Abstract
Introduction
A large proportion of patients experience a wide range of sequelae after acute COVID-19, especially after severe illness. The long-term health sequelae need to be assessed. Our objective was to longitudinally assess persistence of symptoms and clusters of symptoms up to 12 months after hospitalisation for COVID-19 and to assess determinants of the main persistent symptoms.
Methods
In this multicenter prospective cohort study patients with COVID-19 are followed up for 2 years with measurements at 3, 6, 12 and 24 months after hospital discharge. Here, we present interim results regarding persistent symptoms up to 12 months.
Results
We included 492 patients; mean±sd age was 60.2±10.7 years, 335 (68.1%) were males, median length of hospital stay was 11 (6.0–27.0) days. At 3 months after discharge 97.0% of the patients had at least one persisting symptom, this declined to 95.5% and 92.0% at 6 and 12 months, respectively (p=0.010). Muscle weakness, exertional dyspnoea, fatigue, and memory and concentration problems were the most prevalent symptoms with rates over 50% during follow-up. Over time, muscle weakness, hair loss and exertional dyspnoea decreased significantly (p<0.001), while other symptoms such as fatigue, concentration and memory problems, anosmia and ageusia persisted. Symptoms from the physical and respiratory cluster declined significantly over time, in contrast to the fatigue and cognitive symptom clusters.
Conclusion
The majority of patients experienced COVID-19 sequelae up to 12 months after severe infection. Whereas physical and respiratory symptoms showed slow gradual decline, fatigue and cognitive symptoms did not evidently resolve over time.
Short abstract
Over 90% of patients experience #COVID19-related sequelae up to 12 months after hospitalisation. Whereas physical and respiratory symptoms show slow gradual decline, fatigue and cognitive symptoms do not evidently resolve over time. https://bit.ly/3z9I1xK
Introduction
Acute coronavirus disease 2019 (COVID-19) infection in humans is associated with a heterogeneous range of symptoms including respiratory, musculoskeletal, gastrointestinal and neurological symptoms. In 5–14% of patients the respiratory consequences of COVID-19 are severe, requiring hospitalisation for oxygen supplementation or even prolonged ventilatory support [1].
Whereas a proportion of patients fully recover, it becomes increasingly clear that a proportion of patients experience a wide range of long-lasting sequelae after acute COVID-19. Different terms are currently used for describing the presence of post-COVID-19 symptoms, such as long COVID, long haulers, post-COVID-19 syndrome, persistent post-COVID and post-acute sequelae of COVID. Although several definitions are in place, persistent symptoms after COVID-19 are regarded as post-COVID-19 syndrome if they persist or present within 12 weeks of the onset of acute COVID-19 and last for at least 2 months, and are not attributable to alternative diagnoses [2, 3]. The more recent World Health Organization (WHO) definition of post-COVID-19 condition (PCC) is very similar to this definition, adding that symptoms may be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness and must persist for at least 2 months [4]. Symptoms may also fluctuate or relapse over time. These post-acute COVID-19 sequelae encompass a wide range of symptoms and organ systems. Common symptoms include fatigue, shortness of breath and cognitive dysfunction [4].
Although exact overall prevalence of these long-term symptoms remains unclear, it is estimated that between 2.6% and 18.7% of symptomatic patients experience persistent symptoms related to COVID-19 beyond 12 weeks after COVID-19 [5, 6]. This number increases when patients are more severely affected [7]. A recent systematic review described that >50% of all patients (the majority after hospitalisation) experienced post-acute COVID-19 sequelae, even up to 6 months after acute infection [8]. Data from a Chinese cohort demonstrated that symptoms persisted in over 68% of hospitalised patients for COVID-19 at 6 months after disease onset, decreasing to 49% at 12 months [9], whereas recent European results indicated that 91.7% of COVID-19 patients reported at least one symptom at 12 months [10].
The nature of the reported symptoms is diverse and ranges from exertional dyspnoea to sensory overload. Although studies have tried to phenotype the patients with residual symptoms, looked into co-occurrence of pairs of post-COVID-19 symptoms or report on assays of symptoms according to various organ systems, it remains unclear how the various domains of symptoms relate to each other and how frequently certain types of symptoms overlap [11–13].
Currently, most reports on persistent symptoms remain limited to 6 months after infection and little is known regarding the determinants of persistent symptoms. Also, most studies are cross-sectional and studies reporting outcomes across multiple time points are scarce.
The aim of the current study was therefore to assess persistence of symptoms and clusters of symptoms up to 12 months after hospital discharge, to explore how various clusters of symptoms overlap with each other and to assess determinants of the main persistent symptoms after COVID-19.
Methods
Study design
The COvid-19 Follow-up care paths and Long-term Outcomes Within the Dutch healthcare system (CO-FLOW) study is an ongoing multicenter prospective cohort study following COVID-19 patients discharged from hospitals in the Rotterdam–Rijnmond–Delft region in the Netherlands. Detailed description of its protocol can be found elsewhere [14]. In short, up to 2 years after hospitalisation patients with COVID-19 are evaluated at 3, 6, 12 and 24 months after hospital discharge. Here, we present interim results regarding persisting symptoms obtained in the period from 1 July 2020 until 1 December 2021 as part of the CO-FLOW study up to 12 months after discharge. The Medical Ethics Committee of the Erasmus Medical Center (MC) approved this study (MEC-2020–0487). The trial was registered at The Netherlands Trial Register (NL8710) (https://www.trialregister.nl) on 12 June 2020.
Adult patients (≥18 years of age) were eligible to participate in the CO-FLOW study if they had been hospitalised for COVID-19 (diagnosis based on either positive reverse transcription polymerase chain reaction or a clinical diagnosis combined with positive serology for COVID-19) within the previous 6 months and patient or relative had sufficient knowledge of the Dutch or English language. Incapacitated patients were unable to participate given the study procedures. For this study only participants with at least two study visits were included.
