Abstract
The interaction between gut microbiota-derived metabolites and the body plays a significant role in the occurrence and development of cancer. Secondary bile acids (BAs) are the important products produced from gut microbial fermentation of primary BAs, mainly deoxycholic acid (DCA) and lithocholic acid (LCA). In the gut, they can influence the structure of the microbial communities. Several studies have demonstrated that secondary BAs, as signaling molecules, can activate a variety of signaling pathways. They can inhibit the apoptosis of cancer cells, induce the progression of cancer cell cycles, enhance the ability of metastasis and invasion of cancer cells, and promote the transformation of cells into cancer stem cells (CSCs). Moreover, secondary BAs promote cancer by regulating the function of immune cells. Therefore, targeted manipulation of gut microbial and secondary BAs has the potential to be developed as for treatment and prevention of various cancers.
Keywords: secondary bile acids, cancer, Gut microbiota, metabolism, dietary changes
Introduction
Recent years have witnessed a surge in research concerning the relationship between gut microbiota and their metabolites and cancer. Secondary bile acids (BAs) are the products of gut microbiota and can directly or indirectly regulate microbial composition. 1 Studies have revealed that through simple dietary changes, 1 antibiotics, 2 and fecal transplants, 3 gut microbial levels can be regulated, thus regulating secondary BAs’ levels. Many studies indicated that secondary BAs play a specific cancer-promoting effect in colorectal cancer and extra-intestinal cancers, such as liver cancer, pancreatic cancer, esophageal cancer, lung cancer, and gastric cancer.4-8 Therefore, this review describes the interaction between gut microbiota and secondary BAs, highlighting the direct carcinogenic effect of secondary BAs using the above cancer cell lines as examples. This review also examines the regulation of secondary BAs on immune cells.
The Metabolic Process of Secondary BAs
Synthesis of Secondary BAs
Cholesterol is converted to primary bile acids, including cholic acid (CA) and chenodeoxycholic acid (CDCA) in hepatocytes through the classic or alternative bile acid synthesis pathways. 9 The classical pathway is initiated by 7α-hydroxylase (CYP7A1), which is the rate-limiting enzyme, to generate CA and CDCA. The alternative pathway is initiated by CYP27A1, which is followed by CYP7B1 to generate CDCA. 10 BAs bind easily to glycine in vegetarians and taurine in people with a high animal protein or seafood diet. 11 CA and CDCA are conjugated to amino acids taurine or glycine through BA-CoA synthase and BA-amino acid N-acetyl transferase. 9 Amino acid conjugation reduced the pKa of BAs to prevent passive absorption in the duodenum for proper emulsification of dietary lipids, which allows them to be stored in the gallbladder as mixed micelles with cholesterol and phospholipids. The duodenum secretes cholecystokinin in response to food intake, causing primary BAs to enter the small intestine. 9 In the terminal ileum, up to 95% of BAs are reabsorbed back into the liver. 12 In the terminal ileum, the cecum, and upper colon, the primary BAs which are not reabsorbed can produce more than 50 types of secondary BAs. DCA and LCA represent the most common secondary BAs. 13 Microbial bile salt hydrolase (BSH) deconjugates CA and CDCA with glycine or taurine to produce free CA and CDCA. Then microbial 7α-dehydroxylase (7α-HSDH) dehydroxylates them to a lesser extent, converting them respectively to DCA and LCA. 14 As a result of complex microbial biotransformation, numerous derivatives are formed. 15
Absorption of Secondary BAs
DCA is partly reabsorbed in the gut, and LCA is fairly insoluble and little of it is reabsorbed. Part of the unabsorbed secondary BAs are excreted through feces. 16 Moreover, secondary BAs generated in the terminal ileum are actively transported into the epithelial cells of the small intestine, primarily through the apical sodium-dependent bile salt transporter (ASBT) in the apical membrane of the brush margin of the small intestine. Then secondary BAs are inter-intestinal transported to the sinusoidal membrane to exit into the portal circulation by organic solute transporters OSTα and OSTβ of the basement membrane. 12 In addition, secondary BAs generated in the cecum and upper colon are mainly absorbed into the portal circulation through passive diffusion. 17 After that, they are actively transported to the liver cells through sodium taurocholate cotransport. 18
A small fraction of primary BAs can escape the enterohepatic circulation and enter the systemic circulation, 19 and then secondary BAs may be synthesized under the action of bacteria in distant organs, allowing secondary BAs’ signaling to occur in other organs and tissues. 13 In addition, a portion of secondary BAs can be reversely transferred through the intestine to the stomach and esophagus. 20
Regulation of secondary BAs’ metabolism
Regulation of secondary BAs synthesis and absorption is performed mainly by a negative feedback mechanism exerted by Farnesoid × receptor (FXR). In addition, intestinal bacteria and some chemicals may also play a regulatory role.
The FXR suppresses synthesis of secondary BAs. FXR in liver can be activated by hydrophobic primary BAs and secondary BAs, then inhibits the expression of CYP7A1, 12 therefore decreases the levels of primary BAs and then decreases the levels of secondary BAs. Moreover, FXR can also inhibit CYP8B1 and CYP27A1 by complicated mechanisms. 21 The FXR also regulates absorption of secondary BAs. In enterocytes, FXR induces OSTα and OSTβ to efflux secondary BAs into portal blood circulation, therefore limits the uptake of secondary BAs by intestinal cells and reduces their carcinogenic effects on the intestine. 22 The specific mechanism of FXR-mediated regulation of BA synthesis is described in section 3.1.
In addition, hormones and exogenous compounds can also affect secondary BAs synthesis, such as insulin, thyroid hormone, or some drugs, such as phenobarbital and rifampicin, which all affect their synthesis by targeting CYP7A1. Dietary fibers (from vegetables and fruits) can bind to LCA and aid in its excretion in stool. 16 More importantly, a variety of factors can regulate the absorption and synthesis of secondary BA by influencing gut bacteria levels, 23 such as dietary changes and antibiotics. The details will be described in section 4.1.3.
