Abstract
Pathogenic variants in DHDDS have been associated with either autosomal recessive retinitis pigmentosa or DHDDS-CDG. Heterozygous variants in DHDDS have been described in patients with a progressive neurodegenerative disease. Here we report on an individual presenting with a multisystem CDG phenotype who was diagnosed with known homozygous pathogenic DHDDS variants, previously associated with isolated retinitis pigmentosa.
An adult Ashkenazi Jewish female developed multiple symptoms of late onset type 1 CDG including seizures, ataxia, protein losing enteropathy, tremor, and titubation in association with elevated mono-oligo/di-oligo transferrin ratio in blood, and classic retinitis pigmentosa. She was diagnosed by whole exome sequencing with the common Ashkenazi Jewish, homozygous p.K42E variants in DHDDS. She was started on Acetazolamide and responded well to the treatment which improved her titubation, tremor, and generalized edema.
Reviewing the literature, families with DHDDS variants and multisystem presentation were different from our patient's presentation in terms of clinical manifestations, severity, genetic defect, and mode of inheritance. In previously reported patients with neurologic symptoms including seizures, movement abnormalities, and global development delay, the phenotype was caused by heterozygous pathogenic variants in DHDDS. The infant who was reported with a multisystem phenotype and fatal type 1 CDG had compound heterozygosity for a nonsense and a splice site variant in DHDDS, resulting in DHDDS-CDG.
The discovery of the novel phenotype associated with the common p.K42E pathogenic variant in DHDDS expands the spectrum of CDG and further enhances our understanding on the role of DHDDS in glycosylation beyond the retina.
Keywords: Glycosylation, Acetazolamide, Retinitis pigmentosa, Ataxia, Seizures, Intrafamilial variability
Abbreviations: DHDDS, Dehydrodolichyl diphosphate synthase; CDG, Congenital disorders of glycosylation; NGS, Next Generation Sequencing; CNS, Central Nervous System; WES, Whole exome sequencing; PMM2, Phosphomannomutase 2; NPCRS, Nijmegen Pediatric CDG Rating Scale; RP, Retinitis Pigmentosa
Highlights
-
•
We report on the first individual carrying homozygous p.K42E variants in DHDDS associated with protein losing enteropathy, seizures, and ataxia.
-
•
We observed familial variability in association with p.K42E and progressive ataxia in siblings with in DHDDS-CDG.
-
•
The novel DHDDS-CDG patient phenotype broadens the current spectrum of CDG.
-
•
Acetazolamide was successful in treating titubation and recurrent edema in our DHDDS-CDG patient of Ashkenazi Jewish descent.
1. Introduction
The advancement in genetic testing with the broad use of next generation sequencing (NGS) has accelerated the discovery of new patients diagnosed with the rare monogenic disorders called Congenital disorders of glycosylation (CDG).
There are more than 160 different types of CDG, with N-linked glycosylation being its largest sub-group. Most N-linked CDG types are inherited in an autosomal recessive manner, and only very few are inherited in an autosomal dominant or X-linked manner [1,2]. The most common clinical phenotype in N-linked CDG is a multi-systemic manifestation characterized by developmental delay and failure to thrive. Most patients present with dysmorphic features, hypotonia, ataxia, hepatopathy, and coagulopathy. Various cardiac, endocrine, and ophthalmologic abnormalities are also common [3]. Ocular abnormalities are frequently described in Type 1 CDG (an error in the monosaccharide activation, synthesis of lipid-linked oligosaccharides or their transfer to nascent proteins), involving both the anterior and posterior segments of the eye [4]. Characteristic ophthalmologic findings include convergent strabismus, abnormal eye movements, delayed visual maturation, cataract, and hyperopia [5]. The most severe retinal abnormality is retinitis pigmentosa (RP), which has been reported in patients diagnosed with CDG type 1 [4].