Study procedures
In principle, all patients that had been hospitalised were offered outpatient follow-up at one of the participating centres. Patients were recruited during outpatient follow-up after discharge in one of the participating centres, at the inpatient rehabilitation centre, or at the skilled nursing facility. All patients provided written informed consent before the start of the measurements. Recruitment of study participants occurred independent of the patient's recovery status; this was largely based on availability of research personnel to recruit patients and to perform study visits. Study visits were synchronised with the patient's regular follow-up for COVID-19 at each of the participating centres if possible. When patients were discharged from regular follow-up, study visits continued in the Erasmus University MC or, if patients were unable to come to the Erasmus University MC, a research assistant performed the study visit at home. During study visits patients performed noninvasive clinical tests, including physical, psychological and cognitive evaluation. At 3-, 6- and 12-month follow-up visits, patients received questionnaires via e-mail or postal mail. Data were stored in Castor EDC (Castor EDC, Amsterdam, The Netherlands).
Outcomes
A new Corona Symptom Checklist was developed for this study on “novel or worsened symptoms since the onset of COVID-19” during the first 3 months of the study, based on the first experiences with post-COVID-19 patients. All questions are answered with “yes” or “no” (see supplementary appendix 1 for complete questionnaire). During the study visits the Corona Symptom Checklist was administered by a research assistant in a face-to-face interview. As the checklist was still under development when the study started amid the beginning of the COVID-19 pandemic, it was introduced at all study visits after 5 August 2020. As the pandemic evolved and knowledge increased regarding PCC, additional questions were added (sensory overload, headache, chest pain) from June 2021 onwards.
As fatigue is considered as one of the most prevalent symptoms in PCC, we chose to report fatigue not based on the checklist results, but on the validated Fatigue Assessment Scale (FAS) that was assessed in all patients since study onset. The FAS is a 10-item self-report questionnaire and is validated in patients with chronic lung disease [15]. The items are scored on a Likert scale ranging from 1 to 5. A total score of ≥22 is considered to represent substantial fatigue and was used to indicate persisting fatigue [15].
Patient and clinical characteristics were collected at study visits and through electronic patient records. Patient characteristics included age, sex, body mass index (BMI), migration background, pre-COVID educational and employment status, smoking status and comorbidities. Clinical characteristics included baseline laboratory and radiological parameters, complications during hospitalisation including delirium and thrombosis, type and quantity of oxygen support, intensive care unit (ICU) admission, length of stay (LOS) ICU, LOS hospital and COVID-19 directed treatment during hospital admission.
Statistical analyses
We examined descriptive statistics to ensure data met statistical assumptions. Variables were presented as mean ±sd, median with interquartile range or numbers (n) with percentages (%) as appropriate.
Patient-reported symptoms were clustered into one of four clusters according to the nature of the symptom: physical, respiratory, fatigue and cognitive symptom cluster. The physical symptom cluster was composed of the symptoms muscle weakness, balance problems/dizziness, joint pain, tingling/numbness in extremities, hair loss, headache, chest pain, skin rash, vision problems, hoarseness, anosmia, ageusia, stool problems, claudication, hearing problems and miction problems. The respiratory symptom cluster was composed of the symptoms exertional dyspnoea, dyspnoea, cough and phlegm. The fatigue symptom cluster was composed of fatigue and sleeping problems. The cognitive symptom cluster was composed of the symptoms memory problems, concentration problems, sensory overload and anxiety/nightmares. If any of the symptoms in the clusters was present at a time point, persisting symptoms in that cluster were scored as present at that time point. We used generalised estimating equations (GEEs) with an unstructured covariance matrix to assess persistence of symptoms and symptom clusters over time. GEEs account for correlations between patient follow-up measurements and include all observed outcomes despite incomplete data. For longitudinal analyses, a Bonferroni correction was applied and a p-value <0.002 was considered statistically significant. Difference in the distribution of symptom clusters across sexes were assessed with a Chi-Square test. Lastly, we performed multivariable logistic regression analyses with a backward selection procedure to determine which variables are independently associated with the most prevalent symptom per cluster at 3 months after discharge. The dependent variables were muscle weakness, deconditioning/exertional dyspnoea, fatigue and memory problems. We only reported determinants of symptoms at 3 months after discharge, as symptoms were most prevalent at 3 months after discharge and the majority did not decrease significantly over time. Determinants that were examined were age, sex, BMI, migration background (European, Dutch Caribbean, Asian, Turkish and (North) African), pre-COVID educational (low, middle, high) and employment status (employed, not employed, retired), presence of comorbidity, smoking (never versus ever), BMI and C-reactive protein (CRP) at admission, complications of thrombosis or delirium, oxygen supplementation (none, nasal cannula or mask oxygen supplementation, high-flow nasal cannula, mechanical ventilation), LOS hospital and COVID-19 directed treatment with steroids. The covariates BMI and CRP were imputed with their mean value if missing. Variable elimination from the multivariable models was based on goodness of fit using the likelihood ratio test with a p-value of 0.1, and the final models are presented with adjusted odds ratios (ORs) and 95% confidence interval (95% CI). We also assessed clinical characteristics of patients hospitalised for COVID-19 at 3 months follow-up, which are presented across the number of symptoms clusters affected. As numbers per group/characteristic were limited, differences were not statistically assessed, and these trends should be considered as explorative and hypotheses generating. All analyses were performed using Statistical Package for Social Sciences (SPSS) version 25 (IBM SPSS statistics, SPSS Inc, Chicago, IL, USA) and STATA version 8SE (StataCorp LLC, College Station, Texas, USA) and R version 4.1.1 (R-Foundation) were used for graphs.
Results
Characteristics
Between 1 July 2020 and 1 December 2021 patients were recruited in CO-FLOW. The total number of patients hospitalised for COVID-19 during the recruitment period in the region was 4569 of whom 1199 (26%) died during hospitalisation. The number of patients that had been invited is largely unknown due to logistical reasons. From the 3370 survivors, 650 patients (19% of all survivors) were included in this study, of whom 492 participants underwent at least two study measurements and were included in this interim analysis.
Baseline characteristics are presented in table 1. Patients had a mean age of 60.2±10.7 years, 335 (68.1%) were male and 403 (81.9%) had one or more comorbidities: most commonly obesity, cardiovascular or pulmonary disease. Oxygen supplementation during admission was required by 474 (96.3%) patients, 199 (40.4%) had been admitted to the ICU, with a median LOS in ICU of 17 (9.0–30.5) days, and the median total LOS in the hospital was 11 (6.0–27.0) days. Of all patients, 357 (72.6%) received any COVID-19 directed treatment, of whom 330 (70.8%) received any form of steroids and 54 (11.5%) received directed anti-inflammatory treatment.
