Abstract
An 81-year-old woman presented to our hospital due to an abnormal shadow on a chest X-ray and a 4-week-old persistent cough. Laboratory examination revealed increased serum eosinophils and immunoglobulin E. The Asthma Control Test (ACT) score and forced expiratory volume in 1 sec indicated airway obstruction. Chest computed tomography (CT) revealed mucoid impaction in the dilated left-lingular lobar bronchus. She was diagnosed with bronchial asthma and treated with a high-dose inhaled corticosteroid/long-acting β2 agonist. Two months later, her mucoid impaction in the CT image worsened; moreover, bronchoscopy revealed the white mucus plug with Charcot–Leyden crystals and filamentous fungi. The patient was diagnosed with Allergic bronchopulmonary aspergillosis (ABPA) and treatment with 30 mg/day prednisolone was started. Both the blood eosinophil count and the chest image improved almost substantially, and the steroid was discontinued after a year. Sixteen months after cessation of prednisolone treatment, peripheral eosinophilia and mucoid impaction in the left B3b recurred. For the treatment of bronchial asthma and recurrent ABPA, administration of mepolizumab was initiated. Subsequently, although her peripheral eosinophils count decreased, chest CT showed expansion of the mucoid impaction and IgE increased despite mepolizumab treatment. Alternative subcutaneous injection therapy with dupilumab improved chest image, serum IgE level, and her ACT score.
After changing from mepolizumab to dupilumab, her ABPA, asthma, and pulmonary function improved remarkably. This case illustrates the potential utility of dupilumab for ABPA without re-administration of oral prednisolone. Additional research is needed to identify an effective therapy for ABPA with asthma.
Keywords: Allergic bronchopulmonary aspergillosis, Dupilumab, Mepolizumab, Prednisolone, IL-4, IL-13
Abbreviations: ABPA, Allergic bronchopulmonary aspergillosis; ACT, Asthma Control Test; ANCA, Antineutrophil cytoplasmic antibody; CT, Computed tomography; EETosis, Eosinophil extracellular trap cell death; ETosis, Extracellular trap cell death; FEV1, Forced expiratory volume in 1 sec; ICS, Inhaled corticosteroid; IgE, Immunoglobulin E; ILC2, Group-2 innate lymphoid cells; IL-5, Interleukin-5; LABA, Long-acting beta-agonist; PSL, Prednisolone
Highlights
-
•
Systemic corticosteroids are the standard treatment for ABPA.
-
•
ABPA often recurs and requires prolonged courses of corticosteroids.
-
•
We successfully treated a recurrent ABPA case by changing mepolizumab to dupilumab.
-
•
Dupilumab can be used as an alternative for prednisolone for treating ABPA.
1. Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is an eosinophilic pulmonary disease characterized by hypersensitivity to Aspergillus fumigatus. That manifests with asthma [1]. Sensitization to fungal allergens causes severe persistent asthma and low lung function [2]. The standard treatment for ABPA is systemic corticosteroids. However, patients with ABPA often recur and require repeated and prolonged courses of corticosteroids that are associated with many adverse effects, such as osteoporosis, myopathy, cushingoid appearance, insomnia, and increased risk of infection [1,3]. Recently, several studies and case reports have shown that biological treatments, such as humanized monoclonal antibody against immunoglobulin E (IgE) (omalizumab), humanized monoclonal antibody against interleukin-5 (IL-5) (mepolizumab), and humanized monoclonal antibody against IL-5 receptor α (benralizumab), are new approaches to intervene in ABPA cases [4].
Dupilumab is a humanized antibody against IL-4Rα that blocks IL-4 and IL-13 signaling. Dupilumab is approved for severe asthma, atopic dermatotis, and eosinophilic chronic rhinosinusitis with polyps. In clinical trials, dupilumab significantly reduced severe asthma exacerbation and improved lung function [5]. However, there are few reports of treatment with dupilumab for ABPA [6]. We present a case of recurrent ABPA complicated with bronchial asthma in which the patient's asthma symptoms and ABPA rapidly improved after changing from mepolizumab to dupilumab.
