Achieving successful long-term weight loss with lifestyle modification in people with obesity is difficult and underscores the need for effective pharmacotherapy. Since 1947, a total of 18 medications have been approved by the US FDA for treating obesity; however, only 5 remain available for long-term use in the US. Semaglutide, a GLP-1 receptor agonist approved in 2021, demonstrated much greater weight loss than previous medications, which stimulated the development of poly-agonists that combine GLP-1 receptor agonism with GIP and glucagon receptor agonism. The potential of this approach was recently demonstrated by the extraordinary weight loss achieved by tirzepatide, a GLP-1/GIP receptor dual agonist. The therapeutic efficacy of poly-agonists is likely to change the treatment paradigm for obesity. However, the use of medications for obesity, as for other chronic diseases, will likely require life-long treatment, which makes it important to analyze the long-term efficacy, safety, and economic implications of chronic pharmacotherapy.
Obesity is a major public health problem in the United States and many other countries because of its high and increasing prevalence, adverse effects on cardiometabolic function, causal relationship with serious diseases, and economic consequences due to increased health care costs and loss of work. The primary therapy for obesity is weight loss, which requires consuming less energy than expended to decrease body fat mass and body weight, followed by continued consumption of a eucaloric reduced-calorie diet to maintain long-term weight loss and a smaller body size. Moderate 5% weight loss improves multiple cardiometabolic clinical variables and multi-organ insulin sensitivity, and there is a progressive improvement in outcomes with progressive 5% to 15% weight loss (1, 2, 3).
Lifestyle modification that is focused on decreasing energy intake is the cornerstone of weight loss therapy. However, achieving successful weight loss through lifestyle therapy is difficult, and underscores the need for effective and safe pharmacotherapy for obesity. The first medication approved by the US Food and Drug Administration (FDA) for weight loss was desoxyephedrine (methamphetamine) in 1947. Since then, 17 additional medications have been approved by the FDA, but many have been withdrawn because of adverse effects, several are only approved for short-term (less than 12 weeks) use, and only 5 are currently available for long-term obesity therapy in the US. Traditionally, both single and combination FDA-approved drugs for obesity have shown moderate, but clinically-important, weight losses (mean weight loss of 3%–9% greater than placebo at ~1 year of therapy) (4) (Figure 1). In 2021, a new era in obesity pharmacotherapy was established with the FDA approval of semaglutide, a high-dose glucagon-like-peptide-1 receptor (GLP-1R) agonist which causes about a 12% placebo-subtracted weight loss in people with obesity at 68 weeks of treatment (5). An appreciation of the potency of GLP-1R agonists for weight loss has led to the rapid development of agents that combine GLP-1R agonism with agonism at other receptors to enhance their efficacy for weight loss and other metabolic outcomes. Recently, the results of a landmark randomized controlled trial showed treatment with tirzepatide (15 mg subcutaneous/week), a dual GLP-1R/GIP receptor (GIPR) agonist, caused an 18% placebo-subtracted weight loss (21% total weight loss) at 72 weeks in people with obesity (6). Moreover, 40% of participants treated with tirzepatide 15 mg achieved a 25% or greater decrease in body weight. However, the use of this medication requires a prolonged initial treatment period of dose-escalation to attenuate the adverse gastrointestinal effects (nausea, vomiting, diarrhea) typically associated with GLP-1R agonists, which still occurred frequently but rarely resulted in discontinuation of treatment. Other GLP-1R, GIPR, and glucagon receptor (GCGR) poly-agonists are in development and have been tested in phase 1 and phase 2 clinical trials, including GLP-1R/GCGR agonists, GLP-1R/GLP-2R agonists, and triple GLP-1R/GIPR/GCGR agonists (abstracts presented at the American Diabetes Association Annual Meeting in June, 2022 and (7)). In addition, the combination of semaglutide and a long-acting amylin analogue (cagrilintide) achieved a 15%–17% weight loss (7% greater weight loss than semaglutide plus placebo) at 20 weeks of treatment (8).
Figure 1.
