Abstract
Introduction
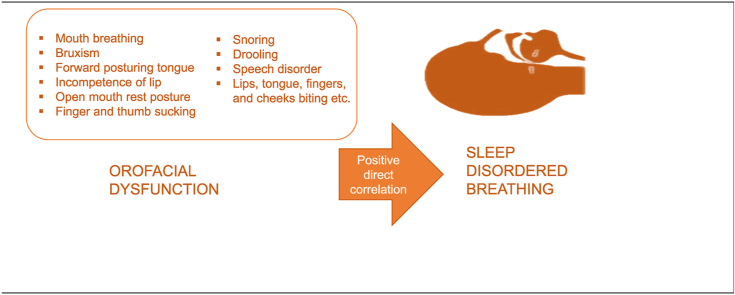
Sleep-disordered breathing (SDB) ranges from partial obstruction of the upper airway resulting in snoring to total upper airway obstruction leading to obstructive sleep apnea. The impairment in the dynamics of the stomatognathic system is termed as orofacial dysfunction. This study investigates the prevalence of orofacial dysfunction and sleep-disordered breathing in primary school children and identifies their correlation.
Methods
A total of 560 forms were distributed to 8 primary schools in Belagavi city. Among them, 482 parents responded (86% response rate), which included 239 boys (49.58%) and 243 girls (50.41%). All the participants were screened for orofacial dysfunction using Nordic Orofacial Dysfunction Test-screening (NOT-S) and sleep-disordered breathing using the Pediatric Sleep Questionnaire (PSQ).
Result
A positive direct correlation of sleep-disordered breathing with orofacial dysfunction (r = 0.47; p ≤ 0.001) was noted. A total of 41(8.58%) children were found to be at risk of sleep-disordered breathing with a score less than or equal to eight, based on (PSQ) Pediatric Sleep Questionnaire, and 156 (32.6%) children showed symptoms of orofacial dysfunction based on Nordic Orofacial Test–Screening (NOT-S).
Conclusion
The study demonstrates that around 32.6% of children had orofacial dysfunction symptoms, and 8.58% of children were at risk for sleep-disordered breathing, girls having a greater risk as compared to boys. There was a positive correlation between orofacial dysfunction and sleep-disordered breathing among children aged 6–12 years.
Keywords: Orofacial dysfunction, Sleep disordered breathing, Indian children, NOT-S, Pediatric sleep questionnaire
Graphical abstract
1. Introduction
Sleep-disordered breathing (SDB) ranges from partial obstruction of the upper airway resulting in snoring to total upper airway obstruction leading to obstructive sleep apnea.1 Three elements contribute to sleep-disordered breathing: anatomical structure, neuromotor tone, and inflammation.2 The critical consequence of pathophysiologies of these elements is upper airway obstruction. Upper airway obstruction during sleep is associated with loud snoring, arousal, sleep fragmentation, intermittent hypoxemia and hypercapnia, nocturnal hypertension, daytime sleepiness, deterioration in academic performance and cognitive abilities.3 Sleep-disordered breathing showed a prevalence of 11.4%–47.5% in North India and 4.8% to 5% in South India.4, 5, 6
Literature suggests that snoring or upper airway obstruction during sleep is associated with craniofacial modification in children.7 Craniofacial transformation and orofacial growth co-occur. The two centers for orofacial development are the intermaxillary synchondrosis and alveolodental ligament that are active at birth and remain so even after maximal orofacial growth.4 The most rapid orofacial growth occurs between birth and two years of age. However, the growth remains operational until the child is six years of age. Around 60% of children gain almost permanent craniofacial structure by the age of 6 years.5 The change in craniofacial structure sometimes causes impairment in orofacial function,8 resulting in nasal obstruction and parafunctional habits such as mouth breathing, bruxism, posturing the tongue forward, incompetence of lip, open mouth rest posture, finger, and thumb sucking, and lips, tongue, fingers, and cheeks biting, etc.6 The orofacial function is a phenomenon in which the central nervous system, neuromuscular system, and stomatognathic system work simultaneously.7 The impairment in the dynamics of the stomatognathic system is termed as orofacial dysfunction.9
Sustained daytime hypertension, increased cardiovascular and cerebrovascular morbidity, and mortality has been reported as long-term consequences of sleep-disordered breathing.8 Various studies have evaluated the association between sleep-disordered breathing and orofacial dysfunction symptoms, which reports that there is a significant relationship between them.1,10 However, the orofacial symptoms in the studies were evaluated either by using a questionnaire or using a tool that measures the physical characteristics of orofacial behavior. None of the studies have evaluated the primary motor and orofacial sensory function, including chewing, swallowing, and speech. Also, the tools used were developed to evaluate orofacial function in obstructive sleep apnea (OSA), which is the most severe form of sleep-disordered breathing. The mildest form of orofacial dysfunction, which has a high potential of progression later in life, is not yet evaluated.
