Abstract
Objectives:
Several studies have demonstrated advantages of the retroperitoneal approach (RP) over the transperitoneal approach (TP) for infrarenal abdominal aortic aneurysm (AAA) repair. We performed a retrospective analysis comparing the outcomes of TP versus RP surgical approach for open complex AAA (cAAA) repair and evaluated their relative use over time.
Methods:
Patients undergoing open repair for intact cAAA (juxtarenal, suprarenal, or type-IV thoracoabdominal aortic aneurysms) between 2011–2019 were identified in the National Surgical Quality Improvement Program (NSQIP). The primary outcome was perioperative death. Secondary outcomes included perioperative complications and approach usage over time. We performed multivariable adjustment by creating propensity scores and using inverse probability-weighted logistic regression.
Results:
Among 1,195 patients identified, 729 (61%) underwent cAAA repair via a TP approach and 466 (39%) underwent repair via an RP approach. Compared with a TP approach, RP patients more frequently had a supracoeliac clamp position (32% vs. 20%, p<.001) and concomitant renal revascularization (30% vs. 18%, p<.001). After adjustment, an RP approach was associated with lower odds of perioperative mortality (4.0% vs. 7.2%; OR:0.54; 95%CI:0.32–0.91; p=.022). Furthermore, an RP approach was associated with lower odds of any major complication (24% vs. 30%; OR:0.73; 95%CI:0.56–0.94), cardiac complications (4.9% vs. 8.2%; OR:0.60; 95%CI:0.37–0.96), wound complications (2.1% vs. 6.0%; OR:0.34; 95%CI:0.17–0.64), and postoperative sepsis (0.8% vs. 2.4%; OR:0.37; 95%CI:0.12–0.99). The proportion of repairs using an RP approach decreased from 2011–2015 to 2016–2019 (42% vs. 35%, p=.020), particularly for supra-renal and type-IV thoracoabdominal aneurysms (49% vs 37%, p=.023).
Conclusion:
In open cAAA repair, the RP approach may be associated with lower perioperative mortality and morbidity compared with the TP approach. However, we found that the relative usage of the RP approach is decreasing over time, even in suprarenal/type-IV TAAA’s, and repairs utilizing a supracoeliac clamping site. Increased utility of the RP approach, when appropriate, may lead to improved outcomes following open cAAA repair.
Keywords: Open Aneurysm Repair, Complex Abdominal Aortic Aneurysms, Surgical Approach, Transperitoneal, Retroperitoneal
INTRODUCTION
There have been inconsistent results in the surgical literature regarding the optimal operative approach to open abdominal aortic aneurysm (AAA) repair1,2. Exposures to open surgical repair include a transperitoneal (TP), or retroperitoneal (RP) approach. Several comparative studies have demonstrated advantages of the RP approach over the TP approach for infrarenal AAA repair, including lower rates of complications and long-term reinterventions3–5. However, the RP approach is used less frequently for infrarenal AAA and more frequently for open repair of complex AAA (cAAA), which involve the renal and visceral segment6.
With the rise of endovascular aneurysm repair (EVAR), a growing proportion of patients who undergo open AAA repairs have aneurysms with short necks or more extensive disease7, raising the question of whether the RP approach may provide clinical benefit for this population. The TP approach offers improved access to the right renal and right iliac arteries and assessment of intra-abdominal disease, and it may be more familiar to surgeons trained in midline (or transverse) laparotomy for general surgery. However, the RP approach may facilitate easier exposure of the suprarenal aorta, and may be preferred in the presence of a hostile abdomen, or repair of an inflammatory aneurysm, or an aneurysm associated with a horseshoe kidney8.
Given the relative advantages of TP and RP approaches and changing clinical profile of the aneurysms being repaired, there may be a benefit to utilizing an RP approach for open cAAA repair. However, little is currently known regarding the impact of these approaches on outcomes for cAAA, which include juxtarenal aneurysms, supra-renal aneurysms, and type-IV thoracoabdominal aneurysms. Therefore, we examined peri-operative outcomes in patients undergoing open cAAA repair via a TP versus an RP approach and evaluated trends in approach usage over time.
METHODS
Data Source
We performed a retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) targeted vascular module. The NSQIP targeted vascular module is a nation-wide multi-institutional collaboration with prospectively collected clinical data of patients undergoing vascular interventions by a majority of vascular surgeons (99%). These data are collected by trained and certified surgical clinical reviewers and include demographics, comorbid conditions, intraoperative variables, as well as 30-day outcomes. Patients who were discharged before 30 days were actively followed by clinical nurses and data abstractors to accomplish complete 30-day outcomes. If patients developed morbidity or mortality during index hospitalization, these were still accounted for, even if hospitalization was >30 days. The NSQIP database has previously been validated and the data are routinely audited for accuracy and reliability.9,10 Further information is available at www.facs.org/qualityprograms/acs-nsqip. These data were analysed retrospectively and reported following the STROBE guidelines.11 The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the requirement for patient consent owing to the retrospective and deidentified nature of the NSQIP database.
Patient Cohort
We included all patients who underwent open cAAA repair between 2011 and 2019 within the vascular targeted NSQIP database (N=2,312). We defined complex aneurysms as juxtarenal, pararenal or supra-renal, and type-IV thoracoabdominal aneurysms (TAAA). We excluded patients undergoing ruptured open cAAA repair (N=477). Furthermore, we excluded all patients undergoing repair for indication other than aneurysm diameter or symptomatic presentation, including dissection (N=28), embolization secondary to an aneurysm (N=7), prior unsatisfactory intervention (N=76), thrombosis (N=24), and undocumented cause for repair (N=40) (Figure 1). Furthermore, patients undergoing open repair with an infrarenal or unknown proximal clamp position (N=456), and patients with missing data on surgical approach (N=9), were also excluded.
Figure 1.
