Abstract
Vascular age is determined by functional and structural changes in the arterial wall. When measured by its proxy, pulse wave velocity, it has been shown to predict cardiovascular and total mortality. Disconcordance between chronological and vascular age might represent better or worse vascular health. Cell senescence is caused by oxidative stress and sustained cell replication. Senescent cells acquire senescence-associated secretory phenotype. Oxidative stress, endothelial dysfunction, dysregulation of coagulation and leucocyte infiltration are observed in the aging endothelium. All of these mechanisms lead to increased vascular calcification and stiffness. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can involve the vascular endothelium. It enters cells using angiotensin-converting enzyme 2 (ACE-2) receptors, which are abundant in endothelial cells. The damage this virus does to the endothelium can be direct or indirect. Indirect damage is caused by hyperinflammation. Direct damage results from effects on ACE-2 receptors. The reduction of ACE-2 levels seen during coronavirus disease 2019 (COVID-19) infection might cause vasoconstriction and oxidative stress. COVID-19 and vascular aging share some pathways. Due to the novelty of the virus, there is an urgent need for studies that investigate its long-term effects on vascular health.
Keywords: vascular aging, coronavirus disease 2019, angiotensin-converting enzyme 2, pulse wave velocity, endothelial senescence
Introduction
The current pandemic of SARS-CoV-2 has been and still is a challenge for all healthcare systems. Symptoms of COVID-19 range from acute respiratory distress syndrome and multi-organ failure to very mild or even asymptomatic cases.1 Recently, the concept that COVID-19 is an endothelial disease has emerged.2 It has been known for some time that various viruses (eg Human immunodeficiency virus, Herpes simplex virus) can induce arterial stiffness and early vascular aging.3,4 This has also been observed for COVID-19.1
In the first part of this narrative review, we present a short overview of the pathology of vascular aging. We then discuss why COVID-19 can be considered an endothelial disease.
Methods
The literature search for this narrative review was conducted using PubMed. Only papers available in English were selected. The literature search used these keywords and their combinations: ‘vascular age’, ‘early vascular aging’, ‘supernormal vascular aging’, ‘endothelial senescence’, ‘oxidative stress’, ‘inflammaging’, ‘vascular calcification’, ‘ACE-2’ and ‘COVID-19’. Both authors reviewed each citation and abstract whenever available. Discrepancies in inclusion were revised and discussed. According to our keyword list, each article was accessed in terms of scientific content, methods used, and completeness in reporting factors.
Vascular Aging
Concepts of Early Vascular Aging and Supernormal Vascular Aging
Vascular age is determined by changes in functional and structural arterial wall properties. Chronological age defines the person’s age. Several tools based on multivariate regression models are available for calculating vascular age.5
Vascular age, when measured by its proxy – carotid-femoral pulse wave velocity (cfPWV), is a better predictor of cardiovascular (CV) mortality as well as all-cause mortality when compared with chronological age.6 Vascular age is a better predictor of CV risk than chronological age when studying patients with type 1 diabetes and for death when studying patients with chronic kidney disease.7,8
Disconcordance between vascular and chronological age might mean two things: either a person is in better vascular health than his peers of the same sex or the opposite – their vascular health is worse than their peers of the same sex. For example, vascular age much higher than chronological age implies early vascular aging (EVA).9 This phenomenon has been observed in patients suffering from inflammatory bowel disease, chronic kidney disease, diabetes mellitus and obesity.10-13 Indeed, EVA can be defined as an abnormally high cfPWV for a person’s age and sex.9 For example, patients with chronic bowel disease have increased cfPWV despite normal blood pressure.11 Chronic low-grade inflammation in these patients mimics that seen in inflammaging.10 Unfortunately, there are no exact cut-off values for EVA yet – neither for cfPWV or other vascular function and structure defining variables – the concept is new, and the studies are ongoing.14
The opposite to EVA, super normal vascular aging (SUPERNOVA), can be attributed to patients if their cfPWV is much lower compared with people of the same sex and age.14 These individuals seem to be protected from vascular aging (measured by cfPWV) by genetic and epigenetic means. Moreover, SUPERNOVA means a reduced risk for CV events.14 The exact mechanism of why these people are protected has not been established yet.
Interestingly, the Lancet Commission on Hypertension has named several avoidable thresholds leading to CV disease: elevated blood pressure, subclinical target organ damage and CV events.15 Since preventive and destiffening strategies are still under development, the subjects with EVA phenotype move through these thresholds faster than those with SUPERNOVA.
