Abstract
Obesity is a complex, chronic disease and global public health challenge. Characterized by excessive fat accumulation in the body, obesity sharply increases the risk of several diseases, such as type 2 diabetes, cardiovascular disease, and nonalcoholic fatty liver disease, and is linked to lower life expectancy. Although lifestyle intervention (diet and exercise) has remarkable effects on weight management, achieving long-term success at weight loss is extremely challenging, and the prevalence of obesity continues to rise worldwide. Over the past decades, the pathophysiology of obesity has been extensively investigated, and an increasing number of signal transduction pathways have been implicated in obesity, making it possible to fight obesity in a more effective and precise way. In this review, we summarize recent advances in the pathogenesis of obesity from both experimental and clinical studies, focusing on signaling pathways and their roles in the regulation of food intake, glucose homeostasis, adipogenesis, thermogenesis, and chronic inflammation. We also discuss the current anti-obesity drugs, as well as weight loss compounds in clinical trials, that target these signals. The evolving knowledge of signaling transduction may shed light on the future direction of obesity research, as we move into a new era of precision medicine.
Subject terms: Metabolic disorders, Molecular medicine
Introduction
Obesity, defined as a body mass index (BMI) ≥30 kg/m2, is a complex chronic disease characterized by an excessive accumulation of fat or adipose tissue in the body.1 According to a report by the Non-Communicable Disease Risk Factor Collaboration, the prevalence of obesity increased worldwide from 1975 to 2016, ranging from 3.7% in Japan to 38.2% in the United States.2 The World Health Organization (WHO) describes obesity as one of the most blatantly visible and under-appreciated public health problems that increase the risk of multiple diseases, such as type 2 diabetes (T2D), cardiovascular disease, hypertension, nonalcoholic fatty liver disease, and certain cancers.3–6 Although the positive relationship between obesity and individual mortality/morbidity has been recognized for more than 20 years, the global prevalence of obesity continues to increase, and the WHO estimates that one out of five adults worldwide will be obese by 2025.4
Usually, obesity occurs when the body’s energy intake exceeds energy expenditure, which is influenced by inherited, physiological, and/or environmental factors.7,8 Indeed, genome-wide association studies have identified more than 300 single-nucleotide polymorphisms and 227 genetic variants related to obesity, although their functional impact on the obese phenotype is still a mystery.9,10 Accumulating evidence shows that unhealthy lifestyles lead to obesity.11–14 Moreover, exposure to environmental endocrine disruptors such as bisphenol A and perfluoroalkyl substances also increases susceptibility to obesity.15–18 Even worse, these acquired factors not only disturb the balance of energy metabolism at the posttranscriptional level,19 but also change the epigenetic inheritance of individuals and thereby make their offspring more susceptible to obesity.20–22
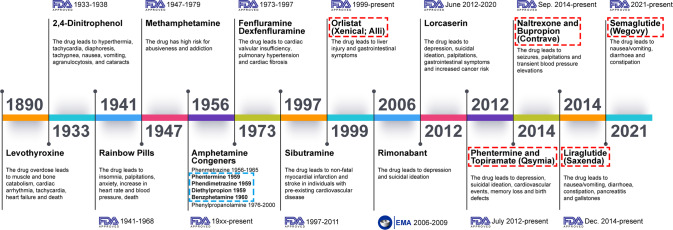
With advances in science and technology as well as the rapid growth of the pharmaceutical industry, tremendous achievements have been made in the fight against obesity;23–25 several strategies, such as calorie restriction, lifestyle management, pharmacotherapy, and bariatric surgery, have been proposed as anti-obesity remedies.26–29 Nonetheless, these interventions are incapable of meeting the global magnitude of medical needs. Recently, numerous factors/signals involved in appetite regulation and peripheral energy absorption, storage, and consumption have been revealed.30–32 These progressions shed light on the understanding of the occurrence of obesity. Some compounds targeting these signals have been translated into clinical uses. For example, appetite regulation, a hotspot of anti-obesity research, is regulated by both the central melanocortin pathway and peripheral signals such as leptin and gut hormones. Glucagon-like peptide 1 (GLP-1), a gut-derived hormone capable of decreasing blood sugar levels and improving glucose tolerance by promoting insulin secretion through cyclic adenosine monophosphate (cAMP)-based signaling pathways,33–35 can also reduce appetite by directly stimulating proopiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) (anorexigenic neurons) but suppressing agouti-related protein (AgRP)/neuropeptide Y (NPY) neurons (orexigenic neurons) through γ-aminobutyric acid (GABA)-dependent signaling.32 These findings make GLP-1 a crucial target for the treatment of obesity and other metabolic disorders.36–38 Indeed, Liraglutide, a kind of GLP-1 analog, has been introduced into the clinical treatment of T2D and obesity.
Although the underpinnings of its pathogenesis are not yet fully understood yet, obesity is well recognized as a heterogeneous disorder regulated by multiple pathways.39–42 The evolving understanding of the signaling pathways involved in obesity occurrence and development allows us to fight obesity in a more precise way. In this review, we summarize the signals/pathways involved in the pathogenesis of obesity, specifically in appetite regulation, adipose tissue metabolism and function, glucose hemostasis, and energy expenditure (Fig. 1), and discuss the current anti-obesity medications (AOMs) in clinical use or under clinical trials, that target these signals.
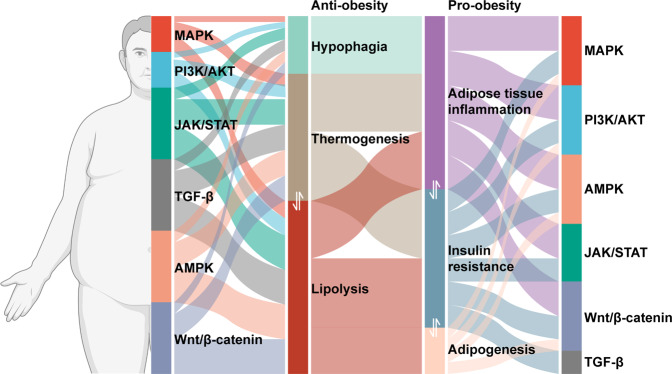
Fig. 1.
Signaling pathways involved in pro-obesity and anti-obesity mechanisms. Insulin resistance, adipose tissue inflammation, and adipogenesis constitute pro-obesity mechanism. Anti-obesity mechanism is composed of thermogenesis, lipolysis, and hypophagia
Signaling pathways in the pathogenesis of obesity
Obesity and the MAPK pathway
Mitogen-activated protein kinases (MAPKs) are critical mediators of signal transduction in mammalian cells.43 MAPK signaling contains a three-tiered kinase cascade composed of a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK), and the MAPK, which connects extracellular stimuli to intracellular signals.44 Upon phosphorylation by MAPK, downstream transcription factors are activated to mediate gene expression and initiate cellular events such as proliferation, inflammation, differentiation, and apoptosis.45,46 MAPK signaling members, including extracellular signal-regulated kinase (ERK) 1/2, c-Jun N-terminal kinase (JNK), and p38 MAPK, play a pivotal role in the regulation of appetite, adipogenesis, glucose homeostasis, and thermogenesis (Fig. 2).47,48
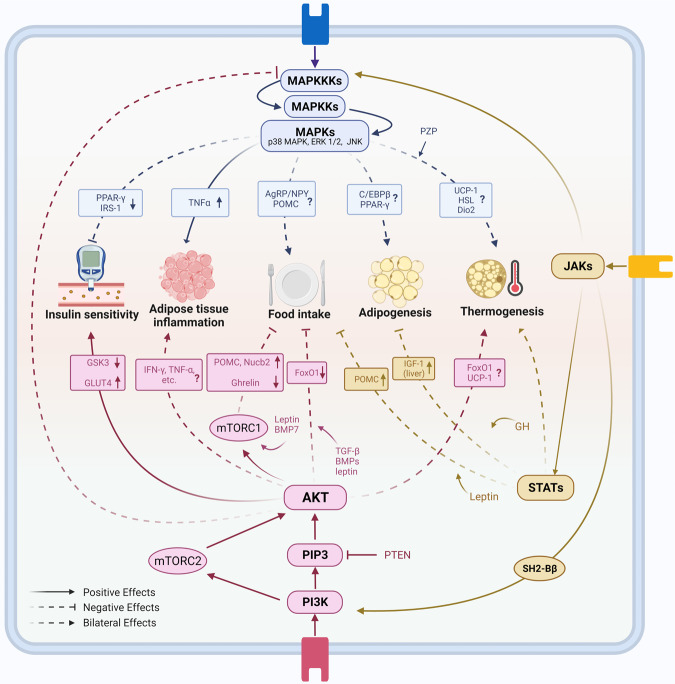
Fig. 2.
MAPK, PI3K, and JAK/STAT signaling pathways in obesity pathogenesis. MAPK signaling pathway includes a three-tiered kinase cascade consisting of MAPK kinase kinases (MAPKKKs), MAPK kinases (MAPKKs), and MAPKs. MAPKs such as ERK1/2, JNK, and p38 MAPK play complicated roles in adipogenesis and appetite regulation. Activation of MAPKs induced adipose tissue inflammation in obesity. MAPKs also cause insulin resistance in obesity by inactivating IRS1 directly and PPAR-γ indirectly. MAPKs signaling pathway plays diverse roles in adipose tissue browning and thermogenesis. PI3K-AKT pathway is closely related to insulin signaling. It increases GLUT4 and downregulates GSK3, resulting in insulin effects. PI3K-AKT signaling in lymphoid cells reduces adipose tissue inflammation to fight against obesity, while it results in the opposite direction in myeloid cells. Besides, PI3K-AKT-mTOR signaling negatively regulates food intake and has a bidirectional effect on thermogenesis. JAK-STAT signaling pathway consists of JAK1, 2, and 3, and STAT family includes STAT1, 2, 3, 4, 5a, 5b, and 6. JAKs cannot only activate STATs, but also MAPKKKs and PI3K. JAK-STAT pathway participates in leptin-mediated anorectic effects. In the liver, the activation of JAK-STAT signaling is negatively related to the accumulation of fat. Notably, there are different impacts from different JAKs and STATs on BAT-related thermogenesis
MAPK-mediated appetite regulation, as well as other MAPK functions in the central nervous system (CNS), contributes to the pathogenesis of obesity. ERK1/2 enhances glucose-stimulated POMC expression in hypothalamic neurons and participates in anorexigenic action.49 Moreover, JNK3 is essential in the effect of the leptin on AgRP neurons in high-fat diet (HFD)-fed mice.50 In addition, JNK1 knockout in the CNS decreases food intake and enhances energy expenditure by blocking the negative feedback of the hypothalamic-pituitary–thyroid axis, and ablation of JNK1 and JNK2 in the pituitary reduces the expression of Dio2, a negative regulator blocking thyroxine-mediated adaptive thermogenesis and lipid accumulation.51,52
ERK signaling is indispensable in the early steps of adipocyte differentiation, as ERK1−/− mice are resistant to the development of adiposity under HFD feeding; preadipocytes from these mice as well as embryo fibroblasts exhibit impaired adipogenesis.53 However, there are in vitro studies with the opposite observation that sustained activation of ERK decreases adipogenesis by inhibiting peroxisome proliferator-activated receptor (PPAR)γ expression via MAPK-mediated phosphorylation.54,55 Considering the different experimental models, these controversial results should be interpreted cautiously. In vitro studies usually lack an appropriate microenvironment for cell interaction, and that may contribute to the inconsistency between in vitro and in vivo results. Similarly, the role of p38 MAPK in adipogenesis is also controversial. In primary embryonic fibroblasts from embryonic mice and preadipocytes from adulthood mice with p38 MAPK subunit knockout or inhibition, the phosphorylation of CCAAT-enhancer binding protein (C/EBP) β is enhanced, and PPARγ expression is increased, suggesting that p38 MAPK suppresses adipogenesis.56 Conversely, increased p38 MAPK activity is observed during human preadipocyte differentiation in vitro, and pharmacological inhibition of p38 MAPK in these cells reduces the accumulation of triglycerides and the expression of PPARγ together with other adipogenesis markers.57 Suppression of p38 MAPK activity also blocks adipogenesis in 3T3-L1 cells.58 In vivo, treatment with a p38 inhibitor reduces C/EBPβ phosphorylation and decreases PPARγ expression.59 In human white adipose tissue (WAT), the increased number of hypertrophic adipocytes is also associated with the upregulated p38 MAPK signals, and the phosphorylated p38 MARK is coupled with fasting levels of triglycerides, insulin, and glucose.60 Together, these findings suggest that p38 MAPK has bifunctional effects on adipocyte differentiation and adipogenesis. There is a possible interpretation that p38 MAPK functions differently in human and mouse preadipocytes.
There is a complex association between obesity and insulin resistance. The MAPK signaling pathway is closely involved in the development of insulin resistance. By dephosphorylating and deactivating multiple MAPKs, dual specificity phosphatase 9 restores the tyrosine phosphorylation level of insulin receptor substrate-1 (IRS1) and its capacity to mediate insulin signal transduction.61 Similarly, deficiency of caspase recruitment domain 9, an endogenous activator of MAPKs, mitigates HFD-induced insulin resistance and adipocyte enlargement.62 Phosphorylation of PPARγ by ERK enhances the ability of transcriptional coactivator with PDZ-binding motif to negatively regulate PPARγ and impair insulin sensitivity.63 JNK1 and JNK2 induce insulin resistance via serine/threonine phosphorylation of IRS, while JNK3 may improve insulin sensitivity in obesity.64 Ablation of MAPK phosphatase-1 in skeletal muscle, which activates both JNK and p38 MAPK, leads to increased insulin sensitivity and elevated energy expenditure, making mice resistant to the development of diet-induced obesity.65 However, the p38 MAPK pathway, through enhancement of the mRNA stability and nuclear migration of X-box binding protein 1 in the liver, maintains glucose homeostasis in the context of obesity, demonstrating its complicated impacts in different models.66 A recent study summarized that regulation of lipid metabolism by p38 MAPK was tightly connected to calcium ions.67 Notably, insulin resistance in adipose tissue may result from the chronic inflammation induced under obese condition. Inhibition of MAPKs is associated with less inflammatory cell infiltration, improved glucose tolerance, and ameliorated adipocyte enlargement.62 In adipose tissues from HFD-fed mice, integrated multiomic analysis shows that the inflammatory genes are enriched in MAPK pathways in macrophages.68 Licochalcone F, a synthetic retrochalcone, was found to inhibit tumor necrosis factor (TNF)α-induced expression of inflammatory factors and further alleviated glucose tolerance, reduced adipocyte size, and decreased macrophage infiltration in WAT, by interacting with MAPK signaling pathway.69
Brown adipose tissue (BAT) thermogenesis alleviates obesity by increasing energy expenditure. This process is regulated by MAPK signaling.70–72 Thermogenic gene expression stimulated by substances including IL-27, irisin, cinnamaldehyde, and withaferin A, is perturbed by p38 MAPK or ERK inhibitors.70,73–75 Overexpression of mitogen-activated protein kinase kinase 6 (MEK6), an upstream repressive factor of p38/ERK, decreases the expression of uncoupling protein 1 (UCP1) and hormone-sensitive lipase (HSL) in adipocytes.76 Other stimuli, such as cold exposure, promote browning by inducing p38 MAPK signaling and secretion of fibroblast growth factor (FGF)21.77 Interestingly, pregnancy zone protein, a novel hepatokine identified in the context of intermittent fasting, can promote p38 MAPK-dependent UCP1 expression in BAT, exhibiting therapeutic potential in the treatment of obesity.78
Obesity and the PI3K/AKT pathway
The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway is a key regulator of cell growth and proliferation, and aberrant activation of this pathway promotes the development of obesity.79–81 PI3K and AKT are two major nodes in this pathway, which are activated by upstream signals such as hormones and growth factors. Upon activation, PI3K converts phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), activates phosphoinositide-dependent kinases and AKT,82,83 and then leverages glycogen synthase kinase (GSK)3, PKCs, and the forkhead box (Fox) family to regulate glycogen synthesis, glucose uptake, and adipogenesis, respectively (Fig. 2).84,85 Mammalian target of rapamycin (mTOR) is one of the key downstream targets of PI3K/AKT pathway, referred to as PI3K/AKT/mTOR pathway together with the upstream sometimes. mTOR forms two distinct complexes, mTORC1 and mTORC2; raptor and PRAS40 are the specific subunits of mTORC1, whereas rictor, mSIN1, and Protor1/2 are the specific subunits of the mTORC2 complex.86,87 mTORC1 and mTORC2 act differently in the PI3K/AKT/mTOR signaling pathway and both are closely associated with the pathogenesis of obesity (Fig. 2).
The PI3K/AKT pathway regulates appetite via the CNS and peripheral tissues. It has been reported that leptin acts on the mediobasal part of the hypothalamus to suppress food intake partially through PI3K-AKT-FoxO1 pathway,88 and selective inhibition of PI3K abolishes the effect of leptin.89 mTOR also contributes to appetite regulation in the central and peripheral systems. Stimulation of mTOR in the hypothalamus decreases food intake and ameliorates age-dependent obesity in animal studies by activating POMC neurons.88,90,91 Transforming growth factor (TGF)-β/bone morphogenetic proteins (BMPs) in the hypothalamus closely interact with PI3K/AKT pathway to reduce appetite and mitigate obesity.92 A study showed that intracerebroventricular administration of BMP7 has an anorectic effect, which could be completely abolished by rapamycin pretreatment, indicating the existence of leptin-independent BMP7-mTOR-p70S6K signaling.93 In peripheral tissues, stimulation of mTOR in gastric X/A-like cells decreases the production of ghrelin, an orexigenic hormone that also decreases UCP1 expression.94,95 Similarly, secretion of Nucb2/nesfatin1, another hormone with anorexigenic effects, is enhanced by activation of mTORC.96
The PI3K/AKT pathway is indispensable to the insulin signaling pathway. Dysregulation of this signaling is associated with the severity of obesity and insulin resistance.97–99 Negative correlation between AKT activity and body fat percentage has been found both in animal models and humans, and AKT may be responsible for insulin resistance in the obese population.100,101 Inhibition of PI3K/AKT signaling leads to degradation of Sort1, an element of the glucose transporter 4 (GLUT4) storage vesicles, and decreases insulin sensitivity.102,103 Similar findings were obtained in mice with overexpressed phosphotyrosine interaction domain containing 1, which impairs PI3K/AKT signaling and directly interacts with low-density lipoprotein receptor-related protein (LRP)1, another part of GLUT4 vesicles.104 Furthermore, repression of PPARγ, the key regulator of adipocyte differentiation, leads to insulin resistance via PI3K/AKT signaling.105 However, it is plausible that manipulation of the PI3K/AKT pathway can regulate early adipogenesis. In support of this point, alchemilla monticola functions its anti-adipogenic effect via inhibiting this pathway.106 As a negative regulator of PI3K-mediated signal transduction, phosphatase and tensin homolog (PTEN) can also impact insulin effects. Metformin was reported to restore insulin resistance via 5′-AMP-activated protein kinase (AMPK)-mediated downregulation of PTEN.107 Notably, loss of PTEN could lead to obesity with preserved insulin sensitivity.108 PTEN haploinsufficiency in humans increases the risk of obesity as a monogenic factor but decreases the risk of T2D because of enhanced insulin sensitivity.109 As the largest insulin-sensitive organ, skeletal muscle has a significant role in glucose and lipid homeostasis. In the muscle of ob/ob mice, the expression of AKT2 was lower, and insulin resistance was observed in vitro.110 The PI3K inhibitor wortmannin fully inhibits insulin-stimulated glucose uptake in skeletal muscle.111 RalGAPα1 mainly exists in skeletal muscle, blunts insulin effects by preventing translocation of GLUT4, and can be inactivated by AKT. When blocking the inactivation process of RalGAPα1 by AKT, mice showed greater fat mass, larger body weight, and elevated levels of lipid in the bloodstream in adulthood.112 Another important organ, liver, also participates in glucose and lipid metabolism. PI3K/AKT/mTOR and PI3K/AKT/FoxO1 pathways in hepatocytes are parts of insulin signaling, and participate in hepatic glucose and lipid metabolism, such as de novo lipogenesis (DNL) and hepatic glucose production (HGP).113 Using specific knockout mice, Titchenell et al. demonstrated that activation of both of the above signaling pathways by insulin was necessary and sufficient for insulin-mediated lipid metabolism in the liver. They also found that PI3K/AKT/FoxO1 pathway contributes to insulin-mediated suppression of HGP.114 GSK3 is one of the substrates of AKT. Proteomics and phosphoproteome analysis revealed a downregulated substrate motif of AKT and hyperactivation of GSK3 in islets of obese diabetic mice, with the latter at least partly contributing to β cell failure.115 Intriguingly, mice carrying mutant GSK3, which blocks phosphorylation by AKT, have higher energy expenditure and are protected from HFD-induced metabolic syndrome.116 Some microRNAs, such as miR-33, miR-143, and miR-153, can inhibit the activity of the PI3K/AKT pathway and induce glucose intolerance in obesity.117
Hyperinsulinemia is both the cause and the consequence of insulin resistance.118 The activation of PI3K and phosphorylation of AKT are blunted in human myoblasts under continuous high insulin exposure.119 PI3K is also inhibited by the activation of glucocorticoid receptor, which contributes to insulin resistance in Cushing’s syndrome.120 Adipose tissue inflammation is another cause of impaired insulin tolerance. CD4+ T cells regulate inflammation in adipose tissue and obesity. A recent study identified Kruppel-like zinc-finger family 10 in CD4+ T cells as an essential regulator of obesity, insulin resistance, and fatty liver, the effects of which are mediated by PI3K-AKT-mTOR signaling.121 Conversely, specific ablation of the insulin receptor in myeloid cells led to reduced obesity-associated inflammation in adipose tissue.122 These opposite results indicate the different roles of PI3K/AKT signaling in lymphoid and myeloid cells.
In addition, mTORC1-p70 ribosomal S6 kinase 1 (S6K1) plays an essential role in insulin action. It is upregulated and has a positive correlation with insulin resistance in human visceral fat tissue.123 Furthermore, deficiency of this signaling results in less adipose tissue mass and enhanced lipolysis.124 The dedicator of cytokinesis 5 is widely expressed in vivo and reinforces insulin sensitivity by inhibiting mTORC1-S6K1.125 On the other hand, mTORC2 is essential in insulin-inhibited hepatic gluconeogenesis, and long-term rapamycin administration impairs insulin sensitivity by disrupting mTORC2 function.126 Whereas classic PI3K/AKT signaling activates mTOR, the subclasses of PI3K, including class II and class III, play different roles in the regulation of mTOR and glycerolipid metabolism. PI3KC2β in class II PI3K and its derivative, PtdIns-(3,4)-P2, promote the interaction between endosomes/lysosomes and mTOR1 and inhibit mTORC1, and class III PI3K stimulates mTORC1 in multiple ways to influence the effects of insulin.127,128
The PI3K/AKT pathway also plays a role in thermogenesis.129 HFD feeding induces the expression of the signaling scaffolding protein Gab2 in adipose tissues. Deletion of Gab2 in mice increases the expression of UCP1 and other thermogenic genes in BAT and attenuates HFD-related weight gain through downregulation of the PI3K-Akt-FoxO1 signaling pathway.130 Whole-body overexpression of PTEN, which counteracts PI3K-mediated signal transduction, activates BAT, decreases body weight, and increases appetite in mice.131 In contrast, PTEN knockout in hypothalamic leptin-sensitive neurons increases PI3K activity and leads to browning of WAT and weight loss.132 A possible explanation is that systemic overexpression of PTEN exerts opposing effects in both the central and peripheral systems, but is more potent in the latter. Notably, upregulation of UCP1 expression by albiflorin is attributed to the activation of AMPK and PI3K/AKT pathways because the effect could be eliminated when cells were cotreated with the AMPK inhibitor Compound C or the PI3K inhibitor LY294002.133 Through the PI3K/AKT pathway, glutamine supplementation reduces waist circumference in overweight volunteers and improves glucose homeostasis in the adipose mass of HFD-fed rats.134 Suppression of mTORC1 in BAT, by ablation of raptor or dissociation of raptor by growth factor receptor binding protein-10, enhances mitochondrial respiration and thermogenesis, suggesting that mTORC1 per se has a negative effect on energy expenditure.135–137 Meanwhile, mTORC1 is also indispensable for β-adrenergic stimulation-induced brown adipogenesis under cold exposure through the phosphorylation of S6K1 to promote protein synthesis.138,139 Similarly, reducing the expression of β-adrenergic receptors via the response gene to complement 32 lowers mTORC1/S6K1 activity and decreases thermogenic gene expression.140 mTORC2 reduces UCP1 expression in BAT, and ablation of rictor, an essential component of mTORC2, increases thermogenesis and alleviates HFD-induced obesity through the Sirtuin 6 (Sirt6)-FoxO1 pathway.141
Obesity and the JAK/STAT pathway
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is one of the major intracellular signal transduction pathways and is an essential downstream mediator for various cytokines, hormones, and growth factors. The whole family of STAT proteins (STAT1, 2, 3, 4, 5a, 5b, and 6) can be activated by tyrosine phosphorylation in response to cytokine and growth factor stimulation.142 The binding of cytokines or growth factors to their cognate receptors activates JAKs (JAK1, JAK2, JAK3, or Tyk2), enabling them to transphosphorylate each other and the cytoplasmic tail of the receptor on tyrosine residues.143,144 The receptor subunits then provide a docking site for STAT proteins, which are in turn phosphorylated as well.142 The phosphorylated STAT proteins translocate to the nucleus, bind to specific DNA elements and regulate the transcription of targeted genes.145 The dysregulation of the JAK/STAT signaling pathway contributes to obesity directly or by interacting with other signaling pathways including MAPK and PI3K (Fig. 2).
