Abstract
Obstructive sleep apnea (OSA) affects nearly 1 billion people worldwide, including approximately 35 million US residents. OSA has detrimental cardiovascular and neurocognitive consequences. Positive airway pressure corrects sleep disordered breathing but is not always tolerated or used sufficiently. Oral appliances and surgery provide alternatives in select populations but are variably effective.
Hypoglossal nerve stimulation can effectively treat obstructive sleep apnea. Targeted hypoglossal nerve stimulation (THN) is simpler than incumbent technology with no sensor and an easier, proximal electrode implantation. The third clinical study of THN, THN3, was the first randomized, controlled trial of hypoglossal nerve stimulation to demonstrate significant improvement of sleep disordered breathing in OSA. The present investigation reports the design of a novel trial of targeted stimulation to provide additional Level 1 evidence in moderate to severe obstructive apnea.
OSPREY is a randomized, parallel-arm, 13-month trial wherein all subjects are implanted, 2/3 are activated at Month 1 (“Treatment”) and 1/3 are activated at Month 7 (“Control”). The primary endpoint is the difference in apnea-hypopnea index response rates between Treatment and Control groups at Month 7. Secondary endpoints include quality of life and oximetry metrics.
OSPREY follows an adaptive “Goldilocks” design which optimizes the number of subjects with the need for high-confidence results. A maximum of 150 subjects is allowed, at which study power of >95% is predicted. Interim analyses begin once 50 patients are randomized and recur after each 20 additional randomizations to detect early success or futility.
OSPREY is a unique, efficient trial that should provide high-confidence confirmation of the safety and efficacy of targeted hypoglossal nerve stimulation for moderate to severe obstructive sleep apnea.
Keywords: Obstructive sleep apnea, Randomized, Controlled trial, Adaptive design, Surgical treatment, Hypoglossal nerve, Targeted hypoglossal nerve stimulation, Neurostimulation, Sleep
1. Background
Obstructive sleep apnea (OSA) is thought to affect up to 1 billion people worldwide although the majority remain undiagnosed and untreated [2]. In the US, conservative estimates suggest that at least 35 million patients are affected [31]. Moderate to severe OSA comprises around half of the global prevalence at approximately 450 million people [2]. OSA is known to have major neurocognitive and cardiovascular sequelae although optimizing treatment to prevent complications remains problematic [14].
Nasal CPAP (continuous positive airway pressure) is first line treatment for most OSA patients supported by evidence from randomized controlled trials (RCTs) [16,32]. However, CPAP effectiveness can be limited by variable adherence to therapy. PAP therapy can provide transformative benefits for some patients and up to 87% of PAP treated patients met Medicare criteria for therapy adherence when assessed wirelessly [24]. However, epidemiologic studies indicate adherence rates closer to 70% with significant disparities between women and men, younger versus older subjects, and with long-term adherence less than 50% for women under 30 [30]. Thus, there is a need for alternative therapeutic approaches [22]. Mandibular advancement devices are an alternative, although their efficacy is variable and long-term benefits are unclear [33]. Surgical upper airway reconstruction is clinically beneficial in selected patients, although surgery usually does not usually normalize apnea-hypopnea index (AHI) and entails short term morbidity and risk [5–7,17,19,21,38]. Pharmacotherapies have been proposed although none has well-established, long-term therapeutic benefit for OSA [18,36]. Hypoglossal nerve stimulation (HGNS) has been FDA approved since 2014, although randomized trials in this context are limited to short-term, randomized withdrawal in responders [23,35].
The third clinical study of Targeted Hypoglossal Nerve stimulation (THN), THN3, was the first completed parallel-arm, randomized, controlled trial of HGNS in OSA. The trial enrolled 138 moderate to severe OSA patients (AHI 20–65 events/h) at 20 international sites. Subjects were implanted and randomized 2:1 to active or inactive therapy treatment, respectively, for 3 months beginning 1 month after surgery. All patients thereafter received active therapy and were followed for a total of 11 months after therapy activation to either 12 or 15 months post-implant. Primary efficacy endpoints were based on AHI and oxygen desaturation index (ODI) response rates, with AHI response defined by the “Sher criteria” (≥50% reduction vs. baseline and AHI < 20 events/h) and ODI response defined as ≥25% reduction versus baseline. The four co-primary endpoints required AHI and ODI response rates to be higher for active therapy versus inactive therapy at the end of the randomization period and the pooled 11-month active therapy AHI and ODI response rates to be >50%. Secondary endpoints consisted of the Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ) and EQ-5D visual analog scale, compared between active and inactive therapy at the conclusion of the randomization period. THN3 met all primary and secondary endpoints with the exception of AHI responder rate after 11 months of therapy. For the randomized portion of the trial, AHI response rates for active and inactive therapy groups were 52% and 20%, respectively, and for ODI 63% and 41%, respectively. Pooled AHI and ODI responses following 11 months of therapy were 41% and 63%, respectively.