The Pathology of Age-Related Vascular Remodelling
Senescence and Endothelial Dysfunction
Vascular aging starts from endothelial senescence; the hallmarks of endothelial cell senescence are dysregulation of vascular tone and stiffness, increased endothelium permeability, altered angiogenesis and mitochondrial biogenesis.16 Cell senescence might be caused by oxidative stress and sustained replication.17 Senescent cells lose their ability to divide and begin to produce pro-inflammatory and matrix-degrading molecules, and this is referred to as senescence-associated secretory phenotype (SASP).16 This paracrine activity of SASP cells leads to inflammation, degradation of extracellular matrix and vascular remodelling.16 Senescent endothelial cells also tend to produce less vasodilator nitric oxide (NO) but more vasoconstrictive endothelin-1.18 Endothelial cell senescence is regulated by the p53 protein pathway, activated in response to telomere dysfunction and deoxyribonucleic acid (DNA) damage.19
Vascular endothelial dysfunction per se is characterized by vasoconstriction, pro-coagulation, pro-inflammation and proliferative effects regulated by endothelium through paracrine or autocrine means.18 All these effects have been observed at a greater level in older people when compared with younger ones, even in the absence of CV disease.18 Endothelial dysfunction is often measured by endothelium-dependent dilation (EDD).20 The endothelium promotes vasodilation through NO synthesized by endothelial NO synthase (eNOS) and other, less critical vasodilators – prostaglandins and hyperpolarizing factors.21 The decreasing EDD with age is attributed to a decrease in NO availability.22 The reduction of NO synthesis could be explained by the decline in tetrahydrobiopterin (BH4), an essential cofactor for NO synthesis in eNOS.23 Vasodilating effects can be modified by reduced eNOS expression, inactivation of NO, and conversion of NO to pro-oxidant molecules.24 As a response to angiotensin II or oxidized low-density lipoproteins, the endothelium produces endothelin-1, which has a robust vasoconstrictive effect.25 These regulatory mechanisms are essential in regulating peripheral blood pressure, but when a disease influences these pathways, it can restrict local blood flow.
Oxidative Stress
When the endothelial cell is not stressed, it has several antioxidant systems, like superoxide dismutase, glutathione peroxidase and heme oxygenase, all of which work against local oxidative stress.26 When influenced by pro-inflammatory cytokines, endothelial cells can produce nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, and this process leads to the synthesis of superoxide anions and local oxidative stress.27 Production of reactive oxygen species (ROS) is associated with hypertension, hyperlipidemia and diabetes.28 Markers of oxidative stress are found in arteries of aging humans and animals.29 Aging-related increase of ROS might be explained by more active NADPH oxidase, heightened mitochondrial production and a process caused by the decrease of BH4, leading eNOS to uncouple and shift from NO production to ROS production.30 ROS can activate a cascade resulting in nuclear factor kappa-B entering the nucleus and activating genes responsible for synthesizing pro-inflammatory cytokines.31 ROS produced during oxidative stress also interact with NO, leading to the accumulation of peroxynitrite – an extremely reactive and toxic metabolite.31 The causal interaction between oxidative stress and reduction of NO levels and endothelial function is further illustrated by experimental data, which shows that the administration of antioxidants improves both NO availability and ED.32 In aged endothelium, a vicious cycle of oxidative stress and inflammation exists, each fuelling another.16
Thrombogenesis
Endothelial cells play a crucial role in preventing the blood from clotting. It produces heparan sulfates, thrombomodulin, NO and prostacyclin to promote anticoagulation and anti-aggregation.33 If these systems fail and a thrombus is formed, endothelium also produces plasminogen activators, which have a profibrinolytic effect.34 Normally, the endothelium has anticoagulant, antiplatelet and profibrinolytic qualities. However, the endothelium can act oppositely. Some triggers (eg, pro-inflammatory cytokines) can promote clot formation through von Willebrand factor and tissue factor expression and inhibit fibrinolysis through plasminogen activator inhibitor-1.35 While the endothelium protects the body from blood clotting under normal circumstances, when exposed to inflammatory signals, it can act oppositely, leading to thrombosis.