The JAK/STAT signaling pathway is correlated with the melanocortin pathway since the energy homeostasis regulated by leptin is mediated by JAK/STAT.146 During leptin signaling, leptin receptor (LEPR), expressed at the plasma membrane as a dimer, activates receptor-associated JAK2 to phosphorylate LEPR, which then binds to STAT3 and STAT5. They are then phosphorylated by JAK2 to function as transcription factors.147,148 Activation of STAT3/STAT5 by LEPR is essential to control food intake.149–152 In addition, phosphorylated STAT3 induces the expression of suppressor of cytokine signaling 3, which acts as a feedback inhibitor of the leptin signaling pathway.153 Binding of leptin to LEPR results in downstream activation of Rho-kinase 1, which phosphorylates and activates JAK2 to maintain energy homeostasis.154 The binding also leads to JAK2 interaction with SH2-Bβ, which in turn promotes IRS1- and IRS2-mediated activation of the PI3K pathway.155,156 Then, it promotes transcription of POMC and increases the expression of carboxypeptidase with increased processing of POMC to α-melanocyte-stimulating hormone (α-MSH), and suppresses food intake.157 In contrast, suppression of JAK/STAT signaling in CNS is associated with decreased leptin sensitivity in POMC neurons.158
The accumulation of fat in the liver (hepatic steatosis) is a feature of obesity.159 This process is regulated in part through JAK/STAT signaling pathway by growth factors and cytokines.160,161 Studies have consistently suggested that hepatocyte-specific deficiency of STAT3 leads to insulin resistance and increased expression of gluconeogenic genes.162–164 Conversely, STAT3 activation in hepatocytes may prevent steatosis. Treatment of obese mice with STAT3-inducing cytokines (IL-6 and IL-22) or overexpression of STAT3 ameliorates hepatic fat accumulation.165,166 The pivotal role of the hepatic growth factor–JAK2–STAT5–IGF1 axis in lipid metabolism has been confirmed. Through activation of JAK2 and STAT5, growth factor plays a key role in the production of hepatic IGF1. The precise mechanism by which low growth factor levels contribute to obesity is controversial but may be attributed to decreased lipolysis in adipose tissue and increased hepatic steatosis.167 Loss of STAT5 signaling results in concurrent activation of STAT1 and STAT3 and intracellular lipid accumulation. Furthermore, there is evidence showing that mice with hepatocyte-specific deletion of JAK2 develop spontaneous steatosis as early as 2 weeks of age but manifest protection against HFD-induced insulin resistance and glucose intolerance.168
Peripheral JAK/STAT signaling pathway can be also activated by leptin.169,170 For instance, HFD-induced leptin secretion in adipose tissue increases the expression of the STAT3 target gene encoding caveolin-1, which decreases leptin signaling in a negative feedback manner.171 To further explore the role of STAT3 in adipocytes, Cernkovich et al. utilized an adipocyte-specific STAT3 mouse colony and observed increased body weight and adipose tissue mass with adipocyte hypertrophy, suggesting that STAT3 promotes lipolysis and inhibits adipogenesis.172 Moreover, mice lacking Tyk2 become progressively obese due to defective differentiation of BAT, indicating that the activation of STAT3 by Tyk2 is essential for BAT function.173 STAT4 also contributes to obesity-related pathophysiology by reducing insulin sensitivity and increasing adipocyte inflammation.174 Similarly, elevated interferon-γ levels and JAK–STAT1 signaling in obesity also lead to adipocyte dysfunction and insulin resistance.175,176 As the major upstream kinases required for STAT activity, JAK proteins also impact adipose function. Adipocyte-specific knockout of JAK2 in mice drives adiposity due to defective lipolysis,177 while pharmacological inhibition of JAK/STAT promotes UCP1 expression and browning of human adipocytes in vitro.178
Obesity and the TGF-β signaling pathway
The TGF-β superfamily consists of TGF-β1-3, activins/inhibins, growth differentiation factors (GDFs), myostatin, and BMPs, playing diverse roles in appetite regulation, lipid metabolism, and glucose homeostasis (Fig. 3).179,180
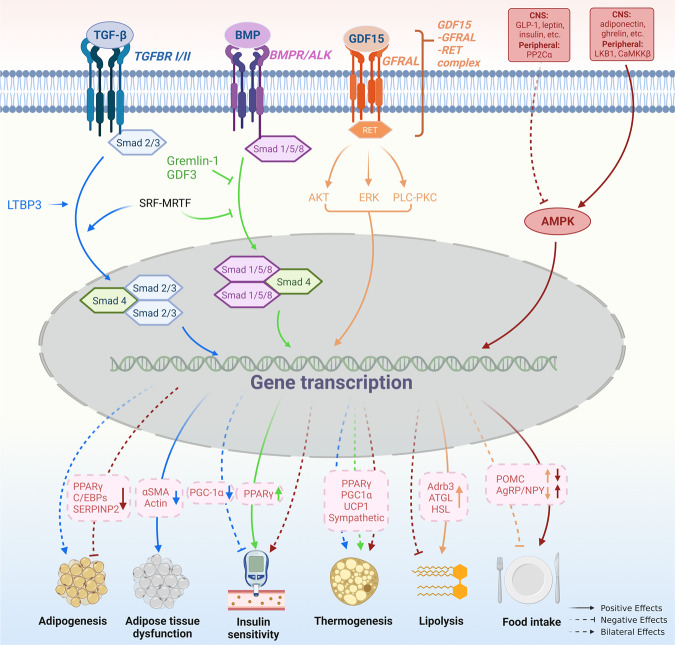
Fig. 3.
TGF-β and AMPK signaling pathways in obesity pathogenesis. The TGF-β superfamily consists of TGF-β1-3, GDFs, BMPs, etc., which play a diverse role in the development of obesity. TGF-β shows dual effects on adipogenesis/adipocyte differentiation. TGF-β inhibits MSC adipocyte commitment by phosphorylating and suppressing PPARγ and C/EBPs expression, through Smad3 signaling. However, pulsed TGF-β1 administration during the commitment phase shows a promotion effect on adipogenesis in MSC by down-regulating SERPINB2 expression. In adipocytes, TGF-β signaling is involved in adipose tissue dysfunction by enhancing the expression of myofibroblast signature genes. The role of TGF-β in BAT-associated thermogenesis is also controversial. Activation of TGF-β signaling by LTBP3 promotes WAT browning by modulating UCP1 expression, while hepatic TGF-β signaling contributes to HFD-induced steatosis and obesity by reducing mitochondrial respiration and inhibiting white-to-beige fat conversion. In addition, SRF - MRTF - axis which transcriptionally enhances the TGF-β but attenuates BMP signaling pathway suppresses brown adipogenesis. TGF-β/Smad3 signaling also plays a negative role in insulin sensitivity by suppressing PGC-1α expression in adipose tissue. BMP seems to play a contrary role to TGF- β in the regulation of insulin sensitivity by up-regulating PPARγ expression. Similar to TGF- β, the role of BMP in BAT-associated thermogenesis is inconsistent. BMP4 promotes WAT browning and this process is inhibited by Gremlin-1. However, BMP-4 signaling during the terminal differentiation phase can impair the acquisition of a mature brown adipocyte phenotype. GDF15, another member of TGF- β superfamily, was identified as a potential target for the treatment of obesity. By interacting with GFRAL and followed by the activation of AKT-, ERK-, and PLC-PKC signaling pathway, GDF15 stimulates lipolysis by up-regulating Adrb3, ATGL, and HSL expressions. It also inhibits food intake in a CNS-dependent manner via an unknown mechanism. AMPK is a heterotrimer complex. It is activated by adiponectin, ghrelin, etc. in CNS and LKB1 and CaMKKβ in peripheral tissue, and inactivated by GLP-1, leptin, etc. in CNS and PP2Cα in peripheral tissue. Activation of AMPK in CNS results in hyperphagia, insulin resistance, decreased thermogenesis, and weight gain. While, in adipocytes, it results in inhibited adipogenesis, insulin sensitiveness, enhanced thermogenesis, and weight loss. However, AMPK limits lipolysis since AMPK is an enzyme in case of energy shortage
GDF15, a member of the TGF-β superfamily, has been identified as a central regulator of appetite and a potential target for the treatment of obesity.181–183 Mice lacking GDF15 exhibit obesity and pharmacological GDF15 administration to mice triggers a taste aversive response, suggesting that GDF15 plays a regulatory role in energy balance.184 Intracerebroventricular injection of GDF15 into the lateral ventricle of mice results in reduced food intake, and this effect requires an intact brainstem area postrema (AP) and nucleus of the solitary tract, suggesting that CNS is one of the targets of GDF15 action.182 Mechanistically, by interaction with glial cell line-derived neurotrophic factor (GDNF)-family receptor α-like (GFRAL) expressed in the brainstem, GDF15 decreases vagal sympathetic nervous system (SNS) activity (vagal efferent) and delays gastric emptying.185 In addition, hGDF-15-expressing xenografts show upregulated lipolytic genes (adrenoceptor beta 3, or Adrb3; adipose triglyceride lipase, or ATGL; HSL) in both WAT and BAT, resulting in decreased adipose tissue mass.186
TGF-β signaling shows dual effects on adipogenesis/adipocyte differentiation. A study by Ahdjoudj et al. found that TGF-β functioned to inhibit mesenchymal stem cell (MSC) adipocyte commitment by phosphorylating and suppressing PPARγ expression as well as the expression of C/EBPs, partially through mothers against decapentaplegic 3 (Smad3) signaling.187,188 Deletion of TGF-β receptor 2 in MSCs resulted in a marked increase in adipocyte expansion in murine bone marrow, which was accompanied by an increase in PPARγ expression.189 However, another study found that continuous TGF-β1 treatment enhanced osteoblast differentiation as evidenced by increased mineralized matrix production, while pulsed TGF-β1 administration during the commitment phase increased mature lipid-filled adipocyte numbers.190 Global gene expression analysis revealed that serpin peptidase inhibitor clade B (ovalbumin) member 2 (SERPINB2) was significantly downregulated in TGF-β1-treated cells, and silencing of SERPINB2 in untreated cells enhanced the adipogenic differentiation capacity of both marrow osteoblast and adipocyte progenitor cells.190 These results suggest that the function of TGF-β in adipogenesis is determined by the mode of administration, and SERPINB2 was identified as the TGF-β1-responsive gene through which it negatively regulates adipogenic differentiation. In adipocytes, TGF-β1 was proven to be involved in obesity-related adipose tissue dysfunction. Adipocytes from HFD-fed mice showed enriched TGF-β1 effector protein Smad at HFD-induced promoters and enhancers and were associated with myofibroblast signature genes.191
Plasma levels of TGF-β1 are elevated in noninsulin-dependent diabetes mellitus.192 TGF-β signaling regulates glucose tolerance and energy homeostasis, and systemic blockade of TGF-β/Smad3 signaling protects mice from obesity, diabetes, and hepatic steatosis by enhancing PPARγ coactivator 1α (PGC-1α) expression in adipose tissue.193 In addition, recent studies have reported that aerobic exercise can inhibit TGF-β to improve insulin resistance,194 and inhibition of TGF-β/Smad3 signaling can prevent β-cell apoptosis,195 which is indicative of the therapeutic potential of TGF-β/Smad3 antagonists in restoring insulin sensitivity and β-cell homeostasis in diabetes. BMP signaling also interacts with the insulin signaling system to coordinately regulate glucose homeostasis. BMP-2 and BMP-6 enhance insulin-mediated glucose uptake in both insulin-sensitive and insulin-insensitive adipocytes.196,197 This function was achieved by inducing the expression and activation of PPARγ, which improves insulin sensitivity.198–201 In addition, another member of the TGF-β superfamily, GDF-3, has been shown to affect glucose uptake in vitro by limiting BMP signaling and inducing insulin resistance in vivo, and GDF-3 expression was associated with obesity-linked PPARγ S273 phosphorylation.202 From the above data, it seems that TGF-β plays a negative role in glucose homeostasis regulation, whereas BMP functions oppositely to improve insulin sensitivity.
Inconsistent results were observed in regard to the role of TGF-β in energy expenditure. Latent TGF-β-binding protein 3 (LTBP3), which regulates TGFβ activity by forming intracellular complexes with the TGF-β pro-peptide, has been demonstrated to promote WAT browning by modulating UCP1 expression and mitochondrial oxygen consumption through TGF-β2 signaling.203 However, hepatic TGF-β signaling was found to contribute to HFD-induced steatosis and obesity by reducing mitochondrial respiration and inhibiting white-to-beige fat conversion, effects that are mediated by hepatocyte-derived exosomal let-7b-5p.204 In addition, the serum response factor (SRF)–myelin-related transcription factor (MRTF) axis transcriptionally enhances TGF-β but attenuates the BMP signaling pathway and thus suppresses brown adipogenesis.205 These results indicate that the TGF-β family may play diverse roles in BAT regulation, which is determined not only by its upstream characteristics but also by its origination and the specific pathways activated.
BMP4, another member of the TGF-β superfamily, is secreted by differentiated preadipocytes and drives a beige/brown adipose phenotype in preadipocytes.206 Expression of BMP4 promotes adipocytes of WAT to present brown fat characteristics, leading to a reduction in adiposity and related metabolic disorders.207 This process can be inhibited by Gremlin-1, an extracellular antagonist of BMPs.206 Knockdown of Gremlin-1 or treatment with BMP4 during adipocyte differentiation induces a shift from a white to a brown-like phenotype.206 Thus BMP4 and its antagonist Gremlin-1 together constitute a feedback cascade to control adipogenic commitment and differentiation. Further study suggests that BMP7 has similar effects on the white-to-brown transition as BMP4 in primary human adipose stem cells.208 In contrast, there are also studies showing that BMP4 signaling during the terminal differentiation phase can instead impair the acquisition of a mature brown adipocyte phenotype, favoring a more white-like phenotype, and likewise, exposure of mature brown adipocytes to BMP4 induces a brown-to-white-like adipocyte shift.209,210 BMP8B is another important regulator of energy balance. BMP8B is expressed in both peripheral tissues including BAT and the hypothalamus. It functions peripherally to increase the response of BAT to adrenergic stimulation while acting centrally to increase sympathetic output to BAT. Bmp8b-KO mice exhibit impaired thermogenesis and reduced metabolic rate, causing weight gain despite hypophagia.211–213 It is worth noting that the effect of BMPs is dependent not only on their own levels but also on levels of cellular BMPs antagonists making the cells resistant to secreted BMPs.206 Several antagonists, such as GREM1, GREM2, and NOGGIN, are expressed in adipose tissue.206 GREMLIN-1 and NOGGIN, two powerful and secreted BMP4 inhibitors, were found to be markedly increased in adipose tissue in obesity, inhibiting BMP4-induced precursor cell commitment/differentiation and white to beige/brown adipocyte conversion.206,214 Thus, WAT becomes resistant to BMP4 action in obesity due to the increased secretion of these antagonists.
Obesity and the AMPK pathway
AMPK is a heterotrimer complex consisting of a catalytic subunit α (α1, α2) and two regulatory subunits β (β1, β2) and γ (γ1, γ2, γ3) and is activated by phosphorylation of the α subunit at Thr172.215 AMPK functions as a “fuel gauge” to monitor cellular energy status and is highly conserved across all eukaryotic species.215,216 Growing evidence suggests that brain AMPK plays a pivotal role in the development of obesity by regulating feeding, insulin sensitivity, BAT thermogenesis, and browning of WAT (Fig. 3).217
Activation of AMPK in CNS results in weight gain. David Carling and Caroline Small groups first demonstrated that hypothalamic AMPK regulates feeding behavior.218 This seminal study found that in vivo administration of leptin decreased hypothalamic AMPK activity and reduced food intake, while in vivo administration of ghrelin stimulated hypothalamic AMPK activity and increased food intake.218 A parallel work in the same year by Barbara Kahn et al revealed that AMPK is highly expressed in many hypothalamic regions and regulation of hypothalamic AMPK is part of a feedback system to the physiological modulation of feeding.219 Therefore, refeeding diminishes but fasting boosts the AMPK activity in the hypothalamus.218,219 From a macro-perspective, activation and inhibition of hypothalamic AMPK increases and decreases body weight, respectively.219 This was subsequently validated by the weight monitoring of mice lacking AMPKα2 in POMC or AgRP neurons of the arcuate nucleus (ARC). POMCα2KO mice developed obesity while AgRPα2KO mice developed an age-dependent lean phenotype.220 AMPK inhibition in both the ARC and the VMH can cause severe and prolonged hypoglycaemia.221,222 In contrast, AMPK activation in the VMH can cause insulin resistance.222 Moreover, accumulating evidence supports that hypothalamic AMPK manages BAT thermogenesis via its modulation of the SNS.215,217 Targeted administration of triiodothyronine in the VMH of the hypothalamus leads to decreased AMPK activity, elevated SNS activity, increased BAT thermogenesis, and reduced weight.223 Besides, central administration of estradiol inactivates AMPK in the VMH of the hypothalamus, resulting in SNS-mediated activation of BAT thermogenesis and weight loss.224 Furthermore, Nogueiras et al found that central injection of liraglutide in mice resulted in weight loss independent of hypophagia. Instead, such reduced weight is caused by AMPK-mediated BAT thermogenesis and adipocyte browning in the VMH of the hypothalamus.225
Intriguingly, the activation of AMPK in adipocytes results in weight loss. First, activated AMPK in brown and beige adipocytes increased non-shivering thermogenesis and improved insulin sensitivity.226 Second, it is reported that reduced body weight and improved insulin sensitivity by a low-calorie diet or bariatric surgery are closely related to increased AMPK activation in adipose tissue.226 Third, AMPK activation diminishes adipogenesis in adipocytes via shutting down eIF2α-dependent translation, activating WNT/β-catenin and Pref-1/ERK1/2/SOX9 pathways, and downregulating adipogenic markers including C/EBPβ, PPARγ, C/EBPα, FAS, aP2 and SREBP-1c.227–232 Fourth, studies have also reported the importance of AMPK substrates in obesity. For instance, both human and mouse studies link a bona fide AMPK substrate TBC1D1 to the development of obesity.233,234 Wang and Chen groups introduced a knockin mutation that prevents the phosphorylation of TBC1D1 by activated AMPK and found that the knockin mice developed obesity on a normal chow diet. Mechanistically, blockade of TBC1D1 phosphorylation in adipocytes promotes insulin-like growth factor 1 (IGF1) secretion and consequently activates the IGF1R/Akt/mTOR pathway, which in turn induces the expressions of lipogenic genes, resulting in weight gain.235 AMPK is activated in the setting of enhanced lipolysis like exercise and fasting. However, in adipocytes, AMPK counterintuitively limits lipolysis since AMPK is an enzyme in case of energy shortage.236 This could be explained by the fact that lipolysis is very demanding for energy homeostasis and the accumulation of free fatty acids from lipolysis into adipocytes may be detrimental to the energy-producing process because they are well-known mitochondrial uncouplers.236,237 The inhibition of lipolysis by activated AMPK served as a feedback mechanism preventing excessive energy consumption.
Obesity and the Wnt/β-catenin signaling pathway
The Wnt/β-catenin pathway is a canonical pathway in Wnt signaling and is composed of Wnt proteins, Frizzled and LRP5/6), Dishevelled proteins, Axin, GSK3, and β-catenin. In addition, there are two other noncanonical Wnt pathways, the Ca2+-dependent pathway, and the planar cell polarity pathway.238,239 The activation/inhibition of the Wnt signaling pathway leads to different effects in obesity pathogenesis, which is determined by the specific pathways of action (Fig. 4).
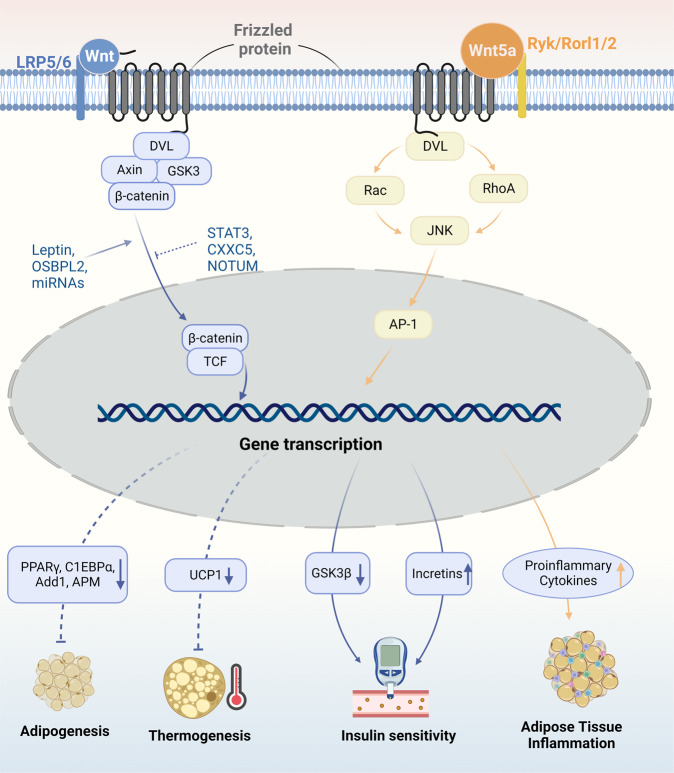
Fig. 4.
Wnt/β-catenin pathways in obesity pathogenesis. In the canonical Wnt pathway, upon activation by Wnt proteins, β-catenin is released and enters the nucleus as a transcription coactivator of TCF to regulate the transcription of target genes. The activation of Wnt/β-catenin pathway leads to, (1) the supersession of adipogenesis by down-regulating the expression of PPARγ, C1EBPα, Add1, APM, etc.; (2) the inhibition of BAT-related thermogenesis by down-regulating UCP-1; and (3) the increase of insulin sensitivity by down-regulating GSK3β expression in CNS while up-regulating incretins within the small intestinal epithelium. The canonical Wnt signaling can be stimulated by factors including leptin, OSBPL2, and miRNAs like miR-23b, miR-148b miR-4269, and miR-4429. It can also be inhibited by JAK/STAT3 pathway, CXXC5, and NOTUM. These factors are all involved in the pathogenesis of obesity by regulating Wnt/β-catenin signaling pathway. Additionally, Wnt5a, a part of the non-canonical Wnt pathway, induces obesity-associated inflammation in WAT in a JNK-dependent manner, which further contributes to the occurrence of insulin resistance in adipose tissue
The Wnt/β-catenin pathway has been suggested to have a negative effect on adipogenesis and obesity.240–243 Wnt/β-catenin induces osteoblastogenesis from MSCs and simultaneously suppresses the expression of adipocyte-related genes including PPARγ and fatty acid synthase, thus inhibiting adipogenesis.244,245 Knockout of oxysterol-binding protein-like 2 (OSBPL2), a transport protein mediating the function of β-catenin, promoted the maturation of preadipocytes and caused an obese phenotype.246 When Wnt signaling was activated within adipose progenitor cells, mice showed significantly reduced visceral fat and a higher degree of fibrosis in subcutaneous WAT due to alternation of the adipocyte into a fibroblastic lineage.247 However, the stimulation of Wnt signaling within mature adipocytes did not yield the same result.247 Conversely, Wnt/β-catenin was found to be upregulated in mature adipocytes within WAT, and ablation of β-catenin in mature adipocytes exhibited resistance against HFD-induced adipose tissue expansion but not chow-diet adipose tissue.248 In another study, adipocyte-specific loss of β-catenin downregulated gene expression related to DNL and protected against HFD-induced obesity and metabolic dysfunction.249 Intriguingly, this study suggests that deficiency of β-catenin in adipocytes can be sensed and compensated for by CD45-/CD31- stromal cells to maintain tissue-wide Wnt signaling homeostasis in chow-fed mice, while with long-term HFD, this compensatory mechanism is overridden.249 Wntless, a chaperone protein for the secretion of Wnts, is essential for DNL in mature adipocytes and induced by HFD. Similarly, knockout of Wntless in adipose tissue did not lead to a lean phenotype under a chow diet because of compensation from surrounding stromal cells but reduced WAT mass in HFD-fed mice.250 Moreover, knockdown of LRP5, an essential protein in canonical Wnt signaling, in either abdominal or gluteal adipose progenitors leads to distinct biological outcomes: enhanced abdominal adipogenesis and suppressed gluteal adipogenesis.251 Therefore, Wnt/β-catenin signaling plays a complicated role in different fat depots, different diets, and different stages of adipogenesis.
The Wnt/β-catenin pathway influences insulin action and systemic glucose homeostasis.252,253 The canonical Wnt transcriptional effector TCF7L2 was found to be closely related to susceptibility to T2D.254 In visceral adipose tissues of patients with obesity-related diabetes and HDF-fed mice, the Wnt/β-catenin pathway is downregulated. Inhibition of CXXC-type zinc-finger protein 5 (CXXC5), a negative feedback regulator of Wnt signaling, alleviates the phenotype of obesity-related diabetes.255 Wnt signaling induces the synthesis of incretins within the small intestinal epithelium and is linked to T2D.256 In addition, Wnt5a, a part of the noncanonical Wnt pathway, has been proven to induce obesity-associated inflammation in WAT and contribute to dysregulation in glucose metabolism in a JNK-dependent manner.257
Wnt/β-catenin signaling contributes to the regulation of energy homeostasis.258 Wnt signaling was downregulated in leptin-deficient mice and this was rescued by leptin treatment.259 A recent study suggested that Wnt/β-catenin signaling mediates leptin effects by suppressing GSK3β, an inhibitor of insulin signaling.260 In addition, via integration of the leptin signal, Wnt/β-catenin signaling is associated with neuroendocrine regulation of body weight.261 Mice lacking β-catenin specifically in osteoblasts exhibit decreased fat accumulation and increased energy expenditure.262 Compared to lean controls, Wnt/β-catenin signaling in exosomes derived from obese visceral adipose tissue emerges as one of the top canonical pathways.263 Activation of Wnt/β-catenin signaling inhibits the browning of adipocytes,264 whereas suppression-enhanced browning is mainly displayed at early adipocyte differentiation, suggesting that Wnt/β-catenin-regulated browning is likely in beige precursor cells.265 Other organs, such as the liver, can promote the browning of WAT by secreting NOTUM, an inhibitor of Wnt signaling.266 The Wnt/β-catenin pathway is also responsible for STAT3-regulated preadipocyte differentiation, suggesting an interaction between the Wnt/β-catenin pathway and the JAK/STAT pathway during the early stage of adipogenesis.267
Other signals/pathways
ER stress factors and the involved pathways
Endoplasmic reticulum (ER) is a critical organelle responsible for vital metabolic functions.268 ER stress refers to a condition in which unfolded or misfolded proteins accumulate in ER and leads to stress conditions.269 A plethora of evidence from animal and clinical studies shows that elevated ER stress in adipose tissue is induced by obesity, which in turn impairs ER functions and leads to metabolic dysfunction within the cell.270
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is primarily retained in the ER under normal conditions. Under ER stress induced by inflammation or the accumulation of reactive oxygen species (ROS), MANF is released in large amounts into the cytoplasm and partially translocated into the nucleus. By activating the unfolded protein response (UPR) signaling cascade and negatively regulating nuclear factor kappa B (NF-κB) signaling, MANF inhibits the transcription of proinflammatory factors and improves ER homeostasis.271 MANF can also interact with multiple signaling molecules including p38, mTOR, AMPK, etc., via unknown mechanisms.272 Although the precise functions of MANF have not been fully clarified, emerging evidence supports that MANF is closely associated with the occurrence of obesity (Fig. 5).273,274 The regulatory role of MANF in energy homeostasis in the CNS and peripheral tissues seems to be discordant. MANF is abundantly expressed in the central neurons regulating appetite,275 and its expression in several hypothalamic nuclei that critically regulate food intake is likely to be affected by feeding state. Upon fasting, MANF expression in the hypothalamus of mice increased markedly. The upregulated MANF in the hypothalamus leads to the development of hyperphagia and obesity, while its reduction in the hypothalamus results in hypophagia and retarded body weight gain.276 Mechanistically, MANF induces the expression of PIP4k2b, an interacting partner of MANF in the ER, to trigger insulin resistance and disrupt insulin signaling in the CNS, leading to hyperphagia and fat mass accumulation.276 In contrast to the upregulation of MANF in the hypothalamus upon fasting, overnutrition leads to a decrease in MANF transcription in the subfornical organ, a forebrain sensory circumventricular organ controlling energy balance and hydration status.277 Although whether MANF also acts to positively regulate energy intake via the subfornical organ is unknown, the above evidence suggests that negative feedback may exist in the regulation of MANF expression patterns in the CNS via food intake. Peripherally, strong expression of MANF was observed in tissues and cells with high energy consumption, such as heart, muscle, and BAT.275 A recent study revealed that MANF is a feeding-induced hepatokine whose expression in the liver is strongly induced by HFD.278 Liver-specific MANF overexpression protected mice against HFD-induced obesity by promoting the browning of inguinal subcutaneous WAT.278 Mechanistically, MANF activates the p38 MAPK pathway to directly promote white adipocyte browning.278 Mice with MANF knockout in the liver showed impaired WAT browning and exacerbated diet-induced obesity, whereas subcutaneous injection of recombinant MANF retarded body weight gain in both diet-induced and genetic obese mouse models.278 These results indicate that peripheral MANF positively regulates thermogenesis and resists obesity. Of note, circulating MANF levels were found to be positively correlated with BMI in humans,278 indicating that obesity may increase the peripheral level of MANF in a compensator manner to relieve excessive weight gain. However, the exact role and mechanism of MANF in regulating energy balance still need further investigation, especially in regard to the different modes of action in the CNS and peripheral tissues.
Fig. 5.
