Abstract
OBJECTIVE:
To assess the efficacy and safety of vaginal progesterone to prevent recurrent preterm birth and adverse perinatal outcomes in singleton gestations with a history of spontaneous preterm birth.
DATA SOURCES:
MEDLINE, EMBASE, LILACS, and CINAHL (from their inception to February 28, 2022), Cochrane databases, Google Scholar, bibliographies, and conference proceedings.
STUDY ELIGIBILITY CRITERIA:
Randomized controlled trials that compared vaginal progesterone to placebo/no treatment in asymptomatic women with a singleton gestation and a history of spontaneous preterm birth.
STUDY APPRAISAL AND SYNTHESIS METHODS:
The primary outcomes were preterm birth <37 and <34 weeks of gestation. Secondary outcomes included adverse maternal and perinatal outcomes. Pooled relative risks (RRs) with 95% confidence intervals (CIs) were calculated. We assessed risk of bias in the included studies, heterogeneity (I2 test), small-study effects, publication bias, and quality of evidence; performed subgroup and sensitivity analyses; and calculated 95% prediction intervals (PIs) and adjusted RRs.
RESULTS:
Ten studies (2958 women) met the inclusion criteria, 7 with a sample size <150 (small studies) and 3 with a sample size >600 (large studies). Among the 7 small studies, 4 were at high risk of bias, 2 were at some concerns of bias, and only 1 was at low risk of bias. All large studies were at low risk of bias. Vaginal progesterone significantly decreased the risk of preterm birth <37 weeks (RR, 0.64; 95% CI, 0.50–0.81; I2=75%; 95% PI, 0.31–1.32; very low-quality evidence) and <34 weeks (RR, 0.62; 95% CI, 0.42–0.92; I2=66%; 95% PI, 0.23–1.68; very low-quality evidence), and admission to the neonatal intensive care unit (RR, 0.53; 95% CI, 0.33–0.85; I2=67%; 95% PI, 0.16–1.79; low-quality evidence). There were no significant differences between the vaginal progesterone and placebo/no treatment groups in other adverse perinatal and maternal outcomes. Subgroup analyses revealed that vaginal progesterone decreased the risk of preterm birth <37 weeks (RR, 0.43; 95% CI, 0.33–0.55; I2=0%) and <34 weeks (RR, 0.27; 95% CI, 0.15–0.49; I2=0%) in the small, but not in the large studies (RR, 0.98; 95% CI, 0.88–1.09; I2=0% for preterm birth <37 weeks; and RR, 0.94; 95% CI, 0.78–1.13; I2=0% for preterm birth <34 weeks). Sensitivity analyses restricted to studies at low risk of bias indicated that vaginal progesterone did not reduce the risk of preterm birth <37 weeks (RR, 0.96; 95% CI, 0.84–1.09) and <34 weeks (RR, 0.90; 95% CI, 0.71–1.15). There was clear evidence of substantial small-study effects in the meta-analyses of preterm birth <37 and <34 weeks of gestation because of funnel plot asymmetry and of the marked difference in pooled RRs obtained from fixed-effect and random-effects models. The adjustment for small-study effects resulted in a markedly reduced and non-significant effect of vaginal progesterone on preterm birth <37 weeks (RR, 0.86; 95% CI, 0.68–1.10) and <34 weeks (RR, 0.92; 95% CI, 0.60–1.42).
CONCLUSION:
There is no convincing evidence supporting the use of vaginal progesterone to prevent recurrent preterm birth or to improve perinatal outcomes in singleton gestations with a history of spontaneous preterm birth.
Keywords: 17α-hydroxyprogesterone caproate, neonatal morbidity, neonatal mortality, prematurity, preterm delivery, prior preterm birth, progestin, progestogen, small-study effects
Condensation:
There is no convincing evidence supporting the use of vaginal progesterone to prevent preterm birth in singleton gestations with a history of spontaneous preterm birth.
INTRODUCTION
In the United States, the rate of preterm birth declined steadily from 2007 to 2014 but then rose continuously up to 2019.1 In 2020, the rate of preterm birth declined to 10.09%, the first decline in the rate since 2014.1 The most recent global estimates of preterm birth showed that 14.8 million live births (10.6% of all live births) were born preterm in 2014.2 In 2019, preterm birth complications were the leading cause of death among children younger than 5 years worldwide, accounting for 17.7% of all deaths, and for 36.1% of neonatal deaths.3 In addition, surviving preterm neonates are at increased risk for short-term complications, long-term neurodevelopmental disabilities, chronic diseases in adulthood, and mortality in early to mid-adulthood.4–14
Preterm labor is a syndrome associated with multiple mechanisms of disease, including infection/inflammation, decidual hemorrhage and vascular disease, uterine overdistention, cervical disease, disruption of maternal-fetal tolerance, decidual senescence, immunologically mediated processes, maternal stress, and decline in progesterone action, among others.15–22 The syndromic nature of preterm labor explains why a single method of intervention does not prevent all, or even predict most, cases of preterm birth.
A history of spontaneous preterm birth (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) is a major risk factor for recurrent preterm birth. Women with a history of spontaneous preterm birth have a 2.5- to 4-fold increased risk of spontaneous preterm birth in a subsequent pregnancy compared to women with no history of spontaneous preterm birth.23–34 In 2017, a meta-analysis of 32 studies, involving just over 55,000 women with at least one prior singleton spontaneous preterm birth, reported that the pooled rate of recurrent spontaneous preterm birth was 30% (95% confidence interval [CI], 27%−34%).35 The risk of recurrent preterm birth increases as the gestational age of the previous preterm birth declines and as the number of previous preterm births increases.28–34 Recurrences often occur at the same gestational age of the previous preterm birth.25,29 Women born spontaneously preterm have an increased risk of spontaneous preterm delivery in their own pregnancies.36–38 Genetic, environmental, and behavioral risk factors shared with two pregnancies could contribute to the increased risk of recurrent preterm birth.28,32,39
Since 2003, the American College of Obstetricians and Gynecologists (ACOG) has recommended the administration of 17α-hydroxyprogesterone caproate (17-OHPC) to patients with a singleton gestation and a history of spontaneous preterm birth aiming to prevent preterm birth.40 In 2021, the ACOG updated its guidelines on the prediction and prevention of spontaneous preterm birth and recommended offering either vaginal progesterone or 17-OHPC.41 These guidelines were endorsed by the Society for Maternal-Fetal Medicine (SMFM). The evidence base for making this recommendation was an individual patient data (IPD) meta-analysis that included 31 trials of vaginal progesterone, 17-OHPC, and oral progesterone in asymptomatic women at increased risk for preterm birth.42 This study reported that vaginal progesterone reduced the risk of preterm birth <34 weeks of gestation in high-risk singleton gestations but did not report results separately for the subgroup of women with a singleton gestation and a history of spontaneous preterm birth. Therefore, an assessment of the efficacy and safety of vaginal progesterone in such women is needed.
The objective of this systematic review and meta-analysis was to evaluate the efficacy and safety of vaginal progesterone to prevent recurrent preterm birth and adverse perinatal outcomes in asymptomatic women with a singleton gestation and a history of spontaneous preterm birth.
MATERIAL AND METHODS
This systematic review and meta-analysis followed a predefined protocol registered with PROSPERO (CRD42021275154) and was performed and reported according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Intervention43 and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines,44 respectively. Both authors independently retrieved and reviewed studies for eligibility, assessed their risk of bias, and extracted data. Any discrepancies were resolved through discussion between the review authors.
Data Sources and Searches
Eligible trials were identified through searches in MEDLINE, EMBASE, LILACS, CINAHL, the Cochrane Central Register of Controlled Trials, and clinical trial registries (all from their inception to February 28, 2022), using the keywords progesterone and preterm birth to be as inclusive as possible. Google Scholar, proceedings of congresses and scientific meetings on obstetrics and maternal-fetal medicine, reference lists of identified studies, previously published systematic reviews, and review articles were also searched. We did not use any language restrictions and translated non-English studies.
Eligibility criteria
We included randomized controlled trials in which asymptomatic women with a singleton gestation and a history of at least one spontaneous preterm birth in any of their previous pregnancies were randomly allocated to receive vaginal progesterone or placebo/no treatment for the prevention of preterm birth. Quasi-randomized trials, trials that assessed vaginal progesterone in women with threatened or arrested preterm labor or second-trimester bleeding, and trials in which vaginal progesterone was administered in the first trimester to prevent miscarriage were excluded from the review. Results of subgroup analyses for women with a history of spontaneous preterm birth in randomized controlled trials, whose primary aim was to prevent preterm birth in singleton gestations with a midtrimester sonographic short cervix, were not included in the meta-analysis.
Outcome measures
The primary outcomes were preterm birth <37 and <34 weeks of gestation. Secondary outcomes included preterm birth <28 weeks of gestation, threatened preterm labor or need for tocolysis, use of antenatal corticosteroids, cesarean delivery, any maternal adverse event, discontinuation of treatment because of adverse events, preterm prelabor rupture of membranes, preeclampsia, gestational hypertension, gestational diabetes mellitus, respiratory distress syndrome (RDS), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), grade III/IV IVH, neonatal sepsis, retinopathy of prematurity, bronchopulmonary dysplasia, periventricular leukomalacia, fetal death, neonatal death, perinatal death, birthweight <1500 g and <2500 g, admission to the neonatal intensive care unit (NICU), use of mechanical ventilation, patent ductus arteriosus, and long-term neurodevelopmental and health outcomes.
Assessment of risk of bias
We assessed the risk of bias in each included study for the primary and secondary outcomes by using the Cochrane risk of bias tool 2 (RoB 2),45 which considers the following domains: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; and (5) bias in the selection of the reported result. For each domain, the tool comprises a series of signaling questions aiming to elicit information about features of the trial relevant to risk of bias. Once the signaling questions had been answered, the next steps were to reach a risk-of-bias judgement and to assign 1 of 3 levels to each domain: low risk of bias, some concerns of bias, or high risk of bias. Finally, an overall risk of bias judgement was reached for each study as follows: low risk of bias (the study is judged to be at low risk of bias for all domains), some concerns of bias (the study is judged to raise some concerns in at least one domain, but not to be at high risk of bias for any domain), and high risk of bias (the study is judged to be at high risk of bias in at least one domain or to have some concerns for multiple domains in a way that substantially lowers confidence in the result).
Data extraction
A data extraction form was used to collect information on authors, title, publication date, language, duplicate publications, trial registration, funding sources, study characteristics (trial design, setting, follow-up period, attrition and exclusions from the analysis, and intention-to-treat analysis), participants (inclusion and exclusion criteria, number of women randomized, baseline characteristics, and country and date of recruitment), interventions (gestational age at trial entry, daily dose of vaginal progesterone, duration, compliance, use of co-interventions, and characteristics of interventions used in the control group) and outcomes (prespecified primary and secondary outcomes, definition of outcomes, number of outcome events and/or mean ± SD for each outcome, and total number of participants in each group). We included in our meta-analysis additional data of included studies that had been provided to previous meta-analyses.
