Abstract
The triad of diabetic ketoacidosis (DKA), severe hypertriglyceridemia, and acute pancreatitis have been occasionally described in severely obese patients with type 2 diabetes mellitus (T2DM). Herein, we present a long-term clinical course of a Thai man with ketosis-prone diabetes mellitus (KPDM) complicated with recurrent pancreatitis due to multifactorial chylomicronemia syndrome. Genetic testing showed no mutation in lipoprotein lipase (LPL) and its co-factors. The patient was referred to multidisciplinary team for lifelong weight loss consultation, limiting intake of fat and simple carbohydrates, and adherence to lipid-lowering medications. Subsequent follow-up 1 year later showed no recurrent pancreatitis. In patients with multifactorial chylomicronemia syndrome, long-term management with dietary modifications together with pharmacotherapy remains the cornerstone of successful treatment.
Keywords: Mutifactorial chylomicronemia, hypertriglyceridemic pancreatitis, ketosis-prone diabetes mellitus (KPDM)
Introduction
Diabetic dyslipidemia is a common feature of type 2 diabetes mellitus (T2DM) characterized by a combination of elevated serum triglyceride (TG) and a decrease in high-density lipoproteins (HDL). 1 However, very severe hypertriglyceridemia defined by serum triglyceride levels ⩾2000 mg/dL are rarely encountered in clinical practice. When fasting serum triglyceride levels >885 mg/dL, the presence of chylomicrons leads to creamy appearance in blood samples and clinical feature of chylomicronemia. 2 Both monogenic and polygenic causes of chylomicronemia could contribute to the same manifestations but differ in age of onset, severity of hypertriglyceridemia, inheritance pattern, and prevalence. While monogenic chylomicronemia has been estimated to develop in 1 per 1000,000 cases, polygenic chylomicronemia has been estimated to occur in 1 per 600 cases in the general population. 3
In patients with T2DM, the triad of diabetic ketoacidosis (DKA), severe hypertriglyceridemia, and acute pancreatitis have been occasionally described in severely obese patients with type 2 diabetes mellitus (T2DM). 4 This triad is a unique subgroup of patients who might have aggravating factors in the background of coexisting genetic predispositions. Hypertriglyceridemia induced acute pancreatitis could induce beta-cell dysfunction and lead to transient insulin deficiency that possibly triggers DKA. 5 High morbidity and mortality rate from multi-organ failure had been observed in these patients. 6 The occurrence of this triad in newly diagnosed ketosis-prone diabetes mellitus (KPDM) is rarely reported. We report a long-term clinical course of a Thai man with KPDM complicated with recurrent pancreatitis due to multifactorial chylomicronemia syndrome. An informed written consent was obtained from the patient for publication of this case report.
Case Presentation
A 33-year-old Thai male patient with a history of oral medications-treated diabetes mellitus, recurrent hypertriglyceridemic pancreatitis, and recurrent DKA (3 times within the past 5 years) came to our hospital in July 2020 for the management of plasma triglyceride levels. At the age of 29 years (height 172 cm, weight 83 kg, and BMI 28.1 kg/m2), he experienced the first hypertriglyceridemia-induced pancreatitis (plasma triglyceride 8230 mg/dL) in association with DKA during his stay in Thailand. He was not taking any medications and had no history of tobacco, alcohol, or illicit drug abuse. The patient had no known family medical history of lipid disorders. Ultrasound ruled out the presence of gallstones. Moderate hypertriglyceridemia (varied from 425 to 638 mg/dL) was documented in previous annual health check-up but T2DM was first diagnosed at the same time of acute pancreatitis in 2016. Initial glycated hemoglobin (A1C) was 10.2% and random plasma glucose was 320 mg/dL No details of pancreatic antibodies or beta-cell function was investigated. After resolution of DKA, insulin therapy was successfully discontinued within 3 months after discharge and he had been treated with linagliptin in combination with metformin for 3 years. Serum fasting triglyceride levels were still elevated ranging from 540 to 5726 mg/dL even after he was prescribed oral fish oil (Omega-3-acid ethyl esters) 2 g per day and fenofibrate 135 mg/day. But he was lost to follow-up and discontinued all medications.
