Abstract
Lepidoptera, butterflies and moths, are significant pollinators and ecosystem health indicators. Therefore, monitoring their diversity, distribution, and extinction risks are of critical importance. We aim to understand drivers of local extinction risks of the butterflies in Bangladesh. We conducted a systematic review to extract local extinction risks of the butterflies of Bangladesh, and possible drivers (e.g., body size and diet breadth) of their extinction. We tested whether body size, larval host plants and adult nectar plants contribute to the local extinction risks of butterflies. We predicted butterflies with larger body size and fewer host and nectar plants would be in greater extinction risk. We showed extinction risk is higher in larger butterflies than smaller butterflies, and in butterflies with fewer number of host and nectar plants than the butterflies with higher number host and nectar plants. Our study identifies body size and diet breadth as a potential driver of the local extinction of butterflies thereby suggesting larger conservation urgency for the larger butterflies with narrow diet breadth.
Keywords: Insects, Biodiversity, Extinction, Diet breadth, South Asia, Conservation
Insects, Conservation, biodiversity, extinction, diet breadth, South Asia
1. Introduction
Biodiversity— the variety of life, including variation among genes, species, and functional traits— is crucial for a balance ecosystem [1, 2]. Despite continuous conservation efforts, most indicators of the state of biodiversity (covering species’ population trends, habitat extent and condition, and community composition) are on the decline. On the other hand, indicators of pressures on biodiversity (including resource consumption, invasive alien species, nitrogen pollution, overexploitation, and climate change impacts) are on the rise [1]. Sustaining biodiversity is a fundamental process to maintain a functional ecosystem, hence, the biodiversity loss which might affect the dynamics and functioning of ecosystems [3]. Continuous monitoring of extinction risks and understanding roles its drivers are essential to conserve species and to maintain ecological balance.
The regional distribution of a species is a combined outcome of colonization and extinction of a set of local populations [4]. Extinction of species and the reduction of species’ distribution areas are primarily caused by anthropogenic activities and because of climate change [5]. Recent studies have shown non-random extinctions across the world [6, 7, 8], with some species are more prone to extinction than others [9]. However, there is a lack of data on species distributions, abundance and associated ecological factors which affects key ecological events including species extinction risk [10].
Butterflies have been a significant focus for ecologists because of their role as pollinators and ecosystem health indicators [11, 12]. Butterflies are a widely distributed insect order that evolved from the early Jurassic period. A total of 180,000 species are distributed worldwide excluding Antarctica [13]. They are found in all global territory and populate in desert land even in tropical forests too [14]. However, many species of this insect group are in risk of extinction because of habitat loss and under the influence of changing climatic condition [15]. Extinction risks of butterflies have been conducted in regional scales as well global scale. Assessing the regional extinction risks and its contributing factors can assist in protecting butterflies in a particular ecogeographic region. Physiological conditions (body size, sexual dimorphism), behaviour (migration), diet breadth (host plants and nectar plants) are some factors that contribute to the extinction risks of butterflies [9]. However, studies resulted in conflicting outcomes: some studies suggested a correlation of body size, the number of host and nectar plants with extinction risks whereas other studies indicated otherwise [9, 16, 17, 18, 19], indicating the demand of further studies.
Here, we aim identify to possible driver of the local extinction risks of butterflies using the butterflies of Bangladesh. Bangladesh, despite its small geographical area, has a high insect diversity [20]. Currently over 400 butterflies are known to occur in Bangladesh [21, 22, 23, 24, 25, 26, 27]. We extracted local extinction risks of the butterflies from IUCN Red List of Bangladesh 2015. We then collated data on the body size, larval host plants, and adult nectar plants of the butterflies of Bangladesh from published literature to determine if these factors contribute to local extinct risks. We predicted that extinction risks of the butterflies with larger body size, fewer host plants, and fewer nectar plants would be greater than that of butterflies with smaller body size, higher host plants, and nectar plants.
2. Methods
2.1. Literature review
We conducted a systematic literature search in the Web of Science database to identify studies on the butterflies of Bangladesh to extract body size, host plant and nectar plant data. The search was conducted on December 01, 2019, using the keywords “butterfly” and “Bangladesh” and articles published since 1971 were extracted. The search was later updated on May 31, 2020, and then the updated findings were merged with the initial search. We further cross-checked retrieved articles to identify further relevant articles that we might have missed during the Web of Science search.