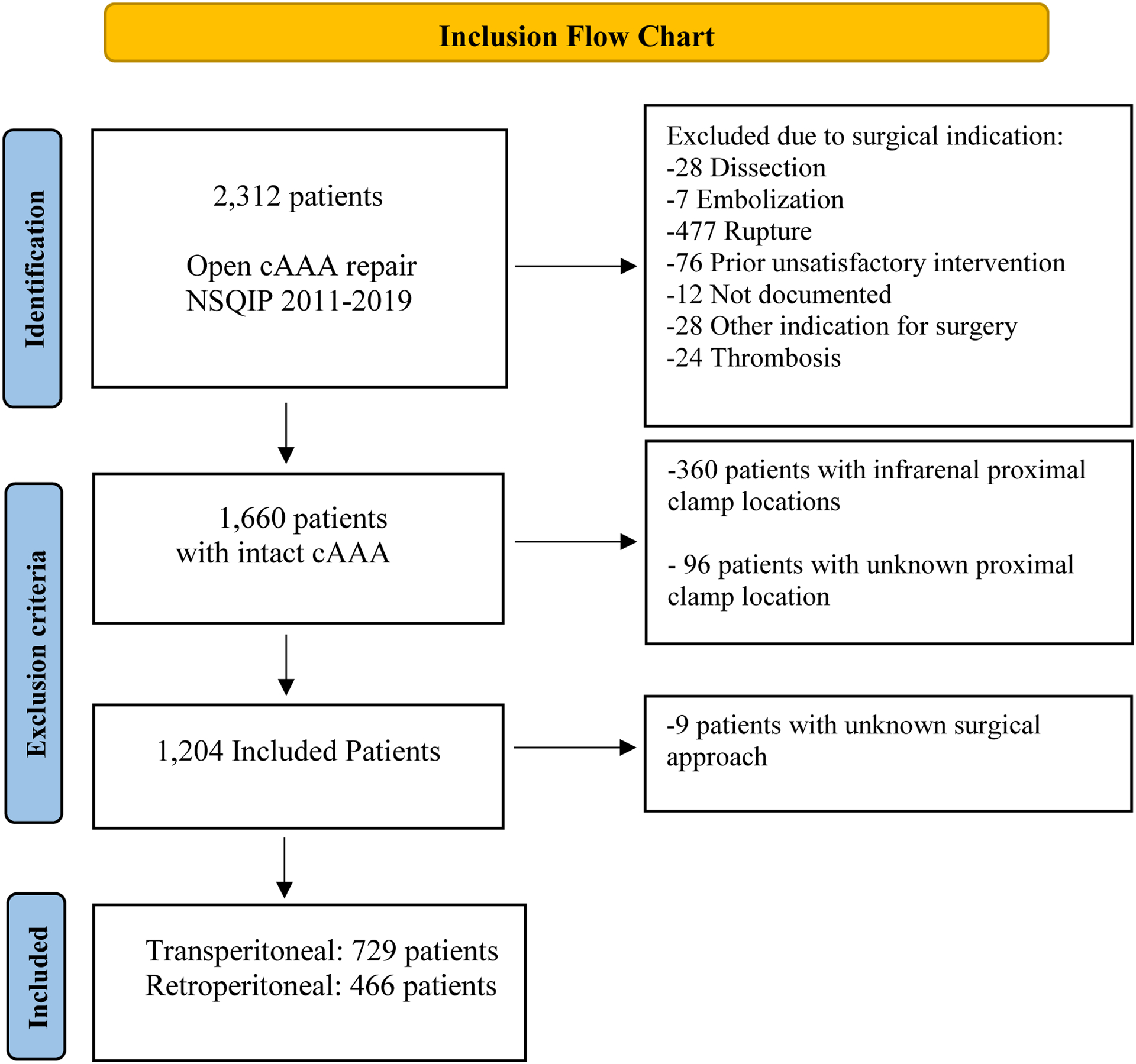
Inclusion Flow Chart
Definitions and Variables
Baseline characteristics included patients’ demographics, comorbidities, and anatomic/procedural characteristics. We calculated the estimated glomerular filtration rate (eGFR) in accordance with the Chronic Kidney Disease Epidemiology Collaboration equation using a single preoperative creatinine value. We categorized preoperative renal function into an eGFR greater than 60 mL/min/1.73m2, between 30–60 mL/min/1.73m2, and less than 30 mL/min/1.73m2 or preoperative dialysis (regardless of eGFR). Prior chronic kidney disease (CKD) was defined as an eGFR <60 mL/min/1.73m2 or preoperative dialysis. We calculated body mass index (BMI) according to the standard weight/height2 (kg/m2), and obesity was defined as a BMI >30 kg/m2. Systemic inflammatory response syndrome (SIRS) was considered if the patient had at least two of the five clinical signs and symptoms of SIRS12. Other comorbidities were defined as given within NSQIP and have been described previously13. A large preoperative AAA diameter was defined as a diameter >6.5cm14, while repair below threshold was defined as repair of an aneurysm with a preoperative diameter below the threshold of current guidelines (male: <5.5cm, female: <5.0cm)15. With regard to proximal aneurysm extent, both pararenal and supra-renal aneurysms were referred to as supra-renal as has been reported previously16.
Our primary outcome was perioperative mortality which was defined as death within 30 days of repair, or during index hospitalization. Secondary outcomes included a composite outcome of any major complication, which included cardiac complications, respiratory complications, renal complications, stroke, ischemic colitis, lower extremity ischemia, sepsis, aneurysm rupture within 30 days, and reoperation during index hospitalization. Cardiac complications included development of cardiac arrest or myocardial infarction. Respiratory complications included prolonged ventilator requirement (>48h), unplanned reintubation, postoperative pneumonia, or development of pulmonary embolism. Renal complications consisted of acute renal insufficiency (a creatinine rise of >2mg/dL from preoperative value, without requirement of dialysis) or acute renal failure requiring new dialysis. Ischemic colitis was defined as postoperative development of symptoms of ischemic colitis (abdominal pain, diarrhea, fever, leukocytosis, or elevated serum lactate), or a sigmoidoscopy or colonoscopy documenting ischemia of the colon wall. In the case of ischemic colitis, treatment type was defined as either medical or surgical. Lower extremity ischemia was defined as the necessity of a reintervention for treatment. Any wound complication was defined as a composite variable inclusive of any surgical site infection (SSI), superficial SSI, deep SSI, organ/space SSI, or dehiscence. Other outcomes such as sepsis were defined as previously described13.
In order to report outcomes over time, patients were stratified into an early and late cohort. Patients within the early cohort underwent cAAA repair between 2011 and 2015, whereas patients in the late cohort underwent repair between 2016 and 2019, and the threshold was created to ensure an equal distribution of patients among groups.
