Summary
Background
CD28-CD8+ T cells represent a differentiated CD8+ T cell subset that is found to be increased in various conditions associated with chronic antigenic stimulation such as aging, chronic viral infections, autoimmune diseases, cancers, and allotransplantation.
Methods
Using multivariate models, we analyzed a large cohort of 1032 kidney transplant patients in whom 1495 kidney graft biopsies were performed concomitant with a peripheral blood leukocyte phenotyping by flow cytometry. We investigated the association between the level of CD28-CD8+ T cells in the blood and the diagnosis of graft rejection according to the recent Banff classification of renal allograft pathology.
Findings
We found that antibody-mediated rejection (ABMR) was associated with a significant increase in the percentage as well as the absolute number of CD28-CD8+ T cells in the peripheral blood of kidney transplant patients at the time of biopsy. The confounder-adjusted mean difference of log percentage and log absolute value between the ABMR group and the normal/subnormal histology group were 0.29 (p=0.0004) and 0.38 (p=0.0004), respectively. Moreover, we showed that CD28-CD8+ T cells from the patients diagnosed with ABMR responded more rigorously to TCR and FcγRIIIA (CD16) engagement compared to their CD28+ counterparts as evidenced by an increase in the expression of IFNγ, TNFα, and CD107a.
Interpretation
Collectively, our data suggest that differentiated CD28-CD8+ T cells, with increased frequency, number, and function, may participate in the pathobiology of ABMR. Further studies are warranted to clarify the immunological role of this T cell subset in kidney graft rejection.
Funding
Agence nationale de la recherche (France).
Keywords: Kidney transplantation, Rejection, Antibody, T lymphocyte, CD8, CD28
Research in context.
Evidence before this study
CD28-CD8+ T cells represent a differentiated CD8+ T cell subset that is found to be increased in various conditions associated with persistent antigen exposure such as chronic infection and allotransplantation. In 2006, we have reported that kidney graft patients with biopsy-proven chronic rejection had a significant increase in the percentage of CD28-CD8+ T cells in the peripheral blood compared to patients with long-term drug-free graft tolerance and healthy individuals. In searching in PubMed publications from 2000 to 2020 containing both key words “CD28-CD8+ T cells” and “transplantation”, we identified 6 other publications in which the percentage or number of CD28-CD8+ T cells was found to be associated with the risk of allograft rejection (Ref 21 to 26). All of them are small studies with a maximum of 200-300 patients per study.
Added value of this study
Using carefully designed multivariable model, we analyzed a large cohort of 1032 kidney transplant patients in whom 1495 kidney graft biopsies were performed and showed that antibody-mediated rejection (ABMR) was associated with a significant increase in the percentage as well as the absolute number of CD28-CD8+ T cells in the peripheral blood. Moreover, we showed that CD28-CD8+ T cells from the patients diagnosed with ABMR responded more rigorously to TCR and FcγRIIIA engagement compared to their CD28+ counterparts in terms of TH1 cytokine secretion and cytotoxicity. These finding suggest that differentiated CD28-CD8+ T cells participate in the pathobiology of ABMR.
Implications of all the available evidence
Our study sheds new insight into the understanding of a potential role of a “cellular component”, namely the CD28-CD8+ T cell population, in the pathobiology of ABMR. Future studies using more comprehensive lymphocyte phenotyping strategies and functional experiments should clarify the role of memory/effector CD8+ T cells in different forms of graft rejection.
Alt-text: Unlabelled box
Introduction
Kidney allograft rejection remains the major cause of renal damage and graft loss. The histological diagnosis graft rejection is complemented by donor-specific antibody (DSA) identification1 and more recently by molecular analyses.2,3 The Banff classification divides kidney graft rejections into two main categories: antibody-mediated rejection (ABMR) and T cell-mediated rejection (TCMR).1, 2, 3 Nevertheless, the biological mechanisms underlying the rejection process are more complicated than it appears in the pathological classification and involve both the adaptive immune system, particularly CD4+ and CD8+ T cells and the innate immune system.4 Among the several cell types involved in graft rejection, CD8+ T cells play an important role, especially in allorecognition.5 Patients with high frequency of donor-specific CD8+ T cells before kidney transplant have increased risk of acute rejection after transplant.6 CD8+ T cells are found in kidney allograft undergoing rejection, both acute and chronic, and the majority of them are cytotoxic T cells.7, 8, 9 Both CD4+ and CD8+ T cell activation by alloantigens require the second or costimulatory signal, most importantly via the interaction between CD28 on T cells and B7 on antigen-presenting cells (APCs).10 Chronic antigenic stimulation of CD8+ T cells, however, can lead to a down-regulation of CD28. The CD28-CD8+ T cell population has been shown to be increased with age and in various diseases associated with chronic immune activation such as chronic viral infection, autoimmune diseases, cancer, and allotransplantation [reviewed in11 and12]. In 2006, we reported that kidney graft patients with biopsy-proven chronic rejection according to the Banff 05 classification13,14 had a significant increase in the percentage of CD28-CD8+ T cells in the peripheral blood compared to patients with long-term drug-free graft tolerance and healthy individuals.15 Although CD28-CD8+ T cells may exhibit regulatory properties in some disease models,16,17 we have shown that CD28-CD8+ T cells from patients with chronic rejection express the cytotoxic molecules granzyme A and perforin and degranulate in response to stimulation with donor MHC.15 Following those observations, we set up a clinical protocol in which a peripheral blood lymphocyte phenotyping was performed each time a kidney graft biopsy was done at our center between 2008 and 2016. Kidney graft biopsies were carried out either for cause or for surveillance (at 3 months and 1 year post-transplantation). We hypothesized that there was an association between peripheral blood T cell phenotypes, especially CD28-CD8+ T cells and the histological diagnosis of kidney graft biopsies. Based on the large cohort of 1495 kidney graft biopsies established from our clinical protocol, we confirmed that ABMR diagnosis was associated with an increase in the percentage as well as the absolute number of CD28-CD8+ T cells in the peripheral blood at the time of graft biopsy. Using in vitro culture assays, we also showed that CD28-CD8+ T cells from recipients diagnosed with ABMR responded vigorously to TCR and CD16 stimulation, suggesting their active role in mediating graft rejection.
Patients, materials and methods
Study design
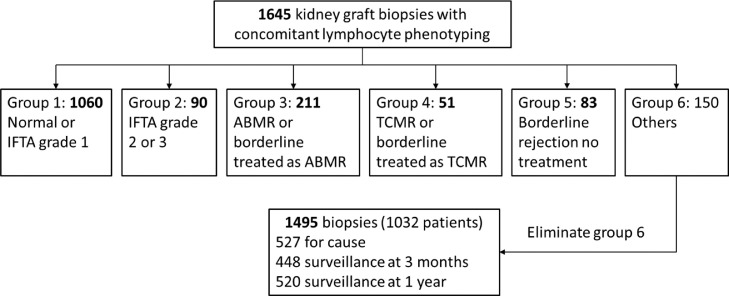
From 2008 to 2016, we established at our institution, Nantes University Hospital (Centre Hospitalier Universitaire or CHU de Nantes), a cohort of 1645 kidney graft biopsies (protocol “Peribiopsy N° RC13_0251”) for which a peripheral lymphocyte phenotyping was performed at the time of biopsy. Renal biopsies were interpreted by our renal pathologist (K.R.) based on the last Banff classification2 except for the diagnosis of chronic cellular rejection which was based exclusively on the presence of chronic allograft arteriopathy.1 The full histological criteria were detailed in the Banff 2015 and 2017 Kidney Meeting Report.1,2 Briefly, ABMR was diagnosed based on the presence histologic evidence of tissue injury (microvascular inflammation [g>0 and/or ptc>0] and/or acute thrombotic microangiopathy and/or acute tubular injury in the absence of any other cause for acute ABMR or transplant glomerulopathy [cg>0] and/or chronic allograft vasculopathy for chronic ABMR), associated with evidence of current antibody interraction with vascular endothelium including significant linear C4d staining in peritubular capillaries or at least moderate microvascular inflammation ([g+ptc]≥2) and serologic evidence of DSA. The diagnosis of acute TCMR was based on a combination of interstitial inflammation (i≥2), tubulitis (t≥2), and/or arteritis (v≥1), whereas chronic TCMR was diagnosed mainly based on the presence of chronic allograft arteriopathy in the absence of DSA, C4d positivity or significant microvascular inflammation. After having taken into account the clinical decision, the histological diagnoses were organized into 6 biopsy groups (Figure 1):
-
-
Group 1 (n=1060): normal/sub-normal or interstitial fibrosis and tubular atrophy (IFTA) grade 1.
-
-
Group 2 (n=90): IFTA grade 2 or 3.
-
-
Group 3 (n=211): ABMR or borderline rejection treated as ABMR with plasma exchanges and intravenous immunoglobulins (IVIg), with or without rituximab.