TABLE 1.
Patient and clinical characteristics of patients hospitalised for COVID-19
| n# | All (n=492) | |
| Patient characteristic | ||
| Age years | 60.2±10.7 | |
| Sex, male | 335 (68.1) | |
| BMI kg·m−2 | 437 | 29.3±5.5 |
| Migration background | 491 | |
| European | 373 (76.0) | |
| Dutch Caribbean | 61 (12.4) | |
| Asian | 25 (5.1) | |
| Turkish | 18 (3.7) | |
| (North) African | 14 (2.9) | |
| Pre-COVID education | 489 | |
| Low | 166 (33.9) | |
| Middle | 172 (35.2) | |
| High | 151 (30.9) | |
| Pre-COVID employment | 490 | |
| Unemployed | 77 (15.7) | |
| Employed | 297 (60.6) | |
| Retired | 116 (23.7) | |
| Smoking status | 491 | |
| Never | 211 (43.0) | |
| Former | 270 (55.0) | |
| Current | 10 (2.0) | |
| Comorbidities | ||
| ≥1 | 403 (81.9) | |
| Obesity (BMI ≥30) | 190 (38.6) | |
| Diabetes | 95 (19.3) | |
| Cardiovascular disease/hypertension | 192 (39.0) | |
| Pulmonary disease | 119 (24.2) | |
| Renal disease | 46 (9.3) | |
| Gastrointestinal disease | 22 (4.5) | |
| Neuromuscular disease | 49 (10.0) | |
| Malignancy | 56 (11.4) | |
| Autoimmune/inflammatory disease | 54 (11.0) | |
| Mental disorder | 25 (5.1) | |
| In-hospital characteristics | ||
| PCR confirmed SARS-CoV-2 | 485 (98.6) | |
| Serology confirmed SARS-CoV-2 | 7 (1.4) | |
| Laboratory values | ||
| Creatinine µmol·L−1 | 471 | 82.0 (69.0–100.0) |
| (CKD-EPI) eGFR mL·min−1 | 456 | 82.0 (66.0–90.0) |
| CRP mg·L−1 | 467 | 85.0 (47.0–154.0) |
| Ferritin µg·L−1 | 284 | 832.5 (443.5–1613.3) |
| ALAT U·L−1 | 457 | 37.0 (26.0–56.0) |
| Haemoglobin mmol·L−1 | 468 | 8.6 (7.9–9.2) |
| MCV fL | 461 | 89.0 (85.0–91.0) |
| Thrombocytes, 10⁹/L | 463 | 211.0 (159.0–276.0) |
| Lymphocytes absolute count, 10⁹/L | 325 | 0.9 (0.6–1.1) |
| D-dimer mg·L−1 | 237 | 1.1 (0.6–380.0) |
| NT-proBNP pmol·mL−1 | 90 | 18.5 (8.8–48.0) |
| IL-6 pmol·mL−1 | 36 | 55.5 (28.0–179.0) |
| Chest radiograph abnormalities | 468 | |
| Normal | 59 (12.6) | |
| Moderate | 99 (21.2) | |
| Severe | 310 (66.2) | |
| Thrombosis | 484 | 79 (16.3) |
| Delirium | 477 | 121 (25.4) |
| Requiring oxygen supplementation | 492 | 474 (96.3) |
| Requiring high-flow nasal cannula | 462 | 150 (32.5) |
| ICU admission | 199 (40.4) | |
| Invasive mechanical ventilation | 175 (35.6) | |
| Length of intubation days | 167 | 14.0 (8.0–27.0) |
| Tracheostomy | 482 | 64 (13.3) |
| Length of ICU stay days | 197 | 17.0 (9.0–30.5) |
| Length of hospital stay days | 11.0 (6.0–27.0) | |
| COVID-19 directed treatment | 466 | |
| None | 109 (23.4) | |
| (Hydroxy)chloroquine | 14 (3.0) | |
| Steroids | 330 (70.8) | |
| Antivirals | 69 (14.8) | |
| Anti-inflammatory (IL-6) treatment | 54 (11.6) | |
| Convalescent plasma | 8 (1.7) | |
| Monoclonal antibodies | 0 (0.0) | |
| Time interval between discharge and follow-up visit | ||
| 3-month visit, days | 385 | 94.7±22.8 |
| 6-month visit, days | 483 | 184.8±27.9 |
| 12-month visit, days | 271 | 368.3±17.3 |
Data are presented as n (%), mean±sd or, for non-normally distributed variables, median (interquartile range). BMI: body mass index; PCR: polymerase chain reaction; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; NT-proBNP: N-terminal pro-brain natriuretic peptide; IL-6: interleukin-6; ICU: intensive care unit. #: adjusted n is presented for variables with a total number of patients <492.
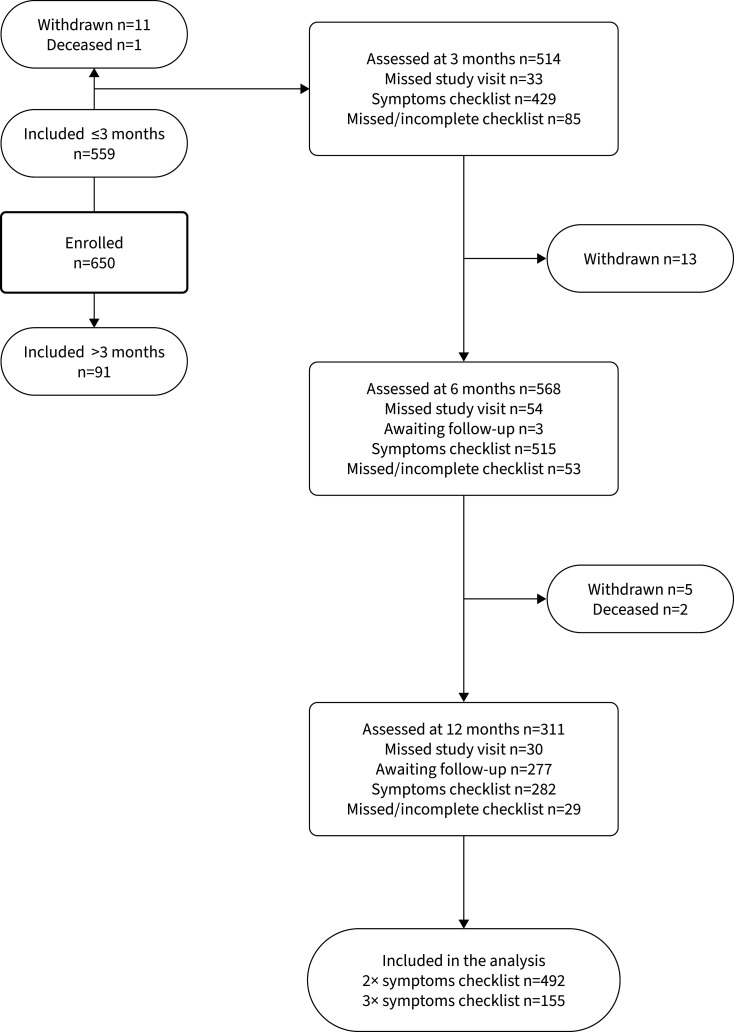
To date, 20 patients withdrew from the study or were deceased during follow-up. Up to 54 patients missed one or more study visits. A flowchart of the included patients and study measurements is shown in figure 1.
