Abstract
Although strong evidence exists for using individual hypnosis to treat pain, evidence regarding group applications is limited. This project evaluated changes in multiple outcome measures in persons with chronic pain treated with 8 weeks of group hypnosis. Eighty-five adults with diverse chronic pain etiologies completed an 8-session, structured group hypnosis treatment. Pain intensity, pain interference, and global health were evaluated at baseline, posttreatment, and 3- and 6-months posttreatment. Linear mixed effects models assessed changes in outcomes over time. In a model testing all 3 outcome measures simultaneously, participants improved substantially from pre- to posttreatment and maintained improvement across follow-up. Analyses of individual outcomes showed significant pre- to posttreatment reductions in pain intensity and interference, which were maintained for pain intensity and continued to improve for pain interference across follow-up. The findings provide compelling preliminary evidence that a group format is an effective delivery system for teaching individuals skills in using hypnosis for chronic pain management. Larger randomized controlled trials are warranted to demonstrate equivalence of outcomes between treatment modes.
Keywords: hypnosis, psychotherapy, group, widespread chronic pain, fibromyalgia, complementary therapies
Zusammenfassung:
Obgleich es starke Beweise für den Einsatz individueller Hypnose zur Schmerzbehandlung gibt, ist die Evidenz für Gruppenbehandlung begrenzt. Mit diesem Projekt wurden Veränderungen bei mehreren Ergebnismessungen an chronischen Schmerzpatienten bewertet, welche in einer 8-wöchigen Gruppenhypnose behandelt wurden. Fünfundachtzig Erwachsene mit verschiedener chronischer Schmerzursache absolvierten in 8 Sitzungen eine strukturierte Gruppenhypnose. Zu Studienbeginn wurden Schmerzintensität, Schmerzinterferenz und allgemeine Gesundheit bewertet, sodann wurden die Werte nach der Behandlung, sowie nach 3 und nach 6 Monaten ermittelt. Linear gemischte Effektmodelle untersuchten Veränderungen von Ergebnissen über die Zeit. In einem Testmodell zur gleichzeitigen Erhebung aller 3 Ergebnismessungen hatten die Teilnehmer sich signifikant von vor zu nach der Behandlung verbessert und die Verbesserung bestand über das Follow-up hin fort. Die Analyse der individuellen Ergebnisse zeigte eine signifikante Schmerzreduktion bezüglich Intensität und Interferenz von der Vor- zur Nachuntersuchung und bestand auch weiterhin für die Schmerzintensität fort, während die Schmerzinterferenz sich im Follow-up weiter verbesserte. Die Ergebnisse liefern überzeugende vorläufige Beweise dafür, dass ein Gruppenformat ein effektives Vermittlungssystem für individuelle Fertigkeiten im Gebrauch von Hypnose zur Behandlung chronischer Schmerzen ist. Größere randomisierte kontrollierte Studien sollten die Gleichwertigkeit der Ergebnisse der verschiedenen Behandlungsmodi nachzuweisen.
Résumé :
Bien qu’il existe des preuves solides de l’utilisation de l’hypnose individuelle pour traiter la douleur, les preuves concernant les applications de groupe sont limitées. Ce projet a évalué les changements dans plusieurs mesures de résultats chez les personnes souffrant de douleurs chroniques traitées avec 8 semaines d’hypnose de groupe. Quatre-vingt-cinq adultes souffrant de diverses étiologies de douleurs chroniques ont suivi un traitement d’hypnose de groupe structuré en 8 séances. L’intensité de la douleur, l’interférence de la douleur et la santé globale ont été évaluées au départ, après le traitement et trois et six mois après le traitement. Des modèles linéaires à effets mixtes ont évalué les changements dans les résultats au fil du temps. Dans un modèle testant simultanément les 3 mesures de résultats, les participants se sont considérablement améliorés entre le pré et le post-traitement et ont maintenu leur amélioration tout au long du suivi. Les analyses des résultats individuels ont montré des réductions significatives avant et après le traitement de l’intensité de la douleur et de l’interférence, qui ont été maintenues pour l’intensité de la douleur et ont continué à s’améliorer pour l’interférence de la douleur tout au long du suivi. Les résultats fournissent des preuves préliminaires convaincantes au fait qu’une présentation de groupe est un système de prestation efficace pour enseigner aux individus des compétences dans l’utilisation de l’hypnose pour la gestion de la douleur chronique. Des essais contrôlés randomisés de plus grande envergure sont justifiés pour démontrer l’équivalence des résultats entre les modes de traitement.
Resumen:
Aunque existe evidencia sólida para el uso de la hipnosis individual en el tratamiento de dolor, la evidencia con respecto a las aplicaciones grupales es limitada. Este proyecto evaluó los cambios usando múltiples medidas para evaluar resultados en personas con dolor crónico tratadas con 8 semanas de hipnosis grupal. Ochenta y cinco adultos con diversas etiologías de dolor crónico completaron un tratamiento estructurado de hipnosis grupal de 8 sesiones. La intensidad e interferencia del dolor, así como la salud global se evaluaron al inicio, terminando el tratamiento y 3 y 6 meses después del tratamiento. Los modelos lineales de efectos mixtos evaluaron los cambios en los resultados a lo largo del tiempo. En un modelo que evaluó las 3 mediciones de resultados simultáneamente, los participantes mejoraron sustancialmente desde el pretratamiento hasta el postratamiento y mantuvieron la mejora durante el seguimiento. Los análisis de los resultados individuales mostraron reducciones significativas antes y después del tratamiento en la intensidad del dolor y la interferencia, que se mantuvieron para la intensidad del dolor y continuaron mejorando para la interferencia del dolor durante el seguimiento. Los resultados proporcionan evidencia preliminar convincente de que un formato grupal es un sistema de entrega eficaz para enseñar a las personas habilidades en el uso de la hipnosis para el tratamiento del dolor crónico. Se justifican ensayos controlados aleatorios más grandes para demostrar la equivalencia de los resultados entre las modalidades de tratamiento.
Chronic pain affects approximately a third of the U.S. population and costs up to $635 billion annually due to its medical cost and associated impact on disability and function (Institute of Medicine [U.S.]. Committee on Advancing Pain Research Care and Education, 2011). Increasingly, people with chronic pain and their healthcare providers are searching for accessible, effective, nonpharmacological treatments for chronic pain that enhance coping and self-management (Institute of Medicine [U.S.], 2011).
Clinical trials show that hypnosis is effective for reducing chronic pain symptoms and can have additional benefits for sleep, energy, mood, and overall quality of life (Jensen & Patterson, 2014). Individuals can learn to apply self-hypnosis as a “skill” through home practice as a method of both coping with symptoms and to increase perceived control over pain (Jensen, 2011). Hypnosis has received increasing empirical attention and clinical use as a self-management tool for patients to incorporate into multimodal pain management regimens. Yet, the application of hypnosis in integrative medicine clinics and particularly in group settings remains underutilized (McKernan et al., 2020).
Integrative medicine clinics serve individuals with chronic pain and high medical complexity who present with diverse pain etiologies, substantial functional limitations, and psychosocial dysfunction (Greeson et al., 2008; Hansen et al., 2019). Hypnosis has been applied to the treatment of musculoskeletal (Tan et al., 2015), neuropathic (Dorfman et al., 2013), and centralized pain (Zech et al., 2017). Along with its secondary benefits to overall well-being (Jensen et al., 2006), hypnosis has been shown to be efficacious in treating state anxiety (e.g., prior to an event like a medical procedure) and anxiety-related conditions such as tension-type headache (Hammond, 2010; Johnson et al., 2016; Schupp et al., 2005). Thus, hypnosis may be well-suited to individuals with diverse pain presentations as well as those for whom stress can trigger acute symptom exacerbations.
Offering psychosocial treatment in group formats has the potential to increase access and provide additional support for individuals with chronic pain. The experience of chronic pain is extremely isolating (Newton et al., 2013). Moreover, studies of group treatments for a variety of conditions report that patients benefit from the social support offered by these interventions, as well as from the positive experience of coping with illness collectively (Finlay et al., 2018; Kissane et al., 2003). As access to nonpharmacological therapies remains a consistent challenge in integrative medicine clinics due to long waiting times (Hansen et al., 2019), applying hypnosis treatment in a group format allows for increased access and decreased waiting times. While recent studies have demonstrated the feasibility and acceptability of group hypnosis for chronic stress (Fisch et al., 2020), the available literature on group hypnosis for pain is sparse.
Despite a growing body of research evaluating its efficacy and mechanisms, key gaps in practical applications of clinical hypnosis include the lack of standardized hypnosis treatment protocols, delivery of hypnosis to representative treatment samples, and empirical evaluation of group-based hypnosis treatment (Milling, 2014). In addition, the majority of existing investigations of hypnosis for chronic pain involve studies providing individual hypnosis with specific diseases that have small samples and limited follow-up (Adachi et al., 2014). For example, a recent meta-analysis reviewing hypnosis for chronic pain indicated that of 12 reviewed clinical studies, only one involved group delivery (Adachi et al., 2014). The single group study was a pilot trial with a small sample size (i.e., N = 47 across three treatment conditions), restricted to participants with fibromyalgia, with no follow-up period (the latter issue making it difficult to discern whether hypnosis benefits were lasting). Restricting study samples to those with very specific pain conditions limits generalizability to clinical settings that serve individuals with etiologically diverse pain presentations and often overlapping pain conditions.
