Abstract
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of high mortality and heavy burden in the world. Unfortunately, emphysema, as an important component of COPD, has no curative treatments currently. Recently, human umbilical cord mesenchymal stem cells-derived exosomes (hUCMSC-Ex) constitute a promising alternative approach for tissue regeneration and repair. However, the roles of hUCMSC-Ex in emphysema and its mechanism are largely unknown. Here, we investigated the effect and the action mechanism of hUCMSC-Ex in repairing emphysema induced by papain in rats.
Methods
SD rats were used to establish a papain-induced emphysema model and estimate the effect and mechanism of hUCMSC-Ex treatment. H&E staining and mean linear intercept (MLI) were used to evaluate the hUCMSC-Ex effect on emphysema. Western blotting, TUNEL and miRNA-seq were used to investigate the molecular mechanisms of hUCMSC-Ex treatment in models of papain-induced emphysema.
Results
Papain treatment led to typical emphysema, while hUCMSC-Ex reversed emphysematous changes effectively. Apoptosis of endothelial cells and other types of cells were observed in models, while hUCMSC-Ex effectively prevented their apoptosis. hUCMSC-Ex repressed active caspase-3, activated VEGF-VEGFR2-mediated AKT pathway and MEK/ERK pathway in emphysematous lungs. Notably, several miRNAs, such as hsa-miR-10a-5p and hsa-miR-146a-5p, were target related to the roles of hUCMSC-Ex in papain-induced emphysema through VEGF-VEGFR2-mediated AKT and MEK/ERK pathways.
Conclusions
hUCMSC-Ex effectively rescued the papain-induced emphysema injury through VEGF-VEGFR2-mediated AKT pathway and MEK/ERK pathway.
Keywords: Emphysema, Papain, hUCMSC-Ex, AKT pathway, MEK/ERK pathway, Apoptosis
Highlights
-
•
Exosomes from human umbilical cord mesenchymal stem cells (hUCMSC-Ex) pro protect against papain-injured emphysema in rats.
-
•
hUCMSC-Ex prevent lung cells apoptosis by activating VEGF-VEGFR2-mediated AKT and MEK/ERK pathways.
-
•
Several miRNAs, such as hsa-miR-10a-5p, were target related to the roles of hUCMSC-Ex.
1. Introduction
Chronic obstructive pulmonary disease (COPD), as a common and frequently occurring disease, is a leading cause of mortality in the world [[1], [2], [3]]. In addition, patients with COPD need to bear severe economic burden in the treatment because they usually suffer from complications such as cardiovascular disease, depression, osteoporosis, muscle wasting, and lung cancer [4]. COPD has posed a major threat to human health and has become a public health concern worldwide. COPD characteristic pathology consists of small airway lesions and emphysema changes. However, current medications only target the airway lesions but not the emphysema. So far, emphysema, as an important component of COPD, has no curative treatments.
Mesenchymal stem cells (MSC)-based therapy constitutes a promising alternative approach for tissue regeneration and repair including COPD and emphysema [[5], [6], [7]]. MSC promote tissue repair and regeneration through anti-inflammation, anti-apoptosis and angiogenesis [[8], [9], [10], [11]]. However, it is now well known that the repair effect of MSC is mediated through paracrine pathway rather than cell engraftment and differentiation [[12], [13], [14]]. As one important form of paracrine pathway, exosomes play critical role in tissue regeneration and may be an alternative to whole-cell therapy. Exosomes, described as 30–200 nm in diameter, are the smallest type of extracellular vesicles constitutively secreted by all most cell types and serve as an important means of cell-to-cell communication [15,16]. Exosomes deliver a variety of biologically active substances such as proteins, DNA, RNA and lipids to adjacent or distant cells to affect cellular functions [17,18]. MSC-derived exosomes have been applied to therapy lung diseases such as various factors-induced acute lung injury and asthma by inhibiting inflammation and several pathways [[19], [20], [21], [22], [23]]. However, there are relatively few studies on the application of MSC exosomes in COPD or emphysema coupled with insufficient data about their mechanism of action. Ridzuan et al. reported that MSC-derived extracellular vesicles ameliorated COPD by inhibiting airway inflammation [24], whereas other aspects such as the anti-apoptosis ability of MSC-exosomes in COPD or emphysema has not been clarified. Human umbilical cord MSC (hUCMSC) are highly suitable for treating emphysema due to their easier access, faster expansion, lower immunogenicity and lower likelihood of infection. Here, we investigated the effect and the action mechanism of hUCMSC-Exosomes (hUCMSC-Ex) in repairing emphysema induced by papain in rats.
2. Materials and methods
2.1. hUCMSC culture, exosome isolation and characterization
This study was approved by the Institutional Ethics Committee of the Affiliated People's Hospital of Jiangsu University. hUCMSC were isolated from fresh umbilical cords obtained from healthy mothers attending the Affiliated People's Hospital of Jiangsu University. All mothers signed informed consent before entering the experiment. hUCMSC were isolated and characterized as previously described [25]. Cells were cultured in human umbilical cord mesenchymal stem cells complete medium (Cyagen, Guangzhou, China) containing 10% FBS and 1% penicillin-streptomycin and were incubated in a humidified incubator at 37 °C with 5% CO2. Cells from the 3rd passage were used in subsequent experiments.
When the cultured hUCMSC reached about 70%–80% confluence, the supernatant was discarded, the cells were washed 3 times with PBS. Then the complete medium was replaced with medium containing 10% exosome-free FBS. After 2 days, the supernatant of hUCMSC were harvested for exosome isolation. The supernatant was centrifuged at 2000×g for 20 min at 4 °C, 10,000×g for 30 min at 4 °C, then passed through a 220 nm filter (Millipore, MA, USA) to remove cell debris and other non-exosome proteins. Then, the supernatant was concentrated using 100-KD molecular weight cutoff hollow fiber membrane (Millipore, MA, USA) at 1000×g for 30 min at 4 °C repeatedly until the supernatant was concentrated to approximate 240 μL. Exosome extraction reagent ExoQuick-TC (SBI, Mountain View, CA, USA) was added into the final concentrated liquid according to the manufacturer's instructions. The mixture was overnight at 4 °C and centrifuged at 1500×g for 30 min to precipitate exosomes. The supernatant was discarded and exosomes were resuspended with PBS. Exosomes were quantified by BCA kit (Beyotime, Shanghai, China) according to the manufacturer's manual.