Statistical Analysis
Categorical variables were presented as counts and as percentages. Continuous variables were presented either as mean or median together with their standard deviation or interquartile range respectively, depending on whether results were normally distributed according to visual aid and the Shapiro-Wilk test. For unadjusted comparisons, Fisher Exact or Pearson’s χ2 tests were used for categorical variables, and independent student T-tests or Mann Whitney U-tests were used for parametric and non-parametric continuous variables, respectively.
Due to low-event rates for the primary outcome and to mitigate confounding by indication, we created propensity scores and calculated inverse probability of treatment weights (IPTW). To avoid extreme weights and subsequently variance inflation, a major limitation of IPTW methods, we removed the lowest 1% and highest 1% of weights.17 We built logistic regression models where surgical approach was the outcome of interest. Covariates were selected a priori and generously introduced into the model, including age, sex, race, smoking status, obesity, hypertension (requiring medication), congestive heart failure, chronic obstructive pulmonary disease, CKD, dialysis, insulin-dependent diabetes mellitus, SIRS, prior abdominal aortic surgery, surgery indication (diameter/non-ruptured symptomatic), proximal aneurysm extent, distal aneurysm extent, proximal clamp zone, renal revascularization, year of procedure, and large preoperative aneurysm diameter (>6.5cm). After weighting the standard mean differences for all adjusted factors were <0.10, indicating adequate balance. The proportions of RP versus TP repair for juxtarenal aneurysms and suprarenal/type-IV TAAA’s were plotted over time. Univariable linear regression analysis was performed to test for statistical significance in change over time and multivariable linear regression analysis was performed to adjust for potential concurrent changes in supracoeliac clamp utility and concomitant renal revascularization over time.
All values except for race (23%), prior abdominal aortic surgery (5.9%), and distal aneurysm extent (9.3%) had <2% missing values. In order to maintain statistical power, missing values were included as missing indicator variables. To assess validity, results were compared with complete data alone, for which the effect of each variable did not change. All tests were two sided, and statistical significance was defined as P<0.05.
RESULTS
Patient Characteristics
We identified 1,195 patients of whom 466 (39%) underwent repair via an RP approach and 729 (61%) via a TP approach. Patients undergoing repair through an RP approach were more often White (RP: 82% TP: 65%; P<.001). The remaining baseline demographics and comorbidities were comparable between surgical approaches. (Table 1)
Table 1.
Baseline Demographics and Comorbidities of all included Patients undergoing Open cAAA Repair
| Retroperitoneal (N=466) | Transperitoneal (N=729) | ‘p-value | |
|---|---|---|---|
| Age, mean | 71 [IQR 66, 70] | 72 [IQR 60, 77] | .63 |
| Female | 155 (33%) | 213 (29%) | .16 |
| Race | <.001 | ||
| Non-Hispanic White | 383 (82%) | 475 (65%) | |
| Black | 10 (2.1%) | 23 (3.2%) | |
| Hispanic | 7 (1.5%) | 9 (1.2%) | |
| Other | 7 (1.5%) | 11 (1.5%) | |
| Unknown | 59 (13%) | 211 (29%) | |
| Obese | 134 (29%) | 186 (26%) | .15 |
| Current Smoker | 225 (48%) | 349 (48%) | .94 |
| Diabetes | 57 (12%) | 77 (11%) | .42 |
| Hypertension | 369 (79%) | 601 (82%) | .18 |
| Prior Congestive Heart Failure | 6 (1.3%) | 15 (2.1%) | .37 |
| Prior COPD | 108 (23%) | 143 (20%) | .16 |
| Preoperative Renal Function | .16 | ||
| eGFR>60 | 294 (63%) | 460 (63%) | |
| eGFR 30–60 | 159 (34%) | 233 (32%) | |
| eGFR<30 | 13 (2.8%) | 36 (4.9%) | |
| Dialysis | 4 (0.9%) | 4 (0.5%) | .72 |
| Steroid use | 10 (2.1%) | 20 (2.7%) | .57 |
| Malignant weight loss | 8 (1.7%) | 9 (1.2%) | .62 |
| Bleeding Disorder | 39 (8.4%) | 52 (7.1%) | .50 |
| SIRS | 11 (2.4%) | 17 (2.3%) | 1 |
| Prior Abdominal Aortic Surgery | 184 (26%) | 134 (32%) | .068 |
BMI = body mass index; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; SIRS = systemic inflammatory response syndrome
Between surgical approaches, there were no differences in symptomatic presentation, aortic diameter >6.5cm, or repair of aneurysms below the threshold guideline. (Table 2) Patients undergoing an RP approach more often had an aortic distal aneurysm extent as opposed to the iliac arteries (57% vs. 50%, p=.047). However, RP approach trended towards higher rates of proximal aneurysm extent (supra-renal/type-IV TAAA: 38% vs. 32%, p=.058). Furthermore, RP patients had a higher rate of supracoeliac clamp placement (32% vs. 20%, p<.001), and a higher rate of renal revascularization (30% vs. 18%, p<.001). There were no differences in the rate of visceral revascularization (6.4% vs. 4.7%, p=.23), lower extremity revascularization (7.7% vs. 7.8%, p>.99), or inferior mesenteric artery reimplantation (3.2% vs. 5.2%, p=.14). Patients undergoing open cAAA repair through an RP approach had longer operative times (247 min [IQR 192–317] vs. 235 min [IQR 178–300], p=.006), however this difference was mitigated for patients undergoing repair without a concomitant procedure (226 [186–284] vs. 227 [174–287], p=.45).
Table 2.