Questions also remain regarding factors that affect treatment response to hypnotic analgesia. Because applying hypnosis to chronic pain of diverse etiologies in a group setting remains a new area of study, here we sought to draw from existing and related literature to explore factors that may influence outcome in this population. In hypnosis trials, assessing hypnotizability can enhance research by evaluating its potential impact on outcomes (Elkins, 2021). There is also some evidence that sex, catastrophizing, and pain type may influence change in symptoms following psychological intervention for chronic pain. For example, previous investigations demonstrated that men, but not women, maintained reduction in pain intensity following multidisciplinary pain intervention (Keogh et al., 2005). In addition, pain catastrophizing has been shown to mediate responses to experimental pain following hypnosis (Kronfli et al., 2012). Regarding type of pain, widespread pain (i.e., increased number of pain sites) has been associated with higher levels of pain interference after psychological intervention (Turner et al., 2007). Further, the presence of neuropathic pain has been associated with greater reductions in pain after hypnosis (Jensen et al., 2009; McKittrick et al., 2021). Due to the diverse nature of pain presentations served in integrative medicine, the type of pain experienced and degree of pain throughout the body may also be relevant to the change process.
Given these considerations, the current project aimed to assess the utility of group hypnosis as an intervention for chronic pain in an integrative medicine setting with a broad range of pain etiologies. In addition to evaluating its overall benefit to participants’ pain and overall health, we explored potential predictors or factors associated with treatment response, including hypnotizability, catastrophizing, sex, degree of widespread pain, and pain type.
Method
Study Design and Procedures
This study was a single-site project and publicly registered clinical trial (NCT #03384953) approved by the Vanderbilt University Medical Center Institutional Review Board (#170652), conducted in accordance with the declaration of Helsinki. This study used a pretest-posttest design to evaluate our primary aim of assessing the overall effect of group training in self-hypnosis for chronic pain across a broad range of pain etiologies.
We recruited individuals to an eight-session group intervention via a large academic medical center integrative medicine clinic and online through ClinicalTrials.gov between 06/2017 and 09/2019, with data collection concluding in 03/2020. Participants were screened for study eligibility via referring medical providers or study personnel using a structured screening form. Consenting participants completed assessments prior to the initiation of treatment, posttreatment, and at 3 and 6 months posttreatment. Figure 1 details study flow.
Figure 1.
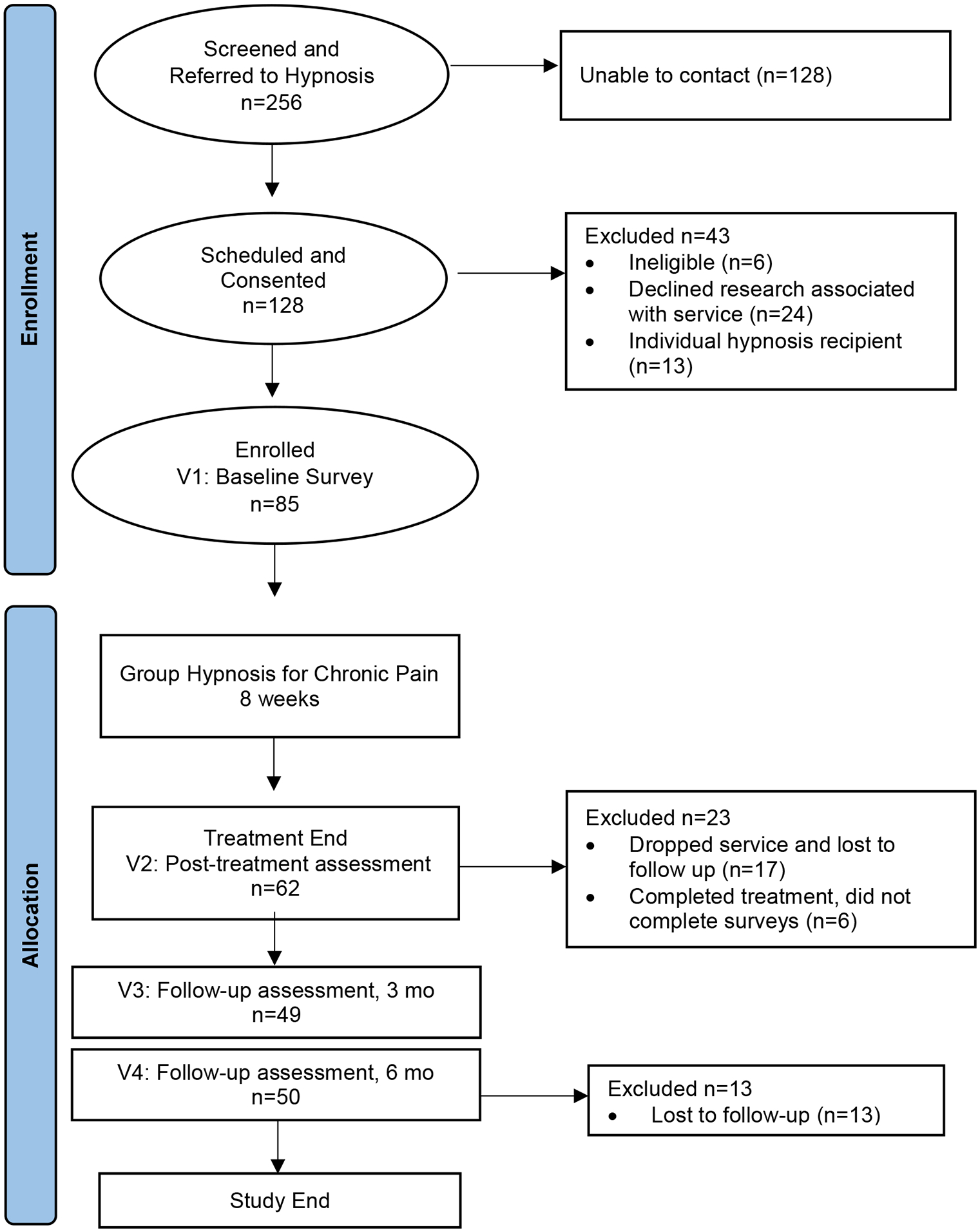
Study Flow
Eligibility Criteria
Participants were recruited when referred to an established clinical hypnosis service (McKernan et al., 2020). Study-eligible individuals were provided with the option to register for research participation in addition to clinical hypnosis treatment in a group setting. Eligibility criteria followed recommendations by pragmatic trialists, who emphasize collecting data under “usual” conditions in real-world settings where participants represent individuals likely to receive this treatment in clinical practice (Ford & Norrie, 2016; Kanzler et al., 2021; Schwartz & Lellouch, 1967). Thus, we included individuals with a diversity of pain etiologies, often with multiple pain conditions in a single individual, who were able to participate in a group setting.
Participants were English-speaking adults with existing chronic pain as defined by pain persisting for 6 months or longer, with current pain levels 4 or greater on the NRS-11 scale (Farrar et al., 2001). Participants needed to be psychologically stable and able to tolerate group interaction. Therefore, at screening all participants were asked questions regarding thoughts of self-harm, recent experiences of severe psychiatric or behavioral conditions, and recent experiences of seeing or hearing things that were not there. Those reporting acute emotional distress, symptoms of severe and persistent mental illness (i.e., active psychosis, delirium, or mania), active suicidality (i.e., endorsing active thoughts of self-harm on the day of screening), or previously noted behavioral issues interfering with group participation were excluded from participation. Last, potential participants taking large doses of opioid medication (defined as morphine equivalent dose 120mg or greater per day) were excluded with the concern that it could interfere with one’s ability to experience a hypnotic induction, reliably complete assessment measures, or would need care specifically addressing opioid use prior to treatment engagement.
Screening Process
Screening occurred via a structured, preapproved embedded clinical referral form reviewed during clinic visits when a patient was interested in being referred to hypnosis services (see measures below). Referring medical providers reviewed eligibility criteria in most cases, informed by chart review and clinical observations with the patient. At the conclusion of the referral and screening process, patients were asked if they were interested in participating in ongoing research associated with hypnosis services. Those who indicated research interest provided contact information for study registration. Study personnel then contacted participants and confirmed eligibility and discussed research procedures. When screening information was missing, unavailable, or when participants requested participation through ClinicalTrials.gov, trained research assistants assessed screening criteria via telephone using the same structured screening form, and those responses were reviewed by the PI prior to research enrollment.
Outcomes Assessment
Eligible participants completed consent procedures followed by a battery of validated assessment measures at baseline, immediately posttreatment, and at 3 months and 6 months posttreatment online via Redcap (Harris et al., 2009) or on paper. Questionnaires were completed within a 7-day period prior to service initiation, and within approximately 1 week of each follow-up timepoint. We formally assessed hypnotizability posttreatment to reduce the potential for expectancy bias related to the outcome of the hypnotizability assessment. Details of each assessment are provided in the Measures section below.
Intervention Description
The intervention followed an 8-week manualized protocol developed by Jensen and colleagues (Jensen, 2011) designed to teach individuals self-hypnosis skills for managing chronic pain. All sessions occurred at a tertiary integrative medicine clinic at an academic medical center and were in person. Sessions occurred in a 90-minute format, with a 5-to-10 minute break in the middle for participants to shift positions and use the restroom prior to receiving an induction. Each session began with a check-in, discussion of expectations (first session) or home practice, and 20 to 30 minutes of formal hypnosis including an induction, suggestions, posthypnotic suggestions, and alerting. Participants then discussed their experience posthypnosis with questions addressed by facilitators. Throughout treatment, suggestions purposefully varied and covered a wide range of experiences from comfort, sense of control over pain and its effects, to future goals, sleep improvement, and sensation alteration. This approach allies with evidence supporting diversifying suggestions to target different brain areas consistent with pain relief (Jensen & Patterson, 2014).
Participants were taught self-hypnosis in the initial session and received new recordings that included hypnotic inductions and suggestions, including posthypnotic suggestions for enhancing the benefits of treatment by practicing self-hypnosis without the recordings. Participants were encouraged to listen to at least one recording every day and to practice self-hypnosis briefly (i.e., 2 to 3 minutes each time, but more if they wish) three to five times daily for home practice. Participants were given workbooks to follow and reference weekly material between appointments. Sessions were offered weekly aside from breaks during holiday weeks.