Statistical analysis
Statistical analysis was performed according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions.46 Data were analyzed according to the intention-to-treat principle. We calculated the pooled relative risk (RR) for dichotomous outcomes with associated 95% CI by using a random-effects model. This approach was chosen in anticipation of significant heterogeneity among included studies. The number needed to treat (NNT) for an additional beneficial or harmful outcome with their 95% CIs was calculated for outcomes for which there was a statistically significant reduction or increase in absolute risk difference.47,48
Heterogeneity of the results among studies was firstly assessed with the visual inspection of forest plots for any lack of overlap of CIs. Then, we quantified statistical heterogeneity by using the I2 statistical test.49 In the presence of statistical heterogeneity (I2 ≥30%), we investigated potential causes through subgroup analyses.46 We also addressed heterogeneity by calculating 95% prediction intervals for meta-analyses that contained at least 5 studies.50 The prediction interval shows the range of true effect size in future studies similar to those in the meta-analysis.51–53
If there were at least 10 studies included in a meta-analysis, we constructed funnel plots to investigate small-study effects and publication biases.54 Funnel plot asymmetry was assessed visually and with Egger’s55 and Harbord’s56 tests. A P value of <0.10 indicated significant asymmetry of the funnel plot and evidence of small-study effects. In the presence of both heterogeneity and funnel plot asymmetry, we compared the fixed-effect and random-effects estimates of the intervention effect since the random-effects model weights small studies higher.54 Different effect sizes strongly suggest small-study effects bias. If the results were inconsistent between the fixed-effect and random-effects models meta-analyses, we presented both results. In case of funnel plot asymmetry, a contour-enhanced funnel plot was constructed to differentiate asymmetry attributed to publication bias from that which owes to other factors.54,57,58 On a contour-enhanced funnel plot, contours lines separating areas of statistical significance from non-significance are superimposed on the funnel plot. If studies appear to be missing in areas of statistical non-significance of the plot, then it is possible that the asymmetry is due to publication bias. Conversely, if studies appear to be missing in areas of high statistical significance, this reduces the plausibility that publication bias is the underlying cause of funnel plot asymmetry. If small-study effects were suspected, we planned to use the iterative non-parametric Trim and Fill method for adjusting treatment effect estimates as a sensitivity analysis.59–61 The basic idea of this method is to add studies to the funnel plot until it becomes symmetric.
We performed subgroup analyses according to study sample size (<150 vs ≥150), study setting (low/middle-income countries vs high-income countries vs both low/middle- and high-income countries), study center status (single center vs multicenter), trial registration status (registered vs not registered), mean gestational age at treatment initiation (<24 weeks vs ≥24 weeks of gestation), and daily dose of vaginal progesterone (90–100 mg vs ≥200 mg). We assessed subgroup differences by an interaction test in which a P value ≥0.05 was considered to indicate that the effect of treatment did not differ significantly between subgroups.62–64 To test the robustness of the meta-analyses, we carried out sensitivity analyses by including only studies at overall low risk of bias.65 Subgroup and sensitivity analyses were restricted to the primary outcomes of preterm birth <37 and <34 weeks of gestation.
Assessment of quality of evidence
The quality (certainty) of the body of evidence for each individual outcome was assessed by using the 5 GRADE criteria (overall risk of bias, consistency of effect, imprecision, indirectness, and publication bias).66 The GRADE approach categorizes the certainty of the evidence into 4 levels: (1) high: we are very confident that the true effect lies close to that of the estimate of the effect; (2) moderate: we are moderately confident in the effect estimate, and the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; (3) low: our confidence in the effect estimate is limited, and the true effect may be substantially different from the estimate of the effect; and (4) very low: we have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect. We estimated an overall GRADE quality rating by taking the lowest quality of evidence from all of the outcomes critical to decision making.67 As all studies included in the review were randomized controlled trials, the starting level for all assessments was high certainty. We downgraded the level of certainty in the presence of high risk of bias in included trials, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, and high probability of publication bias. We downgraded the evidence by 1 level if we considered the limitation to be serious, and 2 levels if we considered it to be very serious.
Statistical analyses were performed by using Review Manager (RevMan [Computer program]. Version 5.4.1 The Cochrane Collaboration, 2020), StatsDirect (Version 3.3.5; StatsDirect Ltd, Wirral, United Kingdom), and Comprehensive Meta-Analysis (Version 3; Biostat, Englewood, NJ, United States). GRADEpro GDT (GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2021) was used to assess the certainty of the evidence and create the summary of findings table.
RESULTS
Selection, characteristics and risk of bias of studies
Our search strategy identified 14 studies for possible inclusion, of which we excluded 4 (2 quasi-randomized trials,68,69 1 non-randomized trial,70 and 1 trial that included women presenting with symptoms or signs of threatened preterm labor71) (Supplemental Figure 1). Ten studies, including 2958 women with a singleton gestation and a history of spontaneous preterm birth, fulfilled inclusion criteria.72–81 Nine studies were published in English72–74,76–81 and 1 in Persian.75
The main characteristics of included trials are presented in Table 1. Four studies included only women with a singleton gestation and a history of spontaneous preterm birth.73,74,77,81 The remaining 6 studies comprised women with a singleton gestation and a history of spontaneous preterm birth and women with other risk factors for preterm birth72,75,76,78,79 or women with a twin gestation with a history of spontaneous preterm birth.80 We obtained data separately for singleton gestations with a history of spontaneous preterm birth from 4 of these studies.76,78–80 Seven studies had a sample size <150 (“small studies”)72,74–78,81 and 3 had a sample size >600 (611 in the study by O’Brien et al,73 912 in the study by Norman et al,79 and 775 in the study by Crowther et al;80 “large studies”). All large studies were double-blind, placebo-controlled, registered (2 prospectively79,80 and 1 retrospectively73), multicenter trials (2 conducted in high-income countries78,80 and 1 conducted in both high-income [67% of recruited women] and low/middle-income [33% of recruited women] countries73). All small studies were conducted in single centers located in low/middle-income countries. Four small studies evaluated vaginal progesterone vs placebo72,76,77,78 and 3 evaluated vaginal progesterone vs no treatment.74,75,81 Only 2 small trials were registered, both retrospectively.77,78 The remaining 5 small trials72,74–76,81 were not registered in a clinical trials registry.
TABLE 1.
Main characteristics of studies included in the systematic review
| First author, year | Trial enrolment | Main inclusion/exclusion criteria | Interventions (No. of women with a singleton gestation and history of spontaneous preterm birth) | Compliance | Trial registration | Primary outcome |
|---|---|---|---|---|---|---|
| Da Fonseca,72 2003 | Single Center in Brazil | •Inclusion: singleton gestation and history of spontaneous preterm birth, or prophylactic cervical cerclage because of cervical insufficiency, or uterine malformation •Exclusion: allergy to progesterone |
•Vaginal progesterone suppository 100 mg/d from 24–34 weeks of gestation (n=65) •Placebo (n=68) |
Unreported | Not registered | Preterm birth <37 weeks |
| O’Brien,73 2007 | 53 Centers in United States, India, South Africa, Czech Republic, Chile, and El Salvador | •Inclusion: singleton gestation and history of spontaneous preterm birth •Exclusion: planned cervical cerclage, preterm labor, preterm premature rupture of membranes, clinical chorioamnionitis or vaginal bleeding, placenta previa, history of adverse reaction to progesterone, treatment with progesterone within 4 weeks before enrollment, treatment for seizure disorder, psychiatric illness or chronic hypertension at time of enrollment, history of acute or chronic congestive heart failure, renal failure, uncontrolled diabetes mellitus, active liver disorder, HIV infection with CD4 count of <350 cells/mm3 and requiring multiple antiviral agents, history or suspicion of breast/genital tract malignancy or thromboembolic disease, Müllerian duct anomaly, major fetal anomaly or chromosomal disorder, or multiple gestation |
•Vaginal progesterone gel 90 mg/d from 18–22 to 37+0 weeks of gestation, rupture of membranes or delivery, whichever occurred first (n=309) •Placebo (n=302) |
96.2% for vaginal progesterone group and 96.4% for placebo group | NCT00086177 | Preterm birth ≤32 weeks |
| Majhi,74 2009 | Single center in India | •Inclusion: singleton gestation and history of spontaneous preterm birth •Exclusion: multifetal gestation, fetal congenital malformation, current or planned cervical cerclage, or any associated medical disorder |
•Vaginal progesterone capsule 100 mg/d from 20–24 to 36 weeks of gestation or delivery, whichever occurred first (n=50) •Standard care (n=50) |
100% for vaginal progesterone group | Not registered | Preterm birth <37 and <34 weeks |
| Akbari,75 2009 | Single Center in Iran | •Inclusion: singleton gestation and history of spontaneous preterm birth, or previous cervical cerclage, or uterine malformation •Exclusion: preterm labor, preterm premature rupture of membranes, allergy to progesterone, fetal growth restriction, major fetal anomaly, unexplained vaginal bleeding, multiple gestation, chronic medical disorders, or cancer |
•Vaginal progesterone suppository 100 mg/d from 24–34 weeks of gestation (n=65) •Standard care (n=67) |
Unreported | Not registered | Mean gestational age at delivery |
| Cetingoz,76 2011 | Single Center in Turkey | •Inclusion: singleton gestation with a history of spontaneous preterm birth or uterine malformation, or twin gestation •Exclusion: in-place or planned cervical cerclage, or serious fetal anomalies |
•Vaginal progesterone suppository 100 mg/d from 24–34 weeks of gestation (n=37) •Placebo (n=34) |
100% for both study groups | Not registered | Preterm birth <37 weeks |
| Modi,77 2014 | Single Center in India | •Inclusion: singleton gestation and history of spontaneous preterm birth •Exclusion: allergy to progesterone, multiple gestation, preterm prelabor rupture of membranes, fetal congenital malformation, diabetes mellitus, chronic renal disease, chronic hypertension, heart disease, severe anemia, cervical incompetence (cervical length <25 mm or funneling on ultrasound), progesterone intake less than two weeks, or therapeutic preterm delivery |
•Vaginal progesterone suppository 100 mg/d from 24–30 to 34 weeks of gestation (n=41) •Placebo (n=40) |
41.5% for vaginal progesterone group and 70.0% for placebo group | CTRI/2008/091/00 0218 | Preterm birth <37 weeks |
| Azargoon,78 2016 | Single Center in Iran | •Inclusion: singleton gestation and history of spontaneous preterm birth, or history of spontaneous preterm birth and cervical length ≤28 mm plus cerclage, or uterine malformation, or uterine intramural myoma ≥7 cm •Exclusion: clinical chorioamnionitis, allergy to progesterone, serious fetal anomalies, multiple gestation, polyhydramnios, fetal growth restriction, hyperthyroidism, gestational diabetes, blood pressure ≥140/90 mm Hg, heart disease, epilepsy, or the use of anticonvulsants |
•Vaginal progesterone suppository 400 mg/d from 16–22 to 36 weeks of gestation (n=28) •Placebo (n=25) |
Unreported | IRCT2010122733 86N2 | Preterm birth <37 weeks |
| Norman,79 2016 | 65 Centers in United Kingdom and one in Sweden | •Inclusion: singleton gestation and history of spontaneous preterm birth, or cervical length ≤25 mm, or positive fetal fibronectin test combined with other clinical risk factors for preterm birth •Exclusion: congenital structural or chromosomal fetal anomaly, known sensitivity, contraindications, or intolerance to progesterone or any excipient, rupture of membranes, or prescription or ingestion of medications known to interact with progesterone |
•Vaginal progesterone capsule 200 mg/d from 22–24 to 34 weeks of gestation or delivery, whichever occurred first (n=457) •Placebo (n=455) |
66.3% for vaginal progesterone group and 70.9% for placebo group | ISRCTN14568373 | Fetal death or delivery occurring before 34 weeks of gestation, a composite of neonatal death, brain injury, or bronchopulmonary dysplasia, and the Bayley-III cognitive composite score at 2 years of age |
| Crowther,80 2017 | 32 Centers in Australia, 5 in New Zealand, and 2 in Canada | •Inclusion: singleton or twin gestation and history of spontaneous preterm birth in the preceding pregnancy •Exclusion: active vaginal bleeding requiring hospital admission at ≥18 weeks of gestation, preterm prelabor rupture of membranes, active labor, known lethal fetal anomaly or fetal demise, progesterone treatment after 16 weeks’ gestation, or any contraindication to continuation of the pregnancy or progesterone therapy |
•Vaginal progesterone pessary 100 mg/d from 20-24 to 34 weeks of gestation or delivery, whichever occurred first (n=390) •Placebo (n=385) |
91.6% for vaginal progesterone group and 90.8% for placebo group | ISRCTN20269066 | Respiratory distress syndrome and severity of respiratory disease |
| Abdou,81 2018 | Single Center in Egypt | •Inclusion: singleton gestation and history of spontaneous preterm birth •Exclusion: uterine malformation, prophylactic cervical cerclage, multiple gestation, fetal malformation, or premature prelabor rupture of membranes |
•Vaginal progesterone suppository 200 mg/d from 20–24 to 36 weeks of gestation or delivery, whichever occurred first (n=45) •Standard care (n=45) |
Unreported | Not registered | Preterm birth <37 weeks |
The daily dose of vaginal progesterone used in the trials was 90 mg in 1 study,73 100 mg in 6 studies,72,74–77,80 200 mg in 2 studies,79,81 and 400 mg in 1 study.78 Most studies administered the treatment from 20–24 to 34–36 weeks of gestation. Compliance >90% was reported in 4 studies.73,74,76,80 In the studies by Modi et al77 and Norman et al,79 the compliance in the vaginal progesterone group was 42% and 66%, respectively, whereas in the placebo group, it was 70% and 71%, respectively. Compliance was not reported in 4 trials.72,75,78,81
Figure 1 shows the risk of bias in each included study. The 3 large studies73,79,80 were at overall low risk of bias for the primary outcomes and most secondary outcomes. Among the 7 small studies, 4 had an overall high risk of bias,72,75,77,81 2 had some concerns of bias,74,78 and only 1 had an overall low risk of bias.76 One study72 was deemed to be at high risk of bias given the deviations from the intended interventions: 13 women with preterm birth due to prelabor rupture of membranes or medically indicated delivery (8 in the vaginal progesterone group and 5 in the placebo group) were inappropriately excluded from analyses after randomization. The inclusion of these women in the analyses (intention-to-treat effect) would become the effect of vaginal progesterone on preterm birth <37 weeks of gestation into a non-statistically significant result. The studies by Akbari et al,75 Modi et al,77 and Abdou et al81 were judged to have an overall high risk of bias because they had some concerns of bias in multiple domains. The studies by Akbari et al75 and Abdou et al81 did not provide information on the methods used to generate the random allocation sequence and to conceal allocation, on the blinding of outcome assessors to intervention status, and on the selection of the reported result. In addition, the study by Akbari et al75 provided insufficient information on deviations from intended interventions. The study by Modi et al77 did not report information on the number of women with missing outcome data and on the selection of the reported result. The study by Majhi et al74 was judged as having some concerns of bias because participants and personnel were aware of intervention and there was no information on blinding of outcome assessors to intervention status. The study by Azargoon et al78 was judged to have some concerns of bias arising from the randomization process because the allocation concealment method was not reported.
