Abstract
Although the role of ethylene in tomato (Solanum lycopersicum) fruit ripening has been intensively studied, its role in tomato fruit growth remains poorly understood. In addition, the relationship between ethylene and the developmental factors NON-RIPENING (NOR) and RIPENING INHIBITOR (RIN) during ripening is under debate. Here, we carried out comprehensive genetic analyses of genome-edited mutants of tomato ETHYLENE INSENSITIVE 2 (SlEIN2), four EIN3-like genes (SlEIL1–4), and three EIN3 BINDING F-box protein genes (SlEBF1–3). Both slein2-1 and the high-order sleil mutant (sleil1 sleil2 sleil3/SlEIL3 sleil4) showed reduced fruit size, mainly due to decreased auxin biosynthesis. During fruit maturation, slein2 mutants displayed the complete cessation of ripening, which was partially rescued by slebf1 but not slebf2 or slebf3. We also discovered that ethylene directly activates the expression of the developmental genes NOR, RIN, and FRUITFULL1 (FUL1) via SlEIL proteins. Indeed, overexpressing these genes partially rescued the ripening defects of slein2-1. Finally, the signal intensity of the ethylene burst during fruit maturation was intimately connected with the progression of full ripeness. Collectively, our work uncovers a critical role of ethylene in fruit growth and supports a molecular framework of ripening control in which the developmental factors NOR, RIN, and FUL1 act downstream of ethylene signaling.
Low-level ethylene production promotes tomato fruit growth by modulating auxin biosynthesis/signaling, and the ethylene burst induces fruit ripening by activating fruit development-related genes.
IN A NUTSHELL.
Background: Fruit development is an intriguing evolutionary strategy that functions in both seed protection and seed dispersal. Tomato (Solanum lycopersicum) has long been used as a model system to uncover mechanisms underlying fruit growth and ripening. The phytohormone ethylene serves as a central regulator of fruit ripening, but whether and how ethylene regulates tomato fruit growth remains elusive. The naturally occurring mutants that initially pointed to the roles of the transcription factors (TFs) RIN and NOR in fruit maturation show complete ripening cessation, but clustered regularly interspaced short palindromic repeats/CRISPR associated9 (CRISPR/Cas9) generated rin and nor knockout mutants display much milder ripening inhibition, suggesting that the current model of the roles of RIN and NOR and their relationships with the ripening hormone ethylene need to be re-evaluated.
Question: Does ethylene regulate tomato fruit growth? Negatively or positively? What is the relationship between ethylene and ripening-related developmental factors? Does ethylene function downstream or upstream of these factors?
Findings: We uncovered four major findings: (1) ethylene positively regulates fruit growth by inducing the accumulation of seed-derived auxin; (2) multiple ETHYLENE INSENSITIVE3-Like proteins (EILs) and EIN3-binding F-box proteins (EBFs) play both redundant and divergent roles in tomato; (3) during ripening, the ethylene signaling pathway involving ETHYLENE INSENSITIVE2 (EIN2)-EBF1-EILs directly activates the transcription of developmental genes RIN, NOR, and FUL1; and (4) Genetic evidence showed that ethylene signaling intensity is intimately associated with the efficiency of achieving full ripeness, which may explain why climacteric fruits such as tomato usually exhibit an ethylene burst during ripening.
Next steps: As overexpressing RIN, NOR, or FUL1 only partially rescued the fruit ripening defect of slein2, we plan to examine whether this defect could be fully restored by simultaneously overexpressing RIN, NOR, FUL1, and/or other TF genes. Moreover, the elaborate mechanism integrating ethylene and auxin signaling during fruit growth requires further investigation.
Introduction
The gaseous phytohormone ethylene plays fundamental roles in various developmental processes and stress responses. Ethylene perception and the downstream signal transduction cascade have been well established in Arabidopsis thaliana (Binder, 2020). In the absence of ethylene, ethylene receptors interact with the endoplasmic reticulum (ER)-anchored Raf-like serine/threonine kinase CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1; Kieber et al., 1993; Gao et al., 2003), which then phosphorylates and inactivates ETHYLENE INSENSITIVE2 (EIN2; Ju et al., 2012; Qiao et al., 2012), a positive regulator of ethylene responses. EIN2 has a central position in relaying the ethylene signal, because loss of EIN2 function in Arabidopsis eliminates all ethylene responses (Alonso et al., 1999). EIN2 protein stability is regulated by two F-box proteins, EIN2-TARGETING PROTEIN1 (ETP1) and ETP2, via ubiquitin/26S proteasome-mediated degradation (Qiao et al., 2009). In the presence of ethylene, both ethylene receptors and CTR1 are inactivated, and the C-terminus of EIN2 becomes dephosphorylated and proteolytically cleaved by an unknown enzyme (Ju et al., 2012; Qiao et al., 2012; Wen et al., 2012).
The released C-terminal fragment of EIN2 then translocates from the ER to the nucleus to activate the transcription factors EIN3 and EIN3-LIKE1 (EIL1; Ju et al., 2012; Qiao et al., 2012; Wen et al., 2012), or to the cytoplasmic processing-body to repress the translation of EIN3-BINDING F-BOX PROTEIN1 (EBF1) and EBF2 mRNA (Li et al., 2015; Merchante et al., 2015). The TFs EIN3/EIL1 are master regulators of ethylene responses in Arabidopsis (Chao et al., 1997). Although EIN3 and EIL1 largely overlap in their functions, they also have distinct roles: EIN3 mainly regulates ethylene responses in seedlings, while EIL1 plays a greater role during leaf expansion and stem elongation in adult plants (An et al., 2010). EIN3 and EIL1 are both degraded via the 26S proteasome by the F-box proteins EBF1 and EBF2 in the absence of an ethylene signal (Guo and Ecker, 2003; Potuschak et al., 2003). As with EIN3/EIL1, these two EBF proteins are engaged in both redundant and specific functions. EBF1 mainly contributes to the initial response to ethylene, whereas EBF2 is more significantly involved in later stages of the ethylene response and the resumption of growth once ethylene is removed (Binder et al., 2007). Interestingly, EBF2 transcript levels are directly induced by EIN3, forming a negative feedback regulatory loop between EIN3 and EBFs (Konishi and Yanagisawa, 2008).
Tomato (Solanum lycopersicum) is a global commercial crop and an ideal model plant to study the molecular basis of fruit quality and ripening control (Giovannoni, 2004). Ethylene serves as the core regulator of climacteric fruit ripening, and many studies have demonstrated that manipulating ethylene biosynthesis and signaling genes in tomato significantly influences fruit maturation (Grierson, 2013). Climacteric fruits like tomato have two distinct ethylene systems: while the ethylene concentrations associated with system-1 are generally low and detected in all tissues, system-2 ethylene concentrations rise 100- to 300-fold specifically during ripening (McMurchie et al., 1972; Li et al., 2019). System-1 is the basal low level of ethylene production in preclimacteric fruits and vegetative tissues and nonclimacteric fruits. System-2 is the high-level ethylene production observed during ripening in climacteric fruits and in certain senescent flowers (Oetiker and Yang, 1995). Remarkably, system-1 is known to be ethylene autoinhibitory, whereas system-2 is autocatalytic (Oetiker and Yang, 1995).
As most studies in tomato have focused on the role of system-2 ethylene in ripening control, our understanding of system-1 ethylene in tomato fruit remains limited. The tomato genome encodes more ethylene biosynthesis and signaling components than that of Arabidopsis, including 13 1-AMINOCYCLOPROPANE-1-CARBOXYLATE (ACC) SYNTHASE (SlACSs), 6 ACC OXIDASE (SlACOs), 7 ETHYLENE RESPONSE (SlETR) receptors, 4 SlCTRs, 1 SlEIN2, 6 SlEILs, 4 SlEBFs, and 77 ETHYLENE RESPONSE FACTORS (SlERFs; Liu et al., 2015), pointing to more flexibility during ethylene signaling in tomato. Indeed, ethylene negatively regulates fruit-set, as demonstrated by the formation of parthenocarpic fruits in the gain-of-function allele Sletr1-1 of the tomato ethylene receptor (Shinozaki et al., 2015).