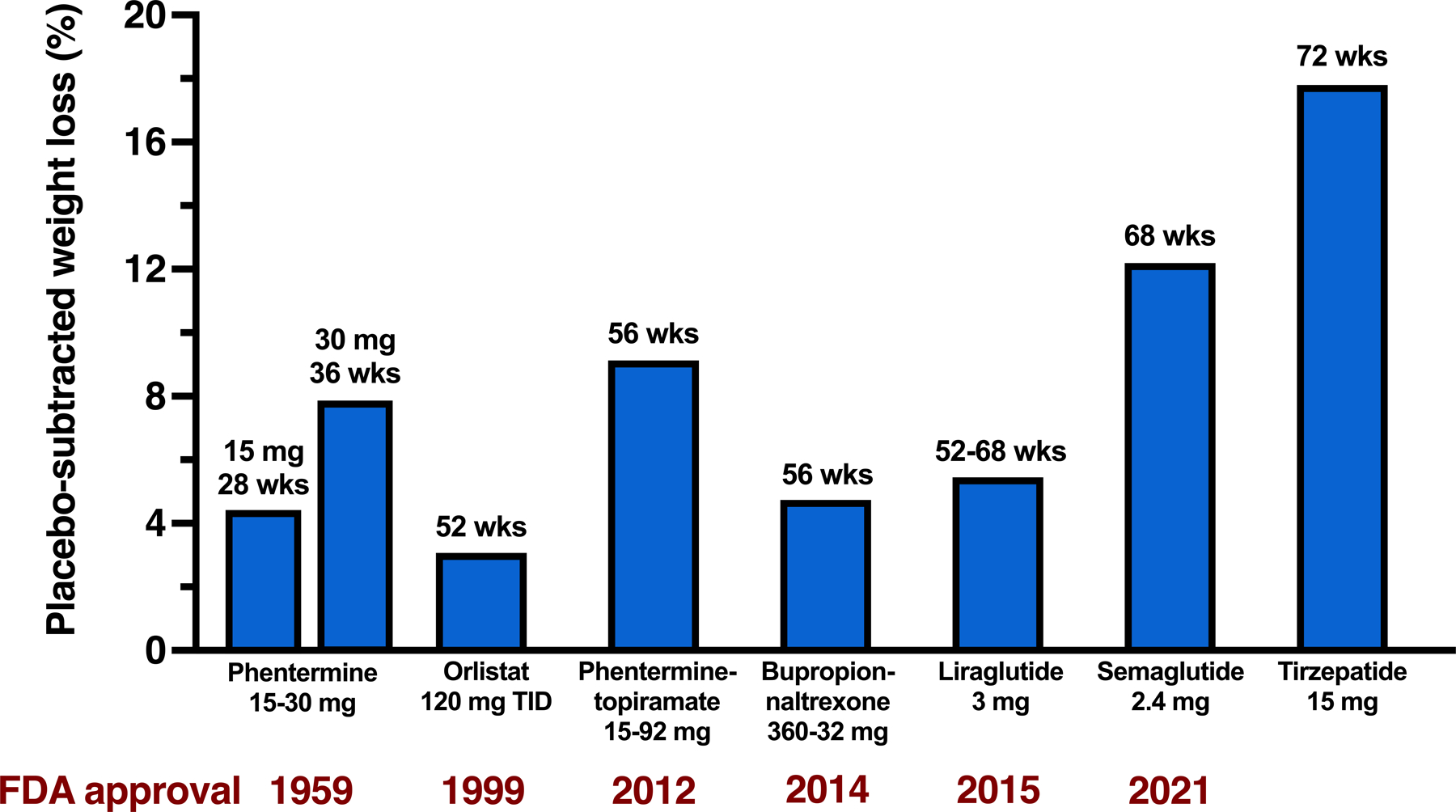
Effect of obesity medications on body weight in people with obesity without diabetes. Data are expressed as the mean of placebo-subtracted weight loss point estimates from randomized placebo-controlled trials, using intention-to-treat, last-observation-carried-forward (ITT-LOCF) or treatment regimen estimand analyses.
The impressive weight loss outcomes of the poly-agonists suggest additive or synergistic effects. The independent effects of GLP-1, GIP, and GCG on energy balance and metabolic function have been determined primarily in studies conducted in rodent models (Figure 2). GLP-1 decreases food intake by acting on GLP1R in the hypothalamus and brainstem, and improves glycemic control by slowing gastric emptying, increasing glucose-stimulated insulin secretion, suppressing glucagon secretion and increasing β-cell mass. It is unclear whether GIPR agonism enhances weight loss because GIP itself does not affect food intake or energy expenditure, GIPR antagonists induce weight loss in mice and non-human primates (9), and tirzepatide does not cause weight loss in obese Glp1r−/− mice (10). GIPR agonism could exert beneficial metabolic effects by enhancing glucose-stimulated insulin secretion, increasing postprandial adipose tissue blood flow and triglyceride storage, and by attenuating weight loss-induced bone loss by decreasing bone resorption and increasing osteoblast activity. GCGR agonism could enhance weight loss by decreasing food intake, which has been shown in rodents and is mediated by the hepatic vagus nerve. In rodents, GCGR agonism increases energy expenditure, primarily by activating brown adipose tissue. However, it seems unlikely that chronic GCGR agonist therapy increases energy expenditure in people. Although acute glucagon infusion increases resting energy expenditure, short-term 72-hour glucagon infusion did not affect sleeping, basal and 24-hour energy expenditure (11). However, glucagon has potent effects on hepatic glucose, lipid and amino acid metabolism. Glucagon stimulates hepatic glucose production, which can have adverse effects on plasma glucose concentrations that are offset by the beneficial effects of weight loss, and decreases intrahepatic triglyceride content (mediated by increased intrahepatic triglyceride lipolysis, fatty acid oxidation, and ketogenesis and decreased de novo lipogenesis) and decreases plasma LDL-cholesterol concentration (mediated by increased hepatic LDL receptor activity).
