Abstract
Cancer is one of the major causes of mortality, accounting for ~9.5 million deaths globally in 2018. The spectrum of conventional treatment for cancer includes surgery, chemotherapy and radiotherapy. Recently, cold plasma therapy surfaced as a novel technique in the treatment of cancer. The FDA approval of the first trial for the use of cold atmospheric plasma (CAP) in cancer therapy in 2019 is evidence of this. This review highlights the mechanisms of action of CAP. Additionally, its applications in anticancer therapy have been reviewed. In summary, this article will introduce the readers to the exciting field of plasma oncology and help them understand the current status and prospects of plasma oncology.
Keywords: Cold plasma, cold atmospheric plasma, cancer, plasma oncology, anticancer therapy
Introduction
The term cancer incorporates >277 different cancer types.1 Currently, it is reported to be one of the deadliest diseases that exist worldwide. In 2018, there were ~9.5 million deaths and ~18.1 million new cancer-related cases reported globally. Moreover, data suggest that, by 2040, the number of cancer-related deaths is anticipated to increase to ~16.4 million and the number of cancer cases could rise to ~29.5 million.2–5 Cancer is a genomic disease that dysregulates the structure and function of genes, arising from the alteration of DNA. A common feature is a genomic damage or altered gene expression. There are six characteristics of cancer starting from the evasion of growth suppressors, activation of invasion and metastasis, followed by facilitating replicative immortality, then the initiation of angiogenesis, leading to cell death resistance and sustained proliferative signaling.6–12
The pathogenesis of cancer is multifactorial and complex. It is associated with infidelity in DNA replication and the DNA repair system. Additionally, radiation, chemicals and viruses can also cause the development of cancer. Hence, there is a need to study the pathogenesis of cancer to develop novel modalities for cancer treatment.13–18
In cancer, the cells escape the normal growth control mechanisms and divide indefinitely. The development of cancer involves the accumulation of several genetic changes over time in a multistep process. A transcription factor: p53, also referred to as the ‘guardian of the genome’, plays a crucial part in cell cycle division, differentiation or cell death. It also has a role in angiogenesis and DNA metabolism. It has been observed that in >50% of the tumor cases the p53 pathway is aberrant. Mutation of the p53 gene results in the production of faulty protein, leading to aberration in the p53 pathway. Thus, the inactivation of p53 protein prevents activation of the cellular apoptotic machinery, resulting in increased survival of cancer cells.1,19–25 Telomerase activity is also increased in cancer cells, which imparts immortalization to the cancer cells by preventing the shortening of telomeres.26,27
Growth factors play an important part in normal growth control. Growth factor receptors transfer signals via signaling pathways, such as the mitogen-activated protein (MAP) kinase pathway, to promote proliferation. However, in cancer these pathways are derailed enabling cells to generate independent internal signals as a result of mutation in the growth receptor factor genes. Another strategy to become independent of the growth factors, which is often used by cancer cells, involves activation of signaling using internal components. For example, the structure of Ras protein is altered in cancer cells. This enables the cancer cells to continuously send internal signals for stimulation of growth in the absence of growth factors.6,19,28
Cancer causes disruption in the functioning of vital genes. The genes responsible for carcinogenesis are broadly categorized as oncogenes and tumor suppressor genes. Protooncogenes are normal genes that are involved in cell division and growth. After mutation or gene amplification, they are converted to oncogenes that promote autonomous cell growth in cancer cells, even in the absence of mitogenic signaling. The most common mutated oncogene in cancer is the Ras gene. It is activated by point mutation. The Ras oncogene stimulates cells continuously without any external trigger. Some examples of protooncogenes activated by overexpression include transforming growth factor (TGF)-α, fibroblast growth factors and cyclins. Platlet-derived growth factor (PDGF) receptors are activated by overexpression.1,29,30 Additionally, the lack of tumor suppressor genes causes uncontrolled cell division. A cancer cell is accompanied with loss of function in at least one tumor suppressor gene. Examples of tumor suppressor genes include APC, CDH1, NF1, TP53, VHL, WT1 and RBA.19,31
For a cell to undergo the complete process of carcinogenesis it must undergo three changes: malignant transformation; invasion of the surrounding cells; and metastasis. For invasion and metastasis to proceed, cell motility, cell invasiveness and angiogenesis are necessary. G proteins regulate cell motility. Various matrix metalloproteinases (MMPs) enhance cell invasiveness. For solid tumors to grow, angiogenesis is essential. It is regulated by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor.19,32–34
A deeper learning and comprehension of the genetic and molecular basis of cancer development, along with the pivotal genes involved, will provide a map for further research strategies in the field of cancer treatment. This will help in developing therapies to target the abnormal cancer cells and spare normal cells; avoiding adverse effects of the conventional treatments.6
Cancer, although considered a modern disease, extends back to ancient Greek and Egyptian civilizations.35,36 Modern oncology was considered to be first identified during the 1700s, when researchers studied the carcinogenic activity of various substances, including soot and tobacco.37 Until the 1890s, surgery or cauterization of tumors was considered to be the only therapeutic option for cancer treatment. In 1900, Marie Curie started using X-rays in tumor treatment. Further, in 1940, several antitumor drugs were developed for the treatment of solid and hematological tumors, together known as chemotherapy.24 Targeted therapy in the form of tyrosine kinase inhibitors and tumor-specific monoclonal antibodies was developed in the 1980s.35,38–40
The National Cancer Institute defines a conventional treatment or therapy for cancer as a process that is widely used and accepted by the majority of healthcare professionals. It is different from the complementary or alternative therapies, which are not used widely. The spectrum of conventional cancer treatment includes surgery, chemotherapy and radiotherapy.41 The operative approach is the most widely employed method for cancer cases. The idea of this treatment is based on the perception that cancer is a localized disease for a respective organ. However, it is not the case. Cancer is a systemic disease, and a tumor is one of the symptoms of its chronic presence. Further, 30–40% of cancer cases are treated with radiotherapy and are mainly combined with other conventional therapies for treating solid tumors. The curative effect of radiotherapy is based upon genetic material damage, preventing growth and proliferation. Unfortunately, radiation also has the potential to damage surrounding healthy tissues, restricting its use even while using modern equipment. Similar to radiotherapy, chemotherapy also has cancerous effects that cause secondary tumors.42,43
There are also other methods that exist but are generally less applicable. Some of them include hormone therapy and immunotherapy. Hormone therapy is applied in prostate and breast cancer, and immunotherapy is majorly applied in the field of experimental research.42,44 Recently, cold atmospheric plasma (CAP) therapy surfaced as a novel therapy in the treatment of cancer, it will be discussed in detail in this review.
CAP and its application in cancer therapy
Matter is present as solid, liquid, gas or plasma in nature; and plasma accounts for 99% of the universe. Plasma was first identified by William Crookes as early as 1879. However, Irving Langmuir gave the substance its name: plasma, in 1928; because it resembled blood plasma which has many constituents.45–47 Plasma is defined as an ionized gas of neutral characteristics consisting of positively charged ions, electrons and neutral particles. The process associated with the phenomenon of separation of electrons from an atom, generating a pair of positively and negatively charged particles, is termed ionization. The ionized gas can conduct an electric current. This property of plasma has helped in achieving various biological outcomes.48,49
Plasma can be classified into either hot or cold atmospheric plasma (CAP; also called cold plasma; both terms are used in this review). Hot plasma is almost completely ionized and the electrons and heavy particles (ions, atoms or molecules) are at high temperatures. By contrast, cold plasma has a low degree of ionization (10−4 to 10−6). Electrons are at relatively higher temperatures and the heavy particles have a temperature closer to the room temperature. CAP has a temperature of <104°F at the application site.45,50,51 Plasma medicine uses room temperature and atmospheric pressure plasma (i.e., CAP) to generate reactive species that interact with biological targets to achieve therapeutic outcomes.45,52
In the first set of experiments, it was found that CAP possesses bactericidal properties.53,54 It was known from the very beginning that CAP produced reactive nitrogen species (RNS) and reactive oxygen species (ROS), which caused the observed biological outcomes.53,55 Eventually, it was observed that CAP could also be employed for disinfecting biological tissues. Thus, CAP was utilized in wound healing.56 These preliminary observations resulted in the anticipation of the potential of CAP by the scientific community, causing the field to develop rapidly. Soon, CAP was applied in the treatment of cancer.57 The timeline for the application of CAP is depicted in Figure 1.
Figure 1.
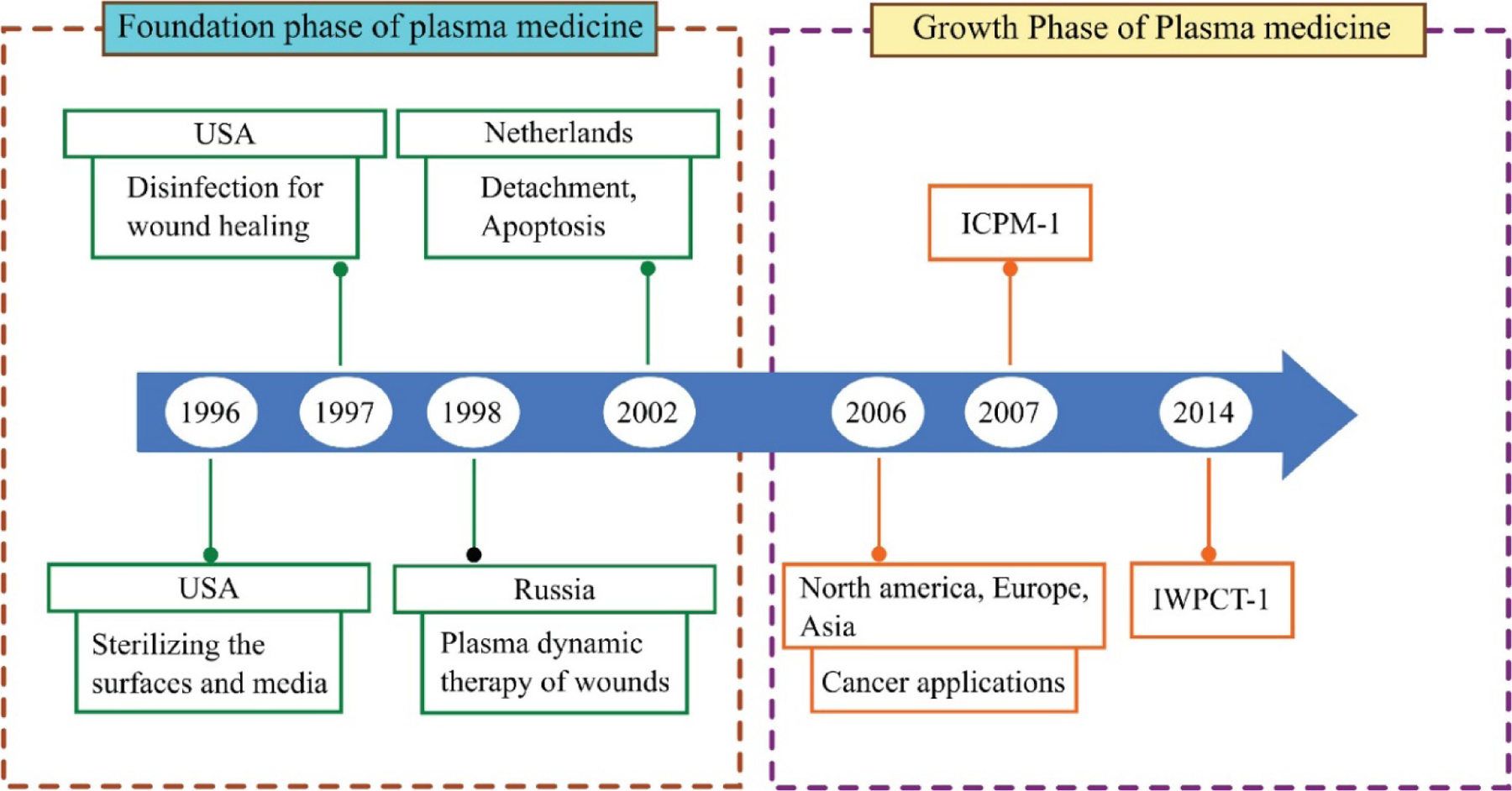
Timeline depicting the major milestones in plasma medicine. The period 1996–2005 can be considered as the foundation phase. The decade from 2006 onward can be considered as the growth phase and is flourishing still. In 2007, the International Conference on Plasma Medicine (ICPM) became the first conference to deal entirely with plasma medicine but The International Workshop on Plasma for Cancer Treatment (IWPCT), established in 2014, was the first workshop on the application of cold atmospheric plasma (CAP) in anticancer therapy.