Procedural Characteristics following Retroperitoneal and Transperitoneal approach in open cAAA repair
| Retroperitoneal (N=466) | Transperitoneal (N=729) | ||
|---|---|---|---|
| p-value | |||
| Surgical Indication | .49 | ||
| Diameter | 416 (89%) | 640 (88%) | |
| Non-ruptured symptomatic | 50 (11%) | 89 (12%) | |
| Large AAA-diameter (>6.5cm) | 134 (30%) | 184 (25%) | .96 |
| Repair below threshold guidelines | 63 (14%) | 103 (14%) | .83 |
| Proximal Aneurysm Extent | .058 | ||
| Juxtarenal | 288 (62%) | 499 (68%) | |
| Supra-renal | 144 (31%) | 183 (25%) | |
| Type-IV Thoracoabdominal aneurysm | 34 (7.3%) | 47 (6.4%) | |
| Proximal Clamp Site | <.001 | ||
| Above one renal | 134 (29%) | 329 (45%) | |
| Above two renals | 181 (39%) | 257 (35%) | |
| Supracoeliac | 151 (32%) | 143 (20%) | |
| Distal Aneurysm Extent | .047 | ||
| Aortic | 267 (57%) | 366 (50%) | |
| Common iliac | 137 (29%) | 264 (36%) | |
| Internal/External iliac | 16 (3.4%) | 34 (4.7%) | |
| Not documented | 46 (9.9%) | 65 (8.9%) | |
| Concomitant Procedure | 164 (35%) | 220 (30%) | .081 |
| Renal revascularization | 140 (30%) | 129 (18%) | <.001 |
| Visceral revascularization | 30 (6.4%) | 34 (4.7%) | .23 |
| Lower extremity revascularization | 36 (7.7%) | 57 (7.8%) | >.99 |
| Inferior Mesenteric Artery Reimplantation | 15 (3.2%) | 38 (5.2%) | .14 |
| Operative time (minutes) | 247 [IQR 192, 317] | 235 [IQR 178, 300] | .006 |
| Operative time with no concomitant procedures (minutes) | 226 [IQR 186, 284] | 227 [IQR 174, 287] | .45 |
| Repair by Vascular Surgeon | 461 (99%) | 721 (99%) | .97 |
Perioperative Outcomes
After adjustment, the RP approach was associated with lower perioperative mortality compared with the TP approach (4.0% vs. 7.2%/OR: 0.54; 95%CI: 0.32–0.91; p=.022; Table 3). The RP approach was also associated with lower risk of any major complication (24% vs. 30%//OR: 0.73; 95%CI: 0.56–0.94; p=.017), specifically cardiac complications (4.9% vs. 8.2%/OR: 0.60; 95%CI: 0.37–0.96; p=.037). Furthermore, the RP approach was associated with lower rates of wound complications (2.1% vs. 6.0%/OR: 0.34; 95%CI: 0.17–0.64; p=.001), particularly with lower rates of wound dehiscence (0.5% vs. 2.2%/OR: 0.22; 95%CI: 0.04–0.71; p=.022). Furthermore, compared with TP, the RP approach was associated with a trend towards lower rate of sepsis (0.8% vs. 2.4%/OR: 0.37; 95%CI: 0.12–1.02; p=.059) and respiratory complications (10% vs. 14%/OR: 0.78; 95%CI: 0.50–1.04; p=.079). There were no differences between groups with regards to renal complications, ischemic colitis, and lower extremity ischemia. (Table 3) Moreover, there was no difference in reoperation rate between groups (11% vs. 14%/OR: 0.77; 95%CI: 0.53–1.08; p=.13). Finally, there were no differences in length of stay between both approaches (Table 3).
Table 3.
Inverse Probability Weight Adjusted Outcomes following open cAAA repair by an RP versus TP approach.
| Odds Ratios (95% Confidence Intervals); Reference TP approach | |||||
|---|---|---|---|---|---|
| Adjusted Rates | Retroperitoneal vs. Transperitoneal | ||||
| Retroperitoneal | Transperitoneal | OR | P-value | 95% CI | |
| Mortality, perioperative | 4.0% | 7.2% | 0.54 | .020 | 0.32–0.90 |
| Major complication | 24% | 30% | 0.73 | .014 | 0.54–0.93 |
| Reoperation | 11% | 14% | 0.77 | .13 | 0.53–1.08 |
| Cardiac complication | 4.9% | 8.2% | 0.60 | .037 | 0.37–0.96 |
| Myocardial Infarction | 4.0% | 5.2% | 0.76 | .33 | 0.43–1.32 |
| Major Respiratory Complication | 10% | 14% | 0.72 | .079 | 0.50–1.04 |
| Reintubation | 6.0% | 8.0% | 0.73 | .18 | 0.46–1.15 |
| Pneumonia | 8.1% | 5.8% | 1.44 | .12 | 0.91–2.28 |
| Major Renal Complication | 9.7% | 9.7% | 1.0 | >.99 | 0.68–1.49 |
| Stroke | 0.4% | 0.8% | 0.58 | .47 | 0.12–2.50 |
| Ischemic Colitis | 4.8% | 5.5% | 0.86 | .57 | 0.51–1.45 |
| Lower Extremity Ischemia | 2.5% | 3.2% | 0.75 | .43 | 0.37–1.53 |
| Sepsis | 0.8% | 2.4% | 0.37 | .059 | 0.12–1.02 |
| Any Wound Complication | 2.1% | 6.0% | 0.34 | .001 | 0.17–0.64 |
| Wound dehiscence | 0.5% | 2.2% | 0.22 | .022 | 0.04–0.71 |
| Superficial SSI | 0.8% | 1.3% | 0.69 | .51 | 0.21–2.11 |
| Deep SSI | 0.1% | 0.9% | 0.13 | .095 | 0.01–1.11 |
| Organ/space SSI | 0.5% | 1.6% | 0.35 | .095 | 0.08–1.11 |
| Unadjusted Rates | |||||
| ICU length of stay >5 days | 25% | 21% | .11 | ||
| Length of stay >10 days | 31% | 31% | .88 | ||
Adjusted outcomes were accounted for: Age, Sex, Race (ref White/Black/Other/Unknown), Obesity, Hypertension, COPD, Congestive Heart Failure, CKD, Dialysis, Diabetes, SIRS, Prior Abdominal Aortic Surgery (Yes/No/Unknown), Surgery Indication (Diameter/Non-ruptured Symptomatic), Large AAA-diameter, Proximal extent (Juxtarenal/Supra-renal/Type-IV TAAA) + Distal extent (Aortic/Iliac/Common Femoral/Unknown), Proximal Clamp Location (Above one renal/Above two renals/Supracoeliac), Renal Revascularization (Yes/No), and year of operation
COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; SIRS = systemic inflammatory response syndrome; SSI = surgical site infection
Use of the Retroperitoneal Approach over Time
For the overall cAAA cohort, we found lower RP usage in the late cohort compared with the early cohort (late: 35% vs. early: 42%, p=.020; Figure 2). There was no difference in use of a supracoeliac clamp (late: 23% vs. early: 26%, p=.44), but there was a lower proportion of concomitant renal revascularization in the late cohort (late: 20% vs. early: 25%, p=.037). In the late cohort, RP approach use was lower for suprarenal and type IV TAAA (late: 37% vs. early: 49%, p=.023), but not for juxtarenal aneurysms (late: 35% vs. early: 38%, p=.32). Among open cAAA repairs utilizing a supracoeliac clamp site, the usage of RP approach decreased over time (late: 44% vs. early: 57%, p=.029). Among patients undergoing concomitant renal revascularization, there was no significant difference in the use of RP approach over time (late: 47% vs. early: 56%, p=.19). There was no significant difference in perioperative mortality following open cAAA repair between the late and early cohort (late vs. early: 5.3% vs. 7.3%, p=.21).
