Abstract
Purpose:
To evaluate the effectiveness of a telephone navigation intervention for increasing use of cancer control services among underserved 2-1-1 callers.
Design:
Randomized controlled trial.
Setting:
2-1-1 call centers in Houston and Weslaco, Texas (located in the Rio Grande Valley near the Mexican border)
Participants:
2-1-1 callers in need of Pap test, mammography, colorectal cancer screening, smoking cessation counseling, and/or HPV vaccination for a daughter (n=1,554). A majority were low-income and described themselves as Black or Hispanic.
Intervention:
Participants were randomly assigned to receive either a cancer control referral for the needed service(s) with telephone navigation from a trained cancer control navigator (n=995) or a referral only (n=559).
Measures:
Uptake of each individual service and any needed service.
Analysis:
Assessed uptake in both groups using bivariate chi-square analyses and multivariable logistic regression analyses, adjusted for sociodemographic covariates. Both per-protocol and intent-to-treat approaches were used.
Results:
Both interventions increased cancer control behaviors. Referral with navigation intervention resulted in significantly greater completion of any needed service (OR=1.38; p=.042), Pap test (OR=1.56; p=.023), and smoking cessation counseling (OR=2.66; p=.044), than referral-only condition. Other outcomes showed the same trend although the difference was not statistically significant: mammography (OR=1.53; p=.106); colorectal cancer screening (OR=1.80; p=.095); and HPV vaccination of a daughter (OR=1.61; p=.331).
Conclusion:
Adding cancer control referrals and navigation to an informational service like the 2-1-1 program can increase overall participation in cancer control services.
Key words and keywords for indexing: Cancer prevention and screening, cancer disparities, HPV vaccination, patient navigation, health promotion, tobacco control, population health, low income, racial minority groups, social support
Purpose
Racial/ethnic minorities are at increased risk of mortality from breast, cervical, and colorectal cancers,1–3 which is largely due to poverty and limited access to care. Minority populations are more likely to be medically underserved due to limited access to quality health care and health insurance compared to whites.4, 5 Also, individuals of lower socioeconomic status are at higher risk for cancer-related mortality regardless of their ethnicity as they are more likely to be diagnosed at later stages.6 Despite the fact that screening for breast, cervical, and colorectal cancers reduce cancer-related mortality rates, racial minorities are screened at disproportionately lower rates compared to others, and thus experience cancer-related health disparities.7 For example, racial/ethnic minorities are less likely to successfully quit smoking,8, 9 less likely to initiate HPV vaccination, and have lower rates of cancer screening.10, 11 Targeted cancer control services in these populations are needed to reduce the cancer burden.
The 2-1-1 helpline is a three-digit dialing code approved for nationwide use by the Federal Communications Commission in 2000. It connects individuals in need to basic health and social services by providing referrals to appropriate community-based and government agencies. There are 2-1-1 call centers in all 50 states serving about 94% of the US population. Callers typically seek assistance with food, housing, bills, employment, social services, free or affordable health care, and information during emergency situations such as hurricanes. 2-1-1 callers are mostly women, racial/ethnic minorities, unemployed, have lower levels of income and are uninsured,12–16 characteristics also common to individuals with disproportionately higher risk for cancers.
Studies have demonstrated that 2-1-1 callers have significant needs and could benefit from cancer control services, and they are willing to answer questions about these needs and be connected to those services.17, 18 A pilot study implemented with 2-1-1 callers in Missouri reported that most callers felt 2-1-1 should ask about health, and all said receiving health information and referrals made using 2-1-1 at least somewhat (67%) or much (33%) more appealing.19 Based on these findings, we expanded the existing 2-1-1 protocol to include screening callers for cancer control needs and referring them to services. We also developed a telephone navigation component to address any barriers.
Navigators are referral and information specialists who have received additional training in screening and client communication related activities. They encourage and assist those needing cancer control services with scheduling and keeping initial and follow-up appointments and address other needs. Other studies have demonstrated the potential of incorporating navigation to screening services for 2-1-1 callers but none have examined the impact of this approach across an array of cancer control outcomes.18, 20–22 Our study aimed to determine the impact of referral to cancer control services and to compare the effectiveness of the use of navigation with referral versus referral only.
Methods
Design
To assess the effectiveness of a navigation-based referral intervention in comparison to the standard 2-1-1 referral protocol, we conducted a randomized controlled trial, using a two-group pre-test post-test design. Eligible participants were randomly assigned by a 2-1-1 information specialist to either referral with navigation (R&N) by a Cancer Control Navigator or the basic referral-only (comparison) condition using a 2:1 ratio of R&N to referral only. Random assignments were conducted using a computer-generated system embedded into the online survey platform (Qualtrics).
Sample
We collaborated with 2-1-1 call centers in Houston and Weslaco, Texas (located in the Rio Grande Valley near the Mexican border) which, together, receive an average of 60,600 calls per month. Between February 2011 and May 2013, we screened for study eligibility (18 years or older and spoke English or Spanish) among a randomly selected sample of individuals calling into the two 2-1-1 centers. Those meeting initial criteria were invited to participate in a five-minute health assessment, which included items asking about breast, cervical, and colorectal cancer screening, smoking behavior, and the presence of a daughter in the household in need of HPV vaccination. After completing the assessment (described in detail in Measures section below), callers were considered eligible to enroll in the study if they were non-adherent to breast, cervical, and/or colorectal cancer screening guidelines in place by the American Cancer Society23 at the time of the study; if they were in need of smoking cessation counseling; or if they had a daughter in need of HPV vaccination. The final study sample consisted of 1,554 individuals who met eligibility criteria, verbally consented to participate, and completed a baseline survey.
Intervention
We developed a cancer control telephone navigation program which consisted of adding cancer control-specific resources to 2-1-1 Texas’ existing referral database to connect callers to appropriate services if they were in need of breast, cervical, or colorectal cancer screening, tobacco cessation counseling, and/or HPV vaccination for a daughter. We used Social Cognitive Theory (SCT)24 as the theoretical framework that informed both intervention development and evaluation of intervention impact. SCT has, as a major foundational principle, the concept of reciprocal determinants that includes constructs related to motivation such as knowledge, self-efficacy, and skills (addressed by phone navigators in the R&N condition) and recognizes that there are environmental factors that influence outcomes such as access to resources (addressed in both conditions). To implement the new cancer control component of the referral system, 2-1-1 information specialists needed to assess cancer control needs among callers and inform them of the importance of adhering to relevant cancer control behaviors. Phone navigators also provided logistical support (e.g., scheduling appointments, arranging transportation to care) and increase callers’ motivation to complete the needed service by addressing both psychosocial (by addressing behavioral capability, skills, and self-efficacy) and structural barriers.
