Introduction
Wolf’s isotopic response (WIR) is the emergence of an unrelated skin eruption at the site of a previously healed skin eruption. While this phenomenon is classically incited by varicella zoster virus reactivation, any dermatosis may precede WIR.1, 2, 3 Possible mechanisms include the immunocompromised district hypothesis, in which the inciting lesion impairs regional skin immunity, thereby increasing the risk of a subsequent dermatosis.4,5 Here, we describe the case of a patient who experienced a de novo eruption of lichen planus (LP) at the site of previously healed allergic contact dermatitis (ACD) in the setting of long-term biologic use.
Case report
A 48-year-old female with a history of psoriasis on guselkumab (anti-interleukin 23 [IL-23] monoclonal antibody), diabetes controlled on metformin, and Roux-en-Y gastric bypass 5 years ago presented to our clinic with a painful, pruritic rash along both arms. Two years before presentation, the patient began guselkumab injections every 8 weeks for chronic psoriasis. Eight months before presentation, the patient experienced an acute episode of urushiol-induced ACD following exposure to poison ivy, with pruritus and linear vesicular streaks along both arms and upper back. This rash was severe enough to require presentation to the emergency department for systemic steroids. Her skin was clear of all rashes before and after this episode, including complete clearing of her psoriasis. Four months later, she developed a new painless papulosquamous rash along her right arm and upper back, in the same distribution as her previous contact dermatitis. She later developed similar lesions along her left wrist. Two months later, she self-discontinued guselkumab. Her papulosquamous rash persisted until presentation, but it never spread to other body sites (including nails or mucosa).
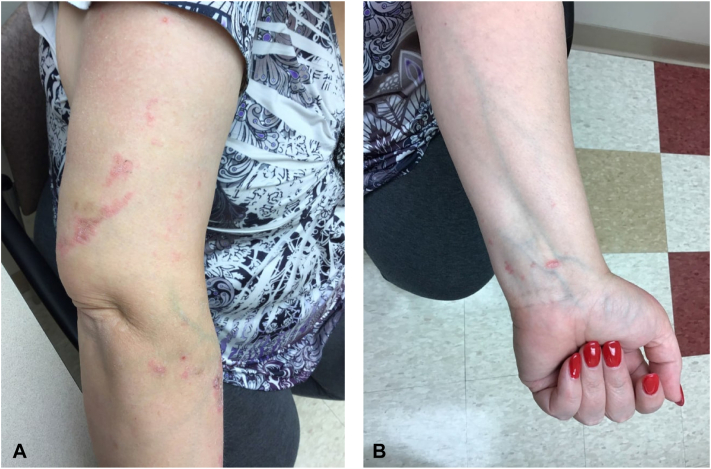
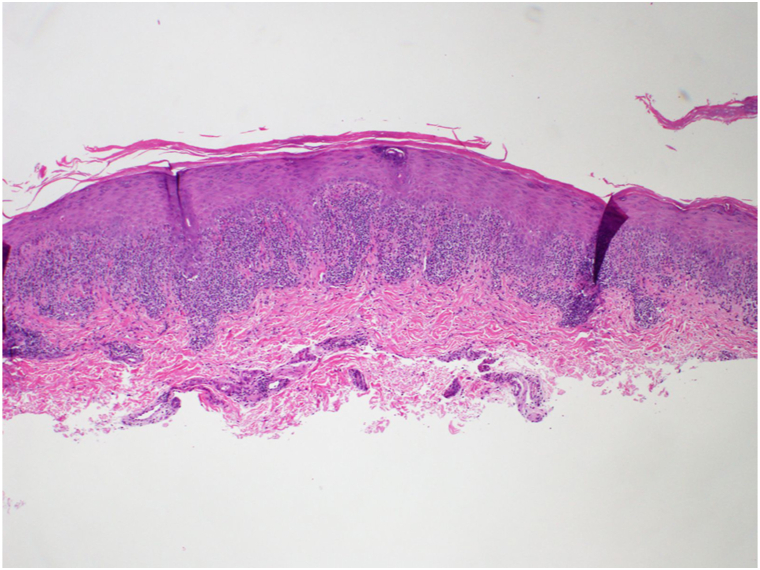
The patient presented to our dermatology clinic with well-defined thin scaly plaques showing koebnerization. This rash extended from the right posterior shoulder down to the extensor and linear surfaces of the right arm and forearm, as well as on the left wrist (Fig 1). A shave biopsy was diagnostic of LP showing wedge-shaped hypergranulosis, lymphohistiocytic infiltrate at the dermoepidermal junction, and necrotic keratinocytes (Fig 2). This biopsy was taken from the left wrist for cosmetic reasons, but the clinical morphology appeared identical bilaterally.
Fig 1.
Lichen planus eruption 4 months after allergic contact dermatitis and 2 months after discontinuing guselkumab. Clinical examination shows scaly papules and plaques across the lateral aspect of the right upper arm (A) and the palmar aspect of the left wrist (B).
Fig 2.
