Abstract
Presbyopia may represent the largest segment of refractive errors that is without an established and effective refractive surgery treatment. Corneal Inlays are materials (synthetic or allogenic) implanted in the stroma of patients’ corneas to improve presbyopia. These inlays, introduced into the United States in 2015 via the small-aperture corneal inlay (KAMRATM, SightLife Surgical/CorneaGen, Seattle, Washington, United States), were met with an initial wave of enthusiasm. Subsequent models like the shape-changing corneal inlay (RAINDROPTM, Revision Optics, Lake Forest, California, United States) offered excellent results for patients, but longer-term research raised questions about patient safety. At the time of this article, no synthetic corneal inlays are available in the United States for the correction of presbyopia. Other options for presbyopia correction include allograft corneal inlays, trifocal synthetic corneal inlays, pharmacologic therapies, scleral incisions or additive techniques and PresbyLASIK. Presently, allograft inlays consist of corneal lenticules removed from patients undergoing Small Incision Lenticule Extraction (SMILE). We will review corneal inlays and other alternative procedures that may provide effective and predictable treatments for patients with presbyopia.
Keywords: corneal inlay, corneal onlay, SMILE, PEARL, PresbyLASIK, refractive surgery, KAMRA, raindrop, flexivue, corneal allografts, presbyopic allogenic refractive lenticule
Introduction
Presbyopia affects roughly 1.8 billion people worldwide, and its surgical or medical management is an important and growing field.1 With the advances in technology and surgical techniques, there is an increasing effort to develop safe and effective strategies for the surgical management of presbyopia that allows patients to be glasses-independent. Patients with myopia, hyperopia, and astigmatism have established treatments, while the treatment for presbyopia remains open for innovation. Corneal inlays represent one effort to bridge the care gap for patients with presbyopia.
This effort is not new, as Jose Ignacio Barraquer was interested in correcting refractive errors in the 1940s when most of his contemporaries felt ametropic eyes were healthy and should be supplemented with glasses. Barraquer felt glasses were nothing more than a prosthetic and that if an organ was not functioning to perfection, it should be improved.2 In 1949, he published his first preliminary paper about the correction of ametropia through modifying the curvature of the cornea.2 After Barraquer’s initial procedures of reshaping the cornea, others in the 1950s and 60s experimented with materials such as polysulfone, polypropylene and silicone oil for corneal implants, but these led to stromal necrosis and extrusion of the implant.3
Today’s corneal implants, made with polyvinylidene fluoride and carbon or hydroxyethyl methacrylate and methyl methacrylate with laser-etched pores, are significant advances from the materials of the 50s and 60s. Common synthetic corneal inlays include the small-aperture corneal inlay (KAMRA), shape-changing corneal inlay (Raindrop), and the refractive corneal inlay (Flexivue Mircolens). The small-aperture corneal inlay uses optics similar to a pinhole occluder which increases a patient’s depth of focus. The shape-changing corneal inlay increases the curvature of the cornea theoretically improving near and intermediate vision. The refractive corneal inlay contains a bifocal design with a central plano zone with no refractive power for distance and a peripheral positive refractive zone for near vision.4 However, the answer for presbyopia may not reside in these novel synthetic materials but in new techniques that use human allograft corneal inlays to improve vision.
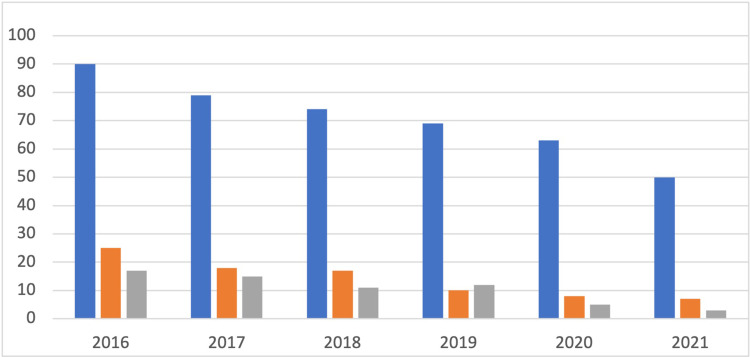
The surgical management of presbyopia has significantly evolved since being introduced by Barraquer.4 Corneal inlays have been experimented with since 1949 and represented a promising treatment for presbyopia due to advancements in biocompatible materials and femtosecond laser technology developed in the 1990s.5 Unfortunately, early complications related to corneal inlays used in the treatment of presbyopia caused them to fall out of favor in the last few years, and at the time of this article, no synthetic corneal inlays are available for the correction of presbyopia in the United States. This decrease in commercial activity was also reflected in the down-trending amount of research over the past few years (Figure 1). Nevertheless, the initial wave of optimism for corneal inlays has spurred innovation and research, affecting a significant and growing market. Synthetic inlays are still available in more than 50 countries around the world despite their falling out of favor in the US.6
Figure 1.
Published scientific literature concerning “Presbyopia” and “Corneal Inlay” based on a search of multiple data bases including Google Scholar (blue), Embase (Orange), and PubMed (grey). This is representative of the decrease in research published in multiple search engines on corneal inlays as a treatment modality for presbyopia since 2016.
Literature Search METHOD
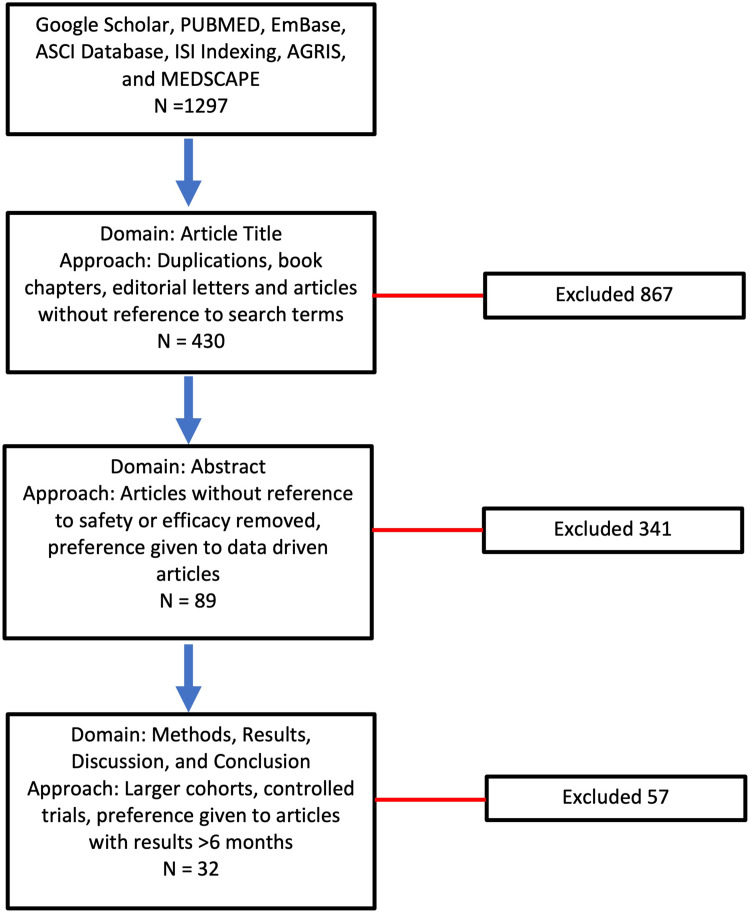
The algorithm for our literature search is shown in Figure 2. Our search parameters included the following words and phrases: Corneal Inlay, small-aperture corneal inlay, KAMRA, RAINDROP corneal inlay, PRESBIA FLEXIVUE, ALLOTEX, Presbyopic Allogenic Refractive Lenticule (PEARL), Corneal Inlay Complications, pilocarpine hydrochloride ophthalmic solution 1.25% (VUITYTM), Review of Corneal Inlays, and Corneal Onlay. After identifying 1297 articles on search engines, including Google Scholar, PUBMED, Embase, ASCI Database, ISI Indexing, AGRIS, and MEDSCAPE, duplications, book chapters, editorial letters, and articles without reference to search terms were excluded. After carefully assessing the abstracts of the remaining 430, articles without reference to safety or efficacy were excluded. At this stage, articles published after the year 2010 were also included. We then included the remaining 89 papers for full review and focused on reports with larger cohorts, controlled trials, and results of studies that were longer than 6 months in duration. For this review, 32 articles focused on corneal inlays were selected. A total of 51 articles are referenced in this paper, with the additional articles representing historical importance and alternative treatments for presbyopia.
