Abstract
Objective
This study aimed to provide feasible suggestions for intraventricular injection of tigecycline to treat intractable Acinetobacter baumannii intracranial infections by studying its pharmacokinetics/pharmacodynamics and neurotoxicity.
Methods
A simple and reliable two-dimensional high-performance liquid chromatography (2D-HPLC) method was used to determine tigecycline concentration. The pharmacokinetics (PK) of tigecycline in cerebrospinal fluid (CSF) was investigated by performing therapeutic drug monitoring (TDM). The pharmacodynamics (PD) of tigecycline was evaluated by its minimum inhibitory concentration (MIC) against XDR A. baumannii. CCK8 assay was used to evaluate the cytotoxicity of different concentrations of tigecycline effect on PC12 cells, and apoptosis assay was analyzed by flow cytometry.
Results
Tigecycline retention time in 2D-HPLC was 7.636 min. The lower limit of quantitation (LLOQ) was 0.1mg/L, which met the requirements of concentration determination for TDM. The MIC50 and MIC90 values of tigecycline for A. baumannii were 2 and 4 mg/L, respectively. After a dose of 5mg tigecycline, Cmax in CSF was 37.894 mg/L which was high above the MIC values. The t1/2 of tigecycline was estimated to be 2.73 hours. Tigecycline significantly decreased cell viability as assessed and induced apoptosis of the PC12 cell. The IC50 value of PC12 cells treated with tigecycline was about 51.35 mg/L.
Conclusion
Intraventricular injection of tigecycline is a promising method for treating XDR A. baumannii intracranial infection. Since a high concentration of tigecycline in CSF may have potential neurotoxicity, and the t1/2 was short, giving small doses of less than 5 mg at least twice a day may be safer and more effective. Intraventricular injection of tigecycline must be selected cautiously and best carried out under TDM.
Keywords: tigecycline, intraventricular injection, XDR A. baumannii, intracranial infection, TDM
Introduction
Intracranial infection is a severe, rapidly progressing, refractory disease with high mortality. Due to the blood–brain barrier, most antibiotics cannot enter the central nervous system (CNS) to play an effective antibacterial role. In recent years, A. baumannii, associated with post-neurosurgical meningitis and ventriculitis, has increasingly been reported.1–3 According to China Antimicrobial Surveillance Network (CHINET), the most frequent organism was A. baumannii (12.5%) in 3157 pathogenic bacteria from cerebrospinal fluid strains.4 It poses severe challenges to the anti-infection treatment given the high disability and mortality rate and few sensitive antibiotics to choose from. About 80% of A. baumannii strains were resistant to imipenem and meropenem. The majority of resistant strains are only sensitive to tigecycline and polymyxin B.4 However, intravenous tigecycline and polymyxin B have poor CSF penetration and cannot treat the intracranial infection. Intraventricular or intrathecal drug administration is a method that bypasses the blood–brain barrier and other mechanisms that limit drug distribution into the brain.5
According to the International Consensus Guidelines for the Optimal Use of the Polymyxins, Colistin Methanesulfonate (CMS) is the preferred polymyxin for intraventricular or intrathecal administration.6 CMS is not approved for sale in China. Polymyxin B is the only alternative for intraventricular or intrathecal administration. Polymyxin B has neurotoxicity. There may be some meningeal irritation symptoms, such as fever, headache, neck stiffness, CSF cell count, and protein increase after intraventricular injection of polymyxin B. These symptoms are similar to an intracranial infection, which can affect evaluation of the curative effect. At the same time, treating XDR A. baumannii infection usually requires an antimicrobial agent combination. Another sensitive antibiotic, tigecycline, may be an alternative for intraventricular injection. It is much cheaper than polymyxin B, promisingly, some successful treatments with intraventricular tigecycline have been reported, and these reports had no serious and irreversible adverse reactions.7–9 However, intraventricular tigecycline is off-label drug use, and there is no internationally recognized guideline on the dosage and frequency of administration. Due to the lack of large sample research, its effectiveness and safety may be biased.
