Abstract
Heart failure (HF), a global health issue characterized by structural or functional cardiac dysfunction, which was found to be associated with the gut microbiome recently. Although multiple studies suggested that the gut microbiome may have an impact on the development of cardiovascular diseases, the underlying mechanism of the gut microbiome in HF remains unclear. The study of metabolites from gut microbiota influenced by dietary nutrition uptake suggested that gut microbiota may affect the process of HF. However, on the basis of the microbiota’s complicated roles and their interactions with metabolites, studies of microbial metabolites in HF had rarely been described so far. In this review, we focused on dietary nutrition-related factors that were involved in the development and progression of HF, such as trimethylamine N-oxide (TMAO), short-chain fatty acids (SCFAs), and bile acids (BAs), to summarize their advances and several potential targets in HF. From a therapeutic standpoint, we discussed microbial metabolites as a potential strategy and their applications in HF as well.
Keywords: heart failure, microbial metabolites, trimethylamine N-oxide (TAMO), diet, therapy
Graphical Abstract
Microbial metabolites that impact heart failure and correlated therapeutic strategies. 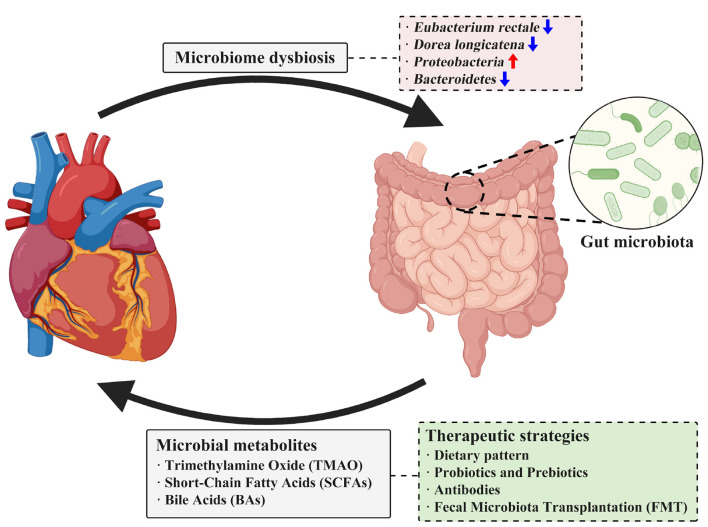
Introduction
Heart failure (HF) is a multifactorial and systemic syndrome with high morbidity and mortality worldwide (Sinnenberg and Givertz, 2020). Although great advances have been made due to the development and achievement in cardiovascular medications and vascular intervention, the incidence of HF is still increasing and is estimated at around 6 cases per 1,000 persons for the last decades in some western countries (DeVore et al., 2021; Seferović et al., 2021). Factors like age, gender, dietary structure, and metabolic diseases are demonstrated to be the risk factors in HF according to the 2021 European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of HF (Heidenreich et al., 2022). In the past few years, researchers have shown that the interactions and communications between the translocation of the bacteria from gut to blood and their subsequent pathogenic responses elicited in HF (Fromentin et al., 2022),
however, the underlying mechanisms of how gut microbiota affect the development of HF remain largely unknown.
Human digestive tracts harbor more than 100 trillion microbes that may contribute to several physiological processes in the host (Brito, 2021; Alberdi et al., 2022). Under physiological context, gut microbiota collaborates with the host to help produce energy, synthesize proteins, and absorb nutrients, as well as maintain gut homeostasis (Alwan and Di Meglio, 2021; Le Bras, 2022; Trevelline and Kohl, 2022). These functions confer gut microbiota on the significance in the development of HF. Yet, when it comes to pathological context, gut microbiota dysbiosis may allow endotoxin (e.g., lipopolysaccharide, LPS) produced by gram-negative bacteria translocated from gut to the blood circulation, which subsequently results in the development of cardiomyopathy (Brainin et al., 2021; Drozd and Cubbon, 2021). The study of metabolites produced by gut microbiota that affect their hosts confirmed this hypothesis. Zhang et al. (2021) pointed out that the trimethylamine N-oxide (TMAO), a newly identified microbial metabolite, may contribute to the occurrence and progression of both acute and chronic HF. Further, they found that TMAO was an early predictor that distinguished individuals from other diseases at the risk of cardiac symptoms. This study led to the promise that gut microbiota may not only be a biomarker but also a therapeutic target in HF. On the basis of these results and the corresponding advances achieved in the past few years, we then want to discuss the communications between microbial metabolites and their roles in the development and progression of HF, and the potential therapeutic strategies based on those interactions are reviewed as well.
Gut microbiome dysbiosis in heart failure
The gut microbiome is a complicated and dynamic system inside the gastrointestinal tract (Kuziel and Rakoff-Nahoum, 2022). The structure of the gut microbiota depends on age, diet, antibiotics, and digestive diseases in humans (Grieneisen et al., 2021). The gut microbiota in healthy individuals belongs to seven major phyla (bacteroidetes, firmicutes, proteobacteria, fusobacteria, verrucomicrobia, cyanobacteria, and actinobacteria) (Adak and Khan, 2019). However, in HF patients, the structure and abundance of gut microbiota altered significantly compared to healthy controls, indicating the potential correlations between the gut microbiome and HF (Spehlmann et al., 2022). Low levels of cardiac output and sympathetic vasoconstriction that result in intestinal ischemia are the major causes of microbial dysbiosis in HF patients (Verbrugge et al., 2013). Intestinal ischemia and hypoxia lead to necrosis of villi cells, which are the principal constituents of the intestinal barrier. Moreover, ischemia can cause intestinal tissue congestion, increasing barrier permeability and thus allowing more bacterial toxins to leak into the circulation.
Heart failure patients are found to have more pathogenic bacterial and less probiotics (Wang et al., 2021). Early in 2017, Kamo et al. (2017) investigated the microbiome of HF patients by 16S rRNA metagenomic analysis and found that Eubacterium rectale and Dorea longicatena were decreased in HF patients. Moreover, they also found that the composition of gut microbiota altered with ages in HF patients, older patients tended to harbor more Proteobacteria but less Bacteroidetes. Another study conducted by Kummen et al. (2018) also analyzed the gut microbiota in HF patients and found that chronic HF patients are more likely to have more Proteobacteria and less Bacteroides as well. Moreover, some specific pathogenic bacteria such as Shigella, Campylobacter, and Candida were also found to be correlated with the severity of HF and even be regarded as potential prognostic indicators in HF (Yuzefpolskaya et al., 2020). The structure of gut microbiota is also different in patients with different subtypes of HF. Microbial structure of heart failure with preserved ejection fraction (HFpEF) patients was demonstrated to be with an increase of Enterococcus while a decrease of Butyricicoccus in fecal samples (Huang et al., 2021). Meanwhile, studies have also revealed that microbial dysbiosis could induce chronic inflammation and immune dysregulation and ultimately aggravate heart failure with reduced ejection fraction (HFrEF) (Madan and Mehra, 2020). This characteristic in HF patients makes microbiota possible in diagnosing or treatment of HF.
