Abstract
Black women diagnosed with epithelial ovarian cancer have poorer survival compared to White women. Factors that contribute to this disparity, aside from socioeconomic status and guideline-adherent treatment, have not yet been clearly identified. We examined data from the Ovarian Cancer in Women of African Ancestry (OCWAA) consortium which harmonized data on 1,074 Black women and 3,263 White women with ovarian cancer from seven U.S. studies. We selected potential mediators and confounders by examining associations between each variable with race and survival. We then conducted a sequential mediation analysis using an imputation method to estimate total, direct, and indirect effects of race on ovarian cancer survival. Black women had worse survival than White women (HR=1.30; 95%CI=1.16–1.47) during study follow-up; 67.9% of Black women and 69.8% of White women died. In our final model, mediators of this disparity include college education, nulliparity, smoking status, body mass index, diabetes, diabetes/race interaction, postmenopausal hormone (PMH) therapy duration, PMH duration/race interaction, PMH duration/age interaction, histotype, and stage. These mediators explained 48.8%(SE=12.1%) of the overall disparity; histotype/stage and PMH duration accounted for the largest fraction. In summary, nearly half of the disparity in ovarian cancer survival between Black and White women in the OCWAA consortium is explained by education, lifestyle factors, diabetes, PMH use, and tumor characteristics. Our findings suggest that several potentially modifiable factors play a role. Further research to uncover additional mediators, incorporate data on social determinants of health, and identify potential avenues of intervention to reduce this disparity is urgently needed.
Keywords: Ovarian cancer, disparities, survival
Graphical Abstract
Introduction
Epithelial ovarian cancer (EOC) is a highly fatal disease and the fifth leading cause of cancer mortality among women in the United States1. Black women have poorer survival compared to White women, with 5-year survival rates of 39% and 48%, respectively1–3. Socioeconomic status (SES) and access to care may explain part4–8 but not all4 of the survival disparity. Most prior studies have examined SES or guideline adherent treatment, but have not accounted for other prognostic factors such as body mass index (BMI) or smoking status. Data on other factors that affect survival are needed to better understand and potentially mitigate survival disparities.
There are several factors that differ in distribution between Black and White women that have been shown to have prognostic significance in ovarian cancer outcomes, and thus warrant investigation as mediators of this relationship. Obesity (BMI>30) before or at ovarian cancer diagnosis has been associated with worse survival47,48 among women with ovarian cancer, as has smoking49,50. Additionally, there is evidence that lower income and education levels are associated with worse survival51, while PMH therapy use is associated with improved survival50,52–54. However, as most prior epidemiologic studies have included small samples of Black women, how these factors may impact the Black-White ovarian cancer survival disparity has not been adequately examined. Further, no previous study has used mediation analyses to investigate the effect of these factors (e.g., BMI, smoking, histotype) on the survival disparities among women with ovarian cancer.
The present study capitalized on harmonized data on Black and White EOC cases from the Ovarian Cancer in Women of African Ancestry (OCWAA) Consortium9. To elucidate which factors may impact EOC survival disparities between Black and White women, we used statistically informed variable selection and mediation analyses on time to event10,11 to examine factors purported to have prognostic significance in women with EOC.
Materials and Methods
Study population
The OCWAA Consortium9 is a U.S. based collaboration formed to understand racial differences in risk and outcomes of EOC. The present analysis includes 1,074 Black and 3,263 White EOC cases from seven contributing studies. These include four case-control studies - the African-American Cancer Epidemiology Study (AACES)12, the Cook County Case-Control Study (CCCCS)13,14, the Los Angeles County Ovarian Cancer Study (LACOCS)15, the North Carolina Ovarian Cancer Study (NCOCS)16 - and three case-control studies nested within prospective cohorts - the Black Women’s Health Study (BWHS)17, Multiethnic Cohort Study (MEC)18, and the Women’s Health Initiative (WHI)19. Eligible cases were diagnosed with incident EOC; tumor histotype was classified according to the 2014 World Health Organization Classification of Tumors of Female Reproductive Organs that combined morphology and grade into the following histotypes: high-grade serous, low-grade serous, endometrioid, clear cell, mucinous, carcinosarcoma, and other epithelial tumors.9,20,21 Questionnaire, medical record, and tumor/cancer registry data were harmonized across studies. Vital status for all-cause mortality and date of death were identified through the National Death Index, state cancer and death registry data, and LexisNexis Accurint, and have been described in detail previously9. To account for potential left truncation in the case-control studies, if the interview date was more than 6 months after the diagnosis date, follow-up time was defined as time between interview and last contact.45 For all other cases, follow-up time was defined as time between diagnosis and last contact.
Defining confounders and mediators
Candidate mediators and confounders were screened by testing exposure (race)-variable and variable-outcome (ovarian cancer survival) relationships22,23 (Table 1). Chi-square tests were used to assess the exposure-mediator and exposure-confounder relationships. Type III ANOVA tests of the potential mediators/confounders in a survival model that controlled for age at diagnosis, year of diagnosis, and study site were used to assess the mediator-outcome and confounder-outcome relationships. If both tests of the exposure-variable and variable-outcome relationship were significant at the p<0.10 level, the variable was selected for inclusion in the analyses. Then, analysis variables were separated into variables judged to be confounders (including variables along a confounding pathway) and mediators. Mediators were variables that could reasonably be assumed to lie on the causal pathway between race and ovarian cancer survival, where race influenced the mediator and not vice-versa. Figure 1 shows the ideal mediation scenario with no unmeasured confounders. We included the Area Deprivation Index (ADI)24, which captures the socioeconomic disadvantage of an area (at the Census tract level in these analyses), as a confounder rather than a mediator. However, in secondary analyses, the ADI was also treated as a mediator as it has been considered a mediator in studies of other health endpoints11,25. In secondary analyses limited to 1) high-grade serous cases and 2) distant stage cases, we rescreened the mediators for inclusion into the respective models (Supplemental Methods).
Table 1.