The morphology of extracted exosomes was observed by a transmission electron microscope (TEM) (FEI Tecnai 12, Philips). The size distribution of extracted exosomes was analyzed by nanosight tracking analysis (NTA) (Malvern Panalytical, Malvern, UK). The CD63 and HSP70 markers of exosomes were detected by western blotting.
2.2. Animal experiments
The animal studies were carried out with the approval of the Institutional Animal Care and Use Committee of Jiangsu University. Our experiments conformed to the effective laws and ethical recommendations currently in China. The male Sprague Dawley rats were housed at an ambient temperature of 25 °C with 12 h for light and 12 h for darkness. Emphysema models were established using papain through intratracheal instillation. Rats were assigned randomly into three groups: (A) Control group (n = 10), (B) Papain group (n = 10), (C) Papain + hUCMSC-Ex group (hUCMSC-Ex, n = 10). Rats in group B and C were administered with papain at a dosage of 40 mg/kg body weight one time a week for 4 weeks. Rats in group A only received saline by intratracheal instillation. On the same day after the fourth administration of papain, 200 μg hUCMSC-Ex resuspended in PBS were infused into each rat in group C through the tail vein, whereas groups A and B received only the same volume of PBS. After 7 days from hUCMSC-Ex infusion, rats were sacrificed and samples were achieved. The left lungs were harvested and the upper lobes were fixed in 4% paraformaldehyde for hematoxylin and eosin (HE) analysis and apoptosis assay, the remaining left lungs were used to western blot analysis.
2.3. Animal live imaging analysis
The hUCMSC-Ex was labeled with DIR and injected into the model group rats in order to observe whether the labeled hUCMSC-Ex could reach the lungs. DIR solid dye was dissolved with DMSO and was added to the hUCMSC-Ex dissolved in PBS according to the instructions. Then mixed them well, placed them at 37 °C in the dark for 30 min. Subsequently, the liquid was transferred to a 100 KD ultrafiltration tube and was centrifuged at 1000 g at 4 °C for 30 min. Then added PBS into the ultrafiltration tube and centrifugated again to remove unbound dye. After 24 h from labeled-hUCMSC-Ex were injected into the model rats by tail vein, rats were anesthetized and a live imaging system was used to track the fluorescence distribution in rats.
2.4. H&E staining and MLI calculation
Fixed lungs were paraffin-embedded, sectioned into 4 um slices and stained with hematoxylin and eosin for histological analysis. Mean linear intercept (MLI) was proposed by Dunnill in 1964 and is now commonly used for quantitative assessment of lung histological analysis [26]. In the present study, MLI was calculated to determine the severe extent of emphysema and the cure effect of hUCMSC-Ex on emphysema. MLI was determined by random slices from each specimen. Briefly, we drew a cross at the center of each vision field avoiding large blood vessels and bronchial at original magnification of × 100, counted the total number of alveolar septa (NS) encountered in all lines, and measured the total length of the crosshairs (L). MLI was calculated according to the formula MLI = L/NS. The MLI value represented the average alveoli diameter.
2.5. TUNEL analysis
TUNEL assay was applied to analyze the apoptosis of lung tissues using paraffin sections with commercially available kit (Sigma–Aldrich, QIA33) following the manufacturer's instructions. Briefly, paraffin sections were deparaffinizated, rehydrated, and digested with proteinase K. They were then covered with Equilibration Buffer for 30 min and incubated with TdT Labeling Reaction Mixture in a humid atmosphere. Slices were then washed with PBS and incubated with conjugate for 30 min. After rinsing with PBS, sections were covered with DAB solution and were counterstained with hematoxylin. Apoptosis extent was determined by dividing the number of TUNEL-positive cells to the total cell number in randomly selected fields at × 400 magnification.
2.6. Western blot analysis
hUCMSC-Ex and left lungs were lysed in RIPA lysis buffer containing 1% PMSF and protein concentration was examined using BCA protein assay kit. For detection of phosphorylated proteins (p-VEGFR2, p-AKT, p-MEK, p-ERK), phosphatase inhibitor (Biosharp) was also added in the lysis buffer to protect the phosphorylation groups. Protein samples were separated by a sodium dodecyl sulfatepolyacrylamide gel electrophoresis, transferred to PVDF membranes, then blocked with 5% BSA for 2 h at room temperature and incubated with primary antibodies overnight at 4 °C. The primary antibodies were as follows: CD63 (Proteintech 25682-1-AP), HSP70 (Proteintech 10995-1-AP), caspase-3 (Proteintech 19677-1-AP), VEGF (R&D MAB564), p-VEGFR2 (Millipore # 07–722), VEGFR2 (Abcam ab39256), p-AKT (Affinity AF0016), AKT (Affinity AF6261), p-MEK (Affinity AF3385), MEK (Affinity AF6385), p-ERK (Affinity AF1015), ERK (Affinity AF0155) and β-actin (Abcam ab8227). All antibodies were diluted according to the instructions. The membranes were then washed with 0.1% PBST and incubated with HRP-conjugated secondary antibody. Finally, they were detected with enhanced chemiluminescence (Millipore, MA, USA). β-actin was used as the internal control for protein loading.
