Abstract
Objective
To study the effects of the “Mirror Effect Plus Protocol” (MEPP) on global facial function in acute and severe Bell's Palsy.
Design
Single blind and randomized controlled trial to compare the effects of basic counseling (control group) versus MEPP (experimental group) over one year.
Setting
Outpatient clinic following referrals from Emergency or Otorhinolaryngology Departments.
Subjects
40 patients (n = 20 per group) with moderately severe to total palsy who received standard medication were recruited within 14 days of onset. Baseline characteristics were comparable between the groups.
Interventions
The experimental group received the MEPP program (motor imagery + manipulations + facial mirror therapy) while the control group received basic counseling. Both groups met the clinician monthly until 6 months and at one-year post-onset for assessments.
Outcome measures
Facial symmetry, synkinesis, and quality of life were measured using standardized scales. Perceived speech intelligibility was rated before and after therapy by naïve judges.
Results
Descriptive statistics demonstrated improvements in favor of the MEPP for each measured variable. Significant differences were found for one facial symmetry score (House-Brackmann 2.0 mean (SD) = 7.40 (3.15) for controls versus 5.1 (1.44) for MEPP), for synkinesis measures (p = 0.008) and for quality-of-life ratings (mean (SD) score = 83.17% (17.383) for controls versus 98.36% (3.608) for MEPP (p = 0.002)). No group difference was found for perceived speech intelligibility.
Conclusion
The MEPP demonstrates promising long-term results when started during the acute phase of moderately severe to total Bell's Palsy.
Keywords: Bell palsy, facial nerve diseases, facial paralysis, rehabilitation, synkinesis, mirror therapy
Introduction
Bell's palsy is a disabling condition for patients affected by it, particularly for those experiencing severe enduring sequelae despite an adequate medication intake.1,2 Recent systematic review reported the advantages of early facial rehabilitation combined with standard medication for moderately severe to total acute Bell's Palsy.3,4 Benefits of early facial rehabilitation and counseling are multifold: it helps guiding patients in not overstimulating their face and it allows for passive and specific soft tissue mobilization. 5 Moreover, as loss of facial nerve activity leads to rapid changes in cortical representations, 6 early intervention is desired to avoid a “learned paralysis.” 7
Contrary to chronic Bell's Palsy, no specific facial therapy seems to stand out for the treatment of acute Bell's Palsy. 8 As demonstrated by recent data,8–11 interesting results could be achieved with mirror therapy. Our team developed an early intervention for patients with acute and moderately severe to total Bell's Palsy, using mirror therapy as the main tool. The “Mirror Effect Plus Protocol” or MEPP (see methods for details and our previous publication about the exact nature of MEPP 12 ) also integrates facial manipulations, 5 motor imagery sessions, 13 principles of motor learning,14,15 and components of facial neuromuscular retraining.4,16,17 Paolucci et al. 8 also recently used mirror therapy and motor imagery with 22 patients suffering from facial palsy secondary to an acoustic neuroma resection. Their experimental (mirror therapy) group showed better recovery and fewer functional impairments than the control group, who received traditional facial neuromuscular retraining.
To date, no studies have reported on the long-terms effects of mirror therapy on patients with acute Bell's Palsy, by assessing simultaneously the perspective of clinicians with standardized scales, perspective of patients with patient-centered outcome measures, or perspective of naïve observers. This randomized controlled trial aimed to investigate the long-term effects of the MEPP in acute Bell's Palsy compared to medical treatment and basic counseling alone, through those three perspectives.
Based on recent evidence,3,4,9,18 we hypothesized that the MEPP would help patients achieve better facial symmetry and a better-perceived quality of life, and that those effects would still be present at one-year post-onset. Also, based on contradictory evidence,4,19,20 we made the weak hypothesis that the MEPP would have a beneficial effect on the long-term development of synkinesis. Finally, based on the findings of Moverare et al., 21 we hypothesized that the MEPP would have no effect on speech intelligibility.
Methods
The study was approved by the ethics committee of Centre de recherche du Centre intégré universitaire de santé et services sociaux du Nord-de-l’île-de-Montréal (MP-32-2017-1365; start date in January 2017 and end date October 2021), which was the organization responsible for the study’ s integrity and process. All patients gave their written, free and informed consent to participate. This study followed the CONSORT guidelines and recommendations for transparent, controlled trials and was registered (ISRCTN93896690). Retrospective registration took place as the study was initially designed to gather pilot data, but then ethic approbation was obtained to increase the number of participants and do a randomized trial.
All patients were recruited from either the Emergency or the Otorhinolaryngology Departments of two tertiary acute care centers. They were examined in ambulatory care settings, where they received information about the project and consented (or not) to be contacted by the research team. They were medically stable before Bell's Palsy onset. Other inclusion criteria were: (1) first episode of Bell's Palsy; (2) moderately severe to total Bell's Palsy (i.e. House-Brackmann 2.0 grade 4, 5 and 6) at baseline (i.e. 10–14 days post-onset); (3) the recommended drug regimen for severe and total Bell's Palsy (1000 mg valacyclovir three times daily for 7 days and 50 mg prednisone once daily for 10 days) within 72 h post onset 22 ; and (4) normal cognitive status based on the Montreal Cognitive Assessment. 23 Exclusion criteria were: (1) active psychiatric interventions; (2) history of head and neck cancer with chemo or radiotherapy; and (3) bilateral facial palsy. The research team set up the first appointment to confirm patient's eligibility. When eligible, allocation to the control group or MEPP group was performed by a researcher (K.M.) who was not in direct contact with the patients, through a sealed email that was open in front of the patient, following computerized balanced (1:1 ratio) block randomization. A power calculation based on a similar study 24 in chronic Bell's Palsy revealed an effect size of 1.2, meaning that 12 patients per group had to be recruited to reach a power of 83%. As our study took place in the acute phase of BP, recruitment was increased up to 20 patients per group.