Figure 2.
The step-by-step algorithm for performing our literature search is demonstrated in this image.
Synthetic Corneal Inlays
Newer biocompatible materials and refractive methods offer a promising approach to treat presbyopia. Synthetic corneal inlays were designed as a surgical treatment for presbyopia. These inlays are placed at varying depths of a patient’s stroma to improve near vision.4,7 The product design, surgical technique, efficacy, and safety are discussed for each type of synthetic corneal inlay. Table 1 summarizes the device mechanisms of action, materials, and dimensions.
Table 1.
| KAMRA | Flexivue | Raindrop | |
|---|---|---|---|
| Mechanism of Action | Small-aperture to increase a patient’s depth of focus | Central zone without refractive power and peripheral positive refractive zone for near vision | Increase the overlying corneal curvature thereby increasing refractive power |
| Material | Polyvinylidene Fluoride | Biocompatible hydrophilic acrylic material | Hydrogel |
| Dimensions | Thickness 6μm | Inner Hole Diameter 0.51mm | Thickness 32μm |
| Inner Diameter 1.6mm | Central Flat Zone Diameter 1.6mm | Diameter 2mm | |
| Outer Diameter 3.6mm | Total Diameter 3.2mm |
KAMRATM
Product Design and Surgical Techniques
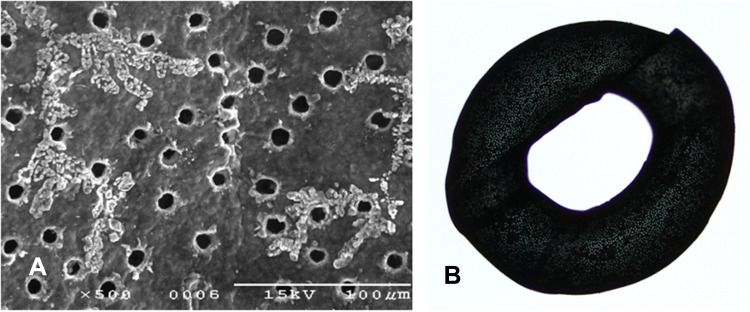
The small-aperture corneal inlay, KAMRA, the first corneal inlay to gain US Food and Drug Administration (FDA) approval in 2015, uses small-aperture optics to increase a patient’s depth of focus. Using a small-aperture inlay effectively blocks bending rays of light, minimizing refraction and improving near vision.4 The mechanism of action is similar to the pinhole technique used when evaluating a patient’s visual acuity. The current model features 8400 laser-etched holes that facilitate the passage of nutrients, aqueous, and oxygen through the cornea (Figure 3). The device is 3.8mm in diameter with a 1.6mm central opening. The device is only 6μm thick.8 As of 2022, KAMRA is no longer in production, but the manufacturer still supports patients with an implanted corneal inlay.
Figure 3.
Close-up image of a KAMRA inlay via scanning electron microscope illustrating amorphous material surrounding holes that were laser drilled into the inlay (A). Image of the KAMRA corneal inlay showing the 8400 laser-etched holes intended to facilitate passage of nutrients, aqueous, and oxygen (B).
The original model (ACI7000) was implanted under a corneal flap between 170–180 μm deep. However, preliminary studies highlighted concerns that a corneal inlay placed superficially was more likely to affect a patient’s uncorrected distance visual acuity (UDVA).9 A summary of the device properties and mechanism of action can be found in Table 1. Figure 4 shows a KAMRA Inlay implanted in a patient’s cornea.
Figure 4.
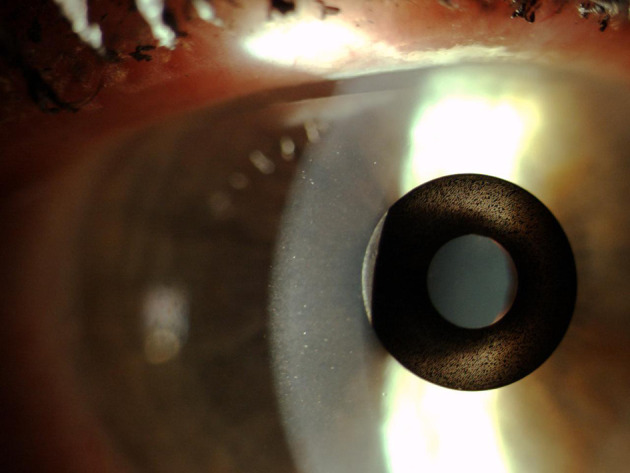
Slit-lamp photograph of KAMRA inlay placed in the stroma of a patient’s cornea. (Courtesy of Hoopes Vision).
Efficacy
In a longitudinal study of the first design, Yilmaz et al found that while uncorrected near visual acuity (UNVA) improved from a mean of J7 preoperatively to J1 in the treated eye, UDVA decreased by one line.
