Highlights
-
•
Clinically useful MRI-based biomarkers for depression treatment response are lacking.
-
•
Structural predictors (hippocampal volume, WMH, and amygdala activation) are most replicated.
-
•
Network-based predictors are highly inconsistent so far.
-
•
Continual technological/methodological advancement may improve circuit-based biomarkers.
Keywords: Major depressive disorder, MRI, Antidepressant, Response, Prediction
Abbreviations: ACC, anterior cingulate cortex; ALFF, amplitude of low frequency fluctuation; BDI, Beck’s depression inventory; BOLD, blood-oxygen level dependent; CEN, central executive network; C-ON, cingulo-opercular network; CSF, cerebral spinal fluid; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; DMN, default mode network; ECN, executive control network; FA, fractional anisotropy; FC, functional connectivity; FPN, fronto-parietal network; fMRI, functional magnetic resonance imaging; HDRS, Hamilton depression rating scale; ICA, independent component analysis; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; LLD, late-life depression; MADRS, Montgomery-Asberg depression rating scale; MDD, major depressive disorder; mPFC, medial prefrontal cortex; MRI, magnetic resonance imaging; MFG, middle frontal gyrus; MTG, middle temporal gyrus; NBS, network-based statistic; OFC, orbital frontal cortex; PCC, posterior cingulate cortex; SMN, somatomotor network; SN, salience network; STG, superior temporal gyrus; VAN, ventral attention network; VBM, voxel-based morphometry; VS, ventral striatum; WMH, white matter hyperintensity
Abstract
Major depressive disorder is among the most prevalent psychiatric disorders, exacting a substantial personal, social, and economic toll. Antidepressant treatment typically involves an individualized trial and error approach with an inconsistent success rate. Despite a pressing need, no reliable biomarkers for predicting treatment outcome have yet been discovered. Brain MRI measures hold promise in this regard, though clinical translation remains elusive. In this review, we summarize structural MRI and functional MRI (fMRI) measures that have been investigated as predictors of treatment outcome. We broadly divide these into five categories including three structural measures: volumetric, white matter burden, and white matter integrity; and two functional measures: resting state fMRI and task fMRI. Currently, larger hippocampal volume is the most widely replicated predictor of successful treatment. Lower white matter hyperintensity burden has shown robustness in late life depression. However, both have modest discriminative power. Higher fractional anisotropy of the cingulum bundle and frontal white matter, amygdala hypoactivation and anterior cingulate cortex hyperactivation in response to negative emotional stimuli, and hyperconnectivity within the default mode network (DMN) and between the DMN and executive control network also show promise as predictors of successful treatment. Such network-focused measures may ultimately provide a higher-dimensional measure of treatment response with closer ties to the underlying neurobiology.
1. Introduction
Major Depressive Disorder (MDD) is among the most ubiquitous psychiatric disorders affecting nearly 300 million people worldwide (Whiteford et al., 2010), with the highest disability-adjusted life years of any mental illness (Murray et al., 2012). Its adverse effects are felt from the individual to the societal level, impacting quality of life (Brenes, 2007), incurring a $210 billion economic burden (Greenberg et al., 2015), and, in up to 8% of depressed individuals, resulting in suicide (Strakowski and Nelson, 2015). As a first-line treatment, pharmacotherapy has an eventual success rate between 54% and 71% (Fava and Davidson, 1996). However, as explicitly stated in one clinician’s guide (Eisenberg Center at Oregon Health & Science University, 2007), “There is no evidence to guide selection of the best initial drug for an individual.” Hence, antidepressant treatment assumes a trial-and-error approach, with medication selection based on physician and patient preferences, an approach that may prolong the course of the disease through unsuccessful trials. Given the negative outcomes associated with depression, there is a pressing need for development of biomarkers of treatment response.
MDD is a highly heterogeneous disorder, both phenomenologically and pathophysiologically (Monroe and Anderson, 2015), with an equally unpredictable treatment outcome. This poses a serious challenge for the development of clinically relevant biomarkers, and it is unlikely that any single biomarker will provide substantial predictive value across the multi-dimensional spectra of depression. Nevertheless, by focusing on specific neurobiological facets of MDD and treatment response, we may be able to advance prediction science toward better clinical outcomes.
One important source of heterogeneity within depression is differentiation across the life span. Adolescence, mid-life, and late-life show marked differences in pathophysiology, clinical profiles and neurobiological makers (Kessler, 2010). Late-life depression (LLD) is particularly pernicious, as it carries a longer treatment response time together with a higher relapse rate (Aizenstein et al., 2014). There is also evidence of distinct neural differences in LLD versus mid-life depression (Aizenstein et al., 2014). Cognitive decline, neurodegeneration, and increased cerebrovascular burden may all play a more important role in LLD.
There is no single modality capable of capturing the complex pathophysiology underlying treatment response in MDD. Neuroimaging, gene expression, proteomics, neuroendocrinology and other modalities all may contribute in varying extents and interrelatedly toward the goal of understanding treatment response (Breitenstein et al., 2014). Magnetic resonance imaging (MRI) is the most widespread neuroimaging method owing to its full brain coverage, spatial resolution, and non-invasiveness. Furthermore, functional MRI (fMRI) gives a window into the temporal dynamics of brain activity through the blood-oxygen level dependent (BOLD) signal. With MRI, structural differences of both grey and white matter, regional activation in response to stimuli or task, or regional correspondence of spontaneous brain activity can provide a window into the neurobiological differences that influence depression treatment response.
This naturalistic review aims to assemble and contextualize the most relevant and recent literature using structural and functional MRI to predict antidepressant treatment outcome. We briefly describe our search constraints and methods and then summarize the literature of three major structural predictors: volumetric analysis, white matter hyperintensity (WHM) burden, and white matter integrity; and two major functional predictors: resting state functional connectivity (FC) and task activation. We conclude with a discussion of future directions in prediction science.
2. Methods
The focus of this review is on imaging studies of pharmacotherapy response in non-psychotic MDD (including adult and geriatric, but not adolescent populations). Primary treatment must have been by pharmacotherapy with FDA-approved antidepressants as this is the most common treatment approach (Practice guideline for the treatment of patients with major depressive disorder (revision), 2000). Ketamine and other less common or investigational drugs are not included. Studies that employ a naturalistic treatment paradigm are included. Psychotherapy is a common and effective alternative to antidepressant treatment. However, given the more direct biological pathway of antidepressants and emerging evidence of differences in neurobiological mechanisms between antidepressants and psychotherapy (Treatment outcomes and neural mechanisms, 2008), we restrict our focus to studies of antidepressant treatment. For all the reviewed studies, at minimum, a baseline pre-treatment MRI scan must have been performed. Finally, for MRI, we include all structural and functional modalities. MR spectroscopy is not considered in this review.
This review considers both outcome prediction from baseline MRI measures alone and prediction from longitudinal changes in MRI measures during treatment. Both have clinical utility and provide insights into neurobiological mechanisms, though they differ in subtle, but important ways. Given equal predictive capacity, baseline prediction is clinically preferable as it does not require additional visits or a time delay in guiding treatment selection. However, the biomarkers are more associative in nature and therefore do not provide information about the neurobiological changes associated with the dynamic clinical course. Longitudinal studies, in contrast, examine MRI-measurable changes that take place over the course of treatment. This approach provides valuable insight about changes in the neurobiological processes associated with successful treatment. However, they are less practical for acute clinical prediction of treatment outcome since they require multiple scans. Throughout this review, we use increase/decrease to specifically imply longitudinal changes.
One primary challenge is the heterogeneity of study designs. While an impediment to data synthesis, it can also provide valuable explanations for seemingly contradictory results. For this reason, we restrict ourselves to a review rather than a meta-analysis. However, we do provide a subjective classification of evidence for specific findings in the literature as none, weak, moderate or strong based off the number, size, and quality of studies reporting 1) an effect, 2) a null finding, and 3) an effect in the opposite direction. One primary source of heterogeneity is in the treatment outcome definition. Many of the studies define remission as achieving a Hamilton Depression Rating Scale (HDRS) score less than or equal to 7 or Montogmery-Asberg Depression Rating Scale (MADRS) score less than 10. This is often accompanied by the requirement that it must remain below those cutoffs for at least 2 weeks. Response is usually defined as at least a 50% reduction in the HDRS or MADRS. As a continuous measure, change in HDRS or MADRS (relative or absolute) is also frequently analyzed and will be referred to as symptomatic improvement. Still other studies report group-level effects for the whole cohort, regardless of clinical outcome. These findings in particular are difficult to interpret. Time to achieve response/remission is yet another source of heterogeneity, with trials of 6 to 12 weeks being the most common. Remission and response will be used without further clarification in this paper if they fall within these guidelines. Furthermore, ‘treatment outcome’ will be used as an umbrella term for remission, response, and symptomatic improvement. Most of the studies included in this review are open-label, so treatment outcome is generally susceptible to placebo effects. As a final nomenclature note, we specifically label studies as small or large if the number of depressed participants does not exceed 20 or is at least 100, respectively. A lack of remark on the study size implies the sample size lies somewhere between these bounds (20< n <100).
The literature search was conducted on MEDLINE with the following queries:
((depression[Abstract] OR depressed[Abstract]) NOT bipolar[Abstract]) AND
(MRI[Abstract] OR volume[Abstract] OR structure[Abstract] OR structural[Abstract]) AND.
treatment[Abstract] NOT
(adolescent[Abstract] OR adolescence[Abstract]).
((depression[Abstract] OR depressed[Abstract]) NOT bipolar[Abstract]) AND
(diffusion[Abstract] OR DTI[Abstract]) AND
treatment[Abstract] NOT
(adolescent[Abstract] OR adolescence[Abstract]).
((depression[Abstract] OR depressed[Abstract]) NOT bipolar[Abstract] AND
(fMRI[Abstract] OR functional magnetic resonance[Abstract] OR functional MRI[Abstract]) AND
treatment[Abstract] NOT
(adolescent[Abstract] OR adolescence[Abstract]).
3. Structural predictors
Structural MRI is particularly appealing for clinical implementation owing to the simplicity of acquisition and (often) ease of interpretation. The association of structural MRI measures with treatment outcome most commonly fall into three broad categories: (1) volumetric analysis, (2) WMH burden, and (3) regional or tract-based white matter integrity.
3.1. Volumetric analysis
Volumetric analysis is the most frequently reported structural measure for predicting treatment outcome. Early approaches typically relied upon manual segmentation of a small number of structures, most often the hippocampus. The focus on the hippocampus is owed to the stress-toxicity hypothesis of depression, which posits that depression-related chronic hypercortisolemia may decrease the hippocampal volume over time (McEwen, 1992). Methodological advances, primarily voxel-based morphometry (VBM), allowed for whole-brain volumetric analysis without manual segmentation (Ashburner and Friston, 2000). Machine learning methods have provided yet another avenue for prediction from structural images without pre-defining the features of interest. All reported volumes are baseline measures before commencing antidepressant treatment unless otherwise noted. Results are summarized in Table 1 and Fig. 1.
Table 1.
Summary of volumetric predictors of treatment outcome.
| Region | Baseline | Longitudinal Changes |
|---|---|---|
| Hippocampus | Strong evidence for association between larger hippocampal volume and positive treatment outcome, effect localized to body/tail, mixed results for lateralization | Moderate evidence for association between increases in hippocampal volume and positive treatment outcome, left lateralization, various time scales. |
| Amygdala | None reported despite targeted investigations | None reported |
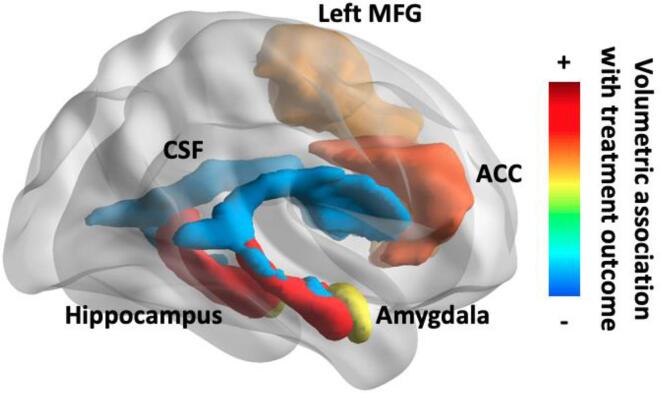
| ACC | Moderate evidence for association between larger ACC volume and positive treatment outcome | None reported |
| CSF | Weak evidence for association between smaller CSF volume and positive treatment outcome | None reported |
| Middle frontal gyrus | Weak evidence for association between larger hippocampal volume and positive treatment outcome | None reported |
| Whole-brain | No association with treatment outcome | None reported |
Fig. 1.