Cancer treatment relies on a combinatorial approach to increase the chances of survival for cancer patients.58 One such approach uses CAP for cancer therapy.59 CAP selectively kills cancer cells without harming the healthy cells even at low doses. This led to the origin of ‘plasma oncology’. Plasma oncology is a focused study of the use of CAP; for cancerous and malignant tumors treatment and has become an emerging branch of plasma medicine.60 CAP is also used as a palliative treatment.59,61 CAP induces physical effects such as the production of UV rays, electromagnetic fields and heat, as well as chemical effects such as the generation of ROS and RNS. The physical effects have a negligible impact on the biological targets but the chemical effects lead to cellular apoptosis.62 This review provides a comprehensive overview of different methods of CAP generation, followed by potential mechanisms of action. The recent applications of CAP in anticancer therapy have also been reviewed.
Advantages of CAP therapy in cancer over conventional therapies
Numerous in vitro and in vivo studies have reported the selectivity of CAP toward tumor cells. CAP has proven to be not only an adjunct therapy but also a successful approach for killing tumor cells. CAP therapy has many advantages over conventional therapies in cancer treatment.49,63 Cancer is a gene mutation disease in which the oncogenes target specific signaling molecules. Mutations and involvement of multiple signaling pathways lead to complexity in cancer treatment.64 Conventional anticancer therapies such as surgery, chemotherapy or radiotherapy are associated with disadvantages including adverse effects, being time consuming and high costs.38 CAP can help to solve these problems because it is fast, reliable and cost-effective.60,64,65 Currently, plasma can be applied directly to the skin for skin cancer treatment without producing any systemic effects. Chen et al. developed a micro-sized CAP, which is a local treatment tool that prevents systemic adverse effects.63 Application of CAP to internal organs can seem technically difficult but the results are encouraging. Focal therapy in the form of CAP can be used for the treatment of prostate cancer to achieve targeted therapy of the tumor foci. This spares the patient from the treatment of the whole prostate gland and results in minimized side effects.66 CAP serves as a potential alternative to electrochemotherapy (ECT). It can also be used as a palliative treatment in melanoma therapy, either alone or with ECT.67 CAP has been used for head and neck carcinoma palliative treatment. Patients reported a reduction in fetid odor and pain without any side effects upon CAP treatment. CAP has the potential to become an evidence-based medicine for head and neck cancer.68 CAP has restored the sensitivity and resistance issues related with temozolomide chemoresistant cancerous tissues69 and tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) therapy.70 CAP can be combined with nanotechnology to potentiate anticancer effects.71,72 Also, no incidence of CAP resistance has been reported yet.62
Earlier, CAP was directly applied to tumor cells. However, for the past few years, plasma-activated media (PAM) has been used. PAM is as effective as direct CAP treatment and can be applied to or injected into the tumor cells. It can retain its anticancer activity for 7 days upon storage.73
Generation of CAP
CAP is referred to as the non-equilibrium plasma in which the electron temperature is very high as compared with the gas temperature. The production of cold plasma at atmospheric pressure removes the handling problems and usage of large vacuum systems. Therefore, CAP sources provide a cost-effective solution compared with other conventional sources. The generation of plasma at atmospheric pressure results in equilibrium conditions (i.e., the electrons transfer most of their energy to the gaseous atoms and as a result of that the gas temperature Tg increases and approaches the electron temperature Te). Therefore, to obtain cold plasma at atmospheric pressure different techniques have been used such as applying short, pulsed voltages to prevent the thermalization, controlling the current using the dielectric barrier and heat transfer by using convection (i.e., gas flow) in the reactor.
During the past few years, various CAP devices have been used to obtain plasma (a combination of varied reactive species) that can be utilized for biomedical purposes. The prerequisite for the generation and maintenance of CAP is energy. Electromagnetic radiation and electrical energy are fed into the CAP sources to ignite the plasma in air, inert gases or a mixture of gases.45,74 In most of the CAP sources, air, N2 and O2 are generally used for the generation of RONS but it requires an input of high energy and most of the energy is used at the vibrational and excitational levels. The breakdown of the noble gases is relatively easier when compared with the molecular gases. Therefore, the CAP sources using the noble gases such as helium and argon are employed to generate the plasma; and air, N2 and O2 are mixed in defined proportions for the production of RONS of the desired concentration. In a study, helium was found to be the most-used carrier gas, followed by air and argon. Since 2015, the use of argon has increased substantially.62
CAP
CAP sources are directly employed in plasma medicine. The main components of cold plasma responsible for the interaction with the surroundings are shown in Figure 2. Electrons and ions are the charged species and the excited atom constituents of the plasma are the neutral species. Apart from these species, other important components are RONS. The biologically active RONS produced by the CAP sources are hydroxyl (OH), ozone (O3), superoxide (O2−), singlet oxygen 1O2, nitric dioxide (NO2) and nitric oxide (NO). On interacting with the liquid, nitrate (NO3−), nitrite (NO2−), peroxynitrite (ONOO−), nitric acid (HNO3) and hydrogen peroxide (H2O2) are also produced. The formation of these species is dependent on the composition of gas, the concentration of which is controlled by using the operating and geometrical parameter like the applied frequency, voltage pulse, gas flow (if gas used is in a flowing condition), gas pressure and proportion of the mixture gases. Radiation of different wavelengths [i.e., visible (VIS), UV and infrared] is also produced from the CAP sources which are not directly biological effective. However, the UV radiation helps in the generation of different species that are indirectly produced from the RONS through different chemical reactions.75
Figure 2.
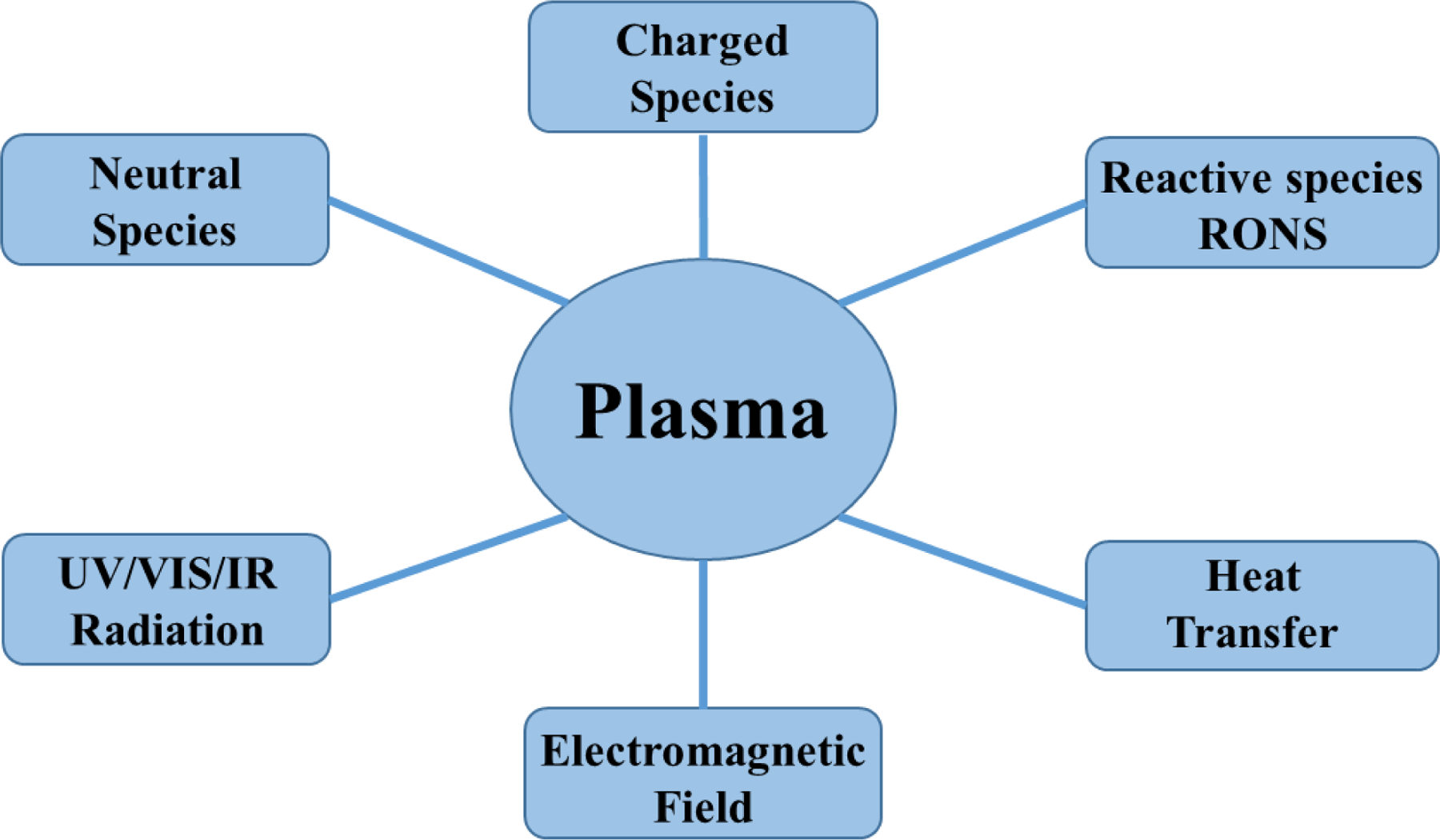
Components of cold atmospheric plasma (CAP) for the interaction with the surroundings.
These components basically expanded the use of CAP in wound healing, cancer treatment, skin treatment, among others; however, a rigorous investigation is still needed before they can be brought into direct use for medical treatment. In general, CAP sources can be divided into direct sources, indirect sources and hybrid sources.
Direct sources.
These plasmas use living tissues or organs as one of the electrodes. The plasma-generated current can pass directly through the biological system in form of displacement current, conduction current or both. The conduction current must be kept in check to prevent any harm to the biological system. Additionally, dielectric barrier discharge (DBD) is a direct plasma source.
Indirect sources.
The plasma in these sources is generated between two electrodes. Reactive ions produced by the plasma are held in a gas flow and transported to the target site. They include a variety of devices including plasma needles to plasma torches. They are commonly known as atmospheric pressure plasma jets (APPJ).
Hybrid sources.
Hybrid sources, as the name suggests, are a combination of the two afore mentioned production techniques: direct sources and indirect sources. A current-free grounded wire electrode is incorporated in a hybrid source. The grounded wire electrode, which has comparatively lesser electrical resistance than skin surface, prevents current passing through and from the skin. MiniFlatPlaster® and HandPlaster® plasma dispensers are examples of hybrid sources.74,76–78
DBD
The first DBD reactor was built by Siemens in 1857.79 DBD consists of two flat electrodes: one which is maintained at high voltage whereas the second electrode is kept grounded. The electrodes are covered by a dielectric material. Glass, ceramic, quartz, plastic, mica, among others, are typically used as the dielectric material. The electrodes are filled with a carrier gas in the middle. Ionization of the carrier gas as a result of the high voltage generates plasma (Figure 3). Generally, DBDs have a frequency in the kHz range whereas the power consumption ranges from 10 to 100 W.60–63 The configuration of the electrodes can vary (e.g., cylindrical electrodes) but the basic concept remains the same.45,62,80 DBD is suitable for intense treatment over a larger area.73
Figure 3.