The 2nd model (ACI7000PDT) has 8400 laser-etched holes compared to 1600 in the 1st model, with each hole smaller in diameter. Additionally, a femtosecond laser is used to create a deeper corneal pocket of at least 220μm for the inlay. This subsequent model, placed at greater depth, is less likely to affect corneal topography and distance visual acuity than its predecessor.9
A recent systematic review using data from 2011–2018 including 18 articles and 2724 eyes found that 78.5% of eyes had an UNVA of 20/32 or better and 90.5% of eyes had an UDVA of 20/25 or better.10
Safety and Outcomes
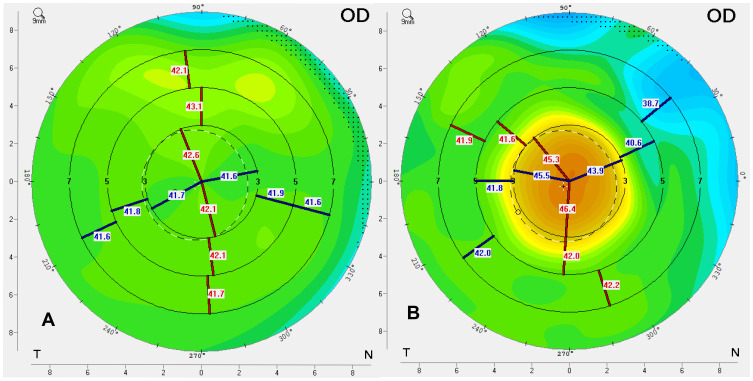
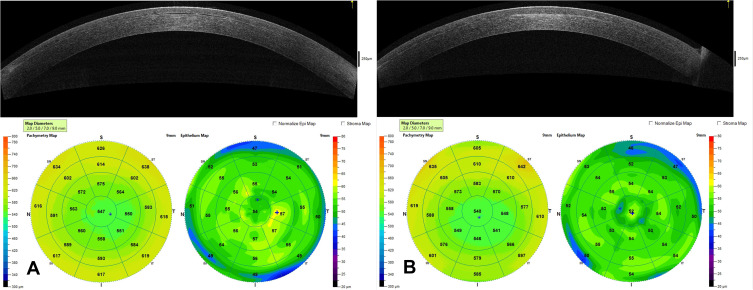
There are now over 20,000 KAMRA inlays currently implanted, and we have a greater understanding of the inlay’s impact as a treatment for presbyopia.4 Longitudinal research reviewing original FDA trials showed that 3.4% of patients with the KAMRA inlay experienced a compromise in CDVA of ≥ 2 lines lost at 24-months of follow-up.11,12 Overall, ~8.5% of the 508 inlays from the FDA trial were removed postoperatively with concerns ranging from cosmetic reasons to corneal haze. Figure 5 demonstrates corneal haze overlying a KAMRA inlay and within the inner ring of the inlay. Figure 6 displays persistent residual haze following explantation of the inlay. Follow-up surgical interventions were required in 11% of patients, which included explantation (8%), additional refractive surgeries, recentration, and lamellar rinse.12 Others experienced a hyperopic shift which was theorized to result from thickening of the stroma overlying the implant. A small minority of patients experienced keratitis, which required removal of the implant.13 Figure 7 demonstrates OCT of the cornea with the KAMRA inlay in place and after explantation of the inlay. Increased fibrosis and haze are apparent on the follow-up imaging after the inlay was explanted.
Figure 5.
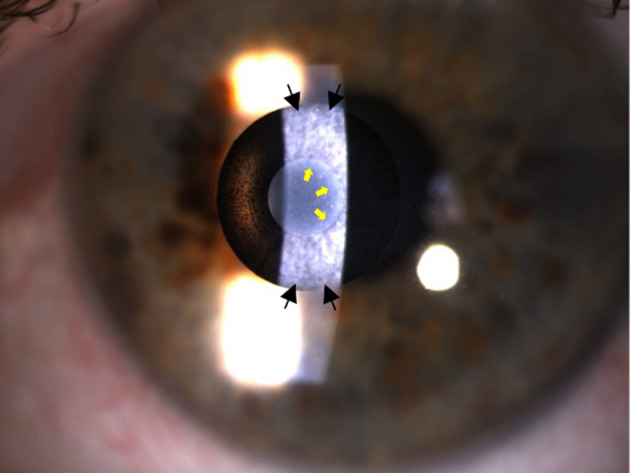
Slit lamp photographs 12-years after implantation showing KAMRA inlay with corneal haze. Black arrows: haze overlying the implant. Yellow Arrows: haze within the inner ring of the implant. (Courtesy of Hoopes Vision).
Figure 6.
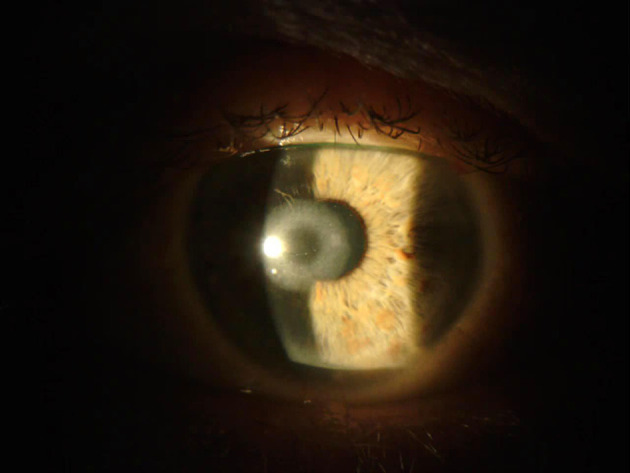
Persistent corneal haze 2-weeks after explantation of KAMRA inlay.
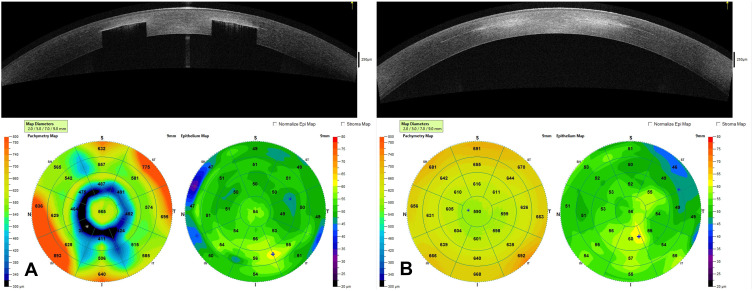
Figure 7.
This image contains an OCT of the cornea and the corneal topography of a patient with the KAMRA inlay in place 12-years after implantation (A) and an OCT of the cornea with corneal topography 1-month after explantation of the inlay (B). (Courtesy of Hoopes Vision).
A systematic review of KAMRA implants from 2011–2018 found that out of 2724 eyes, 101 eyes (3.7%) required explantation due to vision blurriness, development of epithelial microcysts, incorrect implant placement, or hyperopic shift changes.
Raindrop (Revision Optics, Lake Forest, California, United States)
Product Design and Surgical Technique
The product is a permeable hydrogel-based implant 2mm in diameter with a central thickness of 32μm. By placing the product in a pocket of corneal stroma in the non-dominant eye, the curvature of the cornea was slightly increased with the antecedent increase in power by a few diopters. The resulting hyperprolate cornea would then theoretically improve near and intermediate vision. The Raindrop itself has no actual refractive power. However, the induced hyperprolate shape and corneal remodeling creates a multifocal cornea (Figure 8).4 Designers hoped it would negligibly affect UDVA as the paracentral light rays around the implant are unaltered.7 A summary of the device properties and mechanism of action can be found in Table 1. As of 2018, the product is no longer available in the U.S.14
Figure 8.
Corneal topography before (A) and after (B) Raindrop inlay implantation, showing the intended significant increase in central corneal steepness. (Courtesy of Hoopes Vision).