2.7. miRNA sequencing
Total RNA, containing the miRNA fraction, was prepared using mirVana miRNA Isolation kit (Invitrogen), according to the manufacturer's instruction. Sequencing libraries were generated using NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA) following manufacturer's recommendations. First strand cDNA was synthesized using random hexamer primer and M-MuLVReverse Transcriptase (RNase H-). Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. The library fragments were purified with AMPure XP system (Beckman Coulter, Beverly, USA). After cluster generation, the library preparations were sequenced on an Illumina Hiseq platform. FASTQ files were aligned to the human genome (assembly hg19) using STAR and GENCODE v19 transcriptome annotation. Read pairs aligned to gene features were counted and summarized as reads per kilobase of transcript per million mapped reads (RPKM) values at the gene level using feature Count.
2.8. Bioinformatics
The resulting P-values were adjusted using the Benjamini and Hochberg's approach for controlling the false discovery rate. Genes with an adjusted P-value < 0.05 found by DESeq2 were assigned as differentially expressed. GO and KEGG enrichment analysis of differentially expressed genes was implemented by the cluster Profiler R package.
2.9. Statistical analysis
Data were all presented as mean ± SEM. GraphPad Prism software was used for statistical analysis and for figures creation. Unpaired t test and one-way analysis of variance were carried out to compare the difference among two or multiple groups, respectively. The level of statistical significance was set at P < 0.05 for all tests.
3. Results
3.1. Characterization of hUCMSC and hUCMSC-Ex
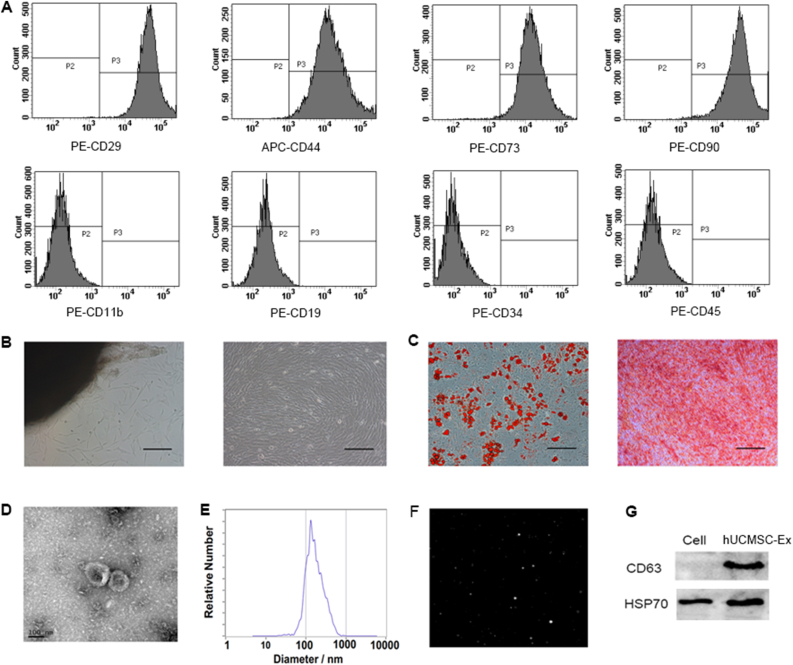
Immunophenotype analysis showed that hUCMSC were positive for CD29, CD44, CD73 and CD90, but negative for CD11b, CD19, CD34 and CD45 (Fig. 1A). Morphologically, hUCMSC were spindle-shaped (Fig. 1B). Differentiation assays showed that hUCMSC retained the ability to differentiate into adipocytes and osteoblasts (Fig. 1C). TEM showed the hUCMSC-Ex were 30–200 nm and typically round, with high density at the periphery and low density at the center (Fig. 1D). The NTA results showed that the average particle size was 134.5 nm, and most of the particles were distributed in the range of 30–200 nm (Fig. 1E) with the vedio image (Fig. 1F). Western blotting showed that the extracted hUCMSC-Ex could expressed the marker proteins CD63 and HSP70 very well, but the expression of the two proteins in hUCMSC lysate was almost no or relative lower (Fig. 1G). This is in line with the main signs of exosomes. The above results indicate that we have successfully extracted hUCMSC-Ex with complete structure and high purity from hUCMSC conditioned medium.
Fig. 1.
Characterization of hUCMSC and hUCMSC-Ex. (A) Immunophenotype analysis of hUCMSC. hUCMSC were positive for CD29, CD44, CD73 and CD90, but negative for CD11b, CD19, CD34 and CD45. (B) Morphology of hUCMSC. (C) Differentiation potential of hUC-MSCs. (a) Adipogenic differentiation. (b) Osteogenic differentiation. (B–C) Magnification, ×100; scale bar, 200 μm. (D) TEM results of hUCMSC-Ex. (E) Diameter ranges of hUCMSC-Ex from NTA. (F) The vedio image of hUCMSC-Ex from NTA. (G) The protein expression of CD63 and HSP70 in hUCMSC-Ex were detected by western blot.