On the basis of these findings, it is possible that HF could be treated by diets or drugs through the gut microbiome. Intake of dietary fiber is related to the incidence of HF. Kaye et al. (2020) found that a high-fiber diet increased the abundance of acetate-producing bacteria and suppresses the development of cardiovascular diseases subsequently. Similarly, Marques et al. (2017) also found that diets with high fiber or acetate could prevent hypertension and HF via modulating gut microbiota. Moreover, anti-inflammatory diets like vegetables, fruit, and nuts are able to reduce HF incidence in cigarette addicted population by regulating gut microbiota (Kaluza et al., 2020). This study followed more than 70 thousand people for 14.9 years and found that intake of anti-inflammatory food was negatively correlated to HF incidence because anti-inflammatory diets could partially inhibit those oxidative stress and cell death caused by smoking, as well as regulate and maintain gut microbial homeostasis. Moreover, drugs such as spermidine are demonstrated to improve cardiac function by regulating microbial abundance and diversity (Chen et al., 2021). Spermidine intake could increase the percentage of Muribaculaceae in HF mice while its antagonist reduced the ratio of firmicutes/bacteroidetes, which is a biomarker of microbial dysbiosis and is associated with cardiac function. Taken together, these observations indicate the possibilities of a new strategy for microbiota by regulating dietary nutrition and probiotics, which will be an alternative in the treatment of HF in the future.
The microbial metabolites associated with heart failure
Trimethylamine N-oxide
The trimethylamine N-oxide, an important molecule from the gut microbial metabolite, whose level is determined by daily uptake of red meat, eggs, dairy food, and saltwater fish that contains choline, lecithin, and L-carnitine, is found to be associated with HF (Naghipour et al., 2021; Li X. et al., 2022). These HF-related substances from daily uptake were catabolized to trimethylamine (TMA) by gut microbiota following digestion. The resulting products are then transformed to TMAO by flavin-containing monooxygenase 3 (FMO3) in the liver (Figure 1). Circulating levels of TMAO in the blood have been suggested to have a positive correlation with HF patients (Suzuki et al., 2019; Drapala et al., 2020), demonstrating its risk role in the development of HF. Indeed, Organ et al. showed that mice fed with TMAO have decreased left ventricular ejection fractions (LVEF) along with increased brain natriuretic peptide (BNP) levels, while dietary elimination of TMAO improves cardiac function and protects the ventricle from remodeling in HF mice (Organ et al., 2020). In addition to this finding, Li et al. reported that cardiac hypertrophy could be induced by TMAO in new-born Sprague–Dawley (SD) rats and inhibition of TMAO by antibiotics alleviates cardiac hypertrophy accordingly (Li et al., 2019). Yet, there have been some contradictory reports on TMAO. Videja et al. (2020) reported that TMAO protects right ventricle cardiac functions from damage in rats by mitochondrial energy synthesis by reducing pyruvate metabolism and preserving fatty acid oxidation. In line with this finding, Gawrys-Kopczynska et al. (2020) reported that TMAO reduces the mortality of HF rats by producing diuresis mechanistically. Thus, the specific role of TMAO remains controversial due to its double-side role in HF, and the corresponding mechanisms of microbiota-derived TMAO in HF are required to be further explored in different contexts (Table 1).
FIGURE 1.
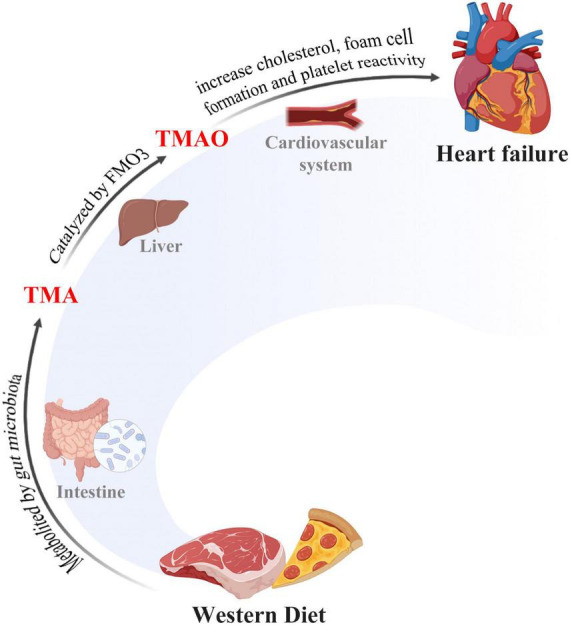
The gut-microbiome-heart axis. Western diet was metabolized to TMA by gut microbiota and then TMA was converted to TMAO in liver tissues. TMAO accumulation triggered cholesterol in many pathological processes, including transportation and foam cell formation, thus inducing heart failure.
TABLE 1.
Studies on predictive values of TMAO for HF clinical outcomes.
| Patients | Clinical outcomes | References |
| Acute HF patients (n = 972) | In-hospital mortality, all-cause mortality and rehospitalization | Suzuki et al., 2016 |
| HFpEF patients (n = 146) | Cardiac-caused mortality and hospitalization | Kinugasa et al., 2021 |
| Acute myocardial infarction (AMI)-related HF patients (n = 985) | Major adverse cardiac events (MACE), including all-cause death, MI recurrence and rehospitalization | Li N. et al., 2022 |
| Stable HF patients (n = 720) | Mortality | Tang et al., 2014 |
| Chronic HF patients (n = 112) | Mortality and heart transplantation | Tang et al., 2015 |
| New-onset or progressively worsening HF patients (n = 2234) | Mortality and rehospitalization | Suzuki et al., 2019 |
Notably, in light of those ambiguities, several studies are conducted to investigate the underlying molecular mechanism of TMAO in HF. Collectively, three mechanisms might be involved in the development of HF, namely, inflammation-induced responses, activation of cell signaling pathway, and interactions among cells or tissues. In detail, inflammation-induced responses in endothelial cells by TMAO promote the development of HF (Brunt et al., 2020). TMAO-triggered activation of NLRP3 canonical inflammasome, leads to the release of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, resulting in endothelial dysfunction that is responsible for vascular calcification (Zhang et al., 2020). Notably, the same results also demonstrated that TMAO promotes the release of those cytokines by activating the NF-κB signaling pathway in atherosclerosis rats (Li et al., 2021). These studies led to the definition of TMAO-induced HF as an inflammatory disease. However, besides, TMAO is also found to aggravate HF by activating the calcium signaling pathway followed by the gain of function in platelets, which is responsible for the ischemic and non-ischemic HF to occur (Fedotcheva et al., 2021). Dysfunction of the calcium signaling pathway caused by TAMO had been found to impair heart function, and subsequently induce T-tubules remodeling (Witkowski et al., 2021). As one of the risk factors in HF, foam cells triggered by TMAO promoted the formation of atherosclerotic plaque (Steinke et al., 2020; Jiang et al., 2021). Recently, a necrotic programmed cell death, pyroptosis, which is characterized by cell swelling, plasma membrane rupture, and release of cytoplasmic contents, was found in endothelial cells (Wu et al., 2020). Upon pyroptosis execution, necrotic endothelial cells triggered potent immune responses that may cause damage to myocardial cells. Recently, TMAO was found to be involved in T-tubule degeneration induced by TMAO via translocation of JPH2 in mouse cardiomyocytes. Collectively, TMAO-induced activation of the calcium signaling pathway was shown to be required for HF to occur, yet, the underlying mechanism of TMAO-induced calcium signaling activation and its association with pyroptosis is required to be further explored.