-
-
Group 4 (n=51): TCMR or borderline rejection treated as TMCR with corticosteroids.
-
-
Group 5 (n=83): borderline rejection without treatment.
-
-
Group 6 (n=150): other changes not considered to be caused by rejection.
Figure 1.
Histological diagnoses of kidney graft biopsies.
Since our study focused on the association between lymphocyte phenotypes and rejection, 150 biopsies from group 6 (other changes) were excluded, leaving 1495 biopsies for the analysis, including 527 for cause biopsies and 448 and 520 three-month and 1-year surveillance biopsies, respectively. The designation of those groups was done a priori before all statistical analyses, which were then performed by an independent biostatistician (FLB). The main objective of our study was to investigate whether there was an association between the percentage and the absolute number of CD28-CD8+ T cells and the five biopsy groups (the principal analysis). In addition, we also analyzed the subgroup of patients with lymphocyte phenotyping at 1 year post-transplantation (patients with 1-year surveillance biopsy) to look for an association between the percentage and absolute number of CD28-CD8+ T cells at 1 year and graft survival (the graft survival analysis).
Lymphocyte phenotyping by flow cytometry
Each time a patient underwent a kidney graft biopsy at CHU de Nantes, a blood sample was drawn for routine laboratory analyses and an EDTA tube containing about 5 ml of blood was sent to the center for immunomonitoring (Centre d'Immunomonitorage Nantes-Atlantique or CIMNA) at CHU de Nantes for lymphocyte phenotyping. Flow cytometry was performed on fresh whole blood within 24 h after sampling and cells were analyzed on a BD FACS Canto II flow cytometer. Several lymphocyte surface markers were stained but in this report, we focused our study on the CD28-CD8+ T cell subset. To determine the percentage of CD28-CD8+ T cells, blood was incubated with the following fluorescence-conjugated monoclonal antibodies: anti-CD45-PerCP Cy5.5 (clone 2D1), anti-CD3-FITC (clone SK7), anti-CD28-APC (clone CD28.2), and anti-CD8-PE (clone B9.11) (all from BD Biosciences except anti-CD8 from Beckman Coulter), lysed with FACS lysing solution, and washed with PBS. CD28-CD8+ T cells were identified as CD45+CD3+CD8+CD28- and the percentage of CD28-CD8+ T cells in total lymphocytes was reported (see Supplementary Figure 1 for gating strategy). CD8+ T cell absolute number was also measured by flow cytometry by staining whole blood with the four-color monoclonal antibody reagent BD Multitest™CD3/CD8/CD45/CD4 in BD Trucount™ Tubes (BD Biosciences) according to the manufacturer's instruction. The absolute number of CD28-CD8+ T cells was calculated by multiplying the absolute number of CD8+ T cells by the percentage of CD28-CD8+ T cells in CD8+ T cells and expressed in thousand/µl.
Functional analysis of CD8+ T cells
Frozen PBMCs of 24 and 46 kidney transplant recipients belonging to group 3 (ABMR) and group 1 (normal/subnormal biopsies), respectively, were retrieved from Centre de Resources Biologiques of Nantes (CRB). Frozen PBMCs were thawed and rested overnight in complete RPMI medium. 106 PBMCs were cultured for 4h in 24-well flat bottom plates at 37°C in 5% CO2 and, when indicated, treated with plate-bound anti-CD3 (clone OKT3; 2 µg/mL), plate-bound anti-CD16 (clone 3G8; 10 µg/mL), soluble anti-CD28 (clone CD28.2; 2 µg/mL) and/or soluble IL-15 (10 ng/mL; Miltenyi Biotec). Anti-CD107a PE (clone H4A3) and Brefeldin A (5 ug/mL, Sigma) were added at culture initiation. After stimulation, cells were first stained for cell surface markers (CD3 [clone UCHT1], CD8 [clone HIT8a], CD45RA [clone HI100], CD28 [clone CD28.2] and CCR7 [clone G043H7]), followed by fixation and permeabilization (Intracellular Fixation & Permeabilization Buffer Set, Thermo Fisher) and stained for intracellular cytokines TNFα [clone Mab11] and IFNγ [Clone B27]). Cells were acquired with a Celesta flow cytometer (BD Immunocytometry Systems) and the data analyzed using FlowJo Version 10.8.0 (TreeStar). The percentage of cells expressing TNFα/IFNγ and CD107a after stimulation were compared using non-parametric tests.
Clinical data
Clinical data required for the analysis were extracted from the DIVAT (for “Données Informatisées et Validées en Transplantation”) database which was carried out prospectively, exhaustively, and independently by clinical research associates on key dates during post-operative follow-up of clinical and biological data of all incident transplanted patients at our institute. The data are subject to an annual audit to warrant quality and completeness. Recipient characteristics include age, gender, transplantation rank (first transplantation or re-transplantation), year of transplantation, the initial kidney disease (possibly recurrent or not), and the cytomegalovirus (CMV) serology, history of diabetes, history of arterial hypertension, history of cardiovascular disease, CMV serology, number of HLA-A-B-DR incompatibilities, ABO mismatch, donor-specific antibodies (DSA), anti-HLA class I and II immunization. Donor features include age, gender, and donor type (living or deceased). Baseline transplantation parameters were cold ischemia time, delayed graft function (DGF), induction therapy, and maintenance treatments [including cyclosporine A (CSA), tacrolimus, mammalian target of rapamycin (mTOR) inhibitors, mycophenolate mofetil (MMF), and corticosteroids]. Parameters collected at the time of biopsy were the reason for biopsy – for cause or surveillance biopsy (at 3 months or 1 year post-transplantation), serum creatinine, the rank of biopsy, post-transplantation time, histological diagnosis according to 2015 Banff classification and the whole details of the Banff elementary lesions, DSA, and type of treatment for each rejection episode, and CD28-CD8+ T cells both in percentage and in absolute value. The follow-up and the collection of data were stopped upon graft failure (defined as return to dialysis or retransplantation) or death.
Statistical analyses
For the principal analysis, the characteristics at the time of biopsy between the five biopsy groups were described using median and quartiles for continuous variables and frequency and proportion for categorical data. Two features were studied: the percentage and the absolute value of CD28-CD8+ T cells. Since these two features were non-Gaussian, they were transformed using the natural logarithm function (Supplementary Figure 2). Linear mixed-effects models (random intercept per transplantation) were used to study the association between the biopsy groups and each feature.18 We systematically included in multivariate models the following clinically important variables: recipient and donor age, recipient and donor sex, re-transplantation, recipient and donor CMV serology, cold ischemia time, time from transplantation to biopsy, reason for biopsy (for cause vs surveillance biopsy), induction therapy at transplantation, creatinine at biopsy, HLA-A, -B and-DR incompatibilities and anti-HLA class I and II immunization. We also included into the models all the covariates significantly associated with the features in univariate models (p-value<0.2). We did not consider interaction. The residuals’ analyses were performed to check the models’ validities. In each model, we first tested if the outcome was significantly different in at least one of the biopsy groups using a likelihood ratio test. If significant, we explored which groups differed by performing the six following comparisons: group 3 versus 1, group 4 versus 1, group 5 versus 3, group 5 versus 4, group 2 versus 1, and group 5 versus 1. Corrected p-values were determined using the Holm-Bonferroni method to control for the inflation of the type I error rate associated with multiple testing.
For the graft survival analysis, in order to determine if there was an association between the log percentage and/or absolute number of CD28-CD8+ T cells at 1 year post-transplantation and graft survival, we included recipients with a 1-year post-transplantation surveillance biopsy and a concomitant lymphocyte phenotyping. The baseline of this analysis was the time at which the 1-year biopsy was performed. The outcome was the graft survival defined by the time to the first event of either return to dialysis or pre-emptive re-transplantation (all-cause death with a functioning graft were right-censored). The median time of follow-up was estimated by using the reverse Kaplan-Meier estimator. The survival curve was obtained by the Kaplan-Meier estimator. To take into consideration confounding factors, we used multivariable cause-specific Cox models. We included in multivariable models all the covariates significantly associated with the outcomes in univariate Cox models (p-value <0.2). The proportional hazard assumption was tested from the Schoenfeld residuals.19 The log-linearity assumption has been checked in unadjusted analysis if the Bayesian Information Criterion was not reduced using natural spline transformation compared to the inclusion of the covariate in its natural scale. In case of violation, variables were transformed. We used R version 3.6.3 and the package ‘base’, ‘dplyr’, ‘survival’, ‘etm’, ‘plotrix’, ‘prodlim’, ‘data.table’, ‘splines’, ‘officer’, ‘flextable’, ‘lattice’, ‘forestplot’, ‘gtools’, ‘lme4’ and ‘lmerTest’ packages for all data analyses (see https://www.r-project.org/).
Ethical statement
This study on organ transplantation was conducted in accordance with the declaration of Istanbul, the declaration of Helsinki, and local regulations. DIVAT has received ethical committee authorization (CNIL decision DR-2015-087, N°914184). All participants gave their informed consent (www.divat.fr).