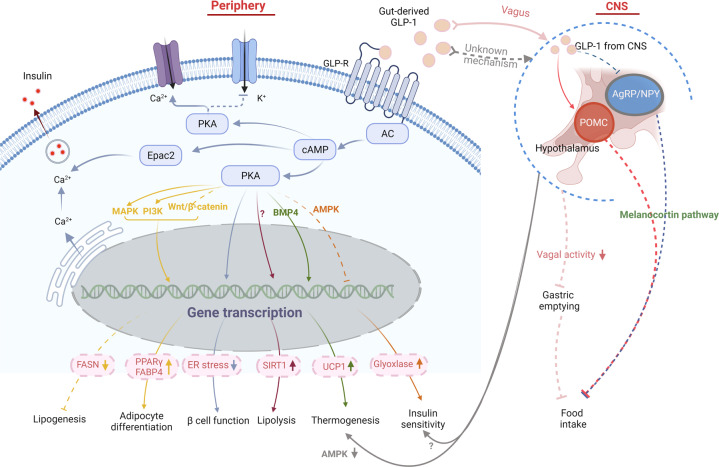
GLP-1 signaling pathway in obesity pathogenesis. The anti-obesity effect of GLP-1 can be mediated by either peripheral or central signals. In the periphery, the activation of GLP-R by gut-derived GLP-1 enhances the glucose-stimulated insulin secretion, through PKA-dependent or Epac2 pathway. By enhanced PKA activity, GLP-1 alleviates insulin resistance and leads to weight loss in obese diabetic mice by reducing ER stress and improving β-cell function. It also improves insulin sensitivity in peripheral tissue by suppressing AMPK-related pathway and elevating glyoxalase. By interacting with multiple signaling pathways including PI3K, MAPK, and Wnt4-β-catenin pathways, GLP-1 promotes pre-adipocyte differentiation by up-regulating PPARγ and FABP4, but suppresses lipogenesis in mature adipocytes by decreasing fatty acid synthase expression. GLP-1 also enhances lipolysis in WAT by increasing the expression and activity of Sirt1, through yet unknown mechanisms. Additionally, GLP-1 participates in the regulation of thermogenesis by inhibiting BMP4-related signaling pathway and thus induces the expression of thermogenic genes like UCP1. Gut-derived GLP-1 also interacts with GLP-R expressed in vagus, through which the information is transmitted upward to the CNS, which in turns suppresses vagal activity and gastric emptying, so as to increase satiety and reduce food intake. Besides, peripheral GLP-1 plays a role in the regulation of insulin sensitivity and BAT-related thermogenesis in a CNS-dependent manner. the latter is partially mediated by suppressing AMPK signaling pathway. Central GLP-1 produced by neurons in the caudal medulla is transmitted into the hypothalamus and functions to reduce food intake by activating POMC neurons while suppressing AgRP/NPY neurons in this area
Inositol-requiring enzyme 1α (IRE1α) is another evolutionarily conserved ER stress sensor that may serve as a critical switch governing energy balance.279 ER stress stimulates IRE1α oligomerization in ER membranes and autophosphorylation of IRE1α’s cytosolic domain.279 Activated IRE1α RNase catalyzes the unconventional splicing of Xbp1 mRNA and helps to generate a transcriptionally active transcription factor XBP1s to initiate the transcription of the key UPR gene to cope with ER stress.280 IRE1α can also function by interacting with TNF receptor-associated factor-2 and apoptosis signal-regulating kinase 1 to form a complex, which further activates downstream of stress kinases JNK and plays a crucial role in the regulatory machinery governing proteostasis and ER’s functional integrity.281–283 IRE1α can be activated by three major types of signals: nutrients, hormones, and immunological stimuli. Nutrients such as saturated fatty acids can activate IRE1α in a manner that does not rely on its unfolded protein-sensing ability.284 This, in turn, activates the NOD-like receptor thermal protein domain associated protein 3 inflammasome in macrophages and drives HFD-induced IL-1β secretion.284 Metabolic hormones such as insulin activate IRE1α–XBP1 pathway in livers as well as in primary hepatocytes and result in the enhanced de novo lipogenic program in an XBP1s-dependent manner.285 Some inflammatory stimuli including lipopolysaccharide (LPS) and IL-4, can also activate the Xbp1 mRNA-splicing activity of IRE1α by interacting with toll-like receptors (TLRs).286 The activation of IRE1α exerts a broad range of tissue- or cell-type-specific functions in energy metabolism. Centrally, IRE1α plays complex roles in appetite regulation. Mouse with exons 16 and 17 of gene encoding IRE1α deletion in POMC neurons shows marginal acceleration of HFD-induced obesity with considerable impairments in leptin and insulin sensitivity in POMC neurons and energy expenditure.287 In contrast, mouse with exon 2 fragment of IRE1 gene deletion in POMC neurons exhibits significant resistance to HFD-induced obesity and improvement of insulin resistance.288 In addition, increased energy expenditure and leptin sensitivity with higher production of α-MSH in the hypothalamus were also observed in mice with POMC neuron-specific ablation of IRE1α.288 Peripherally, mice with myeloid-specific IRE1α abrogation largely reversed HFD-induced M1-M2 imbalance in WAT and blocked HFD-induced obesity, insulin resistance, hyperlipidemia, and hepatic steatosis.289 In addition, myeloid-specific IRE1α abrogation increased WAT browning and energy expenditure in mice.289 These results suggest the multifaceted functions of IRE1α protein between CNS and periphery, and genetic deletion of different regions of IRE1α-encoding gene leads to apparent discrepancy in the phenotypes.
Immune-related pathways
Many of the comorbidities of obesity including T2D and cardiovascular disease are related to the dysimmunity induced by obesity.290 WAT is composed of various types of cells including adipocytes and immune cells.291 As an endocrine organ, WAT produces a variety of proinflammatory cytokines and integrates immune signaling in the dysfunctional metabolic status.292 Despite that the specific primordial trigger for sustained inflammation in obese WAT is unknown, this process is likely to be associated with metabolic stressors (from nucleic acids to lipids, from small compounds to macromolecules) arising from excessive adipocyte hypertrophy and hyperplasia induced by overnutrition, and also external stimuli such as the elevated levels of plasma LPS.292 Under these internal stressors and external stimuli, immune cells infiltrate and produce proinflammatory cytokines locally, resulting in WAT remodeling and insulin resistance. Mechanistically, obesity-related chronic inflammation in WAT is partially mediated, if not all, by TLRs expressed in adipocytes and macrophages.293 TLRs is an evolutionarily ancient family of pattern recognition receptors, which can recognize microbiological components such as the pathogen-associated molecular patterns (PAMPs) like LPS, and also internal stimuli such as nonesterified fatty acid.294 By activating TLR4/TLR2, WAT stressors or LPS stimulate NF-κB and JNK signaling, upregulate the expression of inflammatory cytokines including TNF-α and IL-6, and further induce insulin resistance in adipocytes and macrophages.295,296 TLRs-related pathways are also involved in the locally proinflammatory environment in BAT. The proinflammatory condition in BAT not only decreases the insulin sensitivity of BAT and impairs the uptake of fuel for thermogenesis, but also alters the activity of BAT by disturbing its energy expenditure mechanism. TLR2/4 were upregulated in the BAT from the obese mice, paralleled with the upregulation of inflammatory cytokines and chemokines in this tissue.297 Activation of TLR4 and TLR2 in brown adipocytes induces the activation of NF-κB and MAPK signaling pathways, leading to inflammatory cytokine/chemokine expression and attenuating both basal and isoproterenol-induced UCP1 expression.297 TLR4 activation by LPS also represses β3-adrenergic-mediated WAT browning and caused ROS production and mitochondrial dysfunction, whereas the deletion of TLR4 protects mitochondrial function and thermogenic activation.298
TLRs-related pathways are also involved in the regulation of the microorganism environment in the intestines.299 Given that the highest numbers of microbiomes are found in the gut, the role of gut microorganisms has been extensively studied and its polymorphism was implicated to be associated with obesity.300 Gut microbiological components play a crucial role in human metabolic regulation. With expressions of TLRs, colonocytes and endocrine cells are able to sense and transmit signals from PAMPs and thus functionally regulate inflammation, intestinal nutrient absorption, and insulin and incretins secretion.299 Activated TLRs mainly work through myeloid differentiation factor 88 protein (MyD88)-dependent and MyD88-independent signaling pathways.301 Animal study found that the deletion of MyD88 in intestines partially protects against diet-induced obesity, diabetes, and inflammation,302,303 indicating that the overactivation of MyD88 by some specific microbes may be one of the mechanisms of pathological gut microbial environment-related obesity.
Another pathway closely related to the inflammation status of obesity is the cyclic stimulator of the interferon genes (STING) signaling pathway. Usually, STING senses the presence of cytosolic DNA, either from the nucleus or mitochondria, and in turn, triggers downstream signaling to induce the expression of inflammatory and type I interferon genes in immune cells.304 Emerging evidence suggests that this signaling pathway may have additional functions beyond innate immune surveillance and may contribute to the chronic inflammation observed in obese patients (Fig. 3).305–309 Although the notion that obesity triggers chronic, low-grade inflammation has been recognized for decades, the pivotal role of the STING pathway in obesity has recently been appreciated.310–312 The STING pathway can be activated by palmitic acid, leading to mitochondrial damage and thereby mtDNA leakage. Through the cytosolic DNA sensor cGAS, mtDNA activates the STING-interferon regulatory Factor 3 pathway and induces a chronic sterile inflammatory response in mouse adipose tissue.313,314 In STING-deficient mice, the effects of diet-induced obesity, including endothelial inflammation (in adipose tissue), insulin resistance, and glucose intolerance, were alleviated.314 These findings support the notion that STING signaling plays a critical role in obesity-related adipose inflammation and insulin resistance. Of note, adipose tissue-specific knockout of DsbA-L, a chaperone-like protein identified in the mitochondrial matrix that maintains mitochondrial integrity, activates the cGAS-STING pathway in adipose tissue and exacerbates obesity-related pathology, while fat-specific overexpression of DsbA-L protected mice against HFD-induced activation of the STING pathway and chronic inflammation.313 These results suggest that maintaining mitochondrial homeostasis to target STING activation may be an alternative anti-obesity strategy. After translocation from the ER to the Golgi, STING can activate TANK-binding kinase 1 (TBK1), a downstream target that is essential for STING-dependent signaling.315 Recent studies report that systemic or adipocyte-specific TBK1 knockout attenuates HFD-induced obesity by increasing energy expenditure.316,317 Consistently, pharmacological inhibition of TBK1 enhances insulin sensitivity and reduces chronic inflammation caused by obesity.316,318,319 However, the potential bidirectional roles of TBK1 in regulating inflammation should not be ignored, as it is found to promote STING ubiquitination and degradation and in turn elevate NF-κB activity and inflammation.320 Nevertheless, the crosstalk between TBK1 in the STING pathway and inflammation status and insulin resistance merits further investigation.
Altogether, these results indicate that a positive energy balance and overnutrition lead to abnormal inflammation responses in peripheral tissues/organs such as adipose tissue and intestinal tract, and this, in turn, drives some of the systemic metabolic alterations associated with obesity like impaired insulin sensitivity and decreased thermogenesis. Targeting the key molecules/pathways mediating the abnormal inflammatory status may be crucial for the management of obesity-related inflammation and complications.
Drug-related signaling molecules and pathways
GLP-1
GLP-1 is released by intestinal L‐cells and also by a discrete population of neurons in the caudal medulla.321 As an incretin, the circulating level of GLP-1 elevates severalfold after a meal, which partially depends upon mechanical forces such as gastric distension.322,323 Gastric distension also activates nucleus tractus solitarius (NTS) neurons to release GLP‐1,324 which contributes to the negative energy balance of central GLP‐1.325 In addition, both peripheral GLP-1 secretion and central GLP‐1 cellular activity are regulated by classic satiety factors such as cholecystokinin (CCK) and leptin.326,327 GLP-1 works by activating GLP-1 receptors (GLP-1Rs), which can couple to Gαs, Gαq, Gαi, and Gαo.323,328–330 GLP-1Rs are widely expressed in the CNS, in peripheral organs (such as the pancreas), and in peripheral nerves such as vagal afferents.328,331–333 By stimulating GLP-1R, GLP-1 leads to an increase in intracellular Ca2+ and adenylate cyclase (AC), the activation of cAMP-dependent protein kinase (PKA) and Epac2, and the subsequent activation of multiple signal transduction pathways such as MAPK, PI3K, and BMP4, thus regulating the transcription of target genes.334 The activation of GLP‐1R has potent effects on the regulation of appetite, gastric motility, glucose, lipid metabolism, and even body thermogenesis (Fig. 5). These effects have made GLP-1R a viable target for diabetes mellites and obesity therapies,328 which we will discuss later.
The mechanism involved in GLP-1/GLP-R-mediated satiation is complicated, and there may be two substantially different modes of action between the central and peripheral regions. Within the CNS, activation of NTS GLP‐1 neurons leads to an attenuation of metabolic rate and a reduction in food consumption.335–337 Notably, ablation or inhibition of NTS GLP‐1 neurons increased refeeding after a fast and inhibited stress‐induced hypophagia.338 This phenomenon is considered to be mediated by a “local circuit”. Neuron-produced GLP‐1 is transported to the axon terminals of the producing cells and is stored in synaptic vesicles until it is eventually released into the synaptic cleft or extrasynaptically released into the brain parenchyma.339 Considering that GLP‐1-producing neurons are also projecting neurons with axons containing GLP‐1 vesicles in many distinct regions of the brain, it is speculated that the release and action of GLP‐1 within the CNS is similar to that of other neurotransmitters and modulators, which are locally restricted. From this point of view, GLP‐1 released from a specific neuron only acts at the site of its release, and it is entirely determined by the CNS area to which these neurons project.340 For instance, GLP‐1R is coexpressed with POMC neurons independent of AgRP/NPY expression. Electrophysiological measurements of murine brain slices revealed that GLP-1 can directly stimulate POMC/CART neurons via transient receptor potential channel 5, whereas it indirectly inhibits neurotransmission in neurons expressing NPY and AgRP via GLP‐1R-dependent activation of presynaptic GABAergic neurons.341,342 The involved intracellular signaling is proposed to be that GLP-1R activation increased PKA and MAPK activity and decreased the phosphorylation of AMPK in the NTS.343
In contrast, peripheral GLP-1 potentially works throughout the entire body by acting on, for example, vagal nerve endings embedded into the gut mucosa or is transported freely to most sites in the body accessible from the circulation.344 Currently, strong evidence suggests that the satiation effects of gut-derived GLP-1 are primarily mediated by vagal afferents, which relay the information to the hypothalamus and other forebrain regions by way of ascending second-order neurons.334 Peripheral administration of a GLP-1–albumin recombinant fusion protein, which is much larger and unable to cross the blood–brain barrier, activates neurons in the CNS coupled to feeding and inhibits food intake in mice,345 suggesting that peripheral GLP-1 activates central neurons regulating energy intake without direct interaction with GLP-1R in CNS. In rats, peripheral GLP-1-induced anorexia and neuronal activation of hypothalamic feeding circuits were both precluded by bilateral vagotomy or surgical transection of the brainstem-hypothalamic pathway.346 Likewise, selectively ablating nodose ganglionic neurons and the vagus nerve via systemic treatment with capsaicin completely blocks the anorectic effect of peripherally administered exendin-4 in mice.347 Collectively, these findings indicate that food reduction induced by peripheral GLP-1 is CNS-dependent. It is worth noting that the “brain circuits” mediating satiation induced by GLP-1 originating from either the CNS or periphery have only been described in rodents, but knowledge is limited, and it is not clear whether this circuit exists in humans.
GLP-1 also shows inhibitory effects on pentagastrin- and meal-stimulated gastric acid secretion and gastric emptying. GLP-1-induced gastrointestinal motility inhibition is mediated through GLP-1R at the level of myenteric neurons, followed by downstream signaling of nitrergic and cAMP-dependent mechanisms, resulting in the inhibition of vagal activity.348–350 Targeting GLP-1R signaling via exendin or vagal afferent denervation abolishes the inhibitory effect of centrally or peripherally administered GLP-1 on gastric emptying and acid secretion.351 In addition, intraperitoneal administration of an albumin-linked GLP-1R agonist that is unable to cross the blood–brain barrier can still activate neurons in the CNS that are coupled to gastrointestinal motility and lead to the inhibition of gastric emptying.345 Collectively, these experimental data indicate that the inhibitory effect of GLP-1 on gastric emptying and acid secretion is vagus-dependent and involves GLP-1Rs and/or on vagal afferent fibers that relay sensory information from the digestive tract to the brainstem.
GLP-1 stimulates glucose-dependent insulin secretion by binding to its specific receptor on pancreatic cells. GLP-1R stimulation leads to the activation of AC activity and the production of cAMP,352 which is the primary effector of GLP-1–induced insulin secretion. cAMP stimulates insulin secretion via two distinct mechanisms: PKA-dependent phosphorylation of downstream targets and PKA-independent activation of Epac2.352 In vivo, GLP-1R agonists improve glucose tolerance, enhance β-cell proliferation and neogenesis, and inhibit β-cell apoptosis in experimental rodent models of diabetes, leading to increased β-cell mass.353–355 Obese diabetic db/db mice develop ER stress, and GLP-1R agonists not only decrease the weights of mice but also reduce the levels of ER stress markers and improve β-cell function and survival during ER stress in a PKA-dependent manner.356 In addition to stimulating insulin secretion, GLP-1 also plays an important role in improving insulin sensitivity in insulin-targeting organs/tissues such as the liver and adipose tissue, partially through AMPK-related pathways.357–359 This can be mediated by its direct actions on peripheral tissue by improving glyoxalase activity359 and via CNS signals, which is suggested by the evidence that central GLP-1R antagonism attenuated the remission in HFD-induced insulin resistance caused by peripheral GLP-1 infusion.360 Although some evidence indicates that GLP-1R in the brain is not necessary for physiologic control of glucose regulation, the central actions of GLP-1R signaling should not be ignored given its critical role in lowering weight, which is the primary goal for T2D and also other metabolic disorders.
GLP-1 signaling is also a regulator of adipogenesis. Growing in vitro evidence revealed that GLP-1R activation increased the expression of differentiation marker genes such as PPARγ and FABP4 and lipid accumulation during preadipocyte differentiation.361 Gut-derived GLP-1 also increases adipocyte mass through preadipocyte proliferation and inhibition of apoptosis,362 which is partially mediated by the PI3K, MAPK, and Wnt4-β-catenin pathways.362–364 Notably, although GLP-1 signaling seems to promote preadipocyte differentiation both in vivo and in vitro, it decreased fatty acid synthase expression in mature adipocytes,361 an enzyme closely related to lipogenesis and the development of visceral obesity.365–367 Considering that adipocyte enlargement plays the leading role during lipogenesis and obesity, while adipocyte differentiation can offset the negative metabolic effects of obesity,291,368 the terminal effect of GLP-1 on metabolism may still be positive.
GLP-1R activation was found to directly increase lipolysis and fatty acid oxidation by upregulating Sirt1 expression in differentiated 3T3-L1 adipocytes.369 It also enhances lipolysis by promoting BAT thermogenesis or white adipocyte browning.370,371 Recent studies revealed that GLP-1R located in the epicardial adipose tissue (EAT) was directly correlated with genes promoting beta-oxidation and white-to-brown adipocyte differentiation but inversely correlated with pro-adipogenic genes,372 while EAT is a risk factor for cardiovascular diseases,373 suggesting that GLP-1R may be a new target to modulate cardiovascular risk related to obesity. GLP-1 signaling participates in the process of thermogenesis in BAT by inhibiting the BMP4-related signaling pathway in HFD-induced obese mice.370 Central signaling may play a role in GLP-1-induced thermogenesis since GLP-1 administered into the dorsomedial hypothalamus of rats increases BAT thermogenesis and triglyceride mobilization in the liver, whereas loss of GLP-1 signaling in the dorsomedial hypothalamus area reduces BAT thermogenesis and increases adiposity.374 Similar results were observed in a mouse model; central injection of a clinically used GLP-1R agonist, liraglutide, stimulates BAT thermogenesis and white adipocyte browning independent of nutrient intake.225 Activation of AMPK in the hypothalamic ventromedial nucleus (VMN) blunted both central liraglutide-induced thermogenesis and adipocyte browning.225 These data indicate that GLP-1 lowers body weight by regulating either food intake or energy expenditure through various hypothalamic sites and that these mechanisms might be clinically relevant.
Melanocortin signaling pathway
The melanocortin signaling pathway consists of a set of hormonal and neuropeptidergic networks with three major components: pro-peptide POMC, which is posttranslationally processed by prohormone convertases into a number of biologically active moieties, including α-MSH, β-MSH, γ-MSH, and adrenocorticotrophin (ACTH);375 the five G protein-coupled melanocortin receptors, MC1R-MC5R, that mediate their actions;376 and endogenous antagonists of those receptors, agouti, and AgRP.377,378 Although its mechanism of action is not yet clear, it is certain that the melanocortin signaling pathway plays a key role in the development of obesity by regulating energy homeostasis (Fig. 6), and compounds targeting the melanocortin system have been investigated extensively from basic to clinical research for anti-obesity purpose.
Fig. 6.
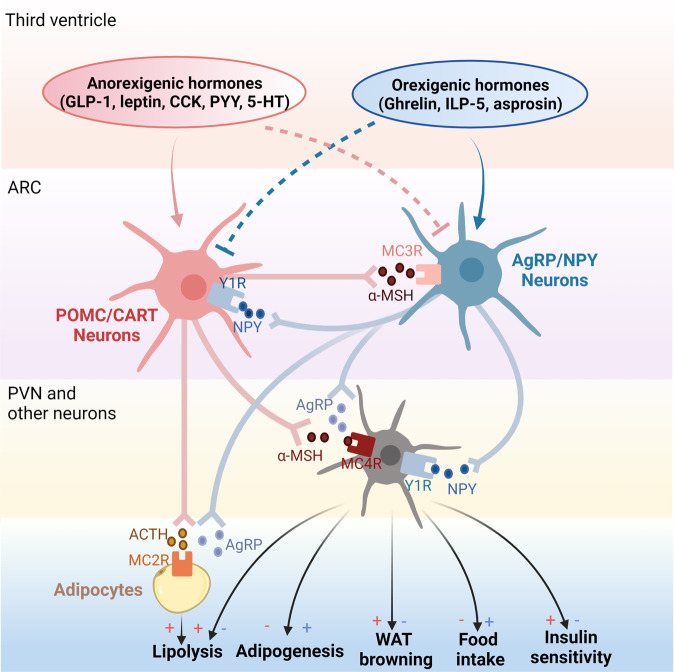
Melanocortin pathway in obesity pathogenesis. The melanocortin pathway consists of POMC; melanocortin receptors MC1R-MC5R; and agouti and AgRP. POMC/CART neurons in ARC are stimulated by anorexigenic hormones in the third ventricle like GLP-1, leptin, CCK, PYY, and 5-HT, while suppressed by orexigenic hormones like Ghrelin, ILP-5, and asprosin. Upon stimulation, POMC/CART neurons secrete POMC including α-MSH and ACTH. α-MSH is released into the PVN. By interacting with MC4R, α-MSH activates PVN neurons and displays anti-obesity effects by inhibiting adipogenesis, promoting lipolysis, inducing WAT browning, reducing food intake, and improving insulin sensitivity. ACTH released by POMC/CART neurons actions on adipocytes directly by binding to MC2R, further promoting lipolysis. However, these effects can be abolished by AgRP, which is the endogenous antagonist of POMC and is secreted by AgRP/NPY neurons in ARC. Conversely, AgRP/NPY neurons can be stimulated by orexigenic hormones in the third ventricle but inhibited by anorexigenic hormones. Notably, POMC/CART and AgRP/NPY neurons interact mutually. NPY receptor Y1R is expressed in POMC/CART neurons and its activation inhibits POMC neurons in the ARC. In contrast, MC3R expressed in AgRP/NPY neurons seems to increase food intake in an “AgRP circuitry”-dependent manner
The melanocortin system serves as a major regulator of food intake and energy balance.378,379 ARC POMC neurons are activated by multiple anorexigenic hormones including leptin, GLP-1, CCK, peptide tyrosine-tyrosine (PYY), etc., but inhibited by orexigenic hormones, such as ghrelin, asprosin, and insulin-like peptide 5 (ILP-5).380–384 In general, POMC neurons are more active in post nutritional repletion states than in fasting states,385 indicating negative feedback exists in the regulation of food intake. Endocrine factors that activate POMC neurons cause anorexia and weight loss.384 These effects are mimicked by local application of α-MSH and are diminished in mice that lack MC4R,386 indicating that α-MSH signaling through the MC4R axis is at least partly responsible for some of these endocrine effects. Over the past 20 years, the role of ARC POMC neurons in response to peripheral factors, particularly the adipocyte-derived hormone leptin, has been extensively studied. Leptin is a hormone secreted by adipocytes to modulate several neuroendocrine functions.387 LEPRs are widely expressed in the CNS, particularly in some regions of the hypothalamus regulating feeding.388 By activating LEPR in ARC, leptin modulates the activity of the melanocortin pathway by enhancing the α-MSH cleaved from POMC but blunting the synthesis of NPY and AgRP, to activate MC4R axis and thus exhibits an anorectic effect.389,390 Population studies have identified that the deficiency of LEPR caused by the mutations in LEPR gene leads to severe obesity.391 Despite several variants of MC4R being associated with significantly lower BMI and lower odds of obesity, most MC4R variants cause loss of function and increase obesity risk.392,393 In contrast, activation of AgRP neurons leads to hyperphagia and weight gain. Notably, a recent study revealed that the chronic activation of AgRP GABA+ neurons or non-AgRP GABA+ neurons both leads to obesity, while inhibition of arcuate GABA+, but not AgRP neurons reduces weight gain, indicating that arcuate GABA+ neurons may be the major mediator to increase food intake.378 In addition, appetite is suppressed in mice lacking MC3R.394 Considering that MC3R is expressed in 97% of AgRP/NPY neurons and pharmacological effects of MC3R compounds on feeding are dependent on intact AgRP circuitry in mice,395 the dominant effect of MC3R appears to be the regulation of the AgRP circuitry. Notably, the NPY receptor Y1R is expressed in POMC/CART neurons,396 and its activation inhibits POMC neurons through the Y1R-mediated activation of G protein-gated inwardly rectifying potassium channel currents.397 These results indicate that there is an interplay between these two peptides at multiple levels.70
It has been recently proposed that leptin and insulin also act on POMC neurons to increase energy expenditure via a pathway that involves protein-tyrosine phosphatases 1B and T-cell protein-tyrosine phosphatase and leads to increased browning differentiation of WAT.158,398 Regarding lipid metabolism, Lede et al. analyzed transcriptome changes and found significant alterations in components of triacylglycerol metabolism, unsaturated fatty acid biosynthesis, PPAR signaling pathways, and lipid transport and storage in MC4R-deficient mice compared to the wild-type condition.399 Furthermore, Iqbal et al. found that LEPR deficiency resulted in lipid accumulation in the intestine, liver, and plasma.400 The molecular mechanism was decreased intestinal microsomal triglyceride transfer protein expression, reduced assembly and secretion of lipoproteins, and elevated triglyceride accumulation.400
The melanocortin system also contributes to the regulation of glucose metabolism. Mouse models and humans with genetic deficiency of POMC or MC4R show significant hyperinsulinemia and insulin resistance.401–403 Conversely, activation of brain MC4R enhances insulin sensitivity.404 Similarly, the direct leptin action on POMC neurons lowers glucagon levels and improves hepatic insulin sensitivity.405 Moreover, nutritional status modulates insulin responsiveness in POMC neurons.406 Another population of hypothalamic POMC neurons that regulates both energy and glucose homeostasis has been found to express the serotonin (5-hydroxytryptamine [5-HT]) receptor 2C receptor, which signals to induce activation of TrpC5 and the mTOR pathway.407–410 A recent study suggested that the 5’−3’ exoribonuclease XRN1 inhibits AgRP neuron function.411 Together, these studies highlight an important role of the melanocortin pathway in the regulation of obesity and glucose homeostasis.