All sessions were standardized and followed a treatment manual that described the material to be covered, discussion points, and hypnosis inductions and suggestions to use each week. Within this standardization, the treatment also allowed for some flexibility to individualize experiences as deemed appropriate by the (supervised) study clinicians. For example, participants were told they could generate personalized “favorite place” imagery, and facilitators routinely elicited and encouraged participants to experience the sensations, words, sounds, and images during the hypnotic sessions that they found most useful. The hypnotic scripts used were permissive; facilitators provided options for varied and nuanced experiences within suggestions and incorporated personalized imagery to enhance the inductions and meet patient needs.
Interventionists, Qualifications, and Intervention Monitoring
Interventionists included clinical psychologists, clinical psychology postdoctoral fellows, psychology interns, and practicum students trained in clinical hypnosis. Minimum training requirements for providers included completing a 2-day, 14-hour workshop on hypnosis provision and engaging in ongoing hypnosis supervision for 1 year. Services offered included those billed to insurance by licensed provider, as well as those delivered at no cost by providers in training under supervision. Most groups offered were conducted with two providers present, as recommended by the protocol (Jensen, 2011). At study outset, the PI (LCM) conducted the first group alone as no trainees were available to colead the group due to the time of year it occurred. Although group services were offered to every referral as a primary treatment option, individual services were made available based on provider availability, patient scheduling conflicts precluding participation, and clinical circumstances that warranted individual intervention. Those completing individual services were not included in analysis (see Figure 1). Intervention fidelity could not be monitored via audio recordings due to groups containing both research participants and other patients who did not consent to research and participated in the clinical service only (see Figure 1).
Measures
Participant Screening and Demographic Information
Structured Screening Form.
All participants were screened with a structured “hypnosis referral” form containing 13 questions at the point of referral to group services. Eight Yes/No questions evaluated patient characteristics and appropriateness for group. These questions included information on patient age, whether they spoke English, and had a history of cognitive impairment or current cognitive limitations, history of psychosis or thought disorder, past psychiatric hospitalizations not related to suicidal ideation, current unstable psychiatric or behavioral needs, active suicidal ideation or acute emotional distress, had a current Morphine Equivalent Dose of 120mg or greater daily, and whether the person had past issues participating in a group at the center. The form contained five additional questions pertaining to pain. Two questions evaluated current and worst pain intensity in the past week using an 11-item numeric rating scale (NRS; Farrar et al., 2001). One question inquired about pain persistence on a 4-point Likert scale (1=occasional pain, 4=pain all the time). The remaining two pain questions asked about duration of symptoms, and referral diagnosis. Those who met criteria evaluating group appropriateness and who had current pain levels of 4 or greater on the NRS-11 scale were research-eligible.
Demographic and Descriptive Information.
Study participants completed a 12-item questionnaire indicating age, sex, race/ethnicity, religious orientation, household income, previous exposure to hypnosis, and information regarding diagnosis and treatment.
Outcome Variables
Pain Intensity.
Four items from the Brief Pain Inventory (BPI-Pain Severity scale; Keller et al., 2004) were used to assess current, worst, least, and average pain over the past week using an 11-point Likert scale, with higher scores indicating increased severity. Responses to these items are averaged to compute a score reflecting characteristic pain intensity. The 0–10 scale has been noted as the measure of pain intensity with the most strengths and fewest weaknesses and is recommended for use in pain clinical trials (Dworkin et al., 2005). The internal consistency of the composite pain intensity score at baseline in the current sample was excellent, Cronbach’s ɑ = .92. This instrument was chosen to assess pain intensity at study outset due to the available data on clinically meaningful differences in similar pain samples that informed study design (Mease et al., 2011).
Pain interference.
The Patient-Reported Outcomes Measurement Information System® (PROMIS®) Pain Interference six-item static short form was used to assess pain interference (Amtmann et al., 2010). The items assess the effects of pain on relevant aspects of a person’s life across multiple domains. Previous research indicates the measure is valid across diverse pain samples (Askew et al., 2016). The internal consistency of the Pain Interference scale in the current sample at baseline was excellent, Cronbach’s ɑ = .96.
Global Health.
The two-item PROMIS Global Health Physical 2A was used to assess overall quality of life. Respondents indicate their perceived quality of their health and ability to carry out everyday activities on a 5-category Likert scale ranging from Poor to Excellent. The two-item global health scale has demonstrated acceptable reliability and is considered a parsimonious measure for estimating self-reported health in large samples (Hays et al., 2017). In this sample, the two Global Health items had a correlation of .32 at baseline.
Potential Treatment Predictors
Pain extent.
We used the Michigan Body Map, second edition (Brummett et al., 2011) to assess the location(s) of chronic pain complaints and the degree of pain extent. It is a two-sided body image with check-box responses for 35 potential body areas where chronic pain (defined as pain longer than 3 months) might exist, and a box for “no pain.” Degree of pain extent was measured continuously by number of pain sites endorsed, with higher numbers indicating greater degree of pain extent. The measure is considered an efficient, reliable, and valid measure in quantifying the overall number of body areas with pain when assessing fibromyalgia-like or centralized pain symptoms (Brummett et al., 2016). The internal consistency in our current sample across timepoints was excellent, Cronbach’s ɑ = .96.
Pain Type.
To assess for the presence of potential neuropathic pain, we used the PainDetect instrument (Freynhagen et al., 2006). The PainDetect is a simple, patient-based, easy-to-use screening tool to assess the presence of neuropathic and nociceptive pain. It consists of nine self-report items that assess for the quality (e.g., burning, tingling), spatial characteristics (direction pain radiates, locations of pain), and individual pain patterns of respondents. Total scores can be calculated from the nine items, with scores greater than 18 indicating likely neuropathic pain. As such, a dichotomous variable of neuropathic (>18) and nonneuropathic pain (≤18) was used in analysis. In our sample, the PainDetect had good internal consistency, Cronbach’s ɑ =.80.
Catastrophizing.
Pain catastrophizing was assessed using the 13-item Pain Catastrophizing Scale (PCS; Sullivan et al., 1995). On this scale, respondents rate the frequency with which they experience different negative thoughts and feelings associated with pain on a 0 (Not at all) to 4 (All the time) Likert scale. The PCS is commonly used in pain research with a great deal of evidence supporting its reliability and validity (Osman et al., 1997). The internal consistency for the PCS in the current sample indicated excellent reliability, Cronbach’s ɑ =.95.
Hypnotizability.
We used the Harvard Group Scale of Hypnotic Susceptibility (HGSHS; Shor & Orne, 1963) to assessed hypnotizability. The HGSHS scale consists of a verbatim spoken hypnotic induction followed by a series of 12 hypnotic suggestions. The total score is a sum of the suggestions responded to (i.e., higher scores indicate greater hypnotizability). It is one of the most commonly applied measures of hypnotizability in group settings, comparable to other standardized hypnotizability measures (Busija et al., 2011) and reliable when delivered in groups or individually (Angelini et al., 1999). In our sample, the HGSHS had adequate internal consistency, Cronbach’s ɑ = .71.
Data Analysis
This study used a pretest-posttest design to evaluate our primary aim of assessing the overall benefit of hypnosis for chronic pain across a broad range of patient etiologies. All analyses were conducted with R statistical software (R Development Core Team, 2010). We constructed a linear mixed effects model to examine overall changes in three outcome measures (pain intensity, pain interference, global health) over time, testing for changes from pretreatment to multiple follow-up points: posttreatment; 3-month follow-up; and 6-month follow-up. Given that the outcome variables were correlated (r’s = .46 to .57 at pretreatment), a multivariate approach allowed for additional statistical power for testing changes in the outcome variables over time, and a coherent framework for testing for treatment predictors (Huberty & Morris, 1989). This approach used a family-wise alpha of 0.05 for testing significance in mean-level differences of each timepoint tested against pretreatment levels, which was 0.05/3 or 0.017. We then explored measure-level differences across assessment time points. Missing values were handled by listwise deletion.
As an exploratory aim, we then evaluated the role of potential predictors or factors associated with degree of change over time: baseline pain extent, baseline pain type (neuropathic vs. nonneuropathic), baseline pain catastrophizing, hypnotizability assessed at posttreatment, and sex. We explored each potential predictor in interaction with timepoint each in their own model, simply adding the predictor of interest to the primary multivariate outcome linear mixed effects model.
Results
Sample Characteristics
We recruited 85 participants with a chronic pain diagnosis for eight sessions of group hypnosis between 06/2017 and 09/2019. We ran a total of 11 group cohorts, ranging from 5 to 10 patients, Mode = 8. Figure 1 represents study flow. Of 85 participants who completed the pretreatment assessment, 62 completed a posttreatment assessment, which reduced to 50 individuals at 6-month follow-up. Of those enrolled, 82% completed treatment, which we defined as participants attending four or more of the eight total sessions. There were no significant differences in any baseline clinical or demographic characteristics in those who did and did not complete treatment (see Appendix I).
Per Table 1, study participants were predominately white (84%) and female (72%) with an average age of 51.19 years (SD = 13.48). About half were married or in a domestic partnership. The majority (58%) had a Bachelor’s degree or higher education with an additional 28% having some college education without a Bachelor’s degree. While some of the participants worked full-time or part-time (36%), many were unable to work (37%) or were retired (19%). Nearly a third of the participants had some previous experience with hypnosis (27%).
Table 1.