2. Case presentation
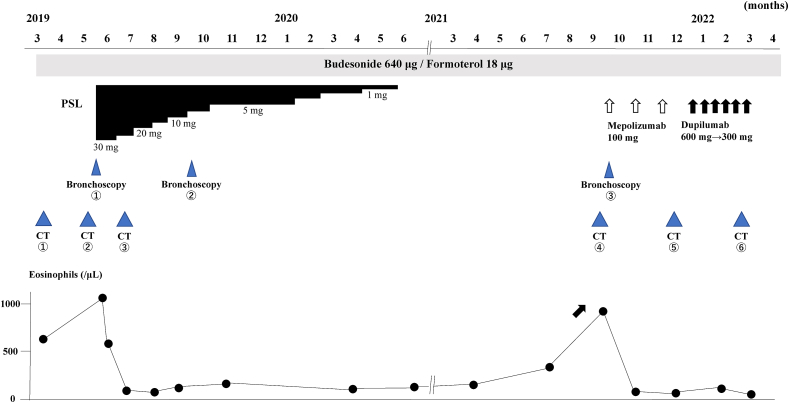
An 81-year-old never-smoker woman was referred to our hospital because of an abnormal shadow on chest X-ray and a 4-week-old persistent cough. She was diagnosed with allergic rhinitis, hyperlipidemia, and hypertention seven years ago. Wheezing on maximal forced exhalation was present. An uncontrolled asthma state was indicated by an Asthma Control Test (ACT) score of 13. The total serum immunoglobulin (Ig)E level was 241 IU/mL (0–148 IU/mL). Inflammatory markers indicated high peripheral blood eosinophilia (642/μL) and elevated FeNO (59 ppb). Pulmonary function test showed a forced expiratory volume in 1 sec (FEV1) of 1720 mL (115.4% of predicted value) and FEV1/forced vital capacity of 80.4%. Serum proteinase-3 anti-neutrophil cytoplasmic antibody (ANCA) and myeloperoxidase-ANCA results were negative (<1.0 U/mL). Serological test results for Aspergillus-specific IgE were positive, and an antibody test against Aspergillus gave a negative result. Fig. 1 shows the patient's clinical course. Chest computed tomography (CT) demonstrated pulmonary infiltrates and left-lingular lung lobe bronchiectasis with high-attenuation mucoid impaction (Fig. 2a and b). On referral, she was diagnosed with bronchial athma; hence, we first administered an inhaled corticosteroid (ICS)/long-acting beta-agonist (LABA) (budesonide 640 μg/formoterol 18 μg). Two months after the referral, pulmonary infiltrates had increased (Fig. 2c).
Fig. 1.
Clinical course of the patient.
With prednisolone (PSL) treatment, peripheral blood eosinophil count decreased. The clinical course remained stable as PSL was gradually tapered and discontinued after 1 year. After discontinuance on September 2021, recurrence of ABPA was observed and mepolizumab was started. After 3 months of mepolizumab treatment, ABPA recurred a second time, so mepolizumab was replaced with dupilumab. Subsequently, dramatic improvement in ABPA was observed. Bronchoscopy was performed 2 months after the original referral (May 2019: Bronchoscopy #1), 4 months after treatment with prednisolone (September 2019: Bronchoscopy #2), and at the recurrence of ABPA (September 2021: Bronchoscopy #3). Chest computed tomography (CT) was performed on referral (March 2019: CT #1), 2 months after referral (May 2019: CT #2), 2 months after treatment with prednisolone (July 2019: CT #3), at recurrence of ABPA before treatment with mepolozumab (September 2021: #4), 3 months after treatment with mepolizumab (November 2021: CT #5), and 3 months after treatment with dupilumab (February 2022: CT #6).
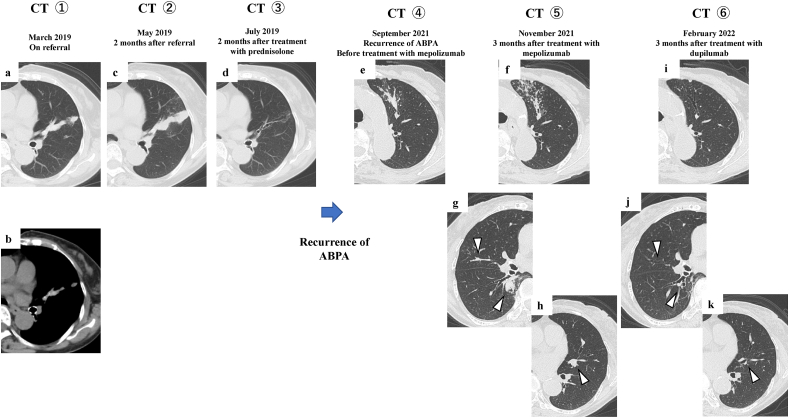
Fig. 2.