Development and implementation of the intervention is described elsewhere.25 Briefly, we trained information specialists from within the 2-1-1 system on standard 2-1-1 protocols regarding referrals, crisis intervention, system software, telephone systems, referral databases, and documentation. Specialists were certified by the Alliance of Information and Referral Systems (AIRS). All specialists participating in the study also received study protocol training in order to conduct the cancer risk assessments (i.e., assess callers’ needs for services) and provide relevant referrals.
Selected information specialists were designated as Cancer Control Navigators (CCN) to follow up with enrolled participants regarding referrals as well as to provide navigation services. They received additional CCN-specific training on breast, cervical, and colorectal cancer screening, smoking cessation and HPV vaccination recommendations. They also received information about available services in the area, and communication strategies and methods to motivate callers to act on referrals. Much of the navigator-specific training focused on building rapport, understanding participants’ perceptions of and reasons for considering a cancer control service, resolving misinformation about a service, and providing support or motivation to bolster participants’ resolve to use the service. They also received an adapted version of Motivation and Problem Solving training.26
To test the effectiveness of the navigation intervention, participants randomized to the referral-only group received referrals specific to their cancer control needs (i.e., name and contact of service provider). Those randomized to the R&N condition received the standard referral plus navigation. The R&N condition consisted of the following: the CCN, housed at 2-1-1, received an electronic summary profile of the participant including responses to the cancer risk assessment, reason for calling 2-1-1, and name and contact information. The CCN called participants within one working day of participant assignment to the R&N condition. These calls included building a collaborative relationship with callers, identifying their needs, working with them to identify barriers to services and coordinate solutions, and providing logistical (e.g., making appointments) and emotional support. The CCNs tracked and documented all caller interactions. Navigation services were provided by telephone only. Blinded data collectors attempted to contact participants in each group to complete follow-up surveys three- and six-months post-baseline to assess use of needed cancer control services.
Measures
Risk assessment survey to identify need for services.
Participants’ need for cancer screening services was established at enrollment based on ACS recommendations23 in place at the time of the study. Female callers who had never had a Pap test or had been tested more than three years ago were considered in need of cervical cancer screening, and women 40 years old or older who had not had a mammogram in the last year were considered in need of mammography. Callers who reported being a current smoker (currently smoked some days or every day) or not sure were considered in need of smoking cessation referral. Additionally, callers 50 years of age or older who were non-adherent to CRCS guidelines (no sigmoidoscopy in the last five years, colonoscopy in the last 10 years, or FOBT in the last year) were considered in need of CRCS. Regardless of their own screening and risk status, callers were eligible for the study if they had a daughter (9–17) who had not been vaccinated against HPV. Participants who were not sure of their history with respect to a given service were classified as in need for that service. In addition to need for each individual service, an overall need variable was created indicating whether a participant required any service.
Baseline and Follow-up Surveys.
In addition to the risk assessment data collected, at baseline, we collected information on age, gender, race/ethnicity, marital status, annual household income, health insurance status, level of education, and number of children (values over 10 were set to 10). While we also collected information, at baseline and follow-up, related to knowledge and attitudes about screening, social support, perceived discrimination, and other variables informed by our theoretical framework, only those variables used for the current analyses, which is focused on assessing intervention impact, are included in Table 1 below. At follow-up, participants were asked if they had received the “needed service” since their last interview. Uptake of any needed service was the primary outcome assessed while uptake of individual services (i.e., Pap test, mammography, colorectal cancer screening, smoking cessation, HPV vaccination of a daughter) were considered secondary outcomes.
Table 1.
Sample Characteristics by Study Group Assignment
| All Participants | Control | Navigation | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | p | |
| Age a | 1554 | 42.5 (12.4) | 559 | 42.9 (12.6) | 995 | 42.3 (12.3) | .338 |
| Number of Children a | 1554 | 2.3 (1.7) | 559 | 2.3 (1.7) | 995 | 2.4 (1.8) | .609 |
| N | Percent | N | Percent | N | Percent | p | |
| Gender b | .317 | ||||||
| Male | 101 | 6.5% | 41 | 7.3% | 60 | 6.0% | |
| Female | 1453 | 93.5% | 518 | 92.7% | 935 | 94.05% | |
| Education b | .405 | ||||||
| Less than high school | 374 | 24.2% | 127 | 22.8% | 247 | 24.9% | |
| High school or GED | 706 | 45.6% | 250 | 45.0% | 456 | 46.0% | |
| Post high school | 468 | 30.2% | 179 | 32.2% | 289 | 29.1% | |
| Marital Status b | .654 | ||||||
| Not married | 1080 | 69.7% | 392 | 70.4% | 688 | 69.3% | |
| Married/Living with someone | 470 | 30.3% | 165 | 29.6% | 305 | 30.7% | |
| Race/Ethnicity b | .860 | ||||||
| White/Not Hispanic | 115 | 7.7% | 40 | 7.4% | 75 | 7.9% | |
| White/Hispanic | 560 | 37.6% | 208 | 38.7% | 352 | 36.9% | |
| Black | 652 | 43.8% | 234 | 43.6% | 418 | 43.9% | |
| Other | 163 | 10.9% | 55 | 10.2% | 108 | 11.3% | |
| Insurance b | .152 | ||||||
| No insurance (or CHIP only) | 850 | 54.8% | 292 | 52.4% | 558 | 56.2% | |
| Public and/or private insurance | 700 | 45.2% | 265 | 47.6% | 435 | 43.8% | |
| Income b | .266 | ||||||
| <$10,000 | 717 | 48.6% | 263 | 49.3% | 454 | 48.2% | |
| $10,000 – $20,000 | 537 | 36.4% | 201 | 37.7% | 336 | 35.7% | |
| $20,000 or more | 220 | 14.9% | 69 | 12.9% | 151 | 16.0% | |
SD, Standard Deviation.
Boldface p-value indicates statistical significance (p<0.05).
