Highlights
-
•
Negative schizophrenic symptoms have been considered to reflect prefrontal cortex dysfunction.
-
•
Functional imaging support for this theory is however weak, perhaps due to the tasks used.
-
•
We examined negative symptom patients using a novel executive task measuring volitional behaviour.
-
•
Comparison to patients without negative symptoms revealed prefrontal hypoactivation.
Keywords: Schizophrenia, Negative symptoms, Frontal lobes, Executive function, fMRI
Abstract
Background
The negative symptoms of schizophrenia have been proposed to reflect prefrontal cortex dysfunction. However, this proposal has not been consistently supported in functional imaging studies, which have also used executive tasks that may not capture key aspects of negative symptoms such as lack of volition.
Method
Twenty-four DSM-5 schizophrenic patients with high negative symptoms (HNS), 25 with absent negative symptoms (ANS) and 30 healthy controls underwent fMRI during performance of the Computerized Multiple Elements Test (CMET), a task designed to measure poor organization of goal directed behaviour or ‘goal neglect’. Negative symptoms were rated using the PANSS and the Clinical Assessment Interview for Negative Symptoms (CAINS).
Results
On whole brain analysis, the ANS patients showed no significant clusters of reduced activation compared to the healthy controls. In contrast, the HNS patients showed hypoactivation compared to the healthy controls in the left anterior frontal cortex, the right dorsolateral prefrontal cortex (DLPFC), the anterior insula bilaterally and the bilateral inferior parietal cortex. When compared to the ANS patients, the HNS patients showed reduced activation in the left anterior frontal cortex, the left DLPFC and the left inferior parietal cortex. After controlling for disorganization scores, differences remained in clusters in the left anterior frontal cortex and the bilateral inferior parietal cortex.
Conclusions
This study provides evidence that reduced prefrontal activation, perhaps especially in the left anterior frontal cortex, is a brain functional correlate of negative symptoms in schizophrenia. The simultaneous finding of reduced inferior parietal cortex activation was unexpected, but could reflect this region’s involvement in cognitive control, particularly the ‘regulative’ component of this.
1. Introduction
Negative symptoms, often characterized as a triad of lack of volition, poverty of speech and flattening of affect, are among the most important clinical features of schizophrenia. There is a consensus that they make a significant contribution to the poor social and occupational functioning seen in the disorder (Foussias et al., 2014), and their presence early in the course of illness is predictive of poor outcome (Milev et al., 2005, Galderisi et al., 2013). Negative symptoms are also challenging from the therapeutic perspective: they are less responsive to antipsychotic treatment than positive symptoms (Leucht et al., 2017) and early indications that glutamatergic drugs might be effective against them (Tuominen et al., 2005) have not been supported in well-controlled trials (Buchanan et al., 2007, Lieberman et al., 2009, Kinon et al., 2011, Bugarski-Kirola et al., 2016).
The leading explanatory proposal for negative symptoms over the years has been that they reflect prefrontal cortex dysfunction. Thus, authors such as Liddle (Liddle, 1987) and Weinberger (Weinberger, 1988) have noted that lack of volition in schizophrenia resembles the apathy seen in neurological patients with the frontal lobe syndrome. Frith (Frith, 1992) went further to argue that lack of volition (and also poverty of speech) reflected a difficulty with the generation of self-initiated or ‘willed’ actions, while performance of actions in response to environmental stimuli remained intact. He linked this difficulty on the one hand to subregions of the frontal cortex and on the other to a theoretical model of executive function, Norman and Shallice’s supervisory attentional system (Shallice, 1988), which is engaged when non-habitual behaviours have to be performed.
To date, empirical testing of the ‘frontal’ hypothesis of negative symptoms has yielded only equivocal support. Neuropsychological studies have found evidence for associations between scores on negative symptom scales and poor performance on a range of executive tests (for meta-analyses see (Dibben et al., 2009, de Gracia et al., 2009). However, while significant, these correlations are generally low (pooled r = -0.21 in 83 studies in Dibben et al (Dibben et al., 2009); also, similar levels of correlation have been found with non-executive tests (de Gracia et al., 2009). With respect to brain structural imaging, Galderisi and Maj (Galderisi and Maj, 2009) reviewed region of interest studies and found no evidence for a relationship between negative symptoms and volume reductions in the frontal lobe, and only inconsistent findings with respect to frontal subregions. More recently, Walton et al., (Walton et al., 2018) examined cortical thickness in 1985 schizophrenic patients from 17 research groups and found an association between negative symptom severity and left medial orbitofrontal thickness. However, the effect was reduced to trend level after covarying for duration of illness.
Functional imaging provides a further way of testing the frontal hypothesis of negative symptoms. A pioneering PET study by Liddle et al. (1992) found that negative symptoms were associated with reduced resting activity in a wide expanse of the prefrontal cortex bilaterally. However, subsequent PET and SPECT studies (Kaplan et al., 1993, Tamminga et al., 1992, Vaiva et al., 2002, Gonul et al., 2003) failed to consistently replicate this finding. A larger body of studies has investigated associations between negative symptoms and brain activations during performance of executive tasks, including working memory (Menon et al., 2001, Perlstein et al., 2001, Honey et al., 2003, Manoach et al., 1999), planning (Andreasen et al., 1992), vigilance (Potkin et al., 2002) and inhibition of prepotent responses (Snitz et al., 2005); activations during non-executive tasks have also been examined (Heckers et al., 1999, Lahti et al., 2001, MacDonald and Carter, 2003, Shaffer et al., 2015) (for a review see (Goghari et al., 2010). The results of these studies have been mixed: some have found associations between negative symptoms and decreased frontal activation (Andreasen et al., 1992, Potkin et al., 2002, Heckers et al., 1999, Lahti et al., 2001, Shaffer et al., 2015), whereas others have had negative or equivocal findings (Menon et al., 2001, Perlstein et al., 2001, Honey et al., 2003, Manoach et al., 1999, Snitz et al., 2005, MacDonald and Carter, 2003).