Efficacy
Initial results with the shape-changing corneal inlay were excellent. Garza et al published the first peer-reviewed paper on the shape-changing inlay. Except for a single patient who had the inlay explanted over concerns for poor UDVA, 100% of patients were very satisfied with their distance vision, and 95% were very satisfied with their intermediate and near vision. Most patients from this study claimed they never relied on glasses over the 12-month follow-up period.4,15 A review of FDA clinical data on the shape-changing inlay revealed that 98% of patients with the inlay achieved near visual acuity of J5 or better, with 67% achieving J1 or better at 24 months.12
Safety and Outcomes
The Raindrop corneal inlay, after FDA approval in August of 2016, was recalled in 2018 over concerns of postoperative corneal haze. Our own initial experience based on a retrospective chart review on 8 patients with a Raindrop corneal inlay found that 50% of these patients required corneal inlay explantation. Prior to explantation, all 4 patients complained of blurry vision, and 3 of the 4 patients had a decrease of UDVA with an average of 4 lines lost. One patient lost one line of CDVA. 3 of the 4 patients had stromal haze following explantation.16 FDA data revealed that 12% of the 373 eyes required surgical interventions following implantation of the hydrogel-based implant. The explantation rate for this inlay was 7%, with the predominant causative factor being corneal haze and dissatisfaction with visual outcomes.12 Figure 9 displays a slit-lamp photograph of the Raindrop corneal inlay 2 years post-implantation with good centration but fibrosis and haze both centrally and along the periphery of the inlay. Corneal OCT images for the same patient after implantation and corneal OCT 4 months after explantation are shown in Figure 10.
Figure 9.
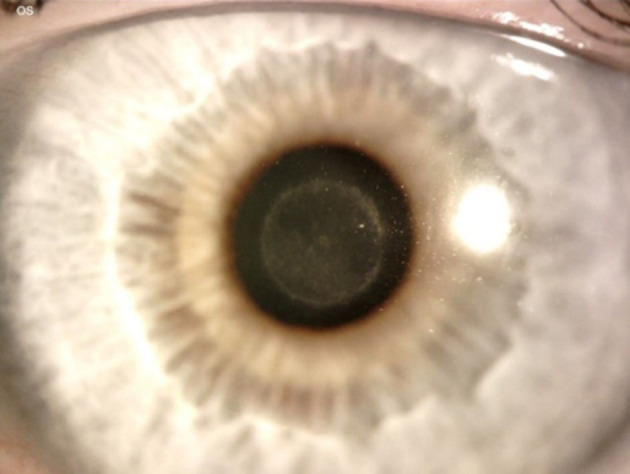
Slit lamp photograph 2 years post-implantation of the RAINDROP inlay showing good centration but with fibrosis and haze both centrally and around the peripheral edge of the inlay. (Courtesy of Hoopes Vision).
Figure 10.
This image contains a corneal OCT and corneal topography 9 months after implantation of Raindrop inlay showing normal appearance of inlay in corneal stroma (A). Corneal OCT and topography 4 months after explantation of the Raindrop inlay demonstrating significant residual haze at the former site of the inlay in the OCT scan and changes in topography (B). (Courtesy of Hoopes Vision).
The FDA released a safety communication to medical providers in 2018 after the 5-year follow-up of patients with the shape-changing corneal inlay revealed a corneal haze incidence of 42%. The produce was subsequently discontinued.14
Flexivue Microlens (Presbia, Irvine, California, United States)
Product Design and Surgical Technique
The Presbia Flexivue Microlens is a transparent refractive corneal inlay of hydroxyethyl methacrylate and methyl methacrylate.7 The device is 3.2mm in diameter with an inner hole diameter of 0.51mm. The central flat zone diameter is 1.6mm. This inlay contains a bifocal design with a central plano zone with no refractive power for distance and a peripheral positive refractive zone for near vision.7,17 This refractive corneal inlay alters the corneal index of refraction to improve near vision performance.4 Centration of the lens is guided by the line of sight (Purkinje Line). A pocket at a depth of 300μm is created with a femtosecond laser, and the lens is inserted with the manufacturer’s tool.17 A summary of the device properties and mechanism of action are in Table 1. The lens remains available in approximately 50 countries worldwide.
Efficacy
In a longitudinal study containing 30 eyes performed in Brazil with a Flexivue corneal inlay, UNVA improved to a mean of J1 in 87% of eyes at one year but decreased to 77% of eyes at 3 years. The number of patients satisfied with their vision decreased from 90% in the first year to 73% at a 3-year follow-up. They reported that UDVA remained stable throughout the study. A total of 8 patients had the device explanted. Four of those patients had a device exchange to improve near vision, and 4 did not undergo reimplantation of the corneal inlay. All 8 patients achieved 20/20 UDVA after corneal inlay explant.17
Safety and Outcomes
Compared to the KAMRA corneal inlay, studies show a more frequent loss of corrected distance visual acuity (CDVA) after implantation. Loss of at least one line in CDVA has been reported as high as 60%, with loss of greater than 3 lines in CDVA in ~10% of eyes.17,18 This device is still commercially available in the Middle East, Europe, and parts of Latin America; however, the company scaled back its efforts in the US in 2019 and is not USFDA approved for clinical use.19,20
Pathophysiology Underlying Complications Associated with Synthetic Corneal Inlays
Because synthetic corneal inlays are a foreign body, some degree of host response may be expected. Unfortunately, the pathway for corneal haze following implantation of corneal inlays has yet to be elucidated. Several suggested theories include inadequate nutrient transfer following implantation, chronic mechanical irritation of keratocytes, and a foreign body response to the inlay material.14 One study found that immune/inflammatory-mediator proteins in the tears of those with corneal haze were significantly different from those without corneal haze. In a study of 34 eyes implanted with the shape-changing corneal inlay (Raindrop), 17 with corneal haze and 17 control eyes, researchers using tear proteomics discovered that 8 proteins were significantly downregulated and one protein significantly upregulated in the eyes with haze compared to controls. These proteins were from several pathways, including protein and energy metabolism, immune functions, wound healing, and platelet degradation.
In patients with the KAMRA corneal inlay, the pathway for corneal haze remains unclear. However, some histopathological findings in a case series provide insight into the pathogenesis. When inlays were examined under a microscope, researchers found thin, acellular fibrous membranes overlaying the pores of the corneal inlay. Chronic inflammatory cells were found on the surface of the inlays, suggesting chronic inflammation in response to the inlay played a role.21
In patients with the Presbia Flexivue Microlens, limited information is available on the pathophysiology underlying patient complications such as corneal necrosis, keratitis, and others. More histopathologic studies are required to understand these pathways and improve future options for overcoming these obstacles to patient care.22
As the pathway for corneal haze is clarified, targeted therapeutics in the future may reduce the risk of corneal haze with reduced side effects when compared to current regimens of steroids and drugs like mitomycin C.14
Corneal Onlays
Based on Barraquer’s original corneal techniques, epikeratophakia (or epikeratoplasty) was introduced in 1980.23 The procedure consists of a lamellar disc from a donor cornea which is sutured into a de-epithelialized groove in the recipient cornea. This procedure has fallen out of favor because of poor predictability and visual outcomes. Compared to donor tissue, synthetic tissue could be shaped more precisely and mass-produced, which may overcome some of the issues with epikeratophakia.24 Corneal onlays garnered some attention for the correction of presbyopia; however, none are currently used in the USA to treat presbyopia. These devices are implanted underneath the epithelium of the cornea as opposed to corneal inlays which are implanted in the stroma.25 Various materials, including human lenticules and synthetic materials, have been used for corneal onlay research.26 At this time, there is limited information about the safety and efficacy of these devices in humans. However, with an even more minimally invasive approach than corneal inlays, these devices may play a role in the future treatment of presbyopia. Because corneal inlays saw improved outcomes when implanted deeper in the stroma and farther from the surface epithelium, corneal onlays may face an increased rate of wound healing complications and scar formation compared to inlays placed in the stroma.
