Abstract
INTRODUCTION:
Non-amnestic presentations of neurodegenerative dementias, including posterior- and visual-predominant cognitive forms, are under-recognized. Specific screening measures for posterior cortical symptoms could allow for earlier, more accurate diagnosis and directed treatment.
METHODS:
Based on clinical experience with posterior cortical atrophy evaluations, high-yield screening questions were collected and organized into a 15-item self-report questionnaire, titled the Colorado Posterior Cortical Questionnaire (CPC-Q). The CPC-Q was then piloted within a longitudinal cohort of cognitive aging, including sixty-three older adults, including healthy older adults (n=33) and adults with either amnestic Alzheimer’s disease (n=21) or posterior cortical atrophy (PCA, n=9).
RESULTS:
The CPC-Q demonstrated acceptable psychometric properties (internal consistency, α =0.89; mean item-total correlation=0.62), correlated strongly with visuospatial measures on cognitive testing (p<0.001), and could distinguish PCA from non-PCA groups (p<0.001; AUC 0.94 (95% CI 0.88, 1.0)).
CONCLUSIONS:
The CPC-Q captured posterior cortical symptoms in older adults, using a gold standard of expert consensus PCA diagnosis. Future studies will validate the CPC-Q in a larger cohort, with recruitment of additional PCA participants, to evaluate its convergent and discriminant validity more thoroughly. As a short, self-report tool, the CPC-Q demonstrates potential to improve detection of non-amnestic neurodegenerative dementias in the clinical setting.
Keywords: cognitive impairment, visuospatial, screening, posterior cortical atrophy, Alzheimer’s disease
Introduction
Non-amnestic cognitive changes are common in aging adults with neurodegenerative syndromes (Possin, 2010) and have been linked with younger ages of onset of Alzheimer’s disease (AD) pathology (Barnes et al., 2015; Blenkinsop et al., 2020). However, these symptoms are frequently under-recognized in clinical evaluations, which can lead to inaccurate diagnosis and incomplete treatment plans. The limited evaluation of non-amnestic symptoms in clinical settings likely stems from both patient and provider factors: patients may not identify these cognitive changes as relevant to their clinical evaluation and providers may not have access to appropriate non-memory screening measures (Bradford et al., 2009; Crutch et al., 2012; Holden et al., 2020). Non-memory symptoms have significant diagnostic and functional implications; enhanced detection of such symptoms would improve accuracy of complex syndrome diagnosis and benefit patient quality of life (de Vugt & Verhey, 2013; Dubois et al., 2016).
Symptoms arising from posterior cortical dysfunction are among the most underappreciated indicators of degenerative disease (Crutch et al., 2017). These symptoms include dysfunction of cognitive processes subserved by occipital, posterior parietal, and posterior temporal regions of the brain and may manifest as visuospatial and visuoperceptual difficulties, as well as dysfunction in non-visual realms including dyspraxia, left/right disorientation, and dyscalculia (Crutch et al., 2012; Possin, 2010; Salimi et al., 2018). Posterior cortical signs and symptoms that are prominent, progressive, and evident early in a disease course, with relative sparing of memory and other cognitive domains, are the core features of posterior cortical atrophy (PCA) syndrome, an atypical neurodegenerative syndrome most commonly due to underlying AD pathology (Crutch et al., 2017). Posterior cortical symptoms are also present in amnestic AD and Lewy body dementia (LBD), with these patients performing worse than healthy aging adults on “dorsal stream” neuropsychological measures of visual localization and angle discrimination, as well as “ventral stream” indicators of object recognition (Possin, 2010; Quental et al., 2009). Moreover, people with AD often display progressive loss of calculation abilities over time (Martin et al., 2003). Symptoms may vary in severity due to the temporal sequence and topographic distribution of neuropathology, but the majority of patients with neurodegenerative dementia will display some degree of posterior cortical dysfunction as their disease progresses.
Currently, few self-report questionnaires incorporate questions to assess the onset of visuospatial and visuoperceptual symptoms in neurodegenerative diseases, instead focusing on early memory difficulties and/or changes in executive functions. To our knowledge, there are only two recently developed questionnaires specifically dedicated to the evaluation of posterior cortical symptoms, neither of which are self-report: one developed within a large dementia registry project based in Taiwan (History-based Artificial Intelligent Clinical Dementia Diagnostic System (HAICDDS)) (Wang et al., 2020), and another designed to characterize symptomology in patients with PCA (Croisile & Mollion, 2011). Within the HAICDDS registry, a seven-question, informant-based visuospatial and visuomotor questionnaire (HAI-VSQ) was deployed, demonstrating good sensitivity and specificity for distinguishing DLB from AD and controls within the registry, though other posterior-predominant cognitive syndromes, including PCA, were not evaluated (Wang et al., 2020). Using a 32-item survey in French for patients and caregivers, Croisile and Mollion (Croisile & Mollion, 2011) were able to differentiate between PCA and typical amnestic AD patients and showed correlations between their measure and an index of global cognitive function. Although an important addition to the assessment of PCA, there are several limitations to implementing this survey in a non-specialty-based setting, including the clinician-administered format, the total number of questions, and the lack of appraisal of some aspects of posterior cortical function (e.g., mathematical ability).
To address this gap in research and clinical care, we developed a brief, self-report measure, with the goal of validating a standardized screening tool for posterior cortical symptoms in clinical evaluations. The 15-item questionnaire, titled the Colorado Posterior Cortical Questionnaire (CPC-Q), was developed based on common posterior cortical symptoms in AD and LBD, as well as anecdotal evidence of questions with high yield from clinical evaluations of patients with PCA through the long-standing partnership between behavioral neurology and neuro-ophthalmology at our institution. We hypothesized that the CPC-Q would 1) demonstrate acceptable psychometric properties in its developed form (i.e., strong internal consistency and limited redundancy using all 15 questions with a 5-point Likert scale format); 2) exhibit strong concurrent validity with a visuospatial composite score from neuropsychological testing; and 3) discriminate PCA from non-posterior predominant presentations (i.e., amnestic AD and healthy older adults).
Methods
Scale Development
Review of the literature revealed no existing self-report questionnaire focusing on early detection of posterior cortical symptomatology in the clinical setting. A list of clinical history questions was generated by the study team based on 1) clinical observation of high yield for detecting posterior cortical dysfunction during clinical assessments, and 2) known symptomatic manifestations of PCA syndrome. Questions were refined by consensus of the study team experts (behavioral neurology, neuro-ophthalmology, and neuropsychology) and a total of fifteen questions were included in the resulting questionnaire, which was titled the Colorado Posterior Cortical Questionnaire (CPC-Q, Table 1). These questions were purposefully kept broad in their wording, given the goal of early detection and wide clinical reach for the CPC-Q. A five-point Likert scale was assigned to each question, ranging from 0 (Never) to 4 (Very Often), leading to total possible scale scores ranging from 0 to 60, with higher scores reflecting greater frequency of posterior cortical symptoms.