Melanocortins circulate throughout the body and exert lipolytic effects on adipocytes via specific melanocortin receptor subtypes.412 Obesity, which is caused by overexpression of AgRP, is generally considered a consequence of antagonism of α-MSH on the hypothalamic melanocortin receptor, given that AgRP stimulates adipogenesis and antagonizes melanocortin-mediated lipolysis in adipocytes.413 Moreover, PPARγ, a critical transcription factor in the regulation of adipocyte differentiation and lipid metabolism,414–416 was reported to regulate transcriptional activation of the MC2R accessory protein gene to stimulate lipolysis induced by ACTH in mature adipocytes.417 Similarly, Mynatt et al. utilized engineered transgenic mice with agouti overexpression in adipose tissue as well as differentiated 3T3-L1 adipocytes and observed an elevation of PPARγ expression in both models, suggesting that PPARγ, probably interacting with ACTH and AgRP, to regulate adipocyte differentiation.418
Drugs/compounds for the treatment of obesity
Lifestyle interventions remain the cornerstone of weight management, but most patients cannot achieve long-term meaningful weight loss simply by changing lifestyles. Thus, pharmacotherapy is appropriate after lifestyle modification failure and is recommended as an adjunct to individuals with BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with obesity-associated comorbidities.419 Currently, the U.S. Food and Drug Administration (FDA) has approved four AOMs that curb appetite (phentermine, phendimetrazine, diethylpropion, and benzphetamine) for short-term (≤12 weeks) use and five AOMs (orlistat, phentermine-topiramate, naltrexone-bupropion, liraglutide, and semaglutide) for long-term use and another drug setmelanotide for people with obesity due to three specific rare genetic conditions (Fig. 7). There has been a long-term effort to develop new weight-loss drugs, while most results have been disappointing, several prominent classes of targets have caught the attention of the scientific community and drugmakers (Table 1).
Fig. 7.
Timeline of anti-obesity medications approved by the FDA or EMA from the late nineteenth century until today (the red dashed line indicates long-term use while the blue dashed line indicates short-term use)
Table 1.
Weight-loss drugs in clinical development after 2015
| Agent type | Agent | Indication | Manufacturer | Trials | ClinicalTrials.gov ID/ref. |
|---|---|---|---|---|---|
| Energy intake | |||||
| MC4R agonist | PL-8905 | Obesity | Palatin Technologies | Announced | Not Recorded |
| NPY5R antagonist | S-237648 | Obesity | Shionogi & Co. | In-house | See R&D Pipeline |
| Triple reuptake inhibitor/SNDRI | Tesofensine/NS-2330 | Obesity | NeuroSearch A/S | Phase 2 | NCT00394667 |
| Peripheral CB1 receptor blocker | GFB-024 (inverse agonist) | Kidney diseases | Goldfinch Bio | Phase 1 | NCT04880291 |
| AM-6545 (antagonist) | Obesity | MAKScientific | Preclinical | See R&D Pipeline | |
| GLP-1R agonist | Beinaglutide/Benaglutide | Obesity | Shanghai Benemae | Phase 3 | NCT03986008 |
| Dulaglutide | T2D | Eli Lilly and Company | Phase 3 | NCT03015220 | |
| Phase 2 | NCT02973100 | ||||
| Phase 4 | NCT02750410 | ||||
| LY3502970 | Obesity, T2D | Eli Lilly and Company | Phase 1 | NCT05086445 | |
| Phase 2 | NCT05051579 | ||||
| Phase 2 | NCT05048719 | ||||
| Efpeglenatide/LAPSExd4 Analog | T2D | Hanmi Pharmaceutical | Phase 3 | NCT03353350 | |
| Exenatide | HO | AstraZeneca | Phase 3 | NCT02860923 | |
| PB-119 | T2D | PegBio Co. | Phase 3 | NCT04504396 | |
| Phase 3 | NCT04504370 | ||||
| Danuglipron/PF-06882961 | Obesity, T2D | Pfizer | Phase 2 | NCT04707313 | |
| Phase 2 | NCT04617275 | ||||
| Phase 2 | NCT03985293 | ||||
| PF-07081532 | Obesity, T2D | Pfizer | Phase 1 | NCT04305587 | |
| RGT001-075 | T2D | Regor Therapeutics | Phase 2 | NCT05297045 | |
| Noiiglutide/SHR20004 | Obesity | Hansoh Pharma | Phase 2 | NCT04799327 | |
| TG103 | Obesity | CSPC Pharmaceutical | Phase 2 | NCT05299697 | |
| TTP273 | T2D | vTv Therapeutics | Phase 2 | NCT02653599 | |
| XW003 | Obesity | Sciwind Biosciences | Phase 2 | NCT05111912 | |
| XW004 | Obesity, T2D | Sciwind Biosciences | Phase 1 | NCT05184322 | |
| GCGR agonist | HM15136/LAPSGlucagon Analog | Obesity, T2D | Hanmi Pharmaceutical | Phase 1 | NCT04167553 |
| NN9030/NNC9204-0530 | Obesity | Novo Nordisk | Phase 1 | NCT02835235 | |
| GIPR agonist | ZP 6590 | Obesity | Zealand Pharma | Preclinical | See R&D Pipeline |
| GLP-1R/GCGR dual agonist | Pemvidutide/ALT-801 | Obesity | Altimmune | Phase 2 | NCT05295875 |
| BI 456906 | Obesity | Boehringer Ingelheim | Phase 2 | NCT04667377 | |
| CT-388 | T2D | Carmot Therapeutics | Phase 1 | NCT04838405 | |
| CT-868 | Obesity, T2D | Carmot Therapeutics | Phase 2 | NCT05110846 | |
| DD01 | Obesity, T2D | D&D Pharmatech | Phase 1 | NCT04812262 | |
| JNJ-64565111 | Obesity, T2D | Johnson & Johnson | Phase 2 | NCT03586830 | |
| Phase 2 | NCT03486392 | ||||
| NN9277/NNC9204-1177 | Obesity | Novo Nordisk | Phase 1 | NCT02941042 | |
| Efinopegdutide/LAPSGLP/GCG | NASH | Hanmi Pharmaceutical | Phase 2 | NCT03486392 | |
| SAR425899 | T2D | Sanofi | Phase 1 | NCT03376802 | |
| OXM analog—Cotadutide/MEDI0382 | T2D | AstraZeneca | Phase 1 | NCT02548585 | |
| Phase 1 | NCT04208620 | ||||
| OXM analog—G3215 | Obesity, T2D | Imperial College London | Phase 1 | NCT02692040 | |
| OXM analog—IBI362/LY3305677 | Obesity, T2D | Eli Lilly and Company | Phase 2 | NCT04904913 | |
| Phase 1 | NCT04440345 | ||||
| OXM analog—MOD-6031 | Obesity, T2D | OPKO Health | Phase 1 | NCT02692781 | |
| OXM analog—OPK-88003/LY2944876 | Obesity, T2D | OPKO Health | Phase 2 | NCT03406377 | |
| GLP-1R/GIPR dual agonist | HS-20094 | T2D | Hansoh Pharma | Phase 1 | NCT05116410 |
| Tirzepatide/LY3298176 | Obesity, T2D | Eli Lilly and Company | Phase 3 | NCT05024032 | |
| Phase 3 | NCT04844918 | ||||
| Phase 3 | NCT04657016 | ||||
| Phase 3 | NCT04660643 | ||||
| Phase 3 | NCT04657003 | ||||
| Phase 3 | NCT04184622 | ||||
| GLP-1R/GIPR/GCGR triple agonist | HM15211/LAPSTriple Agonist | NASH | Hanmi Pharmaceutical | Phase 2 | NCT04505436 |
| LY3437943 | Obesity, T2D | Eli Lilly and Company | Phase 1 | NCT04823208 | |
| Phase 2 | NCT04881760 | ||||
| Phase 2 | NCT04867785 | ||||
| NN9423/NNC9204-1706 | Obesity | Novo Nordisk | Phase 1 | NCT03095807 | |
| NNC0480-0389 | |||||
| SAR441255 | Obesity | Sanofi | Phase 1 | NCT04521738 | |
| GLP-1R agonist and GIPR antagonist | AMG133 | Obesity | Amgen | Phase 1 | NCT04478708 |
| GMA106 | Obesity | Gmax Biopharm | Phase 1 | NCT05054530 | |
| DPP-4 inhibitor | HSK7653 | T2D | Haisco Pharmaceutical | Phase 3 | NCT04556851 |
| Sitagliptin | T2D, NAFLD | Merck & Co. | Phase 4 | NCT05195944 | |
| Phase 3 | NCT02849080 | ||||
| Yogliptin | Obesity, T2D | Easton Biopharmaceuticals | Phase 3 | NCT05318326 | |
| AMYR agonist | Cagrilintide/NN9838/AM833/NNC0174-0833 | Obesity, T2D | Novo Nordisk | Phase 1 | NCT04940078 |
| Phase 1 | NCT05254158 | ||||
| Phase 2 | NCT03856047 | ||||
| ZP8396 | Obesity | Zealand Pharma | Phase 1 | NCT05096598 | |
| AMYR/CTR dual agonist | KBP-042 | T2D | Nordic Bioscience | Phase 2 | NCT03230786 |
| KBP-089 | T2D | Nordic Bioscience | Phase 1 | NCT03907202 | |
| TAS2R agonist | ARD-101 | Obesity | Aardvark Therapeutics | Phase 2 | NCT05121441 |
| PYY/Y2R signaling | NNC0165-1562 | Obesity | Novo Nordisk | Phase 1 | NCT03574584 |
| PYY1875/NNC0165-1875 | Obesity | Novo Nordisk | Phase 1 | NCT03707990 | |
| NN9748/NN9747 | Obesity, T2D | Novo Nordisk | Phase 1 | NCT03574584 | |
| Ghrelin signaling | NOX-B11 | Obesity | NOXXON Pharma | Preclinical | Not Recorded |
| GLWL-01 | PWS | GLWL Research | Phase 2 | NCT03274856 | |
| RM-853/T-3525770 | PWS | Rhythm Pharmaceuticals | Preclinical | See R&D Pipeline | |
| TZP-301 | Obesity | Ocera Therapeutics | Preclinical | Not Recorded | |
| EX-1350 | Obesity, T2D | Elixir Pharmaceuticals | Preclinical | Not Recorded | |
| Leptin analog | Metreleptin | Lipodystrophy | AstraZeneca | Phase 3 | NCT05164341 |
| Leptin sensitizer | Celastrol | Obesity, T2D | Research Use Only | Preclinical | PMID: 26000480420 |
| Withaferin A | Obesity, T2D | Research Use Only | Phase 1 | PMID: 30904387421 | |
| ERX1000 | Obesity | ERX Pharmaceuticals | Phase 1 | NCT04890873 | |
| GDF15 agonist | LA-GDF15 | Obesity | Novo Nordisk | Phase 1 | See R&D Pipeline |
| LY3463251 | Obesity | Eli Lilly and Company | Phase 1 | NCT03764774 | |
| α7-nAChR agonist | GTS-21/DMXB-A | Obesity | Otsuka Pharmaceutical | Phase 1 | NCT02458313 |
| Energy absorption | |||||
| Strain product | WST01 | Obesity, T2D | SJTUSM | Phase 2 | NCT04797442 |
| Xla1 | Obesity | YSOPIA Bioscience | Phase 1 | NCT04663139 | |
| Orlistat and acarbose | EMP16-02 | Obesity | Empros Pharma AB | Phase 1 | NCT04521751 |
| Energy storage | |||||
| MGAT2 inhibitor | BMS-963272 | Obesity | Bristol Myers Squibb | Phase 1 | NCT04116632 |
| S-309309 | Obesity | Shionogi & Co. | In-house | See R&D Pipeline | |
| DGAT2 inhibitor | Ervogastat/PF-06865571 | NASH, NAFLD | Pfizer | Phase 1 | NCT03513588 |
| Sirt1/AMPK/eNOS signaling | NS-0200/Leucine-Metformin-Sildenafil | Obesity | NuSirt Biopharma | Phase 2 | NCT03364335 |
| Labisia pumila extract | SKF7 | Obesity | Medika Natura | Phase 2 | NCT04557267 |
| Stimulating IDE synthesis | Cyclo-Z (cyclo(his-pro) plus zinc) | T2D | NovMetaPharma | Phase 2 | NCT03560271 |
| NCT02784275 | |||||
| αGI inhibitor | Sugardown/BTI320 | Prediabetes | Boston Therapeutics | Phase 2 | NCT02358668 |
| CCR2/CCR5 dual agonist | Cenicriviroc | T2D, NAFLD | AbbVie | Phase 2 | NCT02330549 |
| Energy expenditure | |||||
| SGLT2 inhibitor | Ipragliflozin/ASP1941 | T2D | Astellas Pharma | Phase 3 | NCT02452632 |
| Bexagliflozin/EGT1442 | T2D | Theracos | Phase 3 | NCT02836873 | |
| Phase 3 | NCT02715258 | ||||
| Phase 3 | NCT02558296 | ||||
| Remogliflozin etabonate | T2D | Avolynt | Phase 2 | NCT02537470 | |
| Canagliflozin | Obesity, T2D | Johnson & Johnson | Phase 4 | NCT02360774 | |
| Dapagliflozin | T2D, HF, CKD | AstraZeneca | Phase 2 | NCT05179668 | |
| Phase 4 | NCT04249778 | ||||
| Phase 2 | NCT03968224 | ||||
| Phase 3 | NCT02413398 | ||||
| Empagliflozin | T1D, T2D | Boehringer Ingelheim | Phase 3 | NCT04233801 | |
| Phase 2 | NCT03132181 | ||||
| Phase 4 | NCT03157414 | ||||
| Phase 3 | NCT02863328 | ||||
| Phase 3 | NCT02580591 | ||||
| Phase 3 | NCT02414958 | ||||
| Ertugliflozin | T2D, HF | Merck & Co. | Phase 3 | NCT03717194 | |
| SGLT1/2 inhibitor | Licogliflozin/LIK066 | Obesity | Novartis | Phase 2 | NCT03320941 |
| Phase 2 | NCT03100058 | ||||
| Sotagliflozin | T1D, T2D, CKD | Lexicon Pharmaceuticals | Phase 3 | NCT03242252 | |
| Phase 3 | NCT03242018 | ||||
| Phase 3 | NCT02531035 | ||||
| Phase 3 | NCT02384941 | ||||
| MetAP2 inhibitor | Beloranib/ZGN-440/ZGN-433 | Obesity | Larimar Therapeutics | Phase 2 | NCT01666691 |
| ZGN-1061 | Obesity, T2D | Larimar Therapeutics | Phase 2 | NCT03254368 | |
| FGF21/FGFR1c/β-Klotho signaling | LLF580 | Obesity | Novartis Pharmaceuticals | Phase 1 | NCT03466203 |
| NN9499/NNC0194-0499 | Obesity | Novo Nordisk | Phase 1 | NCT03479892 | |
| MK-3655/NGM313 | Obesity, NASH | Merck & Co. | Phase 1 | NCT02708576 | |
| NCT04583423 | |||||
| BFKB8488A | NAFLD | Genentech | Phase 1 | NCT02593331 | |
| FGFR4 inhibitor | IONIS-FGFR4Rx | Obesity | Ionis Pharmaceuticals | Phase 2 | NCT02476019 |
| FXR agonist | ASC42 | Obesity, NASH | Gannex Pharma | Phase 1 | See R&D Pipeline |
| THR-β agonist | ASC41 | Obesity, NAFLD | Gannex Pharma | Phase 1 | NCT04686994 |
| sGC stimulator | Praliciguat/IW-1973 | T2D | Cyclerion Therapeutics | Phase 2 | NCT02906579 |
| Neutrophil elastase inhibitor | PHP-303 | Obesity | pH Pharma | Phase 1 | NCT03775278 |
| PDE4/5 inhibitor | Roflumilast | Obesity | Altana Pharma | Phase 3 | NCT04800172 |
| Tadalafil | Obesity | Eli Lilly and Company | Phase 2 | NCT02819440 | |
| Glabridin analog | HSG4112 | Obesity | Glaceum | Phase 1 | NCT05310032 |
| Phase 2 | NCT05197556 | ||||
| Phase 1 | NCT04703764 | ||||
| ActRII inhibition | Bimagrumab/BYM338 | T2D | Novartis | Phase 2 | NCT03005288 |
MC4R melanocortin-4 receptor, NPY5R neuropeptide y receptor y5, R&D research and development, SNDRI serotonin–norepinephrine–dopamine reuptake inhibitor, CB1 cannabinoid receptor 1, GLP-1R glucagon-like peptide 1 receptor, T2D type 2 diabetes, LAPS long-acting peptide/protein discovery, HO hypothalamic obesity, GCGR glucagon receptor, GIPR glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide receptor, NASH nonalcoholic steatohepatitis, OXM oxyntomodulin, DPP-4 dipeptidyl peptidase-4, NAFLD nonalcoholic fatty liver disease, AMYR amylin receptor, CTR calcitonin receptor, TAS2R bitter taste receptor class 2, PYY peptide tyrosine-tyrosine, Y2R neuropeptide y receptor type 2, PWS Prader–Willi syndrome, GDF15 growth differentiation factor 15, α7-nAChR alpha7 nicotinic acetylcholine receptor, MGAT2 monoacylglycerol acyltransferase 2, DGAT2 diacylglycerol acyltransferase 2, IDE insulin degrading enzyme, αGI alpha-glucosidase inhibitor, CCR2/5 C-C chemokine receptor type 2/5, SGLT1/2 sodium-glucose cotransporter 1/2, HF heart failure, CKD chronic kidney disease, T1D type 1 diabetes, MetAP2 methionine aminopeptidase 2, FGF21 fibroblast growth factor 21, FGFR1c fibroblast growth factor receptor 1c isoform, FGFR4 fibroblast growth factor receptor 4, FXR farnesoid x receptor, THR-β thyroid hormone receptor beta, sGC soluble guanylate cyclase, PDE4/5 phosphodiesterase-4/5, ActRII activin type II receptors
Zealand Pharma R&D Pipeline: https://www.zealandpharma.com/product-pipeline
Rhythm Pharmaceuticals R&D Pipeline: https://www.rhythmtx.com/our-pipeline-old
Shionogi & Co. R&D Pipeline: https://www.shionogi.com/global/en/innovation/pipeline.html
MAKScientific R&D Pipeline: https://makscientific.com/drug_pipeline.html
Gannex Pharma R&D Pipeline: https://www.gannexpharma.com/portal/list/index/id/9.html
Novo Nordisk R&D Pipeline: https://www.novonordisk.com/science-and-technology/r-d-pipeline.html
The chronology of AOMs
The hunt for AOMs dates back to the late nineteenth century (Fig. 7). A 50-year-old female who was about 160 cm tall and weighed 112 kg died of levothyroxine abuse because of her obsession with losing weight.422 Soon after, there was another case report of using sheep-derived thyroid extract to increase metabolic rate for weight loss.423 The 2,4-dinitrophenol was all the rage for its impressive weight-lowering effect in the 1930s, but it came with lethal side effects and was suspended by the FDA in 1938 (Fig. 7).424–427 Undiscouraged by these failures, the pharmaceutical companies in the weight loss industry have been trying to seek for a panacea to beat obesity. In 1941, the Clark & Clark (Camden, NJ) combined amphetamine, thyroid, and drugs that targeted untoward effects, and named it Clarkotabs, or the rainbow pills, creating the first commercial diet pills.427,428 The rainbow pills enjoyed a high reputation until 1968 when the FDA prohibited their manufacture and marketing due to findings from the U.S. Senate that the rainbow pills killed over 60 persons.428 Methamphetamine and amphetamine congeners (phenmetrazine, phendimetrazine, phentermine, diethylpropion, benzphetamine, cathine, phenylpropanolamine) were well received given their anorectic effect and were approved by the FDA from 1947 to 1976 to manage obesity.427,429–434 Fenfluramine, a serotonergic agent, was approved by the FDA to lower body weight in 1973. The drug was later coupled with phentermine (fen-phen), resulting in an anorectic that exhibited a balanced norepinephrine-serotonin (5-HT) release.426,427,432,433,435,436 Despite fen-phen having never been approved by the FDA, the number of Americans who were prescribed fen-phen exceeded 18 million in 1996.427,437,438 In the same year, the FDA considered dexfenfluramine safe for use and gave a seal of approval.427,429,433 However, fenfluramine and dexfenfluramine were reported to be closely related to valvular heart disease, pulmonary hypertension, and cardiac fibrosis, and were withdrawn from distribution by the FDA in 1997.427,433,435,438–442 That same year, the FDA approved sibutramine for the long-term treatment of obesity and banned the sale of sibutramine in 2011 over concerns about myocardial infarction and stroke.433,443–446 Although the FDA ceased the commercialization of fenfluramine in 1997, phentermine, once used in combination with fenfluramine as fen-phen, is still approved to treat obesity. In July 2012, the FDA approved phentermine/topiramate extended-release (Qsymia) as an adjunct to lifestyle interventions for long-term weight management.447–450 Orlistat (Xenical; Alli), approved for weight loss by the FDA in 1999, inhibits gastrointestinal lipase and thereby blocks the absorption of dietary fat by about 30%.451–457 Cannabinoid receptor type 1 (CB1) is one of the major receptors of the endocannabinoid system and is widespread in the CNS, including regions that control food intake. Rimonabant is a highly selective CB1 receptor blocker that antagonizes CB1 through inverse agonism, thereby modulating neurocircuits controlling homeostatic feeding and hedonic feeding to shed unwanted pounds.458–464 In 2006, the rimonabant is fully accredited by the European Medicines Agency for use in the European Union.465 However, rimonabant antagonized CB1 in the ventral tegmental area (VTA) and amygdala leading to depression and suicidal ideation, prompting its abolition in 2009.466–468 Lorcaserin is a highly selective 5-HT2C receptor agonist, while its affinity for other 5-HT receptors is greatly reduced. In light of the success of lorcaserin in weight-loss trials, the FDA approved it for long-term weight control in June 2012.469–474 In 2020, the FDA called for the discontinuation of lorcaserin as clinical trials had shown increased cancer rates.475 In September and December 2014, naltrexone extended-release/bupropion extended-release (Contrave) and liraglutide (Saxenda) were approved by the FDA for obesity treatment in succession.449,476–486 Semaglutide (Wegovy) is the second FDA-approved GLP-1R agonist targeting obesity after liraglutide.487–495 Compared with an average of about 5–10% of body weight loss achieved with other currently FDA-approved drugs, semaglutide reaches an approximately 15% average weight loss, ushering in a new era against obesity.496 In November 27, 2020, the FDA approved a melanocortin-4 receptor (MC4R) agonist setmelanotide (IMCIVREE) for chronic weight management in the obese aged ≥6 years in the setting of proopiomelanocortin, proprotein convertase subtilisin/kexin type 1, and LEPR deficiency.497,498
Currently approved AOMs
Phendimetrazine, phentermine, diethylpropion, and benzphetamine are amphetamine congeners functionally identical to amphetamine, which can curb appetite and act as FDA-approved sympathomimetic medications for weight management, but only for short-term use due to safety concerns.499 Amphetamine congeners are competitive substrates for the norepinephrine (NE) transporter (NET), dopamine (DA) transporter (DAT), and 5-HT transporter (SERT).500,501 They bind to NET and DAT with 500-fold greater affinity compared with SERT, and therefore harness monoamines as neurotransmitters mostly in catecholamine neurons in the reward and executive function pathways of the brain to exert its behavioral effects.500–504 NE and DA are the prime monoamine neurotransmitters and their concentrations in the synaptic cleft are increased by amphetamine congeners in a dose-dependent manner.503,504 Upon entering the presynaptic neuron, the amphetamine congeners encounter vesicular monoamine transporter type 2. Their interaction collapses the vesicular pH gradient and jeopardizes the acidic environment of the vesicle, preventing the translocation of NE and DA from the axoplasm into vesicles, and causing intracellular accumulation of NE and DA.505,506 Alternatively, amphetamine congeners target monoamine oxidase (MAO) and hinder MAO-mediated NE and DA breakdown, resulting in elevated intracellular concentrations of NE and DA. In fact, the distribution of the two isoenzymes of MAO (MAO-A and MAO-B) varies. The former is located at the synaptic terminals of NE and DA neurons, while the latter is the only isoform that acts at 5-HT terminals and within non-catecholamine neurons. Thus, the effect of amphetamine congeners on the number of extracellular monoamines is significant with regard to NE and DA, but less so for 5-HT.500 Amphetamine congeners further bind to the trace amine-associated receptor 1 and activate PKA and protein kinase C, triggering DA efflux and DAT internalization.501,503 Besides, amphetamine congeners also increase intracellular calcium, leading to DAT phosphorylation and subsequent DA efflux.507,508 All these amphetamine congeners-mediated processes contribute to NE and DA release in reward circuitry and executive functioning via NET and DAT, respectively. Elevated NE and DA produces a sense of satiety by activating postsynaptic NE and DA receptors.501
Qsymia is a combination of phentermine, a sympathomimetic amine anorectic, and topiramate extended-release, an antiepileptic drug. The pharmacologic activity of phentermine is akin to that of its prototype drug, amphetamine, whose mechanism of action has been elucidated in the above paragraph. However, the mechanism by which topiramate is able to manage weight in the long term remains uncharted and requires more in-depth investigations. Topiramate barricades voltage-gated sodium (Na+) and high voltage-activated calcium (Ca+) channels and positively modulates at least one potassium (K+) channel in presynaptic excitatory neurons. Topiramate has inhibitory effects on the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors on postsynaptic neurons, both of which are ionotropic transmembrane receptors for glutamate. Topiramate enhances GABA synthesis and blocks its reuptake or degradation, which augments the activity of GABAergic neurons and positively modulates GABAA receptors. Besides, topiramate inhibits carbonic anhydrase isoenzymes.509–515
Contrave is the trade name of the anti-obesity combination of naltrexone hydrochloride 8 mg and bupropion hydrochloride 90 mg. Bupropion is a weak inhibitor of NE and DA reuptake, generally prescribed for anti-depression and smoking cessation aid.516,517 Naltrexone is a high-affinity antagonist for mu(μ)-opioid receptor (MOPr), primarily prescribed for the management of alcoholism and opioid addiction.516,517 Since bupropion and naltrexone reduce weight in the course of treatment, they were combined as a diet drug.518 Food intake is regulated by the melanocortin and NPY systems in the ARC of the hypothalamus.519 The melanocortin system contains POMC and AgRP cell populations, and adjusts homeostatic energy balance. POMC cells release α-MSH and β-endorphin. α-MSH activates MC4R, which in turn produces anorexic effects that increase energy expenditure and decrease appetite. The attachment of β-endorphin to the inhibitory MOPr on POMC cells spoils the activity of POMC cells. In contrast, AgRP and NPY are orexigenic peptides with appetite-stimulating effects, and ablation and stimulation of AgRP/NPY peptides can lead to decreased and increased food intake, respectively.520 AgRP is an MC4R antagonist that competitively blocks the α-MSH-MC4R cascade, reducing energy expenditure and increasing appetite. Bupropion inhibits the reuptake of NE and DA, provoking POMC neurons in the ARC of the hypothalamus. By releasing both α-MSH and β-endorphin, the β-endorphin-mediated autoinhibitory feedback effect neutralizes the positive signal of weight loss generated by α-MSH, which may interpret the limited long-term weight loss (<5%) of bupropion monotherapy. Intriguingly, naltrexone is able to antagonize MOPr and block its binding with β-endorphin, thereby preventing feedback autoinhibition of POMC neurons. Therefore, the naltrexone/bupropion combination exhibits stronger stimulation of POMC cells than either drug alone. Enhanced POMC signaling underlies the clinically effective weight loss of Contrave.516–518,521
Liraglutide (Saxenda) and semaglutide (Wegovy) are GLP-1R agonists, which help shed pounds via food intake reduction in that they lower appetite and inhibit gastric emptying.322,522 GLP‐1 mainly originates from the intestinal enteroendocrine L cells and the preproglucagon neurons (named after the transcript) or glucagon neurons (named after the gene) located in the hindbrain NTS.523,524 Conventional or habitual thinking holds that the peripheral (gut‐derived and exogenous) and central (brain‐derived) GLP-1 systems are connected, but current shreds of evidence suggest that they are most likely independent entities.524–526 In other words, compared with brain‐derived GLP‐1 released from the preproglucagon neurons, gut‐derived GLP‐1 released from the enteroendocrine L cells and exogenous GLP‐1 act in different modes of action to inhibit feeding behavior. Brierley et al. corroborated this point finding that exogenous liraglutide and semaglutide displayed intact ability for losing weight in the setting of ablative preproglucagon neurons.325 It appears that liraglutide and semaglutide, administered systemically, mimic the function of postprandial gut-derived GLP-1 and directly interact with GLP-1 receptors in the CNS that are not shielded by the blood–brain barrier to exert their slimming effect.524,525 First, liraglutide and semaglutide activate NTS GLP-1R signaling primarily by coupling to Gαs/Gsα, which simultaneously activates AMPK and suppresses ERK1/2 signaling pathways via increasing PKA activity, thereby increasing cAMP response element-binding protein (CREB)-mediated nuclear transcription and protein synthesis to reduce food intake and lose weight.325,527 Besides, increased PKA activity inhibits membrane-bound p-Akt-Ser473 via PI3K/PIP3-mediated translocation of Akt to the membrane, which may promote mTOR/CREB and FoxO signaling pathways.528 Second, GLP-1-producing neurons in the NTS directly project to other nucleus associated with food intake, such as mesolimbic reward system (MRS) nuclei that includes VTA and the nucleus accumbens.529 Injection of the GLP-1R agonist in rat VTA activates GLP-1R and suppresses food intake through AMPA/kainate receptor signaling.530 Likewise, intra-NAc core GLP-1R activation prevents weight gain at least in part via AMPA/kainate receptor signaling.529 These findings provide valuable insights into the negative energy balance mediated by GLP-1R signaling engaged glutamatergic neurotransmission in the MRS. Third, researchers unraveled the neuroanatomical and molecular cascades that modulate feeding behavior in the paraventricular nucleus (PVN) of the hypothalamus, which are NTS–to–PVN glucagon neuronal projection and GLP-1R signaling in the PVN. Specifically, GLP-1R signaling first stimulates the PKA pathway, and then phosphorylates the AMPA receptor subunit glutamate receptor 1 (GluR1, also referred to as GluA1) at S845 to enhance GluA1 membrane trafficking, ultimately augmenting excitatory effects on postsynaptic neurons.531 Fourth, AP is a circumventricular organ that regulates emesis. Electrophysiological effects of GLP-1 on mice AP neurons indicated that GLP-1 directly binds to Gαs and elicits AC that converts adenosine triphosphate (ATP) to cAMP, resulting in the activation of the AC/cAMP/PKA signaling pathway.532
Lipases comprise lingual lipase, gastric lipase, and pancreatic lipase. Lingual lipase has a weak effect on fat degradation, but in infants and young children, it can degrade about 50–70% of ingested fat. It is generally accepted that gastric lipase is a regulator of pancreatic lipase secretion and plays an auxiliary role in lipolysis.533–535 Compared with lingual lipase and gastric lipase, the role of pancreatic lipase in lipolysis is self-evident. The pancreatic lipase directly participates in the regulation of intestinal absorption of fatty acids. When the human body ingests dietary fats, gastric lipase and pancreatic lipase hydrolyze about 10–30% and 50–70% of the lipids, respectively, generating substances including monoglycerides and free fatty acids, which are subsequently absorbed by the intestine. Next, monoacylglycerols are resynthesized into triacylglycerols and stored in the form of adipose tissue for energy deposition.533,536,537 Being the solitary diet drug targeting lipase currently in clinical use, orlistat is available on the market as two different products, Xenical (Roche) and Alli (GlaxoSmithKline), which are prescription and over-the-counter respectively (Fig. 7).538 Orlistat exerts its lipid-inhibiting effect without acting on the CNS or entering the bloodstream.533 Specifically, orlistat localizes active site serine residues of human gastric and pancreatic lipases, which form stoichiometric long-lived acyl-enzyme complexes upon nucleophilic attack on their β-lactone rings. This covalent binding impedes the hydrolysis of dietary triacylglycerols, thereby reducing monoglycerides and free fatty acids, and eventually decreasing fat storage and achieving the purpose of weight loss.533,539–543
AOMs under clinical trial
GLP-1R agonist
Even with the landmark research results of liraglutide in 2009544 and semaglutide in 2021488 and their great success in the market, the enthusiasm for exploration of different GLP-1R agonists does not seem to have faded. We listed the clinical trials concerning GLP-1R agonists after 2015 in Table 1, and their mechanisms of action have been detailed previously.