PresbyLASIK
PresbyLASIK, utilizing excimer laser technology, is another form of refractive surgery for the treatment of presbyopia. PresbyLASIK aims to shape the cornea using a multifocal profile with multi-spheric ablation that allows the patient an acceptable depth of focus for distance, intermediate, and near vision.27 There are currently three dominant methods of PresbyLASIK: Peripheral presbyLASIK (VISX STAR S4TM, VISX, Santa Clara, CA, USA), where the center of the cornea is shaped for distance vision and the periphery is reserved for near vision, central presbyLASIK (AMO Development LLC, Milpitas, CA, USA) where the center of the cornea is shaped for near vision and the periphery is reserved for far vision, and the multifocal transition profile where a transitional vertical multifocal ablation is created to de-center a hyperopic ablation profile.28 The multifocal transition profile using Gaussian excimer laser (SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany) has largely fallen out of favor as surgeons felt it consistently produced a vertical coma.28
There are few clinical trials on presbyLASIK, and future randomized controlled trials are required to produce more robust evidence.27 However, some recent clinical trials offer promising results.
In a recent study comparing femto-presbyLASIK to regular myopic LASIK correction, researchers found that presbyLASIK was as safe and effective as regular LASIK myopia correction and could be recommended to treat presbyopia. Researchers in the study used PresbyLASIK Supracor treatment (B&L Technolas, Munich, Germany).29
In a study of 138 eyes, patients treated with central presbyopic LASIK were followed for one-year and visual outcomes were excellent with a mean UDVA of 20/20 and a mean UNVA of J2. At 6-months postoperatively, 100% of patients said they would recommend the treatment.30 There is current optimism in the field that using this excimer laser to create a multifocal cornea may create an optimized multifocal cornea as the treatment of presbyopia.31
New Developments in the Treatment of Presbyopia
Synthetic corneal inlays over the past decade were met with an initial wave of optimism which has since declined after longitudinal studies showed decreases in postoperative best correct distance visual acuity and postoperative complications like corneal haze.21 The frequency of repeat surgical interventions and long-term complications ultimately stalled the use of corneal inlays in the US However, the principles of synthetic corneal inlays can be applied to human allograft materials and likely with a reduced foreign body response. Human allograft corneal inlays in conjunction with improved eye banking, 3D tissue printing, topography-guided excimer lasers, predictable precision provided by femtosecond laser technology, and potentially other newer technologies may unlock new treatment avenues for patients with presbyopia that allow for spectacle-independence.32–35 New dynamic surgical treatments that allow for customization and flexibility of the treatment after placement may improve patient satisfaction and provider accuracy in regards to visual quality.36 This customization of treatment may provide improved outcomes and reduced complications. However, there remain stumbling blocks that human allograft corneal inlays would need to conquer to become an effective treatment for presbyopia.
Allograft Corneal Inlays
Product Design and Surgical Technique
Two types of allograft corneal inlays are currently available. The TransFormTM Corneal Allograft (TCA) (Allotex, Boston, Massachusetts, United States) is currently pursuing FDA approval in the US The second type consists of a presbyopic allogenic refractive lenticule (PEARL).37–39 Both corneal inlays use small-incision lenticule extraction (SMILE) to harvest the lenticules.37,39
The TCA lenticules undergo a process of sterilization using electron beam radiation and shaping using an excimer laser. After shaping, the final dimensions of the lenticule are 2–3.5mm in diameter, with a central thickness of 15–25μm. These lenticules are acellular, further reducing their antigenicity and risk of tissue rejection.37 In a one-year clinical trial on 28 eyes, Tanriverdi et al described the implantation of allograft corneal inlays for hyperopia and presbyopia. Researchers created a flap in the corneal stroma using a femtosecond laser and inlays were centered on the pupillary axis.40
In addition to the TCA lenticules, harvested lenticules from SMILE procedures have been implanted. SMILE, approved in 2016 for the surgical management of refractive errors, removes a corneal lenticule that can be preserved and transplanted into a recipient eye (either the donor’s other eye or another patient) to correct refractive errors.41 Jacob et al created a pocket 120μm deep in the corneal stroma using a femtosecond laser with a diameter of 7.9mm. A central pocket was then dissected, and the PEARL inlay was implanted. Centration was achieved simply by pressing the inlay down, sliding it to the desired position and verified with an Orbscan (Bausch + Lomb, Bridgewater, New Jersey, United States).39 A summary of these devices, their properties, and mechanisms of action can be found in Table 2.
Table 2.
| Trifocal Corneal Inlay | Allograft Transform Corneal Inlay | Presbyopic Refractive Lenticules | |
|---|---|---|---|
| Mechanism of Action | Combination of small-aperture optics and photon sieve | Shape-changing corneal inlay | Shape-changing corneal inlay |
| Material | Hydrogel-based transparent biocompatible pure phase material | Human allograft corneal tissue | Human allograft corneal tissue |
| Dimensions | Inner Hole Diameter 1.4mm | Total diameter 2–3.5mm | Total diameter 1mm |
| Total Diameter 4.2mm |
Efficacy
Kilic et al reported on 12 presbyopic emmetropic patients who underwent implantation of the TCA inlay. The mean pre-operative UNVA (logMAR) in the treatment group was 0.52 ± 0.14 preoperatively which improved to 0.10 ± 0.06 at a 3-month follow-up. All eyes had a UNVA of 0.20 after a 6-month follow-up. With respect to best corrected distance visual acuity (BCVA), 5 eyes remained unchanged, 4 eyes lost 1 line of BCVA, and 1 eye lost 2 lines of BCVA. No patient lost more than 2 lines.38
In a 1-year clinical trial for patients with hyperopia and presbyopia, Tanriverdi et al reported visual outcomes in 16 patients (28 eyes) with the TCA inlay. Results showed that manifest refraction spherical equivalent (MRSE) decreased from 3.6 D to 0.21 D.40
Additionally, Jacob et al reported promising outcomes with UNVA of J2 in all patients and a UDVA of 20/20 postoperatively after placing presbyopic refractive lenticule inlays.39 Preliminary data suggest that non-synthetic allograft inlays are a safe and effective alternative to their synthetic counterparts.38,39 Lenticules from SMILE have already been implanted in patients with hyperopia, keratoconus, and astigmatism.40,41
Complications
Preliminary results for allograft corneal inlays are promising, but corneal rejection remains a potential risk. Over the past decade, lenticules have been used for conditions such as keratoconus and hyperopia with a relatively low incidence of complications.42,43 TCA inlays show a relatively excellent safety profile. Their reports do not mention lamellar keratitis, epithelial ingrowth, decentralization, corneal necrosis, or other complications. The absence of complications may result from a smaller sample size and shorter follow-up period.38,40
Current studies on presbyopic refractive lenticule inlays by Dr. Soosan Jacobs with a follow-up of 6-months have not reported tissue rejection or explanted lenticules.39 Again, these results may be limited by smaller sample sizes with shorter follow-up times when compared to synthetic corneal inlays.
