Highlights
-
•
Autism and psychopathy are both disorders of social cognition and share numerous of their features but still differ distinctively in their clinical phenotype.
-
•
The lower grey matter volumes in the right temporal pole and the left inferior frontal gyrus are the most prominent findings distinguishing violent offenders with high psychopatic from ASD individuals.
-
•
Violent offenders with high psychopatic traits and individuals with ASD both present similar lower grey matter volumes in the right precentral cortex compared to controls.
Keywords: Social brain networks, Social cognition, Voxel-based morphometry, Pre-motor cortex, Frontotemporal cortex, Empathy
Abstract
The goal of this study was to elucidate the anatomical brain basis of social cognition through two disorders with distinctively different phenotypes of social interaction. We compared structural MR images of 20 individuals with autism spectrum disorder (ASD), 19 violent offenders with high psychopathic traits, and 19 control participants using voxel-based morphometry (VBM). Our earlier study showed lower grey matter volume (GMV) values in the insula, frontal cortex, and sensorimotor cortex of the offender group compared to controls. In the present study, the images of the ASD group revealed lower GMV in the left precuneus, right cerebellum, and right precentral gyrus in comparison with controls. The comparison between the offender and ASD groups showed lower GMV values for the right temporal pole and left inferior frontal gyrus in the offender group. There was also an overlap of both disorders in the right pre-central cortex, showing lower GMV compared to controls. Our findings suggest structural differences between violent offenders with high psychopathy traits and ASD individuals in the frontotemporal social brain network areas, previously associated with empathy. We also provide evidence of similar abnormal structures in the motor cortex for both of these disorders, possibly related to uniting issues of social cognition.
1. Introduction
Brain networks for human social processing encompass large-scale brain networks across all cerebral lobes, the cerebellum, and subcortical areas, such as the striatum and amygdala (Amodio and Frith, 2006, Fernández et al., 2018, Sato and Uono, 2019, Van Overwalle et al., 2015). The somatosensory areas and the inferior occipital gyrus (IOG), fusiform gyrus (FFG), and superior temporal sulcus (STS) are involved, for instance, in recognizing and interpreting different socially relevant cues (Fernández et al., 2018, Lahnakoski et al., 2012, Nummenmaa and Calder, 2009, Sato and Uono, 2019). The posterior STS and temporo-parietal junction (TPJ), along with the temporal poles and several areas of the prefrontal cortex (PFC), are involved in reading others’ thoughts (theory of mind), mentalizing, and empathy (Amodio and Frith, 2006, Blakemore, 2012). The inferior frontal gyrus (IFG) has been linked with emotional empathy and the ability to understand and resonate with the actions of others, and it is functionally connected with STS and the inferior parietal lobule (IPL) (Fitzgerald, 2019, Sato and Uono, 2019). In turn, the ventromedial prefrontal cortex is considered to be involved in the cognitive component of empathy (Fitzgerald, 2019). Autism and psychopathy are two distinctively different disorders involving aberrant socioemotional processing, and structural and functional alterations in the brain networks that support these functions (Sato and Uono, 2019). However, the precise differences in the brain networks across these two disorders remain unknown.
1.1. Abnormalities in frontotemporal, parietal and cerebellar areas of ASD
Autism spectrum disorder (ASD) is a heterogenous neurodevelopmental disorder with deficits in various social cognitive functions. According to DSM-5, the core features of ASD are: A) persistent deficits in social communication and social interaction B) restricted, repetitive patterns of behavior, interests, or activities. The cerebral anatomical findings in ASD have been heterogenous with a limited number of consistent abnormalities (Ecker et al., 2013, Pagnozzi et al., 2018, Riddle et al., 2017). One prominent neuropathologic theory suggests that anatomic abnormalities in ASD are age-specific (Courchesne et al., 2007, Courchesne et al., 2011a, Courchesne et al., 2011b, Courchesne and Pierce, 2005, Ecker et al., 2013, Zielinski et al., 2014). The brain undergoes a period of precocious growth in the early postnatal life of ASD individuals. This is followed by decreased or paused age-related brain growth, so that in later childhood and adolescence there are no differences in total brain volume compared to neurotypical individuals. Moreover, later on in adulthood and older age, there seems to be an acceleration in age-related brain degeneration. This theory has also been supported by findings in longitudinal and cross-sectional MRI studies (Courchesne et al., 2011a, Courchesne et al., 2011b) While the early overgrowth of the ASD in volumetric MRI-studies pertains mostly to the frontal and temporal lobes (Amaral et al., 2008, Carper and Courchesne, 2005, Pagnozzi et al., 2018, Riddle et al., 2017), the abnormal age-related degeneration is also region-specific but focused more in the temporal and parietal lobes (Braden and Riecken, 2019, Wallace et al., 2010). MRI and post-mortem studies also support a similar abnormal growth trajectory in the cerebellum, but with the exception of life-long hypoplasia in the vermis areas (Amaral et al., 2008, Pagnozzi et al., 2018). The cerebellum has traditionally been viewed as related to motor functions, but recent studies have also demonstrated its role in social cognition (Moreno-Rius, 2019, Schmahmann, 2019, Van Overwalle et al., 2015).
1.2. Abnormalities in the frontotemporal, limbic, and cerebellar areas of psychopathy
Psychopathy is a serious disorder of social cognition characterized by grandiosity, callousness, manipulativeness, and lack of remorse, empathy, and guilt. It is often comorbid with conduct disorder and antisocial personality disorder (Johanson et al., 2020). Psychopathy is strongly associated with violence and criminal recidivism (Junewicz and Billick 2020) and is overrepresented in prisoner populations (Johanson et al., 2020). Several biological findings support the theories that conduct disorder in childhood and youth represent a neurodevelopmental precursor to antisocial personality disorder and psychopathy (Junewicz and Billick, 2020, Raine, 2018). Neuroimaging studies in psychopathy research have reported heterogenous results, but a recent systematic review exhibited decreased grey matter volumes in frontotemporal, limbic, paralimbic, and cerebellar structures as the most evident neuroanatomical findings (Johanson et al., 2020). A recent meta-analysis also showed decreased grey matter volumes in dorsolateral cortex and medial orbitofrontal cortex of men with psychopathy (De Brito et al., 2021). Similar consistent findings of reduced grey matter volume in the frontotemporal and limbic regions also occur in youths with conduct problems and aggression (Fairchild et al., 2019). Moreover, these findings are also associated with psychopathic traits in healthy controls and violent offenders (Nummenmaa et al., 2021).