Brain map of volumetric predictors of treatment outcome with subjective scale. ACC = anterior cingulate cortex, CSF = cerebral spinal fluid, MFG = middle frontal gyrus. Created with BrainNet Viewer (Botvinik-Nezer et al., 2020).
3.1.1. Hypothesis-driven volumetric results
We found fourteen studies examining hippocampal volume as a predictor of treatment outcome. The finding that greater baseline hippocampal volume is associated with better outcome has been highly replicated, with only four studies (two large recent studies in mid-life (Bartlett et al., 2018) and LLD (Ahmed et al., 2022), a another LLD study (Khalaf et al., 2015), and a small mid-life study(Toki et al., 2014)) failing to find such an effect, all in the context of wider biomarker investigation. The specifics, however, differ across the studies. A large LLD study reported larger hippocampal volume was associated with remission, as well faster treatment response (Sheline, 2012). The predictive value of hippocampal volume for remission has been localized to the hippocampal body and tail, but not head (MacQueen et al., 2008). More recently, the hippocampal tail finding has been replicated with increasingly finer parcellations of the hippocampus in large samples(Maller, 2018, Nogovitsyn et al., 2020). A recent naturalistic study found both left and right hippocampal volume predicted remission in a treatment-naïve cohort, which removes the confound of antidepressant history (Zarate-Garza, 2021). Hippocampal volume has been examined with and without normalization by the whole-brain volume. Two studies compared normalized and unnormalized hippocampal volumes directly and found that normalization strengthened the differentiation between remitters and non-remitters (Vakili, 2000), (Hsieh, 2002). Interestingly, these are the same two studies that noted a stronger association of right hippocampal volume to remission. In fact, one of the studies found only the right hippocampal volume predicted remission, an effect which was limited to women (Vakili, 2000). The other study, consisting of a geriatric cohort, found both total and right, but not left, hippocampal volume was positively associated with treatment response (Hsieh, 2002). Furthermore, this finding was only for the upper and lower quartiles, indicating that the predictive power of hippocampal volume may be concentrated at the ends of the spectrum.
Longitudinal studies that have examined volumetric changes in depressed participants have reported mixed results. Two studies failed to find an association between remission status and changes in hippocampal volume: one twelve week trial in LLD did, however, report nearly significant increases in hippocampal volume for remitters and decreases for non-remitters (Khalaf et al., 2015) and one year-long trial in mid-life (Frodl, 2004). Three other studies did report such an association, all implicating the left hippocampus. In one study following depressed patients over a three year period, only the left hippocampus showed a significant increase in a subgroup of patients who remained on antidepressant throughout the trial, regardless of remission status (Frodl, 2008). Similarly, a study integrating predictors from structural and functional MRI found that an increase in left hippocampal volume one week after treatment onset predicted remission (Fu et al., 2015). Another study in LLD found that the only significant change in left and total (but not right) hippocampal volume over two years was a decrease in the nonremitters (Taylor, 2014).
Volumetric analysis for predicting treatment outcome has also targeted the amygdala, anterior cingulate cortex (ACC), cerebral spinal fluid (CSF), as well as the whole brain. None of the studies we reviewed found an association between amygdala volume and remission (Frodl, 2004, Frodl, 2008, Fu et al., 2015). This should not be interpreted as an indictment of the amygdala’s role in treatment response (see Functional Predictors section), but merely an indication that functional and structural markers may reflect different pathophysiological features of MDD. Similarly, ACC volume and cortical thickness have been investigated as biomarkers of treatment outcome owing to the region’s key role in emotion regulation (Handbook of emotion regulation, 2014). Two LLD studies focused specifically on ACC volume reported that larger dorsal and rostral (but not subgenual) ACC volumes were associated with remission (Gunning-Dixon, 2009) and larger posterior subgenual ACC volume was associated with improvement of apathy (Yuen, 2014). However, a recent large study in mid-life depression found no baseline difference in rostral or caudal ACC volume between remitters and non-remitters (Bartlett et al., 2018). With enlarged CSF spaces representing a potential marker of brain atrophy, CSF volume has also been explored as a predictor of treatment outcome. A large study reported that greater volume of the third ventricle is associated with non-remission/response (Nogovitsyn et al., 2020), while another study in late-onset depression noted a trend of greater hemispheric and total CSF volume in non-responders that did not rise to the level of statistical significance (Baldwin, 2004). Remission status does not appear to be associated with whole-brain volume (Nogovitsyn et al., 2020) or cortical thickness (Suh, 2020) in mid-life or whole-brain volume in late-life (Khalaf et al., 2015), (Hsieh, 2002), though one study on treatment-resistant depression did find that an increase in whole-brain volume over a 6-month course of treatment was associated with remission (Phillips et al., 2012).
3.1.2. Data-driven volumetric results
A more general approach to identify neuroimaging predictors of treatment outcome is to analyze structural MRI without a predefined region of interest. This avoids the pitfalls associated with the streetlight effect, but presents a more challenging problem, particularly with respect to controlling false positives. One early study employed thresholding and permutation testing to voxel-wise regression and found that faster symptomatic improvement was correlated with larger grey matter volumes in the ACC, insula, and right temporo-parietal cortex in a small sample (Chen, 2007). A study using whole-brain principal component analysis for data-reduction and penalized regression for feature selection found greater cortical thickness in the inferior and middle frontal gyrus predicted remission at 16 weeks (Motter, 2021). Most whole-brain searches, however, have relied upon VBM, which incorporates multiple comparisons corrections via random field theory directly into the methodology (Ashburner and Friston, 2000). The resulting spatial maps have been used in a plethora of ways to predict treatment outcome. Two studies reported that larger right ACC volumes were indicative of clinical remission (Costafreda et al., 2009), (Serra-Blasco, 2016). Higher volume of the left middle frontal gyrus has been associated with remission in small (Costafreda et al., 2009) and large studies (Korgaonkar et al., 2015), whereas lower volume of the right angular gyrus was associated with remission. Of note, a large study found that larger bilateral posterior cingulate cortex (PCC) and left hippocampal volumes and smaller right middle and superior temporal gyrus (STG) volumes predicted faster treatment response (Sämann, 2013). Smaller right inferior and middle temporal gyrus (IFG/MFG) volumes at baseline, as well as decreases in left dorsolateral prefrontal cortex (dlPFC) volume, have also been associated with remission (Li et al., 2010). This latter finding stands in contrast to a small longitudinal study that found increasing dlPFC volume correlated with a reduction in Beck’s Depression Inventory (BDI) scores over the course of antidepressant treatment (Smith et al., 2013). Unfortunately, most other results from the seven VBM studies have not been replicated.
3.2. White matter hyperintensity burden
Vascular depression has been proposed as a subtype of depression (Krishnan et al., 1997) under the hypothesis that “cerebrovascular disease may predispose, precipitate, or perpetuate some geriatric depressive syndromes.” (Alexopoulos, 1997) WMH are a key component of the vascular depression hypothesis for LLD (Taylor et al., 2013) as they are considered a marker of cerebral vasculopathy. However, the pathology associated with WMH is mixed, consisting of microinfarcts, gliosis, increased perivascular spaces, and inflammation (Merino, 2019). Before an automated method quantifying and localizing WMH with T2-weighted FLAIR images was developed (Wu, 2006), analysis required hand-scoring of WMH burden by neuroradiologists. Most studies have found greater regional WMH to be significantly associated with poor outcome, while none have reported greater WMH to be associated with better outcome. Global WMH studies were inconclusive, with two studies reporting higher WMH predicted remission(Gunning-Dixon, 2010, Sneed, 2011) and three reporting no association between global WMH burden and remission (Khalaf et al., 2015), (Iosifescu, 2006, Karim, 2017), one of which was the only WMH study to focus on mid-life depression (Iosifescu, 2006). Two other studies, one large, failed to find an association between global measures of WMH and symptomatic improvement(Salloway et al., 2002, Sneed, 2007), while another large study reported that global WMH burden predicted symptomatic improvement, but not after controlling for baseline depression severity (Sheline, 2010). On the other hand, four studies reported a significant association with poor treatment outcome and deep WMH (Sneed, 2011), (Patankar, 2007, O'Brien et al., 1998, Bella et al., 2010) (generally defined as WMH away from ventricular structures), two others reported an association that was not significant after controlling for age (Baldwin, 2004), (Iosifescu, 2006), and one study found no such association (Motter, 2021). Similarly three studies reported a significant association between periventricular WMH and poor treatment outcome (Baldwin, 2004), (Sneed, 2011, Patankar, 2007), one reported an association that was not significant after controlling for age, and two studies found no such association (Motter, 2021), (O'Brien et al., 1998). The only study we found examining longitudinal changes in WMH reported a significant increase in global WMH in non-remitters, but not in remitters (Khalaf et al., 2015). Results are summarized in Table 2.
Table 2.
Summary of WMH predictors of treatment outcome.
| Region | Baseline Association Treatment | Longitudinal Changes with Treatment |
|---|---|---|
| Deep | Strong evidence for association between lower deep WMH burden and positive treatment outcome in LLD, age effects may be important | None reported. |
| Periventricular | Moderate evidence for association between lower deep WMH burden and positive treatment outcome in LLD | None reported. |
| Whole-brain | Weak evidence for association between lower global WMH burden and positive treatment outcome in LLD, most studies report none | Weak evidence for increases in non-responders in LLD. |
3.3. White matter integrity
Diffusion-weighted MRI quantifies directional coherence of fluid flow that can be used to quantify white matter integrity, most frequently measured in fractional anisotropy (FA). Diffusivity measures may offer complementary information (Alexander et al., 2007), though they have been infrequently investigated in depression treatment response. Early studies tended to examine FA based on regional definitions (e.g., proximity to ACC), which may lack a clear interpretation when the regions are generally defined by grey matter. More recent studies tend to target predefined white matter tracts (e.g., cingulum). Results are summarized in Table 3 and Fig. 2.
Table 3.
Summary of white matter integrity predictors of treatment outcome.
| Region | Baseline Association Treatment | Longitudinal Changes with Treatment |
|---|---|---|
| Frontal | Moderate evidence for association between higher FA of various frontal white matter tracts and positive treatment outcome, may be specific to LLD | None reported |
| Cingulate | Moderate evidence for association between higher FA of cingulum and positive treatment outcome | None reported |
| Whole-brain | None reported | None reported |
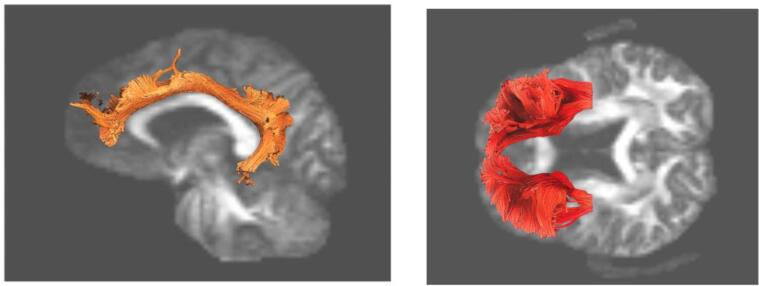
Fig. 2.
Brain map of white matter tract predictors of treatment outcome. Higher FA of both the cingulum (left) and frontal (right) white matter tracts are associated with better treatment outcome. Created with DSI Studio (https://dsi-studio.labsolver.org/).
In a series of LLD studies, remission was shown to be associated with higher FA in frontal white matter 15 mm above the anterior commissure-posterior commissure plane (small study) (Alexopoulos et al., 2002), higher FA in the dlPFC (Alexopoulos, 2008), and lower FA in the superior frontal gyrus (Taylor et al., 2008). Another study found that higher FA of the bilateral inferior fronto-occipital fasciculus, right superior fronto-occipital fasciculus, and internal capsule was associated with greater reduction in HDRS after 6 months of augmentation therapy but not placebo (Krause-Sorio et al., 2020). A longitudinal study of LLD did not find any changes over one year in frontal white matter FA associated with remission, but did report that decreases in FA of the ACC were associated with remission (Taylor, 2011). Higher baseline FA of the ACC has been reported to have both positive (Alexopoulos, 2008) and negative (Taylor et al., 2008) associations with remission status as well as a negative association with residual negative self-referential thinking in a small LLD study (Victoria, 2019). This latter study further reported reduced negative self-referential thinking associated with higher baseline FA of uncinate fasciculus (Victoria, 2019). Changes in whole-brain FA do not appear to differentiate between remitters and non-remitters in LLD (Khalaf et al., 2015).