Instilling drugs directly into the brain’s ventricles must be done carefully and with full consideration of factors that affect the efficacy and safety of the route of administration.10 The efficacy of intraventricular injection of tigecycline is related to its pharmacokinetics (PK) and pharmacodynamics (PD). It means that the concentration of tigecycline in CSF must be higher than the MIC of bacteria and maintain effective concentration for enough time. The safety of intracerebral injection of drugs is most concerned with neurotoxicity. There are only a few successful cases that reported the investigation of tigecycline PK in CSF.11,12 To our knowledge, there are no published data on the neurotoxicity of tigecycline. Therefore, we monitored tigecycline concentration in CSF of a patient with XDR A. baumannii intracranial infection who received an intraventricular tigecycline injection. We evaluated the efficacy of intraventricular injection of tigecycline according to its PK and MIC values. The neurotoxicity of tigecycline was evaluated by the survival rate of PC12 cells under different concentrations of tigecycline detected by the CCK8 assay. Based on the results, we formulated feasible suggestions for intraventricular injection of tigecycline to treat intracranial infections.
Materials and Methods
Concentration Determination of Tigecycline
Quantification of Tigecycline by 2D-HPLC
2D separation of tigecycline was carried out in an FLC2701 2D-HPLC system (ANAX, Changsha, China). The chromatographic conditions (ANAX, Changsha, China) were LC1 column (Aston SC2 C18, 3.5*25 mm, 5 μm), LC2 column (Aston SCB C18, 4.6*125 mm, 5 μm). The mobile phase for LC1 was methanol: ammonium phosphate saline solution in a 45:55 ratio (v/v, pH adjusted to 7.5 using ammonia) and a flow rate of 1.0 mL/min. The mobile phase for LC2 was ammonium phosphate saline solution (pH adjusted to 7.4 using ammonia): ammonium phosphate saline solution (pH adjusted to 3.0 using phosphoric acid): acetonitrile in a 3:5:2 ratio (v/v/v), and a flow rate of 1.5 mL/min. The auxiliary mobile phase was ultra-pure water with a 0.1 mL/min flow rate. The detector cell temperature was 40 °C, UV detection wavelength was 340 nm, and the autosampler injection volume was 200 μL.
CSF Sample Processing
CSF was centrifuged for 5 min (3000 r/min), then 200 μL of supernatant was withdrawn and placed in Eppendorf tubes with 200 μL of perchloric acid (10%) to precipitate proteins. The tubes were vortexed for 1 min and centrifuged for 8 min (14,500 r/min). About 300 μL supernatant and 20 μL ammonium acetate (5.0 mol/L) were added to the sample injection bottle and vortexed for 60s. A final volume of 200 μL was injected into the 2D-HPLC system for quantitative analysis using an external standard.
CSF Sample for TDM
After neurosurgery, a patient infected with XDR A. baumannii has treated with tigecycline 50mg intravenously every 12 hours for three days. Then, tigecycline 5mg was injected through the ventricular drainage tube every 12 hours. After administration, the drainage tube was clamped for 1 hour and opened. CSF was taken at 1h, 6h, 8h, and 12h after administration for TDM.
Antimicrobial Susceptibility
Clinical isolates were isolated from the intracranial infection patient’s CSF and were initially identified by the VITEK®2 automated system. Antimicrobial susceptibility testing was performed by VITEK®2 using the susceptibility card AST-XN01, following Clinical and Laboratory Standards Institute (CLSI) guidelines and the manufacturer’s instructions.13,14 Tigecycline susceptibility testing was performed by Mueller–Hinton broth microdilution. Serial dilutions of tigecycline standard solutions were prepared in triplicate with fresh Mueller–Hinton broth in 96-well microtitre plates. Wells were inoculated with A. baumannii to a 105 cfu/mL final density and incubated at 35°C for 24 hours. A microplate reader measured each well’s optical density (OD) value. MIC50 and MIC90 values (MICs required to inhibit 50% and 90% of the isolates respectively) were calculated.