FIGURE 1.
Flowchart of the patients in the CO-FLOW study during the interim analysis.
Persisting symptoms
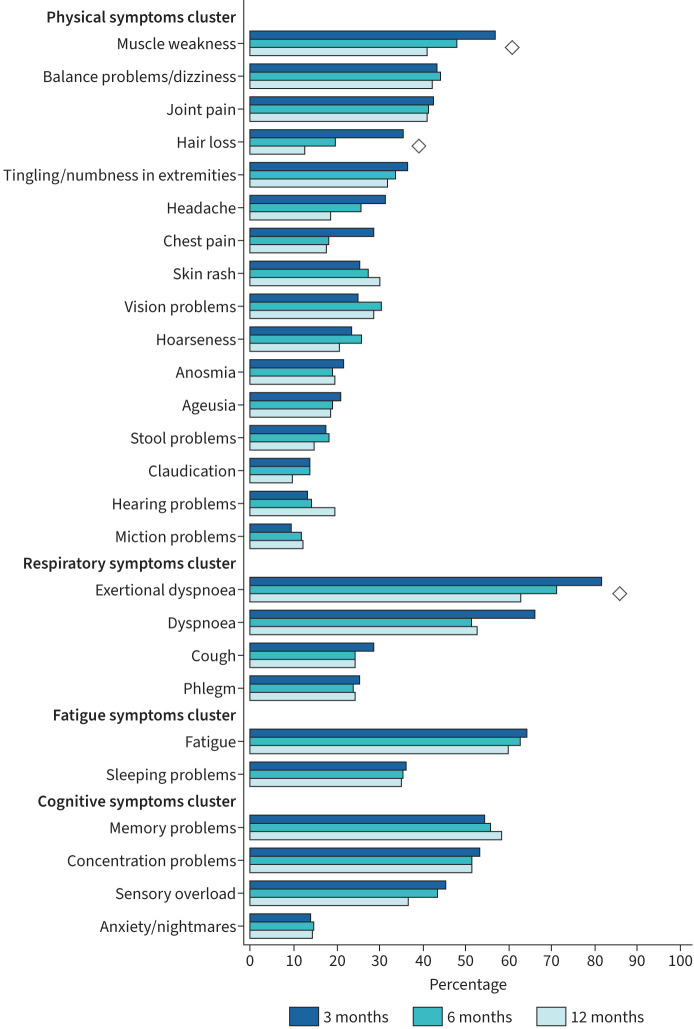
Table 2 presents the number and proportion of patients with persisting symptoms at each follow-up measurement. At 3 months after discharge, 97.0% of the patients had at least one persisting symptom; this proportion of patients declined to 95.5% at 6 months and to 92.0% at 12 months (p=0.010). Presence of a single symptom varied from 9.7% for miction problems to 81.8% for exertional dyspnoea. At all study visits, the most prevalent symptoms were muscle weakness, exertional dyspnoea, fatigue, and memory and concentration problems. These symptoms were reported by >50% of the patients during follow-up; a large number of other persistent symptoms were frequently reported, presented in table 2 and figure 2. Symptoms that significantly declined over time were muscle weakness, hair loss and exertional dyspnoea (p<0.001).
TABLE 2.
Prevalence of COVID-19-related symptoms at 3-, 6- and 12-month follow-up in patients after hospitalisation for COVID-19
| 3 months (n=385) n (%) | 6 months (n=483) n (%) | 12 months (n=271) n (%) | p-value# | |
| Physical symptoms | ||||
| Muscle weakness | 220 (57.1) | 234 (48.4) | 111 (41.0) | <0.001 |
| Balance problems/dizziness | 169 (43.8) | 213 (44.4) | 116 (42.8) | 0.922 |
| Joint pain | 166 (43.2) | 201 (41.6) | 111 (41.0) | 0.352 |
| Tingling/numbness in extremities | 147 (36.8) | 163 (33.9) | 86 (32.1) | 0.291 |
| Hair loss | 138 (35.9) | 98 (20.3) | 35 (12.9) | <0.001 |
| Headache# | 33 (31.4) | 57 (26.1) | 29 (18.6) | 0.579 |
| Chest pain# | 29 (29.0) | 40 (18.4) | 28 (17.8) | 0.069 |
| Skin rash | 99 (25.7) | 132 (27.4) | 82 (30.3) | 0.587 |
| Vision problems | 97 (25.2) | 148 (30.6) | 78 (28.8) | 0.023 |
| Hoarseness | 91 (23.6) | 125 (25.9) | 57 (21.0) | 0.088 |
| Anosmia | 84 (21.9) | 93 (19.3) | 53 (19.6) | 0.369 |
| Ageusia | 82 (21.2) | 94 (19.5) | 52 (19.2) | 0.185 |
| Stool problems | 68 (17.7) | 89 (18.5) | 41 (15.1) | 0.547 |
| Claudication | 54 (14.1) | 68 (14.1) | 27 (10.0) | 0.116 |
| Hearing problems | 52 (13.5) | 70 (14.5) | 53 (19.6) | 0.059 |
| Miction problems | 37 (9.7) | 58 (12.1) | 34 (12.5) | 0.269 |
| Respiratory symptoms | ||||
| Exertional dyspnoea | 315 (81.8) | 345 (71.4) | 171 (63.1) | <0.001 |
| Dyspnoea# | 78 (66.1) | 114 (51.8) | 83 (52.9) | 0.003 |
| Cough | 112 (29.0) | 119 (24.7) | 66 (24.4) | 0.329 |
| Phlegm | 98 (25.5) | 117 (24.2) | 67 (24.7) | 0.727 |
| Fatigue symptoms | ||||
| Fatigue | 243 (64.5) | 277 (63.1) | 156 (60.2) | 0.932 |
| Sleeping problems | 141 (36.5) | 172 (35.6) | 96 (35.4) | 0.777 |
| Cognitive symptoms | ||||
| Memory problems | 211 (54.7) | 271 (56.1) | 158 (58.3) | 0.144 |
| Concentration problems | 206 (53.4) | 249 (51.6) | 140 (51.7) | 0.826 |
| Sensory overload# | 44 (45.5) | 93 (43.9) | 58 (36.7) | 0.503 |
| Anxiety/nightmares | 56 (14.5) | 72 (14.9) | 40 (14.8) | 0.785 |
Data are presented as n (%) indicating the number of patients with symptoms. p-values are obtained from Generalised Estimating Equation analyses, with follow-up visit as fixed factor and symptom (yes/no) at each follow-up visit as dependent variable. Bonferroni correction was applied for multiple testing; a p-value <0.002 was considered statistically significant (printed in bold). #: symptoms headache, chest pain, dyspnoea and sensory overload were added at a later stage, resulting in lower total numbers.