A recent study suggested that TMAO is not only a target but also a potential biomarker in the process of HF. A prospective observational study showed that serum levels of TMAO with its precursor choline and betaine (an oxidative product from choline) were increased in chronic heart failure (CHF) patients. Moreover, the concentration of these metabolites was found to associate with clinical and hemodynamic severity of HF (Papandreou et al., 2021). This was confirmed by a clinical trial, in which a number of 955 chronic HF patients were followed for 7 years, demonstrating the level of TMAO in HF patients as a predictor of HF (Wei et al., 2021). Similarly, another study by Kinugasa et al. (2021) revealed that serum TMAO, choline, and betaine were correlated with left ventricular diastolic function impairment and poor long-term outcomes in HFpEF patients. Notably, TMAO was shown to be a univariate predictor of the death risk of HFpEF patients. However, the sensitivity of TMAO in predicting heart failure with preserved ejection fraction (HFpEF) has differed from heart failure with preserved ejection fraction (HFpEF). Indeed, Berger et al. (2020) found that the predictive value of TMAO in HfrEF patients was better than in HFpEF patients. Accordingly, it is worthwhile to explore the specificity and sensitivity of TMAO in diagnosing HF in the clinic.
Short-chain fatty acids
As the main metabolites from dietary fibers, short-chain fatty acids (SCFAs), such as carboxylic acids (e.g., acetyl acid, propionic acid, and butyric acid), are demonstrated to be associated with HF. A lack of dietary fiber in the feeding of SPF and germ-free mice is reported to promote cardiac remodeling (Kaye et al., 2020). SCFAs belong to the metabolites that are produced by gut microbes in gastrointestinal tracts, from which SCFAs were absorbed and transported to the blood circulation via the portal vein. Interestingly, SCFAs are not only essential to gut homeostasis but also sufficient for the integrity of the epithelial cell barrier to be maintained. SCFAs execute their functions by the activation of G-protein coupled receptors (e.g., GPR41, GPR43, and GPR109A) signaling pathways and downstream transcription factors, followed by the expression of several anti-inflammatory genes accordingly. Notably, SCFAs are also reported to be the inhibition of Th17 and activation of Treg, which lead to the suppression of immune and inflammatory responses.
Recently, Luedde et al. reported that the gut microbiome in HFpEF patients tended to have deficiencies in Ruminococcaceae and Blautia, which were SCFAs producers (Beale et al., 2021). SCFAs were also beneficial to myocardial electrical remodeling for they could partly activate the parasympathetic nerves through the gut-brain axis (Zhou et al., 2021). As for the underlying mechanism, Carley et al. (2021) further revealed that SCFAs were efficient energy sources for HF patients because failing hearts preferred to oxidize SCFA over ketones. Another similar study found that propionic acids could induce vasodilation and thus alleviate heart failure by binding to GPR41, which is very important in regulating lipid metabolism (Muralitharan et al., 2020). Apart from that, SCFAs were also immunomodulators to their hosts. SCFAs such as butyric acid could mediate various immune cells including Tregs, Th1, macrophages, neutrophilic granulocytes, as well as the antigen-presenting cells including dendritic cells and those inflammatory cells, such as neutrophilic granulocytes (Yang et al., 2020). Taken together with all these benefits, SCFAs were hot topics in clinical strategies now, especially in the treatment of HF.
Bile acid
Bile acid (BA) is a metabolite of bile synthesized by gut microbes, which plays a pivotal role in lipid metabolism (Funabashi et al., 2020). Primary bile acids were firstly synthesized in the liver tissues and then converted to secondary bile in the gastrointestinal tracts with the help of gut microbiota (Winston and Theriot, 2020). BA was important in facilitating gut nutrient absorption, regulating lipid, and energy metabolism, as well as maintaining gut homeostasis (Chiang and Ferrell, 2019). Dietary habits, fasting, and circadian rhythms have impacts on the production and re-absorption of BA (Moszak et al., 2020; Yang and Zhang, 2020). Receptors of BA signaling, such as the farnesoid-X receptor (FXR), are expressed in almost all the cardiovascular cells and are closely related to electrical conduction and cellular mechanics in heart tissues. Therefore, the BA signaling is very important in regulating the physiological process and many heart diseases of the host (Vasavan et al., 2018). Recently, studies have focused on the therapeutic potentials of BA on diseases, especially cardiovascular disorders. A prospective cohort study conducted by Mayerhofer et al. (2017) assessed the levels of both primary and secondary BA in CHF patients and then revealed a significant reduction in the level of primary BA and an increase in secondary BA. Doctor Mayerhofer ascribed these findings to the function of microbiota, because microbial metabolism impacts BA synthesis greatly, especially the secondary BA (Mayerhofer et al., 2017). This work revealed a close correlation of BA and the gut microbiota in regulating myocardial functions, but the underlying mechanism remained unknown. Farnesoid X receptor (FXR) and the G-protein coupled receptor 5 (TGR5) are two important molecules in the BA signaling pathway (Chiang and Ferrell, 2020; Xu et al., 2021). A study conducted by Xia et al. (2018) reported that FXR was a potential therapeutic target to HF patients, because FXR could ameliorate cardiac dysfunction and promote myocardial remodeling by increasing adiponectin. Moreover, knock-out of FXR facilitated the restoration of failing heart through inhibiting apoptosis and fibrosis in cardiocytes (Gao et al., 2018).