Sample Demographics and Baseline Characteristics
| Total sample size | 85 |
| Age (mean (SD)) | 51.19 (13.46) |
| Sex = female (%) | 61 (71.8) |
| Self-Identified Race/Ethnicity (%) | |
| African-American/Black | 4 (4.7) |
| Asian/Pacific Islander | 3 (3.5) |
| Hispanic/Latino | 4 (4.7) |
| Multiracial | 0 (0.0) |
| White non-Hispanic | 71 (83.5) |
| Native American/American Indian | 1 (1.2) |
| Prefer not to respond | 2 (2.4) |
| Single, never married | 14 (16.7) |
| Married or in a domestic partnership | 47 (56.0) |
| Divorced | 19 (22.6) |
| Widowed | 4 (4.8) |
| Grade school | 0 (0.0) |
| High school diploma or equivalent | 4 (4.7) |
| Vocational/Technical school | 3 (3.5) |
| Some college | 24 (28.2) |
| Bachelor’s Degree | 25 (29.4) |
| Master’s Degree | 14 (16.5) |
| Doctorate or Professional Degree | 10 (11.8) |
| Other | 5 (5.9) |
| Employed full-time | 24 (28.2) |
| Employed part-time | 7 (8.2) |
| Self-employed | 2 (2.4) |
| Unemployed | 5 (5.9) |
| Retired | 16 (18.8) |
| Unable to work | 31 (36.5) |
| Under $10,000 | 2 (2.4) |
| $10,000–19,999 | 9 (10.6) |
| $20,000–$50,000 | 13 (15.3) |
| $50,000–$100,000 | 29 (34.1) |
| $100,000–$150,000 | 8 (9.4) |
| $150,000 or higher | 5 (5.9) |
| Would rather not say | 19 (22.4) |
| Had Past Experience with Hypnosis (%) | 23 (27.4) |
| Years of pain symptoms (mean (SD)) | 13.59 (11.02) |
| Baseline Clinical Variables (mean (SD)) | |
| Pain Intensity (BPI) | 5.69 (1.52) |
| Pain Interference (PROMIS-PI) | 23.72 (5.04) |
| Pain Catastrophizing (PCS) | 44.88 (22.55) |
| Global Health (PROMIS-GH-2a) | 5.44 (1.57) |
| Hypnotizability (HGSHS) | 7.46 (2.75) |
| Pain Extent (MBM) | 13.14 (13.32) |
Note: SD = standard deviation, BPI = brief pain inventory, PROMIS = patient-reported outcomes measurement information system, PI = pain interference, GH = global health, HGSHS = Harvard group scale of hypnotic susceptibility, MBM = Michigan body map
Table 2 details patients’ primary pain-related condition prompting referral to treatment, organized by specific condition, diagnostic code, and diagnostic grouping. We confirmed this information by using two sources in the electronic medical record, including provider referral forms containing diagnostic information, clinical notes and billed encounters, and problem list data. Results are presented for the total sample. Overall, the most common referral diagnosis was fibromyalgia (n = 22 individuals). Participants had diverse and complex chronic conditions prompting referral, including centralized or diffuse musculoskeletal pain conditions (33%, e.g., fibromyalgia, central pain syndrome), neuropathic pain conditions (6%, e.g., diabetic neuropathy, parasthesias), inflammatory conditions (7%, e.g., Bechet’s disease, psoriatic arthritis), back pain (14%, e.g., chronic low back pain, postlaminectomy syndrome), head pain (9%, e.g., migraine), and pelvic pain (13%, e.g., interstitial cystitis, vulvodynia). Participants reported having longstanding symptoms of their referral condition for an average of M(SD) = 13.59 (11.02) years. Pain duration was comparable between diagnostic conditions.
Table 2.
Primary Referral Diagnosis of Participants, by Diagnostic Grouping
| Group | ICD-10 | Total Sample (N = 85) | |
|---|---|---|---|
| n | % | ||
| Diffuse Musculoskeletal Pain | 28 | 32.9% | |
| Fibromyalgia | M79.7 | 22 | |
| Complex regional pain syndrome-I/II | G90.5, G90.522, G57.71 | 2 | |
| Chronic Pain Syndrome | G89.4 | 3 | |
| Central Pain Syndrome | G89.0 | 1 | |
| Back Pain | 12 | 14.1% | |
| Chronic low back pain | M54.5, M54.42 | 7 | |
| Sacral joint dysfunction | M53.3 | 1 | |
| Post-laminectomy syndrome | M96.1 | 3 | |
| Split spinal cord malformation | Q06.9 | 1 | |
| Neck Pain | 3 | 3.5% | |
| Spinal stenosis – cervical specification | M47.812, M48.02 | 2 | |
| Cervical spondylosis | M47.812 | 0 | |
| Degeneration of intervertebral disc – cervical specification | M50.30 | 1 | |
| Cervicalgia | M54.2 | 0 | |
| Head Pain | 8 | 9.4% | |
| Chronic migraine | G43.119, G43.709, G43.111, G43.109 | 6 | |
| Chronic headache | G44.301, R51.0 | 2 | |
| Extremity Pain | 5 | 5.9% | |
| Arthritis | M15.0 | 1 | |
| Osteoarthritis | M17.0 | 1 | |
| Chronic right shoulder pain | M25.511 | 1 | |
| Chronic Ankle/foot pain | S82.51 | 1 | |
| Chronic left knee pain | M25.562 | 1 | |
| Neuropathic Pain | 5 | 5.9% | |
| Paraganglioma | D44.7 | 0 | |
| Arachnoiditis | G03.9 | 0 | |
| Diabetic neuropathy | E11.40, E09.42 | 2 | |
| Neuropathic pain | M79.2, G62.9 | 1 | |
| Parasthesia | R20.2 | 2 | |
| Transverse myelitis | G37.3 | 0 | |
| Abdominal Pain | 1 | 1.2% | |
| Irritable bowel syndrome | K58.9 | 1 | |
| Pelvic Pain | 11 | 12.9% | |
| Interstitial cystitis/bladder pain syndrome | N30.10 | 8 | |
| Vulvodynia | N94.819 | 2 | |
| Inguinal pain | R10.30 | 1 | |
| Autoimmune/Inflammatory Pain | 6 | 7.1% | |
| Bechet’s Disease | M35.2 | 3 | |
| Still’s Disease | M06.1 | 1 | |
| Inflammatory polyarthropathy | M06.4 | 1 | |
| Psoriatic Arthritis | L40.50 | 1 | |
| Chronic Pain NOS | 4 | 4.7% | |
| Chronic pain due to trauma | G89.21 | 0 | |
| Chronic chest/precordial pain | R02.7, R07.9 | 2 | |
| Rib pain on right side | R07.81 | 1 | |
| Chronic pain NOS | G89.2 | 1 | |
| Unavailable/Missing in Medical Record | N/A | 2 | 2.3% |
| Number of years symptomatic M(SD), Min, Max | 8.03 (6.21) .125, 30 |
||
Note. n = frequency of patients with clinical diagnosis; % = percentage of subgroup; M = mean; SD = standard deviation, Min = minimum, Max = maximum NOS=not otherwise specified.
Baseline Clinical Characteristics
Overall, our sample reported severe and complicated symptoms. Pain severity, averaged M(SD) = 5.69 (1.52) on a 0-to-10 Numeric Rating Scale (Farrar et al., 2001). Participants endorsed feeling persistent or recurrent pain in 40% of the 35 possible pain sites in the Michigan Body Map, M(SD) = 13.14 (13.32). Average pain catastrophizing was M(SD) = 44.88 (22.55) and fell in the clinically relevant range (Sullivan et al., 1995). Average pain interference was high at M(SD) = 23.72 (5.04) and fell between a 65.7 and 66.7 T-score, while average global health M(SD) = 5.44 (1.57) was low and between a 37.3 and 41.1 T-score.
Change in Outcome
We first established the optimal random effects structure to our outcome model before examining the fixed effects. The simplest permissible model was one that allowed random intercepts by participant. A model that included cohort-level random effects did not improve prediction over that model and was discarded, X2 = 0.56, df = 1, p = .46. A subsequent model that allowed for random slopes over time by participant improved prediction over the simplest model and was retained for the random effects structure in the following analyses, X2 = 53.21, df = 9, p < 0.001.
Multivariate effects
In a model testing all three outcome measures at once, the study participants improved from pre- to posttreatment and maintained this improvement through follow-up (Table 3). There was substantial gain from pre- to posttreatment, B = −0.48, SE = 0.11, t(439.67) = −4.37, p < 0.001, d = −0.42, and these gains were maintained at both 3 months, B = −0.37, SE = 0.12, t(287.27) = −3.73, p < .001, d = −0.44, and 6 months, B = −0.41, SE = 0.32, t(222.39) = −4.11, p < 0.001, d = −0.55.
Table 3.
Primary Outcome Measures Over Time, Presented as M(SD)
| Outcome variable | Baseline (N=85) | Posttreatment (n=63) | 3 months (n=53) | 6 months (n=50) |
|---|---|---|---|---|
| Pain Intensity (BPI) | 5.69 (1.52) | 4.73 (1.88) | 4.54 (1.91) | 4.47 (2.19) |
| Pain Interference (PROMIS-PI) | 23.72 (5.04) | 20.48 (6.88) | 18.17 (6.76) | 17.75 (6.74) |
| Global Health (PROMIS-GH) | 5.44 (1.57) | 5.43 (1.78) | 5.79 (1.78) | 5.81 (1.72) |
Note: M=mean, SD=standard deviation, BPI=brief pain inventory, PROMIS=patient-reported outcomes measurement information system, PI=pain interference, GH=global health
Differential treatment effect on outcomes over timepoints
We noted differences in how the three outcome measures changed over time, as there was a significant interaction of variable and timepoint, F(6, 582.11) = 5.02, p < 0.001. This finding led us to explore patterns over time for each variable in post hoc analyses. We conducted three sets of contrasts of each timepoint using the pretreatment as the reference group, adjusting p values within the family of tests for each outcome variable. Both pain intensity and pain interference saw significant improvement relative to pretreatment, adjusted ps < 0.001, see Figure 2. Pre- to posttreatment effect sizes were medium to large for pain intensity, d = −.76, and for pain interference, d = −.73. Pain interference appeared to trend toward increasing improvement as time progressed, with a larger effect size at 3 months compared to pretreatment, d = −1.09, and at 6 months compared to pretreatment, d = −1.27. Posttreatment reductions in pain intensity instead were relatively stable at 3-month follow-up, d = −.74, and 6-month follow-up, d = −.84. Global health saw no improvement at any timepoint relative to pre-treatment, ps > .65.