Figure 2.
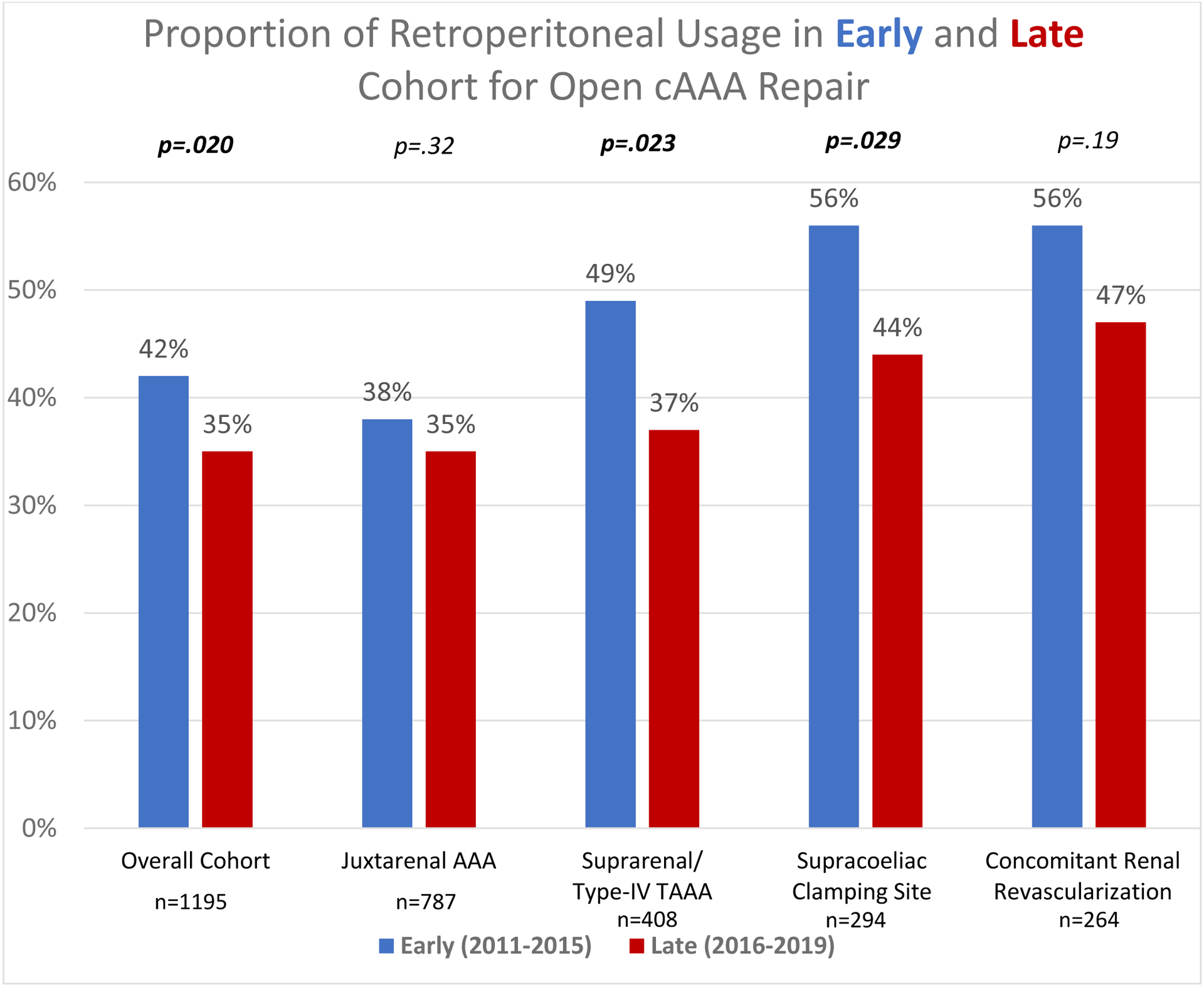
Relative proportion of Retroperitoneal Usage for open repair of complex abdominal aortic aneurysms in the early (before 2015) and late cohort (2015 and after)
Over the study period, there was a trend toward decreased RP usage within the overall cohort (−1.1%/year [95%CI −2.3% − 0.0%], trend-p=.052). There was a decrease in RP usage for suprarenal and type-IV TAAA (−2.4%/year [95%CI −4.5% − 0.0%], trend-p=.031 (Figure 3). RP usage did not change over time for juxtarenal aneurysms (−0.5%/year [95%CI −1.9% − 0.8%], trend-p=.43). Over time there was no difference in supracoeliac clamp usage within the overall cohort (−0.2%/year [95%CI −1.3% − 0.7%], trend-p=.58), but there was a decreasing trend in concomitant renal revascularization (−0.9%/year [95%CI −1.3% − 0.0%], trend-p=.067). Within the subgroup of patients that underwent repair with a supracoeliac cross clamp, there was a decrease in RP usage over time (−2.8%/year [95%CI −5.4% − −0.2%], trend-p=.035). However, within patients undergoing renal revascularization, there was no change in RP usage over time (−1.2%/year [95%CI −3.7% − 1.4%], trend-p=.37).
Figure 3.
