Abstract
In 2018, pegvaliase was approved as the first enzyme substitution treatment for phenylketonuria (PKU) and is now the second medication available for PKU patients since the approval of sapropterin dihydrochloride in 2007. Historically, dietary management has been the mainstay of treatment for PKU. While sapropterin response rate is limited to approximately 50% of PKU patients, pegvaliase has the potential to reduce phenylalanine levels in all PKU patients (Vockley et al., 2014; Longo et al., 2019 [1,3]). Current FDA labeling for pegvaliase includes a dose maximum of 60 mg daily (Longo et al., 2019; BioMarin Pharmaceutical Inc., 2020 [3,4]). We report a case series of four phenylalanine hydroxylase (PAH) deficient patients, previously treated with dietary management only, who initiated treatment with pegvaliase and were titrated to 80 mg daily dosing. The safety profile in these four cases did not differ from lower maintenance dosing (Longo et al., 2019 [3]). Subsequent decreases in Phe levels were observed on 80 mg maintenance dosing, allowing for individualized dietary liberalization in three out of four patients. We conclude that pegvaliase dosing must be personalized to achieve therapeutic goals and that some patients may require higher doses than those included on the product label.
Keywords: Phenylketonuria, Pegvaliase, Dosing
1. Background/intro
Phenylketonuria (PKU) is an inherited autosomal recessive disorder caused by variants in the PAH gene which encodes for phenylalanine hydroxylase (PAH), an enzyme responsible for the conversion of phenylalanine to tyrosine [1]. PAH deficiency leads to phenylalanine (Phe) accumulation, which can cause intellectual disability, microcephaly, delayed speech, seizures, psychiatric symptoms, and behavioral abnormalities [2]. Early detection of elevated Phe through newborn screening allows for rapid initiation of a Phe-restricted diet to prevent severe neurological outcomes [1,2]. However, suboptimal Phe control throughout the lifespan is associated with increased rates of psychiatric illness and deficits in executive function even in patients who were put on treatment early [2]. The American College of Medical Genetics and Genomics (ACMG) guidelines recommend that Phe levels be maintained throughout the lifespan between 120 and 360 uM/L for optimal outcome [1].
Beyond dietary therapy, pharmacologic treatment for PKU became available with the FDA approval of sapropterin dihydrochloride in 2007. However, sapropterin response rate is limited to approximately half of all PKU patients [1]. In 2018, a second pharmacological agent, pegvaliase, was approved as the first enzyme substitution treatment for PKU [3]. Theoretically, this injectable medication has the potential to reduce phenylalanine (Phe) levels in all PKU patients. Due to individual immune response, the length of time to reach efficacy on pegvaliase and the dose required is variable [3]. Pegvaliase received initial label approval for maintenance doses of 20 mg and 40 mg daily [3]. The label was amended in 2020 to include 60 mg daily dosing [4].
While 60 mg dosing increases the likelihood of therapeutic response, patients may require higher dosing to achieve optimal control of Phe levels and dietary liberalization. We report a case series of four PAH deficient patients, historically treated with dietary management, who initiated treatment with pegvaliase and were titrated to 80 mg daily dosing.
2. Case presentations (materials/methods)
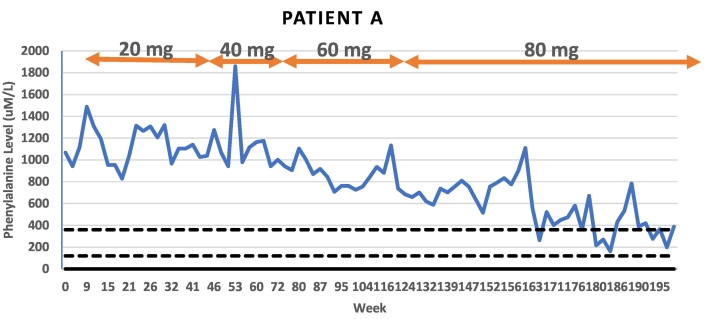
Patient A is a 23-year-old male who was historically inconsistent with formula intake and consumed natural protein beyond his Phe tolerance throughout adolescence. His average Phe level in the year leading into treatment initiation was 1104 uM/L (n = 11) and baseline Phe level at the start of pegvaliase treatment was 1122 uM/L. His BMI at his start visit was 38.88 kg/m2. The patient was increased to 40 mg daily at week 47, after six months on 20 mg daily dosing. The patient remained on 40 mg daily for five months prior increasing to 60 mg daily at week 69. After one year on 60 mg daily dosing, at week 122, the patient was increased to 80 mg daily. He had experienced a 40% reduction in baseline Phe level on 60 mg dosing, yet diet liberalization was not initiated due to levels remaining >600 uM/L. At two years of treatment with pegvaliase, insurance authorization was pursued for 80 mg dosing. The patient has now been on 80 mg daily dosing for 15 months. After 9 months on 80 mg, at week 151, the patient had his first Phe level below 600 uM/L. Since this time point, the patient has maintained lower Phe levels with only one level > 600 uM/L. The majority (55%) of his Phe levels have been within treatment range (120–360 mg/dL) with an average Phe level of 390 uM/L. The patient reports high motivation to continue with treatment on pegvaliase given the reduction in his Phe level. On average, he is consuming the daily recommended natural protein intake although accurate diet records have been a challenge for this patient. He continues to consume one to two servings of formula daily.