Chest CT imaging during the clinical course.
Non-enhanced chest CT revealed high attenuation of the mucoid impaction in the left-lingular lobe on referral (March 2019: CT #1) (a, b). Mucoid impaction in the left-lingular lobe has spread 2 months after referral (May 2019: CT #2) (c). Mucoid impaction in the left-lingular lobe has disappeared 2 months after treatment predonisolone (July 2019: CT #3) (d). Mucoid impaction newly occluded in the left-upper lobe on recurrence of ABPA (September 2021: CT #4) (e). The improvement of mucoid impaction in the left-upper lobe is poor and newly mucoid impactions can be seen in the right-upper lobe, right-lower lobe, and left-upper lobe 3 months after treatment with mepolizumab (November 2021: CT #5) (f, g,and h). All mucoid impactions have disappeared 3 months after treatment with dupilumab (Feburuary 2022: CT #6) (i, j, and k).
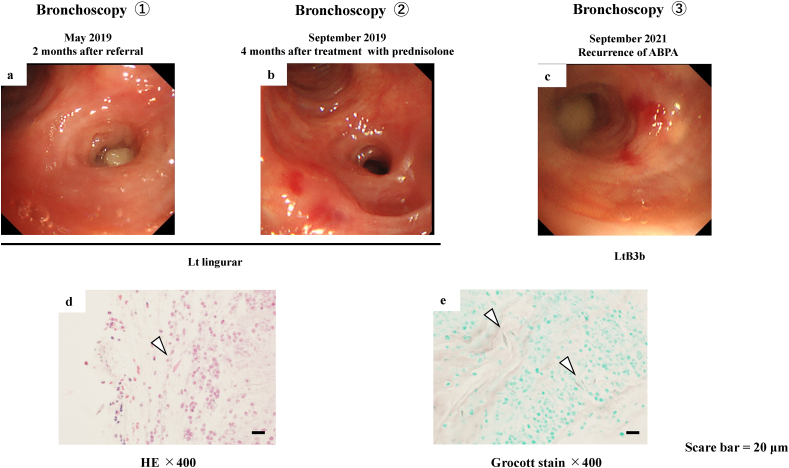
Bronchoscopy revealed that the mucosa of the orifice of the left-lingular lobe bronchus was reddened and edematous, with a mucous plug (Fig. 3a). A biopsy specimen from the lesion was pathologically proven to be a mucoid impaction containing abundant eosinophils and Charcot–Leyden crystals (Fig. 3d). Filamentous fungi suggesting Aspergillus spp. were also observed (Fig. 3e). Bacterial culture did not reveal anything significant in a brushing sample, with no obvious fungi noted on pathological examination. According to the new clinical diagnostic criteria for ABPA/allergic bronchopulmonary mycosis, we diagnosed her with ABPA [7]. After bronchoscopy, systemic oral prednisolone (PSL) 30 mg was administered. Four months later, bronchoscopy showed that the mucoid impaction had disappeared (Fig. 3b). Two months later, her respiratory symptoms and chest radiography findings showed improvement (Fig. 2d). Prednisolone was then gradually tapered and discontinued after 1 year, and her clinical course was stable during the continuous treatment of high-dose ICS/LABA without increased eosinophils.
Fig. 3.
Bronchoscopic and histologic imaging.
Bronchoscopy showing that the orifice of the left-lingular lobe bronchus is occluded by mucoid impaction accompanied by mucosal edema 2 months after referral (May 2019: Bronchoscopy #1) (a). The mucoid impaction has disappeared 4 months after treatment with predonisolone. (September 2019: Bronchoscopy #2) (b). Bronchoscopic findings showing mucoid impaction in the orifice of the left B3b accompanied by mucosal edema on recurrence (September 2021: Bronchoscopy #3) (c). Hematoxylin and eosin staining showing that the biopsies from the mucoid impaction contain abundant eosinophils and Charcot–Leyden crystals (arrowhead) (May 2019) (d). Filamentous fungal hyphae are noted on the Grocott-stained specimen (e).
Scale bar = 20 μm.