Potential Mediators/Confounders
| Potential Mediators/Confoundersa | Black womenb | White womenc | ||||
|---|---|---|---|---|---|---|
| N (%) or Mean (SD) | N (%) or Mean (SD) | pd | HR (95% CI)e |
pf | pg | |
| Study | <0.00001 | <0.00001 | 0.975 | |||
| AACES | 579 (53.9%) | 0 (0.0%) | 1.27 (1.01, 1.59) | |||
| BWHS | 77 (7.2%) | 0 (0.0%) | 1.56 (1.18, 2.08) | |||
| CCCCS | 39 (3.6%) | 208 (6.4%) | 1.61 (1.37, 1.90) | |||
| LACOCS | 124 (11.5%) | 1139 (34.9%) | ref | |||
| MEC | 91 (8.5%) | 145 (4.4%) | 1.62 (1.37, 1.91) | |||
| NCOCS | 117 (10.9%) | 819 (25.1%) | 1.07 (0.96, 1.19) | |||
| WHI | 47 (4.4%) | 952 (29.2%) | 1.09 (0.97, 1.23) | |||
| Year of Diagnosish | <0.00001 | 0.060 | 0.170 | |||
| 1990–1995 | 34 (3.2%) | 272 (8.3%) | ref | |||
| 1995–2000 | 136 (12.7%) | 806 (24.7%) | 0.98 (0.84, 1.14) | |||
| 2000–2005 | 141 (13.1%) | 1070 (32.8%) | 0.95 (0.81, 1.12) | |||
| 2005–2010 | 136 (12.7%) | 897 (27.5%) | 0.84 (0.71, 0.99) | |||
| 2010–2015 | 553 (51.5%) | 172 (5.3% | 0.96 (0.75, 1.23) | |||
| 2015–2020 | 74 (6.9%) | 46 (1.4%) | 1.04 (0.73, 1.48) | |||
| Age at Diagnosis | 58.74 (11.70) | 62.93 (11.95) | <0.00001 | <0.00001 | ||
| + 5 years | 1.15 (1.13, 1.17) | 0.119 | ||||
| Histotype | 0.0003 | <0.00001 | 0.897 | |||
| High-grade serousi | 709 (66.0%) | 2121 (65.0%) | ref | |||
| Clear cell | 38 (3.5%) | 224 (6.9%) | 0.43 (0.36, 0.52) | |||
| Other epithelial | 327 (30.4%) | 918 (28.1%) | 0.64 (0.59, 0.70) | |||
| Stage | 0.038 | <0.00001 | 0.802 | |||
| Distant | 715 (70.5%) | 2321 (71.7%) | ref | |||
| Regional | 97 (9.6%) | 368 (11.4%) | 0.32 (0.28, 0.37) | |||
| Localized | 202 (19.9%) | 546 (16.9%) | 0.21 (0.18, 0.24) | |||
| Body Mass Index (BMI), kg/m2 | <0.00001 | 0.093 | 0.957 | |||
| <25 | 190 (17.8%) | 1609 (49.7%) | ref | |||
| 25–29.9 | 307 (28.8%) | 898 (27.7%) | 1.02 (0.94, 1.12) | |||
| ≥30 | 570 (53.4%) | 731 (22.6%) | 1.10 (1.01, 1.21) | |||
| Diabetes | <0.00001 | 0.001 | 0.008 | |||
| No | 842 (81.4%) | 2878 (94.2%) | ref | |||
| Yes | 193 (18.6%) | 177 (5.8%) | 1.24 (1.09, 1.41) | |||
| Smoking Status | 0.00002 | 0.0008 | 0.885 | |||
| Never | 575 (53.7%) | 1642 (50.7%) | ref | |||
| Former | 359 (33.5%) | 1302 (40.2%) | 1.07 (0.99, 1.15) | |||
| Current | 137 (12.8%) | 296 (9.1%) | 1.27 (1.12, 1.44) | |||
| Education | <0.00001 | 0.008 | 0.893 | |||
| Less than college graduate | 719 (67.1%) | 1637 (50.3%) | ref | |||
| College graduate | 353 (32.9%) | 1618 (49.7%) | 0.90 (0.83, 0.97) | |||
| Nulliparous | 0.008 | 0.013 | 0.479 | |||
| No | 869 (81.0%) | 2513 (77.0%) | ref | |||
| Yes | 204 (19.0%) | 749 (23.0%) | 0.89 (0.81, 0.98) | |||
| Post Menopausal Hormone Durationj | <0.00001 | 0.046 | 0.019 | |||
| Never | 820 (77.4%) | 1513 (47.1%) | ref | |||
| < 5 years | 138 (13.0%) | 563 (17.5%) | 0.94 (0.85, 1.04) | |||
| ≥ 5 years | 101 (9.5%) | 1135 (35.3%) | 0.89 (0.82, 0.98) | |||
| Area Deprivation Index (ADI)k | 109.20 (20.53) | 90.25 (14.94) | <0.00001 | 0.00007 | ||
| + 10 points | 1.05 (1.03, 1.08) | 0.120 |
SD: Standard deviation; AACES: African-American Cancer Epidemiology Study; BWHS, Black Women’s Health Study; CCCCS, Cook County Case-Control Study; LACOCS, Los Angeles County Ovarian Cancer Study; MEC, Multiethnic Cohort Study; NCOCS, North Carolina Ovarian Cancer Study; WHI, Women’s Health Initiative; BMI: body mass index; ADI, Area Deprivation Index
Other variables screened and not selected include health conditions (menopause, prior cancer, prior breast cancer, heart disease, high blood pressure, high cholesterol, osteoporosis, or thyroid condition), pack years of smoking (years), marital status (married/living as married, divorced/separated, widowed, single/never married), ever use and duration (years) of oral contraceptives, and whether a participant ever regularly used aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), or acetaminophen. Unless otherwise noted, all variables were categorized as yes/no.
Among Black participants, ADI is missing at a rate of 13.4%, while the remaining variables are missing at rates of 5.6% or less. No data is missing for site, age at diagnosis, year of diagnosis, or histotype.
Among White participants, ADI is missing at a rate of 36.4%, while the remaining variables are missing at rates of 6.4% or less. No data is missing for site, age at diagnosis, year of diagnosis, or histotype.
p-value for test of the association between race and the variable of interest.
HR and 95% CI of variable of interest in a Cox PH or AFT (if the variable violated the proportional hazard assumption) model while adjusting for site, age at diagnosis, and year of diagnosis.
p-value of the ANOVA test for the variable of interest in a Cox PH or AFT (if the variable violated the proportional hazard assumption) model while adjusting for site, age at diagnosis, and year of diagnosis.
p-value of the ANOVA test for the variable of interest’s interaction with race in a Cox PH model or AFT (if the variable violated the proportional hazard assumption) model adjusting for site, age at diagnosis, and year of diagnosis.
Year of diagnosis is a forced-in confounder (not associated with survival) given differences in year of diagnosis between Black and White women in the included studies
High-grade serous (n=683 Black, n=2052 White) and carcinosarcoma (n=26 Black, n=74 White) were combined into one category based on similar survival observed in this study and the small number of carcinosarcoma cases
Postmenopausal hormone duration includes duration of use of estrogen and progestin either alone or in combination
ADI is the Singh score at census tract level
Figure 1.