Cloning of genes in spontaneous ripening-deficient tomato mutants identified the key TFs NON-RIPENING (NOR) and RIPENING-INHIBITOR (RIN). These TFs, belonging to the NAC (NAM, ATAF, CUC) and MADS (MCM1, AG, DEF, SRF)-box TF families, respectively, control ripening signal induction and ethylene biosynthesis (Vrebalov et al., 2002; United States Patent, No. US6762347B1). In addition to the loss of system-2 ethylene bursts in these two mutants, ChIP-chip and electrophoretic mobility shift assays (EMSAs) revealed the binding capacity of RIN and NOR to a set of ethylene-related genes such as the ethylene biosynthesis genes SlACS2 and SlACS4 (Fujisawa et al., 2013; Gao et al., 2020), the ethylene receptor gene SlETR3 (also named NR, Never-Ripe; Fujisawa et al., 2013), and several SlERFs genes (Lü et al., 2018), supporting the notion that NAC and MADS TFs act upstream of ethylene in the ripening regulatory network.
However, this model has been complicated by the following issues: (1) exogenous application of ethylene does not restore fruit ripening in nor or rin (Giovannoni, 2007); (2) several genome-edited null alleles of ripening-related TF genes exhibit moderate ripening phenotypes, including mutants of NOR, RIN, and FUL1/2 (Ito et al., 2017; Wang et al., 2019); (3) in contrast to spontaneous mutants in genes encoding developmental TFs, loss-of-function null mutants with completely blocked ethylene biosynthesis or signaling have yet to be identified. The Nr and Green-ripe mutants are gain-of-function mutants of SlETR3 and REVERSION-TO-ETHYLENE SENSITIVITY1 (SlRTE1), respectively (Wilkinson et al., 1995; Barry and Giovannoni, 2006), while yellow-fruited tomato1 is a knockdown allele of SlEIN2 (Gao et al., 2016). Hence, the relationship between ethylene and ripening-related TFs needs to be revisited.
In this study, we assessed the role of ethylene signaling throughout the lifecycle of tomato by analyzing loss-of-function mutants in the key ethylene signaling component SlEIN2, the EIL master TF genes SlEIL1–4, and SlEBF1–3, encoding negative regulators of ethylene signaling. Notably, both fruit size and fruit ripening were severely inhibited in slein2 null mutants. Loss-of-function of SlEIN2 resulted in impaired seed development, leading to compromised auxin biosynthesis and signaling. Consequently, the cell division mediated by auxin during fruit early growth was inhibited such that slein2 fruits were smaller than the wild-type (WT). During fruit ripening, ethylene mediates a largely linear transcriptional cascade, wherein the developmental TFs NOR, RIN, and FUL1 act downstream of ethylene signaling. Finally, we showed that the variation in ethylene signal intensity conferred by system-2 ethylene was closely associated with the timing of ripening initiation and the achievement of full ripeness, which may explain why climacteric fruits such as tomato usually exhibit an ethylene burst during fruit ripening.
Results
Generation and phenotypic characterization of tomato slein2 null alleles
An exploration of the full repertoire of ethylene functions in tomato requires genetic materials with fully blocked ethylene signaling. Nr was previously identified as ethylene-insensitive mutant with completely inhibited fruit ripening (Lanahan et al., 1994). However, the inhibited strong ripening phenotype was only observed in tomato cultivar Pearson, while Nr mutants in the cultivar Ailsa Craig (AC) and Micro-Tom (MT) backgrounds showed much moderate ripening inhibition (Li et al., 2019), suggesting that Nr is not generally applicable for studying ethylene function in tomato, which has a great diversity of cultivars worldwide.
The tomato genome possesses a single copy of SlEIN2 (Sloyc09g007870; Liu et al., 2015). We proposed that, like Arabidopsis EIN2 null mutants (Alonso et al., 1999), loss-of-function of this single copy of SlEIN2 would result in completely ethylene-insensitive tomato plants. Therefore, we sought to generate loss-of-function mutants of SlEIN2 with the help of the CRISPR/Cas9 system. To this end, we designed two single-guide RNAs (sgRNAs) targeting the first exon of SlEIN2 (Figure 1A). We then cloned the sgRNAs in a binary vector harboring plant codon-optimized Cas9 (Hu et al., 2019), followed by transformation of tomato cv. “Micro-Tom.” Sequencing of PCR (Polymerase Chain Reaction) amplicons of each independent transformant (T0) and their progenies (T1) led to the identification of two putative null mutant alleles, slein2-1 and slein2-2, each carrying a 1-bp insertion causing a frameshift and premature termination of translation (Figure 1A). We selected slein2-1 for further analysis, as both alleles were completely insensitive to ethylene treatment and exhibited identical phenotypes (Supplemental Figure S1, A–C). Importantly, we generated a transgenic line expressing the SlEIN2 coding region driven by the SlEIN2 promoter in WT (SlEIN2pro:SlEIN2-HA-YFP) and crossed the line with the slein2-1 mutant. This transgenic line fully complemented the petal abscission, fruit ripening, and fruit size shown by slein2-1, demonstrating that these phenotypes are caused by the loss of SlEIN2 function (Supplemental Figure S2A). We detected both full-length and C-terminal fragments of SlEIN2-HA-YFP protein in the transgenic fruits (Supplemental Figure S2B). We also observed the nuclear localization of the SlEIN2-HA-YFP protein when transiently expressed in Nicotiana benthamiana leaves upon ACC treatment (Supplemental Figure S2C).
Figure 1.
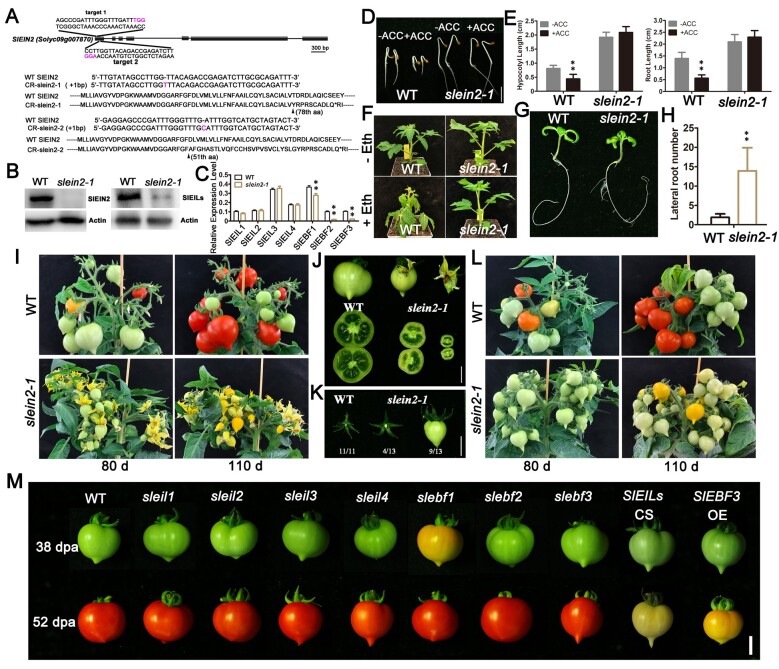
CRISPR/Cas9-mediated mutagenesis of ethylene signaling genes. A, Schematic diagram of the indicated SlEIN2 gene (drawn to scale). Black bars represent exons and lines represent introns. The sequences of the regions targeted by the sgRNAs are shown in the expanded regions. In these sequences, the sgRNAs-target sequences are underlined, and the PAM sequences are colored in purple. Sequences of the isolated mutant alleles are aligned to the WT. The in/del nucleotides are colored. Arrowheads indicate the sites of frameshift in the translated mutant proteins. B, Immunoblotting assay showing the levels of endogenous SlEIN2 and SlEILs proteins in WT and slein2-1 fruits at the MG stage. Actin was used as an internal protein control. C, Relative mRNA levels of four SlEIL (SlEIL1-SlEIL4) genes and three SlEBF (SlEBF1-SlEBF3) genes in WT and slein2-1 fruits at the MG stage. Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. D and E, Triple response assay and statistical analysis. D, WT and slein2-1 seedlings were grown on 1/2 MS medium with or without 5-μM ACC for 3 days in the dark (bar = 1 cm). E, Hypocotyl and root length of 3-day-old seedlings. Error bars indicate sd (n = 24), **P < 0.01, Student’s t test. F, Epinasty assay of WT and slein2-1 plants. One-month-old plants were incubated in 50 ppm ethylene for 24 h. No symptom of epinasty was observed in slein2-1 plants. G and H, Lateral root number of WT and slein2-1. G, 15-day-old seedlings grown on 1/2 MS medium. H, Statistics of lateral root number of WT and slein2-1 seedlings. Error bars indicate sd (n = 24), **P < 0.01, Student’s t test. I, Phenotypes of 80-day-old and 110-day-old WT and slein2-1 plants under natural pollination conditions. J, Representative WT and slein2-1 30-dpa fruits and their respective cross sections (Bar = 1 cm). K, Pollination-independent fruit development of slein2-1. WT and slein2-1 flowers were emasculated at −1 dpa. All 11 emasculated WT ovaries aborted, while 9 of 13 emasculated slein2-1 flowers produced parthenocarpic fruit (Bar = 1 cm). L, Phenotypes of 80-day-old and 110-day-old WT and slein2-1 plants under manual pollination conditions. To eliminate the impact of facultative parthenocarpic fruit, manual pollination was performed for every single WT and slein2-1 flower at the anthesis stage. M, Representative 38 dpa and 52 dpa fruits of WT, sleil1, sleil2, sleil3, sleil4, slebf1, slebf2, slebf3, SlEILs CS, and SlEBF3 OE (Bar = 1 cm).