Figure 1:
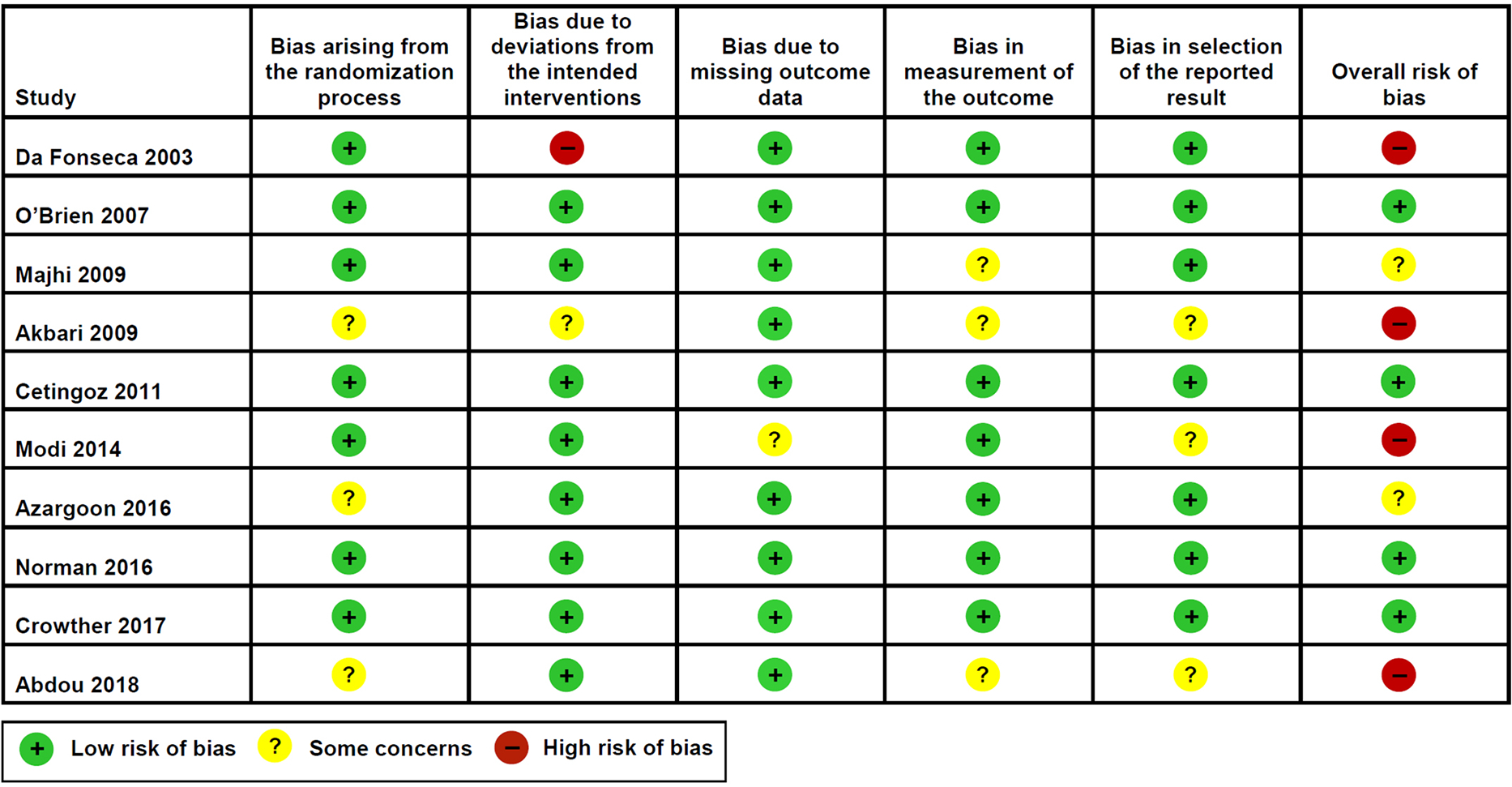
Risk of bias for each included study
Primary outcomes
Vaginal progesterone was associated with a significant decrease in the risk of preterm birth <37 weeks of gestation (32.0% vs 37.8%; pooled RR from random-effects model, 0.64; 95% CI, 0.50–0.81; P = 0.0003; pooled RR from fixed-effect model, 0.85; 95% CI, 0.77–0.93; P = 0.0008; I2 = 75%; 95% prediction interval of the RR, 0.31–1.32; NNT, 17; 95% CI, 11–42) (Figure 2) and preterm birth <34 weeks of gestation (13.5% vs 17.0%; pooled RR from random-effects model, 0.62; 95% CI, 0.42–0.92; P = 0.02; pooled RR from fixed-effect model, 0.79; 95% CI, 0.67–0.94; P = 0.008; I2 = 66%; 95% prediction interval of the RR, 0.23–1.68; NNT, 28; 95% CI, 16–109) (Figure 3).
Figure 2:
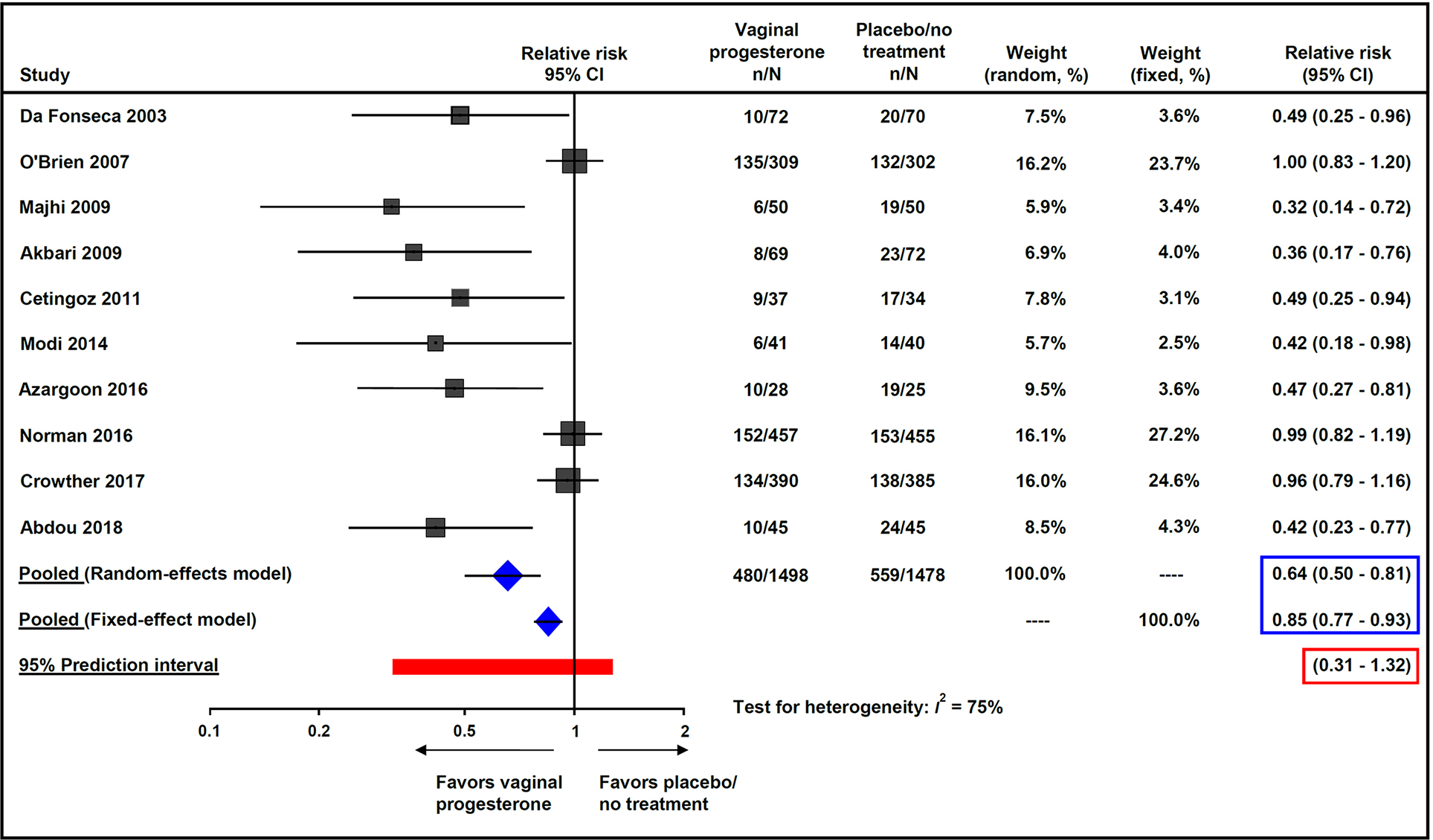
Effect of vaginal progesterone on preterm birth <37 weeks of gestation
Figure 3:
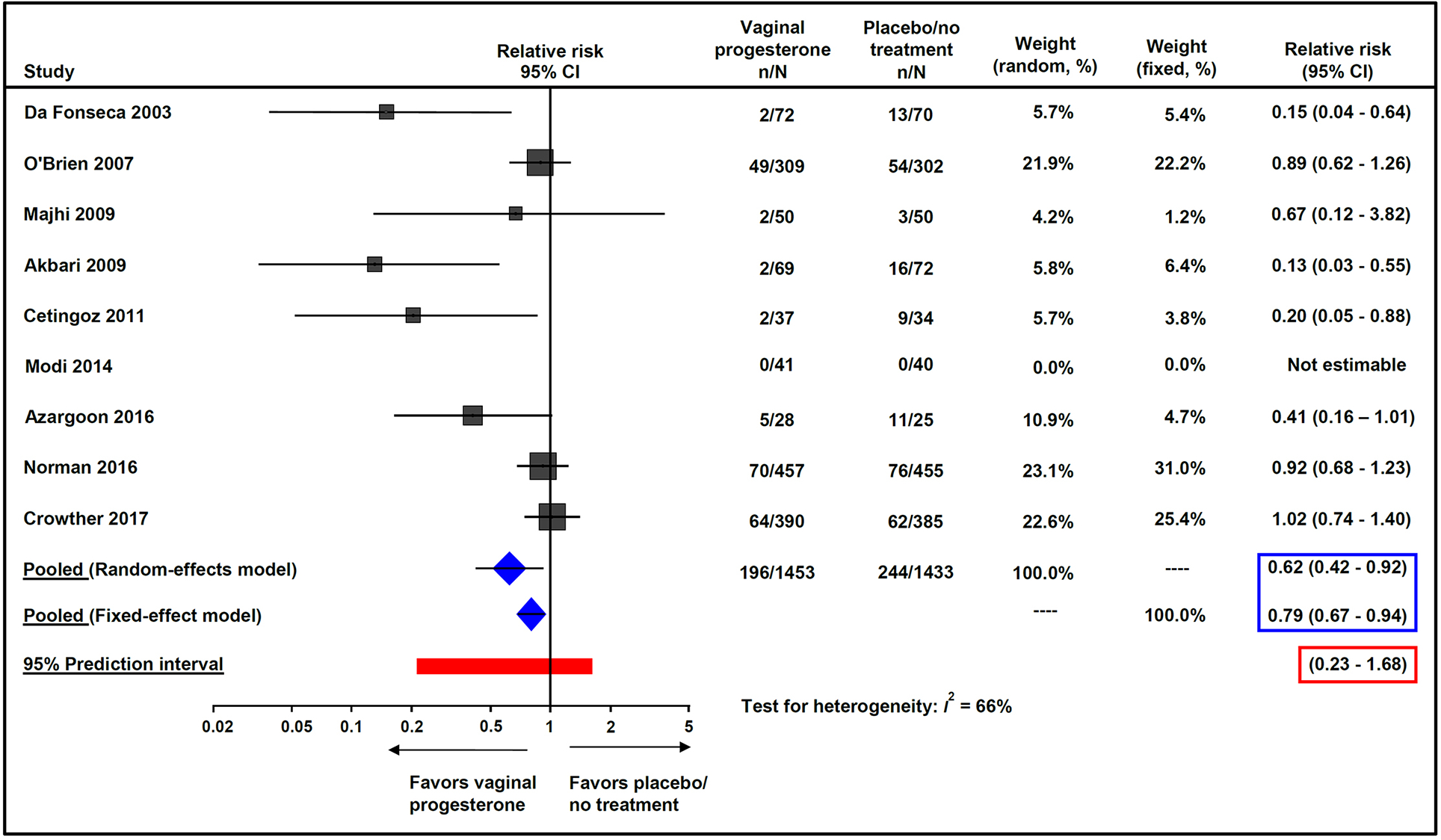
Effect of vaginal progesterone on preterm birth <34 weeks of gestation
Secondary outcomes
Table 2 shows the effect of vaginal progesterone on pregnancy, maternal, and perinatal outcomes. There was no significant difference between the vaginal progesterone and placebo/no treatment groups in the frequency of preterm birth <28 weeks of gestation (4.3% vs 4.0%; pooled RR, 1.12; 95% CI, 0.70–1.78; I2 = 18%). The frequencies of other pregnancy and maternal outcomes did not significantly differ between the study groups. Infants whose mothers received vaginal progesterone had a significantly lower risk of NICU admission (14.4% vs 20.7%; pooled RR from random-effects model, 0.53; 95% CI, 0.33–0.85; P = 0.008; pooled RR from fixed-effect model, 0.70; 95% CI, 0.57–0.85; P = 0.0005; I2 = 67%; 95% prediction interval of the RR, 0.16–1.79; NNT, 16; 95% CI, 10–36). There was no evidence of an effect of vaginal progesterone on RDS, NEC, IVH, grade III/IV IVH, neonatal sepsis, retinopathy of prematurity, bronchopulmonary dysplasia, periventricular leukomalacia, fetal death, neonatal death, perinatal death, birth weight <1500 g and <2500 g, use of mechanical ventilation, and patent ductus arteriosus.
TABLE 2.
Effect of vaginal progesterone on pregnancy, maternal, and perinatal outcomes
| Outcome | No of trials | Vaginal progesterone | Placebo/no treatment | Relative risk (95% CI) | P value | I2, % | 95% Prediction interval for the RR |
|---|---|---|---|---|---|---|---|
| Preterm birth <28 weeks | 972–74,76–81 | 62/1429 (4.3%) | 56/1406 (4.0%) | 1.12 (0.70–1.78) | 0.64 | 18 | 0.51–2.49 |
| Threatened preterm labor or need for tocolysis | 572–74,76,80 | 176/866 (20.3%) | 196/845 (23.2%) | 0.82 (0.63–1.06) | 0.12 | 37 | 0.48–1.42 |
| Use of antenatal corticosteroids | 273,80 | 211/699 (30.2%) | 213/687 (31.0) | 0.98 (0.83–1.14) | 0.76 | 0 | NA |
| Cesarean delivery | 373,74,80 | 215/749 (28.7%) | 191/737 (25.9%) | 1.11 (0.94–1.31) | 0.21 | 0 | NA |
| Any maternal adverse event | 373,76,80 | 385/740 (52.0%) | 369/718 (51.4%) | 1.01 (0.89–1.15) | 0.88 | 41 | NA |
| Discontinuation of treatment because of adverse events | 473,74,76,80 | 44/790 (5.6%) | 31/768 (4.0%) | 1.38 (0.88–2.14) | 0.16 | 0 | NA |
| Preterm prelabor rupture of membranes | 473,74,76,80 | 88/794 (11.1%) | 85/775 (11.0%) | 1.02 (0.77–1.35) | 0.87 | 0 | NA |
| Preeclampsia | 274,80 | 12/440 (2.7%) | 8/435 (1.8%) | 1.48 (0.61–3.58) | 0.38 | NA | NA |
| Gestational hypertension | 274,80 | 6/440 (1.4%) | 5/435 (1.2) | 1.19 (0.37–3.85) | 0.78 | 0 | NA |
| Gestational diabetes mellitus | 274,80 | 45/440 (10.2%) | 40/435 (9.2%) | 1.12 (0.75–1.67) | 0.59 | 0 | NA |
| Respiratory distress syndrome | 673–76,80,81 | 84/896 (9.4%) | 114/883 (12.9%) | 0.62 (0.37–1.04) | 0.07 | 57 | 0.18–2.13 |
| Necrotizing enterocolitis | 473,74,76,80 | 5/782 (0.6%) | 8/766 (1.0%) | 0.64 (0.22–1.90) | 0.42 | 0 | NA |
| Intraventricular hemorrhage | 473,75,76,80 | 17/801 (2.1%) | 15/788 (1.9%) | 1.11 (0.56–2.22) | 0.76 | 0 | NA |
| Grade III/IV intraventricular hemorrhage | 473,74,76,80 | 2/782 (0.3) | 2/766 (0.3) | 0.98 (0.14–6.94) | 0.98 | 0 | NA |
| Neonatal sepsis | 573–76,80 | 15/827 (1.8%) | 22/825 (2.7%) | 0.69 (0.29–1.68) | 0.42 | 26 | 0.14–3.32 |
