Abstract
Objectives
This proof-of-concept study aimed at evaluating the proteolytic profile of histatin 1 and 5 in saliva of adolescents with spastic cerebral palsy (CP) with gingivitis.
Methods
This cross-sectional study included 24 individuals allocated into three groups: G1 (CP with gingivitis; n = 8), G2 (without CP and without gingivitis; n = 8), and G3 (without CP and with gingivitis; n = 8). The gingival index (GI) and simplified oral hygiene index (OHI–S) were evaluated. Whole saliva was collected and used to assess the rate and mode of histatin 1 and 5 at different times. The degradation products were visualized after cationic PAGE and the protein band densities (BDs) were compared with a protein standard. Fragmentation products were collected from the gel, pooled by group and characterized by mass spectrometry. BDs and gingival health parameters were analyzed by One-Way ANOVA or Kruskal Wallis tests, whereas poisson multilevel regression was used to the factors that influenced histatin degradation (α = 5%).
Results
Groups G1 and G3 differed significantly on OHI–S, visible biofilm, oral calculus and GI (p < 0.001). Poisson Regression showed that: 1) CP and gingivitis influenced the degradation of histatin 1 and 5 (p < 0.05); 2) The degradation of histatin 5 was influenced by age and male sex (p < 0.05); and 3) GI influenced significantly the degradation of histatin 1 (p < 0.001). Unique histatin degradation peptides were identified in individuals with gingivitis.
Conslusions
These data demonstrated that both the kinetics and pattern of histatins degradation differ according to the gingival health or disease conditions.
Keywords: Saliva, Proteolysis, Gingivitis, Cerebral palsy, Mass spectrometry
Saliva, Proteolysis, Gingivitis, Cerebral palsy, Mass spectrometry.
1. Introduction
Cerebral Palsy (CP) comprises a group of developmental disorders that affect a person's ability to move and their stability and posture, causing limitations in performing daily life activities. CP is attributed to non-progressive disturbances that occurred during fetal development or in the immature brain [1], and is the most common cause of physical disability in childhood. The estimated prevalence of CP is 2.7/1,000 children, with higher incidence among children of the male sex [2]. The presence of lesion in the cerebral cortex with movement disorder of the spastic type [3] and epilepsy are common comorbidities [1] that have a negative impact on the oral health of subjects with CP. In this regard, it has been reported that individuals with CP have reduced salivary flow rate, a condition that is exacerbated in individuals taking anti-epileptic drugs [4]. Low salivary flow rates lead to a reduction of bacterial clearance from the oral cavity, promoting biofilm formation and the advent of caries and periodontal disease, two oral diseases common in individuals with CP [5]. Furthermore, the persistence of pathological primitive oral reflexes (biting and vomiting), which are more prevalent in subjects with more severe neurological compromise, seriously interfere with an appropriate oral hygiene routine [6], thereby favouring the accumulation of supra- and subgingival biofilm. These features explain why individuals with CP have a notable pattern of gingival inflammation and high prevalence of periodontal disease [7].
The equilibrium of the oral microbiota is maintained by salivary proteins such as lysozyme, cystatins, immunoglobulins and histatin [8]. Histatins are human-specific salivary proteins that participate in the formation of the acquired enamel pellicle [8] and display antifungal and antibacterial activity [9]. Among the 12 different histatins secreted into the oral cavity, 80% of them correspond to histatin 1, 3 and 5 [10]. Histatin 1 is a phosphorylated protein that maintains mineral homeostasis of the tooth mineral by inhibiting the growth of calcium phosphate salt crystals [11]. Histatin 5, derived from the cleavage of histatin 3, has strong antifungal [11] and antimicrobial activity against periodontopathogenic microorganisms such as Porphyromonas gingivalis [12] and Bacteroides gingivalis [13]. Thus, histatins play an important role in the maintenance of oral homeostasis and in the prevention of periodontal disease [14]. However, histatins, as well as other proteins in saliva, are rapidly degraded once secreted into the oral cavity by proteolytic enzymes (proteases) from bacterial origins; the presence and activity of these proteases is greatly increased in the saliva from individulas with periodontitis [15].
The fact that histatins are strongly affected by proteolytic degradation in the oral cavity open avenues for exploring how these proteins are degraded in saliva in individuals with periodontal diseases. The evaluation of the rate of salivary protein degradation may serve as a tool to determine the activity status of periodontitis, whereas the mode of degradation may allow the identification of the proteinaceous fragments (peptides) which could be further used as biomarkers of periodontitis. Considering the high prevalence of periodontitis in individuals with CP, this proof-of-concept study aimed at evaluating the proteolytic profile (rate and mode) of histatins 1 and 5 in saliva of adolescents with CP.
2. Methods
2.1. Ethics statement
This study was reviewed and approved by the Research Ethics Committee of the Cruzeiro do Sul University - Brazil Platform, São Paulo, Brazil (#CE/UCS-2.093.675/2017). Written informed consent was obtained from the parents or guardians of each child after they were informed about the study.
2.2. Study design
This cross-sectional study included adolescents diagnosed with CP who received a physical rehabilitation treatment at a referral center, and subjects without CP who received dental treatment. A total of 24 individuals ranging in age from 12 to 18 years, including males and females, were enrolled in the study and allocated into the following groups (n = 8/group): individuals with CP and with gingivitis (Group 1); individuals with neither CP nor gingivitis (Group 2, negative control); and individuals without CP and with gingivitis (Group 3 – positive control). Gingival Index (GI) and Simplified Oral Hygiene Index (OHI–S) was assessed, and unstimulated whole saliva was collected from all participants. The rate and mode of histatin 1 and 5 degradation in saliva was evaluated at different time points and the degradation products were visualized by Cationic polyacrylamide gel electrophoresis (PAGE) and characterized by Mass Spectrometry (MS). Statistical analysis of the data was performed at a significance level α of 5% (One-Way ANOVA, Kruskal Wallis and Poisson Multilevel Regression).
2.3. Subject selection
The exclusion criteria for all 3 groups included individuals who used antibiotics in the last month; wore orthodontic appliances; smoke; have presence of systemic diseases (symptoms of fever, influenza, pains in the body or diarrhea), or have any inflammatory condition in the oral mucosa, such as aphtha. Subjects who were not able to cooperate during the dental exam and saliva collection were also excluded. Individuals with CP were excluded from the study if they presented with progressive or neurodegenerative lesions or were using medications that would interfere with salivary secretion, such as anticholinergic agents, neuroleptic or benzodiazepine medications within 72 h of saliva collection. Individuals with CP who had undergone surgical procedures for the control of drooling saliva and gastrostomy were also excluded from the study.