Leucocyte Infiltration
Under normal circumstances, the endothelium does not interact with leucocytes for extended periods.36 Various selectins expressed on the endothelial wall help slow leucocytes down and increase the time they spend in contact with the endothelium.37 Pro-inflammatory cytokines (eg interleukin 1 alpha (IL-1α), tumour necrosis factor-alpha (TNF -α)) promote the synthesis of selectins.36 Once slowed down, leucocytes bind to the endothelial wall through adhesion molecules like intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1).37 Leucocytes, bound to the endothelium, can be affected by chemoattractants to enter tissues.38
Inflammation and Inflammaging
Inflammaging is a concept that defines constant chronic low-level inflammation in the absence of apparent infection and is usually attributed to the elderly population.39 During the last decade, inflammaging has been related to cardiovascular pathology in populations with pulmonary diseases, chronic kidney disease, diabetes and obesity.40,41
Inflammatory mediators can worsen vascular endothelial and smooth muscle cell function.42 Accumulation of inflammatory cells in vascular walls is related to hypertension in experimental animal studies.43 Macrophages within the arterial wall produce ROS, which increases adhesion molecule expression, activation of matrix metalloproteinases and reduced amount of NO, leading to vascular remodelling and dysfunction.44 Additionally, increased ROS production and inflammation promote telomere shortening, which is associated with atherosclerosis and major CV events.45 Furthermore, increasing pro-inflammatory mediators and reducing anti-inflammatory mediators might lead to arterial stiffening and vascular calcification.46,47
Inflammaging is closely associated with perturbations in gut microbiota with aging. Based on previous evidence, it is well established that aging is associated with reduced microbiota diversity.48,49 Studies agree that dysbiosis harms the host. On the other end of the spectrum of microbiota diversity stand supercentenarians, whose microbiota tends to be even more biodiverse.50 The type of composition of microbiota determines levels of inflammatory markers, dysfunction of the blood-brain barrier and increased circulating bacterial DNA.51 The well-functioning blood-brain barrier ensures brain health and prevents central nervous system damage.52
Lastly, the lessons about the association between inflammation and CV disease should be learned from pharmaceutical studies. Statins impact low-density lipoprotein cholesterol and inflammation, and statin-related CV event reduction results from both effects.53 Colchicine, an anti-inflammatory drug, was associated with a lower incidence of myocardial infarction.54
Vascular Calcification
There is much evidence that vascular calcification increases with age.55 It is an active process mainly defined by the phenotypic transformation of vascular smooth muscle cells (VSMCs). The osteogenic transformation of VSMCs is preceded by apoptosis, macrophage infiltration and inflammation.56
The phenomenon of arterial wall thickening due to precipitation of calcium phosphate that results in arterial stiffening is referred to as arteriosclerosis.57 It is an essential part of vascular aging and a CV risk factor.58 There are two significant types of arteriosclerosis – calcification of intima and calcification of media.59 Medial sclerosis is prevalent in patients with type 2 diabetes and chronic renal disease and is also associated with aging.60 Oxidative stress is shown to induce aging-associated vascular calcification.61 When medial calcification affects arteries of the extremities, it is referred to as Mönckeberg medial sclerosis.62 It is the most common type of medial sclerosis. Arteriosclerosis in microvasculature leads to an increase in cfPWV and pulse pressure, eventually leading to reduced perfusion.63
Atherosclerosis is mainly defined by intimal thickening that develops as early as the second decade of life and is associated with aging.64 Higher than average amounts of senescent cells, reduced cell proliferation, DNA damage and telomere shortening are found in atherosclerotic lesions, indicating a close connection between aging and atherosclerosis.65,66 As the result of intimal thickening, endothelial barrier becomes more permeable, allowing cholesterol and phospholipids to enter the subendothelium.67 Increased permeability combined with mild hypercholesterolemia or hypertension is thought to be the driving force for early atherosclerotic lesions.68 This has been demonstrated in animal studies, where older rabbits acquired more severe atherosclerotic plaques than younger rabbits when fed an atherogenic diet.69 Coronary artery calcium can be used to measure coronary atherosclerosis. Calcium score measured from computed tomography is a good prognostic tool for adverse CV events though the radiation and economic burden should also be considered.70
How can COVID-19 Damage the Vasculature?