Starting at 16 months after discontinuance of predonisolone, her clinical symptoms gradually recurred with increasing peripheral blood eosinophils (peripheral blood eosinophil count: 958/μL) (Table 1). Chest CT revealed new bronchial mucus impaction in the left-upper-lung lobe without recurrence in the left-lingular lobe (Fig. 2e). Bronchoscopy revealed bronchial mucus plugs in B3b of the left-upper lobe (Fig. 3c). Her poor pulmonary function got worse (Table 1).
Table 1.
Clinical course of the patient.
| March, 2019 Pretreatment with prednisolone |
August, 2019 Posttreatment with prednisolone (after 3 months) |
September 2021 Pretreatment with mepolizumab |
November 2021 Posttreatment with mepolizumab (after 3 months) Pretreatment with dupilumab |
Feburary, 2022 Posttreatment with dupilumab (after 3 months) |
|
|---|---|---|---|---|---|
| FEV1 (mL) | 1720 | 1900 | 1760 | 1720 | 1900 |
| %FEV1 (%) | 115.4 | 131.9 | 123.9 | 121.1 | 134.7 |
| FEV1/FVC (%) | 80.4 | 85.2 | 80.4 | 82.69 | 84.1 |
| FeNO (ppb) | 59 | 15 | 18 | 13 | 13 |
| Eosinophils (cells/μL) | 1137 | 70 | 958 | 91 | 96 |
| IgE (U/L) | 241 | 755 | 929 | 4110 | 1660 |
| ACT | 13 | 24 | 18 | 13 | 25 |
FEV1: Forced expiratory volume in 1 sec, FVC: Forced vital capacity, FeNO: Fractional exhaled nitric oxide, ACT: Asthma control test.
Howerver, she refused to undergo re-administration of prednisolone because of full moon-like face as a side effect. Therefore, to control exacerbation of asthma with ABPA, mepolizumab (100 mg/4 weeks) was started. Her symptoms slightly improved, and the eosinophils dramatically decreased. However, after 3 months on mepolizumab, a second recurrence of ABPA occurred with a remarkable increase in IgE (Fig. 2f, g, h) (Table 1). Therefore, mepolizumab was replaced by dupilumab with a subcutaneous induction dose of 600 mg followed by a maintenance dose of 300 mg/2 weeks. Three months later, she remained in complete remission of ABPA with low serum total IgE levels (1660 U/L) (Table 1). After 3 months of dupilumab treatment, her peripheral eosinophil count had decreased to 96/μL, and mucus impaction was no longer visible on chest CT (Fig. 2i, j, k). Furthermore, pulmonary function test results showed remarkable improvement (Table 1). In this case, administration of dupilumab for 5 months resulted in the absence of ABPA recurrence.
3. Discussion
This was a case of refractory ABPA complicated by asthma with peripheral eosinophilia that was successfully treated after changing mepolizumab to dupilumab.
Eosinophilic inflammation is an important role in mucus plug formation, which is a major pathological component and a characteristic feature of ABPA. Extracellular trap cell death (ETosis) is a critical mechanism of host defense. Eosinophil ETosis (EETosis) is thought to have a pivotal role in mucus plug formation, especially in ABPA [8]. A review of eight cases reported that mepolizumab, a humanized monoclonal antibody targeting human IL-5, had significant effects on ABPA and was considered to be a possible treatment [9]. IL-5 is essential for eosinophil differentiation, activation, migration, and survival [10]. Stimulation of eosinophils with IL-5 and platelet-activating factor has been shown to lead to rapid EETosis [10]. The effects of treatment of ABPA with mepolizumab suggest the importace of EETosis in ABPA [9]. Furthermore, reduction of eosinophilic inflammation by treatment with an anti-IL-5 receptor antibody, benralizumab, is expected to reduce mucus hypersecretion and mucoid impaction. However, a case of a patient who developed massive atelectasis by mucoid impaction during treatment with anti-IL-5 receptor antibody has been reported [11]. Mucoid impaction increased and IgE levels were elevated in this case, despite treatment with the anti-IL-5 antibody mepolizumab.
Dupilumab is an IL-4Rα antibody that inhibits IL-4 and IL-13 signaling. In the present case, dupilumab has shown efficacy against both asthma and ABPA after 3 months of administration. There was a difference in the effects of mepolizumab and dupilumab on the targeted cytokines. IL-4 and IL-13 are major cytokines associated with type 2 inflammation. IL-4 has a pivotal role in differentiation of naive T helper 0 to Th2 cells. Th2 cells produce Th2 cytokines, such as IL-4, IL-5, and IL-13. Furthermore, Th2 cells produce chemokines, such as eotaxin, and regulated on activation normal T cell expressed and secreted, which is a specific chemoattractant for eosinophil cells [12].
