., Melendez M.E., et al. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018;18:85–112. doi: 10.1080/14737159.2017.1404906. [DOI] [PubMed] [Google Scholar]
- 111.Kaczor-Urbanowicz K.E., Wei F., Rao S.L., et al. Clinical validity of saliva and novel technology for cancer detection. Biochim. Biophys. Acta, Rev. Cancer. 2019;1872:49–59. doi: 10.1016/j.bbcan.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chera B.S., Kumar S., Beaty B.T., et al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2019;25:4682–4690. doi: 10.1158/1078-0432.Ccr-19-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Damerla R.R., Lee N.Y., You D., et al. Detection of early human papillomavirus-associated cancers by liquid biopsy. JCO Precis. Oncol. 2019;3 doi: 10.1200/po.18.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cao H., Banh A., Kwok S., et al. Quantitation of human papillomavirus DNA in plasma of oropharyngeal carcinoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:e351–e358. doi: 10.1016/j.ijrobp.2011.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schwarzenbach H., Hoon D.S., Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 116.Jahr S., Hentze H., Englisch S., et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 117.Veyer D., Wack M., Mandavit M., et al. HPV circulating tumoral DNA quantification by droplet-based digital PCR: a promising predictive and prognostic biomarker for HPV-associated oropharyngeal cancers. Int. J. Cancer. 2020;147:1222–1227. doi: 10.1002/ijc.32804. [DOI] [PubMed] [Google Scholar]
- 118.Siravegna G., O'Boyle C.J., Varmeh S., et al. Cell-free HPV DNA provides an accurate and rapid diagnosis of HPV-associated head and neck cancer. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2022;28:719–727. doi: 10.1158/1078-0432.Ccr-21-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rettig E.M., Faden D.L., Sandhu S., et al. Detection of circulating tumor human papillomavirus DNA before diagnosis of HPV-positive head and neck cancer. Int. J. Cancer. 2022 doi: 10.1002/ijc.33996. [DOI] [PubMed] [Google Scholar]
- 120.López R.V., Levi J.E., Eluf-Neto J., et al. Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes Control : CCC. 2014;25:461–471. doi: 10.1007/s10552-014-0348-8. [DOI] [PubMed] [Google Scholar]
- 121.Holzinger D., Wichmann G., Baboci L., et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int. J. Cancer. 2017;140:2748–2757. doi: 10.1002/ijc.30697. [DOI] [PubMed] [Google Scholar]
- 122.Dillner J. The serological response to papillomaviruses. Semin. Cancer Biol. 1999;9:423–430. doi: 10.1006/scbi.1999.0146. [DOI] [PubMed] [Google Scholar]
- 123.Castellsagué X., Pawlita M., Roura E., et al. Prospective seroepidemiologic study on the role of Human Papillomavirus and other infections in cervical carcinogenesis: evidence from the EPIC cohort. Int. J. Cancer. 2014;135:440–452. doi: 10.1002/ijc.28665. [DOI] [PubMed] [Google Scholar]
- 124.Combes J.D., Pawlita M., Waterboer T., et al. Antibodies against high-risk human papillomavirus proteins as markers for invasive cervical cancer. Int. J. Cancer. 2014;135:2453–2461. doi: 10.1002/ijc.28888. [DOI] [PubMed] [Google Scholar]
- 125.Kreimer A.R., Johansson M., Waterboer T., et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2013;31:2708–2715. doi: 10.1200/jco.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anderson K.S., Wong J., D'Souza G., et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br. J. Cancer. 2011;104:1896–1905. doi: 10.1038/bjc.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bertisch B., Franceschi S., Lise M., et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am. J. Epidemiol. 2013;178:877–884. doi: 10.1093/aje/kwt153. [DOI] [PubMed] [Google Scholar]
- 128.Dillner J., Wiklund F., Lenner P., et al. Antibodies against linear and conformational epitopes of human papillomavirus type 16 that independently associate with incident cervical cancer. Int. J. Cancer. 1995;60:377–382. doi: 10.1002/ijc.2910600318. [DOI] [PubMed] [Google Scholar]
- 129.Zumbach K., Kisseljov F., Sacharova O., et al. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in cervical-carcinoma patients from Russia. Int. J. Cancer. 2000;85:313–318. doi: 10.1002/(sici)1097-0215(20000201)85:3<313::aid-ijc3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 130.Kreimer A.R., Johansson M., Yanik E.L., et al. Kinetics of the human papillomavirus type 16 E6 antibody response prior to oropharyngeal cancer. J. Nat. Cancer Inst. 2017;109 doi: 10.1093/jnci/djx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kreimer A.R., Ferreiro-Iglesias A., Nygard M., et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 consortium. Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2019;30:1335–1343. doi: 10.1093/annonc/mdz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith E.M., Ritchie J.M., Pawlita M., et al. Human papillomavirus seropositivity and risks of head and neck cancer. Int. J. Cancer. 2007;120:825–832. doi: 10.1002/ijc.22330. [DOI] [PubMed] [Google Scholar]
- 133.Lang Kuhs K.A., Anantharaman D., Waterboer T., et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: a pooled analysis. Cancer Epidemiol. Biomark. Prev. : Publ. Am. Assoc. Cancer Res. 2015;24:683–689. doi: 10.1158/1055-9965.Epi-14-1217. cosponsored by the American Society of Preventive Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brenner N., Mentzer A.J., Hill M., et al. Characterization of human papillomavirus (HPV) 16 E6 seropositive individuals without HPV-associated malignancies after 10 years of follow-up in the UK Biobank. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hibbert J., Halec G., Baaken D., et al. Sensitivity and specificity of human papillomavirus (HPV) 16 early antigen serology for HPV-driven oropharyngeal cancer: a systematic literature review and meta-analysis. Cancers. 2021:13. doi: 10.3390/cancers13123010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y., Waterboer T., Haddad R.I., et al. Human papillomavirus (HPV) 16 antibodies at diagnosis of HPV-related oropharyngeal cancer and antibody trajectories after treatment. Oral Oncol. 