While THN3 established the safety profile of THN therapy as favorable, the system design was updated to improve functionality and user experience. Therefore, the purpose of the present investigation is to report the design of a confirmatory randomized, controlled trial of THN therapy with the improved system in moderate to severe OSA.
2. Methods/design
2.1. Study design
The “treating Obstructive Sleep Apnea using Targeted Hypoglossal Nerve Stimulation” (OSPREY) study is a multi-center, open-label, prospective, randomized controlled trial of THN therapy via the aura6000® System (LivaNova PLC, London, UK). The trial is sponsored and funded in its entirety by the device manufacturer. The trial has been registered with ClinicalTrials.gov (www.clinicaltrials.gov, NCT04950894). OSPREY is to be conducted in accordance with the Declaration of Helsinki, with all subjects providing informed consent prior to participating in any study-related evaluation. The institutional review board or ethics committee of each site will approve the study protocol, consent forms and other relevant materials.
OSPREY will enroll adult patients with moderate to severe obstructive sleep apnea who report intolerance of or unwillingness to use PAP. During the randomized portion of the study, objective evidence of efficacy will be examined by assessment of improvement in sleep disordered breathing between subjects with active THN therapy (Treatment) versus those with an inactive device (Control). Thereafter, therapy will be initiated in the control group in the non-randomized phase. The Treatment and Control groups will be followed on therapy for a total of 12 and 6 months, respectively, to determine overall safety and efficacy. OSPREY will enroll up to 150 subjects globally at approximately 20 sites.
2.2. Study oversight
Three independent bodies will oversee the OSPREY trial. The Steering Committee (SC), comprising three physician-experts in sleep medicine and sleep surgery, who worked with the sponsor to design the trial, will supervise its operational activities, including site selection, quality of study conduct, enrollment and maintenance of the study protocol. The SC will also participate in analysis of trial results and lead primary publication efforts.
The Data Safety and Monitoring Board (DSMB) also consists of three members, which include a biostatistician and physician specialists in sleep medicine and otorhinolaryngology. The DSMB will be responsible for monitoring the safety and well-being of the subjects participating in the study, ensuring the scientific integrity of OSPREY and recommending action items based on safety issues, including study termination, as warranted.
The Clinical Events Committee (CEC) is composed of three physicians with specialty training in sleep surgery, sleep medicine and otorhinolaryngology. The CEC will adjudicate adverse events from the trial to determine event type, potential impact to study endpoints and relationship of events to the device, stimulation or implant procedure. In this capacity, the CEC will review site-reported unexpected adverse device effects and provide clinical input.
2.3. Study population
Subjects ≥22 years old with moderate to severe OSA (AHI 20–65 events/h in an attended, in-laboratory polysomnogram (PSG)) who have failed or are unwilling to use PAP will be screened. Hypopnea is defined as a decrease in nasal pressure flow by ≥30% of baseline lasting at least 10 s with a ≥ 4% desaturation from pre-event baseline. Subjects will have a body mass index ≤35 kg/m2. Importantly, drug induced sleep endoscopy is not required for qualification due to the different rationale for mechanism of action of THN therapy as compared to distal hypoglossal nerve stimulation technologies [39]. Study candidates will be drawn from each site’s extant and incident patient populations, referrals from collaborating institutions and direction of local, pre-screened exogenous patients to the site by third-party trial recruitment agencies. It will be a goal of the study to distribute enrollment as evenly as feasible across participating sites.
For patients already within a site’s practice, screening will begin with review of medical records, followed by education about THN therapy and the trial, in person or by telephone and will conclude with clinical and home-based evaluation. External patients would first learn of the trial through online portals and undergo pre-screening by telephone interview and medical record review by healthcare professionals employed by the recruiting agencies. Once qualified, they would be referred to the appropriate site study coordinator, at which time screening would continue as with patients who originated within the site’s practice.
Detailed inclusion and exclusion criteria are presented in Tables 1 and 2, respectively. Eligibility criteria derive from several core motivations: (1) selecting subjects within the target population who are willing and able to provide informed consent and fulfill trial requirements, (2) limiting participation to subjects without concomitant conditions, treatments or habits that could confound collection/interpretation of endpoints or effective titration of therapy, and (3) excluding conditions which the device has not been designed to treat or under which its ability to perform safely has not been established. Eligibility criteria also include predictive factors identified in the multicenter feasibility study of THN therapy: BMI < 35 kg/m2, AHI < 65 events/h, apnea index ≤30 events/h, and SpO2 desaturations of ≥10% fewer than 15/h [13].
Table 1.
General inclusion criteria.
|
Table 2.