Cytotoxic Effects of Tigecycline on PC12 Cells
PC12 Cell Culture
PC12 cell is a pheochromocytoma cell line obtained from rat adrenal glands. It was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) in the present study. The passage number of PC12 cells was 10. The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. 1640 medium supplemented with 10% fetal calf serum (FCS), 100 IU/mL penicillin, and 100 mg/L streptomycin were used as the culture medium. Cell monolayers were plated in 96-well plates (Costar).15
CCK8 Assay
PC12 cells were seeded at 5*103 cells per well in 100 μL 1640 medium in 96-well plates and incubated at 37 °C (5% CO2) for 24 hours to the logarithmic growth period. A fresh medium of the appropriate concentration of tigecycline (Wyeth, AMSU/12) was added for 24 hours of incubation. The cell supernatant was removed, and CCK8 was diluted with PBS (1:9) 100 μL and incubated for 1.5 hours. The light absorbance at a wavelength of 450 nm was measured using a microplate reader. The cell viability was expressed as the detected absorbance and compared with control cultures containing no tigecycline. The cells were observed and photographed by an inverted microscope.
Apoptosis Assay
PC12 cells were plated (calculated as 20% of the bottom area) in 6-well plates and incubated at 37 °C (5% CO2) overnight. When the cells grew to around 50% to 60%, different concentrations of tigecycline were added and incubated for 24 hours. After the cells were washed with PBS, they were centrifuged at 1000 rpm for 5 min. The process was repeated three times. The cells were then collected and resuspended in 100 μL phosphate buffer, incubated with 2.5 μL Annexin V-FITC and 5 μL PI (50μg/mL) for 5 min, and analyzed by flow cytometry.
Statistical Analysis
Relevant measurement data is expressed in mean differences ± standard deviation ( ) and analyzed using SPSS software. A T-test was used to assess the data. P < 0.05 was considered statistically significant.
) and analyzed using SPSS software. A T-test was used to assess the data. P < 0.05 was considered statistically significant.
Results
Concentration Determination of Tigecycline
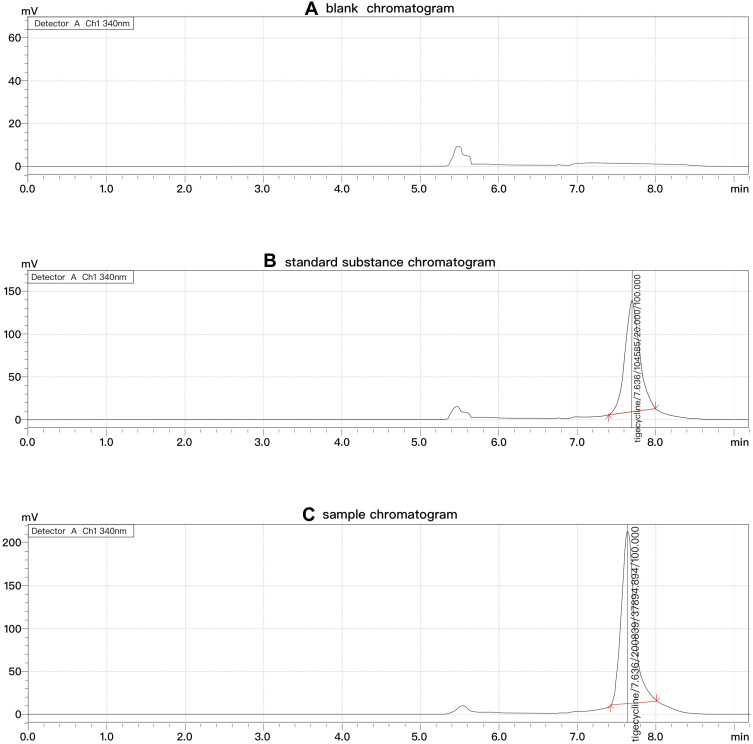
Tigecycline retention time was 7.636 min. In the present method, none of the endogenous matrix components in CSF seemed to interfere with the main peak of tigecycline. The chromatogram is shown in Figure 1.
Figure 1.
Chromatograms of tigecycline in 2D-HPLC (A-C).