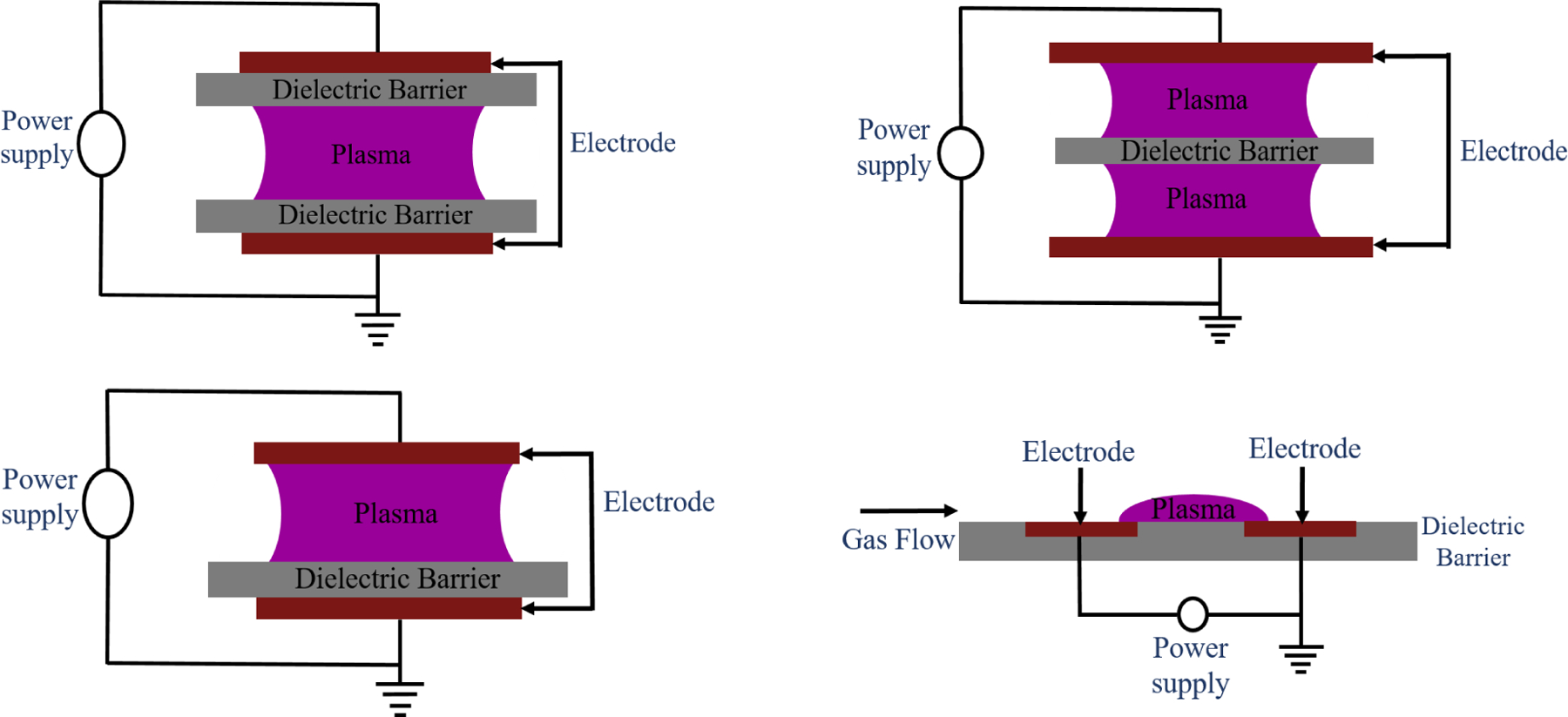
Schematic representation of the different configuration of dielectric barrier discharge (DBD).
The usage of CAP in biomedical applications requires a DBD source that is safe for the patients and doctors and easy to handle. Floating electrode DBD was the first DBD device used for treatment in vivo. DBDs are still the most important CAP sources. They are controllable, robust and scalable devices. Their characterization is however necessary for optimal use.80
CAP plasma jets (C-APPJ)
It was in the mid-2000s when C-APPJs were first developed for their use in plasma medicine. They are meant to maintain temperatures below 40°C and, hence, could be applied to biological tissues.49 The majority of studies conducted use C-APPJ rather than DBD.62 The C-APPJ produces plasma in the open region rather than within the confined gaps, making this device more suitable for the treatment of small and confined areas. There are different types of geometry which are widely used for the production of APPJ, using the ring and pin electrode arrangements.65,66 These C-APPJ sources are powered by the different excitation sources such as microwave, RF, AC and pulsed DC power source and generate the plasma using air, N2, O2, He and Ar as working gases.67,68 The ionized gas exits through a nozzle and is directed a few millimeters downstream of the sample. The plasma column formed outside the nozzle is known as the plasma plume, which is responsible for the interaction with the air and results in the formation of different RONS, which are biologically active. The two main geometrical configurations preferably used in C-APPJs are presented in Figure 4. Most of the developed C-APPJs used for biomedical treatment are based on these types of arrangements. Alternatively, another one is a plasma torch, which consists of a powered electrode with a metal needle of ~1 mm diameter. The needle is inserted in a metal cylinder that is grounded. A quartz tube, which is the dielectric material, is placed between the anode and cathode. The mixture of helium, argon and other species is fed between the metal needle and quartz tube. After ionization, they exit through the torch as a small jet (Figure 5).81,82 Kang et al. made a plasma jet using an outer and inner hollow electrode and porous alumina as a dielectric element. The carrier gas used in this jet was air.77 Mirpour et al. developed a micron plasma jet in which they ionized helium gas using high frequency and voltage.
Figure 4.
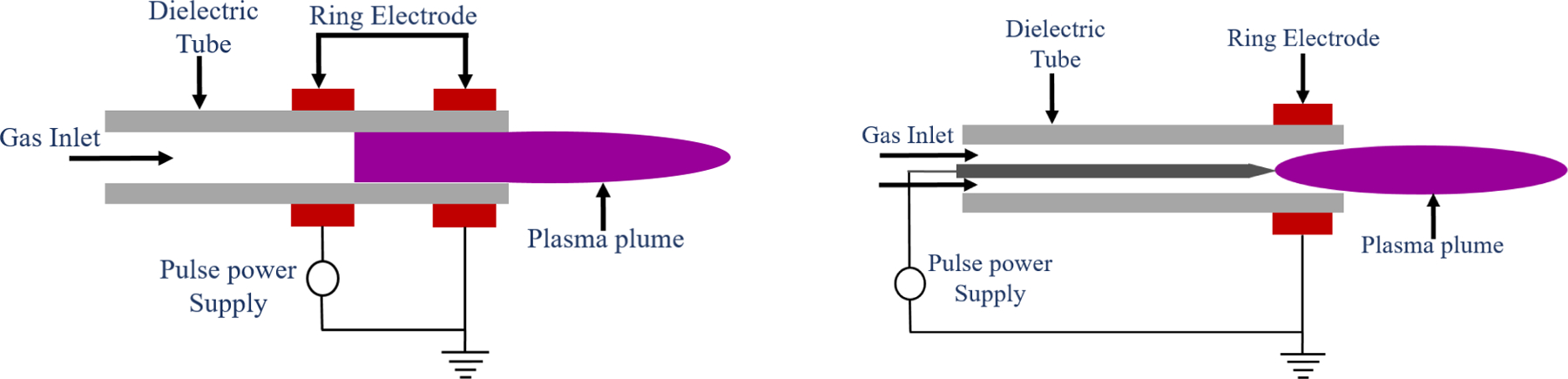
Schematic of the cold atmospheric pressure plasma jet (C-APPJ) with a ring electrode and a centered pin electrode configuration.
Figure 5.
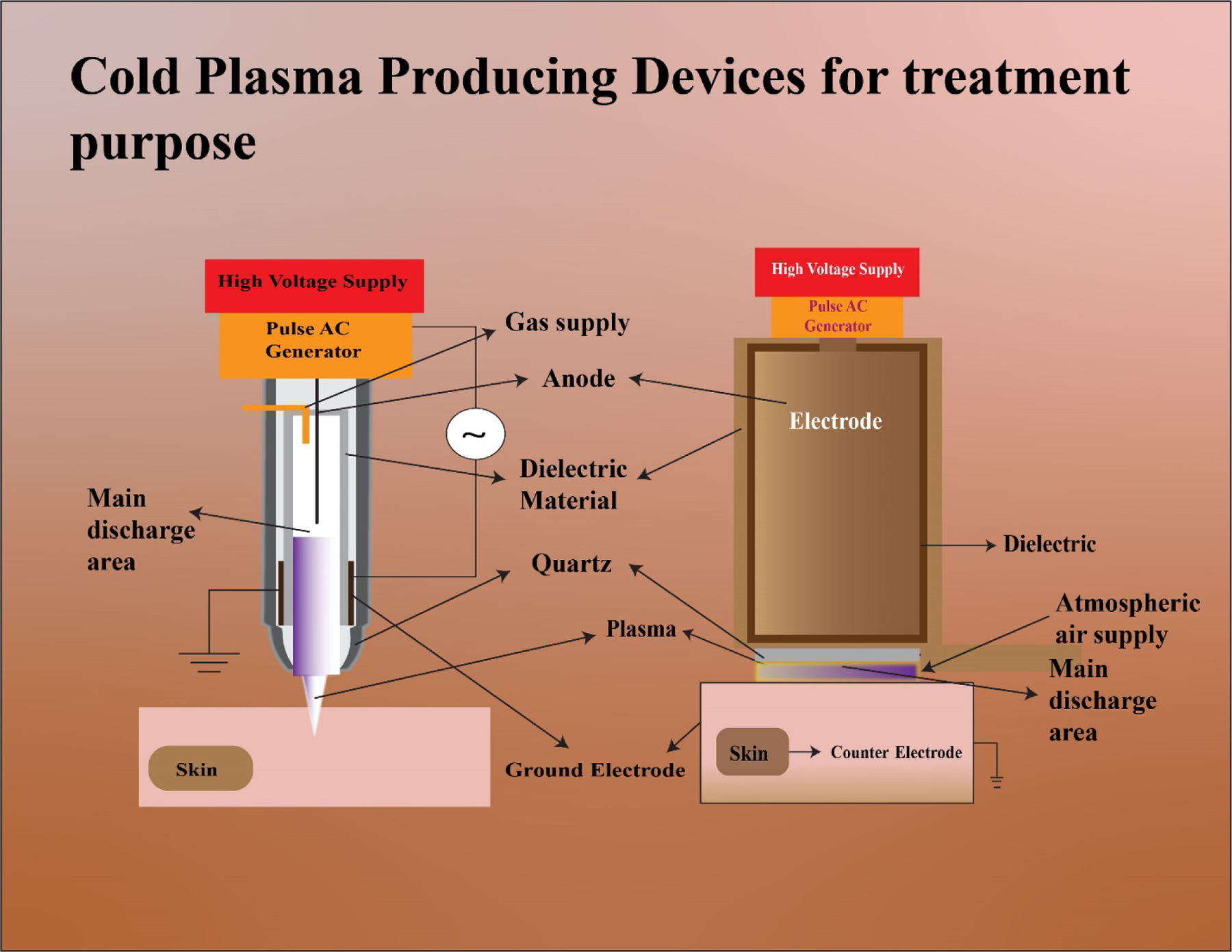
Cold atmospheric plasma (CAP) devices. The two major CAP-producing devices are the plasma jet device on the left and the dielectric barrier discharge (DBD) device depicted on the right.
In the C-APPJ, the formation of the stable and spot-like plasma plume is useful for the delivery of RONS in the confined region.57 The length and properties of the plasma plume can be adjusted by controlling the operating parameters including the applied voltage, pulse frequency, gas flow rate, mixutes, among others. Interaction of the plasma plume with the surroundings leads to the generation of different reactive species and, in turn, enhances applicability in cancer treatment.
A variety of CAP sources have been investigated for their use in plasma medicine. Three CAP sources have been CE-certified by MEDCERT in Germany for medical purposes under ISO 13485 to date. First, kINPen® MED is a APPJ developed by INP Greifswald/Neoplas Tools, Germany. It was CE-certified as a class IIa medical device in 2013,67,83,84 second, plasmaDerm® VU-2010 is a DBD developed by CINOGY System Plasma Technology for Health, Germany, and third SteriPlas® is a device for the treatment of wounds and reduction of microbial load. It was developed by Adtec, UK.67
Arndt et al. compared kINPen® MED and SteriPlas®. In terms of their cellular effects, they were found to be almost comparable. They differed with respect to their effects on cell proliferation and migration because of differences in their treatment modalities and times.85 Standardization of requirements for the development of CAP sources, especially in the field of medicine, is necessary. In 2014, DIN SPEC 91315 was published to certify requirements for CAP sources for their use in plasma medicine. The tests described in this specification are simple and can be performed using common laboratory equipment.67,86
Mechanism of action of CAP in cancer
Is it safe to expose cells and tissues to CAP? To answer this, the mechanism of action of CAP must be understood. Because this in itself is a huge research field, comprehending the mechanism of action of CAP is beneficial not only for the scientific community but also for the healthcare community to obtain optimal therapeutic effects.75 It is currently known that CAP is selective toward cancer cells. It does not produce any deleterious effects on noncancer cells. This has ultimately resulted in reduced side effects compared with conventional therapies. However, biological factors such as cancer type, cell type and culture medium must be accounted for before claiming the selectivity of CAP.51,87
CAP has two major roles in vitro. Primarily, cancer cell activation, which results in a reduction in threshold value of cancerous cells to cytotoxicity of RONS. The second role is the provision of abundant RONS in the extracellular environment of cancer cells.88,89 It must be noted that RONS need to accumulate during the duration of CAP treatment for a few minutes or longer.90 Over the years, numerous mechanisms have been suggested by various researchers to describe the biological action of CAP. However, the exact mechanism remains hazy. The most accepted mechanism of action for CAP in cancer treatment involves the production of RONS. Plasma elicits its effect on the cancer cells through factors such as ions, electrons, photons, UV radiation, free radicals and RONS. RONS include atomic oxygen (O), atomic nitrogen (N), nitric oxide (NO), hydrogen peroxide (H2O2) and hydroxyl radical (OH). When this cocktail of reactive species is directly exposed to cancer cells it induces apoptosis in cancer cells. When PAM is used for indirect exposure to cancer cells, secondary RONS including nitrite (NO2−) and peroxynitrite (ONOO−) are produced, which are the main factors that kill cancer cells. The cellular pathways that these RONS targets are complex.81,90–92 Bruno et al. reported reactive sulfur species that could produce harmful effects on cancer cells. Reactive sulfur species were formed by CAP-induced oxidation of cysteine.93 Bogaerts et al. demonstrated the penetration of CAP-originated RONS through the cell membrane using computer modeling.94 The RONS originating from CAP cause a rise in the intracellular concentration of RONS, which weakens the intracellular antioxidant system and results in breaking of the DNA double-strand. As a result, apoptosis occurs based on the tumor necrosis factor receptor (TNFR) pathway or mitochondrion pathway.81 These pathways have been discussed in detail by Faramarzi and colleagues.95 Rehman et al. conducted a study to compare ROS formation induced by ultrasound, CAP and ionizing radiation. They observed that CAP produced relatively large amounts of ROS in the liquid phase.96 Other reported mechanisms have been listed in Table 2. The mechanisms of application of CAP in cancer are shown in Figure 5. The effects of plasma are dose-dependent. It has been pointed out by many researchers that plasma at lower doses induces cell-cycle arrest, whereas the higher doses result in apoptosis or necrosis in cancer cells.92 The definite mechanism of CAP action is still under speculation. A better understanding of cell signaling events is necessary to enhance the clinical use of CAP in cancer treatment.