In mid-life depression, one large study (iSPOT-DWilliams et al. (2011)) identified the cingulum as the primary predictor of remission through a series of analyses. Using half the sample for testing, decision trees applied to 46 major white matter tracts showed that larger left cingulum FA was the primary predictor of remission, though lower FA of the right superior fronto-occipital fasciculus and higher FA of the right superior longitudinal fasciculus also predicted remission (Korgaonkar et al., 2015). A more focused study on ACC-limbic tracts found that remission status was positively associated with FA in the cingulum and negatively associated with FA in the stria terminalis (Korgaonkar et al., 2014), then followed this up by showing that the ratio of FA in the stria terminalis and cingulum predicts nonremission in another sample (Grieve et al., 2016). A large study examining mean, axial, and radial diffusivity as well as FA found that higher axial diffusivity of the left external capsule, FA of the right superior corona radiata, and FA of the peripheral skeleton were significantly associated with treatment response (Davis, 2019). FA of specific hippocampal white matter tracts linking to the PCC (Korgaonkar et al., 2014) and raphe nuclei (DeLorenzo et al., 2013), (Pillai, 2019) were not associated with treatment response. One of these studies did find higher FA of white matter between the amygdala and raphe nuclei predicted remission status (DeLorenzo et al., 2013), but a replication study in a much larger cohort by the same group found the opposite effect, which was even greater in the placebo group (Pillai, 2019). Longitudinal studies have found that increases in FA (and associated decreases in radial diffusivity) of the forceps minor and superior longitudinal fasciculus were associated with treatment response over 12 weeks (Vieira, 2021) and increases in FA of the right cingulum bundle were associated with symptomatic improvement (Bracht et al., 2015).
4. Functional predictors
Functional MRI adds a temporal layer of variability over structural MRI by examining the blood-oxygen-level-dependent signal as a proxy for neuronal activity. While this increases the complexity of acquisition and analysis, it adds a very informative dimension to the data. fMRI acquisition may be unconstrained, as in resting state, or task-oriented to target specific brain functions and associated regions/networks. This allows a more targeted probing of specific neural circuitry expected to underlie treatment response. We focus on papers published from 2010 onward, after the infamous dead salmon paper shined a very bright (and refreshingly lighthearted) spotlight on the multiple comparisons problem in neuroimaging (Bennett et al., n.d.). Studies from prior to 2010 are discussed if they made a reasonable attempt to control for false positive errors and the findings have since been replicated.
4.1. Resting state fMRI
Resting state fMRI has been a particularly fruitful tool for exploring the intrinsic functional organization of the brain (Smith, 2009), despite challenges regarding reproducibility (Botvinik-Nezer et al., 2020). We broadly divide resting state analysis into two major classes: seed-based and network-based. The results of seed-based analysis are easily interpretable, though if the number of regions is large, multiple comparisons corrections can limit the power of detection. Network-based analysis relies on various data reduction techniques, most popularly independent component analysis (ICA) (McKeown et al., 2003) and graph theory approaches (Wang et al., 2010). These approaches typically limit the number of statistical tests, circumventing the multiple comparisons problem. However, interpretation of the results requires more nuance. It is important to note that intrinsic brain networks are not concretely defined. Nomenclature varies across the literature with significant overlap. While the default mode network (DMN) as a construct is ubiquitous in the literature, other networks have competing nomenclature. Of particular relevance to depression are the other two networks that, with the DMN, comprise the triple network model (Menon, 2011): the executive control network (ECN) and salience network (SN). There is considerable overlap both spatially and functionally of the ECN with the central executive network (CEN), cognitive control network, and fronto-parietal network (FPN) and also of the SN with the cingulo-opercular network (C-ON) and ventral attention network (VAN) (Uddin et al., 2019). Results are summarized in Table 4 and Fig. 3.
Table 4.
Summary of resting state predictors of treatment outcome.
| Region | Baseline Association Treatment | Longitudinal Changes with Treatment |
|---|---|---|
| DMN (PCC) | Mixed evidence for association between both higher and lower DMN FC and positive treatment outcome, heavily investigated | Mixed evidence for association between both increased and decreased FC and positive treatment outcome, heavily investigated |
| SN (ACC) | None reported | None reported |
| ECN (dlPFC) | Weak evidence for association between higher ECN FC and positive treatment outcome | None reported |
| DMN – ECN | Moderate evidence for association between higher DMN-ECN FC and positive treatment outcome, consistent results but few studies | Moderate evidence for association between decreased DMN-ECN FC and positive treatment outcome, consistent results but few studies |
| ECN – SN | Weak evidence for association between higher ECN-SN FC and positive treatment outcome, LLD specific | None reported |
| SN – DMN | Moderate evidence for association between higher SN-DMN FC and positive treatment outcome | None reported |
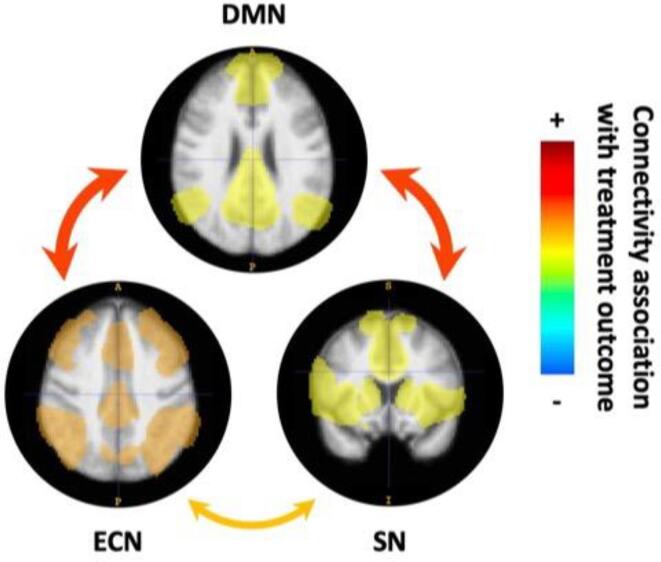
Fig. 3.
Resting state network fMRI predictors of treatment outcome with subjective scale. Within network connectivity is indicated by the network maps, between network connectivity is indicated by arrows. DMN = default mode network, ECN = executive control network, SN = salience network. Created with itk-SNAP (Yushkevich et al., 2006).
4.1.1. Seed-based resting state results
Posterior Cingulate Cortex. All studies but four investigating seed-based resting state biomarkers for treatment outcome included a seed in the PCC, a central DMN hub. Unfortunately, there is little consistency in the results. Lower baseline FC between the PCC and the striatum in LLD (Andreescu et al., 2013); pregenual ACC and medial prefrontal cortex (mPFC) (Goldstein-Piekarski et al., 2018), and right STG in a large study (van der Wijk et al., 2022) predicted remission, as did greater FC between the PCC and the precuneus, cerebellum, STG, orbital frontal cortex (OFC), dorsal and pregenual ACC, and left fusiform gyrus (van der Wijk et al., 2022). Lower FC between the PCC and the subcallosal cortex has also corresponded to symptomatic improvement in a small study (Kozel et al., 2011). Longitudinal studies in LLD have found remission status is associated with increasing FC between the PCC and subgenual ACC/dorsomedial prefrontal cortex (dmPFC) (small study) (Wu et al., 2011) and bilateral ITG/MTG/fusiform gyrus (Karim, 2017) as well as decreasing FC between the PCC and right inferior frontal and middle gyrus, and right supramarginal gyrus (Karim, 2017). Additionally, a mid-life study reported decreasing FC between the PCC and the right lateral parietal lobe and right ITG with antidepressant treatment but not placebo, regardless of clinical outcome (Posner et al., 2013). These heterogeneous findings may reflect the instability of finding in small samples, the challenges in mapping a highly heterogenous clinical construct on a region, or the multiple functions supported by PCC beyond its role as a hub of the DMN.
Dorsolateral Prefrontal Cortex. As a central hub of the ECN, FC of the dlPFC has also been explored, though far less extensively than the PCC. One LLD study reported greater connectivity between the dlPFC and the dorsal ACC and inferior parietal lobe predicted remission (Alexopoulos, 2012), while another large study in mid-life found no significant association between the dlPFC FC and remission (van der Wijk et al., 2022). A longitudinal study in LLD, however, did find that increasing FC between the dlPFC and the right pre- and postcentral gyrus and decreasing FC between the dlPFC and right MTG and occipital gyrus were associated with remission (Karim, 2017).
Anterior Cingulate Cortex. Given that the ACC has been heavily implicated in task-based indicators of treatment outcome, it is surprising that it has not received as much attention as the PCC in the resting state literature. Complicating matters, the ACC has a particularly diverse set of functions and connections within the brain (Rolls, 2019), requiring finer subdivisions. For example, the dorsal ACC is considered a primary hub of the SN (Menon and Uddin, 2010), while the pregenual and subgenual ACC are strongly affiliated with the DMN (Greicius, 2007), and the posterior ACC is considered to be a minor region of the ECN (Uddin et al., 2019). Successful treatment has been associated with lower baseline connectivity between the subgenual ACC and the dorsal ACC (small study) (Kozel et al., 2011), ventromedial PFC (large study) (Dunlop, 2017), and PCC (Goldstein-Piekarski et al., 2018). One large study found that higher connectivity between the pregenual ACC and the cerebellum, PCC, and right occipital cortex and lower connectivity to the dlPFC, anterior medial PFC, right subgenual ACC, right caudate nucleus, right STG, left insula, and right extrastriate cortex differentiates early remitters from late remitters (van der Wijk et al., 2022). Another study reported deficits in subgenual ACC to dlPFC and dorsal ACC to right amygdala connectivity between depressed participants and healthy controls that normalized with treatment, but did not directly relate this to remission or response status (Zhang, 2021, Zhang, 2021). Finally, examination of dynamic FC in a large first-episode study reported that the pregenual ACC maintains a more stable connectivity pattern in responders than non-responders (Tian, 2019).
Limbic Regions. Connectivity of the limbic regions has also been investigated for prediction of treatment outcome. FC between the insula, a hub of the SN, and cerebellum, extrastriate cortex, and right operculum was negatively associated with remission status, while connectivity to the left angular gyrus, putamen, mPFC, and parahippocampal gyrus was positively associated in a large study (van der Wijk et al., 2022). Additionally, a study in LLD reported higher baseline FC between the insula and left inferior/middle frontal gyrus was associated with remission (Karim, 2017). A study focused on reward circuitry reported a normalizing (compared to healthy controls) decrease of FC between the right striatum and left superior frontal gyrus, right caudate nucleus and left precuneus, and right superior ventral striatum (VS) and left inferior parietal lobe, as well as a normalizing increase of FC between the right inferior VS and left cerebellum after treatment, regardless of treatment outcome (Wang, 2019). Finally, one study focused solely on hippocampal connectivity reported FC between the left hippocampus and left inferior frontal gyrus and precuneus was associated with response after two weeks (Xiao, 2021).
4.1.2. Network-based resting state results
ICA offers a fundamentally different approach to FC, focusing on data-driven networks rather than region-wise connectivity. One study identified higher baseline FC in anterior and posterior DMN in depressed compared to healthy controls; regardless of outcome, antidepressant treatment normalized the connectivity in the posterior, but not in the anterior DMN (Li, 2013). Another study found that lower baseline FC of a subgenual ACC/orbitofrontal DMN subnetwork was associated with greater symptomatic improvement. Further, this study reported an overall decrease in connectivity between the DMN and bilateral prefrontal regions, auditory processing cortex, and visual/extrastriate cortex as well as increasing connectivity with the rostral ACC, frontal pole, right hippocampus, parahippocampus, angular gyrus, and middle occipital gyrus (Fu et al., 2015), irrespective of outcome. The ECN also differentiated treatment response, with higher FC to the posterior DMN, somatomotor network (SMN), and somatosensory association cortex indicating better response (Martens et al., 2021). Finally, a study on placebo effects found that SN connectivity in the rostral ACC predicted symptomatic improvement, though the effect was demonstrated to be primarily due to the placebo lead-in (Sikora, 2016).