While COVID-19 is still a new entity, we already have some studies on the relationship between COVID-19 and arterial aging. Evidence suggests that the SARS-CoV-2 virus can spread into the cardiovascular endothelium.71 Further evidence shows that epitheliopathy is present in COVID-19 and is related to the severity of the disease and death.72
SARS-CoV-2 enters cells using ACE-2 receptors; increased ACE-2 production makes it easier for the virus to enter the cell.73 The disease course is worse in individuals with comorbidities like endothelial dysfunction, diabetes, hypertension and CV disease – all of which are associated with elevated ACE-2 receptor expression.74 Once infected, various pathways discussed later lead to a decrease of ACE-2 receptors in endothelial cells.73
ACE-2 receptors are found in various tissues and are targeted as a binding protein for different viruses.75 ACE-2 messenger ribonucleic acid (mRNA) is found in most human cells, mainly in alveolar epithelial cells, enterocytes in the small intestine and vascular endothelial cells, and arterial smooth muscle cells.76 ACE-2 plays a role in anti-inflammation by promoting vasodilation.77 Under normal conditions, a physiologic equilibrium exists between opposingly acting angiotensin derivatives synthesized by ACE and ACE-2. If the concentration of ACE-2 receptors is reduced (as seen in COVID-19), the balance shifts towards vasoconstrictive, oxidative and pro-inflammatory responses.74
ACE-2 typically reduces vasoconstriction and promotes vasodilation, thus reducing hypertension.78 Reduction in ACE-2 levels in peripheral vasculature disturbs the anti-hypertensive role of these enzymes in small vessels.79 The decrease of surface levels of ACE-2 leads to an increase of angiotensin-II, which leads to vasoconstriction.80 This has been a proven pathological response to the SARS virus.80
The damage SARS-CoV-2 does to the endothelial wall can be split into two parts: direct and indirect damage. Indirect damage is caused by hyper inflammation and an increase in circulating cytokine levels.81 Previous studies have shown that acute infection can result in increased cfPWV, possibly by decreasing NO bioavailability.82 In vivo studies have shown that C-reactive protein reduces eNOS expression and activity in endothelial cells, thus leading to functional stiffening of the arteries.83 cfPWV increase during acute infection also strongly correlates with C-reactive protein, IL-6 and matrix metalloproteinase-9 (MMP-9) levels.84 The increase in MMP-9 levels leads to reduced elastin synthesis and fragmentation.85 Healthy elastin prevents vascular smooth muscle cells from changing their phenotype from normal contractile phenotype to pathologic secretory phenotype.86 Increased arterial stiffness leads to increased damage to the arterial wall due to changes in pulse pressure.87 This arterial damage itself leads to atherosclerosis and inflammation, and these effects both contribute to arterial stiffening.88,89 Thus, a vicious cycle is formed.1 Increased arterial stiffness has been proven to cause target organ damage, it is also used to predict CV events and mortality. Infection of the endothelial cell leads to endothelial dysfunction through impaired smooth muscle cell function and vascular extracellular matrix remodelling.83
Direct damage results from SARS-CoV-2 infecting vascular endothelial cells. The SARS-CoV-2 virus uses the ACE-2 receptor to infect its host.90 These receptors are also present on endothelial cells.91 It has also been shown that in vitro grown human capillary organoids can be infected by SARS-CoV-2.90 Diffuse endotheliitis, infiltration of mononuclear cells into endothelium and evidence of endothelial cell death was found in post-mortem studies of patients who died from COVID-19.92 All these findings prove that the virus can infect endothelial cells. Infected cells become a target for recruited immune cells, which may lead to apoptosis and endothelial dysfunction.92
A recent study shows that SARS-CoV-2 S protein alone can be sufficient to cause endothelial cell injury, even without genetic material typically found in the virus. The ways in which S protein damages endothelial cells are various. When affected by S protein, endothelial cells undergo mitochondrial fission, which might lead to apoptosis. Endothelial cells, when exposed to S protein, undergo reduced eNOS activity. This leads to decreased NO bioavailability and endothelial dysfunction. Authors also noticed increased glycolysis in endothelial cells, which led to increased ROS and inflammation. The increase in ROS leads to ACE-2 destabilization and a decrease in ACE-2.93
Systemic inflammation and direct viral damage could be responsible for the progression of atherosclerotic plaques or rupture of older plaques, as seen in Influenza infections.94 SARS-CoV-2 infects endothelium.92 Cells infected by SARS-CoV-2 have been shown to produce increased amounts of MMP-9.95 MMP-9 is associated with increased cfPWV and elastin fragmentation.84 Healthy elastin protects vascular smooth muscle cells from phenotype shift from the normal contractile phenotype to SASP.86 MMP-9 has been shown to promote the formation of new atherosclerotic plaques and instability of plaques.96,97 In vitro studies have demonstrated that SARS-CoV-2 spike protein is enough to induce the synthesis of adhesion molecules (VCAM-1 and ICAM-1) and pro-inflammatory cytokines (TNFα, IL-1β and IL-6) in human umbilical vein endothelial cells.98 Pro-inflammatory cytokines contribute to the progression of atherosclerosis, and the expression of adhesion molecules is crucial for the development of atherosclerosis.99,100 This shows a reasonable theoretical pathway of SARS-CoV-2-induced atherosclerosis.