Even when functional imaging studies have used executive tasks, it is not clear that these would be sensitive to the kind of executive dysfunction relevant to negative symptoms. Thus, it is known that patients with the frontal lobe syndrome may show severe impairment of volitional behaviour in everyday life but still perform normally on many standard tests of executive function (Burgess et al., 2009, Shallice and Burgess, 1991). In response to this problem, Shallice and Burgess (Shallice and Burgess, 1991) devised the Six Elements Task, which was aimed specifically at capturing poor organization of goal-directed behaviour, or ‘goal neglect’ (Duncan et al., 1996). Subjects are required to carry out parts of six different tasks in a 10-minute time period in circumstances where it would be impossible to complete all of them in the allotted time; keeping in mind the overall goal of the task they therefore have to periodically switch from task to task.
The present study took advantage of a recently developed fMRI-adapted version of the Six Elements Task, the Computerised Multiple Elements Test (CMET) (Cullen et al., 2016, Fuentes-Claramonte et al., 2021) to further examine the hypothesis that negative symptoms reflect frontal/executive dysfunction, specifically goal neglect. Given that correlational analyses have been argued to have limited power to detect brain:behaviour correlations in fMRI studies (Yarkoni, 2009), we compared well-matched groups of schizophrenic patients who were selected for either showing or not showing negative symptoms. Healthy controls were also employed.
2. Material and methods
2.1. Participants
The patient sample consisted of two groups of right-handed patients who showed either high or low levels of negative symptoms. All patients had a DSM-5 diagnosis of schizophrenia or schizoaffective disorder, made on the basis of clinical interview and review of casenotes. The patients were selected from a larger group of patients recruited from four different hospitals in the Barcelona area (Benito Menni CASM, Hospital de Sant Rafael, Hospital Sagrat Cor de Martorell, Hospital Mare de Déu de la Mercè) who took part in this and a number of other imaging studies.
Patients were excluded if they (a) were younger than 18 or older than 65 years, (b) had a history of brain trauma or neurological disease, or (c) had shown alcohol/substance abuse within 12 months prior to participation. They were also required to have a premorbid IQ in the normal range (≥70), as estimated using the Word Accentuation Test (Test de Acentuación de Palabras, TAP; (Del Ser et al., 1997, Gomar et al., 2011)). This test requires pronunciation of low-frequency Spanish words whose accents have been removed and is resistant to illness-related cognitive decline. Patients with a current IQ < 70 based on four subtests from the WAIS-III (Vocabulary, Similarities, Matrix Reasoning and Block Design) were also excluded. All patients were taking antipsychotic medication.
The control sample consisted of 30 right-handed healthy individuals recruited from non-medical hospital staff, their relatives and acquaintances, plus independent sources in the community. They were selected to be matched with the patients in terms of age, sex and TAP-estimated IQ. The controls met the same exclusion criteria as the patients. Controls were also excluded if they reported a history of mental illness and/or treatment with psychotropic medication, or had a history of major mental illness in a first-degree relative.
All participants gave written informed consent prior to participation. All the study procedures complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the ethics committee of the Hermanas Hospitalarias group of hospitals (Comité de Ética e Investigación Clínica de Hermanas Hospitalarias). Healthy controls received a gift-card as a compensation for their participation in the study.
2.2. Clinical assessment
Negative symptoms were rated using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) and the Clinical Assessment Interview for Negative Symptoms (CAINS, (Kring et al., 2013, Valiente-Gomez et al., 2015). Identification of PANSS items rating negative symptoms was based on Wallwork et al’s (Wallwork et al., 2012) review of factor analytic studies of this scale. Individual items on the CAINS are summed to give an overall negative symptoms score, and also yield two subscale scores: motivation and pleasure (CAINS-MAP, 9 items) and expressivity (CAINS-EXP, 4 items) – the latter includes items for lack of facial expression, expressive gestures, prosody and amount of speech.
Patients were assigned to ‘high negative symptom’ (HNS) and ‘absent negative symptom’ (ANS) groups based on PANSS negative symptom scores, according to criteria defined by Bucci and Galderisi (Bucci and Galderisi, 2017) (currently there is no method for using CAINS scores to separate high and low negative symptom groups). Patients in the HNS group rated at least moderate (i.e., score of 4 out of a maximum of 7) on three or more PANSS negative symptom items, or moderately severe (i.e., 5 or higher) on two or more items. In contrast, patients in the ANS group had scores of either absent or minimal (i.e., a maximum of 2) on all PANSS negative symptom items.