| Retinopathy of prematurity | 276,80 | 12/422 (2.8%) | 9/414 (2.2%) | 1.32 (0.56–3.09) | 0.53 | NA | NA |
| Bronchopulmonary dysplasia | 374,76,80 | 10/473 (2.1%) | 5/464 (1.1%) | 1.97 (0.68–5.71) | 0.21 | NA | NA |
| Periventricular leukomalacia | 276,80 | 0/423 (0.0) | 1/414 (0.2) | 0.33 (0.01–8.03) | 0.49 | NA | NA |
| Fetal death | 673,74,76,78–80 | 16/1271 (1.3%) | 15/1251 (1.2%) | 1.05 (0.52–2.13) | 0.89 | 0 | 0.52–2.13 |
| Neonatal death | 773–76,78–80 | 20/1340 (1.5%) | 32/1323 (2.4%) | 0.65 (0.36–1.15) | 0.14 | 0 | 0.36–1.15 |
| Perinatal death | 673,74,76,78–80 | 33/1271 (2.6%) | 37/1251 (3.0%) | 0.90 (0.56–1.45) | 0.67 | 0 | 0.56–1.45 |
| Birthweight <1500 g | 473,74,76,80 | 55/781 (7.0%) | 42/762 (5.5%) | 1.28 (0.87–1.89) | 0.21 | 0 | NA |
| Birthweight <2500 g | 573–76,80 | 206/850 (24.2%) | 229/834 (27.5%) | 0.77 (0.54–1.10) | 0.15 | 64 | 0.33–1.82 |
| Admission to NICU | 673,74,75,76,80,81 | 129/896 (14.4%) | 183/883 (20.7%) | 0.53 (0.33–0.85) | 0.01 | 67 | 0.16–1.79 |
| Use of mechanical ventilation | 573–76,80 | 78/825 (9.5%) | 104/825 (12.6%) | 0.65 (0.39–1.08) | 0.10 | 44 | 0.21–2.00 |
| Patent ductus arteriosus | 373,74,80 | 18/721 (2.5%) | 15/718 (2.1%) | 1.19 (0.61–2.36) | 0.61 | 0 | NA |
Data are n/N.
CI, confidence interval; NA, not applicable; NICU, neonatal intensive care unit; RR, relative risk.
To date, only 2 studies reported the effects of prenatal exposure to vaginal progesterone on long-term neurodevelopmental and health outcomes in singleton gestations with a history of spontaneous preterm birth.73,79 O’Brien et al73 assessed neurodevelopmental outcomes at 2 years of age in 293 children born to women enrolled in their trial.82 The frequency of suspected developmental delay, assessed by the Denver II Developmental Screening Test, was similar in the vaginal progesterone and placebo groups (10.3% vs 10.4%, P = 0.95). Moreover, there were no significant differences between the study groups in mean weight, length and head circumference, chronic morbid conditions, and congenital abnormalities not detected at birth. Norman et al79 reported that the Bayley-III cognitive composite score at 2 years of age (N=656 children) did not differ significantly between the vaginal progesterone and placebo groups (mean difference, −0.14; 95% CI, −2.79 to 2.52; P = 0.92). There were no significant between-group differences in the risk of moderate or severe neurodevelopmental impairment, visual or hearing impairment, and disability in renal, gastrointestinal, or respiratory function.
Subgroup and sensitivity analyses
Subgroup analyses of the effect of vaginal progesterone on primary outcomes are depicted in Table 3. Pooled treatment effect estimates from small studies showed that vaginal progesterone significantly reduced the risk of preterm birth <37 weeks of gestation (RR, 0.43; 95% CI, 0.33–0.55; I2 = 0%; 7 trials, 678 women) and <34 weeks of gestation (RR, 0.27; 95% CI, 0.15–0.49; I2 = 0%; 6 trials, 588 women). By contrast, pooled treatment effect estimates from large studies showed little or no difference between the vaginal progesterone and placebo/no treatment groups in the risk of preterm birth <37 weeks of gestation (RR, 0.98; 95% CI, 0.88–1.09; I2 = 0%; 3 trials, 2298 women) and <34 weeks of gestation (RR, 0.94; 95% CI, 0.78–1.13; I2 = 0%; 3 trials, 2298 women). Of note, the RRs and 95% CIs of the 3 large studies for preterm birth <37 weeks of gestation were very similar to each other (Figure 2). Interaction P values <0.0001 for subgroup differences indicated that the effect of vaginal progesterone significantly differed between small and large studies. For the outcome of NICU admission, we undertook a post-hoc subgroup analysis according to sample size and the results were similar to the previous ones: vaginal progesterone significantly reduced the risk of NICU admission in the subgroup of small studies (RR, 0.30; 95% CI, 0.18–0.51; I2 = 0%; 4 trials, 402 women), whereas in the subgroup of large studies it had no effect (RR, 0.87; 95% CI, 0.69–1.09; I2 = 0%; 2 trials, 1377 women) with an interaction P value of 0.0003.
TABLE 3.