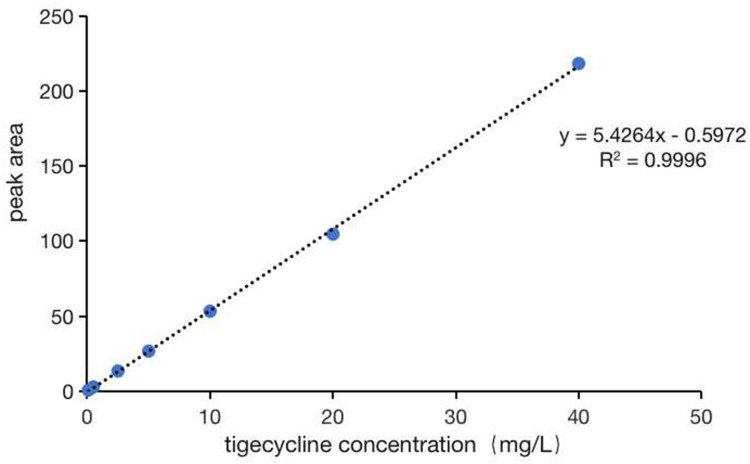
In our study, the linear concentration range of tigecycline was set at 0.1–40.0 mg/L, and the LLOQ was 0.1mg/L. The chromatogram is shown in Figure 2.
Figure 2.
The standard curve of verification result of tigecycline.
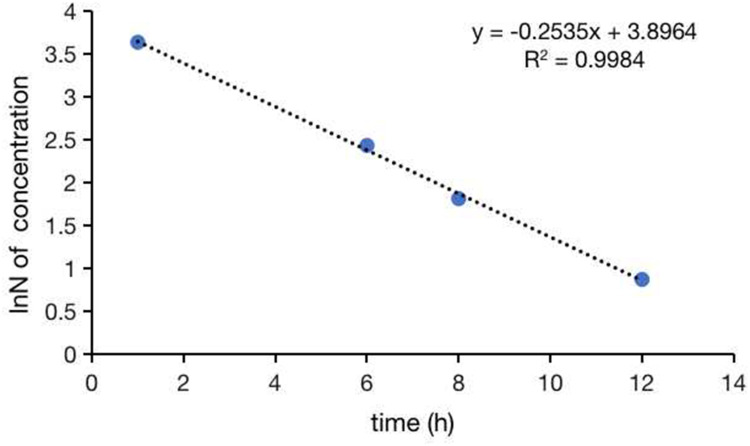
The concentrations of tigecycline in CSF collected 1, 6, 8, and 12 hours after intraventricular injection were 37.89, 11.36, 6.11, and 2.38 mg/L, respectively. The curve of tigecycline concentration exponential decay with time is shown in Figure 3. The t1/2 of tigecycline in CSF was estimated to be 2.73 hours.
Figure 3.
Drug exponential decay curve of tigecycline.
Antimicrobial Susceptibility
CSF culture was positive for XDR A. baumannii (Table 1), which was sensitive to minocycline and TMP/SMZ; intermediate tobramycin based on the Clinical and Laboratory Standards Institute (CLSI). (Table 1) The MIC50 and MIC90 values of tigecycline for A. baumannii were 2 and 4 mg/L performed by Mueller–Hinton broth microdilution, respectively.
Table 1.