GCGR agonist
Glucagon acts through the coupling of glucagon receptor (GCGR) to Gαs and Gq proteins, which trigger the activities of AC and phospholipase C (PLC), respectively.545 It is generally believed that AC catalyzes the conversion of ATP to cAMP and PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate the secondary messengers diacylglycerol and inositol 1,4,5-triphosphate (IP3). Glucagon participates in lipid metabolism by inhibiting and promoting hepatic lipogenesis and β-oxidation, respectively.545 Specifically, glucagon stimulates AC on the adipocyte’s membrane of the liver, leading to increased activity of the cAMP/PKA signaling pathway and phosphorylation/activation of downstream HSL. Phosphorylated HSL converts diglycerides to monoglycerides and yields free fatty acids via monoacylglycerol lipase, thereby attenuating lipogenesis. The activated cAMP/PKA signaling pathway phosphorylates/inactivates acetyl-CoA carboxylase (ACC) to inhibit the conversion of acetyl-CoA to malonyl-CoA, thereby inhibiting the de novo synthesis of fatty acids. Furthermore, the activated cAMP/PKA signaling activates CREB, PPARα, and FoxA2 to induce the transcription of genes required for β-oxidation.545 In a recent article published in Nature, Perry et al. unraveled an IP3 receptor type I (IP3R-I/INSP3R1)-dependent signaling pathway of glucagon-induced lipolysis in the liver. They found that INSP3R1 integrates signals from Gαs/cAMP/PKA and Gq/PLC/IP3 cascades and releases Ca2+ into the mitochondria and cytosol to increase β-oxidation and Ca2+/calmodulin (CaM)-dependent protein kinase II/ATGL-mediated lipolysis, respectively.546,547 Glucagon enhances FGF21 release in the liver to stimulate thermogenesis in BAT or directly stimulates BAT and browning of WAT to raise energy consumption.548 Glucagon induces satiety by regulating the PKA/CaM-dependent protein kinase kinase β (CaMKKβ)/AMPK/AgRP signaling pathway through the liver-brain axis, which triggers satiety signals in the liver and maps to AP and NTS through the hepatic branch of the vagus nerve, and then transmits to the hypothalamic ARC.549–551 Long-acting glucagon is more suitable for use in weight loss in that it has an extremely short half-life in rodents.551,552 HM15136 is a novel long-acting glucagon analog developed by Hanmi PharmaceuticalTM that treats obesity by regulating liver-targeted signaling pathways, energy expenditure, and satiety (Table 1).553
GIPR agonist and antagonist
Currently, the field regarding glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide (GIP) is still in the phenomenological stage and both positive and negative modulation of GIP receptor (GIPR) activity can lead to weight loss.554 This mechanism is backed by admittedly preclinical research and unique among clinical-stage assets. Therefore, there are existing dual agonists in which GLP-1R agonist is combined with GIPR agonist or antagonist. AMG133 is a drug consisting of GLP-1R agonist and GIPR antagonist and is currently under phase 1 clinical trial (Table 1). It might take time to determine whether such a mechanism works in humans.
GLP-1R-based dual or triple agonists
GLP-1R agonists such as liraglutide and semaglutide have been in clinical use to treat obesity for nearly 8 years and 1 year, respectively. However, even with great success, it is undeniable that there is still a huge gap in weight management between GLP-1R agonist monotherapy and bariatric surgery. To achieve equivalent efficacy using a noninvasive strategy, researchers have envisioned a “co-agonist” blueprint combining GLP-1R agonists with GCGR agonists or GIPR agonists, or both.555 In rodents, combined GLP-1 and glucagon administration increased c-Fos expression in the brainstem and amygdala and exhibited a synergistic effect on food intake reduction.556 In humans, coadministration of GLP-1 and glucagon results in a superior reduction in food intake than either GLP-1 or glucagon alone.557 Besides, GLP-1 can neutralize hyperglycemia caused by glucagon,558 and polypharmacy can achieve the same reduction in food intake or increase in energy expenditure at fewer doses.559 Cotadutide (MEDI0832) is the first GLP-1R/GCGR dual agonist under clinical trial and progresses well.560,561 By contrast, other dual agonists have shown mixed results.562 Efinopegdutide (JNJ-64565111) and liraglutide resulted in placebo-adjusted weight loss of 6.7–10.0 and 5.8%, respectively, and gastrointestinal adverse events occurred in 89 and 60%, respectively.563 Besides, the clinical trials of SAR425899 were halted owing to severe gastrointestinal adverse events.564 Several other GLP-1R/GCGR dual agonists are currently in development including pemvidutide/ALT-801, BI 456906, CT-388, CT-868, DD01, and NN9277/NNC9204-1177 (Table 1). Similarly, the observation that GLP-1R/GIPR dual agonist enhanced weight loss in mice has successfully set off an upsurge in the study of GLP-1R/GIPR dual agonists.565,566 For example, tirzepatide (LY3298176) has been shown to surpass semaglutide in glucose and body weight control.567 Therefore, it is rational to step up the pace for the development of GLP-1R/GIPR/GCGR triple agonist. LY3437943 is a novel GLP-1R/GIPR/GCGR triple agonist and showed superior weight loss in mice compared to other incretin receptor-targeting molecules.568 Furthermore, Bossart and Konkar developed SAR441255 and found that treatment of the novel peptide triagonist showed greater metabolic outcomes in mice and monkeys (Table 1).569
Oxyntomodulin (OXM) analog
OXM, as well as GLP-1 and PYY, are intestinal anorectic hormones secreted from the enteroendocrine L cells.570 OXM stands for a weak but glucagon-dominant GLP-1R/GCGR dual agonist as it is 3- and 100-fold less potent than the cognate ligands glucagon and GLP-1 concerning cAMP accumulation, respectively.562,571 OXM has exhibited stronger efficacy in weight and glucose management compared to pure GLP-1R agonists in several preclinical studies.572 Central and peripheral OXM administration can reduce food intake in rodents and humans or rodents, respectively.573
Dipeptidyl peptidase-4 (DPP-4) inhibitor
Like glucagon, both GLP-1 and GIP have characteristically short circulating half-lives, suggesting that they are rapidly proteolytically hydrolyzed by several peptidases in plasma, leading to restricted therapeutic utility and widespread use.574,575 DPP-4 plays a quantitatively pivotal role among these peptidases and its active inhibitors that augments incretin levels by delaying clearance of GLP-1 and GIP are developed to abrogate this pharmacokinetic limitation.576 In addition to weight loss by indirectly increasing the expression of GLP-1 and GIP, DPP-4 inhibitors also achieve weight control in other ways. Catalán et al. are the first to find that caveolin-1 (CAV-1), an integral membrane protein most abundantly distributed in adipose tissue,577 is upregulated in visceral and subcutaneous adipose tissue in obese patients compared to lean controls, regardless of glucose levels.578 They also revealed a significant correlation between CAV-1 mRNA expression and several inflammatory markers.578 In adipocytes, CAV-1 modulates insulin transduction via the Akt signaling pathway.579 Intriguingly, active site in DPP-4 was indispensable in the interaction between DPP-4 and CAV-1.580 These pieces of evidence raise a possible way to beat obesity, that is blocking the interaction between DPP-4 and CAV-1 to improve adipocyte insulin sensitivity. The burning of glucose and fat in brown and beige adipose cells for heat production is primarily mediated by UCP1.581 Takeda et al. deciphered that the DPP-4 inhibitor upregulates UCP1 expression via the inhibition of the ERK1/2 signaling pathway, indicating that long-term use of the DPP-4 inhibitor could significantly improve body weight and energy homeostasis by modulating BAT activity and is a possible option to cure obesity.582
Amylin receptor (AMYR) and calcitonin receptor (CTR) agonists
Amylin has been reported to inhibit gastric emptying through specific binding to AMYR in the gastric fundus and mapping of corresponding neuronal signals to AP and NAc in the hindbrain.583 Amylin induces an anorectic effect based on its positive stimuli on AP neurons through cGMP, c-Fos, and ERK1/2 signaling pathways.584–586 Amylin also induces anorexia by increasing brain 5-HT, stimulating histamine H1 and dopamine D2 receptors, and inhibiting NPY-induced feeding.587–590 Amylin serves as one of the few molecules owning the ability to restore leptin sensitivity in diet-induced obesity by potentiating leptin-induced p-STAT3 in ARC and VMN of the hypothalamus.591,592 Moreover, amylin increases IL-6 to enhance the activation of leptin-induced p-STAT3 in the VMN.593 The human AMYR isoforms are CTR-based complexes incorporating receptor activity-modifying proteins.594,595 Studies have shown that calcitonin induces signaling pathways similar to those of amylin in the hindbrain.594
PYY/NPY receptor type 2 (Y2R) signaling
PYY is co-secreted with GLP-1 and OXM from L cells as PYY1-36 and hereupon rapidly converted to its predominant active form PYY3–36 by cleavage mediated by DPP-4. PYY3–36 is a high-affinity Y2R agonist.432 It is demonstrated that postprandial elevation of PYY3-36 inhibits food intake and reduces weight gain through PYY/Y2R signaling on both AgRP/NPY and POMC neurons in a gut–hypothalamic projection manner.596
Ghrelin signaling
Ghrelin is an endogenous ligand of the growth hormone secretagogue receptor type 1a (GHS-R1a).597 Prior to secretion into the bloodstream, ghrelin requires post-translational serine octanoylation/acylation by ghrelin O-acyltransferase (GOAT) to bind and activate the GHS-R1a signaling for its orexigenic actions. Human genetic studies have identified that rare mutations and single-nucleotide polymorphisms of GHSR gene might be associated with obesity.598 In circulation, esterases can remove the octanoyl group of acylated ghrelin and switch it to unacylated ghrelin. Intriguingly, membrane-anchored GOAT can reacylate unacylated ghrelin to acylated one, which can still function through GHS-R1a signaling.599–601 In the VMN of the hypothalamus, the ghrelin-GHS-R1a axis activates AMPK via PLC/IP3/Ca2+/CaMKKβ and Sirt1/p53 signaling pathways.599,600 Besides, CB1 is required for ghrelin to activate AMPK. Activated AMPK inhibits ACC, leading to reduced malonyl-CoA and subsequent carnitine palmitoyltransferase 1A (CPT1A) and 1C (CPT1C) accumulation. AMPK-CPT1A-uncoupling protein 2 and AMPK-CPT1C-ceramide axes potentiate glutamate release from the presynaptic terminals onto AgRP/NPY neurons in ARC and are essential mediators of the effect of ghrelin on feeding. In the ARC of the hypothalamus, the ghrelin upregulates GHS-R1a/mTORC1/S6K and κ-opioid receptor signaling pathways. These two cascades, as well as the effect of glutamate on AgRP/NPY neurons, upregulate key transcription factors pCREB, FoxO1, and BSX to increase mRNA expressions of AgRP and NPY and induce feeding.602–605
SGLT1/2 inhibitor
There are two sodium-glucose co-transporters (SGLTs) that reabsorb renal glucose, with SGLT2 responsible for more than 90% and the remaining 10% by SGLT1.606 SGLT2 inhibitors directly reduce whole body weight by enabling energy expenditure through glucose excretion.607 It was observed that SGLT2 inhibition resulted in a significant energy loss of approximately 75 g glucose per day (300 kcal/day).608 Besides, osmotic diuresis (107–470 ml/day) brought by SGLT2 inhibitor dapagliflozin in drug-naive patients with T2D may contribute to some weight loss.609,610 SGLT2 inhibition activates AMPK signaling and phosphorylates/inactivates ACC to decrease malonyl-CoA, thereby inhibiting the fatty acid synthesis and enhancing β-oxidation.611–613 SGLT2 inhibition also motivates adipose thermogenesis and lipolysis via the β-adrenoceptor/cAMP/PKA signaling pathway.614 Besides, downregulated SGLT2 promotes the browning of WAT by polarizing M2 adipose tissue macrophages and increasing adiponectin expression in WAT and FGF21 expression in the liver and circulation.613 However, the magnitude of weight loss by SGLT2 inhibitors is modest with an average of 1.5–2 kg (placebo-adjusted). The combined administration of SGLT2 inhibitors and anorectics represents a promising way to lose weight.607
FGF21/FGF receptor 1c isoform (FGFR1c)/β-Klotho signaling
The FGFs form a family of 22 members that regulate a plethora of biological processes including growth, differentiation, development, and metabolism.615 Most FGFs function locally as autocrine or paracrine factors, whilst the endocrine FGFs including FGF15/19, FGF21, and FGF23 possess the ability to enter the circulation and function as hormones.616 Among them, FGF15/19 and FGF21 hold tremendous potential for medicinal purposes in counteracting obesity because they are important in metabolic regulation.616,617 An in vivo study revealed that systemic administration of FGF21 lowered mice body weight by 20%.618 FGF21 is a fasting-induced pleiotropic hormone holding pivotal roles in energy balance and glucose and lipid homeostasis via triggering a heterodimeric receptor complex assembled by FGFR1c and β-Klotho.619 FGF21 activates the FGFR1c/β-Klotho complex on the membrane of adipocytes, triggering MAPK/mTORC1/S6K signaling and subsequent adiponectin secretion and UCP1 upregulation, improving insulin sensitivity and body weight.620 Several lines of evidence have proven that FGF21 administration promotes energy expenditure and losses weight through PGC-1α and CCL11 mediated WAT browning621 and central thermogenic hormones-mediated BAT activation.622 Furthermore, central infusion of FGF21 in lean rats activates the hypothalamic-pituitary-thyroid axis to induce UCP1 expression in WAT, leading to weight loss.623 In contrast, central infusion of FGF21 in obese rats failed to reduce body weight.624 Undoubtedly, such a phenomenon and the notion that obesity is an FGF21-resistant state attract us. Emerging in vivo evidence has monitored a dampened ERK1/2/Elk-1/SRF signaling response induced by FGF21/FGFR1c/β-Klotho signaling.625,626 Therefore, a deeper investigation of the molecular mechanism whereby obesity impairs FGF21/FGFR1c/β-Klotho signaling may offer novel insights for the FGF21-based obesity drug development.
GDF15 agonist
GDF15 is a distant member of the TGF-β superfamily.627 Circulating levels of GDF15 are at a low concentration ranging from 0.1 to 1.2 ng/ml under normal physiological conditions. Once the human body is exposed to stress caused by diseases such as tissue damage, cancer, metabolism, and inflammation, the circulation concentration of GDF15 rises by 10- to 100-fold.628 Surprisingly, obese individuals have significantly elevated serum GDF15 levels compared to healthy controls,629 which may be caused by the liver.185 In 2007, four independent studies uncovered that in neurons in AP and NTS, GDF15 binds to GFRAL and then recruits RET, forming a GDF15-GFRAL-RET trimer that induces the phosphorylation of ERK1/2, Akt, and PLC.630–633 In the ARC, GDF15 suppresses appetite via upregulating p-ERK1/2 and p-STAT3 and reducing and increasing the mRNA level of NPY and POMC, respectively.183,185
Perspectives
From mechanistic evidence to clinical observations, the causal link between obesity and morbidity/mortality has long been established. Although considerable progress has been made in the understanding of the etiology and pathophysiology of obesity, our evolving knowledge about obesity pathogenesis and personalized therapies are not satisfactory yet. The prescription of moving more and eating less for tackling obesity has now been proven as a crude oversimplification of this complex disease.634 Decoding of cellular signaling networks enables us to move towards more precise medicine, enriching our arsenal in the fight against obesity. Indeed, precision therapy can be achieved by targeting specific signals/pathways in different obese populations. Of note, this personalized treatment strategy can be largely enhanced with the help of high-performance computing and artificial intelligence, based on the growing clinical and biological datasets. Nevertheless, owing to the complexity of signaling transductions, identifying the molecular culprits of individual patients is still challenging, which may hamper translation to clinical practice. The success of semaglutide has established a solid foundation for the development of GLP-1R agonists. However, there are more questions than answers. Of primary interest is why GLP-1R agonist works so well, and why there is a huge difference between liraglutide and semaglutide concerning weight control,487 which are both GLP-1R agonists. The difference is difficult to attribute to the molecular basis, a situation that seems to exemplify our relatively primitive understanding of the bridge between in vivo efficacy and mechanisms. In addition, we should break the inherent mindset and focus on accelerating the development of energy-consuming drugs, on the basis of understanding the importance of balancing energy intake and energy expenditure. Weight-loss surgery remains the best option for severely obese patients. In this regard, there exists a brilliant future to decipher the signals or pathways involved in obesity through bariatric surgery. In all, the path to seek and develop AOMs remains challenging, and the in-depth learning of known signals and the development and utilization of efficient tools, such as artificial intelligence, are important parts of achieving precision obesity treatment in the future.
Acknowledgements
This study was supported in part by grants from the Key Research and Development Program of Sichuan Province (22ZDYF2649 and 22ZDYF2138), the National Natural Science Foundation of China (81772079 and 81970715), and the Innovation Spark Project of Sichuan University (2018SCUH0065). Figures within this paper were created with BioRender.com.
Author contributions
T.L. and X.X. helped with the conception, design, and organization of the draft. X.W., B.Z., B.W. and H.X. searched the information, materials, and updates and wrote the drafts. Z.L. and R.L. helped with writing, editing, checking, and formatting. X.W., B.Z., and B.W. edited and formatted the figures. All authors have read and approved the article.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xue Wen, Bohan Zhang, Beiyi Wu, Haitao Xiao
Change history
10/21/2022
A Correction to this paper has been published: 10.1038/s41392-022-01188-4
Contributor Information
Xuewen Xu, Email: xxw_0826@163.com.
Tao Li, Email: scutaoli1981@scu.edu.cn.