Subgroup analyses of effect of vaginal progesterone on preterm birth <37 and 34 weeks of gestation
| Preterm birth <37 weeks of gestation |
Preterm birth <34 weeks of gestation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of trials | Vaginal progesterone | Placebo/no treatment | Relative risk (95% CI) | I2, % | Interaction P value | No. of trials | Vaginal progesterone | Placebo/no treatment | Relative risk (95% CI) | I2, % | Interaction P value |
| Study sample size, n | <0.00001 | 0.0001 | ||||||||||
| <150 | 772,74–78,81 | 59/342 (17.3%) | 136/336 (40.5%) | 0.43 (0.33–0.55) | 0 | 6160,161,162,163 | 13/297 (4.4%) | 52/291 (17.9%) | 0.27 (0.15–0.49) | 0 | ||
| ≥150 | 373,79,80 | 421/1156 (36.4%) | 423/1142 (37.0%) | 0.98 (0.88–1.09) | 0 | 3162–164 | 183/1156 (15.8%) | 192/1142 (16.8%) | 0.94 (0.78–1.13) | 0 | ||
| Study setting | <0.00001 | 0.0003 | ||||||||||
| Low/middle-income countries | 772,74–78,81 | 59/342 (17.3%) | 136/336 (40.5%) | 0.43 (0.33–0.55) | 0 | 6162 | 13/297 (4.4%) | 52/291 (17.9%) | 0.27 (0.15–0.49) | 0 | ||
| High-income countries | 279,80 | 286/847 (33.8%) | 291/840 (34.6%) | 0.97 (0.85–1.11) | 0 | 2162 | 134/847 (15.8%) | 138/840 (16.4%) | 0.96 (0.77–1.20) | 0 | ||
| Both low/middle- and high-income countries | 173 | 135/309 (43.7%) | 132/302 (43.7%) | 1.00 (0.83–1.20) | NA | 1162 | 49/309 (15.9%) | 54/302 (17.9%) | 0.89 (0.62–1.26) | NA | ||
| Study center status | <0.00001 | 0.0003 | ||||||||||
| Single center | 772,74–78,81 | 59/342 (17.3%) | 136/336 (40.5%) | 0.43 (0.33–0.55) | 0 | 6160,161,162,163 | 13/297 (4.4%) | 52/291 (17.9%) | 0.27 (0.15–0.49) | 0 | ||
| Multicenter | 373,79,80 | 421/1156 (36.4%) | 423/1142 (37.0%) | 0.98 (0.88–1.09) | 0 | <0.0001 | 3162–164 | 183/1156 (15.8%) | 192/1142 (16.8%) | 0.94 (0.78–1.13) | 0 | |
| Trial registration status | 0.0001 | 0.0002 | ||||||||||
| Registered | 573,77–80 | 437/1225 (35.7%) | 456/1207 (37.8%) | 0.88 (0.72–1.07) | 62 | 5162 | 188/1225 (15.3%) | 203/1207 (16.8%) | 0.90 (0.74–1.11) | 15 | ||
| Not registered | 572,74–76,81 | 43/273 (15.8%) | 103/271 (38.0%) | 0.42 (0.31–0.57) | 0 | 4162 | 8/228 (3.5%) | 41/226 (18.1%) | 0.21 (0.10–0.44) | 0 | ||
| Mean gestational age at treatment initiation, weeks | 0.01 | <0.0001 | ||||||||||
| <24 | 673,74,78–81 | 447/1279 (35.0%) | 485/1262 (38.4%) | 0.76 (0.60–0.98) | 75 | 5162 | 190/1234 (15.4%) | 206/1217 (16.9%) | 0.91 (0.76–1.09) | 0 | ||
| ≥24 | 472,75–77 | 33/219 (15.1%) | 74/216 (34.3%) | 0.44 (0.31–0.63) | 0 | 4162 | 6/219 (2.7%) | 38/216 (17.6%) | 0.16 (0.07–0.36) | 0 | ||
| Daily dose of vaginal progesterone, mg | 0.97 | 0.54 | ||||||||||
| 90–100 | 772,73–77,80 | 308/968 (31.8%) | 363/953 (38.1%) | 0.62 (0.45–0.85) | 75 | 7162 | 121/968 (12.5%) | 157/953 (16.5%) | 0.51 (0.28–0.92) | 72 | ||
| ≥200 | 378,79,81 | 172/530 (32.5%) | 196/525 (37.3%) | 0.61 (0.32–1.14) | 84 | 2162 | 75/485 (15.5%) | 87/480 (18.1%) | 0.69 (0.32–1.47) | 64 | ||
Data are n/N.
CI, confidence interval; NA, not applicable.
Other subgroup analyses showed that vaginal progesterone significantly decreased the risk of preterm birth <37 and <34 weeks of gestation in studies conducted in low/middle-income countries, single-center studies, and unregistered studies, whereas its administration had no effect in studies conducted in high-income countries and both low/middle- and high-income countries, multicenter trials, and registered studies (all interaction P values <0.0003). Treatment effect estimates were significantly greater in studies for which vaginal progesterone administration was initiated at or after 24 weeks of gestation than in those for which it was initiated before 24 weeks of gestation (interaction P values ≤0.01). There was no evidence of a different effect related to daily dose of vaginal progesterone (interaction P values >0.50).
Sensitivity analyses restricted to the 4 trials at overall low risk of bias73,76,79,80 showed that vaginal progesterone did not reduce the risk of preterm birth <37 weeks of gestation (RR, 0.96; 95% CI, 0.84–1.09; I2 = 31%) and <34 weeks of gestation (RR, 0.90; 95% CI, 0.71–1.15; I2 = 34%). A similar post-hoc sensitivity analysis showed that vaginal progesterone did not significantly decrease the risk of NICU admission (RR, 0.77; 95% CI, 0.53–1.14; I2 = 54%).
Small-study effects and publication bias
There was a strong suggestion of small-study effects because the treatment effect estimates in fixed-effect meta-analyses were noticeably smaller than those in random-effects meta-analyses (Figures 2 and 3). Small-study effects were confirmed after visual inspection of the funnel plot for the outcome preterm birth of <37 weeks of gestation showing pronounced asymmetry (Figure 4A), which was statistically significant according to the Egger’s and Harbord’s tests (P <0.0001 for both). The contour-enhanced funnel plot (Figure 4B) indicates that missing studies would be on the right-hand side of the plot in areas of high statistical significance, which suggests that publication bias is a less likely cause of the funnel asymmetry. After applying the Trim and Fill method to adjust for small-study effects, the overall effect of vaginal progesterone on preterm birth <37 weeks of gestation was considerably reduced and turned into a non-statistically significant result (pooled RR from random-effects model, 0.86; 95% CI, 0.68–1.10; pooled RR from fixed-effect model, 0.94; 95% CI, 0.86–1.03).
Figure 4:
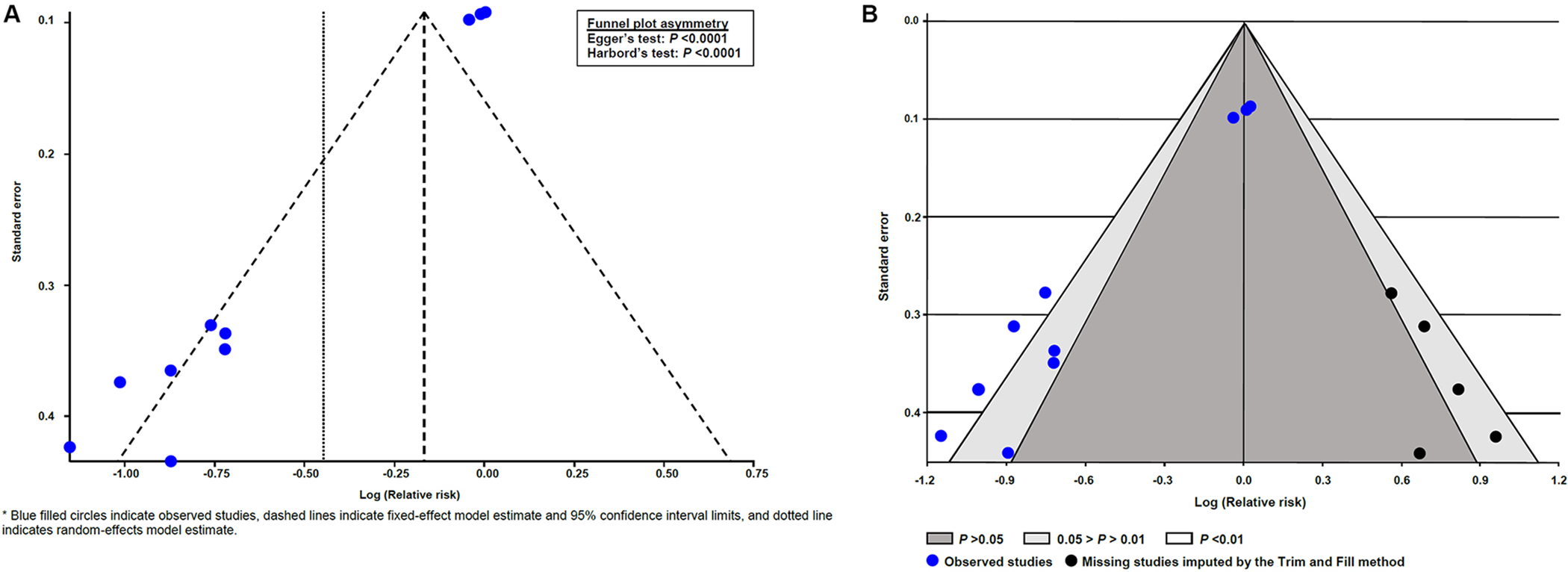
Funnel plots for the outcome of preterm birth <37 weeks of gestation
Although the meta-analysis of the effect of vaginal progesterone on preterm birth <34 weeks of gestation did not include the recommended minimal number of 10 studies but rather 9, we assessed the presence of small-study effects and publication bias in this meta-analysis. Small-study effects were also detected by visual and statistically significant funnel plot asymmetry (Supplemental Figure 2). The adjustment for small-study effects by the Trim and Fill method resulted in a markedly reduced and non-significant effect of vaginal progesterone on preterm birth <34 weeks of gestation (pooled RR from random-effects model, 0.92; 95% CI, 0.60–1.42; pooled RR from fixed-effect model, 0.94; 95% CI, 0.79–1.11).
Quality of evidence based on GRADE
The assessment of the quality of evidence for primary and secondary outcomes is shown in Table 4. Evidence was judged to be of “very low quality” for the primary outcomes of preterm birth <37 and <34 weeks of gestation. We downgraded the quality of evidence 2 levels for very serious inconsistency because of considerable or substantial heterogeneity, probably a result of small-study effects, and 1 level for serious risk of bias in more than one-half of studies contributing to these outcomes. The quality of evidence was considered either as low or as very low for 18 secondary outcomes, moderate for 6, and high for 2. Most secondary outcomes were downgraded for serious or very serious imprecision and/or inconsistency. Considering that the quality of evidence for outcomes critical in decision making, such as preterm birth <37 and <34 weeks of gestation and RDS, was very low, we considered that the overall quality of evidence was very low.
Table 4.