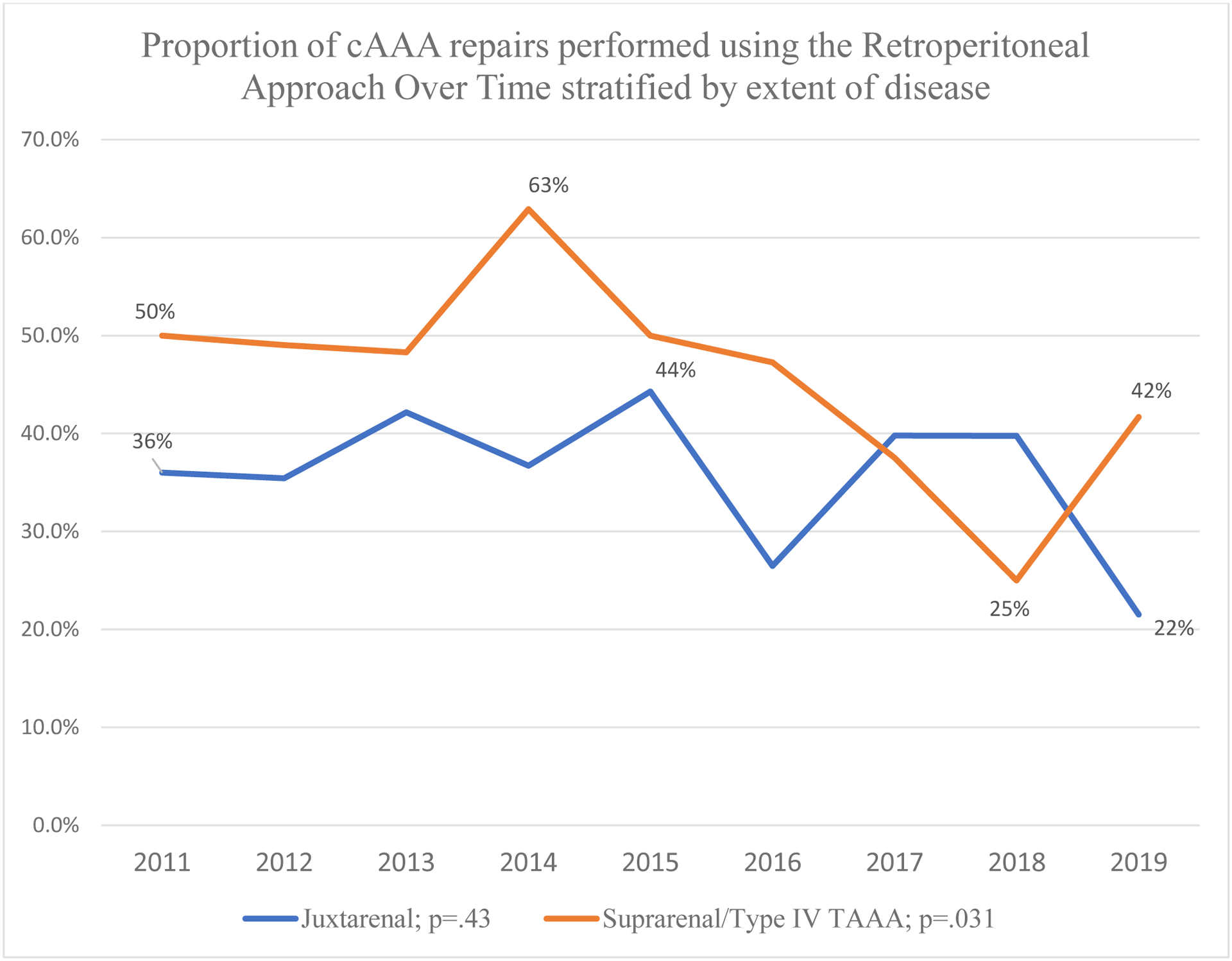
Proportion of cAAA repairs performed using the Retroperitoneal Approach Over Time stratified by extent of disease
DISCUSSION
Our study demonstrates that for open cAAA repair, use of the RP approach was associated with lower risk of perioperative mortality compared with the TP approach. Furthermore, the RP approach was associated with lower risk of any major complication, specifically cardiac complications, and wound complications. However, use of the RP approach decreased over time in patients with supra-renal and type-IV TAAA, and in repairs utilizing a supracoeliac cross clamp.
Our finding of lower risk-adjusted odds of perioperative mortality in patients undergoing an RP versus TP approach for cAAA corroborate findings from a prior study comparing both approaches in open cAAA utilizing the Vascular Quality Initiative.18 Nevertheless, these findings are in contrast with prior studies in infrarenal AAA4–6, which found no perioperative mortality difference between the two approaches. This discrepancy may reflect the relative advantages of each surgical approach with respect to their exposure and thus ease of arterial clamping needed to repair the extent of aneurysmal disease. Notably, patients undergoing an RP approach had significantly higher proximal clamp locations and higher distal aneurysm extents compared with patients undergoing a TP approach; such differences may be expected, as an RP approach may be more suited for aneurysms with more proximal disease, while outcomes are comparable when the aneurysm extent is infrarenal. These differences are in line with previous reports comparing both approaches, which postulate the RP approach to be more commonly used for more proximal aneurysms due to better exposure of the suprarenal abdominal aorta6. However, further hypotheses regarding the cause of the perioperative mortality difference are limited, as NSQIP does not provide specific cause of death.
Besides a difference in perioperative mortality, we also found that the RP approach was associated with lower rates of major complications, including cardiac complications, postoperative sepsis, and wound complications. Within infrarenal AAA, Teixeira et al. found an RP approach to be associated with a lower risk-adjusted incidence of cardiac events compared with the TP approach within the Vascular Quality Initiative (VQI)5. In 1987, Hudson et al. found fewer deleterious hemodynamic changes among patients undergoing the RP approach, citing elevated blood levels of prostacyclin secondary to manipulation of the abdominal contents as a possible cause of hemodynamic instability with the TP approach19. Other studies examining cardiac morbidity following open aortic surgery have found associations with advanced age at surgery, abnormal preoperative serum creatinine level, and valvular heart disease20,21. Sicard et al. attributed differences in complications between the two approaches to entering the abdominal cavity as an unmeasurable physiologic stress2. Though there was no difference in rates of ischemic colitis between groups, the RP approach may reduce the risk of bowel manipulation (consistent with lower incident of paralytic ileus with the RP approach for infrarenal AAA4). The lower adjusted rate of wound complications among patients with cAAA undergoing an RP approach has not been previously found in most previous infrarenal AAA studies. This may reflect NSQIP’s variable definitions, as Buck et al. found lower rates of wound dehiscence among infrarenal AAA within the RP group using the same database6, but were not found in studies utilizing other cohorts4.