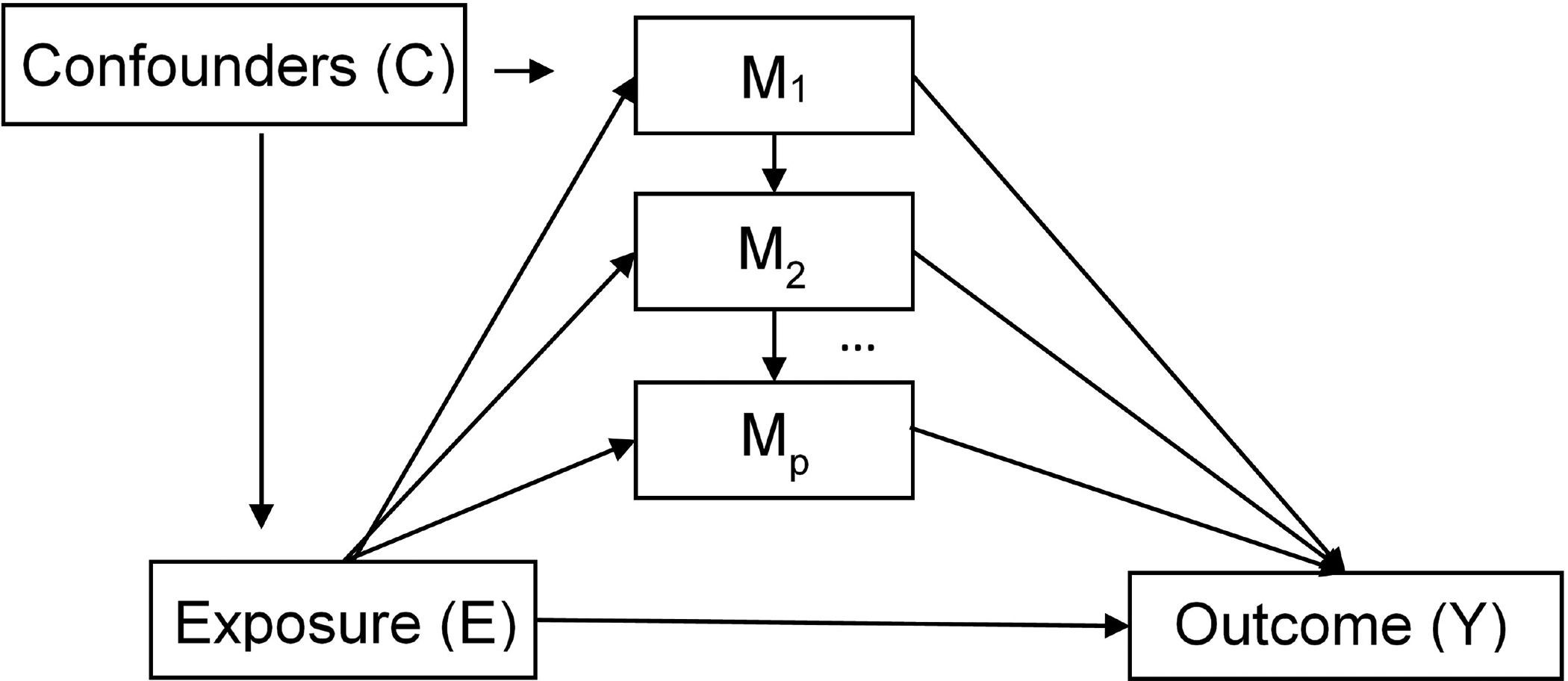
Mediation Scenario Path Diagram. Each mediator (M) can represent a set of joint mediators or an individual mediator.
After mediators were identified as described above, interaction with race was assessed by inserting an interaction term into a model with adjustment for study site, age at diagnosis, and diagnosis year. We set an a priori threshold of p<0.10 for an interaction term to be included in the pool of mediators. Several pairs of mediators (i.e., BMI-diabetes, histotype-stage) were a priori treated jointly for the remainder of the analysis due to their strong association with each other and uncertainty regarding their causal relationship (i.e., the direction of the association, potential unmeasured confounders affecting the association). Lastly, a causal order was imposed to the screened mediators considering temporality and investigator feedback. Ultimately, we assumed the order based on what mediators would reasonably precede the others. For example, college education would typically occur earliest, followed by birth of a woman’s last child. Similarly, hormone therapy would occur later in life, and histotype and stage would be the most recent mediator. The effect of different ordering was examined with sensitivity analyses considering other potential causal orders.
Sequential Mediation Analysis
We used an imputation method documented in Vansteelandt et al.26 and Lange et al.10,46 to estimate total, direct, and indirect effects of variables on survival in a sequential manner. The conditional direct effect estimated by the imputation method can be described as the effect of changing the exposure (e.g., race) while keeping the mediators fixed at the level of the opposite exposure (e.g., how much the hazard changes if we kept the mediator levels that exist among White women but apply them to Black women).
This method10,26,46 fits a Weibull time-to-event model on original data with the confounders and mediator to predict the survival time if the exposure was set to the counterfactual level. The original data is concatenated with the counterfactual data to fit a Cox proportional hazards model on only the confounders, exposure, and counterfactual exposure. The indirect and direct effects are pulled from the hazard ratios of the coefficients of the exposure and counterfactual exposure, respectively. The total effect is calculated as the product of the indirect and direct effects, and the proportion mediated (PM) is calculated as the ratio of the log of the indirect effect and the log of the total effect. Bootstrapping is used to calculate standard errors and 95% confidence intervals.
We extended this method in two ways: we first performed multilevel multiple imputation (MMI)30 to account for missing data (Supplemental Table 1), and we also included multiple ordered mediators. Joint indirect effects for these multiple ordered mediators were successively assessed, calculating the various effects with only the first mediator, then calculating the various effects with the first and second mediators included together, and so on until all of the mediators were included together27,28. In addition, individual path-specific effects were calculated for each sequential mediator (i.e. the first path-specific effect would simply be the indirect effect of the first mediator, the second path-specific effect is the ratio of the indirect effect of the first and second mediators together compared to the indirect effect of the first mediator alone, and so on).
The MI-bootstrap pooled sample (“MI-boot PS”) method29 was used to estimate endpoints of the 95% confidence intervals (CIs) of the mediation effects. For each imputed dataset, 1000 datasets were created by randomly sampling with replacement and effects were calculated for each bootstrap/imputation sample. The endpoints for the CI were then calculated by choosing the 2.5% and 97.5% percentiles for each effect from the pooled distribution of bootstrap effects across the 25 imputations. Due to the wide nature of the percentage mediated confidence intervals, standard error was shown for clarity.
In a secondary analysis, we used a multiple mediator method proposed by Yu et al.11 which estimates an averaged direct effect when controlling the mediators by simulating a random intervention on them according to their marginal distributions. This method uses a different approach to implement the counterfactuals to estimate indirect effects, but still uses hazard ratios from Cox models, as well as the “MI-boot PS” method for confidence intervals and standard errors. Because our approach is novel, we used this secondary method to assess the robustness of our findings.
Because Cox models may be subject to misspecification due to violation of the proportional hazards assumption, we additionally fit accelerated failure time (AFT) outcome models for comparison purposes. Both of the methods used are alternatives to the product-coefficient method, and as such, do not require the rare-events assumption to use a Cox model outcome. Assumptions, interpretations, and implementation details for each method are further described in Supplemental Methods.
Results
Of the 1,074 Black women and 3,263 White women with EOC, 729 (67.9%) Black women and 2,277 (69.8%) White women died. Median follow-up was 3.9 (standard deviation [SD]=4.3) years for Black women and 4.1 (SD=5.9) years for White women. Maximum follow-up was 27.9 years for Black women and 24.1 years for White women. In unadjusted Kaplan Meier curves, Black women had worse survival than White women (Figure 2; p=0.003) while having a younger age at diagnosis (mean=58.7 vs 62.9, respectively) (Table 1). After controlling for age at diagnosis, year of diagnosis, and study site, the survival differences were more pronounced between the two racial groups (Supplemental Figure 1; p<0.001); the HR for Black women compared to White women was 1.30 (95% CI=1.16–1.47).
Figure 2.