N-linked glycosylation defects derange the normal glycosylation process of proteins by disrupting the attachment of glycans to the asparagine residue. This leads to hypoglycosylation of essential glycoproteins (e.g. coagulation factors, enzymes, endocrine and transport proteins, etc) and membrane glycoproteins. The most common N-linked glycosylation defect is PMM2-CDG; a multisystem disease affecting the central nervous system (CNS), vision, hearing, muscle-, endocrine, cardiac, and skeletal function. Deficiency in the phosphomannomutase 2 (PMM2) enzyme activity leads to abnormal protein glycosylation, which also underlies the common retinal pigment anomalies in PMM2-CDG [6]. Other type I CDG that show RP as a common feature include alpha-1,3-glucosyltransferase deficiency (ALG6-CDG) and alpha-1,3-mannosyltransferase deficiency (ALG3-CDG) (3,4).
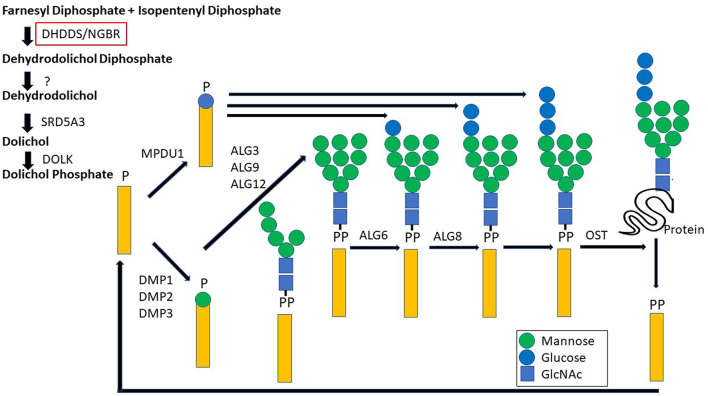
Dehydrodolichyl diphosphate synthase (DHDDS) is a crucial enzyme in the dolichol synthesis pathway (which plays an important role in the N-glycosylation of many glycoproteins) (7). Genetic defects in this pathway lead to abnormal lipid linked oligosaccharide (LLO) synthesis. LLOs are built as Dol-P-Man gives the necessary substrate for all luminal endoplasmic reticulum (ER) mannosylation (Fig. 1) [7]. DHDDS dysfunction results in an increase of misfolded, under-glycosylated glycoproteins in the ER, which elicits an abnormal ER response that triggers apoptosis [8].
Fig. 1.
Schematic figure on the role of DHDDS in N-linked glycosylation.
The pathogenic variant c.124A > G (p.K42E) in DHDDS has been known as a founder mutation causing autosomal recessive RP in families of Ashkenazi Jewish origin (MIM 613861) [9]. Multisystem presentation in association with DHDDS variants have been linked to various genetic mechanisms. Hamdan et al. (2017) and Galosi et al. (2021) identified de novo pathogenic variants (R37H and R211Q) in DHDDS which have been reported in multiple families with developmental delay, seizures, myoclonus, and adult-onset ataxia (MIM 617836) [[10], [11], [12]]. Sabry et al. (2016) identified biallelic loss of function pathogenic variants (c.192G > A; p.W64X and the splicing variant c.441-24A > G) in DHDDS in a young male with hypotonia and lethal epileptic encephalopathy (13). Additionally, he was also identified to carry the homozygous variant c.911 T > C (p.F304S; a common polymorphism) in ALG6. As p.F304S has been considered as a modifier that exacerbates the expression in individuals carrying pathogenic variants in other CDG genes, the authors suggested that the ALG6 variant might have had a role in enhancing phenotypic severity in this case (MIM 613861). This patient (with DHDDS-CDG) had an early onset and exhibited very severe multisystem organ involvement such as bradycardia, axial hypotonia, peripheral hypertonia, an enlarged liver with high transaminases levels, renal failure, and status epilepticus resulting in death at 8 months of age [10,11,13].
Here we present an individual with later onset, multisystemic type 1 CDG in addition to the classic RP manifestation, which is a novel phenotype associated with the DHDDS K42E pathogenic variant.
2. Case presentation
We report on a 64-year-old female, born at term via induced vaginal delivery, with normal birth weight and length. Parents were healthy, and of Ashkenazi Jewish ancestry. The brother of our patient was known with adult-onset RP and mild ataxia; however, his Carbohydrate deficient transferrin testing was normal.