Interventions targeted at microbial metabolites
Dietary intervention
Dietary interventions are effective strategies for maintaining microbial homeostasis and treating diseases because the structure and diversity of the gut microbiota are closely correlated with dietary habits (Holscher, 2020; Trefflich et al., 2020; Rautmann and de La Serre, 2021). Dietary modifications including reducing salt intake and maintaining water-electrolyte balance, were very helpful to HF treatment (Jayachandran et al., 2020). Dietary Approaches to Stop Hypertension (DASH), a designed dietary approach characterized by a high intake of grains, vegetables, and low intake of fat, was strongly recommended to those individuals at the risk of cardiovascular diseases (Filippou et al., 2020; Wickman et al., 2021). A study that followed up 412 participants revealed that those individuals who followed DASH diets were less likely to develop HF (Juraschek et al., 2021), and DASH diets were an effective non-pharmacologic strategy to prevent HF (Fu et al., 2020). Apart from DASH, Mediterranean diets, characterized by large proportions of fish, fruits, nuts, and relatively low proportions of red meat, fat, and sugar, are also proved to be effective in preventing cardiovascular disease (Barrea et al., 2021; Khan et al., 2021). A randomized clinical trial served 209 participants diagnosed with CHF with Mediterranean diets and found that Mediterranean diets significantly decreased the severity of HF (Tuttolomondo et al., 2020). Hence, adherence to healthy dietary patterns, such as DASH and the Mediterranean diet, may have benefits in preventing HF.
Probiotic and prebiotic treatment
Probiotics and prebiotics could maintain and restore microbial homeostasis and protect the host from myocardial damage (Wieërs et al., 2019; Collinson et al., 2020). In probiotics, the microorganisms that are reported to be beneficial to health, several types such as Bifidobacteria, Lactobacillus, Akkermansia, and yeasts have been demonstrated to play a protective role in HF (Suez et al., 2019). Recently, a randomized clinical trial, in which 90 participants had been followed up, revealed that the probiotic yogurt was able to reduce the serum oxLDL and suppress the oxidative reaction in HF patients (Pourrajab et al., 2020). Further, another study found that the intestinal barrier was a potential therapeutic target for HF, and a combined probiotic intervention is capable of repairing gut mucosa (Yu et al., 2021). Besides, in an animal model, the cardioprotective effect of probiotics was also identified. Gan et al. (2014) fed HF rats with Lactobacillus rhamnosus GR-1, and a suppression of myocardial remodeling was found finally in HF. As a fermentation product that may alter the microbial composition and/or the activity of hosts, prebiotics are shown to have a protective effect on HF as well (Peng et al., 2020). However, a study conducted by Jama et al. (2020) made a controversial claim that although dietary fiber modulates gut microbiota, the prebiotic fiber is unable to override mice’s genetic predisposition to HF. Both probiotics and prebiotics are capable of protecting the host from HF, while the controversial role of prebiotics needs more attention in the future.
Antibiotic treatment
The antibiotic treatment provides a strategy for restoring the gut microbiome when microbial dysbiosis occurred in HF. On the basis of the correlation between microbial dysbiosis and HF (Gutiérrez-Calabrés et al., 2020; Han et al., 2021), antibiotics, which, as a commonly used drug in clinics, showed the most effective and conventional way to restore the gut microbiome (Ramirez et al., 2020). By using this strategy, Riba et al. (2017) showed that doxycycline, an antibiotic useful for the treatment of a number of infections, not only improved the left ventricular systolic function but also reduced the severity of HF, and thus protected HF rats from cardiomegaly, cardiac remodeling and fibrosis in an isoproterenol-induced HF model in rat. Similarly, doxycycline was also demonstrated to exert these functions by inhibiting mitochondrial fission and depolarization in cardiomyocytes. Infective endocarditis (IE), especially infection with Methicillin-resistant Staphylococcus aureus (MRSA), is also an important risk factor for congestive HF (Fernández-Hidalgo et al., 2018). Martí-Carvajal et al. (2020) have conducted a comparison of different types of antibiotics for IE treatment and found vancomycin was very effective in inhibiting MRSA infection. However, patients with congestive HF sometimes exhibit reduced vancomycin clearance (Shimamoto et al., 2013). Therefore, given the adverse effects that antibiotics may induce in microecological dysfunction and drug resistance in infectious diseases, it is of great importance to balance their clinical efficacy and adverse effects before using antibiotics (de Gunzburg et al., 2018; Palleja et al., 2018).
Fecal microbiota transplantation
As a promising and fascinating medical intervention, by transferring gut microbiota from healthy donors to recipients (El-Salhy et al., 2020), fecal microbiota transplantation (FMT) helps recipients restore their microbial homeostasis significantly and was proved to be very effective in Clostridium difficile infection (Aira et al., 2021; Fujimoto et al., 2021). Apart from bowel diseases, FMT is also a promising strategy in many cardiac disorders. Zhang et al. (2022) revealed that colonization with youthful gut microbes could recover the microbial homeostasis in aged rats, thus preventing aged-related atrial fibrillation. Similarly, Chen et al. (2020) also found that transplantation of microbes to germ-free mice could restore the arterial remodeling and promote neointimal hyperplasia. However, it is still unclear that whether FMT could treat HF patients in the clinic and the feasibility of FMT application is also needed to be further elucidated in the future.
Trimethylamine N-oxide inhibitors
Apart from those interventions targeted at gut microbes, drugs targeting metabolic signaling are promising as well. Wang et al. found an analog of choline that could inhibit the key enzyme cutC/D during TMAO formation and was effective in preventing cardiometabolic diseases (Wang et al., 2015). Similarly, Roberts et al. also synthesized two inhibitors, which could inactivate cutC/D permanently, and found that these two inhibitors were effective in reducing the level of TMAO in mice (Roberts et al., 2018). Based on these findings, developing and using novel drugs that targeting at TMAO signaling, are potential strategies in HF treatment.
Conclusion
Over the past few years, more and more researchers are participating and interested in the field of gut microbiota and cardiovascular systems, especially in the interactions and communications between gut microbiota and cardiovascular diseases. Yet, the specific factors that may responsible for the HF to occur are still confusing. Although emerging studies revealed that gut microbiota may have an impact on the development of cardiovascular disease by microbiota diversity and metabolites, direct evidence on their communications and consequences on HF is still unclear. Current studies showed the evidence in lab and epidemiology that gut microbiota is found to be associated with HF. However, its communications and cross-talk pattern is needed to be elucidated in the future. On the basis of its metabolism nature, gut microbiota metabolite was found to execute their functions by TMAO and SCFAs in HF. Although a substantial number of metabolites from gut microbiota had been investigated in HF, TMAO as a primary metabolite is thought to be required in the development and progression of HF. In line with this rationale, recent studies showed that a diet containing more fibers is associated with higher TMAO levels, suggesting the protectie effects of TMAO in cardiovascular disease. These studies led to the assumption that targeting TMAO, SCFAs, and gut microbiota, as a new strategy in the treatment of HF, will make sense in the future. It is therefore fascinating and promising to explore the role of microbial metabolites in HF, and this will help us to understand the mechanism of gut microbiota on HF and to facilitate the development of optimized therapeutic strategies in HF.