In the Indian scenario, Orofacial dysfunction in children is the almost untouched topic of research to date. No studies regarding the correlation between orofacial dysfunction and sleep-disordered breathing have been conducted in India to the best of our knowledge. Furthermore, studies report a lack of awareness about the child's sleep health and breathing disorder among parents and health professionals in India.11 This lack of awareness may lead to Indian children being undiagnosed and untreated. Therefore, this study investigates the prevalence of orofacial dysfunction and sleep-disordered breathing among primary school children in Belagavi and identifies the correlation between them during their craniofacial development.
2. Methods
A cross-sectional study among school children was conducted from December 2019 to March 2020 in Belagavi, Karnataka, India, after obtaining ethical approval from the Institutional Ethical Committee.
The sample size of 426 was arrived at with a statistical power calculation of 90% and 5% level of significance. A stratified random sampling technique was performed to ensure that the sample is representative of all primary schools of Belagavi. A number was allotted for all the primary schools from East, west, north, and south of Belagavi. Eight schools were selected using the randomization table method.
The principal investigator approached the school principals for permission to conduct the study, and the rationale of the study was explained. After acquiring their approval, they were provided with the consent forms to be distributed to the students in the age group of 6–12 years, i.e., children from first to the seventh standard. Ten students (5 male and five female) assuming a 20% non-response rate; from each standard, students were randomly selected using the lottery method from all the schools. Class teachers distributed the forms and requested the students to get them filled by their parents. In total, 560 forms were distributed to 8 primary schools in Belagavi city. Among them 482 parents responded (86% response rate); which included 239 boys (49.58%) and 243 girls (50.41%). The class teachers were then requested to fix an appointment with those parents who consented to their and their child's participation in the study. On the day of the examination, the parents were first interviewed by the research assistant seeking information; like if their child is diagnosed with a known developmental disability and craniofacial anomalies and whether they have any cough and cold symptoms. If the answer was “yes,” the child was excluded from the study. Four children (one female and three male) were excluded from the study due to cold and cough symptoms on the day of examination. Thus, 478 children, 236 boys (49.4%), and 242 girls (50.6%) were included for further evaluation. NOT-S interview (Figure 1) and PSQ (Figure 2) were administered to the parents through an interview by the research assistant.5 The principal investigator performed the evaluation of children for orofacial dysfunction using NOT-S examination subsequently on the same day.
3. Outcome measure
PSQ is a valid and reliable instrument with 0.88 and 0.86 validity and reliability, respectively. It is used to evaluate the symptoms of disordered sleep breathing. It has 22 items: snoring, breathing problems, mouth breathing, daytime sleepiness, and behavior problems. Each item has three response options; Yes, No, and don't know. Yes, response scores one, and no or don't know response scores zero. Children with eight or more scores were considered as high risk for SDB, whereas children with scores less than eight were considered as low-risk for SDB.12
NOT-S is a screening tool to identify the area of orofacial dysfunction. It is a freely available screening tool in various languages. It has two parts composed of a structured interview and clinical examination. The structured interview consists of six domains with verbal 'Yes' and ‘No’ response format. If the response to one or more items within a domain is ‘yes,’ the dysfunction criterion is fulfilled; if the response is ‘No,’ it means there is no dysfunction. The examination part also consists of six domains; each domain contains a variable number of items. ‘Yes' in any item of the domain indicates dysfunction in the scored domain.5
4. Data analysis
The characteristics of participants and prevalence rates were described using descriptive statistics. A Chi-square cross-tabulation test was performed to assess the differences between Sleep-disordered breathing and or facial dysfunction in association with gender. Spearman correlation test was performed to evaluate the correlation between sleep-disordered breathing and orofacial dysfunction with age and correlation between (NOT-S) and (PSQ). The P-values of <0.05 were considered significant.
5. Results
Among 478 children recruited, 236 (49.4%) were boys, and 242 (50.6%) were girls with a mean age of 8.92 ± 2.05 years and an age range of 6–12 years (Table 1).
Table 1.