Table 2.
Stated mechanisms of action of CAP on the neoplastic cells
| Mechanism of action | Cancer type | Refs |
|---|---|---|
| Activation of p21 CDK inhibitor | Hepatic carcinoma | 134 |
| Dysfunction of mitochondria | Cervical cancer | 135 |
| Activation of p53 protein | Colon cancer | 136 |
| Alteration of the function of receptors present on the cancer cell surface | Breast cancer | 137 |
| Breaking of DNA double strands owing to increased intracellular RONS | Glioblastoma | 69 |
| Downregulation of gene expression (VEGF, MMP2, MMP9 and MTDH) associated with metastasis | Glioblastoma | 71 |
| Induction of senescence | Melanoma | 132 |
| Disorganization of actin cytoskeleton decreasing cell adhesion; inhibition of cell migration | Melanoma | 138 |
| Selectivity of RONS to cancer cells | Osteosarcoma | 139 |
| Activation of cancer cells to make them sensitive to RONS and accumulation of RONS in the extracellular environment | Pancreatic carcinoma | 140 |
| Inhibition of proliferation of cancer cells by modulating H3K4 methylation | Breast cancer | 141 |
| p53 mutation increases vulnerability to redox crisis; hyperactivation of JNK and NF-κB pathways in cancer cells increases the PAM sensitivity | Breast cancer | 142 |
| Production of RNS; an increase in levels of intracellular calcium induces caspase-3/7 activation | Kidney cancer | 143 |
| Immunogenic cell death | Pancreatic ductal adenocarcinoma | 144 |
| Ozone formation leading to antiproliferative effects on cancer cells | a | 145 |
| Oxidation of cysteine to produce reactive sulfur species | a | 93 |
| Change in the metabolism of D-glutamine and D-glutamate; reduction in glutaminase activity | Acute myeloid leukemia | 146 |
| Induction of HMGB1 expression and immunogenic cell death | Colon cancer Thyroid cancer | 147 |
| An increase in Sestrin2 expression initiates downstream iNOS, Fas and p38 MAPK signaling inducing cancer cell apoptosis | Melanoma | 148 |
| At the transcriptional level, PGNO upregulates M1-type macrophages activation and downregulates M2-type macrophages | a | 131 |
| Activation of stress fiber formation, resulting in alteration of cell morphology and increase in cell motility | Fibrosarcoma | 149 |
| Reduced cysteine–glutamate antiporter xCT expression results in decreased glutathione levels leading to apoptosis | Melanoma | 150 |
| Decreased secretion of MMP-9 results in metastasis inhibition in cancer cells | Ovarian cancer | 151 |
No specific cancer type was mentioned.
Abbreviations: CDK, cyclin-dependent kinase; HMGB1, high mobility group box 1 protein; JNK, c-Jun N-terminal kinase; iNOS, inducible nitric oxide synthase; MMP, matrix metallopeptidases; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK, microtubule-associated protein kinase.
Applications of CAP in cancer treatment
From the earliest applications to inactivate bacteria, CAP has come a long way. Today, a variety of CAP devices are available for the treatment of cancer. CAP can be applied in two ways: direct and indirect treatment (Figure 7). In direct treatment, the CAP is in direct contact with the targeted biological site. All the agents generated by CAP act on the biological tissues or cells. DBD and plasma jets are used in the direct approach, whereas indirect treatment utilizes the ‘afterglow’ of CAP. First, CAP is used to activate liquid media. Then, the CAP-activated liquid media, known as PAM, is applied to or injected into the biological target. PAM is found to be as effective as direct treatment, serving as an alternative for CAP devices. PAM can maintain its anticancer activity for at least 7 days, after storage at 4°C. Sometimes, the plasma jet is unable to penetrate the cancer cells. Injection of PAM in the tumor cells can produce effective results in such cases. However, PAM media has been researched less compared with direct treatments.49,62,73
Figure 7.
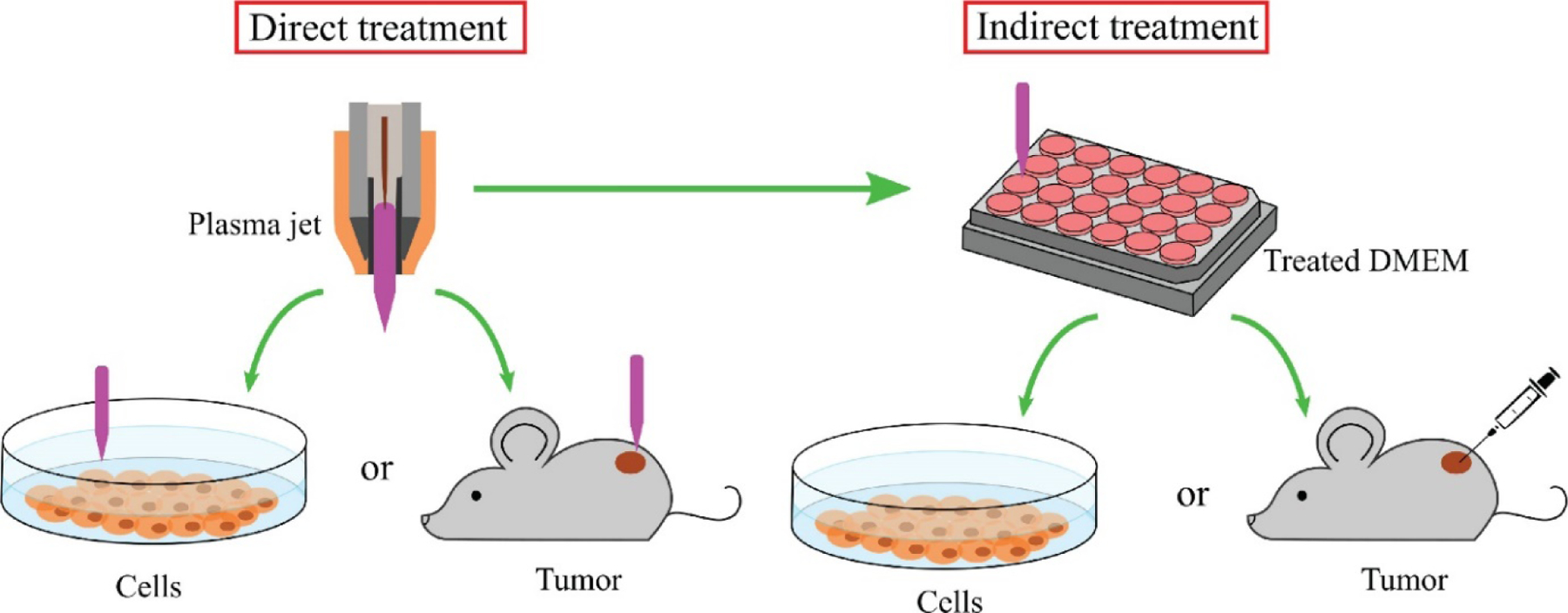
Application of cold atmospheric plasma (CAP). CAP can be applied to the biological target in two ways: direct treatment and indirect treatment.
CAP treatment has produced desirable results in many types of cancers. It can be used as a replacement for conventional therapies or in combination with conventional therapies producing a synergistic effect.62 CAP can be used as a palliative treatment to improve the wellbeing of cancer patients. It has the potential to become an evidence-based medicine in the coming years.59,61,68 Many in vitro and in vivo assays have been conducted to study the effects of CAP in anticancer treatment. The majority of in vitro assays utilize cell lines derived from humans97 or mice. In vivo studies performed help in bringing CAP to the clinical setting to treat cancer. They provide a piece of direct evidence of the effect of CAP on normal noncancerous cells.98 However, when the number of in vivo studies conducted is compared with the in vitro studies, a huge difference is observed. There are only a few in vivo studies, even fewer in human subjects. Most of the studies use mice as the in vivo model. The in vitro and in vivo studies indicating the anticancer activity of CAP are listed in Table 3. Preliminary studies have been conducted on human subjects for curing head and neck carcinoma.68,99–101 These efforts resulted in the approval of the first clinical trial of a CAP ‘scalpel’ for cancer treatment by the FDA in 2019. This CAP device is called the Canady Helios Cold Plasma Scalpel. It is a type of plasma jet and kills tumor cells upon exposure for 2–7 min, as reported in previous studies. It was earlier used in compassionate cases approved by the FDA. The Phase I clinical trial (NCT04267575) for the scalpel was approved for 20 subjects. The conditions and disease listed in the trial are different types of stage IV cancers. The clinical trial started on 30 July 2019 and the estimated study completion date was 28 February 2021.49,102,103
Table 3.
In vitro and in vivo assay studies demonstrating the anticancer activity of CAP
| Study | Treatment | Cancer | The model used in the study | Outcomes | Refs |
|---|---|---|---|---|---|
| In vitro | Direct | Glioblastoma | Human U87MG cells | Increased access to the tumor cells; significant effect on cell viability | 63 |
| Breast cancer | Human MDA-MB-231 cells | ||||
| Melanoma | Murine B16F10 cells | Increased destruction of cancer cells when combined with chemotherapy | 64 | ||
| Breast carcinoma | Human MDA-MB-231 cells | Evidence of potential of CAP in the therapy of breast carcinoma | 137 | ||
| Pancreatic cancer | Murine (C57BL/6 mice) 6606PDA cells and primary fibroblasts | A combination of CAP and gemcitabine is more effective than either of them used alone | 106 | ||
| Pancreatic adenocarcinoma | Murine (transgenic C57BL/6 KrasD12G knock-in mouse) 6606PDA cells and C57BL/6 embryos | CAP can be used as an intraoperative method after surgical resection for the treatment of microscopic residual tumors | 83 | ||
| Cervical cancer | Human SiHa, CaSki, C-33A and DoTc2–4510 cells | Thermal plasma sources can be used as an alternative to invasive tumor lesions ablation | 152 | ||
| Glioblastoma | Human LN18, LN229 and U87MG cells | CAP can be used as combination therapy for patients with glioblastoma who are resistant to temozolomide | 69 | ||
| Prostate cancer | Human LNCaP, PC3 and P69 cells | Selectivity of CAP on prostate cells; the surface temperature rises only by a few °C during CAP treatment | 153 | ||
| Glioblastoma multiforme | Human U373MG-CD14 cells | The toxicity of gold nanoparticles increased 25-fold upon the combination with CAP | 154 | ||
| Breast cancer | Human MDA-MB 231 and MSC cells | CAP combined with nanoparticle approach can be used as a novel dual cancer therapy | 71 | ||
| Indirect | Glioblastoma | Human U87 cell lines | Breast cancer cells were more vulnerable in CAP media; ROS were consumed faster by glioblastoma cells than breast cancer cells | 73 | |
| Breast cancer | Human MCF-7 cells and MDA-MB-231 cells | ||||
| Melanoma | Mel Ei, Mel Juso, Mel Ho, Mel Ju. Mel Im & HTZ19 & neonatal epidermal melanocytes | The mechanism of CAP-induced senescence was firstly described and has great therapeutic potential | 132 | ||
| Squamous cell carcinoma | HGF-1 cells and SCC-15 | Selectivity of CAP toward cancer cells when a small volume of PAM and moderate CAP treatment time was used | 155 | ||
| Osteosarcoma | SaOS-2, human mesenchymal stem cells, human osteoblasts | Minimally invasive approach and selective treatment toward osteosarcoma cells | 139 | ||
| Lung cancer | Human A549 cells, skin keratinocytes: HaCaT and human smooth muscle cells of aorta | The mitochondrial network in cancer cells was affected because of ROS | 156 | ||
| Ovarian cancer | SKOV3, ES2 and WI-38 | Intraperitoneal administration of PAM can be used in ovarian cancer therapy; it acts by hindering the metastasis in cancerous cells | 151 | ||
| Gastric cancer | Human SC-2-NU and GCIY-EGFP cells | PAM can be used for peritoneal metastases treatment in gastric cancer | 157 | ||
| a | Mouse RAW 264.7 and B16F10 cells | PGNO solution can be used as a supportive method to control macrophage function in the cancer cell microenvironment | 131 | ||
| In vivo | Direct | Head and neck cancer | Humans | CAP is clinically relevant and can be used as a palliative treatment | 101 |
| Head and neck cancer | Humans | Visible response to tumor surface | 100 | ||
| Head and neck cancer | Humans | Hyperspectral imaging can help to understand CAP effects in the treatment of cancer | 99 | ||
| Melanoma | C57Bl6 mice Nude mice | CAP selectivity was established and mid-sized tumors in nude mice was eliminated only following single 2 min CAP treatment | 51 | ||
| Bladder cancer | |||||
| Melanoma | Female C57 mice | Increased destruction of cancer cells when combined with chemotherapy | 64 | ||
| Ovarian cancer | Female nude mice (NOS2 and NOS2TR cells) | PAM produced anticancer effects on chemoresistant cancer cells; indirect plasma treatment can lead to a better prognosis | 158 | ||
| Glioblastoma | Female nude mice | CAP activated Ringer’s lactate solution could be employed for use in cancer therapy | 159 | ||
| Breast cancer | |||||
| Ovarian cancer | Mice (ES2 cells) | Intraperitoneal administration of PAM can be used in ovarian cancer treatment | 151 | ||
| Gastric carcinoma | Male BALB/c Slc-nu/nu mice | PAM can be used for peritoneal metastases treatment in tissues of gastric carcinoma | 157 |
No specific cancer type was mentioned.