Early development and growth were normal in our patient, apart from numerous respiratory and urinary tract infections around grade school age. She presented with retinopathy (retinitis pigmentosa) at 20 years of age and subsequently developed cataracts. Three years later, she was diagnosed with grand mal seizure disorder, her seizures are controlled with levetiracetam (last seizure was in 2012). In her late 40s, she underwent radioactive iodine treatment for thyroid cancer. Twelve years later (in her 50s), she was hospitalized due to progressive lower extremity edema, fatigue, some right-sided abdominal discomfort, and abdominal fullness. She was found to have an albumin level of 1.9 g/dl (control range 3.4–5.4 g/dL), low total protein level of 4.7 g/dl (control range 6.0–8.3 g/dL), stool sample was negative for alpha-1 antitrypsin, and there was no evidence of abnormal clonal protein production or proteinuria. There was no sign of synthetic liver dysfunction based on normal coagulation factor levels and normal ammonia level in blood. Her CT scan (abdomen and pelvis) showed pancolitis, mild ascites, small bilateral pleural effusions. She was subsequently diagnosed with protein losing enteropathy, which required administration of IV albumin. At that time, she also experienced episodes of hypoglycemia.
In addition, she underwent an upper endoscopy with extensive biopsies which were normal. Colonoscopy and ileoscopy were unrevealing and did not reveal significant pathology on focused biopsies. Due to her complex phenotype, a genetic evaluation was ordered.
The patient appeared well developed, nourished, and without dysmorphic features. She had severely decreased visual acuity. She was only able to perceive variations in the degree of light or brightness on the left eye and contours on the other. Other symptoms included horizontal nystagmus, titubation, moderate gait ataxia, diadochokinesis, and significant lower extremity edema. No other abnormalities were detected by physical examination.
In parallel with the progression of ataxia symptoms the patient underwent a brain MRI at the age of 60 years, which showed no specific findings, and the cerebellum appeared to be normal. She also underwent a Dopamine transporter scan, a diagnostic method to investigate a possible loss of dopaminergic neurons in striatum, which showed symmetric normal uptake in the bilateral basal ganglia.
Congenital Disorders of Glycosylation Genetic Panel (performed on a whole exome sequencing backbone) revealed the homozygous variant c.124A > G (p.K42E, Chr1:26764719) in exon 3 in the DHDDS gene. Raw whole exome sequencing (WES) data was reviewed in detail. Nine primers were developed going 100 bp into the introns in DHDDS, and we re-sequenced the gene by Sanger sequencing. No additional reportable variants were identified in any other genes.
She then underwent further blood and biochemical based testing given the results of her WES. At this time, laboratory studies showed a borderline low albumin level at 3.5 g/dl (control range 3.5–5.4 g/dL), low total serum protein at 5.4 g/dl (control range 6–8.3 g/dl), and low immunoglobin G (IgG) level at 357 mg/dl (control range 767–1590 mg/dl). Other immunoglobulins (IgM, IgA, and IgE) were normal. There was leukopenia of 2.7 × 10^ (9)/L (control range 4.5 to 11.0 × 10^(9)/L). She also had high total cholesterol levels at 285 mg/dl (desirable <200 mg/dl) and high LDL (207 mg/dl), while triglyceride and HDL levels remained within normal ranges. Alanine transaminase (ALT), Aspartate transaminase (AST), alkaline phosphate (AP), glucose, antithrombin III, factor XI, and factor XI activity were all within normal ranges. Copper and ceruloplasmin levels were also reported to be normal. CT enterography with careful assessment of the liver, small bowel, colon, and retroperitoneum showed improvement compared to the prior study (CT scan of the abdomen and pelvis) with resolution of ascites as well as of the mild colonic changes previously noted. Both ultrasound of the abdomen and liver elastography were normal as well.
Carbohydrate deficient transferrin testing was minimally abnormal with a mono-oligo/di-oligo ratio of 0.09 (≤0.06) and normal a-oligo/di-oligo ratio of 0.006 (≤0.011).
According to the Nijmegen Progression CDG Rating Scale (NPCRS), she had a moderate severity score of 18 at her initial visit.