2.4. Clinical examinations
All the clinical evaluations were performed by a single examiner and using the Gingival Index (GI, kappa = 0.92) and the Simplified Oral Hygiene Index (OHI–S, kappa = 0.86).
The clinical exams were performed in compliance with biosafety standards, with the individuals seated in a dental chair, using mouth openers, clinical mirror and millimetre periodontal probe (Hu-Friedy Colorvue® PerioScreen® probe, Chicago, IL, USA). No radiographic exams were performed. The exams were performed in the following sequence: 1) evaluation of GI, 2) OHI–S, 3) pocket probing depth, and 4) whole saliva collection.
For GI evaluation, four posterior and two anterior teeth were probed at six sites per tooth: disto-vestibular, vestibular, mesio-vestibular, mesio-lingual, lingual, and disto-lingual [16]. For the assessment of posterior teeth, the first completely erupted tooth distal to the second premolar or primary second molar was examined in each quadrant. Gingivitis was diagnosed based on the following criteria: bleeding on probing at ≥ 10% of the sites probed, probing depth ≤3 mm in all the sites evaluated, and absence of attachement loss [17]. The healthy control group was composed of individuals who had bleeding on probing at < 10% of the sites probed, probing depth of ≤3 mm at all sites evaluated, and no attachment loss. All individuals had to have at least 20 teeth in the mouth.
The OHI–S evaluation examined the same six teeth that were sampled on the GI, and oral hygiene was assessed according to the OHI–S criteria [18, 19], which considers the amount of visible biofilm and calculus [(0) = absence of biofilm/calculus or detectable extrinsic stains; (1) = biofilm/calculus covering no more than 1/3 of the cervical portion of the tooth or extrinsic stains; (2) = biofilm/calculus covering more than 1/3 and less than 2/3 of the tooth surface; (3) = biofilm/calculus covering more than 2/3 of the tooth surface]. For the assessment of upper jaw molars, scores were attributed to the vestibular surfaces, and for the mandibular molars, to the lingual surfaces. The individual scores of the tooth indexes were added and divided by six, for both visible biofilm and calculus. The final result of the OHI–S was the sum of the mean value of visible biofilm and the mean value of calculus.
2.5. Whole saliva collection and processing
To prevent blood contamination, unstimulated whole saliva samples were collected between 9:00 and 11:00 AM, followed by the periodontal evaluation, using Salivette® (Sarstedt, Nümbrecht, Germany) saliva collection device. The collection was performed in a ventilated and illuminated room, with the participants comfortably seated in the dental chair. Saliva samples were kept on ice during the collection procedure and up to a maximum period of 3 h after collection. To extract the saliva from the Salivette®, the device was centrifuged at 1,500×g for 2 min at 4 °C and the retrieved saliva was transferred to new microcebtrifuge tubes. Next, saliva was centrifuged at 14,000×g for 20 min at 4 °C (Eppendorf Centrifuge 5415R, Eppendorf, Hauppauge, NY USA). Whole saliva supernatant (WSS) was separated from the pellet and transferred to 1.5 mL tubes and stored at -80 °C until analysis [20].
2.6. Degradation of histatins 1 and 5 in WSS
Individual and pooled analysis were used to examine the degradation rate of histatin 1 and 5 in saliva. WSS from each individual was diluted 1:10 in distilled water. Next, 12.5 μL of diluted WSS from each individual was used to prepare one pool per group, for a final volume of 100 μL of diluted WSS/group. The dilution of the saliva retards the degradation process and allows visualization of all the stages of degradation [21]. Synthetic histatin 1 or 5 (American Peptide Company, Sunnyvale, CA, USA) were added to the pooled samples to a final concentration of 100 μg/mL and were incubated at 37 °C in a water bath for the following time points (t): 0, 0.5, 1.5, 4, 6, 8, 24 and 48 h. Immediately after the addition of the proteins (t = 0) and at the different time points of incubation, an aliquot of 50 μL from each sample was removed and boiled for 5 min to inhibit any proteolytic activity [21]. Samples were then dried and stored for further analysis.
2.7. Cationic polyacrylamide gel electrophoresis
Cationic polyacrylamide gel electrophoresis (PAGE) was performed as described by Oppenheim et al. [11]. After the histatin degradation test, samples were desalted and reconstituted in 20 μL of sucrose buffer and visualized after cationic PAGE. To characterize the proteolytic products, the gels were scanned (Bio-Rad ChemiDrop MP, Bio-Rad Inc., Hercules, USA) and the intensity of the pixels was analyzed with Image LabTM 5.2 software (Bio-Rad Inc., Hercules, USA). The extent of degradation (band intensity, %) was relatively quantified by comparing the intensity of the resulting electrophoretic digest products with the control sample histatins 1, 3 or 5 at time zero. After cationic PAGE and gel scanning for image analysis, the degradation products of histatins 1 and 5 obtained in the pooled samples were individually excised from the gels and placed into microcentrifuge tubes. Proteolytic fragments of histatins 1 and 5 were extracted intact from the gel and desalted before MS analysis.
Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry (LC-ESI-MS/MS).
Mass spectrometry analyses were performed with a LTQ-Velos (Thermo Scientific, San Jose, CA, USA), which allowed liquid chromatography to be performed in line with a fused-silica capillary column (column length 10 mm, column ID 75 μm) filled with C18 resin with 3 μm diameter and 200 Å pore size (Michrom BioResources, Auburn, CA, USA), linked to a mass spectrometer using electrospray ionization mode, in the range of values m/z 390–2000 in MS/MS tandem. For this, samples were reconstituted in 15 μL of 97.5% H2O/2.4% acetonitrile/0.1% formic acid (v/v) and subsequently submitted to reverse phase LC-ESI-MS/MS. The High-Performance Liquid Chromatography (HPLC) reverse flow phase was developed with a linear gradient of 85 min ranging from 50% to 100% of solvent B (0.1% formic acid in acetonitrile), flow of 200 nL/min, with maximum pressure of 280 bar. The voltage by electrospray and temperature of the ion transfer capillary was 1.9 kV and 250 °C, respectively.