SlEIN2 was undetectable in mature green (MG) stage fruits of the slein2-1 mutant, and the levels of its downstream SlEIL proteins were also dramatically reduced (Figure 1B). As expected, the transcript levels of SlEIL1–4 were unchanged, while those of SlEBF1–3 were markedly reduced in the mutant (Figure 1C). Compared to WT, etiolated slein2-1 seedlings had longer hypocotyls/roots and displayed insensitivity to treatment with the ethylene precursor ACC (Figure 1, D and E). Symptoms of petiole epinasty did not appear in slein2-1 when treated with ethylene gas, in contrast to WT (Figure 1F). In addition, many more lateral roots developed in light-grown slein2-1 seedlings versus the WT (Figure 1, G and H), which is in agreement with the negative role of ethylene in lateral root development in Arabidopsis (Negi et al., 2008). slein2-1 petals remained attached to the fruit even at the ripening stage, and the fruits failed to turn red (Figure 1I). We observed ∼70% parthenocarpic fruits in slein2-1 (Figure 1, J and K;Supplemental Figure S3D), which is consistent with prior findings for the gain-of-function Sletr1-1 mutant (Shinozaki et al., 2015).
Notably, fruit number per plant was much higher in slein2-1 compared to WT (Supplemental Figure S3A), in which the fruit-set rate in slein2-1 was arbitrarily set to 100% compared to ∼48% in WT (Supplemental Figure S3B). However, the greater number of fruits per plant was largely offset by the high frequency of facultative parthenocarpic fruits in slein2-1, resulting in greatly reduced fruit diameter and seed number per fruit (Supplemental Figure S3, C and E). This led to a much lower fruit yield (in weight) per plant in slein2-1 compared to WT, although the compromised fruit yield was recovered by manual pollination (Figure 1L;Supplemental Figure S3F). Notably, during vegetative growth, there was no visible difference between WT and slein2-1 plants (Supplemental Figure S4A). During later reproductive growth stages, WT and slein2-1 had comparable inflorescence architecture (Supplemental Figure S4B). In line with the phenotype of the gain-of-function Sletr1-1 mutant (Shinozaki et al., 2015), the protruded stigma in slein2-1 flowers indicated that precocious fruit set occurred prior to anthesis, preventing successful pollination and fertilization (Supplemental Figure S4C). Moreover, leaf and petal senescence, as well as fruit ripening, were delayed or blocked in the slein2-1 mutant (Supplemental Figure S4, D–F). These results suggest that the genome-edited slein2-1 mutant is indeed a loss-of-function allele with completely impaired ethylene signaling.
Redundant and divergent functions of tomato EIL and EBF proteins
EIL and EBF proteins play redundant roles in tomato fruit ripening (Tieman et al., 2001; Yokotani et al., 2009; Yang et al., 2010). To gain a more detailed understanding of ethylene function in tomato, we also generated loss-of-function mutants for SlEIL1–4 and SlEBF1–3 by CRISPR/Cas9-mediated mutagenesis, designated sleil1, sleil2, sleil3, sleil4, slebf1, slebf2, and slebf3 (Supplemental Figure S5A). We did not edit SlEIL5, SlEIL6, or SlEBF4 because these three genes are expressed at very low levels and are thus thought to exert rather limited functions during fruit growth and ripening (Liu et al., 2015). Among all single mutants, only slebf1 exhibited accelerated ripening, whereas all other mutants showed normal growth and developmental phenotypes (Figure 1M). These knockout (KO) alleles provides more explicit information about the distinctive functions of tomato SlEIL and SlEBF than previously generated knockdown mutants. For instance, silencing of SlEBF2 in tomato fruits by virus-induced gene silencing conferred ripening acceleration (Yang et al., 2010), and silencing of SlEBF3 via RNA-interference (RNAi) generated plants with strong leaf epinasty, a typical constitutive ethylene response (Deng et al., 2018).
Next, we generated double, triple, and quadruple mutants by genetic crosses. As SlEIL1 (Solyc06g073720) and SlEIL4 (Solyc06g073730) are two duplicated genes separated by less than 10 kb (https://solgenomics.net/), it is impossible to obtain the sleil1 sleil4 double mutant through genetic crossing. Therefore, we utilized CRISPR/Cas9 to mutagenize both genes simultaneously by designing sgRNAs targeting a shared sequence (Supplemental Figure S5B). We thus obtained the following genotypes: sleil1 sleil2, sleil1 sleil3, sleil1 sleil4, sleil2/SlEIL2 sleil3, sleil2 sleil4, sleil3 sleil4, slebf1 slebf2, slebf1 slebf3, slebf2 slebf3, sleil1 sleil2/SlEIL2 sleil3, sleil1 sleil2 sleil4, sleil1 sleil3 sleil4, sleil2/SlEIL2 sleil3 sleil4, slebf1/SlEBF1 slebf2 slebf3, slebf1 slebf2/SlEBF2 slebf3, slebf1 slebf2 slebf3/SlEBF3, sleil1 sleil2/SlEIL2 sleil3 sleil4, and sleil1 sleil2 sleil3/SLEIL3 sleil4 (Supplemental Figure S6A). We were unable to obtain homozygous mutants for the sleil2 sleil3, sleil1 sleil2 sleil3, sleil2 sleil3 sleil4, and sleil1 sleil2 sleil3 sleil4 combinations (Supplemental Figure S6A), suggesting that sleil2 sleil3 led to embryo lethality. Similarly, the slebf1 slebf2 slebf3 triple mutant also led to embryo lethality. In accordance with these observations, in silico mining of the Tomato Expression Atlas pipeline (SGN-TEA, http://tea.solgenomics.net/) and the TomExpress platform (http://tomexpress.toulouse.inra.fr/) indicated that SlEIL2 and SlEBF3 are preferentially expressed in seeds (Supplemental Figure S7, A–C), supporting the distinctive roles of individual components in regulating tomato embryo/seed development.