General exclusion criteria.
|
Having fulfilled general eligibility criteria, subjects will be screened for concomitant sleep issues that might interfere with therapy delivery. The goal is to exclude characteristics adverse to effective therapy titration, indicative of comorbid sleep pathology unrelated to OSA and/or implicated by previous studies as unfavorable to response (Table 2, items 25 and 26). All subjects will begin by completing home sleep tests (HSTs) on two nights in close succession (WatchPat, Itamar Medical, Caesarea, Israel). HSTs will be processed by the vendor, interpreted by a sleep board-certified physician and reported to the site and sponsor. HSTs will be considered valid for screening purposes when approved by the interpreting physician and total recording time is at least 4 h. To qualify as eligible, subjects must demonstrate a repeatable sleep test outcome defined as HST-estimated AHI values (using 4% desaturation criteria) within 20 events/h of one another. HST results are not used for other screening purposes (e.g., HST results are not used to formally confirm moderate to severe OSA).
Adverse events (AEs) will be collected throughout the course of the study from the time of consent until study exit. AEs will be reported by the site to the sponsor via the electronic data capture system. AE definitions are derived from ISO 14155:2020 - Clinical investigation of medical devices for human subjects.
2.4. Baseline assessment
Subjects who have completed the HSTs and other Screening assessments successfully will proceed to Baseline assessment, at which the Baseline PSG and patient-reported outcome measures (PROMs) will be collected. PROMs will include ESS, FOSQ-10, the Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance Index (SDI-8) and Sleep-Related Impairment (SRI-8) inventories and SF6-D (derived from SF-36) and EQ-5D-5L quality-of-life instruments. Subjects have the option to complete PROM questionnaires during baseline and endpoint visits electronically or in paper form. Paper results will be entered into the electronic data capture system by the study coordinator. PSGs will be submitted to an independent central laboratory for review and scoring using filenames generated with an undivulged code. Scoring will be conducted manually (Alice with Sleepware G3, Philips Respironics Inc., Murrysville, Pennsylvania) and adhere to the American Academy of Sleep Medicine guidelines [1,3] for scoring hypopneas based on a nasal pressure drop of 30% or greater and a desaturation of 4%. Oxygen desaturation index (ODI) will likewise be scored according to a 4% desaturation threshold. Due to the visibility of stimulation artifact in PSG recordings, it is not possible to fully blind the core laboratory scorer. Specific inclusion/exclusion criteria will be applied (Table 2, criteria 25 and 26) and subjects who fulfill all eligibility criteria will proceed to implant.
2.5. Implant
The THN therapy system may be implanted either as an inpatient or outpatient procedure. The implant process has been previously described [13]. Briefly, the implantable portion of the THN therapy system consists of two components: a multi-programmable and rechargeable implanted pulse generator (IPG) and a hypoglossal nerve cuff electrode lead (Fig. 1). The system is implanted unilaterally, typically on the right side. The cuff portion of the lead includes six independent contacts arranged circumferentially in a helical pattern to provide multiple loci for stimulating the nerve. The cuff is implanted around the proximal hypoglossal nerve, in the submandibular region. After being secured in place and looped for strain relief, the remainder of the lead is delivered using a hollow canula subcutaneously to the pectoral pocket for the IPG. The lead is connected to the IPG, and tongue muscle activity and impedance measurements within the expected range are confirmed for each contact. The IPG is then placed in the pocket and the surgical incisions are closed. An experienced implanter completes the procedure in approximately 1–1.5 h. Didactic and practical training (cadaver laboratory and/or proctored procedures) is required for each surgeon to qualify as an implanter. In addition, a sponsor representative attends each surgery to support the procedure and perform testing of the device.