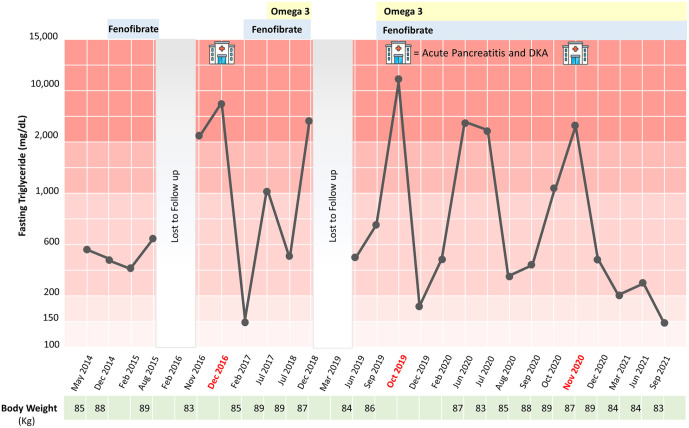
Then, recurrent hypertriglyceridemic pancreatitis in association with multiple organ failures was developed during his travel to India in October 2019. Initial investigations showed plasma glucose 357 mg/dL, serum ketone 4.5 mmol/L (normal <0.6 mmol/L), A1C 8.3%, serum amylase 880 units/L (normal 25-115 units/L), and serum lipase 11 590 units/L (normal 73-393 units/L). No plasma triglyceride level was measured at the time of his arrival to emergency room in India. He was intubated due to drowsiness and transferred to intensive care unit. Continuous intravenous insulin infusion and aggressive hydration were given. Abdominal computed tomography revealed marked swollen pancreas as shown in Figure 1A and B. The patient developed acute respiratory distress syndrome that required mechanical ventilation for 1 week. Later on, fasting hypertriglyceridemia (serum triglyceride level of 2530 mg/dL) was discovered at the third day after admission. Hypertriglyceridemia induced acute pancreatitis was diagnosed and he was treated with plasmapheresis 3 times in conjunction with total parenteral nutrition. After the plasmapheresis treatment together with heparin and continuous intravenous insulin infusion, he was able to extubate at 1 week later but progressive hypoxemia was detected after extubation. Pulmonary embolism at both pulmonary arteries was discovered as shown in Figure 1C. Subcutaneous low molecular weight heparin was given and the patient was transferred back to Thailand after a 2 weeks admission in India. Daily subcutaneous insulin injection was discontinued after DKA resolution for 1 month and the previous oral anti-diabetic medications was re-initiated. Before the patient came to our hospital, his glycemic control was well managed at A1C less 7% but moderate to very severe hypertriglyceridemia was persistently documented (ranging from 487 to 5673 mg/dL) as demonstrated in Figure 2. Low-density lipoprotein (LDL) levels were under 100 mg/dL without statin treatment. His body weight was fluctuated between 83 and 87 kg in the past 2 years and his dietary habits was still high in carbohydrate and fat.
Figure 1.