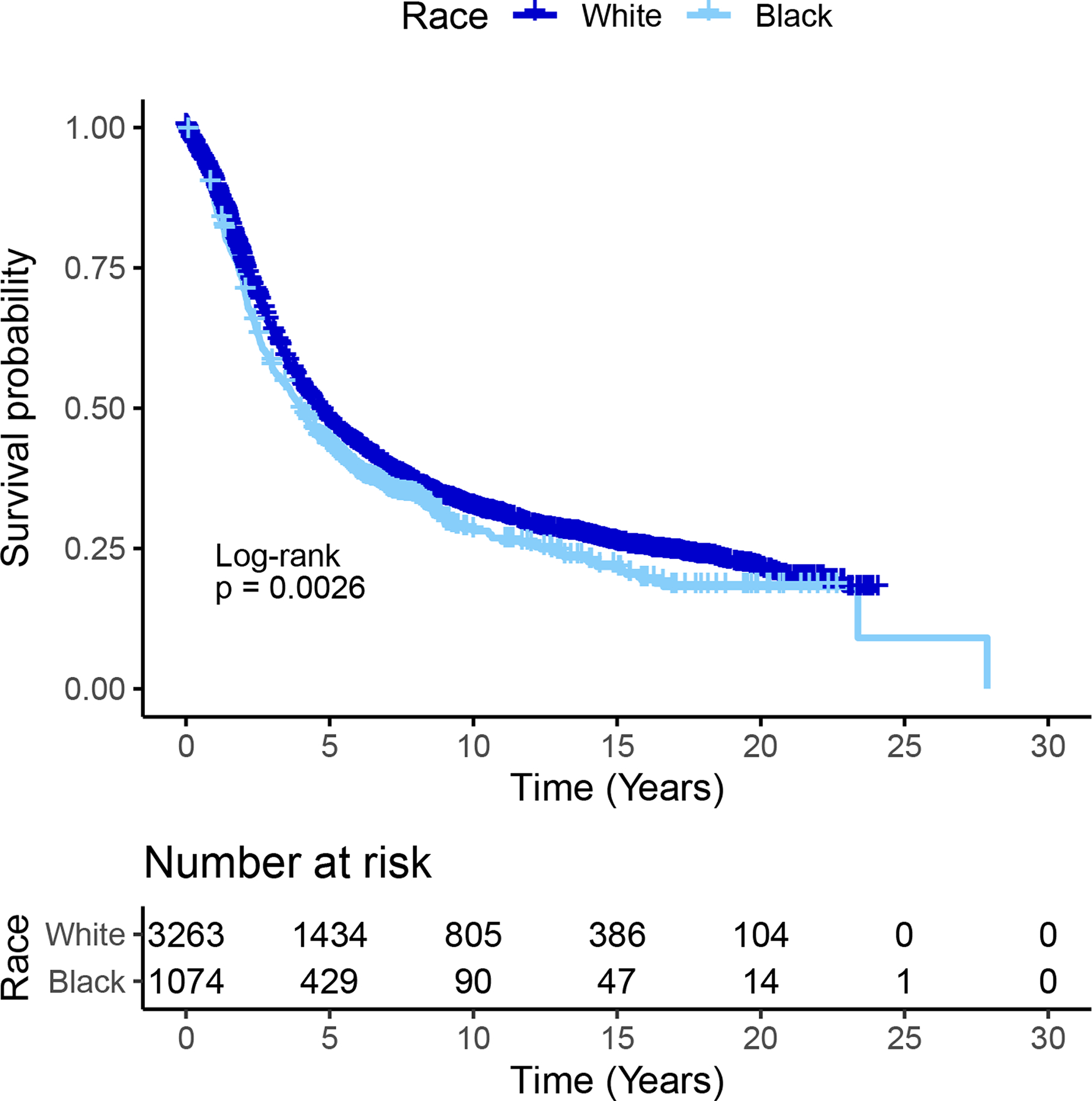
Kaplan-Meier Curves by Race Among Women with Epithelial Ovarian Cancer (N=1,074) Black Women and 3,263 White Women
The most common histotype in both Black and White women was high-grade serous (63.5% vs 62.7%, respectively) while clear cell was more commonly diagnosed among White women (6.9%) than Black women (3.5%) (Table 1). When survival curves were examined by histotype (high-grade serous, clear cell, and all other epithelial), Black women had worse survival in each histotype group, although differences in survival by race were only statistically significant among the high-grade serous histotype (Figure 3).
Figure 3.
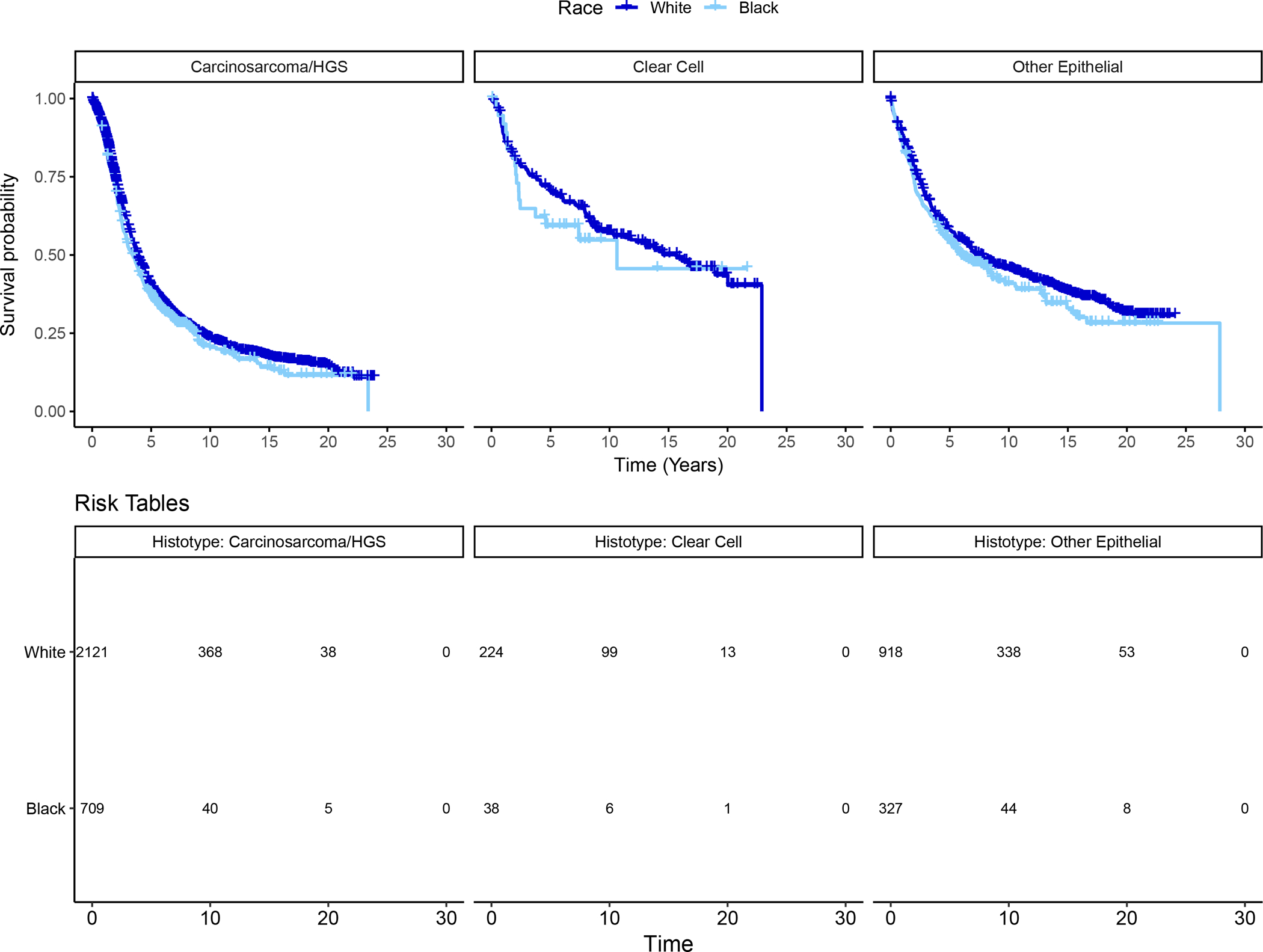
Kaplan-Meier Curves by Epithelial Ovarian Cancer Histotype, Stratified by Race (N = 1,074 Black Women and 3,263 White Women). Panel A: High-grade serous (N=709 Black women, 2,121 White women); log-rank p=0.03. Panel B: Clear cell (N=38 Black women, 224 White women); log-rank p=0.47. Panel C: Other Epithelial (n=327 Black women, 918 White women); log-rank p=0.14
Selection of potential mediators/confounders that may explain the racial disparity in survival
We identified several potential mediators (i.e., college education, nulliparity, smoking status, BMI, diabetes, postmenopausal hormone use [PMH] duration, histotype, and stage) and confounders (i.e., age at diagnosis, year of diagnosis, site, ADI) based on our screening method. Due to the non-linear distribution of BMI, we categorized the BMI variable based on the WHO definitions, which allow for meaningful clinical implications and is consistent with prior literature examine BMI and ovarian cancer survival. Each mediator or confounder met our threshold for inclusion of p<0.10 with both self-reported race and survival time after adjusting for site, age at diagnosis, and year of diagnosis (Table 1). Among the significantly associated mediators/ confounders, interactions of race with diabetes and PMH duration were observed while still adjusting for site, age at diagnosis, and year of diagnosis (p<0.02). In addition, because age is strongly associated with PMH, a PMH duration/age interaction term was included in the final model. Variables screened but not selected as potential mediators/confounders are included in the footnote of Table 1.