In contrast with prior studies involving infrarenal AAA6, our study did not find a significant difference between RP and TP approaches in incidence of pulmonary complications such as pneumonia. Although few observational studies have displayed a decrease in pulmonary complications following an RP approach22,23, these findings were not reproduced in later randomized trials, and a meta-analysis found that the RP approach was associated with significantly lower rates of postoperative pneumonia, with significance lost on high quality study sensitivity analysis4. The authors propose less pain, improved ventilation, and subsequent reduction in atelectasis due to the crossing of fewer dermatomes during an RP incision as a possible explanation4, though it remains unclear whether the RP approach results in a lower incidence of pneumonia following surgery. However, just as with the outcomes for reoperation and postoperative sepsis, these data must be interpreted with caution, as our study might be underpowered to find significant differences in pulmonary complications between groups.
Previously it has been demonstrated that laparotomy related complications are an important cause for long-term reinterventions following open AAA repair, just as graft-related complications are in EVAR24,25. The RP approach has subsequently also demonstrated to be advantageous with regard to lower risk of incisional hernia, or any vascular or abdominal reintervention or readmission3,26,27.
Our findings demonstrated a decrease in the use of the RP approach over time for the overall cohort, as well as for patients with supra-renal/type-IV TAAA, and for repairs utilizing a supracoeliac clamp. We hypothesize that this is a result of the increased utilization of fenestrated and branched endovascular aortic repairs for supra-renal AAA, concurrently decreasing the number of open repairs for cAAA16,28. The decrease in high complexity open repairs, particularly those involving renal revascularization, might also be contributing to the decrease in RP usage. However, the fact that we see a decrease in RP usage within this population might be a concern, as it could potentially demonstrate a loss of RP expertise within the endovascular era. As vascular surgeons might prefer the TP approach due to its familiarity, the TP approach may therefore be performed more frequently for open complex AAA repairs, subsequently decreasing the relative utilization of the RP approach over time as well.
Our study needs to be interpreted within the context of its retrospective design. First, the retrospective non-randomized design carries significant potential for selection bias, as participation to the ACS-NSQIP is voluntary and as certain anatomic factors influence the surgeon’s choice of approach. Furthermore, a retrospective analysis without a prior power analysis is always at risk of type-II errors, though to the best of our knowledge, our study includes the largest sample size comparing both approaches in context of open cAAA repair. Also, our findings might not be nationally representative as the ACS-NSQIP only makes up of 7% to 8% of all AAA repairs in the National Inpatient Sample29. This prior mentioned proportion has been increasing over time and will probably even be higher for all hospitals performing open cAAA repair. However, as NSQIP likely consists of higher volume centers with a demonstrated interest in quality improvement, the use of the RP approach across the United States is likely to be substantially lower. Second, the ACS-NSQIP withholds any surgeon or center or physician specific identification for anonymization reasons, limiting any understanding of differences at this level. Third, the ACS-NSQIP does not provide any information on coronary artery status, proximal clamping time, renal perfusion/protection strategies, or the indication for repair as to why a certain surgical approach was selected. Furthermore, the variable ischemic colitis is limited as it does not provide an elaborate understanding of the postoperative intra-peritoneal status such as the occurrence of post-operative ileus or bowel ischemia. Also, NSQIP does not provide outcomes more than 30 days after repair and following discharge. As a result, we are unable to draw conclusions on the effects of open surgical approach on long-term complications (such as intra-abdominal or abdominal wall complications) or survival. Finally, the centers included in NSQIP may have changed over time, which could confound trend analyses of approach usage over time.
CONCLUSION
In open cAAA repair, the RP approach may be associated with lower perioperative mortality and morbidity compared with the TP approach. However, we found that the relative usage of the RP approach is decreasing over time, even in suprarenal/type-IV TAAA’s, and repairs utilizing a supracoeliac clamping site. Increased utility of the RP approach, when appropriate, may lead to improved outcomes following open cAAA repair.
Supplementary Material
WHAT THIS PAPER ADDS.
The retroperitoneal (RP) approach for open complex abdominal aortic aneurysm (cAAA) repair is associated with lower rates of perioperative mortality and morbidity compared with a transperitoneal (TP) approach. However, usage of the RP approach has decreased over time, including for repairs of suprarenal and type-IV thoracoabdominal aneurysms, and repairs utilizing a supracoeliac clamp site. Increased adoption of the RP approach, when appropriate, may lead to improved outcomes following open repair of cAAA.
ACKNOWLEDGEMENTS
This work was conducted with statistical support from Jue Hou, PhD | Department of Biostatistics Harvard T. H. Chan School of Public Health
CM is supported by grant number F32HS027285 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. PP is supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cambria RP, Brewster DC, Abbott WM, Freehan M, Megerman J, Lamuraglia G, et al. Transperitoneal versus retroperitoneal approach for aortic reconstruction: A randomized prospective study. J Vasc Surg 1990;11(2):314–25. Doi: 10.1016/0741-5214(90)90275-F. [DOI] [PubMed] [Google Scholar]
- 2.Sicard GA, Reilly JM, Rubin BG, Thompson RW, Allen BT, Flye MW, et al. Transabdominal versus retroperitoneal incision for abdominal aortic surgery: Report of a prospective randomized trial. J Vasc Surg 1995;21(2):174–83. Doi: 10.1016/S0741-5214(95)70260-1. [DOI] [PubMed] [Google Scholar]
- 3.Deery SE, Zettervall SL, O’Donnell TFX, Goodney PP, Weaver FA, Teixeira PG, et al. Transabdominal open abdominal aortic aneurysm repair is associated with higher rates of late reintervention and readmission compared with the retroperitoneal approach. J Vasc Surg 2020;71(1):39–45.e1. Doi: 10.1016/j.jvs.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twine CP, Humphreys AK, Williams IM. Systematic review and meta-analysis of the retroperitoneal versus the transperitoneal approach to the abdominal aorta. Eur J Vasc Endovasc Surg 2013;46(1):36–47. Doi: 10.1016/j.ejvs.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Teixeira PGR, Woo K, Abou-Zamzam AM, Zettervall SL, Schermerhorn ML, Weaver FA. The impact of exposure technique on perioperative complications in patients undergoing elective open abdominal aortic aneurysm repair. J Vasc Surg 2016;63(5):1141–6. Doi: 10.1016/j.jvs.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Buck DB, Ultee KHJ, Zettervall SL, Soden PA, Darling J, Wyers M, et al. Transperitoneal versus retroperitoneal approach for open abdominal aortic aneurysm repair in the targeted vascular National Surgical Quality Improvement Program. J Vasc Surg 2016;64(3):585–91. Doi: 10.1016/j.jvs.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albuquerque FC, Tonnessen BH, Noll RE, Cires G, Kim JK, Sternbergh WC. Paradigm shifts in the treatment of abdominal aortic aneurysm: Trends in 721 patients between 1996 and 2008. J Vasc Surg 2010;51(6):1348–53. Doi: 10.1016/j.jvs.2010.01.078. [DOI] [PubMed] [Google Scholar]
- 8.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67(1):2–77.e2. Doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Davis CL, Pierce JR, Henderson W, Spencer CD, Tyler C, Langberg R, et al. Assessment of the Reliability of Data Collected for the Department of Veterans Affairs National Surgical Quality Improvement Program 2007:550–60. Doi: 10.1016/j.jamcollsurg.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Shiloach M, Jr SKF, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, et al. Toward Robust Information : Data Quality and Inter-Rater Reliability in the American College of Surgeons National Surgical Quality Improvement Program. ACS 2010;210(1):6–16. Doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 11.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. 2020. [DOI] [PMC free article] [PubMed]
- 12.Kaukonen KM, Bailey M, Pilcher D, Cooper J, Bellomo R. Systemic Inflammatory Response Syndrome Criteria in Defining Severe Sepsis. N Engl J Med 2015;372(17):1629–38. Doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 13.American College of Surgeons - National Surgical Quality Improvement Program. User Guide for the 2019 ACS NSQIP Procedure Targeted Participant Use Data File (PUF) 2020.