1.3. Similarities and differences in socioemotional processing in ASD and psychopathy
The co-occurrence of psychopathy in autism is possible but rare (Rogers et al., 2006). However, although the clinical appearances of ASD and psychopathy differ considerably, these two disorders also display numerous shared features. A lack of empathy is common in both disorders. However, impairments in the cognitive aspects of empathy are more pronounced in ASD whereas the emotional aspects are more pronounced in psychopathy (Blair, 2008, Lockwood et al., 2013, O'Nions et al., 2014). Both disorders are overrepresented in forensic settings, but despite the elevated risk of violence in psychopathy, there is no evidence supporting a higher risk of violence or offenses in ASD (Howlin, 2000, Im, 2016, Johanson et al., 2020, King and Murphy, 2014, Siponmaa et al., 2001). Both of these disorders have their origin in the early childhood and can be viewed as neurodevelopmental disorders (Raine, 2018). When ASD and psychopathy both have a significant hereditary component (Blonigen et al., 2005, Larsson et al., 2006, Tuvblad et al., 2016, Tuvblad et al., 2014, Woodbury-Smith and Scherer, 2018), they also share some of their genetic basis (O’Nions et al., 2015, Tick et al., 2016, Tiihonen et al., 2020).
Only a few imaging studies have compared the brain basis of these two disorders directly. In a study of 323 typically developing and normally functioning adolescents, higher psychopathic traits were associated with lower cortical thickness in the anterior prefrontal cortex and higher ASD traits were associated with lower cortical thickness in the superior temporal and temporoparietal regions. The only overlapping region of cortical thinning as a function of psychopathic and autistic traits was seen in the left medial parietal cortex (Wallace et al., 2012). One fMRI study using empathy tasks showed reduced responses of brain regions involved in cognitive empathy in youths with ASD; in turn, youths with conduct disorder and callous-unemotional traits showed reduced responses in brain regions involved in emotional empathy compared to typically developing individuals (Klapwijk et al., 2016). In a study using theory of mind tasks, the children with ASD showed atypical neural processing compared to typically developing children, but there was no difference between children with conduct problems and high callous-unemotional traits and typically developing children (O'Nions et al., 2014). In our recent fMRI study comparing these disorders in tasks of social cognition, we found reduced responses in the motor cortex areas of both of these disorders, suggesting an aberrant motor contagion (Sun et al., 2021).
1.4. The current study
The purpose of this study was to provide insight to the structural differences in ASD and criminal psychopathy. We used voxel-base morphometry (VBM) to compare the regional grey matter volume between participants belonging to either of these study groups as well as a group of controls. In a recent structural brain imaging study using the same participants, the main findings were decreased grey matter volumes of the insula, the frontal cortex, and the sensorimotor cortex in violent offenders with psychopathic traits compared to control participants (Nummenmaa et al., 2021). Based on prior studies, we predicted lower grey matter volume (GMV) values in temporo-parietal and cerebellar areas of the ASD compared to control participants. In comparison with the previously studied violent offenders with high psychopathy traits, we predicted lower GMV values in the parietal social cognition areas of the ASD, while the offenders would in turn show lower values in frontal social cognition areas.
2. Materials and methods
2.1. Subjects
Twenty individuals with high functioning ASD (mean age 28 years, range 20–40 years), 19 convicted violent offenders (mean age 31 years, range 23–45 years), and 19 controls (mean age 29 years, range 21–47 years) participated in the study. All subjects gave informed, written consent and were compensated for their participation. The ethics board of the Hospital District of Southwest Finland approved the protocol, and the study was conducted in accordance with the Declaration of Helsinki. The study also obtained a research permit from the Hospital District of Helsinki and Uusimaa since the ASD group participants were largely recruited from this area.
The general inclusion criteria for all groups were male sex and age of 20–50 years. The general exclusion criteria for all groups were current use of narcotics, current abusive use of alcohol (over 14 U /week), severe axis I psychiatric illnesses, autoimmune illnesses, current medical conditions, and the standard MRI exclusion criteria.
Inclusion criterion for the ASD group was a valid autism spectrum disorder diagnosis, and inclusion criterion for the group of violent offenders with psychopathy traits was a current sentence for at least one violent crime (murder, manslaughter, rape, assault). Inclusion criteria for control group were a clean criminal record and no history of neurological or psychiatric disorders.
Offender group participants were inmates of Turku Prison who had been sentenced for murder (n = 5), manslaughter (n = 5), attempted manslaughter (n = 3), or grievous bodily harm (n = 6). They were each escorted by two prison guards to the local research institute for the brain imaging study. Psychiatric diagnoses of the offender group were based on prison health care and forensic psychiatric violence risk assessments, or most thorough forensic psychiatric examination reports concerning legal responsibility, two recruitment interviews, and semi-structured PCL-R interviews. Only two of the offenders refused to be evaluated with the PCL-R interview. In the Scandinavian countries the PCL-R cut-off limit for psychopathy has been suggested to be lower than the North American one and in the range of 25–26 (Jüriloo et al., 2014). Final consensus diagnoses were made by two medical specialists both in psychiatry and forensic psychiatry with 13–25 years of experience in the field of prison psychiatry, who were assisted by a psychologist with a 15-year working history in a Psychiatric Hospital for Prisoners. Possible opioid and dopaminergic medications were mostly withdrawn before the measurements, but it was not possible to execute a total discontinuation of the medications. Clinical information of offenders is found in supplement S1.
Subjects in the ASD group were volunteers from the Helsinki and Turku University Hospital Neuropsychiatric Clinics, with one subject also recruited from Neuropsychiatric Clinic Proneuron, in Espoo. The ASD group diagnoses were verified by a research psychologist, neurologist, and psychiatrist following DSM-5 criteria based on patient history, all accessible information from births records, well-baby clinics, and school healthcare. Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) assessment (Lord et al., 2012) was also used to clarify the autism diagnostics. Possible opioid and dopaminergic medications were withdrawn before measurements, but it was not possible to execute a total discontinuation of the medications. Clinical information of the ASD subjects is found in supplement S2.
The control participants were screened for medical conditions from their patient histories and their use of prescribed medication was double-checked from the Finnish medical database. Clinical information of the control subjects is found in supplement S3.