Independent Samples T-test, two-tailed
Pearson Chi-square test, two-tailed
Analysis
The primary outcome was the uptake of any needed service and secondary outcomes were for the uptake of each needed service. For our primary outcome, we determined a priori that a sample of 1,681 total participants would provide adequate power (80%) to detect a 10% difference in participation for two-sided Chi-square test (α=0.05), assuming a 20% attrition due to lost to follow-up. A series of bivariate tests was conducted to assess the presence of baseline differences in sample characteristics between treatment conditions. Differences were assessed using Pearson chi-square tests for categorical variables and t tests for continuous variables. A significance level of p<.05 (two-tailed) was used for these analyses.
A two-step procedure was used to identify potential covariates of uptake for any and each needed service. Among cases needing a particular service and with follow-up data, we first conducted bivariate tests of the association between sample characteristics and study group assignment with uptake. We used these analyses to help inform what variables to include in the multivariable logistic models below. We considered variables with a p<0.2527 as well as those with previous evidence of association between candidate variables and cancer control outcomes.
For any needed service, and each needed individual service, we conducted bivariate Chi-square tests to assess the association of uptake with intervention group assignment. We then conducted logistic regression analyses with uptake as the dichotomous dependent variable and variables identified in the first step as covariates. Dummy variables for categorical measures were created as necessary. We calculated the odds ratios (OR) with 95% confidence intervals (95%CI) as an adjusted measure of the impact of the intervention. A two-sided significance level of p<0.05 was used for these analyses. For each analysis, all variables were entered and then removed in a stepwise manner to achieve a parsimonious model. Each set of analyses were conducted using a per-protocol approach in which uptake was assessed using cases with follow-up data. We also conducted an intent-to-treat approach in which cases lacking follow-up data were treated as having not received any or each needed service. The Committee for the Protection of Human Subjects at The University of Texas Health Science Center at Houston approved all study procedures (Study HSC-SPH-10-0241), and the study was registered and approved at www.ClinicalTrials.gov (NCT04638010).
Results
A total of 4,961 callers completed the cancer risk assessment (assessed for study eligibility). Of the 4,961 callers assessed, 3,390 (68.3%) were identified as in need of at least one cancer control service. Of the 3,390 callers eligible to participate in the study, 1,554 (45.8%) agreed to participate, completed a baseline survey, and were randomized to a study condition (Figure 1).
Figure 1:
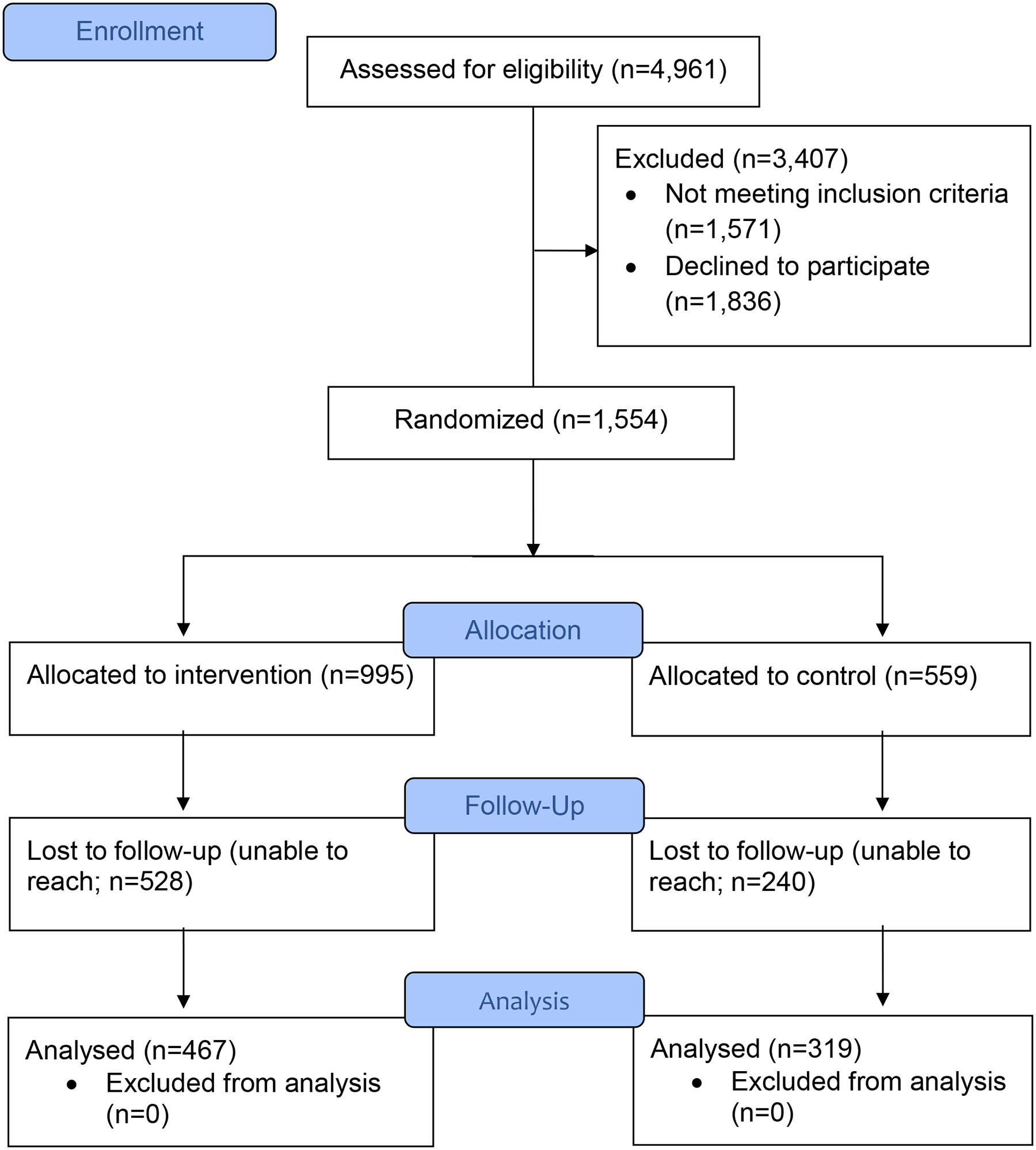
Study Enrollment for Need of Any Service
As shown in Table 1, most of the study participants were female. The mean age of participants was 42.5 years (standard deviation=12.4). Most were currently single. Participants had an average of 2.3 children. Most described themselves as Black or White/Hispanic. Over half were uninsured or had CHIP coverage only. Most participants had a total household income of less than $20,000. These sociodemographic variables did not differ between study groups.