The network-based statistic (NBS) (Zalesky et al., 2010), a graph-based approach to control the family-wise error rate for testing the connectome conceptually similar to cluster thresholding, has become an increasingly popular tool for discriminating important connections. A large study employing NBS found the DMN was the primary distinguisher between remitters and non-remitters with the following associated with remission: greater within DMN connectivity, greater connectivity between the DMN, FPN, and SMN, greater connectivity between the DMN and visual, limbic, auditory and VAN, and greater connectivity between the FPN/SMN and C-ON/dorsal attention network (Korgaonkar et al., 2020). Another study employing NBS noted that the DMN, VAN, and FPN were the primary predictors remission and response, though the method precluded an overall directionality of the effect (Klöbl, 2020). A large study investigating intra- and inter-network connectivity strength reported that greater internal DMN connectivity and lower external ECN connectivity predicted symptom improvement (Fan et al., 2020). In the same large cohort, one week decreases in DMN connectivity, increases in CEN to SMN connectivity, increases in the global connectivity of the bilateral caudate nucleus and right rostral ACC, and decreases in the global connectivity of the left SMN were associated with treatment response compared to placebo (Nemati et al., 2020). A few studies have used other traditional graph theoretic summary measures. A study in LLD reported that whole-brain eigenvector centrality, a measure of a region’s influence/degree of connectivity in a given network, was positively associated with remission in the left inferior frontal gyrus and negatively associated in the right parahippocampus, right fusiform gyrus, and left caudate nucleus (Karim, 2018). It has also been reported that degree centrality, a measure of total FC strength, increases in the hippocampus and decreases in the dmPFC over the course of treatment are associated with symptomatic improvement (Wang, 2015) and that global characteristic path length, a measure of network efficiency, is negatively associated with reduction in depressive symptoms (large study) (Zhang, 2021, Zhang, 2021). Finally, a study investigating longitudinal changes in DMN subsystems reported that average connectivity within the core DMN network increased with treatment (normalized relative to healthy controls) without regard to treatment outcome (Cui et al., 2021).
4.1.3. Miscellaneous resting state results
We found five studies that do not fit into the above methodological categories. A large study examining pair-wise FC as a moderator between treatment and placebo arms found that higher connectivity within the DMN and between the DMN and ECN was predictive of symptomatic improvement (Chin Fatt, 2020). Normalized amplitude of low frequency fluctuations (ALFF), a measure of regional activity strength in the canonical resting state frequency range, in the right lingual gyrus was reported to be positively associated with symptomatic improvement, while cerebellar ALFF showed a negative association (Wang et al., 2014). Another study in LLD employing ALFF reported that responders had greater ALFF in the dmPFC and lower ALFF in the ventromedial PFC and subgenual ACC compared to non-responders (Emam et al., 2019). Baseline functional stability, a measure of the consistency of FC over time, of the ACC, calcarine sulcus, and middle occipital gyrus was reported to be associated with remission status in a large study (Li et al., 2021). Finally, voxel-mirrored homotopic connectivity, a measure of inter-hemispheric coordination, was employed to find a positive association between higher inter-hemispheric coordination in the precuneus and ITG and remission status and a negative association in the middle frontal gyrus and caudate nucleus (Hou et al., 2016).
4.2. Task-based fMRI
Task-based imaging putatively engages specific neural processes of interest, eliciting a more targeted neural response. In depression, tasks typically invoke emotion reactivity and regulation, cognitive control, and reward processing. Analysis can focus either on activation (increased BOLD signal) during a particular aspect of the task or on identifying contrasts between different portions of the task. Resulting activation or contrast maps are typically fed into a general linear model to identify associations with variables of interest, such as treatment outcome. Results are summarized in Table 5 and Fig. 4.
Table 5.
Summary of task activation predictors of treatment outcome.
| Region | Baseline Association Treatment | Longitudinal Changes with Treatment |
|---|---|---|
| Amygdala | Strong evidence for association between lower amygdala activation and positive treatment outcome in emotional tasks with negative stimuli | Moderate evidence for association between decreased amygdala activation and positive treatment outcome in emotional tasks with negative stimuli |
| subgenual ACC | Weak evidence for association between higher sgACC activation and positive treatment outcome in emotional tasks with negative stimuli | None reported |
| pregenual ACC | Moderate evidence for association between higher pgACC activation and positive treatment outcome in emotional tasks with negative stimuli and cognitive tasks | None reported |
| dorsal ACC | Weak evidence for association between lower dACC activation and positive treatment outcome in emotional tasks with negative stimuli | Mixed evidence for association between increased and decreased dACC activation and positive treatment outcome in emotional tasks with negative stimuli |
| PCC | Weak evidence for association between higher PCC activation and positive treatment outcome in emotional tasks with negative stimuli | Weak evidence for association between decreased PCC activation and positive treatment outcome in emotional tasks with negative stimuli |
| dlPFC | Weak evidence for association between higher dlPFC activation and positive treatment outcome in cognitive tasks | Weak evidence for association between decreased dlPFC activation and positive treatment outcome in cognitive tasks |
| Insula | None reported | None reported |
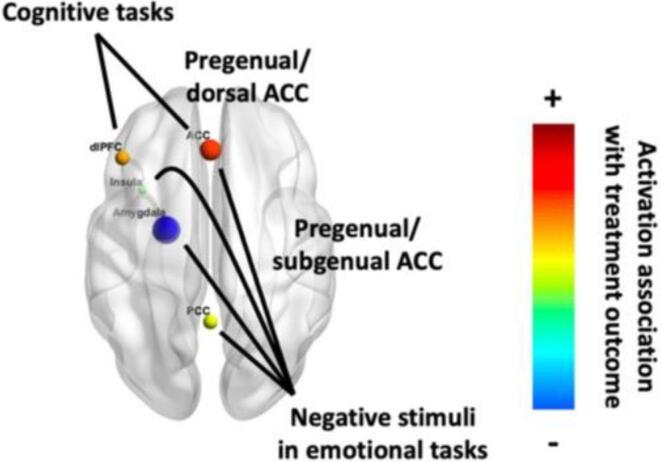
Fig. 4.
Brain map of task fMRI predictors of treatment outcome. Strength of effect is indicated by both color and size; color also indicates direction of effect. Created with BrainNet Viewer (Xia et al., 2013).
4.2.1. Emotion-regulation task results
Tasks targeting the emotion regulation circuitry have been employed most frequently to identify depression treatment biomarkers.
Amygdala. Two studies associated response with lower amygdala activation when viewing negative emotional faces (Ruhé et al., 2011) and subliminal happy and threatening faces (Williams, 2015). The latter study also reported that lower amygdala activation while viewing subliminal sad faces was associated with response to venlafaxine, but not escitalopram or sertraline, while noting that these associations were only present for response, not remission (Williams, 2015). A small study invoking a word relevance task did not find a significant difference between responders and non-responders at baseline, but did report amygdala activity increased for positive words and decreased for negative words compared to non-responders after treatment (Young, 2020). Other longitudinal studies have found decreases in right amygdala activation to masked sad faces and increases in left amygdala activation to masked happy faces in a small study regardless of clinical outcome (Victor et al., 2010), similarly outcome-agnostic decreases in amygdala activation to sad but not fearful faces (Arnone, 2012), increases in amygdala activation to subliminal happy and threatening faces for responders and decreases in amygdala activation to subliminal sad faces for non-responders (Williams, 2015), and outcome-agnostic decreases in response to fearful vs. happy faces after one week of antidepressant treatment (Godlewska et al., 2016). This trend is also supported by numerous small studies from before 2010 that found bilateral decreases in amygdala activation to masked fearful faces after treatment (Sheline, 2001), decreases in left amygdala activation during a sad facial recognition task (Fu, 2004), and bilateral decreases amygdala activation to negative versus neutral emotional stimuli (Anand et al., 2007). In all three of the aforementioned studies, the trends were observed in the entire depressed cohort regardless of remission/response status, though it is worth noting that all three report a significant group decrease in HDRS across the studies. Still, another study noted changes in amygdala activation while viewing emotional faces were not associated with treatment response (Ruhé et al., 2011). Overall, high baseline amygdala activation and post-treatment decreases in amygdala activation to negative or sad stimuli seems be robustly associated with positive treatment outcome, suggesting a potentially reliable biomarker.
Anterior Cingulate Cortex. Improved treatment outcomes have been associated with higher activation of the subgenual ACC in a small study employing a gender discrimination task of sad faces (Keedwell, 2010), higher activation of the pregenual ACC for sad vs. happy faces in a backward masking paradigm (Godlewska, 2018), higher activation of the pregenual ACC to negative pictures (Preuss et al., 2020), and lower activation in the dorsal ACC during negative word processing (small study) (Miller, 2013). Additionally, activation decreases of the dorsal ACC while viewing fearful compared to happy faces one week after starting treatment predicted treatment response (Godlewska et al., 2016). Small studies from before 2010 also found treatment response was associated with activation increases in negative versus neutral contrasts of affective picture viewing in an unspecified portion of the ACC (Davidson et al., 2003), higher baseline pregenual and subgenual ACC activation in negative versus neutral contrasts of emotional face viewing (Chen, 2007), and decreases in right subgenual ACC activation during sad face viewing (Keedwell, 2009). Lower activation of the bilateral mid-cingulate during emotion regulation was associated with remission in LLD, as was activation increases of the mid-cingulate during emotion regulation after a first dose of medication (Karim, 2018).
Posterior Cingulate Cortex. Higher activation of the PCC while viewing negative faces (Samson, 2011), more general negative images(Rizvi, 2013, Preuss et al., 2020), and general positive images (Preuss et al., 2020) was associated with response/symptomatic improvement, as was lower PCC activation in an emotion discrimination task, but only at two weeks and not four (Spies et al., 2017). Irrespective of treatment outcome, longitudinal studies have reported increases in PCC activation during emotion regulation in LLD (Khalaf, 2016), increases of PCC activation (normalizing relative to healthy controls) while viewing medium and high-intensity facial expressions (Fu et al., 2015), and decreases in PCC activation for the negative minus neutral contrast in the emotional Stroop task (Fu et al., 2015).
Frontal regions. Findings are generally limited to tasks that require active participation from the participants. In two small studies, symptomatic improvement was reported to be associated with lower ventrolateral PFC activation to sad or happy faces during a gender discrimination task (Keedwell, 2010) and lower dlPFC activation during negative word processing (Miller, 2013). Further, greater activation of the left middle frontal gyrus and lateral OFC during a faces/shapes task were negatively associated with remission in LLD (Karim, 2018). On the other hand, dmPFC and superior frontal gyrus activation while viewing negative faces (Samson, 2011), right dlPFC activation while viewing all emotional faces (Ruhé et al., 2011), and left superior and middle frontal gyrus activation while engaging in emotion regulation (Karim, 2018) were positively associated with treatment outcome. Further, one day increases in bilateral inferior frontal gyrus, left middle frontal gyrus, and left rectus gyrus activation during a faces/shapes task after a first dose of medication were indicative of remission in LLD (Karim, 2018).
Insula. One study found that higher activation during negative face viewing compared to neutral face viewing was associated with treatment response at baseline (Samson, 2011) and one week (Williams et al., 2021), while another found that lower activation while viewing negative images predicted remission (Rizvi, 2013). Decreased activation of the insula while viewing fearful compared to happy faces one week after starting treatment predicted treatment response (Godlewska et al., 2016).
Other subcortical regions. Lower activation of the thalamus during negative word processing in a small study (Miller, 2013) and higher activation during positive picture viewing (Preuss et al., 2020) was associated with treatment outcome. Three longitudinal studies reported decreased thalamic activity was associated with remission in LLD after one day in an emotion regulation task (Karim, 2018) and response after one week when viewing fearful versus happy faces (Godlewska et al., 2016). Decreased thalamic activity was reported during an explicit sad/angry face matching task after four weeks on venlafaxine but not mirtazapine, regardless of outcome (Frodl, 2011). A small study from 2007 also found an outcome-agnostic decrease in thalamic activity when viewing negative versus neutral emotional stimuli (Anand et al., 2007). The caudate nucleus has also been associated with treatment outcome with inconclusive results. Activation of the caudate nucleus when viewing negative faces has been reported to have a positive association with treatment response (Samson, 2011) and negative association with remission in LLD (Karim, 2018), and lower activation during negative word processing was associated with symptomatic improvement in a small study (Miller, 2013).
4.2.2. Cognitive task results
Studies of depression treatment have also focused on tasks that engage cognitive control as this may be an important process to engage for treatment response. We report six such studies.
Dorsolateral prefrontal cortex. As the main hub of the cognitive control network, many of these studies predefine the dlPFC as a region of interest. One study employing the go/no-go paradigm reported that remission was associated with right dlPFC activation during the inhibitory no-go condition, as well as right inferior parietal lobe activation for SSRI but not SNRI (Gyurak, 2016). A continuous performance test task also implicated higher dlPFC activation with remission, though only in participants without childhood maltreatment (Miller, 2015). Another study employing the n-back task found dlPFC activation and low connectivity between dlPFC and the anterior mPFC, PCC, and parietal lobe, as well as deactivation of the anterior mPFC, correlated with symptom improvement (Meyer et al., 2019). Further, two studies from 2009 reported post-treatment increases in activity of the dlPFC during an emotional interference task (Fales, 2009) and a preparing to overcome potency task in a small LLD cohort (Aizenstein, 2009), regardless of treatment outcome.