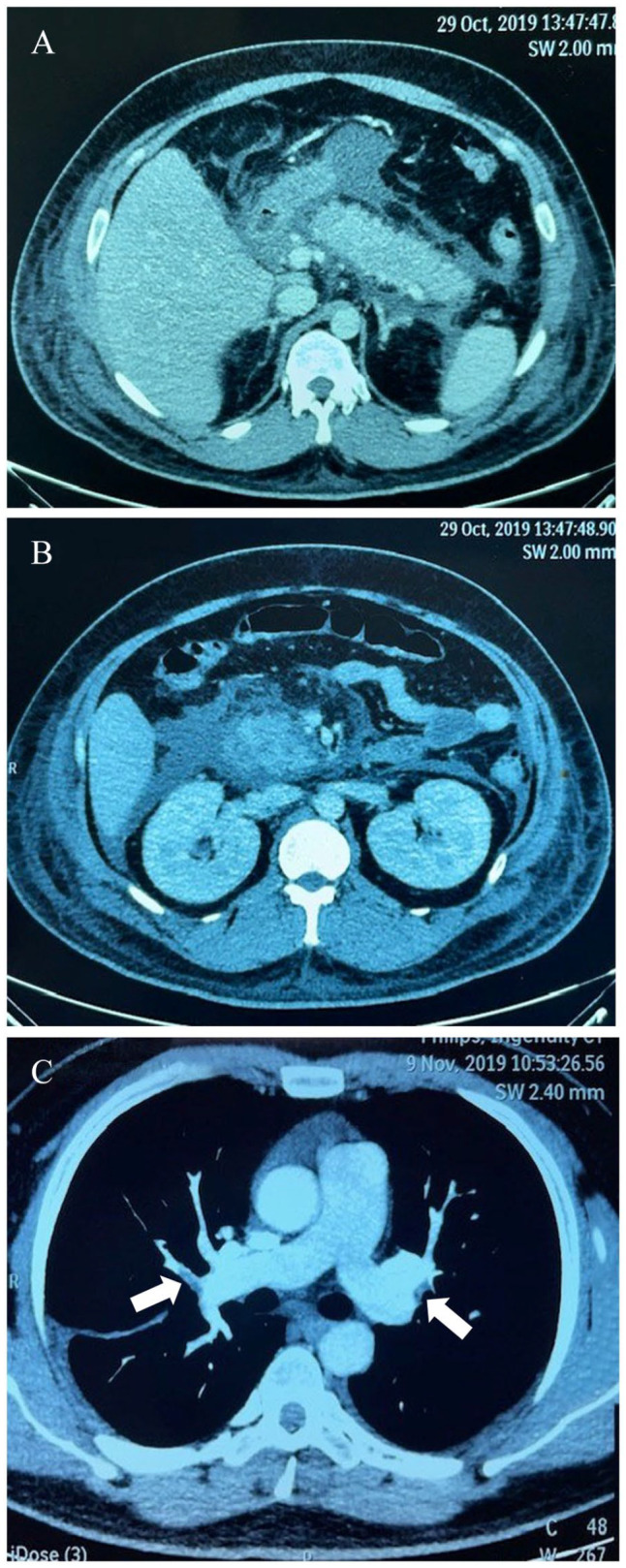
Computed tomography scan of the abdomen revealed: (A) diffuse swollen pancreas with surrounding retroperitoneal fat stranding, (B) liquefactive necrosis of pancreatic parenchyma, and (C) CT angiography revealed intraluminal filling defects (arrowhead) in subsegmental pulmonary arteries, compatible with acute pulmonary embolism.
Figure 2.
Clinical course and laboratory data of this patient from 2014 to 2021.
On initial visit at our hospital in 2020, physical examination revealed no xanthomas or lipemia retinalis. Fasting serum triglyceride level was 3747 mg/dL and A1C was 6.6%. Thyroid function tests showed normal results. Further investigations revealed preserved beta-cell function by mixed meal stimulation test (peak stimulated plasma C-peptide at 8.7 ng/mL) and negative pancreatic autoantibodies (anti GAD and anti-IA2). Genetic testing by whole exome sequencing showed no mutation in lipoprotein lipase (LPL), its co-factors and TG-related genes (Apo A5, Apo C-II, Apo C-III, ApoE, GCKR, GPIHBP1, LMF1, LMNA, and PPARG). 7 Therefore, multifactorial chylomicronemia syndrome with KPDM was diagnosed and dietary management with a dietitian was done. Oral fish oil (Omega-3-acid ethyl esters) was titrated up to 4 g per day and fenofibrate was continued. Unfortunately, the repeated episode of hypertriglyceridemia-induced mild pancreatitis attack in association with DKA was aggravated again by a high fat buffet meal 1 month after his visit to our hospital. The patient was referred to a multidisciplinary team for lifelong weight management, limiting intake of fat and simple carbohydrates, and adherence to lipid-lowering medications. Subsequent follow-up 1 year later showed no recurrent pancreatitis. The patient was advised to lose his bodyweight and healthy eating behavior together with regular OPD follow-ups.