Pegvaliase side effects for patient A included injection site reactions, arthralgia, and an episode of anaphylaxis at 13 weeks into therapy with reported flushing, tingling, vomiting and diarrhea immediately following pegvaliase injection. Due to these side effects, the patient underwent a prolonged titration phase. The patient had a subsequent episode of anaphylaxis at week 80 on 60 mg maintenance dosing with flushing, headache, vomiting and diarrhea. Both episodes resolved without administration of epinephrine and without sequelae. While on 80 mg maintenance dosing, he experienced mild arthralgia and a rash which resolved with a short steroid course. The patient has not reported further side effects in six subsequent months of therapy.
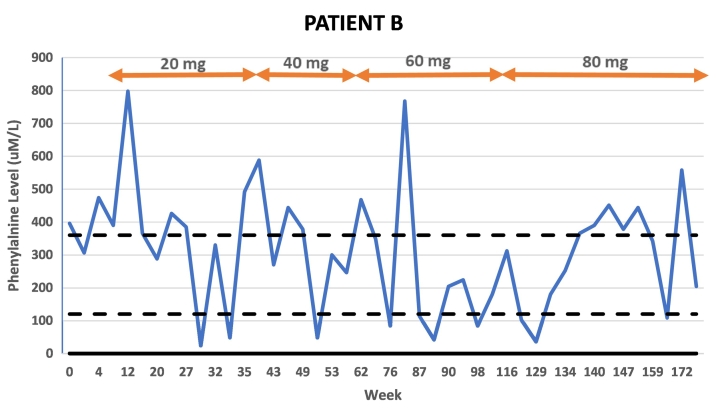
Patient B is a 27-year-old male with a history of variable metabolic control. The patient attributed elevated levels to excess Phe intake beyond his 250 mg daily tolerance because of portion sizes rather than consuming high protein foods. There were also concerns of inadequate caloric intake affecting Phe control in the year prior to pegvaliase initiation. The patient consumed formula daily as prescribed. The patient's average Phe level in the year prior to pegvaliase initiation was 528 uM/L (n = 4) and was 396 uM/L at treatment baseline. His BMI at his start visit was 22.4 kg/m2. Patient B's first Phe level below 120 uM/L occurred at week 29 of treatment on 20 mg daily dosing. However, subsequent Phe levels were above treatment range, and he was dose escalated to 40 mg daily at week 41 of treatment. Phe levels remained outside of the treatment range until 11 weeks on 40 mg dosing when a Phe level of 48 uM/L was obtained. However, once again subsequent Phe levels were outside the treatment range and the patient's dose was escalated to 60 mg daily at week 64. The patient's pegvaliase dose was then reduced over the next 20 weeks due to side effects. The patient resumed 60 mg daily dosing at week 81 and remained at this dose for 15 weeks. Despite a 73% reduction in average Phe on 60 mg daily dosing from baseline average Phe, at week 109 the dose was increased to 80 mg daily to allow for diet liberalization. On 80 mg dosing, the intact protein prescription has been increased by 10 g. The patient's average Phe level on 80 mg dosing is 288 uM/L which is a 45% reduction from his baseline average Phe in the setting of increased natural protein intake.
Pegvaliase side effects for Patient B included injection site reactions, arthralgia, and rashes primarily in the first 68 weeks of treatment. The patient was managed with a combination of dose reduction and additional premedication. He experienced one episode of urticaria on day 3 of 80 mg dosing which resolved after one week of every other day dosing. The patient has continued 80 mg daily injections without subsequent side effects.