Other regions. Among its many functions, the ACC is noted for its role in the conflict monitoring/resolution process, which may be important for depression treatment response (Pizzagalli, 2011). A study employing the go/no-go task noted greater activation in the rostral and dorsal ACC, mid-cingulate, dmPFC, and lateral OFC during commission errors predicted symptomatic improvement (Crane et al., 2017). Two small studies from before 2010 also reported that treatment response was associated with higher rostral ACC activity during commission errors in the parametric go/no-go task (Langenecker, 2007) and higher ACC activity during an n-back task (Marquand et al., 2008). Another important region probed by cognitive tasks is the hippocampus, which has a vital role in memory and learning and is highly susceptible to neurodegeneration in depression (Sapolsky, 2001), as reviewed in Section 3.1 (Volumetric Analysis). A small longitudinal study using the Stroop color-word test reported symptomatic improvement associated with decreases in activation of the right amygdala and hippocampus for citalopram, but not reboxetine, and decreases of the left medial temporal lobe, right inferior parietal lobe, right ventrolateral PFC, and bilateral superior parietal lobe for both medications (Wagner et al., 2010). Finally, a small study employing a word pair memory-encoding task focused on the hippocampus and found that lower activation of the left hippocampus while encoding positive words was associated with a poor treatment response, while the right hippocampus did not show an association with response (Toki et al., 2014).
4.2.3. Reward task results
Despite an explosion of studies focused on reward paradigm, only four of these studies have investigated treatment outcome prediction, all focusing on the ventral striatum. One large study included a balanced placebo arm and used a monetary reward, surprisingly finding that VS activity at odds with reinforcement learning predictions is associated with symptomatic improvement to sertraline treatment versus placebo (Greenberg, 2020). A study employing the monetary incentive delay task used generalized psychophysiological interaction to investigate VS connectivity during the task, reported that VS activity during anticipation, but not consumption, of reward and frontostriatal connectivity was associated with symptomatic improvement (Dunlop, 2020). Another small study using the monetary incentive delay task found that symptomatic improvement was associated with a normalizing increase in the left VS activity during loss anticipation (Stoy, 2012). However, a study focused on the striatum, mPFC, and anterior insula activation during a card guessing task failed to find any association with treatment response (Brandt et al., 2021).
5. Discussion
A substantial body of work has examined structural and functional MRI-derived biomarkers of response to pharmacological treatment of depression. While the results are informative, the overall lack of replicability and discriminatory power have so far been insufficient for clinical application. Larger hippocampal volume, lower WMH burden in LLD, lower amygdala activation and higher ACC activation to negative emotional stimuli, and higher dlPFC activation during cognitive tasks have all been associated with improved treatment outcome, yet each possesses its own limitations. While hippocampal volume as a predictor of treatment outcome has been replicated in numerous studies, a fair number have failed to do so. This may be the result of considering overall volume instead of just the body/tail, which seems to carry more predictive power, or a lateralization effect, on which mixed findings have been reported. Similarly, greater WMH burden has been consistently associated with poor treatment outcome, but it is unclear how this result may influence clinical practice, particularly considering the lack of regional specificity in most WMH studies. Amygdala, ACC, and dlPFC activation are all task-dependent, which introduces yet another challenge for clinical translation. Notably absent from this list are white matter integrity and functional connectivity biomarkers. FA of cingulate and frontal white matter tracts, and DMN and ECN functional connectivity show potential as predictors of treatment outcome but require further investigation to demonstrate their utility. As of now it is unclear if the inconsistency of these findings is due to very small effect size, methodological heterogeneity, or other factors.
The need for larger study sizes, increased replicability, placebo-controlled randomized trials, open data, shared models, multimodal measures, and even things as simple as consistent terminology have been repeatedly addressed in other reviews (Gillett et al., 2020, Fu et al., 2013, Fonseka et al., 2018). We endorse these recommendations but will not recapitulate them here, instead focusing on the role of methodological choices, broadly. One major challenge is that most studies are associative in nature rather than predictive. Participants are typically sorted based on a predefined clinical response (e.g., remitters vs. non-remitters, responders vs. non-responders, or decrease in symptom severity) and then neuroimaging differences between the groups or along the axis of interest are reported. Measures that differ meaningfully at the group level may not always provide useful predictors of clinical outcomes at the individual level(Shmueli, 2010, Calhoun et al., 2017). While more recent studies have increasingly addressed this concern by explicitly focusing on predictive models, feature selection, model specification, and external validation all demand significantly more data (Scheinost et al., 2019). The trade-off between predictive power and generalizability only further exacerbates this demand.
The inherent high-dimensional nature of establishing MRI-based biomarkers for prediction of treatment outcome is wrought with challenges. Foremost among them is the heterogeneity of MDD, which is defined based on a broad collection of symptoms, not some defining pathophysiology. As such, many neural paths may lead to depression or may capture downstream effects associated with the illness. Focusing on a single predictor may be akin to trying to summarize a road map with a single direction. While such an approach may never provide a highly discriminative biomarker for treatment outcome, the “summary direction” may still reveal valuable insights into the neurobiology of depression and its treatment course.
Another challenge is the rapid progress in the field of neuroimaging. On the hardware side, the standard has shifted from 1.5T MRI to 3T MRI and is currently moving to 7T. With respect to study design, early fMRI studies rarely had more than 20 participants. Recent studies have generally analyzed more appropriate sample sizes, but this puts a larger onus on recruitment and typically involves multiple sites. While multi-site studies offer clear benefits for generalizability, they raise further complications for data harmonization (Fortin et al., 2018). Processing methods are constantly evolving, leaving a myriad of choices (Botvinik-Nezer et al., 2020), (Carp, 2012) with little normative guidance (Bowring et al., 2019). As previously mentioned, prior to ∼2010, multiple comparisons were often improperly accounted for (Bennett et al., 2009). Thus, one of the most widely employed methods, cluster thresholding, was shown to have inflated false positive rates (Eklund et al., 2016). Non-parametric permutation testing has now become the gold standard for calculating P values (Nichols and Holmes, 2002). All of this adds up to a (swiftly) moving target where every advance calls into question previously hard-won ground.
This moving-target problem also manifests in the choice of analytic methods. The field of neuroimaging is still relatively young. Before neuroimaging, lesion studies were used primarily to localize brain function and behavior. Early fMRI studies mostly took a similar approach, focusing on localization. While this has provided myriad insights, it is very limited when addressing processes like emotion that most likely involve complex interactions across multiple brain regions. The discovery of coherent functional networks (Biswal et al., 1995) shifted much of the field toward a systems perspective that may better capture these higher order processes. However, the quantification of a network is inherently more complex than that of region of interest activation, which manifests as a drastic increase in the methodological degrees of freedom. Compounding this, we have very little in the way of ground truth to guide our analytic choices. As a result, there is very little true replication in the field; almost every study differs from others in some meaningful way. It can be argued that these differences carry advantages—each study offers a (perhaps slightly) different angle from which to view the underlying question, revealing subtleties that might be hidden from a single vantage point. However, it also makes it exceedingly difficult to develop any unifying models. With so little replication, it is unclear which studies provide meaningful constraints for such models. Meta-analyses make a valiant attempt to fill this gap but are faced with the unenviable task of post hoc data synthesis (Wager et al., 2007). So far, the pace of methodological advances for neuroimaging has far outpaced our ability to consolidate the information gained from them.
A final limitation from these studies is that they do not prescribe a course of action based on the predictions. If a patient is unlikely to remit, what is the best course of action? Fortunately, large multi-site studies are beginning to answer these questions (Cristancho, 2019). Another promising approach is investigating differential biomarkers that may predict which type of treatment (antidepressant class, pharmacotherapy vs. psychotherapy, etc.) is most likely to result in improvement of depressive symptoms (Fonseka et al., 2018). Such biomarkers have tremendous capacity to improve clinical outcomes in line with the “precision psychiatry” approach (Williams, 2016) while also shedding light on possible mechanistic pathways that facilitate further discovery.
The rapid advances of the field mentioned as challenges above also provide reason for optimism. However, more targeted hypotheses may be required. For example, consider a typical hypothesis of the form “increased functional connectivity of the ECN will predict remission.” Functional connectivity of the ECN is a construct requiring additional clarifications. For instance, how is the ECN defined? Does the hypothesis benefit from a predefined network definition (if so, which one?) or would a data-driven approach (e.g. ICA) be more appropriate? How will functional connectivity of a network be quantified? While broad measures can capture network-wide changes, they can also be subject to cancellation effects (e.g. increased connectivity between some regions and decreased connectivity between other regions leads to no effect at the network level) that obscure real differences (Gerlach et al., 2021). These analytic choices have different neurobiological ramifications (e.g., fractional anisotropy of the cingulum is a more robust, but less specific measure than fiber tract count between the anterior and posterior cingulate). The answers to these questions and the resulting implications should be carefully considered and tailored to a neurobiological model. We believe that precise selection and application of the available analytic tools is a key component in advancing the field. Evermore specific probes enabled by methodological advancements may allow us to untangle the complex neural circuitry one piece of the puzzle at a time, providing a more holistic view of pathophysiology of depression and correlates of treatment outcome.
Declaration of Competing Interest
Dr. Olusola Ajilore is the co-founder of KeyWise AI and serves on the advisory boards of Embodied Labs and Blueprint. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This work was completed in thanks to funding from NIMH grants R01 MH076079, R01 MH108509, R01 MH121619, RO1 MH121620, R01 MH102246, K01MH122741, and T32 MH019986.