In the present case, the symptoms of asthma and bronchial mucus plugs improved, followed by improvement in lung airflow obstruction caused by a decrease in the peripheral blood eosinophil count after changing mepolizumab to dupilumab administration. CT revealed a reduction in mucus secretion in the bronchus after changing mepolizumab to dupilumab. In the present case, total IgE levels at several stages of therapy affect the efficacy. After the administration of mepolizumab, total IgE level was elevated; on the CT image, the patient's mucoid impaction did not improve. Clinically, ABPA is characterized by specific IgE/IgG antibodies to Aspergillus fumigatus due to type I and III hypersensitivity reactions [8]. The change in IgE is reported to be important in monitoring therapy for recurrent ABPA exacerbations [13].
After the administration of dupilumab, total IgE level was decreased and mucoid impaction was improved. Blockade of IL-4 inhibits IgE production. Moreover, IL-4 is essential for the induction of B cell-dependent IgE production [14]. Change in blood eosinophils and levels of IgE suggested that dupilumab not only improved eosinophilic inflammation but also mepolizumab-resistant levels of IgE. In addition, omalizumab, an anti-IgE antibody, is reported to be effective in ABPA, and it is one of selective therapies for ABPA [15]. However, after mepolizumab administration, serum IgE was significantly elevated (4110 U/L). Omalizumab's indication is restricted to cases showing IgE levels of <1500 U/L [15]. Mucus plugs in patients with asthma contribute to eosinophilia and airflow obstruction associated with an upregulated IL-4/IL-13 pathway [16]. In recent years, it has been reported that group-2 innate lymphoid cells (ILC2), a type of lung-resident innate effector cell, were strongly mediated by allergic inflammation through production of IL-5 and IL-13 [17]. Dupilumab suppressed ILC2 and reduced the production of IL-5 and IL-13 [18]. Airway eosinophils and mucus were associated with upregulated IL-4/IL-13 [16]. These reports suggest that dupilumab suppressed not only the IL4/IL-13 pathway but also the IL-5 pathway, resulting in suppressed production of mucus. Eight cases of ABPA treated with dupilumab were previously reported [6]. Dupilumab appears to have good potential as a therapeutic agent for ABPA.
To the best of our knowledge, only one case of ABPA successfully treated by changing mepolizumab to dupilumab has been previously reported [19]. Our case of recurrent ABPA with asthma also was successfully treated after changing mepolizumab to dupilumab. Dupilumab therapy improves the quality of life of patients with asthma and ABPA by regulating eosinophilic inflammation in ABPA. Dupilumab may be a treatment option for patients with ABPA who have difficulty in using prednisolone because of its side effects. Further studies are required to explore the general and specific roles of dupilumab in the treatment of asthma and recurrent ABPA.
4. Conclusion
-
•
Dupilumab therapy benefits patients with asthma and ABPA by regulating eosinophilic inflammation in ABPA
-
•
Dupilumab also may benefit patients with ABPA who have difficulty in using prednisolone because of its side effects.
-
•
The general and specific roles of dupilumab in the treatment of asthma and recurrent ABPA would be good subjects for future studies.
Authors’ contributions
Y.K. wrote the manuscript. All authors contributed to editing the manuscript and approved the final version.
Funding
No funding was received for this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank Enago (www.enago.jp) for English language editing.