2.3. Task description
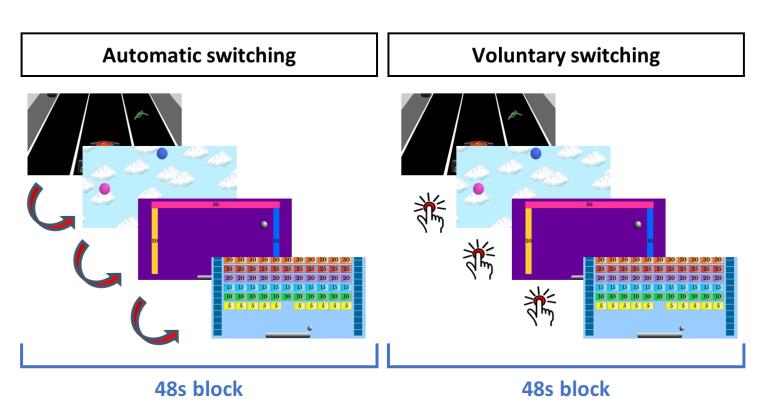
The Computerised Multiple Elements Test (CMET) (Cullen et al., 2016, Fuentes-Claramonte et al., 2021) requires subjects to sequentially play four different video-type games presented in pseudorandom order (see Fig. 1). The games are all similar and involve moving an interactive element on the screen to the left or to the right (with their left or right index fingers) to earn points: in the first game (car), participants have to move a car to pick up fuel from the road; in the second (catch), they have to move a tube to receive balloons that fall from the sky; in the third (ball), they have to move a bar to keep a ball bouncing off walls; in the fourth (brick), they have to move a bar to use a ball to break bricks. The four games are played in two conditions: in the control condition (automatic switching), the games switch automatically from one to another every 12 s until all games have been played once. In the executive condition (voluntary switching) participants have to decide when to switch from one game to the other by pressing a button with their right thumb, with the aim of playing all of them in each block. In this condition the subjects are instructed to divide their time equally to spend approximately the same on each game, although no time information is shown in either condition.
Fig. 1.
Schematic view of the CMET. Participants sequentially play four games during each 48 s block. In the automatic switching condition, the game changes every 12 s without the intervention of the participant. In the voluntary switching condition, the participant has to actively switch games by button press, with approximately the same frequency as in the automatic condition. No time information is shown during either condition.
Stimuli were presented via MRI-compatible goggles (VisuaStim, Resonance Technology), and participants performed the task with an MRI-compatible response system (ResponseGrips, NordicNeuroLab). Four blocks of each condition were presented in alternating order, starting with the automatic condition. Instructions were presented just before each block started for 3 s and indicated whether the subsequent block would be the automatic or the voluntary condition. Between blocks, a fixation cross was presented for 9 s. Total task duration was 8 min, 10 s. Before scanning, the participants underwent a practice session where they learned how to play and switch games.
2.4. Behavioural measures
CMET measures of interest included the total number of voluntary switches across all four blocks and deviation from optimal playing time. This latter measure consists of the sum of deviations from the ideal strategy in the voluntary switching condition of playing each game for 12 s (time underplaying and overplaying a game are complementary, so only overplaying was penalized to avoid double counting).
2.5. Image acquisition and analysis
Images were acquired with a 3 T Philips Ingenia scanner (Philips Medical Systems, Best, The Netherlands). Functional data were acquired using a T2*-weighted echo-planar imaging (EPI) sequence with 245 volumes and the following acquisition parameters: TR = 2000 ms, TE = 30 ms, flip angle = 70°, in-plane resolution = 3.5 × 3.5 mm, FOV = 238 × 245 mm, slice thickness = 3.5 mm, inter-slice gap = 0.75 mm. Slices (32 per volume) were acquired with an interleaved order parallel to the AC-PC plane. We also acquired a high-resolution anatomical volume with a FFE (Fast Field Echo) sequence for anatomical reference and inspection (TR = 9.90 ms; TE = 4.60 ms; Flip angle = 8°; voxel size = 1 × 1 mm; slice thickness = 1 mm; slice number = 180; FOV = 240 mm).
Preprocessing and analysis were carried out with the FEAT module included in the FSL (FMRIB Software Library) software (Smith et al., 2004). The first 10 s (5 volumes) of the sequence, corresponding to signal stabilization, were discarded. Preprocessing included motion correction (using the MCFLIRT algorithm), co-registration and normalization to a common stereotactic space (MNI, Montreal Neurological Institute, template). For accurate registration, a two-step process was used. First, brain extraction was applied to the structural image, and the functional sequence was registered to it. Then the structural image was registered to the standard template. These two transformations were used to finally register the functional sequence to the standard space. Before group analyses, normalized images were spatially filtered with a Gaussian filter (FWHM = 5 mm). To minimize unwanted movement-related effects, individuals with an estimated maximum absolute movement > 3.0 mm or an average absolute movement > 0.3 mm were excluded from the study.
Statistical analysis was performed by means of a General Linear Model (GLM) approach. At the first level, we defined two regressors coding for the automatic and voluntary switching blocks. Fixation periods were not modelled and constituted an implicit baseline. The contrast of interest was voluntary > automatic switching, which should reflect the increase in brain activity when the participant needs to deal with goal-management demands.
GLMs were fitted to generate individual activation maps for the contrast of interest and second level (group) analyses were performed within the FEAT module by means of mixed-effects GLMs (Beckmann et al., 2003), to obtain mean activation maps for each group, and two-sample t-tests for pairwise group comparison. All statistical tests were carried out at the cluster level with a corrected p value of 0.05 using Gaussian random field methods, with a threshold of z > 3.1 (p < 0.001) to define the initial set of clusters.
3. Results
3.1. Clinical and demographic data
Demographic and clinical data for the patient and control groups are shown in Table 1. The two patient groups did not differ significantly from the healthy controls in terms of sex, age or estimated premorbid IQ; however, as expected, both groups had a significantly lower current IQ than the controls (ANS vs HC p = 0.01; HNS vs HC p = 0.003). Although not preselected for, the two patient groups were also found to be matched for length of illness and current IQ.
Table 1.