Author contributions
XL and JjL drafted this manuscript. BZ, ZL, FM, and SW collected the references. YC and JL revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank to Zhenzhen Wang for her help in revising this article.
Funding
This work was supported by the General Research Program in Medicine and Health of Zhejiang Province (2020KY417).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adak A., Khan M. R. (2019). An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 76 473–493. 10.1007/s00018-018-2943-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aira A., Rubio E., Vergara Gómez A., Fehér C., Casals-Pascual C., González B., et al. (2021). rUTI resolution after FMT for clostridioides difficile infection: a case report. Infect. Dis. Ther. 10 1065–1071. 10.1007/s40121-020-00365-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi A., Andersen S. B., Limborg M. T., Dunn R. R., Gilbert M. T. P. (2022). Disentangling host-microbiota complexity through hologenomics. Nat. Rev. Genet. 23 281–297. 10.1038/s41576-021-00421-0 [DOI] [PubMed] [Google Scholar]
- Alwan W., Di Meglio P. (2021). Guardians of the barrier: microbiota engage AHR in keratinocytes to mantain skin homeostasis. Cell Host Microbe 29 1213–1216. 10.1016/j.chom.2021.07.007 [DOI] [PubMed] [Google Scholar]
- Barrea L., Muscogiuri G., Frias-Toral E., Laudisio D., Pugliese G., Castellucci B., et al. (2021). Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 61 3066–3090. 10.1080/10408398.2020.1792826 [DOI] [PubMed] [Google Scholar]
- Beale A. L., O’Donnell J. A., Nakai M. E., Nanayakkara S., Vizi D., Carter K., et al. (2021). The gut microbiome of heart failure with preserved ejection fraction. J. Am. Heart Assoc. 10 e020654. 10.1161/JAHA.120.020654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Kleber M. E., Delgado G. E., März W., Andreas M., Hellstern P., et al. (2020). Trimethylamine N-oxide and adenosine diphosphate-induced platelet reactivity are independent risk factors for cardiovascular and all-cause mortality. Circ. Res. 126 660–662. 10.1161/CIRCRESAHA.119.316214 [DOI] [PubMed] [Google Scholar]
- Brainin P., Mohr G. H., Modin D., Claggett B., Silvestre O. M., Shah A., et al. (2021). Heart failure associated with imported malaria: a nationwide Danish cohort study. ESC Heart Fail 8 3521–3529. 10.1002/ehf2.13441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito I. L. (2021). The comings and goings of the healthy human gut microbiota. Cell Host Microbe 29 1163–1164. 10.1016/j.chom.2021.06.011 [DOI] [PubMed] [Google Scholar]
- Brunt V. E., Gioscia-Ryan R. A., Casso A. G., VanDongen N. S., Ziemba B. P., Sapinsley Z. J., et al. (2020). Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 76 101–112. 10.1161/HYPERTENSIONAHA.120.14759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley A. N., Maurya S. K., Fasano M., Wang Y., Selzman C. H., Drakos S. G., et al. (2021). Short-chain fatty acids outpace ketone oxidation in the failing heart. Circulation 143 1797–1808. 10.1161/CIRCULATIONAHA.120.052671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. B., Shapiro K. E., Wun K., Kuntz T., Theriault B. R., Nooromid M. J., et al. (2020). Microbial colonization of germ-free mice restores neointimal hyperplasia development after arterial injury. J. Am. Heart Assoc. 9:e013496. 10.1161/JAHA.119.013496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guo Z., Li S., Liu Z., Chen P. (2021). Spermidine affects cardiac function in heart failure mice by influencing the gut microbiota and cardiac galectin-3. Front. Cardiovasc. Med. 8:765591. 10.3389/fcvm.2021.765591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J. Y. L., Ferrell J. M. (2019). Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr. 39 175–200. 10.1146/annurev-nutr-082018-124344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J. Y. L., Ferrell J. M. (2020). Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 318 G554–G573. 10.1152/ajpgi.00223.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson S., Deans A., Padua-Zamora A., Gregorio G. V., Li C., Dans L. F., et al. (2020). Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst. Rev. 12 Cd003048. 10.1002/14651858.CD003048.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gunzburg J., Ghozlane A., Ducher A., Le Chatelier E., Duval X., Ruppé E., et al. (2018). Protection of the human gut microbiome from antibiotics. J. Infect. Dis. 217 628–636. 10.1093/infdis/jix604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVore A. D., Granger B. B., Fonarow G. C., Al-Khalidi H. R., Albert N. M., Lewis E. F., et al. (2021). Effect of a hospital and postdischarge quality improvement intervention on clinical outcomes and quality of care for patients with heart failure with reduced ejection fraction: the CONNECT-HF randomized clinical trial. JAMA 326 314–323. 10.1001/jama.2021.8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapala A., Szudzik M., Chabowski D., Mogilnicka I., Jaworska K., Kraszewska K., et al. (2020). Heart failure disturbs gut-blood barrier and increases plasma trimethylamine, a toxic bacterial metabolite. Int. J. Mol. Sci. 21:6161. 10.3390/ijms21176161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd M., Cubbon R. M. (2021). Infection and adverse outcomes in people with chronic heart failure: highlighting a neglected problem. J. Am. Coll. Cardiol. 78:760. 10.1016/j.jacc.2021.04.106 [DOI] [PubMed] [Google Scholar]
- El-Salhy M., Hatlebakk J. G., Gilja O. H., Bråthen Kristoffersen A., Hausken T. (2020). Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 69 859–867. 10.1136/gutjnl-2019-319630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedotcheva N., Olenin A., Beloborodova N. (2021). Influence of microbial metabolites on the nonspecific permeability of mitochondrial membranes under conditions of acidosis and loading with calcium and iron ions. Biomedicines 9:558. 10.3390/biomedicines9050558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Hidalgo N., Ribera A., Larrosa M. N., Viedma E., Origüen J., de Alarcón A., et al. (2018). Impact of Staphylococcus aureus phenotype and genotype on the clinical characteristics and outcome of infective endocarditis. A multicentre, longitudinal, prospective, observational study. Clin. Microbiol. Infect. 24 985–991. 10.1016/j.cmi.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Filippou C. D., Tsioufis C. P., Thomopoulos C. G., Mihas C. C., Dimitriadis K. S., Sotiropoulou L. I., et al. (2020). Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 11 1150–1160. 