2.2. Study selection criteria
We included publications for this study if 1) studies were conducted inside the geographical distribution of Bangladesh 2) articles reported body size data of butterflies 3) publications focused on host plants of the larval stage of butterflies and food sources of adult butterflies, 4) articles, written in English, 5) studies published between 1971 to 2020, and 7) publications available in full-text. We retrieved the full-text of the articles and selected articles based on the inclusion criteria. Furthermore, we also used full-text for cross-referencing and extracted relevant articles.
2.3. Body size, food plants, and extinction risk
We extracted the wingspan of the butterflies as a proxy of body size from the published sources (S1). We took the middle value when wing size was provided in a range. All values were recorded in millimeters (mm). Values were converted to the millimeter when they were presented in other units. We extracted data for butterflies’ host plants — plants in which, butterflies lay eggs and butterflies larva complete their life cycle. We also extracted data on nectar plants – plants from which adult butterflies collect nectar. We collected the threat status of the butterflies of Bangladesh from the IUCN Red List of Bangladesh (2015) [28]. The IUCN Red List of Threatened Species categorizes extinction risk with the following categories: least concern, near threatened, vulnerable, endangered, and critically endangered. We converted the risk categories to a numeric index from least concern (1) to critically endangered (5).
2.4. Statistical analysis
We tested the relation between body size, host plant numbers and nectar plant numbers of the butterflies with the extinction risk categories (response variable) using Cumulative Linked Mixed Models (CLMM). Our response variable, extinction risk categories, is a categorical variable which is also ordered (extinction risks increase from least concern to critically endangered). These type of ordered categorical data are best analysed using models with “ordinal” family [29, 30]. We build cumulative linked mixed model with extinction risk as response variable, body size, host plant numbers, and nectar plants number as fixed effects, and butterfly families as random factor. Family of the butterflies were considered as a random effect in the model to account for any broad scale phylogenetic effects as the phylogenetic relationships among the butterflies of Bangladesh are not apparent. We applied family = “ordinal” and used clmm function of the R package ‘ordinal’ for building the model. Body sizes of the butterflies may influence host plant and nectar plant diversity. To determine the effect of body size on host plant, and nectar plants numbers, we applied (GLMs) with host plant, and nectar plants numbers as response variables and body size, and family as fixed effects (model: glm (log (host_plant_number) ∼ body_size + family, family = gaussian), and glm (log (nectar_plant_number) ∼ body_size + family, family = gaussian). Model assumptions of GLMs were tested and R square values of the models were determined by using r.squaredGLMM and r2 function of MuMIn and performance packages [31, 32]. All the Data were analyzed and visualized in RStudio ver 3.6.2 (R Core Team, 2019) [33] using dplyr package [34].
3. Results
3.1. Study selection
The systematic search returned a total of 92 publications (Figure 1). After an initial review of the titles and abstracts, 18 publications were excluded because they did not meet the selection criteria (studies did not focus on butterflies or conducted outside of the geographical distribution of Bangladesh) (Figure 1). The full text of the 74 publications was obtained and screened for the selection criteria (Figure 1). Of these, 36 publications were excluded because those studies did not provide data for the wing size, host plants, and food plants of the butterflies of Bangladesh (Figure 1). Finally, 38 publications fulfilled the selection criteria (Figure 1). A full list of all publications included in this study is provided in the supplementary data (Supplementary file S1).
Figure 1.
Schematic overview of the systematic literature search.