Demographic and clinical data for the patients and controls.
| HC (N = 30) |
ANS (N = 25) | HNS (N = 24) | Differences | |
|---|---|---|---|---|
| M/F |
16/14 | 16/9 | 18/6 | Χ2 = 2.7, p = 0.26 |
| Age | 39.13 (13.82) Range = 18–61 |
41.24 (12.03) Range = 22–62 |
42.12 (10.86) Range = 26–60 |
F = 0.42, p = 0.66 |
| Estimated premorbid IQ | 101.3 (8.93) Range = 79–114 |
98.79 (8.70) Range = 79–114 |
98.05 (9.42) Range = 85–114 |
F = 0.96, p = 0.39 |
| Current IQ (WAIS-III) | 103.7 (11.42) Range = 81–134 |
94.92 (15.02) Range = 70–123 |
93.04 (11.57) Range = 71–122 |
F = 5.48, p = 0.006 |
| PANSS total score | – | 42.00 (8.06) Range = 30–60 |
68.00 (13.17) Range = 53–103 |
U = 14.5, p < 0.001 |
| PANSS negative syndrome | – | 6.88 (0.93) Range = 6–9 |
23.58 (3.01) Range = 17–28 |
Not performed* |
| PANSS reality distortion | – | 7.00 (2.75) Range = 4–13 |
8.25 (3.35) Range = 4–17 |
U = 238.5, p = 0.22 |
| PANSS disorganization | – | 4.96 (1.67) Range = 3–9 |
6.88 (2.92) Range = 3–14 |
U = 180, p = 0.02 |
| CAINS Total Score | – | 13.28 (7.17) Range = 0–29 |
33.46 (6.78) Range = 20–45 |
t = 10.13, p < 0.001 |
| CAINS-MAP | – | 12.64 (7.10) Range = 0–29 |
24.29 (5.46) Range = 16–33 |
t = 6.46, p < 0.001 |
| CAINS-EXP | – | 0.64 (1.04) Range = 0–3 |
9.17 (3.09) Range = 3–16 |
U = 1.5, p < 0.001 |
| Duration of illness (years) | – | 16.36 (12.07) Range = 2–51 |
18.79 (9.99) Range = 3–38 |
U = 254, p = 0.36 |
| Antipsychotic dose (CPZ equivalents) | – | 340.86 (261.22) Range = 1.2–1100 |
458.41 (290.69) Range = 150–1275 |
U = 214.5, p = 0.09 |
Values are means (SD) or medians (IQR) depending on whether scores were normally distributed. Analysis of variance and t-tests were used to compare normally distributed variables; for non-normally distributed variables, Kruskal-Wallis and Mann-Whitney U tests were used. HC: Healthy controls; ANS: Absent negative symptoms; HNS: High negative symptoms; M/F: Male/Female; PANSS: Positive and Negative Symptoms Scale; CAINS: Clinical Assessment Interview for Negative Symptoms; CAINS-MAP: CAINS motivation and pleasure subscale; CAINS-EXP: CAINS expressivity subscale; CPZ: chlorpromazine.
Groups were preselected on this variable.
3.2. Behavioural performance on the CMET
During the voluntary switching blocks the healthy controls performed significantly better than the two patient groups in terms of number of switches, but the patient groups did not differ from each other (controls: median 12, IQR 5.75, range 0–32; ANS patients: median 9, IQR 5, range 0–39; HNS patients: 9, IQR 10, range = 0–19; Kruskal-Wallis Χ2 = 13.7, p = 0.001; HC vs ANS p = 0.006; HC vs HNS p < 0.001, ANS vs HNS p = 0.39). Deviation time was similarly significantly smaller in the controls than in the patients, with the two patient groups not differing significantly (controls: median 14.47, IQR 9.36, range = 3.99–144; ANS patients: median 60.56, IQR 44.69, range 13.71–144; HNS patients: median 73.12, IQR 82.70, range 19.91–144; Kruskal-Wallis Χ2 = 39.6; HC vs ANS p < 0.001; HC vs HS p < 0.001, ANS vs HNS p = 0.41).
Considering the whole group of patients, there was no correlation between PANSS reality distortion score (based on items in Wallwork et al (Wallwork et al., 2012) and number of switches (ρ = 0.04, p = 0.78) or deviation times (ρ = 0.06, p = 0.69). However, PANSS disorganization scores (again based on Wallwork et al (Wallwork et al., 2012) were significantly associated with longer deviation times (ρ = 0.43, p = 0.002), although not with number of switches (ρ = -0.19, p = 0.18).
3.3. Imaging findings
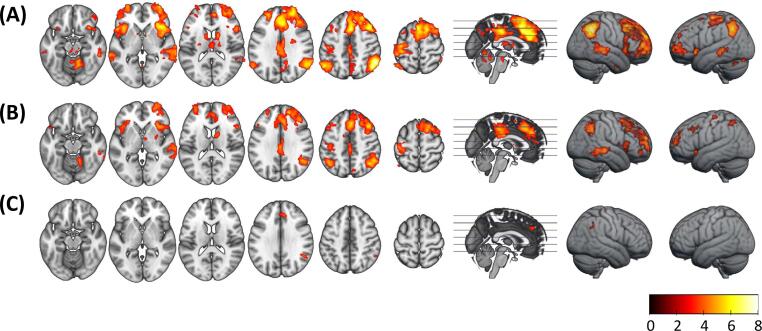
The healthy controls showed significant activation in the voluntary > automatic contrast in the anterior frontal and dorsolateral prefrontal cortex (DLPFC), as well as the inferior frontal cortex and anterior insula and the inferior parietal cortex (all bilateral but more marked in the right hemisphere). There was also a large cluster of activation spanning the dorsal anterior cingulate cortex (ACC) and supplementary motor area (SMA), and another in the middle/posterior cingulate cortex. Additional clusters of activation were seen in the inferior temporal cortex, the left postcentral gyrus, the bilateral caudate nucleus, putamen and thalamus and the cerebellum (see Fig. 2A and Supplementary Table 1).
Fig. 2.
Group mean activation maps. (A) healthy controls, (B) ANS patients, (C) HNS patients. Right side of the image corresponds to right side of the brain. Colour bar shows z values.