2.8. Bioinformatics analysis
Tandem mass spectra were extracted from raw data and queried against a customized human database (Swiss Prot and TrEMBL, Swiss Institute of Bioinformatics, Geneva, Switzerland, http://ca.expasy.org/sprot/) using SEQUEST algorithm in Proteome Discoverer 1.3 software (Thermo Scientific, San Jose, CA, USA). Proteome Discoverer results were validated at peptide and protein levels (1% false discovery rate) and by manually inspecting the MS/MS spectra to confirm the identity of signature b- and y-fragment ions. The individual proteinaceous fragments produced after the degradation of histatin 1 and histatin 5 in diluted WSS were named as “histatin 1 x/y” or “histatin 5 x/y”, respectively, where x corresponds to the residue at the N-terminal region of the fragment, and y to the residue at the C-terminal region of the fragment.
2.9. Statistical analysis
Data analyses were performed using PASW Statistics software (SPSS Inc, Chicago, IL, USA). Assumptions for normal distribution of errors and homogeneity of the variances were tested and, once verified, band densities (BD) at each time point and the gingival health parameters were analyzed using one-way ANOVA or the Kruskal-Wallis test. Multilevel Poisson Regression was performed (considering the Subject level) to evaluate the factors that influenced the percentage of BD of histatin degradation values. All statistical analyses were performed at a significance level α = 5%.
3. Results
Table 1 shows the data for demographic and clinical findings. No significant differences were found amongst the variables age, sex, and salivary flow among groups (p > 0.05). However, significant differences in periodontal health parameters (values from GI, OHI–S), visible biofilm, and calculus were found, with groups G1 and G3 being significantly different from group G2 (p < 0.001).
Table 1.
Descriptive and analytical analysis of demographic data and periodontal health parameters (mean ± S.D., n = 8/group).
| Variables | Groups |
p-value | ||
|---|---|---|---|---|
| G1 | G2 | G3 | ||
| Age (years) | 17.0 ± 1.07 | 17.3 ± 0.80 | 17.3 ± 0.97 | >0.05 |
| Number of individuals by sex | M:5; F:3 | M:2; F:6 | 3 M; 5 F | >0.05 |
| Salivary flow (mL/min) | 0.53 (0.15) | 0.67 (0.13) | 0.69 (0.17) | >0.05 |
| OHI–Sa | 0.79 (0.47)B | 0.22 (0.09)A | 1.00 (0.30)B | <0.001 |
| Visible biofilma | 0.64 (0.31)B | 0.22 (0.92)A | 0.63 (0.14)B | <0.001 |
| Calculusb | 0.14 (0.24)B | 0 (0)A | 0.37 (0.21)B | <0.001 |
| GIa | 0.74 (0.26)B | 0 (0)A | 0.56 (0.09)B | <0.001 |
G1: CP with gingivitis; G2: without CP and without gingivitis; G3: without CP with gingivitis.
One-way ANOVA
Kruskal-Wallis tests
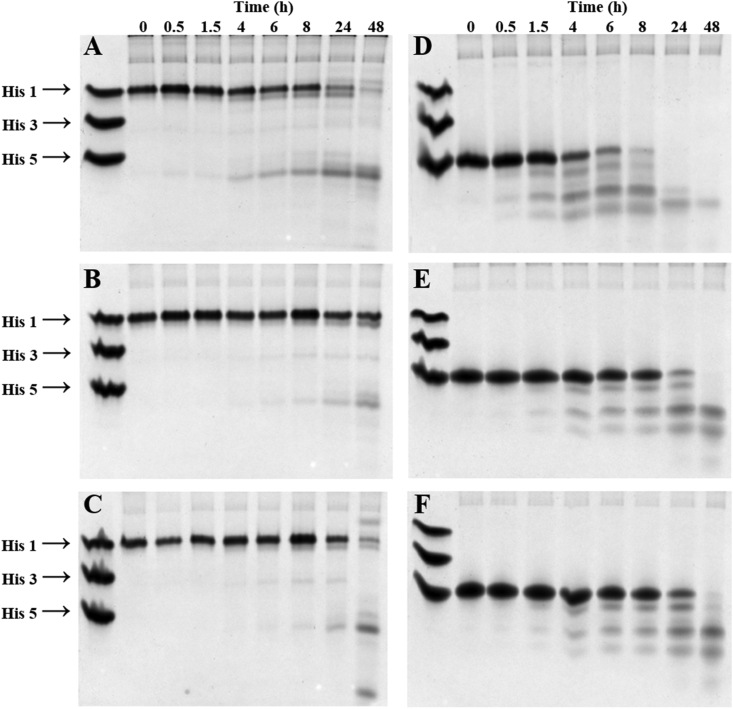
Distinct upper-case letters in the rows show significant differences among groups.Figure 1 shows the results from the analysis of the degradation mode of histatin 1 and histatin 5 in diluted WSS from individuals from G1 to G3 at different time points. On the one hand, histatin 1 degradation fragments formed in 1:10 diluted WSS from individuals in G1 were observed after 4 h (Figure 1A), whereas degradation fragments were visualized after 8 h in G2 (Figure 1B) and G3 (Figure 1C). On the other hand, histatin 5 degradation products were observed after 0.5 h of incubation with 1:10 diluted WS from individuals in G1 (Figure 1D), while the degradation products from the same protein were observed after 1.5 h in G2 (Figure 1E) and G3 (Figure 1F).
Figure 1.
Cationic PAGE analysis of histatin 1 and histatin 5 degradation profile in pooled 1:10 diluted WSS as function of time. A. Cationic PAGE showing the degradation mode of histatin 1 by WSS from G1 individuals. B. Cationic PAGE showing the degradation mode of histatin 1 by WSS from G2 individuals. C. Cationic PAGE showing the degradation mode of histatin 1 by WSS from G3 individuals. D. Cationic PAGE showing the degradation mode of histatin 5 by WSS from G1 individuals. E. Cationic PAGE showing the degradation mode of histatin 5 by WSS from G2 individuals. F. Cationic PAGE showing the degradation mode of histatin 5 by WSS from G3 individuals. Study groups = G1: CP with gingivitis; G2: without CP and without gingivitis; G3: without CP with gingivitis.