References
- 1.Gregg EW, et al. Global health effects of overweight and obesity. N. Engl. J. Med. 2017;377:80–81. doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet390, 2627–2642 (2017). [DOI] [PMC free article] [PubMed]
- 3.Font-Burgada J, et al. Obesity and cancer: the oil that feeds the flame. Cell Metab. 2016;23:48–62. doi: 10.1016/j.cmet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Mohammed MS, et al. Systems and WBANs for controlling obesity. J. Health. Eng. 2018;2018:1564748. doi: 10.1155/2018/1564748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Y, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ. Res. 2021;128:232–245. doi: 10.1161/CIRCRESAHA.120.317933. [DOI] [PubMed] [Google Scholar]
- 6.Piché ME, et al. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020;126:1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 7.Caballero B. Humans against obesity: who will win? Adv. Nutr. 2019;10:S4–S9. doi: 10.1093/advances/nmy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green, M. et al. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int. J. Mol. Sci.21, 2890 (2020). [DOI] [PMC free article] [PubMed]
- 9.Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6:223–236. doi: 10.1016/S2213-8587(17)30200-0. [DOI] [PubMed] [Google Scholar]
- 10.Pigeyre M, et al. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci. (Lond.) 2016;130:943–986. doi: 10.1042/CS20160136. [DOI] [PubMed] [Google Scholar]
- 11.Felső R, et al. Relationship between sleep duration and childhood obesity: systematic review including the potential underlying mechanisms. Nutr. Metab. Cardiovasc. Dis. 2017;27:751–761. doi: 10.1016/j.numecd.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Her TK, et al. Dietary carbohydrates modulate metabolic and β-cell adaptation to high-fat diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2020;318:E856–E865. doi: 10.1152/ajpendo.00539.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedenreich CM, et al. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021;15:790–800. doi: 10.1002/1878-0261.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, et al. Mitochondrial protein hyperacetylation underpins heart failure with preserved ejection fraction in mice. J. Mol. Cell Cardiol. 2022;165:76–85. doi: 10.1016/j.yjmcc.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Bermejo, M. et al. The role of the bisphenol A in diabetes and obesity. Biomedicines9, 666 (2021). [DOI] [PMC free article] [PubMed]
- 16.Kahn LG, et al. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. 2020;8:703–718. doi: 10.1016/S2213-8587(20)30129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darbre PD. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017;6:18–27. doi: 10.1007/s13679-017-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen X, et al. Exposure to per- and polyfluoroalkyl substances and mortality in U.S. adults: a population-based cohort study. Environ. Health Perspect. 2022;130:67007. doi: 10.1289/EHP10393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Petrakis, D. et al. Endocrine disruptors leading to obesity and related diseases. Int. J. Environ. Res. Public Health14, 1282 (2017). [DOI] [PMC free article] [PubMed]
- 20.Samblas M, et al. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 2019;14:421–444. doi: 10.1080/15592294.2019.1595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolaidis S. Environment and obesity. Metabolism. 2019;100s:153942. doi: 10.1016/j.metabol.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Shafei AE, et al. Prenatal exposure to endocrine disruptors and reprogramming of adipogenesis: an early-life risk factor for childhood obesity. Child Obes. 2018;14:18–25. doi: 10.1089/chi.2017.0180. [DOI] [PubMed] [Google Scholar]
- 23.Fock KM, et al. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013;28(Suppl 4):59–63. doi: 10.1111/jgh.12407. [DOI] [PubMed] [Google Scholar]
- 24.Jackson VM, et al. Latest approaches for the treatment of obesity. Expert Opin. Drug Discov. 2015;10:825–839. doi: 10.1517/17460441.2015.1044966. [DOI] [PubMed] [Google Scholar]
- 25.Haywood C, et al. Treatment of obesity in older persons–a systematic review. Obes. Rev. 2019;20:588–598. doi: 10.1111/obr.12815. [DOI] [PubMed] [Google Scholar]
- 26.Trepanowski JF, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern. Med. 2017;177:930–938. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan DH, et al. Guideline recommendations for obesity management. Med. Clin. North Am. 2018;102:49–63. doi: 10.1016/j.mcna.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Narayanaswami V, et al. Obesity: current and potential pharmacotherapeutics and targets. Pharm. Ther. 2017;170:116–147. doi: 10.1016/j.pharmthera.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe BM, et al. Treatment of obesity: weight loss and bariatric surgery. Circ. Res. 2016;118:1844–1855. doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Q, et al. Sleeve gastrectomy ameliorates diabetes-induced cardiac hypertrophy correlates with the MAPK signaling pathway. Front. Physiol. 2021;12:785799. doi: 10.3389/fphys.2021.785799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhari SN, et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat. Chem. Biol. 2021;17:20–29. doi: 10.1038/s41589-020-0604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang R, et al. Potential mechanisms of sleeve gastrectomy for reducing weight and improving metabolism in patients with obesity. Surg. Obes. Relat. Dis. 2019;15:1861–1871. doi: 10.1016/j.soard.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 33.D’Alessio D. Is GLP-1 a hormone: whether and when? J. Diabetes Investig. 2016;7(Suppl 1):50–55. doi: 10.1111/jdi.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutch CR, et al. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology. 2017;158:4139–4151. doi: 10.1210/en.2017-00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lønsmann I, et al. Potential role of adenylyl cyclase 8 signaling complexes in regulating insulin secretion from pancreatic beta cells. Cell Signal. 2020;72:109635. doi: 10.1016/j.cellsig.2020.109635. [DOI] [PubMed] [Google Scholar]
- 36.Grill, H. J. A role for GLP-1 in treating hyperphagia and obesity. Endocrinology161, bqaa093 (2020). [DOI] [PMC free article] [PubMed]
- 37.Brown E, et al. Newer GLP-1 receptor agonists and obesity-diabetes. Peptides. 2018;100:61–67. doi: 10.1016/j.peptides.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Chadda KR, et al. GLP-1 agonists for obesity and type 2 diabetes in children: systematic review and meta-analysis. Obes. Rev. 2021;22:e13177. doi: 10.1111/obr.13177. [DOI] [PubMed] [Google Scholar]
- 39.Finlayson G. Food addiction and obesity: unnecessary medicalization of hedonic overeating. Nat. Rev. Endocrinol. 2017;13:493–498. doi: 10.1038/nrendo.2017.61. [DOI] [PubMed] [Google Scholar]
- 40.Berthoud HR, et al. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152:1728–1738. doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman JM. Leptin and the endocrine control of energy balance. Nat. Metab. 2019;1:754–764. doi: 10.1038/s42255-019-0095-y. [DOI] [PubMed] [Google Scholar]
- 42.Winer DA, et al. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016;23:413–426. doi: 10.1016/j.cmet.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Liang YJ, et al. Kinesins in MAPK cascade: how kinesin motors are involved in the MAPK pathway? Gene. 2019;684:1–9. doi: 10.1016/j.gene.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 44.Pudewell S, et al. Accessory proteins of the RAS-MAPK pathway: moving from the side line to the front line. Commun. Biol. 2021;4:696. doi: 10.1038/s42003-021-02149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanucco E, et al. Elimination of B-RAF in oncogenic C-RAF-expressing alveolar epithelial type II cells reduces MAPK signal intensity and lung tumor growth. J. Biol. Chem. 2014;289:26804–26816. doi: 10.1074/jbc.M114.558999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept Signal Transduct. Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 47.Yaribeygi H, et al. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell Longev. 2020;2020:8609213. doi: 10.1155/2020/8609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kassouf, T. et al. Impact of conventional and atypical MAPKs on the development of metabolic diseases. Biomolecules10, 1256 (2020). [DOI] [PMC free article] [PubMed]
- 49.Zhang J, et al. ERK1/2 mediates glucose-regulated POMC gene expression in hypothalamic neurons. J. Mol. Endocrinol. 2015;54:125–135. doi: 10.1530/JME-14-0330. [DOI] [PubMed] [Google Scholar]
- 50.Vernia S, et al. Excitatory transmission onto AgRP neurons is regulated by cJun NH2-terminal kinase 3 in response to metabolic stress. Elife. 2016;5:e10031. doi: 10.7554/eLife.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabio G, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vernia S, et al. Diet-induced obesity mediated by the JNK/DIO2 signal transduction pathway. Genes Dev. 2013;27:2345–2355. doi: 10.1101/gad.223800.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bost F, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- 54.Hu E, et al. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 55.Font de Mora J, et al. Mitogen-activated protein kinase activation is not necessary for, but antagonizes, 3T3-L1 adipocytic differentiation. Mol. Cell Biol. 1997;17:6068–6075. doi: 10.1128/mcb.17.10.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aouadi M, et al. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes. 2006;55:281–289. doi: 10.2337/diabetes.55.02.06.db05-0963. [DOI] [PubMed] [Google Scholar]
- 57.Aouadi M, et al. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett. 2007;581:5591–5596. doi: 10.1016/j.febslet.2007.10.064. [DOI] [PubMed] [Google Scholar]
- 58.Engelman JA, et al. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- 59.Engelman JA, et al. Constitutively active mitogen-activated protein kinase kinase 6 (MKK6) or salicylate induces spontaneous 3T3-L1 adipogenesis. J. Biol. Chem. 1999;274:35630–35638. doi: 10.1074/jbc.274.50.35630. [DOI] [PubMed] [Google Scholar]
- 60.Bashan N, et al. Mitogen-activated protein kinases, inhibitory-kappaB kinase, and insulin signaling in human omental versus subcutaneous adipose tissue in obesity. Endocrinology. 2007;148:2955–2962. doi: 10.1210/en.2006-1369. [DOI] [PubMed] [Google Scholar]
- 61.Khoubai, F. Z. et al. DUSP9, a dual-specificity phosphatase with a key role in cell biology and human diseases. Int. J. Mol. Sci.22, 11538 (2021). [DOI] [PMC free article] [PubMed]
- 62.Zeng X, et al. The essential function of CARD9 in diet-induced inflammation and metabolic disorders in mice. J. Cell Mol. Med. 2018;22:2993–3004. doi: 10.1111/jcmm.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Ouarrat D, et al. TAZ is a negative regulator of PPARγ activity in adipocytes and TAZ deletion improves insulin sensitivity and glucose tolerance. Cell Metab. 2020;31:162–173.e165. doi: 10.1016/j.cmet.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solinas G, et al. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017;6:174–184. doi: 10.1016/j.molmet.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawan A, et al. Skeletal muscle-specific deletion of MKP-1 reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance. Diabetes. 2018;67:624–635. doi: 10.2337/db17-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, et al. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat. Med. 2011;17:1251–1260. doi: 10.1038/nm.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song, Z. et al. Calcium signaling pathways: key pathways in the regulation of obesity. Int. J. Mol. Sci.20, 2768 (2019). [DOI] [PMC free article] [PubMed]
- 68.Wang Z, et al. Integrated multiomic analysis reveals the high-fat diet induced activation of the MAPK signaling and inflammation associated metabolic cascades via histone modification in adipose tissues. Front. Genet. 2021;12:650863. doi: 10.3389/fgene.2021.650863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bak EJ, et al. Licochalcone F alleviates glucose tolerance and chronic inflammation in diet-induced obese mice through Akt and p38 MAPK. Clin. Nutr. 2016;35:414–421. doi: 10.1016/j.clnu.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–525. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 71.Matesanz N, et al. MKK6 controls T3-mediated browning of white adipose tissue. Nat. Commun. 2017;8:856. doi: 10.1038/s41467-017-00948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim AK, et al. Role of MKK3-p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52:347–358. doi: 10.1007/s00125-008-1215-5. [DOI] [PubMed] [Google Scholar]
- 73.Wang Q, et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature. 2021;600:314–318. doi: 10.1038/s41586-021-04127-5. [DOI] [PubMed] [Google Scholar]
- 74.Jiang J, et al. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metabolism. 2017;77:58–64. doi: 10.1016/j.metabol.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee DH, et al. Withaferin A exerts an anti-obesity effect by increasing energy expenditure through thermogenic gene expression in high-fat diet-fed obese mice. Phytomedicine. 2021;82:153457. doi: 10.1016/j.phymed.2020.153457. [DOI] [PubMed] [Google Scholar]
- 76.Lee, S. et al. MEK6 overexpression exacerbates fat accumulation and inflammatory cytokines in high-fat diet-induced obesity. Int. J. Mol. Sci.22, 13559 (2021). [DOI] [PMC free article] [PubMed]
- 77.Ng R, et al. miRNA-32 drives brown fat thermogenesis and trans-activates subcutaneous white fat browning in mice. Cell Rep. 2017;19:1229–1246. doi: 10.1016/j.celrep.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin J, et al. Hepatokine pregnancy zone protein governs the diet-induced thermogenesis through activating brown adipose tissue. Adv. Sci. (Weinh.) 2021;8:e2101991. doi: 10.1002/advs.202101991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aoki M, et al. Oncogenic roles of the PI3K/AKT/mTOR axis. Curr. Top. Microbiol. Immunol. 2017;407:153–189. doi: 10.1007/82_2017_6. [DOI] [PubMed] [Google Scholar]
- 80.Huang X, et al. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J. Biol. Sci. 2018;14:1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun F, et al. Interleukin-8 promotes integrin β3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019;38:449. doi: 10.1186/s13046-019-1455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corti F, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives. Cancer Treat. Rev. 2019;72:45–55. doi: 10.1016/j.ctrv.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Huang XF, et al. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009;10:610–616. doi: 10.1111/j.1467-789X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 84.Taniguchi CM, et al. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 85.Hemmings BA, et al. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deleyto-Seldas N, et al. The mTOR-autophagy axis and the control of metabolism. Front. Cell Dev. Biol. 2021;9:655731. doi: 10.3389/fcell.2021.655731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee K, et al. Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood. 2013;122:2369–2379. doi: 10.1182/blood-2013-01-477505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kwon O, et al. Leptin signalling pathways in hypothalamic neurons. Cell Mol. Life Sci. 2016;73:1457–1477. doi: 10.1007/s00018-016-2133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hill JW, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J. Clin. Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 91.Yang SB, et al. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–436. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He Y, et al. Hypothalamic BMP9 suppresses glucose production by central PI3K/Akt/mTOR pathway. J. Endocrinol. 2021;248:221–235. doi: 10.1530/JOE-19-0591. [DOI] [PubMed] [Google Scholar]
- 93.Townsend KL, et al. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012;26:2187–2196. doi: 10.1096/fj.11-199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsubone T, et al. Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regul. Pept. 2005;130:97–103. doi: 10.1016/j.regpep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 95.Li Z, et al. mTOR signaling in X/A-like cells contributes to lipid homeostasis in mice. Hepatology. 2019;69:860–875. doi: 10.1002/hep.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Folgueira C, et al. Pharmacological inhibition of cannabinoid receptor 1 stimulates gastric release of nesfatin-1 via the mTOR pathway. World J. Gastroenterol. 2017;23:6403–6411. doi: 10.3748/wjg.v23.i35.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saltiel AR, et al. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 98.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J. Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li T, et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017;25:374–385. doi: 10.1016/j.cmet.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedrichsen M, et al. Differential aetiology and impact of phosphoinositide 3-kinase (PI3K) and Akt signalling in skeletal muscle on in vivo insulin action. Diabetologia. 2010;53:1998–2007. doi: 10.1007/s00125-010-1795-8. [DOI] [PubMed] [Google Scholar]
- 101.Mackenzie RW, et al. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014;7:55–64. doi: 10.2147/DMSO.S48260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhong X, et al. LNK deficiency decreases obesity-induced insulin resistance by regulating GLUT4 through the PI3K-Akt-AS160 pathway in adipose tissue. Aging (Albany NY) 2020;12:17150–17166. doi: 10.18632/aging.103658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, et al. Inhibition of insulin/PI3K/AKT signaling decreases adipose Sortilin 1 in mice and 3T3-L1 adipocytes. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:2924–2933. doi: 10.1016/j.bbadis.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L, et al. PID1 in adipocytes modulates whole-body glucose homeostasis. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:125–132. doi: 10.1016/j.bbagrm.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Chen T, et al. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging (Albany NY) 2019;11:7510–7524. doi: 10.18632/aging.102263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mladenova SG, et al. Anti-adipogenic effect of alchemilla monticola is mediated via PI3K/AKT signaling inhibition in human adipocytes. Front. Pharm. 2021;12:707507. doi: 10.3389/fphar.2021.707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SK, et al. Metformin sensitizes insulin signaling through AMPK-mediated PTEN down-regulation in preadipocyte 3T3-L1 cells. J. Cell Biochem. 2011;112:1259–1267. doi: 10.1002/jcb.23000. [DOI] [PubMed] [Google Scholar]
- 108.Venniyoor A. PTEN: a thrifty gene that causes disease in times of plenty? Front. Nutr. 2020;7:81. doi: 10.3389/fnut.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pal A, et al. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N. Engl. J. Med. 2012;367:1002–1011. doi: 10.1056/NEJMoa1113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sylow L, et al. Akt and Rac1 signaling are jointly required for insulin-stimulated glucose uptake in skeletal muscle and downregulated in insulin resistance. Cell Signal. 2014;26:323–331. doi: 10.1016/j.cellsig.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Lee AD, et al. Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett. 1995;361:51–54. doi: 10.1016/0014-5793(95)00147-2. [DOI] [PubMed] [Google Scholar]
- 112.Chen Q, et al. Targeting RalGAPα1 in skeletal muscle to simultaneously improve postprandial glucose and lipid control. Sci. Adv. 2019;5:eaav4116. doi: 10.1126/sciadv.aav4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wan M, et al. A noncanonical, GSK3-independent pathway controls postprandial hepatic glycogen deposition. Cell Metab. 2013;18:99–105. doi: 10.1016/j.cmet.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Titchenell PM, et al. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 2016;23:1154–1166. doi: 10.1016/j.cmet.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sacco F, et al. Phosphoproteomics reveals the GSK3-PDX1 axis as a key pathogenic signaling node in diabetic islets. Cell Metab. 2019;29:1422–1432.e1423. doi: 10.1016/j.cmet.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Chen H, et al. PI3K-resistant GSK3 controls adiponectin formation and protects from metabolic syndrome. Proc. Natl Acad. Sci. USA. 2016;113:5754–5759. doi: 10.1073/pnas.1601355113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chakraborty C, et al. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip. Rev. RNA. 2014;5:697–712. doi: 10.1002/wrna.1240. [DOI] [PubMed] [Google Scholar]
- 118.Cusi K, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bertacca A, et al. Continually high insulin levels impair Akt phosphorylation and glucose transport in human myoblasts. Metabolism. 2005;54:1687–1693. doi: 10.1016/j.metabol.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 120.Meng Z, et al. Highly bioavailable berberine formulation improves glucocorticoid receptor-mediated insulin resistance via reduction in association of the glucocorticoid receptor with phosphatidylinositol-3-kinase. Int J. Biol. Sci. 2020;16:2527–2541. doi: 10.7150/ijbs.39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wara AK, et al. KLF10 deficiency in CD4(+) T cells triggers obesity, insulin resistance, and fatty liver. Cell Rep. 2020;33:108550. doi: 10.1016/j.celrep.2020.108550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mauer J, et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 2010;6:e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Catalán V, et al. Expression of S6K1 in human visceral adipose tissue is upregulated in obesity and related to insulin resistance and inflammation. Acta Diabetol. 2015;52:257–266. doi: 10.1007/s00592-014-0632-9. [DOI] [PubMed] [Google Scholar]
- 124.Dann SG, et al. mTOR complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 125.Lai Y, et al. DOCK5 regulates energy balance and hepatic insulin sensitivity by targeting mTORC1 signaling. EMBO Rep. 2020;21:e49473. doi: 10.15252/embr.201949473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lamming DW, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bilanges B, et al. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 2019;20:515–534. doi: 10.1038/s41580-019-0129-z. [DOI] [PubMed] [Google Scholar]
- 128.Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem. J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 129.Hinoi E, et al. PI3K/Akt is involved in brown adipogenesis mediated by growth differentiation factor-5 in association with activation of the Smad pathway. Biochem. Biophys. Res. Commun. 2014;450:255–260. doi: 10.1016/j.bbrc.2014.05.108. [DOI] [PubMed] [Google Scholar]
- 130.Wang X, et al. Gab2 deficiency suppresses high-fat diet-induced obesity by reducing adipose tissue inflammation and increasing brown adipose function in mice. Cell Death Dis. 2021;12:212. doi: 10.1038/s41419-021-03519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ortega-Molina A, et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 2012;15:382–394. doi: 10.1016/j.cmet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 132.Plum L, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 133.Jeong MY, et al. Albiflorin ameliorates obesity by inducing thermogenic genes via AMPK and PI3K/AKT in vivo and in vitro. Metabolism. 2017;73:85–99. doi: 10.1016/j.metabol.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 134.Perna, S. et al. The role of glutamine in the complex interaction between gut microbiota and health: a narrative review. Int. J. Mol. Sci.20, 5232 (2019). [DOI] [PMC free article] [PubMed]
- 135.Albert V, et al. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015;33:55–66. doi: 10.1016/j.ceb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 136.Polak P, et al. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 137.Liu M, et al. Grb10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab. 2014;19:967–980. doi: 10.1016/j.cmet.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu D, et al. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J. Clin. Invest. 2016;126:1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laplante M, et al. mTOR signaling at a glance. J. Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen S, et al. Response gene to complement 32 suppresses adipose tissue thermogenic genes through inhibiting β3-adrenergic receptor/mTORC1 signaling. FASEB J. 2018;32:4836–4847. doi: 10.1096/fj.201701508R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung SM, et al. Non-canonical mTORC2 signaling regulates brown adipocyte lipid catabolism through SIRT6-FoxO1. Mol. Cell. 2019;75:807–822.e808. doi: 10.1016/j.molcel.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shuai K. Modulation of STAT signaling by STAT-interacting proteins. Oncogene. 2000;19:2638–2644. doi: 10.1038/sj.onc.1203522. [DOI] [PubMed] [Google Scholar]
- 143.Shuai K. The STAT family of proteins in cytokine signaling. Prog. Biophys. Mol. Biol. 1999;71:405–422. doi: 10.1016/s0079-6107(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 144.Corry J, et al. Activation of STAT transcription factors by the Rho-family GTPases. Biochem. Soc. Trans. 2020;48:2213–2227. doi: 10.1042/BST20200468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem. Sci. 2000;25:496–502. doi: 10.1016/s0968-0004(00)01624-8. [DOI] [PubMed] [Google Scholar]
- 146.Harris M, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J. Clin. Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Banks AS, et al. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 148.Li C, et al. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc. Natl Acad. Sci. USA. 1999;96:9677–9682. doi: 10.1073/pnas.96.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 150.Buettner C, et al. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cui Y, et al. Essential role of STAT3 in body weight and glucose homeostasis. Mol. Cell Biol. 2004;24:258–269. doi: 10.1128/MCB.24.1.258-269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang J, et al. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br. J. Pharm. 2009;158:475–482. doi: 10.1111/j.1476-5381.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galic S, et al. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. Biochem. J. 2014;461:177–188. doi: 10.1042/BJ20140143. [DOI] [PubMed] [Google Scholar]
- 154.Huang H, et al. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat. Neurosci. 2012;15:1391–1398. doi: 10.1038/nn.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anderwald C, et al. Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2. Mol. Endocrinol. 2002;16:1612–1628. doi: 10.1210/mend.16.7.0867. [DOI] [PubMed] [Google Scholar]
- 156.Kim YB, et al. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology. 2000;141:2328–2339. doi: 10.1210/endo.141.7.7536. [DOI] [PubMed] [Google Scholar]
- 157.Plum L, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat. Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang ZY, et al. Protein tyrosine phosphatases in hypothalamic insulin and leptin signaling. Trends Pharm. Sci. 2015;36:661–674. doi: 10.1016/j.tips.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 159.Perry RJ, et al. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wei CC, et al. Magnesium reduces hepatic lipid accumulation in yellow catfish (Pelteobagrus fulvidraco) and modulates lipogenesis and lipolysis via PPARA, JAK-STAT, and AMPK pathways in hepatocytes. J. Nutr. 2017;147:1070–1078. doi: 10.3945/jn.116.245852. [DOI] [PubMed] [Google Scholar]
- 161.Dai Z, et al. Depletion of suppressor of cytokine signaling-1a causes hepatic steatosis and insulin resistance in zebrafish. Am. J. Physiol. Endocrinol. Metab. 2015;308:E849–E859. doi: 10.1152/ajpendo.00540.2014. [DOI] [PubMed] [Google Scholar]
- 162.Inoue H, et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat. Med. 2004;10:168–174. doi: 10.1038/nm980. [DOI] [PubMed] [Google Scholar]
- 163.Inoue H, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 164.Moh A, et al. STAT3 sensitizes insulin signaling by negatively regulating glycogen synthase kinase-3 beta. Diabetes. 2008;57:1227–1235. doi: 10.2337/db06-1582. [DOI] [PubMed] [Google Scholar]
- 165.Hong F, et al. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933–941. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- 166.Ki SH, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lichanska AM, et al. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet. 2008;24:41–47. doi: 10.1016/j.tig.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 168.Shi SY, et al. Hepatocyte-specific deletion of Janus kinase 2 (JAK2) protects against diet-induced steatohepatitis and glucose intolerance. J. Biol. Chem. 2012;287:10277–10288. doi: 10.1074/jbc.M111.317453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li X, et al. Microfluidic systems for studying dynamic function of adipocytes and adipose tissue. Anal. Bioanal. Chem. 2018;410:791–800. doi: 10.1007/s00216-017-0741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Seth M, et al. Leptin and obesity. Physiol. Int. 2020;107:455–468. doi: 10.1556/2060.2020.00038. [DOI] [PubMed] [Google Scholar]
- 171.Singh P, et al. Leptin signaling in adipose tissue: role in lipid accumulation and weight gain. Circ. Res. 2012;111:599–603. doi: 10.1161/CIRCRESAHA.112.273656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cernkovich ER, et al. Adipose-specific disruption of signal transducer and activator of transcription 3 increases body weight and adiposity. Endocrinology. 2008;149:1581–1590. doi: 10.1210/en.2007-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Derecka M, et al. Tyk2 and Stat3 regulate brown adipose tissue differentiation and obesity. Cell Metab. 2012;16:814–824. doi: 10.1016/j.cmet.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dobrian AD, et al. STAT4 deficiency reduces obesity-induced insulin resistance and adipose tissue inflammation. Diabetes. 2013;62:4109–4121. doi: 10.2337/db12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun S, et al. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev. Nutr. 2012;32:261–286. doi: 10.1146/annurev-nutr-071811-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Richard AJ, et al. The role of JAK-STAT signaling in adipose tissue function. Biochim. Biophys. Acta. 2014;1842:431–439. doi: 10.1016/j.bbadis.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shi SY, et al. Adipocyte-specific deficiency of Janus kinase (JAK) 2 in mice impairs lipolysis and increases body weight, and leads to insulin resistance with ageing. Diabetologia. 2014;57:1016–1026. doi: 10.1007/s00125-014-3185-0. [DOI] [PubMed] [Google Scholar]
- 178.Moisan A, et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat. Cell Biol. 2015;17:57–67. doi: 10.1038/ncb3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zamani N, et al. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr. Rev. 2011;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee MJ. Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1160–1171. doi: 10.1016/j.bbadis.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 181.Fung E, et al. Fc-GDF15 glyco-engineering and receptor binding affinity optimization for body weight regulation. Sci. Rep. 2021;11:8921. doi: 10.1038/s41598-021-87959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tsai VW, et al. The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS One. 2014;9:e100370. doi: 10.1371/journal.pone.0100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Johnen H, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 2007;13:1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 184.Patel S, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29:707–718.e708. doi: 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang D, et al. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021;17:592–607. doi: 10.1038/s41574-021-00529-7. [DOI] [PubMed] [Google Scholar]
- 186.Chrysovergis K, et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J. Obes. (Lond.) 2014;38:1555–1564. doi: 10.1038/ijo.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ahdjoudj S, et al. Transforming growth factor-beta inhibits CCAAT/enhancer-binding protein expression and PPARgamma activity in unloaded bone marrow stromal cells. Exp. Cell Res. 2005;303:138–147. doi: 10.1016/j.yexcr.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 188.Li SN, et al. TGF-β/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Res. Ther. 2020;11:41. doi: 10.1186/s13287-020-1552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abou-Ezzi G, et al. TGF-β signaling plays an essential role in the lineage specification of mesenchymal stem/progenitor cells in fetal bone marrow. Stem Cell Rep. 2019;13:48–60. doi: 10.1016/j.stemcr.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Elsafadi M, et al. SERPINB2 is a novel TGFβ-responsive lineage fate determinant of human bone marrow stromal cells. Sci. Rep. 2017;7:10797. doi: 10.1038/s41598-017-10983-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roh HC, et al. Adipocytes fail to maintain cellular identity during obesity due to reduced PPARγ activity and elevated TGFβ-SMAD signaling. Mol. Metab. 2020;42:101086. doi: 10.1016/j.molmet.2020.101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pfeiffer A, et al. Elevated plasma levels of transforming growth factor-beta 1 in NIDDM. Diabetes Care. 1996;19:1113–1117. doi: 10.2337/diacare.19.10.1113. [DOI] [PubMed] [Google Scholar]
- 193.Yadav H, et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang X, et al. Aerobic exercise improves pulmonary fibrosis by improving insulin resistance and inflammation in obese mice. Front. Physiol. 2021;12:785117. doi: 10.3389/fphys.2021.785117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee JH, et al. Protection from β-cell apoptosis by inhibition of TGF-β/Smad3 signaling. Cell Death Dis. 2020;11:184. doi: 10.1038/s41419-020-2365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang H, et al. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol. Cell Biol. 2010;30:4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schreiber I, et al. BMPs as new insulin sensitizers: enhanced glucose uptake in mature 3T3-L1 adipocytes via PPARγ and GLUT4 upregulation. Sci. Rep. 2017;7:17192. doi: 10.1038/s41598-017-17595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bouhlel MA, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 199.Cipolletta D, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hevener AL, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J. Clin. Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hall JA, et al. Obesity-linked PPARγ S273 phosphorylation promotes insulin resistance through growth differentiation factor 3. Cell Metab. 2020;32:665–675.e666. doi: 10.1016/j.cmet.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Halbgebauer D, et al. Latent TGFβ-binding proteins regulate UCP1 expression and function via TGFβ2. Mol. Metab. 2021;53:101336. doi: 10.1016/j.molmet.2021.101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao, J. et al. Hepatocyte TGF-β signaling inhibiting WAT browning to promote NAFLD and obesity is associated with Let-7b-5p. Hepatol. Commun. 6, 1301–1321 (2022). [DOI] [PMC free article] [PubMed]
- 205.Liu R, et al. SRF-MRTF signaling suppresses brown adipocyte development by modulating TGF-β/BMP pathway. Mol. Cell Endocrinol. 2020;515:110920. doi: 10.1016/j.mce.2020.110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gustafson B, et al. BMP4 and BMP antagonists regulate human white and beige adipogenesis. Diabetes. 2015;64:1670–1681. doi: 10.2337/db14-1127. [DOI] [PubMed] [Google Scholar]
- 207.Qian SW, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl Acad. Sci. USA. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Elsen M, et al. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am. J. Physiol. Cell Physiol. 2014;306:C431–C440. doi: 10.1152/ajpcell.00290.2013. [DOI] [PubMed] [Google Scholar]