Mediation analysis results
Using the imputation method, the included mediators explained 48.8% (SE=12.1%) (Table 2) of the overall Black/White disparity; histotype/stage and PMH duration accounted for the largest fraction of the cumulative percent mediated. The path-specific effect of PMH duration on the disparity was 1.03 (95% CI:0.94–1.12), meaning that after the path-specific effects of college education, nulliparity, smoking, BMI, and diabetes were accounted for, Black women are 3% more likely to die from ovarian cancer than White women when looking specifically at differences in PMH duration. This increased hazard translates to 14.2% (SE=25.5%) of the overall Black/White disparity. For histotype and stage, the path-specific effect was 1.04 (95% CI: 0.96–1.13), indicating that Black women are 4% more likely to die from ovarian cancer than White women when looking specifically at differences in histotype and stage. This increased hazard translates to 22.3% (SE=23.5%) of the overall Black/White disparity. Each mediator contributed a path-specific indirect effect ranging from 0.99 to 1.04, while a cumulative indirect effect from all mediators was significant (1.10, 95% CI: 1.03, 1.16). This suggests that Black women are 10% more likely to die from ovarian cancer than White women when considering differences in college education, nulliparity, smoking, BMI, diabetes, PMH duration, histotype and stage.
Table 2.
Sequential Multiple Mediationa of the Effect of Race on Ovarian Cancer Survival using an imputation method10
| Stepb | Mediator(s) Added | Path Specific IE HR (95% CI)c | Indirect Effect HR (95% CI) d | Direct Effect HR (95% CI)e | Total Effect HR (95% CI)f | % Mediated (SE)g | Path Specific % Mediated (SE)h |
|---|---|---|---|---|---|---|---|
| 1 | College | 0.99 (0.94, 1.06) | 0.99 (0.94, 1.06) | 1.24 (1.18, 1.30) | 1.23 (1.16, 1.31) | −5.6 (16.4) | −5.6 (16.4) |
| 2 | Nulliparity | 1.02 (0.92, 1.10) | 1.02 (0.94, 1.07) | 1.23 (1.18, 1.29) | 1.25 (1.16, 1.31) | 5.7 (16.0) | 9.3 (22.1) |
| 3 | Smoking | 0.99 (0.92, 1.09) | 1.00 (0.94, 1.07) | 1.23 (1.18, 1.29) | 1.24 (1.16, 1.31) | 0.0 (16.0) | −7.9 (22.4) |
| 4 | BMI | 1.02 (0.93, 1.11) | 1.02 (0.96, 1.09) | 1.21 (1.16, 1.27) | 1.24 (1.16, 1.31) | 7.8 (14.9) | 5.9 (21.3) |
| Diabetes | |||||||
| Diabetes/Race Interaction | |||||||
| 5 | PMH Duration | 1.03 (0.94, 1.12) | 1.05 (0.99, 1.12) | 1.14 (1.09, 1.19) | 1.20 (1.12, 1.28) | 25.8 (16.0) | 14.2 (25.5) |
| PMH Duration/Race Interaction | |||||||
| PMH Duration/Age Interaction | |||||||
| 6 | Histotype | 1.04 (0.96, 1.13) | 1.10 (1.03, 1.16) | 1.10 (1.06, 1.14) | 1.20 (1.13, 1.27) | 48.8 (12.1) | 22.3 (23.5) |
| Stage |
BMI: body mass index; CI: Confidence Interval; HR: hazard ratio; IE: indirect effect; PMH: post-menopausal hormone.
Confounders adjusted for in the model include year of diagnosis, age at diagnosis, site, and area deprivation index (ADI).
Bold numbers reflect statistically significant values.
Sequential order in which the selected mediators were added to the model. Model also includes variables in all preceding steps.
Path specific indirect effect is the indirect effect through the new variable(s) added only and not through other mediators or through the joint pathways of the new variable(s) with prior mediators.
The indirect effect is the cumulative indirect effect of the variables selected as mediators in a given step and all preceding steps.
The direct effect is the remaining direct effect through all other non-mediated pathways.
Total effect is calculated as log(Indirect Effect HR) + log(Direct Effect HR).
Percent (%) mediated is calculated as log(indirect effect HR)/log(total effect HR).
Path Specific (%) mediated is calculated as log(path specific indirect effect HR)/log(total effect HR).
Under the multiple mediator method, the cumulative PM was 46.7% (SE=23.8%) (Table 3). When investigating ADI/college as a combined mediator (in contrast to the primary analysis where ADI was considered a confounder), significant indirect effects were detected (1.06, 95% CI: 1.00, 1.14), increasing the cumulative PM to 63.5% (SE=8.8%) (Supplemental Table 2). Similar results were observed with the multiple mediator method (data not shown). In sensitivity analyses using AFT models, the results were similar (Supplemental Table 3). We also considered different ordering of the mediators with similar results (Supplemental Table 4).
Table 3.