Patients in the ANS group showed a broadly similar mean activation map, although with slightly less extensive activations and only right-sided activity in the inferior temporal cortex, basal ganglia and thalamus. In contrast, the HNS patients only showed significant activation in small clusters in the ACC and inferior parietal cortex (angular gyrus) (see Fig. 2B and 2C, and Supplementary Table 1).
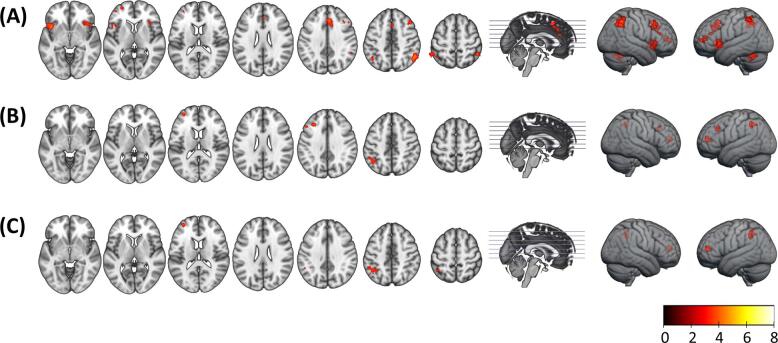
Group comparison between the healthy controls and the ANS patients did not reveal any clusters of significant difference. However, the HNS patients showed significant hypoactivation relative to the healthy controls in the bilateral anterior insula, the left anterior frontal cortex, the right DLPFC and the bilateral inferior parietal cortex. Direct comparison of the ANS and HNS patients revealed clusters of reduced activation in the latter in the left anterior frontal cortex, the left DLPFC and the left inferior parietal cortex (see Fig. 3A and 3B, and Supplementary Table 2).
Fig. 3.
(A) Clusters of hypoactivation in the HNS group relative to the control group (HNS < HC). (B) Clusters of hypoactivation in the HNS group relative to the ANS group (HNS < ANS); there were no clusters of significant difference in the comparison ANS > HC. (C) Regions of hypoactivation in the HNS group relative to the ANS group (HNS < ANS) after covarying for PANSS disorganization scores. Right side of the image is the right side of the brain. Colour bar depicts z values.
3.4. Examination of potential confounding factors
As noted, the ANS and HNS patients differed significantly in disorganization scores. Repeating the above comparisons with disorganization scores as a covariate led to disappearance of the cluster in the left DLPFC, but the clusters in the left anterior frontal cortex and the bilateral inferior parietal cortex remained (see Fig. 3C). Differences with the healthy controls remained essentially unchanged.
The ANS and HNS patients also differed in mean antipsychotic dose at trend level. Repeating the analysis with this variable as a covariate resulted in similar findings to the original comparison but with an additional cluster in the left anterior insula (see Supplementary Fig. 1).
4. Discussion
During performance of an fMRI-adapted task sensitive to goal neglect, schizophrenic patients with negative symptoms, but not those without negative symptoms, showed reduced activation compared to healthy controls in the left anterior frontal cortex, the right DLPFC and the anterior insula bilaterally. Direct comparison between the two patient groups revealed clusters of reduced activation associated with negative symptoms in the left anterior frontal cortex, the left DLPFC and the left inferior parietal cortex. Accordingly, our findings provide support for the frontal hypothesis of negative symptoms, although this was accompanied by a non-hypothesized reduction in parietal CMET-related activation.
While we found that prefrontal cortex hypoactivation was associated with negative symptoms, the areas affected did not unequivocally include the prefrontal region most implicated in schizophrenia, the DLPFC. In particular, a cluster of reduced activation was seen in this location in the initial comparison between patients with high and absent negative symptoms, but it disappeared when disorganization scores (which also differed between the two patients groups) were controlled for. The role of the DLPFC in many different aspects of higher cognitive functioning is well established (e.g. (Duncan and Owen, 2000), but its involvement in performance of willed actions specifically has been relatively little investigated. The relevant evidence consists of a handful of PET and fMRI studies carried out in the 1990s; these all found DLPFC activation, among other prefrontal and non-prefrontal areas, in healthy subjects during paradigms that contrasted intentional and externally-triggered actions and/or verbal responses (Frith et al., 1991, Jahanshahi et al., 1995, Jenkins et al., 2000, Hyder et al., 1997, Phelps et al., 1997). The numbers in these studies were small (N = 6–9) and they did not always employ full correction for multiple comparisons; however, the unanimity of their findings tends to argue in favour of - DLPFC involvement in willed actions being genuine. On these grounds, discounting the role of this prefrontal region in negative symptoms might be considered premature.
On the other hand, we found robust evidence for reduced negative symptom-related activation in another prefrontal region, the lateral portion of the left anterior frontal cortex (BA10). This rostral prefrontal region, which also includes the frontal pole and extends medially, is sometimes considered to form part of the DLPFC, but has been more studied in its own right. A meta-analysis of functional imaging studies (Gilbert et al., 2006) found that its more lateral regions were involved in cognitive as opposed to emotional tasks, particularly those requiring working memory, episodic retrieval and – of particular relevance from the perspective of our study – multi-tasking.
We also found reduced activation in our high negative symptom patients in a region outside the frontal lobe, the left parietal cortex. Based on resting state connectivity findings (Seeley et al., 2007, Menon and Uddin, 2010), as well as other lines of evidence (Marek and Dosenbach, 2018), the parietal cortex has been considered to form part of a ‘frontoparietal network’ that contributes to cognitive control. The concept of cognitive control (Ridderinkhof et al., 2004, Gratton et al., 2018) is essentially similar to that of executive function, and it specifically proposes two major and at least partially dissociated functions – on the one hand a ‘regulative’ function, responsible for the generation of task goals and their adjustment during execution, and on the other an ‘evaluative’ function responsible for monitoring the former processes and signaling when adjustments are necessary. It may be of relevance to our findings that the frontoparietal network has been argued to be particularly involved in the former of these functions (Marek and Dosenbach, 2018, Dosenbach et al., 2008).