Shave biopsy taken from the left wrist. Histopathologic examination demonstrates hyperkeratosis, wedge-shaped hypergranulosis, irregular epidermal hyperplasia, and lymphohistiocytic inflammatory cell infiltrate obscuring the dermoepidermal junction with necrotic keratinocytes. These results are diagnostic of lichen planus.
Six weeks later, the case was presented at grand rounds where a consensus was reached to favor an isotopic response without involvement of herpes zoster as the most likely etiology. At that time, the patient had a linear distribution of erythematous scaly plaques along the right arm and forearm as well as hyperpigmented macules on the left wrist. She began treatment with narrow band ultraviolet B therapy weekly and clobetasol ointment as needed and subsequently added calcipotriene and betamethasone dipropionate foam. She achieved full resolution over the following 6 months.
Discussion
In summary, our patient developed bilateral LP at the site of previously healed ACD in the setting of chronic guselkumab use; this falls within the definition of WIR. A search of the literature did not identify any reported WIR cases where ACD or long-term biologics have been implicated. Although neither a skin biopsy nor a herpes virus polymerase chain reaction test was performed during the evaluation of our patient’s initial eruption, the patient's bilateral pruritic rash with linear streaks across multiple dermatomes favors our clinical diagnosis of ACD as the inciting dermatosis of a WIR. As such, we believe this report supports an expansion of the scope of the circumstances preceding WIR, namely ACD and guselkumab.
We favor the immunocompromised district hypothesis to explain these clinical findings. Specifically, we hypothesize our patient’s long-term exposure to guselkumab led to chronic dysregulation of her IL-23/IL-17 immune axis, which was then exacerbated by her episode of urushiol-induced ACD. This combination of insults may have impaired local immune function in regions recovering from the delayed hypersensitivity response, promoting an autocytotoxic reaction pathway which led to the development of LP.
While the etiopathogenesis of LP is not completely understood, its mechanism involves inflammatory injury to the epidermal basal cell compartment by persistently activated cluster of differentiation (CD)8+ lymphocytes amplified by plasmacytoid dendritic cell-derived interferon alpha.6 Previous studies have identified associations between lichenoid reactions and increased CD4+/CD8+ infiltrates with a decreased CD4+ to CD8+ ratio, as well as increased expression of IL-23 and IL-17.7,8 Other investigations have linked LP to impaired neutrophil/lymphocyte function and an increased neutrophil-to-lymphocyte ratio.9,10 The results of these studies suggest that our patient’s biologic therapy may underlie the phenotypic switch between her eczematous inciting dermatosis and her later papulosquamous WIR. We hope this case report will inspire future studies to further characterize the complex immunologic underpinnings of both LP and WIR.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Exempt.
References
- 1.Wolf R., Wolf D. “Wolf's isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35(4):416–418. doi: 10.1016/j.clindermatol.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Ruocco V., Sanguiliano S., Brunetti G., Ruocco E. Beyond zoster: sensory and immune changes in zoster-affected dermatomes: a review. Acta Derm Venereol. 2012;92(4):378–382. doi: 10.2340/00015555-1284. [DOI] [PubMed] [Google Scholar]
- 3.Wolf R., Wolf D., Ruocco V., Ruocco E. The role of skin trauma (isotopic and isomorphic) in the distribution of morphea. J Am Acad Dermatol. 2015;72(3):560–561. doi: 10.1016/j.jaad.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 4.McCoy W.H., IV, Otchere E., Musiek A.C., Anadkat M.J. Granulomatous dermatitis as a postherpetic isotopic response in immunocompromised patients: a report of 5 cases. JAAD Case Rep. 2018;4(8):752–760. doi: 10.1016/j.jdcr.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruocco V., Brunetti G., Puca R.V., Ruocco E. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23(12):1364–1373. doi: 10.1111/j.1468-3083.2009.03345. [DOI] [PubMed] [Google Scholar]
- 6.Sontheimer R.D. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives. J Invest Dermatol. 2009;129(5):1088–1099. doi: 10.1038/sj.jid.2009.42. [DOI] [PubMed] [Google Scholar]
- 7.Qian H., Jiao L., Fan Z., Wang L., Liu B., Miao G. Analysis of immunologic function changes in lichen planus after clinical treatment. Med Sci Monit. 2018;24:8716–8721. doi: 10.12659/MSM.910931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R., Zeng X., Han Q., et al. Overexpression and selectively regulatory roles of IL-23/IL-17 axis in the lesions of oral lichen planus. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/701094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T., Yoneda K., Ueta E., Osaki T. Cellular immunosuppression in oral lichen planus. J Oral Pathol Med. 1990;19(10):464–470. doi: 10.1111/j.1600-0714.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 10.Ataş H., Cemil B.Ç., Kurmuş G.I., Gönül M. Assessment of systemic inflammation with neutrophil-lymphocyte ratio in lichen planus. Postepy Dermatol Alergol. 2016;33(3):188–192. doi: 10.5114/pdia.2016.56930. [DOI] [PMC free article] [PubMed] [Google Scholar]