Sequential Multiple Mediationa of the Effect of Race on Ovarian Cancer Survival using a multiple mediator method11
| Stepb | Mediator(s) Added | Individual IE HRc | Cumulative IE HRd | Direct Effect HRe | Total Effect HRf | % Mediatedg | Individual % Mediatedh |
|---|---|---|---|---|---|---|---|
| 1 | College | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.21 (1.05, 1.40) | 1.21 (1.05, 1.40) | −0.1 (1.9) | −0.1 (1.9) |
| 2 | Nulliparity | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.20 (1.04, 1.39) | 1.21 (1.05, 1.40) | 3.9 (4.3) | 4.1 (4.0) |
| 3 | Smoking | 1.00 (0.99, 1.01) | 1.01 (1.00, 1.02) | 1.20 (1.04, 1.38) | 1.20 (1.05, 1.39) | 3.5 (4.6) | −0.6 (2.2) |
| 4 | BMI | 1.02 (1.00, 1.04) | 1.02 (1.00, 1.05) | 1.17 (1.01, 1.35) | 1.19 (1.04, 1.38) | 12.2 (12.9) | 9.0 (10.2) |
| Diabetes | |||||||
| Diabetes/Race Interaction | |||||||
| 5 | PMH Duration | 1.03 (1.01, 1.05) | 1.05 (1.02, 1.09) | 1.13 (0.98, 1.32) | 1.19 (1.03, 1.39) | 28.8 (22.3) | 15.2 (13.5) |
| PMH Duration/Race Interaction | |||||||
| PMH Duration/Age Interaction | |||||||
| 6 | Histotype | 1.04 (1.00, 1.09) | 1.11 (1.05, 1.18) | 1.13 (0.98, 1.32) | 1.26 (1.09, 1.47) | 46.7 (23.8) | 18.5 (13.5) |
| Stage |
BMI: body mass index; CI: Confidence Interval; HR: hazard ratio; IE: indirect effect; PMH; post-menopausal hormone.
Confounders adjusted for in the model include year of diagnosis, age at diagnosis, site, and area deprivation index (ADI).
Bold numbers reflect statistically significant values.
Sequential order in which the selected mediators were added to the model. Model also includes variables in all preceding steps.
Individual indirect effect is the indirect effect of the new variable(s) added into each step.
The cumulative indirect effect is the cumulative indirect effect of the variables selected as mediators in a given step and all preceding steps.
The direct effect is the remaining direct effect through all other non-mediated pathways.
Total effect is calculated as log(Indirect Effect HR) + log(Direct Effect HR).
Percent (%) mediated is calculated as log(indirect effect HR)/log(total effect HR).
Individual (%) mediated is calculated as log(individual indirect effect HR)/log(total effect HR).
In analyses limited to high-grade serous cases, results were similar to the main results with only slightly different screened mediators. They included college education, smoking status, BMI, diabetes, PMH duration, PMH duration/age interaction, prior breast cancer, and stage. The percent mediated was 37.8% (SE=13.5%) (Supplemental Table 5), with BMI/diabetes and stage accounting for the largest fraction. When distant stage cases were examined, the mediators included college education, smoking status, BMI, diabetes, diabetes/race interaction, PMH duration, and PMH duration/age interaction and the percent mediated was 28.0% (SE=12.2%) (Supplemental Table 6). When analyzing the ordering of the mediators, we found that despite some variation, each mediator’s average path-specific effect was very close to their path-specific effects in Table 2 (Supplemental Table 4). The mediator with the most variation was nulliparity, with a path-specific effect of 1.02 and an average path-specific effect from the sensitivity analysis of 1.00. The path-specific proportion is a much more variable measure, with the average path-specific proportion mediated being more consistent for BMI and diabetes together, PMH duration, and together, histotype and stage.
Discussion
In the large and geographically diverse OCWAA Consortium9, survival after ovarian cancer diagnosis was shorter in Black women than White women. The association of race with survival can be separated into contributions due to confounding plus the total effect, and the total effect can be divided into a direct effect and indirect effect through our measured mediators. Our results suggest that 40–50% of the total effect may be explained by the list of mediators considered in this analysis. Importantly, many of the variables identified as mediators herein are modifiable (e.g., BMI, smoking status), therefore, interventions targeted at these factors, particularly among Black women, could potentially reduce the survival disparities observed herein. In addition, targeting interventions towards structural inequalities that lead to differences in these modifiable factors could be as or even more crucial than individual-level interventions. Due to the smaller contributions of each mediator individually, this would suggest that structural interventions would be the most beneficial. Despite some individual mediators presenting with a hazard ratio < 1.0, the overlapping confidence intervals do not necessarily suggest a suppressive effect in explaining the disparity. It is more likely that the individual impact on the disparities in ovarian cancer survival is low and that the causes of these disparities have complex relationships with each other, or are themselves a result of society-level factors55.
Prior studies have identified several potential reasons for poorer ovarian cancer survival among Black women. Factors identified as potential contributors to disparities in ovarian cancer outcomes between Black and White women include access to care31,32, neighborhood disadvantage33, and comorbidities34,35. Most4,8,31,35, but not all36, studies have observed that receipt of guideline adherent treatment is a significant contributor. A recent analysis by Chen et. al, using data from the National Cancer Database, estimated that 59.1% of the racial disparity in survival was explained by differences in adherence to treatment guidelines, access to care, and comorbidities; these factors accounted for 36.4%, 22.7%, and 18.2%, respectively, of the differential survival by race31. While our study was not able to examine access to care or detailed treatment information, we did examine use of NSAIDS and comorbidities (i.e., diabetes, overweight/obesity, heart disease, high blood pressure, high cholesterol, osteoporosis, thyroid conditions). Of the comorbidities examined, only diabetes and BMI screened into our final model, together accounting for 6% of the effect of race on survival. The impact of BMI on survival could be due to chemotherapy dosing reduction37 and/or direct effects on survival.
Our study used a novel mediation method by Lange et al. in order to measure both the natural direct effects (how much the hazard changes if we kept the mediator levels that exist among White women but apply them to Black women) and indirect effects (how much the hazard changes if the mediators changed to the levels that exist among Black women but apply them to White women) to more fully understand which factors mediate the impact of race on ovarian cancer survival10. Underlying all analyses was the need to determine whether a variable was a confounder and/or a mediator. The ADI, which we used in these analyses as a proxy for the socioeconomic disadvantage of an area, could have been treated as either, and, in our primary analyses, we considered the ADI a confounder. This decision was made on the basis that, while the ADI is associated with race, it is unlikely that race would impact ADI (meaning that ADI was not on the causal pathway between race and survival). However, ADI may also merit consideration as an important mediator. In secondary analyses, we found that including ADI/college as a mediator impacted the survival disparity by ≈22% (Supplemental Table 2) and increased the overall PM to ≈60%. The ADI has been proposed to capture one domain of systemic racism, specifically residential segregation and housing discrimination38. While we could not capture systemic racism using the individual-level variables available in each study, we acknowledge that it likely plays an important role in ovarian cancer survival disparities between Black and White women.
The present study had many strengths. The OCWAA Consortium includes a large sample size of Black and White cases from across the United States. This study capitalized on data collected by existing epidemiologic studies, which is both efficient and provides detailed data on patient-level characteristics (e.g., parity, PMH duration) that are not always available in registry-based studies. The median follow-up, 4.0 years post-diagnosis, is long compared to many previous studies and allows for a more comprehensive examination of survival differences by race. To the best of our knowledge, this study is the first to conduct mediation analyses to examine the effect of race on ovarian cancer survival disparities. As such, we aimed to be comprehensive in our approach and considered both a primary mediation method based on counterfactual models10 as well as a complementary approach that framed mediation as intervention effects11. In the presence of unmeasured confounders and ordering uncertainty between mediators, sequential mediation analysis may provide insight into the most relevant pathways in regards to survival differences, while other mediation methods that do not account for the influence of mediators on one another may yield biased results due to violation of assumptions39. The results of the two methods we used were consistent, confirming the robustness of our mediation estimates. We also considered the impact of the order in which mediators were added to the model and found that the overall findings were largely consistent. Lastly, we evaluated the “MI-boot” competing method29, in addition to the primary method, the MI-boot PS, for estimation of 95% CIs; estimates were generally very similar, and, although the MI-boot PS was slightly less efficient, it was more robust to the presence of outliers.