3.2. Extinction risks
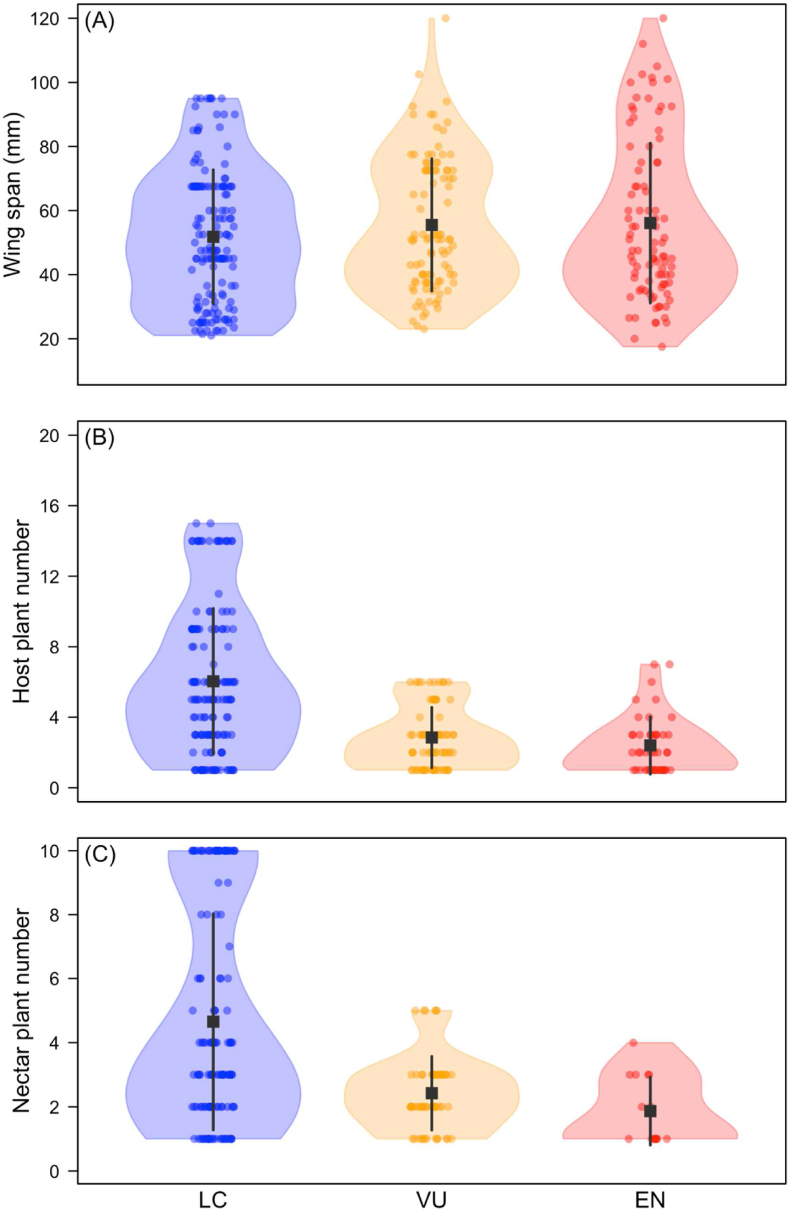
The extinction risk of the butterflies was correlated with their size; larger butterflies were in higher extinction risk than smaller butterflies (CLMM: estimate: 0.045 ± 0.01, Z = 2.9, P < 0.005, Figure 2a). Butterflies with lower number of host plants were in greater extinction risks than butterflies with higher host plants (CLMM: estimate: -0.42 ± 0.09, Z = -4.36, P < 0.0001, Figure 2b). Similarly, butterflies with lower number of nectar plants were in greater extinction risks than butterflies with higher host plants (CLMM: estimate: -0.46 ± 0.13, Z = -3.46, P < 0.001, Figure 2b). Body size of the butterflies did not have a significant effect on the number host plant (GLM: χ2 = 0.12, df = 1, p = 0.91, R2 = 0.30) and the number nectar plant (GLM: χ2 = 0.27, df = 1, p = 0.60, R2 = 0.29).
Figure 2.
Relation between body size, and diet breadth to local extinction risk of butterflies. A) wing size and local extinction risks of butterflies, B) host plant number and local extinction risk of butterflies, C) nectar plant number and local extinction risk of butterflies. Violin plots show the kernel probability density of the data, squares indicate mean of data; vertical lines connecting squares indicate standard deviation of data; circles indicate data point of each species. LC = least concern, VU = vulnerable, and EN = endangered.
4. Discussion
Monitoring regional extinction risks and understanding the ecological factors that contribute to such extinctions are essential to develop conservation strategies for the protection of a species. We tested contributions of the body size and food plants of the butterflies to their extinction risks. We found that body size and larval host plants are significant contributors of the extinction of butterflies.