At the age of 63 years, she began to take Acetazolamide (oral 125 mg BID) for her edema ataxia and tremor. Her blood gas, electrocytes, liver and kidney functions were monitored closely without significant alterations. Both her tremor and edema improved significantly. She has been on Acetazolamide treatment for approximately two years and her NPCRS score improved from 18 to 14.
3. Discussion
Our patient, diagnosed with the common homozygous Ashkenazi variant (p.K42E) in the DHDDS-gene, is quite unique. Direct re-sequencing of DHDDS by Sanger sequencing including 100 bp deep into the intronic regions showed no additional intronic variants. One might suspect that another genetic variant in a different CDG gene could modulate the phenotype, but so far, we have not found any VUS in the same pathway, or pathogenic variants in any other genes.
She initially showed a gradual onset of vision loss and seizures starting at 20-years of age. Two decades later she developed an acute presentation with protein losing enteropathy (PLE), generalized edema and hypoglycemia, which are common signs of type I CDG disorders. This was then followed by the onset of progressive tremors and ataxia. She developed severe progressive RP, which is a hallmark feature amongst Ashkenazi Jewish families with p.K42E variants in DHDDS. Interestingly, the brother of our patient carries the same homozygous p.K42E variants and has mild ataxia in addition to RP.
PLE has been reported in many types of CDG, including PMM2-CDG, MPI-CDG and ALG6-CDG [14]. Abnormal protein glycosylation and aberrant folding of the intestinal membrane and secretory proteins affect the secretory pathway. This then fills the endoplasmic reticulum with insoluble proteins in enterocytes, leading to villous atrophy. However, this is the first report of pathogenic variants found in DHDDS in association with PLE, especially presenting at an older age than usually observed in other type I CDG patients.
DHDDS enzyme coded by the DHDDS gene is vital for dolichol synthesis pathway in the ER. Dolichol importance rises from being the main carrier lipid for N-glycosylation, O-mannosylation, and C-mannosylation reactions, and GPI anchor biosynthesis.
DHDDS enzyme is one of two subunits that form cis-prenyltransferase (cis-PTase), the other subunit is NgBR (Nogo-B receptor) which serves as a stabilizer. cis-PTase leads the primary step in the dolichol synthesis pathway by increasing the chain length of farnesyl pyrophosphate, resulting in the production of polyprenol [15,16]. Polyprenol is a key element of dolichol. The dolichol pathway is essential in the normal function and adequate glycosylation of proteins in the retina. In fact, the activity of the DHDDS enzyme has been detected in many parts of the retina, most prominently in the myoid region of the rod and in the inner segment of the cone photoreceptors. Also, rhodopsin (the primary photoreceptor molecule of vision) is a single chain polypeptide that is coupled by two unusually short N-linked oligosaccharide chains. Thus, the normal function and formation of the retina is dependent on protein N-glycosylation [17].
Homozygous p.K42E variants in DHDDS led to gait ataxia, titubation and tremor in our patient and a milder presentation in our patient's younger brother, who also had RP and CNS involvement (ataxic gait). Normal dolichol synthesis is important in protein transport between the membranes of the ER and lysosomes. Any flaws in the dolichol pathway could cause accumulation of dolichol precursors and proteins in the ER and lysosomes in neurons. This accumulation is similar to that observed in neurodegenerative diseases. This is further supported by the fact that patients with DHDDS related neurodegenerative disorders were found to have a lipid-like-substance in the lysosomes of fibroblast samples. Variable glycosylation of the different lysosomal enzymes could also explain some of the neurological manifestations such as movement disorders and seizures. Furthermore, abnormalities in the dolichol pathway can result in low dolichol levels underlying some of the clinical features in DHDDS related disorders [10].