3.2. hUCMSC-Ex protected against lung injury in papain-induced emphysema
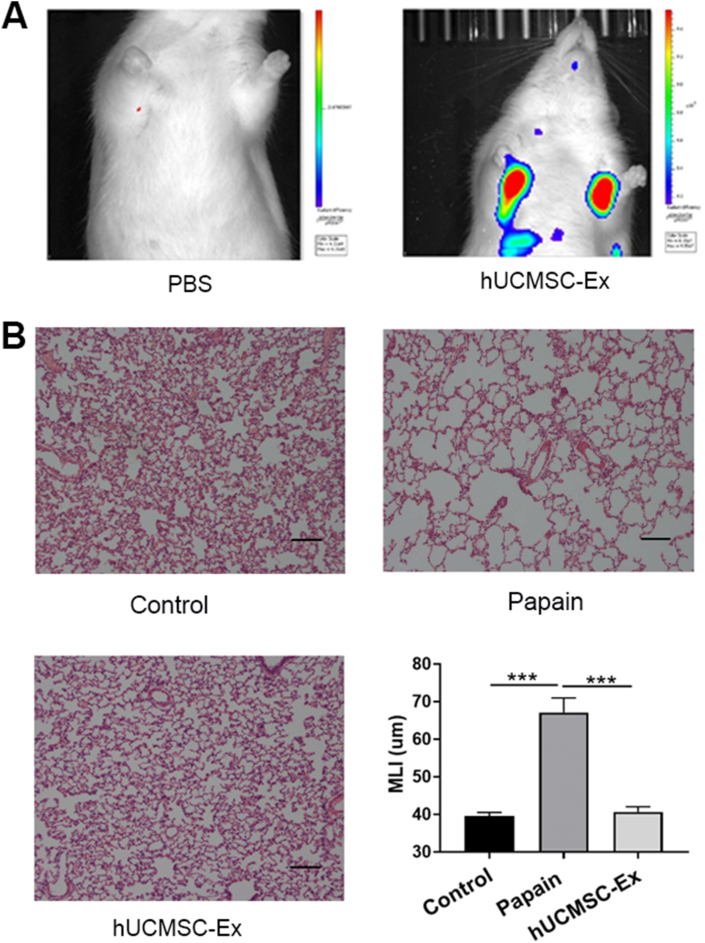
The hUCMSC-Ex were labeled with DIR and injected into the model rats to observe whether the labeled exosomes could reach the lungs, which is a prerequisite for hUCMSC-Ex to function in emphysema. Animal live imaging analysis showed the red fluorescence signal was accumulated in corresponding position of the bilateral lungs of rats in the DIR-labeled hUCMSC-Ex group, while there was no red fluorescence signal in the lungs of the PBS-injected model rats (Fig. 2A), indicating that the labeled hUCMSC-Ex could reach the emphysematous lungs.
Fig. 2.
hUCMSC-Ex reversed papain-induced emphysema. (A) Fluorescence distribution of DIR-labeled hUCMSC-Ex in emphysematous rat lungs after injection 24 h. (B) Representative results of HE staining in lung sections chosen from each group. Typical emphysematous changes were observed in the Papain group. hUCMSC-Ex reversed the papain-induced emphysema. Magnification, x100; scale bar, 100 μm. MLI showed changes of the average alveoli diameter in control, model and treatment groups. Values are presented as mean ± SEM. ∗∗∗P < 0.001. n = 10.
H&E staining indicated that rats in the model group showed severe alveolar destruction and enlargement of alveolar spaces compared with the Control group, suggesting the lungs had become emphysematous. However, hUCMSC-Ex reversed emphysematous changes markedly (Fig. 2B). MLI was determined to quantify enlargement of the alveolar spaces. MLI was significantly higher in the Papain group compared to the Control group. However, MLI was restored to the normal level in the hUCMSC-Ex group. These findings indicate that hUCMSC-Ex can effectively repair papain-induced emphysema injury.
3.3. hUCMSC-Ex prevented apoptosis in papain-induced emphysema
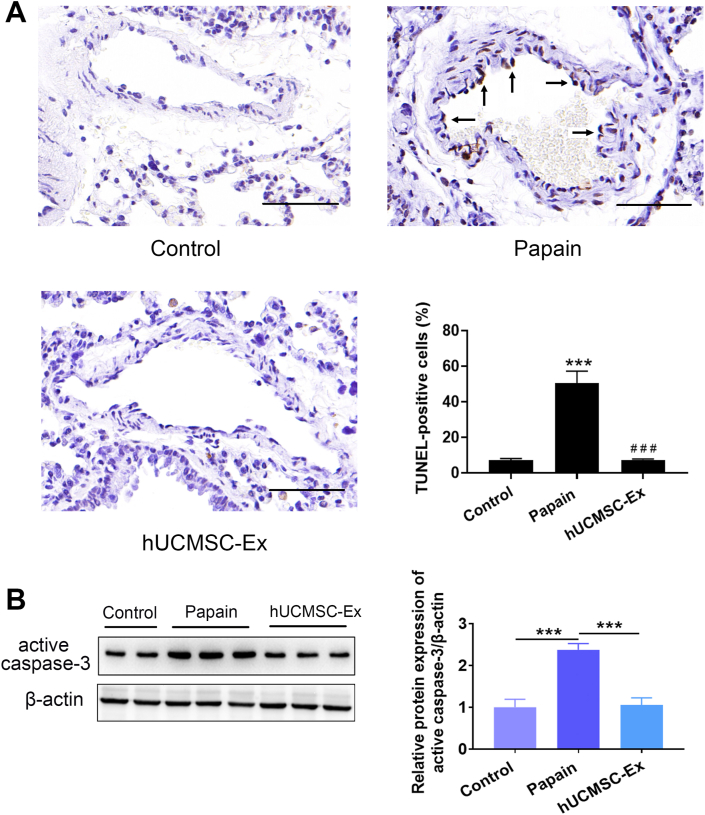
TUNEL staining was performed to analyze the apoptosis of lung tissues. TUNEL results showed that endothelial cell apoptosis was observed companied with apoptosis of other types of cells. Papain group had a significantly higher percentage of TUNEL-positive cells than Control group. Notably, hUCMSC-Ex significantly decreased the percentage of TUNEL-positive cells in the papain group (Fig. 3A). Western blotting showed Papain group had enhanced active caspase-3 levels, while hUCMSC-Ex significantly decreased the activity of active caspase-3 (Fig. 3B).
Fig. 3.
hUCMSC-Ex prevented endothelial cell apoptosis in papain-treated lungs. (A) Representative TUNEL results using lung sections from each group. Increased TUNEL-positive endothelial cells were observed in the Papain group. hUCMSC-Ex reduced TUNEL-positive endothelial cells. Magnification, ×400; scale bar, 50 μm. Quantitative results showed percentage of TUNEL-positive cells in the three groups. Data are presented as mean ± SEM. ∗∗∗P < 0.001 compared with the Control group, ###P < 0.001 compared with the Papain group. n = 3. (B) The expression of active caspase-3 was determined by western blotting. hUCMSC-Ex repressed enhanced active caspase-3 activity in the model group.