Several limitations should be noted. The use of all-cause mortality may introduce a small amount of bias from deaths due to other causes, but due to the severity of ovarian cancer, most deaths among women in this study will have been from the disease and this bias will be minimal. Despite the cumulative measures showing statistical significance, variation in the path-specific effects and proportion mediated estimates is high. Half of the disparity in outcome was not explained by our examined factors. The analyses were limited to those that were available from all studies and which could be harmonized. Treatment data were incomplete among the individual studies that comprise OCWAA and thus could not be examined. Treatment would be a mediator, not a confounder, of the association between race and survival and therefore our findings are significant and valid despite the absence of treatment data. Specifically, findings from our primary analysis indicate that approximately half (49%) of the differential survival between Black and White women can be explained by the included variables. This leaves approximately half (51%) of the disparity to be explained by treatment or other factors, which allows for unexamined treatment factors to contribute a sizable portion of the mediation of the remaining disparity. This percentage is in line with recent work by Chen et al.31 which estimated that nonadherence to National Comprehensive Cancer Network (NCCN) treatment guidelines accounted for 36.4% of the survival disparity by race. While we do not have comprehensive treatment data, we examined several of the factors that Chen et al.31 identified as most strongly associated with nonadherence to NCCN treatment guidelines including education, age, and comorbidity. ADI at the Census tract level was an approximation for socioeconomic disadvantage in this study. More detailed data on socioeconomic disadvantage and access to care, both of potential importance but not available in OCWAA, should be collected and investigated by future studies. Lastly, despite having a large sample size, we were underpowered to examine racial disparities in survival within the rarer histotypes.
In this first mediation study of racial disparities in ovarian cancer survival to date, we used detailed data from seven epidemiologic studies to identify modifiable factors that mediate the disparity in survival between Black and White women. Almost half of the disparity in ovarian cancer survival between Black and White women was explained by education, lifestyle factors, diabetes, PMH use, and tumor characteristics. Future studies should build upon the research presented herein and incorporate data on social determinants of health. A more complete understanding of the remaining disparity is needed in order to ultimately improve outcomes and reduce cancer disparities.
Supplementary Material
Novelty and Impact.
Prior research has identified few factors that contribute to but do not account for the entirety of the ovarian cancer survival disparity between Black and White women. We use a novel statistical approach to conduct the first mediation study to identify factors that mediate the racial disparities in ovarian cancer survival. Such identification and characterization of mediating factors, as demonstrated herein, is critical in order to ultimately reduce these survival disparities.
Acknowledgments
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Long-List.pdf We thank the writing group which included Fabian Camacho, Kristin Guertin, Holly Harris, Courtney Johnson, Trish Moorman, Lynn Rosenberg, Joellen Schildkraut, and Anna Wu.
Pathology data on some of the cancer cases was obtained from the following state cancer registries (AZ, CA, CO, CT, DE, DC, FL, GA, IL, IN, KY, LA, MD, MA, MI, NJ, NY, NC, OK, PA, SC, TN, TX, VA), and results reported do not necessarily represent their views. Approval from the IRBs of participating institutions and cancer registries was obtained, as required.
Funding
This study is supported by the National Institutes of Health (R01-CA207260 to Schildkraut and Rosenberg and K01-CA212056 to Bethea). AACES was funded by NCI (R01-CA142081 to Schildkraut); BWHS is funded by NIH (R01-CA058420,UM1-CA164974, and U01-CA164974 to Rosenberg); CCCCS was funded by NIH/NCI (R01-CA61093 to Rosenblatt); LACOCS was funded by NCI (R01-CA17054 to Pike, R01-CA58598 to Goodman and Wu, and Cancer Center Core Grant P30-CA014089 to Henderson and Wu) and by the California Cancer Research Program (2II0200 to A. Wu); MEC is funded by NCI (U01-CA164973 to Le Marchand, Haiman, and Wilkens); and NCOCS was funded by NCI (R01-CA076016 to Schildkraut). The WHI program is funded by the National Heart, Lung, and Blood Institute through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C and HHSN268201600004C. Additional grants to support WHI inclusion in OCWAA include UM1-CA173642-05 (to Anderson), NHLBI-CSB-WH-2016-01-CM and NHLBI-75N92021D00002.
Abbreviations
- ADI
Area Deprivation Index
- AFT
Accelerated failure time
- BMI
Body Mass Index
- DE
Direct effect
- EOC
Epithelial ovarian cancer
- IE
Indirect effect
- “MI-boot PS”
MI-bootstrap pooled sample
- MMI
Multilevel Multiple Imputation
- NCCN
National Comprehensive Cancer Network
- NDE
Natural direct effect
- NSAID
Non-steroidal anti-inflammatory drug
- OCWAA
Ovarian Cancer in Women of African Ancestry
- PM
Proportion mediated
- SD
Standard deviation
Footnotes
The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Conflict of Interest
The authors declare no potential conflicts of interest.
Ethics Statement
Each study obtained informed consent from its participants; the individual studies and the OCWAA Consortium were approved by the relevant Institutional Review Boards.