Role of funders
Funders had no role in study design, data analyses, interpretation, or writing of reports.
Results
Patient characteristics
A total of 1495 kidney graft biopsies performed on 1032 patients having received kidney or combined kidney/pancreas allografts were included in the principal analysis. On average, we observed 1.5 biopsies per recipient (range from 1 to 5, median at 1). The median time from transplantation to biopsy was one year (range from 0 to 31 years). Seventy percent (1060) of biopsies were normal or sub-normal (group 1), 6% (90) were grade 2/3 IFTA (group 2), 14% (211) were ABMR (group 3), 3.4% (51) were TCMR (group 4), and 5.6% (83) of biopsies and untreated borderline rejection (group 5). The characteristics of the whole sample (1495 biopsies) as well as of each histological group from 1 to 5 were presented in Table 1.
Table 1.
Characteristics of 1495 renal biopsies included in the analysis according their biopsy groups, including categorical and continuous variables.
| Categorical variables | Whole sample (n=1495) |
Biopsy group 1 (n=1060) |
Biopsy group 2 (n=90) |
Biopsy group 3 (n=211) |
Biopsy group 4 (n=51) |
Biopsy group 5 (n=83) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA | n | % | NA | n | % | NA | n | % | NA | n | % | NA | n | % | NA | n | % | |
| Transplantation after 2008 | 0 | 1174 | 78.5 | 0 | 899 | 84.8 | 0 | 55 | 61.1 | 0 | 117 | 55.5 | 0 | 36 | 70.6 | 0 | 67 | 80.7 |
| Male recipient | 0 | 927 | 62.0 | 0 | 670 | 63.2 | 0 | 48 | 53.3 | 0 | 130 | 61.6 | 0 | 28 | 54.9 | 0 | 51 | 61.4 |
| Retransplantation | 0 | 238 | 15.9 | 0 | 165 | 15.6 | 0 | 13 | 14.4 | 0 | 51 | 24.2 | 0 | 3 | 5.9 | 0 | 6 | 7.2 |
| Renal transplantation | 0 | 1343 | 89.8 | 0 | 946 | 89.2 | 0 | 86 | 95.6 | 0 | 192 | 91.0 | 0 | 44 | 86.3 | 0 | 75 | 90.4 |
| Recurrent initial disease | 0 | 350 | 23.4 | 0 | 241 | 22.7 | 0 | 20 | 22.2 | 0 | 57 | 27.0 | 0 | 9 | 17.6 | 0 | 23 | 27.7 |
| DGF | 29 | 484 | 33.0 | 19 | 325 | 31.2 | 2 | 39 | 44.3 | 5 | 68 | 33.0 | 2 | 20 | 40.8 | 1 | 32 | 39.0 |
| History of diabetes | 0 | 356 | 23.8 | 0 | 260 | 24.5 | 0 | 14 | 15.6 | 0 | 44 | 20.9 | 0 | 15 | 29.4 | 0 | 23 | 27.7 |
| History of hypertension | 0 | 1328 | 88.8 | 0 | 946 | 89.2 | 0 | 81 | 90.0 | 0 | 185 | 87.7 | 0 | 47 | 92.2 | 0 | 69 | 83.1 |
| History of cardiovascular disease | 0 | 524 | 35.1 | 0 | 350 | 33.0 | 0 | 38 | 42.2 | 0 | 84 | 39.8 | 0 | 23 | 45.1 | 0 | 29 | 34.9 |
| Recipient/Donor CMV serology | 6 | 0 | 2 | 3 | 0 | 1 | ||||||||||||
| 0 | 514 | 34.5 | 383 | 36.1 | 22 | 25.0 | 65 | 31.2 | 18 | 35.3 | 26 | 31.7 | ||||||
| 1 | 324 | 21.8 | 220 | 20.8 | 33 | 37.5 | 45 | 21.6 | 10 | 19.6 | 16 | 19.5 | ||||||
| 2 | 330 | 22.2 | 234 | 22.1 | 14 | 15.9 | 49 | 23.6 | 13 | 25.5 | 20 | 24.4 | ||||||
| 3 | 321 | 21.6 | 223 | 21.0 | 19 | 21.6 | 49 | 23.6 | 10 | 19.6 | 20 | 24.4 | ||||||
| Male donor | 1 | 861 | 57.6 | 1 | 617 | 58.3 | 0 | 61 | 67.8 | 0 | 110 | 52.1 | 0 | 28 | 54.9 | 0 | 45 | 54.2 |
| Deceased donor | 0 | 1340 | 89.6 | 0 | 941 | 88.8 | 0 | 85 | 94.4 | 0 | 190 | 90.0 | 0 | 47 | 92.2 | 0 | 77 | 92.8 |
| HLA-A-B-DR mismatches > 4 | 0 | 330 | 22.1 | 0 | 233 | 22.0 | 0 | 11 | 12.2 | 0 | 52 | 24.6 | 0 | 15 | 29.4 | 0 | 19 | 22.9 |
| ABO mismatch | 10 | 30 | 2.0 | 7 | 25 | 2.4 | 1 | 0 | 0.0 | 2 | 4 | 1.9 | 0 | 0 | 0.0 | 0 | 1 | 1.2 |
| Depleting induction | 0 | 601 | 40.2 | 0 | 424 | 40.0 | 0 | 31 | 34.4 | 0 | 112 | 53.1 | 0 | 16 | 31.4 | 0 | 18 | 21.7 |
| CSA | 0 | 137 | 9.2 | 0 | 63 | 5.9 | 0 | 13 | 14.4 | 0 | 38 | 18.0 | 0 | 8 | 15.7 | 0 | 15 | 18.1 |
| Tacrolimus | 0 | 1354 | 90.6 | 0 | 995 | 93.9 | 0 | 77 | 85.6 | 0 | 173 | 82.0 | 0 | 41 | 80.4 | 0 | 68 | 81.9 |
| Steroid | 0 | 1256 | 84.0 | 0 | 886 | 83.6 | 0 | 78 | 86.7 | 0 | 182 | 86.3 | 0 | 38 | 74.5 | 0 | 72 | 86.7 |
| Positive anti-class I immunization | 1 | 445 | 29.8 | 0 | 309 | 29.2 | 1 | 23 | 25.8 | 0 | 94 | 44.5 | 0 | 9 | 17.6 | 0 | 10 | 12.0 |
| Positive anti-class II immunization | 1 | 390 | 26.1 | 0 | 264 | 24.9 | 1 | 18 | 20.2 | 0 | 87 | 41.2 | 0 | 10 | 19.6 | 0 | 11 | 13.3 |
| Positive DSA | 1 | 100 | 6.7 | 1 | 62 | 5.9 | 0 | 2 | 2.2 | 0 | 34 | 16.1 | 0 | 1 | 2.0 | 0 | 1 | 1.2 |
| Biopsy rank | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| 1 | 982 | 65.7 | 733 | 69.2 | 41 | 45.6 | 120 | 56.9 | 36 | 70.6 | 52 | 62.7 | ||||||
| 2 | 425 | 28.4 | 293 | 27.6 | 40 | 44.4 | 55 | 26.1 | 10 | 19.6 | 27 | 32.5 | ||||||
| 3 | 79 | 5.3 | 32 | 3.0 | 9 | 10.0 | 30 | 14.2 | 4 | 7.8 | 4 | 4.8 | ||||||
| 4 | 8 | 0.5 | 2 | 0.2 | 0 | 0.0 | 5 | 2.4 | 1 | 2.0 | 0 | 0.0 | ||||||
| 5 | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | ||||||
| For cause biopsy | 0 | 527 | 35.3 | 0 | 264 | 24.9 | 0 | 53 | 58.9 | 0 | 155 | 73.5 | 0 | 37 | 72.5 | 0 | 18 | 21.7 |
| Continuous variables | NA | Md | Q1 Q3 | NA | Md | Q1Q3 | NA | Md | Q1Q3 | NA | Md | Q1Q3 | NA | Md | Q1Q3 | NA | Md | Q1 Q3 |
| Log of CD28-CD8+ T cell percentage | 17 | 1.9 | 1.1 2.8 | 12 | 1.8 | 1.0 2.6 | 1 | 2.5 | 1.6 3.0 | 4 | 2.3 | 1.4 3.2 | 0 | 2.2 | 1.4 2.9 | 0 | 2.3 | 1.2 2.9 |
| Log of CD28-CD8+ T cell absolute value | 19 | -2.7 | -3.6 -1.7 | 14 | -2.9 | -3.7 -1.9 | 1 | -2.1 | -3.0 -1.4 | 4 | -2.1 | -3.2 -1.3 | 0 | -2.6 | -3.3 -1.6 | 0 | -2.4 | -3.4 -1.4 |
| Recipient age (years) | 0 | 51.0 | 39.0 62.0 | 0 | 51.0 | 40.0 62.0 | 0 | 51.0 | 37.0 63.0 | 0 | 49.0 | 33.0 60.0 | 0 | 51.0 | 35.0 63.5 | 0 | 51.0 | 38.5 61.5 |
| Cold ischemia time (hours) | 0 | 15.5 | 12.0 20.1 | 0 | 15.1 | 11.7 19.3 | 0 | 18.3 | 12.8 24.7 | 0 | 17.5 | 13.5 23.9 | 0 | 16.4 | 13.2 18.8 | 0 | 15.9 | 13.0 19.9 |
| Donor age (years) | 0 | 52.0 | 40.0 64.0 | 0 | 52.0 | 42.0 63.0 | 0 | 56.0 | 43.0 65.0 | 0 | 51.0 | 35.0 62.5 | 0 | 50.0 | 36.0 65.0 | 0 | 51.0 | 40.5 64.5 |
| Creatininemia at biopsy (µmol/l) | 22 | 144 | 113 193 | 14 | 136 | 108 176 | 4 | 178 | 145 247 | 2 | 172 | 134 238 | 0 | 225 | 156 360 | 2 | 129 | 108 168 |
| Time from transplant to biopsy (years) | 0 | 1.0 | 0.3 1.1 | 0 | 0.9 | 0.3 1.0 | 0 | 1.1 | 1.0 5.2 | 0 | 2.0 | 1.0 6.6 | 0 | 0.6 | 0.3 2.3 | 0 | 1.0 | 0.3 1.0 |
CMV, cytomegalovirus; CSA, cyclosporin A; DGF, delayed graft function; DSA, donor specific antibodies; HLA, human leucocyte antigens; Md, median; Q, quartile; Q1, 25th percentile; Q3, 75th percentile; NA: not available (missing). Recipient/Donor CMV serology definition, 0: negative donor and recipient; 1: negative donor and positive recipient; 2: positive donor and negative recipient; 3: positive donor and recipient; Log: natural logarithm.