3.4. hUCMSC-Ex activated VEGF/VEGFR2 mediated AKT and MEK/ERK pathways in papain-induced emphysema
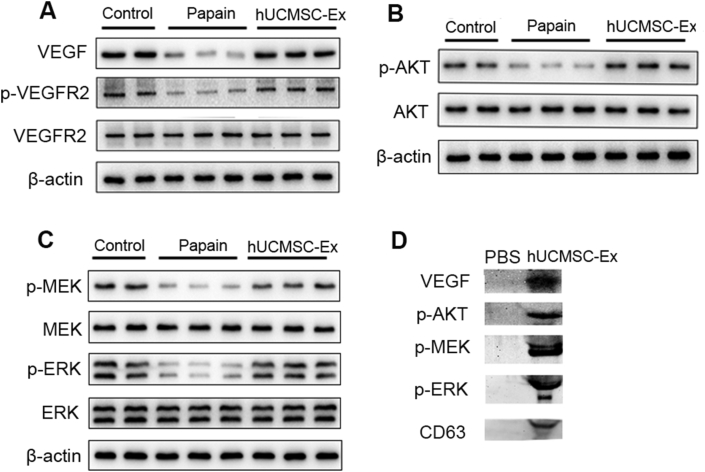
Damage of VEGF/VEGFR2 and their downstream AKT and MEK/ERK survival signal pathways have been implicated in apoptosis of endothelial cells in emphysema. In the current study, we detected proteins expression of pathways-related key molecules such as VEGF, p-VEGFR2, p-AKT, p-MEK and p-ERK by western blotting. The results showed these active molecules VEGF, p-VEGFR2, p-AKT, p-MEK and p-ERK were significantly lower in the Papain group compared to the Control group, while hUCMSC-Ex recovered their expressions in the treatment group (Fig. 4A–C), indicating that hUCMSC-Ex intervention could rescue the VEGF-VEGFR2 mediated AKT and MEK/ERK pathways to prevent cell apoptosis and induce cell survival in emphysematous lungs. The expressions of total VEGFR2, AKT, MEK and ERK were not significantly altered in damaged or treatment groups. Further analysis showed hUCMSC-Ex contained active VEGF, p-AKT, p-MEK and p-ERK proteins (Fig. 4D), which may contribute to the restoration of the pathways.
Fig. 4.
hUCMSC-Ex activated VEGF-VEGFR2-mediated AKT and MEK/ERK pathways in papain-induced emphysema. The protein expressions of pathways-related molecules in the lungs were determined by western blotting. Compared with the Control group, VEGF, p-VEGFR2, p-AKT, p-MEK, p-ERK were significantly inactivated in the Papain group, but all of them were activated in the hUCMSC-Ex group and returned to the level of the Control group. Further analysis showed hUCMSC-Ex contained active proteins VEGF, p-AKT, p-MEK and p-ERK.
3.5. Several miRNAs may be involved in the protective effects of hUCMSC-Ex in papain-induced emphysema
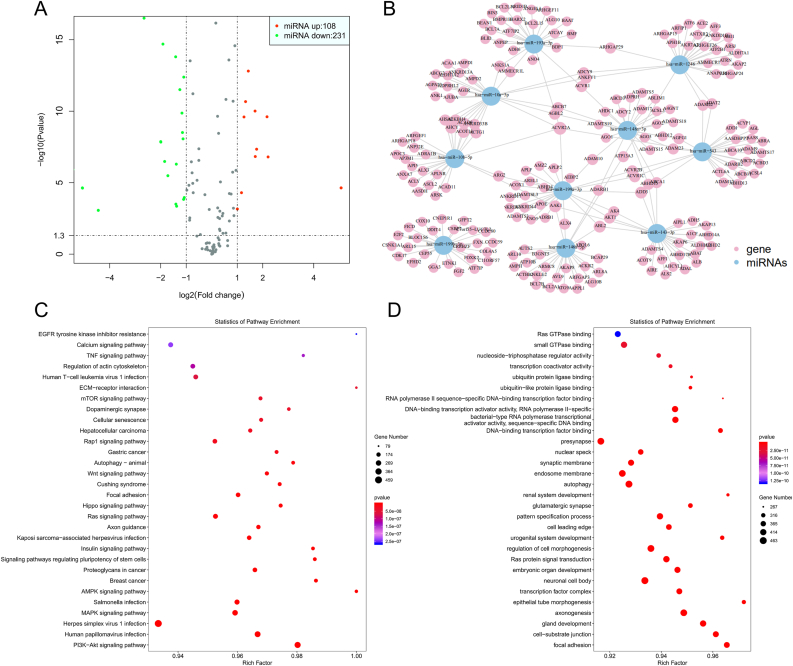
To uncover mechanisms underlying hUCMSC-Ex-mediated protective effects of papain-induced emphysema, we performed unbiased miRNA-seq analysis and found 108 miRNAs were up-regulated and 231 miRNAs were down-regulated in hUCMSC-Ex (Fig. 5A). Then, the top 10 miRNAs including hsa-miR-10a-5p, hsa-miR-146a-5p, hsa-miR-193a-5p, hsa-miR-199a-3p, etc. were used for miRNAs target genes network prediction (Fig. 5B). The result of transcriptome analysis showed that the top 10 miRNA-related target genes were enriched according to the KEGG annotations of cell apoptosis, proliferation and differentiation, including PI3K-Akt signaling pathway (hsa04151), MAPK signaling pathway (hsa04010) and AMPK signaling pathway (hsa04152) (Fig. 5C). Interestingly, GO analysis of these differentially expression transcripts show that the overrepresented biological processes were mostly associated with the regulation of cell morphogenesis (GO:0022,604), and autophagy (GO:0006914) (Fig. 5D). Thus, these results indicated that several miRNAs, such as hsa-miR-10a-5p and hsa-miR-146a-5p, may be involved in the protective effects of hUCMSC-Ex in papain-induced emphysema through AKT and MEK/ERK pathways.
Fig. 5.