The use of Allograft corneal inlays for the correction of presbyopia is a natural progression of the use of SMILE lenticules. Nonetheless, more longitudinal results are required to evaluate long-term complications of allograft corneal inlays. Preliminary results suggest a lower likelihood of postoperative complications when compared to synthetic corneal inlays.39 Still, this non-synthetic corneal inlay approach may face a similar challenge of centration that synthetic corneal inlays faced. The pupil is a dynamic aperture that changes in scotopic and mesopic environments. There are already reports of repeat surgeries required to center implants, and the technique for centering implants, while described in the literature, may have poor inter-surgeon consistency. A standardized approach to centration may reduce the likelihood of decentration for patients with allograft corneal inlays.
Additionally, we have yet to deduce the optimal profile for corneal inlays and how they can be individualized for patients with varying degrees of refractive errors. For example, how would the allograft corneal inlay be shaped for a patient with high myopia and presbyopia compared to a patient with mild hyperopia and presbyopia? Insight into this customization and optimization is essential for quality outcomes in patient care.
Trifocal Corneal Inlays
Product Design and Surgical Technique
Trifocal intraocular lenses are a well-established (albeit premium) IOL typically used for patients following cataract removal.44 However, to our knowledge, corneal inlays are yet to venture into the trifocal design. In their 2021 paper, Furlan et al proposed the first trifocal corneal inlay (TCI) design. This device was evaluated based on computer models and analysis, not in vivo cornea tissue models or live animals. The trifocal is intended to be implanted in the corneal stroma of the non-dominant eye, similar to other corneal inlays. The product is made of transparent biocompatible pure phase material with drilled micro-holes. This is significant as Furlan et al previously proposed a diffractive corneal inlay which was a bifocal design. Unfortunately, that design had a low light throughput due to its relatively larger opaque area. This trifocal corneal inlay with completely transparent material rectifies the decrease in light that previous bifocal models had.45 The total area of the lens is 5.5mm2 compared to the 8.0mm2 and 9.3mm2 of the Flexivue and KAMRA lenses.44 A summary of the device properties and mechanism of action can be found in Table 2.
Efficacy
Using the Liou-Brennan model eye, the authors employed Zemax OpticStudio to analyze their trifocal corneal inlay compared to other corneal inlays. They demonstrated that their TCI led to good visual performance at intermediate distances, with comparable visual acuity at near and far distances to lenses that are currently commercially available. As the design is still experimental, more proof-of-concept investigation and clinical trials will be necessary. An important factor of this design will be whether or not the device can be implanted without compromising distance visual acuity (DVA). The authors’ initial analysis suggests it does not compromise DVA.44 In a follow up analysis of their TCI, they evaluated how spherical aberration effects visual results. They found that spherical aberration extends the focal depth of the intermediate focus which may allow for customized focal distribution that fits the needs of each patient.46
Safety and Outcomes
The study did not discuss general safety information as the authors have yet to implant the device. However, the dramatically decreased total area of the lens combined with the micro-drilled holes and advances in biocompatible material may mitigate the host reaction to the material.44 Since the material for the device is not native corneal tissue, the concern remains that it may lead to a foreign body reaction causing corneal haze or other adverse reactions similar to the TCI’s bifocal counterparts. While the TCI is still in the experimental stages, it offers an interesting technology that may improve how we treat presbyopia in the coming years.
Other Alternative Options
Pharmacologic Therapies
At the time of this article, there is only one FDA-approved eyedrop for the treatment of presbyopia, with another on the horizon. The FDA approved pilocarpine hydrochloride ophthalmic solution 1.25% (VUITYTM) (Allergan, Dublin, Ireland) for the treatment of presbyopia in 2021. In 2 randomized double-blind trials with over 700 patients, approximately 30% of patients gained 3 or more lines of binocular distance corrected near visual acuity, and no patient lost even a single line of corrected distance visual acuity. The most common side effects were headaches and conjunctival hyperemia.47 Another rare but serious side effect was retinal detachment which was noted in two patients shortly after beginning the use of pilocarpine eye drops for the treatment of presbyopia.48 Other pharmaceutical options such as pilocarpine 2% ophthalmic spray (EyeNoviaTM New York, New York, USA), pilocarpine drop with undisclosed concentration (OrasisTM Ponte Vedra, Florida, USA) carbachol/brimonidine tartrate drops (BRIMOCHOLTM Seattle, Washington, USA) and others are currently under research and without FDA approval at the time of this article.49 Pharmaceuticals may be a powerful treatment for patients with presbyopia and have limited side effect profiles in the future. Additionally, patient adoption of this treatment may be easier than surgical techniques as they are less invasive.
Scleral Surgery
Scleral surgery for the correction of presbyopia has historically played a smaller role in the correction of presbyopia than corneal or lens procedures. Scleral laser excision procedures for the treatment of presbyopia was first introduced in 1998 via laser radial sclerectomy. 61 eyes in Argentina were treated via laser radial sclerectomy and 90% of eyes were J2 or better at a 6-month follow up and patients experienced a subjective 2.00 D accommodative shift. However, this procedure is no longer available.50 While a detailed discussion of scleral procedures for presbyopia is beyond the scope of this article, it is worth noting that scleral laser anterior ciliary excision (LaserACE, Ace Vision Group, Newark, CA, USA) has recently completed phase 3 clinical trials for the treatment of presbyopia. Scleral laser anterior ciliary excision is performed by making radial incisions in 3 zones of the sclera near the ciliary muscle to increase the plasticity and compliance during contraction of the ciliary muscle.51 The VisAbility Micro-Insert scleral implant (Refocus Group, Dallas, Texas, United States) is also currently undergoing clinical trials. The scleral implant uses four injection-molded implants about the size of a grain of rice placed at a depth of about 400μm within the sclera and aims to lift the sclera and tighten the zonular fibers holding the lens. More data is required before interpreting the long-term outcomes in these patients.51
Conclusion
The development of corneal inlays is an important building block for future presbyopia treatment. The KAMRA corneal inlay, produced consistent results and was the most common corneal inlay used in the United States. Unfortunately, patients experienced a relatively high rate of > 2 lines of CDVA loss over time.10,12 The Raindrop corneal inlay had high patient satisfaction rates but left patients with corneal haze that did not resolve even after explantation of the device.4,15 The Flexivue Microlens corneal inlay did not receive FDA approval for use in the US. However, the device had relatively high rates of satisfaction and patients consistently had good near vision results at one-year. However, those results declined at 3-year follow ups.17 Even if the use of synthetic inlays has declined over the past few years because of patient safety concerns, future treatments such as allograft corneal inlays, trifocal inlays, presbyLASIK, pharmacologic therapies and others have undoubtedly benefited from the groundwork provided by the invention of the different corneal inlays. PresbyLASIK using an excimer laser, is an exciting new technology that may allow us to produced highly customized care to a variety of patients with presbyopia and other refractive errors. However, there is a still a wide range of reported spectacle independence following this procedure and more long-term follow-up studies are needed to assess its long-term efficacy.28 Allograft corneal inlays may offer the excellent results of synthetic corneal inlays, with increased sample sizes and further follow-up needed to understand more about patient safety and long-term efficacy. Additionally, the process for harvesting lenticules and curating the correct implant takes longer and is rather costly.40 While trifocal corneal inlays may allow for improved visual quality at near, intermediate, and distance, these inlays face similar concerns that past synthetic corneal inlays faced such as reaction to a foreign body and epithelial remodeling that does not reverse with removal of the implant.44 Pharmacologic therapies such as pilocarpine drops are inexpensive, non-invasive, and effective. However, they come with complications such as headache, reduced distance vision especially at night, and as noted earlier have rare associated complications such as retinal detachments. Future treatment for presbyopia will likely feature allogenic tissue implants, newer biocompatible materials, new lens designs, scleral techniques, presbyLASIK, pharmaceuticals with minimal side effects or some combination of these techniques that increase spectacle-free opportunities for patients.