FIGURE 2.
Symptom prevalence over time. Prevalence of COVID-19-related symptoms at 3-, 6- and 12-month follow-up in patients after hospitalisation for COVID-19, sorted by symptoms cluster and from most to least frequently reported. Data are presented as percentage of patients with symptoms. Symptoms marked with ◊ declined significantly over time based on generalised estimating equation analyses, with follow-up visit as fixed factor and symptom (yes/no) at each follow-up visit as dependent variable.
Symptom clusters
The prevalence of symptoms and the overlap between symptom clusters at 3 months follow-up are shown in figure 2. At 3 months, 90.7% of patients reported at least one symptom from the physical symptom cluster; this declined significantly to 86.8% at 6 months and to 84.5% at 12 months (p=0.025). Respiratory symptoms were reported by 87.3%, 79.1% and 76.0% of the patients at 3, 6 and 12 months, respectively (p<0.001). In the fatigue symptom cluster, 68.3% of the patients reported a symptom at 3 months, 67.8% at 6 months and 67.6% at 12 months (p=0.082). A symptom from the cognitive symptom cluster was reported in 71.8% of the patients at 3 months, 70.0% at 6 months and 74.2% at 12 months (p=0.452).
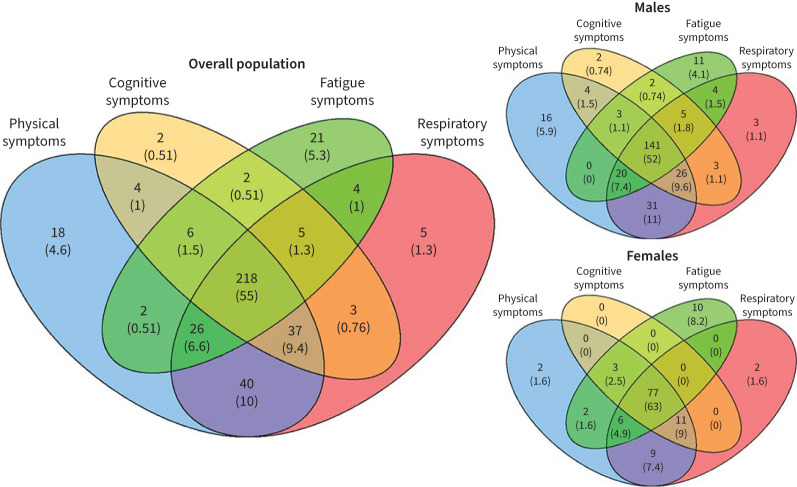
At 3 months after hospital discharge, 218 (56.3%) reported symptoms in all four symptoms clusters and 292 (75.5%) in three clusters. Symptoms in the physical and respiratory symptom clusters most frequently overlapped (figure 3a). The majority of patients with fatigue also experienced cognitive symptoms (86.8%), and vice versa (83.4%). Isolated symptoms were rare but concerned most frequently fatigue in 21 (5.3%) patients or physical symptoms in 18 (4.6%) patients. Females more frequently report symptoms in all four clusters than males (63% versus 52%, p<0.001) (figure 3b and 3c). Fatigue and cognitive symptoms were more frequent in females than in males (80.2% versus 68.6% (p=0.002) and 74.5% versus 68.6% (p=0.009), respectively), and so were the frequency of respiratory (85.9% versus 85.5%, p=0.022) and physical symptoms (90% versus 88.5%, p=0.002).
FIGURE 3.
Venn diagrams showing overlap between the symptom clusters (physical symptoms, cognitive symptoms, fatigue symptoms and respiratory symptoms) for the entire cohort, males and females. Data are presented as n (%) indicating the number of patients with symptoms.
In supplementary table S1 patient and clinical characteristics of patients hospitalised for COVID-19 at 3 months follow-up are presented across the number of symptom clusters affected. The majority of patients (89%) experienced symptoms in two or more clusters. Several trends can be noticed with increasing number of symptom clusters affected: more in females, patients with non-European background, employment, comorbidities and with lower CRP, lower D-dimer and more severely affected chest radiograph upon admission. No association seems present with LOS, ICU admission and ventilation and with COVID-19 directed treatment.