Do our findings provide specific support for Frith’s theory of negative symptoms – that lack of volition reflects a selective impairment in the ability to carry out willed actions while leaving intact actions in response to environmental stimuli? At the practical level, the CMET contrasts activations to responses (i.e., task switching) that are made voluntarily with those that are elicited by external stimuli, and so the task can be considered to model activations to volitional behaviour. At the conceptual level, the picture becomes more complicated: the Six Elements Task – which the CMET is an adaptation of – aims to capture the symptom of goal neglect, where active problem solving is replaced by performance that seems passive, inert, stereotyped, or fragmented (Duncan et al., 1996). Clearly, there is on the face of it a relationship between ‘inert’ and ‘passive’ modes of responding in the frontal lobe syndrome and lack of volition in schizophrenia. However, this similarity does not obviously extend to fragmentation of responding, although it is interesting to note that this aspect of goal neglect is potentially relatable to the disorganization syndrome in schizophrenia, which has also been argued to reflect frontal/executive dysfunction (eg (Liddle, 1987).
We found reduced CMET-associated activations related to negative symptoms despite an association not being seen at the behavioural level – scores on measures of CMET performance did not differ between high negative symptom and absent negative symptom patients. Something that may go some way towards explaining this inconsistency is that the correlations between negative symptoms and executive test scores in schizophrenia are typically weak. Thus, Dibben et al’s (Dibben et al., 2009) meta-analysis found a pooled correlation between negative symptoms and all executive tasks of −0.21, with the values ranging from −0.13 for the Stroop and related tests to −0.27 for verbal fluency. This compares with the behavioural findings in our study: considering the schizophrenic patients as a group (i.e., the ANS + the HNS patients), the correlations between CMET behavioural measures and PANSS negative symptom scores were r = -0.18 for number of switches and r = 0.17 for deviation time.
An unexpected finding in our study was that the high and absent negative symptom groups did not differ in duration of illness or current IQ, even though we did not attempt to match the two groups on these variables. The fact that we prospectively matched the two patient groups for age probably accounts for the lack of difference in duration of illness between them – duration of illness will inevitably be strongly correlated with age. An association between negative symptoms and cognitive impairment is well documented in schizophrenia (Harvey et al., 2006), and so might have been expected to be reflected in a lower current IQ in the high negative symptoms group. Here, the fact that we matched the high negative symptom and low negative symptom patients for estimated premorbid IQ may have played a role, since estimated premorbid IQ will remain correlated with current IQ even in the face of cognitive decline.
In conclusion our findings suggest that prefrontal hypofunction can be demonstrated in association with negative schizophrenic symptoms when an appropriate task is used, in this case one that is sensitive to goal neglect. The findings implicate two subregions, the DLPFC and the anterior frontal cortex, of which the latter might be more important, given that the DLPFC cluster disappeared when disorganization symptoms were controlled for. Some limitations need to be acknowledged. The sample sizes for the high and absent negative symptom groups were relatively small. The lateralization of the findings also differed from analysis to analysis, something that may have reflected these relatively small sample sizes. Our paradigm could not control for the effects of button pressing, which only occurred in the voluntary switching trials. Perhaps the most important limitation is the confounding factor of disorganization; it is quite possible that this also has a basis in disturbed prefrontal cortex function and is clearly something that needs to be addressed further in future studies. What does not seem to be a limitation is the fact that the patients were taking antipsychotic medication; while this could have contributed to the differences found in comparison to healthy controls, it seems unlikely that it would have influenced the findings between the two patient groups, given that they were both on treatment and differences remained after controlling for minor differences in mean antipsychotic dosage.
CRediT authorship contribution statement
Conceptualization: Paola Fuentes-Claramonte, Edith Pomarol-Clotet, Raymond Salvador and Peter McKenna. Investigation: Núria Ramiro, Llanos Torres, Isabel Argila-Plaza, Pilar Salgado-Pineda, Joan Soler-Vidal, María Ángeles Garcia, Auria Albacete, Clara Bosque, Francesco Panicalli, Ester Boix, and Josep Tristany, Josep Munuera. Data curation: Salvador Sarró. Formal analysis: Salvador Sarró, Pilar Salgado-Pineda, Paola Fuentes-Claramonte, Edith Pomarol-Clotet, Raymond Salvador and Peter McKenna. Writing – original draft: Paola Fuentes-Claramonte, Edith Pomarol-Clotet, Raymond Salvador and Peter McKenna. Writing – review and editing: Paola Fuentes-Claramonte, Edith Pomarol-Clotet, Raymond Salvador, Peter McKenna and Miquel Bernado Supervision: Edith Pomarol-Clotet, Paola Fuentes-Claramonte, Miquel Bernado.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Janssen-Cilag, Menarini, Rovi and Takeda. The other authors declare no conflicts of interest.