During vascular aging, vascular smooth muscle cells transition from normal constrictive phenotype to pathological SASP. This transition is irreversible and is driven by the p53 protein pathway as a response to telomere shortening or DNA damage.101 DNA can be damaged by oxidative stress resulting from increased ROS production, associated with SARS-CoV-2 infection, and telomere shortening was noticed in patients after COVID-19.102
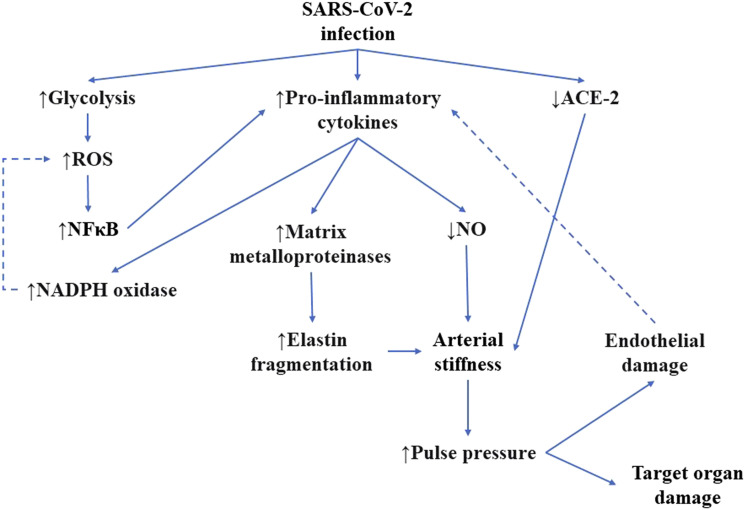
SARS-CoV-2 endothelial infection and pathological changes present in aged vasculature share common pathways. Oxidative stress and pro-inflammatory cytokines activate nuclear factor kappa-B, leading to pro-inflammatory cytokine gene transcription, and increased ROS production in NADPH oxidases.16 Pro-inflammatory cytokines reduce the bioavailability of NO and increase the expression of matrix metalloproteinases, both of which lead to arterial stiffness.83,85 Another common pathway leading to arterial stiffness is a decreased number of ACE-2 receptors, typical for both COVDI-19 and aging.103 Arterial stiffness is associated with increased pulse pressure and endothelial damage.104 The arterial damage triggers the production of pro-inflammatory cytokines.16 The common pathways of arterial aging and SARS-CoV-2 infection have been summed up in Figure 1.
Figure 1.
Common pathways for SARS-CoV-2 infection and arterial aging. Dotted lines represent the possible formation of the vicious cycle. ACE-2, angiotensin-converting enzyme 2; NADPH, nicotinamide adenine dinucleotide phosphate; NFκB, nuclear factor-kappa B; NO, nitric oxide; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Conclusions
Aging vasculature undergoes a variety of biochemical changes and structural remodelling. Certain risk factors and genetic and epigenetic factors can influence the rate at which vascular aging occurs. Together these known and unknown factors are responsible for EVA and SUPERNOVA individuals. SARS-CoV-2 has been proven to, directly and indirectly, damage endothelium and promote changes like those seen in vascular aging. Vascular aging and COVID-19 share common pathways. COVID-19 could lead to early vascular aging. However, due to the novelty of the virus, there is still an urgent need for studies that investigate its long-term effects on vascular health. Also, there is a need to establish if certain medications could decrease any premature arterial aging. Fundamental studies are needed to identify possible therapeutic targets for the prevention and treatment of early vascular aging induced by COVID-19.