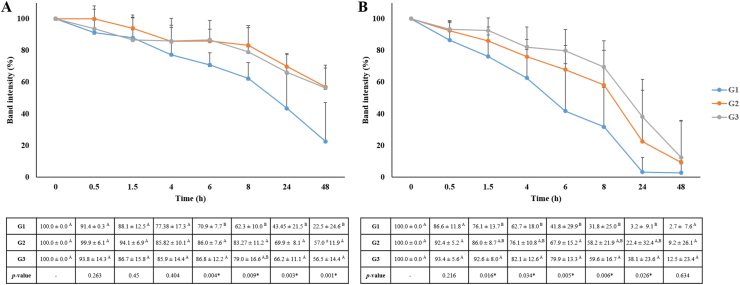
The image analysis of BD obtained after cationic PAGE allowed us to determine the degradation rate of each protein as function of time (Figure 2). The analysis of the degradation rate of histatin 1 in 1:10 diluted WSS showed that G1 exhibited the highest level of histatin 1 degradation at 6, 24 and 48 h, compared with the other two groups (p < 0.05) (Figure 2A). At 48, it was still possible to observe 56% of intact histatin 1 in G2 and G3 while in G1, only 22.54% of intact histatin 1 still remained (Figure 2A). Regarding the degradation rate of histatin 5, G1 exhibited the highest degradation rate compared to G2 and G3 after 1.5 h (p < 0.05), except at 48 h (Figure 2B). Also, G1 showed the highest level of histatin 5 degradation compared with G2 and G3 at 6 h (p = 0.005) (Figure 2B).
Figure 2.
Degradation rate of individual histatin 1 (A) and histatin 5 (B) in 1:10 diluted WSS determined by the mean ± S.D. of the percentage of BD. Distinct upper-case letters in the rows show significant differences in band densities among groups at each time point (ANOVA and Tukey test, p < 0.05). Study groups = G1: CP with gingivitis; G2: without CP and without gingivitis; G3: without CP with gingivitis (n = 8/group).
Table 2 and Table 3 show results from the Multilevel Poisson Regression analysis for histatin 1 and histatin 5, respectively, that was performed to identify factors influencing histatin degradation, measured as a percentage of BD. It was observed that CP influenced the degradation of histatin 1 (p < 0.001, Table 2) and histatin 5 (p = 0.022, Table 3). GI and time had a significant effect on histatin 1 degradation (p < 0.001, Table 2); whereas age (p = 0.015), male sex (p = 0.024), and time (p < 0.001) have a significant effect on histatin 5 degradation (Table 3).
Table 2.
Multilevel Poisson Regression Analysis (considering the subject level) to assess the factors that influenced the percentage of BDs of histatin 1 degradation.
| Univariate |
Adjusted |
||||
|---|---|---|---|---|---|
| Variable |
% BD (mean ± S.D.) |
IRR (CI 95%) |
P-value |
IRR (CI 95%) |
p-value |
| Groups | |||||
| G2 -Control (ref) | 84.5 ± 16.1 | ||||
| G3 -Gingivitis | 81.9 ± 18.4 | 0.96 (0.88–1.06) | 0.495 | 0.96 (0.88–1.06) | 0.495 |
| G1 -CP + Gingivitis | 69.5 ± 28.3 | 0.82 (0.74–0.91) | <0.001∗ | 0.82 (0.74–0.91) | <0.001∗ |
| Sex | |||||
| Female (ref) | 79.3 ± 23.6 | ||||
| Male | 77.6 ± 20.9 | 0.97 (0.88–1.08) | 0.664 | - | - |
| Age (years) | |||||
| Continuous | - | 1.00 (0.95–1.06) | 0.759 | - | - |
| Salivary flow | |||||
| Continuous | - | 1.14 (0.83–1.56) | 0.413 | - | - |
| OHI–S | |||||
| Continuous | - | 0.94 (0.80–1.09) | 0.452 | - | - |
| Visible biofilm | |||||
| Continuous | - | 0.87 (0.71–1.06) | 0.188 | ||
| Calculus | |||||
| Continuous | - | 0.96 (0,69–1,34) | 0,845 | - | - |
| GI | |||||
| Continuous | - | 0.84 (0.76–0.92) | <0.001∗ | - | - |
| Time | |||||
| Continuous | - | 0.91 (0.89–0.93) | <0.001∗ | 0.91 (0.89–0.93) | <0.001∗ |
CP + gingivitis in relation to Gingivitis: IRR = 0.84; CI = 0.75–0.96; p = 0.009∗. CI = 95%; p < 0.05∗
S.D.: Standard deviation; ref: Reference; G1: CP with gingivitis; G2: without CP and without gingivitis; G3: without CP with gingivitis (n = 8/group).
Table 3.
Multilevel Poisson Regression Analysis (considering the subject level) to assess the factors that influenced the percentage of bands densities (BDs) of histatin 5 degradation (n = 8/group).
| Univariate |
Adjusted |
||||
|---|---|---|---|---|---|
| Variable |
% BD (mean ± S.D.) |
IRR (CI 95%) |
p-value |
IRR (CI 95%) |
p-value |
| Groups | |||||
| G2 -Control (ref) | 64.04 (35.35) | ||||
| G3 -Gingivitis | 71.02 (32.07) | 1.10 (0.94–1.29) | 0.196 | 1.10 (0.95–1.29) | 0.184 |
| G1 -CP + Gingivitis | 50.61 (38.25) | 0.79 (0.64–0.96) | 0.022∗ | 0.81 (0.67–0.98) | 0.037∗ |
| Sex | |||||
| Female (ref) | 66.66 (34.67) | ||||
| Male | 55.21 (37.31) | 0.82 (0.70–0.97) | 0.024∗ | - | - |
| Age (years) | |||||
| Continuous | - | 1.12 (1.02–1.22) | 0.015∗ | 1.08 (1.00–1.17) | 0.037∗ |
| Salivary flow | |||||
| Continuous | - | 0.81 (0.47–1.42) | 0.482 | - | - |
| OHI–S | |||||
| Continuous | - | 0.99 (0.79–1.25) | 0.996 | - | - |
| Visible biofilm | |||||
| Continuous | - | 0.90 (0.62–1.32) | 0.615 | - | - |
| Calculus | |||||
| Continuous | - | 1.13 (0.78–1.66) | 0.499 | - | - |
| GI | |||||
| Continuous | - | 0.80 (0.60–1.06) | 0.126 | - | - |
| Time | |||||
| Continuous | - | 0.80 (0.76–0.83) | <0.001∗ | 0.80 (0.76–0.83) | <0.001∗ |
CP + gingivitis in relation to Gingivitis: IRR = 0.73; CI = 0.61–0.87; p = 0.001∗. CI = 95%; p < 0.05∗
S.D.: Standard deviation; ref: Reference; G1: CP with gingivitis; G2: without CP and without gingivitis; G3: without CP with gingivitis.