Future perspectives
CAP has outlined an entirely new frontier in cancer therapy: plasma oncology. Various in vivo and in vitro assay-based research programs performed in the past have established significant and selective activity of CAP on tumor cells.60,104 The use of CAP in cancer started somewhere around 2010 and has progressed ever since. After all, the studies conducted now suggest that the time has come to finally pave a way for more clinical trials in the future. The preliminary steps have been taken, which was reflected by the launch of the first clinical trial by the FDA in 2019.57,102,103 Another recent advance is in the in vivo treatment of glioblastoma.105
CAP can either be used alone or in synergy with other conventional therapies for effective cancer treatment. A combination of pulsed electric field and CAP can be used successfully as palliative therapy for cancer patients.59 CAP and chemotherapeutic drugs have the potential to produce synergistic effects; for example, CAP and gemcitabine.106 CAP can also be used to reinstate the susceptivity of cancer chemoresistant cells.69 Additionally, it can be used to improve the efficacy of current immunotherapies for cancer treatment. Recently, the potential of CAP as a supplement to immunotherapy has been established particularly for the treatment of glioblastoma.107 CAP combined with nanotechnology can be very promising.108,109 Currently, plasma is used as a palliative treatment and is EBM level III and IV for head and neck cancer. CAP has the potential to be used in the clinic for manageable tumor stem cells or small tumors soon.68
Self-organization has been a focus of research recently. The automatic transition from a homogeneous state to a regular or transitional pattern is self-organization. Self-organization in CAP can lead to coherent structure formation. These coherent structures can be used to make adaptive CAP devices. The adaptive CAP approach can be used to obtain real-time data, which can help in modifying the composition and power of plasma to obtain optimal therapeutic effects. This can serve as a tool in personalized medication. CAP treatment can be tailormade for every patient based on his or her requirements and genotype.110,111
CAP has great potential in pharmaceutical applications but it still faces opportunities and challenges because it is still at the exploratory stage. The molecular mechanism of CAP remains unclear and there is no explanation for the synergistic effect of CAP and chemotherapy in cancer. Moreover, there is also a lack of sufficient and definite in vivo data. Thus, strenuous efforts are required for CAP to become a truly novel drug.110,112
Concluding remarks
Plasma has come a long way from being used to kill bacteria to its use in cancer therapy. Since 2010, there has been a huge surge in research in plasma oncology. Today, it has become one of the trending topics in cancer research. The anticipation of the immense societal impact that CAP could one day have justifies the awareness, funding and efforts of researchers in this direction. The FDA approval of the very first clinical trials in 2019 is evidence thereof. CAP selectivity in cancer treatment, dose-dependent activity and synergism with conventional therapies has proven CAP to be an adjunct in cancer therapy. CAP can be employed for treating various cancer types including melanoma, glioblastoma, cervical cancer, breast cancer and prostate cancer. The newer approaches of combining nanotechnology and CAP will only result in better results. Overall, further progress in plasma medicine demands a clear understanding in chemistry, technology, microbiology and engineering induced by CAP.
Figure 6.
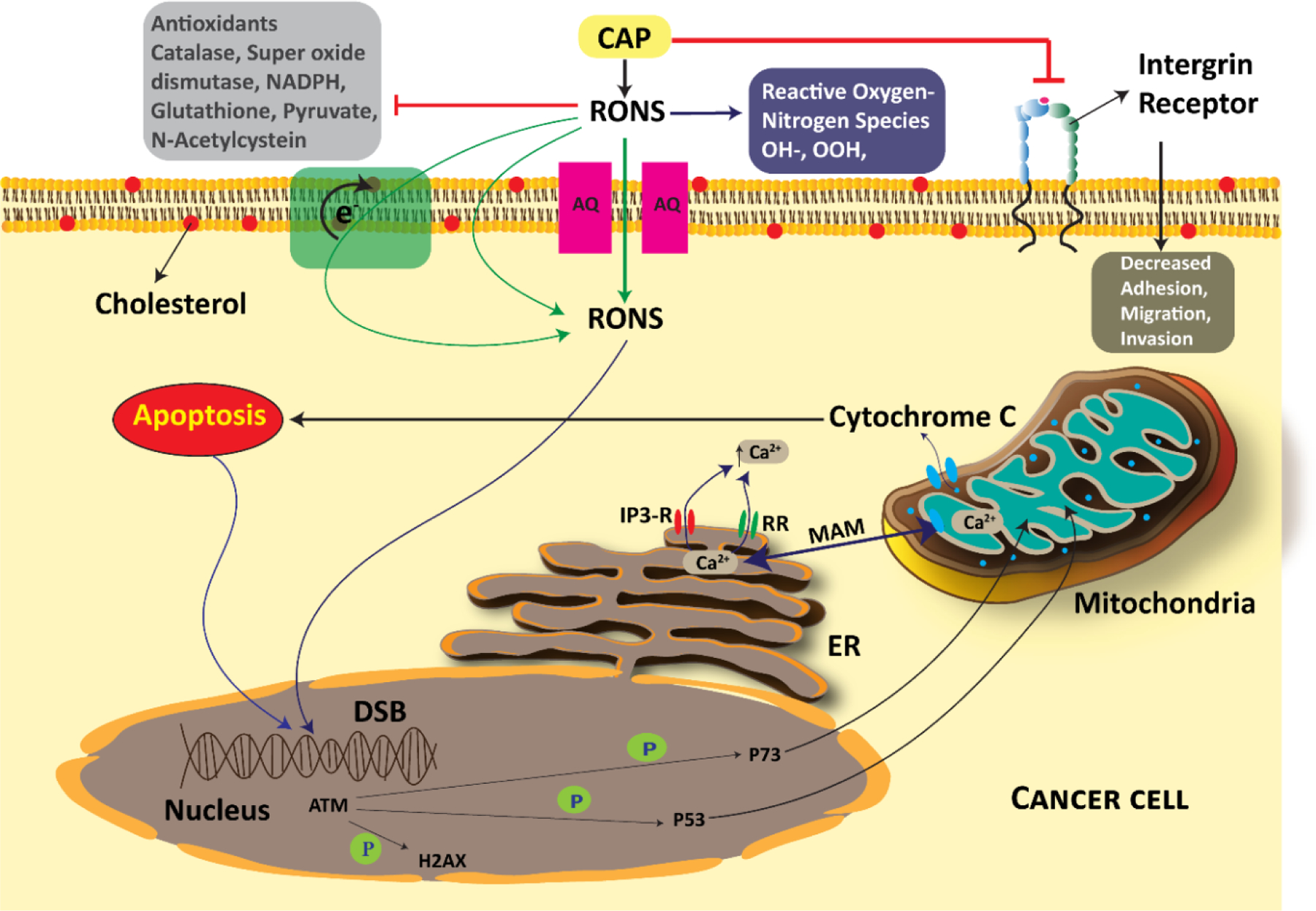
Cold atmospheric plasma (CAP) an its mechanisms of action in cancer cells.
Table 1.
Overview of CAP sources used in cancer treatment
| Plasma source type | Plasma source | Device | Used gas | Frequency range | Power/discharge voltage | Treatment |
|---|---|---|---|---|---|---|
| Direct source | DBD | Floating electrode DBD | Air | 35 MHz | 4 ±1 W | Melanoma113 |
| DBD plasma needle | Argon | AC 8–40 kHz |
30 kV | Lung adenocarcinoma114 | ||
| PlasmaDerm® VU-2010 | Argon | DC pulsed 100–400 Hz |
14 kV | Melanoma115,116 | ||
| Upper electrode: silver paste Lower electrode: steel mesh Dielectric material: glass |
Air | AC 60 Hz |
5.7 W | Human cancer cell lines: KB, MCF-7, HeLa, H460, SNU-80 and T98G117 | ||
| Electrodes: copper Dielectric material: quartz | Air | Pulse-like 50 kHz, 5 μs pulse width |
370–1000 V/ 0.6–7 W |
Colorectal cancer118 | ||
| Miniature DBD device | Air | Pulsed 1000 Hz, 10 μs pulse width |
12 kV | Lung adenocarcinoma119 | ||
| DBD inside 96-well plate (planar electrode) | Air | AC 50 Hz | 15 kV | Cervical cancer120 | ||
| DBD plasma source was used to prepare PAM | Air | AC 15 kHz |
6.8 ±0.6 W | Breast cancer121 | ||
| Indirect source | Jets | Plasma jet | Helium | 25 kHz | 4 kV | Breast cancer122 |
| Atmospheric pressure microplasma jet (APMPJ) | Helium | 60 kHz | 5–5.5 kV | Lung cancer123 | ||
| APMPJ | Helium | 6 kHz | 4 kV | Breast cancer124 | ||
| APPJ | Helium | 50 kHz | 6.38 kV | Skin cancer125 | ||
| kINPen® | Argon | 1–1.5 MHz | 1–6 kV | Pancreatic cancer83,126 Melanoma127 Colon cancer128 |
||
| J-Plasma System® (Bovie Medical Corporation) | Helium | 492 kHz | 40 W/6.5 kV | Prostate cancer129 | ||
| invivoPen | Helium | AC 8.8 kHz |
5 kV | Breast cancer130 | ||
| Torches | Plasma-generated nitric oxide (PGNO)-generating microwave plasma | Nitrogen and oxygen mixture | Microwave 2.45 GHz |
400 W | Melanoma131 | |
| Hybrid source | Surface micro discharge | MiniFlatPlaster | Air | Pulsed 6.75 kHz |
7 kV | Melanoma132 Colorectal cancer133 |
Teaser:
This review will help readers to peek into the interesting world of plasma oncology and the way it has revolutionized cancer therapy, understanding its mechanism, applications and its future prospects.
Highlights:
Plasma oncology is an emerging field in treatment of diseases including cancer
Plasma has wide applications in biomedical, food and agriculture fields
Cold atmospheric plasma (CAP) is a selective and cell-specific antitumor modality
FDA approval of the first trial for use of CAP in cancer therapy came in 2019
CAP can also be used synergistically with conventional therapy against cancers
Acknowledgments
We would like to thanks to Achalla Vaishnav Pavan Kumar, Ram Prakash Lamba and Vanshikha Singh for their contribution in proofreading and design of the figures.
Biographies

Sunil Kumar Dubey
Dr Sunil Kumar Dubey is presently working as a General Manager, R&D Healthcare Division, Emami, Kolkata, India. He has >15 years of industrial, teaching, research and administrative experience. Before this he was Assistant Professor in the Department of Pharmacy, Birla Institute of Technology and Science (BITS), Pilani, India. He was also a visiting Assistant Research Professor in Department of Chemical and Biomolecular Engineering at University of Maryland, USA. He has extensive research experience in the area of pharmacokinetic and pharmacodynamic modeling and simulations, nanomedicines, preclinical and clinical studies. He has supervised PhD, postgraduate and undergraduate students. He has published several research articles, book chapters in renowned journals and presented papers at conferences in India and elsewhere. He has successfully completed various projects related to new product development, analytical method development, validation and pharmacokinetic and pharmacodynamic investigations.