We showed that larger butterflies were in greater extinction risk compared to the smaller butterflies, a pattern also found in butterflies [35] and other insects i.e., damselflies [30], and ground beetles [36]. Larger insects require more resources for growth and development, have lower reproduction rates, and smaller population sizes [37, 38]; all of which contribute to the extinction risk. A few previous studies, on the other hand, did not find significant effect of body sizes on extinction risks of butterflies [9, 39]. Larger body size of insects are often associated larger geographical distribution and greater dispersion capacity which can provide larger insects to access greater number of food resources, thereby reducing extinction risks [40]. We, however, did not find any evidence in support that larger butterflies can access greater number of nectar plant and host plants which probably further justify our findings. Overall, our study support global and regional extinction risks in the trend that invertebrates [41, 42, 43, 44] and vertebrates [45, 46] that larger species are usually more vulnerable to extinction.
Our findings showed that extinction risks of butterflies with fewer host plants are greater than butterflies with higher host plants. The diversity of butterflies, like many other insects, depends on the abundance of food sources and habitats [47, 48]. Butterfly larvae depend on host plants for their growth, development and therefore higher plant richness promotes greater butterfly diversity [49]. Butterflies that can use resources from greater number of host plants are expected to have a greater likelihood of fulfilling their resource requirements in a greater number of habitat patches [50]. Therefore, highly host-specific butterflies are more likely to be vulnerable to localized fragmentation of resources [9]. Our study corroborated previous findings that larval host plant richness are the most important determinant regional extinction of butterflies [9, 51]. Butterflies with very limited and threatened host plants are also likely to co-extinction their host plants [19, 52]. Furthermore, this greater extinction risks of herbivore insects with fewer host plants are also applicable to other insects such as moths [39], aphids [53], leaf beetles [54], and weevils [55].
Nectar feeding of adult butterflies plays a critically important role for somatic maintenance and reproduction, and population persistence [56, 57], especially for the species that emerge with unyoked eggs [58]. Butterfly morphology such as proboscis length, and flower morphology such as corolla depth restrict butterflies to extract nectar from a limited number of plant species [59], therefore nectar plant resources are predicted to contribute to the extinction of butterflies. Similarly, nectar plants diversity have been found as a significant contributor of extinction risk in butterflies [60]. Our result corroborates previous findings that nectar plants breadth may contribute to the extinction risk of butterflies [61, 62]. The impact of the nectar plants on butterfly extinction risks is strong as host plants probably because adults butterflies, like the butterflies larva, adults use only limited number of plants for feeding because of the length of proboscis, and specific nutritional requirements for egg maturation [63]. In comparison to host plants, nectar plants of Bangladeshi butterflies are less studied. Our study indicates the importance of nectar plants for butterflies and thereby suggesting further studies are required to understand requirements of specific nectar plants to develop conservation strategies to protect the vulnerable butterflies of Bangladesh.
Understanding extinction risks and identifying its potential drivers are essential step to determine the conservation demand for the protection insects. In this study, we showed that butterflies of Bangladesh with larger wingspan, and fewer host and nectar plants are in greater extinction risk. Our study highlights the drivers of local extinction risks of the butterflies. Recent studies showed that the butterflies of Bangladesh are weekly protected [64]. Our study will provide resources for conservation biologists to develop conservation strategies to protect the butterflies of Bangladesh.
Declarations
Author contribution statement
Anwar Palash, Shatabdi Paul: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sabrina Karim Resha: Performed the experiments.