ABMR is associated with an increase in the percentage and absolute number of peripheral blood CD28-CD8+ T cells
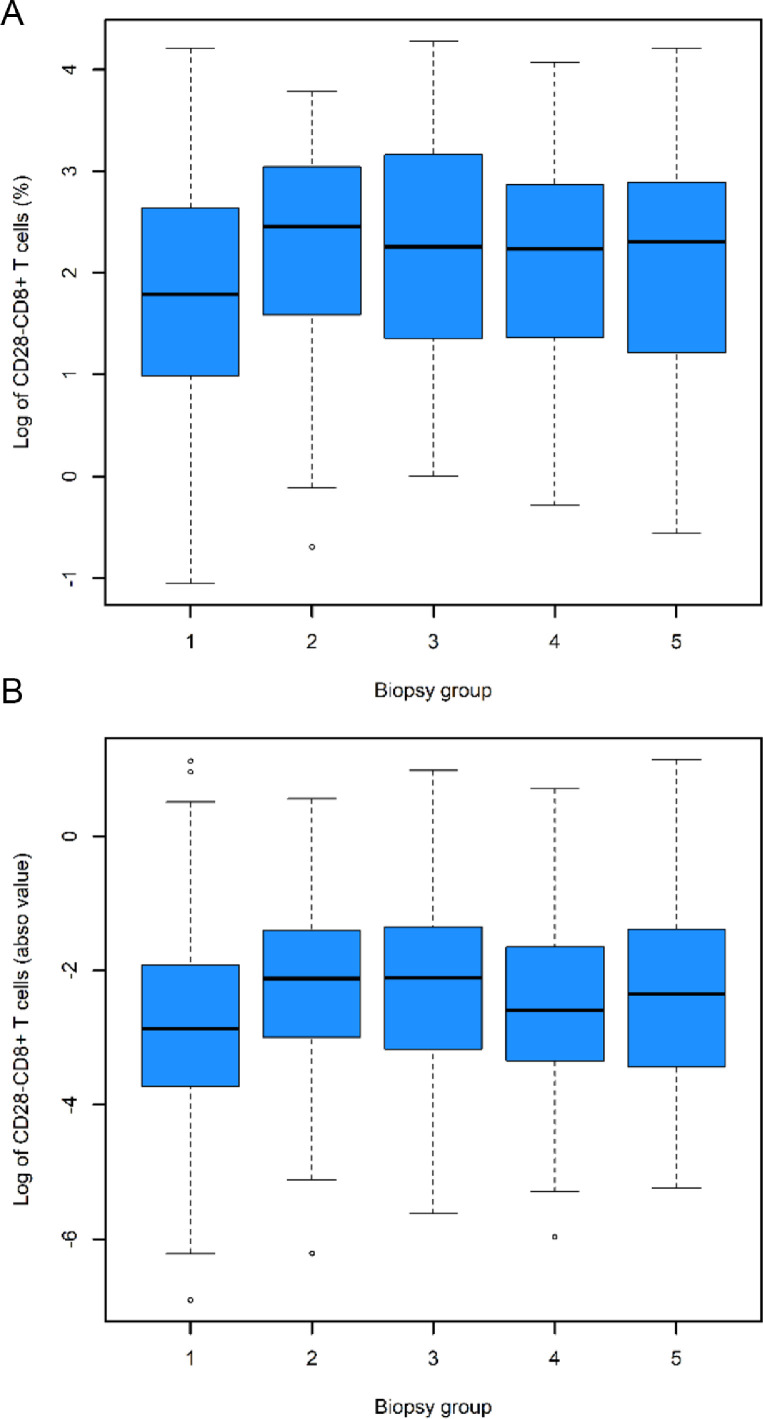
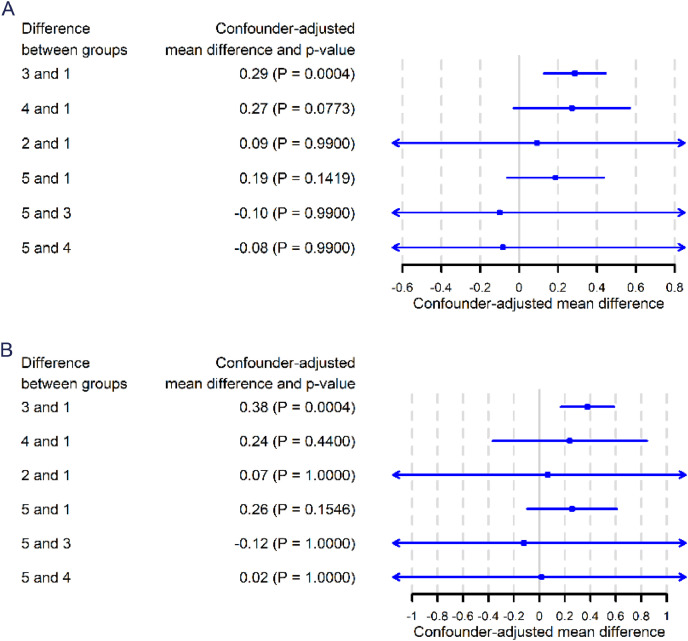
The distribution of the log percentage and log absolute number of CD28-CD8+ T cells in the 5 biopsy groups are shown in Figure 2A and 2B, respectively. We first analyzed the unadjusted effect of baseline covariates on the log percentage of CD28-CD8+ T cells using linear mixed models and found that biopsy groups were significant associated with the log percentage of CD28-CD8+ T cells (p<0.0001) (Supplementary Table 1). In addition to the predetermined clinically important variables as mentioned in Material and Methods (Statistical Analyses), the following covariates were also included into the multivariate model because they were found to be associated with the log percentage of CD28-CD8+ T cells (p<0.2) in the linear mix model: transplantation after 2008, delay graft function (DGF), history of cardiovascular diseases, deceased donor, ABO mismatch, cyclosporine A (CSA), tacrolimus, and biopsy rank. Next, we performed multivariate linear mixed model (Table 2) and confirmed a significant association between biopsy groups and the log percentage of CD28-CD8+ T cells (p = 0.0003). The confounder-adjusted mean difference of log percentage of CD28-CD8+ T cells between group 3 (ABMR) and group 1 (normal or sub-normal) was 0.29 (p = 0.0004) (Figure 3A). In other words, it means that the percentage of CD28-CD8+ T cells is 34% higher in ABMR patients compared with patients with normal/sub-normal histology. Other comparisons were not statistically significant (Figure 3A).
Figure 2.
Distribution of the natural logarithm of the percentage (A) and absolute number (B) of CD28-CD8+ T cells according to histological groups. In each boxplot, the central line indicates the median of the data set, the shaded box represents the interquartile range (IQR) which means 25 percentile to 75 percentile or quartile 1 (Q1) to quartile 3 (Q3), and the lower and upper bars indicate Q1 - 1.5 IQR and Q3 + 1.5 IQR, respectively. Biopsy group 1: normal/sub-normal, group 2: IFTA grade 2/3, group 3: ABMR, group 4: TCMR, and group 5: borderline rejection. See also Supplementary Tables 2 and 3 for the unadjusted mean difference in the log percentage and log absolute number, respectively, between biopsy groups.