References
- H.A. Whiteford A.J. Ferrari L. Degenhardt V. Feigin T. Vos G. Forloni The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010 PloS One 10 2 2015 e0116820 e0116820. [DOI] [PMC free article] [PubMed]
- Murray C.J.L., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S.Y., Ali M.K., AlMazroa M.A., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Bahalim A.N., Barker-Collo S., Barrero L.H., Bartels D.H., Basáñez M.-G., Baxter A., Bell M.L., Benjamin E.J., Bennett D., Bernabé E., Bhalla K., Bhandari B., Bikbov B., Abdulhak A.B., Birbeck G., Black J.A., Blencowe H., Blore J.D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T.S., Bryan-Hancock C., Bucello C., Buchbinder R., Buckle G., Budke C.M., Burch M., Burney P., Burstein R., Calabria B., Campbell B., Canter C.E., Carabin H., Carapetis J., Carmona L., Cella C., Charlson F., Chen H., Cheng A.-A., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahiya M., Dahodwala N., Damsere-Derry J., Danaei G., Davis A., Leo D.D., Degenhardt L., Dellavalle R., Delossantos A., Denenberg J., Derrett S., Des Jarlais D.C., Dharmaratne S.D., Dherani M., Diaz-Torne C., Dolk H., Dorsey E.R., Driscoll T., Duber H., Ebel B., Edmond K., Elbaz A., Ali S.E., Erskine H., Erwin P.J., Espindola P., Ewoigbokhan S.E., Farzadfar F., Feigin V., Felson D.T., Ferrari A., Ferri C.P., Fèvre E.M., Finucane M.M., Flaxman S., Flood L., Foreman K., Forouzanfar M.H., Fowkes F.G.R., Fransen M., Freeman M.K., Gabbe B.J., Gabriel S.E., Gakidou E., Ganatra H.A., Garcia B., Gaspari F., Gillum R.F., Gmel G., Gonzalez-Medina D., Gosselin R., Grainger R., Grant B., Groeger J., Guillemin F., Gunnell D., Gupta R., Haagsma J., Hagan H., Halasa Y.A., Hall W., Haring D., Haro J.M., Harrison J.E., Havmoeller R., Hay R.J., Higashi H., Hill C., Hoen B., Hoffman H., Hotez P.J., Hoy D., Huang J.J., Ibeanusi S.E., Jacobsen K.H., James S.L., Jarvis D., Jasrasaria R., Jayaraman S., Johns N., Jonas J.B., Karthikeyan G., Kassebaum N., Kawakami N., Keren A., Khoo J.-P., King C.H., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Laden F., Lalloo R., Laslett L.L., Lathlean T., Leasher J.L., Lee Y.Y., Leigh J., Levinson D., Lim S.S., Limb E., Lin J.K., Lipnick M., Lipshultz S.E., Liu W., Loane M., Ohno S.L., Lyons R., Mabweijano J., MacIntyre M.F., Malekzadeh R., Mallinger L., Manivannan S., Marcenes W., March L., Margolis D.J., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGill N., McGrath J., Medina-Mora M.E., Meltzer M., Memish Z.A., Mensah G.A., Merriman T.R., Meyer A.-C., Miglioli V., Miller M., Miller T.R., Mitchell P.B., Mock C., Mocumbi A.O., Moffitt T.E., Mokdad A.A., Monasta L., Montico M., Moradi-Lakeh M., Moran A., Morawska L., Mori R., Murdoch M.E., Mwaniki M.K., Naidoo K., Nair M.N., Naldi L., Narayan K.M.V., Nelson P.K., Nelson R.G., Nevitt M.C., Newton C.R., Nolte S., Norman P., Norman R., O'Donnell M., O'Hanlon S., Olives C., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Page A., Pahari B., Pandian J.D., Rivero A.P., Patten S.B., Pearce N., Padilla R.P., Perez-Ruiz F., Perico N., Pesudovs K., Phillips D., Phillips M.R., Pierce K., Pion S., Polanczyk G.V., Polinder S., Pope C.A., Popova S., Porrini E., Pourmalek F., Prince M., Pullan R.L., Ramaiah K.D., Ranganathan D., Razavi H., Regan M., Rehm J.T., Rein D.B., Remuzzi G., Richardson K., Rivara F.P., Roberts T., Robinson C., De Leòn F.R., Ronfani L., Room R., Rosenfeld L.C., Rushton L., Sacco R.L., Saha S., Sampson U., Sanchez-Riera L., Sanman E., Schwebel D.C., Scott J.G., Segui-Gomez M., Shahraz S., Shepard D.S., Shin H., Shivakoti R., Silberberg D., Singh D., Singh G.M., Singh J.A., Singleton J., Sleet D.A., Sliwa K., Smith E., Smith J.L., Stapelberg N.JC., Steer A., Steiner T., Stolk W.A., Stovner L.J., Sudfeld C., Syed S., Tamburlini G., Tavakkoli M., Taylor H.R., Taylor J.A., Taylor W.J., Thomas B., Thomson W.M., Thurston G.D., Tleyjeh I.M., Tonelli M., Towbin J.A., Truelsen T., Tsilimbaris M.K., Ubeda C., Undurraga E.A., van der Werf M.J., van Os J., Vavilala M.S., Venketasubramanian N., Wang M., Wang W., Watt K., Weatherall D.J., Weinstock M.A., Weintraub R., Weisskopf M.G., Weissman M.M., White R.A., Whiteford H., Wiebe N., Wiersma S.T., Wilkinson J.D., Williams H.C., Williams S.RM., Witt E., Wolfe F., Woolf A.D., Wulf S., Yeh P.-H., Zaidi A.KM., Zheng Z.-J., Zonies D., Lopez A.D. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Brenes G.A. Anxiety, Depression, and Quality of Life in Primary Care Patients. Prim. Care Companion J. Clin. Psychiatry. 2007;9:437–443. doi: 10.4088/pcc.v09n0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P.E., Fournier A.-A., Sisitsky T., Pike C.T., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J. Clin. Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Strakowski S., Nelson E. Oxford University Press; 2015. Major Depressive Disorder. [Google Scholar]
- Fava M., Davidson K.G. Definition and epidemiology of treatment-resistant depression. Psychiatr. Clin. North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- Eisenberg Center at Oregon Health & Science University. Choosing Antidepressants for Adults: Clinician’s Guide. in Comparative Effectiveness Review Summary Guides for Clinicians (Agency for Healthcare Research and Quality (US), 2007). [PubMed]
- Monroe S.M., Anderson S.F. Depression: The Shroud of Heterogeneity. Curr. Dir. Psychol. Sci. 2015;24:227–231. [Google Scholar]
- Kessler R.C., et al. Age Differences in Major depression: Results from the National Comorbidity Surveys Replication (NCS-R) Psychol. Med. 2010;40:225. doi: 10.1017/S0033291709990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein H.J., Khalef A., Walker S.E., Andreescu C. MRI Predictors of Treatment Response in Late-Life Depression. J. Geriatr. Psychiatry Neurol. 2014;27:24–32. doi: 10.1177/0891988713516541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein B., Scheuer S., Holsboer F. Are there meaningful biomarkers of treatment response for depression? Drug Discov. Today. 2014;19:539–561. doi: 10.1016/j.drudis.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am. J. Psychiatry157, 1–45 (2000). [PubMed]
- Treatment outcomes and neural mechanisms DeRubeis, R. J., Siegle, G. J. & Hollon, S. D. Cognitive therapy vs. medications for depression. Nat. Rev. Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., et al. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol. Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. NeuroImage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bartlett E.A., DeLorenzo C., Sharma P., Yang J., Zhang M., Petkova E., Weissman M., McGrath P.J., Fava M., Ogden R.T., Kurian B.T., Malchow A., Cooper C.M., Trombello J.M., McInnis M., Adams P., Oquendo M.A., Pizzagalli D.A., Trivedi M., Parsey R.V. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology. 2018;43(11):2221–2230. doi: 10.1038/s41386-018-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Ryan C., Christman S., Elson D., Bermudez C., Landman B.A., Szymkowicz S.M., Boyd B.D., Kang H., Taylor W.D. Structural MRI-Based Measures of Accelerated Brain Aging do not Moderate the Acute Antidepressant Response in Late-Life Depression. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2022;30(9):1015–1025. doi: 10.1016/j.jagp.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf A., Edelman K., Tudorascu D., Andreescu C., Reynolds C.F., Aizenstein H. White Matter Hyperintensity Accumulation During Treatment of Late-Life Depression. Neuropsychopharmacology. 2015;40(13):3027–3035. doi: 10.1038/npp.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S., Okamoto Y., Onoda K., Matsumoto T., Yoshimura S., Kunisato Y., Okada G.o., Shishida K., Kobayakawa M., Fukumoto T., Machino A., Inagaki M., Yamawaki S. Hippocampal activation during associative encoding of word pairs and its relation to symptomatic improvement in depression: a functional and volumetric MRI study. J. Affect. Disord. 2014;152-154:462–467. doi: 10.1016/j.jad.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., et al. Treatment Course With Antidepressant Therapy in Late-Life Depression. Am. J. Psychiatry. 2012;169:1185–1193. doi: 10.1176/appi.ajp.2012.12010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G.M., Yucel K., Taylor V.H., Macdonald K., Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol. Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Maller J.J., et al. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol. Psychiatry. 2018;23:1737–1744. doi: 10.1038/mp.2017.224. [DOI] [PubMed] [Google Scholar]
- Nogovitsyn N., Muller M., Souza R., Hassel S., Arnott S.R., Davis A.D., Hall G.B., Harris J.K., Zamyadi M., Metzak P.D., Ismail Z., Downar J., Parikh S.V., Soares C.N., Addington J.M., Milev R., Harkness K.L., Frey B.N., Lam R.W., Strother S.C., Rotzinger S., Kennedy S.H., MacQueen G.M. Hippocampal tail volume as a predictive biomarker of antidepressant treatment outcomes in patients with major depressive disorder: a CAN-BIND report. Neuropsychopharmacology. 2020;45(2):283–291. doi: 10.1038/s41386-019-0542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate-Garza P.P., et al. Hippocampal volume as treatment predictor in antidepressant naïve patients with major depressive disorder. J. Psychiatr. Res. 2021;140:323–328. doi: 10.1016/j.jpsychires.2021.06.008. [DOI] [PubMed] [Google Scholar]
- Vakili K., et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol. Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Hsieh M.-H., et al. Hippocampal volume and antidepressant response in geriatric depression. Int. J. Geriatr. Psychiatry. 2002;17:519–525. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- Frodl T., et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J. Clin. Psychiatry. 2004;65:492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- Frodl T., et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. JPN. 2008;33:423–430. [PMC free article] [PubMed] [Google Scholar]
- Fu C.HY., Costafreda S.G., Sankar A., Adams T.M., Rasenick M.M., Liu P., Donati R., Maglanoc L.A., Horton P., Marangell L.B. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry. 2015;15(1) doi: 10.1186/s12888-015-0457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W.D., et al. Hippocampus atrophy and the longitudinal course of late-life depression. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2014;22:1504–1512. doi: 10.1016/j.jagp.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handbook of emotion regulation, 2nd ed. xviii, 669 (The Guilford Press, 2014).
- Gunning-Dixon F.M., et al. Anterior Cingulate Cortical Volumes and Treatment Remission of Geriatric Depression. Int. J. Geriatr. Psychiatry. 2009;24:829–836. doi: 10.1002/gps.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen G.S., et al. Neuroanatomical correlates of apathy in late-life depression and antidepressant treatment response. J. Affect. Disord. 2014;166:179–186. doi: 10.1016/j.jad.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R., et al. Treatment response in late-onset depression: relationship to neuropsychological, neuroradiological and vascular risk factors. Psychol. Med. 2004;34:125–136. doi: 10.1017/s0033291703008870. [DOI] [PubMed] [Google Scholar]
- Suh J.S., et al. An investigation of cortical thickness and antidepressant response in major depressive disorder: A CAN-BIND study report. NeuroImage Clin. 2020;25 doi: 10.1016/j.nicl.2020.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.L., Batten L.A., Aldosary F., Tremblay P., Blier P. Brain-volume increase with sustained remission in patients with treatment-resistant unipolar depression. J. Clin. Psychiatry. 2012;73:625–631. doi: 10.4088/JCP.11m06865. [DOI] [PubMed] [Google Scholar]
- Chen C.-H., et al. Brain Imaging Correlates of Depressive Symptom Severity and Predictors of Symptom Improvement After Antidepressant Treatment. Biol. Psychiatry. 2007;62:407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Motter J.N., et al. Cortical thickness predicts remission of depression with antidepressants in patients with late-life depression and cognitive impairment. J. Affect. Disord. 2021;295:438–445. doi: 10.1016/j.jad.2021.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda S.G., Chu C., Ashburner J., Fu C.H.Y., Domschke K. Prognostic and Diagnostic Potential of the Structural Neuroanatomy of Depression. PLoS ONE. 2009;4(7):e6353. doi: 10.1371/journal.pone.0006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Blasco M., et al. NATURALISTIC COURSE OF MAJOR DEPRESSIVE DISORDER PREDICTED BY CLINICAL AND STRUCTURAL NEUROIMAGING DATA: A 5-YEAR FOLLOW-UP. Depress. Anxiety. 2016;33:1055–1064. doi: 10.1002/da.22522. [DOI] [PubMed] [Google Scholar]