Table 1:
Colorado Posterior Cortical Questionnaire (CPC-Q)
| Item | Question | Symptom Assessed | Hit Rate PCA | Hit Rate AD | Hit Rate HC | Item-Total Correlation | Visuospatial Composite Correlation | Executive Composite Correlation | Memory Composite Correlation | Language Composite Correlation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Do you feel your vision has worsened? | Broad visual changes | 67% | 29% | 42% |
r=0.57
p<0.001 |
r=0.36
p=0.003 |
r=0.19 p=0.13 |
r=0.01 p=0.96 |
r=0.04 p=0.78 |
| 2 | Do you have any problems finding things that turn out to be directly in your sight? | Simultanagnosia | 56% | 5% | 6% |
r=0.65
p<0.001 |
r=0.44
p<0.001 |
r=0.43
p<0.001 |
r=0.25
p=0.04 |
r=0.32
p=0.01 |
| 3 | Do you have any depth perception problems? | Binocular depth perception | 67% | 19% | 18% |
r=0.6
p<0.001 |
r=0.31
p=0.01 |
r=0.33
p=0.007 |
r=0.18 p=0.14 |
r=0.24 p=0.06 |
| 4 | Have you experienced any problems with driving? | Visuoperception, motion, depth perception | 33% | 10% | 21% |
r=0.57
p<0.001 |
r=0.35
p=0.004 |
r=0.25
p=0.04 |
r=0.05 p=0.66 |
r=0.26
p=0.04 |
| 5 | Are you having any problems with reading? | Visual crowding, visual tracking, alexia | 100% | 10% | 15% |
r=0.79
p<0.001 |
r=0.67
p<0.001 |
r=0.43
p<0.001 |
r=0.24 p=0.052 |
r=0.37
p=0.003 |
| 6 | When reading, do you have to follow the lines with your finger to stay on the correct line? | Visual tracking | 89% | 5% | 12% |
r=0.78
p<0.001 |
r=0.72
p<0.001 |
r=0.54
p<0.001 |
r=0.27
p=0.03 |
r=0.46
p<0.001 |
| 7 | Are you having any problems working with numbers (i.e. calculations)? | Gerstmann syndrome: Acalculia | 67% | 29% | 24% |
r=0.77
p<0.001 |
r=0.55
p<0.001 |
r=0.36
p=0.003 |
r=0.3
p=0.01 |
r=0.37
p=0.003 |
| 8 | Are you having any problems mentally calculating a tip at a restaurant? | Gerstmann syndrome: Acalculia | 67% | 19% | 12% |
r=0.82
p<0.001 |
r=0.6
p<0.001 |
r=0.47
p<0.001 |
r=0.28
p=0.02 |
r=0.45
p<0.001 |
| 9 | Have you seen shadows in your periphery, and then discovered that nothing is there? | Visual illusions | 44% | 14% | 12% |
r=0.59
p<0.001 |
r=0.39
p=0.001 |
r=0.33
p=0.007 |
r=0.18 p=0.16 |
r=0.07 p=0.6 |
| 10 | Have you had experiences where you thought something was moving, but it was actually still? | Positive perceptual phenomenon | 44% | 14% | 6% |
r=0.53
p<0.001 |
r=0.44
p<0.001 |
r=0.41
p<0.001 |
r=0.26
p=0.03 |
r=0.25
p=0.048 |
| 11 | Are you having difficulty recognizing familiar faces? | Prosopagnosia | 44% | 10% | 9% |
r=0.73
p<0.001 |
r=0.38
p=0.002 |
r=0.37
p=0.002 |
r=0.2 p=0.11 |
r=0.35
p=0.005 |
| 12 | Have you found it more challenging to learn new faces compared to a year ago? | Prosopagnosia | 44% | 33% | 21% |
r=0.56
p<0.001 |
r=0.24 p=0.06 |
r=0.33
p=0.006 |
r=0.16 p=0.2 |
r=0.28
p=0.03 |
| 13 | Have you experienced any visual hallucinations (i.e. seeing something that is not there)? | Visual hallucinations | 22% | 5% | 0% |
r=0.64
p<0.001 |
r=0.48
p<0.001 |
r=0.37
p=0.002 |
r=0.3
p=0.01 |
r=0.26
p=0.045 |
| 14 | Have you had any difficulties recognizing a tool, utensil, or device when you see it? | Visual agnosia | 11% | 5% | 3% |
r=0.45
p<0.001 |
r=−0.05 p=0.72 |
r=−0.22 p=0.08 |
r=−0.12 p=0.34 |
r=−0.15 p=0.3 |
| 15 | Are you having any problems knowing left from right? | Gerstmann syndrome: Left-right confusion | 11% | 0% | 6% |
r=0.32
p=0.01 |
r=0.15 p=0.24 |
r=0.24
p=0.048 |
r=0.07 p=0.57 |
r=0.18 p=0.16 |
PCA: Posterior cortical atrophy; AD: Alzheimer’s disease; HC: Healthy control; Hit rate includes any response for that scale item that was not 0 (Never). Bold indicates p<0.05. Shades of gray in cells indicate strength of correlation: darkest gray r>0.6, medium gray r=0.4–0.6, light gray r=0.2–0.4, white r=0–0.2.
Participants
The developed 15-item CPC-Q was piloted within an ongoing longitudinal, observational study of cognitive aging at the University of Colorado Alzheimer’s and Cognition Center, the Longitudinal Biomarker and Clinical Phenotyping (Bio-AD) study. Sixty-three participants independently completed the CPC-Q by self-report on a paper form. All participants underwent clinical interview, neurological examination, and neuropsychological testing. Participants were classified as healthy controls if they were a) community dwelling older adults, b) had no self or informant report of significant current or recent cognitive changes in the past year, and c) no diagnosis of mild cognitive impairment (MCI) or dementia. Participants were excluded if they had a major psychiatric disorder, a co-existing non-AD neurological condition known to affect cognition (e.g., Parkinson’s disease; large vessel infarct; multiple sclerosis), current evidence or history in the past two years of a focal brain lesion, current substance abuse, significant systemic medical illness or active neoplastic disease, significant sensory or motor deficits that would interfere with cognitive testing, or traumatic brain injury with loss of consciousness greater than five minutes.
Clinical syndrome (i.e., control, AD, PCA) and stage of severity (normal, MCI, dementia) were reviewed at a case consensus conference with a board-certified neuropsychologist, two board-certified behavioral neurologists, and clinical research coordinator. Cognitive diagnoses were made based on NIA-AA clinical criteria for MCI and AD dementia (Albert et al., 2011; McKhann et al., 2011) and published consensus classification criteria for PCA (Crutch et al., 2017). A subset of cognitive measures from the research protocol were reviewed in a consensus conference for this classification; however, to reduce circularity in our methodological approach, cognitive measures that were reviewed in the consensus conference for differential diagnosis were separate from those used as primary outcomes in the research study. All participants provided written informed consent and the study protocol was approved by the Colorado Multiple Institutional Review Board (Protocol #15–1774).