Several miRNAs may be involved in the protective effects of hUCMSC-Ex in papain-induced emphysema. (A) miRNA-seq showed that 108 miRNAs were up-regulated and 231 miRNAs were down-regulated in hUCMSC-Ex. Volcano plot graphs of differentially expressed genes (as assessed by the –log10 (padj)). (B) Significant Target gene network prediction of the top 10 miRNAs. (C) The result of transcriptome analysis showed that the top 10 miRNA-related target genes were enriched according to the KEGG annotations. (D) GO analysis of the top 10 miRNA-related target genes show that the overrepresented biological processes (P values: Benjamini-Hochberg corrected).
4. Discussion
Several animal models have been established and used in order to explore the pathogenesis and interventions of emphysema. As early as 1965, Gross et al. first discovered that intratracheal injection of papain could induce emphysema [27]. Papain has broad proteolytic activity, and its enzymatic attack on proteins in the lung can lead to alveolar expansion [28], causing emphysema. To date, papain-induced models have been widely used in the study of COPD and emphysema [[29], [30], [31]]. In the current investigation, we successfully induced emphysema model in rats using papain because alveolar spaces were enlarged in model group. In this papain-induced emphysema models, we showed the repair effect of hUCMSC-Ex and its ability of opposing apoptosis in SD rats.
Apoptosis of pulmonary parenchymal cells is increasing recognized as an important contributor to the pathogenic mechanisms of emphysema. Increased apoptosis of endothelial and epithelial cells has been reported in human emphysematous lungs [32]. Enhanced apoptosis was also found in animal models induced by cigarette smoke, papain and VEGFR2 blocker [[33], [34], [35]]. More direct evidence is that intratracheal instillation of active caspase-3 could induce lung cell apoptosis and emphysema in animals [36] and administration with caspase inhibitor markedly alleviated the apoptosis-dependent emphysema [35]. Furthermore, alveolar septal cell apoptosis was correlated with the decrease of alveolar surface area in human emphysema [37]. Notably, despite smoking cessation, cell apoptosis persists in COPD patients [38]. These findings provided further evidences that the key role of apoptosis in emphysema and COPD. In our papain-induced emphysema, excessive apoptosis in lungs was illustrated by TUNEL analysis and increased active caspases-3 activity. Administration with hUCMSC-Ex greatly inhibited increased apoptosis and increased active caspase-3, suggesting hUCMSC-Ex have remarkably great ability of preventing apoptosis in the papain-induced emphysema.
Previous studies have reported that endothelial cell apoptosis dominated in patients with COPD and emphysema. Among the apoptotic cells in sections of COPD lung tissues, most were endothelial cells [39]. There were also observed increased endothelial cell apoptosis in cigarette smoke-damaged lungs in animal models [33]. Moreover, Tuder and his colleagues reported that lung endothelial cells underwent a greater extent of apoptosis than lung epithelial cells when they were exposed to cigarette smoke extract in vitro [40]. In our papain-induced models, endothelial cell apoptosis was also observed companied with apoptosis of other types of cells. Numerous literatures report VEGF and its receptor VEGFR2 mediated survival signaling pathways are essential for the survival of endothelial cells, the maintenance of the vascular system and maintenance of alveolar structural homeostasis. Decreased expression of VEGF and VEGFR2 in human emphysematous lungs are related to increased endothelial cell death. The reduction of VEGF/VEGFR2 expression can lead to endothelial cell apoptosis, which leads to emphysema changes [35,41]. These findings further demonstrated that VEGF-VEGFR2-mediated signaling pathways implicated in the lung cells apoptosis and emphysema development. It is proposed that reduced VEGF or VEGFR2 would lead to endothelial cell death and microcirculation damage, subsequently promoting further epithelial cell death and emphysema development. VEGF has been proved as a well characterized anti-apoptotic growth factor. VEGF specifically binds to Flk-1/KDR (VEGFR2 receptor) of endothelial cell and activates PI3K/AKT pathway [42]. During this process, VEGFR2 needs to be activated to phosphorylated form. Subsequently, activated-AKT suppresses BAD and caspase-9 expression to reduce apoptosis [43,44]. In addition, VEGF represses endothelial cell apoptosis trough activating ERK pathway [45]. AKT pathway and ERK pathway are essential for cell survival. To evaluate whether hUCMSC-Ex promoted activation of the AKT cascade and ERK cascade in emphysema, we examined the activation of proteins involved in the two signaling pathways in lungs and hUCMSC-Ex. In this papain-induced emphysema, we observed significantly decreased expression of VEGF signaling-related key molecules include VEGF, p-VEGFR2, p-AKT, p-MEK and p-ERK by western blotting, while hUCMSC-Ex reversed this reduction by increasing their expressions, accompanied by decreased active caspase-3 and decreased TUNEL-positive cells in the lungs of the treatment group, suggesting hUCMSC-Ex can prevent apoptosis through the VEGF/VEGFR2 mediated AKT pathway and ERK pathway. Furthermore, we found hUCMSC-Ex contain the VEGF, p-AKT, p-MEK and p-ERK active proteins, which may contribute to the restoration of the pathways.