Three-hundred nineteen of 559 (57.1%) participants in the referral-only condition whereas 467 of 995 (46.9%) in the R&N condition completed a follow-up survey (χ2=14.70; df=1; p<.001). Participants who completed a follow-up were on average older than those who did not (44.5 years vs. 40.5 years, respectively; t=−6.44; df =1552; p<.001) and were more likely to be female than male (51.8% vs. 33.7%, respectively; χ2=12.37; df=1; p<.001).
While follow-up interviews were originally planned at one and three months following the baseline survey, because, in the R&N condition, delivery of the intervention often extended beyond one month, we modified the protocol to follow-up at 3 and 6 months for all services for each study arm. The actual time from the baseline survey to follow-up survey varied considerably with a median of 176 days (5.9 months) and was longer for those in the R&N condition than for those in the referral-only condition (311 vs. 140 days, respectively; independent-samples median test = 4.04; df = 1; p=.044).
The 319 participants in the referral arm with follow-up data reported a total of 568 needed services or 1.8 services per participant. The 467 participants in the R&N arm with follow-up data reported a total of 881 needed services or 1.9 services per participant (t=−1.74; df=784; p=.083). Participants in the referral arm received a total of 178 services or 31.3% of reported needed services. Participants in the R&N arm received a total of 322 services or 36.5% of reported needed services (test of proportions Z=2.04; p=.041). Results from analyses assessing intervention impact on any needed service and on each service individually are presented below.
Uptake of Any Needed Service
Overall, 1,554 participants needed one or more cancer control services at baseline. Follow-up data were available for 786 (50.6%) of those in need. In the bivariate analyses, participants who received R&N were significantly more likely to participate in any cancer control behavior compared to those who received referral only (49.7% vs. 42.0%, respectively; p=.034; Table 2). In the logistic regression model adjusted for gender, race/ethnicity, income, health insurance status, and age, participants who received R&N were significantly more likely to participate in any cancer control behavior (OR=1.38; 95%CI=1.01–1.89; p=.042) compared to referral only (Table 2). As shown in Table 3, females were more likely to participate in any cancer control behavior (OR=4.43; 95%CI=1.85–10.57, p=.001) compared to men. Participants aged 36–45 years (OR=1.95; 95%CI=1.27–2.98; p=.002), 46–54 years (OR=2.20; 95%CI=1.42–3.40; p<.001), and 55 years and older (OR=1.75; 95%CI=1.09–2.81; p=.022) were more likely to participate in any cancer control behavior compared to those aged 18–35 years.
Table 2.
Unadjusted and Adjusted Uptake of Cancer Control Services at Follow-Up by Study Group Assignment (Per Protocol)
| Unadjusted ** | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Navigation | p | OR | 95% CI | p | |||
| N | N (Percent) Uptake |
N | N (Percent) Uptake |
|||||
| Any service (n=786) | 319 | 134 (42.0%) | 467 | 232 (49.7%) | .034 | 1.38a | 1.01–1.89 | .042 |
| Pap test (n=514) | 197 | 58 (29.4%) | 317 | 124 (39.1%) | .026 | 1.56b | 1.06–2.29 | .023 |
| Mammogram (n=343) | 132 | 46 (34.8%) | 211 | 87 (41.2%) | .238 | 1.53c | .91–2.56 | .106 |
| Colorectal cancer screening (n=181) | 78 | 18 (23.1%) | 103 | 34 (33.0%) | .144 | 1.80d | .90–3.59 | .095 |
| Smoking cessation (n=111) | 50 | 8 (16.0%) | 61 | 19 (31.1%) | .064 | 2.66e | 1.03–6.90 | .044 |
| HPV vaccination for daughter (n=89) | 40 | 12 (30.0%) | 49 | 19 (38.8%) | .387 | 1.61f | .62–4.22 | .331 |
OR, odds ratio; 95%CI, 95% confidence interval
Boldface p-value indicates statistical significance (p<0.05)
Pearson Chi-square test, two-tailed
full model shown in Table 3
full model shown in Table 4
full model shown in Table 5
full model shown in Table 6
full model shown in Table 7
full model shown in Table 8
Table 3.
Adjusted Uptake of Any Needed Service at Follow-Up among Participants in Need of at Least One Service at Baseline (Per Protocol)
| All | Females Only | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |
| Intervention | ||||||
| Referral Only (reference category) | 1.00 | --- | 1.00 | --- | ||
| Referral & Navigation | 1.38 | 1.01–1.89 | .042 | 1.42 | 1.03–1.95 | .032 |
| Gender | ||||||
| Male (reference category) | 1.00 | --- | ||||
| Female | 4.43 | 1.85–10.57 | .001 | |||
| Race/Ethnicity | ||||||
| White/Not Hispanic (reference category) | 1.00 | --- | 1.00 | --- | ||
| White/Hispanic | 1.16 | .61–2.19 | .649 | .70 | .33–1.51 | .364 |
| Black | 1.66 | .90–3.01 | .103 | .79 | .46–1.35 | .392 |
| Other Race/Ethnicity | 1.44 | .69–3.01 | .337 | 1.17 | .68–2.01 | .564 |
| Income | ||||||
| None-$10,000(reference category) | 1.00 | --- | 1.00 | --- | ||
| $10,000–$20,000 | 1.37 | .98–1.92 | .064 | 1.46 | 1.04–2.05 | .031 |
| $20,000 or more | 1.55 | .99–2.42 | .053 | 1.52 | .96–2.39 | .072 |
| Public and/or private insurance | ||||||
| No (reference category) | 1.00 | --- | 1.00 | --- | ||
| Yes | 1.34 | .95–1.89 | .094 | 1.37 | .97–1.95 | .074 |
| Age (Years) | ||||||
| 18–35 (reference category) | 1.00 | --- | 1.00 | --- | ||
| 36–45 | 1.95 | 1.27–2.98 | .002 | 2.02 | 1.31–3.10 | < .001 |
| 46–54 | 2.20 | 1.42–3.40 | < .001 | 2.39 | 1.53–3.72 | < .001 |
| 55 or older | 1.75 | 1.09–2.81 | .022 | 1.78 | 1.10–2.89 | .020 |
OR, odds ratio; 95%CI, 95% confidence interval
Boldface p-value indicates statistical significance (p<0.05)
Uptake of Pap Test
Of 1,453, (93.5%%) women enrolled in the study, 984 (67.7%) were in need of a Pap test at baseline based on ACS recommendations. Follow-up data on Pap uptake were available for 514 (52.2%) in-need women. Women who received R&N were more likely to get a Pap than those who received referral only (39.1% vs. 29.4%, respectively, p=.026) in the bivariate analysis (Table 2). In the logistic regression model adjusted for health insurance status, women who received R&N were more likely to get a Pap compared to referral only (OR=1.56; 95%CI=1.06–2.29; p=.023). Women with health insurance were also more likely to get a Pap than those without health insurance (OR=1.64; 95%CI=1.13–2.37; p=.010). Full adjusted model results are shown in Table 4.