All the participants completed the Autism-Spectrum Quotient (AQ) questionnaire for the evaluation of ASD (Baron-Cohen et al., 2001, Bishop et al., 2004). AQ comprises 50 questions measuring five different areas of autism spectrum disorder: social skill, attention switching, attention to detail, communication, and imagination. As all the participants in control group scored below the 32 cut-off limit in the AQ, they were considered non-autistic and ADOS-2 evaluation was not considered necessary (Baron-Cohen et al., 2001). Three of the offender group did not complete the AQ questionnaire. One participant in the offender group scored 35 but showed no evidence of ASD in clinical evaluations and ADOS-2 evaluations in this group were also not considered necessary. Psychopathy was evaluated with Levenson Self-Report Psychopathy Scale (LSRP) questionnaire across all the participants (Levenson et al., 1995). LSRP measures two dimensions of psychopathy: primary psychopathy, indicating inclination to lie, lack of remorse, and callousness, and secondary psychopathy indicating impulsivity, short temper, and low tolerance for frustration. As all the participants in control and the ASD groups scored below 49 in the LSRP, they were considered non-psychopathic and PCL-R evaluation was not considered necessary (Brinkley et al., 2001). None of the participants had previous or current severe mental disorders, as assessed by SCID-I interview (Spitzer et al., 1992). The education of all the participants was classified in three different categories: (1) primary school, (2) secondary degree, (3) university degree. All the participants were also screened for their clinical status, with comprehensive general blood laboratory exams, including a urinary test for the use of narcotics. Subject characteristics can be found in Table 1A & B.
Table 1A.
Subject characteristics.
| Controls Mean (Std.) | ASD Mean (Std.) | Offenders Mean (Std.) | |
|---|---|---|---|
| Age | 29 (8) | 28 (6) | 31 (7) |
| n (males) | 19 | 20 | 19 |
| Education* | |||
| No graduation | 0 | 0 | 2 |
| Primary School | 0 | 3 | 11 |
| Second degree | 10 | 14 | 5 |
| University | 9 | 3 | 0 |
| Psychopathy | |||
| PCL-R | – | – | 26 (5) n = 17 |
| LSRP primary 1 | 22 (3) | 23 (4) | 31 (6) n = 15 |
| LSRP secondary* | 13 (3) | 17 (3) | 20 (3) n = 15 |
| Autism | |||
| AQ* | 11 (4) | 28 (6) | 20 (6) n = 16 |
| ADOS-2 | – | 11 (4) | – |
| Handedness | |||
| Right | 16 | 13 | 16 |
| Left | 3 | 7 | 3 |
PCL-R = Psychopathy Checklist-Revisited, LSRP = Levenson Self-Report Psychopathy Scale, AQ = Autism Spectrum Quotient, ADOS-2 = Autism Diagnostic Observation Schedule-Second Edition.
*Significant p < 0.05 differences between all groups in ONE-WAY ANOVA analyses.
Significant p < 0.05 differences between groups except between ASD and Healthy controls in ONE-WAY ANOVA analyses.
Table 1B.
Subject characteristics.
| Controls Number of participants | ASD Number of participants | Offenders Number of participants | |
|---|---|---|---|
| Current diagnoses: | |||
| Autism Spectrum disorder | – | 20 | – |
| Antisocial personality disorder | – | – | 16 |
| Borderline personality disorder | – | – | 4 |
| ADHD | – | 6 | 5 |
| Previous diagnoses: | |||
| Mood and Anxiety disorder | – | 8 | 4 |
| Substance abuse | – | – | 18 |
| Alcohol abuse | – | – | 13 |
| Medication: | |||
| SSRIs, SNRIs | – | 4 | 5 |
| Tricyclic antidepressants | – | – | 4 |
| Other antidepressants | – | 1 | – |
| Hypnotics and Anxiolytics | – | 3 | 6 |
| Neuroleptics | – | – | 6 |
| Stimulants | – | 2 | 1 |
| Betablockers | – | – | 2 |
| Antihistamines | – | 1 | 9 |
| Levothyroxine | – | 1 | 1 |
2.2. MR imaging and analysis
Structural MRI data were collected using a Phillips Ingenuity TF PET/MR 3 T scanner. We used a T1-weighted sequence (TR 9.8 ms, TE 4.6 ms, flip angle 7°, 250 mm FOV, 256 × 256 voxel matrix) with (1 mm3) isotropic voxel size. The T1 images were screened for structural abnormalities by a radiologist. We used SPM12 (Wellcome Trust Center for Imaging, London, UK, https://www.fil.ion.ucl.ac.uk/spm) to conduct the voxel-based morphometry, applying automated spatial normalization, tissue classification, and radio-frequency bias correction combined with the segmentation step. Medium affine regularization 0.01 was used with a 25 mm cut-off for spatial normalization. Following normalization and segmentation into grey matter and white matter, we incorporated a non-linear transformation step, as implemented in CAT12 toolbox, to take into account volume changes caused by spatial normalization. Similar non-linear transformation step was also used to account for the differences in total brain size across subjects. Finally, all the segmented, normalized, and modulated GM and WM images were smoothed using a Gaussian kernel of 8 mm full width at half maximum (FWHM).
In the full volume analysis, the normalized and smoothed VBM images were analyzed with SPM12 using General Linear Models. We firstly disclosed regional variations of grey matter volume among all groups using ANOVA F-tests, which was also used to find out group differences in the total intracranial volume and total grey matter volume. The further pair-wise comparisons between the groups were performed with T-tests in six different t-contrasts: 1) Offenders < ASD, 2) ASD < Controls, 3) Offenders < Controls, 4) ASD < Offenders, 5) Controls < ASD, 6) Controls < Offenders. The primary p-value threshold was set at < 0.01 with a cluster level extent threshold set at qFDR-corr < 0.05. To pinpoint the most robust differences between groups, we also assessed the effects using stricter statistical thresholds (primary p-value thresholds at 0.005 and 0.001 with cluster level extent threshold set at qFDR-corr < 0.05.). To better elucidate the between-groups differences, we also evaluated GMV in a priori regions of interest (ROI). The ROIs were set based on the previous anatomical imaging studies at frontal, temporal, insular and amygdala areas defined by the AAL atlas (Tzourio-Mazoyer et al., 2002) (supplement S4). As our earlier study showed fMRI differences in motor cortex function, we also evaluated an AAL-defined ROI at the precentral gyrus for any anatomical differences between these groups (Sun et al., 2021). In addition to the group comparisons, we conducted continuous analyses based on the LSRP and AQ questionnaire scores.