Bile acid receptor-mediated signaling pathways
Studies have found that BAs realize their effects through nuclear farnesoid × receptor (FXR), membrane TGR5 receptor, vitamin D receptor (VDR), pregnane × receptor (PXR) and constitutive androstane receptor (CAR). 21 Among them, primary BAs mainly activate FXR and secondary BAs mainly activate TGR5. Therefore, we mainly introduce these 2 receptor-mediated bile acid signaling pathways.
FXR-Mediated Signaling Pathways
FXR is expressed in several organs, including the liver, intestine, kidneys, and immune cells. The FXR-mediated signaling pathways can regulate the metabolism of BAs, sugar and lipid and regulate inflammatory response. 23
The FXR-mediated signaling pathways suppress the synthesis of BAs. FXR in liver can be activated by hydrophobic primary BAs and secondary BAs, then induces small heterodimer partner (SHP) to inhibit the expression of CYP7A1. 12 In addition, in human primary hepatocytes, activated FXR induces fibroblast growth factor 19(FGF19), an intestinal hormone, to activate extracellular signal-regulated kinase 1/2 (ERK1/2) of the MAPK pathway and inhibit CYP7A1 gene transcription. 24 Activated FXR can also induce FGF19 in human and FGF15 in mice, and then activate hepatic FGF receptor 4 (FGFR4) signaling to inhibit CYP7A1. 12 Moreover, BAs induce pro-inflammatory cytokines TNFα and IL-1β in macrophages/Kupffer cells to activate TLR4 in hepatocytes, and then inhibit CYP7A1 and CYP8B1 through ERK1/2 /JNK signaling. 24
Studies have found that activation of FXR could activate the AKT and ERK1/2 signaling pathways, and then collaborate with insulin in regulating the metabolism of sugar in the liver. 25 In addition, FXR activation may reduce NAFLD, as it reduces steatosis by inhibiting lipogenesis, decreases chemically-induced hepatic inflammation and fibrosis. 25 What’s more, FXR activation has been reported to repress the NF-κB signaling pathway and inhibit the IL-6/STAT3 signaling pathway, 26 and thus reduces the production of pro-inflammatory cytokines and exerts anti-inflammatory effects.
TGR5-Mediated Signaling Pathways
TGR5 is highly represented in the gastrointestinal tract, non-parenchymal liver cells, and macrophages, overseeing a variety of homeostatic and regulatory functions, thus preventing the occurrence of diabetes, inflammatory bowel disease and other diseases. 27 However, during dysbiosis, its activation may play a pro-cancer effect in a variety of cancers.
In the enteroendocrine cells, TGR5 stimulates glucagon-like peptide 1, thereby exerting anti-diabetic activity. 19 Secondary BAs are also reported to inhibit the activation of NLRP3 inflammasome via the TGR5–cAMP–PKA axis, thereby exerting anti-inflammatory effects. 27
Intestinal metaplasia (IM) increases the risk of gastric cancer. A recent study found that DCA is involved in the development of IM through the TGR5-ERK1/2-HNF4α axis. 28 What’s more, liver fibrosis increases the risk of liver cancer. A recent study revealed that by acting on TGR5 in HSCs in mice, taurodeoxycholate (TDCA) and glycodeoxycholate (GDCA) activated p38MAPK and ERK1/2 signaling pathways, thus significantly promoting liver fibrosis. 10 Studies have shown that DCA can also activate EGFR, mitogen-activated protein kinase (MAPK) and STAT3 signaling pathways by acting on TGR5, and play carcinogenic effects in colorectal cancer and other cancers. 29 The specific anti-cancer mechanism of secondary BAs will be illustrated later.
The Interaction Between Gut Microbiota and Secondary BAs
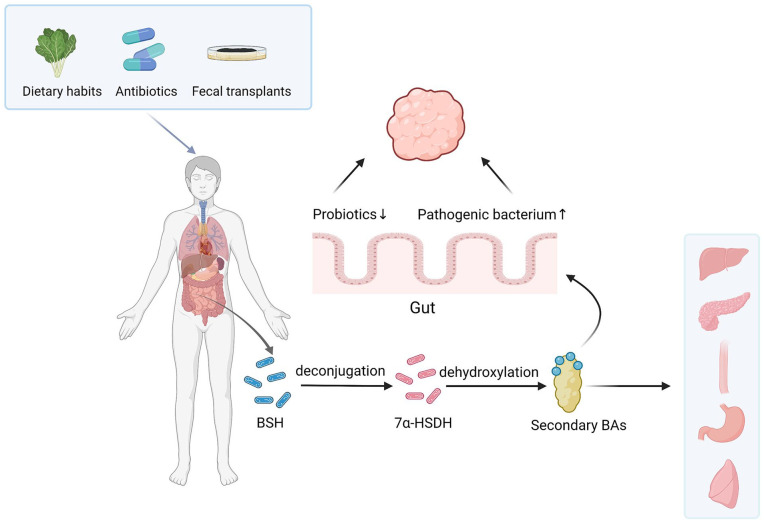
The gut houses the largest and most diverse population of microbes among the body’s many organs. 30 Secondary BAs are produced in the terminal ileum, the caecum, and upper colon through deconjugation, dehydroxylation, oxidation, isopropylation, desulfurization, and esterification. BSH induces deconjugation, and 7α-HSDH induces dehydroxylation, the 2 most important reactions in the conversion process.13,31 In addition, secondary BAs can shape the structure of intestinal microbial communities after being produced 9 (Figure 1).
Figure 1.
Metabolic processes of secondary BAs and their interactions with intestinal bacteria.
In the terminal ileum, the cecum, and upper colon, secondary bile acids (BAs) are produced by the deconjugation of BSH and the dehydroxylation of 7α-HSDH from primary BAs. Through simple dietary changes, antibiotics, and fecal transplants, intestinal microbes can be regulated, alongside regulating levels of secondary BAs. Secondary BAs can regulate gut microbial composition, increase the levels of pathogenic bacterium and reduce the levels of probiotics, thus promoting the occurrence of intestinal tumors. In addition, their signaling can occur in the liver, pancreas, esophagus, stomach, and lung.