Neha Dabholkar
Neha Dabholkar is presently working in Formulation R&D Injectables at IPDO, Dr Reddy’s Laboratories, Hyderabad, India. For the past 5 years, she has been working as an editorial assistant for Pharma Times – the newsmagazine of Indian Pharmaceutical Association (IPA). She holds her post-graduation qualification with specialization in pharmaceutics from Birla Institute of Technology and Science (BITS), Pilani. She has an eclectic research experience in the area of nanomedicine – particularly lipidic nanocarriers. She has several publications in respected international journals in her field of study. She has just started her pharmaceutical career and aspires to create a mark with her scientific approach and innovative ideas.

Prashant Kesharwani
Prashant Kesharwani is an assistant professor of pharmaceutics in the School of Pharmaceutical Education and Research, Jamia Hamdard University, New Delhi, India. An overarching goal of his current research is the development of nanoengineered drug delivery systems for various diseases with a focus on dendrimer-based drug delivery systems. He has numerous publications in respected international journals and books and has presented talks and presentations at international conferences. He is a recipient of many research grants from various funding bodies. He is also a receipt of several internationally acclaimed awards viz. the most prestigious ‘Ramanujan Fellowship Award’ from the Science and Engineering Research Board (SERB), Government of India. He actively participates in outreach and scientific dissemination for the service of the wider community.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
There are no conflicts of interest or disclosures associated with the manuscript.
References
- [1].Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. J Cancer Res Pract 2017;4:127–9. 10.1016/j.jcrpr.2017.07.001. [DOI] [Google Scholar]
- [2].National Cancer Institute. Cancer Statistics 2021. [Google Scholar]
- [3].Pashirzad M, Sathyapalan T, Sheikh A, Kesharwani P, Sahebkar A. Cancer stem cells: An overview of the pathophysiological and prognostic roles in colorectal cancer. Process Biochem 2022;115:19–29. 10.1016/J.PROCBIO.2022.02.006. [DOI] [Google Scholar]
- [4].Sheikh A, Md S, Kesharwani P. Aptamer grafted nanoparticle as targeted therapeutic tool for the treatment of breast cancer. Biomed Pharmacother 2022;146:112530. 10.1016/J.BIOPHA.2021.112530. [DOI] [PubMed] [Google Scholar]
- [5].Nitheesh Y, Pradhan R, Hejmady S, Taliyan R, Singhvi G, Alexander A, et al. Surface engineered nanocarriers for the management of breast cancer. Mater Sci Eng C 2021;130:112441. 10.1016/J.MSEC.2021.112441. [DOI] [PubMed] [Google Scholar]
- [6].Cullen JM, Breen M. An Overview of Molecular Cancer Pathogenesis, Prognosis, and Diagnosis. Tumors Domest Anim 2016:1–26. 10.1002/9781119181200.ch1. [DOI] [Google Scholar]
- [7].Dobson JM, Samuel S, Milstein H, Rogers K, Wood JLN. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J Small Anim Pract 2002. 10.1111/j.1748-5827.2002.tb00066.x. [DOI] [PubMed] [Google Scholar]
- [8].Adams VJ, Evans KM, Sampson J, Wood JLN. Methods and mortality results of a health survey of purebred dogs in the UK. J Small Anim Pract 2010. 10.1111/j.1748-5827.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- [9].Modi D, Nirmal J, Warsi MH, Bhatia M, Hasan N, Kesharwani P, et al. Formulation and development of tacrolimus-gellan gum nanoformulation for treatment of dry eye disease. Colloids Surfaces B Biointerfaces 2022;211:112255. 10.1016/J.COLSURFB.2021.112255. [DOI] [PubMed] [Google Scholar]
- [10].Singh D, Kesharwani P, Alhakamy NA, Siddique HR. Accentuating CircRNA-miRNA-Transcription Factors Axis: A Conundrum in Cancer Research. Front Pharmacol 2022;12:3904. 10.3389/FPHAR.2021.784801/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sheikh A, Md S, Kesharwani P. RGD engineered dendrimer nanotherapeutic as an emerging targeted approach in cancer therapy. J Control Release 2021;340:221–42. 10.1016/J.JCONREL.2021.10.028. [DOI] [PubMed] [Google Scholar]
- [12].Singh V, Kesharwani P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. J Control Release 2021;338:394–409. 10.1016/J.JCONREL.2021.08.054. [DOI] [PubMed] [Google Scholar]
- [13].Nenclares P, Harrington KJ. The biology of cancer. Med (United Kingdom) 2020. 10.1016/j.mpmed.2019.11.001. [DOI] [Google Scholar]
- [14].Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003. 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- [15].Singh V, Md S, Alhakamy NA, Kesharwani P. Taxanes loaded polymersomes as an emerging polymeric nanocarrier for cancer therapy. Eur Polym J 2022;162:110883. 10.1016/J.EURPOLYMJ.2021.110883. [DOI] [Google Scholar]
- [16].Madamsetty VS, Tavakol S, Moghassemi S, Dadashzadeh A, Schneible JD, Fatemi I, et al. Chitosan: A versatile bio-platform for breast cancer theranostics. J Control Release 2021;341:733–52. 10.1016/J.JCONREL.2021.12.012. [DOI] [PubMed] [Google Scholar]
- [17].Singh V, Kesharwani P. Dendrimer as a promising nanocarrier for the delivery of doxorubicin as an anticancer therapeutics 10.1080/09205063.2021.1938859 2021:1–29. 10.1080/09205063.2021.1938859. [DOI] [PubMed] [Google Scholar]
- [18].Singh A, Handa M, Ruwali M, Flora SJ., Shukla R, Kesharwani P. Nanocarrier mediated autophagy: An emerging trend for cancer therapy. Process Biochem 2021;109:198–206. 10.1016/J.PROCBIO.2021.07.011. [DOI] [Google Scholar]
- [19].Cancer council Australia. Cancer biology: molecular and genetic basis 2014. [Google Scholar]
- [20].Muller PAJ, Vousden KH. P53 mutations in cancer. Nat Cell Biol 2013. 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- [21].Chae SW, Sohn JH, Kim DH, Choi YJ, Park YL, Kim K, et al. Overexpressions of cyclin B1, cdc2, p16 and p53 in human breast cancer: The clinicopathologic correlations and prognostic implications. Yonsei Med J 2011. 10.3349/ymj.2011.52.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Singh V, Sahebkar A, Kesharwani P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur Polym J 2021;158:110683. 10.1016/J.EURPOLYMJ.2021.110683. [DOI] [Google Scholar]
- [23].Chadar R, Afsana, Kesharwani P. Nanotechnology-based siRNA delivery strategies for treatment of triple negative breast cancer. Int J Pharm 2021;605:120835. 10.1016/J.IJPHARM.2021.120835. [DOI] [PubMed] [Google Scholar]
- [24].Surekha B, Kommana NS, Dubey SK, Kumar AVP, Shukla R, Kesharwani P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surfaces B Biointerfaces 2021;204:111837. 10.1016/j.colsurfb.2021.111837. [DOI] [PubMed] [Google Scholar]
- [25].Sheikh A, Alhakamy NA, Md S, Kesharwani P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front Pharmacol 2022;12:4024. 10.3389/FPHAR.2021.803304/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blasco MA. Telomeres and human disease: Ageing, cancer and beyond. Nat Rev Genet 2005. 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- [27].Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis 2009. 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Witsch E, Sela M, Yarden Y. Roles for Growth Factors in Cancer Progression. Physiology 2010. 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cigudosa JC, Parsa NZ, Louie DC, Filippa DA, Jhanwar SC, Johansson B, et al. Cytogenetic analysis of 363 consecutively ascertained diffuse large B-cell lymphomas. Genes Chromosom Cancer 1999. . [DOI] [PubMed] [Google Scholar]
- [30].Chial BH, Write PD, Right S, Education N. Proto-oncogenes to Oncogenes to Cancer. Nat Educ 2008. 10.1038/315758a0. [DOI] [Google Scholar]
- [31].Lee EYHP, Muller WJ. Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol 2010. 10.1101/cshperspect.a003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carmeliet P VEGF as a key mediator of angiogenesis in cancer. Oncology 2005. 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- [33].Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013. 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kessenbrock K, Plaks V, Werb Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010. 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol 2018;9. 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sudhakar A History of Cancer, Ancient and Modern Treatment Methods. J Cancer Sci Ther 2009. 10.4172/1948-5956.100000e2. [DOI] [PubMed] [Google Scholar]
- [37].Faguet GB. A brief history of cancer: Age-old milestones underlying our current knowledge database. Int J Cancer 2015. 10.1002/ijc.29134. [DOI] [PubMed] [Google Scholar]
- [38].Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M, et al. Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 2011. 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hajdu SI. Pathfinders in oncology from ancient times to the end of the Middle Ages. Cancer 2016. 10.1002/cncr.29955. [DOI] [PubMed] [Google Scholar]
- [40].Hajdu SI, Vadmal M, Tang P. A note from history: Landmarks in history of cancer, part 7. Cancer 2015. 10.1002/cncr.29365. [DOI] [PubMed] [Google Scholar]
- [41].National Cancer Institute. NCI Dictionaries 2020. [Google Scholar]
- [42].Damyanov CA, Maslev IK, Pavlov VS. Conventional Treatment of Cancer Realities and Problems. Ann Complement Altern Med 2018;1:1–9. [Google Scholar]
- [43].Tannock IF. Conventional cancer therapy: Promise broken or promise delayed? Lancet 1998;351. 10.1016/S0140-6736(98)90327-0. [DOI] [PubMed] [Google Scholar]
- [44].Dubey SK, Kali M, Hejmady S, Saha RN, Alexander A, Kesharwani P. Recent advances of dendrimers as multifunctional nano-carriers to combat breast cancer. Eur J Pharm Sci 2021;164:105890. 10.1016/j.ejps.2021.105890. [DOI] [PubMed] [Google Scholar]
- [45].Hoffmann C, Berganza C, Zhang J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med Gas Res 2013;3:1–15. 10.1186/2045-9912-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Langmuir I Oscillations in Ionized Gases. Proc Natl Acad Sci 1928. 10.1073/pnas.14.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tonks L The Birth of “Plasma.” Am J Phys 1967. 10.1119/1.1974266. [DOI] [Google Scholar]
- [48].Keidar M, Yan D, Sherman JH, Keidar M, Yan D, Sherman JH. Plasma as a fourth state of matter. Cold Plasma Cancer Ther 2019. 10.1088/2053-2571/aafb9cch1. [DOI] [Google Scholar]
- [49].Laroussi M Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front Phys 2020;8:1–7. 10.3389/fphy.2020.00074. [DOI] [Google Scholar]
- [50].Milella A, Palumbo F. Encyclopedia of Membranes. Encycl Membr 2015. 10.1007/978-3-642-40872-4. [DOI] [Google Scholar]
- [51].Keidar M, Walk R, Shashurin A, Srinivasan P, Sandler A, Dasgupta S, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer 2011;105:1295–301. 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Graves DB. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Process Polym 2014. 10.1002/ppap.201400068. [DOI] [Google Scholar]