References
- 1.Agarwal R., Chakrabarti A., Shah A., Gupta D., Meis J.F., Guleria R., Moss R., Denning D.W. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy: J. Br. Soc. Allergy Clin. Immunol. 2013;43(8):850–873. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 2.Fukutomi Y., Taniguchi M. Sensitization to fungal allergens: resolved and unresolved issues. Allergol. Int.: Off. J. Japanese Soc. Allergol. 2015;64(4):321–331. doi: 10.1016/j.alit.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Eraso I.C., Sangiovanni S., Morales E.I., Fernández-Trujillo L. vol. 14. 2020. (Use of Monoclonal Antibodies for Allergic Bronchopulmonary Aspergillosis in Patients with Asthma and Cystic Fibrosis: Literature Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewington-Gower E., Chan L., Shah A. vol. 12. 2021. (Review of Current and Future Therapeutics in ABPA). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro M., Corren J., Pavord I.D., Maspero J., Wenzel S., Rabe K.F., Busse W.W., Ford L., Sher L., FitzGerald J.M., et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura T., Okano T., Naito M., Tsuji C., Iwanaka S., Sakakura Y., Yasuma T., Fujimoto H., D'Alessandro-Gabazza C.N., Oomoto Y., et al. Complete withdrawal of glucocorticoids after dupilumab therapy in allergic bronchopulmonary aspergillosis: a case report. World J.Clin. cases. 2021;9(23):6922–6928. doi: 10.12998/wjcc.v9.i23.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano K., Hebisawa A., Ishiguro T., Takayanagi N., Nakamura Y., Suzuki J., Okada N., Tanaka J., Fukutomi Y., Ueki S., et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J. Allergy Clin. Immunol. 2021;147(4):1261–1268. doi: 10.1016/j.jaci.2020.08.029. e1265. [DOI] [PubMed] [Google Scholar]
- 8.Ueki S., Hebisawa A., Kitani M., Asano K., Neves J.S. Allergic bronchopulmonary aspergillosis-A luminal hypereosinophilic disease with extracellular trap cell death. Front. Immunol. 2018;9:2346. doi: 10.3389/fimmu.2018.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolebeyan A., Mohammadi O., Vaezi Z., Amini A. Mepolizumab as possible treatment for allergic bronchopulmonary aspergillosis: a review of eight cases. Cureus. 2020;12(8) doi: 10.7759/cureus.9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagase H., Ueki S., Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int.: Off. J. Japanese Soc. Allergol. 2020;69(2):178–186. doi: 10.1016/j.alit.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Takimoto T., Kagawa T., Tachibana K., Arai T. vol. 8. 2020. (Massive Atelectasis by Mucoid Impaction in an Asthma Patient during Treatment with Anti-interleukin-5 Receptor Antibody). 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson D., Humbert M., Buhl R., Cruz A.A., Inoue H., Korom S., Hanania N.A., Nair P. vol. 47. 2017. pp. 161–175. (Revisiting Type 2-high and Type 2-low Airway Inflammation in Asthma: Current Knowledge and Therapeutic Implications). 2. [DOI] [PubMed] [Google Scholar]
- 13.Ricketti A.J., Greenberger P.A., Patterson R. Serum IgE as an important aid in management of allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 1984;74(1):68–71. doi: 10.1016/0091-6749(84)90089-7. [DOI] [PubMed] [Google Scholar]
- 14.Kariyawasam H.H., James L.K. vol. 14. 2020. pp. 1757–1769. (Dupilumab: Clinical Efficacy of Blocking IL-4/IL-13 Signalling in Chronic Rhinosinusitis with Nasal Polyps). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J.X., Fan L.C., Li M.H., Cao W.J., Xu J.F. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literature. Respir. Med. 2017;122:33–42. doi: 10.1016/j.rmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Svenningsen S., Haider E., Boylan C., Mukherjee M., Eddy R.L., Capaldi D.P.I., Parraga G., Nair P. CT and functional MRI to evaluate airway mucus in severe asthma. Chest. 2019;155(6):1178–1189. doi: 10.1016/j.chest.2019.02.403. [DOI] [PubMed] [Google Scholar]
- 17.Jin J., Sunusi S., Lu H. Group 2 innate lymphoid cells (ILC2s) are important in typical type 2 immune-mediated diseases and an essential therapeutic target. J. Int. Med. Res. 2022;50(1) doi: 10.1177/03000605211053156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel G., Pan J., Ye L., Shen X., Rosloff D., D'Souza S.S., Fung I.T.H., Celstin J., Sun W., Sankar P., et al. vol. 50. 2020. pp. 267–270. (Blockade of IL-4Rα Inhibits Group 2 Innate Lymphoid Cell Responses in Asthma Patients). 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikura S., Saraya T., Yoshida Y., Oda M., Ishida M., Honda K., Nakamoto K., Tamura M., Takata S., Shimoyamada H., et al. Successful treatment of mepolizumab- and prednisolone-resistant allergic bronchopulmonary aspergillosis with dupilumab. Inten. Med (Tokyo, Japan) 2021;60(17):2839–2842. doi: 10.2169/internalmedicine.6679-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.