Figure 2.
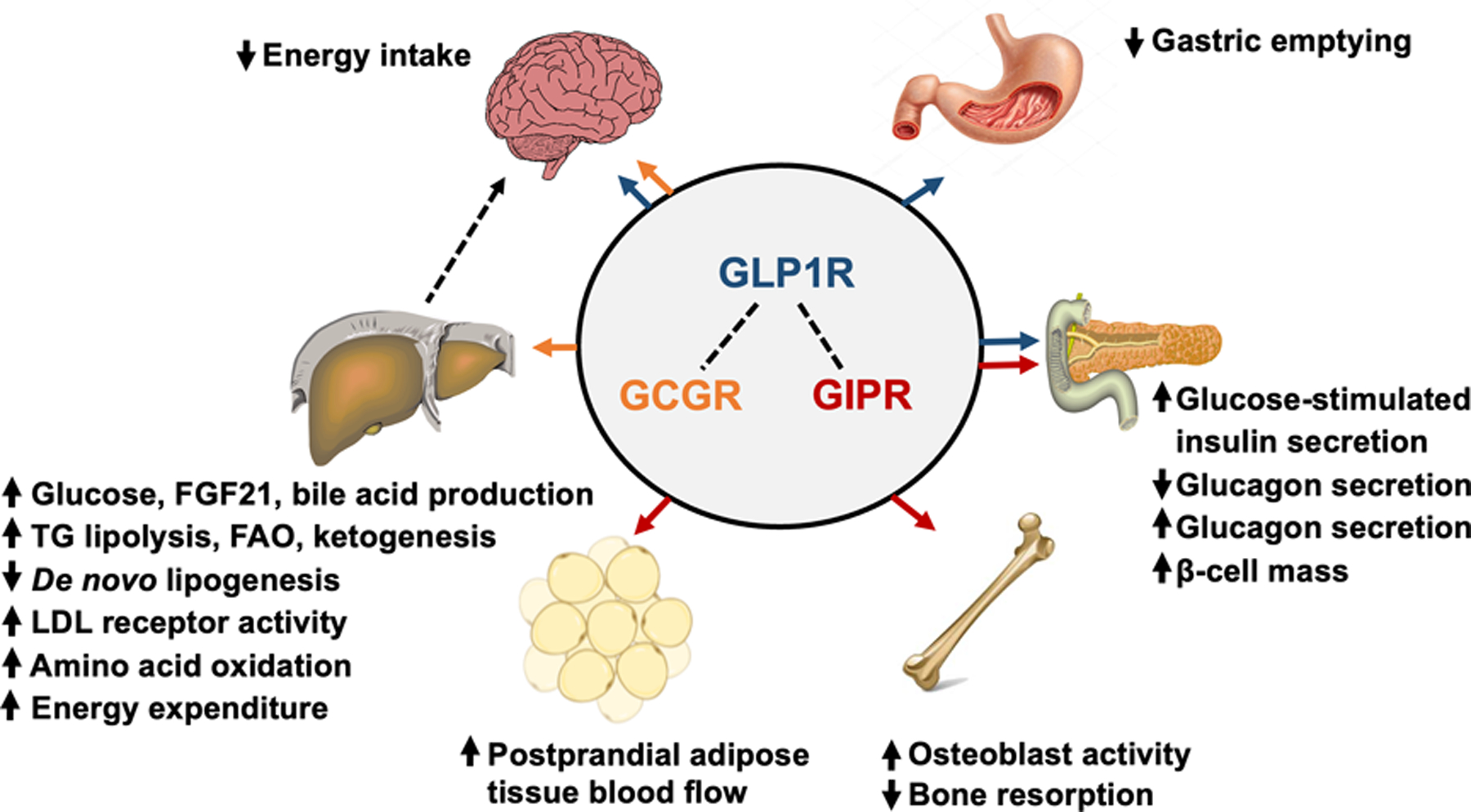
Multi-organ effects of GLP-1 (blue arrows), GIP (orange arrows), and glucagon (red arrows) receptor agonism. GLP1R agonists decrease energy intake through actions in the hypothalamus and brainstem, slow the rate of gastric emptying, enhance glucose-stimulated insulin secretion, inhibit glucagon secretion, and increase β-cell mass. GIPR agonists stimulate glucose-stimulated insulin secretion (however, the incretin effect of GIP is diminished or abolished in people with type 2 diabetes), stimulate glucagon secretion, increase β-cell mass, stimulate osteoblast activity while inhibiting bone resorption, and increase postprandial adipose tissue blood flow. GCGR agonists primarily act on the liver, and: i) increase glucose production by stimulating glycogenolysis and gluconeogenesis, ii) increase FGF21 production, iii) increase bile acid production, iv) decrease de novo lipogenesis, v) increase intrahepatic triglyceride lipolysis, fatty acid oxidation and ketogenesis, vi) increase amino acid oxidation, and vii) increase LDL receptor activity which decreases plasma LDL-C concentration. Although GCGR agonism increases energy expenditure in rodent models, short-term (72-hours) GCG infusion does not affect energy expenditure in people. GCGR agonism also decreases energy intake, and this effect requires the hepatic vagus nerve. Abbreviations: GLP-1, glucagon-like-peptide-1; GIP, glucose-dependent insulinotropic peptide; GLP1R, GLP-1 receptor; GIPR, GIP receptor; GCGR, glucagon receptor; FGF21, fibroblast growth factor-21; LDL, low-density lipoprotein; LDL-C, LDL cholesterol.
The development of GLP-1R, GIPR, and GCGR poly-agonists marks a new era in obesity pharmacotherapy. The impressive weight loss induced by tirzepatide is a “game-changer” and demonstrates that obesity medications can achieve considerable clinical benefits that will likely be embraced by patients, physicians and, hopefully, payors. Effective pharmacotherapy for obesity is likely to require long-term, if not life-long, treatment, which makes it important to consider long-term adherence and weight loss efficacy, and the effects on obesity co-morbidities (particularly cardiovascular outcomes), safety (including potential adverse effects on muscle mass and bone health), and cost of poly-agonist therapy. A greater understanding of the mechanisms responsible for the clinical and metabolic effects of the poly-agonists, the balance of receptor agonism needed for optimal effects, and the heterogeneity in response among individual patients and different patient populations will be important to guide future drug development and clinical application.