All patients underwent eight assessments spread over 12 months. The first assessment took place 10–14 days after Bell's Palsy onset and before any facial therapy. Each subsequent assessments were performed at 1, 2, 3, 4, 5, 6, and 12 months after onset. Assessments were video-recorded using a Samsung Galaxy S5-Neo placed approximately 1 m in front of the patient or with the institutional Zoom application during the COVID-19 pandemic. The electronic video files were transferred to a PC and then converted to mp4 video files.
At each assessment timepoints, different measures were taken. First, the severity of facial palsy, the global symmetry and the degree of synkinesis were assessed through the perspective of clinicians with the House-Brackmann 2.0 scale 25 (also known as the Facial Nerve Grading Scale 2.0) and the Sunnybrook Facial Grading System. 26 Both were chosen because of their high inter-observer agreement and validity. 27 The House-Brackmann 2.0 scale gives a global score from 4 (normal facial function) to 24 (total palsy). It is also widely used for its grading from 1 to 6 (1 = normal function; 6 = total palsy). The Sunnybrook scale gives a global score, from 0% (total palsy) to 100% (normal function). Specific subscores (e.g. synkinesis, symmetry at rest) can be calculated from either scale to allow for more specific analyses.
Second, the perspective of patients was assessed with the validated Canadian French version of the Facial Clinimetric Evaluation questionnaire (FaCE-F). 28 It was administered at each assessment timepoint to measure the evolution of the patients regarding quality-of-life and the functional consequences of their Bell's Palsy. This patient-reported outcome measure instrument is valid, reliable, and assesses facial palsy's functional and social impact. 28 The questionnaire was added to the protocol when it became available (starting with patient #12), so 12 patients from the control group and 14 from the experimental group completed the questionnaire.
Finally, to assess perceived speech intelligibility, the perspective of naïve listeners was adopted (see data analysis for details). Patients were asked to talk about their past weekend for one to 2 min to gather a sample of spontaneous speech at each assessment timepoint. Samples of spontaneous speech were extracted for patients who were native speakers of French (n = 25) at the initial and 5 months-post onset assessments.
In sum, considering the hypotheses and the above-mentioned measures, study outcomes were as follows. Based on the work of Nicastri et al., 4 the primary outcomes were the improvement in global symmetry as measured by House-Brackmann 2.0 grades, House-Brackmann 2.0 scores, and Sunnybrook scores at each Bell's Palsy phase. Recovery was defined as a House-Brackmann 2.0 grade of 2 or less (House-Brackmann 2.0 score of 9 or less) 4 and a Sunnybrook score of 80 or more. 29 The secondary outcome was the development of synkinesis as measured by the synkinesis subscores of both scales. The third outcome was the perceived change of quality-of-life with the FaCE-F questionnaire at each assessment timepoint. Finally, the fourth outcome was the change in percentage of patients'speech intelligibility.
The intervention regimen for patients in the MEPP group was the MEPP, as described in detail in Martineau et al.10,12 MEPP patients were met for four in-clinic sessions during the first 2 weeks following initial assessment, and then once per month for follow-ups. Briefly, the MEPP uses modified visual feedback (mirror therapy) as the main rehabilitation component, and integrates relevant components of other approaches (motor imagery, mime therapy, and neuromuscular retraining). Specifically, education about the anatomy and function of the face, soft tissue manipulations, and motor imagery sessions10,12 is first provided. Then, controlled facial movements are taught, using specific instructions that consider the particular morphology of facial muscles. 12 Patients are invited to execute different facial movements while looking at their face through a free website (https://webcamtoy.com/fr/ or https://mepp.marcottelab.ca/auth/signin) that provide modified visual biofeedback. In other words, the website generates a symmetrical facial movement, by duplicating the healthy hemiface of the patient. Facial exercises are continued at home twice daily until recovery is achieved. Two daily and short sessions of 15 min of therapy are privileged to promote motor learning and avoid muscular fatigue. 12
Participants in the control group did not attend therapy sessions. Nonetheless, the control group received instructions for avoiding excessive facial movements during the assessments: for example, they were instructed not to over-activate their faces, try not to compensate for lack of mobility by trying high amplitude and non-specific movements. They also received responses to their questions if needed.
Data analysis included following procedures: facial movement recordings were analyzed for each patient at each timepoint by three independent judges, who were blind to group assignment and assessment time. A mean score of the three independent judges’ ratings was computed for continuous variables (the House-Brackmann 2.0 and Sunnybrook total scores), whereas a median score was calculated for ordinal variables (synkinesis scores and House-Brackmann 2.0 grades). To account for the clinical differences between each phase of Bell's Palsy evolution, assessment timepoints were grouped in phases 30 for our analysis: baseline as flaccid phase (10–14 days post-onset); 1-, 2- and 3-months post-onset as paretic phase; 4-, 5- and 6- months post-onset as the synkinetic phase, and 1-year post-onset as the chronic phase. For perceived speech intelligibility, forty French-speaking naïve judges, blind to group assignment and time of assessment, were asked to listen to spontaneous speech samples and to rate intelligibility, using a visual analog scale from 0% to 100% (perceptual auditory analysis was 0% = not intelligible at all and 100% = perfectly intelligible). For each patient, a mean score based on ratings of the blind judges was obtained at the two selected timepoints.
Statistical analyses were all performed with SPSS (IBM, version 27) with the significance level set at p < 0.05. Two-way random effects intraclass correlation coefficients and their respective 95% confidence interval (CI) were calculated to determine consistency between raters for measures of facial symmetry, synkinesis, and perceptual analysis of speech intelligibility. Results are reported in Supplemental Material 1. All variables were above the threshold of high reliability (intraclass correlation > 0.80). 31
For the first and second outcomes (facial symmetry and synkinesis), descriptive statistics were obtained, and the data distribution was inspected to verify normality. A LOG transformation was applied to non-normal variables, and a mixed model analysis (α = 0.05 and CI, 95%) was conducted to observe differences in House-Brackmann 2.0 global score and grade. Bonferroni corrections were applied to account for multiple testing. Synkinesis was treated as a dichotomic variable with a chi-square analysis because of the predominance of 0 values, and only a few of every other value (0, 1, 2, and 3 for House-Brackmann 2.0, and 0 to 10 for Sunnybrook).