Fig. 1.
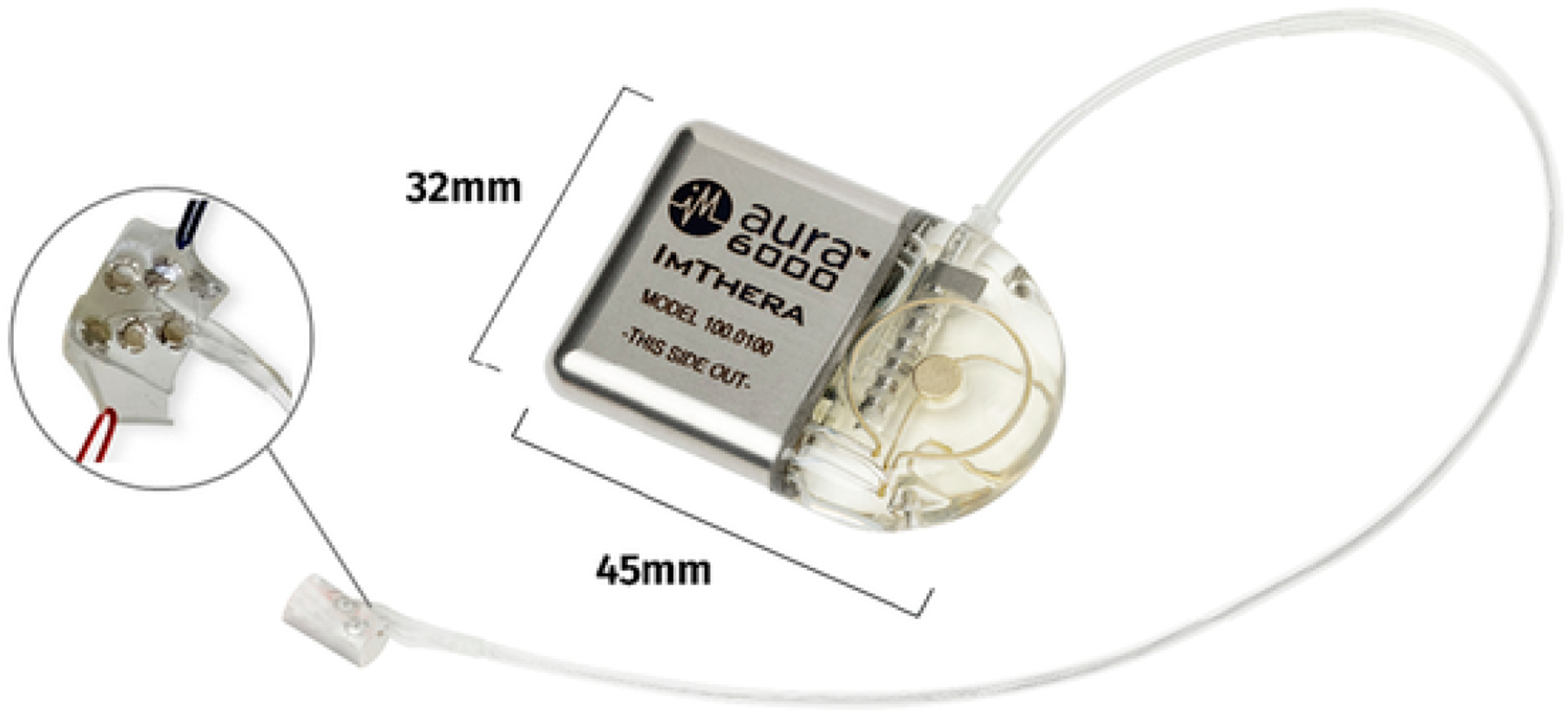
The Aura6000® targeted hypoglossal nerve (THN) therapy system with inset image detailing arrangement of electrode contacts.
2.6. Randomization
Seven to 20 days following implant, subjects will report to the study site to assess surgical healing and to receive a randomization assignment (Fig. 2). Allocation to Treatment and Control groups will follow a 2:1 ratio to obtain greater experience with active therapy and to facilitate patient enrollment and retention. Subjects will be preferentially allocated using a process of minimization by means of an automated, computerized algorithm [8]. The process will seek to ensure balance between the two study groups in Baseline AHI, BMI and tonsil size. The procedure allocates the first subject to Treatment or Control at random. Subsequent subjects are dynamically allocated according to whichever study arm leads to better balance. That is, the allocation seeks to minimize differences in AHI, BMI and Tonsil Size. At the time of randomization, the study coordinator requests the assignment for the patient through the electronic data capture system, at which time the assignment is automatically generated by computer according to the process described above. Subject blinding is not possible as they manually control therapy and can perceive its effects.
Fig. 2.

OSPREY trial schematic. Positive airway pressure therapies are not allowed at any time during the study. During the majority of the randomization period, the control group may continue using oral appliances, positional therapy, supplemental oxygen, upper airway exercises and lifestyle management strategies.
2.7. Concomitant treatments
Following consent and during participation in OSPREY, subjects will not be allowed to use adjunct treatments for their OSA, with the exception of the control group. The control group may continue using OSA treatments (except PAP or undergoing upper airway surgery) until 14 days prior to the Month 7 PSG. Permissible treatments include oral appliances, positional therapy, supplemental oxygen, upper airway muscle exercises, weight loss and lifestyle management (e.g., abstinence from alcohol, caffeine and heavy meals before sleep).
2.8. Randomized phase
The OSPREY follow-up schedule is illustrated in Fig. 3. One month following implant, all subjects present for general examination and review of current treatments. Treatment group subjects undergo additional procedures, including evaluation of stimulation impedances and awake stimulation with each contact to determine amplitudes at which patients sense stimulation (sensory threshold) and discomfort (sensory limit). Treatment group patients will be provided with training on using the THN therapy system at home. Finally, Treatment subjects undergo PSG for activation of THN therapy and initial titration.
Fig. 3.

OSPREY time and events schedule.
After Month 1, subjects assigned to both trial arms return for monthly follow-up. Control subjects return for office visits at Months 3, 5 and 7 and receive telephone follow-up at Months 2, 4 and 6. Treatment arm subjects attend office visits monthly through Month 7 and undergo PSG with the opportunity for titration at Months 2, 3 (two successive nights) and 6, while PSGs optionally occur at Months 4 and 5. The primary endpoint PSG is collected in both arms at Month 7.