Gut Microbiota and Formation of Secondary BAs
Bacteria with BSH activity
Bacteria with BSH activity mainly include Firmicutes and phyla Bacteroidetes. Gram-positive bacteria are Clostridium, Bacteroidetes, Lactobacillus, Bifidobacterium, Enterococcus, Escherichia, and Listeria.13, 31 Gram-negative bacteria are primarily Methanobrevibacter smithii and Methanosphera stadtmanae of the domain Archaea. 32 Furthermore, some aerobic bacteria, such as Actinobacteria and Proteobacteria, can also produce BSH. 12
Bacteria with 7α-HSDH activity
Bacteria can remove the hydroxyl groups from unbound BAs, but only a few have 7α-HSDH activity. These bacteria are mainly Clostridium and Eubacterium in the phylum Firmicutes.13,33 In addition, Ruminococcus and Trichospiraceae also have 7α-HSDH activity, 34 and the content of Ruminococcus is positively correlated with the content of DCA. 35 A recent study demonstrated that Desulfovibrionale also has 7α-HSDH activity, and mice carrying Desulfovibrionale produce more secondary BAs. 36
Steerability of gut microbiota
Multiple factors can increase the levels of bacteria that produce secondary BAs. A study found that after feeding wild-type C57BL/6 mice a high-fat diet for 20 weeks, the proportion of Clostridium in the intestinal tract of the mice increased concomitantly with the levels of DCA. 2 Compared to healthy rural Africans on a low-fat, high-fiber diet, healthy Africans on a high-fat, low-fiber diet had higher fecal 7α-dehydroxylated bacteria and higher levels of secondary BAs. 37 In addition patients with active ulcerative colitis, who received a fecal transplant had higher levels of Escherichia and secondary BAs than those given a placebo. 3
Multiple factors can decrease the levels of bacteria that produce secondary BAs. A study found that 2 the levels of Clostridium and DCA in wild-type C57BL/6 mice decreased significantly after vancomycin treatment at week 9. In liver cancer models driven either by the hepatocarcinogen diethylnitrosamine or overexpression of c-Myc, using vancomycin can play the same role, thus preventing liver cancer progression. 38 In addition, the activity of many bacteria is affected by the gut’s pH, such as Clostridium c-25, which only performs well in neutral or slightly alkaline gut environments. 39 When healthy subjects took lactulose, their gut pH and DCA levels decreased. 40 Taking Pu ‘erh tea, water extract of Ganoderma lucidum, walnut, and vitamin B6 can also reduce the levels of bacteria that produce secondary BAs, thereby reducing secondary BAs.13,40-43
Secondary BAs Change the Composition of Gut Microbiota
Pathogenic bacteria
Opportunistic pathogens such as Ruminococcus, Shigella, Desulforvibrio, and Dorea are known to play an essential role in developing gut tumors. However, studies have disclosed that DCA can promote the growth of these bacteria. 44 Moreover, Akkermansia muciniphila and Bacteroidetes are mucin degrading bacteria. Excess mucin degradation can damage the integrity of the intestinal mucosal barrier and promote the colonization and carcinogenesis of intestinal pathogens. However, a study has found that the levels of DCA and Akkermansia muciniphila, and Bacteroidetes increased in CA-treated APCmin/+ mice. 4 It is known that Apcmin/+ mice can develop intestinal adenomas spontaneously, which are good animal models of colorectal cancer precancerous lesions. 4
Probiotics
Probiotics such as Lactobacillus and Bifidobacterium in the small intestine are known to promote health by mediating host immune homeostasis. 45 Moveover, nisin, a polycyclic peptide produced during bacterial fermentation, has shown cytotoxic effects on colorectal cancer cells and head and neck squamous cell carcinomas. So far, Lactobacillus has been found to contain 4 types of nisin. 46 DCA is one of the most effective anti-bacterial BAs. Its anti-bacterial activity is 10 times that of CA. It can significantly inhibit the growth of Lactobacillus and Bifidobacterium. 31 In addition, Clostridium butyricum is known to reduce the content of secondary BAs and increase the quantity of short-chain fatty acids (SCFAs) in the cecum. 47 SCFAs have been shown to have anti-cancer effects in colorectal, gastric, breast, prostate, and liver cancers. 48 However, study has shown that the level of Clostridium butyricum decreased in APCmin/ + mice treated with DCA. 44
Mechanism of Secondary BAs Promoting Cancer
Secondary BAs are strong signal molecules that control various physiological and pathological processes and can activate various signaling pathways. Then they can inhibit the apoptosis of cancer cells, induce the progression of cancer cell cycle, enhance the ability of metastasis and invasion of cancer cells, and promote the transformation of cells into cancer stem cells (CSCs). Through these mechanisms, secondary BAs can directly promote cancer. In addition, secondary BAs can indirectly promote cancer by regulating the function of immune cells (Figure 2).
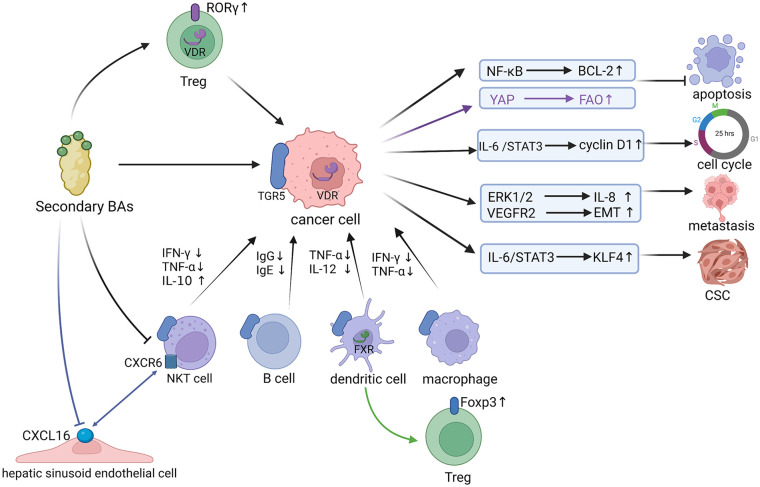
Figure 2.