Summary of findings on the quality of evidence for primary and secondary outcomes
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with placebo/no treatment | Risk with vaginal progesterone | ||||
| Preterm birth <37 weeks | 378 per 1000 | 242 per 1000 (189 to 306) | RR 0.64 (0.50 to 0.81) | 2976 (10 studies) | ⊕⊝⊝⊝ Very lowb,c |
| Preterm birth <34 weeks | 170 per 1000 | 106 per 1000 (72 to 57) | RR 0.62 (0.42 to 0.92) | 2886 (9 studies) | ⊕⊝⊝⊝ Very lowc,d |
| Preterm birth <28 weeks | 40 per 1000 | 45 per 1000 (28 to 71) | RR 1.12 (0.70 to 1.78) | 2835 (9 studies) | ⊕⊕⊕⊝ Moderatee |
| Respiratory distress syndrome | 129 per 1000 | 80 per 1000 (48 to 134) | RR 0.62 (0.37 to 1.04) | 1779 (6 studies) | ⊕⊝⊝⊝ Very lowc,f,g |
| Necrotizing enterocolitis | 10 per 1000 | 7 per 1000 (2 to 20) | RR 0.64 (0.22 to 1.90) | 1548 (4 studies) | ⊕⊕⊝⊝ Lowh |
| Intraventricular hemorrhage | 19 per 1000 | 21 per 1000 (11 to 42) | RR 1.11 (0.56 to 1.22) | 1589 (4 studies) | ⊕⊕⊝⊝ Lowh |
| Grade III/IV intraventricular hemorrhage | 3 per 1000 | 3 per 1000 (0 to 18) | RR 0.98 (0.14 to 6.94) | 1548 (4 studies) | ⊕⊕⊝⊝ Lowh |
| Neonatal sepsis | 27 per 1000 | 18 per 1000 (8 to 45) | RR 0.69 (0.29 to 1.68) | 1652 (5 studies) | ⊕⊝⊝⊝ Very lowc,h |
| Retinopathy of prematurity | 22 per 1000 | 29 per 1000 (12 to 67) | RR 1.32 (0.56 to 3.09) | 836 (2 studies) | ⊕⊕⊝⊝ Lowh |
| Bronchopulmonary dysplasia | 11 per 1000 | 21 per 1000 (7 to 62) | RR 1.97 (0.68 to 5.71) | 937 (3 studies) | ⊕⊕⊝⊝ Lowh |
| Periventricular leukomalacia | 2 per 1000 | 1 per 1000 (0 to 19) | RR 0.33 (0.01 to 8.03) | 837 (2 studies) | ⊕⊕⊝⊝ Lowh |
| Fetal death | 12 per 1000 | 13 per 1000 (6 to 26) | RR 1.05 (0.52 to 2.13) | 2522 (6 studies) | ⊕⊕⊝⊝ Lowh |
| Neonatal death | 24 per 1000 | 16 per 1000 (9 to 28) | RR 0.65 (0.36 to 1.15) | 2663 (7 studies) | ⊕⊕⊝⊝ Lowi |
| Perinatal death | 30 per 1000 | 27 per 1000 (17 to 43) | RR 0.90 (0.56 to 1.45) | 2522 (6 studies) | ⊕⊕⊝⊝ Lowh |
| Birthweight <1500 g | 55 per 1000 | 71 per 1000 (48 to 104) | RR 1.28 (0.87 to 1.89) | 1543 (4 studies) | ⊕⊕⊕⊝ Moderatej |
| Birthweight <2500 g | 275 per 1000 | 211 per 1000 (148 to 302) | RR 0.77 (0.54 to 1.10) | 1684 (5 studies) | ⊕⊕⊝⊝ Lowf,g |
| Admission to NICU | 207 per 1000 | 110 per 1000 (68 to 176) | RR 0.53 (0.33 to 0.85) | 1779 (6 studies) | ⊕⊕⊝⊝ Lowc,f |
| Use of mechanical ventilation | 126 per 1000 | 82 per 1000 (49 to 136) | RR 0.65 (0.39 to 1.08) | 1650 (5 studies) | ⊕⊕⊝⊝ Lowg,k |
| Patent ductus arteriosus | 21 per 1000 | 25 per 1000 (13 to 49) | RR 1.19 (0.61 to 2.36) | 1439 (3 studies) | ⊕⊕⊝⊝ Lowh |
| Threatened preterm labor or need for tocolysis | 232 per 1000 | 190 per 1000 (146 to 246) | RR 0.82 (0.63 to 1.06) | 1711 (5 studies) | ⊕⊕⊝⊝ Lowg,k |
| Use of antenatal corticosteroids | 310 per 1000 | 304 per 1000 (257 to 353) | RR 0.98 (0.83 to 1.14) | 1386 (2 studies) | ⊕⊕⊕⊕ High |
| Cesarean delivery | 259 per 1000 | 288 per 1000 (244 to 339) | RR 1.11 (0.94 to 1.31) | 1486 (3 studies) | ⊕⊕⊕⊝ Moderatej |
| Any maternal adverse event | 514 per 1000 | 519 per 1000 (457 to 591) | RR 1.01 (0.89 to 1.15) | 1458 (3 studies) | ⊕⊕⊕⊕ High |
| Discontinuation of treatment because of adverse events | 40 per 1000 | 56 per 1000 (36 to 86) | RR 1.38 (0.88 to 2.14) | 1558 (4 studies) | ⊕⊕⊕⊝ Moderatej |
| Preterm prelabor rupture of membranes | 110 per 1000 | 112 per 1000 (84 to 148) | RR 1.02 (0.77 to 1.35) | 1569 (4 studies) | ⊕⊕⊕⊝ Moderatej |
| Preeclampsia | 18 per 1000 | 27 per 1000 (11 to 66) | RR 1.48 (0.61 to 3.58) | 875 (2 studies) | ⊕⊕⊝⊝ Lowh |
| Gestational hypertension | 11 per 1000 | 14 per 1000 (4 to 44) | RR 1.19 (0.37 to 3.85) | 875 (2 studies) | ⊕⊕⊝⊝ Lowh |
| Gestational diabetes mellitus | 92 per 1000 | 103 per 1000 (69 to 154) | RR 1.12 (0.75 to 1.67) | 875 (2 studies) | ⊕⊕⊕⊝ Moderatej |
CI, confidence interval; NICU, neonatal intensive care unit; RR, relative risk
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded two levels due to very serious inconsistency: considerable heterogeneity (I2=75%), probably due to small-study effects.
Downgraded one level due to serious risk of bias: ≥50% of studies contributing to the outcome are at “high risk of bias” or “some concern of bias”.
Downgraded two levels due to very serious inconsistency: substantial heterogeneity (I2=66%), probably due to small-study effects.
Downgraded one level due to serious imprecision: 95% CI included no effect and is imprecise (lower and upper bounds <0.75 and>1.25, respectively).
Downgraded one level due to serious inconsistency: substantial heterogeneity (I2 ≥50%).
Downgraded one level due to serious imprecision: 95% CI included no effect and is imprecise (lower bound <0.75).
Downgraded two levels due to very serious imprecision: small total number of events; 95% CI included no effect and is imprecise (lower and upper bounds <0.75 and>1.25, respectively).
Downgraded two levels due to very serious imprecision: small total number of events; 95% CI included no effect and is imprecise (lower bound <0.75).
Downgraded one level due to serious imprecision: 95% CI included no effect and is imprecise (upper bound >1.25).
Downgraded one level due to serious inconsistency: moderate heterogeneity with some studies suggesting substantial benefit, and others no effect.
COMMENT
Principal findings
First, 2 meta-analyses that included data from all trials showed that the administration of vaginal progesterone to asymptomatic women with a singleton gestation and a history of spontaneous preterm birth significantly reduced the risk of preterm birth <37 and <34 weeks of gestation. However, evidence was highly conflicting because vaginal progesterone was associated with a large decrease in the risk of both outcomes in small studies, whereas in large studies, it had no effect. The quality of the body of evidence for both outcomes was very low, which means that the true effect is probably markedly different from the estimated effect. Second, low-quality evidence from 1 meta-analysis indicated that neonates of mothers who received vaginal progesterone had a significantly lower risk of NICU admission. There was no evidence for a beneficial effect on the other adverse perinatal outcomes evaluated. Third, there was clear evidence of substantial small-study effects in the meta-analyses of the primary outcomes of preterm birth <37 and <34 weeks of gestation. Fourth, treatment effect estimates of vaginal progesterone on preterm birth <37 and <34 weeks of gestation and on NICU admission were substantially reduced and changed to non-statistically significant after performing sensitivity analyses restricted to studies at overall low risk of bias. The treatment effect estimates were also considerably reduced and turned into non-statistical significance after meta-analyses were adjusted for small-study effects. Five, the 95% prediction intervals for the meta-analyses of the effect of vaginal progesterone on preterm birth <37 and <34 weeks of gestation and NICU admission contained values ≥1, which indicates that vaginal progesterone could have no effect or even an effect in the opposite direction in some patient populations or in a new study. Six, evidence from 2 studies showed that, at 2 years of age, there were no significant differences in neurodevelopmental and health outcomes between children exposed prenatally to vaginal progesterone and those exposed to placebo. Finally, there were no significant differences in the frequency of adverse maternal outcomes between the vaginal progesterone and placebo/no treatment groups.
Methodological issues
There was substantial between-trial heterogeneity (I2 ≥66%) in the 3 meta-analyses that showed a significant beneficial effect of vaginal progesterone administration, which affects the extent to which generalizable conclusions can be made. We explored the reasons for heterogeneity among results of studies through subgroup analyses and identified plausible explanations. Subgroup analyses showed that the intervention effects significantly differed between small and large studies. Indeed, the beneficial effects of vaginal progesterone substantially increased in the meta-analyses of small studies, but they disappeared in the meta-analyses restricted to large studies. All small studies were conducted at a single center in low/middle-income countries, and most were not registered. All these characteristics were individually associated with significantly larger treatment effect estimates of vaginal progesterone in the subgroup analyses. These findings are in accordance with previous evidence from meta-epidemiological studies showing that small trials and single-center trials reported larger beneficial effects of treatment than the large trials and multicenter trials, respectively.83–91 Interestingly, in 1 of these studies,85 a dose-effect relationship was found: the smaller the trial size, the larger the difference in treatment effect estimates. Moreover, there is some evidence indicating that treatment effect estimates are significantly larger in unregistered than in registered trials, in trials conducted in less developed than in more developed countries, and in trials that deviated from the intention-to-treat analysis than in trials that reported the standard approach.92–94
Small-study effects, defined as the tendency of small trials to report larger benefits of treatment than large trials do, is a well-known critical and challenging issue that may threaten the validity of the results of a meta-analysis.95 Erroneous conclusions can arise from a meta-analysis if small-study effects are not properly accounted for. We found clear evidence of small-study effects in the meta-analyses of the effect of vaginal progesterone on preterm birth <37 and <34 weeks of gestation. Small-study effects may be a result of publication bias, true clinical heterogeneity, or low-quality studies reporting inflated effect sizes.54 Contour-enhanced funnel plots suggested that small-study effects were more likely to be due to factors other than publication bias. Clinical heterogeneity of patients when small studies focus on high-risk patients for whom the treatment might be more effective also does not appear to be a plausible explanation for small-study effects in our meta-analysis because the frequencies of preterm birth <37 and <34 weeks of gestation in the placebo/no treatment groups were comparable between the small and large trials (40.5% vs 37.0% for preterm birth <37 weeks and 17.9% vs 16.8% for preterm birth <34 weeks). Differences in compliance and daily dose of vaginal progesterone used between the small and large trials also did not appear to explain small-study effects. Another reason that may explain small-study effects is the lower methodological quality leading to spuriously inflated effects in smaller studies. There is strong evidence indicating that trials at high or unclear risk of bias, mainly those arising from the randomization process and those due to deviations from the intended interventions, are significantly associated with exaggerated beneficial intervention effect estimates and increases in between-trial heterogeneity as compared to trials at low risk of bias.87,96–103 In our systematic review, 6 of 7 small trials were judged to be at overall high risk of bias (N=4) or some concerns of bias (N=2).