For the third outcome (quality of life) and the fourth outcome (perceived speech intelligibility), descriptive statistics and repeated measures analysis of variances (ANOVAs) with Greenhouse-Geisser correction were conducted. Visual inspection of quantile plots (Q–Q) for the difference between the time points did not reveal any notable deviations from normality. Every assessment timepoint was included in the analysis for quality of life. For perceived speech intelligibility, a comparison was made between the pre-determined 2 timepoints (at baseline and 5 months post-onset) to assess speech differences following the intervention. The synkinetic phase (5 months post-onset) was chosen for the post-intervention measure because it is generally the moment where patients reach a relatively stable level in their facial evolution.
Results
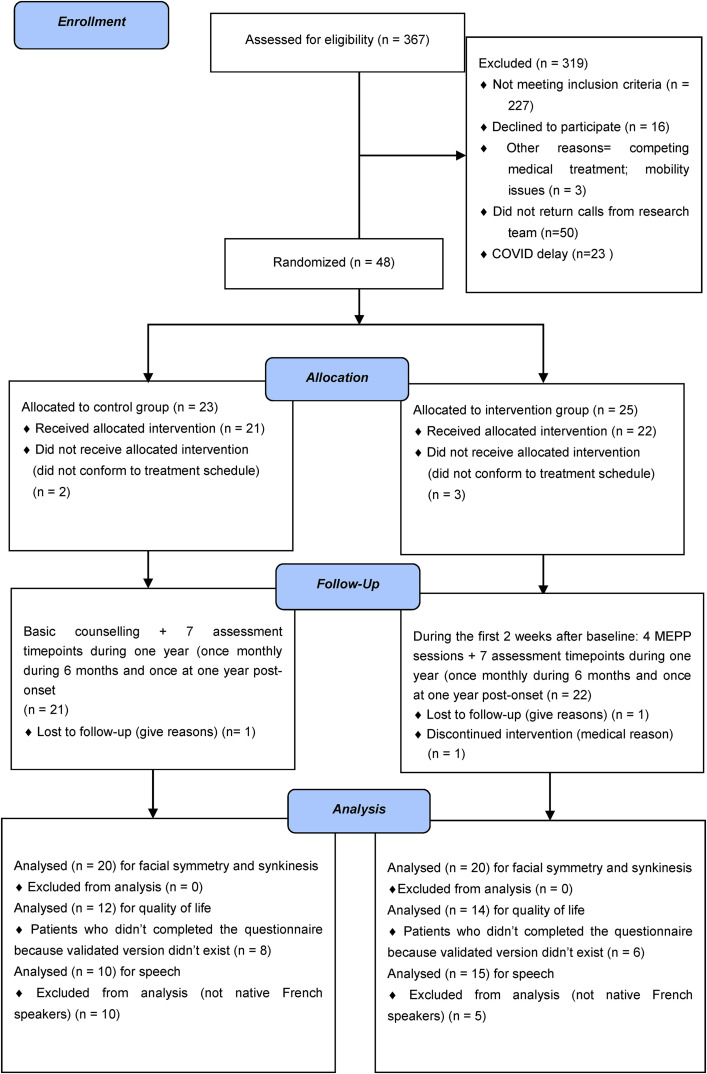
Three-hundred-sixty-seven patients were referred from January 2017 to October 2020. From that number, 48 patients were enrolled and attributed to either the intervention group (MEPP) (n = 23) or the control group (n = 25). Two patients from the control group did not follow the treatment schedule, and one was lost to follow-up. From the treatment group, three did not follow the treatment schedule, one was lost during follow-up, and one was excluded for medical reasons. The resultant 40 participants were evenly separated per-protocol between the two conditions and completed the study over a 12-month follow-up period (see CONSORT study flow diagram in Figure 1).
Figure 1.
CONSORT 2010 Flow Diagram of this controlled randomized longitudinal trial.
Patient characteristics at baseline assessment and characteristics of lost or excluded patients after allocation (n = 8) are reported in Table 1. As can be seen in Table 1, at baseline, patients from both groups did not differ in terms of any of the measured demographic characteristics. Also, excluded patients were similar to the included ones, which confirm that there was no abandonment effect in the study.
Table 1.
Baseline characteristics of the participants in each group and of the lost/excluded patients after allocation, along with results of testing for differences between the groups.