Carcinogenic mechanisms of secondary Bas.
After acting on TGR5 on the surface of the cancer cells or VDR in the nucleus of the cancer cells, secondary BAs can activate various signaling pathways, inhibit the apoptosis of cancer cells, induce the progression of the cancer cell cycle, enhance the ability of metastasis and invasion of cancer cells, and promote the transformation of cells into cancer stem cells (CSCs). The YAP signaling pathway is mediated by VDR in the nucleus, while the others are mediated by TGR5. What’s more, secondary BAs can inhibit the functions of NKT cells, B cells, dendritic cells, and macrophages via acting on TGR5. In addition, the expression of Foxp3 was influenced by FXR on DCs’ surface, but not by FXR on CD4 + T cells’ surface, while secondary BAs up-regulated the level of RORγ + Treg cells in mouse colon through the BA receptor VDR. Besides, secondary BAs can reduce the level of CXCL16 chemokine in hepatic sinusoid endothelial cells, thereby inhibiting the accumulation of CXCR6 + NKT cells.
Direct Cancer-Promoting Effect
Inhibiting the apoptosis of cancer cells
Secondary BAs can inhibit the apoptosis of cancer cells by regulating the mitochondrial apoptosis pathway and metabolic pathway.
The mitochondrial apoptosis pathway is mainly regulated by the B-cell lymphoma factor 2 (BCL-2) family in the outer membrane of mitochondria. 49 BCL-XL and BCL-2 are members of anti-apoptotic proteins. 50 Researchers have found that DCA activated the IL-6/STAT3 signaling pathway in human esophageal adenocarcinoma cell line OE33, then up-regulated the expression of anti-apoptotic protein BCL-XL and improved the anti-apoptotic activity of OE33. 7
A recent study has shown that in subjects from the community-based Cameron County Hispanic Cohort, DCA, and total unconjugated secondary BAs were positively associated with liver steatosis, which is a risk factor for HCC. 51 In addition, the intestinal microbial receptors of Toll-like receptor 5 deficient (T5KO) mice were disabled and the mice were immunodeficient, and inulin could induce liver cancer in these mice. A study has revealed that in vancomycin-treated T5KO mice, the levels of gut microbiota that produces secondary BAs decreased, including Lachnospiraceae, Ruminococcaceae, and Clostridium XIVa, and then protected T5KO mice from inulin-induced HCC. 38 Moreover, studies have found that DCA can inhibit the apoptosis of liver cancer cells. Golgi protein 73 (GP73) is a type II transmembrane glycoprotein, 52 and its increased expression in hepatocytes has been associated with advanced liver disease. 53 However, a study 54 has found that in the human liver cancer line Hep G2, reduced expression of GP73 might lead to a reduction in BCL-2. In human liver cancer line Huh-7 and SMMC7721, DCA activated the NF-κB pathway to destroy the Golgi structure by binding to TGR5, thereby promoting the release of GP73. 55 Besides, a study 56 has shown that NF-κB can reduce apoptosis by activating the anti-apoptotic protein BCL-2. However, DCA has been demonstrated to significantly activate the NF-κB signaling pathway, thereby enhancing the anti-apoptotic ability of cancer cells. 57 It’s worth noting that secondary BAs have also been shown to promote apoptosis in cancer cells. A study has found that in human prostate cancer cell line PC-3, LCA triggered both intrinsic and extrinsic pathways of apoptotic cell death that were caspase-dependent, 58 however, the specific mechanism remains to be further studied.
Fatty acid oxidation (FAO) protects cancer cells from the loss-of-nest apoptosis by maintaining redox homeostasis. 59 However, a study has found that in metastatic lymph node tumors, conjugated DCA taurodeoxycholic acid (TDCA) can activate Yes-associated protein (YAP) signaling pathway by acting on nuclear VDR. Then, TDCA can promote the transformation of cancer cells into FAO metabolism mode. In this way, cancer cells can be adapted to tumor microenvironment and inhibit their apoptosis. 60 In addition, in human gastric carcinoma cell line AGS, DCA, through binding to TGR5, significantly activated EGFR or ERK1/2, then inhibited their apoptosis. 8
Inducing the progression of cancer cell cycle
The cell cycle includes 4 discrete phases: Gap 0/Gap 1 (G0/G1), Synthesis (S), Gap 2 (G2), and Mitosis (M). Cyclin regulates its progression, promoting the cell cycle by binding to cyclin-dependent kinases. 61 A study has found that in human pancreatic adenocarcinoma cell line BxPC3, DCA activated STAT3 signaling pathway and increased the expression of cyclin D1 after acting on TGR5. 29 In addition, the level of DCA increased in the CA-treated Apcmin/+ mice. Subsequently, DCA, through binding to TGR5, activated the IL-6 /STAT3 signaling pathway and increased the expression of cyclin D1. 4 Thus, DCA can promote the transition of the cell cycle from G1 to S. What’s more, study has demonstrated that in human lung cancer cell line H1975, the transcription activity of JAK2/STAT3 was significantly inhibited by TGR5 knockout. Flow cytometry results revealed that G1/S transition was significantly inhibited. 6
Enhancing the ability of metastasis and invasion of cancer cells
Studies of colon cancer patients have shown that their levels of fecal secondary BAs were elevated. 16 In addition, fecal samples from African Americans at high risk for colon cancer were more abundant in bacteria that encoded secondary BA production. 62 Moreover, studies have found that LCA can promote the metastasis of colon cancer cells. It is known that tumor angiogenesis is one of the key steps in tumor growth and metastasis, and IL-8 is an important angiogenic factor. 63 A study has shown that 1 day after LCA treatment of human colon cancer cell line HCT116, LCA activated ERK1/2 signal and increased IL-8 level by acting on TGR5 on the surface of HCT116. As a result of treating endothelial cells ECV304 with the supernatant of the cells mentioned above, the result showed that the endothelial cells proliferated obviously. This result indicates that LCA induces IL-8 expression in tumor microenvironment by activating ERK1/2 signaling pathway, then stimulates endothelial cell proliferation and tubular cell formation. 64