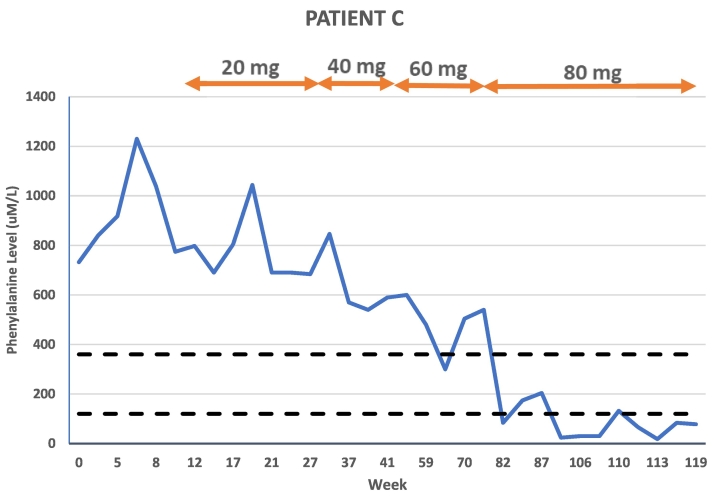
Patient C is a 40-year-old female with a similar history to Patient B in terms of metabolic control and reported diet. She was historically managed on a dietary restriction of 250 mg Phe daily and attributed poorly controlled Phe levels to excess intake of vegetables with higher Phe content versus eating high protein foods. In the year prior to pegvaliase treatment, the patient had several life stressors that she reported contributed to her struggle with following the PKU diet. Her average Phe level in the year prior to treatment initiation was 588 uM/L (n = 5) and her baseline Phe level at her pegvaliase start visit was 732 uM/L. Her BMI at treatment start was 34.27 kg/m2. At 32 total weeks of treatment, after approximately five months on 20 mg daily dosing, the patient was increased to 40 mg daily. The patient was then increased to 60 mg daily at 53 weeks of treatment. At 80 weeks of treatment, after almost seven months on 60 mg daily dosing, the patient was increased to 80 mg daily. She achieved a partial response at each maintenance dose on pegvaliase, including a 26% reduction in Phe level with the increase to 40 mg daily. After increasing to 60 mg daily, the patient's Phe level was further reduced by 28% from the average Phe on 40 mg daily. It was not until she escalated to 80 mg daily, however, that the patient achieved and maintained Phe levels in the treatment range. Her average Phe on 80 mg daily dosing is 90 uM/L which represents a 78% decrease from her average Phe level on 60 mg. Given multiple Phe levels <120 uM/L on 80 mg maintenance dosing, the patient's diet was able to be fully liberalized within eight weeks. The patient has discontinued all medical food and now consumes 70–80 g of intact protein daily.
Pegvaliase side effects for Patient C included injection site reactions only.
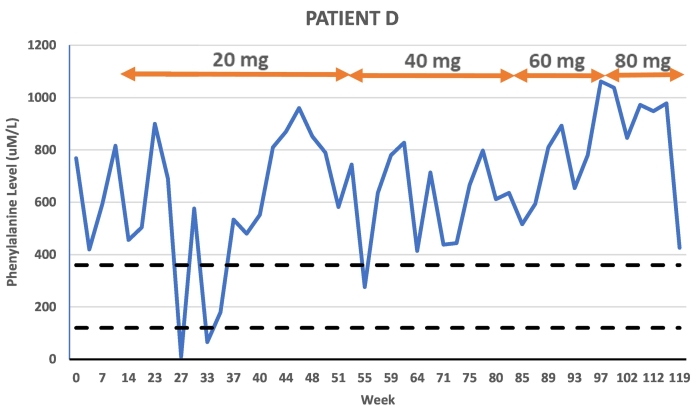
Patient D was an 18-year-old male patient in poor Phe control prior to initiating treatment with pegvaliase. The patient was consistent with formula intake but during his adolescence began to consume excess Phe beyond his tolerance of 250 mg daily. The patient followed a low protein diet but was loosely tracking Phe intake. The patient's average Phe level in the year prior to pegvaliase was 636 uM/L (n = 9) and his baseline Phe at his start visit was 690 uM/L. His BMI at treatment initiation was 23.1 kg/m2. This patient had a delayed titration schedule due to side effects and anxiety around injecting. The patient reached 20 mg daily dosing at week 25 of treatment. After 7 months of 20 mg daily dosing, the patient was increased to 40 mg daily where the patient remained until week 81 when he was increased to 60 mg daily. After 15 weeks of 60 mg daily dosing, the patient was increased to 80 mg daily. He discontinued pegvaliase at week 126, following 30 weeks on 80 mg daily dosing with an average Phe level of 858 uM/L. His average Phe level during the entirety of his pegvaliase treatment was 660 uM/L prior to increasing to 80 mg. However, the patient transitioned to living on a college campus within six weeks of increasing to 80 mg daily and subsequently reported significantly higher Phe intake. Due to side effects and reported anxiety over injections, the patient injected inconsistently throughout the entirety of his pegvaliase treatment. No consistent decrease in Phe levels was noted at any maintenance dose.
Pegvaliase side effects for Patient D included injection site reactions and arthralgia. The primary barrier for this patient was anxiety related to self-injecting, which resulted in prolonged episodes of intermittent dosing and limited reporting to clinic.