Our patient had distinctive neurological findings that severely affected her quality of life. She suffered from progressive intention and postural tremor in her upper extremities and head with titubation. She had moderate ataxia with abnormal gait, could not walk without support, coupled with frequent falls in the past. To address her lower limb edema, she was started on a low dose of oral Acetazolamide (a carbonic anhydrase inhibitor that is conventionally used as a diuretic). It was recently discovered that Acetazolamide had the potential to improve movement abnormalities and possibly improve glycosylation (as it has been shown to have positive effects on CNS symptoms in type 1 CDG patients) [18]. It also has effect on episodic ataxia related to genetic metabolic disorders by altering the pH in the neuronal cell. Lowering the pH can cause a decrease in the electronic flow of the neuronal membranes. The decrease in electronic flow then disables the abnormally activated calcium channels, due to abnormal CaV2.1 N-glycosylation, hence, making the membranes less excitable to stimulation [18,19]. Our patient had no adverse effects while on the drug. Acetazolamide had multiple therapeutic benefits including improvement in her titubation, extremity tremor, and in pen holding and writing, which are not directly captured by NPCRS, but were significant in her quality of life. However, she still had mild ataxia that was affecting her mobility. The improvement of the movement disorder was demonstrated by her NCPRS severity score (improvement in self-care section (Table 1).
Table 1.
NCPRS scores before and after starting oral therapy with 250 mg Acetazolamide daily.
| Nijmegen Progression CDG Rating Scale (NPCRS)⁎ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section I |
Section II |
Section III |
Overall Score | ||||||||||
| Vision | Feeding | Self-care | Mobility | Hearing Commu-nication Education |
Blood | Gastro-intestinal | Endo-crine | Seizures Encephalopathy Pulmonary Cardiac Renal Liver Coagulation |
Vision | Ataxia | Growth Development Strabismus Myopathy Pyramidal Dystonia Neuropathy |
||
| Before Acetazolamide treatment | 3 | 1 | 2 | 2 | 0 | 1 | 3 | 1 | 0 | 3 | 2 | 0 | 18 |
| 8 | 6 | 5 | |||||||||||
| After Acetazolamide treatment | 3 | 0 | 1 | 2 | 0 | 0 | 1 | 2 | 0 | 3 | 2 | 0 | 14⁎⁎ |
| 6 | 3 | 5 | |||||||||||
NPCRS is based around three domains, with each domain containing specific items relevant to the assessment of CDG patients. The three domains are as follows: Section I (Current Function) is a domain consisting of five questions regarding five general functions (vision, hearing, communication, feeding and mobility); Section II (System Specific Involvement) includes questions directly assessing CNS, blood, gastrointestinal, endocrine, respiratory, cardiovascular, renal and liver function over the preceding 6 months; Section III (Current Clinical Assessment) includes questions related to status. For each item, there are four possible responses reflecting normal (0), mild (1), moderate (2) and severe (3) impairment.
We only included the NCPRS section that were relevant to our case (The most significant improvement was a decrease in involuntary head movements (titubation), and in pen holding and writing, which are significant in quality of life, but not captured by the NPCRS.
4. Conclusion
We described biallelic homozygous pathogenic variants in the DHDDS gene, presenting with a multisystemic, late onset type 1 CDG with intrafamilial variability. DHDDS gene variants have been reported to cause RP, several neurodevelopment disorders, and a dominant form of epileptic encephalopathy. This case adds a new clinical presentation to the phenotypic spectrum of DHDDS-CDG and a potential off label treatment option for ataxia related to glycosylation disorders.
Author contributions
JM, LV, DDG and EM all contributed to writing the manuscript, AM revised the manuscript and designed the figure.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Acknowledgement
EM is funded by the grant titled Frontiers in Congenital Disorders of Glycosylation (1U54NS115198-01) from the National Institute of Neurological Diseases and Stroke (NINDS) and the National Center for Advancing Translational Sciences (NCATS), and the Rare Disorders Clinical Research Network (RDCRN), at the National Institute of Health. DDG was supported by a Fellowship of the Belgian American Educational Foundation.
Data availability
Data will be made available on request.