Table 2.
Results of the multivariable linear mixed model of log percentage of CD28-CD8+ T cells measured at time of biopsy.
| Adjusted mean difference | 95% CI | p-value | |
|---|---|---|---|
| Biopsy group | 0.0003 | ||
| 2 (vs. 1) | 0.09 | [-0.09; 0.28] | |
| 3 (vs. 1) | 0.29 | [0.15; 0.43] | |
| 4 (vs. 1) | 0.27 | [0.05; 0.49] | |
| 5 (vs. 1) | 0.19 | [0.01; 0.36] | |
| Transplantation after 2008 | -0.25 | [-0.41; -0.08] | 0.0033 |
| Recipient age (years) | 0.00 | [-0.00; 0.01] | 0.5457 |
| Male recipient | 0.01 | [-0.11; 0.12] | 0.9157 |
| Retransplantation | 0.35 | [0.16; 0.53] | 0.0003 |
| DGF | 0.03 | [-0.09; 0.15] | 0.6582 |
| Cold ischemia time (hours) | 0.00 | [-0.00; 0.01] | 0.2846 |
| History of cardiovascular disease | 0.00 | [-0.11; 0.12] | 0.9378 |
| Recipient/Donor CMV serology | <0.0001 | ||
| 1 (vs. 0) | 1.19 | [1.04; 1.33] | |
| 2 (vs. 0) | 0.66 | [0.51; 0.80] | |
| 3 (vs. 0) | 1.24 | [1.09; 1.38] | |
| Donor age (years) | 0.01 | [0.00; 0.01] | 0.0305 |
| Male donor | 0.02 | [-0.09; 0.14] | 0.6653 |
| Deceased donor | -0.02 | [-0.26; 0.22] | 0.8721 |
| HLA-A-B-DR mismatches > 4 | -0.01 | [-0.14; 0.13] | 0.9103 |
| ABO mismatch | -0.50 | [-0.92; -0.08] | 0.0194 |
| Depleting induction | 0.00 | [-0.13; 0.14] | 0.9541 |
| CSA | 0.14 | [-0.49; 0.77] | 0.6632 |
| Tacrolimus | -0.02 | [-0.65; 0.62] | 0.9542 |
| Positive anti-class I immunization | 0.01 | [-0.13; 0.14] | 0.9216 |
| Positive anti-class II immunization | -0.12 | [-0.27; 0.03] | 0.1151 |
| Biopsy rank | <0.0001 | ||
| 2 (vs. 1) | 0.32 | [0.24; 0.39] | |
| 3 (vs. 1) | 0.38 | [0.22; 0.53] | |
| 4 (vs. 1) | 0.85 | [0.40; 1.29] | |
| 5 (vs. 1) | 0.46 | [-0.74; 1.65] | |
| For cause biopsy | -0.03 | [-0.14; 0.07] | 0.5267 |
| Creatininemia at biopsy (µmol/l) | -0.00 | [-0.00; 0.00] | 0.1940 |
| Post-transplantation time of the biopsy (years) | 0.01 | [-0.02; 0.03] | 0.5772 |
CI, confidence interval; CMV, cytomegalovirus; CSA, cyclosporin A; DGF, delayed graft function; HLA, human leucocyte antigens. Recipient/Donor CMV serology definition, 0: negative donor and recipient; 1: negative donor and positive recipient; 2: positive donor and negative recipient; 3: positive donor and recipient.
Figure 3.
Confounder-adjusted mean difference, 95% confidence intervals and p-value adjusted by Holm–Bonferroni method for the natural logarithm of the percentage (A) and the absolute number (B) of CD28-CD8+ T cells.
Similarly, we analyzed the unadjusted effect of baseline covariates on the log absolute number of CD28-CD8+ T cells using linear mixed models and found that biopsy groups were also significant associated with the log absolute number of CD28-CD8+ T cells (p<0.0001) (Supplementary Table 2). The following covariates were found to be associated with the log absolute number of CD28-CD8+ T cells (p<0.2) in the linear mix model and were included into the multivariate model (in addition to the predetermined clinically important variables): transplantation after 2008, renal transplantation, recurrent initial disease, DGF, deceased donor, ABO mismatch, CSA, tacrolimus, steroid, positive DSA, and biopsy rank. Next, we performed multivariate linear mixed model (Table 3) and observed a significant association between biopsy groups and the log absolute number of CD28-CD8+ T cells (p=0.0006). The confounder-adjusted mean difference between group 3 and group 1 was 0.38 (p = 0.0004) (Figure 3B), meaning that the absolute number of CD28-CD8+ T cells is 46% higher in the ABMR group compared to the normal/sub-normal histology group. The confounder-adjusted differences between the others groups were not statistically significant (Figure 3B). Taken together, those analyses show that ABMR is associated with a significant increase in the percentage and absolute number of CD28-CD8+ T cells in the patient's blood at the time of biopsy.
Table 3.
Results of the multivariable linear mixed model of log absolute number of CD28-CD8+ T cells measured at time of biopsy.
| Adjusted mean difference | 95% CI | p-value | |
|---|---|---|---|
| Biopsy group | 0.0006 | ||
| 2 (vs. 1) | 0.07 | [-0.18; 0.31] | |
| 3 (vs. 1) | 0.38 | [0.19; 0.56] | |
| 4 (vs. 1) | 0.24 | [-0.05; 0.53] | |
| 5 (vs. 1) | 0.26 | [0.02; 0.49] | |
| Transplantation after 2008 | -0.40 | [-0.61; -0.19] | 0.0002 |
| Recipient age (years) | -0.01 | [-0.02; -0.00] | 0.0215 |
| Male recipient | 0.03 | [-0.11; 0.17] | 0.6869 |
| Retransplantation | 0.41 | [0.16; 0.65] | 0.0011 |
| Renal transplantation | -0.03 | [-0.31; 0.24] | 0.8055 |
| Recurrent initial disease | 0.07 | [-0.09; 0.24] | 0.3728 |
| DGF | 0.01 | [-0.14; 0.16] | 0.8637 |
| Cold ischemia time (hours) | 0.01 | [-0.00; 0.02] | 0.2286 |
| Recipient/Donor CMV serology | <0.0001 | ||
| 1 (vs. 0) | 1.30 | [1.12; 1.49] | |
| 2 (vs. 0) | 0.72 | [0.53; 0.90] | |
| 3 (vs. 0) | 1.38 | [1.19; 1.56] | |
| Donor age (years) | 0.01 | [0.00; 0.02] | 0.0089 |
| Male donor | 0.05 | [-0.10; 0.19] | 0.5286 |
| Deceased donor | -0.06 | [-0.36; 0.24] | 0.7066 |
| HLA-A-B-DR mismatches > 4 | -0.04 | [-0.20; 0.13] | 0.6558 |
| ABO mismatch | -0.88 | [-1.41; -0.36] | 0.0010 |
| Depleting induction | -0.61 | [-0.80; -0.42] | <0.0001 |
| CSA | 0.29 | [-0.49; 1.08] | 0.4622 |
| Tacrolimus | 0.14 | [-0.65; 0.93] | 0.7305 |
| Steroid | -0.04 | [-0.23; 0.15] | 0.6902 |
| Positive anti-class I immunization | -0.02 | [-0.18; 0.15] | 0.8527 |
| Positive anti-class II immunization | -0.15 | [-0.34; 0.04] | 0.1262 |
| Positive DSA | -0.04 | [-0.34; 0.25] | 0.7783 |
| Biopsy rank | <0.0001 | ||
| 2 (vs. 1) | 0.50 | [0.39; 0.61] | |
| 3 (vs. 1) | 0.64 | [0.42; 0.86] | |
| 4 (vs. 1) | 1.06 | [0.44; 1.68] | |
| 5 (vs. 1) | 1.02 | [-0.65; 2.69] | |
| For cause biopsy | -0.05 | [-0.19; 0.09] | 0.4612 |
| Creatininemia at biopsy (µmol/l) | -0.00 | [-0.00; -0.00] | 0.0110 |
| Post-transplantation time of the biopsy (years) | 0.04 | [0.01; 0.07] | 0.0202 |
CI, confidence interval; CMV, cytomegalovirus; CSA, cyclosporin A; DGF, delayed graft function; HLA, human leucocyte antigens; NA: not available (missing). Recipient/Donor CMV serology definition, 0: negative donor and recipient; 1: negative donor and positive recipient; 2: positive donor and negative recipient; 3: positive donor and recipient.