Antibiotics Susceptibility Tests for A. baumannii in CSF
| Antibiotics | Susceptibility | MIC (mg/L) |
|---|---|---|
| Ticarcillin/Clavulanic Acid | Resistant | ≥128.0 |
| Piperacillin/Tazobactam | Resistant | ≥128.0 |
| Ceftazidime | Resistant | ≥64.0 |
| Cefoperazone/Sulbactam | Resistant | ≥64.0 |
| Trimethoprim/Sulfamethoxazole | Susceptible | ≤20.0 |
| Imipenem | Resistant | ≥16.0 |
| Meropenem | Resistant | ≥16.0 |
| Tobramycin | Intermediate | 8.0 |
| Cefepime | Resistant | ≥32.0 |
| Colistin | Susceptible | ≤0.5 |
Cytotoxic Effects of Tigecycline on PC12 Cells
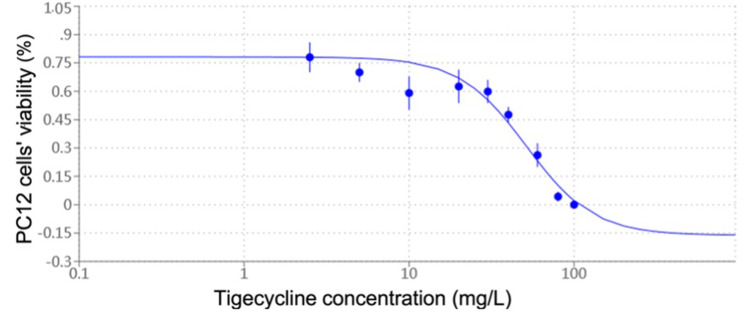
As shown in Figure 4, tigecycline induced a marked decrease in cell viability in a dose-dependent manner in PC12 cells in a certain concentration range. There is an obvious linear relationship between 20 and 100 mg/L of tigecycline. The IC50 value determined after 24 hours of cell treatment was about 51.35 mg/L.
Figure 4.
Cytotoxic effects of tigecycline on PC12 cells. It was expressed as percentages of control cells’ viability. Data are expressed as the mean ± SD of six independent experiments. Values are significantly different (p < 0.05) from control.
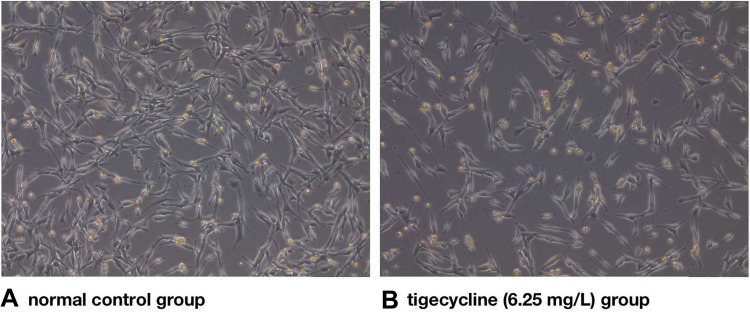
PC12 cells in the normal control group were in a sticky situation with a uniform morphology (Figure 5A). The number of cells decreased significantly in the tigecycline-treated group (Figure 5B) compared to the control group (Figure 5A). In contrast, tigecycline-treated cells had apoptosis and more floating cells. The volume became smaller and round, and the axons became shorter and smaller (Figure 5B).
Figure 5.
Effect of tigecycline on the change in PC12 morphology.
Notes: (A) Normal control group, (B) Tigecycline (6.25 mg/L) group.
As shown in Table 2, PC12 cells in the control group also had a certain proportion of apoptosis. After treatment with different concentrations of tigecycline for 24 hours, the apoptosis rate of PC12 cells increased to varying degrees, which was statistically significant compared with the control group (P < 0.05).
Table 2.
Changes in Apoptosis Rate (%) of PC12 Cells Induced by Tigecycline in Flow Cytometry via Annexin V-FITC Staining
| The Concentration of Tigecycline (mg/L) | The Apoptosis Rate of PC12 Cells (%,  ) ) |
|---|---|
| 0 (control group) | 2.27±0.26 |
| 10 | 2.72±0.14 |
| 20 | 4.48±0.13ab |
| 40 | 4.58±0.15 ab |
| 80 | 7.12±0.19 abcd |
| 160 | 12.59±0.43 abcde |
Notes: aCompared with the control group (P<0.05), b Compared with the 10 mg/L group (P<0.05), cCompared with the 20 mg/L group (P<0.05), dCompared with the 40 mg/L group (P<0.05), eCompared with the 80 mg/L group (P<0.05).