miRNAs are a large family of post-transcriptional regulators of gene expression, which are small non-coding RNAs that usually inhibit the translation and stability of messenger RNAs (mRNAs), which are about 20–24 nucleotides in length, and control many developmental and cellular processes in eukaryotes, such as inflammation, cell cycle regulation, stress response, differentiation, apoptosis and migration, etc. [46,47]. miRNAs are involved in the regulation of almost all signaling circuits in cells, and their dysregulation has been shown to play a crucial role in the development of certain diseases [46]. To uncover mechanisms underlying hUCMSC-Ex-mediated protective effects of papain-induced emphysema, we performed miRNA sequencing and predicted target genes for the top 10 miRNAs, such as hsa-miR-10a-5p, hsa-miR-146a-5p, hsa-miR-193a-5p, hsa-miR-199a-3p, as well as KEGG analysis and GO analysis of target genes. Several cancer cells can secrete exosomal granules containing miR-146a-5p, while overexpression of miR-146a target genes affects cell proliferation, invasion, metastasis and cell survival [48]. miR-199a-3p/145-5p are relatively highly expressed miRNAs in exosomes, which can promote PC12 cell differentiation by regulating the NGF/TrkA pathway [49]. miR10a-5p can be directly bound by MIR22HG, and MIR22HG inhibits growth, migration and invasion by regulating the miR-10a-5p/NCOR2 axis in hepatocellular carcinoma cells [50]. Expression of miR-193a-5p in hepatoma cells mimics reduced proliferation, survival, migration and invasion and their growth as xenograft tumors in nude mice [51]. Here, the results of miRNA-seq showed that hUCMSC-Ex was enriched according to the KEGG annotations of cell apoptosis, proliferation and differentiation, including PI3K-Akt signaling pathway, MAPK signaling pathway and AMPK signaling pathway, indicating that several miRNAs, such as hsa-miR-10a-5p and hsa-miR-146a-5p, may be involved in the protective effects of hUCMSC-Ex in papain-induced emphysema through AKT and MEK/ERK pathways. The specific miRNAs and target genes in this process remains to be further studied.
In conclusion, this investigation reports the role of the exosomes derived from hUCMSC in repairing papain-induced emphysema by preventing apoptosis through VEGF and VEGFR2 mediated AKT pathway and MEK/ERK pathway, for the first time. This study provides a basis for application of hUCMSC-Ex in intervening emphysema and COPD.
Declaration of competing interest
The authors have no conflicting financial interest.
Acknowledgements
This work was supported by the Youth Medical Talents Project of “Ke Jiao Qiang Wei” project of Jiangsu province (Grant number: QNRC2016449) and the “Innovative and Entrepreneurial Elite Team” Program (2016), Jiangsu, China.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.May S.M., Li J.T. Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy Asthma Proc. 2015;36(1):4–10. doi: 10.2500/aap.2015.36.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García Castillo E., Alonso Pérez T., Ancochea J., Pastor Sanz M.T., Almagro P., Martínez-Camblor P., et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2015 and GOLD 2019 staging: a pooled analysis of individual patient data. ERJ Open Res. 2020;6(4):253–2020. doi: 10.1183/23120541.00253-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestbo J., Hurd S.S., Agustí A.G., Jones P.W., Vogelmeier C., Anzueto A., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 4.Rabe K.F., Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- 5.Coppolino I., Ruggeri P., Nucera F., Cannavò M.F., Adcock I., Girbino G., et al. Role of stem cells in the pathogenesis of chronic obstructive pulmonary disease and pulmonary emphysema. COPD. 2018;15(5):536–556. doi: 10.1080/15412555.2018.1536116. [DOI] [PubMed] [Google Scholar]
- 6.Huh J.W., Kim S.Y., Lee J.H., Lee J.S., Van Ta Q., Kim M., et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011;301(3):L255–L266. doi: 10.1152/ajplung.00253.2010. [DOI] [PubMed] [Google Scholar]
- 7.Le Thi Bich P., Nguyen Thi H., Dang Ngo Chau H., Phan Van T., Do Q., Dong Khac H., et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res Ther. 2020;11(1):60. doi: 10.1186/s13287-020-1583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X., Liu L., Wang Y., Yu Y., Li Z., Liu Y., et al. Human umbilical cord-derived mesenchymal stem cells alleviate acute lung injury caused by severe burn via secreting TSG-6 and inhibiting inflammatory response. Stem Cell Int. 2022;2022 doi: 10.1155/2022/8661689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S., Cui G., Peng C., Lavin M.F., Sun X., Zhang E., et al. Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res Ther. 2018;9(1):110. doi: 10.1186/s13287-018-0846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X., Jiang X., Qu C., Chang P., Zhang C., Qu Y., et al. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy. 2015;17(5):560–570. doi: 10.1016/j.jcyt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Amani S., Shahrooz R., Hobbenaghi R., Mohammadi R., Baradar Khoshfetrat A., Karimi A., et al. Angiogenic effects of cell therapy within a biomaterial scaffold in a rat hind limb ischemia model. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-99579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierro M., Ionescu L., Montemurro T., Vadivel A., Weissmann G., Oudit G., et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68(5):475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 13.Bollini S., Gentili C., Tasso R., Cancedda R. The regenerative role of the fetal and adult stem cell secretome. J Clin Med. 2013;2(4):302–327. doi: 10.3390/jcm2040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratajczak M.Z., Kucia M., Jadczyk T., Greco N.J., Wojakowski W., Tendera M., et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26(6):1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 15.Bang C., Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Camussi G., Deregibus M.C., Bruno S., Cantaluppi V., Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 17.Maas S.L.N., Breakefield X.O., Weaver A.M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y., Liu J., Chen P., Lin L., Luo Y., Ma X., et al. Exosomal miR-22-3p from human umbilical cord blood-derived mesenchymal stem cells protects against lipopolysaccharid-induced acute lung injury. Life Sci. 2021;269 doi: 10.1016/j.lfs.2020.119004. [DOI] [PubMed] [Google Scholar]