| Group | Included patients | Excluded/lost patients | ||
|---|---|---|---|---|
| Controls (n = 20) | MEPP (n = 20) | Controls (n = 3) | MEPP (n = 5) | |
| Sex, n (%) | ||||
| Female | 8 (40%) | 10 (50%) | 2 | 1 |
| Male | 12 (60%) | 10 (50%) | 1 | 4 |
| Δ Controls vs MEPP: | X2 = 0.886; p = 0.345 | |||
| Δ Included vs excluded: | X2 = 0.152; p = 0.69 | |||
| Age, mean (SD) in years | 48.2 (15.7) | 47.9 (18.1) | 58.0 (9.84) | 51 (14.6) |
| Δ Controls vs MEPP: | t = −0.047; p = 0.963 | |||
| Δ Included vs Excluded: | t = −1.84; p = 0.207 | |||
| Medical history, n (%) | ||||
| Tobacco | 4 (20%) | 3 (15%) | 1 | 0 |
| Hypertension | 7 (35%) | 7 (35%) | 2 | 1 |
| Diabetes | 5 (25%) | 3 (15%) | 0 | 1 |
| Dyslipidemia | 5 (25%) | 1 (5%) | 0 | 0 |
| Hypothyroidism | 2 (10%) | 3 (15%) | 0 | 0 |
| Facial Nerve Grading System 2.0 grade 10–14 D.P.O, n (%) | ||||
| 4 | 9 (45%) | 11 (55%) | 0 | 0 |
| 5 | 10 (50%) | 7 (35%) | 3 | 5 |
| 6 | 1 (5%) | 2 (10%) | 0 | 0 |
| Δ Controls vs MEPP: | U = 213.5; p = 0.774 | |||
| Δ Included vs Excluded: | U = 89; p = 0.060 | |||
| SB score, mean (SD) | 27.9 (13.2) | 30.7 (13.2) | 19.0 (14.9) | 20.8 (5.8) |
| Δ Controls vs MEPP: | t = −0.623; p = 0.537 | |||
| Δ Included Vs Excluded: | t = 1.904; P = 0.063 | |||
Note. Facial Nerve Grading Scale 2.0 (House-Brackman 2.0) scores: 6 = total palsy; 5 = severe palsy; 4 = moderate-to-severe palsy. D.P.O.: Days post onset; Sunnybrook (SB) scores: Minimum possible = 0 or total palsy; maximum possible = 100% or normal; MEPP: mirror effect plus protocol; Δ: differences; X2: bilateral asymptomatic Chi-Square analysis (alpha = 0.05) for sex differences; U: bilateral Mann-Whitney U test (alpha = 0.05) for differences in grades for facial symmetry; t: Bilateral t-test for 2 samples (alpha = 0.05) for differences in age or score for facial symmetry.
Table 2 presents descriptive results about facial symmetry. Specifically, descriptive means and standard deviations for House-Brackmann 2.0 global scores and Sunnybrook global scores are presented for each Bell's Palsy phase, based on group assignment. In Supplemental Material 2, Figure S2.1 presents this data visually. The recovery criterion (House-Brackmann 2.0 grade 2 (scores 4 to 9) or Sunnybrook 80%) was reached at the beginning of the paretic phase for the MEPP group and during the synkinetic phase for the control group. Thus, faster recovery is observed in the MEPP group compared to the control group. Results of the mixed model analysis are also presented in Table 2, whereas the post-hoc analysis results are reported in Supplemental Material 2. In summary, a significant difference between the groups was found for House-Brackmann 2.0 global score and grade, but no group effect was found on Sunnybrook global score. Post hoc analysis for House-Brackmann 2.0 grade and global score demonstrated that a significant difference was found between the two groups for the paretic, synkinetic, and chronic phases, but not at baseline. In other words, patients from the two groups evolved significantly differently after baseline, in favor of the MEPP group, when considering the House-Brackmann 2.0 grade and global scores.
Table 2.
Descriptive results on SB global score and House-Brackmann 2.0 (FNGS 2.) global score per group and assessment timepoints with mix model analysis results on SB global score, FNGS 2.0 global score and FNGS 2.0 grade.
| Descriptive analysis | ||||
|---|---|---|---|---|
| MEPP group | ||||
| Assessment timepoints | Baseline (Flaccid phase) | 1-2-3 MPO (Paretic phase) | 4-5-6 MPO (Synkinetic phase) | 1 YPO (Chronic phase) |
| FNGS 2.0 mean scores (SD) | 19.65 (2.53) | 7.90 (5.21) | 6.14 (3.94) | 5.10 (1.44) |
| SB mean scores (SD) | 30.75 (13.22) | 79.76 (23.21) | 88.85 (16.65) | 93.85 (8.39) |
| Control group | ||||
| FNGS 2.0 mean scores (SD) | 19.15 (2.45) | 9.50 (4.94) | 7.60 (4.54) | 7.40 (3.15) |
| SB mean scores (SD) | 27.95 (13.18) | 72.45 (23.75) | 82.35 (20.49) | 83.80 (14.08) |
| Mix model analysis | ||||
| Variable of interest | Time effect | Condition effect | Interaction effect | |
| SB global mean score | F = 217.44 (p < 0.001)* | F = 4.44 (p = 0.04)* | F = 1.12 (p = 0.346) | |
| FNGS 2.0 mean score | F = 185.37 (p < 0.001)* | F = 6.55 (p = 0.013)* | F = 3.93 (p = 0.010)* | |
| FNGS 2.0 median grade | F = 104.04 (p < 0.001)* | F = 9.445 (p < 0.003)* | F = 4.28 (p = 0.007)* | |
Note. FNGS 2.0: House-Brackmann 2.0 or Facial Nerve Grading Scale 2.0; SB: Sunnybrook; SD: standard deviation; MPO: months post-onset; YPO: year post-onset.
*significant differences.
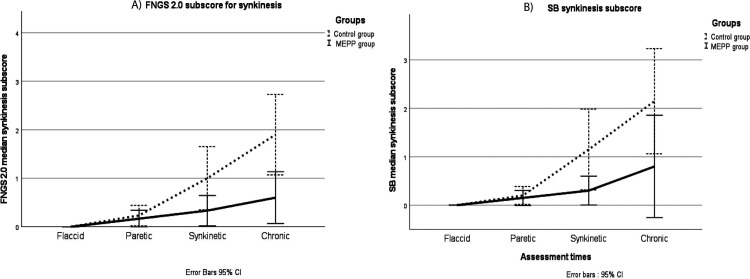
Table 3 presents the number of individuals who developed synkinesis in each group at each assessment timepoints. Figure 2 shows the longitudinal changes of the synkinesis scores of both House-Brackmann 2.0 and Sunnybrook scales. A bilateral asymptomatic Chi-square test allowed to compare the number of participants who developed synkinesis with the ones who did not develop synkinesis, between the groups. Significant differences between the groups were found for synkinesis scores on House-Brackmann 2.0 (X2 = 7.117, P = 0.008) as well as on Sunnybrook (X2 = 3.772, P ≤ 0.05).
Table 3.