Cognitive Assessment
Participants completed cognitive testing with the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) and the Spanish and English Neuropsychological Assessment Scales (SENAS) (Mungas, Reed, Haan, et al., 2005). An informant-based interview was also conducted (Clinical Dementia Rating Scale [CDR] {Morris, 1993, The Clinical Dementia Rating (CDR): current version and scoring rules}), which was used to assess and rate severity of functional impairment.
The SENAS battery was based on item response theory (IRT), and psychometrically matched measures were created across different scales, thus assuring reliability across the full ability continuum (Mungas et al., 2004; Mungas, Reed, Haan, et al., 2005; Mungas et al., 2000; Mungas, Reed, Tomaszewski Farias, et al., 2005; Mungas et al., 2011). For the purposes of this study, IRT composite scores were used for each of the domains described below. These IRT scores do not have floor or ceiling effects and are normally distributed. IRT scores may be interpreted as unadjusted standard scores (Mean=0; SD=1) based on a demographically diverse sample of older adults aged over 60 (Mungas et al., 2004).
Cognitive domains of interest included visuospatial, executive, memory, and language functions. Visuospatial function was measured using the SENAS spatial IRT composite (i.e., Spatial Localization scale), which evaluates the ability to perceive and reproduce two-dimensional spatial relationships. Executive function was assessed with the SENAS executive IRT composite (digit span backward, visual span backward, list sorting, fluency). Memory was assessed with a multi-trial list-learning measure from the SENAS (five learning trials, 15 items) and the memory IRT composite incorporated both learning trials and delayed recall. Language was assessed with the language/semantic knowledge IRT composite, based on scores from a nonverbal picture associate measure and a verbal object naming task. Administration procedures, measure development, and psychometric characteristics of the SENAS battery are described in detail elsewhere (Mungas et al., 2004).
Statistical Analyses
Demographic and clinical characteristics of participants were evaluated using descriptive statistics. For the purposes of these analyses, clinical syndrome was used as the primary diagnostic grouping. Normality of data was tested using Shapiro-Wilk W tests by diagnostic group. Depending on these results, either one-way ANOVA or Kruskal-Wallis one-way ANOVA analyses were performed to evaluate for groupwise differences in demographic and clinical characteristics between diagnostic groups. Groupwise differences were then tested by either t-tests or Mann Whitney U tests as appropriate. Frequency of responses for individual CPC-Q items were tabulated for the overall cohort and by diagnostic group. Internal consistency of the CPC-Q was assessed using Cronbach’s alpha and item-total correlations (i.e., the correlation between an item and the total score without that item). For internal consistency using Cronbach’s alpha, a value of 0.7 and greater, and for item-total correlation, a value of at least 0.5, were considered acceptable. As a measure of redundancy in scale items, item-item correlations were calculated and averaged, with an acceptable item-item correlation set between 0.15 and 0.5. An exploratory factory analysis was also performed to test dimensionality of the CPC-Q.
For concurrent validity of the CPC-Q with established cognitive measures, pairwise correlations were calculated between Montreal Cognitive Assessment (MoCA), CDR, and visuospatial, executive, memory, and language composite scores. Correlations for both total CPC-Q score and individual CPC-Q scale items were evaluated. A linear regression model, with CPC-Q total score as the dependent variable, was also used to evaluate the contribution from each cognitive composite score, with relevant covariates of participant age, education, and disease duration. For discriminant validity of the CPC-Q, sensitivity, specificity, and receiver operating characteristic (ROC) curves with the area under the curve (AUC) were determined for the classification of PCA versus non-PCA (healthy older adults, AD) using CPC-Q scores. Statistical significance was set at an alpha of 0.05. Statistical analyses were performed using the Stata statistical software package (StataCorp 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Participant Characteristics
Demographic and clinical characteristics of the sixty-three participants (33 healthy older adults, 21 with amnestic AD, 9 with PCA) are presented in Table 2. The healthy older adult group was not significantly different in terms of age, sex, or years of education compared to the amnestic AD and PCA groups. The PCA group was significantly younger than the amnestic AD group, with significantly lower MoCA and visuospatial composite scores.
Table 2:
Clinical characteristics of participants by diagnosis
| Healthy Older Adult (n=33) |
Amnestic AD (n=21) |
PCA (n=9) |
|
|---|---|---|---|
| Age (y) | 69.5 ± 6.5 (55–82) | 75.2 ± 4.4 (63–83) | 65.8 ± 8.6 (54–78) |
| Gender (%F) | 67% | 43% | 56% |
| Education (y) | 17.2 ± 1.9 (13–20) | 17.0 ± 2.2 (13–20) | 16.7 ± 3.0 (12–20) |
| Disease duration (y) | NA | 4.3 ± 3.6 (2–18) | 5.3 ± 1.7 (3–9) |
| MoCA | 26 ± 2.2 (22–30) | 20 ± 4 (7–27) | 16 ± 4 (7–21) |
| CDR (%) |
0: 100%
0.5: 0% 1: 0% 2: 0% |
0: 0% 0.5: 68% 1: 16% 2: 16% |
0: 0% 0.5:33% 1: 33% 2: 33% |
| Visuospatial Composite Score | 0.7 ± 0.5 (−0.4–2.1) | 0.3 ± 0.9 (−2.4–2.3) | −2.3 ± 0.7 (−2.9 to −1.2) |
| Executive Composite Score | 0.6 ± 0.4 (−0.3–1.3) | −0.1 ± 0.5 (−1.4–0.6) | −0.5 ± 0.8 (−1.8–0.7) |
| Verbal Memory Composite Score | 1.1 ± 0.6 (−0.2–2) | −0.5 ± 0.8 (−2.1–1.3) | −0.5 ± 0.9 (−1.6–1.5) |
| Language Composite Score | 1.8 ± 0.6 (0.4–3) | −0.5 ± 0.7 (−2.5–0.7) | −0.8 ± 0.9 (−2.3–0.7) |
| Total CPC-Q: Likert (max score=60) | 3.5 ± 3.8 (0–14) | 4.5 ± 4.7 (0–14) | 24.2 ± 14.8 (7–44) |
| Total CPC-Q: Y/N (max score=15) | 2.1 ± 2.3 (0–9) | 2.1 ± 2.1 (0–6) | 8 ± 4.7 (3–14) |
Data presented as mean ± SD (range). AD: Alzheimer’s disease; PCA: Posterior cortical atrophy; MoCA: Montreal Cognitive Assessment; CDR: Clinical Dementia Rating Scale; CPC-Q: Colorado Posterior Cortical Questionnaire. CPC-Q: Likert: scores include 5-point Likert scale for each item; CPC-Q: Y/N: scores include binary “yes/no” scores for each item. Bold indicates significant groupwise differences (p<0.05). SENAS Item Response Theory (IRT) based cognitive composite scores (i.e., visuospatial, executive, verbal memory, language) are presented as unadjusted standard scores based on a demographically diverse normative sample.