Discussion
Our present case highlights the importance of comprehensive approach in patients with severe hypertriglyceridemia in the setting of KPDM. Typically, patients with diabetes who develop hypertriglyceridemia-induced acute pancreatitis are in long-standing poor glycemic control or in the state of insulin-deficiency. However, this case is a typical case of KPDM with preserved β-cell function (B+) and absence of pancreatic antibodies (A−) as described in mostly African Americans patients. 8 In this patient, transient episode of insulin-deficiency state aggravated the recurrence of DKA and also high-fat foods could precipitate severe hypertriglyceridemic pancreatitis. Maintenance of serum TG below the threshold level (probably at less than 2000 mg/dL) has been associated with less likelihood to develop acute pancreatitis. 3
It is important to differentiate the cause of chylomicronemia as familial chylomicronemia syndrome (FCS) that is very resistant to treatment and requires lifelong very-low-fat diet (total fat intake <10%-15% daily calories). 2 Most cases with chylomicronemia belong to the polygenic form of chylomicronemia called multifactorial chylomicronemia syndrome (MCS) as in the present case. Rare heterozygous variants in TG-related genes or common variants with small effects in more than 40 genes had been identified recently as a cause of MCS. 9 Based on the proposal diagnostic score of FCS, 10 our patient was very unlikely to have FCS from calculated score at only 4 points (+5 from fasting TG > 1130 mg/dL for 3 consecutive blood analyses, −5 from previous fasting TG < 177 mg/dL, +1 from history of pancreatitis, +1 from unexplained abdominal pain, +1 from no history of familial combined hyperlipidemia, +1 from onset of symptoms at age <40 years). However, the genetic confirmation should be performed to reliably exclude the presence of pathogenic variants. Dietary modifications in MCS should be focused on low-carbohydrate and limited simple sugars rather than very-low-fat diet. 3 Therefore, strict dietary control and weight management are essential to prevent pancreatitis in patients with MCS. Currently available pharmacologic treatments to lower TG levels might not be effective unless aggravating dietary factors are controlled.
Severe hypertriglyceridemia might play a role in the development of ketosis from lipotoxicity which cause impairment in insulin secretion and biosynthesis. 11 The inhibitory effects of free fatty acid on glucose-induced insulin secretion had been reported at the level of glucose oxidation. 12 A recent clinical study also confirmed that very high triglyceride levels were associated with KPDM patients. 13 Weight loss and lifelong commitment to dietary modifications could be difficult to these patients and a comprehensive approach to address the complex network of underlying risk factors are required. 14 In morbidly obese patients with MCS, bariatric surgery had been reported to successfully treat and control acute pancreatitis.15,16 However, this option should be reserved for patients with indications for metabolic surgery. Recently, evinacumab (anti-ANGPLT3 monoclonal antibody) which increase LPL activity emerged as a promising therapy for the management of individuals with MCS in phase 2 of clinical trials. 17
Several treatment modalities have been proposed for the rapid reduction of TG such as plasmapheresis, heparin, and continuous insulin infusion in non-diabetic patients.18,19 But the use of plasmapheresis is controversial and should be carefully selected only for suitable patients. 20 Pro-thrombotic state due to systemic inflammatory response and hypercoagulable state associated with severe hypertriglyceridemia had been reported to provoke thrombosis complications during the clinical course of pancreatitis as in our patient.21,22 Early detection of these fatal complications should be promptly recognized in all patients with hypertriglyceridemia-induced acute pancreatitis.
Conclusion
The triad of acute pancreatitis, severe hypertriglyceridemia and DKA constitutes a unique subgroup of patients in which insulin deficiency is the core defect in association with underlying genetic predisposition. Molecular diagnosis of underlying genetic defect should be pursued to exclude familial chylomicronemia syndrome. We report an unusual case of MCS in the setting of newly diagnosed KPDM which long-term management with dietary modifications together with pharmacotherapy remains the cornerstone of successful treatment.
Clinical Pearls
The triad of diabetic ketoacidosis (DKA), severe hypertriglyceridemia, and acute pancreatitis have been occasionally described in severely obese patients with type 2 diabetes mellitus (T2DM).
However, the occurrence of this triad in newly diagnosed ketosis-prone diabetes mellitus (KPDM) has rarely been reported. Herein, we present a long-term clinical course of a Thai man with KPDM complicated with recurrent pancreatitis due to multifactorial chylomicronemia syndrome.
Long-term management with dietary modifications together with pharmacotherapy remains the cornerstone of successful treatment.