Number of individuals with synkinesis at House-Brackmann 2.0 (FNGS 2.0) and SB synkinesis subscore along with Chi-square analysis for individuals with and without synkinesis between the groups.
| MEPP group | Control group | Chi-square analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessment timepoints | Baseline (Flaccid phase) | 1-2-3 MPO (Paretic phase) | 4-5-6 MPO (Synkinetic phase) | 1 YPO (Chronic phase) | Baseline (Flaccid phase) | 1-2-3 MPO (Paretic phase) | 4-5-6 MPO (Synkinetic phase) | 1 YPO (Chronic phase) | |
| Number of individuals with synkinesis at FNGS 2.0 ‘s synkinesis subscore | 0 | 1 | 3 | 5 | 0 | 5 | 8 | 13 | X2 = 7.117 P = 0.008* |
| Number of individuals with synkinesis at SB ‘s synkinesis subscore | 0 | 2 | 4 | 5 | 0 | 4 | 9 | 12 | X2 = 3.772 P ≤ 0.05* |
Note. FNGS 2.0: Facial Nerve Grading Scale 2.0 or House-Brackmann 2.0; SB: Sunnybrook; SD: standard deviation; MPO: months post-onset; YPO: year post-onset
*Significant differences between the groups at the Chi-Square analysis (alpha = 0.05).
Figure 2.
Longitudinal changes in (a) median House-Brackmann 2.0 (FNGS 2.0) and (b) SB synkinesis scores, according to the treatment group for the complete cohort (n = 40). Legend: House-Brackmann 2.0 or FNGS 2.0 synkinesis scores: 4 = severe synkinesis; 0 = no synkinesis. Sunnybrook synkinesis scores: 15 = severe synkinesis; 0 = no synkinesis Assessment times: Flaccid phase = 10–14 days post-onset; paretic phase: 1-, 2-, and 3- months post onset; Synkinetic phase: 4-, 5-, and 6-months post-onset; chronic phase: 1-year post-onset; Black line: MEPP participants; Dash line: Controls. SB: Sunnybrook; MEPP: Mirror Effect Plus Protocol; FNGS: Facial Nerve Grading Scale.
Table 4 shows the results about quality-of-life and functional consequences of Bell's Palsy. Specifically, mean scores (with standard errors and CI) are presented for the FaCE-F questionnaire 28 for the two groups at each phase. The repeated measures ANOVA showed a significant difference between the groups for the global score of FaCE-F (F = 12.831; p = 0.002). The post-hoc analysis confirmed no differences between the groups at baseline, but significant differences in favor of the experimental group at every other assessment timepoints. Those results demonstrate that long-lasting functional improvements for quality of life are larger in the MEPP group. A figure illustrating the evolution of the global score of FaCE-F of both groups over every phase of the study is presented in Supplemental Material 3.
Table 4.
Mean changes of quality of life and functional impacts measured with face-F for each group (n = 26) and assessment timepoints along with standard errors and confidence intervals.
| MEPP group | ||||
|---|---|---|---|---|
| Assessment timepoints | Baseline (Flaccid phase) | 1-2-3 MPO (Paretic phase) | 4-5-6 MPO (Synkinetic phase) | 1 YPO (Chronic phase) |
| FaCE mean scores (SE) | 30.83 (4.95) | 90.78 (4.23) * | 96.69* | 98.35 (3.22) * |
| [CI 95%] | [20.60–41.63] | [81.99–99.48] | [3.57–89.23] | [91.70–105.01] |
| Control group | ||||
| FaCE mean scores (SE) | 43.571 (4.58) | 68.00 (4.57) * | 84.02 (3.85) * | 83.16 (3.48) * |
| [CI 95%] | [34.10–53.04] | [76.07–91.98] | [76.07–91.98] | [75.97–90.35] |
FaCE-F scores: 100 = no functional impact; 0 = severe functional impact. Assessment times: Flaccid phase = 10–14 days post-onset; Paretic phase: 1-, 2-, and 3-months post onset; Synkinetic phase: 4-, 5- and 6- months post-onset; chronic phase: 1 year post-onset; YPO = Year post-onset; SE = standard errors; CI 95%: confidence interval; ANOVA: analysis of variance.
*significant differences between the groups at the post hoc repeated measures ANOVA (alpha = 0.05; p = 0.002).
Finally, results about the perceived speech intelligibility are reported in Supplemental Material 4. Descriptive statistics of the perceptual analysis of 40 French-speaking naïve judges were included in this analysis. Only French-speaking judges were chosen to avoid confounding variables in the analysis (such as understandability problems because of a foreign accent). Twenty-five (25) French-speaking patients (control group n = 10; experimental n = 15) were included in the analysis, and both groups received high intelligibility ratings at the first assessment (mean (SD) of 86.9% (4.91) for the control group and 86.6 (6.33) for the MEPP group), and at 5 months post-onset (mean (SD) of 91.5 (5.45) for the control group and 90.9 (4.7) for the MEPP group). The repeated measures ANOVA demonstrated a significant improvement of perceived intelligibility over time (F = 12.24; p = 0.002), without a group effect (F = 0.057; p = 0.814).
Discussion
Globally, descriptive results in this study showed a trend toward a better and faster recovery of the MEPP group for every tested outcome measure, except for intelligibility, which was in line with our initial hypothesis. Statistical differences between the groups could be found in House-Brackmann 2.0 scores and grades, as well as on synkinesis assessments and quality-of-life measures, which suggests that the MEPP positively influenced those parameters. Also, the differences between the groups were larger in the chronic phase for most outcome measures, suggesting a long-lasting effect of early intervention. To understand the operant mechanisms of the MEPP, an in-depth look of the main study's results compared with relevant literature will be undertaken.