A study revealed that transfer of fecal microorganisms from DCA-treated mice to another group of Apcmin/+ mice increased colon cancer diversity. 44 In addition, in the azoxymethane (AOM) and dextran sodium sulfate (DSS)-induced murine colitis-associated colon cancer model, vancomycin treatment could decrease the levels of gut microbiota that produce secondary BAs and dramatically suppresses tumor development. 65 Moreover, studies have found that DCA can promote the metastasis of colon cancer cells. It is known that epithelial-mesenchymal transition (EMT) is closely related to cancer metastasis, and its main characteristics include decreased expression of E-cadherin and increased expression of vimentin. 66 A study has revealed that in Apcmin/+ mice and human colon cancer cell line HCT116, compared with the control groups, DCA upregulated VEGFR2 signaling pathway by acting on TGR5 on the surface of cancer cells, and then significantly inhibited e-cadherin expression and upregulated vimentin expression. 67
Giving mice a high-fat diet induced changes in gut microbiota and increased levels of DCA. Elevated DCA levels promoted the senescence-associated secretory phenotype (SASP) phenotype of hepatic stellate cell (HSC), which in turn secretes various pro-tumor factors in the liver, thereby promoting the development of HCC in mice exposed to chemical carcinogens. 68 In addition, a study treated human HSC line LX2 with DCA for 1 week, then treated human hepatoma cell line HuH with the supernatant of the above-mentioned cells. The wound healing test and the in vitro invasion test showed that the metastasis and invasion ability of HuH were higher than those of the control groups without DCA. Besides, the ERK1/2 and Smad3 signaling pathways were activated, and the expression of E-cadherin decreased and vimentin increased. 69 Furthermore, western blot assay showed that after treatment of the human non-small cell lung cancer cell line H1975 with increasing DCA concentrations (from 20 to 40 µM), the expression level of P-STAT3Tyr705 was significantly upregulated, and the migration and invasion of H1975 cells were greatly increased in a dose-dependent manner. 6 However, a recent study has also revealed that in mice that were grafted with breast cancer cell line 4T1, LCA acted on TGR5 on the surface of 4T1, then inhibitedβ-catenin signaling pathway, therefore reduced cellular proliferation and inhibited metastasis of 4T1. This may be due to the absence of acute toxic effects due to the use of LCA at concentrations of 100 to 1000 nM, which are closer to LCA concentrations reported in the breast.70,71
Promoting the transformation of cells into CSCs
It is well-known that CSCs are self-renewing cells that can generate heterogeneous tumor cells. 72 In addition, they are resistant to treatment, critical for cancer development, maintenance and recurrence.73,74
Secondary BAs can promote the transformation of non-cancer cells into CSCs. It is known that adenomatous polyps are the most common precursor to colon cancer. However, a study has found that the level of secondary BA producing bacteria in adenoma patients was higher than that in normal controls. Moreover, a study has shown that adding DCA into the feed of WT mice has the potential to induce colon cancer. 75 In addition, in human normal colon cells (HCoEpiC), DCA or LCA activated the Wnt/β-catenin signaling pathway and increased the levels of the oncogenic protein c-Myc by 12 to 15 times, thereby transforming normal colon cells into CSCs and triggering the occurrence of colon cancer. 76 A study has also shown that after 12 months of exposure to DCA, c-Myc expression in Barrett’s esophagus cells increased in a time-dependent manner, 77 then initiating and maintaining esophageal adenocarcinoma or esophageal squamous carcinoma. 78 These results suggest that LCA and DCA have the potential to transform non-cancer cells into CSCs.
Secondary BAs can promote the transformation of cancer cells into CSCs. A study has shown that in human esophageal adenocarcinoma cell line OE33, DCA, through binding to TGR5, upregulated the expression of the reprogramming factor Kruppel-like factor 4 (KLF4) by activating IL-6/STAT3 signaling pathway, leading to OE33 reprogramming into multi-differentiated CRCs, thereby increasing the chances of malignancy. 7 However, it is known that risk factors for ovarian cancer include loss-of-function mutations in the BRCA1 (breast cancer 1, early onset) gene. However, DCA resulted in a greater than four-fold induction of BRCA1 transcript levels, thereby reducing the risk of ovarian cancer. 79
Regulating Immune Cells to Promote Cancer Indirectly
Secondary BAs can mediate the communication between gut microbiota and the immune system, regulate the function of innate and specific immune cells, and shape the local intestinal and systemic immune environment, contributing to cancer promotion.
Regulating innate immune cells
Secondary BAs can inhibit the function of macrophages. It is known that macrophages can secrete IL-6 to promote B cell precursors to become antibody producing cells, secrete IFN-γ and TNF-α to promote apoptosis of cancer cells, secrete MCP-1 to chemotactic anti-cancer cells such as natural killer cells and T lymphocytes. 80 However, studies have found that DCA and LCA can inhibit the activation of spleen and intestinal macrophages which is induced by the toll-like receptor-4. 81 They can also inhibit the secretion of IL-6, IFN-γ, and TNF-α, and induce the polarization of anti-cancer M1 macrophages to pro-cancer M2 macrophages.82,83 Furthermore, macrophages and Kupffer cells lacking secondary BA receptor GPBAR1 showed increased expression of IL-6 and MCP-1 in response to LPS. 84
Secondary BAs can inhibit the function of dendritic cells (DCs). It is known that DCs can secrete TNF-α to promote apoptosis of cancer cells and secrete IL-12 to activate Th1 cells to participate in anti-tumor immune response. 85 However, a study has demonstrated that DCA and LCA can inhibit DCs to secrete TNF-α and IL-12, 82 and then play a role in promoting cancer. In addition, a study has shown that when DCs are co-cultured with naïve CD4 + T cells, adding 3β -hydroxy-deoxycholic acid (isoDCA) can induce the expression of forkhead box protein 3 (Foxp3) in T cells and induce immunosuppression, thereby reducing the immune stimulation properties of DCs. 86 Interestingly, the expression of Foxp3 was influenced by FXR on DCs’ surface, but not by FXR on CD4 + T cells’ surface. 86
Regulating specific immune cells
Secondary BAs can inhibit the function of B cells. B cells exert their anti-cancer effects mainly through secreting antibodies and then through antibody-dependent cytotoxicity, phagocytosis, and complement activation. 87 However, a study has found that DCA and LCA can inhibit IL-6 secretion, 82 and then inhibit B cell precursors to become antibody-producing cells, thus down-regulating the levels of IgE and IgG.