Titration during PSG is guided by capture and sensory thresholds and subjective responses to stimulation from awake subjects and is based on prior clinical experience. During sleep, the process begins with evaluation of each of the 6 electrode cuff contacts to determine the potential to increase airway patency at a given stimulation intensity. Stimulation is initiated below capture threshold and gradually escalated until an effect is noted on the nasal pressure (tidal airflow) signal or the sensory limit is reached with an arousal. Contacts demonstrating the greatest increase in airflow and improvement in sleep disordered breathing without causing arousals are selected for further evaluation. Usually 2–3 of 6 contacts are included in the therapeutic regimen. Beneficial contacts are next combined in repeating sequences of stimulation. Therapy parameters (amplitude, pulse duration, frequency and contact on time) are adjusted to achieve maximum efficacy. The impact of candidate sequences on airway patency is assessed throughout sleep stages and body positions over the course of the night. If necessary, parameters are further optimized to maintain stable ventilation, oxygenation and sleep. The most beneficial sequence and stimulation parameters are selected as the chronic therapeutic program. All titrations in OSPREY are performed by trained representatives of the sponsor. Titration may occur during any PSG session except Baseline and the endpoints at Months 7 and 13.
2.9. Nonrandomized phase
Following collection of the primary efficacy data at Month 7, subjects enter the nonrandomized phase of the trial. Control subjects immediately undergo a second night of polysomnography to initiate therapy as part of their follow-up schedule, which mirrors the Treatment group schedule of from Month 1 to Month 7. For treatment arm subjects, telephone follow-up occurs at Month 8, 10 and 12 with office visits at Month 9, 11 and 13. An optional PSG with the opportunity for titration occurs at Month 9 and a mandatory PSG is performed at Month 11. All subjects have a final endpoint PSG performed at Month 13 and thereafter exit the study.
2.10. Objectives
The primary objective of OSPREY is to demonstrate the safety and effectiveness of the THN therapy system in ameliorating sleep disordered breathing associated with OSA after 6 months of stimulation compared to a control group with no stimulation.
2.11. Outcomes
The primary safety endpoint is a descriptive evaluation of all reported serious adverse device/procedure related events through Month 7 for the Treatment and Control groups. Relationship to procedure, device and study will be adjudicated by the CEC.
The primary efficacy endpoint is to demonstrate that the AHI responder rate of subjects with active device stimulation (Treatment Group) is statistically significantly higher than subjects with the inactive device (Control Group) at Month 7. Responder status is defined as reduction in AHI from Baseline of at least 50% and an AHI of less than 20 events/h.
Secondary endpoints include measurements of improvement in the Treatment group from Baseline to Month 7 in ODI, ESS, FOSQ, PROMIS SDI and SRI, SF-6D, EQ-5D and T90, the proportion of time per hour of sleep spent with arterial blood oxygen saturation less than 90%. THN therapy compliance will also be reported.
2.12. Statistical analysis
2.12.1. Maximum sample size
OSPREY is designed to have approximately 97% power at its maximum sample size of 150 evaluable subjects to detect a significant difference (one-sided p-value <0.025) in the AHI response rate between the Treatment and Control groups (Table 3). The sample size is based on the results of the THN3 study (NCT02263859), in which the AHI responder rate following 3 months of THN therapy in the Treatment group (N = 88) was 52% while the Control group (N = 45) responder rate was 20%. A superiority evaluation of clinically significant response rates is utilized because no criteria for minimum clinically important difference in response rate has been established.
Table 3.
Statistical properties of trial design.
| Fixed Sample Size Estimated Primary Endpoint Statistical Power | ||||||
| Assumes Previous Trial Response Rates: 52% (Treatment) vs. 20% (Control) – Observed Response Rates Determine Actual Power | ||||||
| Primary endpoint power | 49.2% | 65.9% | 79.3% | 88.2% | 92.7% | 96.7% |
| No. Randomized Subjects | 50 | 70 | 90 | 110 | 130 | 150 |
| Bayesian Operational Characteristics | ||||||
| Size-dependent Thresholds for Early Success and Futility | ||||||
| Early success if PPn > | 0.990 | 0.990 | 0.975 | 0.975 | 0.950 | 0.950 |
| Early futility if PPmax < | 0.005 | 0.005 | 0.010 | 0.010 | 0.025 | 0.025 |
2.12.2. Bayesian design and data analysis
OSPREY is designed according to Bayesian principles, employing a “Goldilocks” design [4] which allows optimization of enrollment accrual to predict early success or early futility with high confidence while minimizing the exposure of subjects to experimental therapy. Data analysis will consist of a series of interim analyses beginning when the number of randomized subjects reaches 50. Interim analyses are scheduled to take place after every additional 20 subjects are randomized, up to the maximum sample size of 150.