Functional analysis of CD8+ T cells in ABMR patients
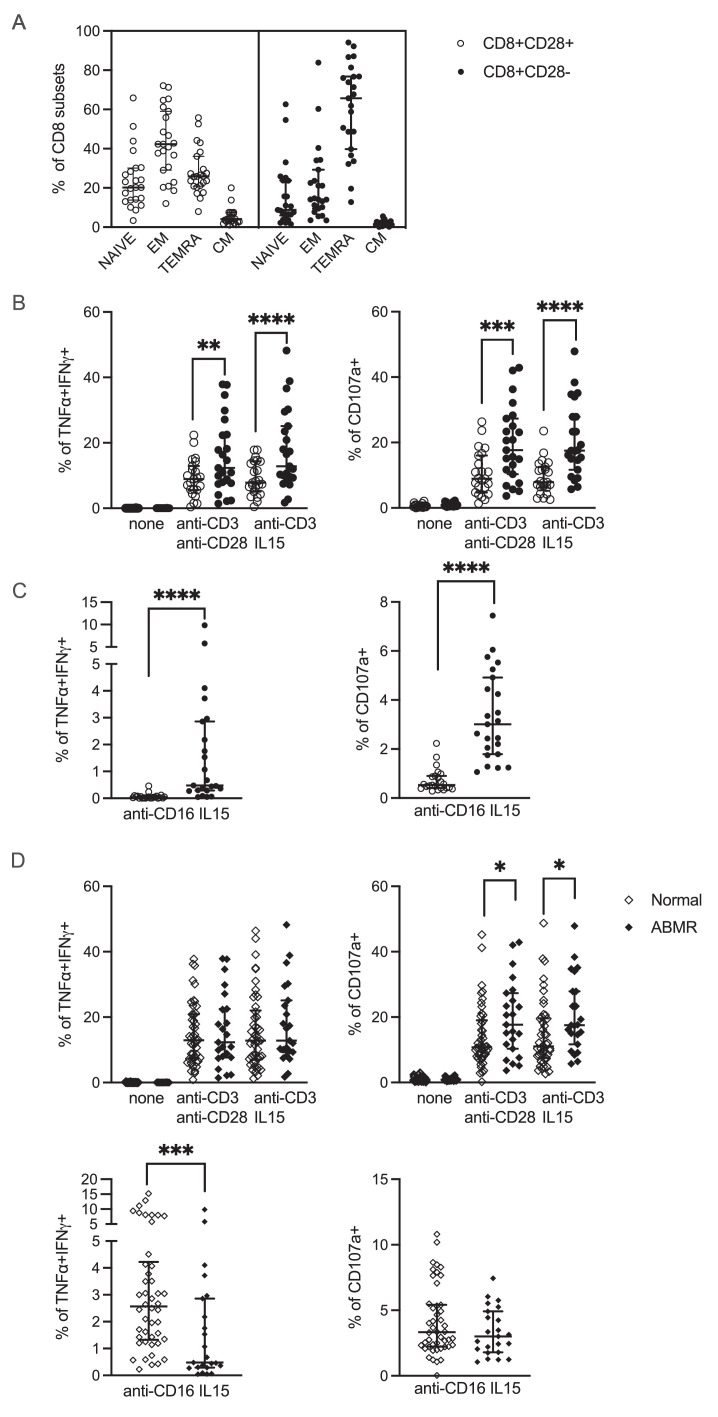
In order to decipher the mechanisms underlying the association between CD28-CD8+ T cells and ABMR, we retrieved 24 frozen PBMC samples from 24 patients belonging to biopsy group 3 (ABMR) to be used for further functional studies. A detailed flow cytometry analysis of those samples revealed that CD28-CD8+ T cells contained higher percentage of the effector-memory CD8+ T cells expressing CD45RA (TEMRA) defined as CCR7-CD45RA+ compared to CD28-CD8+ T cells (Figure 4A). We stimulated PBMCs with plate-bound anti-CD3 mAb and either soluble anti-CD28 or IL-15 and then stained for intracellular TNFα and IFNγ or surface CD107a, a marker of cytotoxic degranulation. As shown in Figure 4B, compared to CD28+CD8+ T cells, significantly higher percentages of CD28-CD8+ T cells were double-positive for TNFα and IFNγ (left) and positive for CD107a (right), suggesting that CD28-CD8+ T cells produce more TH1 cytokines and have stronger cytotoxic potential in response to TCR stimulation. Because the CD28-CD8+ T cells can include a subpopulation of T cells that expresses FcγRIIIA (CD16),20 we replaced anti-CD3 by anti-CD16 mAb and obtained the same result, i.e. significantly higher percentages of CD28-CD8+ T cells were double-positive for TNFα and IFNγ (left) and positive for CD107a (right) (Figure 4C).
Figure 4.
Functional studies of CD8+ T cells in ABMR. Frozen PBMCs (n=24) from patients diagnosed with ABMR (biopsy group 3) was retrieved from our biocollection for functional studies. (A) Flow cytometry staining for CD45RA and CCR7 divides CD8+ T cells into 4 subpopulations: naïve (CD45RA+CCR7+), central memory (CM) (CD45RA-CCR7+), effector memory (EM) (CD45RA-CCR7-), and effector memory expressing CD45RA (TEMRA) (CD45RA+CCR7-). The percentage of these 4 subpopulations in CD28+CD8+ and CD28-CD8+ T cells are shown. (B) and (C) PBMCs were stimulated with plate-bound anti-CD3 mAb, soluble anti-CD28 mAb or IL-15, and plate-bound anti-CD16 mAb as indicated for 4 h. Cells were stained for surface CD8, CD28, and CD107a, followed by intracellular staining for TNFα and IFNγ and analyzed by flow cytometry. The percentage of cells double positive for TNFα and IFNγ (left) and positive for CD107a (right) among CD28+CD8+ (open circles) and CD28-CD8+ (filled circles) T cells are shown. (D) Frozen PBMCs (n=46) were also retrieved from patients with normal/subnormal biopsies (group 1) and stimulated as in (B) and (C). The functional responses of the CD28-CD8+ T cell subpopulation from patients with ABMR and normal/subnormal biopsies were compared (see also Supplementary Figure 3). *: p<0.05; **: p<0.01; ***:p<0.001; ****: p<0.0001.
We asked whether the finding that CD28-CD8+ T cells respond more vigorously to TCR or FcγRIIIA stimulation compared to their CD28+CD8+ counterparts is specific to patients diagnosed with ABMR. To answer this question, we retrieved 46 PBMC samples from patients belonging to biopsy group 1 (normal or subnormal) and performed the same functional tests. We found that this finding is not restricted to ABMR because CD28-CD8+ T cells from patients with normal/subnormal biopsies also showed higher responses to TCR or FcγRIIIA stimulation compared to their CD28+CD8+ counterparts (Supplementary Figure 3). Next, we compared the functional responses of the CD28-CD8+ T cell subpopulation between these two groups of patients, ABMR and normal/subnormal biopsies. Interestingly, we found that CD28-CD8+ T cells from patients with ABMR showed higher cytotoxic potential in response to TCR stimulation than those from patients with normal/subnormal biopsies, as evidenced by significantly higher percentages of cells with CD107a expression (Figure 4D, upper right). On the other hand, the percentages of CD28-CD8+ T cells expressing both TNFα and IFNγ in response to TCR stimulation (Figure 4D, upper left) or expressing CD107a in response to FcγRIIIA stimulation (Figure 4D, lower right) were not different between these two groups of patients. Surprisingly, lower percentages of CD28-CD8+ T cells from ABMR patients expressed TNFα and IFNγ in response to FcγRIIIA stimulation compared to those from patients with normal/subnormal biospsies (Figure 4D, lower left).
The percentage and absolute number of CD28-CD8+ T cells at 1 year are not associated with graft survival
For the graft survival analysis, we asked whether there was an association between the percentage and absolute number of CD28-CD8+ T cells at 1 year and graft survival. To this end, we used data from the subgroup of 510 patients who underwent a 1 year surveillance biopsy because they also had a lymphocyte phenotyping at the time of biopsy. The median follow-up time was 5.0 years (range from 0 to 11.1 years). During follow-up, we observed 61 returns to dialysis and 51 deaths with a functioning graft. The characteristics of the 510 patients included in this analysis are shown in Supplementary Table 3. As in the principle analysis, the percentage and absolute number of CD28-CD8+ T cells also underwent log transformation. First, we established the death-censor graft survival curve using the Kaplan-Meier method (Supplementary Figure 4). Survival rates at 5 and 8 years (the baseline being the date of the 1-year screening biopsy) were 88.4% (95% CI: 85.2%-91.8%) and 78.3% (95% CI: 72.6%-84.5%), respectively. For robustness analysis, we estimated the cumulative incidence curves of the outcome from both the Kaplan-Meier estimator and the Aalen-Johansen estimator considering deaths with functioning graft as competing events and found that the two curves were close (Supplementary Figure 5). Unadjusted Cox models did not found any significant association between the log percentage and log absolute number of CD28-CD8+ T cells and graft survival (Supplementary Table 4). The multivariable Cox models confirmed the absence of significant association between the log percentage (Table 4) and the log absolute number (Table 5) of CD28-CD8+ T cells and graft survival (HR = 1.15, 95% CI: 0.88-1.51, p = 0.30 and HR = 1.17, 95% CI: 0.93-1.46, p = 0.18, respectively).