Determinants of persisting symptoms
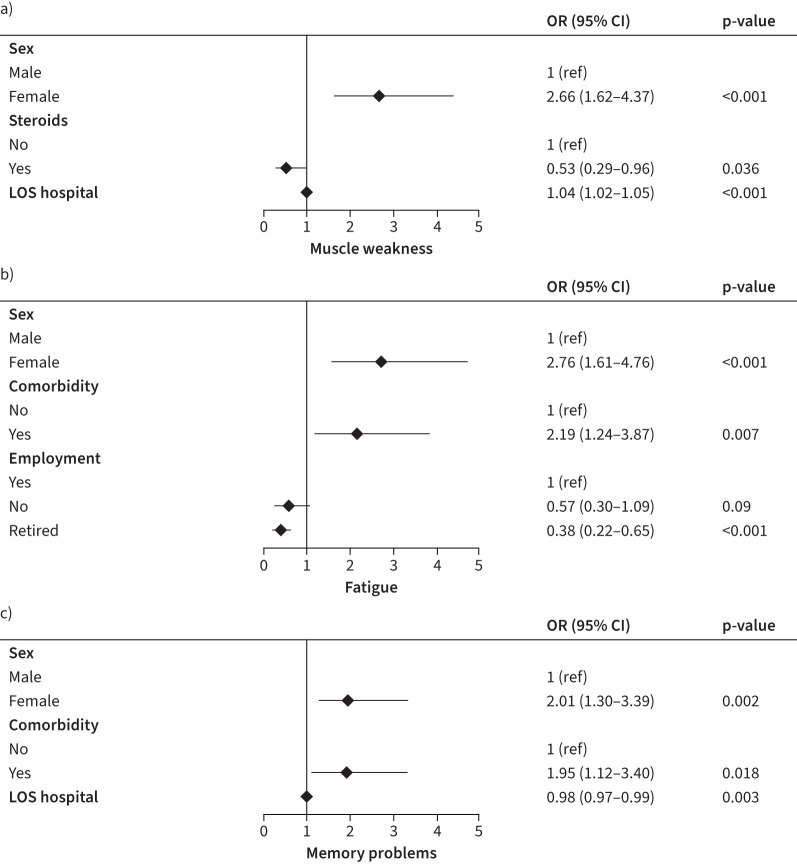
Out of the physical symptom cluster, muscle weakness was the most frequently reported symptom at 3 months after hospital discharge. Patients who were female (OR 2.66, 95% CI 1.62–4.37, p<0.001) and had a longer LOS hospital (OR 1.04, 95% CI 1.02–1.05, p<0.001) were more likely to experience muscle weakness at 3 months after hospital discharge, and patients who received steroids as treatment during hospitalisation (OR 0.53, 0.29–0.96, p=0.036) were less likely to experience muscle weakness (figure 4). Out of the respiratory symptom cluster, exertional dyspnoea was the most prevalent symptom. We were unable to perform valid multivariable logistic regression on this outcome given the high prevalence of this symptom (81.5%) at 3 months after hospital discharge.
FIGURE 4.
Forest plot of the patient and admission characteristics associated with the most prevalent symptoms for the a) physical, b) fatigue and c) cognitive symptoms clusters obtained by multivariable logistic regression analyses. LOS: length of stay.
Fatigue was the most prevalent symptom in the fatigue symptom cluster. Patients who were female (OR 2.76, 1.61–4.76, p<0.001) and/or had comorbidities (OR 2.19, 1.24–3.87, p=0.007) were more likely to develop fatigue symptoms, while patients who were retired (OR 0.38, 0.22–0.65, p=0.001) were less likely to develop fatigue symptoms at 3 months after hospital discharge (figure 4).
At 3 months, memory problems were the most frequently reported symptom in the cognitive symptom cluster. Patients who were female (OR 2.01, 1.30–3.39, p=0.002), had a shorter LOS (OR 0.98, 0.97–0.99, p=0.003) and/or had comorbidities (OR 1.95, 1.12–3.40, p=0.018) were more likely to experience memory problems at 3 months after hospital discharge (figure 4).
Discussion
Up to 12 months after hospitalisation for COVID-19 over 90% of patients suffer from at least one persisting symptom. Muscle weakness, exertional dyspnoea, fatigue, and memory and concentration problems were the most prevalent symptoms with reporting rates of over 50% of the patients at one of the time points. Although several physical and respiratory symptoms (muscle weakness, hair loss, exertional dyspnoea) declined significantly over time, others – including fatigue and cognitive symptoms – persisted.
Our findings support the observation from a recent meta-analysis that the short-term prevalence of persisting symptoms was similar to long-term prevalence of symptoms up to 6 months after hospital discharge [8]. Persisting symptoms are a common feature of COVID-19, especially after hospitalisation but may also occur after mild or even asymptomatic infection. To date, long-term data regarding persisting symptoms at 12 months and beyond are limited. In a cohort study from Wuhan, the proportion of persistent symptoms was shown to decrease from 68% at 6 months to 49% at 12 months after hospitalisation and 55% at 24 months after hospitalisation [9].
Although this finding appears to contradict our findings, their cohort contained only 1% of patients that had received mechanical ventilation compared to 35.6% in our cohort. The severity of acute COVID-19 is increasingly recognised to be associated with a larger proportion and longer duration of persisting symptoms and should thus be taken into account when comparing studies [16, 17]. Our study unfortunately shows a much less optimistic picture regarding recovery over time, with a high prevalence of persisting symptoms that is much more in line with other European outcomes that demonstrate numbers of incomplete recovery between 71.1 and 91.7% of patients after severe or critical COVID-19 infection, without evident improvement over time [10, 18]. We do have to acknowledge that symptoms may have resided in severity over time or could be in part attributed to other causes, but we did not have data on this. More and longer-term follow-up results will be collected to obtain even more insight into the future outlook of these patients. Also, one has to take into account that our study only addressed hospitalised patients, and sequelae in this group will differ substantially from non-hospitalised patients.