At each interim analysis, the study primary endpoint is evaluated according to intention-to-treat principles. Sher criteria are to be used to assess AHI response for subjects who have completed 7 months of follow-up and is the only parameter used for interim analyses. For subjects who have not yet reached 7 months of follow-up, a Bayesian model will be used to impute whether a patient is an AHI responder or non-responder for each follow up visit. The modeling is based on a beta-binomial distribution derived from a non-informative prior distribution which has been updated with the number of responding and non-responding subjects at each follow-up within the trial. The interim analysis involves calculation of two predictive probabilities which relate to the possibility of trial success. The predictive probability of trial success with the present subjects randomized (PPn) is defined as the predictive probability that the Treatment group AHI responder rate will be significantly higher than the Control group if the trial stops accrual immediately and all enrolled subjects complete the Month 7 follow-up. PPn is calculated by applying the Bayesian modeling framework thousands of times. The proportion of imputed complete datasets that achieve statistical significance for the primary efficacy endpoint is the predictive probability of trial success. Early success is declared if PPn exceeds a size-dependent (number of patients randomized) threshold which conservatively restricts the potential of a false positive result (Table 3). The predictive probability of trial success at the maximum sample size, PPmax, is defined as the predictive probability that the AHI responder rate of the Treatment group will be significantly higher than the Control group if the study randomizes the maximum sample size of 150 evaluable subjects. Futility is declared if PPmax is less than a size-dependent threshold that restricts the potential for a false negative trial result. Interim analyses will be conducted by an independent statistician and the results reported to the DSMB for review and sponsor notification.
In the final analysis, the primary efficacy endpoint will be analyzed on an intention-to-treat basis using the one-sided Fisher’s exact test (test of Treatment vs. Control AHI response rate superiority) at the α = 0.025 significance level. For other efficacy endpoints, changes from Baseline in the Treatment group and treatment effect differences between the Treatment and Control arms, together with two-tailed 95% confidence intervals, will be generated and reported with relevant p-values. Secondary endpoints are not individually powered and no corrections are planned for multiple comparisons. However, they will be hierarchically evaluated in the order previously listed. Month 13 efficacy outcomes will be reported relative to Baseline to provide evidence of long-term, sustained therapeutic benefit. Likewise, AE data to Month 13 will establish the long-term safety profile of THN therapy. Results of the trial will be reported according to the Consolidated Standards of Reporting Trials (CONSORT) and the adaptive designs CONSORT extension.
3. Discussion
We view the OSPREY study as an important advance for the treatment of OSA for several reasons. First, the study design is unique for HGNS trials since it is based on a parallel group randomized controlled trial with an adaptive design. In contrast, the current literature is primarily focused on single arm treatment trials, observational studies and patient registries and various responder analyses [15,35,37]. Second, we have produced an efficient design that should allow enrollment of patients and a relatively rapid definitive conclusion. Third, our design will minimize the exposure of patients to experimental therapies through a 6 month randomized phase in accordance with precedent and expert consensus [11,26].
The optimal study design for alternative therapies for OSA is under debate. Based on existing literature, some have argued that withholding of CPAP may be unethical. However, controlled studies of CPAP have failed to show benefits in many cases, perhaps due to suboptimal therapy adherence in relatively asymptomatic patients [20,27]. Thus, surgical therapies which do not require treatment compliance may be helpful given the limitations of existing therapy [21,38]. One study design could include a head-to-head comparison of HGNS vs. CPAP with the assumption that CPAP may have good efficacy but limited effectiveness based on adherence to treatment. Although comparative effectiveness studies would clearly have value [10], we do not view HGNS as competitive with CPAP but rather a useful alternative for select patients. As a result, we have designed OSPREY to target patients who cannot or will not tolerate PAP therapy to facilitate rescue approaches in the future.
The STAR trial of HGNS was published in 2014 [35]. The authors reported a prospective observational case series in which improvements in AHI were observed in association with HGNS. The investigators performed a randomized withdrawal study in which patients who responded to HGNS were randomized to continued therapy versus discontinuation of HGNS. The results provided compelling evidence for HGNS use in some patients but the design of the randomized withdrawal is limited to assessments in the ‘responder’ group. Longitudinal observational studies can also be limited by changes over time such as those secondary to diet and exercise or other health behaviors. The Hawthorne effect can likewise be relevant where health outcomes can improve in the context of a clinical study when participants are aware that their health status is being assessed. Although observational studies have value, randomized trials such as OSPREY may better handle certain biases or confounding factors and may allow drawing of causal inferences [28,29].
Despite the strengths of OSPREY, we acknowledge a number of limitations. First, it is not powered or of sufficient duration for clinical outcomes such as myocardial infarction or cerebrovascular events [34]. However, the endpoints include important objective and subjective outcomes which would provide compelling rationale for future studies. We recognize the limitations of the AHI [25] and AHI responder rate, but they are required metrics for regulatory approval and comparison with other HGNS trials. Second, given that HGNS is FDA approved, one cannot be confident that OSPREY is without selection bias by the candidates or investigators since some candidates may opt for commercially available HGNS treatment rather than enrolling in the study. However, since the benefits of HGNS are still debated, as is the cost versus benefit, further evidence will be useful. Third, there are additional HGNS approaches under investigation, a fact that may be important to consider in the context of the OSPREY results [12]. Since head-to-head comparisons with other HGNS devices or with pharmacotherapy is not being performed in OSPREY, future comparative effectiveness studies would be needed to draw rigorous conclusions [9]. Finally, as with other trials of HGNS, restrictive eligibility criteria will result in substantial attrition in the patient funnel but are important to reduce heterogeneity that would confound assessment of outcomes. Despite these limitations, we view OSPREY as well designed and likely to yield important conclusions.