3. Results
3.1. Voxel-based morphometry
ANOVA (F-test) analyses revealed significant group differences in the right temporal pole, left fusiform cortex, left insular cortex, right culmen of vermis, left frontal pole, left inferior frontal gyrus, cingulate gyrus, left precuneus, and right precentral gyrus, with FDR-thresholded at p < 0.01. In the pair-wise comparisons the offenders versus ASD group, GMV was lower in the right temporal pole and left inferior frontal gyrus (Fig. 1A). These findings were still significant when the p-threshold was tightened all the way from 0.01 to 0.001 (supplement S5). AQ and LSRP scores had no correlation with GMV in any of these areas. In the ASD versus control group, GMV was lower in the right precentral cortex, left precuneus, right culmen of vermis, and the right cerebellum areas (Fig. 1B). These findings were still significant when the p-threshold was tightened from 0.01 to 0.001 (supplement S5). The AQ scores had a negative correlation with GMV in all of these areas, with an FDR threshold at p < 0.01. In the offender versus control group, GMV was found to be lower in the bilateral insular cortex, left frontal pole, right precentral cortex, cingulate gyrus, left inferior temporal gyrus, and left fusiform cortex (Fig. 1C). These findings were still significant when the p-threshold was tightened from 0.01 to 0.001 (supplement S5). The total LSRP and primary psychopathy domain scores had a negative correlation with GMV (FDR corrected at p < 0.01) only in the left frontal pole. Secondary psychopathy domain was not associated with GMV using the same threshold. There was an overlapping region in the precentral cortex where both offender and ASD groups showed lower GMV in comparison with controls (Fig. 2). When education, total intracranial volume, and age were used as covariates, the results remained essentially unchanged (FDR corrected, p < 0.01). We also found no differences in the total intracranial volume (p = 0.1553) and total grey matter volume (p = 0.0732) in ANOVA analyses. No significant voxels were found in the opposite contrasts (Offender > ASD, ASD > Controls, and Offender > Control). Stereotactic coordinates and t-values for the results can be found in supplement S4.
Fig. 1.
A. Brain regions with lower GMV in Offender versus ASD group. The data is FDR-thresholded at p < 0.01. Tpole = Temporal pole, IFGtriang = Inferior Frontal Gyrus, Triangular part B. Brain regions with lower GMV in ASD versus Controls. The data is FDR-thresholded at p < 0.01 PreCG = Precentral Gyrus, PCUN = Precuneus, CE = Cerebellum C. Brain regions with lower GMV in Offenders vs Controls. The data is FDR-thresholded at p < 0.01 PreCG = Precentral Gyrus, Fpole = Frontal pole, INS = Insula, Cg = Cingulate Gyrus, ITG = Inferior Temporal Gyrus, TFUS = Temporal Fusiform Gyrus. The range from FDR correction to T-value of 4 is used for better visualization of the variance in the scale.
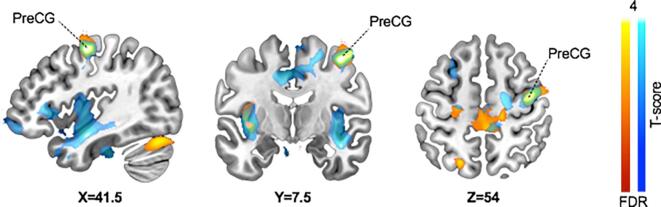
Fig. 2.
Overlapping (green) brain regions with lower grey matter volume in ASD (red) and Offender groups (blue) compared to control group. The data is FDR-thresholded at p < 0.01. PreCG = Precentral Gyrus. The range from FDR correction to T-value of 4 is used for better visualization of the variance in the scale. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. ROI analyses
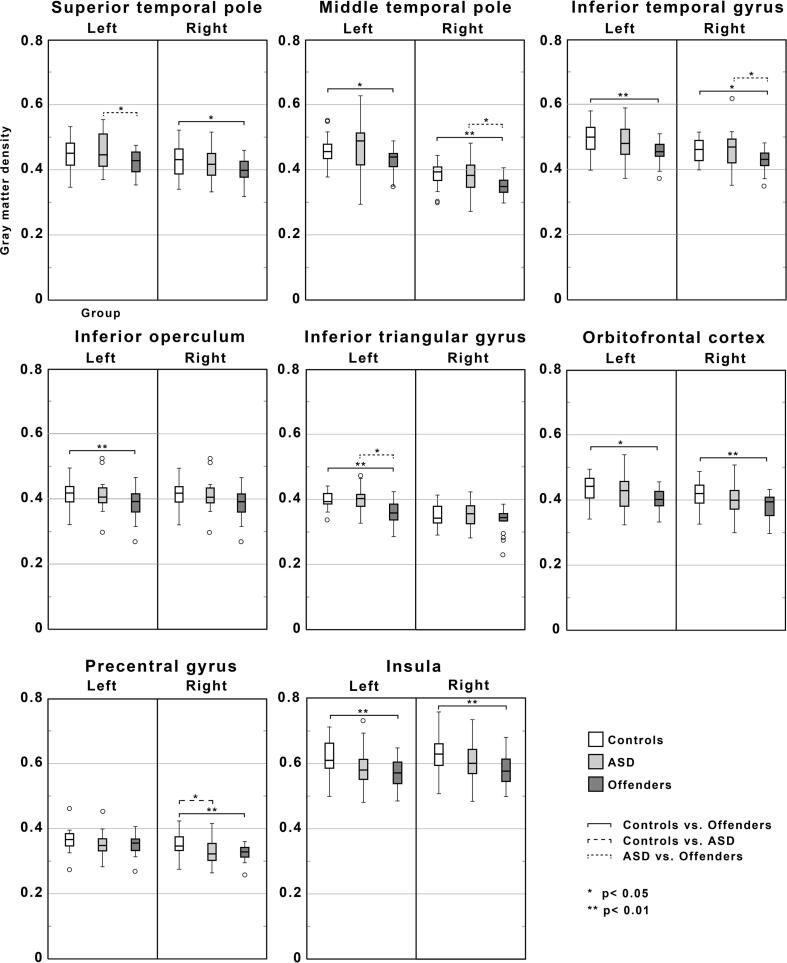
The ROI analyses of the temporal areas showed lower GMV in the right temporal inferior, right middle temporal pole, and left temporal pole superior regions of the offenders versus ASD group. In frontal areas, only the left frontal inferior triangular region of the offender group showed significantly lower GMV compared to the ASD group (Fig. 3). In the ASD group, only the precentral region showed lower GMV compared to controls, with no significant difference in the temporal, frontal or insular areas (Fig. 3). The offender group had significantly lower GMV of the bilateral temporal inferior, right temporal pole, left middle temporal pole, and right temporal pole superior regions compared with the control group. In the frontal areas, the offender group had lower GMV of the left frontal inferior operculum, left frontal inferior triangular, bilateral middle orbital, left frontal superior orbital, left frontal inferior orbital, left frontal medial orbital, left frontal middle, and bilateral frontal superior medial regions. There was also lower GMV in bilateral insula and right precentral gyrus regions of the offenders compared to control group (Fig. 3). The amygdala area did not show any significant differences between groups. Statisics for regional tests are shown in supplement S6.