Figure 2.
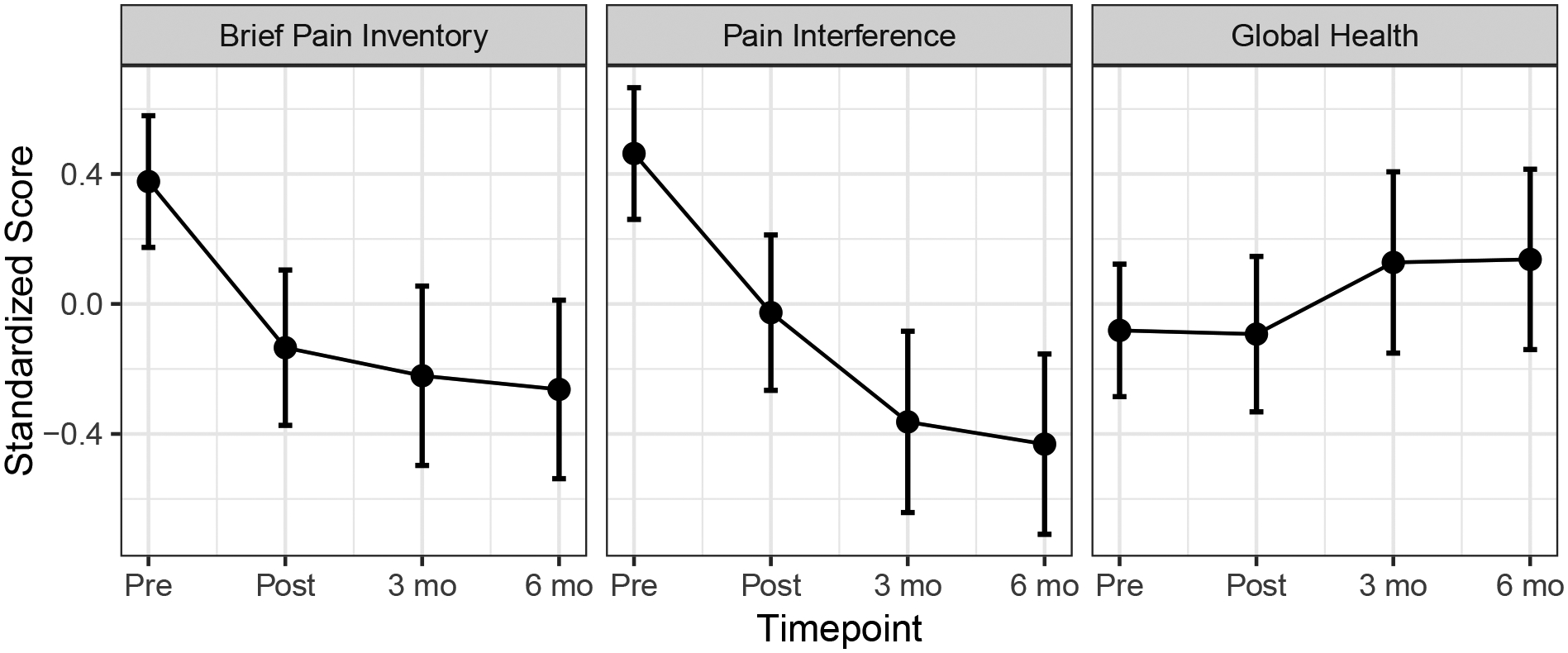
Differential treatment effects of primary outcome measures over time
Note. 95% confidence intervals are presented around modelled estimates.
Exploring Predictors of Treatment Outcomes
While there was a main effect of baseline pain extent on mean level outcome variables, F(1, 72.47) = 8.78, p = .004 (i.e., more pain extent was associated with more pain intensity and pain interference and worse general health), we found no evidence that the degree of baseline pain extent predicted degree of improvement in outcomes, F(3,100.4) = 0.35, p = .79. Similarly, baseline pain catastrophizing had an overall main effect on level of outcome variables, F(1,76.47) = 16.09, p < .001, and did not impact degree of improvement, F(1, 112.04) = .78, p = .51. The presence of neuropathic pain at baseline had a small main effect on mean level outcome variables, such that neuropathic pain was associated with greater pain intensity, interference, and worse general health overall, F(1, 49.16) = 3.68, p = .06. There was not evidence that the presence of neuropathic pain predicted improvement following treatment, F(3, 430.06) = 1.31, p = .27.
Hypnotizability assessed posttreatment was not associated significantly with outcomes, F(1, 55.72) = 0.29, p = .59. Prior hypnosis exposure appeared to have a small main effect on overall level of outcome variables, such that those with prior hypnosis experience had less severe symptoms overall, F(1, 76.83) = 3.62, p = .06); however, prior hypnosis experience was not associated with treatment gains over time. There was no overall main effect of sex on level of outcome variables, F(1, 80.32) = 1.10, p = .30, nor differences between sexes over time, F(1, 116.60) = 1.88, p = .14. An exploratory analysis of all timepoints suggested that women had greater reduction in overall symptoms immediately posttreatment, B = −.36, SE = .15, t(178.71) = −2.34, p = .02, but this gap reduced over the follow-up period, i.e., six months, B = −.19, SE = .20, t(60.90) = −.93, p = .36.. As suggested in a plot of the interaction over time (Figure 3), women saw an immediate improvement that stabilized over time while men tended to experience more gradual improvement over time.
Figure 3.
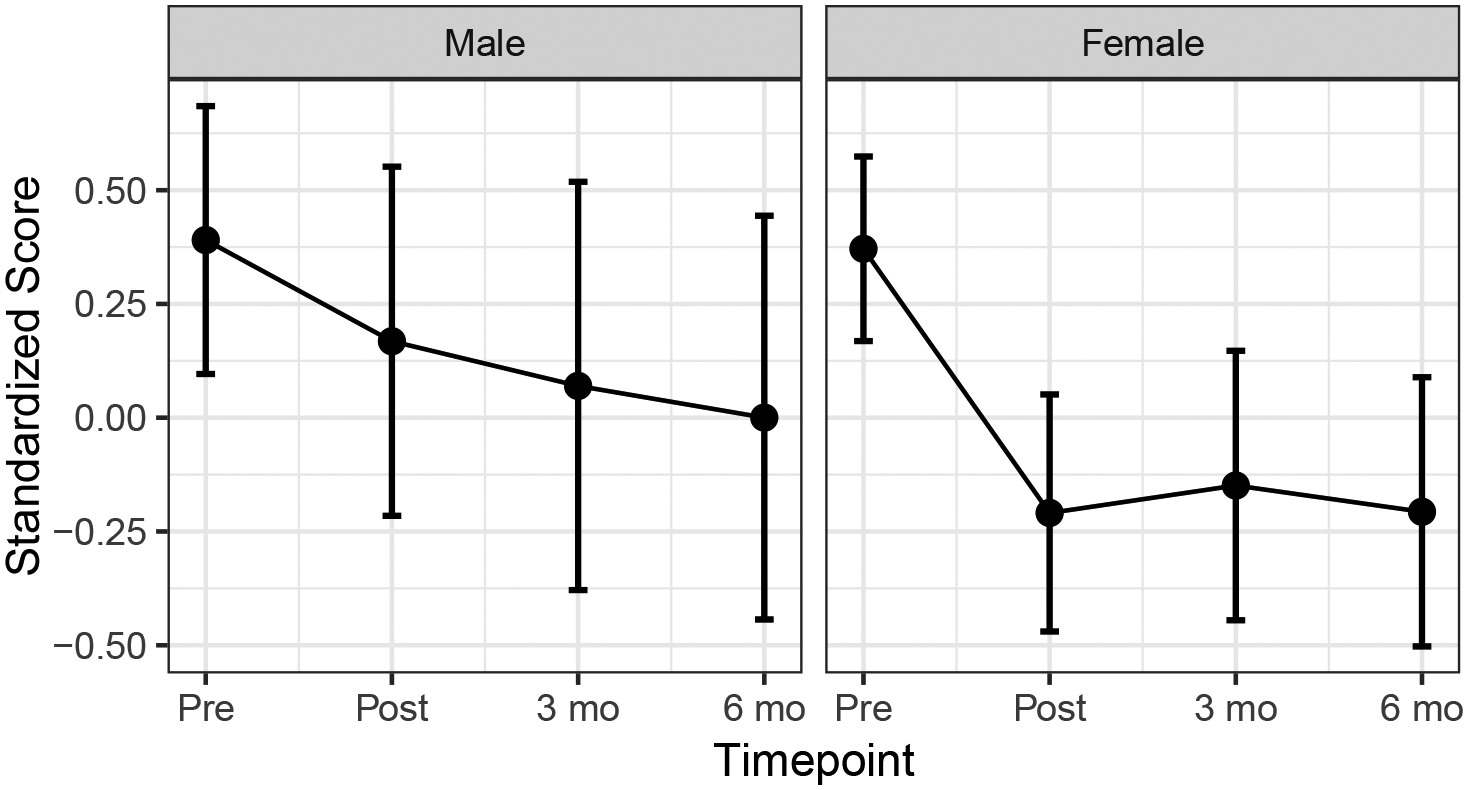
Patterns of multivariate outcome over time by sex
Discussion
This study provides additional evidence for the potential for individuals with chronic pain to benefit from group training in self-hypnosis. In a sample of individuals with diverse, longstanding pain conditions, group hypnosis participation was associated with significant improvements, including reductions in average pain intensity and pain interference. These benefits were maintained through 6 months. Analysis of change over time revealed a pattern of emerging effects at follow-up. Specifically, while participants experienced no significant changes in global health relative to pretreatment at any assessment time point, immediate posttreatment significant reductions in average pain intensity were maintained at 6 months, and participants’ initial significant posttreatment reductions in pain interference appeared to improve further at 3 and again at 6 months.