Footnotes
Declaration of Competing Interest
None.
References
- [1].AASM, International classification of sleep disorders, in: Diagnostic and Coding Manual, 2nd ed, Westchester, Illinois, American Academy of Sleep Medicine, 2005, 10.1007/s13311-012-0145-6. [DOI] [Google Scholar]
- [2].Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A, Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis, Lancet Respir. Med 7 (2019) 687–698, 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep M, Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine, J. Clin. Sleep Med 8 (2012) 597–619, 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Broglio KR, Connor JT, Berry SM, Not too big, not too small: a goldilocks approach to sample size selection, J. Biopharm. Stat 24 (2014) 685–705, 10.1080/10543406.2014.888569. [DOI] [PubMed] [Google Scholar]
- [5].Browaldh N, Nerfeldt P, Lysdahl M, Bring J, Friberg D, SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea, Thorax 68 (2013) 846–853, 10.1136/thoraxjnl-2012-202610. [DOI] [PubMed] [Google Scholar]
- [6].Browaldh N, Bring J, Friberg D, SKUP(3) RCT; continuous study: changes in sleepiness and quality of life after modified UPPP, Laryngoscope 126 (2016) 1484–1491, 10.1002/lary.25642. [DOI] [PubMed] [Google Scholar]
- [7].Browaldh N, Bring J, Friberg D, SKUP(3) : 6 and 24 months follow-up of changes in respiration and sleepiness after modified UPPP, Laryngoscope 128 (2018) 1238–1244, 10.1002/lary.26835. [DOI] [PubMed] [Google Scholar]
- [8].Brown S, Thorpe H, Hawkins K, Brown J, Minimization--reducing predictability for multi-centre trials whilst retaining balance within centre, Stat. Med 24 (2005) 3715–3727, 10.1002/sim.2391. [DOI] [PubMed] [Google Scholar]
- [9].Carberry JC, Grunstein RR, Eckert DJ, The effects of zolpidem in obstructive sleep apnea - an open-label pilot study, J. Sleep Res 28 (2019) e12853, 10.1111/jsr.12853. [DOI] [PubMed] [Google Scholar]
- [10].Carson SS, Goss CH, Patel SR, Anzueto A, Au DH, Elborn S, Gerald JK, Gerald LB, Kahn JM, Malhotra A, Mularski RA, Riekert KA, Rubenfeld GD, Weaver TE, Krishnan JA, American Thoracic Society Comparative Effectiveness Research Working G, An official American Thoracic Society research statement: comparative effectiveness research in pulmonary, critical care, and sleep medicine, Am. J. Respir. Crit. Care Med 188 (2013) 1253–1261, 10.1164/rccm.201310-1790ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, Abraham WT, remede System Pivotal Trial Study G, Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial, Lancet 388 (2016) 974–982, 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
- [12].Eastwood PR, Barnes M, MacKay SG, Wheatley JR, Hillman DR, Nguyen XL, Lewis R, Campbell MC, Petelle B, Walsh JH, Jones AC, Palme CE, Bizon A, Meslier N, Bertolus C, Maddison KJ, Laccourreye L, Raux G, Denoncin K, Attali V, Gagnadoux F, Launois SH, Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea, Eur. Respir. J 55 (2020), 10.1183/13993003.01320-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Friedman M, Jacobowitz O, Hwang MS, Bergler W, Fietze I, Rombaux P, Mwenge GB, Yalamanchali S, Campana J, Maurer JT, Targeted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea: six-month results, Laryngoscope 126 (2016) 2618–2623, 10.1002/lary.25909. [DOI] [PubMed] [Google Scholar]
- [14].Gottlieb DJ, Punjabi NM, Diagnosis and management of obstructive sleep apnea: a review, JAMA 323 (2020) 1389–1400, 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- [15].Heiser C, Steffen A, Hofauer B, Mehra R, Strollo PJ Jr, Vanderveken OM, Maurer JT, Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial, J. Clin. Med 10 (2021), 10.3390/jcm10132880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jenkinson C, Davies RJ, Mullins R, Stradling JR, Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial, Lancet 353 (1999) 2100–2105, 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- [17].Joar S, Danielle F, Johan B, Arne L, Roberta N, Nanna B, Sleep quality after modified Uvulopalatopharyngoplasty: results from the SKUP3 randomized controlled trial, Sleep 41 (2018), 10.1093/sleep/zsx180. [DOI] [PubMed] [Google Scholar]
- [18].Jordan AS, McSharry DG, Malhotra A, Adult obstructive sleep apnoea, Lancet 383 (2014) 736–747, 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kezirian EJ, Weaver EM, Yueh B, Deyo RA, Khuri SF, Daley J, Henderson W, Incidence of serious complications after uvulopalatopharyngoplasty, Laryngoscope. 114 (3) (2004. Mar) 450–453, 10.1097/00005537-200403000-00012. [DOI] [PubMed] [Google Scholar]
- [20].Kohler M, Stradling JR, Does continuous positive airway pressure therapy improve non-alcoholic fatty liver disease? Respirology 21 (2016) 209–210, 10.1111/resp.12720. [DOI] [PubMed] [Google Scholar]