Acknowledgements
This work was supported by CIBERSAM and the Catalonian Government (2014-SGR-1573 and 2017-SGR-1271 to FIDMAG). Also by the Instituto de Salud Carlos III, co-funded by European Union (ERDF/ESF, “Investing in your future”): Miguel Servet Research contracts (CPII13/00018 to RS and MS10/00596 to EP-C), Sara Borrell contract (CD19-00149 to PF-C), and Research Project Grants (PI14/01151 to RS, PI14/01148 to EP-C and PI14/01691 to PM). María Ángeles García-León and Salvador Sarró were supported by Júlia Gil research fellowships.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103119.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andreasen N.C., Rezai K., Alliger R., Swayze V.W., 2nd, Flaum M., Kirchner P., et al. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch. Gen. Psychiatry. 1992;49(12):943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bucci P., Galderisi S. Categorizing and assessing negative symptoms. Current Opinion in Psychiatry. 2017;30(3):201–208. doi: 10.1097/YCO.0000000000000322. [DOI] [PubMed] [Google Scholar]
- Buchanan R.W., Javitt D.C., Marder S.R., Schooler N.R., Gold J.M., McMahon R.P., et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am. J. Psychiatry. 2007;164(10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Bugarski-Kirola D., Iwata N., Sameljak S., Reid C., Blaettler T., Millar L., et al. Efficacy and safety of adjunctive bitopertin versus placebo in patients with suboptimally controlled symptoms of schizophrenia treated with antipsychotics: results from three phase 3, randomised, double-blind, parallel-group, placebo-controlled, multicentre studies in the SearchLyte clinical trial programme. Lancet Psychiatry. 2016;3(12):1115–1128. doi: 10.1016/S2215-0366(16)30344-3. [DOI] [PubMed] [Google Scholar]
- Burgess P.W., Alderman N., Volle E., Benoit R.G., Gilbert S.J. Mesulam's frontal lobe mystery re-examined. Restor. Neurol. Neurosci. 2009;27(5):493–506. doi: 10.3233/RNN-2009-0511. [DOI] [PubMed] [Google Scholar]
- Cullen B., Brennan D., Manly T., Evans J.J. Towards validation of a new computerised test of goal neglect: Preliminary evidence from clinical and neuroimaging pilot studies. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0148127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gracia D.M., Viechtbauer W., Simons C.J., van Os J., Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol. Bull. 2009;135(1):157–171. doi: 10.1037/a0014415. [DOI] [PubMed] [Google Scholar]
- Del Ser T., Gonzalez-Montalvo J.I., Martinez-Espinosa S., Delgado-Villapalos C., Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain Cogn. 1997;33(3):343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Dibben C.R., Rice C., Laws K., McKenna P.J. Is executive impairment associated with schizophrenic syndromes? A meta-analysis. Psychological Medicine. 2009;39(3):381–392. doi: 10.1017/S0033291708003887. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Emslie H., Williams P., Johnson R., Freer C. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cogn. Psychol. 1996;30(3):257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Foussias G., Agid O., Fervaha G., Remington G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur. Neuropsychopharmacol. 2014;24(5):693–709. doi: 10.1016/j.euroneuro.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Friston K., Liddle P.F., Frackowiak R.S. Willed action and the prefrontal cortex in man: a study with PET. Proceedings of the Royal Society London B: Biological Sciences. 1991;244(1311):241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Frith CD. The cognitive neuropsychology of schizophrenia. Hove: Erlbaum (UK), Taylor & Francis; 1992.
- Fuentes-Claramonte P., Santo-Angles A., Argila-Plaza I., Lechon M., Guardiola-Ripoll M., Almodovar-Paya C., et al. Brain imaging of executive function with the computerised multiple elements test. Brain Imaging and Behavior. 2021;15(5):2317–2329. doi: 10.1007/s11682-020-00425-0. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Maj M. Deficit schizophrenia: an overview of clinical, biological and treatment aspects. European psychiatry. 2009;24(8):493–500. doi: 10.1016/j.eurpsy.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Mucci A., Bitter I., Libiger J., Bucci P., Fleischhacker W.W., et al. Persistent negative symptoms in first episode patients with schizophrenia: results from the european first episode schizophrenia trial. Eur. Neuropsychopharmacol. 2013;23(3):196–204. doi: 10.1016/j.euroneuro.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Spengler S., Simons J.S., Steele J.D., Lawrie S.M., Frith C.D., et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cognit. Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Goghari V.M., Sponheim S.R., MacDonald A.W., 3rd. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci. Biobehav. Rev. 2010;34(3):468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomar J.J., Ortiz-Gil J., McKenna P.J., Salvador R., Sans-Sansa B., Sarro S., et al. Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr. Res. 2011;128(1–3):175–176. doi: 10.1016/j.schres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Gonul A.S., Kula M., Esel E., Tutus A., Sofuoglu S. A Tc-99m HMPAO SPECT study of regional cerebral blood flow in drug-free schizophrenic patients with deficit and non-deficit syndrome. Psychiatry Res. 2003;123(3):199–205. doi: 10.1016/s0925-4927(03)00067-2. [DOI] [PubMed] [Google Scholar]
- Gratton G., Cooper P., Fabiani M., Carter C.S., Karayanidis F. Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology. 2018;55(3) doi: 10.1111/psyp.13016. [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Koren D., Reichenberg A., Bowie C.R. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr. Bull. 2006;32(2):250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S., Goff D., Schacter D.L., Savage C.R., Fischman A.J., Alpert N.M., et al. Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch. Gen. Psychiatry. 1999;56(12):1117–1123. doi: 10.1001/archpsyc.56.12.1117. [DOI] [PubMed] [Google Scholar]
- Honey G.D., Sharma T., Suckling J., Giampietro V., Soni W., Williams S.C., et al. The functional neuroanatomy of schizophrenic subsyndromes. Psychol. Med. 2003;33(6):1007–1018. doi: 10.1017/s0033291703007864. [DOI] [PubMed] [Google Scholar]