First, the MEPP was developed to help patients avoiding uncalibrated facial motor execution, by decreasing sensory-motor discrepancies during the flaccid phase of severe and acute Bell's Palsy.12,32 This is mainly achieved through modified visual feedback during facial movements (known as mirror therapy), as well as with optimal instructions that augment proprioceptive awareness of the face. Thus, despite the facial flaccidity, neural signature similar to the pre-morbid motor execution could be activated through the MEPP,13,33–35 avoiding a “learned paralysis”, 11 and resulting in a better facial symmetry during volitional movement.12,36,37
Second, significant differences between the groups were found in favor of the MEPP regarding synkinesis, contrary to the findings by Nicastri et al., 4 but similar to others.19,20 It must be highlighted that the differences between the groups in the present study were larger in the chronic phase of the facial palsy (i.e. at one-year post-onset), whereas Nicastri et al. followed their patients only until 6 months post-onset. Synkineses’ universally reproducible and repetitive patterns suggest that their development could be triggered by disturbed topography in the facial nerve nucleus, after regeneration of facial nerve lesions. 38 The facial nerve nucleus acts as a turntable, sending motor efferences from the central nervous system to the peripheral nervous system, and adjusting these efferences with perceived responses. By modulating the intensity of the signal through early therapy, aberrant peripheral fiber activation could be lowered, and synkinesis could be lessened. 39
Third, regarding the quality of life and functional impacts of Bell's Palsy, the present study found a significant improvement for the MEPP group compared to the control group. Those results align with our initial hypothesis and with many informal comments received by the patients, who stated that they appreciated being involved and engaged very early on in their recovery process. This also supports the hypothesis that facial rehabilitation in the acute phase probably has positive effects on anxiety, and reinforces patients’ feelings of empowerment.5,8
Forth, an improvement was observed over time in perceived speech intelligibility, but the effect was similar for both groups, as hypothesized. Data about intelligibility in early Bell's Palsy is relatively scarce and somewhat contradictory.21,40–42 Until now, speech intelligibility was mostly assessed through patient-centered questionnaires.40–42 Thus, even though patient-related outcome measures are primordial in facial palsy assessment, they are also subject to a certain variability caused by personal factors. 43 Using the perspective of naïve listeners, we showed that the intelligibility of patients with acute and severe Bell's Palsy seems to remain relatively good despite the context of severe facial palsy. Similarly, a previous study reported that patients with facial palsy did not differ from normative data using objective results of intelligibility through standardized assessment. 21 Clinically, this could mean that intelligibility is not a factor to address specifically in therapy (except if explicitly requested by the patient).
Overall, one of the strengths of this study is the follow-up of every patient until 1-year post-onset. Until now, very little literature has longitudinally studied the effects of facial palsy for as long as one-year post-onset. Above all, quality evidence-based studies about the effects of early facial retraining on synkinesis continue to be scarce.3,44 This study suggests that early retraining could potentially reduce synkinesis of moderately severe to total Bell's Palsy. Another strength of the study consists in its multidimensional assessment of facial palsy, including clinicians’ outcomes, patient-centered outcomes and observers’ outcomes (i.e., naïve judges). The observed consistency of the results through these different perspectives and measurements strongly suggests that early facial retraining is beneficial for the population of interest.
One of the weaknesses in this study relates to the relatively small sample size. A higher number of participants would have helped to generalize our results about the efficacy of the MEPP, ruling out any false positives or negatives in the statistics. Unfortunately, eight participants could not be included in the analysis after allocation. Thus, absence of significant differences between groups for the Sunnybrook global score could maybe be explained by a potential lack of statistical power, in the context of a high variance for this particular score. Another weakness of this study concerns the fact that the quality-of-life questionnaire has not been completed by our full cohort. When we started the study, no validated patient-centered questionnaire for facial palsy was available in Canadian French. This measure has thus been added to the study after the validated FaCE-F scale was made available. 28 Finally, the randomization procedure led to the inclusion of two more patients with a grade 4 severity rating (at House-Brackmann 2.0) in the MEPP group at baseline. As grade 4 patients are more likely to recover completely, 45 it cannot be excluded that it affected our results. However, it is noteworthy to mention that both groups did not statistically differ in terms of severity of facial palsy at baseline, meaning that a similar evolution could reasonably be expected as the null hypothesis, which was not the case.
In summary, the results of this study suggest that a mirror therapy, within an early facial retraining protocol as the MEPP, could support the recovery of patients suffering from acute and severe Bell's Palsy. Specifically, early facial retraining with the MEPP could support improvement in facial symmetry and reduction of synkinesis in the long run, with measurable impact at one-year post-onset. When provided in the acute phase, the MEPP also could also help patients achieving a better quality of life than counseling alone. From the perspective of naïve observers, patients with severe facial palsy did not demonstrate intelligibility issues, and the MEPP did not significantly change intelligibility ratings compared to controls. In future research, a bigger sample size with more patients rated with severity grades 5 and 6 at House-Brackman 2.0 should be recruited. Also, a direct comparison between the MEPP and other interventions (such as standard individualized facial rehabilitation) would be interesting to conduct. Finally, to overcome subjectivity caused by human-generated facial ratings using scales such as House-Brackmann 2.0 or Sunnybrook, it would be interesting to evaluate facial symmetry with objective instrumental facial metrics tools such as Emotrics. 46
Clinical messages.
Patients who receive appropriate medication but still suffer of severe Bell's Palsy after 14 days seem to benefit from the MEPP.
The MEPP significantly improved patient's quality-of-life during recovery and most probably contributed to decrease synkinesis at one-year post-onset.
The MEPP supports recovery of the facial symmetry.