- 14.de Guerre LEVM, Dansey K, Li C, Lu J, Patel PB, van Herwaarden JA, et al. Late outcomes after endovascular and open repair of large abdominal aortic aneurysms. J Vasc Surg 2021;74(4):1152–60. Doi: 10.1016/j.jvs.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019;57(1):8–93. Doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Ultee KHJ, Zettervall SL, Soden PA, Darling J, Verhagen HJM, Schermerhorn ML. Perioperative outcome of endovascular repair for complex abdominal aortic aneurysms. J Vasc Surg 2017;65(6):1567–75. Doi: 10.1016/j.jvs.2016.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: A primer for practitioners. BMJ 2019;367:1–10. Doi: 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi V, Marcaccio CL, Patel PB, Varkevisser RRB, Patel VI, Soden PA, et al. A retroperitoneal operative approach is associated with improved perioperative outcomes compared with a transperitoneal approach in open repair of complex abdominal aortic aneurysms. J Vasc Surg 2022:1–11. Doi: 10.1016/j.jvs.2022.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson JC, Heinrich Wurm W, O’Donnell TF, Shoenfeld NA, Mackey WC, Callow AD, et al. Hemodynamics and prostacyclin release in the early phases of aortic surgery: Comparison of transabdominal and retroperitoneal approaches. J Vasc Surg 1988;7(2):190–8. Doi: 10.1016/0741-5214(88)90136-X. [DOI] [PubMed] [Google Scholar]
- 20.Troisi N, Dorigo W, Lo Sapio P, Pratesi G, Pulli R, Gensini GF, et al. Preoperative Cardiac Assessment in Patients Undergoing Aortic Surgery: Analysis of Factors Affecting the Cardiac Outcomes. Ann Vasc Surg 2010;24(6):733–40. Doi: 10.1016/J.AVSG.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 21.West CA, Noel AA, Bower TC, Cherry KJ, Gloviczki P, Sullivan TM, et al. Factors affecting outcomes of open surgical repair of pararenal aortic aneurysms: A 10-year experience. J Vasc Surg 2006;43(5):921–9. Doi: 10.1016/j.jvs.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Peck JJ, McReynolds DG, Baker DH, Eastman AB. Extraperitoneal approach for aortoiliac reconstruction of the abdominal aorta. Am J Surg 1986;151(5):620–3. Doi: 10.1016/0002-9610(86)90571-4. [DOI] [PubMed] [Google Scholar]
- 23.Arko FR, Bohannon WT, Mark M, LS D, Patterson DE, Manning LG, et al. Retroperitoneal Approach for Aortic Surgery: Is it Worth it? Vascular 2002;10(2):185. Doi: 10.1177/096721090201000215. [DOI] [Google Scholar]
- 24.Schermerhorn ML, O’Malley JA, Jhaveri A, Philip C, Pomposelli F, Landon B. Endovascular Versus Open Repair of Abdominal Aortic Aneurysms in the Medicare Population. N Engl J Med 2008;52(5):222–3. Doi: 10.1097/01.sa.0000307957.69920.56. [DOI] [PubMed] [Google Scholar]
- 25.Bensley RP, Schermerhorn ML, Hurks R, Sachs T, Boyd CA, O’Malley AJ, et al. Risk of late-onset adhesions and incisional hernia repairs after surgery. J Am Coll Surg 2013;216(6):1–32. Doi: 10.1016/j.jamcollsurg.2013.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover K, Lyden S, Bena JF, Smolock C, Parodi F. Mid-Term Outcomes of Retroperitoneal and Transperitoneal Exposures in Open Aortic Aneurysm Repair. Ann Vasc Surg 2020;66:35–43.e1. Doi: 10.1016/j.avsg.2019.10.074. [DOI] [PubMed] [Google Scholar]
- 27.DeCarlo C, Manxhari C, Boitano LT, Mohebali J, Schwartz SI, Eagleton MJ, et al. Transabdominal approach associated with increased long-term laparotomy complications after open abdominal aortic aneurysm repair. J Vasc Surg 2021;73(5):1603–10. Doi: 10.1016/j.jvs.2020.08.154. [DOI] [PubMed] [Google Scholar]
- 28.Varkevisser RRB, O’Donnell TFX, Swerdlow NJ, Liang P, Li C, Ultee KHJ, et al. Fenestrated endovascular aneurysm repair is associated with lower perioperative morbidity and mortality compared with open repair for complex abdominal aortic aneurysms. J Vasc Surg 2019;69(6):1670–8. Doi: 10.1016/j.jvs.2018.08.192. [DOI] [PubMed] [Google Scholar]
- 29.Dansey K, de Guerre L, Li C, Lu JJ, Patel PB, Scali ST, et al. Not All Databases Are Created Equal, a Comparison of Administrative Data and Quality Improvement Registries for Abdominal Aortic Aneurysm Repair. J Vasc Surg 2020;72(1):e196–7. Doi: 10.1016/j.jvs.2020.04.338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