- Hyder F., Phelps E.A., Wiggins C.J., Labar K.S., Blamire A.M., Shulman R.G. “Willed action”: a functional MRI study of the human prefrontal cortex during a sensorimotor task. PNAS. 1997;94(13):6989–6994. doi: 10.1073/pnas.94.13.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M., Jenkins I.H., Brown R.G., Marsden C.D., Passingham R.E., Brooks D.J. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118(Pt 4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jenkins I.H., Jahanshahi M., Jueptner M., Passingham R.E., Brooks D.J. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123(Pt 6):1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Kaplan R.D., Szechtman H., Franco S., Szechtman B., Nahmias C., Garnett E.S., et al. Three clinical syndromes of schizophrenia in untreated subjects: relation to brain glucose activity measured by positron emission tomography (PET) Schizophr. Res. 1993;11(1):47–54. doi: 10.1016/0920-9964(93)90037-j. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kinon B.J., Zhang L., Millen B.A., Osuntokun O.O., Williams J.E., Kollack-Walker S., et al. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 2011;31(3):349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- Kring A.M., Gur R.E., Blanchard J.J., Horan W.P., Reise S.P. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am. J. Psychiatry. 2013;170(2):165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti A.C., Holcomb H.H., Medoff D.R., Weiler M.A., Tamminga C.A., Carpenter W.T., Jr. Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am. J. Psychiatry. 2001;158(11):1797–1808. doi: 10.1176/appi.ajp.158.11.1797. [DOI] [PubMed] [Google Scholar]
- Leucht S., Leucht C., Huhn M., Chaimani A., Mavridis D., Helfer B., et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: Systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry. 2017;174(10):927–942. doi: 10.1176/appi.ajp.2017.16121358. [DOI] [PubMed] [Google Scholar]
- Liddle P.F. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol. Med. 1987;17(1):49–57. doi: 10.1017/s0033291700012976. [DOI] [PubMed] [Google Scholar]
- Liddle P.F., Friston K.J., Frith C.D., Hirsch S.R., Jones T., Frackowiak R.S. Patterns of cerebral blood flow in schizophrenia. Br. J. Psychiatry. 1992;160(2):179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Lieberman J.A., Papadakis K., Csernansky J., Litman R., Volavka J., Jia X.D., et al. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology. 2009;34(5):1322–1329. doi: 10.1038/npp.2008.200. [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., 3rd, Carter C.S. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J. Abnorm. Psychol. 2003;112(4):689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- Manoach D.S., Press D.Z., Thangaraj V., Searl M.M., Goff D.C., Halpern E., et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol. Psychiatry. 1999;45(9):1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Marek S., Dosenbach N.U.F. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues in Clinical Neuroscience. 2018;20(2):133–140. doi: 10.31887/DCNS.2018.20.2/smarek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Anagnoson R.T., Mathalon D.H., Glover G.H., Pfefferbaum A. Functional neuroanatomy of auditory working memory in schizophrenia: relation to positive and negative symptoms. NeuroImage. 2001;13(3):433–446. doi: 10.1006/nimg.2000.0699. [DOI] [PubMed] [Google Scholar]
- Milev P., Ho B.C., Arndt S., Andreasen N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Perlstein W.M., Carter C.S., Noll D.C., Cohen J.D. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am. J. Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Hyder F., Blamire A.M., Shulman R.G. FMRI of the prefrontal cortex during overt verbal fluency. NeuroReport. 1997;8(2):561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Potkin S.G., Alva G., Fleming K., Anand R., Keator D., Carreon D., et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Positron emission tomography. Am. J. Psychiatry. 2002;159(2):227–237. doi: 10.1176/appi.ajp.159.2.227. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., van den Wildenberg W.P., Segalowitz S.J., Carter C.S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer J.J., Peterson M.J., McMahon M.A., Bizzell J., Calhoun V., van Erp T.G., et al. Neural correlates of schizophrenia negative symptoms: Distinct subtypes impact dissociable brain circuits. Molecular Neuropsychiatry. 2015;1(4):191–200. doi: 10.1159/000440979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Cambridge University Press; Cambridge [England]; New York: 1988. From neuropsychology to mental structure. [Google Scholar]
- Shallice T., Burgess P.W. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23 Suppl 1:S208-19. [DOI] [PubMed]
- Snitz B.E., MacDonald A., 3rd, Cohen J.D., Cho R.Y., Becker T., Carter C.S. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am. J. Psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Thaker G.K., Buchanan R., Kirkpatrick B., Alphs L.D., Chase T.N., et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch. Gen. Psychiatry. 1992;49(7):522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- Tuominen H.J., Tiihonen J., Wahlbeck K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 2005;72(2–3):225–234. doi: 10.1016/j.schres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Vaiva G., Cottencin O., Llorca P.M., Devos P., Dupont S., Mazas O., et al. Regional cerebral blood flow in deficit/nondeficit types of schizophrenia according to SDS criteria. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2002;26(3):481–485. doi: 10.1016/s0278-5846(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Valiente-Gomez A., Mezquida G., Romaguera A., Vilardebo I., Andres H., Granados B., et al. Validation of the Spanish version of the Clinical Assessment for Negative Symptoms (CAINS) Schizophr. Res. 2015;166(1–3):104–109. doi: 10.1016/j.schres.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Wallwork R.S., Fortgang R., Hashimoto R., Weinberger D.R., Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr. Res. 2012;137(1–3):246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E., Hibar D.P., van Erp T.G.M., Potkin S.G., Roiz-Santianez R., Crespo-Facorro B., et al. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol. Med. 2018;48(1):82–94. doi: 10.1017/S0033291717001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger D.R. Schizophrenia and the frontal lobe. Trends Neurosci. 1988;11(8):367–370. doi: 10.1016/0166-2236(88)90060-4. [DOI] [PubMed] [Google Scholar]
- Yarkoni T. Big Correlations in Little Studies: Inflated fMRI Correlations Reflect Low Statistical Power-Commentary on Vul et al. (2009). Perspectives on Psychological Science. 2009;4(3):294-8. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.