Age and gender distribution.
| Characteristics | n (%) |
|---|---|
| Gender | |
| Female | 236(49.4) |
| Male | 242(50.6) |
| Age | |
| 6 | 79(16.5) |
| 7 | 65(13.6) |
| 8 | 72(15.1) |
| 9 | 61(12.8) |
| 10 | 65(13.6) |
| 11 | 69(14.4) |
| 12 | 67(14.0) |
A total of 41(8.58%) children were found to be at risk of sleep-disordered breathing with a score less than or equal to eight, based on (PSQ) Pediatric Sleep Questionnaire, and 156 (32.6%) children showed symptoms of orofacial dysfunction based on Nordic Orofacial Test–Screening (NOT-S). Among them, the number of children with at least one domain affected was 44, followed by breathing and nose breathing and nose breathing under which 28 children were affected, followed by sensory function and drooling under which 27 children were affected, and the least affected domain was facial expression under which one child was affected.
Chi-square cross-tabulation test on PSQ score and NOT-S scores with the gender revealed that there was a significant difference between PSQ score and gender (χ2 = 21.699: p = 0.027). NOT-S and gender did not show significant difference (χ2 = 0.545: p = 0.969) (Table 2). There is a negative indirect relationship between age and PSQ (r = −0.136: p = 0.003) and between age and NOT-S (r = −0.133: p = 0.004). There was a positive direct correlation of sleep-disordered breathing based on (PSQ) with orofacial dysfunction based on Nordic Orofacial Test–Screening (r = 0.47: p = 0.001) (Table 3).
Table 2.
Correlation between genders with sleep disordered breathing and orofacial dysfunction based on PSQS & NOTS Score.
| Score | Gender |
Chi Square value | p-value | ||
|---|---|---|---|---|---|
| Male | Female | ||||
| PSQS | 0 | 47 | 45 | 21.699 | 0.027* |
| 1 | 17 | 36 | |||
| 2 | 44 | 28 | |||
| 3 | 31 | 32 | |||
| 4 | 19 | 25 | |||
| 5 | 29 | 18 | |||
| 6 | 24 | 20 | |||
| 7 | 8 | 14 | |||
| 8 | 5 | 10 | |||
| 9 | 6 | 8 | |||
| 10 | 3 | 6 | |||
| 11 | 3 | 0 | |||
| NOTS | 0 | 160 | 162 | 0.545 | 0.969 |
| 1 | 43 | 42 | |||
| 2 | 20 | 21 | |||
| 3 | 12 | 16 | |||
| 4 | 1 | 1 | |||
*Denotes statistically significant at p < 0.05.
Table 3.
Association between Age and sleep disordered breathing, age and orofacial dysfunction and sleep disordered breathing & orofacial dysfunction based on PSQS & NOTS Score.
| variables | r-value | p-value |
|---|---|---|
| Sleep disordered breathing and orofacial dysfunction | 0.47a | 0.001* |
| Age and orofacial dysfunction | −0.133b | 0.004* |
| Age and sleep disordered breathing | −0.136c | 0.003* |
*Denotes statistically significant at p < 0.05.
Spearman's correlation test (rs = 0.47).
Spearman's correlation test (rs = −0.136).
Spearman's correlation test (rs = −0.133).
6. Discussion
The present study used a valid and reliable PSQS with a 0.88 and 0.86 validity and reliability to evaluate sleep-disordered breathing symptoms and NOT-S to evaluate orofacial dysfunction among 478 children aged 6–12 years in Belagavi city.