Psychometric Properties
Internal consistency of the 15-item CPC-Q is strong (Cronbach’s alpha=0.89). Item-total correlations were all statistically significant and ranged from 0.32 (Item #15: Recognizing Tools) to 0.82 (Item #8: Calculating Tip), with a mean item-total correlation of 0.62 (SD 0.14) (Table 1). Item-item correlations for the CPC-Q are presented in Table 3. The mean item-item correlation was 0.35 (SD 0.17), ranging from 0.02–0.85. Scale item #8 (Are you having any problems mentally calculating a tip at a restaurant?) correlated significantly with eight other scale items (items regarding driving (#4), reading (#5, 6), numbers (#7), faces (#11, 12), hallucinations (#13), and illusions (#9)). The two scale items questioning problems with reading (Item #5 and 6) and with calculations (Item #7 and 8) also correlated significantly with one another. The two calculation items also correlated significantly with the question on visual hallucinations (Item #13), as did those on illusions (#9) and recognizing faces (#11). The Cronbach’s alpha for the CPC-Q remained strong (0.81) when items #6 and 8 were removed. Factor analysis of the CPC-Q revealed a total of nine factors, with the first factor explaining 60 % of the variance (eigenvalue 5.9, Figure 1). Primary loading factors for CPC-Q items ranged from 0.3 (item #15: Left-Right Confusion) to 0.83 (item #8: Calculating Tip).
Table 3:
Item-item correlations for the CPC-Q
| Item 1 Vision Worse |
Item 2 In Front |
Item 3 Depth |
Item 4 Driving |
Item 5 Reading |
Item 6 Reading Line |
Item 7 Number |
Item 8 Tip |
Item 9 Shadow |
Item 10 Moving |
Item 11 Faces |
Item 12 New Faces |
Item 13 VH |
Item 14 Tool |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item 1 | 1 | |||||||||||||
| Item 2 | 0.31 p=0.01 |
1 | ||||||||||||
| Item 3 | 0.2 p=0.1 |
0.61
p<0.001 |
1 | |||||||||||
| Item 4 | 0.44 p<0.001 |
0.44 p<0.001 |
0.2 p=0.1 |
1 | ||||||||||
| Item 5 |
0.54
p<0.001 |
0.45 p=0.001 |
0.35 p=0.004 |
0.42 p<0.001 |
1 | |||||||||
| Item 6 | 0.43 p<0.001 |
0.53
p<0.001 |
0.33 p=0.006 |
0.4 p<0.001 |
0.85
p<0.001 |
1 | ||||||||
| Item 7 | 0.3 p=0.01 |
0.43 p<0.001 |
0.43 p=0.002 |
0.5 p<0.001 |
0.5 p<0.001 |
0.46 p<0.001 |
1 | |||||||
| Item 8 | 0.3 p=0.01 |
0.37 p=0.005 |
0.41 p<0.001 |
0.51
p<0.001 |
0.55
p<0.001 |
0.58
p<0.001 |
0.86
p<0.001 |
1 | ||||||
| Item 9 | 0.26 p=0.03 |
0.19 p=0.12 |
0.45 p<0.001 |
0.09 p=0.47 |
0.3 p=0.01 |
0.35 p=0.004 |
0.47 p<0.001 |
0.55
p<0.001 |
1 | |||||
| Item 10 | 0.15 p=0.24 |
0.35 p=0.004 |
0.32 p=0.009 |
0.11 p=0.37 |
0.46 p<0.001 |
0.50 p<0.001 |
0.24 p=0.05 |
0.27 p=0.03 |
0.54
p<0.001 |
1 | ||||
| Item 11 | 0.29 p=0.02 |
0.44 p<0.001 |
0.33 p=0.007 |
0.37 p=0.002 |
0.49 p<0.001 |
0.49 p<0.001 |
0.52
p<0.001 |
0.55
p<0.001 |
0.38 p=0.002 |
0.34 p=0.005 |
1 | |||
| Item 12 | 0.14 p=0.24 |
0.3 p=0.02 |
0.25 p=0.04 |
0.22 p=0.07 |
0.29 p=0.03 |
0.28 p=0.02 |
0.43 p<0.001 |
0.51
p<0.001 |
0.32 p=0.009 |
0.21 p=0.09 |
0.64
p<0.001 |
1 | ||
| Item 13 | 0.36 p=0.003 |
0.22 p=0.07 |
0.35 p=0.003 |
0.02 p=0.84 |
0.47 p<0.001 |
0.48 p<0.001 |
0.53
p<0.001 |
0.59
p<0.001 |
0.66
p<0.001 |
0.26 p=0.03 |
0.57
p<0.001 |
0.41 p<0.001 |
1 | |
| Item 14 | 0.14 p=0.3 |
0.26 p=0.03 |
0.34 p=0.005 |
0.25 p=0.04 |
0.37 p=0.002 |
0.41 p<0.001 |
0.19 p=0.13 |
0.36 p=0.002 |
0.2 p=0.11 |
0.33 p=0.006 |
0.27 p=0.02 |
0.18 p=0.14 |
0.03 p=0.84 |
1 |
| Item 15 | 0.06 p=0.61 |
0.26 p=0.03 |
0.07 p=0.57 |
0.32 p=0.01 |
0.08 p=0.51 |
0.14 p=0.26 |
0.35 p=0.004 |
0.27 p=0.03 |
0.05 p=0.67 |
0.13 p=0.29 |
0.42 p<0.001 |
0.34 p=0.005 |
0.09 p=0.46 |
0.05 p=0.66 |
VH: visual hallucinations. BOLD indicates significant item-item correlation coefficients outside defined bounds of 0.15–0.5.