Secondary BAs can inhibit the function of natural killer T (NKT) cells. NKT cells are known to secrete IFN-γ and TNF-α to promote apoptosis of cancer cells.80,88 However, DCA and LCA were found inhibit NKT cells from secreting IFN-γ and TNF-α. 82 In addition, DCA and LCA can promote NKT cells to secrete IL-10, 82 which is known to down-regulate the activity of T lymphocytes and TNF-α. 89 Gut microbiota-bile acid axis participates in the regulation of NKT cells’ anti-cancer function. It is known that ABX, consisting of vancomycin, neomycin, and primaxin, had confirmed antibacterial efficacy and was not toxic to the liver. 90 In addition, spontaneous HCC was induced using MYC transgenic mice. A recent study has found that in ABX-treated MYC mice bearing HCC, the levels of TDCA and taurolithocholic acid (TLCA) were decreased, the accumulation of hepatic NKT cells was observed, and fewer and smaller HCC were found. In addition, more hepatic NKT cells were found in germ-free mice. Specifically, secondary BAs can reduce the level of CXCL16 chemokine in hepatic sinusoid endothelial cells, thereby inhibiting the accumulation of CXCR6+NKT cells and promoting hepatocellular carcinoma progression. 5 However, after using vancomycin to kill secondary BA-producing bacterium, the level of CXCL16 increased, and the number of CXCR6+NKT cells increased. 5
Secondary BAs can enhance the function of regulatory T cells (Tregs), which are known to promote immunosuppressive microenvironment formation and tumor progression. Foxp3 is one of the key transcription factors controlling the development and function of Tregs. 91 However, a study has shown that the LCA derivative isoalloLCA can increase Foxp3 expression in naïve CD4+T cells by producing mitochondrial reactive oxygen species. 92 In addition, when DCs were co-cultured with naïve CD4+T cells, isoDCA addition increased Foxp3 expression and subsequently enhanced the function of Tregs. 86 Gut microbiota-bile acid axis participates in the regulation of Treg’s cancer-promoting functions. It is known that RORγt+ Treg has a strong inhibitory effect on T cell and is preferentially amplified in colon cancer and increases with tumor stage, while RORγt+ T inhibitors may prevent and treat colon cancer. 93 However, a recent study has found that through enzymatic steps performed by Clostridium species (Clostridium scindens) and the engineered Bacteroides sp. (B. thetaiotamicron, B. fragilis, and B. ovatus), which are isoDCA-producing bacteria, the level of RORγt+ pTreg cells increased, 86 hence exerting a cancer-promoting effect indirectly. Further, lithocholic/3-oxo-lithocholic acids also up-regulated the level of RORγ+ Treg cells in mouse colon through the BA receptor VDR. 94
Conclusion and Outlook
Secondary BAs are the essential metabolites of the gut microbiota and have been proved to contribute to colorectal cancer, liver cancer, pancreatic cancer, esophageal cancer and other types of cancers. Targeted manipulation of the gut microbial and their metabolites to inhibit cancer progression is an emerging field of study. However, regulating gut microbial populations accurately and effectively remains a challenge. Research and development of microbiota-oriented foods to selectively regulate the bacterial abundance and secondary BAs levels have great potential for application.
Acknowledgments
The Figures in this review were created with BioRender.com.
Thanks to Home for Researchers (www.home-for-researchers.com) for the language editing.
Footnotes
Author Contributions: Original concept, performing the literature search and writing the paper [Rong Yang]; revising the paper [Li Qian].
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No.81771689, No.81373130), the Six Talent Peak Projects in Jiangsu Province(No.YY-050), the Key Projects of Innovation and Entrepreneurship Training Program for College Students in Jiangsu Province (No.202011117002Z), the Young Academic Leaders Project of Yangzhou University(No.201714).