- [53].Laroussi M Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans Plasma Sci 1996. 10.1109/27.533129. [DOI] [Google Scholar]
- [54].Kelly-Wintenberg K, Montie TC, Brickman C, Roth JR, Carr AK, Sorge K, et al. Room temperature sterilization of surfaces and fabrics with a One Atmosphere Uniform Glow Discharge Plasma. J Ind Microbiol Biotechnol 1998. 10.1038/sj.jim.2900482. [DOI] [PubMed] [Google Scholar]
- [55].Laroussi M Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans Plasma Sci 2002. 10.1109/TPS.2002.804220. [DOI] [Google Scholar]
- [56].Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol 2010. 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- [57].Laroussi M From killing bacteria to destroying cancer cells: 20 years of plasma medicine. Plasma Process Polym 2014. 10.1002/ppap.201400152. [DOI] [Google Scholar]
- [58].Vaishnav Pavan Kumar A, Dubey SK, Tiwari S, Puri A, Hejmady S, Goraine B, et al. Recent advances in nanoparticles mediated photothermal therapy induced tumor regression. Int J Pharm 2021:120848. 10.1016/J.IJPHARM.2021.120848. [DOI] [PubMed] [Google Scholar]
- [59].Wolff CM, Steuer A, Stoffels I, von Woedtke T, Weltmann KD, Bekeschus S, et al. Combination of cold plasma and pulsed electric fields – A rationale for cancer patients in palliative care. Clin Plasma Med 2019. 10.1016/j.cpme.2020.100096. [DOI] [Google Scholar]
- [60].Schlegel J, Köritzer J, Boxhammer V. Plasma in cancer treatment. Clin Plasma Med 2013. 10.1016/j.cpme.2013.08.001. [DOI] [Google Scholar]
- [61].Metelmann HR, Nedrelow DS, Seebauer C, Schuster M, von Woedtke T, Weltmann KD, et al. Head and neck cancer treatment and physical plasma. Clin Plasma Med 2015. 10.1016/j.cpme.2015.02.001. [DOI] [Google Scholar]
- [62].Dubuc A, Monsarrat P, Virard F, Merbahi N, Sarrette JP, Laurencin-Dalicieux S, et al. Use of cold-atmospheric plasma in oncology: a concise systematic review. Ther Adv Med Oncol 2018. 10.1177/1758835918786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen Z, Lin L, Zheng Q, Sherman JH, Canady J, Trink B, et al. Micro-Sized Cold Atmospheric Plasma Source for Brain and Breast Cancer Treatment. Plasma Med 2018. 10.1615/plasmamed.2018026588. [DOI] [Google Scholar]
- [64].Saadati F, Mahdikia H, Abbaszadeh HA, Abdollahifar MA, Khoramgah MS, Shokri B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci Rep 2018;8:1–15. 10.1038/s41598-018-25990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Plasmas L, Schlegel J. Cancer Treatment using Articles : Cold Atmospheric Plasma and Cancer Articles : Cold Atmospheric Plasma and Cancer 2012;6:1295–301. [Google Scholar]
- [66].Hirst AM, Frame FM, Maitland NJ, O’Connell D. Low temperature plasma: A novel focal therapy for localized prostate cancer? Biomed Res Int 2014. 10.1155/2014/878319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bernhardt T, Semmler ML, Schäfer M, Bekeschus S, Emmert S, Boeckmann L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid Med Cell Longev 2019. 10.1155/2019/3873928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Metelmann HR, Seebauer C, Rutkowski R, Schuster M, Bekeschus S, Metelmann P. Treating cancer with cold physical plasma: On the way to evidence-based medicine. Contrib to Plasma Phys 2018. 10.1002/ctpp.201700085. [DOI] [Google Scholar]
- [69].Köritzer J, Boxhammer V, Schäfer A, Shimizu T, Klämpfl TG, Li YF, et al. Restoration of Sensitivity in Chemo - Resistant Glioma Cells by Cold Atmospheric Plasma. PLoS One 2013. 10.1371/journal.pone.0064498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ishaq M, Han ZJ, Kumar S, Evans MDM, Ostrikov K. Atmospheric-pressure plasma- and TRAIL-induced apoptosis in TRAIL-resistant colorectal cancer cells. Plasma Process Polym 2015. 10.1002/ppap.201400207. [DOI] [Google Scholar]
- [71].Zhu W, Lee SJ, Castro NJ, Yan D, Keidar M, Zhang LG. Synergistic Effect of Cold Atmospheric Plasma and Drug Loaded Core-shell Nanoparticles on Inhibiting Breast Cancer Cell Growth. Sci Rep 2016;6:1–11. 10.1038/srep21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hejmady S, Pradhan R, Alexander A, Agrawal M, Singhvi G, Gorain B, et al. Recent advances in targeted nanomedicine as promising antitumor therapeutics. Drug Discov Today 2020;25:2227–44. 10.1016/j.drudis.2020.09.031. [DOI] [PubMed] [Google Scholar]
- [73].Yan D, Talbot A, Nourmohammadi N, Cheng X, Canady J, Sherman J, et al. Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci Rep 2015. 10.1038/srep18339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gay-Mimbrera J, García MC, Isla-Tejera B, Rodero-Serrano A, García-Nieto AV, Ruano J. Clinical and Biological Principles of Cold Atmospheric Plasma Application in Skin Cancer. Adv Ther 2016. 10.1007/s12325-016-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dubey SK, Parab S, Alexander A, Agrawal M, Achalla VPK, Pal UN, et al. Cold atmospheric plasma therapy in wound healing. Process Biochem 2022;112:112–23. 10.1016/J.PROCBIO.2021.11.017. [DOI] [Google Scholar]
- [76].Keidar M, Shashurin A, Volotskova O, Ann Stepp M, Srinivasan P, Sandler A, et al. Cold atmospheric plasma in cancer therapy. Phys Plasmas 2013. 10.1063/1.4801516. [DOI] [Google Scholar]
- [77].Kang WS, Hong YC, Hong YB, Kim JH, Uhm HS. Atmospheric-pressure cold plasma jet for medical applications. Surf Coatings Technol 2010. 10.1016/j.surfcoat.2010.08.138. [DOI] [Google Scholar]
- [78].Keidar M, Yan D, Sherman JH, Keidar M, Yan D, Sherman JH. Introduction to the non-thermal plasmas. Cold Plasma Cancer Ther 2019. 10.1088/2053-2571/aafb9cch2. [DOI] [Google Scholar]
- [79].Bárdos L, Baránková H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010. 10.1016/j.tsf.2010.07.044. [DOI] [Google Scholar]
- [80].Brandenburg R Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci Technol 2017. 10.1088/1361-6595/aa6426. [DOI] [Google Scholar]
- [81].Yan D, Sherman JH, Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017. 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schütze A, Jeong JY, Babayan SE, Park J, Selwyn GS, Hicks RF. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans Plasma Sci 1998. 10.1109/27.747887. [DOI] [Google Scholar]
- [83].Liedtke KR, Diedrich S, Pati O, Freund E, Flieger R, Heidecke CD, et al. Cold physical plasma selectively elicits apoptosis in murine pancreatic cancer cells in vitro and in ovo. Anticancer Res 2018. 10.21873/anticanres.12901. [DOI] [PubMed] [Google Scholar]
- [84].Reuter S, Von Woedtke T, Weltmann KD. The kINPen - A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J Phys D Appl Phys 2018;51. 10.1088/1361-6463/aab3ad. [DOI] [Google Scholar]
- [85].Arndt S, Schmidt A, Karrer S, von Woedtke T. Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clin Plasma Med 2018. 10.1016/j.cpme.2018.01.002. [DOI] [Google Scholar]
- [86].Mann MS, Tiede R, Gavenis K, Daeschlein G, Bussiahn R, Weltmann KD, et al. Introduction to DIN-specification 91315 based on the characterization of the plasma jet kINPen® MED. Clin Plasma Med 2016. 10.1016/j.cpme.2016.06.001. [DOI] [Google Scholar]
- [87].Biscop E, Lin A, Van Boxem W, Van Loenhout J, De Backer J, Deben C, et al. Influence of cell type and culture medium on determining cancer selectivity of cold atmospheric plasma treatment. Cancers (Basel) 2019. 10.3390/cancers11091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dubey SK, Salunkhe S, Agrawal M, Kali M, Singhvi G, Tiwari S, et al. Understanding the pharmaceutical aspects of dendrimers for the delivery of anticancer drugs. Curr Drug Targets 2019;20:1–13. 10.2174/1389450120666191031092259. [DOI] [PubMed] [Google Scholar]
- [89].Dubey SK, Pradyuth SK, Saha RN, Singhvi G, Alexander A, Agrawal M, et al. Application of photodynamic therapy drugs for management of glioma. Https://DoiOrg/101142/S1088424619300192 2020:162–74. 10.1142/S1088424619300192. [DOI] [Google Scholar]
- [90].Keidar M, Yan D, Sherman JH, Keidar M, Yan D, Sherman JH. The anti-cancer mechanism of cold atmospheric plasma in vitro. Cold Plasma Cancer Ther 2019. 10.1088/2053-2571/aafb9cch4. [DOI] [Google Scholar]
- [91].Turrini E, Laurita R, Stancampiano A, Catanzaro E, Calcabrini C, Maffei F, et al. Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T-Lymphoblastoid Leukemia Cells. Oxid Med Cell Longev 2017;2017. 10.1155/2017/4271065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kim SJ, Chung TH. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci Rep 2016. 10.1038/srep20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bruno G, Heusler T, Lackmann JW, von Woedtke T, Weltmann KD, Wende K. Cold physical plasma-induced oxidation of cysteine yields reactive sulfur species (RSS). Clin Plasma Med 2019. 10.1016/j.cpme.2019.100083. [DOI] [Google Scholar]
- [94].Bogaerts A, Yusupov M, Razzokov J, Van der Paal J. Plasma for cancer treatment: How can RONS penetrate through the cell membrane? Answers from computer modeling. Front Chem Sci Eng 2019. 10.1007/s11705-018-1786-8. [DOI] [Google Scholar]
- [95].Faramarzi F, Zafari P, Alimohammadi M, Moonesi M, Rafiei A, Bekeschus S. Cold Physical Plasma in Cancer Therapy: Mechanisms, Signaling, and Immunity. Oxid Med Cell Longev 2021;2021:1–19. 10.1155/2021/9916796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rehman MU, Jawaid P, Uchiyama H, Kondo T. Comparison of free radicals formation induced by cold atmospheric plasma, ultrasound, and ionizing radiation. Arch Biochem Biophys 2016. 10.1016/j.abb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- [97].Niu N, Wang L. In vitro human cell line models to predict clinical response to anticancer drugs. Pharmacogenomics 2015. 10.2217/pgs.14.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Keidar M, Yan D, Sherman JH, Keidar M, Yan D, Sherman JH. The anti-cancer effect of CAP treatment in vivo. Cold Plasma Cancer Ther 2019. 10.1088/2053-2571/aafb9cch5. [DOI] [Google Scholar]
- [99].Rutkowski R, Schuster M, Unger J, Seebauer C, Metelmann HR, Woedtke T v., et al. Hyperspectral imaging for in vivo monitoring of cold atmospheric plasma effects on microcirculation in treatment of head and neck cancer and wound healing. Clin Plasma Med 2017. 10.1016/j.cpme.2017.09.002. [DOI] [Google Scholar]
- [100].Schuster M, Seebauer C, Rutkowski R, Hauschild A, Podmelle F, Metelmann C, et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J Cranio-Maxillofacial Surg 2016. 10.1016/j.jcms.2016.07.001. [DOI] [PubMed] [Google Scholar]
- [101].Metelmann HR, Seebauer C, Miller V, Fridman A, Bauer G, Graves DB, et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin Plasma Med 2018. 10.1016/j.cpme.2017.09.001. [DOI] [Google Scholar]
- [102].ClinicalTrials.gov. Canady Helios Cold Plasma Scalpel Treatment at the Surgical Margin and Macroscopic Tumor Sites 2019.
- [103].Michael Irving NEW ATLAS. FDA approves clinical trial of cold plasma “scalpel” for cancer treatment 2019.