3. Results/conclusion
Lifelong management of PKU is challenging, and it is well documented that many adult patients become lost to follow-up (LTFU) [5,6]. Despite the ACMG recommendation that PKU treatment continue throughout the lifespan, studies show that more than half of PKU patients over the age of 18 stop attending clinic and become LFTU [5]. While this is more common in adult patients, PKU treatment can be challenging for patients of any age and their families. Advancements in drug development have provided PKU patients with alternative treatment methods to control Phe levels and optimize function. Pegvaliase approval in 2018 provided patients the first opportunity to manage their blood Phe levels by enzyme substitution treatment. The FDA expanded the dosing label for pegvaliase in 2020 to allow for an increase in maintenance dosing to 60 mg, but there are patients who do not respond even at that dose. Insurance authorization for 80 mg dosing was initially denied for three out of four patients. All denials were appealed successfully with clinical justification for dose increase. The safety profile of 80 mg dosing in the 4 cases presented was not different than that observed at lower maintenance dosing. There have been no anaphylactic episodes on 80 mg dosing in this patient cohort. Indeed, the frequency of side effects on 80 mg daily dosing was less than these patients experienced on lower doses. All patients were initiated on a premedication regimen of H1 and H2 receptor antagonists at the start of pegvaliase therapy and continued those medications throughout dose escalation to 80 mg daily. In three of the four reported patients, this higher dose resulted in improved blood Phe control despite diet liberalization. The fourth patient did not achieve efficacy on increased dosing prior to discontinuation, however his treatment course was complicated by inconsistent dosing, anxiety around injections, and his transition to independent care of his PKU. This patient was the only one escalated to the 80 mg dose who experienced anxiety and hesitation around adding a fourth injection. Assessing a patient's motivation and willingness to do four injections daily is paramount in the decision to dose escalate knowing the impact these factors have on adherence. We conclude that pegvaliase dosing must be individualized to achieve therapeutic goals and that some patients may require higher doses than those included on the product label (Fig. 1, Fig. 2, Fig. 3, Fig. 4).
Fig. 1.
Patient A Phe levels in relation to time and dose
Fig. 2.
Patient B Phe levels in relation to time and dose
Fig. 3.
Patient C Phe levels in relation to time and dose
Fig. 4.
Patient D Phe levels in relation to time and dose
Funding
The authors did not receive any financial compensation or support for this publication.
CRediT authorship contribution statement
Erika R. Vucko: Conceptualization, Writing – original draft, Writing – review & editing. Kirsten E. Havens: Conceptualization, Writing – original draft, Writing – review & editing. Joshua J. Baker: Writing – review & editing. Barbara K. Burton: Writing – review & editing.
Declaration of Competing Interest
EV, JB, and BB are investigators of BioMarin Pharmaceutical Inc. clinical trials. EV and BB are on the BioMarin Pharmaceutical Inc. Speakers Bureau.
Data availability
The data that has been used is confidential.
References
- 1.Vockley J., Andersson H.C., Antshel K.M., Braverman N.E., Burton B.K., Frazier D.M., Mitchell J., Smith W.E., Thompson B.H., Berry S.A. American college of medical genetics, genomics therapeutics committee, phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. 2014;16:188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 2.Brumm V.L., Bilder D., Waisbren S.E. Psychiatric symptoms and disorders in phenylketonuria. Mol. Genet. Metab. 2010;99(1):S59–S63. doi: 10.1016/j.ymgme.2009.10.182. [DOI] [PubMed] [Google Scholar]
- 3.Longo N., Dimmock D., Levy H., Viau K., Bausell H., Bilder D.A., Burton B., Gross C., Northrup H., Rohr F., Sacharow S., Sanchez-Valle A., Stuy M., Thomas J., Vockley J., Zori R., Harding C.O. Evidence- and consensus-based recommendations for the use of pegvaliase in adults with phenylketonuria. Genet. Med. 2019;21(8):1851–1867. doi: 10.1038/s41436-018-0403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BioMarin Pharmaceutical Inc . 2020. Pegvaliase Prescribing Information. (San Rafael, CA) [Google Scholar]
- 5.Berry S.A., Brown C., Grant M., Greene C.L., Jurecki E., Koch J., Moseley K., Suter R., Van Calcar S.C., Wiles J., Cederbaum S. Newborn screening 50 years later: access issues faced by adults with PKU. Genet. Med. 2013;15(8):591–599. doi: 10.1038/gim.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurecki E.R., Cederbaum S., Kopesky J., Perry K., Rohr F., Sanchez-Valle A., et al. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017;120:190–197. doi: 10.1016/j.ymgme.2017.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.