Acknowledgments
The authors wish to thank the patient for allowing them to share his story with the medical community and to Dr.Tinapa Himathongkam for English language editing.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Thewjitcharoen Yotsapon  https://orcid.org/0000-0002-2317-4041
https://orcid.org/0000-0002-2317-4041
References
- 1. Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150-159. [DOI] [PubMed] [Google Scholar]
- 2. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dron JS, Hegele RA. Genetics of hypertriglyceridemia. Front Endocrinol. 2020;11:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang Z, Xu Z, Xu R, Huang L, Xu X, Lai X. Whole exome sequencing identifies three novel gene mutations in patients with the triad of diabetic ketoacidosis, hypertriglyceridemia, and acute pancreatitis. J Diabetes. 2021;13:200-210. [DOI] [PubMed] [Google Scholar]
- 5. Winzell MS, Svensson H, Enerbäck S, et al. Pancreatic beta-cell lipotoxicity induced by overexpression of hormone-sensitive lipase. Diabetes. 2003;52:2057-2065. [DOI] [PubMed] [Google Scholar]
- 6. Shemesh E, Zafrir B. Hypertriglyceridemia-related pancreatitis in patients with type 2 diabetes: links and risks. Diabetes Metab Syndr Obes. 2019;12:2041-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown EE, Sturm AC, Cuchel M, et al. Genetic testing in dyslipidemia: a scientific statement from the national lipid association. J Clin Lipidol. 2020;14:398-413. [DOI] [PubMed] [Google Scholar]
- 8. Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hegele RA, Ginsberg HN, Chapman MJ, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014;2:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moulin P, Dufour R, Averna M, et al. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): expert panel recommendations and proposal of an “FCS score”. Atherosclerosis. 2018;275:265-272. [DOI] [PubMed] [Google Scholar]
- 11. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Investig. 1994;93:870-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ye S, Ran H, Zhang H, et al. Elevated serum triglycerides are associated with ketosis-prone type 2 diabetes in young individuals. Diabetes Metab Syndr Obes. 2021;14:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gavish D, Eisenberg S, Berry EM, et al. Bulimia. An underlying behavioral disorder in hyperlipidemic pancreatitis: a prospective multidisciplinary approach. Arch Intern Med. 1987;147:705-708. [DOI] [PubMed] [Google Scholar]
- 15. Hsu SY, Lee WJ, Chong K, Ser KH, Tsou JJ. Laparoscopic bariatric surgery for the treatment of severe hypertriglyceridemia. Asian J Surg. 2015;38:96-101. [DOI] [PubMed] [Google Scholar]
- 16. Maraninchi M, Padilla N, Béliard S, et al. Impact of bariatric surgery on apolipoprotein C-III levels and lipoprotein distribution in obese human subjects. J Clin Lipidol. 2017;11:495-506.e3. [DOI] [PubMed] [Google Scholar]
- 17. Rosenson RS. A phase 2 trial of the efficacy and safety of evinacumab in patients with severe hypertriglyceridemia. Paper presented at: ACC 2021. [cited 2022 May 12]. https://www.tctmd.com/news/evinacumab-lowers-triglycerides-severe-hypertriglyceridemia
- 18. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rawla P, Sunkara T, Thandra KC, Gaduputi V. Hypertriglyceridemia-induced pancreatitis: updated review of current treatment and preventive strategies. Clin J Gastroenterol. 2018;11:441-448. [DOI] [PubMed] [Google Scholar]
- 20. Joglekar K, Brannick B, Kadaria D, Sodhi A. Therapeutic plasmapheresis for hypertriglyceridemia-associated acute pancreatitis: case series and review of the literature. Ther Adv Endocrinol Metab. 2017;8:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simpson HC, Mann JI, Meade TW, Chakrabarti R, Stirling Y, Woolf L. Hypertriglyceridaemia and hypercoagulability. Lancet. 1983;1:786-790. [DOI] [PubMed] [Google Scholar]
- 22. Zheng C, Zhong X, Ma M, Zheng X, Jiang B, Zheng YP. Hyperlipidaemic acute pancreatitis complicated with multiple deep vein thromboses and pulmonary embolism: a case successfully salvaged by radiologic intervention. Curr Med Res Opin. 2021;37:53-57. [DOI] [PubMed] [Google Scholar]