Fig. 3.
Regional GMV in frontal, temporal, insular and precentral regions of interest (ROI) across the three groups. Asterisks denote statistically significant differences between groups.
4. Discussion
Our main finding was that violent offenders with psychopathic traits have lower GMV in frontotemporal social cognition areas when compared with ASD individuals, but compared to controls, they both present similar lower GMV in motor areas. In comparison with controls, the offender group had widespread lower grey matter volume in frontotemporal areas, while the ASD group showed lower volume in the precuneus and cerebellum. In addition to these divergent patterns, both ASD and offender groups had lower grey matter volume in the pre-central motor cortex when compared to the control group. Altogether these results suggest that ASD and psychopathy involve distinct patterns of structural alterations in the brain, the commonalities and shared patterns of lowered GMV may explain some of the shared phenotypical features of these groups. The ANOVA analyses revealed GMV differences in similar areas as found in the pair-wise T-test comparisons; the only exceptions were additional effects in the right cerebellar and right insular areas.
4.1. Lower grey matter volume values in the frontotemporal areas of violent offenders with psychopathy traits compared to autism spectrum disorder individuals
When comparing the offenders and ASD groups, we found significantly lower GMV values in the right temporal pole and left frontal inferior triangular gyrus of the offenders. The differences were robust and significant with a primary threshold of 0.001. Both of these areas are associated with social cognition and related to emotional aspects of empathy (Leigh et al., 2013, Sato and Uono, 2019). In the ROI analyses we also found lower GMV values for the offenders versus ASD group in regions involved in social cognition (superior temporal pole, middle and inferior temporal pole, and inferior triangular gyrus). In ASD the frontotemporal areas are impaired by overgrowth in childhood and accelerated degeneration in adulthood and older age (Courchesne et al., 2011a, Courchesne et al., 2011b, Zielinski et al., 2014). In psychopathy, these frontotemporal areas are already seen to be affected at neurodevelopmental precursor stages of childhood and subsequently throughout adulthood (Fairchild et al., 2019, Johanson et al., 2020, Rogers and De Brito, 2016). Along with these divergent growth trajectories, our findings suggest different pathological mechanisms behind the frontotemporal defects in these disorders, but this needs further research.
4.2. Similar lower grey matter volume areas in the motor cortex of violent offenders with psychopathy traits and autism spectrum disorder individuals
The full-volume analysis revealed lower GMV in right precentral cortex of both offender and ASD groups compared with the controls. Importantly, GMV in this region did not differ between these two groups (ASD and Offenders). This effect was also confirmed in the ROI analyses. Aberrant structure of this precentral area might suggest a similar impairment of motor functioning in both groups. Deficits in motor areas are consistently found in ASD (Fournier et al., 2010, Mosconi and Sweeney, 2015, Nebel et al., 2014), but there is also evidence of functional and anatomical deviancies of motor cortex in psychopathy (De Brito et al., 2021, Robinson and Bresin, 2014, Tillem et al., 2019). Similarly as mentioned before, there is evidence of abnormal growth trajectory of the ASD also in the motor cortex regions (Mostofsky et al., 2007, Riddle et al., 2017). As our ASD participants were all adults, our findings could reflect this abnormal age-related degeneration of the motor cortex. The functional organization of the motor cortex is also disrupted in children with ASD (Nebel et al., 2012), and significant and widespread alterations in motor performance have even been argued to precede the core symptoms of ASD (Fournier et al., 2010, Mosconi and Sweeney, 2015). There are also reports of increased motor and premotor cortex white matter volumes positively correlating with poor motor function in ASD (Mostofsky et al., 2007). A study of ASD individuals in a high security prison has also interestingly shown a positive correlation between motor clumsiness and antisocial lifestyle in PCL-R evaluations (Murphy, 2007). To our knowledge, there is only one study that has observed decreased grey matter values in the motor cortex of children with conduct problems and aggression (Fairchild et al., 2019). Slightly similar overlapping findings have earlier been seen in the cortical thickness of psychopathy and ASD traits compared to healthy controls but these were located more in the parietal cortex (Wallace et al., 2012). In our latest fMRI study with the same participants, we found uniting altered somatosensory and motor responses to emotional expressions in the ASD and offender group, reflecting functional problems in these same brain areas (Sun et al., 2021). Here our anatomical findings also suggest similar aberrancies in the motor areas in these two groups. Along with motor functioning problems, these findings could also be possibly related to issues of social cognition such as motor imagination, motor observation, and motor empathy (Bhattacharjee et al., 2021, Bons et al., 2013).
4.3. Lower grey matter volume values in ASD compared to controls
In the ASD group we found lower grey matter volume values in the right cerebellum, left precuneus, and right pre-motor cortex compared to controls. Comparable areas also had a negative correlation with AQ scores. The lower grey matter values of the cerebellum in the ASD group is a well-defined finding and can be seen as reflecting deficits in motor control but also social cognition in the ASD group (Moreno-Rius, 2019, Schmahmann, 2019, Van Overwalle et al., 2015). The precuneus is a part of the social cognition network and is interestingly linked to default-mode networks and sensing of self and others, which may be seen as reflecting deficits in the theory of mind of the ASD group (Cavanna and Trimble, 2006). All in all, the differences in the grey matter volume of social cognition areas in the ASD group were surprisingly small when compared to controls. This may be due to the phenotypical and genetic heterogeneity of the disorder and our relatively small sample size (Pelphrey et al., 2011).
4.4. Lower grey matter volume values in violent offenders with psychopathy traits compared to healthy controls
In an earlier study with the same offender and control participants, the main findings were lower grey matter volumes in the anterior insula, frontal cortex, and the sensorimotor areas in the violent offenders compared to controls (Nummenmaa et al., 2021). Following these earlier main findings, we found lower grey matter values in the bilateral insular cortex, left frontal pole, right precentral cortex, cingulate gyrus, left inferior temporal gyrus, and left fusiform cortex in the offender group compared to the control group. The total and primary psychopathy domain scores of the LSRP questionnaire correlated negatively with the left frontal pole area. All these results are comparable with earlier findings in systematic reviews of psychopathy (De Brito et al., 2021, Johanson et al., 2020). These whole brain findings in this group were also robust and still clear with a p-value threshold of 0.001. In the ROI analyses the offender group also showed vastly lower GMV values in the frontal, temporal, and insular areas as well as the precentral cortex areas.