Md Kawsar Khan: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
MKK thanks Payal Barua and Leelaboti Khona for their support.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Butchart S.H., Walpole M., Collen B., Van Strien A., Scharlemann J.P., Almond R.E., Baillie J.E., Bomhard B., Brown C., Bruno J. Global biodiversity: indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 2.Gonçalves J., Alves P., Pôças I., Marcos B., Sousa-Silva R., Lomba Â., Honrado J.P. Exploring the spatiotemporal dynamics of habitat suitability to improve conservation management of a vulnerable plant species. Biodivers. Conserv. 2016;25:2867–2888. [Google Scholar]
- 3.Cardinale B.J., Duffy J.E., Gonzalez A., Hooper D.U., Perrings C., Venail P., Narwani A., Mace G.M., Tilman D., Wardle D.A. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 4.Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Am. Entomol. 1969;15:237–240. [Google Scholar]
- 5.Tsahar E., Izhaki I., Lev-Yadun S., Bar-Oz G. Distribution and extinction of ungulates during the Holocene of the southern Levant. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinney M.L. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Systemat. 1997;28:495–516. [Google Scholar]
- 7.Russell G.J., Brooks T.M., McKinney M.M., Anderson C.G. Present and future taxonomic selectivity in bird and mammal extinctions. Conserv. Biol. 1998;12:1365–1376. [Google Scholar]
- 8.Purvis A., Gittleman J.L., Cowlishaw G., Mace G.M. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh L.P., Sodhi N.S., Brook B.W. Ecological correlates of extinction proneness in tropical butterflies. Conserv. Biol. 2004;18:1571–1578. [Google Scholar]
- 10.Pimm S.L., Jenkins C.N., Abell R., Brooks T.M., Gittleman J.L., Joppa L.N., Raven P.H., Roberts C.M., Sexton J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344 doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 11.New T.R. John Wiley & Sons; 2013. Lepidoptera and Conservation. [Google Scholar]
- 12.Proctor M., Yeo P., Lack A. HarperCollins Publishers; 1996. The Natural History of Pollination. [Google Scholar]
- 13.Capinera J.L. Springer Science & Business Media; 2008. Encyclopedia of Entomology. [Google Scholar]
- 14.Gullan P.J., Cranston P.S. John Wiley & Sons; 2014. The Insects: an Outline of Entomology. [Google Scholar]
- 15.Franzén M., Johannesson M. Predicting extinction risk of butterflies and moths (Macrolepidoptera) from distribution patterns and species characteristics. J. Insect Conserv. 2007;11:367–390. [Google Scholar]
- 16.Nieminen M. Risk of population extinction in moths: effect of host plant characteristics. Oikos. 1996:475–484. [Google Scholar]
- 17.Johst K., Brandl R. Body size and extinction risk in a stochastic environment. Oikos. 1997:612–617. [Google Scholar]
- 18.Hanski I., Singer M.C. Extinction-colonization dynamics and host-plant choice in butterfly metapopulations. Am. Nat. 2001;158:341–353. doi: 10.1086/321985. [DOI] [PubMed] [Google Scholar]
- 19.Koh L.P., Sodhi N.S., Brook B.W. Co-extinctions of tropical butterflies and their hostplants. Biotropica. 2004;36:272–274. [Google Scholar]
- 20.Shah M.N.A., Khan M.K. OdoBD: an online database for the dragonflies and damselflies of Bangladesh. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen T. 2004. Butterflies of Bangladesh : an Annotated Checklist.https://portals.iucn.org/library/node/8591 accessed. [Google Scholar]
- 22.Khan M.K. Three new records of butterfly from university of Chittagong and Shahjalal University of science and technology in Bangladesh. Int. J. Fauna Biol. Stud. 2014;1:30–33. [Google Scholar]
- 23.Khan M.K., Neogi A.K. Confirmation, distribution and updated status of Tarucus venosus Moore, 1882 in Bangladesh. J. Entomol. Zool. Stud. 2014;2:125–128. [Google Scholar]