Looking into determinants, female sex was the most important predictor of persistent symptoms. Earlier, we demonstrated a relationship between female sex and increased risk for fatigue up to 6 months after discharge [7]. Now we extend these findings to other symptoms and show that females more frequently experience symptoms from multiple symptom clusters 3 months after hospitalisation. Previous studies have also demonstrated that female sex was associated with an increased number of persisting symptoms [10, 18, 19]. It is frequently stated that, while acute cases of COVID-19 tend to be most severe in older males, PCC seems to be more frequent in younger females. Age, however, was not found to be a determinant of persistent symptoms in our cohort, nor in other studies after adjustment for confounders [10, 19]. Also, it is necessary to bear in mind that there may be a bias in symptoms reporting between males and females [20]. We also found presence of comorbidity to be associated with increased fatigue and memory problems, but this was not found by others [10]. Obesity was previously described as a major risk factor for not fully recovering [18]. It is quite possible that pre-existent health problems make patients more vulnerable to unfavourable outcome after severe illness. We found that patients treated with steroids during hospital stay were less likely to report muscle weakness during follow-up. This finding seems counter-intuitive at first. A potential explanation for this, although speculative, may be that as COVID-19 is known to cause long-term immunological dysfunction that may relate to (part of) the persisting symptoms, immunomodulation with corticosteroids in the acute phase may positively affect development of some of the symptoms such as muscle weakness. The relationship between in-hospital treatments and long-term outcomes has been little studied and should be addressed in future studies.
Although numbers of acute COVID-19 may eventually decrease with increasing immunity in the population, our findings point out that consequences will be long felt by many. As challenges in vaccination programmes worldwide continue to hinder effective control measures, the number of people with PCC will only continue to increase. Vaccination may play an important role not only to prevent new infections, but also in preventing PCC, as it was recently demonstrated that post-vaccination breakthrough infections are less likely to be associated with symptoms persisting for >28 days [21].
The best approach to PCC is unclear. As symptoms range from mild to severe and are very diverse in nature, there is no one size fits all treatment possible. Although we grouped symptoms into clusters, there are currently no universally recognised phenotypes, diagnostic criteria, minimal severity scores or diagnostic tests to establish a diagnosis of PCC. Establishing more objective and evidence-based definitions and phenotypes of PCC will be necessary to compare findings across cohorts and settings, and to establish evidence-based interventions. Also, the impact of prior symptoms and prior comorbidity needs to be taken into account, just as the expected effects of hospitalisation.
There are currently many unknowns regarding PCC, including the underlying mechanisms. Current theories on PCC include virus-specific pathophysiological changes, immunological aberrations and inflammatory damage in response to the acute infection and expected sequelae of post-critical illness [22]. Indeed, it is very hard to differentiate between the expected sequelae, such as described in the post-intensive care syndrome, and the sequelae that are specific for COVID-19 [23]. Nonetheless, in a large analysis on electronic health records data, key features of long COVID (e.g. breathlessness and fatigue) were more frequently reported after COVID-19 than in matched controls after influenza virus infection [13]. Overall, it is becoming increasingly clear that the sequelae after COVID-19 are more prevalent than after most other types of infections, persist for a long time, and have a major impact on the burden of disease and healthcare.
Our study has several strengths and limitations. We followed a large cohort of patients in a longitudinal design at 3, 6 and 12 months after hospital discharge. Currently, long-term follow-up data are scarce. As the study is still ongoing, data were not complete for the entire cohort; also, the initial patients were generally recruited between 3 and 6 months after hospital discharge, resulting in unequal groups at different time points. We therefore included only participants with data of at least two study measurements and used GEE models to make maximal use of all data and to investigate how symptoms developed over time.
As we included patients at the outpatient clinic after discharge, a selection bias of patients with lingering symptoms cannot be excluded. Therefore, it is useful to have some more insights in the recruitment procedure of participants. All patients that had been hospitalised were offered outpatient follow-up, unless this was logistically not possible. Recruitment of study participants occurred independent of the patient's recovery status. Inclusion in this study was largely based on availability of research personnel to recruit patients and to perform study visits, which was the most limitative step for inclusion. Although consent rate to participation was very high, the exact number of patients approached for participation is unknown, which is a limitation of this study. Our final study cohort was representative of the overall admitted population that received aftercare (data not shown). Nonetheless, the extent of selection bias cannot be quantified.
One inclusion criterion was that patients or their relatives had to be able to communicate in Dutch or English. Therefore, there is underrepresentation of individuals with a migration background in this study compared to the community where this cohort was established. Nonetheless, 24% of the participants in this cohort had a migration background; migration background was not a predictor of residual symptoms in this study. Another limitation is that our results are only generalisable to hospitalised patients and we did not have a control group. Also, we did not have patient scores on the severity of complaints. Even though symptoms may persist for considerable time, the severity may very well decrease over time, as was also shown in other studies [16]. Also, we cannot exclude that symptoms had other aetiology than post-COVID.
To summarise, a large number of post-COVID-19 patients experienced persistent symptoms up to 12 months after hospitalisation for COVID-19. Whereas physical and respiratory symptoms showed slow gradual decline, fatigue and cognitive symptoms did not evidently resolve over time. This finding stresses the importance of finding the underlying causes and effective treatments for PCC on the one hand, and adequate COVID-19 prevention on the other hand. Large and long-term cohort studies are urgently needed to help better understand persistent symptoms after COVID-19 and its biological drivers.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00355-2022.supplement (245.5KB, pdf)
Acknowledgements
We would like to extend our gratitude to all the participants of the ongoing CO-FLOW study for their efforts, as well as all the entire group of measurement assistants that currently assists in CO-FLOW: G.W.M. Broeren, R.M.B. Imkamp, J. Andela, L. Bierman, T. Huijboom, A. Luijckx, S. Roovers, I. Simons and L. van Veggel.
Provenance: Submitted article, peer reviewed.