- 20.Liu J.S., Du J., Cheng X., Zhang X.Z., Li Y., Chen X.L. Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J Chin Med Assoc. 2019;82(12):895–901. doi: 10.1097/JCMA.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Deng X., Ji X., Shi X., Ying Z., Shen K., et al. Mesenchymal stem cell exosomes reverse acute lung injury through Nrf-2/ARE and NF-κB signaling pathways. PeerJ. 2020;8:e9928. doi: 10.7717/peerj.9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren J., Liu Y., Yao Y., Feng L., Zhao X., Li Z., et al. Intranasal delivery of MSC-derived exosomes attenuates allergic asthma via expanding IL-10 producing lung interstitial macrophages in mice. Int Immunopharm. 2021;91 doi: 10.1016/j.intimp.2020.107288. [DOI] [PubMed] [Google Scholar]
- 23.Dong B., Wang C., Zhang J., Zhang J., Gu Y., Guo X., et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res Ther. 2021;12(1):204. doi: 10.1186/s13287-021-02244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridzuan N., Zakaria N., Widera D., Sheard J., Morimoto M., Kiyokawa H., et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD) Stem Cell Res Ther. 2021;12(1):54. doi: 10.1186/s13287-020-02088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao C., Xu W., Zhu W., Hu J., Qian H., Yin Q., et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32(1):8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Dunnill M.S. Evaluation of a simple method of sampling the lung for quantitative histological analysis. Thorax. 1964;19(5):443–448. doi: 10.1136/thx.19.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross P., Pfitzer E.A., Tolker E., Babyak M.A., Kaschak M. Experimental emphysema: its production with papain in normal and silicotic rats. Arch Environ Health. 1965;11:50–58. doi: 10.1080/00039896.1965.10664169. [DOI] [PubMed] [Google Scholar]
- 28.Johanson W.G., Jr., Pierce A.K. Effects of elastase, collagenase, and papain on structure and function of rat lungs in vitro. J Clin Invest. 1972;51(2):288–293. doi: 10.1172/JCI106813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado M.N., Mazzoli-Rocha F., Casquilho N.V., Maron-Gutierrez T., Ortenzi V.H., Morales M.M., et al. Bone marrow-derived mononuclear cell therapy in papain-induced experimental pulmonary emphysema. Front Physiol. 2018;9:121. doi: 10.3389/fphys.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machado M.N., Figueirôa S.F., Mazzoli-Rocha F., Valença Sdos S., Zin W.A. Papain-induced experimental pulmonary emphysema in male and female mice. Respir Physiol Neurobiol. 2014;200:90–96. doi: 10.1016/j.resp.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Pastor L.M., Sánchez-Gascón F., Girona J.C., Bernal-Mañas C.M., Morales E., Beltrán-Frutos E., et al. Morphogenesis of rat experimental pulmonary emphysema induced by intratracheally administered papain: changes in elastic fibres. Histol Histopathol. 2006;21(12):1309–1319. doi: 10.14670/HH-21.1309. [DOI] [PubMed] [Google Scholar]
- 32.Kasahara Y., Tuder R.M., Cool C.D., Lynch D.A., Flores S.C., Voelkel N.F. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163(3 Pt 1):737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 33.Guan X.J., Song L., Han F.F., Cui Z.L., Chen X., Guo X.J., et al. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem. 2013;114(2):323–335. doi: 10.1002/jcb.24377. [DOI] [PubMed] [Google Scholar]
- 34.Zhen G., Xue Z., Zhao J., Gu N., Tang Z., Xu Y., et al. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy. 2010;12(5):605–614. doi: 10.3109/14653241003745888. [DOI] [PubMed] [Google Scholar]
- 35.Kasahara Y., Tuder R.M., Taraseviciene-Stewart L., Le Cras T.D., Abman S., Hirth P.K., et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106(11):1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoshiba K., Yokohori N., Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003;28(5):555–562. doi: 10.1165/rcmb.2002-0090OC. [DOI] [PubMed] [Google Scholar]
- 37.Imai K., Mercer B.A., Schulman L.L., Sonett J.R., D'Armiento J.M. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005;25(2):250–258. doi: 10.1183/09031936.05.00023704. [DOI] [PubMed] [Google Scholar]
- 38.Hodge S., Hodge G., Holmes M., Reynolds P.N. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur Respir J. 2005;25(3):447–454. doi: 10.1183/09031936.05.00077604. [DOI] [PubMed] [Google Scholar]
- 39.Segura-Valdez L., Pardo A., Gaxiola M., Uhal B.D., Becerril C., Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117(3):684–694. doi: 10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 40.Tuder R.M., Wood K., Taraseviciene L., Flores S.C., Voekel N.F. Cigarette smoke extract decreases the expression of vascular endothelial growth factor by cultured cells and triggers apoptosis of pulmonary endothelial cells. Chest. 2000;117(5 Suppl 1):241S–242S. doi: 10.1378/chest.117.5_suppl_1.241s. [DOI] [PubMed] [Google Scholar]
- 41.Giordano R.J., Lahdenranta J., Zhen L., Chukwueke U., Petrache I., Langley R.R., et al. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem. 2008;283(43):29447–29460. doi: 10.1074/jbc.M804595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerber H.P., McMurtrey A., Kowalski J., Yan M., Keyt B.A., Dixit V., et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273(46):30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 43.Cardone M.H., Roy N., Stennicke H.R., Salvesen G.S., Franke T.F., Stanbridge E., et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 44.del Peso L., González-García M., Page C., Herrera R., Nuñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278(5338):687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 45.Gupta K., Kshirsagar S., Li W., Gui L., Ramakrishnan S., Gupta P., et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247(2):495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 46.Di Leva G., Garofalo M., Croce C.M. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 48.Iacona J.R., Lutz C.S. miR-146a-5p: expression, regulation, and functions in cancer. Wiley Interdiscip Rev RNA. 2019;10(4):e1533. doi: 10.1002/wrna.1533. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y., Lai X., Wu D., Liu B., Wang N., Rong L. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res Ther. 2021;12(1):117. doi: 10.1186/s13287-021-02148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Y., Zhou Y., Huan L., Xu L., Shen M., Huang S., et al. LncRNA MIR22HG inhibits growth, migration and invasion through regulating the miR-10a-5p/NCOR2 axis in hepatocellular carcinoma cells. Cancer Sci. 2019;110(3):973–984. doi: 10.1111/cas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy S., Hooiveld G.J., Seehawer M., Caruso S., Heinzmann F., Schneider A.T., et al. microRNA 193a-5p regulates levels of nucleolar- and spindle-associated protein 1 to suppress hepatocarcinogenesis. Gastroenterology. 2018;155(6):1951–1966. doi: 10.1053/j.gastro.2018.08.032. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]