In the present study, 32.6% of children reported orofacial dysfunction symptoms, among which the number of children with at least one domain affected was 44. The highest affected domain was ‘habits,’ under which 9.2% of children were seen. The number of children with the least affected domain ranges from 0.2% to 1.5%. This result is in accordance with the previous reports that revealed that the most commonly affected NOT-S domain in normal children was ‘Habit.'13,14
Sleep-disordered breathing has previously been reported to be (51.1%) among preschool and school-aged children in rural India.15 This rate is higher than reported in Dutch children (25%), Italian children (4.9%), Turkish children (7%), and urban Indian children (11.4%).13,14,16 In this study, the prevalence of sleep-disordered breathing reported is 8.58%, which is less than that reported in rural Indian children, as this study was also conducted in the urban area. A higher prevalence of sleep-disordered symptoms among children in rural areas than in urban areas has been reported previously.13
The present study showed a higher risk of SDB among female children, with 58% being affected as compared to 41% of male children, which is in agreement with a previous study that reported a higher risk of sleep disorders in females than males.17 The reason for this finding may be attributed to added descending mandibular growth, lower facial height, and anteriorly positioned hyoid bone in females than in males. Studies also show that these craniofacial features are more prevalent in severe sleep-disordered breathing.18 However, some of the other studies reported a lower risk of sleep-disordered breathing in females than males.19, 20, 21 This difference in the prevalence can also be attributed to gender bias that may influence the parents' reporting regarding snoring in boys and girls.18
The result also shows a positive direct association between sleep-disordered breathing and orofacial dysfunction among school children. This result agrees with Baidas et al., which concludes that there is a strong relationship between orofacial symptoms and sleep-disordered breathing and found that the history of digit sucking habit is associated with sleep-disordered breathing.9 They have assessed orofacial dysfunction symptoms using a questionnaire that included questions like digit sucking habits, facial muscle pain, temporomandibular joint pain, and bruxism symptoms. In the present study, we have used a standardized tool for assessing orofacial dysfunction, which contains a wider orofacial function area. Our study result is also in agreement with the study by Huynh et al., which reported that the association between thumb/finger sucking histories is statistically significant with heavy breathing at night.19
Mouth breathing during the early stage of life impairs the temporomandibular joint,21,22 and the prevalence of SDB is high in individuals with temporomandibular dysfunction.23 The mild orofacial dysfunction symptoms can progress into severe obstructive sleep apnea.24,25 In addition, the findings of this study suggest that majority of mild orofacial dysfunction symptoms remain unnoticed in the growing age of the children. This issue can be resolved by routine evaluation of orofacial functions in children and using various intervention strategies during the early life stages. Therefore, screening children for early identification of orofacial dysfunction is necessary to recognize and intervene the orofacial dysfunction and prevent its adverse consequences in later life.
The study did have some inherent limitations as the results for sleep-disordered breathing and orofacial dysfunction interviews were based on parents' opinions, which may be subjective to a certain extent. The accuracy of the results could be enhanced by the use of comprehensive and objective sleep-disordered breathing tools like polysomnography and contact devices. Furthermore, clinical evaluation of the children along with questionnaire may yield more objective information and take imminent studies to an advanced level, which we plan to undertake subsequently.
7. Conclusion
We found that 32.6% of children have symptoms of orofacial dysfunction, and 8.58%, children are at risk of sleep-disordered breathing, with girls being at higher risk than boys. There is a positive correlation between sleep-disordered breathing and orofacial dysfunction in the age group of 6–12 years. This study underscores the importance of screening children in the early stage of life for orofacial dysfunction and SDB, which is vital to prevent various adverse consequences during childhood and later in life. Further studies, including larger sample sizes with broader age groups and more comprehensive tools for sleep-disordered breathing, are required to generalize the result in a larger population.
Contributor Information
Deepa Metgud, Email: drdeepa_metgud@yahoo.com.
Punnya V. Angadi, Email: punnya_angadi@rediffmail.com.
Anjana Panthee, Email: aanzanapanthee@gmail.com.
Annexure I
Fig. 1.
NOT-S Screening and Examination tool
Fig. 2.
Pediatric Sleep Questionaire tool
References