Table 4.
Adjusted Uptake of Pap Test at Follow-Up Among Age-Eligible Participants Non-Adherent at Baseline (Per Protocol)
| OR | 95%CI | p | |
|---|---|---|---|
| Intervention | |||
| Referral Only (reference category) | 1.00 | --- | |
| Referral & Navigation | 1.56 | 1.06–2.29 | .023 |
| Public and/or private insurance | |||
| No (reference category) | 1.00 | --- | |
| Yes | 1.64 | 1.13–2.37 | .010 |
OR, odds ratio; 95%CI, 95% confidence interval
Boldface p-value indicates statistical significance (p<0.05)
Uptake of Mammography
Of 824 women aged 40 years and older, 594 (72.1%) needed a mammogram at baseline based on ACS recommendations. Follow-up data on mammography were available for 343 (57.7%) in-need women. In the bivariate analyses, women who received R&N were more likely to get a mammogram than those who received referral only, however, the difference was not statistically significant (41.2% vs. 34.8%, respectively; p=.238; Table 2). In the logistic regression model adjusted for race/ethnicity, income, health insurance, and age, women who received R&N were non-significantly more likely to get a mammogram (OR=1.53; 95%CI=.91–2.56; p=.106) compared to those who received referral only (Table 2). Black women (OR=3.32; 95%CI=1.26–8.75; p=.015) and women of other race/ethnicity (OR=3.47; 95%CI=1.02-11.87; p=.047) were more likely to get a mammogram compared to white women. Women with an annual income of $20,000 or more were more likely to get a mammogram than women with an annual income of <$10,000 (OR=4.01; 95%CI=1.96–8.23; p<.001). Women with health insurance were more likely to get a mammogram than those without health insurance (OR=2.86; 95%CI=1.60–5.09; p<.001). Women aged 49–54 years were more likely to get a mammogram than those aged 40–43 years (OR=2.18; 95%CI=1.05–4.52; p<.037)). Full adjusted model results are shown in Table 5.
Table 5.
Adjusted Uptake of Mammography at Follow-Up Among Age-Eligible Participants Non-Adherent at Baseline (Per Protocol)
| OR | 95%CI | p | |
|---|---|---|---|
| Intervention | |||
| Referral Only (reference category) | 1.00 | --- | |
| Referral & Navigation | 1.53 | .91–2.56 | .106 |
| Race/Ethnicity | |||
| White/Not Hispanic (reference category) | 1.00 | --- | |
| White/Hispanic | 1.82 | .64–5.19 | .260 |
| Black | 3.32 | 1.26–8.75 | .015 |
| Other Race/Ethnicity | 3.47 | 1.02–11.87 | .047 |
| Income | |||
| None-$10,000(reference category) | 1.00 | --- | |
| $10,000–$20,000 | 1.69 | .96–2.98 | .068 |
| $20,000 or more | 4.01 | 1.96–8.23 | < .001 |
| Public and/or private insurance | |||
| No (reference category) | |||
| Yes | 2.86 | 1.60–5.09 | < .001 |
| Age (Quartiles) (Years) | |||
| 40–43 (reference category) | 1.00 | --- | |
| 44–48 | 1.02 | .48–2.15 | .968 |
| 49–54 | 2.18 | 1.05–4.52 | .037 |
| 55 or older | 1.33 | .63–2.81 | .456 |
OR, odds ratio; 95%CI, 95% confidence interval
Boldface p-value indicates statistical significance (p<0.05)
Uptake of Colorectal Cancer Screening
Of 484 participants aged 50 years or older, 316 (65.3%) needed colorectal cancer screening based on ACS recommendations. Follow-up data on colorectal cancer screening were available for 181 (57.3%) in-need participants. Participants who received R&N were more likely to get colorectal cancer screening compared to the those who received referral only, however, the difference was not statistically significant (33.0% vs. 23.1%, respectively; p=.144) (Table 2). In a logistic regression model adjusted for gender and health insurance coverage, participants who received R&N were non-significantly more likely to get colorectal cancer screening (OR=1.80; 95%CI=.90–3.59; p=.095) compared to those who received referral only (Table 2). Colorectal cancer screening uptake was not significantly associated with other factors. .Full adjusted model results shown in Table 6.
Table 6.
Adjusted Uptake of Colorectal Cancer Screening at Follow-Up Among Age-Eligible Participants Non-Adherent at Baseline (Per Protocol)
| OR | 95%CI | p | |
|---|---|---|---|
| Intervention | |||
| Referral Only (reference category) | 1.00 | --- | |
| Referral & Navigation | 1.80 | .90–3.59 | .095 |
| Gender | |||
| Male (reference category) | 1.00 | --- | |
| Female | 4.27 | .93–19.51 | .061 |
| Public and/or private insurance | |||
| No (reference category) | 1.00 | --- | |
| Yes | 2.04 | .98–4.23 | .056 |
OR, odds ratio; 95%CI, 95% confidence interval
Boldface p-value indicates statistical significance (p<0.05)
Uptake of Smoking Cessation Counseling
Of 1,554 participants, 533 (34.3%) needed a referral to a smoking cessation program. Follow-up data on smoking cessation were available for 111 (20.8%) in-need participants. Those who received R&N were more likely to get smoking cessation counseling compared to those who received referral only (31.1% vs. 16.0%, respectively, p=.064), however, the difference was not statistically significant (Table 2). In the adjusted logistic regression model that included health insurance coverage, participants who received R&N were more likely to get smoking cessation counseling (OR=2.66; 95%CI=1.03–6.90; p=.044) compared to referral only (Table 2). Uptake of smoking cessation counseling was not significantly associated with other factors. Full adjusted model results shown in Table 7.