Table 4.
Results of the multivariable cause-specific Cox model studying the associations between the percentage of CD28-CD8+ T cells and graft survival.
| HR | 95% CI | p-value | |
|---|---|---|---|
| Log of CD28-CD8+ T cells (%) | 1.15 | [0.88; 1.51] | 0.2963 |
| Male recipient | 1.57 | [0.84; 2.94] | 0.1605 |
| Retransplantation | 1.14 | [0.48; 2.70] | 0.7731 |
| DGF | 1.17 | [0.66; 2.09] | 0.5954 |
| Cold ischemia time (hours) | 0.0548 | ||
| Between 12 and 24 h (vs. less than 12 h) | 3.76 | [1.15; 12.31] | |
| 24 h or more (vs. less than 12 h) | 2.34 | [0.58; 9.49] | |
| History of hypertension | 0.68 | [0.31; 1.48] | 0.3267 |
| Donor older than 55 years | 0.97 | [0.55; 1.71] | 0.9077 |
| HLA-A-B-DR mismatches > 4 | 1.92 | [1.01; 3.62] | 0.0453 |
| CSA | 1.98 | [0.79; 4.97] | 0.1451 |
| Positive anti-class II immunization | 1.60 | [0.82; 3.10] | 0.1683 |
| Creatininemia at biopsy (µmol/l) | 1.02 | [1.01; 1.02] | <0.0001 |
CI, confidence interval; CSA, cyclosporin A; DGF, delayed graft function; HLA, human leucocyte antigens; HR, hazard ratio; Log: natural logarithm.
Table 5.
Results of the multivariable cause-specific Cox model studying the associations between the absolute number of CD28-CD8+ T cells and graft survival.
| HR | 95% CI | p-value | |
|---|---|---|---|
| Log of CD28-CD8+ T cells (absolute value) | 1.17 | [0.93; 1.46] | 0.1791 |
| Male recipient | 1.55 | [0.82; 2.90] | 0.1742 |
| Retransplantation | 1.20 | [0.51; 2.83] | 0.6730 |
| DGF | 1.16 | [0.65; 2.08] | 0.6128 |
| Cold ischemia time (hours) | 0.0536 | ||
| Between 12 and 24 h (vs. less than 12 h) | 3.76 | [1.15; 12.31] | |
| 24 h or more (vs. less than 12 h) | 2.32 | [0.57; 9.42] | |
| History of hypertension | 0.67 | [0.31; 1.47] | 0.3201 |
| Donor older than 55 years | 0.95 | [0.54; 1.69] | 0.8692 |
| HLA-A-B-DR mismatches > 4 | 1.86 | [0.99; 3.52] | 0.0555 |
| CSA | 1.97 | [0.79; 4.95] | 0.1483 |
| Positive anti-class II immunization | 1.61 | [0.83; 3.14] | 0.1617 |
| Creatininemia at biopsy (µmol/l) | 1.02 | [1.01; 1.02] | <0.0001 |
CI, confidence interval; CSA, cyclosporin A; DGF, delayed graft function; HLA, human leucocyte antigens; HR, hazard ratio; Log: natural logarithm.
Discussion
Our analyses of a cohort of 1495 kidney graft biopsies performed on 1032 patients have shown that ABMR is significantly associated with an increase in the percentage as well as the absolute number of the differentiated CD28-CD8+ T cells in the peripheral blood measured at the time of graft biopsy. This large cohort study corroborates the findings of our previous study performed on a small number of kidney graft recipients showing an increase in the percentage of CD28-CD8+ T cells in patients with chronic rejection.15 Increased CD28-CD8+ T cells have been shown to be associated with graft rejection in several studies by other groups based on smaller numbers of patients. In a cross-sectional study of 121 kidney transplant recipients, CD28-CD57+CD8+ T cells were found to be increased in patients with TCMR compared to those with normal biopsy.21 In another study including 25 living donor kidney transplant recipients, patients with borderline or acute rejection were found to have higher pretransplant frequencies of CD28-CD8+ T cells compared to rejection-free kidney graft recipients.22 CD28-CD8+ T cells were also shown to be increased in patients with bronchiolitis obliterans syndrome (BOS) compared to stable patients in a study including 68 lung transplant recipients.23 On the contrary, some studies showed that high numbers of CD28-CD8+ T cells before transplant were associated with a lower risk of early24 and late kidney graft rejection and graft loss.25 Since the interaction between CD28 on T cells and B7 on APC is the principal target for the costimulatory blocking drug belatacept, CD28-CD8+ T cells have also been implicated in belatacept-resistant kidney graft rejection.26
However, CD28-CD8+ T cells are likely a heterogeneous population containing both cells with effector/memory properties and those with regulatory capacities. In vitro expanded CD28-CD8+ T cells were shown to have suppressive effect on CD4+ T cell proliferation in response to allogenic stimulation.27 On the contrary, we and others have shown that CD28-CD8+ T cells in patients with allograft rejection have effector properties as they express cytotoxic molecules such as granzyme A, granzyme B, and perforin, as well as inflammatory cytokines such as IFN-γ, TNF-α, and IL-2.8,15,28 The result of our analyses showing an association between an increase in CD28-CD8+ T cells and antibody-mediated rejection is interesting but not surprising. The Banff classification of renal allograft pathology might give an impression that kidney transplant rejections are caused by 2 distinct underlying mechanisms, either T cell-mediated or antibody-mediated. In reality, both mechanisms are usually operational at the same time. For instance, T cell relative frequency among graft infiltrating cells is similar between ABMR and TCMR.29 Since MHC as well as minor histocompatibility antigens are T cell-dependent antigens, recipient B cell activation and differentiation leading to the generation of donor-specific antibodies (DSA) requires T cell help. In addition, cytotoxic CD8+ T cells are found in renal biopsies with ABMR, either by immunohistological staining or gene expression analyses.8,9,30,31
Moreover, we have demonstrated in the current study that compared to their CD28+ counterparts, CD28-CD8+ T cells produce more TH1 cytokines and have stronger cytotoxic potential in response to TCR stimulation or FcγRIIIA (CD16) engagement. Those results are in concordance with our previous report on TEMRA CD8+ T cells.20 The potent effector functions of the CD28-CD8+ T cell subpopulation is not specific to ABMR because we obtained the same results in recipients with normal biopsies. However, we found that CD28-CD8+ T cells from ABMR patients have stronger cytotoxicity in response to TCR stimulation than those from patients with normal biopsies. Although the increase in CD28-CD8+ T cells might be a marker of the overall strength of alloimmune reaction, taken together, our experimental data suggest that CD28-CD8+ T cells may also participate in the pathobiology of ABMR, especially through their potent cytotoxic response upon TCR stimulation.
We next performed multivariable Cox models to investigate whether an increase in CD28-CD8+ T cells can help to predict kidney graft failure. We did not find any significant association between the percentage and the absolute number of CD28-CD8+ T cells at 1 year post-transplantation and graft survival. Nevertheless, in one of our previous studies using a more sophisticated gating strategy, we have shown that increased levels of TEMRA CD8+ T cells defined as CD45RA+CCR7- CD8+ were associated with a 2-fold higher risk of long-term graft dysfunction.32
Our study has some limitations. First of all, it is a single-center study. Secondly, based on our publication in 2006,15 we selected only a few markers so that the test could be performed routinely on fresh blood samples at the immunophenotyping platform of our hospital at the time of kidney graft biopsy and the results of this study could validate the findings of our previous study. This lymphocyte phenotyping strategy was designed more than a decade ago, now it becomes clear that more lymphocyte markers are necessary to better identify effector/memory CD8+ T cells.20,32, 33, 34 Thirdly, polyclonal TCR stimulation was used in our functional studies of CD8+ T cells, stimulation with donor antigens could further confirm our findings. Fourthly, although we have included many covariates in the multivariable statistical models, we cannot exclude unobserved confounders, for example, ethnicity and socioeconomic status, which were not collected in our database.
Finally, in multivariable analyses, we did not consider individuals with missing data on the covariates, which might reduce the statistical power leading to non-significant results. However, our effort to limit missing data was considerable. As shown in Table 1, 20/30 variables studied contained no missing data. Of the 10 variables with missing data, 4 variables contained only 1 case with missing data. Overall, about 5% of total cases were excluded from the multivariable analysis because of at least 1 missing data. We have looked at variables with missing data and found no overt reason to suspect the presence of « missing not at random » (MNAR), where the cause of missing data is directly linked with the value of those data. Given the small percentage of cases with missing data and the probable absence of MNAR, our approach to analyze only cases with complete data would unlikely induce significant bias.