Footnotes
Author Contribution: All authors contributed to: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and, (3) final approval of the version to be published.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ignas Badaras https://orcid.org/0000-0002-9423-6641
Abbreviations
- ACE-2
angiotensin-converting enzyme 2
- BH4
tetrahydrobiopterin
- cfPWV
carotid-femoral pulse wave velocity
- COVID-19
coronavirus disease 2019
- CV
cardiovascular
- DNA
deoxyribonucleic acid
- EDD
endothelium-dependent dilatation
- eNOS
endothelial nitric oxide synthase
- EVA
early vascular aging
- IL
interleukin
- ICAM-1
intercellular adhesion molecule-1
- MMP-9
matrix metalloproteinase-9
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- ROS
reactive oxygen species
- SARS
severe acute respiratory syndrome
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SASP
senescence-associated secretory phenotype
- TNFα
tumour necrosis factor-alpha
- SUPERNOVA
Super normal vascular aging
- VCAM-1
vascular cell adhesion molecule-1
- VSMCs
vascular smooth muscle cells
References
- 1.Saeed S, Mancia G. Arterial stiffness and COVID-19: a bidirectional cause-effect relationship. J Clin Hypertens. 2021;23:1099-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fourie CMT, Schutte AE. Early vascular aging in the HIV infected: Is arterial stiffness assessment the ideal tool? Virulence. 2017;8:1075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotronias D, Kapranos N. Herpes simplex virus as a determinant risk factor for coronary artery atherosclerosis and myocardial infarction. Vivo Athens Greece. 2005;19:351-7. [PubMed] [Google Scholar]
- 5.Laurent S, Boutouyrie P, Cunha PG, et al. Concept of extremes in vascular aging. Hypertension. 2019;74:218-28. [DOI] [PubMed] [Google Scholar]
- 6.Sequí-Domínguez I, Cavero-Redondo I, Álvarez-Bueno C, et al. Accuracy of pulse wave velocity predicting cardiovascular and all-cause mortality. a systematic review and meta-analysis. J Clin Med. 2020;9:2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Andrade CRM, Silva ELC, da Matta M de FB, et al. Vascular or chronological age: which is the better marker to estimate the cardiovascular risk in patients with type 1 diabetes? Acta Diabetol. 2016;53:925-33. [DOI] [PubMed] [Google Scholar]
- 8.Lin M, Chan GC, Chan KW, et al. Vascular age is associated with the risk of dialysis or death in chronic kidney disease. Nephrology. 2020;25:314-22. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson PM. Early vascular ageing – a concept in development. Eur Endocrinol. 2015;11:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanoli L, Rastelli S, Granata A, et al. Arterial stiffness in inflammatory bowel disease: a systematic review and meta-analysis. J Hypertens. 2016;34:822-9. [DOI] [PubMed] [Google Scholar]
- 11.Lioufas N, Hawley CM, Cameron JD, et al. Chronic kidney disease and pulse wave velocity: a narrative review. Int J Hypertens. 2019;2019:9189362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhammad IF, Borné Y, Östling G, et al. Arterial stiffness and incidence of diabetes: a population-based cohort study. Diabetes Care. 2017;40:1739-45. [DOI] [PubMed] [Google Scholar]
- 13.Jordan J, Nilsson PM, Kotsis V, et al. Joint scientific statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and early vascular ageing. J Hypertens. 2015;33:425-34. [DOI] [PubMed] [Google Scholar]
- 14.Bruno RM, Nilsson PM, Engström G, et al. Early and supernormal vascular aging: clinical characteristics and association with incident cardiovascular events. Hypertens Dallas Tex. 2020;76:1616-24. [DOI] [PubMed] [Google Scholar]
- 15.Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet Lond Engl. 2016;388:2665-712. [DOI] [PubMed] [Google Scholar]
- 16.Donato AJ, Morgan RG, Walker AE, et al. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia G, Aroor AR, Jia C, et al. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta BBA - Mol Basis Dis 2019;1865:1802-9. [DOI] [PubMed] [Google Scholar]
- 20.Chia PY, Teo A, Yeo TW. Overview of the assessment of endothelial function in humans. Front Med. 2020;7:542567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identifcation as nitric oxide (Nobel Lecture). Angew Chem Int Ed. 1999;38:1870-80. [DOI] [PubMed] [Google Scholar]
- 22.Torregrossa AC, Aranke M, Bryan NS. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J Geriatr Cardiol JGC. 2011;8:230-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashi Y, Noma K, Yoshizumi M, et al. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411-8. [DOI] [PubMed] [Google Scholar]
- 24.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxidants Redox Signal. 2007;9:955-69. [DOI] [PubMed] [Google Scholar]
- 25.Houde M, Desbiens L, D’Orléans-Juste P. Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol San Diego Calif. 2016;77:143-75. [DOI] [PubMed] [Google Scholar]
- 26.Sena CM, Leandro A, Azul L, et al. Vascular oxidative stress: impact and therapeutic approaches. Front Physiol; 9:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubos E, Kelly NJ, Oldebeken SR, et al. Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation. J Biol Chem. 2011;286:35407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paneni F, Costantino S, Battista R, et al. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2015;8:150-8. [DOI] [PubMed] [Google Scholar]