Funding:
Supported by NIH grants P30 DK056341 (Washington University Nutrition and Obesity Research Center), P30 DK020579 (Washington University Diabetes Research Center), UL1 TR000448 (Washington University Institute of Clinical and Translational Sciences), and T32 DK007120, and the Barnes-Jewish Hospital Foundation.
Footnotes
Disclosure: SK has a sponsored research agreement with Janssen Pharmaceuticals Inc., serves on a scientific advisory board for Altimmune, and has served as a consultant for Eli Lilly and B2M Medical.
REFERENCES
- 1.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab 2016;23: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34: 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391: 541–551. [DOI] [PubMed] [Google Scholar]
- 4.Garvey WT. New Horizons. A New Paradigm for Treating to Target with Second-Generation Obesity Medications. J Clin Endocrinol Metab 2022;107: e1339–e1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021;384: 989–1002. [DOI] [PubMed] [Google Scholar]
- 6.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med 2022. [DOI] [PubMed]
- 7.Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, et al. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021;44: 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enebo LB, Berthelsen KK, Kankam M, Lund MT, Rubino DM, Satylganova A, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2.4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet 2021;397: 1736–1748. [DOI] [PubMed] [Google Scholar]
- 9.Killion EA, Wang J, Yie J, Shi SD, Bates D, Min X, et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci Transl Med 2018;10: eaat3392. [DOI] [PubMed] [Google Scholar]
- 10.Samms RJ, Christe ME, Collins KA, Pirro V, Droz BA, Holland AK, et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J Clin Invest 2021;131: e146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whytock KL, Carnero EA, Vega RB, Tillner J, Bock C, Chivukula K, et al. Prolonged Glucagon Infusion Does Not Affect Energy Expenditure in Individuals with Overweight/Obesity: A Randomized Trial. Obesity (Silver Spring) 2021;29: 1003–1013. [DOI] [PubMed] [Google Scholar]