- [104].Jain R, Pradhan R, Hejmady S, Singhvi G, Dubey SK. Fluorescence-Based Method for Sensitive and Rapid Estimation of Chlorin e6 in Stealth liposomes for Photodynamic therapy against Cancer. Spectrochim Acta Part A Mol Biomol Spectrosc 2020;244:118823. 10.1016/j.saa.2020.118823. [DOI] [PubMed] [Google Scholar]
- [105].Keidar M, Yan D, Sherman JH, Almeida ND, Sack K, Sherman JH. Clinical applications of cold atmospheric plasma for glioblastoma. Cold Plasma Cancer Ther 2019:0–15. 10.1088/2053-2571/aafb9cch8. [DOI] [Google Scholar]
- [106].Masur K, Von Behr M, Bekeschus S, Weltmann KD, Hackbarth C, Heidecke CD, et al. Synergistic Inhibition of Tumor Cell Proliferation by Cold Plasma and Gemcitabine. Plasma Process. Polym, 2015. 10.1002/ppap.201500123. [DOI] [Google Scholar]
- [107].Almeida ND, Klein AL, Hogan EA, Terhaar SJ, Kedda J, Uppal P, et al. Cold Atmospheric Plasma as an Adjunct to Immunotherapy for Glioblastoma Multiforme. World Neurosurg 2019. 10.1016/j.wneu.2019.06.209. [DOI] [PubMed] [Google Scholar]
- [108].Singhvi G, Rapalli VK, Nagpal S, Dubey SK, Saha RN. Nanocarriers as Potential Targeted Drug Delivery for Cancer Therapy. Nanosci Med 2020;1:51–88. 10.1007/978-3-030-29207-2_2. [DOI] [Google Scholar]
- [109].Jain R, Sarode I, Singhvi G, Dubey SK. Nanocarrier Based Topical Drug Delivery-A Promising Strategy for Treatment of Skin Cancer. Curr Pharm Des 2020;26:4615–23. 10.2174/1381612826666200826140448. [DOI] [PubMed] [Google Scholar]
- [110].Keidar M A prospectus on innovations in the plasma treatment of cancer. Phys Plasmas 2018. 10.1063/1.5034355. [DOI] [Google Scholar]
- [111].Keidar M, Yan D, Beilis II, Trink B, Sherman JH. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol 2018. 10.1016/j.tibtech.2017.06.013. [DOI] [PubMed] [Google Scholar]
- [112].Gao L, Shi X, Wu X. Applications and challenges of low temperature plasma in pharmaceutical field. J Pharm Anal 2020. 10.1016/j.jpha.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, et al. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in Melanoma skin cancer cell lines. Plasma Chem Plasma Process 2007. 10.1007/s11090-007-9048-4. [DOI] [Google Scholar]
- [114].Huang J, Chen W, Li H, Wang XQ, Lv GH, Khohsa ML, et al. Deactivation of A549 cancer cells in vitro by a dielectric barrier discharge plasma needle. J Appl Phys 2011. 10.1063/1.3553873. [DOI] [Google Scholar]
- [115].Daeschlein G, Scholz S, Lutze S, Arnold A, von Podewils S, Kiefer T, et al. Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Exp Dermatol 2013. 10.1111/exd.12201. [DOI] [PubMed] [Google Scholar]
- [116].CINOGY®. The PlasmaDerm® Product Family 2020. [Google Scholar]
- [117].Panngom K, Baik KY, Ryu YH, Uhm HS, Choi EH. Differential responses of cancer cell lines to non-thermal plasma from dielectric barrier discharge. Curr Appl Phys 2013. 10.1016/j.cap.2012.12.025. [DOI] [Google Scholar]
- [118].Han D, Cho JH, Lee RH, Bang W, Park K, Kim MS, et al. Antitumorigenic effect of atmospheric-pressure dielectric barrier discharge on human colorectal cancer cells via regulation of Sp1 transcription factor. Sci Rep 2017. 10.1038/srep43081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Karki SB, Yildirim-Ayan E, Eisenmann KM, Ayan H. Miniature Dielectric Barrier Discharge Nonthermal Plasma Induces Apoptosis in Lung Cancer Cells and Inhibits Cell Migration. Biomed Res Int 2017. 10.1155/2017/8058307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gerber IC, Mihai CT, Gorgan L, Ciorpac M, Nita A, Pohoata V, et al. Air Dielectric Barrier Discharge Plasma Source For In Vitro Cancer Studies. Clin Plasma Med 2018. 10.1016/j.cpme.2017.12.006. [DOI] [Google Scholar]
- [121].Subramanian PSG, Jain A, Shivapuji AM, Sundaresan NR, Dasappa S, Rao L. Plasma-activated water from a dielectric barrier discharge plasma source for the selective treatment of cancer cells. Plasma Process Polym 2020:1–13. 10.1002/ppap.201900260. [DOI] [Google Scholar]
- [122].Akhlaghi M, Rajayi H, Mashayekh AS, Khani M, Hassan ZM, Shokri B. On the design and characterization of a new cold atmospheric pressure plasma jet and its applications on cancer cells treatment. Biointerphases 2015. 10.1116/1.4918806. [DOI] [PubMed] [Google Scholar]
- [123].Zuo X, Wei Y, Wei Chen L, Dong Meng Y. Non-equilibrium atmospheric pressure microplasma jet: An approach to endoscopic therapies. Phys Plasmas 2013. 10.1063/1.4817958. [DOI] [Google Scholar]
- [124].Mirpour S, Piroozmand S, Soleimani N, Jalali Faharani N, Ghomi H, Fotovat Eskandari H, et al. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci Rep 2016;6:1–10. 10.1038/srep29048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kim DY, Kim SJ, Joh HM, Chung TH. Characterization of an atmospheric pressure plasma jet array and its application to cancer cell treatment using plasma activated medium. Phys Plasmas 2018. 10.1063/1.5037249. [DOI] [Google Scholar]
- [126].Bekeschus S, Freund E, Spadola C, Privat-Maldonado A, Hackbarth C, Bogaerts A, et al. Risk assessment of kINPen plasma treatment of four human pancreatic cancer cell lines with respect to metastasis. Cancers (Basel) 2019. 10.3390/cancers11091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rajasekaran P, Mertmann P, Bibinov N, Wandke D, Viöl W, Awakowicz P. DBD plasma source operated in single-filamentary mode for therapeutic use in dermatology. J Phys D Appl Phys 2009. 10.1088/0022-3727/42/22/225201. [DOI] [Google Scholar]
- [128].Bekeschus S, Lin A, Fridman A, Wende K, Weltmann KD, Miller V. A Comparison of Floating-Electrode DBD and kINPen Jet: Plasma Parameters to Achieve Similar Growth Reduction in Colon Cancer Cells Under Standardized Conditions. Plasma Chem Plasma Process 2018. 10.1007/s11090-017-9845-3. [DOI] [Google Scholar]
- [129].Barekzi N, Laroussi M. Effects of low temperature plasmas on cancer cells. Plasma Process Polym 2013. 10.1002/ppap.201300083. [DOI] [Google Scholar]
- [130].Zhou X, Cai D, Xiao S, Ning M, Zhou R, Zhang S, et al. Invivopen: A novel plasma source for in vivo cancer treatment. J Cancer 2020. 10.7150/jca.38613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lee CB, Seo IH, Chae MW, Park JW, Choi EH, Uhm HS, et al. Anticancer Activity of Liquid Treated with Microwave Plasma-Generated Gas through Macrophage Activation. Oxid Med Cell Longev 2020. 10.1155/2020/2946820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Arndt S, Wacker E, Li YF, Shimizu T, Thomas HM, Morfill GE, et al. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp Dermatol 2013. 10.1111/exd.12127. [DOI] [PubMed] [Google Scholar]
- [133].Schneidera C, Arndta S, Zimmermann JL, Li Y, Karrer S, Bosserhoff AK. Cold atmospheric plasma treatment inhibits growth in colorectal cancer cells. Biol Chem 2018. 10.1515/hsz-2018-0193. [DOI] [PubMed] [Google Scholar]
- [134].Yan X, Zou F, Zhao S, Lu X, He G, Xiong Z, et al. On the mechanism of plasma inducing cell apoptosis. IEEE Trans Plasma Sci 2010. 10.1109/TPS.2010.2056393. [DOI] [Google Scholar]
- [135].Ahn HJ, Kim K Il, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One 2011. 10.1371/journal.pone.0028154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Tuhvatulin AI, Sysolyatina EV, Scheblyakov DV, Logunov DY, Vasiliev MM, Yurova MA, et al. Non-Thermal Plasma Causes P53-Dependent Apoptosis in Human Colon Carcinoma Cells. Acta Naturae 2012. 10.32607/20758251-2012-4-3-82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang M, Holmes B, Cheng X, Zhu W, Keidar M, Zhang LG. Cold Atmospheric Plasma for Selectively Ablating Metastatic Breast Cancer Cells. PLoS One 2013. 10.1371/journal.pone.0073741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Schmidt A, Bekeschus S, von Woedtke T, Hasse S. Cell migration and adhesion of a human melanoma cell line is decreased by cold plasma treatment. Clin Plasma Med 2015. 10.1016/j.cpme.2015.05.003. [DOI] [Google Scholar]
- [139].Canal C, Fontelo R, Hamouda I, Guillem-Marti J, Cvelbar U, Ginebra MP. Plasma-induced selectivity in bone cancer cells death. Free Radic Biol Med 2017. 10.1016/j.freeradbiomed.2017.05.023. [DOI] [PubMed] [Google Scholar]
- [140].Yan D, Xu W, Yao X, Lin L, Sherman JH, Keidar M. The Cell Activation Phenomena in the Cold Atmospheric Plasma Cancer Treatment. Sci Rep 2018. 10.1038/s41598-018-33914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lee S, Park S, Lee H, Jeong D, Ham J, Choi EH, et al. ChIP-seq analysis reveals alteration of H3K4 trimethylation occupancy in cancer-related genes by cold atmospheric plasma. Free Radic Biol Med 2018. 10.1016/j.freeradbiomed.2018.08.004. [DOI] [PubMed] [Google Scholar]
- [142].Xiang L, Xu X, Zhang S, Cai D, Dai X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic Biol Med 2018. 10.1016/j.freeradbiomed.2018.06.001. [DOI] [PubMed] [Google Scholar]
- [143].Iuchi K, Morisada Y, Yoshino Y, Himuro T, Saito Y, Murakami T, et al. Cold atmospheric-pressure nitrogen plasma induces the production of reactive nitrogen species and cell death by increasing intracellular calcium in HEK293T cells. Arch Biochem Biophys 2018. 10.1016/j.abb.2018.07.015. [DOI] [PubMed] [Google Scholar]
- [144].Van Loenhout J, Deben C, Jacobs J, De Waele J, Van Audenaerde J, Marcq E, et al. Immunogenic Potential Of Cold Atmospheric Plasma For The Treatment Of Pancreatic Cancer. Clin Plasma Med 2018;9:26. 10.1016/j.cpme.2017.12.041. [DOI] [Google Scholar]
- [145].Mokhtari H, Farahmand L, Yaserian K, Jalili N, Majidzadeh-A K. The antiproliferative effects of cold atmospheric plasma-activated media on different cancer cell lines, the implication of ozone as a possible underlying mechanism. J Cell Physiol 2019. 10.1002/jcp.27428. [DOI] [PubMed] [Google Scholar]
- [146].Xu D, Ning N, Xu Y, Wang B, Cui Q, Liu Z, et al. Effect of cold atmospheric plasma treatment on the metabolites of human leukemia cells. Cancer Cell Int 2019. 10.1186/s12935-019-0856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Yoon Y, Ku B, Lee K, Jung YJ, Baek SJ. Cold atmospheric plasma induces HMGB1 expression in cancer cells. Anticancer Res 2019;39:2405–13. 10.21873/anticanres.13358. [DOI] [PubMed] [Google Scholar]
- [148].Xia J, Zeng W, Xia Y, Wang B, Xu D, Liu D, et al. Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2-mediated nitric oxide synthase signaling. J Biophotonics 2019. 10.1002/jbio.201800046. [DOI] [PubMed] [Google Scholar]
- [149].Chang CH, ichi Yano K, Sato T. Nanosecond pulsed current under plasma-producing conditions induces morphological alterations and stress fiber formation in human fibrosarcoma HT-1080 cells. Arch Biochem Biophys 2020. 10.1016/j.abb.2020.108252. [DOI] [PubMed] [Google Scholar]
- [150].Bekeschus S, Eisenmann S, Sagwal SK, Bodnar Y, Moritz J, Poschkamp B, et al. xCT (SLC7A11) expression confers intrinsic resistance to physical plasma treatment in tumor cells. Redox Biol 2020. 10.1016/j.redox.2019.101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Nakamura K, Peng Y, Utsumi F, Tanaka H, Mizuno M, Toyokuni S, et al. Novel Intraperitoneal Treatment With Non-Thermal Plasma-Activated Medium Inhibits Metastatic Potential of Ovarian Cancer Cells. Sci Rep 2017. 10.1038/s41598-017-05620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Feil L, Koch A, Brucker S, Wallwiener D, Weiss M. Inhibition of cervical cancer cell growth by non-thermal atmospheric plasma application utilizing a thermal argon plasma source. Eur J Obstet Gynecol Reprod Biol 2019. 10.1016/j.ejogrb.2018.08.446. [DOI] [Google Scholar]
- [153].Fofana M, Buñay J, Judée F, Baron S, Menecier S, Nivoix M, et al. Selective treatments of prostate tumor cells with a cold atmospheric plasma jet. Clin Plasma Med 2020. 10.1016/j.cpme.2020.100098. [DOI] [Google Scholar]
- [154].He Z, Liu K, Manaloto E, Casey A, Cribaro GP, Byrne HJ, et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci Rep 2018;8:1–11. 10.1038/s41598-018-23262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Pereira S, Pinto E, Ribeiro PA, Sério S. Study of a Cold Atmospheric Pressure Plasma jet device for indirect treatment of Squamous Cell Carcinoma. Clin Plasma Med 2019. 10.1016/j.cpme.2018.09.001. [DOI] [Google Scholar]
- [156].Adachi T, Nonomura S, Horiba M, Hirayama T, Kamiya T, Nagasawa H, et al. Iron stimulates plasma-activated medium-induced A549 cell injury. Sci Rep 2016. 10.1038/srep20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Takeda S, Yamada S, Hattori N, Nakamura K, Tanaka H, Kajiyama H, et al. Intraperitoneal Administration of Plasma-Activated Medium: Proposal of a Novel Treatment Option for Peritoneal Metastasis From Gastric Cancer. Ann Surg Oncol 2017. 10.1245/s10434-016-5759-1. [DOI] [PubMed] [Google Scholar]
- [158].Utsumi F, Kajiyama H, Nakamura K, Tanaka H, Mizuno M, Ishikawa K, et al. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One 2013. 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Tanaka H, Nakamura K, Mizuno M, Ishikawa K, Takeda K, Kajiyama H, et al. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci Rep 2016. 10.1038/srep36282. [DOI] [PMC free article] [PubMed] [Google Scholar]