ORCID iD: Rong Yang  https://orcid.org/0000-0003-4525-7881
https://orcid.org/0000-0003-4525-7881
References
- 1. Wu X, Yin S, Cheng C, Xu C, Peng J. Inclusion of soluble fiber during gestation regulates gut Microbiota, improves bile acid homeostasis, and enhances the reproductive performance of Sows. Front Vet Sci. 2021;8:756910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang L, Gong Z, Zhang X, et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes. 2020;12:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paramsothy S, Nielsen S, Kamm MA, et al. Specific bacteria and metabolites associated with response to fecal Microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019;156:1440-1454.e2. [DOI] [PubMed] [Google Scholar]
- 4. Wang S, Dong W, Liu L, et al. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis. Mol Carcinog. 2019;58:1155-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu X, Chen B, You W, Xue S, Qin H, Jiang H. The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer. Cancer Lett. 2018;412:194-207. [DOI] [PubMed] [Google Scholar]
- 7. Chen M, Ye A, Wei J, Wang R, Poon K. Deoxycholic acid upregulates the reprogramming factors KFL4 and OCT4 through the IL-6/STAT3 pathway in esophageal adenocarcinoma cells. Technol Cancer Res Treat. 2020;19:1533033820945302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354:154-159. [DOI] [PubMed] [Google Scholar]
- 9. Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The role of the gut Microbiota in bile acid metabolism. Ann Hepatol. 2017;16 Suppl 1:S21-S26. [DOI] [PubMed] [Google Scholar]
- 10. Xie G, Jiang R, Wang X, et al. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine. 2021;66:103290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ridlon JM, Devendran S, Alves JM, et al. The ‘in vivo lifestyle’ of bile acid 7α-dehydroxylating bacteria: comparative genomics, metatranscriptomic, and bile acid metabolomics analysis of a defined microbial community in gnotobiotic mice. Gut Microbes. 2020;11:381-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101:47-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kühn T, Stepien M, López-Nogueroles M, et al. Prediagnostic plasma bile acid levels and colon cancer risk: a prospective study. J Natl Cancer Inst. 2020;112:516-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ocvirk S, O’Keefe SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 2021;73:347-355. [DOI] [PubMed] [Google Scholar]
- 16. Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014;12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ridlon JM, Wolf PG, Gaskins HR. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes. 2016;7:201-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014;86:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quilty F, Freeley M, Gargan S, Gilmer J, Long A. Deoxycholic acid induces proinflammatory cytokine production by model oesophageal cells via lipid rafts. J Steroid Biochem Mol Biol. 2021;214:105987. [DOI] [PubMed] [Google Scholar]
- 21. Šarenac TM, Mikov M. Bile Acid Synthesis: from nature to the chemical modification and synthesis and their applications as drugs and nutrients. Front Pharmacol. 2018;9:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Yang M, Dong W, et al. Gut dysbiosis and abnormal bile acid metabolism in colitis-associated cancer. Gastroenterol Res Pract. 2021;2021:6645970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Zhang S, Zhou W, Hu D, Xu H, Ji G. Secondary bile acids and tumorigenesis in colorectal cancer. Front Oncol. 2022;12:813745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554-g573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsuei J, Chau T, Mills D, Wan YJ. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med. 2014;239:1489-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun R, Xu C, Feng B, Gao X, Liu Z. Critical roles of bile acids in regulating intestinal mucosal immune responses. Therap Adv Gastroenterol. 2021;14:17562848211018098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carino A, Graziosi L, D’Amore C, et al. The bile acid receptor GPBAR1 (TGR5) is expressed in human gastric cancers and promotes epithelial-mesenchymal transition in gastric cancer cell lines. Oncotarget. 2016;7:61021-61035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ni Z, Min Y, Han C, et al. TGR5-HNF4α axis contributes to bile acid-induced gastric intestinal metaplasia markers expression. Cell Death Discov. 2020;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagathihalli NS, Beesetty Y, Lee W, et al. Novel mechanistic insights into ectodomain shedding of EGFR ligands amphiregulin and TGF-α: impact on gastrointestinal cancers driven by secondary bile acids. Cancer Res. 2014;74:2062-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci. 2019;20:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhan K, Zheng H, Li J, et al. Gut microbiota-bile acid crosstalk in diarrhea-irritable bowel syndrome. Biomed Res Int. 2020;2020:3828249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Funabashi M, Grove TL, Wang M, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582:566-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fukui H. Leaky gut and Gut-Liver axis in liver cirrhosis: clinical studies update. Gut Liver. 2021;15:666-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu H, Shao W, Liu Q, et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat Commun. 2022;13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, Fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21:62. [DOI] [PubMed] [Google Scholar]
- 38. Singh V, Yeoh BS, Abokor AA, et al. Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota. Gut Microbes. 2020;11:1077-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song I, Gotoh Y, Ogura Y, Hayashi T, Fukiya S, Yokota A. Comparative genomic and physiological analysis against Clostridium scindens reveals Eubacterium sp. c-25 as an atypical deoxycholic acid producer of the human gut Microbiota. Microorganisms. 2021;9:2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagengast FM, Hectors MP, Buys WA, van Tongeren JH. Inhibition of secondary bile acid formation in the large intestine by lactulose in healthy subjects of two different age groups. Eur J Clin Invest. 1988;18:56-61. [DOI] [PubMed] [Google Scholar]
- 41. Huang F, Zheng X, Ma X, et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat Commun. 2019;10:4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naumann S, Schweiggert-Weisz U, Eglmeier J, Haller D, Eisner P. in vitro interactions of dietary fibre enriched food ingredients with primary and secondary bile acids. Nutrients. 2019;11:1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Nirmagustina DE, Kumrungsee T, Okazaki Y, Tomotake H, Kato N. Feeding of the water extract from Ganoderma lingzhi to rats modulates secondary bile acids, intestinal microflora, mucins, and propionate important to colon cancer. Biosci Biotechnol Biochem. 2017;81:1796-1804. [DOI] [PubMed] [Google Scholar]
- 44. Cao H, Xu M, Dong W, et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer. 2017;140:2545-2556. [DOI] [PubMed] [Google Scholar]
- 45. Alrubaye B, Abraha M, Almansour A, et al. Microbial metabolite deoxycholic acid shapes microbiota against Campylobacter jejuni chicken colonization. PLoS One. 2019;14:e0214705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jaye K, Li CG, Chang D, Bhuyan DJ. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes. 2022;14:2038865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen D, Jin D, Huang S, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020;469:456-467. [DOI] [PubMed] [Google Scholar]