An important consideration is assessing the relative clinical value of these findings. Our baseline results indicate severe, chronic, and diffuse pain in our sample, which resembles individuals with chronic widespread pain conditions such as fibromyalgia. On average, participants experienced chronic widespread pain in 40% of their body (approximately 13 body sites). This finding indicates far more diffuse pain than previous studies of a mixed chronic pain sample showing approximately M(SD) = 3.90 (2.90) sites in average (Brummett et al., 2016). When considering what type of pain reduction would be clinically meaningful in this population, a clinically meaningful reduction in pain intensity for individuals with fibromyalgia is approximately a 2-point reduction in average pain intensity as measured by the BPI, which is consistent with what is considered moderately clinically meaningful in chronic pain trials more broadly (Dworkin et al., 2008; Mease et al., 2011). In chronic pain populations, it is important to evaluate both levels of pain intensity and the impact of pain on daily life when assessing the impact of an intervention (Dworkin et al., 2005). A clinically meaningful improvement in pain interference for individuals with chronic pain is reflected by a 2-to-3-point reduction on the PROMIS-PI (Chen et al., 2018). Per Table 3, while the average (but statistically significant) reductions in pain intensity found in this investigation do not meet the threshold for clinical significance in the sample as a whole, the improvements in pain interference exceed them. Together, these findings suggest that in addition to slight overall reduction in pain intensity, training in self-hypnosis might reduce the negative impact of pain on function over time. Given the known impact of pain on daily life and well-being, and that these are core domains to living with chronic pain (Dworkin et al., 2005), this finding aligns with qualitative reports in previous long-term investigations that show individuals with longstanding, complex pain of diverse etiologies can benefit from some of the positive effects of hypnosis outside of pain intensity, even in instances where overall health may not improve (Jensen et al., 2006), as we observed in this study.
We examined numerous factors that have been shown to affect treatment outcomes in prior research, including pain extent at baseline, baseline pain type, baseline catastrophizing, hypnotizability assessed a posttreatment, and sex. Although certain factors were associated with increased symptomology in our sample, we did not find evidence that any of these variables predicted change following hypnosis. For example, hypnotizability assessed at posttreatment was not associated with treatment outcomes, nor was baseline catastrophizing, pain extent, or presence versus absence of neuropathic pain. Women experienced more immediate benefits of hypnosis posttreatment than men initially, although this difference abated during the follow-up period. It may be that women benefit from the group process more than men, which is consistent with previous research suggesting women have more positive subjective experiences and outcomes in group therapy and its supportive elements—whereas men can feel disconnected in groups that are predominantly female (Ogrodniczuk, 2006; Ogrodniczuk et al., 2006).
In contrast to a recent meta-analysis detailing associations between hypnotizability and acutely induced, experimental pain reduction in mostly healthy subjects (Thompson et al., 2019), these study findings concur with previous research showing that hypnotizability shows weak and generally nonsignificant associations with treatment outcome in chronic pain clinical trials (Jensen & Patterson, 2014; Patterson & Jensen, 2003). Most recently, a follow-up meta-analysis examining the effects of hypnosis on clinical pain (including acute, procedural, and chronic pain) found a mean weighted effect of hypnotizability on pain reduction of r = .53 over six trials (Milling et al., 2021). However, only two trials of the six available for analysis were chronic pain samples. Thus, additional research is warranted to better understand the factors that influence the associations between hypnotizability and outcomes in clinical populations.
Although the distribution of hypnotizability in our sample appeared elevated compared to other published (Benham et al., 2002) average scores in the 1990s (M = 7.48 vs M = 6.73), hypnotizability scores have been increasing over time (Költő et al., 2014), and a linear model built on HGSHS scores from data spanning from 1973 to 2010 would predict 7.56 as an average score for females in 2019 and 6.87 as an average score for males. Given that our sample was predominately female, although they may appear slightly elevated, our sample’s scores are generally in line with trends in the general inflation of hypnotizability scores over time.
We previously reported the feasibility and financial viability of hypnosis services for chronic pain in real-world settings (McKernan et al., 2020). Ultimately, widening the evidence base for hypnosis is key to increase its support among healthcare providers and organizations. The lack of standardized hypnosis treatment protocols for chronic pain has slowed its adoption, uptake, and recognition as an empirically supported treatment (Elkins et al., 2007). Recent efforts by experts in the field have led to several manualized protocols to standardize approaches to hypnosis, including the one used in this investigation (Jensen, 2011; Palsson, 2006; Williams et al., 2020). It is important to note the potential for research integration into clinical services. In our study, when referred to hypnosis services, 76% of eligible participants approached for participation enrolled in research. Our high enrollment may be a positive reflection of our study being embedded in clinical care and fewer participation barriers with the use of online assessments (Rengerink et al., 2017). We also provided brief education to our referring providers about hypnosis, dispelling myths and correcting misconceptions about its usage and boundaries when initiating hypnosis services (McKernan et al., 2020). Doing so may have improved our referral rate. As we demonstrated and others have noted (Yeh et al., 2014), appropriate hypnosis education can improve adoption, where informed patients and providers are willing and open to participating in treatment and research process.
We conducted this study at a low cost with limited resources. Our primary limitation was with the study design, in particular the lack of a control group or randomized assignment to treatment. Thus, we cannot draw conclusions regarding the role that treatment per se had in the improvements observed. Both time (which can create a regression to the mean confound) and nonspecific effects (e.g., therapist attention, placebo effects) provide alternative explanations for the benefits observed. Lack of randomization also limited the control of confounding factors that may have influenced treatment outcomes. We also did not collect data on medication intake of participants throughout the study, which may have influenced outcomes as an unmeasured confounding factor. Because this research was conducted through ongoing clinical care, we were limited to a pre-to-posttreatment design with convenience sampling. As some participants in the hypnosis clinical service did not consent to research and elected to engage in clinical treatment (24 of the 128 who qualified, per Figure 1), we could not formally monitor intervention fidelity through audio recordings for privacy reasons and were limited to monitoring implementation via direct clinical observation and ongoing group and individual supervision of trainees conducting hypnosis.
Data collection was limited at follow-up, where we experienced significant data loss by 6 months. In our clinical experience, approximately one third of participants may drop group services after initiation, which is consistent with the 62 of 85 individuals completing surveys posttreatment (approximately 73% of the sample, per Figure 1). Analysis of dropout rates from group services report average attrition at 19%, which can be as high as 63% (McDermut et al., 2001). However, our methods of telephone and email contact reminders to complete surveys within the 7-day window of each follow-up assessment point may not have been sufficient to maintain higher participant engagement at follow-up. We were able to examine any potential differences between those who completed and did not complete treatment, and we are encouraged by the lack of statistically tested differences between those who did and did not complete treatment in baseline characteristics. However, it remains possible that individuals who benefitted more from treatment were biased in their follow-up reports.
One study strength included our success in enrolling a relatively large sample with a proportion that was able to provide extended follow-up. The study also allowed us to assess translation of services in real-world settings in patients with diverse pain etiologies and extended duration of symptoms. We measured hypnotizability posttreatment; there is a small possibility that individuals with lower hypnotizability levels were more likely to drop out of treatment—however, this is speculative at this point.
Questions remain about the optimal duration of hypnosis, as treatment length has varied across studies. Significant benefits of hypnosis can occur in as few as two sessions (Tan et al., 2015). A recent, large, randomized trial showed that individuals with chronic pain can have significant and lasting pain reduction after only four hypnosis sessions (Jensen et al., 2020). Eighty percent of our sample completed at least four treatment sessions, and the average number of sessions attended in this study was 5.42 (SD = 2.11). To learn whether patients may benefit from a shorter duration of treatment, future studies may examine the optimal “dose” of treatment by varying the level of in-person sessions and closely tracking participant self-hypnosis practice outside of session. Given that some treatment gains appear to increase over time, studies with extensive (e.g., at least 6 months) follow-up appear to be ideal. As intervention delivery rapidly evolves with the use of technological platforms and applications (Shore et al., 2020), gathering information about the benefits of hypnosis delivered electronically through telemedicine or enhanced by technology is a much-needed future area of study (McKernan, 2020).
Summary and Conclusions
Although achieved with a pre-posttreatment design, the effect sizes in our study provided compelling preliminary evidence that a group format is an effective delivery system for hypnosis used to manage severe chronic pain from a wide range of pain etiologies. For our sample of individuals with chronic pain, training in self-hypnosis was associated with immediate significant reductions in pain and meaningful change in pain interference that maintained or improved over the course of 6 months. Research that uses a randomized controlled study design with greater numbers of participants is needed to evaluate outcome equivalence between group and individual intervention, and the potential for these interventions to be delivered via telemedicine formats.