4.5. Limitations
The groups were matched by their age and sex, but there were statistically significant differences in education between groups. Even though the education as a covariate did not show any essential changes in our results, we recommend formal IQ evaluations in future studies with similar comparison setups. Also, since we only included male subjects, our findings may not be generalized to females. Although there was no evidence of current alcohol or drug abuse in any participants as a confounding factor, the history of abuse in the offender group was still more common. The MR images and patient histories of our subjects did not indicate any brain injuries, but these should be considered as a more common confounding factor in violent offenders (Katzin et al., 2020). Since the screening questionnaires raised a justified suspicion of co-occurrent psychopathy and ASD in one of our participants (Rogers et al., 2006), we recommend more diagnostic ADOS-2 and PCL-R evaluations across all groups in future studies with similar comparison setups. Finally, the sample size was limited due to the complicated recruitment and measurement protocols in the prison environment. However, we used strict statistical significance and were nevertheless able to establish consistent differences between the groups.
4.6. Conclusion
We conclude that lower grey matter volume of the right temporal pole and left inferior frontal gyrus in violent offenders with high psychopathy traits is the most salient structural difference in comparison with ASD individuals. These brain areas have been linked with both psychopathy (Johanson et al., 2020) and ASD in previous studies of these social cognition deficits (Courchesne et al., 2011a, Courchesne et al., 2011b). Interestingly, these areas are linked with emotional aspects of empathy, which is seen as more defective in psychopathy (Blair, 2008, Fitzgerald, 2019, Jones et al., 2010, Lockwood et al., 2013). The ASD group showed surprisingly little grey matter loss in any of the areas typically associated with social cognition, possibly due to the phenotypical and genetical heterogeneity of the disorder. We also found decreased grey matter volume values in similar areas of the right precentral cortex in both groups. As motor function impairment is a well-known early onset deficit in the ASD group, there is only little knowledge of this issue on the psychopathy trait. Considering the nature of these disorders, the motor cortex finding could possibly be also more closely related to similar issues of social cognition such as motor imagination, motor observation, and motor empathy.
Declaration of competing interest
Jari Tiihonen has participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to his employing institution, and has been a consultant and/or advisor to and/or has received honoraria from: Eli Lilly, Evidera, Janssen-Cilag, Lundbeck, Orion, Otsuka, Mediuutiset, Sidera, and Sunovion.
CRediT authorship contribution statement
Tuomo Noppari: Formal analysis, Investigation, Writing – original draft, Visualization. Lihua Sun: Software, Formal analysis, Investigation, Data curation, Writing – review & editing. Lasse Lukkarinen: Formal analysis, Investigation. Vesa Putkinen: Software, Formal analysis, Investigation, Data curation, Writing – review & editing. Pekka Tani: Conceptualization, Validation, Resources, Writing – review & editing, Supervision. Nina Lindberg: Resources, Writing – review & editing, Funding acquisition. Emma Saure: Investigation, Writing – review & editing. Hannu Lauerma: Conceptualization, Validation, Resources, Writing – review & editing, Supervision. Jari Tiihonen: Conceptualization, Validation, Resources, Writing – review & editing, Supervision. Niina Venetjoki: Investigation, Resources, Writing – review & editing. Marja Salomaa: Investigation, Resources, Writing – review & editing. Päivi Rautio: Investigation, Resources. Jussi Hirvonen: Investigation, Resources. Juha Salmi: Conceptualization, Software, Validation, Resources, Writing – review & editing, Visualization, Supervision. Lauri Nummenmaa: Conceptualization, Methodology, Software, Validation, Resources, Data curation, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study was supported by the Academy of Finland (grants numbers 294897 and 332225, to L.N.), Valon Vuoksi Foundation (grants to L.S. and L.L.) and Turku Collegium for Science and Medicine, University of Turku (to L.S.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103116.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
References
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Malesand Females, Scientists and Mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/A:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S., Kashyap R., Abualait T., Annabel Chen S.-H., Yoo W.-K., Bashir S. The Role of Primary Motor Cortex: More Than Movement Execution. J. Mot. Behav. 2021;53:258–274. doi: 10.1080/00222895.2020.1738992. [DOI] [PubMed] [Google Scholar]
- Bishop D.V.M., Maybery M., Maley A., Wong D., Hill W., Hallmayer J. Using self-report to identify the broad phenotype in parents of children with autistic spectrum disorders: A study using the Autism-Spectrum Quotient. J. Child Psychol. Psychiatry. 2004;45(8):1431–1436. doi: 10.1111/j.1469-7610.2004.00849.x. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R. Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Q. J. Exp. Psychol. 2008;2006(61):157–170. doi: 10.1080/17470210701508855. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. Development of the social brain in adolescence. J. R. Soc. Med. 2012;105:111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen D.M., Hicks B.M., Krueger R.F., Patrick C.J., Iacono W.G. Psychopathic personality traits: heritability and genetic overlap with internalizing and externalizing psychopathology. Psychol. Med. 2005;35:637–648. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons D., van den Broek E., Scheepers F., Herpers P., Rommelse N., Buitelaaar J.K. Motor, Emotional, and Cognitive Empathy in Children and Adolescents with Autism Spectrum Disorder and Conduct Disorder. J. Abnorm. Child Psychol. 2013;41:425–443. doi: 10.1007/s10802-012-9689-5. [DOI] [PubMed] [Google Scholar]
- Braden B.B., Riecken C. Thinning faster? Age-related cortical thickness differences in adults with autism spectrum disorder. Res. Autism Spectr. Disord. 2019;64:31–38. doi: 10.1016/j.rasd.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley C.A., Schmitt W.A., Smith S.S., Newman J.P. Construct validation of a self-report psychopathy scale: does Levenson’s self-report psychopathy scale measure the same constructs as Hare’s psychopathy checklist-revised? Personal. Individ. Differ. 2001;31:1021–1038. doi: 10.1016/S0191-8869(00)00178-1. [DOI] [Google Scholar]