Table 7.
Adjusted Uptake of Smoking Cessation Counseling at Follow-Up Among Smokers at Baseline (Per Protocol)
| OR | 95%CI | p | |
|---|---|---|---|
| Intervention | |||
| Referral Only (reference category) | 1.00 | --- | |
| Referral & Navigation | 2.66 | 1.03–6.90 | .044 |
| Public and/or private insurance | |||
| No (reference category) | 1.00 | --- | |
| Yes | 2.18 | .77–6.19 | .142 |
OR, odds ratio; 95%CI, 95% confidence interval
Boldface p-value indicates statistical significance (p<0.05)
Uptake of HPV Vaccination of a Daughter
Of 1,554 participants, 327 (21.0%) participants had a daughter 9–17 years of age in need of HPV vaccination. Follow-up data on HPV vaccination were available for 89 (27.2%) of these participants with an in-need daughter. Participants who received R&N were non-significantly more likely to get their daughter vaccinated against HPV compared to participants who received referral only (38.8% vs. 30.0%, respectively; p=.387) (Table 2). In a logistic regression model adjusted for gender and annual income, participants who received R&N were more likely to get their daughter vaccinated against HPV (OR=1.61; 95%CI=0.62–4.22; p=.331) compared to referral only (Table 2), however, this difference was not statistically significant. Participants with an annual income of $10,000-$20,000 were more likely to have their daughters vaccinated against HPV (OR=3.17; 95%CI=1.10–9.14; p=.033) than those with an annual income of <$10,000. Full adjusted model results shown in Table 8.
Table 8.
Adjusted Uptake of HPV Vaccination of a Daughter at Follow-Up Among Age-Eligible Participants Non-Adherent at Baseline (Per Protocol)
| OR | 95%CI | p | |
|---|---|---|---|
| Intervention | |||
| Referral Only (reference category) | 1.00 | --- | |
| Referral & Navigation | 1.61 | .62–4.22 | .331 |
| Gender | |||
| Male (reference category) | 1.00 | --- | |
| Female | .23 | .02–2.95 | .257 |
| Income | |||
| None-$10,000 (reference category) | 1.00 | --- | |
| $10,000–$20,000 | 3.17 | 1.10–9.14 | .033 |
| $20,000 or more | 1.04 | .26–4.16 | .955 |
OR, odds ratio; 95%CI, 95% confidence interval
Boldface p-value indicates statistical significance (p<0.05)
Given the preponderance of females in the study sample and the inclusion of female-specific services (Pap test and mammography), we conducted the logistic regression analysis excluding males. There were 1,453 women in need of any service, 752 (51.8%) of whom had a follow-up survey. As shown in Table 3, results for the women were similar to those for the entire study population. Women who received R&N were more likely to participate in any cancer control behavior (OR=1.42; 95%CI=1.03–1.95; p=.032) compared to referral only. Women with an income of $10,000–20,000 were significantly more likely to participate in any cancer control behavior compared to women with an income of <$10,000 (OR=1.46; 95%CI=1.04–2.05; p=.031). Women 36–45 years old were significantly more likely to report uptake than women 18–35 years old (OR=2.02; 95% CI=1.31–3.10; p<.001). The same was true for women 46–54 years old (OR=2.39; 95%CI=1.53–3.72; p<.001) and women 55 years old or older (OR=1.78; 95%CI=1.10–2.89; p=.020).
We conducted an intent to treat analysis assuming that those lost to follow-up had not received the needed service. In these analyses, although, in general, the direction of the relationship indicated higher completion among those in the R&N arm, the results were not statistically significant for either “any needed service” or individual services. Nevertheless, since there were no differences in demographic characteristics among individuals who were lost to follow-up and those with follow-up data, we assumed that individuals lost were “missing at random” thus increasing confidence in the per protocol analyses presented above.
Discussion
The use of navigators to improve access to cancer control services was borne out of the need to reduce the health disparities in populations that are medically underserved. They have provided comprehensive services and education and referral services to populations in need, and this has proven to be important in reducing health disparities. A systematic review of the use of community health workers and patient navigators to improve cancer outcomes among patients reported that programs using patient navigators or community health workers can improve the completion and timeliness of breast, cervical, and colorectal cancer screening among medically underserved populations.28
This is the first study conducted in the 2-1-1 population to demonstrate the impact of using referral with navigation versus referral only to increase uptake of cancer control services among disadvantaged minorities in need of these services in Texas. Our findings show that the R&N intervention resulted in significantly higher use of any needed cancer control service as well as Pap test screening and smoking cessation counseling. Uptake of the other services (mammogram, colorectal cancer screening services, and HPV vaccination for daughter) were also higher in the R&N group but these were not statistically significant at the p<.05 level.
Another study conducted among 2-1-1 callers in Missouri randomized participants into three groups: referral only, tailored materials and navigation.18 Their findings showed that navigation was more effective than verbal referrals in getting participants to contact referrals for breast and cervical cancer screenings and smoking cessation. Also, navigation was more effective than tailored reminders in getting participants to contact referrals for breast and cervical cancer screening and smoke free home policies but not as effective for colon cancer screening.
Previous studies have demonstrated the utility of community health workers providing cancer control navigation in person29 including the effectiveness of navigation for increasing cancer control behaviors among those with the greatest number of barriers.30, 31 Our intervention, which aimed to address such barriers and provide logistical and emotional support to scheduling and completing cancer control behaviors, supports the effectiveness of cancer control navigation delivered by phone.
Limitations
There were limitations to the current study. The study was conducted in one state. Generalizability to other locations will be limited if our sample of 2-1-1 callers differ significantly from those in other sites. However, our call centers included one located in a major urban center and one in a border community. Our sample was diverse in terms of race/ethnicity but was predominantly female. This was expected given most 211 callers are women. Another limitation was that participants in the referral-only comparison group were more likely to have completed a follow-up and those in the R&N condition. This was due to the nature of the R&N intervention that sometimes extended over several months. Although this limitation does not impact the primary analyses, it does make the intent to treat analyses (that assumes no service completion for those lost to follow-up) even more stringent in that more individuals in the R&N condition were assumed to have no cancer control service completion. Nevertheless, as noted above, since we assume missing at random based on an examination of demographic characteristics among those lost and those followed up that showed no differences, we focus on the per protocol analyses.