Acknowledgments
This project was supported by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Appendix 1: Sample Demographics and Baseline Characteristics by Completer Status
| Overall | Noncompleters | Completers | p | |
|---|---|---|---|---|
| Total sample size | 85 | 19 | 66 | |
| Age (mean (SD)) | 51.19 (13.46) | 48.37 (12.13) | 52.00 (13.80) | 0.303 |
| Sex = female (%) | 61 (71.8) | 15 (78.9) | 46 (69.7) | 0.617 |
| Race (%) | 0.146 | |||
| African-American/Black | 4 (4.7) | 3 (15.8) | 1 (1.5) | |
| Asian/Pacific Islander | 3 (3.5) | 0 (0.0) | 3 (4.5) | |
| Hispanic/Latino | 4 (4.7) | 1 (5.3) | 3 (4.5) | |
| Multiracial | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Native American/American Indian | 71 (83.5) | 15 (78.9) | 56 (84.8) | |
| White non-Hispanic | 1 (1.2) | 0 (0.0) | 1 (1.5) | |
| Prefer not to respond | 2 (2.4) | 0 (0.0) | 2 (3.0) | |
| Marital status (%) | 0.718 | |||
| Single, never married | 14 (16.7) | 3 (15.8) | 11 (16.9) | |
| Married or in a domestic partnership | 47 (56.0) | 11 (57.9) | 36 (55.4) | |
| Divorced | 19 (22.6) | 5 (26.3) | 14 (21.5) | |
| Widowed | 4 (4.8) | 0 (0.0) | 4 (6.2) | |
| Education Status (%) | ||||
| Grade school | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.388 |
| High school diploma or equivalent | 4 (4.7) | 1 (5.3) | 3 (4.5) | |
| Vocational/Technical school | 3 (3.5) | 2 (10.5) | 1 (1.5) | |
| Some college | 24 (28.2) | 3 (15.8) | 21 (31.8) | |
| Bachelor’s Degree | 25 (29.4) | 5 (26.3) | 20 (30.3) | |
| Master’s Degree | 14 (16.5) | 5 (26.3) | 9 (13.6) | |
| Doctorate or Professional Degree | 10 (11.8) | 2 (10.5) | 8 (12.1) | |
| Other | 5 (5.9) | 1 (5.3) | 4 (6.1) | |
| Employment status (%) | 0.290 | |||
| Employed full-time | 24 (28.2) | 5 (26.3) | 19 (28.8) | |
| Employed part-time | 7 (8.2) | 0 (0.0) | 7 (10.6) | |
| Self-employed | 2 (2.4) | 0 (0.0) | 2 (3.0) | |
| Unemployed | 5 (5.9) | 2 (10.5) | 3 (4.5) | |
| Retired | 16 (18.8) | 2 (10.5) | 14 (21.2) | |
| Unable to work | 31 (36.5) | 10 (52.6) | 21 (31.8) | |
| Household Income (%) | 0.338 | |||
| Under $10,000 | 2 (2.4) | 1 (5.3) | 1 (1.5) | |
| $10,000–19,999 | 9 (10.6) | 1 (5.3) | 8 (12.1) | |
| $20,000–$50,000 | 13 (15.3) | 4 (21.1) | 9 (13.6) | |
| $50,000–$100,000 | 29 (34.1) | 3 (15.8) | 26 (39.4) | |
| $100,000–$150,000 | 8 (9.4) | 2 (10.5) | 6 (9.1) | |
| $150,000 or higher | 5 (5.9) | 1 (5.3) | 4 (6.1) | |
| Would rather not say | 19 (22.4) | 7 (36.8) | 12 (18.2) | |
| Had Past Experience with Hypnosis (%) | 23 (27.4) | 3 (15.8) | 20 (30.8) | 0.319 |
| Years of pain symptoms (mean(SD)) | 13.59 (11.02) | 12.61 (7.60) | 13.86 (11.83) | 0.674 |
| Baseline Clinical Variables (mean (SD)) | ||||
| Pain Intensity (BPI) | 5.69 (1.52) | 5.92 (1.40) | 5.62 (1.56) | 0.458 |
| Pain Interference (PROMIS-PI) | 23.72 (5.04) | 24.89 (4.92) | 23.38 (5.07) | 0.251 |
| Global Health (PROMIS-GH-2a) | 5.44 (1.57) | 5.33 (1.19) | 5.47 (1.67) | 0.746 |
| Pain Catastrophizing (PCS) | 44.88 (22.55) | 39.88 (17.23) | 46.18 (23.69) | 0.308 |
| Hypnotizability (HGSHS) | 7.46 (2.75) | 8.00 (2.00) | 7.43 (2.79) | 0.728 |
| Pain Extent (MBM) | 13.14 (13.32) | 9.95 (12.41) | 14.06 (13.52) | 0.238 |
Note: SD = standard deviation, BPI = brief pain inventory, PROMIS = patient-reported outcomes measurement information system, PI = pain interference, GH = global health, HGSHS = Harvard group scale of hypnotic susceptibility, MBM = Michigan body map.
Footnotes
Disclosure statement: The authors have no relevant disclosures to note.
Data sharing statement:
a de-identified data set associated with this study may be available upon written request to the corresponding author, and established in accordance with institutional data sharing policies.
References
- Adachi T, Fujino H, Nakae A, Mashimo T, & Sasaki J (2014). A meta-analysis of hypnosis for chronic pain problems: a comparison between hypnosis, standard care, and other psychological interventions. International Journal of Clinical and Experimental Hypnosis, 62(1), 1–28. 10.1080/00207144.2013.841471 [DOI] [PubMed] [Google Scholar]
- Amtmann D, Cook KF, Jensen MP, Chen7 WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, & Lai JS (2010). Development of a PROMIS item bank to measure pain interference. Pain, 150(1), 173–182. 10.1016/j.pain.2010.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini FJ, Kumar VK, & Chandler L (1999). The Harvard Group Scale of Hypnotic Susceptibility and related instruments: individual and group administrations. International Journal of Clinical and Experimental Hypnosis, 47(3), 236–250. 10.1080/00207149908410035 [DOI] [PubMed] [Google Scholar]
- Askew RL, Cook KF, Revicki DA, Cella D, & Amtmann D (2016). Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. Journal of Clinical Epidemiology, 73, 103–111. 10.1016/j.jclinepi.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham G, Smith N, & Nash MR (2002). Hypnotic susceptibility scales: Are the mean scores increasing? International Journal of Clinical and Experimental Hypnosis, 50(1), 5–16. 10.1080/00207140208410087 [DOI] [PubMed] [Google Scholar]
- Brummett CM, Bakshi RR, Goesling J, Leung D, Moser SE, Zollars JW, Williams DA, Clauw DJ, & Hassett AL (2016). Preliminary validation of the Michigan Body Map (MBM). Pain, 157(6), 1205–1212. 10.1097/j.pain.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett CM, Hassett AL, Brummett KA, Clauw DJ, & Williams DA (2011). The Michigan Body Map and its use in assessing the American College of Rheumatology survey criteria for Fibromyalgia. Arthritis & Rheumatism, 62, 744. [Google Scholar]
- Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, & Osborne RH (2011). Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQOL). Arthritis Care & Research, 63(S11), S383–S412. 10.1002/acr.20541 [DOI] [PubMed] [Google Scholar]
- Chen CX, Kroenke K, Stump TE, Kean J, Carpenter JS, Krebs EE, Bair MJ, Damush TM, & Monahan PO (2018). Estimating minimally important differences for the PROMIS pain interference scales: Results from 3 randomized clinical trials. Pain, 159(4), 775–782. 10.1097/j.pain.0000000000001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman D, George MC, Schnur J, Simpson DM, Davidson G, & Montgomery G (2013). Hypnosis for treatment of HIV neuropathic pain: A preliminary report. Pain Medicine, 14(7), 1048–1056. 10.1111/pme.12074 [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDemott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wenicke J, & Witter J (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain, 113(1–2), 9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, & Zavisic S (2008). Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain, 9(2), 105–121. 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Elkins G, Jensen MP, & Patterson DR (2007). Hypnotherapy for the management of chronic pain. International Journal of Clinical and Experimental Hypnosis, 55(3), 275–287. 10.1080/00207140701338621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins GR (2021). Hypnotizability: Emerging perspectives and research. International Journal of Clinical and Experimental Hypnosis, 69(1), 1–6. 10.1080/00207144.2021.1836934 [DOI] [PubMed] [Google Scholar]
- Farrar JT, Young JP, LaMoreaux L, Werth JL, & Poole RM (2001). Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain, 94(2), 149–158. [DOI] [PubMed] [Google Scholar]
- Finlay KA, Peacock S, & Elander J (2018). Developing successful social support: An interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychology and Health, 33(7), 846–871. 10.1080/08870446.2017.1421188 [DOI] [PubMed] [Google Scholar]
- Fisch S, Binting S, Roll S, Cree M, Brinkhaus B, & Teut M (2020). Group hypnosis for stress reduction – A feasibility study. International Journal of Clinical and Experimental Hypnosis, 68(4), 493–510. 10.1080/00207144.2020.1781537 [DOI] [PubMed] [Google Scholar]
- Ford I, & Norrie J (2016). Pragmatic trials. New England Journal of Medicine, 375(5), 454–463. 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- Freynhagen R, Baron R, Gockel U, & Tolle TR (2006). painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Current Medical Research and Opinion, 22(10), 1911–1920. 10.1185/030079906x132488 [DOI] [PubMed] [Google Scholar]
- Greeson JM, Rosenzweig S, Halbert SC, Cantor IS, Keener MT, & Brainard GC (2008). Integrative medicine research at an academic medical center: Patient characteristics and health-related quality-of-life outcomes. The Journal of Alternative and Complementary Medicine, 14(6), 763–767. 10.1089/acm.2008.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond DC (2010). Hypnosis in the treatment of anxiety and stress-related disorders. Expert Review of Neurotherapeutics, 10(2), 263–273. 10.1586/ern.09.140 [DOI] [PubMed] [Google Scholar]
- Hansen KA, McKernan LC, Carter SD, Allen C, & Wolever RQ (2019). A replicable and sustainable whole person care model for chronic pain. The Journal of Alternative and Complementary Medicine (New York, N.Y.), 25(S1), S86–S94. 10.1089/acm.2018.0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Schalet BD, Spritzer KL, & Cella D (2017). Two-item PROMIS® global physical and mental health scales. Journal of Patient-Reported Outcomes, 1(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberty CJ, & Morris JD (1989). Multivariate analysis versus multiple univariate analyses. Psychological Bulletin, 105(2), 302–308. 10.1037/0033-2909.105.2.302 [DOI] [Google Scholar]
- Institute of Medicine (U.S.). Committee on advancing pain research care and education. (2011). Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, D.C.: National Academies Press (US). Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK92522/ [PubMed] [Google Scholar]
- Jensen MP (2011). Hypnosis for chronic pain management: Therapist guide. Oxford University Press. [Google Scholar]