CO-FLOW collaboration Group: Michel E. van Genderen, Jasper van Bommel, Diederik A.M.P.J. Gommers (all Department of Adult Intensive Care Medicine, Erasmus MC, University Medical Center Rotterdam, The Netherlands), Erwin Ista (Departments of Pediatrics and Pediatric Surgery, Intensive Care Unit, Erasmus MC Sophia Children's Hospital Rotterdam, and Department of Internal Medicine, section Nursing Science, Erasmus MC, Erasmus University Medical Center Rotterdam, The Netherlands), Robert van der Stoep (Department of Physical Therapy, Erasmus MC, University Medical Center Rotterdam, The Netherlands), Rutger Osterthun (Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, and Rijndam Rehabilitation, Rotterdam, The Netherlands), Markus P.J.M. Wijffels, Marieke M. Visser (both Rijndam Rehabilitation, Rotterdam, The Netherlands), Janette J. Tazmi-Staal, Eva G. Willems (both Laurens Intermezzo, Rotterdam, The Netherlands), Roxane Heller (Department of Respiratory Medicine, Ikazia Hospital, Rotterdam, The Netherlands), Shai A. Gajadin (Department of Respiratory Medicine, IJsselland Hospital, Capelle aan de IJssel, The Netherlands), Wouter J.B. Blox (Department of Respiratory Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands), Laurien Oswald, Sieshem Bindraban (both Department of Respiratory Medicine, Franciscus Gasthuis & Vlietland, Rotterdam, The Netherlands), Rob Slingerland (Department of Respiratory Medicine, Maasstad Hosptial, Rotterdam, The Netherlands), Herbert J. van de Sande (Aafje Nursing Home, Rotterdam, The Netherlands), Ronald N. van Rossem, Stephanie van Loon-Kooij (both Department of Respiratory Medicine, Reinier de Graaf Gasthuis, Delft, The Netherlands), L. Martine Bek (Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, The Netherlands), Julia C. Berentschot, Merel E. Hellemons, Susanne M. Huijts, Joachim G.J.V. Aerts (all Department of Respiratory Medicine, Erasmus MC, University Medical Center Rotterdam, The Netherlands), Majanka H. Heijenbrok-Kal (Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, and Rijndam Rehabilitation, Rotterdam, The Netherlands), Rita J.G. van den Berg-Emons (Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, The Netherlands) and Gerard M. Ribbers (Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, and Rijndam Rehabilitation, Rotterdam, The Netherlands).
Author contributions: L.M. Bek, J.C. Berentschot, M.H. Heijenbrok-Kal, R.J.G. van den Berg-Emons and M.E. Hellemons devised the main conceptual ideas and proof outline for this manuscript; L.M. Bek and J.C. Berentschot included the patients; L.M. Bek and J.C. Berentschot contributed to data collection and aggregation; M.E. Hellemons and J.H. Vlake designed the figures; L.M. Bek, J.C. Berentschot and M.H. Heijenbrok-Kal performed the statistical analyses; L.M. Bek, J.C. Berentschot, M.H. Heijenbrok-Kal, R.J.G. van den Berg-Emons and M.E. Hellemons wrote the manuscript. All authors were involved in the main study design, discussed the results, commented on the manuscript and approved the final manuscript.
Conflict of interest: L.M. Bek, J.C. Berentschot and M.H. Heijenbrok-Kal have received support for the present manuscript from ZonMw COVID-19 program, Rijndam Rehabilitation and Laurens. M.E. van Genderen has received a research grant from stichting Theia and stichting SGS, outside the submitted work. R.J.G. van den Berg-Emons has received a grant from ZonMw (Dutch organisation for health research) outside the submitted work. M.E. Hellemons has received a grant from ZonMw outside the submitted work and is an associate editor of this journal. The remaining authors have nothing to disclose.
Support statement: The study is funded by the COVID-19 Program Care and Prevention of The Netherlands Organization for Health Research and Development (ZonMw, grant number 10430022010026), and Rijndam Rehabilitation and Laurens (both in Rotterdam, The Netherlands). The COVID-19 review committee of the funding body has independently reviewed the study protocol. None of the funders have a role in the study design, nor are involved in analysis and interpretation of data or writing of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19). JAMA 2020; 324: 782–793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2.Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute covid-19 in primary care. BMJ 2020, 370: m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 3.Shah W, Hillman T, Playford ED, et al. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021; 372: n136. doi: 10.1136/bmj.n136 [DOI] [PubMed] [Google Scholar]
- 4.WHO . A clinical case definition of post COVID-19 condition by a Delphi consensus. www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [DOI] [PMC free article] [PubMed]
- 5.Sudre CH, Murray BA-OX, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Maaden T, Mutubuki EN, de Bruijn S, et al. Prevalence and severity of symptoms 3 months after infection with SARS-CoV-2 compared to test-negative and population controls in the Netherlands. medRxiv 2022; preprint [. 10.1101/2022.06.15.22276439]. [DOI] [PubMed] [Google Scholar]
- 7.Hellemons ME, Huijts S, Bek L, et al. Persistent health problems beyond pulmonary recovery up to 6 months after hospitalization for SARS-CoV-2; a longitudinal study of respiratory, physical and psychological outcomes. Ann Am Thorac Soc 2022; 19: 551–561. doi: 10.1513/AnnalsATS.202103-340OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 2021; 4: e2128568-e. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 2022; in press [ 10.1016/S2213-2600(22)00126-6]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comelli A, Viero G, Bettini G, et al. Patient-reported symptoms and sequelae 12 months after COVID-19 in hospitalized adults: a multicenter long-term follow-up study. Front Med (Lausanne) 2022; 9: 834354. doi: 10.3389/fmed.2022.834354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38: 101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estiri H, Strasser ZH, Brat GA, et al. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med 2021; 19: 249. doi: 10.1186/s12916-021-02115-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021; 18: e1003773. doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bek LM, Berentschot JC, Hellemons ME, et al. CO-FLOW: COvid-19 Follow-up care paths and Long-term Outcomes Within the Dutch health care system: study protocol of a multicenter prospective cohort study following patients 2 years after hospital discharge. BMC Health Serv Res 2021; 21: 847. doi: 10.1186/s12913-021-06813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Kleijn WPE, De Vries J, Wijnen PAHM, et al. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med 2011; 105: 1388–1395. doi: 10.1016/j.rmed.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 16.Wynberg E, van Willigen H, Dijkstra M, et al. Evolution of COVID-19 symptoms during the first 9 months after illness onset. medRxiv 2021; preprint [ 10.1101/2021.05.05.21256710]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logue JK, Franko NM, Mcculloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021; 4: e210830. doi: 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PHOSP-COVID Collaborative Group . Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med 2022; 10: 761–775. doi: 10.1016/S2213-2600(22)00127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27: 1607–1613. doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardel A, Wallander MA, Wallman T, et al. Age and sex related self-reported symptoms in a general population across 30 years: patterns of reporting and secular trend. PLoS One 2019; 14: e0211532. doi: 10.1371/journal.pone.0211532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2021; 22: 43–55. doi: 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014; 370: 1626–1635. doi: 10.1056/NEJMra1209390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00355-2022.supplement (245.5KB, pdf)