Previous Presentations
Portions of this work have been previously presented at the AACR 2021 annual meeting and ASPO.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7–30, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Park HK, Ruterbusch JJ, Cote ML: Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes. Cancer Epidemiol Biomarkers Prev 26:1511–1518, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart SL, Harewood R, Matz M, et al. : Disparities in ovarian cancer survival in the United States (2001–2009): Findings from the CONCORD-2 study. Cancer 123 Suppl 24:5138–5159, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandera EV, Lee VS, Rodriguez-Rodriguez L, et al. : Racial/Ethnic Disparities in Ovarian Cancer Treatment and Survival. Clin Cancer Res 22:5909–5914, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer KC, Peterson CE, Davis FG, et al. : The influence of neighborhood socioeconomic status and race on survival from ovarian cancer: a population-based analysis of Cook County, Illinois. Ann Epidemiol 25:556–63, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peres LC, Schildkraut JM: Racial/ethnic disparities in ovarian cancer research. Adv Cancer Res 146:1–21, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Taylor JS, Rajan SS, Zhang N, et al. : End-of-Life Racial and Ethnic Disparities Among Patients With Ovarian Cancer. J Clin Oncol 35:1829–1835, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karanth S, Fowler ME, Mao X, et al. : Race, Socioeconomic Status, and Health-Care Access Disparities in Ovarian Cancer Treatment and Mortality: Systematic Review and Meta-Analysis. JNCI Cancer Spectr 3:pkz084, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schildkraut JM, Peres LC, Bethea TN, et al. : Ovarian Cancer in Women of African Ancestry (OCWAA) consortium: a resource of harmonized data from eight epidemiologic studies of African American and white women. Cancer Causes Control 30:967–978, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange T, Hansen KW, Sørensen R, et al. : Applied mediation analyses: a review and tutorial. Epidemiol Health 39:e2017035, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Q, Wu X, Li B, et al. : Multiple mediation analysis with survival outcomes: With an application to explore racial disparity in breast cancer survival. Stat Med 38:398–412, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schildkraut JM, Alberg AJ, Bandera EV, et al. : A multi-center population-based case-control study of ovarian cancer in African-American women: the African American Cancer Epidemiology Study (AACES). BMC Cancer 14:688, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Dolecek TA, Davis FG: Racial differences in stage at diagnosis and survival from epithelial ovarian cancer: a fundamental cause of disease approach. Soc Sci Med 71:274–281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson CE, Rauscher GH, Johnson TP, et al. : The association between neighborhood socioeconomic status and ovarian cancer tumor characteristics. Cancer Causes Control 25:633–7, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Wu AH, Pearce CL, Tseng CC, et al. : Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer 124:1409–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schildkraut JM, Moorman PG, Halabi S, et al. : Analgesic drug use and risk of ovarian cancer. Epidemiology 17:104–7, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bethea TN, Palmer JR, Adams-Campbell LL, et al. : A prospective study of reproductive factors and exogenous hormone use in relation to ovarian cancer risk among Black women. Cancer Causes Control 28:385–391, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolonel LN, Henderson BE, Hankin JH, et al. : A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 151:346–57, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hays J, Hunt JR, Hubbell FA, et al. : The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 13:S18–77, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kurman R, Carcangiu M, Herrington C, et al. : WHO classification of tumours of femal reproductive organs, in IARC (ed). Lyon, 2014 [Google Scholar]
- 21.Peres LC, Cushing-Haugen KL, Köbel M, et al. : Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J Natl Cancer Inst 111:60–68, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Woodward M, Perkovic V, et al. : Mediators of the Effects of Canagliflozin on Heart Failure in Patients With Type 2 Diabetes. JACC Heart Fail 8:57–66, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Yu Q, Li B: mma: An r package for multiple mediation analysis. Journal of Open Research Software 5:11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh GK: Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health 93:1137–43, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green CR, Hart-Johnson T: The association between race and neighborhood socioeconomic status in younger Black and White adults with chronic pain. J Pain 13:176–86, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Vansteelandt S, Bekaert M, Lange T: Imputation strategies for the estimation of natural direct and indirect effects. Epidemiol Methods 1:131–158, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Steen J, Loeys T, Moerkerke B, et al. : Flexible Mediation Analysis With Multiple Mediators. Am J Epidemiol 186:184–193, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Vanderweele TJ, Vansteelandt S, Robins JM: Effect decomposition in the presence of an exposure-induced mediator-outcome confounder. Epidemiology 25:300–6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schomaker M, Heumann C: Bootstrap inference when using multiple imputation. Stat Med 37:2252–2266, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White IR, Royston P: Imputing missing covariate values for the Cox model. Stat Med 28:1982–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen F, Bailey CE, Alvarez RD, et al. : Adherence to treatment guidelines as a major determinant of survival disparities between black and white patients with ovarian cancer. Gynecol Oncol 160:10–15, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Bristow RE, Zahurak ML, Ibeanu OA: Racial disparities in ovarian cancer surgical care: a population-based analysis. Gynecol Oncol 121:364–8, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Vieira VM, Villanueva C, Chang J, et al. : Impact of community disadvantage and air pollution burden on geographic disparities of ovarian cancer survival in California. Environ Res 156:388–393, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chase DM, Fedewa S, Chou TS, et al. : Disparities in the allocation of treatment in advanced ovarian cancer: are there certain patient characteristics associated with nonstandard therapy? Obstet Gynecol 119:68–77, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Hildebrand JS, Wallace K, Graybill WS, et al. : Racial disparities in treatment and survival from ovarian cancer. Cancer Epidemiol 58:77–82, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Cronin KA, Howlader N, Stevens JL, et al. : Racial Disparities in the Receipt of Guideline Care and Cancer Deaths for Women with Ovarian Cancer. Cancer Epidemiol Biomarkers Prev 28:539–545, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Griggs JJ, Bohlke K, Balaban EP, et al. : Appropriate Systemic Therapy Dosing for Obese Adult Patients With Cancer: ASCO Guideline Update. J Clin Oncol 39:2037–2048, 2021 [DOI] [PubMed] [Google Scholar]
- 38.Alson JG, Robinson WR, Pittman L, et al. : Incorporating Measures of Structural Racism into Population Studies of Reproductive Health in the United States: A Narrative Review. Health Equity 5:49–58, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanderWeele TJ, Vansteelandt S: Mediation Analysis with Multiple Mediators. Epidemiol Methods 2:95–115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Q, Fan Y, Wu X: General mulple mediation analysis with an applicaiton to explore racial disparities in breast cancer survival. J Biomet Biostat 5:189, 2014 [Google Scholar]
- 41.VanderWeele TJ: Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology 21:540–51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naimi AI, Kaufman JS, MacLehose RF: Mediation misgivings: ambiguous clinical and public health interpretations of natural direct and indirect effects. Int J Epidemiol 43:1656–61, 2014 [DOI] [PubMed] [Google Scholar]
- 43.van Buuren S, Groothuis-Oudshoom K: mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 45:1–67, 2011 [Google Scholar]
- 44.Azur MJ, Stuart EA, Frangakis C, et al. : Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 20:40–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azzato E, Greenberg D, Shah M et al. Prevalent cases in observational studies of cancer survival: do they bias hazard ratio estimates?. Br J Cancer 100, 1806–1811, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176(3):190–195. [DOI] [PubMed] [Google Scholar]
- 47.Nagle CM, Dixon SC, Jensen A, et al. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer. Sep 1 2015;113(5):817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Protani MM, Nagle CM, Webb PM (2012) Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila) 5(7): 901–910. [DOI] [PubMed] [Google Scholar]
- 49.Praestegaard C, Jensen A, Jensen SM, et al. Cigarette smoking is associated with adverse survival among women with ovarian cancer: Results from a pooled analysis of 19 studies. Int J Cancer. Jun 1 2017;140(11):2422–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SJ, Rosen B, Fan I, et al. Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer. Mar 28 2017;116(7):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibfelt EH, Dalton SO, Høgdall C, Fagö-Olsen CL, Steding-Jessen M, Osler M, Johansen C, Frederiksen K, & Kjær SK (2015). Do stage of disease, comorbidity or access to treatment explain socioeconomic differences in survival after ovarian cancer? - A cohort study among Danish women diagnosed 2005–2010. Cancer epidemiology, 39(3), 353–359. [DOI] [PubMed] [Google Scholar]
- 52.Eeles RA, Morden JP, Gore M, et al. Adjuvant Hormone Therapy May Improve Survival in Epithelial Ovarian Cancer: Results of the AHT Randomized Trial. J Clin Oncol. Dec 10 2015;33(35):4138–44. [DOI] [PubMed] [Google Scholar]
- 53.Shafrir AL, Babic A, Tamimi RM, Rosner BA, Tworoger SS, Terry KL. Reproductive and hormonal factors in relation to survival and platinum resistance among ovarian cancer cases. Br J Cancer. Nov 22 2016;115(11):1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mascarenhas C, Lambe M, Bellocco R, et al. Use of hormone replacement therapy before and after ovarian cancer diagnosis and ovarian cancer survival. Int J Cancer. Dec 15 2006;119(12):2907–15. [DOI] [PubMed] [Google Scholar]
- 55.Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