In summary, this is the first time the differentiated CD28-CD8+ T cell phenotype is shown to be associated with ABMR in a large cohort of more than 1000 kidney graft recipients with nearly 1500 kidney graft biopsies. Our study sheds new insight into the understanding of an intriguing subpopulation of CD8+ T cells in the transplant setting. Future studies using more comprehensive lymphocyte phenotyping strategies on large cohorts of renal transplant patients together with functional experiments using preserved donor cells as stimulators are needed to clarify the role of memory/effector CD8+ T cells in different forms of graft rejection.
Contributors
HLM, ND, MR, KR, FLB, SB, and MG participate in the writing and revision of the manuscript; SB, MG, HLM, ND, SLB, RD, and FLB participate in the conceptualization and methodology; MR provides and verifies lymphocyte phenotyping data; KR interpretes kidney graft biopsies; FD and AW provide and verify patients’ laboratory data; FLB perform statistical analyses; ND, GT, and AV design and perform in vitro functional studies; CK perform data extraction from the DIVAT database; SLB, RD, CK, HLM, SB, and MG verify the extracted data; SB and MG acquire funding and supervise the study. All authors approved the final version of the manuscript.
Data sharing statement
All the data supporting the findings of this study are available within the article and its Supplemental Information files or from the corresponding author upon reasonable request.
Declaration of interests
All authors declared no conflict of interest.
Acknowledgements
This study has been registered with French Research Ministry: RC13_0251, last agreement N°13334, CNIL N° for the cohort: 891735. This work was performed in the context of the DHU Oncogreffe, the LabEx IGO (ANR-11- LABX-0016-01), the ANR project BIKET (ANR-17-CE17-0008) and the ANR project KTD-innov (ANR-17-RHUS-0010) thanks to French government financial support managed by the National Research Agency. We thank the biological resource centre for biobanking (Nantes Université, CHU Nantes, Centre de Ressources Biologiques (CRB), F-44000 Nantes, France) (BRIF: BB-0033-00040). We also thank S. Le Floch, clinical research assistant and the CIMNA platform for blood lymphocyte phenotyping.
*DIVAT Cohort Collaborators (Medical Doctors, Surgeons, HLA Biologists) in Nantes: Gilles Blancho, Julien Branchereau, Diego Cantarovich, Agnès Chapelet, Jacques Dantal, Clément Deltombe, Lucile Figueres, Claire Garandeau, Magali Giral, Caroline Gourraud-Vercel, Maryvonne Hourmant, Georges Karam, Clarisse Kerleau, Christophe Masset, Delphine Kervela, Sabine Lebot, Aurélie Meurette, Simon Ville, Christine Kandell, Anne Moreau, Karine Renaudin, Anne Cesbron, Florent Delbos, Alexandre Walencik, and Anne Devis.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104226.
Contributor Information
Magali Giral, Email: magali.giral@chu-nantes.fr.
Sophie Brouard, Email: sophie.brouard@univ-nantes.fr.
Appendix. Supplementary materials
References
- 1.Loupy A, Haas M, Solez K, et al. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17(1):28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell–mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. doi: 10.1111/ajt.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell– and antibody-mediated rejection. Am J Transplant. 2020;20(9):2318–2331. doi: 10.1111/ajt.15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363(15):1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 5.Siu JHY, Surendrakumar V, Richards JA, Pettigrew GJ. T cell allorecognition pathways in solid organ transplantation. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.02548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Berg PJ, Yong SL, Koch SD, et al. Characteristics of alloreactive T cells measured before renal transplantation. Clin Exp Immunol. 2012;168(2):241–250. doi: 10.1111/j.1365-2249.2011.04551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen CB. Acute kidney graft rejection morphology and immunology. APMIS Suppl. 1997;105(S67):5–35. [PubMed] [Google Scholar]
- 8.Ashton-Chess J, Dugast E, Colvin RB, et al. Regulatory, effector, and cytotoxic T cell profiles in long-term kidney transplant patients. J Am Soc Nephrol. 2009;20(5):1113–1122. doi: 10.1681/ASN.2008050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sablik KA, Jordanova ES, Pocorni N, Clahsen-van Groningen MC, Betjes MGH. Immune cell infiltrate in chronic-active antibody-mediated rejection. Front Immunol. 2020;10 doi: 10.3389/fimmu.2019.03106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338(25):1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 11.Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14(11):2460–2466. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solez K, Colvin RB, Racusen LC, et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN') Am J Transplant. 2007;7(3):518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 14.Colvin RB. Pathology of chronic humoral rejection. Contrib Nephrol. 2009;162:75–86. doi: 10.1159/000170814. [DOI] [PubMed] [Google Scholar]
- 15.Baeten D, Louis S, Braud C, et al. Phenotypically and functionally distinct CD8+ lymphocyte populations in long-term drug-free tolerance and chronic rejection in human kidney graft recipients. J Am Soc Nephrol. 2006;17(1):294–304. doi: 10.1681/ASN.2005020178. [DOI] [PubMed] [Google Scholar]
- 16.Najafian N, Chitnis T, Salama AD, et al. Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest. 2003;112(7):1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davila E, Kang YM, Park YW, et al. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005;174(11):7292–7301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 18.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 19.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 20.Jacquemont L, Tilly G, Yap M, et al. Terminally Differentiated effector memory CD8+T cells identify kidney transplant recipients at high risk of graft failure. J Am Soc Nephrol. 2020;31(4):876–891. doi: 10.1681/ASN.2019080847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko EJ, Seo J-W, Kim KW, et al. Phenotype and molecular signature of CD8+ T cell subsets in T cell- mediated rejections after kidney transplantation. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vondran FW, Timrott K, Kollrich S, et al. Pre-transplant immune state defined by serum markers and alloreactivity predicts acute rejection after living donor kidney transplantation. Clin Transplant. 2014;28(9):968–979. doi: 10.1111/ctr.12399. [DOI] [PubMed] [Google Scholar]
- 23.Hodge G, Hodge S, Ahern J, Holmes-Liew CL, Reynolds PN, Holmes M. Up-regulation of alternate co-stimulatory molecules on proinflammatory CD28null T cells in bronchiolitis obliterans syndrome. Clin Exp Immunol. 2013;173(1):150–160. doi: 10.1111/cei.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedeoglu B, Meijers RW, Klepper M, et al. Loss of CD28 on peripheral T cells decreases the risk for early acute rejection after kidney transplantation. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betjes MGH, Litjens NHR. High numbers of differentiated CD28null CD8+ T cells are associated with a lowered risk for late rejection and graft loss after kidney transplantation. PLoS One. 2020;15(2) doi: 10.1371/journal.pone.0228096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castro-Rojas CM, Godarova A, Shi T, et al. mTOR inhibitor therapy diminishes circulating CD8+ CD28- effector memory T cells and improves allograft inflammation in belatacept-refractory renal allograft rejection. Transplantation. 2020;104(5):1058–1069. doi: 10.1097/TP.0000000000002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbon CM, Davies JK, Voskertchian A, et al. Alloanergization of human T cells results in expansion of alloantigen-specific CD8(+) CD28(-) suppressor cells. Am J Transplant. 2014;14(2):305–318. doi: 10.1111/ajt.12575. [DOI] [PubMed] [Google Scholar]
- 28.Lo DJ, Weaver TA, Stempora L, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvani J, Terada M, Lesaffre C, et al. In situ multiplex immunofluorescence analysis of the inflammatory burden in kidney allograft rejection: a new tool to characterize the alloimmune response. Am J Transplant. 2020;20(4):942–953. doi: 10.1111/ajt.15699. [DOI] [PubMed] [Google Scholar]
- 30.Yazdani S, Callemeyn J, Gazut S, et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int. 2019;95(1):188–198. doi: 10.1016/j.kint.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Homs S, Mansour H, Desvaux D, et al. Predominant Th1 and cytotoxic phenotype in biopsies from renal transplant recipients with transplant glomerulopathy. Am J Transplant. 2009;9(5):1230–1236. doi: 10.1111/j.1600-6143.2009.02596.x. [DOI] [PubMed] [Google Scholar]
- 32.Yap M, Boeffard F, Clave E, et al. Expansion of highly differentiated cytotoxic terminally differentiated effector memory CD8+ T cells in a subset of clinically stable kidney transplant recipients: a potential marker for late graft dysfunction. J Am Soc Nephrol. 2014;25(8):1856–1868. doi: 10.1681/ASN.2013080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap M, Tilly G, Giral M, Brouard S, Degauque N. Benefits of using CD45RA and CD28 to investigate CD8 subsets in kidney transplant recipients. Am J Transplant. 2016;16(3):999–1006. doi: 10.1111/ajt.13581. [DOI] [PubMed] [Google Scholar]
- 34.Cossarizza A, Chang HD, Radbruch A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur J Immunol. 2019;49(10):1457–1973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.