- Korgaonkar M.S., Rekshan W., Gordon E., Rush A.J., Williams L.M., Blasey C., Grieve S.M. Magnetic Resonance Imaging Measures of Brain Structure to Predict Antidepressant Treatment Outcome in Major Depressive Disorder. EBioMedicine. 2015;2(1):37–45. doi: 10.1016/j.ebiom.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sämann P.G., et al. Prediction of antidepressant treatment response from gray matter volume across diagnostic categories. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2013;23:1503–1515. doi: 10.1016/j.euroneuro.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Li C.-T., Lin C.-P., Chou K.-H., Chen I.-Y., Hsieh J.-C., Wu C.-L., Lin W.-C., Su T.-P. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. NeuroImage. 2010;50(1):347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Smith R., Chen K., Baxter L., Fort C., Lane R.D. Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex. J. Affect. Disord. 2013;146:414–419. doi: 10.1016/j.jad.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Botvinik-Nezer R., Holzmeister F., Camerer C.F., Dreber A., Huber J., Johannesson M., Kirchler M., Iwanir R., Mumford J.A., Adcock R.A., Avesani P., Baczkowski B.M., Bajracharya A., Bakst L., Ball S., Barilari M., Bault N., Beaton D., Beitner J., Benoit R.G., Berkers R.M.W.J., Bhanji J.P., Biswal B.B., Bobadilla-Suarez S., Bortolini T., Bottenhorn K.L., Bowring A., Braem S., Brooks H.R., Brudner E.G., Calderon C.B., Camilleri J.A., Castrellon J.J., Cecchetti L., Cieslik E.C., Cole Z.J., Collignon O., Cox R.W., Cunningham W.A., Czoschke S., Dadi K., Davis C.P., Luca A.D., Delgado M.R., Demetriou L., Dennison J.B., Di X., Dickie E.W., Dobryakova E., Donnat C.L., Dukart J., Duncan N.W., Durnez J., Eed A., Eickhoff S.B., Erhart A., Fontanesi L., Fricke G.M., Fu S., Galván A., Gau R., Genon S., Glatard T., Glerean E., Goeman J.J., Golowin S.A.E., González-García C., Gorgolewski K.J., Grady C.L., Green M.A., Guassi Moreira J.F., Guest O., Hakimi S., Hamilton J.P., Hancock R., Handjaras G., Harry B.B., Hawco C., Herholz P., Herman G., Heunis S., Hoffstaedter F., Hogeveen J., Holmes S., Hu C.-P., Huettel S.A., Hughes M.E., Iacovella V., Iordan A.D., Isager P.M., Isik A.I., Jahn A., Johnson M.R., Johnstone T., Joseph M.J.E., Juliano A.C., Kable J.W., Kassinopoulos M., Koba C., Kong X.-Z., Koscik T.R., Kucukboyaci N.E., Kuhl B.A., Kupek S., Laird A.R., Lamm C., Langner R., Lauharatanahirun N., Lee H., Lee S., Leemans A., Leo A., Lesage E., Li F., Li M.Y.C., Lim P.C., Lintz E.N., Liphardt S.W., Losecaat Vermeer A.B., Love B.C., Mack M.L., Malpica N., Marins T., Maumet C., McDonald K., McGuire J.T., Melero H., Méndez Leal A.S., Meyer B., Meyer K.N., Mihai G., Mitsis G.D., Moll J., Nielson D.M., Nilsonne G., Notter M.P., Olivetti E., Onicas A.I., Papale P., Patil K.R., Peelle J.E., Pérez A., Pischedda D., Poline J.-B., Prystauka Y., Ray S., Reuter-Lorenz P.A., Reynolds R.C., Ricciardi E., Rieck J.R., Rodriguez-Thompson A.M., Romyn A., Salo T., Samanez-Larkin G.R., Sanz-Morales E., Schlichting M.L., Schultz D.H., Shen Q., Sheridan M.A., Silvers J.A., Skagerlund K., Smith A., Smith D.V., Sokol-Hessner P., Steinkamp S.R., Tashjian S.M., Thirion B., Thorp J.N., Tinghög G., Tisdall L., Tompson S.H., Toro-Serey C., Torre Tresols J.J., Tozzi L., Truong V., Turella L., van ‘t Veer A.E., Verguts T., Vettel J.M., Vijayarajah S., Vo K., Wall M.B., Weeda W.D., Weis S., White D.J., Wisniewski D., Xifra-Porxas A., Yearling E.A., Yoon S., Yuan R., Yuen K.S.L., Zhang L., Zhang X.u., Zosky J.E., Nichols T.E., Poldrack R.A., Schonberg T. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582(7810):84–88. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K.R., Hays J.C., Blazer D.G. MRI-defined vascular depression. Am. J. Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., et al. Vascular Depression. Hypothesis. Arch. Gen. Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Taylor W.D., Aizenstein H.J., Alexopoulos G.S. The Vascular Depression Hypothesis: Mechanisms Linking Vascular Disease with Depression. Mol. Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino J.G. White Matter Hyperintensities on Magnetic Resonance Imaging: What Is a Clinician to Do? Mayo Clin. Proc. 2019;94(3):380–382. doi: 10.1016/j.mayocp.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Wu M., et al. A Fully Automated Method for Quantifying and Localizing White Matter Hyperintensities on MR Images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., et al. MRI signal hyperintensities and treatment remission of geriatric depression. J. Affect. Disord. 2010;126:395–401. doi: 10.1016/j.jad.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed J.R., et al. MRI signal hyperintensities and failure to remit following antidepressant treatment. J. Affect. Disord. 2011;135:315–320. doi: 10.1016/j.jad.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Iosifescu D.V., et al. Brain white-matter hyperintensities and treatment outcome in major depressive disorder. Br. J. Psychiatry J. Ment. Sci. 2006;188:180–185. doi: 10.1192/bjp.188.2.180. [DOI] [PubMed] [Google Scholar]
- Karim H.T., et al. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol. Psychiatry. 2017;22:450–457. doi: 10.1038/mp.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S., Correia S., Boyle P., Malloy P., Schneider L., Lavretsky H., Sackheim H., Roose S., Krishnan K.R.R. MRI subcortical hyperintensities in old and very old depressed outpatients: the important role of age in late-life depression. J. Neurol. Sci. 2002;203-204:227–233. doi: 10.1016/s0022-510x(02)00296-4. [DOI] [PubMed] [Google Scholar]
- Sneed J.R., et al. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., et al. Support for the Vascular Depression Hypothesis in Late Life Depression: Results from a Two Site Prospective Antidepressant Treatment Trial. Arch. Gen. Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patankar T.F., et al. Virchow-Robin space dilatation may predict resistance to antidepressant monotherapy in elderly patients with depression. J. Affect. Disord. 2007;97:265–270. doi: 10.1016/j.jad.2006.06.024. [DOI] [PubMed] [Google Scholar]
- O'Brien J., Ames D., Chiu E., Schweitzer I., Desmond P., Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ. 1998;317(7164):982–984. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bella R., Pennisi G., Cantone M., Palermo F., Pennisi M., Lanza G., Zappia M., Paolucci S. Clinical Presentation and Outcome of Geriatric Depression in Subcortical Ischemic Vascular Disease. Gerontology. 2010;56(3):298–302. doi: 10.1159/000272003. [DOI] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos G.S., Kiosses D.N., Choi S.J., Murphy C.F., Lim K.O. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am. J. Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., et al. Microstructural White Matter Abnormalities and Remission of Geriatric Depression. Am. J. Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Taylor W.D., Kuchibhatla M., Payne M.E., MacFall J.R., Sheline Y.I., Krishnan K.R., Doraiswamy P.M., García A.V. Frontal White Matter Anisotropy and Antidepressant Remission in Late-Life Depression. PLoS ONE. 2008;3(9):e3267. doi: 10.1371/journal.pone.0003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Sorio B., Siddarth P., Milillo M.M., Vlasova R., Ercoli L., Narr K.L., Lavretsky H. Regional White Matter Integrity Predicts Treatment Response to Escitalopram and Memantine in Geriatric Depression: A Pilot Study. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.548904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W.D., et al. One-year change in anterior cingulate cortex white matter microstructure: relationship with late-life depression outcomes. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2011;19:43–52. doi: 10.1097/JGP.0b013e3181e70cec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria L.W., et al. White Matter Abnormalities Predict Residual Negative Self-Referential Thinking Following Treatment of Late-Life Depression with Escitalopram: A Preliminary Study. J. Affect. Disord. 2019;243:62–69. doi: 10.1016/j.jad.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Rush A.J., Koslow S.H., Wisniewski S.R., Cooper N.J., Nemeroff C.B., Schatzberg A.F., Gordon E. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials. 2011;12(1) doi: 10.1186/1745-6215-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Williams L.M., Song Y.J., Usherwood T., Grieve S.M. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br. J. Psychiatry J. Ment. Sci. 2014;205:321–328. doi: 10.1192/bjp.bp.113.140376. [DOI] [PubMed] [Google Scholar]
- Grieve S.M., Korgaonkar M.S., Gordon E., Williams L.M., Rush A.J. Prediction of nonremission to antidepressant therapy using diffusion tensor imaging. J. Clin. Psychiatry. 2016;77:e436. doi: 10.4088/JCP.14m09577. e443. [DOI] [PubMed] [Google Scholar]
- Davis A.D., et al. White Matter Indices of Medication Response in Major Depression: A Diffusion Tensor Imaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4:913–924. doi: 10.1016/j.bpsc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- DeLorenzo C., Delaparte L., Thapa-Chhetry B., Miller J.M., Mann J.J., Parsey R.V. Prediction of Selective Serotonin Reuptake Inhibitor Response Using Diffusion-Weighted MRI. Front. Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R.L.I., et al. Examining raphe-amygdala structural connectivity as a biological predictor of SSRI response. J. Affect. Disord. 2019;256:8–16. doi: 10.1016/j.jad.2019.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira R., et al. White Matter Microstructure Alterations Associated With Paroxetine Treatment Response in Major Depression. Front. Behav. Neurosci. 2021;15 doi: 10.3389/fnbeh.2021.693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Jones D.K., Müller T.J., Wiest R., Walther S. Limbic white matter microstructure plasticity reflects recovery from depression. J. Affect. Disord. 2015;170:143–149. doi: 10.1016/j.jad.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Bennett, C. M., Baird, A. A., Miller, M. B. & L, G. Journal of Serendipitous and Unexpected Results Neural Correlates of Interspecies Perspective Taking in the Post-Mortem Atlantic Salmon: An Argument For Proper Multiple Comparisons Correction.
- Smith S.M., et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M.J., Hansen L.K., Sejnowski T.J. Independent component analysis of functional MRI: what is signal and what is noise? Curr. Opin. Neurobiol. 2003;13:620–629. doi: 10.1016/j.conb.2003.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zuo X., He Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Yeo B.T.T., Spreng R.N. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 2019;32:926–942. doi: 10.1007/s10548-019-00744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C., Tudorascu D.L., Butters M.A., Tamburo E., Patel M., Price J., Karp J.F., Reynolds C.F., Aizenstein H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 2013;214(3):313–321. doi: 10.1016/j.pscychresns.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein-Piekarski A.N., Staveland B.R., Ball T.M., Yesavage J., Korgaonkar M.S., Williams L.M. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl. Psychiatry. 2018;8(1) doi: 10.1038/s41398-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wijk G., Harris J.K., Hassel S., Davis A.D., Zamyadi M., Arnott S.R., Milev R., Lam R.W., Frey B.N., Hall G.B., Müller D.J., Rotzinger S., Kennedy S.H., Strother S.C., MacQueen G.M., Protzner A.B. Baseline Functional Connectivity in Resting State Networks Associated with Depression and Remission Status after 16 Weeks of Pharmacotherapy: A CAN-BIND Report. Cereb. Cortex N. Y. 2022;32(6):1223–1243. doi: 10.1093/cercor/bhab286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel F.A., Rao U., Lu H., Nakonezny P.A., Grannemann B., McGregor T., Croarkin P.E., Mapes K.S., Tamminga C.A., Trivedi M.H. Functional Connectivity of Brain Structures Correlates with Treatment Outcome in Major Depressive Disorder. Front. Psychiatry. 2011;2 doi: 10.3389/fpsyt.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Andreescu C., Butters M.A., Tamburo R., Reynolds C.F., Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. Neuroimaging. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Hellerstein D.J., Gat I., Mechling A., Klahr K., Wang Z., McGrath P.J., Stewart J.W., Peterson B.S. Antidepressants Normalize the Default Mode Network in Patients With Dysthymia. JAMA Psychiatry. 2013;70(4):373. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos G.S., et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019;224(9):3001–3018. doi: 10.1007/s00429-019-01945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop B.W., et al. Functional Connectivity of the Subcallosal Cingulate Cortex Identifies Differential Outcomes to Treatment with Cognitive Behavior Therapy or Antidepressant Medication for Major Depressive Disorder. Am. J. Psychiatry. 2017;174:533–545. doi: 10.1176/appi.ajp.2016.16050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., et al. Functional impairment-based segmentation of anterior cingulate cortex in depression and its relationship with treatment effects. Hum. Brain Mapp. 2021;42:4035–4047. doi: 10.1002/hbm.25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., et al. Predicting escitalopram monotherapy response in depression: The role of anterior cingulate cortex. Hum. Brain Mapp. 2019;41:1249–1260. doi: 10.1002/hbm.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., et al. Duloxetine effects on striatal resting-state functional connectivity in patients with major depressive disorder. Hum. Brain Mapp. 2019;40:3338–3346. doi: 10.1002/hbm.24601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., et al. Functional connectivity of the hippocampus in predicting early antidepressant efficacy in patients with major depressive disorder. J. Affect. Disord. 2021;291:315–321. doi: 10.1016/j.jad.2021.05.013. [DOI] [PubMed] [Google Scholar]
- Li B., et al. A Treatment-Resistant Default Mode Subnetwork in Major Depression. Biol. Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Martens M.A.G., Filippini N., Harmer C.J., Godlewska B.R. Resting state functional connectivity patterns as biomarkers of treatment response to escitalopram in patients with major depressive disorder. Psychopharmacology. 2021 doi: 10.1007/s00213-021-05915-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora M., et al. Salience Network Functional Connectivity Predicts Placebo Effects in Major Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:68–76. doi: 10.1016/j.bpsc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Korgaonkar M.S., Goldstein-Piekarski A.N., Fornito A., Williams L.M. Intrinsic connectomes are a predictive biomarker of remission in major depressive disorder. Mol. Psychiatry. 2020;25:1537–1549. doi: 10.1038/s41380-019-0574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöbl M., et al. Predicting Antidepressant Citalopram Treatment Response via Changes in Brain Functional Connectivity After Acute Intravenous Challenge. Front. Comput. Neurosci. 2020;14 doi: 10.3389/fncom.2020.554186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S. Fan S. Nemati T.J. Akiki J. Roscoe C.L. Averill S. Fouda L.A. Averill C.G. Abdallah Pretreatment Brain Connectome Fingerprint Predicts Treatment Response in Major Depressive Disorder Chronic Stress 4 2020 247054702098472. [DOI] [PMC free article] [PubMed]