Acknowledgments
Rachel Huynh, James Ellis, Matt Conley.
Funding Statement
No funding was received for this article.
Abbreviations
PEARL, PrEsbyopic Allogenic Refractive Lenticule; FDA, Food and Drug Administration; UDVA, Uncorrected Distance Visual Acuity; UNVA, Uncorrected Near Visual Acuity; US, United States; CDVA, Corrected Distance Visual Acuity; SMILE, Small-Incision Lenticule Extraction; LASIK, Laser Assisted In-Situ Keratomileusis; TCI, Trifocal Corneal Inlay; DVA, Distance Visual Acuity.
Disclosure
The authors have no competing interests to report.
References
- 1.Katz JA, Karpecki PM, Dorca A, et al. Presbyopia - A review of current treatment options and emerging therapies. Clin Ophthalmol. 2021;15:2167–2178. doi: 10.2147/OPTH.S259011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barraquer JI. Modification of refraction by means of intracorneal inclusions. Int Ophthalmol Clin. 1966;6(1):53–78. doi: 10.1097/00004397-196606010-00004 [DOI] [PubMed] [Google Scholar]
- 3.Lane SL, Lindstrom RL, Cameron JD, et al. Polysulfone corneal lenses. J Cataract Refract Surg. 1986;12(1):50–60. doi: 10.1016/S0886-3350(86)80057-8 [DOI] [PubMed] [Google Scholar]
- 4.Moarefi A, Shamik M, Wiley BW. A review of presbyopia treatment with corneal inlays. Ophthalmol Ther. 2017;6(1):55–65. doi: 10.1007/s40123-017-0085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aristeidou A, Taniguchi EV, Tsatsos M, et al. The evolution of corneal and refractive surgery with the femtosecond laser. Eye Vis. 2015;2(2):12. doi: 10.1186/s40662-015-0022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-González JM, Borroni D, Rachwani-Anil R, Rocha-de-Lossada C. Refractive corneal inlay implantation outcomes: a preliminary systematic review. Int Ophthalmol. 2022;42(2):713–722. doi: 10.1007/S10792-021-02024-4 [DOI] [PubMed] [Google Scholar]
- 7.Fenner BJ, Moriyama AS, Mehta JS. Inlays and the cornea. Exp Eye Res. 2021;205:108474. doi: 10.1016/J.EXER.2021.108474 [DOI] [PubMed] [Google Scholar]
- 8.Sane MFB, Birdsong O, Reddy V. Corneal Inlays. EyeWiki; 2021. Available from: https://eyewiki.aao.org/Corneal_Inlays#Corneal_inlays. Accessed February 18, 2022.
- 9.Naroo SA, Bilkhu PS. Clinical utility of the KAMRA corneal inlay. Clin Ophthalmol. 2016;10:913–919. doi: 10.2147/OPTH.S89132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pluma-Jaramago I, Rocha-de-Lossada C, Rachwani-Anil R, et al. Small-aperture intracorneal inlay implantation in emmetropic presbyopic patients: a systematic review. Eye. 2022. doi: 10.1038/s41433-022-02032-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dexl AK, Jell G, Strohmaier C, et al. Long-term outcomes after monocular corneal inlay implantation for the surgical compensation of presbyopia. J Cataract Refract Surg. 2015;41(3):566–575. doi: 10.1016/J.JCRS.2014.05.051 [DOI] [PubMed] [Google Scholar]
- 12.Moshirfar M, Desautels JD, Wallace RT, et al. Comparison of FDA safety and efficacy data for KAMRA and Raindrop corneal inlays. Int J Ophthalmol. 2017;10(9):1446–1451. doi: 10.18240/ijo.2017.09.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwood M, Bafna S, Thompson V. Surgical correction of presbyopia: lenticular, corneal, and scleral approaches. Int Ophthalmol Clin. 2016;56(3):149–166. doi: 10.1097/IIO.0000000000000124 [DOI] [PubMed] [Google Scholar]
- 14.Fenner BJ, Liu YC, Koh SK, et al. Mediators of corneal haze following implantation of presbyopic corneal inlays. Invest Ophthalmol Vis Sci. 2019;60(4):868–876. doi: 10.1167/IOVS.18-25761 [DOI] [PubMed] [Google Scholar]
- 15.Garza EB, Gomez S, Chayet A, et al. One-year safety and efficacy results of a hydrogel inlay to improve near vision in patients with emmetropic presbyopia. J Refract Surg. 2013;29(3):166–172. doi: 10.3928/1081597X-20130129-01 [DOI] [PubMed] [Google Scholar]
- 16.Moshirfar M, Skanchy DF, Rosen DB, et al. Visual prognosis after explantation of small-aperture corneal inlays in presbyopic eyes: a case series. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):129–133. [PMC free article] [PubMed] [Google Scholar]
- 17.Beer SMC, Werner L, Nakaon EM, et al. Clinical science A 3-year follow-up study of a new corneal inlay: clinical results and outcomes. Br J Ophthalmol. 2020;104(5):723–728. doi: 10.1136/bjophthalmol-2019-314314 [DOI] [PubMed] [Google Scholar]
- 18.Han G, Lim DH, Yang CM, et al. Refractive corneal inlay for presbyopia in emmetropic patients in Asia: 6-month clinical outcomes. BMC Ophthalmol. 2019;19(1):1–12. doi: 10.1186/S12886-019-1069-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perriello B Presbia abandons Flexivue microlens, lays off all but 3 – massDevice; 2019. Available from: https://www.massdevice.com/presbia-abandons-flexivue-microlens-lays-off-all-but-3/. Accessed February 22, 2022.
- 20.Presbia. Presbia’s flexivue microlens® receives approval for patient implantation in Brazil; 2011. Available from: https://presbia.com/media/press-brazil-registration-approval-and-distributor-agreement.pdf2011. Accessed February 22, 2022.
- 21.Ong HS, Chan AS, Yau CW, et al. Corneal inlays for presbyopia explanted due to corneal haze. J Refract Surg. 2018;34(5):357–360. doi: 10.3928/1081597X-20180308-01 [DOI] [PubMed] [Google Scholar]
- 22.Duignan ES, Farrell S, Treacy MP, et al. Corneal inlay implantation complicated by infectious keratitis. Br J Ophthalmol. 2016;100(2):269–273. doi: 10.1136/bjophthalmol-2015-306641 [DOI] [PubMed] [Google Scholar]
- 23.Azar DT. Corneal Inlays. In: Gatinel D, Hoang-Xuan T, editors. Refractive Surgery. 2nd ed. Mosby; 2006. [Google Scholar]
- 24.American Academy of Ophthalmology. Basic and clinical science course. chapter 4: onlays and inlays; 2020. Available from: https://www.aao.org/bcscsnippetdetail.aspx?id=9e64e294-28e5-4eed-b91a-17940e70deb9. Accessed April 12, 2022.