Supplemental Material
Supplemental material, sj-doc-1-cre-10.1177_02692155221107090 for The “Mirror Effect Plus Protocol” for acute Bell's palsy: A randomized controlled trial with 1-year follow-up by Sarah Martineau, Akram Rahal, Eric Piette, Sami Moubayed and Karine Marcotte in Clinical Rehabilitation
Supplemental material, sj-docx-2-cre-10.1177_02692155221107090 for The “Mirror Effect Plus Protocol” for acute Bell's palsy: A randomized controlled trial with 1-year follow-up by Sarah Martineau, Akram Rahal, Eric Piette, Sami Moubayed and Karine Marcotte in Clinical Rehabilitation
Acknowledgments
The authors deeply thank our participants and their families for their commitment to this project. We also thank our precious collaborators, who participated either in recruitment, analysis or as blind judges: Anne-Marie Chouinard, Camille Rivest, Marie Julien, Khawla Kharra, Nadim T. Saydy, Laurence Gascon, Ariane Poulin and Stéphanie Des Ormeaux
Footnotes
Author note: Akram Rahal, Faculté de Médecine, Université de Montréal, Montréal, Québec, Canada.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SM holds a doctoral training scholarship and KM holds a research scholar (Junior1) both from the “Fonds de Recherche du Québec – Santé”.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Réseau Provincial de Recherche en Adaptation-Réadaptation, Fonds de Recherche du Québec – Santé (grant number 5080, 266532, 33310).
ORCID iD: Sarah Martineau https://orcid.org/0000-0001-7444-4649
Supplemental material: Supplemental material for this article is available online.
References
- 1.Luijmes RE, Pouwels S, Beurskens CH, et al. Quality of life before and after different treatment modalities in peripheral facial palsy: a systematic review. Laryngoscope 2017; 127: 1044–1051. 2016/11/20. [DOI] [PubMed] [Google Scholar]
- 2.Gyori E, Przestrzelski C, Pona I, et al. Quality of life and functional assessment of facial palsy patients: a questionnaire study. Int J Surg 2018; 55: 92–97. 2018/05/23. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira M, Marques EE, Duarte JA, et al. Physical therapy with drug treatment in Bell palsy: a focused review. Am J Phys Med Rehabil 2015; 94: 331–340. 2015/03/19. [DOI] [PubMed] [Google Scholar]
- 4.Nicastri M, Mancini P, De Seta D, et al. Efficacy of early physical therapy in severe Bell’s palsy: a randomized controlled trial. Neurorehabil Neural Repair 2013; 27: 542–551. [DOI] [PubMed] [Google Scholar]
- 5.Infante-Cossio P, Prats-Golczer V-E, Lopez-Martos R, et al. Effectiveness of facial exercise therapy for facial nerve dysfunction after superficial parotidectomy: a randomized controlled trial. Clin Rehabil 2016; 30: 1097–1107. [DOI] [PubMed] [Google Scholar]
- 6.Song W, Cao Z, Lang C, et al. Disrupted functional connectivity of striatal sub-regions in Bell’s palsy patients. NeuroImage: Clinical 2017; 14: 122–129. 2017/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature 1995; 377: 489–490. [DOI] [PubMed] [Google Scholar]
- 8.Paolucci T, Cardarola A, Colonnelli P, et al. Give me a kiss! An integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur J Phys Rehabil Med 2020; 56: 58–67. 2019/03/28. [DOI] [PubMed] [Google Scholar]
- 9.Barth JM, Stezar GL, Acierno GC, et al. Mirror book therapy for the treatment of idiopathic facial palsy. Ear Nose Throat J 2020: 145561320913211. 2020/07/25. DOI: 10.1177/0145561320913211. [DOI] [PubMed] [Google Scholar]
- 10.Martineau S, Rahal A, Piette É, et al. The mirror effect plus protocol for acute Bell’s palsy: a randomised and longitudinal study on facial rehabilitation. Acta Otolaryngol 2020: 1–6. DOI: 10.1080/00016489.2020.1842905. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain 2009; 132: 1693–1710. 2009/06/10. [DOI] [PubMed] [Google Scholar]
- 12.Martineau S, Martel-Sauvageau V, Piette E, et al. A pilot study on the mirror effect PLUS protocol: a standardized and adapted facial rehabilitation for acute Bell’s palsy. Canadian Journal of Speech-Language-Pathology and Audiology 2020; 44: 57–72. [Google Scholar]
- 13.Eaves DL, Riach M, Holmes PS, et al. Motor imagery during action observation: a brief review of evidence, theory and future research opportunities. Front Neurosci 2016; 10: 514. 2016/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maas E, Robin DA, Austermann Hula SN, et al. Principles of motor learning in treatment of motor speech disorders. Am J Speech Lang Pathol 2008; 17: 277–298. 2008/07/30. [DOI] [PubMed] [Google Scholar]
- 15.Bislick LP, Weir PC, Spencer K, et al. Do principles of motor learning enhance retention and transfer of speech skills? A systematic review. Aphasiology 2012; 26: 709–728. [Google Scholar]
- 16.Barbara M, Antonini G, Vestri A, et al. Role of Kabat physical rehabilitation in Bell’s palsy: a randomized trial. Acta Otolaryngol 2010; 130: 167–172. [DOI] [PubMed] [Google Scholar]
- 17.Diels J, Combs D. Neuromuscular retraining for facial paralysis. Otolaryngol Clin North Am 1997; 30: 727–743. 1997/10/01. [PubMed] [Google Scholar]
- 18.Monini S, Buffoni A, Romeo M, et al. Kabat rehabilitation for Bell’s palsy in the elderly. Acta Otolaryngol 2016; 137: 646–650. 2016/12/15. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara K, Furuta Y, Yamamoto N, et al. Factors affecting the effect of physical rehabilitation therapy for synkinesis as a sequela to facial nerve palsy. Auris Nasus Larynx 2018; 45: 732–739. 2017/11/05. [DOI] [PubMed] [Google Scholar]
- 20.Penteado TCB, Testa JRG, Antunes ML, et al. Évaluation de la technique Chevalier pour la prévention des séquelles dans la paralysie faciale périphérique: evaluation of the Chevalier method for the prevention of sequelae after peripheral facial nerve palsy. Kinésithérapie, la Revue 2009; 9: 40–47. [Google Scholar]