We did not observe substantial ripening cessation in the double or triple sleil mutant combinations (Supplemental Figure S6A). However, a co-suppression line for four SlEIL genes (SlEILs CS) and a line overexpressing SlEBF3 (SlEBF3 OE) did show severe defects in ripening (Figure 1M), which is consistent with the results of previous studies in which SlEIL or SlEBF genes were manipulated in tomato (Tieman et al., 2001; Yokatani et al., 2009; Deng et al., 2018). The above data thus support the notion that the four SlEIL genes play highly redundant roles, as also evidenced by the finding that plants with only one functional copy of SlEIL2 (sleil1 sleil2/SlEIL2 sleil3 sleil4) or SlEIL3 (sleil1 sleil2 sleil3/SLEIL3 sleil4) displayed relatively normal petal abscission and eventually produced ripe fruits (Supplemental Figure S6A). We also tested the sensitivity of all genotypes to the classical ethylene triple response using etiolated seedlings, which revealed weak ethylene insensitivity for sleil1, sleil4, sleil1 sleil2, sleil1 sleil3, sleil2 sleil4, and sleil3 sleil4 in terms of hypocotyl elongation, but stronger ethylene insensitivity for sleil1 sleil4, sleil1 sleil3 sleil4, SlEIL CS, and SlEBF3 OE (Supplemental Figure S6, B and C). In contrast, slebf1 slebf2, slebf1 slebf3, and slebf2 slebf3 etiolated seedlings showed a constitutive ethylene response in the absence of ACC and were hypersensitive to ACC (Supplemental Figure S6, B and C).
We next crossed slein2-1 with the three individual slebf mutants and found that slebf1, but not slebf2 or slebf3, fully rescued the petal abscission and partially rescued fruit ripening of the slein2 mutant (Supplemental Figure S6A). This finding confirms the specific role of SlEBF1, and it also demonstrates that slebf1 is genetically epistatic to slein2. Interestingly, the sleil1 sleil2/SlEIL2 sleil3 sleil4 higher-order mutant had fruits of similar size to WT but with a visible deficiency in fruit ripening, while the sleil1 sleil2 sleil3/SlEIL3 sleil4 higher-order mutant produced much smaller fruits like slein2 but with relatively normal fruit ripening (Supplemental Figure S6A), supporting to the functional specialization among SlEIL members in regulating fruit growth and ripening. Collectively, these results demonstrate that SlEIL and SlEBF proteins play both redundant and specialized functions in tomato.
Ethylene plays a positive role in tomato fruit growth
Despite the previous observation that treatment with high levels of ACC suppressed fruit growth (Shinozaki et al., 2015), how endogenous ethylene affects fruit growth remains to be addressed. The obviously reduced fruit size observed in the slein2-1 and sleil1 sleil2 sleil3/SlEIL3 sleil4 mutants suggested a positive role for ethylene signaling in controlling fruit growth in tomato (Supplemental Figure S4, E and A). To further verify this idea, we conducted manual pollination and cross-fertilization assays to eliminate the influence of facultative parthenocarpic fruit development in WT and slein2-1 (Figure 2A). Manual pollination using slein2-1 as the pollen recipient failed to restore normal fruit size using pollen from either slein2-1 or WT. In contrast, fruit size was normal when slein2-1 pollen was used to pollinate WT pistils (Figure 2A). Remarkably, the viable seed number significantly decreased when slein2-1 or WT pollen was used to pollinate slein2-1 pistils, suggesting that the deletion of SlEIN2 resulted in reduced seed set and/or impaired seed development (Figure 2B). However, pollen viability, pollen germination, and pollen tube attraction were comparable between WT and slein2-1 (Supplemental Figure S8, A–C), suggesting that pollen development is unaffected in slein2-1. Meanwhile, the comparable ovule number in 0-day postanthesis (dpa) and 3-dpa WT and slein2-1 fruits (Supplemental Figure S8D) implies that seed development, but not seed set, is impaired in slein2 fruits.
Figure 2.
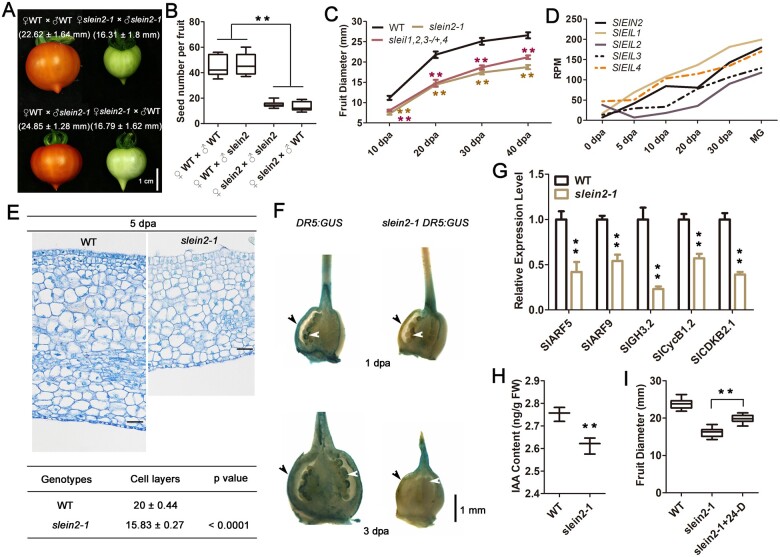
Ethylene positively regulates fruit growth. A, Cross-fertilization assay. Emasculated WT flowers were fertilized with WT or slein2-1 pollen. Conversely, WT or slein2-1 pollen was used to fertilize emasculated slein2-1 flowers. Each plant bears the same number of fruits. The 45-dpa fruits and statistics of fruit diameters were shown (n = 12, sd). B, Statistics of viable seed number of fruits harvested in (A). The seed number was determined at 55 dpa. For each genotype, n = 12. **P < 0.01, Student’s t test. C, Statistics of WT, slein2-1, sleil1 sleil2 sleil3/SlEIL3 sleil4 fruit diameter at different growth stages (10, 20, 30, and 40 dpa) with manual pollination. Each plant bears the same number of fruits. For each genotype, n = 12. **P < 0.01, Student’s t test. D, Expression data of SlEIN2, SlEIL1, SlEIL2, SlEIL3, and SlEIL4 in pericarps at different growth stages (0, 5, 10, 20, and 30 dpa, MG). Data were extracted from the Tomato Expression Atlas platform (https://tea.solgenomics.net/). RPM, reads per million mapped reads. E, Histological analysis of transverse sections of 5-dpa WT and slein2-1 fruit pericarps, and statistics of their respective number of cell layers (n = 10, SD). Student’s t test. Bar = 20 μm. F, GUS staining analysis of the auxin-responsive promoter DR5 fused to the GUS reporter gene (DR5:GUS) in WT and slein2-1 young fruits (1 and 3 dpa). Black arrowheads indicate pericarps. White arrowheads indicate seeds. G, Relative mRNA levels of three auxin-related genes (SlARF5, SlARF9, and SlGH3.2) and two cell cycle genes (SlCycB1.2 and SlCDKB2.1) in WT and slein2-1 young fruits (3 dpa). Error bars indicate sd (n = 3), **P < .01. Student’s t test. H, IAA content in WT and slein2-1 young fruits (3 dpa). Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. I, Statistics of WT and slein2-1 fruit diameter at 30 dpa and the diameter of 30-dpa slein2-1 fruits treated with 20-μM 2,4-D once a week after fertilization. Error bars indicate sd (n = 12), **P < 0.01, Student’s t test.
A detailed kinetic analysis of fruit diameter under manual pollination conditions demonstrated the suppressed fruit growth of slein2-1 and sleil1 sleil2 sleil3/SlEIL3 sleil4 throughout the fruit growth and maturation cycle (Figure 2C). Notably, when we measured fruit size under manual pollination conditions, WT and mutant plants had comparable fruit set, without additional attached flowers or parthenocarpic fruits in the mutants. As tomato is source-limited, additional organs including the attached flowers and the higher rates of parthenocarpic fruits, as seen in slein2 plants under self-pollination conditions, could act as strong sinks for nutrient consumption, which would lead to smaller fruit size due to limited nutrients. To clarify this issue, we performed manual pollination of the first two flowers in the first inflorescence of WT and slein2-1 plants with all other flowers removed, a condition where nutrient levels are theoretically adequate for the remaining two fruits to grow. Compared to WT, slein2-1 had smaller fruit size as well as decreased seed number (Supplemental Figure S8, E–G), suggesting that EIN2-mediated ethylene signaling promotes tomato fruit growth per se. Consistently, data from the SGN-TEA pipeline indicated that SlEIN2 and four SlEIL genes displayed increasing expression levels in the pericarps throughout fruit growth (Figure 2D), further supporting the notion that SlEIN2 and SlEIL genes exert a regulatory function in pericarps during fruit growth.