- 25.Evans MD, Xie RZ, Fabbri M, et al. Progress in the development of a synthetic corneal onlay. Invest Ophthalmol Vis Sci. 2002;43(10):3196–3201. [PubMed] [Google Scholar]
- 26.Oelker AM, Grinstaff MW. Synthesis, characterization, and in vitro evaluation of a hydrogel-based corneal onlay. IEEE Trans Nanobioscience. 2012;11(1):37–45. doi: 10.1109/TNB.2011.2166978 [DOI] [PubMed] [Google Scholar]
- 27.Fernández J, Molina-Martín A, Rocha-de-Lossada C, Rodríguez-Vallejo M, Piñero DP. Clinical outcomes of presbyopia correction with the latest techniques of presbyLASIK: a systematic review. Eye. 2022;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vargas-Fragoso V, Alió JL. Corneal compensation of presbyopia: PresbyLASIK: an updated review. Eye Vis. 2017;4:11. doi: 10.1186/s40662-017-0075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajic B, Cvejic Z, Massa H, et al. Laser vision correction for regular myopia and supracor presbyopia: a comparison study. Appl Sci. 2020;10:873. doi: 10.3390/app10030873 [DOI] [Google Scholar]
- 30.Wang Yin GH, McAlinden C, Pieri E, et al. Surgical treatment of presbyopia with central presbyopic keratomileusis: one-year results. J Cataract Refract Surg. 2016;42(10):1415–1423. doi: 10.1016/j.jcrs.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 31.Tamayo GE Consider latest excimer laser techniques for presbyopia; 2016. Available from: https://www.ophthalmologytimes.com/view/consider-latest-excimer-laser-techniques-presbyopia. Accessed February 18, 2022.
- 32.Sommer AC, Blumenthal EZ. Implementations of 3D printing in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1815–1822. doi: 10.1007/s00417-019-04312-3 [DOI] [PubMed] [Google Scholar]
- 33.Moshirfar M, Odayar VS, McCabe SE, Ronquillo YC. Corneal donation: current guidelines and future direction. Clin Ophthalmol. 2021;15:2963–2973. doi: 10.2147/OPTH.S284617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiwari NN, Sachdev GS, Ramamurthy S, Dandapani R. Comparative analysis of visual outcomes and ocular aberrations following wavefront optimized and topography-guided customized femtosecond laser. Indian J Ophthalmol. 2018;66(11):1558–1561. doi: 10.4103/ijo.IJO_507_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercer RN, Milliken CM, Waring GO, Rocha KM. Future trends in presbyopia correction. J Refract Surg. 2021;37(S1):S28–S34. doi: 10.3928/1081597X-20210408-06 [DOI] [PubMed] [Google Scholar]
- 36.Charman WN. Developments in the correction of presbyopia II: surgical approaches. Ophthalmic Physiol Opt. 2014;34(4):397–426. doi: 10.1111/opo.12129 [DOI] [PubMed] [Google Scholar]
- 37.ALLOTEX. TransForm allograft lenticule product design and manufacturing process; 2019. Available from: https://allotex.com/science/clinical-data/. Accessed February 18, 2022.
- 38.Kılıç A, Tabakcı BN, Özbek M, Muller D, Mrochen M. Excimer laser shaped allograft corneal inlays for presbyopia: initial clinical results of a pilot study. J Clin Exp Ophthalmol. 2019;10. doi: 10.4172/2155-9570.1000799 [DOI] [Google Scholar]
- 39.Jacob S, Kumar DA, Agarwal A, Aravind R, Saijimol AI, Saijimol AI. Preliminary evidence of successful near vision enhancement with a new technique: prEsbyopic Allogenic Refractive Lenticule (PEARL) corneal inlay using a SMILE lenticule. J Refract Surg. 2017;33(4):224–229. doi: 10.3928/1081597X-20170111-03 [DOI] [PubMed] [Google Scholar]
- 40.Tanriverdi C, Ozpinar A, Haciagaoglu S, Kilic A. Sterile excimer laser shaped allograft corneal inlay for hyperopia: one-year clinical results in 28 eyes. Curr Eye Res. 2021;46(5):630–637. doi: 10.1080/02713683.2021.1884728 [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Zhou Y. Small incision lenticule extraction (SMILE) combined with allogeneic intrastromal lenticule inlay for hyperopia with astigmatism. PLoS One. 2021;16(9):e0257667. doi: 10.1371/journal.pone.0257667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganesh S, Brar S. Femtosecond intrastromal lenticular implantation combined with accelerated collagen cross-linking for the treatment of keratoconus - Initial clinical result in 6 eyes. Cornea. 2015;34(10):1331–1339. doi: 10.1097/ICO.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 43.Sun L, Yao P, Li M, Shen Y, Zhao J, Zhou X. The safety and predictability of implanting autologous lenticule obtained by SMILE for hyperopia. J Refract Surg. 2015;31(6):374–379. doi: 10.3928/1081597X-20150521-03 [DOI] [PubMed] [Google Scholar]
- 44.Furlan WD, Montagud-Martínez D, Ferrando V, García-Delpech S, Monsoriu JA. A new trifocal corneal inlay for presbyopia. Sci Rep. 2021;11(1):6620. doi: 10.1038/s41598-021-86005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furlan WD, García-Delpech S, Udaondo P, Remón L, Ferrando V, Monsoriu JA. Diffractive corneal inlay for presbyopia. J Biophotonics. 2017;10(9):1110–1114. doi: 10.1002/jbio.201600320 [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Espert A, Montagud-Martínez D, Ferrando V, Furlan WD, Monsoriu JA. Assessment of a new trifocal diffractive corneal inlay for presbyopia correction using an adaptive optics visual simulator. Photonics. 2022;9(3):135. doi: 10.3390/photonics9030135 [DOI] [Google Scholar]
- 47.Federal Drug Administration. VUITY (pilocarpine hydrochloride ophthalmic solution) 1.25%, for topical ophthalmic use; 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214028s000lbl.pdf. Accessed March 24, 2022.
- 48.Al-Khersan H, Jr HWF, Townsend JH. Retinal detachments associated with topical pilocarpine use for presbyopia: pilocarpine-associated retinal detachments. Am J Ophthalmol. 2022;242:52–55. doi: 10.1016/j.ajo.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 49.Korenfeld MS, Robertson SM, Stein JM, et al. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: a safety and preliminary efficacy trial. Eye. 2021;35(12):3292–3301. doi: 10.1038/s41433-020-01391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin JT, Mallo O. Treatment of presbyopia by infrared laser radial sclerectomy. J Refract Surg. 2003;19(4):465–467. doi: 10.3928/1081-597X-20030701-18 [DOI] [PubMed] [Google Scholar]
- 51.Hipsley A, Hall B, Rocha KM. Scleral surgery for the treatment of presbyopia: where are we today? Eye Vis. 2018;5:4. doi: 10.1186/s40662-018-0098-x [DOI] [PMC free article] [PubMed] [Google Scholar]