Figure 1:
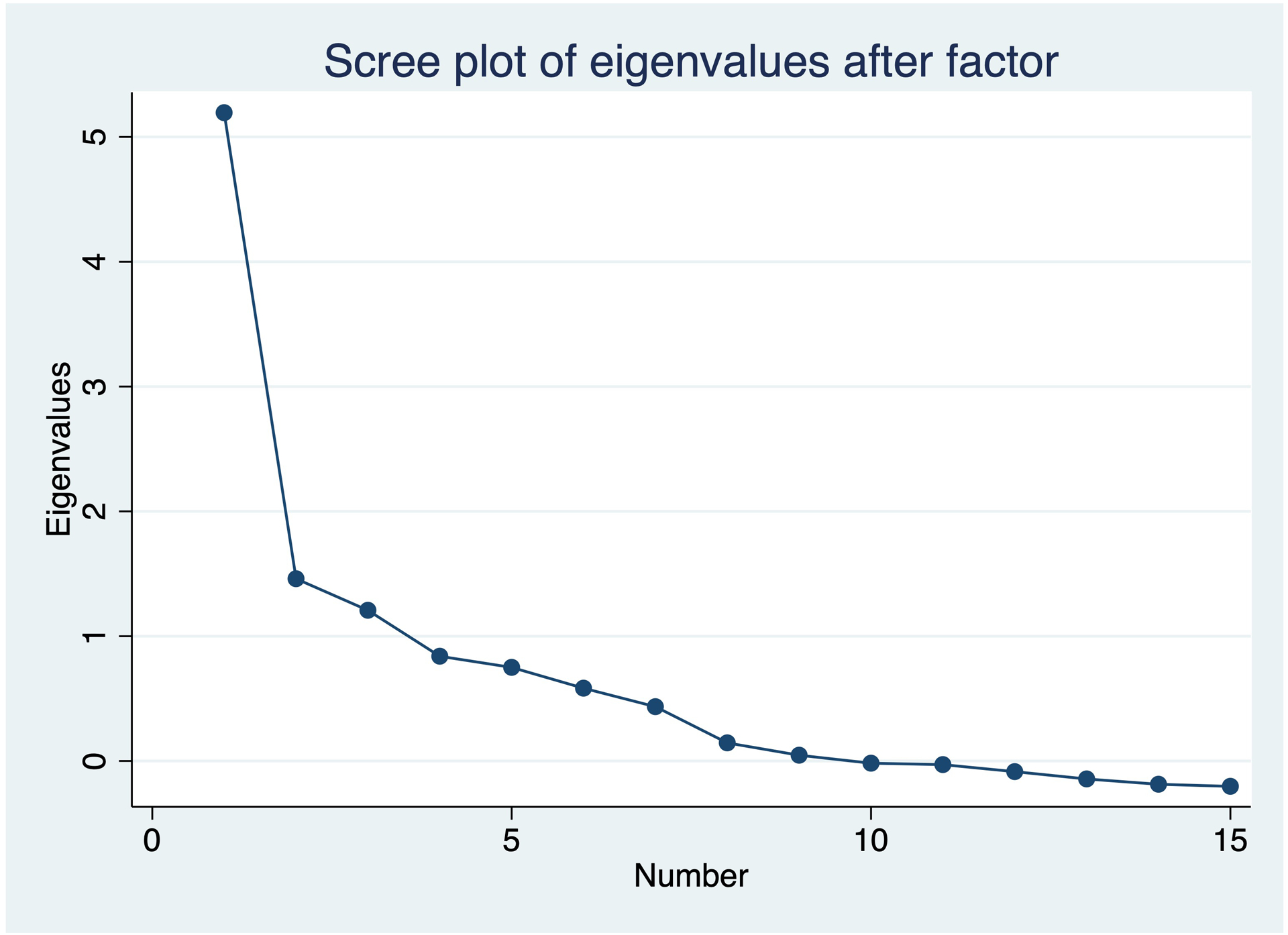
Scree plot for CPC-Q factor analysis
Concurrent Validity
Univariately, higher CPC-Q total scores correlated significantly with lower MoCA scores (r=−0.46, p=0.002), higher CDR scores (r=0.53, p<0.001), lower visuospatial composite scores (r=−0.66, p<0.001), and lower executive composite scores (r=−0.55, p<0.001). CPC-Q total scores were also significantly, though less strongly, correlated with language (r=−0.43, p<0.001) and memory (r=−0.28, p=0.03) composite scores. Twelve individual scale items correlated significantly with visuospatial composite scores (correlation coefficients ranging from 0.31 to 0.72; Table 1). Thirteen scale items correlated significantly with executive composite scores (correlation coefficients ranging from 0.24 to 0.54). Fewer CPC-Q items correlated significantly with the language (10 items) and memory (6 items) composite scores, and correlations were weaker overall (range 0.25 to 0.46).
A multivariate linear regression model including the four cognitive composite scores, adjusting for age education, and disease duration, explained 73.9% of the variance in CPC-Q total score (F (7, 21)=8.51, p=0.0001). Only the visuospatial composite score significantly predicted CPC-Q score (β=−3.8, p=0.03); no other cognitive domain scores were significantly related to the CPC-Q outcome in this complete model.
Discriminant Validity
CPC-Q total scores were not significantly different between healthy older adults and amnestic AD participants (mean diff: −1.0, 95% CI −3.3, 1.4; t=-0.8, p=0.4). Total CPC-Q scores were significantly higher for the PCA group than both the amnestic AD group (mean diff: 19.7, 95% CI 12.5, 27, t=5.6, p<0.001) and the healthy older adult group (mean diff: 20.7, 95% CI 15.1, 26.4, t=7.8, p<0.001). When binarizing CPC-Q scale items by collapsing all non-zero item responses, these significant differences were maintained (Table 2, Figure 2).