In summary, we believed that small-study effects in the meta-analyses of the effect of vaginal progesterone on preterm birth <37 and <34 weeks of gestation are mainly explained by the poor methodological quality of most small trials. Regardless of whether publication bias or lower methodological quality of small trials or a combination of both are the main cause of small-study effects, they have the same effect on meta-analyses: lead to an exaggeration of the pooled treatment effect. It has been claimed that in the presence of small-study effects restriction of analyses to high-quality, large trials might provide more valid estimates than overall analyses of trials.84 The Cochrane Handbook for Systematic Reviews of Interventions recommends restricting the primary analysis to studies judged to be at overall low risk of bias65,66 or considering reporting the results of meta-analyses restricted to the larger, more rigorous studies.54
Comparison with existing literature
In 2021, the EPPPIC group published an IPD meta-analysis that assessed the efficacy of progestogens (vaginal progesterone, 17α-hydroxyprogesterone caproate [17-OHPC], and oral progesterone) to prevent preterm birth in asymptomatic high-risk women and reported that vaginal progesterone was associated with a significant reduction in the risk of preterm birth <34 weeks of gestation in singleton pregnancies.42 There were no significant differences between the vaginal progesterone and placebo/no treatment groups in the risk of the remaining primary outcomes (preterm birth <37 and <28 weeks of gestation, perinatal death, serious neonatal complications, and maternal complications). The main shortcoming of this IPD meta-analysis was that it grouped together women with a history of spontaneous preterm birth, short cervix, congenital uterine anomalies, uterine leiomyomas, pregnancy after assisted reproductive technologies, or a positive fetal fibronectin test combined with other clinical risk factors into a single category. This limitation was highlighted by the U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) after the publication of this study as follows: “The EPPPIC meta-analysis grouped together HPC [hydroxyprogesterone caproate] trials of patients with differences in their risk profiles, including combining women with a prior PTB [preterm birth] and those without a prior PTB [preterm birth], and women with and without a short cervix. Because of this grouping, the meta-analysis does not provide relevant information regarding Makena’s effectiveness for its approved use”.104 The same applies for the use of vaginal progesterone in women with a history of spontaneous preterm birth. Importantly, this IPD meta-analysis did not address small-study effects nor did it perform sensitivity analyses according to risk of bias despite the fact that 3 included trials74,78,105 that compared vaginal progesterone vs placebo/no treatment were judged to be at high risk of bias in at least one domain.
Recently, a network meta-analysis compared the efficacy of several interventions to prevent preterm birth in high-risk women with a singleton gestation and reported that vaginal progesterone appeared to be the most effective in decreasing the risk of preterm birth.106 Unfortunately, this study also inappropriately combined patients with several risk factors for preterm birth (history of spontaneous preterm birth, midtrimester loss, cervical insufficiency due to cervical surgery, uterine anomalies, and short cervix) into a single group. This limitation seriously threatens the reliability and validity of this network meta-analysis, which requires that the different sets of randomized controlled trials are similar, on average, in all important factors other than the intervention comparison being made.107 Moreover, this study included a quasi-randomized trial,68 which was excluded from our meta-analysis, and did not assess small-study effects.
There are several reasons why meta-analyses evaluating the efficacy of any proposed intervention to prevent preterm birth in high-risk populations should not group women with different risk factors into a single category: (1) preterm birth is a complex syndrome caused by multiple etiological factors with different mechanisms of disease and significant individual heterogeneity.15–22 This is the rationale for assessing separately the proposed interventions to prevent preterm birth in multiple gestations; (2) the groups of patients with a sonographic short cervix and those with a history of spontaneous preterm birth do not overlap in clinical practice. In the IPD meta-analysis by Romero et al,108 only 29% of women with a short cervix (cervical length ≤25 mm) had a history of spontaneous preterm birth, whereas in the trial by O’Brien et al73 only 5.1% of women with a history of spontaneous preterm birth had a short cervix (cervical length ≤25 mm). In the PROLONG trial,109 which assessed 17-OHPC to prevent recurrent preterm birth, only 1.4% of women with a history of spontaneous preterm birth had a short cervix (cervical length <25 mm); and (3) the risks for preterm birth and adverse perinatal outcomes substantially differ between patients with a short cervix and those with a history of spontaneous preterm birth. For example, in the IPD meta-analysis by Romero et al108 the frequencies of preterm birth <34 weeks of gestation and perinatal death among women with a midtrimester sonographic short cervix in the placebo group were 26.5% and 4.8%, respectively, in comparison to 17.0% and 3.0%, respectively, among women with a history of spontaneous preterm birth in the placebo/no treatment group of our current meta-analysis.
Strengths and limitations
The major strengths of this study are the rigorous methodology used in its conduct and the strict adherence to the updated Cochrane’s guidelines.43 Taken together, they comprise the inclusion of a larger number of studies assessing vaginal progesterone vs placebo/no treatment in women with a history of spontaneous preterm birth (N=10) in comparison to those included in the EPPPIC meta-analysis42 (N=5); the risk of bias assessment that was based on the Cochrane RoB 2 tool;45 the thorough investigation of sources of heterogeneity and causes of small-study effects; the calculation of 95% prediction intervals and adjusted treatment effect estimates for small-study effects; and the careful assessment of quality of evidence, among others.
Our study has some limitations: (1) various trials, mainly the small ones, did not report results for several adverse maternal and perinatal outcomes that were assessed in our systematic review, which could lead to changes in the results of some meta-analyses; (2) given a lack of data, we were unable to perform the prespecified subgroup analyses according to the number of previous spontaneous preterm births and the gestational age of previous spontaneous preterm birth; (3) the studies by Da Fonseca et al72 and Akbhari et al,75 which also included women with uterine malformations, cervical insufficiency, and previous cerclage, did not report results separately for the women with a history of spontaneous preterm birth. However, participants with these risk factors accounted for only 6% of the total number of women who were recruited in these trials; (4) only 2 trials reported data on the long-term effects of prenatal exposure to vaginal progesterone; and (5) some authors have suggested that the Trim and Fill method does not perform well when the between-study heterogeneity is large.110,111 Hence, the adjusted estimates for small-study effects should be taken with some caution.
Conclusions and implications
After examining the conflicting results between small and large trials on the efficacy of vaginal progesterone to prevent recurrent preterm birth in women with a singleton gestation and a history of spontaneous preterm birth, we concluded that there is no convincing evidence supporting its use in these patients. Evidence strongly suggests that the claimed beneficial effects of vaginal progesterone are attributable to methodological limitations in most of the included small trials. Our results do not support the ACOG current guideline’s41 recommendation of offering vaginal progesterone to patients with a singleton gestation and a history of spontaneous preterm birth. On the other hand, the FDA’s comprehensive analysis112 of the PROLONG trial109 also does not support ACOG’s41 and SMFM’s113 recommendation to offer 17-OHPC to these patients because this intervention did not demonstrate a statistically significant treatment benefit vs. placebo on preterm birth <37, <35 and <32 weeks of gestation, and a neonatal morbidity composite index. In addition, the FDA’s analysis112 did not find relevant differences in the treatment effect when analyzed by race (Black vs non-Black), region (US vs non-US), history of spontaneous preterm birth (1 previous spontaneous preterm birth vs >1 previous spontaneous preterm birth), and “composite” risk level (no risk factor vs ≥ 1 risk factor vs ≥ 2 risk factors). Findings from our study along with those from the FDA’s analysis112f and recent observational studies114–118 suggest that ACOG’s and SMFM’s guidelines41,113 should be revised.
Previously, we reported that vaginal progesterone administered to women with a singleton gestation and a midtrimester sonographic short cervix significantly reduces the risk of preterm birth and improves perinatal outcomes.108 The beneficial effects of vaginal progesterone were demonstrated in women with or without a history of spontaneous preterm birth.108,119,120 In summary, vaginal progesterone should be offered to patients with a singleton gestation and a history of spontaneous preterm birth only if they are diagnosed with a sonographic short cervix (cervical length ≤25 mm) in the midtrimester.
Supplementary Material
AJOG at a Glance
Why was the study conducted?
Some professional organizations recommend offering vaginal progesterone to women with a singleton gestation and a history of spontaneous preterm birth despite conflicting evidence about its efficacy.
Key findings
Meta-analyses including data from 10 trials showed that vaginal progesterone reduced the risk of preterm birth <37 and <34 weeks of gestation and admission to the neonatal intensive care unit. However, subgroup analyses revealed that this intervention was beneficial only in small (N=7), but not in large (N=3), trials. Sensitivity analyses restricted to studies at low risk of bias and adjustment for small-study effects both resulted in a markedly reduced and non-significant effect of vaginal progesterone on preterm birth <37 and <34 weeks of gestation.
What does this study add to what is known?
There is no convincing evidence supporting the use of vaginal progesterone to prevent recurrent preterm birth in singleton gestations with a history of spontaneous preterm birth.
Financial support:
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Role of the funding source:
The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review or approval of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Registration: This systematic review and meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021275154) on September 23, 2021.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2020. Natl Vital Stat Rep 2022;70:1–50. [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health 2022;6:106–115. Erratum in: Lancet Child Adolesc Health 2022;6:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. The National Academies Collection: reports funded by National Institutes of Health. In: Behrman RE, Butler AS, eds. Preterm birth: causes, consequences, and prevention. Washington (DC): National Academies Press (US), National Academy of Sciences; 2007. [Google Scholar]
- 5.Manuck TA, Rice MM, Bailit JL, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 2016;215:103.e1–103.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catov JM, Scifres CM, Caritis SN, Bertolet M, Larkin J, Parks WT. Neonatal outcomes following preterm birth classified according to placental features. Am J Obstet Gynecol 2017;216:411.e1–411.e14. [DOI] [PubMed] [Google Scholar]
- 7.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9. [DOI] [PubMed] [Google Scholar]
- 8.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 2012;379:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández de Gamarra-Oca L, Ojeda N, Gómez-Gastiasoro A, et al. Long-term neurodevelopmental outcomes after moderate and late preterm birth: a systematic review. J Pediatr 2021;237:168–76.e11. [DOI] [PubMed] [Google Scholar]
- 10.Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol 2014;210:285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 2020;63:508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crump C, Sundquist J, Sundquist K. Risk of hypertension into adulthood in persons born prematurely: a national cohort study. Eur Heart J 2020;41:1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangla A, Kandasamy Y. Effects of prematurity on long-term renal health: a systematic review. BMJ Open 2021;11:e047770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crump C Preterm birth and mortality in adulthood: a systematic review. J Perinatol 2020;40:833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29. [DOI] [PubMed] [Google Scholar]
- 16.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113(Suppl):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotsch F, Romero R, Erez O, et al. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J Matern Fetal Neonatal Med 2009;22(Suppl 2):5–23. [DOI] [PubMed] [Google Scholar]
- 18.Villar J, Papageorghiou AT, Knight HE, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol 2012;206:119–23. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Gravett MG, Iams J, et al. The preterm birth syndrome: issues to consider in creating a classification system. Am J Obstet Gynecol 2012;206:113–8. [DOI] [PubMed] [Google Scholar]
- 20.Esplin MS. Overview of spontaneous preterm birth: a complex and multifactorial phenotype. Clin Obstet Gynecol 2014;57:518–30. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esplin MS, Manuck TA, Varner MW, et al. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. Am J Obstet Gynecol 2015;213:429.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercer BM, Goldenberg RL, Das A, et al. The preterm prediction study: a clinical risk assessment system. Am J Obstet Gynecol 1996;174:1885–93. [DOI] [PubMed] [Google Scholar]
- 24.Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm prediction study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1998;178:1035–40. [DOI] [PubMed] [Google Scholar]