- Jensen MP, Barber J, Romano JM, Hanley MA, Raichle KA, Molton IR, Engel JM, Osborne TL, Stoelb BL, Cardenas DD, & Patterson DR (2009). Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. International Journal of Clinical and Experimental Hypnosis, 57(3), 239–268. 10.1080/00207140902881007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, McArthur KD, Barber J, Hanley MA, Engel JM, Romano JM, Cardenas DD, Kraft GH, Hoffman AJ, & Patterson DR (2006). Satisfaction with, and the beneficial side effects of, hypnotic analgesia. International Journal of Clinical and Experimental Hypnosis, 54(4), 432–447. 10.1080/00207140600856798 [DOI] [PubMed] [Google Scholar]
- Jensen MP, Mendoza ME, Ehde DM, Patterson DR, Molton IR, Dillworth TM, Gertz KJ, Chan J, Hakimian S, Battalio SL, Ciol MA (2020). Effects of hypnosis, cognitive therapy, hypnotic cognitive therapy, and pain education in adults with chronic pain: A randomized clinical trial. Pain, 161(10), 2284–2298. 10.1097/j.pain.0000000000001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, & Patterson DR (2014). Hypnotic approaches for chronic pain management: Clinical implications of recent research findings. The American Psychologist, 69(2), 167–177. 10.1037/a0035644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Marcus J, Hickman K, Barton D, & Elkins GR (2016). Anxiety reduction among breast-cancer survivors receiving hypnotic relaxation therapy for hot flashes. International Journal of Clinical and Experimental Hypnosis, 64(4), 377–390. 10.1080/00207144.2016.1209042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzler KE, McGeary DD, McGeary C, Blankenship AE, Young-McCaughan S, Peterson AL, Buhrer JC, Cobos BA, Dobmeyer AC, Hunter CL, Bhagwat A, Blue Star JA, & Goodie JL (2021). Conducting a pragmatic trial in integrated primary care: Key decision points and considerations. Journal of Clinical Psychology in Medical Settings, 1–10. 10.1007/s10880-021-09790-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, & Cleeland CS (2004). Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clinical Journal of Pain, 20(5), 309–318. [DOI] [PubMed] [Google Scholar]
- Keogh E, McCracken LM, & Eccleston C (2005). Do men and women differ in their response to interdisciplinary chronic pain management? Pain, 114(1–2), 37–46. 10.1016/j.pain.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Kissane DW, Bloch S, Smith GC, Miach P, Clarke DM, Ikin J, Love A, Ranieri N, & McKenzie D (2003). Cognitive-existential group psychotherapy for women with primary breast cancer: A randomised controlled trial. Psycho-Oncology, 12(6), 532–546. 10.1002/pon.683 [DOI] [PubMed] [Google Scholar]
- Költő A, Gősi-Greguss AC, Varga K, & Bányai EI (2014). The influence of time and gender on hungarian hypnotizability scores. International Journal of Clinical and Experimental Hypnosis, 62(1), 84–110. 10.1080/00207144.2013.841487 [DOI] [PubMed] [Google Scholar]
- Kronfli T, Goodin B, & McGuire L (2012). Pain catastrophizing as an underlying mechanism examining the hypoalgesic effects of clinical hypnosis. Journal of Pain, 13(4). [Google Scholar]
- McDermut W, Miller IW, & Brown RA (2001). The efficacy of group psychotherapy for depression: A meta-analysis and review of the empirical research. Clinical Psychology: Science and Practice, 8(1), 98–116. 10.1093/clipsy.8.1.98 [DOI] [Google Scholar]
- McKernan LC (2020). The positive message in negative findings: brief psychosocial intervention can lead to lasting pain reduction using hypnosis, education, or cognitive approaches. Pain, 161(10), 2227–2228. doi: 10.1097/j.pain.0000000000001942 [DOI] [PubMed] [Google Scholar]
- McKernan LC, Finn MTM, Patterson DR, Williams RM, & Jensen MP (2020). Clinical hypnosis for chronic pain in outpatient integrative medicine: An implementation and training model. Journal of Alternative and Complementary Medicine (New York, N.Y.), 26(2), 107. 10.1089/acm.2019.0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKittrick ML, Connors EL, & McKernan LC (2021). Hypnosis for chronic neuropathic pain: A scoping review. Pain Medicine, ePublication ahead of print. 10.1093/pm/pnab320 [DOI] [PubMed] [Google Scholar]
- Mease PJ, Spaeth M, Clauw DJ, Arnold LM, Bradley LA, Russell IJ, Kajdasz DK, Walker DJ, & Chappell AS (2011). Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care & Research, 63(6), 821–826. 10.1002/acr.20449 [DOI] [PubMed] [Google Scholar]
- Milling LS (2014). Hypnosis in the treatment of headache pain: A methodological review. Psychology of Consciousness: Theory, Research, and Practice, 1(4), 431–444. 10.1037/cns0000031 [DOI] [Google Scholar]
- Milling LS, Valentine KE, LoStimolo LM, Nett AM, & McCarley HS (2021). Hypnosis and the Alleviation of Clinical Pain: A comprehensive meta-analysis. International Journal of Clinical and Experimental Hypnosis, 69(3), 297–322. 10.1080/00207144.2021.1920330 [DOI] [PubMed] [Google Scholar]
- Newton NJ, Southall JL, Raphael JH, Ashford RL, & LeMarchand K (2013). A narrative review of the impact of disbelief in chronic pain. Pain Management Nursing, 14(3), 161–171. 10.1016/j.pmn.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Ogrodniczuk JS (2006). Men, women, and their outcome in psychotherapy. Psychotherapy Research, 16(4), 453–462. 10.1080/10503300600590702 [DOI] [Google Scholar]
- Ogrodniczuk JS, Piper WE, & Joyce AS (2006). Treatment compliance in different types of group psychotherapy: Exploring the effect of age. Journal of Nervous and Mental Disease, 194(4), 287–293. 10.1097/01.nmd.0000207366.49820.85 [DOI] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, & O’Neill E (1997). Factor structure, reliability, and validity of the Pain Catastrophizing Scale. Journal of Behavioral Medicine, 20(6), 589–605. 10.1023/a:1025570508954 [DOI] [PubMed] [Google Scholar]
- Palsson OS (2006). Standardized hypnosis treatment for irritable bowel syndrome: The North Carolina protocol. International Journal of Clinical and Experimental Hypnosis, 54(1), 51–64. 10.1080/00207140500322933 [DOI] [PubMed] [Google Scholar]
- Patterson DR, & Jensen MP (2003). Hypnosis and clinical pain. Psychological Bulletin, 129(4), 495–521. 10.1037/0033-2909.129.4.495 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2010). R: A language and environment for statistical computing. In R Foundatoin for Statistical Computing. [Google Scholar]
- Rengerink KO, Kalkman S, Collier S, Ciaglia A, Worsley SD, Lightbourne A, Eckert L, Groenwold RH, Grobbee DE, & Irving EA (2017). Series: Pragmatic trials and real world evidence: Paper 3. Patient selection challenges and consequences. Journal of Clinical Epidemiology, 89, 173–180. 10.1016/j.jclinepi.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Schupp CJ, Berbaum K, Berbaum M, & Lang EV (2005). Pain and anxiety during interventional radiologic procedures: Effect of patients’ state anxiety at baseline and modulation by nonpharmacologic analgesia adjuncts. Journal of Vascular and Interventional Radiology, 16(12), 1585–1592. 10.1097/01.RVI.0000185418.82287.72 [DOI] [PubMed] [Google Scholar]
- Schwartz D, & Lellouch J (1967). Explanatory and pragmatic attitudes in therapeutical trials. Journal of Chronic Diseases, 20(8), 637–648. 10.1016/0021-9681(67)90041-0 [DOI] [PubMed] [Google Scholar]
- Shor RE, & Orne EC (1963). Harvard Group Scale of Hypnotic Susceptibility. Consulting Psychologists Press, INC. [DOI] [PubMed] [Google Scholar]
- Shore JH, Schneck CD, & Mishkind MC (2020). Telepsychiatry and the coronavirus disease 2019 Pandemic—Current and future outcomes of the rapid virtualization of psychiatric care. JAMA Psychiatry, 77(12), 1211–1212. 10.1001/jamapsychiatry.2020.1643 [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Bishop SR, & Pivik J (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7(4), 524. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- Tan G, Rintala DH, Jensen MP, Fukui T, Smith D, & Williams W (2015). A randomized controlled trial of hypnosis compared with biofeedback for adults with chronic low back pain. European Journal of Pain, 19(2), 271–280. 10.1002/ejp.545 [DOI] [PubMed] [Google Scholar]
- Thompson T, Terhune DB, Oram C, Sharangparni J, Rouf R, Solmi M, Veronese N, & Stubbs B (2019). The effectiveness of hypnosis for pain relief: A systematic review and meta-analysis of 85 controlled experimental trials. Neuroscience & Biobehavioral Reviews, 99, 298–310. 10.1016/j.neubiorev.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Turner JA, Holtzman S, & Mancl L (2007). Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain, 127(3), 276–286. 10.1016/j.pain.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Williams RM, Ehde DM, Day M, Turner AP, Hakimian S, Gertz K, Ciol M, McCall A, Kincaid C, Pettet MW, Patterson D, Suri P, & Jensen MP (2020). The chronic pain skills study: Protocol for a randomized controlled trial comparing hypnosis, mindfulness meditation and pain education in veterans. Contemporary Clinical Trials, 90, 105935. 10.1016/j.cct.2020.105935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh VM, Schnur JB, & Montgomery GH (2014). Disseminating hypnosis to health care settings: Applying the RE-AIM framework. Psychology of consciousness (Washington, D.C.), 1(2), 213–228. 10.1037/cns0000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech N, Hansen E, Bernardy K, & Häuser W (2017). Efficacy, acceptability and safety of guided imagery/hypnosis in fibromyalgia – A systematic review and meta-analysis of randomized controlled trials. European Journal of Pain, 21(2), 217–227. 10.1002/ejp.933 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
a de-identified data set associated with this study may be available upon written request to the corresponding author, and established in accordance with institutional data sharing policies.