- 48. Mirzaei R, Afaghi A, Babakhani S, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139:111619. [DOI] [PubMed] [Google Scholar]
- 49. Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hafezi S, Rahmani M. Targeting BCL-2 in cancer: advances, challenges, and perspectives. Cancers. 2021;13:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kwan SY, Jiao J, Qi J, et al. Bile acid changes associated with liver fibrosis and steatosis in the Mexican-American population of South Texas. Hepatol Commun. 2020;4:555-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wright LM, Yong S, Picken MM, Rockey D, Fimmel CJ. Decreased survival and hepato-renal pathology in mice with C-terminally truncated GP73 (GOLPH2). Int J Clin Exp Pathol. 2009;2:34-47. [PMC free article] [PubMed] [Google Scholar]
- 53. Kladney RD, Cui X, Bulla GA, Brunt EM, Fimmel CJ. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology. 2002;35:1431-1440. [DOI] [PubMed] [Google Scholar]
- 54. Zhang YL, Zhang YC, Han W, et al. Effect of GP73 silencing on proliferation and apoptosis in hepatocellular cancer. World J Gastroenterol. 2014;20:11287-11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang D, Yao M, Yan Y, et al. Deoxycholic acid upregulates serum Golgi protein 73 through activating NF-κB pathway and destroying Golgi structure in liver disease. Biomolecules. 2021;11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sasaki CT, Doukas SG, Doukas PG, Vageli DP. Weakly acidic bile is a risk factor for hypopharyngeal carcinogenesis evidenced by DNA damage, antiapoptotic function, and premalignant dysplastic lesions in vivo. Cancers. 2021;13:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gafar AA, Draz HM, Goldberg AA, et al. Lithocholic acid induces endoplasmic reticulum stress, autophagy and mitochondrial dysfunction in human prostate cancer cells. PeerJ. 2016;4:e2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma Y, Temkin SM, Hawkridge AM, et al. Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett. 2018;435:92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee CK, Jeong SH, Jang C, et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644-649. [DOI] [PubMed] [Google Scholar]
- 61. Mens MMJ, Ghanbari M. Cell cycle regulation of stem cells by MicroRNAs. Stem Cell Rev Rep. 2018;14:309-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan P, Zhu H, Yin L, et al. Integrin αvβ6 promotes lung cancer proliferation and metastasis through upregulation of IL-8-Mediated MAPK/ERK signaling. Transl Oncol. 2018;11:619-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nguyen TT, Lian S, Ung TT, Xia Y, Han JY, Jung YD. Lithocholic acid stimulates IL-8 expression in human colorectal cancer cells via activation of erk1/2 MAPK and suppression of STAT3 activity. J Cell Biochem. 2017;118:2958-2967. [DOI] [PubMed] [Google Scholar]
- 65. Tanaka Y, Ito S, Isobe K. Vancomycin-sensitive bacteria trigger development of colitis-associated colon cancer by attracting neutrophils. Sci Rep. 2016;6:23920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang J, Antin P, Berx G, et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Song X, An Y, Chen D, et al. Microbial metabolite deoxycholic acid promotes vasculogenic mimicry formation in intestinal carcinogenesis. Cancer Sci. 2022;113:459-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97-101. [DOI] [PubMed] [Google Scholar]
- 69. Nguyen PT, Kanno K, Pham QT, et al. Senescent hepatic stellate cells caused by deoxycholic acid modulates malignant behavior of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:3255-3268. [DOI] [PubMed] [Google Scholar]
- 70. Mikó E, Vida A, Kovács T, et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim Biophys Acta Bioenerg. 2018;1859:958-974. [DOI] [PubMed] [Google Scholar]
- 71. Mikó E, Kovács T, Sebő, et al. Microbiome-microbial metabolome-cancer cell interactions in breast Cancer-Familiar, but unexplored. Cells. 2019;8:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walcher L, Kistenmacher AK, Suo H, et al. Cancer Stem Cells-origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. 2020;11:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Desai A, Yan Y, Gerson SL. Concise reviews: cancer stem cell targeted therapies: toward clinical success. Stem Cells Transl Med. 2019;8:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang X, Meng S, Zhang R, et al. GP73-regulated oncolytic adenoviruses possess potent killing effect on human liver cancer stem-like cells. Oncotarget. 2016;7:29346-29358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bernstein C, Holubec H, Bhattacharyya AK, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011;85:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Farhana L, Nangia-Makker P, Arbit E, et al. Bile acid: a potential inducer of colon cancer stem cells. Stem Cell Res Ther. 2016;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xu Y, Surman DR, Diggs L, et al. Bile acid-induced “Minority MOMP” promotes esophageal carcinogenesis while maintaining apoptotic resistance via mcl-1. Oncogene. 2020;39:877-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Testa U, Castelli G, Pelosi E. Esophageal cancer: genomic and molecular characterization, Stem Cell Compartment and clonal evolution. Medicines. 2017;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jin Q, Noel O, Nguyen M, Sam L, Gerhard GS. Bile acids upregulate BRCA1 and downregulate estrogen receptor 1 gene expression in ovarian cancer cells. Eur J Cancer Prev. 2018;27:553-556. [DOI] [PubMed] [Google Scholar]
- 80. Yang P, Peng Y, Feng Y, et al. Immune cell-derived extracellular vesicles - new strategies in Cancer Immunotherapy. Front Immunol. 2021;12:771551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol. 2018;9:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fiorucci S, Carino A, Baldoni M, et al. Bile acid signaling in inflammatory bowel diseases. Dig Dis Sci. 2021;66:674-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [DOI] [PubMed] [Google Scholar]
- 84. Visekruna A, Luu M. The role of short-chain fatty acids and bile acids in intestinal and liver function, inflammation, and carcinogenesis. Front Cell Dev Biol. 2021;9:703218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gardner A, de Mingo Pulido Á, Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. 2020;11:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Campbell C, McKenney PT, Konstantinovsky D, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581:475-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Davis SK, Selva KJ, Kent SJ, Chung AW. Serum IgA fc effector functions in infectious disease and cancer. Immunol Cell Biol. 2020;98:276-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Banerjee S, Hamzoui K, Safari A, Borhani-Haghighi A. The cerebrospinal fluid presentations of neuro-Bechet Disease, a way to know the etiopathogenesis and improve armamentarium. Iran J Immunol. 2021;18:170-178. [DOI] [PubMed] [Google Scholar]
- 89. Mirlekar B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: its implications in cancer immunotherapy. SAGE Open Med. 2022;10:20503121211069012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. He B, Liu Y, Hoang TK, et al. Antibiotic-modulated microbiome suppresses lethal inflammation and prolongs lifespan in Treg-deficient mice. Microbiome. 2019;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hang S, Paik D, Yao L, et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature. 2019;576:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blatner NR, Mulcahy MF, Dennis KL, et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Song X, Sun X, Oh SF, et al. Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis. Nature. 2020;577:410-415. [DOI] [PMC free article] [PubMed] [Google Scholar]