- Carper R.A., Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol. Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain J. Neurol. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Campbell K., Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Karns C., Davis H., Ziccardi R., Carper R., Tigue Z., Chisum H.J., Moses P., Pierce K., Lord C., Lincoln A., Pizzo S., Schreibman L., Haas R., Akshoomoff N., Courchesne R. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2011;76 doi: 10.1212/01.wnl.0000399191.79091.28. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Pierce K., Schumann C.M., Redcay E., Buckwalter J.A., Kennedy D.P., Morgan J. Mapping Early Brain Development in Autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- De Brito S.A., McDonald D., Camilleri J.A., Rogers J.C. Cortical and subcortical gray matter volume in psychopathy: A voxel-wise meta-analysis. J. Abnorm. Psychol. 2021;130:627–640. doi: 10.1037/abn0000698. [DOI] [PubMed] [Google Scholar]
- Ecker C., Spooren W., Murphy D.G.M. Translational approaches to the biology of Autism: false dawn or a new era? Mol. Psychiatry. 2013;18:435–442. doi: 10.1038/mp.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G., Hawes D.J., Frick P.J., Copeland W.E., Odgers C.L., Franke B., Freitag C.M., De Brito S.A. Conduct disorder. Conduct disorder primer. 2019;5(1) doi: 10.1038/s41572-019-0095-y. [DOI] [PubMed] [Google Scholar]
- Fernández M., Mollinedo-Gajate I., Peñagarikano O. Neural Circuits for Social Cognition: Implications for Autism. Neuroscience. 2018;370:148–162. doi: 10.1016/j.neuroscience.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. In: Empathy Study. Kondo M., Nikku B., editors. IntechOpen; 2019. Empathy: Autism and Psychopathy. [Google Scholar]
- Fournier K.A., Hass C.J., Naik S.K., Lodha N., Cauraugh J.H. Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. J. Autism Dev. Disord. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Howlin P. Outcome in Adult Life for more Able Individuals with Autism or Asperger Syndrome. Autism. 2000;4:63–83. doi: 10.1177/1362361300004001005. [DOI] [Google Scholar]
- Im D.S. Template to Perpetrate: An Update on Violence in Autism Spectrum Disorder. Harv. Rev. Psychiatry. 2016;24:14–35. doi: 10.1097/HRP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson M., Vaurio O., Tiihonen J., Lähteenvuo M. A Systematic Literature Review of Neuroimaging of Psychopathic Traits. Front. Psychiatry. 2020;10 doi: 10.3389/fpsyt.2019.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.P., Happé F.G.E., Gilbert F., Burnett S., Viding E. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. J. Child Psychol. Psychiatry. 2010;51:1188–1197. doi: 10.1111/j.1469-7610.2010.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junewicz A., Billick S.B. Conduct Disorder: Biology and Developmental Trajectories. Psychiatr. Q. 2020;91:77–90. doi: 10.1007/s11126-019-09678-5. [DOI] [PubMed] [Google Scholar]
- Jüriloo A., Lauerma H., Holmalahti T., Tyni S., Aarnio J., Viitanen P., Wuolijoki T., Mattila A., Lintonen T., Joukamaa M., Vartiainen H. Psychopathic traits in a representative sample of Finnish male prisoners. Nord J Psychiatry. 2014;68:117–122. doi: 10.3109/08039488.2013.780259. [DOI] [PubMed] [Google Scholar]
- Katzin S., Andiné P., Hofvander B., Billstedt E., Wallinius M. Exploring Traumatic Brain Injuries and Aggressive Antisocial Behaviors in Young Male Violent Offenders. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.507196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C., Murphy G.H. A Systematic Review of People with Autism Spectrum Disorder and the Criminal Justice System. J. Autism Dev. Disord. 2014;44:2717–2733. doi: 10.1007/s10803-014-2046-5. [DOI] [PubMed] [Google Scholar]
- Klapwijk E.T., Aghajani M., Colins O.F., Marijnissen G.M., Popma A., van Lang N.D.J., van der Wee N.J.A., Vermeiren R.R.J.M. Different brain responses during empathy in autism spectrum disorders versus conduct disorder and callous-unemotional traits. J. Child Psychol. Psychiatry. 2016;57:737–747. doi: 10.1111/jcpp.12498. [DOI] [PubMed] [Google Scholar]
- Lahnakoski J., Glerean E., Salmi J., Jääskeläinen I., Sams M., Hari R., Nummenmaa L. Naturalistic fMRI Mapping Reveals Superior Temporal Sulcus as the Hub for the Distributed Brain Network for Social Perception. Front. Hum. Neurosci. 2012;6:233. doi: 10.3389/fnhum.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H., Andershed H., Lichtenstein P. A genetic factor explains most of the variation in the psychopathic personality. J. Abnorm. Psychol. 2006;115:221–230. doi: 10.1037/0021-843X.115.2.221. [DOI] [PubMed] [Google Scholar]
- Leigh R., Oishi K., Hsu J., Lindquist M., Gottesman R.F., Jarso S., Crainiceanu C., Mori S., Hillis A.E. Acute lesions that impair affective empathy. Brain. 2013;136:2539–2549. doi: 10.1093/brain/awt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson M.R., Kiehl K.A., Fitzpatrick C.M. Assessing Psychopathic Attributes in a Noninstitutionalized Population. J. Pers. Soc. Psychol. 1995;68(1):151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Lockwood P.L., Bird G., Bridge M., Viding E. Dissecting empathy: high levels of psychopathic and autistic traits are characterized by difficulties in different social information processing domains. Front. Hum. Neurosci. 2013;7:760. doi: 10.3389/fnhum.2013.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S. Los Angeles; California: 2012. Autism Diagnostic Observation Schedule, (ADOS-2) Modules 1–4. [Google Scholar]
- Moreno-Rius J. Is there an “antisocial” cerebellum? Evidence from disorders other than autism characterized by abnormal social behaviours. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;89:1–8. doi: 10.1016/j.pnpbp.2018.08.025. [DOI] [PubMed] [Google Scholar]
- Mosconi M.W., Sweeney J.A. Sensorimotor dysfunctions as primary features of autism spectrum disorders. Sci. China Life Sci. 2015;58(10):1016–1023. doi: 10.1007/s11427-015-4894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky S.H., Burgess M.P., Gidley Larson J.C. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Murphy D. Hare Psychopathy Checklist Revised profiles of male patients with Asperge’s syndrome detained in high security psychiatric care. J. Forensic Psychiatry Psychol. 2007;18:120–126. doi: 10.1080/14789940601014777. [DOI] [Google Scholar]