Next, we sought to investigate how ethylene signaling regulates fruit growth. Histology analysis revealed that the pericarp of slein2-1 fruits at 5 dpa had fewer cell layers than that of WT (Figure 2E), suggesting an impairment of cell division during the early stage of growth. Given that tomato fruit growth is mainly controlled by auxin-mediated cell division in young fruits (McAtee et al., 2013; Fenn and Giovannoni, 2021), it is likely that auxin signaling is impaired in slein2-1 fruit. To test this hypothesis, we examined β-GLUCURONIDASE (GUS) expression driven by the synthetic auxin-responsive promoter DR5 in WT and slein2-1. In WT fruits, we observed a strong auxin signal following fertilization at 1 dpa and 3 dpa, whereas this auxin signal was much less intense in slein2-1 fruits, particularly in pericarps and seeds (Figure 2F). In line with this observation, the transcript levels of auxin-responsive genes AUXIN RESPONSE FACTOR 5 (SlARF5), SlARF9, and GRETCHEN HAGEN 3.2 (SlGH3.2) decreased two- to four-fold in slein2-1 compared to WT (Figure 3G). Notably, SlARF5 and SlARF9 have been implicated as regulators of fruit development in tomato (de Jong et al., 2015; Liu et al., 2018a, 2018b), and SlGH3.2 has been identified as a regulator of auxin-ethylene levels (Sravankumar et al., 2018). Similarly, the transcript levels of cell division-related genes CYCLIN B1.2 (SlCycB1.2) and CYCLIN-DEPENDENT KINSAE 2.1 (SlCDKB2.1) were also lower in slein2-1 compared to WT (Figure 2G).
Figure 3.
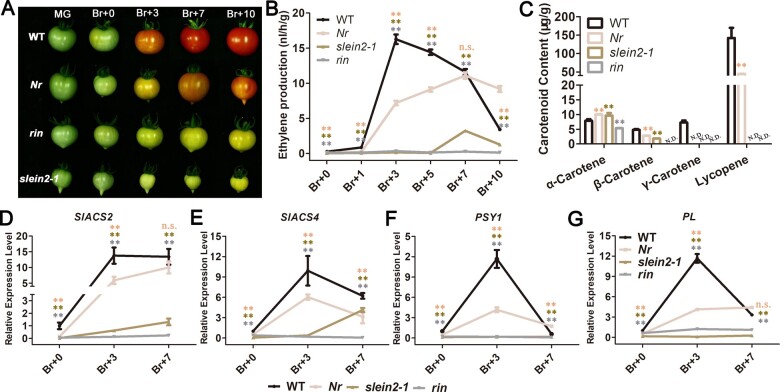
rin and slein2-1 fruits show comparable ripening features. A, Different stages of fruit ripening of WT, Nr, rin, and slein2-1. Fruits of rin and slein2-1 show complete ripening cessation. The first signs of carotenoid accumulation on the external surface of the fruit were taken to define the breaker stage. B, Ethylene production of WT, Nr, rin, and slein2-1 fruits at different ripening stages. Error bars indicate sd (n = 12). **P < 0.01, Student’s t test; n.s., not significantly different. C, Carotenoid levels in WT, Nr, rin, and slein2-1 fruits at the Br+10 stage. Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. n.d., not detectable. D–G, Relative mRNA levels of tomato SlACS2 (D), SlACS4 (E), PSY (F), and Pl (G) in WT, Nr, rin, and slein2-1 fruits at different ripening stages. Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. Br + 0, color breaker stage; Br + 3, 3-day postcolor breaker stage; Br + 7, 7-day postcolor breaker stage; Br + 10, 10-day postcolor breaker stage.
We then measured IAA contents in WT and slein2 mutant fruits at 3 dpa. The slein2 fruits contained significantly lower levels of this auxin than WT (Figure 2H). The application of 2,4-dichlorophenoxyacetic acid (2,4-D), an auxin analog, partially restored the normal fruit size of slein2-1 (Figure 2I). These data suggest that auxin accumulation is impaired in slein2-1. Auxin accumulation could be attributed to auxin biosynthesis and/or transport. Two genes, TRYPTOPHAN AMINOTRANSFERASE RELATED1 (TAR1) and the YUCCA gene tomato FLOOZY5 (ToFZY5), encoding enzymes in the major auxin biosynthetic pathway (Expósito-Rodríguez et al., 2011; Pattison et al., 2015), were downregulated in slein2 fruits compared to WT (Supplemental Figure S9A). However, three genes, TAR2, ToFZY3, and ToFZY6, were upregulated in slein2 fruits (Supplemental Figure S9A). Meanwhile, the auxin efflux carrier genes SlPIN3 and SlPIN9 and the auxin influx carrier genes SlLAX3 and SlLAX5 displayed reduced expression levels in slein2 fruits, while the expression of SlPIN2, SlPIN4, SlPIN5, SlPIN8, SlLAX1, and SlLAX4 increased in slein2 fruits (Supplemental Figure S9, B and C).
Taken together, these data indicate that ethylene signaling is important for normal fruit growth. As seeds are a rich source of auxin in developing fruit (Ozga et al., 2002, Dorcey et al., 2009), the impaired seed development of slein2-1 fruits (fewer seeds) is likely to decrease auxin accumulation (by affecting auxin biosynthesis and/or transport) as well as auxin-mediated cell division, leading to smaller fruit size. In addition, the tomato cultivar MT is known to carry a mutation in a brassinosteroid biosynthesis gene (Marti et al., 2006), sometimes hampering phenotypic analysis using this cultivar. To further verify the role of ethylene signaling in fruit growth, we generated a loss-of-function mutant of SlEIN2 in the AC cultivar background using CRISPR/Cas9. In line with the smaller fruits of slein2 in the MT background, we also observed drastically reduced fruit size of slein2 in the AC background (Supplemental Figure S10), supporting the conclusion that ethylene positively regulates fruit growth in different tomato cultivars.
slein2 shows comparable ripening inhibition and molecular features as rin
slein2-1, which shows completely blocked ethylene signaling, provides the ideal genetic background to better understand the role of ethylene in tomato fruit ripening. To clarify the similarities and differences between slein2-1 and spontaneous ripening-deficient mutants, we compared ripening progression of WT, Nr, rin, and slein2-1 fruits. Compared to WT, we observed a complete inhibition of ripening in slein2-1 and rin fruits and a partial inhibition of ripening in Nr fruits (Figure 3A). Next, we assessed the changes in ethylene production in WT, Nr, rin, and slein2-1 fruits during ripening (Figure 3B). Compared to WT, ethylene production in rin fruit remained at a basal level without any increase from the color breaker stage (Br, fruit showing visible yellow color) Br + 0 to Br + 10, as previously reported (Vrebalov et al., 2002). Similarly, we observed a drastic inhibition of ethylene production in slein2-1 fruits, corroborating the notion that system-2 ethylene biosynthesis is largely regulated by an autocatalytic system (Yokotani et al., 2009). slein2-1 fruits still accumulated a small amount of ethylene at the Br + 7 stage, confirming the existence of a nonautocatalytic system pathway regulating system-2 ethylene biosynthesis during ripening (Yokotani et al., 2009). Meanwhile, we extracted and quantified the carotenoids from Br + 10 stage fruits of WT, Nr, rin, and slein2-1. β-carotene, γ-carotene, and lycopene accumulated to much lower levels in rin and slein2-1 mutants relative to WT, wherein γ-carotene and lycopene were almost undetectable in rin and slein2-1 fruits (Figure 3C).