Discussion
Tigecycline is an important agent for carbapenem-resistant Gram-negative bacilli, especially for XDR A. baumannii. The treatment of XDR A. baumannii infection usually requires a combination of two antimicrobial agents or even three antimicrobial agents. According to the recommendations of the infectious diseases society of America and the European Society of clinical microbiology and infectious diseases (ESCMID), combination therapy including at least two in-vitro active antibiotics among the available antibiotics (polymyxin, aminoglycoside, tigecycline, sulbactam combinations) was suggested for patients with severe and high-risk carbapenem-resistant A. baumannii (CRAB) infections (conditional recommendation for use, very low certainty evidence).16,17 Some studies have shown that tigecycline-based therapy combined with sulbactam compound preparation, carbapenem antibiotics, and polymyxin B synergistically antibacterial effect on XDR A. baumannii.18,19 However, due to the limitation of the blood–brain barrier, intravenous tigecycline cannot be used to treat XDR A. baumannii intracranial infection. In the Consensus of Chinese Experts on Diagnosing and Treating Central Nervous System Infection in Neurosurgery (2021 Edition), intravenous combined with intrathecal or intraventricular injection is recommended to treat the nervous system infection caused by A. baumannii (strong recommendation for use, low certainty evidence). Polymyxin B 5mg per day for intrathecal injection is internationally recognized as the recommended dose. However, there is no internationally recognized recommended dose of tigecycline. To the consensus of Chinese experts, the recommended dose of tigecycline for intraventricular injection is 1 to 10 mg per 12 hours. Based on a few case reports, the evidence level of this dosing scheme is relatively low. The safety and effectiveness of the drug delivery scheme also lack sufficient and evidence-based evaluation. We proposed to analyze the feasibility of tigecycline intraventricular injection in treating A. baumannii intracranial infection and the safe and effective dosage and frequency of administration through a series of in vivo and in vitro studies.
The antibiotics’ efficacy is related to their PK/PD for time-dependent antibiotics. PD means that the concentration of antibiotics must exceed the MIC to inhibit bacterial growth or kill bacteria. PK means how long the antibiotics can maintain the concentration above the MIC after metabolism and excretion in the body. After administration, we performed TDM after a single intraventricular injection of 5mg tigecycline and took the samples at 1 h, 6 h, 8 h, and 12 h after administration. Since the drainage tube was clamped for 1 hour after injection, we can take the concentration 1 hour after administration as C0. Cmax is the concentration of C0, up to 37.894 mg/L. The concentration 12 hours later was 2.38 mg/L. In some previous antimicrobial activity studies, tigecycline MIC90s were < or = 2 mg/L for A. baumannii.20,21 But in our study, tigecycline MIC50/90 was 2/4 mg/L. It indicated that the sensitivity of tigecycline against XDR A. baumannii was decreased. After intraventricular injection of tigecycline 5mg for 12 hours, the drug concentration decreased between MIC50 and MIC90. Since the patient was transferred only twice an intraventricular injection, we did not measure the steady-state trough concentrations after multiple injections. In Wu’s study,12 the trough concentrations of tigecycline in CSF for the three different dosages of tigecycline intravenous injection–intraventricular injection (IV–ICV) combined administration were 2.886, 1.290, and 0.313 mg/L for 40 mg IV/10 mg ICV, 45 mg IV/5 mg ICV and 50 mg IV/1 mg ICV tigecycline, respectively. Our concentration measured 12 hours after intraventricular injection was similar to the trough concentrations of 45 mg IV/5 mg ICV and 40 mg IV/10 mg ICV. The half-life calculated from the exponential decay of tigecycline concentration was 2.73 hours. Some guidelines suggest that dosages and intervals of intraventricular antimicrobial therapy should be adjusted based on CSF antimicrobial concentrations to 10–20 times the MIC of the causative microorganism (strong, low).22 According to the above results, we considered that 5mg tigecycline for intraventricular injection was required at least twice a day to maintain the effective inhibitory concentration against XDR A. baumannii in CSF. Unfortunately, we did not collect CSF from the drainage bag to assess tigecycline loss through the drainage tube. In future research, we will continue to improve the data collection.