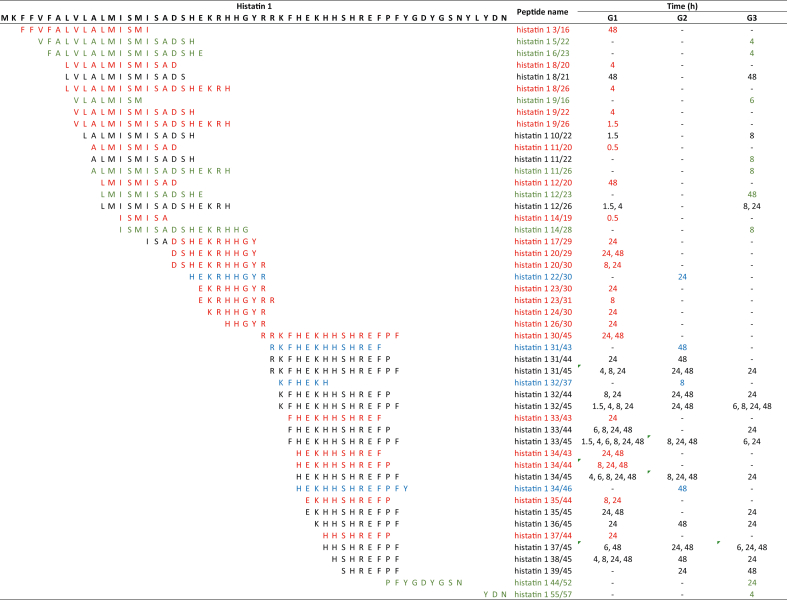
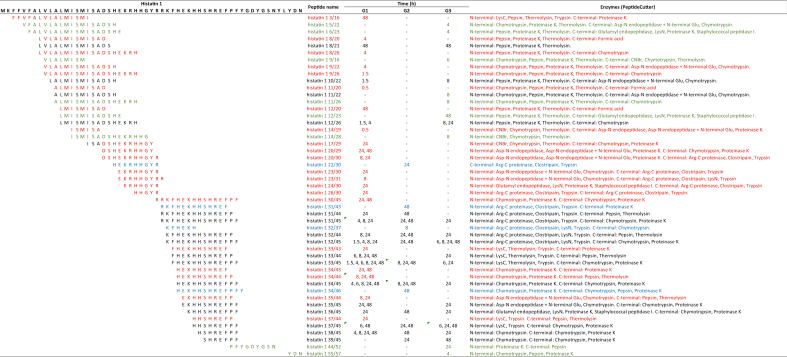
To characterize the degradation products of histatin 1 and histatin 5 in diluted WSS, the bands obtained after cationic PAGE were excised from the gel and processed for mass spectrometry analyses. In total, 49 proteinaceous fragments derived from histatin 1 (peptides) were identified, from which 21 peptides were exclusive to G1, four to G2, and eight to G3 (Table 4). In G1, peptides produced by the degradation of histatin 1 were detected at 0.5 h and 1.5 h. Degradation products of histatin 1 were identified at 4 h in G3. Only the peptide “histatin 1 55/57” is derived from the C-terminal region of histatin 1 protein, while the remaining 40 histatin 1 peptides had two proteolytic cleavages (Table 4).
Table 4.
Amino acid sequences of 49 histatin 1 fragments generated upon its incubation with WSS from individuals in G1, G2, and G3, at different time points (0, 0.5, 1.5, 4, 6, 8, 24 and 48 h). Red represents unique peptides identified for G1; blue represents unique peptides identified for G2; green represents unique peptides for G3.
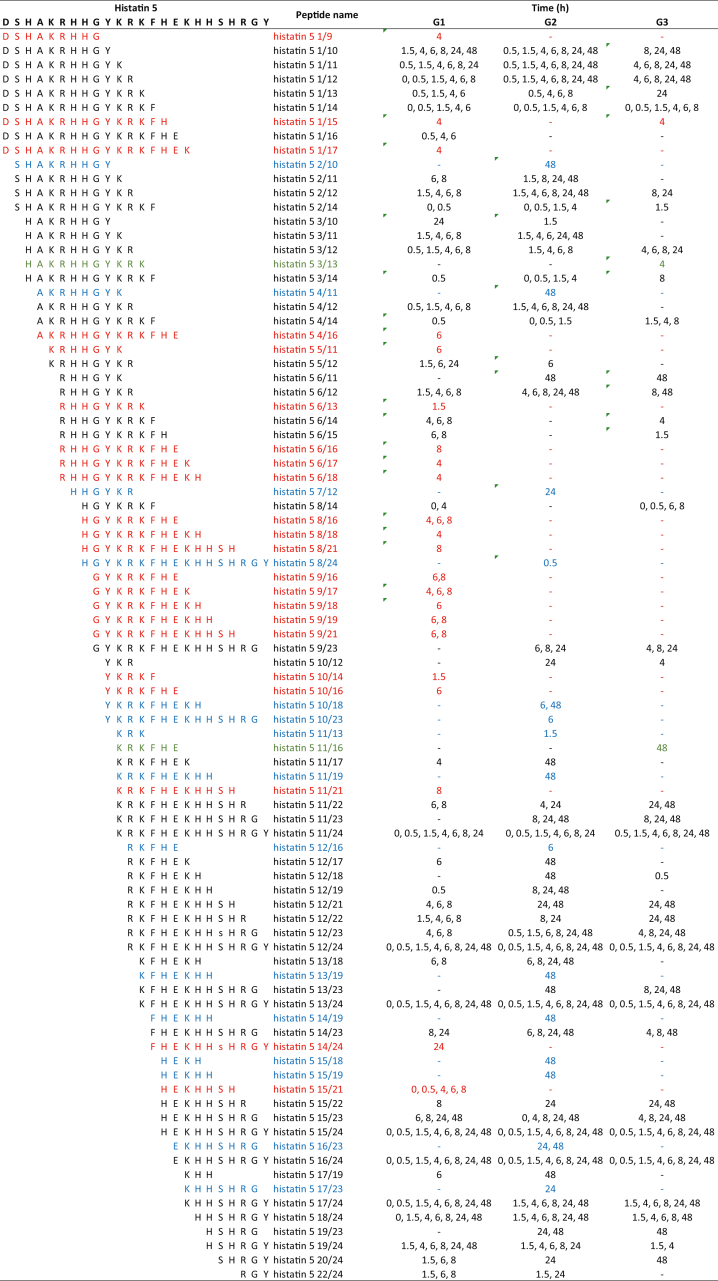
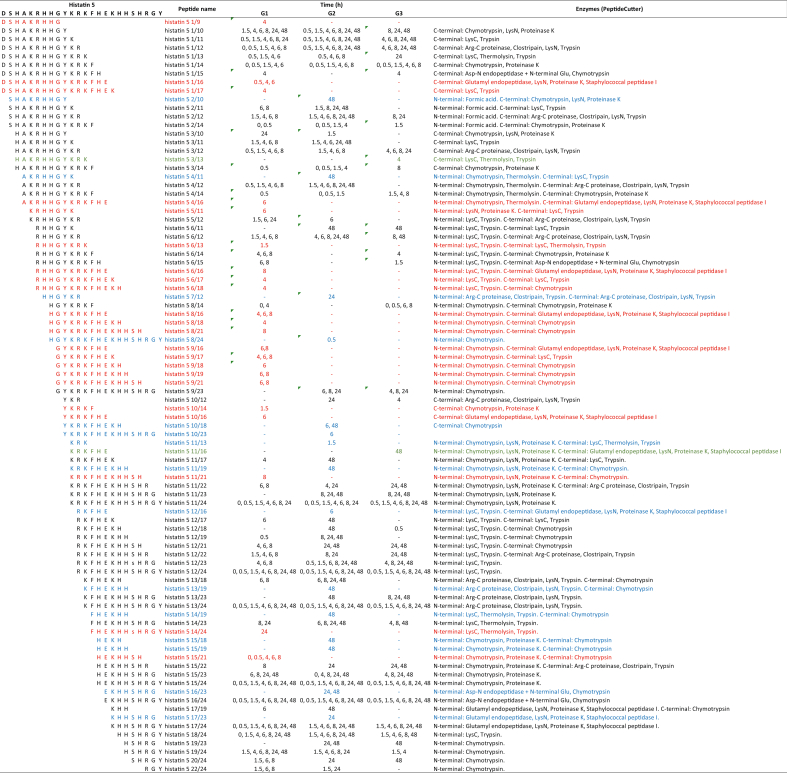
After the proteolysis of histatin 5, it was possible to identify 88 peptides; of those peptides, 22 were exclusively found in G1, 15 in G2, and two in G3 (Table 5). Interestingly, peptides derived from histatin 5 degradation were detected at time point 0. From the identified peptides, nine correspond to fragments at the N-terminal region of histatin 5, whereas 12 peptides correspond to the C-terminal region of the protein.
Table 5.
Amino acid sequences of 88 histatin 5 fragments generated upon its incubation with WSS from individuals in G1, G2, and G3, at different time points (0, 0.5, 1.5, 4, 6, 8, 24 and 48 h). Red represents unique peptides identified for G1; blue represents unique peptides identified for G2; green represents unique peptides for G3.
4. Discussion
This is the first study to evaluate the degradation of histatin 1 and histatin 5 in the saliva of individuals with CP and gingivitis. Saliva is a dynamic fluid in which the proteolytic reactions between salivary enzymes and proteins results in the formation of a series of new components [22]. Although proteolytic events may already occur in the salivary glands during proteolytic post-translational processing [11], these reactions are triggered by proteolytic enzymes from human and bacterial origin present in the oral cavity [23], and activity is increased in individuals with periodontal diseases [15]. In this study, the results confirmed this fact by demonstrating that histatin 1 and histatin 5 were degraded more rapidly in individuals with CP with gingivitis.