We next explored the molecular features underlying the decreased ethylene production and carotenoid accumulation in the mutants. During fruit ripening, SlACS2 and SlACS4 are thought to mainly contribute to system-2 ethylene production (Barry et al., 2000). The transcript levels of the ethylene biosynthesis genes SlACS2 and SlACS4 (Figure 3, D and E), the carotenoid biosynthesis gene PHYTOENE SYNTHASE (PSY1; Figure 3F), and the softening control gene PECTATE LYASE (PL; Figure 3G) were significantly lower in rin and slein2-1 fruits than in WT. The four genes that are evidently downregulated when fruit ripening is defective have been widely used as marker genes during fruit ripening (Barry et al., 2000). Interestingly, as observed in SlEILs RNAi fruits (Yokotani et al., 2009), SlACS2 and SlACS4 transcript levels in slein2-1 fruits somewhat increased from Br + 0 to Br + 7 (Figure 3, D and E), which could explain the slightly increased ethylene production in slein2-1 fruits at Br + 7. Compared with rin and slein2-1, Nr fruits showed less pronounced defects in ripening, ethylene production, and carotenoid accumulation, as well as reduced expression of SlACS42/4, PSY1, and PL (Figure 3), pointing to strong residual ethylene signaling in this gain-of-function mutant. Together, these results reveal that slein2-1 and rin fruits display comparable ripening-deficient phenotypes and share similar underlying molecular features.
Unlike slein2, rin fruits are still responsive to exogenous ethylene treatment
Given that slein2 and rin fruits show similarly severe ripening defects, we next investigated the relationship between ethylene signaling and developmentally related TFs, such as RIN, which has become controversial in recent years (reviewed by Wang et al., 2020; Brumos, 2021). The external application of ethylene gas did not restore the fruit ripening of rin (Giovannoni, 2007), while E4, a typical ethylene-responsive gene, can be induced by external ethylene treatment in rin (Barry et al., 2000). To determine whether this apparent lack of responsiveness of rin is due to the ethylene insensitivity or dis-regulation of downstream pathways, we examined the physiological and molecular aspects of the ethylene response exhibited by rin fruits. To this end, we collected MG (full-size green fruit) stage fruits from WT, Nr, rin, and slein2-1 plants and exposed them to exogenous ethylene (Figure 4A). Using days from treatment to color breaker as the scorable index, we established that rin fruits have a shorter color breaker time than Nr or slein2-1 fruits (Figure 4B), suggesting that rin fruits are at least partially responsive to ethylene.
Figure 4.
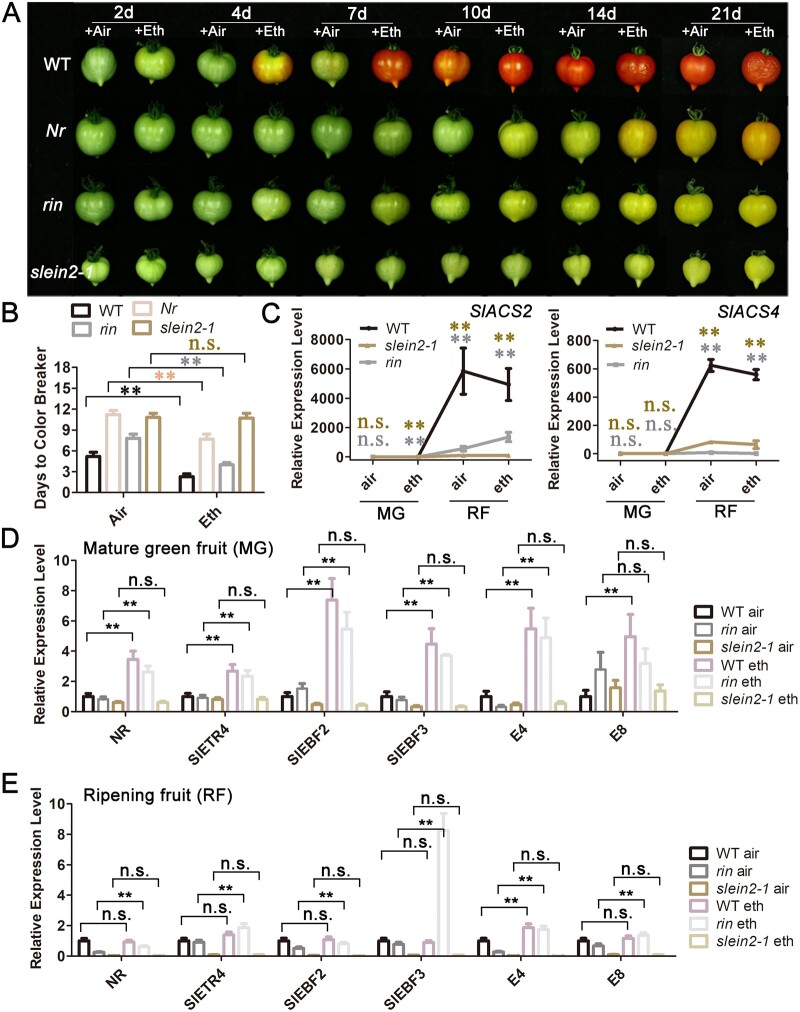
rin but not slein2 responds to exogenous ethylene treatment. A, Effect of exogenous ethylene treatment on WT, Nr, rin, and slein2-1 fruits. MG fruits were incubated in a sealed chamber with air or 50 ppm ethylene. Daily observation was performed to identify the days to color breaker, and photographs were taken at the indicated times. B, Statistics of days to color breaker of fruits in (A). Error bars indicate sd (n = 12), **P < 0.01, Student’s t test. C, Relative mRNA levels of SlACS2 and SlACS4 in WT, rin, and slein2-1 fruits responding to ethylene at different stages (RF, Br + 3 fruits). Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. D, Relative mRNA levels of NR, SlETR4, SlEBF2, SlEBF3, E4, and E8 in WT, rin, and slein2-1 fruits responding to ethylene at the MG stage. Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. E, Relative mRNA levels of NR, SlETR4, SlEBF2, SlEBF3, E4, and E8 in WT, rin, and slein2-1 fruits responding to ethylene at the Br + 3 ripening stage. Error bars indicate sd (n = 3), **P < 0.01, Student’s t test.
We also compared the transcript levels of several ethylene biosynthesis genes in WT, rin, and slein2-1 fruits at the MG and RF (ripening fruits collected at stage Br + 3) stages, with or without exogenous ethylene treatment for 24 h. Consistent with the burst of ethylene production at Br + 3 during fruit ripening (Figure 3B), WT fruits showed a hundred-fold and a thousand-fold increase in SlACS2 and SlACS4 transcript levels, respectively, from MG to RF (Figure 4C). However, such a vast induction of SlACS2 and SlACS4 was not observed in rin or slein2-1 fruits, regardless of ethylene treatment (Figure 4C), suggesting that the system-2 ethylene biosynthesis pathway is severely impaired in rin and slein2-1 fruits. The transcript levels of NR, SlETR4, SlEBF2, SlEBF3, and E4, all of which were induced by ethylene in WT, were unaltered in slein2-1, but were upregulated in rin upon ethylene treatment (Figure 4, D and E), pointing to the existence of active ethylene signaling in rin fruits. Interestingly, upon ethylene treatment of WT fruits, NR, SlETR4, SlEBF2, SlEBF3, and E8 transcript levels increased in MG fruits but remained constant in RF fruits (Figure 4, D and E), suggesting a saturated endogenous ethylene response in WT fruits at the RF stage. In contrast, the expression of these ethylene signaling genes was significantly induced by ethylene treatment in rin fruits at both the MG and RF stages (Figure 4E). These results indicate that the endogenous ethylene response remained at a basal level in rin fruits at both stages, which is consistent with the basal level of ethylene production measured in rin fruits at different ripening stages (Figure 3B). Collectively, these results imply that rin fruits can respond to ethylene, but that system-2 ethylene biosynthesis is severely impaired in this background.