Another potential limitation is that need for Pap test, mammography, and colorectal cancer screening was based on ACS recommendations in place at the time of the study. However, other national recommendations existed at the time and breast, cervical, and colorectal cancer screening recommendations have changed over time. These differences in recommendations may have affected our results. Another limitation is that participation in cancer control services outcome was based on self-report, however, the validity of self-report for most of the cancer control behavioral outcomes has been previously established.32–34 Our study sample of 1,554 participants was slightly less than the estimated number required (1,681) for our desired power of .80, and the observed attrition rates were higher than the assumed 20%. While there were significant differences between the two groups for our primary outcome (i.e., uptake of any needed service) and two of the secondary outcomes (i.e., uptake of Pap test and smoking cessation counseling), the degree of lost-to-follow-up impacted power to detect significant differences for our remaining secondary outcomes (i.e., mammography, update of colorectal cancer screening, HPV vaccination of a daughter). Nevertheless, these non-significant associations trended in the positive direction similar to our other outcomes. Our participants were recruited from phone callers. However, several options exist for contacting 2-1-1, including text messaging, dedicated websites, on-line chat, and mobile apps. The feasibility of intervening through these modalities should be considered in future projects.
Conclusions
This study showed the effectiveness of a telephone navigation program for increasing cancer control behaviors overall (considering any needed service), and for Pap test screening and smoking cessation counseling among low income, minority 2-1-1 callers above what can be achieved by referral alone. It expands on previous cancer control efforts that incorporate the use of navigators in a social service resource. This study demonstrates the potential of collaboration with 211 call centers across the country to address cancer-related health disparities.
So What? (Implications for Health Promotion Practitioners and Researchers).
What is already known on this topic?
A systematic review of the use of community health workers and patient navigators to improve cancer outcomes among patients reported that programs using patient navigators or community health workers can improve the completion and timeliness of breast, cervical, and colorectal cancer screening among medically underserved populations. However, the reach of these programs is limited. Studies have demonstrated that 2-1-1 callers have significant needs and could benefit from cancer control services.
What does this article add?
This manuscript describes the first study, conducted in the 2-1-1 population, to demonstrate the impact of using referral with navigation versus referral only to improve cancer control behaviors among a medically underserved population. Our findings show using referral with navigation can significantly increase the use of cancer control services overall within this high-need population.
What are the implications for health promotion practice or research?
Combining an effective strategy for increasing cancer control behaviors (patient navigation) with a high-reach program, the 2-1-1 helpline can have great impact for cancer control and reducing cancer related health inequities.
Funding Acknowledgements:
This research was funded through the Cancer Prevention Research Institute of Texas: Increasing Breast, Cervical, and Colorectal Cancer Screening and HPV Vaccination among Underserved Texans: A Collaboration with the United Way’s 2-1-1 Program (#PP100077 and #PP120086). Lynn Ibekwe was supported through a predoctoral fellowship (T32CA057712: Cancer Prevention and Control Research Training and Career Development Program; Mullen, PI) at The University of Texas Health Science Center at Houston (UTHealth) School of Public Health and partially funded by funded by the Department of Health Promotion and Behavioral Sciences and the Center for Health Promotion and Prevention Research at UTHealth School of Public Health. Disclaimer: The opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
Contributor Acknowledgements:
We would like to express our gratitude to our United Way 2-1-1 partners in Houston and Weslaco for their collaboration and invaluable contributions by navigators and information specialists throughout program development and implementation.
Footnotes
Clinical Trial Registration: This study was registered at www.Clinicaltrials.gov and received approval on November 20, 2020 (NCT04638010).
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
Ethical Approval:
This study received approval for all study procedures from the Committee for the Protection of Human Subjects at The University of Texas Health Science Center at Houston (Study HSC-SPH-10–0241). All study participants verbally provided informed consent to participate in the study.
References
- 1.National Cancer Institute. Cancer Health Disparities. Updated November 17, 2020. Accessed January 3, 2020. https://www.cancer.gov/about-nci/organization/crchd/cancer-health-disparities-fact-sheet#q11
- 2.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. Journal of Environmental and Public Health. 03/2012/01/received 02/27/accepted 2017;2017:2819372. doi: 10.1155/2017/2819372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA: a cancer journal for clinicians. Nov 2017;67(6):439–448. doi: 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 4.Adepoju OE, Preston MA, Gonzales G. Health Care Disparities in the Post–Affordable Care Act Era. American Journal of Public Health. 11/01/24/accepted 2015;105(Suppl 5):S665–S667. doi: 10.2105/AJPH.2015.302611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Keefe EB, Meltzer JP, Bethea TN. Health Disparities and Cancer: Racial Disparities in Cancer Mortality in the United States, 2000–2010. Frontiers in Public Health. 04/1501/07/received 03/11/accepted 2015;3:51. doi: 10.3389/fpubh.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer causes & control : CCC. 11/12 2009;20(4):10.1007/s10552–008-9256–0. doi: 10.1007/s10552-008-9256-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention; US Dept of Health and Human Services. Increasing Population-based Breast and Cervical Cancer Screenings: An Action Guide to Facilitate Evidence-based Strategies. 2014. 2014. https://stacks.cdc.gov/view/cdc/40706