- [21].Lee HM, Kim HY, Suh JD, Han KD, Kim JK, Lim YC, Hong SC, Cho JH, Uvulopalatopharyngoplasty reduces the incidence of cardiovascular complications caused by obstructive sleep apnea: results from the national insurance service survey 2007–2014, Sleep Med. 45 (2018) 11–16, 10.1016/j.sleep.2017.12.019. [DOI] [PubMed] [Google Scholar]
- [22].Lin HS, Zuliani G, Amjad EH, Prasad AS, Badr MS, Pan CJ, Rowley JA, Treatment compliance in patients lost to follow-up after polysomnography, Otolaryngol. Head Neck Surg 136 (2007) 236–240, 10.1016/j.otohns.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [23].Malhotra A, Hypoglossal-nerve stimulation for obstructive sleep apnea, N. Engl. J. Med 370 (2014) 170–171, 10.1056/NEJMe1314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV, Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis, Chest 153 (2018) 843–850, 10.1016/j.chest.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Malhotra A, Gottlieb DJ, The AHI is useful but limited: how can we do better? Sleep. 44 (9) (2021. Sep 13), zsab150 10.1093/sleep/zsab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mann EA, Nandkumar S, Addy N, Demko BG, Freedman NS, Gillespie MB, Headapohl W, Kirsch DB, Phillips BA, Rosen IM, Schneider LD, Stepnowsky CJ, Yaremchuk KL, Eydelman MB, Study design considerations for sleep-disordered breathing devices, J. Clin. Sleep Med 16 (2020) 441–449, 10.5664/jcsm.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, Investigators S, Coordinators., CPAP for prevention of cardiovascular events in obstructive sleep apnea, N. Engl. J. Med 375 (2016) 919–931, 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- [28].Pack AI, Magalang UJ, Singh B, Kuna ST, Keenan BT, Maislin G To RCT or not to RCT? Depends on the question. A response to McEvoy et al, Sleep 44 (2021), 10.1093/sleep/zsab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pack AI, Magalang UJ, Singh B, Kuna ST, Keenan BT, Maislin G, Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: understanding and overcoming bias, Sleep 44 (2021), 10.1093/sleep/zsaa229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patel SR, Bakker JP, Stitt CJ, Aloia MS, Nouraie SM, Age and sex disparities in adherence to CPAP, Chest. 159 (1) (2021. Jan) 382–389 10.1016/j.chest.2020.07.017, Epub 2020 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM, Increased prevalence of sleep-disordered breathing in adults, Am. J. Epidemiol (2013), 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pepperell J, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling J, Davies R, Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial, Lancet 359 (2002) 204–210, 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- [33].Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB, Cistulli PA, Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial, Am. J. Respir. Crit. Care Med 187 (2013) 879–887, 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- [34].Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM, Sleep-disordered breathing and mortality: a prospective cohort study, PLoS Med. 6 (2009) e1000132, 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Strollo PJ Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP, Group ST, Upper-airway stimulation for obstructive sleep apnea, N. Engl. J. Med 370 (2014) 139–149, 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- [36].Taranto-Montemurro L, Messineo L, Sands SA, Azarbarzin A, Marques M, Edwards BA, Eckert DJ, White DP, Wellman A, The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial, Am. J. Respir. Crit. Care Med 199 (2019) 1267–1276, 10.1164/rccm.201808-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thaler E, Schwab R, Maurer J, Soose R, Larsen C, Stevens S, Stevens D, Boon M, Huntley C, Doghramji K, Waters T, Kominsky A, Steffen A, Kezirian E, Hofauer B, Sommer U, Withrow K, Strohl K, Heiser C, Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy, Laryngoscope 130 (2020) 1333–1338, 10.1002/lary.28286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Weaver EM, Maynard C, Yueh B, Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery, Otolaryngol. Head Neck Surg 130 (2004) 659–665, 10.1016/j.otohns.2003.12.012. [DOI] [PubMed] [Google Scholar]
- [39].Zaidi FN, Meadows P, Jacobowitz O, Davidson TM, Tongue anatomy and physiology, the scientific basis for a novel targeted neurostimulation system designed for the treatment of obstructive sleep apnea, Neuromodulation. (2013) 10.1111/j.1525-1403.2012.00514.x, Jul-Aug;16(4):376–86; discussion 386. [DOI] [PubMed] [Google Scholar]