Ethylene regulates ripening by transcriptionally activating the developmental factor genes NOR, RIN, and FUL1
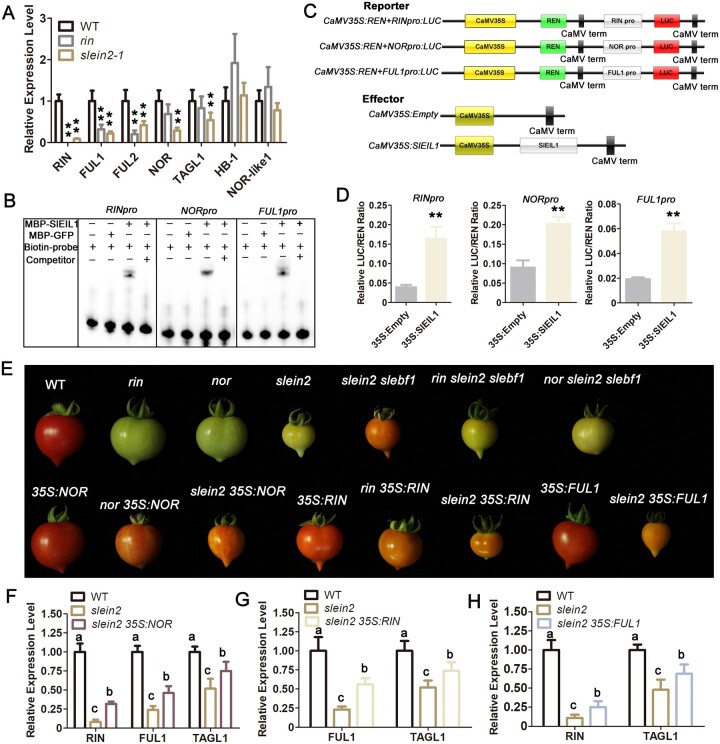
Recent studies revealed that the naturally occurring rin and nor mutants are dominant-negative (gain-of-function) instead of loss-of-function mutants (Ito et al., 2017; Wang et al., 2019; Gao et al., 2020). However, the strong ripening-cessation phenotype of these natural mutant alleles provides compelling genetic evidence for the essential roles of RIN-type MADS-box and NOR-type NAC TFs in fruit ripening control. rin fruits exhibited ethylene responsiveness, while fruit ripening was not restored by exogenous ethylene treatment, raising the possibility that the MADS-box TFs including RIN might act downstream of ethylene during fruit ripening. In accordance with this hypothesis, the ripening-related developmental factor genes, including RIN, FUL1/2, NOR, and TOMATO AGAMOUS-LIKE 1 (TAGL1), were expressed at reduced levels in slein2-1 fruits during ripening (Figure 5A;Supplemental Figure S11, A and B). As RIN was previously reported as a direct target gene of tomato EIL proteins by ChIP-seq (Lü et al., 2018), we conducted EMSAs and determined that SlEIL1 binds to the promoter regions of RIN, NOR, and FUL1 in vitro (Figure 5B). We then performed a dual-luciferase reporter assay in Arabidopsis protoplasts co-transfected with reporter constructs consisting of the firefly luciferase (LUC) gene driven by the RIN, NOR, or FUL1 promoters, and an effector construct overexpressing SlEIL1 together with the Renilla (REN) LUC gene driven by the cauliflower mosaic virus (CaMV) 35S promoter for normalization. Relative LUC/REN activity derived from the RINpro:LUC, NORpro:LUC, and FUL1pro:LUC reporters was higher than that of 35S:SlEIL1 co-transfected with the empty effector construct (35S:Empty; Figure 5, C and D), indicating that SlEIL1 is a transcriptional activator of RIN, NOR, and FUL1 expression.
Figure 5.
Ripening-related TF genes act downstream of ethylene signaling during fruit ripening. A, Relative mRNA levels of RIN, FUL1, FUL2, NOR, TAGL1, HB-1, and NOR-like1, in WT, rin, and slein2-1 fruits at the Br + 3 ripening stage. Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. B, EMSA showing the binding of tomato EIL protein SlEIL1 to the promoters of RIN, NOR, and FUL1. Biotin-labeled probes (10 fmol) were used in each reaction. Cold probe indicates unlabeled probe, and 5 pmol of cold probe was used for competition with biotin-labeled probe. C and D, Transient dual-LUC reporter assay illustrating the activation of RIN, NOR, and FUL1 transcription by SlEIL1. Error bars indicate sd (n = 3), **P < 0.01, Student’s t test. E, Representative fruits of the indicated genotypes at the ripening stage. F–H, Relative mRNA levels of ripening-related TF genes (RIN, FUL1, or TAGL1) in WT, slein2, and slein2 35S:NOR (F), slein2 35S:RIN (G), and slein2 35S:FUL1 (H) fruits at the Br + 3 ripening stage. Error bars indicate sd. Different letters above the bars indicate statistically significant differences between the samples (n = 3, P < 0.01, Student’s t test).
The inconsistency between the phenotypes of the natural rin and CRISPR KO-rin mutants led to a revised model of ripening control that places RIN downstream of ethylene (Lü et al., 2018; Li et al., 2020), although adequate genetic evidence is still lacking. To provide genetic evidence to further confirm that the developmental factors RIN, NOR, and FUL1 are downstream components of ethylene signaling during fruit ripening, we crossed the slein2 slebf1 double mutant with nor or rin to generate the respective triple mutants. In both cases, the partial rescue of fruit ripening conferred by the loss of SlEBF1 in the slein2 background was eliminated upon the introduction of the nor or rin mutation (Figure 5E). Considering the recently proposed dominant-negative nature of the rin and nor mutations (Ito et al., 2017; Wang et al., 2019; Gao et al., 2020), we interpreted these results as an indication that the ethylene-mediated control of ripening in tomato requires the function of a set of MADS-box and NAC TFs rather than single family member. This scenario somewhat differs from the previous model in which three distinct regulatory loops control ripening-associated genes in different fruit types, with MADS-RIN playing a central role in some fruits (like tomato) while NAC-NOR taking on this role in others (like melon; Lü et al., 2018).
We then generated transgenic tomato plants overexpressing NOR (35S:NOR), RIN (35S:RIN), or FUL1 (35S:FUL1) and crossed them with the rin, nor, and slein2-1 mutants (Figure 5E;Supplemental Figure S12, A–F). Overexpression of NOR and RIN partially restored fruit ripening of nor and rin, respectively (Figure 5E), which is consistent with a previous report of transgenic complementation of the nor mutant by NOR (Jiang et al., 2020). Remarkably, we established that the overexpression of NOR, RIN, or FUL1 in the slein2 mutant background partially restored fruit ripening, confirming the notion that NOR, RIN, and FUL1 are positive regulators of ripening that function downstream of ethylene signaling. Taken together, the genetic evidence demonstrates that rin and nor are genetically epistatic to the slein2 ebf1 double mutant and that ethylene controls ripening by regulating NOR, RIN, and FUL1 expression.
The partial, but not complete, rescue of ripening seen in slein2 35Spro:NOR, slein2 35Spro:FUL1, and slein2 35Spro:RIN fruits prompted us to analyze the expression of homologous MADS-box genes in light of previous studies suggesting that the MADS-box TF RIN homodimerizes and/or heterodimerizes with other ripening-related MADS-box members such as FUL1/2 and TAGL1 (Fujisawa et al., 2014; Ito et al., 2017). Accordingly, we compared the transcript levels of the three ripening-related TF genes, RIN, FUL1, and TAGL1 in WT, slein2-1, and slein2 35Spro:NOR fruits at the Br + 3 stage: their transcript levels were all higher in slein2 35Spro:NOR than in the slein2-1 mutant, but lower than in WT (Figure 5F). We obtained similar results when overexpressing RIN or FUL1 in the slein2-1 background, with higher transcript levels for FUL1 and TAGL1 (in slein2 35Spro:RIN) or for RIN and TAGL1 (in slein2 35Spro:FUL1), but lower than in WT (Figure 5, G and H). These results highlight the importance of direct regulation of ripening-related TFs by ethylene signaling. In addition, they suggest that the concerted actions of these ripening-related TFs might be crucial for controlling tomato fruit ripening.