Figure 2:
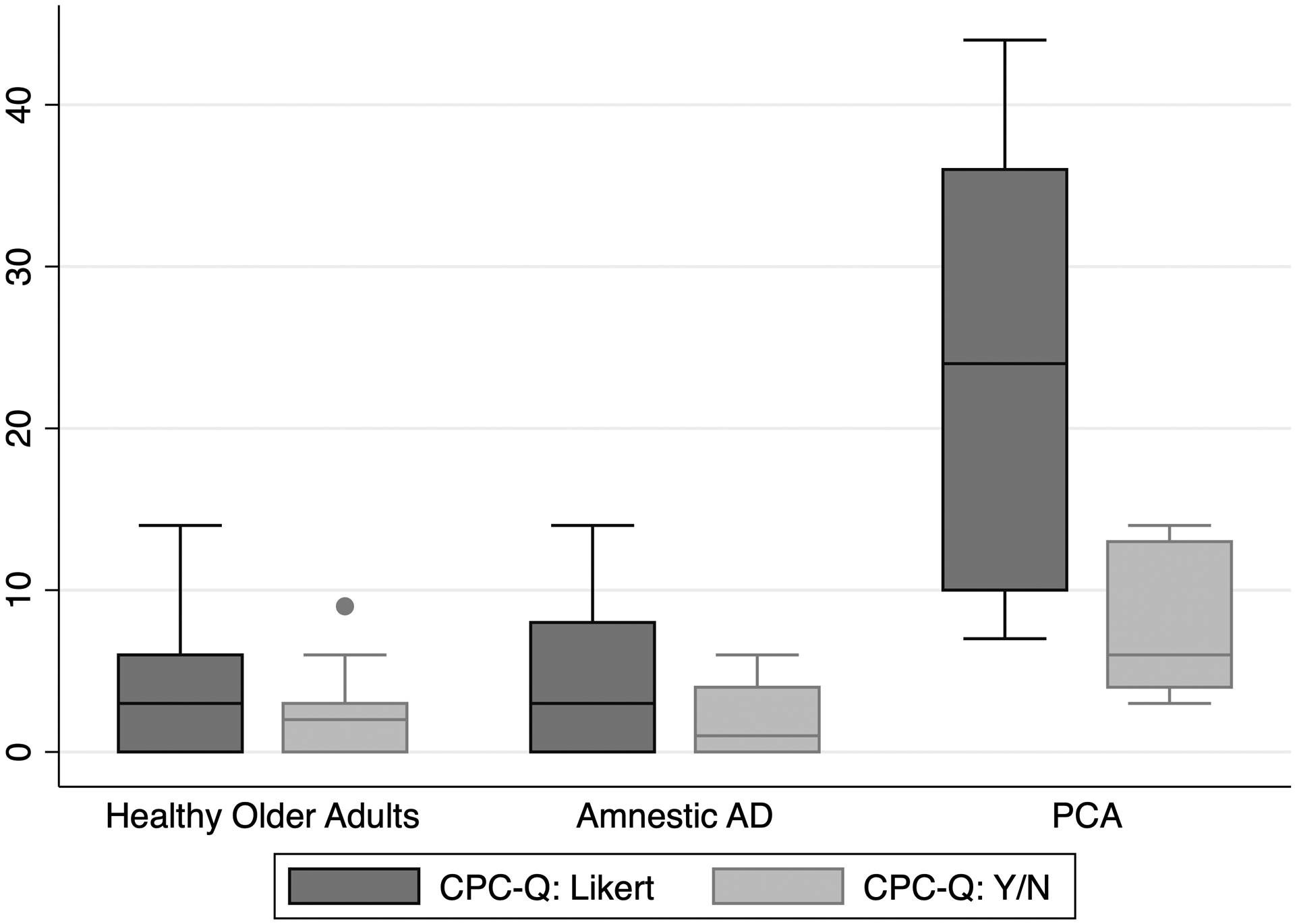
CPC-Q total scores by diagnostic group
The area under the curve for distinguishing PCA from non-PCA with the CPC-Q was 0.94 (95% CI 0.88, 1.0) (Figure 3). A CPC-Q cut-off score of ≥7/60 demonstrated a sensitivity of 100% and specificity of 77.8% for PCA diagnosis, with 81% of participants correctly classified. The area under the curve for distinguishing PCA from amnestic AD with the CPC-Q was 0.92 (95% CI 0.83, 1.0). A cut-off score of ≥7/60 demonstrated a sensitivity of 100% and specificity of 71.4% for PCA, with 80% of participants correctly classified.
Figure 3:
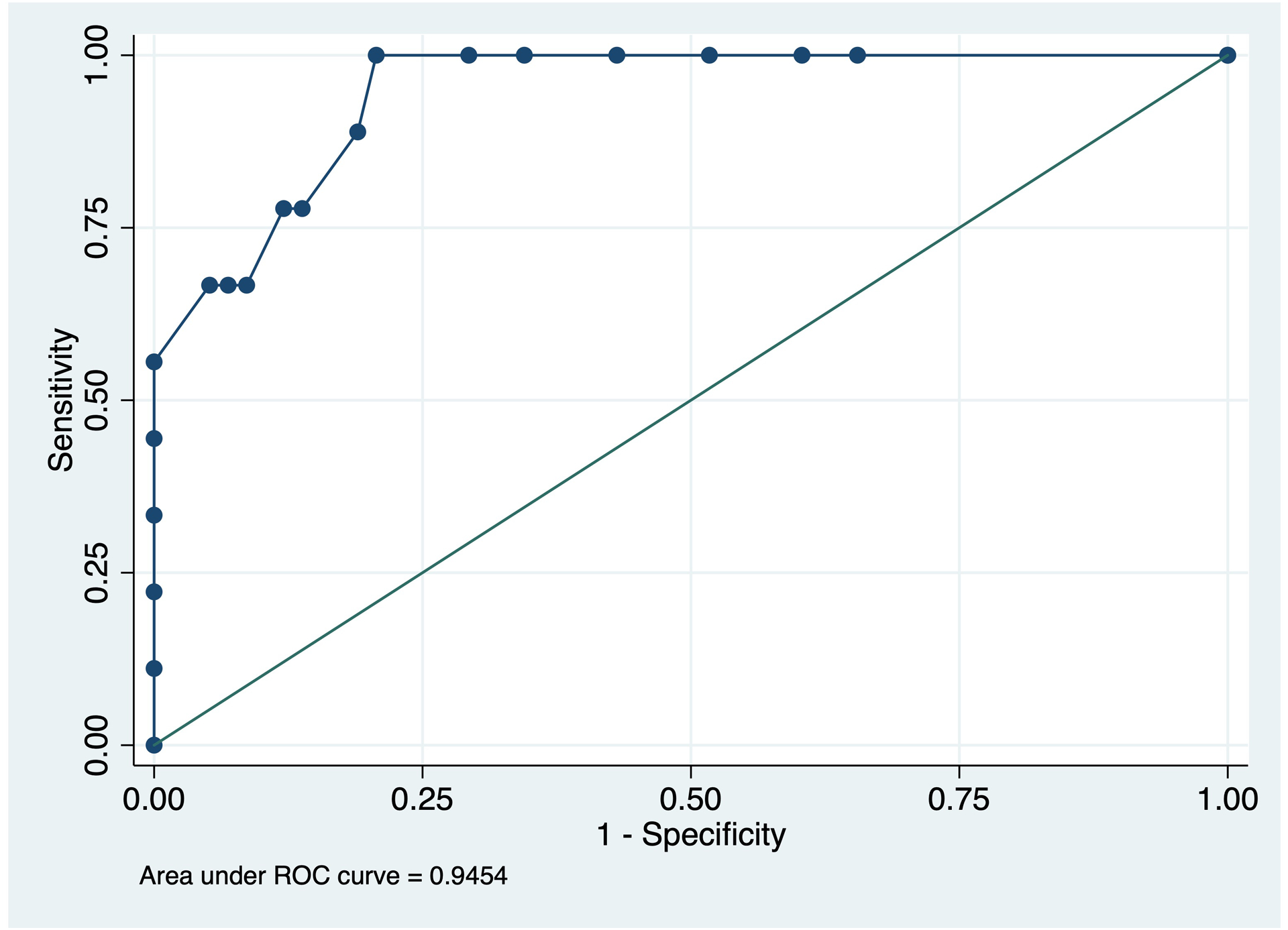
Receiver Operating Characteristic curve for PCA diagnosis by CPC-Q total score
Discussion
By leveraging extensive clinical experience with diagnosing and managing patients with PCA, combined with thorough cognitive classification through a longitudinal aging and cognition study (Bio-AD study), we developed a novel, brief questionnaire for posterior cortical symptomatology. The Colorado Posterior Cortical Questionnaire (CPC-Q) demonstrates acceptable psychometric properties in a self-report format, using a 5-point Likert scale. At least two scale items can likely be removed due to high inter-item correlations (reading: item #5 or 6 and calculation: item #7 or 8); items with more specific examples of impairments (#6 and 8) are did demonstrate stronger correlation coefficients with the visuospatial composite score and may be more useful. We also demonstrated that the CPC-Q is capturing posterior cortical symptoms, as defined by correlation with visuospatial and executive neuropsychological tests, and it can reliably distinguish PCA from non-PCA groups in the clinical research setting.