- 1.Baidas L., Al-Jobair A., Al-Kawari H., AlShehri A., Al-Madani S., Al-Balbeesi H. Prevalence of sleep-disordered breathing and associations with orofacial symptoms among Saudi primary school children. BMC Oral Health. 2019;19(1):43. doi: 10.1186/s12903-019-0735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha D., Guilleminault C. Sleep disordered breathing in children. Indian J Med Res. 2010;131(2):311. [PubMed] [Google Scholar]
- 3.Gozal D., O'Brien L.M. Snoring and obstructive sleep apnoea in children: why should we treat? Paediatr Respir Rev. 2004;5:S371–S376. doi: 10.1016/s1526-0542(04)90066-8. [DOI] [PubMed] [Google Scholar]
- 4.Krogman W.M., Mazaheri M., Harding R.L., et al. A longitudinal study of the craniofacial growth pattern in children with clefts as compared to normal, birth to six years. Cleft Palate J. 1975;12(1):59–84. [PubMed] [Google Scholar]
- 5.Guilleminault C., Sullivan S.S., Huang Y.S. Sleep-disordered breathing, orofacial growth, and prevention of obstructive sleep apnea. Sleep med clin. 2019;14(1):13–20. doi: 10.1016/j.jsmc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Bakke M., Bergendal B., McAllister A., Sjoegreen L., Asten P. Development and evaluation of a comprehensive screening for orofacial dysfunction. Swed Dent J. 2007;31(2):75–84. [PubMed] [Google Scholar]
- 7.Miller A.J. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med. 2002;13(5):409–425. doi: 10.1177/154411130201300505. [DOI] [PubMed] [Google Scholar]
- 8.Hla K.M., Skatrud J.B., Finn L., Palta M., Young T. The effect of correction of sleep-disordered breathing on BP in untreated hypertension. Chest. 2002;122(4):1125–1132. doi: 10.1378/chest.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 9.Alaçam A., Yılmaz B.C.Ç., Incioğlu A.S. Assessment of orofacial dysfunction using the NOT-S method in a group of Turkish children with cerebral palsy. Eur Arch Paediatr Dent. 2019:1–7. doi: 10.1007/s40368-019-00475-z. [DOI] [PubMed] [Google Scholar]
- 10.de Felício C.M., da Silva Dias F.V., Folha G.A., et al. Orofacial motor functions in pediatric obstructive sleep apnea and implications for myofunctional therapy. Int J Pediatr Otorhinolaryngol. 2016;90:5–11. doi: 10.1016/j.ijporl.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Manzar M.D., Hussain M.E. Lack of awareness and apathy to sleep health issues. Indian J Sci Commun. 2014;13:7–10. [Google Scholar]
- 12.Chervin R.D., Hedger K., Dillon J.E., Pituch K.J. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 13.Leme M.S., Barbosa T.D.S., Gavião M.B.D. Assessment of orofacial functions in Brazilian children using the Nordic Orofacial Test-Screening (NOT-S) Rev Odonto Ciência. 2012;27(2):108–114. [Google Scholar]
- 14.Bergendal B., Bakke M., McAllister A., Sjögreen L., Åsten P. Profiles of orofacial dysfunction in different diagnostic groups using the Nordic Orofacial Test (NOT-S)—a review. Acta Odontol Scand. 2014;72(8):578–584. doi: 10.3109/00016357.2014.942874. [DOI] [PubMed] [Google Scholar]
- 15.Brunetti L., Rana S., Lospalluti M.L., et al. Prevalence of obstructive sleep apnea syndrome in a cohort of 1,207 children of southern Italy. Chest. 2001;120(6):1930–1935. doi: 10.1378/chest.120.6.1930. [DOI] [PubMed] [Google Scholar]
- 16.Ersu R., Arman A.R., Save D., et al. Prevalence of snoring and symptoms of sleep-disordered breathing in primary school children in Istanbul. Chest. 2004;126(1):19–24. doi: 10.1378/chest.126.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Bixler E.O., Vgontzas A.N., Lin H.M., Liao D., Calhoun S., Vela-Bueno A., Graff G. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brockmann P.E., Koren D., Kheirandish-Gozal L., Gozal D. Gender dimorphism in pediatric OSA: is it for real? Respir Physiol Neurobiol. 2017;245:83–88. doi: 10.1016/j.resp.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Li S., Jin X., Yan C., Wu S., Jiang F., Shen X. Habitual snoring in school-aged children: environmental and biological predictors. Respir Res. 2010;11(1):144. doi: 10.1186/1465-9921-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh N.T., Morton P.D., Rompre P.H., Papadakis A., Remise C. Associations between sleep-disordered breathing symptoms and facial and dental morphometry, assessed with screening examinations. Am J Orthod Dentofacial Orthop. 2011;140(6):762–770. doi: 10.1016/j.ajodo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z., Sun H., Wu Y., et al. Mouth breathing impairs the development of temporomandibular joint at a very early stage. Oral Dis. 2020;26(7):1502–1512. doi: 10.1111/odi.13377. [DOI] [PubMed] [Google Scholar]
- 22.Mp S.K., Duraisamy R. Evaluation of association between parafunctional habits and temporomandibular joint disorders among dental patients. J Contemp Issues Bus Govern. 2020;26(2):77–84. [Google Scholar]
- 23.Smith M.T., Wickwire E.M., Grace E.G., et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32(6):779–790. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martynowicz H., Gac P., Brzecka A., et al. The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings. J Clin Med. 2019;8(10):1653. doi: 10.3390/jcm8101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E.J., Choi J.H., Kim K.W., et al. The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur Arch Oto-Rhino-Laryngol. 2011;268(4):533–539. doi: 10.1007/s00405-010-1397-6. [DOI] [PubMed] [Google Scholar]