- 209.Modica S, et al. The dual role of BMP4 in adipogenesis and metabolism. Adipocyte. 2017;6:141–146. doi: 10.1080/21623945.2017.1287637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Modica S, et al. Bmp4 promotes a brown to white-like adipocyte shift. Cell Rep. 2016;16:2243–2258. doi: 10.1016/j.celrep.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 211.Whittle AJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Martins L, et al. A functional link between AMPK and Orexin mediates the effect of BMP8B on energy balance. Cell Rep. 2016;16:2231–2242. doi: 10.1016/j.celrep.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pellegrinelli V, et al. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat. Commun. 2018;9:4974. doi: 10.1038/s41467-018-07453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hoffmann JM, et al. BMP4 gene therapy in mature mice reduces BAT activation but protects from obesity by browning subcutaneous adipose tissue. Cell Rep. 2017;20:1038–1049. doi: 10.1016/j.celrep.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 215.López M. EJE PRIZE 2017: hypothalamic AMPK: a golden target against obesity? Eur. J. Endocrinol. 2017;176:R235–R246. doi: 10.1530/EJE-16-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Garcia D, et al. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell. 2017;66:789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.López M, et al. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 218.Andersson U, et al. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 219.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 220.Claret M, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Han SM, et al. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- 222.McCrimmon RJ, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57:444–450. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 223.López M, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Martínez de Morentin PB, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beiroa D, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 226.Desjardins EM, et al. Emerging role of AMPK in brown and beige adipose tissue (BAT): implications for obesity, insulin resistance, and type 2 diabetes. Curr. Diab Rep. 2018;18:80. doi: 10.1007/s11892-018-1049-6. [DOI] [PubMed] [Google Scholar]
- 227.Dagon Y, et al. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem. Biophys. Res. Commun. 2006;340:43–47. doi: 10.1016/j.bbrc.2005.11.159. [DOI] [PubMed] [Google Scholar]
- 228.Lee H, et al. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int J. Mol. Med. 2011;28:65–71. doi: 10.3892/ijmm.2011.674. [DOI] [PubMed] [Google Scholar]
- 229.Lin F, et al. The Ca2+/calmodulin-dependent protein kinase kinase, CaMKK2, inhibits preadipocyte differentiation. Endocrinology. 2011;152:3668–3679. doi: 10.1210/en.2011-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bijland S, et al. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. (Lond.) 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 231.Habinowski SA, et al. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2001;286:852–856. doi: 10.1006/bbrc.2001.5484. [DOI] [PubMed] [Google Scholar]
- 232.Giri S, et al. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr. Metab. (Lond.) 2006;3:31. doi: 10.1186/1743-7075-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stone S, et al. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum. Mol. Genet. 2006;15:2709–2720. doi: 10.1093/hmg/ddl204. [DOI] [PubMed] [Google Scholar]
- 234.Chadt A, et al. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat. Genet. 2008;40:1354–1359. doi: 10.1038/ng.244. [DOI] [PubMed] [Google Scholar]
- 235.Chen L, et al. Disruption of the AMPK-TBC1D1 nexus increases lipogenic gene expression and causes obesity in mice via promoting IGF1 secretion. Proc. Natl Acad. Sci. USA. 2016;113:7219–7224. doi: 10.1073/pnas.1600581113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Daval M, et al. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim. Biophys. Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 238.Abou Azar F, et al. Metabolic contributions of Wnt signaling: more than controlling flight. Front. Cell Dev. Biol. 2021;9:709823. doi: 10.3389/fcell.2021.709823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Komiya Y, et al. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Laudes M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J. Mol. Endocrinol. 2011;46:R65–R72. doi: 10.1530/JME-10-0169. [DOI] [PubMed] [Google Scholar]
- 241.Lagathu C, et al. Dact1, a nutritionally regulated preadipocyte gene, controls adipogenesis by coordinating the Wnt/beta-catenin signaling network. Diabetes. 2009;58:609–619. doi: 10.2337/db08-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Doğan A, et al. A new hope for obesity management: Boron inhibits adipogenesis in progenitor cells through the Wnt/β-catenin pathway. Metabolism. 2017;69:130–142. doi: 10.1016/j.metabol.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 243.Xie X, et al. Exposure to HBCD promotes adipogenesis both in vitro and in vivo by interfering with Wnt6 expression. Sci. Total Environ. 2020;705:135917. doi: 10.1016/j.scitotenv.2019.135917. [DOI] [PubMed] [Google Scholar]
- 244.Iyer S, et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Invest. 2013;123:3409–3419. doi: 10.1172/JCI68049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Matsushita K, et al. Nuclear hormone receptor LXRα inhibits adipocyte differentiation of mesenchymal stem cells with Wnt/beta-catenin signaling. Lab. Invest. 2016;96:230–238. doi: 10.1038/labinvest.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang T, et al. Oxysterol-binding protein-like 2 contributes to the developmental progression of preadipocytes by binding to β-catenin. Cell Death Discov. 2021;7:109. doi: 10.1038/s41420-021-00503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zeve D, et al. Wnt signaling activation in adipose progenitors promotes insulin-independent muscle glucose uptake. Cell Metab. 2012;15:492–504. doi: 10.1016/j.cmet.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen M, et al. CTNNB1/β-catenin dysfunction contributes to adiposity by regulating the cross-talk of mature adipocytes and preadipocytes. Sci. Adv. 2020;6:eaax9605. doi: 10.1126/sciadv.aax9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bagchi DP, et al. Wnt/β-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol. Metab. 2020;42:101078. doi: 10.1016/j.molmet.2020.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bagchi DP, et al. Wntless regulates lipogenic gene expression in adipocytes and protects against diet-induced metabolic dysfunction. Mol. Metab. 2020;39:100992. doi: 10.1016/j.molmet.2020.100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Loh NY, et al. LRP5 regulates human body fat distribution by modulating adipose progenitor biology in a dose- and depot-specific fashion. Cell Metab. 2015;21:262–273. doi: 10.1016/j.cmet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rulifson IC, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proc. Natl Acad. Sci. USA. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Das B, et al. The role of Wnt pathway in obesity induced inflammation and diabetes: a review. J. Diabetes Metab. Disord. 2021;20:1871–1882. doi: 10.1007/s40200-021-00862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jin T. Current understanding on role of the Wnt signaling pathway effector TCF7L2 in glucose homeostasis. Endocr. Rev. 2016;37:254–277. doi: 10.1210/er.2015-1146. [DOI] [PubMed] [Google Scholar]
- 255.Seo SH, et al. Inhibition of CXXC5 function reverses obesity-related metabolic diseases. Clin. Transl. Med. 2022;12:e742. doi: 10.1002/ctm2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.García-Martínez JM, et al. WNT/beta-catenin increases the production of incretins by entero-endocrine cells. Diabetologia. 2009;52:1913–1924. doi: 10.1007/s00125-009-1429-1. [DOI] [PubMed] [Google Scholar]
- 257.Fuster JJ, et al. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64:1235–1248. doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Helfer G, et al. Hypothalamic Wnt signalling and its role in energy balance regulation. J. Neuroendocrinol. 2016;28:12368. doi: 10.1111/jne.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Benzler J, et al. Hypothalamic WNT signalling is impaired during obesity and reinstated by leptin treatment in male mice. Endocrinology. 2013;154:4737–4745. doi: 10.1210/en.2013-1746. [DOI] [PubMed] [Google Scholar]
- 260.Boucsein A, et al. Central signalling cross-talk between insulin and leptin in glucose and energy homeostasis. J. Neuroendocrinol. 2021;33:e12944. doi: 10.1111/jne.12944. [DOI] [PubMed] [Google Scholar]
- 261.Boucsein A, et al. Photoperiodic and diurnal regulation of WNT signaling in the arcuate nucleus of the female djungarian hamster, Phodopus sungorus. Endocrinology. 2016;157:799–809. doi: 10.1210/en.2015-1708. [DOI] [PubMed] [Google Scholar]
- 262.Yao Q, et al. Wnt/β-catenin signaling in osteoblasts regulates global energy metabolism. Bone. 2017;97:175–183. doi: 10.1016/j.bone.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 263.Ferrante SC, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr. Res. 2015;77:447–454. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fan Q, et al. Ginsenoside Rb1 facilitates browning by repressing Wnt/β-catenin signaling in 3T3-L1 adipocytes. Med. Sci. Monit. 2021;27:e928619. doi: 10.12659/MSM.928619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lo KA, et al. Wnt inhibition enhances browning of mouse primary white adipocytes. Adipocyte. 2016;5:224–231. doi: 10.1080/21623945.2016.1148834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Guo F, et al. NOTUM promotes thermogenic capacity and protects against diet-induced obesity in male mice. Sci. Rep. 2021;11:16409. doi: 10.1038/s41598-021-95720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cantwell MT, et al. STAT3 suppresses Wnt/β-catenin signaling during the induction phase of primary Myf5+ brown adipogenesis. Cytokine. 2018;111:434–444. doi: 10.1016/j.cyto.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Di Conza, G. et al. ER stress responses: an emerging modulator for innate immunity. Cells9, 695 (2020). [DOI] [PMC free article] [PubMed]
- 269.Rashid HO, et al. ER stress: autophagy induction, inhibition and selection. Autophagy. 2015;11:1956–1977. doi: 10.1080/15548627.2015.1091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lemmer IL, et al. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021;47:101169. doi: 10.1016/j.molmet.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Li-Na Z, et al. Mesencephalic astrocyte-derived neurotrophic factor and its role in nervous system disease. Neurol. Sci. 2017;38:1741–1746. doi: 10.1007/s10072-017-3042-2. [DOI] [PubMed] [Google Scholar]
- 272.Tang Q, et al. MANF: an emerging therapeutic target for metabolic diseases. Trends Endocrinol. Metab. 2022;33:236–246. doi: 10.1016/j.tem.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 273.Yang S, et al. MANF: a new player in the control of energy homeostasis, and beyond. Front. Physiol. 2018;9:1725. doi: 10.3389/fphys.2018.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Galli E, et al. Mesencephalic astrocyte-derived neurotrophic factor is upregulated with therapeutic fasting in humans and diet fat withdrawal in obese mice. Sci. Rep. 2019;9:14318. doi: 10.1038/s41598-019-50841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Danilova T, et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is highly expressed in mouse tissues with metabolic function. Front. Endocrinol. (Lausanne) 2019;10:765. doi: 10.3389/fendo.2019.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yang S, et al. MANF regulates hypothalamic control of food intake and body weight. Nat. Commun. 2017;8:579. doi: 10.1038/s41467-017-00750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Peterson CS, et al. The transcriptome of the rat subfornical organ is altered in response to early postnatal overnutrition. IBRO Rep. 2018;5:17–23. doi: 10.1016/j.ibror.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wu, T. et al. Feeding-induced hepatokine, Manf, ameliorates diet-induced obesity by promoting adipose browning via p38 MAPK pathway. J. Exp. Med.218, e20201203 (2021). [DOI] [PMC free article] [PubMed]
- 279.Hwang J, et al. Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018;43:593–605. doi: 10.1016/j.tibs.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chopra, S. et al. IRE1α-XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science365, eaau6499 (2019). [DOI] [PubMed]
- 281.Song S, et al. Crosstalk of ER stress-mediated autophagy and ER-phagy: involvement of UPR and the core autophagy machinery. J. Cell Physiol. 2018;233:3867–3874. doi: 10.1002/jcp.26137. [DOI] [PubMed] [Google Scholar]
- 282.Huang S, et al. Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease. J. Biol. Chem. 2019;294:18726–18741. doi: 10.1074/jbc.REV119.007036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Xu B, et al. Roflumilast prevents ischemic stroke-induced neuronal damage by restricting GSK3β-mediated oxidative stress and IRE1α/TRAF2/JNK pathway. Free Radic. Biol. Med. 2021;163:281–296. doi: 10.1016/j.freeradbiomed.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 284.Robblee MM, et al. Saturated fatty acids engage an IRE1α-dependent pathway to activate the NLRP3 inflammasome in myeloid cells. Cell Rep. 2016;14:2611–2623. doi: 10.1016/j.celrep.2016.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ning J, et al. Constitutive role for IRE1α-XBP1 signaling pathway in the insulin-mediated hepatic lipogenic program. Endocrinology. 2011;152:2247–2255. doi: 10.1210/en.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Martinon F, et al. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yao T, et al. Ire1α in POMC neurons is required for thermogenesis and glycemia. Diabetes. 2017;66:663–673. doi: 10.2337/db16-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Xiao, Y. et al. Knockout of inositol-requiring enzyme 1α in pro-opiomelanocortin neurons decreases fat mass via increasing energy expenditure. Open Biol.6, 160131 (2016). [DOI] [PMC free article] [PubMed]
- 289.Shan B, et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 2017;18:519–529. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 290.Petrus P, et al. Glutamine links obesity to inflammation in human white adipose tissue. Cell Metab. 2020;31:375–390.e311. doi: 10.1016/j.cmet.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 291.Longo, M. et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci.20, 2358 (2019). [DOI] [PMC free article] [PubMed]
- 292.Vishvanath L, et al. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Invest. 2019;129:4022–4031. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang C, et al. Correlation of TLR4 and KLF7 in. Inflamm. Induc. Obes. Inflamm. 2017;40:42–51. doi: 10.1007/s10753-016-0450-z. [DOI] [PubMed] [Google Scholar]
- 294.de Heredia FP, et al. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 295.Peixoto LG, et al. Metformin attenuates the TLR4 inflammatory pathway in skeletal muscle of diabetic rats. Acta Diabetol. 2017;54:943–951. doi: 10.1007/s00592-017-1027-5. [DOI] [PubMed] [Google Scholar]
- 296.Korbecki J, et al. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm. Res. 2019;68:915–932. doi: 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bae J, et al. Activation of pattern recognition receptors in brown adipocytes induces inflammation and suppresses uncoupling protein 1 expression and mitochondrial respiration. Am. J. Physiol. Cell Physiol. 2014;306:C918–C930. doi: 10.1152/ajpcell.00249.2013. [DOI] [PubMed] [Google Scholar]
- 298.Okla M, et al. Inhibitory effects of Toll-like receptor 4, NLRP3 inflammasome, and interleukin-1β on white adipocyte browning. Inflammation. 2018;41:626–642. doi: 10.1007/s10753-017-0718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Burgueño JF, et al. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:263–278. doi: 10.1038/s41575-019-0261-4. [DOI] [PubMed] [Google Scholar]
- 300.Liu R, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 301.Takeda K, et al. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 302.Takeda K, et al. Microbial recognition by Toll-like receptors. J. Dermatol Sci. 2004;34:73–82. doi: 10.1016/j.jdermsci.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 303.Everard A, et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat. Commun. 2014;5:5648. doi: 10.1038/ncomms6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhang X, et al. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity. 2020;53:43–53. doi: 10.1016/j.immuni.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 305.Andrade WA, et al. Type I interferon induction by Neisseria gonorrhoeae: dual requirement of cyclic GMP-AMP synthase and Toll-like receptor 4. Cell Rep. 2016;15:2438–2448. doi: 10.1016/j.celrep.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Petrasek J, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl Acad. Sci. USA. 2013;110:16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bai J, et al. cGAS‒STING signaling and function in metabolism and kidney diseases. J. Mol. Cell Biol. 2021;13:728–738. doi: 10.1093/jmcb/mjab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Li T, et al. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018;215:1287–1299. doi: 10.1084/jem.20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Oduro PK, et al. The cGAS-STING signaling in cardiovascular and metabolic diseases: future novel target option for pharmacotherapy. Acta Pharm. Sin. B. 2022;12:50–75. doi: 10.1016/j.apsb.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kawai T, et al. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021;320:C375–C391. doi: 10.1152/ajpcell.00379.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Esser N, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pr. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 312.Wang T, et al. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 313.Bai J, et al. DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc. Natl Acad. Sci. USA. 2017;114:12196–12201. doi: 10.1073/pnas.1708744114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mao Y, et al. STING-IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler Thromb. Vasc. Biol. 2017;37:920–929. doi: 10.1161/ATVBAHA.117.309017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Yum, S. et al. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl Acad. Sci. USA118, e2100225118 (2021). [DOI] [PMC free article] [PubMed]
- 316.Cruz VH, et al. Loss of Tbk1 kinase activity protects mice from diet-induced metabolic dysfunction. Mol. Metab. 2018;16:139–149. doi: 10.1016/j.molmet.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhao P, et al. TBK1 at the crossroads of inflammation and energy homeostasis in adipose tissue. Cell. 2018;172:731–743.e712. doi: 10.1016/j.cell.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Reilly SM, et al. An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice. Nat. Med. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Oral EA, et al. Inhibition of IKKɛ and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell Metab. 2017;26:157–170.e157. doi: 10.1016/j.cmet.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Prabakaran, T. et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J.37, e97858 (2018). [DOI] [PMC free article] [PubMed]
- 321.Smith NK, et al. GLP-1: molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019;128:94–105. doi: 10.1016/j.neuint.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Campbell JE, et al. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 323.Müller TD, et al. Glucagon-like peptide 1 (GLP-1) Mol. Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vrang N, et al. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 325.Brierley DI, et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 2021;3:258–273. doi: 10.1038/s42255-021-00344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hisadome K, et al. CCK stimulation of GLP-1 neurons involves α1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes. 2011;60:2701–2709. doi: 10.2337/db11-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Anini Y, et al. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 328.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 329.Weston C, et al. Investigating G protein signalling bias at the glucagon-like peptide-1 receptor in yeast. Br. J. Pharm. 2014;171:3651–3665. doi: 10.1111/bph.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Bavec A, et al. Different role of intracellular loops of glucagon-like peptide-1 receptor in G-protein coupling. Regul. Pept. 2003;111:137–144. doi: 10.1016/s0167-0115(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 331.Muscogiuri G, et al. Glucagon-like peptide-1 and the central/peripheral nervous system: crosstalk in diabetes. Trends Endocrinol. Metab. 2017;28:88–103. doi: 10.1016/j.tem.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 332.Nakamura T, et al. PSCs and GLP-1R: occurrence in normal pancreas, acute/chronic pancreatitis and effect of their activation by a GLP-1R agonist. Lab Invest. 2014;94:63–78. doi: 10.1038/labinvest.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Krieger JP, et al. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65:34–43. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 334.Baggio LL, et al. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 335.Gabery, S. et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight5, e133429 (2020). [DOI] [PMC free article] [PubMed]
- 336.Patel V, et al. Central GLP-1 receptor activation improves cholesterol metabolism partially independent of its effect on food intake. Can. J. Physiol. Pharm. 2016;94:161–167. doi: 10.1139/cjpp-2014-0457. [DOI] [PubMed] [Google Scholar]
- 337.NamKoong C, et al. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem. Biophys. Res. Commun. 2017;490:247–252. doi: 10.1016/j.bbrc.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 338.Holt MK, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68:21–33. doi: 10.2337/db18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zheng H, et al. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct. Funct. 2015;220:3011–3022. doi: 10.1007/s00429-014-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kabahizi A, et al. Glucagon-like peptide-1 (GLP-1) signalling in the brain: From neural circuits and metabolism to therapeutics. Br. J. Pharm. 2022;179:600–624. doi: 10.1111/bph.15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Secher A, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.He Z, et al. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons – implications for energy balance and glucose control. Mol. Metab. 2019;28:120–134. doi: 10.1016/j.molmet.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hayes MR, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Berthoud, H. R. et al. Gut-brain communication and obesity: understanding functions of the vagus nerve. J. Clin. Invest.131, e143770 (2021). [DOI] [PMC free article] [PubMed]
- 345.Baggio LL, et al. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 346.Abbott CR, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 347.Talsania T, et al. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 348.Grunddal, K. V. et al. Expression profile of the GLP-1 receptor in the gastrointestinal tract and pancreas in adult female mice. Endocrinology163, bqab216 (2022). [DOI] [PubMed]
- 349.Baggio LL, et al. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J. Clin. Invest. 2014;124:4223–4226. doi: 10.1172/JCI78371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Halim MA, et al. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J. Clin. Endocrinol. Metab. 2018;103:575–585. doi: 10.1210/jc.2017-02006. [DOI] [PubMed] [Google Scholar]
- 351.Imeryüz N, et al. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am. J. Physiol. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 352.Jones B, et al. Control of insulin secretion by GLP-1. Peptides. 2018;100:75–84. doi: 10.1016/j.peptides.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 353.Wang C, et al. Puerarin ameliorates hyperglycemia in HFD diabetic mice by promoting β-cell neogenesis via GLP-1R signaling activation. Phytomedicine. 2020;70:153222. doi: 10.1016/j.phymed.2020.153222. [DOI] [PubMed] [Google Scholar]
- 354.Portha B, et al. Activation of the GLP-1 receptor signalling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp. Diabetes Res. 2011;2011:376509. doi: 10.1155/2011/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kapodistria K, et al. Liraglutide, a human glucagon-like peptide-1 analogue, stimulates AKT-dependent survival signalling and inhibits pancreatic β-cell apoptosis. J. Cell Mol. Med. 2018;22:2970–2980. doi: 10.1111/jcmm.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yusta B, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 357.Ayala JE, et al. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology. 2009;150:1155–1164. doi: 10.1210/en.2008-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kim, E. R. et al. A GLP-1/GLP-2 receptor dual agonist to treat NASH: targeting the gut-liver axis and microbiome. Hepatology75, 1523–1538 (2021). [DOI] [PubMed]
- 359.Rodrigues T, et al. GLP-1 improves adipose tissue glyoxalase activity and capillarization improving insulin sensitivity in type 2 diabetes. Pharm. Res. 2020;161:105198. doi: 10.1016/j.phrs.2020.105198. [DOI] [PubMed] [Google Scholar]
- 360.Parlevliet ET, et al. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2010;299:E318–E324. doi: 10.1152/ajpendo.00191.2010. [DOI] [PubMed] [Google Scholar]
- 361.Chen J, et al. GLP-1/GLP-1R signaling in regulation of adipocyte differentiation and lipogenesis. Cell Physiol. Biochem. 2017;42:1165–1176. doi: 10.1159/000478872. [DOI] [PubMed] [Google Scholar]
- 362.Challa TD, et al. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem. 2012;287:6421–6430. doi: 10.1074/jbc.M111.310342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sancho V, et al. The action of GLP-1 and exendins upon glucose transport in normal human adipocytes, and on kinase activity as compared to morbidly obese patients. Int J. Mol. Med. 2007;19:961–966. [PubMed] [Google Scholar]
- 364.Liu R, et al. Glucagon like peptide-1 promotes adipocyte differentiation via the Wnt4 mediated sequestering of beta-catenin. PLoS One. 2016;11:e0160212. doi: 10.1371/journal.pone.0160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Beysen C, et al. Inhibition of fatty acid synthase with FT-4101 safely reduces hepatic de novo lipogenesis and steatosis in obese subjects with non-alcoholic fatty liver disease: Results from two early-phase randomized trials. Diabetes Obes. Metab. 2021;23:700–710. doi: 10.1111/dom.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Syed-Abdul MM, et al. Fatty acid synthase inhibitor TVB-2640 reduces hepatic de novo lipogenesis in males with metabolic abnormalities. Hepatology. 2020;72:103–118. doi: 10.1002/hep.31000. [DOI] [PubMed] [Google Scholar]
- 367.Berndt J, et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50:1472–1480. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- 368.Ghaben AL, et al. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019;20:242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 369.Xu F, et al. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia. 2016;59:1059–1069. doi: 10.1007/s00125-016-3896-5. [DOI] [PubMed] [Google Scholar]
- 370.Wang X, et al. Glucagon-like peptide-1 improves fatty liver and enhances thermogenesis in brown adipose tissue via inhibiting BMP4-related signaling pathway in high-fat-diet-induced obese mice. Int. J. Endocrinol. 2021;2021:6620289. doi: 10.1155/2021/6620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.López M, et al. Hypothalamic GLP-1: the control of BAT thermogenesis and browning of white fat. Adipocyte. 2015;4:141–145. doi: 10.4161/21623945.2014.983752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Dozio E, et al. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: a target to modulate cardiovascular risk? Int J. Cardiol. 2019;292:218–224. doi: 10.1016/j.ijcard.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 373.Ansaldo AM, et al. Epicardial adipose tissue and cardiovascular diseases. Int J. Cardiol. 2019;278:254–260. doi: 10.1016/j.ijcard.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 374.Lee SJ, et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol. Metab. 2018;11:33–46. doi: 10.1016/j.molmet.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bertagna X. Proopiomelanocortin-derived peptides. Endocrinol. Metab. Clin. North Am. 1994;23:467–485. [PubMed] [Google Scholar]
- 376.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 377.Yeo GSH, et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol. Metab. 2021;48:101206. doi: 10.1016/j.molmet.2021.101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhu C, et al. Profound and redundant functions of arcuate neurons in obesity development. Nat. Metab. 2020;2:763–774. doi: 10.1038/s42255-020-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.da Silva AA, et al. CNS regulation of glucose homeostasis: role of the leptin-melanocortin system. Curr. Diab. Rep. 2020;20:29. doi: 10.1007/s11892-020-01311-1. [DOI] [PubMed] [Google Scholar]
- 380.Knudsen LB, et al. Long-acting glucagon-like peptide-1 receptor agonists have direct access to and effects on pro-opiomelanocortin/cocaine- and amphetamine-stimulated transcript neurons in the mouse hypothalamus. J. Diabetes Investig. 2016;7(Suppl 1):56–63. doi: 10.1111/jdi.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wang Q, et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 2014;3:64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Duerrschmid C, et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 2017;23:1444–1453. doi: 10.1038/nm.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yuan M, et al. Asprosin: a novel player in metabolic diseases. Front. Endocrinol. (Lausanne) 2020;11:64. doi: 10.3389/fendo.2020.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.He Y, et al. Barbadin potentiates long-term effects of lorcaserin on POMC neurons and weight loss. J. Neurosci. 2021;41:5734–5746. doi: 10.1523/JNEUROSCI.3210-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wang C, et al. AgRP neurons trigger long-term potentiation and facilitate food seeking. Transl. Psychiatry. 2021;11:11. doi: 10.1038/s41398-020-01161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Chen AS, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 387.Obradovic M, et al. Leptin and obesity: role and clinical implication. Front. Endocrinol. (Lausanne) 2021;12:585887. doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Butiaeva LI, et al. Leptin receptor-expressing pericytes mediate access of hypothalamic feeding centers to circulating leptin. Cell Metab. 2021;33:1433–1448.e1435. doi: 10.1016/j.cmet.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 389.Ilnytska O, et al. The role of the agouti-related protein in energy balance regulation. Cell Mol. Life Sci. 2008;65:2721–2731. doi: 10.1007/s00018-008-8104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 391.Wasim M, et al. Role of leptin deficiency, inefficiency, and leptin receptors in obesity. Biochem. Genet. 2016;54:565–572. doi: 10.1007/s10528-016-9751-z. [DOI] [PubMed] [Google Scholar]
- 392.Lotta LA, et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. 2019;177:597–607.e599. doi: 10.1016/j.cell.2019.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Farooqi IS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 394.Chen AS, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 395.Sweeney, P. et al. The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci. Transl. Med.13, eabd6434 (2021). [DOI] [PMC free article] [PubMed]
- 396.Broberger C, et al. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 397.Roseberry AG, et al. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 398.Dodd GT, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Lede V, et al. Altered hepatic lipid metabolism in mice lacking both the melanocortin type 4 receptor and low density lipoprotein receptor. PLoS One. 2017;12:e0172000. doi: 10.1371/journal.pone.0172000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Iqbal J, et al. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J. Lipid Res. 2010;51:1929–1942. doi: 10.1194/jlr.M005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Tallam LS, et al. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 402.Lukas RJ. Pharmacological distinctions between functional nicotinic acetylcholine receptors on the PC12 rat pheochromocytoma and the TE671 human medulloblastoma. J. Pharm. Exp. Ther. 1989;251:175–182. [PubMed] [Google Scholar]
- 403.Kühnen P, et al. Melanocortin-4 receptor signalling: importance for weight regulation and obesity treatment. Trends Mol. Med. 2019;25:136–148. doi: 10.1016/j.molmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 404.Obici S, et al. Central melanocortin receptors regulate insulin action. J. Clin. Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Berglund ED, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Dodd, G. T. et al. Insulin regulates POMC neuronal plasticity to control glucose metabolism. Elife7, e38704 (2018). [DOI] [PMC free article] [PubMed]
- 407.Barone I, et al. Fluoxetine modulates the activity of hypothalamic POMC neurons via mTOR signaling. Mol. Neurobiol. 2018;55:9267–9279. doi: 10.1007/s12035-018-1052-6. [DOI] [PubMed] [Google Scholar]
- 408.Berglund ED, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest. 2013;123:5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Gao Y, et al. TrpC5 mediates acute leptin and serotonin effects via POMC neurons. Cell Rep. 2017;18:583–592. doi: 10.1016/j.celrep.2016.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Lam DD, et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 2011;13:584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Takaoka S, et al. Neuronal XRN1 is required for maintenance of whole-body metabolic homeostasis. iScience. 2021;24:103151. doi: 10.1016/j.isci.2021.103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Boston BA. The role of melanocortins in adipocyte function. Ann. N. Y Acad. Sci. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 413.Xue B, et al. The agouti gene product inhibits lipolysis in human adipocytes via a Ca2+-dependent mechanism. FASEB J. 1998;12:1391–1396. [PubMed] [Google Scholar]
- 414.Hamm JK, et al. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann. N.Y. Acad. Sci. 1999;892:134–145. doi: 10.1111/j.1749-6632.1999.tb07792.x. [DOI] [PubMed] [Google Scholar]
- 415.Morrison, R. F. et al. Insights into the transcriptional control of adipocyte differentiation. J. Cell Biochem.Suppl 32–33, 59–67 (1999). [DOI] [PubMed]
- 416.Morrison RF, et al. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J. Biol. Chem. 1999;274:17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 417.Kim NS, et al. Transcriptional activation of melanocortin 2 receptor accessory protein by PPARγ in adipocytes. Biochem. Biophys. Res. Commun. 2013;439:401–406. doi: 10.1016/j.bbrc.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 418.Mynatt RL, et al. Regulation of PPARgamma and obesity by agouti/melanocortin signaling in adipocytes. Ann. N.Y Acad. Sci. 2003;994:141–146. doi: 10.1111/j.1749-6632.2003.tb03173.x. [DOI] [PubMed] [Google Scholar]
- 419.Wharton S, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Liu J, et al. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Pires N, et al. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: a phase I trial. J. Ayurveda Integr. Med. 2020;11:68–72. doi: 10.1016/j.jaim.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Bhasin S, et al. Sudden death associated with thyroid hormone abuse. Am. J. Med. 1981;71:887–890. doi: 10.1016/0002-9343(81)90392-2. [DOI] [PubMed] [Google Scholar]
- 423.McCone J. Thyroid extract in obesity, with report of a case. Pac. Rec. Med. Sur. 1897;12:288–289. [Google Scholar]
- 424.Tainter M, et al. Use of dinitrophenol in obesity and related conditions: a progress report. J. Am. Med. Assoc. 1933;101:1472–1475. [Google Scholar]
- 425.Council on Pharmacy and Chemistry. JAMA105, 31–33 (1935).