- 8.King G, Polednak A, Bendel RB, Vilsaint MC, Nahata SB. Disparities in smoking cessation between African Americans and Whites: 1990–2000. Am J Public Health. Nov 2004;94(11):1965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Dept of Health and Human Services. Tobacco use among U.S. racial/ethnic minority groups--African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. A Report of the Surgeon General. Executive summary. Vol. 47. 1998:v-xv, 1–16. MMWR: Recommendations and reports. Oct 9. https://www.cdc.gov/mmwr/preview/mmwrhtml/00055081.htm [PubMed] [Google Scholar]
- 10.Gelman A, Miller E, Schwarz EB, Akers AY, Jeong K, Borrero S. Racial disparities in human papillomavirus vaccination: Does access matter? The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 08/27 2013;53(6):756–762. doi: 10.1016/j.jadohealth.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA: a cancer journal for clinicians. 2013;63(3):151–166. doi: 10.3322/caac.21173 [DOI] [PubMed] [Google Scholar]
- 12.Savas LS, Fernandez ME, Jobe D, Carmack CC. Human papillomavirus vaccine: 2-1-1 helplines and minority parent decision-making. American journal of preventive medicine. Dec 2012;43(6 Suppl 5):S490–6. doi: 10.1016/j.amepre.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcaraz KI, Arnold LD, Eddens KS, et al. Exploring 2-1-1 service requests as potential markers for cancer control needs. American journal of preventive medicine. Dec 2012;43(6 Suppl 5):S469–74. doi: 10.1016/j.amepre.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortinois AA, Glazier RH, Caidi N, Andrews G, Herbert-Copley M, Jadad AR. Toronto’s 2-1-1 healthcare services for immigrant populations. American journal of preventive medicine. Dec 2012;43(6 Suppl 5):S475–82. doi: 10.1016/j.amepre.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 15.Rodgers JT, Purnell JQ. Healthcare navigation service in 2-1-1 San Diego: guiding individuals to the care they need. American journal of preventive medicine. Dec 2012;43(6 Suppl 5):S450–6. doi: 10.1016/j.amepre.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 16.Roux AM, Herrera P, Wold CM, Dunkle MC, Glascoe FP, Shattuck PT. Developmental and autism screening through 2-1-1: reaching underserved families. American journal of preventive medicine. Dec 2012;43(6 Suppl 5):S457–63. doi: 10.1016/j.amepre.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eddens KS, Alcaraz KI, Kreuter MW, Rath S, Greer R. A 2-1-1 Research Collaboration: Participant Accrual and Service Quality Indicators. American journal of preventive medicine. 2012;43(6 Suppl 5):S483–S489. doi: 10.1016/j.amepre.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreuter MW, Eddens KS, Alcaraz KI, et al. Use of cancer control referrals by 2-1-1 callers: a randomized trial. American journal of preventive medicine. Dec 2012;43(6 Suppl 5):S425–34. doi: 10.1016/j.amepre.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddens KS, Kreuter MW. Proactive screening for health needs in United Way’s 2-1-1 information and referral service. J Soc Serv Res. 2011;37(2):113–123. doi: 10.1080/01488376.2011.547445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kegler MC, Bundy L, Haardörfer R, et al. A Minimal Intervention to Promote Smoke-Free Homes Among 2-1-1 Callers: A Randomized Controlled Trial. American Journal of Public Health. 03/07/29/accepted 2015;105(3):530–537. doi: 10.2105/AJPH.2014.302260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen PD, Savas LS, Bundy ŁT, et al. Minimal Intervention Delivered by 2-1-1 Information and Referral Specialists Promotes Smoke-Free Homes among 2-1-1 Callers: A Texas Generalization Trial. Tobacco control. 2016;25(Suppl 1):i10–i18. doi: 10.1136/tobaccocontrol-2016-053045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams RS, Stollings JH, Bundy L, et al. A Minimal Intervention to Promote Smoke-Free Homes among 2-1-1 Callers: North Carolina Randomized Effectiveness Trial. PloS one. 2016;11(11):e0165086. doi: 10.1371/journal.pone.0165086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Cancer Society. History of ACS Recommendations for the Early Detection of Cancer in People Without Symptoms. American Cancer Society. Updated July 30, 2020. Accessed June 3, 2021. https://www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/overview/chronological-history-of-acs-recommendations.html [Google Scholar]
- 24.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice Hall; 1986. [Google Scholar]
- 25.Ibekwe LN, Walker TJ, Ebunlomo E, et al. Using Implementation Mapping to Develop Implementation Strategies for the Delivery of a Cancer Prevention and Control Phone Navigation Program: A Collaboration With 2-1-1. Health Promotion Practice. 2020;0(0):1524839920957979. doi: 10.1177/1524839920957979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidrine JI, Reitzel LR, Figueroa PY, et al. Motivation and Problem Solving (MAPS): Motivationally Based Skills Training for Treating Substance Use. Cognitive and Behavioral Practice. 2013/11/01/ 2013;20(4):501–516. doi: 10.1016/j.cbpra.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code for Biology and Medicine. 2008/12/16 2008;3(1):17. doi: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roland KB, Milliken EL, Rohan EA, et al. Use of Community Health Workers and Patient Navigators to Improve Cancer Outcomes Among Patients Served by Federally Qualified Health Centers: A Systematic Literature Review. Health Equity. 05/01 2017;1(1):61–76. doi: 10.1089/heq.2017.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez ME, Gonzales A, Tortolero-Luna G, et al. Effectiveness of Cultivando la Salud: A breast and cervical cancer screening promotion program for low-income Hispanic women. American Journal of Public Health. 2009;99(5):936–943. NOT IN FILE. doi:doi: 10.2105/AJPH.2008.136713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freund KM, Battaglia TA, Calhoun E, et al. Impact of Patient Navigation on Timely Cancer Care: The Patient Navigation Research Program. JNCI: Journal of the National Cancer Institute. 2014;106(6):dju115. doi: 10.1093/jnci/dju115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodday AM, Parsons SK, Snyder F, et al. Impact of patient navigation in eliminating economic disparities in cancer care. Cancer. 2015;121(22):4025–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplan LS, McQueen DV, Qualters JR, Leff M, Garrett C, Calonge N. Validity of Women’s Self-Reports of Cancer Screening Test Utilization in a Managed Care Population. Cancer Epidemiology Biomarkers & Prevention. 2003;12(11):1182–1187. [PubMed] [Google Scholar]
- 33.Baier M, Calonge N, Cutter G, et al. Validity of Self-Reported Colorectal Cancer Screening Behavior. Cancer Epidemiology Biomarkers & Prevention. 2000;9(2):229–232. [PubMed] [Google Scholar]
- 34.Mandelson MT, LaCroix AZ, Anderson LA, Nadel MR, Lee NC. Comparison of Self-reported Fecal Occult Blood Testing with Automated Laboratory Records among Older Women in a Health Maintenance Organization. American Journal of Epidemiology. 1999;150(6):617–621. doi: 10.1093/oxfordjournals.aje.a010060 [DOI] [PubMed] [Google Scholar]