- 21.Moverare T, Lohmander A, Hultcrantz M, et al. Peripheral facial palsy: speech, communication and oral motor function. Eur Ann Otorhinolaryngol Head Neck Dis 2017; 134: 27–31. 2016/11/12. [DOI] [PubMed] [Google Scholar]
- 22.Gagyor I, Madhok VB, Daly F, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev 2015: Cd001869. 2015/11/13. DOI: 10.1002/14651858.CD001869.pub8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 24.Beurskens CH, Heymans PG. Mime therapy improves facial symmetry in people with long-term facial nerve paresis: a randomised controlled trial. Aust J Physiother 2006; 52: 177–183. [DOI] [PubMed] [Google Scholar]
- 25.Vrabec JT, Backous DD, Djalilian HR, et al. Facial nerve grading system 2.0. Otolaryngoly and Head and Neck Surgery 2009; 140: 445–450. [DOI] [PubMed] [Google Scholar]
- 26.Ross B, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngol Head Neck Surg 1996; 114: 380–386. 1996/03/01. [DOI] [PubMed] [Google Scholar]
- 27.Fattah AY, Gurusinghe AD, Gavilan J, et al. Facial nerve grading instruments: systematic review of the literature and suggestion for uniformity. Plastic Reconstructive Surgery 2015; 135: 569–579. [DOI] [PubMed] [Google Scholar]
- 28.Gascon L, Martineau S, Saltychev M, et al. French Canadian translation, cultural adaptation, and validation of facial clinimetric evaluation scale and facial disability Index questionnaires for patients with peripheral facial paralysis. 2021. DOI: 10.1089/fpsam.2020.0608. [DOI] [PubMed] [Google Scholar]
- 29.Neely JG, Cherian NG, Dickerson CB, et al. Sunnybrook facial grading system: reliability and criteria for grading. Laryngoscope 2010; 120: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay RW, Robinson M, Hadlock TA. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts eye and ear infirmary. Phys Ther 2010; 90: 391–397. [DOI] [PubMed] [Google Scholar]
- 31.Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. 4th ed. Oxford: Oxford University Press, 2008. [Google Scholar]
- 32.Klingner CM, Volk GF, Brodoehl S, et al. The effects of deefferentation without deafferentation on functional connectivity in patients with facial palsy. Neuroimage: Clinical 2014; 6: 26–31. 2014/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macuga KL, Frey SH. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage 2012; 59: 2798–2807. 2011/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt S, Di Rienzo F, Collet C, et al. Multiple roles of motor imagery during action observation. Front Hum Neurosci 2013; 7: 807. 2013/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright DJ, Williams J, Holmes PS. Combined action observation and imagery facilitates corticospinal excitability. Front Hum Neurosci 2014; 8: 951. 2014/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanchin T, Martin F, Labbe D. Lengthening temporalis myoplasty: a new approach to facial rehabilitation with the “mirror-effect” method. Ann Chir Plast Esthet 2013; 58: 632–637. [DOI] [PubMed] [Google Scholar]
- 37.Garmi R, Labbe D, Coskun O, et al. Lengthening temporalis myoplasty and brain plasticity: a functional magnetic resonance imaging study. Ann Chir Plast Esthet 2013; 58: 271–276. 2013/04/30. [DOI] [PubMed] [Google Scholar]
- 38.Slattery WH, Azizzadeh B. The facial nerve. New York: Thieme, 2014, p.236. [Google Scholar]
- 39.Angelov DN, Ceynowa M, Guntinas-Lichius O, et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol Dis 2007; 26: 229–242. 2007/02/14. [DOI] [PubMed] [Google Scholar]
- 40.Barry P, Mancini J, Alshukry A, et al. Validation of French versions of the facial disability index and the facial clinimetric evaluation scale, specific quality of life scales for peripheral facial palsy patients. Clin Otolaryngol 2019; 44: 313–322. [DOI] [PubMed] [Google Scholar]
- 41.de Swart BJ, Verheij JC, Beurskens CH. Problems with eating and drinking in patients with unilateral peripheral facial paralysis. Dysphagia 2003; 18: 267–273. 2003/10/23. [DOI] [PubMed] [Google Scholar]
- 42.Kahn JB, Gliklich RE, Boyev KP, et al. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope 2001; 111: 387–398. 2001/02/27. [DOI] [PubMed] [Google Scholar]
- 43.Kleiss IJ, Hohman MH, Susarla SM, et al. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE scale: a retrospective cohort study. Clin Otolaryngol 2015; 40: 651–656. 2015/04/11. [DOI] [PubMed] [Google Scholar]
- 44.Pereira LM, Obara K, Dias JM, et al. Facial exercise therapy for facial palsy: systematic review and meta-analysis. Clin Rehabil 2011; 25: 649–658. 2011/03/09. [DOI] [PubMed] [Google Scholar]
- 45.Marsk E, Bylund N, Jonsson L, et al. Prediction of nonrecovery in Bell’s palsy using Sunnybrook grading. Laryngoscope 2012; 122: 901–906. 20120228. [DOI] [PubMed] [Google Scholar]
- 46.Guarin D, Dusseldorp J, Hadlock T, et al. A machine learning approach for automated facial measurements in facial palsy. 2018; 20: 335–337. DOI: 10.1001/jamafacial.2018.0030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-cre-10.1177_02692155221107090 for The “Mirror Effect Plus Protocol” for acute Bell's palsy: A randomized controlled trial with 1-year follow-up by Sarah Martineau, Akram Rahal, Eric Piette, Sami Moubayed and Karine Marcotte in Clinical Rehabilitation
Supplemental material, sj-docx-2-cre-10.1177_02692155221107090 for The “Mirror Effect Plus Protocol” for acute Bell's palsy: A randomized controlled trial with 1-year follow-up by Sarah Martineau, Akram Rahal, Eric Piette, Sami Moubayed and Karine Marcotte in Clinical Rehabilitation