The damage of drugs to nerve cells is irreversible. The intraventricular injection can make the drug reach a high concentration in CSF. It is very necessary to study the neurotoxicity of tigecycline. PC12 cells are derived from a transplantable mouse pheochromocytoma and differentiated into neuroendocrine cells induced by nerve growth factor (NGF). It is a common nerve cell strain for the study of drug neurotoxicology. In the present study, we evaluated the action of different concentrations of tigecycline-induced cytotoxicity in cultured PC12 cells. Our results showed that exposure of PC12 cells to tigecycline leads to cell death with an IC50 of 51.35 mg/L, as shown by the CCK8 assay. Tigecycline induced a marked decrease in cell viability in a dose-dependent manner in PC12 cells. The cell viability decreased linearly, especially when the concentration was between 20 and 100 mg/L. And flow cytometry analysis showed that the apoptosis rate increased gradually with the increase of tigecycline concentration. Thus, tigecycline may have potential toxicity to nerve cells. Compared with the IC50 of nerve cells, the lower the peak concentration of tigecycline in CSF, the safer the intraventricular injection of tigecycline. The peak concentration of 5mg tigecycline intraventricular injection was 37.894 mg/L. We calculated that the apparent CSF distribution volume was about 132 mL, equivalent to the total amount of human CSF (130 ~ 150mL) under normal conditions. Therefore, we infer that the peak concentration of intraventricular injection can be estimated according to the single-dose and CSF volume quotient, which is very meaningful for us to study the maximum single dose of drug safety. Because the peak concentration is close to the IC50, we considered that 5mg might not be a safe enough dose for intraventricular injection. We may need to reduce the peak concentration by reducing a single dose to reduce neurotoxicity. Admittedly, the nervous system is complex and contains many cells that interact differently. One type of cell does not represent this network,23 and we need to study more types of nerve cells to determine the neurotoxicity of tigecycline.
In conclusion, intraventricular injection of tigecycline is a promising method for treating XDR A. baumannii intracranial infection. But the high concentration of tigecycline may have irreversible neurotoxic effects. We do not recommend tigecycline as the preferred agent for intraventricular injection in treating intracranial infection of XDR A. baumannii. If it is added as a last resort, giving small doses of less than 5 mg and more than twice a day may be safer and more effective. The intraventricular injection is best performed under TDM to ensure safety and effectiveness. In further research, we will investigate the neurotoxicity of tigecycline in more types of human nerve cells and obtain more data on individualized precision therapy by tigecycline TDM to provide more objective evidence for the treatment of XDR A. baumannii intracranial infection.
Funding Statement
This study was supported by the Health Commission of Hunan Province of China (No.202113011112).
Ethics Approval
The study was approved by the Ethics Committee of Hunan Provincial People’s Hospital (2022 Scientific Research Ethics Review NO: 15). All procedures performed in this study involving human participants followed the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The participant’s legal guardian has signed the informed consent before treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hussein K, Rabino G, Feder O., et al. Risk factors for meningitis in neurosurgical patients with cerebrospinal fluid drains: prospective observational cohort study. Acta Neurochir. 2019;161(3):517–524. doi: 10.1007/s00701-019-03801-y [DOI] [PubMed] [Google Scholar]
- 2.Long W, Yuan J, Liu J, et al. Multidrug Resistant Brain Abscess Due to Acinetobacter baumannii Ventriculitis Cleared by Intraventricular and Intravenous Tigecycline Therapy: a Case Report and Review of Literature. Front Neurol. 2018;9:518. doi: 10.3389/fneur.2018.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurdyumova NV, Danilov GV, Ershova ON. Features of Nosocomial Meningitis in Patients of a Neurosurgical Critical Care Unit. Zh Vopr Neirokhir Im N N Burdenko. 2015;79:55–59. doi: 10.17116/neiro201579347-51 [DOI] [PubMed] [Google Scholar]