10.1093/advances/nmaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromentin S., Forslund S. K., Chechi K., Aron-Wisnewsky J., Chakaroun R., Nielsen T., et al. (2022). Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 28 303–314. 10.1038/s41591-022-01688-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Liu Y., Zhang L., Zhou L., Li D., Quan H., et al. (2020). Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J. Am. Heart Assoc. 9 e016804. 10.1161/JAHA.120.016804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K., Kimura Y., Allegretti J. R., Yamamoto M., Zhang Y. Z., Katayama K., et al. (2021). Functional restoration of bacteriomes and viromes by fecal microbiota transplantation. Gastroenterology 160 2089.e12–2102.e12. 10.1053/j.gastro.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi M., Grove T. L., Wang M., Varma Y., McFadden M. E., Brown L. C., et al. (2020). A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 582 566–570. 10.1038/s41586-020-2396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X. T., Ettinger G., Huang C. X., Burton J. P., Haist J. V., Rajapurohitam V., et al. (2014). Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail 7 491–499. 10.1161/CIRCHEARTFAILURE.113.000978 [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhao Y., Yuan A., Xu L., Huang X., Su Y., et al. (2018). Effects of farnesoid-X-receptor SUMOylation mutation on myocardial ischemia/reperfusion injury in mice. Exp. Cell Res. 371 301–310. 10.1016/j.yexcr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Gawrys-Kopczynska M., Konop M., Maksymiuk K., Kraszewska K., Derzsi L., Sozanski K., et al. (2020). TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. Elife 9:e57028, 10.7554/eLife.57028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen L., Dasari M., Gould T. J., Björk J. R., Grenier J. C., Yotova V., et al. (2021). Gut microbiome heritability is nearly universal but environmentally contingent. Science 373 181–186. 10.1126/science.aba5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Calabrés E., Ortega-Hernández A., Modrego J., Gómez-Gordo R., Caro-Vadillo A., Rodríguez-Bobada C., et al. (2020). Gut microbiota profile identifies transition from compensated cardiac hypertrophy to heart failure in hypertensive rats. Hypertension 76 1545–1554. [DOI] [PubMed] [Google Scholar]
- Han Z. L., Chen M., Fu X. D., Yang M., Hrmova M., Zhao Y. H., et al. (2021). Potassium alginate oligosaccharides alter gut microbiota, and have potential to prevent the development of hypertension and heart failure in spontaneously hypertensive rats. Int. J. Mol. Sci. 22:9823. 10.3390/ijms22189823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich P. A., Bozkurt B., Aguilar D., Allen L. A., Byun J. J., Colvin M. M., et al. (2022). 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 145 e895–e1032. 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- Holscher H. D. (2020). Diet affects the gastrointestinal microbiota and health. J. Acad. Nutr. Diet. 120 495–499. 10.1016/j.jand.2019.12.016 [DOI] [PubMed] [Google Scholar]
- Huang Z., Mei X., Jiang Y., Chen T., Zhou Y. (2021). Gut microbiota in heart failure patients with preserved ejection fraction (GUMPTION study). Front. Cardiovasc. Med. 8:803744. 10.3389/fcvm.2021.803744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jama H. A., Fiedler A., Tsyganov K., Nelson E., Horlock D., Nakai M. E., et al. (2020). Manipulation of the gut microbiota by the use of prebiotic fibre does not override a genetic predisposition to heart failure. Sci. Rep. 10:17919. 10.1038/s41598-020-73614-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M., Chung S. S. M., Xu B. (2020). A critical review on diet-induced microbiota changes and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 60 2914–2925. 10.1080/10408398.2019.1666792 [DOI] [PubMed] [Google Scholar]
- Jiang S., Shui Y., Cui Y., Tang C., Wang X., Qiu X., et al. (2021). Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 46:102115. 10.1016/j.redox.2021.102115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraschek S. P., Kovell L. C., Appel L. J., Miller E. R., III, Sacks F. M., Chang A. R., et al. (2021). Effects of diet and sodium reduction on cardiac injury, strain, and inflammation: the DASH-sodium trial. J. Am. Coll. Cardiol. 77 2625–2634. 10.1016/j.jacc.2021.03.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza J., Levitan E. B., Michaëlsson K., Wolk A. (2020). Anti-inflammatory diet and risk of heart failure: two prospective cohort studies. Eur. J. Heart Fail 22 676–682. 10.1002/ejhf.1746 [DOI] [PubMed] [Google Scholar]
- Kamo T., Akazawa H., Suda W., Saga-Kamo A., Shimizu Y., Yagi H., et al. (2017). Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One 12:e0174099. 10.1371/journal.pone.0174099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D. M., Shihata W. A., Jama H. A., Tsyganov K., Ziemann M., Kiriazis H., et al. (2020). Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation 141 1393–1403. 10.1161/CIRCULATIONAHA.119.043081 [DOI] [PubMed] [Google Scholar]
- Khan M. S., Khan F., Fonarow G. C., Sreenivasan J., Greene S. J., Khan S. U., et al. (2021). Dietary interventions and nutritional supplements for heart failure: a systematic appraisal and evidence map. Eur. J. Heart Fail 23 1468–1476. 10.1002/ejhf.2278 [DOI] [PubMed] [Google Scholar]
- Kinugasa Y., Nakamura K., Kamitani H., Hirai M., Yanagihara K., Kato M., et al. (2021). Trimethylamine N-oxide and outcomes in patients hospitalized with acute heart failure and preserved ejection fraction. ESC Heart Fail 8 2103–2110. 10.1002/ehf2.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummen M., Mayerhofer C. C. K., Vestad B., Broch K., Awoyemi A., Storm-Larsen C., et al. (2018). Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J. Am. Coll. Cardiol. 71 1184–1186. 10.1016/j.jacc.2017.12.057 [DOI] [PubMed] [Google Scholar]
- Kuziel G. A., Rakoff-Nahoum S. (2022). The gut microbiome. Curr. Biol. 32 R257–R264. 10.1016/j.cub.2022.02.023 [DOI] [PubMed] [Google Scholar]
- Le Bras A. (2022). Impact of microbiota on CNS macrophages. Lab. Anim. 51:7. 10.1038/s41684-021-00898-6 [DOI] [PubMed] [Google Scholar]