Posterior cortical symptoms are underreported by patients and families, as well as underrecognized by clinicians (Holden et al., 2020). With more focus on typical amnestic presentations of cognitive decline, both among the community and within the medical field, non-amnestic symptoms remain underappreciated (Wong et al., 2019). This limits our ability to fully appraise the frequency and severity of non-amnestic syndromes, including visuospatial and dysexecutive cognitive presentations, especially in earlier stages. PCA is likely more common than currently thought due to lack of adequate screening measures for early symptomatic detection (Beh et al., 2015). By instituting broad screening methods, partnering with ophthalmology and optometry colleagues, there is significant opportunity to improve detection of posterior cortical variants of cognitive decline. A simple screening tool would also circumvent perceived barriers to recognition of higher cortical dysfunction due to neurodegeneration, such as lack of time, knowledge, and adequate resources (Liu et al., 2020). Not only will such efforts contribute to earlier and more specific cognitive diagnoses, but there is also potential to improve functionality and independence for those affected by posterior cortical symptoms detected by such a screening tool.
Posterior cortical symptoms have a substantial impact on patients’ daily lives, as these neurological functions play key roles in activities of daily living, ranging from completing community-based tasks (e.g., making change or tipping at a restaurant) to engaging in social interactions (e.g., recognizing familiar faces). Impairments in posterior cortical functions have significant implications for both personal and public safety, as visuospatial difficulties have been linked to increased risk of tripping and falling due to the contributions of these cognitive processes to gait stability (Amboni et al., 2013; Martin et al., 2009; Martin et al., 2013). Visual processes are also part of a complex cognitive network that underlies the ability to drive competently and safely (Jacobs et al., 2017; Yamasaki & Tobimatsu, 2018). Drivers with AD are over three times more likely to be involved in a motor vehicle accident at intersections compared to healthy older controls and visuospatial impairments are specifically linked to safety errors on the road (Aksan et al., 2015; Stinchcombe et al., 2016). Collectively, these studies highlight the need for early detection and standardized assessment of posterior cortical symptoms to mitigate risk, address safety issues, and improve patient quality of life. Our initial findings validating the CPC-Q will help address this current gap and we will expand upon this work to optimize the performance of this scale and work towards dissemination.
Although our results did not suggest differences in performance on the CPC-Q between healthy controls and amnestic AD, strong associations were observed between the CPC-Q and performance on validated neuropsychological measures of visuospatial functioning. One consideration is that self-report measures require adequate insight, which may be more commonly preserved with PCA than with typical AD (Mendez et al., 2002; Tang-Wai et al., 2004). Furthermore, though spatial difficulties are also common in typical AD, individual patients vary in the temporal course of their visuospatial symptom onset. Our results suggest that visual cognitive difficulties detected by neuropsychological testing are also captured by the CPC-Q, indicating that this self-report tool may still be useful for typical AD and/or other neurodegenerative syndromes (e.g., LBD) in the context of measurable visuospatial impairment. Future studies should address the utility of the CPC-Q in typical AD, LBD, and other neurodegenerative disorders in larger clinical settings.
Univariately, the CPC-Q was associated with all cognitive domains, although associations were strongest for visuospatial function and executive functions. Nonetheless, only the visuospatial composite was predictive of the CPC-Q total score when appraising all cognitive composites simultaneously as predictors in the complete model. Although this is consistent with our hypotheses, it is worth noting that many neuropsychological measures of non-visuospatial domains are confounded by visual stimuli. This is the case both in our study with research-specific measures and in clinical assessments, wherein measures of language often include visual pictures (i.e., SENAS confrontation naming; SENAS nonverbal semantic association) and executive control measures frequently incorporate visuomotor processing or visual working memory (i.e., SENAS visual span backwards). Although removing such items from neuropsychological protocols (both research and clinical) may help disentangle aspects of visuospatial vs non-visuospatial function, there is also data to suggest that posterior cortical dysfunction may meaningfully disrupt frontoparietal connections and parietal-occipital-ventral temporal connections. We elected to not remove these measures from our composite scores, given that the a) IRT scores are more robust than individual raw scores, and b) language measures that do not include visual stimuli (e.g., fluency) have some overlap with executive functions. This is a broader debate outside the scope of the current research study; however, moving forward, studies addressing the role of the CPC-Q in early detection of posterior cortical dysfunction should further investigate the benefit versus detriment of including cognitive measures outside the canonical visuospatial domain that include visual stimuli.
Strengths of this study include a participant pool that has been thoroughly evaluated and cognitively characterized, with adjudication of clinical syndrome and severity of cognitive impairment through consensus conference procedures. Limitations include a relatively small sample of PCA participants, though large effect sizes were demonstrated in most of our analyses even with this small sample. Further, the deployment of the CPC-Q took place in a clinical research setting, rather than in a clinical setting, where the tool would ultimately be utilized. While this initial validation study demonstrates a potential role for the CPC-Q, it is not adequate for establishing the utility of the CPC-Q for its ultimate purpose of distinguishing the earliest stages of a neurodegenerative process that has a predilection for posterior cortical regions and corresponding symptoms.
Towards this end, future studies should include validation of the CPC-Q in a larger cohort, particularly with larger numbers of PCA participants to both confirm and expand the insights presented herein. Application of the CPC-Q to patients with PCA syndrome due to underlying AD, as well as those due to underlying LBD, will also be necessary to fully evaluate convergent and discriminant validity of the CPC-Q. Comparisons with traditional neurodegenerative biomarkers, including structural neuroimaging and serum and cerebrospinal fluid results, must also be explored. In addition, longitudinal assessment will be important to determine if those healthy older adults with higher baseline CPC-Q scores are at higher risk of future posterior cortical cognitive decline.
The CPC-Q has potential to provide a brief, yet comprehensive, self-report measure of posterior cortical symptoms that could lead to improved identification and early diagnosis of non-amnestic cognitive syndromes, leading to improved management and outcomes for people living with neurodegenerative disorders.
Acknowledgements:
The authors would like to thank the professional research assistants of the University of Colorado Alzheimer’s and Cognition Center (CUACC), including Tanner Gustavsen, Rini Kaplan, Katherine Varley, Katrina Bengston, Jada Boyd, Erika Dallman, Grace Fishback, Jennifer Krupa, Natalie Lopez-Esquibel, Abigail Simpson, and Michelle Stocker, as well as the Behavioral Neurology fellows, including Dr. James Bateman, Dr. Tara Carlisle, Dr. Isaiah Kletenik, Dr. Zachary Macchi, Dr. Jakob Mrozewski, Dr. Justin Otis, and Dr. Justin Persson, who performed histories and examinations on participants. We thank Dr. Huntington Potter, director and founder of the CUACC, for his support of this longitudinal cohort. We also sincerely thank the Bio-AD study participants and their families for their donation of their time, mental energy, and support of this work.