References
- 1.Wilson M.P., et al. Active site variants in STT3A cause a dominant type I congenital disorder of glycosylation with neuromusculoskeletal findings. Am. J. Hum. Genet. 2021;108(11):2130–2144. doi: 10.1016/j.ajhg.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng B.G., et al. Predominant and novel de novo variants in 29 individuals with ALG13 deficiency: clinical description, biomarker status, biochemical analysis, and treatment suggestions. J. Inherit. Metab. Dis. 2020;43(6):1333–1348. doi: 10.1002/jimd.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang I.J., He M., Lam C.T. Congenital disorders of glycosylation. Ann. Transl. Med. 2018;6(24):477. doi: 10.21037/atm.2018.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altassan R., et al. International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: diagnosis, treatment and follow up. J. Inherit. Metab. Dis. 2019;42(1):5–28. doi: 10.1002/jimd.12024. [DOI] [PubMed] [Google Scholar]
- 5.Morava E., et al. Ophthalmological abnormalities in children with congenital disorders of glycosylation type I. Br. J. Ophthalmol. 2009;93(3):350–354. doi: 10.1136/bjo.2008.145359. [DOI] [PubMed] [Google Scholar]
- 6.Vaes L., et al. Genotype-phenotype correlations in PMM2-CDG. Genes (Basel) 2021;12(11) doi: 10.3390/genes12111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefeber D.J., et al. Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am. J. Hum. Genet. 2009;85(1):76–86. doi: 10.1016/j.ajhg.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandra Rao S., et al. Retinal degeneration caused by rod-specific Dhdds ablation occurs without concomitant inhibition of protein N-glycosylation. iScience. 2020;23(6):101198. doi: 10.1016/j.isci.2020.101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Züchner S., et al. Whole-exome sequencing links a variant in DHDDS to retinitis pigmentosa. Am. J. Hum. Genet. 2011;88(2):201–206. doi: 10.1016/j.ajhg.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galosi S., et al. De novo DHDDS variants cause a neurodevelopmental and neurodegenerative disorder with myoclonus. Brain. 2021 doi: 10.1093/brain/awab299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng B.G., Freeze H.H. Perspectives on glycosylation and its congenital disorders. Trends Genet. 2018;34(6):466–476. doi: 10.1016/j.tig.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdan F.F., et al. High rate of recurrent De Novo mutations in developmental and epileptic encephalopathies. Am. J. Hum. Genet. 2017;101(5):664–685. doi: 10.1016/j.ajhg.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabry S., et al. A case of fatal Type I congenital disorders of glycosylation (CDG I) associated with low dehydrodolichol diphosphate synthase (DHDDS) activity. Orphanet J. Rare Dis. 2016;11(1):84. doi: 10.1186/s13023-016-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westphal V., et al. Reduced heparan sulfate accumulation in enterocytes contributes to protein-losing enteropathy in a congenital disorder of glycosylation. Am. J. Pathol. 2000;157(6):1917–1925. doi: 10.1016/S0002-9440(10)64830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantagrel V., Lefeber D.J. From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases. J. Inherit. Metab. Dis. 2011;34(4):859–867. doi: 10.1007/s10545-011-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edani B.H., et al. Structural elucidation of the cis-prenyltransferase NgBR/DHDDS complex reveals insights in regulation of protein glycosylation. Proc. Natl. Acad. Sci. U. S. A. 2020;117(34):20794–20802. doi: 10.1073/pnas.2008381117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelinger L., et al. A missense mutation in DHDDS, encoding dehydrodolichyl diphosphate synthase, is associated with autosomal-recessive retinitis pigmentosa in Ashkenazi Jews. Am. J. Hum. Genet. 2011;88(2):207–215. doi: 10.1016/j.ajhg.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Monseny A.F., et al. AZATAX: acetazolamide safety and efficacy in cerebellar syndrome in PMM2 congenital disorder of glycosylation (PMM2-CDG) Ann. Neurol. 2019;85(5):740–751. doi: 10.1002/ana.25457. [DOI] [PubMed] [Google Scholar]
- 19.Garone G., et al. Clinical and genetic overview of paroxysmal movement disorders and episodic ataxias. Int. J. Mol. Sci. 2020;21(10) doi: 10.3390/ijms21103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 1.Ligezka A.N., Mohamed A., Pascoal C., Ferreira V.D.R., Boyer S., Lam C., Edmondson A., Krzysciak W., Golebiowski R., Perez-Ortiz J., Morava E. Patient-reported outcomes and quality of life in PMM2-CDG. Mol. Genet. Metab. 2022;136(2):145–151. doi: 10.1016/j.ymgme.2022.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.