- 24.Rahman M.S., Al Haidar I.K., Neogi A.K., Ul Hasan M.A., Rahman M.M., Shaburul Imam S.M. First record of six species and subspecies of butterflies (Insecta: Lepidoptera) in Bangladesh. J. Insect Biodivers. Syst. 2016;2:373–380. [Google Scholar]
- 25.Khan A.K.M.M.A., Khan T., Khan M.K. Three new records of butterfly from north-east region of Bangladesh, festschr. 50th anniv. IUCN red list threat Species. 2014:35–38. [Google Scholar]
- 26.Neogi A.K., Baki M.A., Sadat M.N., Selim S.R., Bhouiyan N.A. Five new records of butterfly species from dhaka, pirojpur and Cox`S bazar districts in Bangladesh. J. Entomol. Zool. Stud. 2014;2:197–200. [Google Scholar]
- 27.Chowdhury S., Hesselberg T., Böhm M., Islam M.R., Aich U. Butterfly diversity in a tropical urban habitat (Lepidoptera: papilionoidea), Orient. Insects. 2017;51:417–430. [Google Scholar]
- 28.Red List of Bangladesh : Volume 7 : Butterflies. IUCN; 2016. https://www.iucn.org/content/red-list-bangladesh-volume-7-butterflies [Google Scholar]
- 29.Suárez-Tovar C.M., Rocha-Ortega M., González-Voyer A., González-Tokman D., Córdoba-Aguilar A. The larger the damselfly, the more likely to be threatened: a sexual selection approach. J. Insect Conserv. 2019;23:535–545. [Google Scholar]
- 30.Rocha-Ortega M., Rodríguez P., Bried J., Abbott J., Córdoba-Aguilar A. Why do bugs perish? Range size and local vulnerability traits as surrogates of Odonata extinction risk. Proc. R. Soc. B Biol. Sci. 2020;287 doi: 10.1098/rspb.2019.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartoń K. 2019. MuMIn: Multi-Model Inference. R Package Version 1.42.1.https://CRAN.R-project.org/package=MuMIn [Google Scholar]
- 32.Lüdecke D., Makowski D., Ben-Shachar M., Patil I., Waggoner P., Wiernik B.W., Arel-Bundock V., Jullum M. 2021. Assessment of Regression Models Performance. [Google Scholar]
- 33.R.C. Team, R: A . 2019. Language and Environment for Statistical Computing. [Google Scholar]
- 34.Wickham H., François R., Henry L., Müller K., RStudio dplyr. 2019. A Grammar of Data Manipulation.https://CRAN.R-project.org/package=dplyr [Google Scholar]
- 35.Purvis A., Gittleman J.L., Cowlishaw G., Mace G.M. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolte D., Boutaud E., Kotze D.J., Schuldt A., Assmann T. Habitat specialization, distribution range size and body size drive extinction risk in carabid beetles. Biodivers. Conserv. 2019;28:1267–1283. [Google Scholar]
- 37.Matern A., Drees C., Meyer H., Assmann T. Population ecology of the rare carabid beetle Carabus variolosus (Coleoptera: carabidae) in north-west Germany, J. Insect Conserv. 2008;12:591–601. [Google Scholar]
- 38.Chichorro F., Juslén A., Cardoso P. A review of the relation between species traits and extinction risk. Biol. Conserv. 2019;237:220–229. [Google Scholar]
- 39.Nieminen M. Risk of population extinction in moths: effect of host plant characteristics. Oikos. 1996;76:475–484. [Google Scholar]
- 40.Bommarco R., Biesmeijer J.C., Meyer B., Potts S.G., Pöyry J., Roberts S.P.M., Steffan-Dewenter I., Öckinger E. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. R. Soc. B Biol. Sci. 2010;277:2075–2082. doi: 10.1098/rspb.2009.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattila N., Kotiaho J.S., Kaitala V., Komonen A. The use of ecological traits in extinction risk assessments: a case study on geometrid moths. Biol. Conserv. 2008;141:2322–2328. [Google Scholar]
- 42.Seibold S., Brandl R., Buse J., Hothorn T., Schmidl J., Thorn S., Müller J. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conserv. Biol. 2015;29:382–390. doi: 10.1111/cobi.12427. [DOI] [PubMed] [Google Scholar]
- 43.Shahabuddin G., Ponte C.A. Frugivorous butterfly species in tropical forest fragments: correlates of vulnerability to extinction. Biodivers. Conserv. 2005;14:1137–1152. [Google Scholar]