- Nebel M.B., Joel S.E., Muschelli J., Barber A.D., Caffo B.S., Pekar J.J., Mostofsky S.H. Disruption of functional organization within the primary motor cortex in children with autism. Hum. Brain Mapp. 2012;35:567–580. doi: 10.1002/hbm.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel M.B., Eloyan A., Barber A.D., Mostofsky S.H. Precentral gyrus functional connectivity signatures of autism. Front. Syst. Neurosci. 2014;8:80. doi: 10.3389/fnsys.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L., Calder A.J. Neural mechanisms of social attention. Trends Cogn. Sci. 2009;13:135–143. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Lukkarinen L., Sun L., Putkinen V., Seppälä K., Karjalainen T., Karlsson H.K., Hudson M., Venetjoki N., Salomaa M., Rautio P., Hirvonen J., Lauerma H., Tiihonen J. Brain Basis of Psychopathy in Criminal Offenders and General Population. Cereb. Cortex bhab072. 2021;31(9):4104–4114. doi: 10.1093/cercor/bhab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Nions E., Tick B., Rijsdijk F., Happé F., Plomin R., Ronald A., Viding E., Jones A. Examining the Genetic and Environmental Associations between Autistic Social and Communication Deficits and Psychopathic Callous-Unemotional Traits. PLoS ONE. 2015;10(9):e0134331. doi: 10.1371/journal.pone.0134331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Nions E., Sebastian C.L., McCrory E., Chantiluke K., Happé F., Viding E. Neural bases of Theory of Mind in children with autism spectrum disorders and children with conduct problems and callous-unemotional traits. Dev. Sci. 2014;17(5):786–796. doi: 10.1111/desc.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnozzi A.M., Conti E., Calderoni S., Fripp J., Rose S.E. A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2018;71:68–82. doi: 10.1016/j.ijdevneu.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Shultz S., Hudac C.M., Wyk B.C.V. Constraining Heterogeneity: The Social Brain and its Development in Autism Spectrum Disorder. J. Child Psychol. Psychiatry. 2011;52:631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Antisocial Personality as a Neurodevelopmental Disorder. Annu. Rev. Clin. Psychol. 2018;14:259–289. doi: 10.1146/annurev-clinpsy-050817-084819. [DOI] [PubMed] [Google Scholar]
- Riddle K., Cascio C.J., Woodward N.D. Brain structure in autism: a voxel-based morphometry analysis of the Autism Brain Imaging Database Exchange (ABIDE) Brain Imaging Behav. 2017;11:541–551. doi: 10.1007/s11682-016-9534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., Bresin K. Higher Levels of Psychopathy Predict Poorer Motor Control: Implications for Understanding the Psychopathy Construct. J. Psychopathol. Behav. Assess. 2014;36:201–210. doi: 10.1007/s10862-013-9388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.C., De Brito S.A. Cortical and Subcortical Gray Matter Volume in Youths With Conduct Problems: A Meta-analysis. JAMA Psychiatry. 2016;73:64–72. doi: 10.1001/jamapsychiatry.2015.2423. [DOI] [PubMed] [Google Scholar]
- Rogers J., Viding E., James Blair R., Frith U., Happé F. Autism spectrum disorder and psychopathy: shared cognitive underpinnings or double hit? Psychol. Med. 2006;36:1789–1798. doi: 10.1017/S0033291706008853. [DOI] [PubMed] [Google Scholar]
- Sato W., Uono S. The atypical social brain network in autism: advances in structural and functional MRI studies. Curr. Opin. Neurol. 2019;32:617–621. doi: 10.1097/WCO.0000000000000713. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. The cerebellum and cognition. Neurosci. Lett. 2019;688:62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Siponmaa L., Kristiansson M., Jonson C., Nydén A., Gillberg C. Juvenile and young adult mentally disordered offenders: the role of child neuropsychiatric disorders. J. Am. Acad. Psychiatry Law. 2001;29:420–426. [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B.W., Gibbon M., First M.B. The Structured Clinical Interview for DSM-III-R (SCID): I: History, Rationale, and Description. Arch. Gen. Psychiatry. 1992 doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Sun, L., Lukkarinen, L., Noppari, T., Nazari-Farsani, S., Putkinen, V., Seppälä, K., Hudson, M., Tani, P., Lindberg, N., Karlsson, H.K., Hirvonen, J., Salomaa, M., M, N.V., Lauerma, H., Tiihonen, J., Nummenmaa, L., 2021. Aberrant motor contagion of emotions in psychopathy and high-functioning autism. https://doi.org/10.1101/2021.07.05.450842. [DOI] [PMC free article] [PubMed]
- Tick B., Bolton P., Happé F., Rutter M., Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J. Child Psychol. Psychiatry. 2016;57:585–595. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J., Koskuvi M., Lähteenvuo M., Virtanen P.L.J., Ojansuu I., Vaurio O., Gao Y., Hyötyläinen I., Puttonen K.A., Repo-Tiihonen E., Paunio T., Rautiainen M.-R., Tyni S., Koistinaho J., Lehtonen Š. Neurobiological roots of psychopathy. Mol. Psychiatry. 2020;25:3432–3441. doi: 10.1038/s41380-019-0488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillem S., Brennan G., Wu J., Mayes L., Baskin-Sommers A. Alpha response reveals attention abnormalities in psychopathy. Personal. Disord. Theory Res. Treat. 2019;10:291–296. doi: 10.1037/per0000314. [DOI] [PubMed] [Google Scholar]
- Tuvblad C., Bezdjian S., Raine A., Baker L.A. The Heritability of Psychopathic Personality in 14 to 15 year Old Twins: A Multi-Rater, Multi-Measure Approach. Psychol. Assess. 2014;26:704–716. doi: 10.1037/a0036711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvblad C., Wang P., Bezdjian S., Raine A., Baker L.A. Psychopathic personality development from ages 9 to 18: Genes and environment. Dev. Psychopathol. 2016;28:27–44. doi: 10.1017/S0954579415000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., D’aes T., Mariën P. Social cognition and the cerebellum: A meta-analytic connectivity analysis. Hum. Brain Mapp. 2015;36:5137–5154. doi: 10.1002/hbm.23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.L., Dankner N., Kenworthy L., Giedd J.N., Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–3754. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.L., Shaw P., Lee N.R., Clasen L.S., Raznahan A., Lenroot R.K., Martin A., Giedd J.N. Distinct Cortical Correlates of Autistic versus Antisocial Traits in a Longitudinal Sample of Typically Developing Youth. J. Neurosci. 2012;32:4856–4860. doi: 10.1523/JNEUROSCI.6214-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury-Smith M., Scherer S.W. Progress in the genetics of autism spectrum disorder. Dev. Med. Child Neurol. 2018;60:445–451. doi: 10.1111/dmcn.13717. [DOI] [PubMed] [Google Scholar]
- Zielinski B.A., Prigge M.B.D., Nielsen J.A., Froehlich A.L., Abildskov T.J., Anderson J.S., Fletcher P.T., Zygmunt K.M., Travers B.G., Lange N., Alexander A.L., Bigler E.D., Lainhart J.E. Longitudinal changes in cortical thickness in autism and typical development. Brain J. Neurol. 2014;137:1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.