2017;67:77–82. doi: 10.1016/j.oraloncology.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Y., Springer S., Mulvey C.L., et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ekanayake Weeramange C., Liu Z., Hartel G., et al. Salivary high-risk human papillomavirus (HPV) DNA as a biomarker for HPV-driven head and neck cancers. J. Mol. Diagn. : J. Mod. Dynam. 2021;23:1334–1342. doi: 10.1016/j.jmoldx.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gillison M.L., Broutian T., Pickard R.K., et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bettampadi D., Villa L.L., Ponce E.L., et al. Oral human papillomavirus prevalence and type distribution by country (Brazil, Mexico and the United States) and age among HPV infection in men study participants. Int. J. Cancer. 2020;146:3026–3033. doi: 10.1002/ijc.32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.D'Souza G., McNeel T.S., Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2017;28:3065–3069. doi: 10.1093/annonc/mdx535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao M., Rosenbaum E., Carvalho A.L., et al. Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. Int. J. Cancer. 2005;117:605–610. doi: 10.1002/ijc.21216. [DOI] [PubMed] [Google Scholar]
- 143.Martin-Gomez L., Fulp W.J., Schell M.J., et al. Oral gargle-tumor biopsy human papillomavirus (HPV) agreement and associated factors among oropharyngeal squamous cell carcinoma (OPSCC) cases. Oral Oncol. 2019;92:85–91. doi: 10.1016/j.oraloncology.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fakhry C., Blackford A.L., Neuner G., et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019;5:985–992. doi: 10.1001/jamaoncol.2019.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.D'Souza G., Clemens G., Troy T., et al. vol. 12. Cancer prevention research; Philadelphia, Pa: 2019. pp. 689–700. (Evaluating the Utility and Prevalence of HPV Biomarkers in Oral Rinses and Serology for HPV-Related Oropharyngeal Cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chai R.C., Lim Y., Frazer I.H., et al. A pilot study to compare the detection of HPV-16 biomarkers in salivary oral rinses with tumour p16(INK4a) expression in head and neck squamous cell carcinoma patients. BMC Cancer. 2016;16:178. doi: 10.1186/s12885-016-2217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahn S.M., Chan J.Y., Zhang Z., et al. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2014;140:846–854. doi: 10.1001/jamaoto.2014.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Masterson L., Sorgeloos F., Winder D., et al. Deregulation of SYCP2 predicts early stage human papillomavirus-positive oropharyngeal carcinoma: a prospective whole transcriptome analysis. Cancer Sci. 2015;106:1568–1575. doi: 10.1111/cas.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stanley M. Prophylactic HPV vaccines: prospects for eliminating ano-genital cancer. Br. J. Cancer. 2007;96:1320–1323. doi: 10.1038/sj.bjc.6603695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muñoz N., Kjaer S.K., Sigurdsson K., et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J. Nat. Cancer Inst. 2010;102:325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 151.Lehtinen M., Paavonen J., Wheeler C.M., et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/s1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 152.Harper D.M., DeMars L.R. HPV vaccines - a review of the first decade. Gynecol. Oncol. 2017;146:196–204. doi: 10.1016/j.ygyno.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 153.Dillner J., Kjaer S.K., Wheeler C.M., et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ (Clinical research ed) 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel C., Brotherton J.M., Pillsbury A., et al. vol. 23. 2018. (The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Falcaro M., Castañon A., Ndlela B., et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet (London, England) 2021;398:2084–2092. doi: 10.1016/s0140-6736(21)02178-4. [DOI] [PubMed] [Google Scholar]
- 156.Giuliano A.R., Palefsky J.M., Goldstone S., et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palefsky J.M., Giuliano A.R., Goldstone S., et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 158.Schlecht N.F., Masika M., Diaz A., et al. Risk of oral human papillomavirus infection among sexually active female adolescents receiving the quadrivalent vaccine. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Castillo A., Osorio J.C., Fernández A., et al. Effect of vaccination against oral HPV-16 infection in high school students in the city of Cali, Colombia. Papillomavirus Res. (Amsterdam, Netherlands) 2019;7:112–117. doi: 10.1016/j.pvr.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herrero R., Quint W., Hildesheim A., et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Suzich J.A., Ghim S.J., Palmer-Hill F.J., et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fakhry C., Rosenthal B.T., Clark D.P., et al. vol. 4. Cancer prevention research; Philadelphia, Pa: 2011. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal "pap-test equivalent"; pp. 1378–1384. (High-Risk Populations). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kent L.N., Leone G. The broken cycle: E2F dysfunction in cancer. Nat. Rev. Cancer. 2019;19:326–338. doi: 10.1038/s41568-019-0143-7. [DOI] [PubMed] [Google Scholar]
- 164.Sherr C.J., Roberts J.M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 165.Tommasino M., Crawford L. Human papillomavirus E6 and E7: proteins which deregulate the cell cycle. Bioessays : News Rev. Mol. Cell. Dev. Biol. 1995;17:509–518. doi: 10.1002/bies.950170607. [DOI] [PubMed] [Google Scholar]
- 166.Tommasino M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014;26:13–21. doi: 10.1016/j.semcancer.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 167.Reinstein E., Scheffner M., Oren M., et al. Degradation of the E7 human papillomavirus oncoprotein by the ubiquitin-proteasome system: targeting via ubiquitination of the N-terminal residue. Oncogene. 2000;19:5944–5950. doi: 10.1038/sj.onc.1203989. [DOI] [PubMed] [Google Scholar]