- Li N., Zhou J., Wang Y., Chen R., Li J., Zhao X., et al. (2022). Association between trimethylamine N-oxide and prognosis of patients with acute myocardial infarction and heart failure. ESC Heart Fail Online ahead of print 10.1002/ehf2.14009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Fan Z., Cui J., Li D., Lu J., Cui X., et al. (2022). Trimethylamine N-oxide in heart failure: a meta-analysis of prognostic value. Front. Cardiovasc. Med. 9:817396. 10.3389/fcvm.2022.817396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang L., Ren P., Yang Y., Li S., Qin X., et al. (2021). Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine 93:153812. 10.1016/j.phymed.2021.153812 [DOI] [PubMed] [Google Scholar]
- Li Z., Wu Z., Yan J., Liu H., Liu Q., Deng Y., et al. (2019). Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Invest. 99 346–357. 10.1038/s41374-018-0091-y [DOI] [PubMed] [Google Scholar]
- Madan S., Mehra M. R. (2020). Gut dysbiosis and heart failure: navigating the universe within. Eur. J. Heart Fail 22 629–637. 10.1002/ejhf.1792 [DOI] [PubMed] [Google Scholar]
- Marques F. Z., Nelson E., Chu P. Y., Horlock D., Fiedler A., Ziemann M., et al. (2017). High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135 964–977. 10.1161/CIRCULATIONAHA.116.024545 [DOI] [PubMed] [Google Scholar]
- Martí-Carvajal A. J., Dayer M., Conterno L. O., Gonzalez Garay A. G., Martí-Amarista C. E. (2020). A comparison of different antibiotic regimens for the treatment of infective endocarditis. Cochrane Database Syst. Rev. 5 Cd009880. 10.1002/14651858.CD009880.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer C. C. K., Ueland T., Broch K., Vincent R. P., Cross G. F., Dahl C. P., et al. (2017). Increased secondary/primary bile acid ratio in chronic heart failure. J. Card Fail 23 666–671. 10.1016/j.cardfail.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Moszak M., Szulińska M., Bogdański P. (2020). You are what you eat-the relationship between diet. microbiota, and metabolic disorders-a review. Nutrients 12:1096. 10.3390/nu12041096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralitharan R. R., Jama H. A., Xie L., Peh A., Snelson M., Marques F. Z. (2020). Microbial peer pressure: the role of the gut microbiota in hypertension and its complications. Hypertension 76 1674–1687. 10.1161/HYPERTENSIONAHA.120.14473 [DOI] [PubMed] [Google Scholar]
- Naghipour S., Cox A. J., Peart J. N., Du Toit E. F., Headrick J. P. (2021). Trimethylamine N-oxide: heart of the microbiota-CVD nexus? Nutr. Res. Rev. 34 125–146. 10.1017/S0954422420000177 [DOI] [PubMed] [Google Scholar]
- Organ C. L., Li Z., Sharp T. E., III, Polhemus D. J., Gupta N., Goodchild T. T., et al. (2020). Nonlethal inhibition of gut microbial trimethylamine N-oxide production improves cardiac function and remodeling in a murine model of heart failure. J. Am. Heart Assoc. 9:e016223. 10.1161/JAHA.119.016223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleja A., Mikkelsen K. H., Forslund S. K., Kashani A., Allin K. H., Nielsen T., et al. (2018). Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 3 1255–1265. 10.1038/s41564-018-0257-9 [DOI] [PubMed] [Google Scholar]
- Papandreou C., Bulló M., Hernández-Alonso P., Ruiz-Canela M., Li J., Guasch-Ferré M., et al. (2021). Choline Metabolism and Risk of Atrial Fibrillation and Heart Failure in the PREDIMED Study. Clin. Chem. 67 288–297. 10.1093/clinchem/hvaa224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Tabashsum Z., Anderson M., Truong A., Houser A. K., Padilla J., et al. (2020). Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 19 1908–1933. 10.1111/1541-4337.12565 [DOI] [PubMed] [Google Scholar]
- Pourrajab B., Naderi N., Janani L., Mofid V., Hajahmadi M., Dehnad A., et al. (2020). Comparison of probiotic yogurt and ordinary yogurt consumption on serum Pentraxin3, NT-proBNP, oxLDL, and ApoB100 in patients with chronic heart failure: a randomized, triple-blind, controlled trial. Food Funct. 11 10000–10010. 10.1039/d0fo01014f [DOI] [PubMed] [Google Scholar]
- Ramirez J., Guarner F., Bustos Fernandez L., Maruy A., Sdepanian V. L., Cohen H. (2020). Antibiotics as major disruptors of gut microbiota. Front. Cell Infect. Microbiol. 10:572912. 10.3389/fcimb.2020.572912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautmann A. W., de La Serre C. B. (2021). Microbiota’s role in diet-driven alterations in food intake: satiety, energy balance, and reward. Nutrients 13:3067. 10.3390/nu13093067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba A., Deres L., Eros K., Szabo A., Magyar K., Sumegi B., et al. (2017). Doxycycline protects against ROS-induced mitochondrial fragmentation and ISO-induced heart failure. PLoS One 12:e0175195. 10.1371/journal.pone.0175195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Gu X., Buffa J. A., Hurd A. G., Wang Z., Zhu W., et al. (2018). Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 24 1407–1417. 10.1038/s41591-018-0128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferović P. M., Vardas P., Jankowska E. A., Maggioni A. P., Timmis A., Milinković I., et al. (2021). The heart failure association atlas: heart failure epidemiology and management statistics 2019. Eur. J. Heart Fail. 23 906–914. 10.1002/ejhf.2143 [DOI] [PubMed] [Google Scholar]
- Shimamoto Y., Fukuda T., Tominari S., Fukumoto K., Ueno K., Dong M., et al. (2013). Decreased vancomycin clearance in patients with congestive heart failure. Eur. J. Clin. Pharmacol. 69 449–457. 10.1007/s00228-012-1340-4 [DOI] [PubMed] [Google Scholar]
- Sinnenberg L., Givertz M. M. (2020). Acute heart failure. Trends Cardiovasc. Med. 30 104–112. 10.1016/j.tcm.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Spehlmann M. E., Rangrez A. Y., Dhotre D. P., Schmiedel N., Chavan N., Bang C., et al. (2022). Heart failure severity closely correlates with intestinal dysbiosis and subsequent metabolomic alterations. Biomedicines 10:809. 10.3390/biomedicines10040809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke I., Ghanei N., Govindarajulu M., Yoo S., Zhong J., Amin R. H. (2020). drug discovery and development of novel therapeutics for inhibiting TMAO in models of atherosclerosis and diabetes. Front. Physiol. 11:567899. 10.3389/fphys.2020.567899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J., Zmora N., Segal E., Elinav E. (2019). The pros, cons, and many unknowns of probiotics. Nat. Med. 25 716–729. 10.1038/s41591-019-0439-x [DOI] [PubMed] [Google Scholar]
- Suzuki T., Heaney L. M., Bhandari S. S., Jones D. J., Ng L. L. (2016). Trimethylamine N-oxide and prognosis in acute heart failure. Heart 102 841–848. 10.1136/heartjnl-2015-308826 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Yazaki Y., Voors A. A., Jones D. J. L., Chan D. C. S., Anker S. D., et al. (2019). Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure: results from BIOSTAT-CHF. Eur. J. Heart Fail 21 877–886. 10.1002/ejhf.1338 [DOI] [PubMed] [Google Scholar]