- Nemati S., Akiki T.J., Roscoe J., Ju Y., Averill C.L., Fouda S., Dutta A., McKie S., Krystal J.H., Deakin J.F.W., Averill L.A., Abdallah C.G. A Unique Brain Connectome Fingerprint Predates and Predicts Response to Antidepressants. iScience. 2020;23(1):100800. doi: 10.1016/j.isci.2019.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim H.T., et al. Acute trajectories of neural activation predict remission to pharmacotherapy in late-life depression. NeuroImage Clin. 2018;19:831–839. doi: 10.1016/j.nicl.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., et al. The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum. Brain Mapp. 2015;36:768–778. doi: 10.1002/hbm.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., et al. Global topology alteration of the brain functional network affects the 8-week antidepressant response in major depressive disorder. J. Affect. Disord. 2021;294:491–496. doi: 10.1016/j.jad.2021.07.078. [DOI] [PubMed] [Google Scholar]
- Cui J., Wang Y., Liu R., Chen X., Zhang Z., Feng Y., Zhou J., Zhou Y., Wang G. Effects of escitalopram therapy on resting-state functional connectivity of subsystems of the default mode network in unmedicated patients with major depressive disorder. Transl. Psychiatry. 2021;11(1) doi: 10.1038/s41398-021-01754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Fatt C.R., et al. Effect of Intrinsic Patterns of Functional Brain Connectivity in Moderating Antidepressant Treatment Response in Major Depression. Am. J. Psychiatry. 2020;177:143–154. doi: 10.1176/appi.ajp.2019.18070870. [DOI] [PubMed] [Google Scholar]
- Wang L.-J., Kuang W.-H., Xu J.-J., Lei D., Yang Y.-C. Resting-state brain activation correlates with short-time antidepressant treatment outcome in drug-naïve patients with major depressive disorder. J. Int. Med. Res. 2014;42:966–975. doi: 10.1177/0300060514533524. [DOI] [PubMed] [Google Scholar]
- Emam H., Steffens D.C., Pearlson G., Wang L. Increased ventromedial prefrontal cortex activity and connectivity predict poor sertraline treatment outcome in late-life depression. Int. J. Geriatr. Psychiatry. 2019;34:730–737. doi: 10.1002/gps.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang Y.u., Meng C., Zhang C., Zhao W., Zhu D.-M., Zhu J. Functional stability predicts depressive and cognitive improvement in major depressive disorder: A longitudinal functional MRI study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;111:110396. doi: 10.1016/j.pnpbp.2021.110396. [DOI] [PubMed] [Google Scholar]
- Hou Z., Song X., Jiang W., Yue Y., Yin Y., Zhang Y., Liu Y., Yuan Y. Prognostic value of imbalanced interhemispheric functional coordination in early therapeutic efficacy in major depressive disorder. Psychiatry Res. Neuroimaging. 2016;255:1–8. doi: 10.1016/j.pscychresns.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Yushkevich P.A., Piven J., Hazlett H.C., Smith R.G., Ho S., Gee J.C., Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Ruhé H.G., Booij J., Veltman D.J., Michel M.C., Schene A.H. Successful Pharmacologic Treatment of Major Depressive Disorder Attenuates Amygdala Activation to Negative Facial Expressions: A Functional Magnetic Resonance Imaging Study. J. Clin. Psychiatry. 2011;72 doi: 10.4088/JCP.10m06584. [DOI] [PubMed] [Google Scholar]
- Williams L.M., et al. Amygdala Reactivity to Emotional Faces in the Prediction of General and Medication-Specific Responses to Antidepressant Treatment in the Randomized iSPOT-D Trial. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015;40:2398–2408. doi: 10.1038/npp.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D., et al. Response to SSRI intervention and amygdala activity during self-referential processing in major depressive disorder. NeuroImage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T.A., Furey M.L., Fromm S.J., Öhman A., Drevets W.C. Relationship of Emotional Processing to Masked Faces in the Amygdala to Mood State and Treatment in Major Depressive Disorder. Arch. Gen. Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D., et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am. J. Psychiatry. 2012;169:841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Godlewska B.R., Browning M., Norbury R., Cowen P.J., Harmer C.J. Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl. Psychiatry. 2016;6(11):e957. doi: 10.1038/tp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., et al. Attenuation of the Neural Response to Sad Faces in Major Depressionby Antidepressant Treatment: A Prospective, Event-Related Functional Magnetic Resonance ImagingStudy. Arch. Gen. Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Gardner K., Lowe M.J. Reciprocal Effects of Antidepressant Treatment on Activity and Connectivity of the Mood Regulating Circuit: An fMRI Study. J. Neuropsychiatry Clin. Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell P.A., et al. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J. Affect. Disord. 2010;120:120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Godlewska B.R., et al. Predicting Treatment Response in Depression: The Role of Anterior Cingulate Cortex. Int. J. Neuropsychopharmacol. 2018;21:988–996. doi: 10.1093/ijnp/pyy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss A., Bolliger B., Schicho W., Hättenschwiler J., Seifritz E., Brühl A.B., Herwig U. SSRI Treatment Response Prediction in Depression Based on Brain Activation by Emotional Stimuli. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.538393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.M., et al. fMRI response to negative words and SSRI treatment outcome in major depressive disorder: a preliminary study. Psychiatry Res. 2013;214:296–305. doi: 10.1016/j.pscychresns.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Irwin W., Anderle M.J., Kalin N.H. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am. J. Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Keedwell P., et al. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J. Psychopharmacol. Oxf. Engl. 2009;23:775–788. doi: 10.1177/0269881108093589. [DOI] [PubMed] [Google Scholar]
- Samson A.C., et al. Brain activation predicts treatment improvement in patients with major depressive disorder. J. Psychiatr. Res. 2011;45:1214–1222. doi: 10.1016/j.jpsychires.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Rizvi S.J., et al. Neural response to emotional stimuli associated with successful antidepressant treatment and behavioral activation. J. Affect. Disord. 2013;151:573–581. doi: 10.1016/j.jad.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Spies M., Kraus C., Geissberger N., Auer B., Klöbl M., Tik M., Stürkat I.-L., Hahn A., Woletz M., Pfabigan D.M., Kasper S., Lamm C., Windischberger C., Lanzenberger R. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl. Psychiatry. 2017;7(1):e1008. doi: 10.1038/tp.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf A., et al. Altered Functional Magnetic Resonance Imaging Markers of Affective Processing During Treatment of Late-Life Depression. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2016;24:791–801. doi: 10.1016/j.jagp.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.J., Brown E.C., Clark D.L., Pike G.B., Ramasubbu R. Early post-treatment blood oxygenation level-dependent responses to emotion processing associated with clinical response to pharmacological treatment in major depressive disorder. Brain Behav. 2021;11:e2287. doi: 10.1002/brb3.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., et al. Different effects of mirtazapine and venlafaxine on brain activation: an open randomized controlled fMRI study. J. Clin. Psychiatry. 2011;72:448–457. doi: 10.4088/JCP.09m05393blu. [DOI] [PubMed] [Google Scholar]
- Gyurak A., et al. Frontoparietal Activation During Response Inhibition Predicts Remission to Antidepressants in Patients With Major Depression. Biol. Psychiatry. 2016;79:274–281. doi: 10.1016/j.biopsych.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Miller S., et al. Cognition – Childhood Maltreatment Interactions in the Prediction of Antidepressant Outcomes in Major Depressive Disorder Patients: Results from the iSPOT-D Trial. Depress. Anxiety. 2015;32:594–604. doi: 10.1002/da.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B.M., Rabl U., Huemer J., Bartova L., Kalcher K., Provenzano J., Brandner C., Sezen P., Kasper S., Schatzberg A.F., Moser E., Chen G., Pezawas L. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl. Psychiatry. 2019;9(1) doi: 10.1038/s41398-019-0395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales C.L., et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J. Affect. Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein H.J., et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A. Frontocingulate Dysfunction in Depression: Toward Biomarkers of Treatment Response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane N.A., Jenkins L.M., Bhaumik R., Dion C., Gowins J.R., Mickey B.J., Zubieta J.-K., Langenecker S.A. Multidimensional prediction of treatment response to antidepressants with cognitive control and functional MRI. Brain. 2017;140(2):472–486. doi: 10.1093/brain/aww326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker S.A., et al. Frontal and Limbic Activation During Inhibitory Control Predicts Treatment Response in Major Depressive Disorder. Biol. Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand A.F., Mourão-Miranda J., Brammer M.J., Cleare A.J., Fu C.H.Y. Neuroanatomy of verbal working memory as a diagnostic biomarker for depression. NeuroReport. 2008;19:1507–1511. doi: 10.1097/WNR.0b013e328310425e. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Depression, antidepressants, and the shrinking hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2001;98(22):12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G., Koch K., Schachtzabel C., Sobanski T., Reichenbach J.R., Sauer H., Schlösser R.G.M. Differential effects of serotonergic and noradrenergic antidepressants on brain activity during a cognitive control task and neurofunctional prediction of treatment outcome in patients with depression. J. Psychiatry Neurosci. JPN. 2010;35(4):247–257. doi: 10.1503/jpn.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg T., et al. Reward related ventral striatal activity and differential response to sertraline versus placebo in depressed individuals. Mol. Psychiatry. 2020;25:1526–1536. doi: 10.1038/s41380-019-0490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K., et al. Clinical, behavioral, and neural measures of reward processing correlate with escitalopram response in depression: a Canadian Biomarker Integration Network in Depression (CAN-BIND-1) Report. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020;45:1390–1397. doi: 10.1038/s41386-020-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M., et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J. Psychopharmacol. (Oxf.) 2012;26:677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Brandt I.M., Köhler-Forsberg K., Ganz M., Ozenne B., Jorgensen M.B., Poulsen A., Knudsen G.M., Frokjaer V.G., Fisher P.M. Reward processing in major depressive disorder and prediction of treatment response – Neuropharm study. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2021;44:23–33. doi: 10.1016/j.euroneuro.2020.12.010. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y., Csermely P. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett G., Tomlinson A., Efthimiou O., Cipriani A. Predicting treatment effects in unipolar depression: A meta-review. Pharmacol. Ther. 2020;212 doi: 10.1016/j.pharmthera.2020.107557. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Steiner H., Costafreda S.G. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol. Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Fonseka T.M., MacQueen G.M., Kennedy S.H. Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder. J. Affect. Disord. 2018;233:21–35. doi: 10.1016/j.jad.2017.10.049. [DOI] [PubMed] [Google Scholar]
- Shmueli G. To Explain or to Predict? Stat. Sci. 2010;25:289–310. [Google Scholar]
- Calhoun V.D., Lawrie S.M., Mourao-Miranda J., Stephan K.E. Prediction of Individual Differences from Neuroimaging Data. NeuroImage. 2017;145:135–136. doi: 10.1016/j.neuroimage.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Noble S., Horien C., Greene A.S., Lake E.MR., Salehi M., Gao S., Shen X., O'Connor D., Barron D.S., Yip S.W., Rosenberg M.D., Constable R.T. Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage. 2019;193:35–45. doi: 10.1016/j.neuroimage.2019.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J.-P., Cullen N., Sheline Y.I., Taylor W.D., Aselcioglu I., Cook P.A., Adams P., Cooper C., Fava M., McGrath P.J., McInnis M., Phillips M.L., Trivedi M.H., Weissman M.M., Shinohara R.T. Harmonization of cortical thickness measurements across scanners and sites. NeuroImage. 2018;167:104–120. doi: 10.1016/j.neuroimage.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. On the plurality of (methodological) worlds: estimating the analytic flexibility of FMRI experiments. Front. Neurosci. 2012;6:149. doi: 10.3389/fnins.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowring A., Maumet C., Nichols T.E. Exploring the impact of analysis software on task fMRI results. Hum. Brain Mapp. 2019;40:3362–3384. doi: 10.1002/hbm.24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.M., Wolford G.L., Miller M.B. The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 2009;4:417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Lindquist M., Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect. Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristancho P., et al. Optimizing Outcomes of Treatment-Resistant Depression in Older Adults (OPTIMUM): Study Design and Treatment Characteristics of the First 396 Participants Randomized. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2019;27:1138–1152. doi: 10.1016/j.jagp.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Williams L.M. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3(5):472–480. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach A.R., Karim H.T., Kazan J., Aizenstein H.J., Krafty R.T., Andreescu C. Networks of worry-towards a connectivity-based signature of late-life worry using higher criticism. Transl. Psychiatry. 2021;11(1) doi: 10.1038/s41398-021-01648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]