- 4.Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi: 10.1007/s10096-019-03673-1 [DOI] [PubMed] [Google Scholar]
- 5.Cook AM, Robert KDM, Owen RD, Pesaturo AB, Hatton J. Intracerebroventricular Administration of Drugs. Pharmacotherapy. 2009;29:832–845. doi: 10.1592/phco.29.7.832 [DOI] [PubMed] [Google Scholar]
- 6.Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39:10–39. doi: 10.1002/phar.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauretti L, D’Alessandris QG, Fantoni M, et al. First reported case of intraventricular tigecycline for meningitis from extremely drug-resistant Acinetobacter baumannii. J Neurosurg. 2017;127(2):370–373. doi: 10.3171/2016.6.JNS16352 [DOI] [PubMed] [Google Scholar]
- 8.Deng ZW. A case report of intraventricular and intrathecal tigecycline infusions for an extensively drug-resistant intracranial Acinetobacter baumannii infection. Medicine. 2019;98:e15139. doi: 10.1097/MD.0000000000015139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Li -D-D, Yin B, et al. Successful treatment of pyogenic ventriculitis caused by extensively drug-resistant Acinetobacter baumannii with multi-route tigecycline: a case report. World J Clin Cases. 2021;9:651–658. doi: 10.12998/wjcc.v9.i3.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook AM, Robert KDM, Owen RD, Pesaturo AB, Hatton J. Pesaturo, Jimmi Hatton. Intracerebroventricular administration of drugs. Pharmacotherapy. 2009;29:832–845. doi: 10.1592/phco.29.7.832 [DOI] [PubMed] [Google Scholar]
- 11.Xing H, Cheng C, Zhang Y, et al. Successful Treatment With Intrathecal and Intravenous Polymyxin B-Based Combination Against MDR Acinetobacter baumannii Meningitis in Pediatric Patient: a Case Report. Front Pediatr. 2021;9:564991. doi: 10.3389/fped.2021.564991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Chen K, Zhao J, Wang Q, Zhou J. Intraventricular administration of tigecycline for the treatment of multidrug-resistant bacterial meningitis after craniotomy: a case report. J Chemother. 2018;30:49–52. doi: 10.1080/1120009X.2017.1338846 [DOI] [PubMed] [Google Scholar]
- 13.Romney Humphries AMB, Hindler JA, Schuetzd AN. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100. J Clin Microbiol. 2021;59:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters Version 12.0[EB/OL];2020. Available from: https://www.eucast.org/. Accessed August 17, 2022.
- 15.Chen J, Tang M, Liu M, et al. Neferine and lianzixin extracts have protective effects on undifferentiated caffeine-damaged PC12 cells. BMC Complement Med Ther. 2020;20(1):76. doi: 10.1186/s12906-020-2872-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamma PD. Infectious Diseases Society of America Guidance on the Treatment of AmpC beta-lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab1013 [DOI] [PubMed] [Google Scholar]
- 17.Paul M. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2021. doi: 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 18.Petersen PJ, Labthavikul P, Jones CH, Bradford PA. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemother. 2006;57(3):573–576. doi: 10.1093/jac/dki477 [DOI] [PubMed] [Google Scholar]
- 19.Sopirala MM, Mangino JE, Gebreyes WA, et al. Synergy Testing by Etest, Microdilution Checkerboard, and Time-Kill Methods for Pan-Drug-Resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:4678–4683. doi: 10.1128/AAC.00497-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sader HS, Jones RN, Dowzicky MJ, Fritsche TR. Antimicrobial activity of tigecycline tested against nosocomial bacterial pathogens from patients hospitalized in the intensive care unit. Diagn Microbiol Infect Dis. 2005;52(3):203–208. doi: 10.1016/j.diagmicrobio.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Waites KB, Duffy LB, Dowzicky MJ. Antimicrobial susceptibility among pathogens collected from hospitalized patients in the United States and in vitro activity of tigecycline, a new glycylcycline antimicrobial. Antimicrob Agents Chemother. 2006;50(10):3479–3484. doi: 10.1128/AAC.00210-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017;64(6):e34–e65. doi: 10.1093/cid/ciw861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annabi E, Ben salem I, Abid-Essefi S. Acetamiprid, a neonicotinoid insecticide, induced cytotoxicity and genotoxicity in PC12 cells. Toxicol Mech Methods. 2019;29(8):580–586. doi: 10.1080/15376516.2019.1624907 [DOI] [PubMed] [Google Scholar]