- 29.Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gielis J, Quirynen L, Briedé J, et al. Pathogenetic role of endothelial nitric oxide synthase uncoupling during lung ischaemia-reperfusion injury†. Eur J Cardio-Thorac Surg. 2017;52(2):256-263. [DOI] [PubMed] [Google Scholar]
- 31.Tesauro M, Mauriello A, Rovella V, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. 2017;281:471-82. [DOI] [PubMed] [Google Scholar]
- 32.Bhayadia R, Schmidt BMW, Melk A, et al. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci. 2016;71:161-9. [DOI] [PubMed] [Google Scholar]
- 33.Marcus AJ, Broekman MJ, Drosopoulos JH, et al. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawdey MS, Loskutoff DJ. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest. 1991;88:1346-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost JTH. 2005;3:1590-6. [DOI] [PubMed] [Google Scholar]
- 36.McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol. 2013;50:7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noels H, Weber C, Koenen RR. Chemokines as therapeutic targets in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2019;39:583-92. [DOI] [PubMed] [Google Scholar]
- 39.Franceschi C, Garagnani P, Parini P, et al. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576-90. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Richardson G, Benli FM, et al. Inflammageing in the cardiovascular system: mechanisms, emerging targets, and novel therapeutic strategies. Clin Sci. 2020;134:2243-62. [DOI] [PubMed] [Google Scholar]
- 41.Prattichizzo F, De Nigris V, Spiga R, et al. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev. 2018;41:1-17. [DOI] [PubMed] [Google Scholar]
- 42.Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: the biological basis of CANTOS and beyond. J Am Coll Cardiol. 2017;70:2278-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison DG, Guzik TJ, Lob H, et al. Inflammation, immunity and hypertension. Hypertension. 2011;57:132-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Ciuceis C, Amiri F, Brassard P, et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106-13. [DOI] [PubMed] [Google Scholar]
- 45.Samani NJ, Boultby R, Butler R, et al. Telomere shortening in atherosclerosis. Lancet Lond Engl. 2001;358:472-3. [DOI] [PubMed] [Google Scholar]
- 46.Tomiyama H, Shiina K, Matsumoto‐Nakano C, et al. The contribution of inflammation to the development of hypertension mediated by increased arterial stiffness. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2017;6:e005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchis P, Ho CY, Liu Y, et al. Arterial ‘inflammaging’ drives vascular calcification in children on dialysis. Kidney Int. 2019;95:958-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shintouo CM, Mets T, Beckwee D, et al. Is inflammageing influenced by the microbiota in the aged gut? A systematic review. Exp Gerontol. 2020;141:111079. [DOI] [PubMed] [Google Scholar]
- 49.Biagi E, Franceschi C, Rampelli S, et al. Gut microbiota and extreme longevity. Curr Biol CB. 2016;26:1480-5. [DOI] [PubMed] [Google Scholar]
- 50.Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fransen F, van Beek AA, Borghuis T, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang W, Zhu H, Feng Y, et al. The impact of gut microbiota disorders on the blood&brain barrier. Infect Drug Resist. 2020;13:3351-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195-207. [DOI] [PubMed] [Google Scholar]
- 54.Crittenden DB, Lehmann RA, Schneck L, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. 2012;39:1458-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66:1643-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.New SEP, Aikawa E. The role of extracellular vesicles in de novo mineralization: an additional novel mechanism of cardiovascular calcification. Arterioscler Thromb Vasc Biol. 2013;33:1753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalampogias A, Siasos G, Oikonomou E, et al. Basic mechanisms in atherosclerosis: the role of calcium. Med Chem Shariqah United Arab Emir. 2016;12:103-13. [DOI] [PubMed] [Google Scholar]
- 58.Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010;10:S213-20. [DOI] [PubMed] [Google Scholar]
- 59.Mackey RH, Venkitachalam L, Sutton-Tyrrell K. Calcifications, arterial stiffness and atherosclerosis. Adv Cardiol. 2007;44:234-44. [DOI] [PubMed] [Google Scholar]
- 60.Fok P-W, Lanzer P. Media sclerosis drives and localizes atherosclerosis in peripheral arteries. PLoS One. 2018;13:e0205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pescatore LA, Gamarra LF, Liberman M. Multifaceted mechanisms of vascular calcification in aging. Arterioscler Thromb Vasc Biol. 2019;39:1307-16. [DOI] [PubMed] [Google Scholar]
- 62.Lanzer P, Boehm M, Sorribas V, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.London GM, Guérin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731-40. [DOI] [PubMed] [Google Scholar]
- 64.Stary HC. Macrophages, macrophage foam cells, and eccentric intimal thickening in the coronary arteries of young children. Atherosclerosis. 1987;64:91-108. [DOI] [PubMed] [Google Scholar]
- 65.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245-59. [DOI] [PubMed] [Google Scholar]
- 67.Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262-75. [DOI] [PubMed] [Google Scholar]