Funding Sources:
This work was supported by grants from the National Institute on Aging (NIA; PI; R01 AG058772, B. Bettcher, PI), NIH/NCATS Colorado CTSA UL1 TR002535 (R. Sokol, PI), and support from the State of Colorado and many generous philanthropists. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Conflicts: None
References
- Aksan N, Anderson SW, Dawson J, Uc E, & Rizzo M (2015). Cognitive functioning differentially predicts different dimensions of older drivers’ on-road safety. Accid Anal Prev, 75, 236–244. 10.1016/j.aap.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 270–279. https://doi.org/S1552-5260(11)00104-X [pii] 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amboni M, Barone P, & Hausdorff JM (2013). Cognitive contributions to gait and falls: evidence and implications. Mov Disord, 28(11), 1520–1533. 10.1002/mds.25674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, & van der Flier WM (2015). Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimers Dement, 11(11), 1349–1357. 10.1016/j.jalz.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh SC, Muthusamy B, Calabresi P, Hart J, Zee D, Patel V, & Frohman E (2015). Hiding in plain sight: a closer look at posterior cortical atrophy. Pract Neurol, 15(1), 5–13. 10.1136/practneurol-2014-000883 [DOI] [PubMed] [Google Scholar]
- Blenkinsop A, van der Flier WM, Wolk D, Lehmann M, Howard R, Frost C, & Barnes J (2020). Non-memory cognitive symptom development in Alzheimer’s disease. Eur J Neurol. 10.1111/ene.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford A, Kunik ME, Schulz P, Williams SP, & Singh H (2009). Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord, 23(4), 306–314. 10.1097/WAD.0b013e3181a6bebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisile B, & Mollion H (2011). [Q-ACP: a questionnaire for evaluating visual and gestural complaints in patients with posterior cortical atrophy]. Rev Neurol (Paris), 167(6–7), 485–494. 10.1016/j.neurol.2010.11.003 [DOI] [PubMed] [Google Scholar]; (Q-ACP: un questionnaire d’evaluation des plaintes visuelles et gestuelles des patients ayant une atrophie corticale posterieure.)
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, & Fox NC (2012). Posterior cortical atrophy. Lancet Neurol, 11(2), 170–178. 10.1016/S1474-4422(11)70289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, … Associated Syndromes Professional Interest, A. (2017). Consensus classification of posterior cortical atrophy. Alzheimers Dement, 13(8), 870–884. 10.1016/j.jalz.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vugt ME, & Verhey FR (2013). The impact of early dementia diagnosis and intervention on informal caregivers. Prog Neurobiol, 110, 54–62. 10.1016/j.pneurobio.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Dubois B, Padovani A, Scheltens P, Rossi A, & Dell’Agnello G (2016). Timely Diagnosis for Alzheimer’s Disease: A Literature Review on Benefits and Challenges. J Alzheimers Dis, 49(3), 617–631. 10.3233/JAD-150692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden SK, Bettcher BM, & Pelak VS (2020). Update on posterior cortical atrophy. Curr Opin Neurol, 33(1), 68–73. 10.1097/WCO.0000000000000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Hart EP, & Roos RAC (2017). Driving with a neurodegenerative disorder: an overview of the current literature. J Neurol, 264(8), 1678–1696. 10.1007/s00415-017-8489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pelak VS, van Stavern G, & Moss HE (2020). Higher Cortical Dysfunction Presenting as Visual Symptoms in Neurodegenerative Diseases. Front Neurol, 11, 679. 10.3389/fneur.2020.00679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K, Thomson R, Blizzard L, Wood A, Garry M, & Srikanth V (2009). Visuospatial ability and memory are associated with falls risk in older people: a population-based study. Dement Geriatr Cogn Disord, 27(5), 451–457. 10.1159/000216840 [DOI] [PubMed] [Google Scholar]
- Martin KL, Blizzard L, Wood AG, Srikanth V, Thomson R, Sanders LM, & Callisaya ML (2013). Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci, 68(6), 726–732. 10.1093/gerona/gls224 [DOI] [PubMed] [Google Scholar]
- Martin RC, Annis SM, Darling LZ, Wadley V, Harrell L, & Marson DC (2003). Loss of calculation abilities in patients with mild and moderate Alzheimer disease. Arch Neurol, 60(11), 1585–1589. 10.1001/archneur.60.11.1585 [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, … Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Ghajarania M, & Perryman KM (2002). Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord, 14(1), 33–40. 10.1159/000058331 [DOI] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, & Gonzalez H (2004). Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess, 16(4), 347–359. 10.1037/1040-3590.16.4.347 [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Haan MN, & Gonzalez H (2005). Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology, 19(4), 466–475. 10.1037/0894-4105.19.4.466 [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Marshall SC, & Gonzalez HM (2000). Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology, 14(2), 209–223. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Tomaszewski Farias S, & DeCarli C (2005). Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic Whites. J Int Neuropsychol Soc, 11(5), 620–630. 10.1017/S1355617705050745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Widaman KF, Reed BR, & Tomaszewski Farias S (2011). Measurement invariance of neuropsychological tests in diverse older persons. Neuropsychology, 25(2), 260–269. 10.1037/a0021090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Possin KL (2010). Visual spatial cognition in neurodegenerative disease. Neurocase, 16(6), 466–487. 10.1080/13554791003730600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quental NBM, Brucki SMD, & Bueno OFA (2009). Visuospatial function in early Alzheimer’s disease: Preliminary study. Dement Neuropsychol, 3(3), 234–240. 10.1590/S1980-57642009DN30300010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, & Burrell JR (2018). Can visuospatial measures improve the diagnosis of Alzheimer’s disease? Alzheimers Dement (Amst), 10, 66–74. 10.1016/j.dadm.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe A, Paquet S, Yamin S, & Gagnon S (2016). Assessment of Drivers with Alzheimer’s Disease in High Demand Driving Situations: Coping with Intersections in a Driving Simulator. Geriatrics (Basel), 1(3). 10.3390/geriatrics1030021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, … Petersen RC (2004). Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S.]. Neurology, 63(7), 1168–1174. [DOI] [PubMed] [Google Scholar]
- Wang CT, Hung GU, Wei CY, Tzeng RC, & Chiu PY (2020). An Informant-Based Simple Questionnaire for Visuospatial Dysfunction Assessment in Dementia. Front Neurosci, 14, 44. 10.3389/fnins.2020.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B, Lucente DE, MacLean J, Padmanabhan J, Quimby M, Brandt KD, … Dickerson BC (2019). Diagnostic evaluation and monitoring of patients with posterior cortical atrophy. Neurodegener Dis Manag, 9(4), 217–239. 10.2217/nmt-2018-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, & Tobimatsu S (2018). Driving Ability in Alzheimer Disease Spectrum: Neural Basis, Assessment, and Potential Use of Optic Flow Event-Related Potentials. Front Neurol, 9, 750. 10.3389/fneur.2018.00750 [DOI] [PMC free article] [PubMed] [Google Scholar]