- 25.Bloom SL, Yost NP, McIntire DD, Leveno KJ. Recurrence of preterm birth in singleton and twin pregnancies. Obstet Gynecol 2001;98:379–85. [DOI] [PubMed] [Google Scholar]
- 26.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol 2006;195:643–50. [DOI] [PubMed] [Google Scholar]
- 27.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med 2006;19:773–82. [DOI] [PubMed] [Google Scholar]
- 28.Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Recurrent preterm birth. Semin Perinatol 2007;31:142–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esplin MS, O’Brien E, Fraser A, et al. Estimating recurrence of spontaneous preterm delivery. Obstet Gynecol 2008;112:516–23. [DOI] [PubMed] [Google Scholar]
- 30.Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecutive Pregnancies Study: recurrent preterm delivery by subtype. Am J Obstet Gynecol 2014;210:131.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Baer RJ, Berghella V, et al. Recurrence of preterm birth and early term birth. Obstet Gynecol 2016;128:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha AS, de Cássia Ribeiro-Silva R, Paixao ES, et al. Recurrence of preterm births: A population-based linkage with 3.5 million live births from the CIDACS Birth Cohort. Int J Gynaecol Obstet 2021. Dec 1. doi: 10.1002/ijgo.14053. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyama R, Makino S, Nojiri S, et al. Retrospective study of the recurrence risk of preterm birth in Japan. J Matern Fetal Neonatal Med 2022;35:515–19. [DOI] [PubMed] [Google Scholar]
- 34.Adane AA, Shepherd CCJ, Farrant BM, White SW, Bailey HD. Patterns of recurrent preterm birth in Western Australia: A 36-year state-wide population-based study. Aust N Z J Obstet Gynaecol 2022. Feb 14. doi: 10.1111/ajo.13492. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open 2017;7:e015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter TF, Fraser AM, Hunter CY, Ward RH, Varner MW. The risk of preterm birth across generations. Obstet Gynecol 1997;90:63–7. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol 2008;167:474–9. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya S, Amalraj Raja E, Ruiz Mirazo E, et al. Inherited predisposition to spontaneous preterm delivery. Obstet Gynecol 2010;115:1125–33. [DOI] [PubMed] [Google Scholar]
- 39.Ananth CV. Epidemiologic approaches for studying recurrent pregnancy outcomes: challenges and implications for research. Semin Perinatol 2007;31:196–201. [DOI] [PubMed] [Google Scholar]
- 40.American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol 2003;102:1115–6. [DOI] [PubMed] [Google Scholar]
- 41.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Prediction and Prevention of Spontaneous Preterm Birth: ACOG Practice Bulletin, Number 234. Obstet Gynecol 2021;138:e65–e90. [DOI] [PubMed] [Google Scholar]
- 42.EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet 2021;397:1183–94. Erratum in: Lancet 2021;397:1446. [DOI] [PubMed] [Google Scholar]
- 43.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane. [Google Scholar]
- 44.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane. [Google Scholar]
- 46.Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane, 2021. [Google Scholar]
- 47.Saver JL, Lewis RJ. Number needed to treat: conveying the likelihood of a therapeutic effect. JAMA 2019;321:798–9. [DOI] [PubMed] [Google Scholar]
- 48.Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane. [Google Scholar]
- 49.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Partlett C, Riley RD. Random effects meta-analysis: Coverage performance of 95% confidence and prediction intervals following REML estimation. Stat Med 2017;36:301–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 53.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane. [Google Scholar]
- 55.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–57. [DOI] [PubMed] [Google Scholar]
- 57.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991–6. [DOI] [PubMed] [Google Scholar]
- 58.Palmer TM, Peters JL, Sutton AJ, Moreno SG. Contour-enhanced funnel plots for meta-analysis. Stata J 2008;8:242–54. [Google Scholar]
- 59.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 60.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- 61.Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore) 2019;98:e15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klebanoff MA. Subgroup analysis in obstetrics clinical trials. Am J Obstet Gynecol 2007;197:119–22. [DOI] [PubMed] [Google Scholar]
- 63.Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci 2013;14:134–43. [DOI] [PubMed] [Google Scholar]
- 64.Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405–11. [DOI] [PubMed] [Google Scholar]
- 65.Boutron I, Page MJ, Higgins JPT, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane. [Google Scholar]
- 66.Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane. [Google Scholar]
- 67.Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66:151–7. [DOI] [PubMed] [Google Scholar]
- 68.Ahuja R, Sood A, Pal A, Mittal R. Role of micronized progesterone in prevention of preterm labour in women with previous history of one or more preterm births: a research study at a tertiary care hospital. Int J Reprod Contracept Obstet Gynecol 2015;4:1176–80. [Google Scholar]
- 69.Villalba MM, Vallega RJD, Soyao MJM. Effectiveness of prophylactic vaginal micronized progesterone in prevention of preterm birth among high risk women seen at the tertiary government hospital outpatient department: a randomized controlled trial. J Obstet Gynaecol Res 2020;46(Suppl 1):40. [Google Scholar]
- 70.Mohamed MA, Salama KM, Eldeen AAS, Saafan NA The efficacy of vaginal progesterone in reducing preterm birth in high-risk pregnancies. Benha J Appl Sci 2020;5:317–22. [Google Scholar]
- 71.Deshpande H, Sharma MM, Reyaz A, Madkar C, Huzurbazar S. Assessment of efficacy of micronized progesterone by vaginal route for prevention of preterm labour. Int J Clin Obstet Gynecol 2019;3:294–8. [Google Scholar]
- 72.Da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003;188:419–24. [DOI] [PubMed] [Google Scholar]
- 73.O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30:687–96. [DOI] [PubMed] [Google Scholar]
- 74.Majhi P, Bagga R, Kalra J, Sharma M. Intravaginal use of natural micronised progesterone to prevent pre-term birth: a randomised trial in India. J Obstet Gynaecol 2009;29:486–91. [DOI] [PubMed] [Google Scholar]
- 75.Akbari S, Birjandi M, Mohtasham N. Evaluation of the effect of progesterone on prevention of preterm delivery and its complications [in Persian]. Sci J Kurdistan Univ Med Sci 2009;14:11–9. [Google Scholar]
- 76.Cetingoz E, Cam C, Sakallı M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet 2011;283:423–9. [DOI] [PubMed] [Google Scholar]
- 77.Modi R, Rathore AM, Arora R. Randomized trial of natural progesterone in prevention of preterm birth in high risk women. J Pediatr Obstet Gynecol 2014. (Jul/Aug);101–107. [Google Scholar]
- 78.Azargoon A, Ghorbani R, Aslebahar F. Vaginal progesterone on the prevention of preterm birth and neonatal complications in high risk women: a randomized placebo-controlled double-blind study. Int J Reprod Biomed (Yazd) 2016;14:309–16. [PMC free article] [PubMed] [Google Scholar]
- 79.Norman JE, Marlow N, Messow CM, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet 2016;387:2106–16. Erratum in: Lancet 2019;393:228. Erratum in: Lancet 2019;393:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crowther CA, Ashwood P, McPhee AJ, et al. Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS study): a multicentre, randomised, placebo-controlled trial. PLoS Med 2017;14:e1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdou AM. Role of vaginal progesterone in prevention of preterm labor in women with previous history of one or more previous preterm births. Open J Obstet Gynecol 2018;8:329–37. [Google Scholar]
- 82.O’Brien JM, Steichen JJ, Phillips JA, Creasy GW. Two year infant outcomes for children exposed to supplemental intravaginal progesterone gel in utero: secondary analysis of a multicenter, randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol 2012;206:S223. [Google Scholar]
- 83.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. Erratum in: Ann Intern Med 2008;149:219. [DOI] [PubMed] [Google Scholar]
- 84.Nüesch E, Trelle S, Reichenbach S, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care 2013;17:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dechartres A, Trinquart L, Faber T, Ravaud P. Empirical evaluation of which trial characteristics are associated with treatment effect estimates. J Clin Epidemiol 2016;77:24–37. [DOI] [PubMed] [Google Scholar]
- 88.Unverzagt S, Prondzinsky R, Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol 2013;66:1271–80. [DOI] [PubMed] [Google Scholar]
- 89.Papageorgiou SN, Antonoglou GN, Tsiranidou E, Jepsen S, Jäger A. Bias and small-study effects influence treatment effect estimates: a meta-epidemiological study in oral medicine. J Clin Epidemiol 2014;67:984–92. [DOI] [PubMed] [Google Scholar]
- 90.Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med 2011;155:39–51. [DOI] [PubMed] [Google Scholar]
- 91.Bafeta A, Dechartres A, Trinquart L, Yavchitz A, Boutron I, Ravaud P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ 2012;344:e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dechartres A, Ravaud P, Atal I, Riveros C, Boutron I. Association between trial registration and treatment effect estimates: a meta-epidemiological study. BMC Med 2016;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panagiotou OA, Contopoulos-Ioannidis DG, Ioannidis JP. Comparative effect sizes in randomised trials from less developed and more developed countries: meta-epidemiological assessment. BMJ 2013;346:f707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abraha I, Cherubini A, Cozzolino F, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. BMJ 2015;350:h2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29. [DOI] [PubMed] [Google Scholar]
- 96.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–12. [DOI] [PubMed] [Google Scholar]
- 97.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–13. [DOI] [PubMed] [Google Scholar]
- 98.Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 2007;36:847–57. [DOI] [PubMed] [Google Scholar]
- 99.Nüesch E, Reichenbach S, Trelle S, et al. The importance of allocation concealment and patient blinding in osteoarthritis trials: a meta-epidemiologic study. Arthritis Rheum 2009;61:1633–41. [DOI] [PubMed] [Google Scholar]
- 100.Savović J, Jones H, Altman D, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess 2012;16:1–82. [DOI] [PubMed] [Google Scholar]
- 101.Page MJ, Higgins JP, Clayton G, Sterne JA, Hróbjartsson A, Savović J. Empirical evidence of study design biases in randomized trials: systematic review of meta-epidemiological studies. PLoS One 2016;11:e0159267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Savovic J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, Sterne JAC. Association between risk-of-bias assessments and results of randomized trials in Cochrane reviews: The ROBES meta-Epidemiologic study. Am J Epidemiol 2018;187:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amer MA, Herbison GP, Grainger SH, Khoo CH, Smith MD, McCall JL. A meta-epidemiological study of bias in randomized clinical trials of open and laparoscopic surgery. Br J Surg 2021;108:477–483. [DOI] [PubMed] [Google Scholar]
- 104.CDER perspective on recently published results of EPPPIC meta-analysis. 2021. Available at https://www.fda.gov/drugs/drug-safety-and-availability/cder-perspective-recently-published-results-epppic-meta-analysis. Accessed February 25, 2022.
- 105.Aboulghar MM, Aboulghar MA, Amin YM, Al-Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reprod Biomed Online 2012;25:133–8. [DOI] [PubMed] [Google Scholar]
- 106.Care A, Nevitt SJ, Medley N, et al. Interventions to prevent spontaneous preterm birth in women with singleton pregnancy who are at high risk: systematic review and network meta-analysis. BMJ 2022;376:e064547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. Cochrane. [Google Scholar]
- 108.Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol 2018;218:161–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol 2020;37:127–36. [DOI] [PubMed] [Google Scholar]
- 110.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–62. [DOI] [PubMed] [Google Scholar]
- 111.Moreno SG, Sutton AJ, Ades AE, et al. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol 2009;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Makena (hydroxyprogesterone caproate injection). New Drug Application 021945/Supplement 023. 2019. Available at https://www.fda.gov/media/132431/download. Accessed February 25, 2022.
- 113.Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Electronic address: pubs@smfm.org. SMFM Statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol 2020;223:B16–B18. [DOI] [PubMed] [Google Scholar]
- 114.Nelson DB, McIntire DD, McDonald J, Gard J, Turrichi P, Leveno KJ. 17-alpha Hydroxyprogesterone caproate did not reduce the rate of recurrent preterm birth in a prospective cohort study. Am J Obstet Gynecol 2017;216:600.e1–600.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Massa K, Childress K, Vricella LK, et al. Pregnancy duration with use of 17-α-hydroxyprogesterone caproate in a retrospective cohort at high risk of recurrent preterm birth. Am J Obstet Gynecol MFM 2020;2:100219. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Garcia SM, Kellom KS, Boelig RC, Matone M. Eligibility, utilization, and effectiveness of 17-alpha hydroxyprogesterone caproate (17OHPC) in a statewide population-based cohort of medicaid enrollees. Am J Perinatol 2021. Nov 16. doi: 10.1055/s-0041-1739504. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 117.Hakim JB, Zhou A, Hernandez-Diaz S, Hart JM, Wylie BJ, Beam AL. Effectiveness of 17-OHP for prevention of recurrent preterm birth: a retrospective cohort study. Am J Perinatol 2021. Dec 31. doi: 10.1055/s-0041-1740512. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 118.Nelson DB, McIntire DD, Leveno KJ. A chronicle of the 17-alpha hydroxyprogesterone caproate story to prevent recurrent preterm birth. Am J Obstet Gynecol 2021;224:175–86. [DOI] [PubMed] [Google Scholar]
- 119.Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol 2018;219:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanchez-Ramos L Vaginal progesterone is an alternative to cervical cerclage in women with a short cervix and a history of preterm birth. Am J Obstet Gynecol 2018;219:5–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