- 44.Terzopoulou S., Rigal F., Whittaker R.J., Borges P.A., Triantis K.A. Drivers of extinction: the case of Azorean beetles. Biol. Lett. 2015;11 doi: 10.1098/rsbl.2015.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett P.M., Owens I.P. Variation in extinction risk among birds: chance or evolutionary predisposition? Proc. R. Soc. Lond. B Biol. Sci. 1997;264:401–408. [Google Scholar]
- 46.Fritz S.A., Bininda-Emonds O.R., Purvis A. Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol. Lett. 2009;12:538–549. doi: 10.1111/j.1461-0248.2009.01307.x. [DOI] [PubMed] [Google Scholar]
- 47.Burghardt K.T., Tallamy D.W., Gregory Shriver W. Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conserv. Biol. 2009;23:219–224. doi: 10.1111/j.1523-1739.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 48.Ricketts T.H., Daily G.C., Ehrlich P.R. Does butterfly diversity predict moth diversity? Testing a popular indicator taxon at local scales. Biol. Conserv. 2002;103:361–370. [Google Scholar]
- 49.Bashar M.A., Mamun M.A., Aslam A.F.M., Chowdhury A.K. Biodiversity maintenance and conservation of butterfly-plant association in some forests of Bangladesh. Bangladesh J. Zool. 2006;34:55. [Google Scholar]
- 50.Swihart R.K., Gehring T.M., Kolozsvary M.B., Nupp T.E. Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: the importance of niche breadth and range boundaries. Divers. Distrib. 2003;9:1–18. [Google Scholar]
- 51.León-Cortés J.L., Lennon J.J., Thomas C.D. Ecological dynamics of extinct species in empty habitat networks. 2. The role of host plant dynamics. Oikos. 2003;102:465–477. [Google Scholar]
- 52.Stork N.E., Lyal C.H. Extinction or’co-extinction’rates? Nature. 1993;366:307. 307. [Google Scholar]
- 53.Thacker J.I., Hopkins G.W., Dixon A.F.G. Aphids and scale insects on threatened trees: co-extinction is a minor threat. Oryx. 2006;40:233–236. [Google Scholar]
- 54.Fox J.W., Vasseur D., Cotroneo M., Guan L., Simon F. Population extinctions can increase metapopulation persistence. Nat. Ecol. Evol. 2017;1:1271–1278. doi: 10.1038/s41559-017-0271-y. [DOI] [PubMed] [Google Scholar]
- 55.Tol R.W.H.M.V., Dijk N.V., Sabelis M.W. Host plant preference and performance of the vine weevil Otiorhynchus sulcatus. Agric. For. Entomol. 2004;6:267–278. [Google Scholar]
- 56.Murphy D.D., Menninger M.S., Ehrlich P.R. Nectar source distribution as a determinant of oviposition host species in Euphydryas chalcedona. Oecologia. 1984;62:269–271. doi: 10.1007/BF00379025. [DOI] [PubMed] [Google Scholar]
- 57.Tudor O., Dennis R.L.H., Greatorex-Davies J.N., Sparks T.H. Flower preferences of woodland butterflies in the UK: nectaring specialists are species of conservation concern. Biol. Conserv. 2004;119:397–403. [Google Scholar]
- 58.Wheeler D. The role of nourishment in oogenesis. Annu. Rev. Entomol. 1996;41:407–431. doi: 10.1146/annurev.en.41.010196.002203. [DOI] [PubMed] [Google Scholar]
- 59.Tiple A.D., Khurad A.M., Dennis R.L.H. Adult butterfly feeding–nectar flower associations: constraints of taxonomic affiliation, butterfly, and nectar flower morphology. J. Nat. Hist. 2009;43:855–884. [Google Scholar]
- 60.Wallisdevries M.F., Van Swaay C.A.M., Plate C.L. Changes in nectar supply: a possible cause of widespread butterfly decline. Curr. Zool. 2012;58:384–391. [Google Scholar]
- 61.Schultz C.B., Dlugosch K.M. Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia. 1999;119:231–238. doi: 10.1007/s004420050781. [DOI] [PubMed] [Google Scholar]
- 62.Brommer Jon.E., Fred M.S. Movement of the Apollo butterfly Parnassius apollo related to host plant and nectar plant patches. Ecol. Entomol. 1999;24:125–131. [Google Scholar]
- 63.Thomas R.C., Schultz C.B. Resource selection in an endangered butterfly: females select native nectar species. J. Wildl. Manag. 2016;80:171–180. [Google Scholar]
- 64.Chowdhury S., Alam S., Chowdhury S.U., Rokonuzzaman Md., Shahriar S.A., Shome A.R., Fuller R.A. Butterflies are weakly protected in a mega-populated country, Bangladesh. Glob. Ecol. Conserv. 2021;26 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.