The analysis of the degradation rate and mode of histatins showed, on one hand, that the degradation products of histatin 1 started to appear at 0.5 h only in the pooled saliva from G1, and at 4 h in pooled saliva from G3 (Figure 1). After measuring the BDs (%), significant differences were found between the groups only after 4 h of incubation with diluted saliva, with a reduction in band density of 29.14% for G1 and approximately 14% for G2 and G3 (Figure 2). The degradation rate increased over time, reaching 77.46% in G1 and approximately 43.3% in G2 and G3 at 48 h, with the speed of histatin 1 degradation being 1.78 times faster in G1 during a 48 h interval (Figure 2). However, these results diverged from results of other studies that showed approximately 50% of histatin 1 had degraded at 1.5 h of incubation with WSS and had totally degraded after 40 h [24]. This may have occurred because of differences in the concentration of polyacrilamyde used during cationic PAGE. In this study, we used 20% polyacrylamide, differing from other studies that used a concentration of 15%. The intent of increasing polyacrilamyde concentration was to increase the likelihood of visualization of low molecular weight proteins, such as histatins in the gel, as well as their degradation products. On the contrary, using HPLC, a more sensitive technique, McDonald et al. [8] observed that the degradation of histatin 1 occurs soon after 0.5 h of incubation in diluted WSS from periodontally healthy individuals, with 28% of the intact protein being degraded at 0.5 h, and 83% at 2 h. Besides, we observed that GI had an influence on the degradation of histatin 1 (Table 2), indicating that these proteins protect against the development of periodontal disease [12, 13, 14]. On the other hand, it was observed that histatin 5 degrades faster than histatin 1, as degradation products were observed as early as time point zero in saliva pools from all groups (Figure 1). After 6 h, 58.2% of histatin 5 was degraded in G1, an this protein was almost completely degraded at 48 h (Figure 2). In G2 and G3, the degradation of histatin 5 was approximately 26.12% at 6 h and 89.13% at 48 h of incubation. These results indicate that histatin 5 degrades 2.23 times faster in G1 than in G2 and G3 during the first 6 h (Figure 2). Given this finding, it is expected that histatin 5 would be completed degraded if incubated for periods longer than 48 h [25]. Regarding the finding that age has an effect on histatin 5 degradation (Table 3), we previously demonstrated that the salivary proteome and specific peptides of the oral cavity showed important variations with age up to adolescence, contributing to the understanding of the physiological variability that occurs in human saliva [26].
The characterization of the proteinaceus fragments of histatin 1 and histatin 5, produced after the degradation of the intact proteins by proteolytic enzymes present in diluted WSS, allowed us to identify peptides that are unique to each group being studied. We found that 29 out of 49 proteinaceous fragments derived from histatin 1 (peptides) were unique to individuals with gingivitis (G1 and G3, Table 4), and 24 out of 88 histatin 5 peptides were exclusively found in the same groups (G1 and G3, Table 5). These peptide sequences are important because they may be considered as fingerprints of periodontal diseases, opening avenues for the development of novel approaches to diagnose and assess the activity of periodontal disease, and not only the history of the disease, as done with the current diagnostic criteria of periodontal disease.