- Tang W. H., Wang Z., Fan Y., Levison B., Hazen J. E., Donahue L. M., et al. (2014). Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 64 1908–1914. 10.1016/j.jacc.2014.02.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Wang Z., Shrestha K., Borowski A. G., Wu Y., Troughton R. W., et al. (2015). Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card Fail 21 91–96. 10.1016/j.cardfail.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trefflich I., Jabakhanji A., Menzel J., Blaut M., Michalsen A., Lampen A., et al. (2020). Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit. Rev. Food Sci. Nutr. 60 2990–3004. 10.1080/10408398.2019.1676697 [DOI] [PubMed] [Google Scholar]
- Trevelline B. K., Kohl K. D. (2022). The gut microbiome influences host diet selection behavior. Proc. Natl. Acad. Sci. U.S.A. 119 e2117537119. 10.1073/pnas.2117537119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttolomondo A., Di Raimondo D., Casuccio A., Velardo M., Salamone G., Cataldi M., et al. (2020). Mediterranean diet adherence and congestive heart failure: relationship with clinical severity and ischemic pathogenesis. Nutrition 70:110584. 10.1016/j.nut.2019.110584 [DOI] [PubMed] [Google Scholar]
- Vasavan T., Ferraro E., Ibrahim E., Dixon P., Gorelik J., Williamson C. (2018). Heart and bile acids - clinical consequences of altered bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 1864 1345–1355. 10.1016/j.bbadis.2017.12.039 [DOI] [PubMed] [Google Scholar]
- Verbrugge F. H., Dupont M., Steels P., Grieten L., Malbrain M., Tang W. H., et al. (2013). Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 62 485–495. 10.1016/j.jacc.2013.04.070 [DOI] [PubMed] [Google Scholar]
- Videja M., Vilskersts R., Korzh S., Cirule H., Sevostjanovs E., Dambrova M., et al. (2020). Microbiota-derived metabolite trimethylamine N-oxide protects mitochondrial energy metabolism and cardiac functionality in a rat model of right ventricle heart failure. Front. Cell Dev. Biol. 8:622741. 10.3389/fcell.2020.622741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Cai Z., Ferrari M. W., Liu Y., Li C., Zhang T., et al. (2021). The correlation between gut microbiota and serum metabolomic in elderly patients with chronic heart failure. Mediators Inflamm. 2021:5587428. 10.1155/2021/5587428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Roberts A. B., Buffa J. A., Levison B. S., Zhu W., Org E., et al. (2015). Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163 1585–1595. 10.1016/j.cell.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Zhao M., Huang M., Li C., Gao J., Yu T., et al. (2021). FMO3-TMAO axis modulates the clinical outcome in chronic heart-failure patients with reduced ejection fraction: evidence from an Asian population. Front. Med. 16 295–305. 10.1007/s11684-021-0857-2 [DOI] [PubMed] [Google Scholar]
- Wickman B. E., Enkhmaa B., Ridberg R., Romero E., Cadeiras M., Meyers F., et al. (2021). Dietary management of heart failure: DASH diet and precision nutrition perspectives. Nutrients 13:4424. 10.3390/nu13124424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieërs G., Belkhir L., Enaud R., Leclercq S., Philippart de Foy J. M., Dequenne I., et al. (2019). How probiotics affect the microbiota. Front. Cell Infect Microbiol. 9:454. 10.3389/fcimb.2019.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J. A., Theriot C. M. (2020). Diversification of host bile acids by members of the gut microbiota. Gut Microbes 11 158–171. 10.1080/19490976.2019.1674124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski M., Witkowski M., Friebel J., Buffa J. A., Li X. S., Wang Z., et al. (2021). Vascular endothelial tissue factor contributes to trimethylamine N-oxide-enhanced arterial thrombosis. Cardiovasc. Res. Online ahead of print 10.1093/cvr/cvab263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Chen J., Chen J., Tao J., Wu S., Xu G., et al. (2020). Trimethylamine N-oxide promotes apoE(-/-) mice atherosclerosis by inducing vascular endothelial cell pyroptosis via the SDHB/ROS pathway. J. Cell Physiol. 235 6582–6591. 10.1002/jcp.29518 [DOI] [PubMed] [Google Scholar]
- Xia Y., Zhang F., Zhao S., Li Y., Chen X., Gao E., et al. (2018). Adiponectin determines farnesoid X receptor agonism-mediated cardioprotection against post-infarction remodelling and dysfunction. Cardiovasc. Res. 114 1335–1349. 10.1093/cvr/cvy093 [DOI] [PubMed] [Google Scholar]
- Xu M., Shen Y., Cen M., Zhu Y., Cheng F., Tang L., et al. (2021). Modulation of the gut microbiota-farnesoid X receptor axis improves deoxycholic acid-induced intestinal inflammation in mice. J. Crohns Colitis 15 1197–1210. 10.1093/ecco-jcc/jjab003 [DOI] [PubMed] [Google Scholar]
- Yang W., Yu T., Huang X., Bilotta A. J., Xu L., Lu Y., et al. (2020). Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 11:4457. 10.1038/s41467-020-18262-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang J. (2020). Bile acid metabolism and circadian rhythms. Am. J. Physiol. Gastrointest. Liver Physiol. 319 G549–G563. 10.1152/ajpgi.00152.2020 [DOI] [PubMed] [Google Scholar]
- Yu H., Dong A., Zhao L., Li P., Zhang Q., Lu J., et al. (2021). Intervention effect of probiotics in gastric cancer patients with complications of coronary heart disease and heart failure. J. Oncol. 2021:1620891. 10.1155/2021/1620891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzefpolskaya M., Bohn B., Nasiri M., Zuver A. M., Onat D. D., Royzman E. A., et al. (2020). Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J. Heart Lung Transplant. 39 880–890. 10.1016/j.healun.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Li Y., Yang P., Liu X., Lu L., Chen Y., et al. (2020). Trimethylamine-N-oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κB) signals. Arterioscler. Thromb. Vasc. Biol. 40 751–765. 10.1161/ATVBAHA.119.313414 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Ke B., Du J. (2021). TMAO: how gut microbiota contributes to heart failure. Transl. Res. 228 109–125. 10.1016/j.trsl.2020.08.007 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang S., Li B., Luo Y., Gong Y., Jin X., et al. (2022). Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 118 785–797. 10.1093/cvr/cvab114 [DOI] [PubMed] [Google Scholar]
- Zhou M., Li D., Xie K., Xu L., Kong B., Wang X., et al. (2021). The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 12 12580–12593. 10.1039/d1fo02040d [DOI] [PubMed] [Google Scholar]