- 426.Daneschvar HL, et al. FDA-approved anti-obesity drugs in the United States. Am. J. Med. 2016;129:879.e871–876. doi: 10.1016/j.amjmed.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 427.Müller TD, et al. Anti-obesity therapy: from rainbow pills to polyagonists. Pharm. Rev. 2018;70:712–746. doi: 10.1124/pr.117.014803. [DOI] [PubMed] [Google Scholar]
- 428.Cohen PA, et al. The return of rainbow diet pills. Am. J. Public Health. 2012;102:1676–1686. doi: 10.2105/AJPH.2012.300655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann. Intern Med. 2005;143:380–385. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- 430.Xia Y, et al. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015;23:1721–1728. doi: 10.1002/oby.21136. [DOI] [PubMed] [Google Scholar]
- 431.Hauner H, et al. Efficacy and safety of cathine (nor-pseudoephedrine) in the treatment of obesity: a randomized dose-finding study. Obes. Facts. 2017;10:407–419. doi: 10.1159/000478098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Müller TD, et al. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 2022;21:201–223. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Stăcescu, Ș. et al. A historical overview upon the use of amphetamine derivatives in the treatment of obesity. J. Pharm. Care7, 72–79 (2019).
- 434.Kernan WN, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N. Engl. J. Med. 2000;343:1826–1832. doi: 10.1056/NEJM200012213432501. [DOI] [PubMed] [Google Scholar]
- 435.Carvajal A, et al. Efficacy of fenfluramine and dexfenfluramine in the treatment of obesity: a meta-analysis. Methods Find. Exp. Clin. Pharm. 2000;22:285–290. doi: 10.1358/mf.2000.22.5.796647. [DOI] [PubMed] [Google Scholar]
- 436.Li Z, et al. Body weight loss with phentermine alone versus phentermine and fenfluramine with very-low-calorie diet in an outpatient obesity management program: a retrospective study. Curr. Ther. Res. Clin. Exp. 2003;64:447–460. doi: 10.1016/S0011-393X(03)00126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Goodrick GK, et al. The fen-phen controversy. Eat. Disord. 1997;5:343–348. [Google Scholar]
- 438.Connolly HM, et al. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 439.Cannistra LB, et al. Valvular heart disease associated with dexfenfluramine. N. Engl. J. Med. 1997;337:636. doi: 10.1056/nejm199708283370912. [DOI] [PubMed] [Google Scholar]
- 440.Centers for Disease Control and Prevention (CDC). Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations, November 1997. MMWR Morb. Mortal. Wkly Rep.46, 1061–1066 (1997). [PubMed]
- 441.Rasmussen S, et al. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 1997;337:1773. [PubMed] [Google Scholar]
- 442.Kurz X, et al. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 1997;337:1772–1773. [PubMed] [Google Scholar]
- 443.James WP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Engl. J. Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 444.Fujioka K, et al. Weight loss with sibutramine improves glycaemic control and other metabolic parameters in obese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2000;2:175–187. doi: 10.1046/j.1463-1326.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 445.Caterson ID, et al. Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes. Metab. 2012;14:523–530. doi: 10.1111/j.1463-1326.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 446.Scheen AJ. Sibutramine on cardiovascular outcome. Diabetes Care. 2011;34(Suppl 2):S114–S119. doi: 10.2337/dc11-s205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Gadde KM, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 448.Garvey WT, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Bray GA, et al. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 450.Smith SM, et al. Phentermine/topiramate for the treatment of obesity. Ann. Pharmacother. 2013;47:340–349. doi: 10.1345/aph.1R501. [DOI] [PubMed] [Google Scholar]
- 451.Hauptman JB, et al. Initial studies in humans with the novel gastrointestinal lipase inhibitor Ro 18-0647 (tetrahydrolipstatin) Am. J. Clin. Nutr. 1992;55:309s–313s. doi: 10.1093/ajcn/55.1.309s. [DOI] [PubMed] [Google Scholar]
- 452.Padwal RS, et al. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 453.Hill JO, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1-y study. Am. J. Clin. Nutr. 1999;69:1108–1116. doi: 10.1093/ajcn/69.6.1108. [DOI] [PubMed] [Google Scholar]
- 454.Zhi J, et al. Retrospective population-based analysis of the dose-response (fecal fat excretion) relationship of orlistat in normal and obese volunteers. Clin. Pharm. Ther. 1994;56:82–85. doi: 10.1038/clpt.1994.104. [DOI] [PubMed] [Google Scholar]
- 455.Davidson MH, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 456.Sjöström L, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 457.Chanoine JP, et al. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–2883. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 458.Lambert DM, et al. The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. J. Med. Chem. 2005;48:5059–5087. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- 459.Di Marzo V, et al. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 460.Di Marzo V, et al. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- 461.Rinaldi-Carmona M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 462.Rinaldi-Carmona M, et al. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- 463.Van Gaal L, et al. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31(Suppl 2):S229–S240. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- 464.Cota D, et al. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res. Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 465.McLaughlin, P. J. Rimonabant. In Reference Module in Biomedical Sciences (Elsevier, 2017). 10.1016/B978-0-12-801238-3.96545-0
- 466.Sherafat-Kazemzadeh R, et al. Pharmacotherapy for childhood obesity: present and future prospects. Int J. Obes. (Lond.) 2013;37:1–15. doi: 10.1038/ijo.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Sam AH, et al. Rimonabant: from RIO to Ban. J. Obes. 2011;2011:432607. doi: 10.1155/2011/432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Meye FJ, et al. Neutral antagonism at the cannabinoid 1 receptor: a safer treatment for obesity. Mol. Psychiatry. 2013;18:1294–1301. doi: 10.1038/mp.2012.145. [DOI] [PubMed] [Google Scholar]
- 469.Smith SR, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 470.Fidler MC, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J. Clin. Endocrinol. Metab. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 471.O’Neil PM, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 472.Bohula EA, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N. Engl. J. Med. 2018;379:1107–1117. doi: 10.1056/NEJMoa1808721. [DOI] [PubMed] [Google Scholar]
- 473.Bohula EA, et al. Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial. Lancet. 2018;392:2269–2279. doi: 10.1016/S0140-6736(18)32328-6. [DOI] [PubMed] [Google Scholar]
- 474.Kelly EM, et al. Formulary management of 2 new agents: lorcaserin and phentermine/topiramate for weight loss. J. Manag Care Pharm. 2013;19:642–654. doi: 10.18553/jmcp.2013.19.8.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Sharretts J, et al. Cancer risk associated with lorcaserin – the FDA’s review of the CAMELLIA-TIMI 61 Trial. N. Engl. J. Med. 2020;383:1000–1002. doi: 10.1056/NEJMp2003873. [DOI] [PubMed] [Google Scholar]
- 476.Greenway FL, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 477.Apovian CM, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21:935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Wadden TA, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Hollander P, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Yanovski SZ, et al. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA. 2015;313:1213–1214. doi: 10.1001/jama.2015.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Pi-Sunyer X, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 482.le Roux CW, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 483.Davies MJ, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314:687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 484.Wadden TA, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J. Obes. (Lond.) 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 485.Kelly AS, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 2020;382:2117–2128. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 486.Drucker DJ, et al. Liraglutide. Nat. Rev. Drug Discov. 2010;9:267–268. doi: 10.1038/nrd3148. [DOI] [PubMed] [Google Scholar]
- 487.O’Neil PM, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 488.Wilding JPH, et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 489.Davies M, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 490.Wadden TA, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 Randomized Clinical Trial. JAMA. 2021;325:1403–1413. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Rubino D, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 Randomized Clinical Trial. JAMA. 2021;325:1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Novo Nordisk A/S. Two-year Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity (STEP 5). https://clinicaltrials.gov/ct2/show/NCT03693430
- 493.Kadowaki T, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10:193–206. doi: 10.1016/S2213-8587(22)00008-0. [DOI] [PubMed] [Google Scholar]
- 494.Novo Nordisk A/S. Research Study of How Well Semaglutide Works in People Living With Overweight orObesity (STEP 7). https://clinicaltrials.gov/ct2/show/NCT04251156
- 495.Rubino DM, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 Randomized Clinical Trial. JAMA. 2022;327:138–150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Abbasi J. Semaglutide’s success could usher in a “new dawn” for obesity treatment. JAMA. 2021;326:121–123. doi: 10.1001/jama.2021.10307. [DOI] [PubMed] [Google Scholar]
- 497.Clément K, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 498.Markham A. Setmelanotide: first approval. Drugs. 2021;81:397–403. doi: 10.1007/s40265-021-01470-9. [DOI] [PubMed] [Google Scholar]
- 499.Joo JK, et al. Pharmacotherapy for obesity. J. Menopausa. Med. 2014;20:90–96. doi: 10.6118/jmm.2014.20.3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Ferrucci M, et al. The effects of amphetamine and methamphetamine on the release of norepinephrine, dopamine and acetylcholine from the brainstem reticular formation. Front. Neuroanat. 2019;13:48. doi: 10.3389/fnana.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Stemmer K, et al. CNS-targeting pharmacological interventions for the metabolic syndrome. J. Clin. Invest. 2019;129:4058–4071. doi: 10.1172/JCI129195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Rothman RB, et al. Monoamine transporters and psychostimulant drugs. Eur. J. Pharm. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 503.Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011;116:164–176. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Grandy DK, et al. “TAARgeting Addiction”—The Alamo bears witness to another revolution: an overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference. Drug Alcohol Depend. 2016;159:9–16. doi: 10.1016/j.drugalcdep.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Eiden LE, et al. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y Acad. Sci. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Sulzer D, et al. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. 2016;6:123–148. doi: 10.1016/j.baga.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Vaughan RA, et al. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharm. Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Underhill SM, et al. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron. 2014;83:404–416. doi: 10.1016/j.neuron.2014.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Angehagen M, et al. Topiramate reduces AMPA-induced Ca(2+) transients and inhibits GluR1 subunit phosphorylation in astrocytes from primary cultures. J. Neurochem. 2005;94:1124–1130. doi: 10.1111/j.1471-4159.2005.03259.x. [DOI] [PubMed] [Google Scholar]
- 510.Porter RJ, et al. Mechanisms of action of antiseizure drugs. Handb. Clin. Neurol. 2012;108:663–681. doi: 10.1016/B978-0-444-52899-5.00021-6. [DOI] [PubMed] [Google Scholar]
- 511.Greenfield LJ., Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure. 2013;22:589–600. doi: 10.1016/j.seizure.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Kim GW, et al. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin. Pharm. Ther. 2014;95:53–66. doi: 10.1038/clpt.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Halpern B, et al. Safety assessment of combination therapies in the treatment of obesity: focus on naltrexone/bupropion extended release and phentermine-topiramate extended release. Expert Opin. Drug Saf. 2017;16:27–39. doi: 10.1080/14740338.2017.1247807. [DOI] [PubMed] [Google Scholar]
- 514.Lei XG, et al. Efficacy and safety of phentermine/topiramate in adults with overweight or obesity: a systematic review and meta-analysis. Obesity (Silver Spring) 2021;29:985–994. doi: 10.1002/oby.23152. [DOI] [PubMed] [Google Scholar]
- 515.Harte R, et al. Topiramate. Practical Diabetes. 2020;37:34–35a. [Google Scholar]
- 516.Greig SL, et al. Naltrexone ER/Bupropion ER: a review in obesity management. Drugs. 2015;75:1269–1280. doi: 10.1007/s40265-015-0427-5. [DOI] [PubMed] [Google Scholar]
- 517.Christou GA, et al. The efficacy and safety of the naltrexone/bupropion combination for the treatment of obesity: an update. Hormones (Athens) 2015;14:370–375. doi: 10.14310/horm.2002.1600. [DOI] [PubMed] [Google Scholar]
- 518.Pilitsi E, et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019;92:170–192. doi: 10.1016/j.metabol.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 519.Schwartz MW, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 520.Vohra MS, et al. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharm. 2022;915:174611. doi: 10.1016/j.ejphar.2021.174611. [DOI] [PubMed] [Google Scholar]
- 521.Billes SK, et al. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharm. Res. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 522.Drucker DJ, et al. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 2017;127:4217–4227. doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Larsen PJ, et al. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 524.Trapp S, et al. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br. J. Pharm. 2022;179:557–570. doi: 10.1111/bph.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Williams DL. The diverse effects of brain glucagon-like peptide 1 receptors on ingestive behaviour. Br. J. Pharm. 2022;179:571–583. doi: 10.1111/bph.15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Brierley DI, et al. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. Br. J. Pharm. 2022;179:584–599. doi: 10.1111/bph.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Pabreja K, et al. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br. J. Pharm. 2014;171:1114–1128. doi: 10.1111/bph.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Rupprecht LE, et al. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am. J. Physiol. Endocrinol. Metab. 2013;305:E751–E759. doi: 10.1152/ajpendo.00367.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Mietlicki-Baase EG, et al. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J. Neurosci. 2014;34:6985–6992. doi: 10.1523/JNEUROSCI.0115-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Mietlicki-Baase EG, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. Endocrinol. Metab. 2013;305:E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Liu J, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. 2017;96:897–909.e895. doi: 10.1016/j.neuron.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kawatani M, et al. Glucagon-like peptide-1 (GLP-1) action in the mouse area postrema neurons. Peptides. 2018;107:68–74. doi: 10.1016/j.peptides.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 533.Liu TT, et al. Lipase inhibitors for obesity: a review. Biomed. Pharmacother. 2020;128:110314. doi: 10.1016/j.biopha.2020.110314. [DOI] [PubMed] [Google Scholar]
- 534.Basque JR, et al. Establishment of culture systems of human gastric epithelium for the study of pepsinogen and gastric lipase synthesis and secretion. Microsc. Res. Tech. 2000;48:293–302. doi: 10.1002/(SICI)1097-0029(20000301)48:5<293::AID-JEMT6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 535.Aloulou A, et al. Gastric lipase: an extremophilic interfacial enzyme with medical applications. Cell Mol. Life Sci. 2008;65:851–854. doi: 10.1007/s00018-008-7546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Lowe ME. Molecular mechanisms of rat and human pancreatic triglyceride lipases. J. Nutr. 1997;127:549–557. doi: 10.1093/jn/127.4.549. [DOI] [PubMed] [Google Scholar]
- 537.Lowe ME. The triglyceride lipases of the pancreas. J. Lipid Res. 2002;43:2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 538.Bénarouche A, et al. Using the reversible inhibition of gastric lipase by Orlistat for investigating simultaneously lipase adsorption and substrate hydrolysis at the lipid-water interface. Biochimie. 2014;101:221–231. doi: 10.1016/j.biochi.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 539.Borgström B. Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochim. Biophys. Acta. 1988;962:308–316. doi: 10.1016/0005-2760(88)90260-3. [DOI] [PubMed] [Google Scholar]
- 540.Hadváry P, et al. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem. J. 1988;256:357–361. doi: 10.1042/bj2560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Gargouri Y, et al. Inactivation of pancreatic and gastric lipases by THL and C12:0-TNB: a kinetic study with emulsified tributyrin. Biochim. Biophys. Acta. 1991;1085:322–328. doi: 10.1016/0005-2760(91)90136-6. [DOI] [PubMed] [Google Scholar]
- 542.Hadváry P, et al. The lipase inhibitor tetrahydrolipstatin binds covalently to the putative active site serine of pancreatic lipase. J. Biol. Chem. 1991;266:2021–2027. [PubMed] [Google Scholar]
- 543.Lüthi-Peng Q, et al. Identification of the active-site serine in human pancreatic lipase by chemical modification with tetrahydrolipstatin. FEBS Lett. 1992;299:111–115. doi: 10.1016/0014-5793(92)80112-t. [DOI] [PubMed] [Google Scholar]
- 544.Astrup A, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 545.Galsgaard KD, et al. Glucagon receptor signaling and lipid metabolism. Front. Physiol. 2019;10:413. doi: 10.3389/fphys.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Perry RJ, et al. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nature. 2020;579:279–283. doi: 10.1038/s41586-020-2074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Hayashi Y. Glucagon regulates lipolysis and fatty acid oxidation through inositol triphosphate receptor 1 in the liver. J. Diabetes Investig. 2021;12:32–34. doi: 10.1111/jdi.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.González-García, I. et al. Glucagon, GLP-1 and thermogenesis. Int. J. Mol. Sci.20, 3445 (2019). [DOI] [PMC free article] [PubMed]
- 549.Geary N, et al. Glucagon acts in the liver to control spontaneous meal size in rats. Am. J. Physiol. 1993;264:R116–R122. doi: 10.1152/ajpregu.1993.264.1.R116. [DOI] [PubMed] [Google Scholar]
- 550.Al-Massadi, O. et al. Glucagon control on food intake and energy balance. Int. J. Mol. Sci.20, 3905 (2019). [DOI] [PMC free article] [PubMed]
- 551.Del Prato S, et al. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obes. Rev. 2022;23:e13372. doi: 10.1111/obr.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Wewer Albrechtsen NJ, et al. Dynamics of glucagon secretion in mice and rats revealed using a validated sandwich ELISA for small sample volumes. Am. J. Physiol. Endocrinol. Metab. 2016;311:E302–E309. doi: 10.1152/ajpendo.00119.2016. [DOI] [PubMed] [Google Scholar]
- 553.Pharmaceutical, Hanmi. “Pipeline: R&D.” Pipeline R&D, https://www.hanmipharm.com/ehanmi/handler/Rnd-FocusedPipelineC.
- 554.Campbell JE. Targeting the GIPR for obesity: to agonize or antagonize? Potential mechanisms. Mol. Metab. 2021;46:101139. doi: 10.1016/j.molmet.2020.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Sánchez-Garrido MA, et al. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60:1851–1861. doi: 10.1007/s00125-017-4354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Parker JA, et al. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J. Obes. (Lond.) 2013;37:1391–1398. doi: 10.1038/ijo.2012.227. [DOI] [PubMed] [Google Scholar]
- 557.Cegla J, et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63:3711–3720. doi: 10.2337/db14-0242. [DOI] [PubMed] [Google Scholar]
- 558.Tan TM, et al. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62:1131–1138. doi: 10.2337/db12-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Bagger JI, et al. Effect of oxyntomodulin, glucagon, GLP-1, and combined glucagon +GLP-1 infusion on food intake, appetite, and resting energy expenditure. J. Clin. Endocrinol. Metab. 2015;100:4541–4552. doi: 10.1210/jc.2015-2335. [DOI] [PubMed] [Google Scholar]
- 560.Ambery PD, et al. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, Phase 1 study. Br. J. Clin. Pharm. 2018;84:2325–2335. doi: 10.1111/bcp.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Ambery P, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607–2618. doi: 10.1016/S0140-6736(18)30726-8. [DOI] [PubMed] [Google Scholar]
- 562.Hope DCD, et al. Striking the balance: GLP-1/glucagon co-agonism as a treatment strategy for obesity. Front. Endocrinol. (Lausanne) 2021;12:735019. doi: 10.3389/fendo.2021.735019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Alba M, et al. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose-ranging study. Clin. Obes. 2021;11:e12432. doi: 10.1111/cob.12432. [DOI] [PubMed] [Google Scholar]
- 564.Tillner J, et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes. Metab. 2019;21:120–128. doi: 10.1111/dom.13494. [DOI] [PubMed] [Google Scholar]
- 565.Finan B, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 566.Tan Q, et al. Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes. Front. Endocrinol. (Lausanne) 2022;13:838410. doi: 10.3389/fendo.2022.838410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Coskun T, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol. Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Coskun, T. et al. 679-P: The Novel GIP, GLP-1, and Glucagon Triple Receptor Agonist LY3437943 Exhibits Robust Efficacy in Preclinical Models of Obesity and Diabetes. Diabetes. 70 (Supplement_1), 679–P. (2021).
- 569.Bossart M, et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab. 2022;34:59–74.e10. doi: 10.1016/j.cmet.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 570.Busetto, L. et al. Gastrointestinal hormones and their regulation of food intake. In Encyclopedia of Endocrine Diseases 2nd edn (eds Huhtaniemi, I. & Martini, L.) 398–405 (Academic Press, Oxford, 2019).
- 571.Jorgensen R, et al. Oxyntomodulin differentially affects glucagon-like peptide-1 receptor beta-arrestin recruitment and signaling through Galpha(s) J. Pharm. Exp. Ther. 2007;322:148–154. doi: 10.1124/jpet.107.120006. [DOI] [PubMed] [Google Scholar]
- 572.Muppidi A, et al. Design of potent and proteolytically stable oxyntomodulin analogs. ACS Chem. Biol. 2016;11:324–328. doi: 10.1021/acschembio.5b00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Murphy KG, et al. Gut peptides in the regulation of food intake and energy homeostasis. Endocr. Rev. 2006;27:719–727. doi: 10.1210/er.2006-0028. [DOI] [PubMed] [Google Scholar]
- 574.Mentlein R, et al. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 575.Muscelli E, et al. Mechanisms for the antihyperglycemic effect of sitagliptin in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2012;97:2818–2826. doi: 10.1210/jc.2012-1205. [DOI] [PubMed] [Google Scholar]
- 576.Kim GW, et al. Regulation of appetite to treat obesity. Expert Rev. Clin. Pharm. 2011;4:243–259. doi: 10.1586/ecp.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Scherer PE, et al. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol. 1994;127:1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Catalán V, et al. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin. Endocrinol. (Oxf.) 2008;68:213–219. doi: 10.1111/j.1365-2265.2007.03021.x. [DOI] [PubMed] [Google Scholar]
- 579.Palacios-Ortega S, et al. Effects of high glucose on caveolin-1 and insulin signaling in 3T3-L1 adipocytes. Adipocyte. 2016;5:65–80. doi: 10.1080/21623945.2015.1122856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Ohnuma K, et al. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc. Natl Acad. Sci. USA. 2004;101:14186–14191. doi: 10.1073/pnas.0405266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Kajimura S, et al. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Takeda K, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin enhances brown adipose tissue function, thereby preventing obesity in mice. FEBS Open Bio. 2018;8:1782–1793. doi: 10.1002/2211-5463.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Reda TK, et al. Amylin, food intake, and obesity. Obes. Res. 2002;10:1087–1091. doi: 10.1038/oby.2002.147. [DOI] [PubMed] [Google Scholar]
- 584.Riediger T, et al. Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1833–R1843. doi: 10.1152/ajpregu.2001.281.6.R1833. [DOI] [PubMed] [Google Scholar]
- 585.Potes CS, et al. Noradrenergic neurons of the area postrema mediate amylin’s hypophagic action. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R623–R631. doi: 10.1152/ajpregu.00791.2009. [DOI] [PubMed] [Google Scholar]
- 586.Potes CS, et al. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin’s eating inhibitory effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R340–R351. doi: 10.1152/ajpregu.00380.2011. [DOI] [PubMed] [Google Scholar]
- 587.Chance WT, et al. Amylin increases transport of tyrosine and tryptophan into the brain. Brain Res. 1992;593:20–24. doi: 10.1016/0006-8993(92)91257-f. [DOI] [PubMed] [Google Scholar]
- 588.Mollet A, et al. Histamine H1 receptors mediate the anorectic action of the pancreatic hormone amylin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1442–R1448. doi: 10.1152/ajpregu.2001.281.5.R1442. [DOI] [PubMed] [Google Scholar]
- 589.Lutz TA, et al. Dopamine D(2) receptors mediate amylin’s acute satiety effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1697–R1703. doi: 10.1152/ajpregu.2001.280.6.R1697. [DOI] [PubMed] [Google Scholar]
- 590.Morris MJ, et al. Does neuropeptide Y contribute to the anorectic action of amylin? Peptides. 2001;22:541–546. doi: 10.1016/s0196-9781(01)00348-5. [DOI] [PubMed] [Google Scholar]
- 591.Roth JD, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc. Natl Acad. Sci. USA. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Turek VF, et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology. 2010;151:143–152. doi: 10.1210/en.2009-0546. [DOI] [PubMed] [Google Scholar]
- 593.Le Foll C, et al. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes. 2015;64:1621–1631. doi: 10.2337/db14-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Zakariassen HL, et al. Central control of energy balance by amylin and calcitonin receptor agonists and their potential for treatment of metabolic diseases. Basic Clin. Pharm. Toxicol. 2020;127:163–177. doi: 10.1111/bcpt.13427. [DOI] [PubMed] [Google Scholar]
- 595.Bailey RJ, et al. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br. J. Pharm. 2012;166:151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Batterham RL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 597.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 598.Wang W, et al. Ghrelin receptor mutations and human obesity. Prog. Mol. Biol. Transl. Sci. 2016;140:131–150. doi: 10.1016/bs.pmbts.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 599.Cui H, et al. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017;13:338–351. doi: 10.1038/nrendo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Yanagi S, et al. The homeostatic force of ghrelin. Cell Metab. 2018;27:786–804. doi: 10.1016/j.cmet.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 601.Abizaid A, et al. Ghrelin signaling: GOAT and GHS-R1a take a LEAP in complexity. Trends Endocrinol. Metab. 2020;31:107–117. doi: 10.1016/j.tem.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Kola B, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Kola B, et al. The CB1 receptor mediates the peripheral effects of ghrelin on AMPK activity but not on growth hormone release. FASEB J. 2013;27:5112–5121. doi: 10.1096/fj.13-232918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Liu T, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Romero-Picó A, et al. Hypothalamic κ-opioid receptor modulates the orexigenic effect of ghrelin. Neuropsychopharmacology. 2013;38:1296–1307. doi: 10.1038/npp.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Kanai Y, et al. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J. Clin. Invest. 1994;93:397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Pereira MJ, et al. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79:219–230. doi: 10.1007/s40265-019-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Rajeev SP, et al. Energy balance and metabolic changes with sodium-glucose co-transporter 2 inhibition. Diabetes Obes. Metab. 2016;18:125–134. doi: 10.1111/dom.12578. [DOI] [PubMed] [Google Scholar]
- 609.List JF, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.List, J. F. et al. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. Suppl. S20–S27 (2011). [DOI] [PubMed]
- 611.Hawley SA, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65:2784–2794. doi: 10.2337/db16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Xu L, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Xu L, et al. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: focus on fat browning and macrophage polarization. Adipocyte. 2018;7:121–128. doi: 10.1080/21623945.2017.1413516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Yang X, et al. Inhibition of the sodium-glucose co-transporter SGLT2 by canagliflozin ameliorates diet-induced obesity by increasing intra-adipose sympathetic innervation. Br. J. Pharm. 2021;178:1756–1771. doi: 10.1111/bph.15381. [DOI] [PubMed] [Google Scholar]
- 615.Beenken A, et al. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Owen BM, et al. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015;26:22–29. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Guo, J. Y. et al. Fibroblast growth factor 19 and fibroblast growth factor 21 regulation in obese diabetics, and non-alcoholic fatty liver disease after gastric bypass. Nutrients14, 645 (2022). [DOI] [PMC free article] [PubMed]
- 618.Coskun T, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 619.Geng L, et al. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat. Rev. Endocrinol. 2020;16:654–667. doi: 10.1038/s41574-020-0386-0. [DOI] [PubMed] [Google Scholar]
- 620.Minard AY, et al. mTORC1 is a major regulatory node in the FGF21 signaling network in adipocytes. Cell Rep. 2016;17:29–36. doi: 10.1016/j.celrep.2016.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Huang Z, et al. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 2017;26:493–508.e494. doi: 10.1016/j.cmet.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 622.Fisher FM, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Yilmaz U, et al. Effects of central FGF21 infusion on the hypothalamus-pituitary-thyroid axis and energy metabolism in rats. J. Physiol. Sci. 2018;68:781–788. doi: 10.1007/s12576-018-0595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Sarruf DA, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Fisher FM, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Ge X, et al. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J. Biol. Chem. 2011;286:34533–34541. doi: 10.1074/jbc.M111.248591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Hale C, et al. Growth differentiation factor 15 as a potential therapeutic for treating obesity. Mol. Metab. 2021;46:101117. doi: 10.1016/j.molmet.2020.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Tsai VW, et al. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. Int J. Obes. (Lond.) 2018;42:561–571. doi: 10.1038/ijo.2017.258. [DOI] [PubMed] [Google Scholar]
- 629.Dostálová I, et al. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur. J. Endocrinol. 2009;161:397–404. doi: 10.1530/EJE-09-0417. [DOI] [PubMed] [Google Scholar]
- 630.Hsu JY, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550:255–259. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 631.Yang L, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017;23:1158–1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 632.Mullican SE, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017;23:1150–1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 633.Emmerson PJ, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017;23:1215–1219. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 634.Levy RL, et al. Behavioral intervention for the treatment of obesity: strategies and effectiveness data. Am. J. Gastroenterol. 2007;102:2314–2321. doi: 10.1111/j.1572-0241.2007.01342.x. [DOI] [PubMed] [Google Scholar]