To better understand the origin of histatin degradation products, we further explored the potential proteolytic cleavages based on in silico observations and the type of enzyme cleaving the protein (Table 6 and Table 7). The results showed that 6 out of 21 peptides derived from the proteolysis of histatin 1 in diluted WSS from individuals with gingivitis (G1 and G3) were produced by enzymes with trypsin-like activities (Table 6). Additionally, similar enzymes were responsible for generating 7 out of 22 unique histatin 5 fragments in the same groups (Table 7). These findings highlight the unique role of periodontopathogenic bacteria associated with gingivitis, such as Porphyromonas gingivalis, Tanerella forsythia and Treponema denticola [27, 28], on the degradation of histatin 1 and 5 in whole saliva from individuals with gingivitis, since these microorganisms produce proteolytic enzymes with trypsin-like activities [29].
Table 6.
Amino acid sequences of 49 histatin 1 fragments generated upon its incubation with WSS from individuals in G1, G2, and G3, at different time points (0, 0.5, 1.5, 4, 6, 8, 24 and 48 h), and list of proteolytic enzymes responsible for each cleavage. Red represents unique peptides identified for G1; blue represents unique peptides identified for G2; green represents unique peptides for G3.
Table 7.
Amino acid sequences of 88 histatin 5 fragments generated upon its incubation with WSS from individuals in G1, G2, and G3, at different time points (0, 0.5, 1.5, 4, 6, 8, 24 and 48 h), and list of proteolytic enzymes responsible for each cleavage. Red represents unique peptides identified for G1; blue represents unique peptides identified for G2; green represents unique peptides for G3.
The results from this study also allowed us to identify unique histatin peptides in the healthy subjects (G2). Despite the rapid degradation of histatin 5, its proteolysis does not have an inhibitory effect on its activity. In fact, some of the degradation products of histatin 5 in saliva from healthy individuals (G2) conserved the zinc-binding motive (HEXXH), necessary for zinc chelation at the local catalytic site of host proteases, such as matrix metalloproteases (MMP) MMP-2 and MMP-9 [24], inhibiting their activity [30]. These enzymes actively participate in bone resorption and the breakdown of periodontal tissues that occur in periodontal diseases [31]. Therefore, the potential therapeutic use of the peptides “histatin 5 8/24” and “histatin 5 10/23” should be further explored; they were the only two peptides unique to G2 that belong to the C-terminal region of the protein and that have an intact HEKHH sequence. Moreover, the combined use of histatin-5-deirved peptides with antibiotics may potentialize the bactericidal activity of histatin 5 [32].
One methodological limitation of the study was the absence of a group with CP without gingivitis. However, due to the high prevalence of gingivitis among individuals with CP, it was not feasible to recruit individuals with CP without gingivitis for this study. The goal of rehabilitation in individuals with CP is to achieve independence, but the motor compromise of the upper limbs limits the execution of proper oral hygiene procedures and leads to the development of gingivitis. The use of 1:10 diluted whole saliva supernatant allows the assessment of the degradation of synthetic histatins because this biofluid does not contain detectable levels of cationic proteins that may mask or alter the results obtained by cationic PAGE [23].
In summary, this study demonstrated that the degradation of histatin 1 was more resistant to proteolysis when compared with histatin 5, and the degradation of histatin 1 and histatin 5 occurred more rapidly in subjects with CP. The differences in the kinetics of protein degradation among healthy and gingivitis groups indicates that the level and type of proteolysis done by the enzymes secreted in health/disease conditions are highly specific. Altogether, our findings demonstrate that the degradation rate and mode of histatind degradation may serve as useful determinants of disease activity, and may generate the fingerprint of periodontitis. The translational applicability of our findings may lead to the development of a novel, innovative and promising chair-side approaches to diagnose and asses the activity of periodontitis, allowing clinicians to propose patient-orineted management plans.
Declarations
Author contribution statement
Gabriela M. de Gutierrez, Lina M. Marin, Yizhi Xiao, Andrea Escalante-Herrera, Maria T. Santos, Walter L. Siqueira: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Gabriela M. de Gutierrez: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lina M. Marin, Yizhi Xiao, Andrea Escalante-Herrera: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Maria T. Santos, Walter L. Siqueira: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr. Walter L. Siqueira was supported by Canadian Institutes of Health Research [106657 and 400347], Canada Foundation for Innovation [25116].
Gabriela M. de Gutierrez was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [CAPES-PDSE].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Bearden D.R., Monokwane B., Khurana E., Baier J., Baranov E., Westmoreland K., Mazhani L., Steenhoff A.P. Pediatric cerebral palsy in Botswana: etiology, outcomes, and comorbidities. Pediatr. Neurol. 2016;59:23–29. doi: 10.1016/j.pediatrneurol.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maenner M.J., Blumberg S.J., Kogan M.D., Christensen D., Yeargin-Allsopp M., Schieve L.A. Prevalence of cerebral palsy and intellectual disability among children identified in two U.S. National Surveys, 2011-2013. Ann. Epidemiol. 2016;26(3):222–226. doi: 10.1016/j.annepidem.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos M.T.B.R., Manzano F.S., Chamlian T.R., Masiero D., Jardim J.R. Effect of spastic cerebral palsy on jaw-closing muscles during clenching. Spec. Care Dent. 2010;30(4):163–167. doi: 10.1111/j.1754-4505.2010.00143.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira A.C.F.M., Mayer M.P.A., Kawamoto D., Santos M.T.B.R. Constipation, antiepileptic drugs, and gingivitis in children and adolescents with cerebral palsy. Int. J. Paediatr. Dent. 2019;29(5):635–641. doi: 10.1111/ipd.12488. [DOI] [PubMed] [Google Scholar]