- 68.Zhu X-Y, Bentley MD, Chade AR, et al. Early changes in coronary artery wall structure detected by microcomputed tomography in experimental hypercholesterolemia. Am J Physiol-Heart Circ Physiol. 2007;293:H1997-2003. [DOI] [PubMed] [Google Scholar]
- 69.Spagnoli LG, Orlandi A, Mauriello A, et al. Age-dependent increase of rabbit aortic atherosclerosis a morphometric approach. Pathol - Res Pract 1992;188:637-42. [DOI] [PubMed] [Google Scholar]
- 70.Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol. 2009;6:681-8. [DOI] [PubMed] [Google Scholar]
- 71.Gustafson D, Raju S, Wu R, et al. Overcoming barriers. Arterioscler Thromb Vasc Biol. 2020;40:1818-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scialo F, Daniele A, Amato F, et al. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198:867-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abassi Z, Higazi AAR, Kinaneh S, et al. ACE2, COVID-19 infection, inflammation, and coagulopathy: missing pieces in the puzzle. Front Physiol. 2020;11:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Datta PK, Liu F, Fischer T, et al. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10:7448-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W, McKinnie SMK, Farhan M, et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-Apelin-13 and Apelin-17. Hypertension. 2016;68:365-77. [DOI] [PubMed] [Google Scholar]
- 79.Galán M, Jiménez-Altayó F. Small resistance artery disease and ACE2 in hypertension: a new paradigm in the context of COVID-19. Front Cardiovasc Med. 2020;7:588692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan LY, Komarasamy TV, RMT Balasubramaniam V. Hyperinflammatory immune response and COVID-19: a double edged sword. Front Immunol; 12:742941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clapp BR, Hingorani AD, Kharbanda RK, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172-8. [DOI] [PubMed] [Google Scholar]
- 83.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vasc Pharmacol. 2016;77:1-7. [DOI] [PubMed] [Google Scholar]
- 84.Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193-200. [DOI] [PubMed] [Google Scholar]
- 85.Bouvet C, Moreau S, Blanchette J, et al. Sequential activation of matrix metalloproteinase 9 and transforming growth factor β in arterial elastocalcinosis. Arterioscler Thromb Vasc Biol. 2008;28:856-62. [DOI] [PubMed] [Google Scholar]
- 86.Orr AW, Lee MY, Lemmon JA, et al. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2009;29:225-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steppan J, Barodka V, Berkowitz DE, et al. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Constantinides P. The role of arterial wall injury in atherogenesis and arterial thrombogenesis. Zentralblatt Allg Pathol U Pathol Anat. 1989;135:517-30. [PubMed] [Google Scholar]
- 89.Davis C, Fischer J, Ley K, et al. The role of inflammation in vascular injury and repair. J Thromb Haemostasis. 2003;1:1699-709. [DOI] [PubMed] [Google Scholar]
- 90.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605-10. [DOI] [PubMed] [Google Scholar]
- 92.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet Lond Engl. 2020;395:1417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei Y, Zhang J, Schiavon CR, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE2. BioRxiv Prepr Serv Biol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gopal R, Marinelli MA, Alcorn JF. Immune mechanisms in cardiovascular diseases associated with viral infection. Front Immunol; 11:570681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gelzo M, Cacciapuoti S, Pinchera B, et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci Rep. 2022;12:1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li T, Li X, Feng Y, et al. The role of matrix metalloproteinase-9 in atherosclerotic plaque instability. Mediat Inflamm. 2020;2020:3872367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Florence JM, Krupa A, Booshehri LM, et al. Metalloproteinase-9 contributes to endothelial dysfunction in atherosclerosis via protease activated receptor-1. PLoS One. 2017;12:e0171427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robles JP, Zamora M, Adan-Castro E, et al. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling. J Biol Chem. 2022;298:101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fatkhullina AR, Peshkova IO, Koltsova EK. The role of cytokines in the development of atherosclerosis. Biochem Biokhimiia. 2016;81:1358-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292-301. [DOI] [PubMed] [Google Scholar]
- 101.Katsuumi G, Shimizu I, Yoshida Y, et al. Vascular senescence in cardiovascular and metabolic diseases. Front Cardiovasc Med; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mongelli A, Barbi V, Gottardi Zamperla M, et al. Evidence for biological age acceleration and telomere shortening in COVID-19 survivors. Int J Mol Sci. 2021;22:6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.AlGhatrif M, Tanaka T, Moore AZ, et al. Age-associated difference in circulating ACE2, the gateway for SARS-COV-2, in humans: results from the InCHIANTI study. GeroScience. 2021;43:619-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Said MA, Eppinga RN, Lipsic E, et al. Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J Am Heart Assoc; 7:e007621. [DOI] [PMC free article] [PubMed] [Google Scholar]