- 5.Leite M.F., Aznar L.C.A., Ferreira M.C.D., Guaré R.O., Santos M.T.B. Increased salivary immunoglobulin A and reduced α-amylase activity in whole saliva from spastic cerebral palsy individuals. J. Oral Pathol. Med. 2013;42(6):480–485. doi: 10.1111/jop.12047. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues Dos Santos M.T.B., Nogueira M.L.G. Infantile reflexes and their effects on dental caries and oral hygiene in cerebral palsy individuals. J. Oral Rehabil. 2005;32(12):880–885. doi: 10.1111/j.1365-2842.2005.01518.x. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso A.M.R., Gomes L.N., Silva C.R.D., Soares R.d.S.C., Abreu M.H.N.G.d., Padilha W.W.N., Cavalcanti A.L. Dental caries and periodontal disease in Brazilian children and adolescents with cerebral palsy. Int. J. Environ. Res. Publ. Health. 2015;12(1):335. doi: 10.3390/ijerph120100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald E.E., Goldberg H.A., Tabbara N., Mendes F.M., Siqueira W.L. Histatin 1 resists proteolytic degradation when adsorbed to hydroxyapatite. J. Dent. Res. 2011;90(2):268–272. doi: 10.1177/0022034510388653. [DOI] [PubMed] [Google Scholar]
- 9.Puri S., Edgerton M. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot. Cell. 2014;13(8):958–964. doi: 10.1128/EC.00095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamkin M.S., Oppenheim F.G. Structural features of salivary function. Crit. Rev. Oral Biol. Med. : an official publication of the American Association of Oral Biologists. 1993;4(3-4):251–259. doi: 10.1177/10454411930040030101. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheim F.G., Xu T., McMillian F.M., Levitz S.M., Diamond R.D., Offner G.D., Troxler R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988;263(16):7472–7477. [PubMed] [Google Scholar]
- 12.Borgwardt D.S., Martin A.D., Van Hemert J.R., Yang J., Fischer C.L., Recker E.N., Nair P.R., Vidva R., Chandrashekaraiah S., Progulske-Fox A., Drake D., Cavanaugh J.E., Vali S., Zhang Y., Brogden K.A. Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B (HagB) and alters HagB-induced chemokine responses. Sci. Rep. 2014;4 doi: 10.1038/srep03904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikata M., Kanehira T., Oh H., Tani H., Tazaki M., Kuboki Y. Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem. Biophys. Res. Commun. 1991;174(2):625–630. doi: 10.1016/0006-291x(91)91463-m. [DOI] [PubMed] [Google Scholar]
- 14.Imatani T., Kato T., Minaguchi K., Okuda K. Histatin 5 inhibits inflammatory cytokine induction from human gingival fibroblasts by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2000;15(6):378–382. doi: 10.1034/j.1399-302x.2000.150607.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M., Slots J. Salivary enzymes. Origin and relationship to periodontal disease. J. Periodontal. Res. 1983;18(6):559–569. doi: 10.1111/j.1600-0765.1983.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 16.Löe H., Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol. Scand. 1963;21(6):533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 17.Chapple I.L.C., Mealey B.L., Van Dyke T.E., Bartold P.M., Dommisch H., Eickholz P., Geisinger M.L., Genco R.J., Glogauer M., Goldstein M., Griffin T.J., Holmstrup P., Johnson G.K., Kapila Y., Lang N.P., Meyle J., Murakami S., Plemons J., Romito G.A., Shapira L., Tatakis D.N., Teughels W., Trombelli L., Walter C., Wimmer G., Xenoudi P., Yoshie H. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89(Suppl 1):S74–s84. doi: 10.1002/JPER.17-0719. [DOI] [PubMed] [Google Scholar]
- 18.Greene J.C., Vermillion J.R. The simplified oral hygiene index. JADA (J. Am. Dent. Assoc.) 1964;68(1):7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 19.Santos M.T., Guare R.O., Celiberti P., Siqueira W.L. Caries experience in individuals with cerebral palsy in relation to oromotor dysfunction and dietary consistency. Spec. Care Dent. 2009;29(5):198–203. doi: 10.1111/j.1754-4505.2009.00092.x. [DOI] [PubMed] [Google Scholar]
- 20.Siqueira W.L., de Oliveira E., Mustacchi Z., Nicolau J. Electrolyte concentrations in saliva of children aged 6-10 years with Down syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004;98(1):76–79. doi: 10.1016/j.tripleo.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 21.McDonald E.E., Goldberg H.A., Tabbara N., Mendes F.M., Siqueira W.L. Histatin 1 resists proteolytic degradation when adsorbed to hydroxyapatite. J. Dent. Res. 2011;90(2):268–272. doi: 10.1177/0022034510388653. [DOI] [PubMed] [Google Scholar]
- 22.Helmerhorst E.J., Oppenheim F.G. Saliva: a dynamic proteome. J. Dent. Res. 2007;86(8):680–693. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 23.Helmerhorst E.J., Alagl A.S., Siqueira W.L., Oppenheim F.G. Oral fluid proteolytic effects on histatin 5 structure and function. Arch. Oral Biol. 2006;51(12):1061–1070. doi: 10.1016/j.archoralbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Sun X., Salih E., Oppenheim F.G., Helmerhorst E.J. Activity-based mass spectrometric characterization of proteases and inhibitors in human saliva. Proteonomics Clin. Appl. 2009;3(7):810–820. doi: 10.1002/prca.200800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomadaki K., Bosch J., Oppenheim F., Helmerhorst E. The diagnostic potential of salivary protease activities in periodontal health and disease. Oral Dis. 2013;19(8):781–788. doi: 10.1111/odi.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabras T., Pisano E., Boi R., Olianas A., Manconi B., Inzitari R., Fanali C., Giardina B., Castagnola M., Messana I. Age-dependent modifications of the human salivary secretory protein complex. J. Proteome Res. 2009;8(8):4126–4134. doi: 10.1021/pr900212u. [DOI] [PubMed] [Google Scholar]
- 27.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 28.Haffajee A.D., Socransky S.S., Patel M.R., Song X. Microbial complexes in supragingival plaque. Oral Microbiol. Immunol. 2008;23(3):196–205. doi: 10.1111/j.1399-302X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 29.Bretz W.A., Loesche W.J. Characteristics of trypsin-like activity in subgingival plaque samples. J. Dent. Res. 1987;66(11):1668–1672. doi: 10.1177/00220345870660111301. [DOI] [PubMed] [Google Scholar]
- 30.Gusman H., Lendenmann U., Grogan J., Troxler R.F., Oppenheim F.G. Is salivary histatin 5 a metallopeptide? Biochim. Biophys. Acta. 2001;1545(1-2):86–95. doi: 10.1016/s0167-4838(00)00265-x. [DOI] [PubMed] [Google Scholar]
- 31.Franco C., Patricia H.R., Timo S., Claudia B., Marcela H. Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 2017;18(2) doi: 10.3390/ijms18020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du H., Puri S., McCall A., Norris H.L., Russo T., Edgerton M. Human salivary protein histatin 5 has potent bactericidal activity against ESKAPE pathogens. Front. Cell. Infect. Microbiol. 2017;7:41. doi: 10.3389/fcimb.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.