The signal intensity of system-2 ethylene mediates the efficiency of the tomato ripening machinery
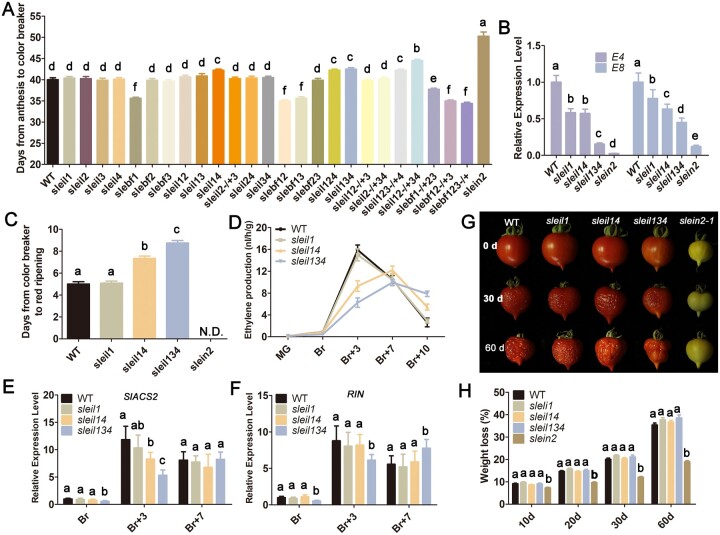
A large body of literature demonstrates the essential role of system-2 ethylene in fruit ripening (Oeller et al., 1991; Picton et al., 1993; Oetiker and Yang, 1995; Grierson, 2013; Liu et al., 2015; Li et al., 2019). As system-2 ethylene production is largely autocatalytically regulated, we sought to explore the connection between ethylene signal intensity and the efficiency of the ripening machinery. The complete block of ethylene signaling in the slein2-1 mutant, combined with the quantitative changes seen in other mutants (enhanced or partially blocked signaling), allowed us to comprehensive dissect their influence on fruit ripening. Color breaker time (days from anthesis to visible yellow color) of sleil1 sleil4, sleil1 sleil2 sleil4, sleil1 sleil3 sleil4, sleil1 sleil2/SlEIL2 sleil3 sleil4, sleil1 sleil2 sleil3/SlEIL3 sleil4, and slein2-1 fruits occurred later than in WT, while slebf1, slebf1 slebf2, slebf1 slebf3, slebf1/SlEBF1 slebf2 slebf3, slebf1 slebf2/SlEBF2 slebf3, and slebf1 slebf2 slebf3/SlEBF3 fruits reached the same stage more quickly than the WT (Figure 6A), suggesting that the intensity of the ethylene signal is positively associated with the timing of ripening initiation, which is consistent with the results of prior studies in which the expression of tomato SlEIL or SlEBF genes was manipulated (Tieman et al., 2001; Yang et al., 2010). Notably, although slein2-1 fruits were the most delayed in color breaker time, they did eventually undergo a color change at ∼50 dpa, suggesting the existence of an ethylene-independent pathway for the initiation of fruit ripening.
Figure 6.
The signal intensity of system-2 ethylene mediates the efficiency of the tomato ripening machinery. A, Statistics of days from anthesis to the color breaker stage in fruits of the WT and corresponding mutant alleles. Error bars indicate sd. Different letters above the bars indicate statistically significant differences between the samples (n = 20, P < 0.01, Student’s t test). B, Relative mRNA levels of ripening-associated ethylene signaling marker genes E4 and E8 in WT, sleil1, sleil1 sleil4, sleil1 sleil3 sleil4, and slein2-1 fruits at the Br + 10 stage. Error bars indicate sd. Different letters above the bars indicate statistically significant differences between the samples (n = 3, P < 0.01). C, Statistics of days from the color breaker to red ripening stage in WT, sleil1, sleil1 sleil4, sleil1 sleil3 sleil4, and slein2-1 fruits. Error bars indicate sd. Different letters above the bars indicate statistically significant differences between the samples (n = 20, P < 0.01, Student’s t test). n.d., no data available. D, Ethylene production of WT, sleil1, sleil1 sleil4, and sleil1 sleil3 sleil4 fruits at different ripening stages. Error bars indicate sd (n = 12). E and F, Relative mRNA levels of SlACS2 (E) and RIN (F) of WT, sleil1, sleil1 sleil4, and sleil1 sleil3 sleil4 fruits at different ripening stages. Error bars indicate sd. Different letters above the bars indicate statistically significant differences between the samples (n = 3, P < 0.01, Student’s t test). G, Br + 10 fruits that were harvested from WT, sleil1, sleil1 sleil4, sleil1 sleil3 sleil4, and slein2-1 plants and stored at room temperature for two months. H, Physiological loss of water (weight loss %) in WT, sleil1, sleil1 sleil4, sleil1 sleil3 sleil4, and slein2-1 fruits during different stages of storage. The weight loss per fruit was calculated at 10, 20, 30, and 60 days after storage. Error bars indicate sd. Different letters above the bars indicate statistically significant differences between the samples (n = 20, P < 0.01, Student’s t test).
To assess the relationship between ethylene signal intensity and the ripening program after the color breaker stage, we selected WT, sleil1, sleil1 sleil4, sleil1 sleil3 sleil4, and slein2 fruits, as they exhibited gradually decreasing expression of the system-2 ethylene response marker genes E4 and E8 (Figure 6B) and thus represent a continuum of endogenous ethylene signal intensity. Intriguingly, compared to WT fruits, which reached the red ripening stage ∼5 days after the color breaker stage, sleil1 sleil4 and sleil1 sleil3 sleil4 fruits took ∼7 and 9 days, respectively, to achieve red ripening (Figure 6C). In contrast, slein2-1 fruits never reached the red ripening stage (Figure 6C). In agreement with their observed delayed ripening, sleil1 sleil4 and sleil1 sleil3 sleil4 fruits produced less ethylene than WT (Figure 6D) and showed lower expression of ACS2 and RIN (Figure 6, E and F). These results indicate that, once ripening has been initiated, reaching the fully ripe stage proportionally depends on ethylene signaling, whereby the number of days (duration) required to reach full ripeness depends on the signal intensity conferred by system-2 ethylene. In support of this notion, sleil1 sleil2/SlEIL2 sleil3 sleil4 fruits, which had a slightly weaker ethylene response than sleil1 sleil3 sleil4 fruits, took even longer (∼10 days) to achieve the red ripening stage (Supplemental Figure S13A); likewise, SlEIL CS fruits, which exhibited a more severe inhibition of ripening and an even weaker ethylene response than sleil1 sleil2/SlEIL2 sleil3 sleil4 fruits, did not turn red until ∼90 dpa (or the Br + 40 stage) (Supplemental Figure S13B).
Finally, to better understand the role of ethylene in postclimacteric fruits, we carried out postharvest behavior assessments using WT, sleil1, sleil1 sleil4, sleil1 sleil3 sleil4, and slein2-1 fruits harvested at the Br + 10 stage. Only slein2-1 fruits showed clear resistance to postharvest deterioration, as determined by measuring water/weight loss (Figure 6, G and H). In contrast, WT, sleil1, sleil1 sleil4, and sleil1 sleil3 sleil4 fruits shared the same postharvest behavior (Figure 6, G and H). Therefore, we conclude that once full ripeness has been achieved, the signal intensity conferred by system-2 ethylene is no longer a determinant of the subsequent fruit deterioration. The conclusion helps explain (to a certain extent) the existence of a shut-down mechanism of ethylene biosynthesis in postclimacteric red tomato to confer fruit preservation (van de Poel et al., 2012). Taken together, these results demonstrate that the signal intensity of system-2 ethylene is intimately associated with the timely onset of ripening and, more importantly, that an ethylene burst following ripening initiation is critical for the achievement of full ripeness in due course.
Discussion
Fruit development comprises three stages: fruit setting, fruit growth, and fruit ripening. Plant hormones are tightly associated with all three stages (McAtee et al., 2013). Auxin and gibberellin are generally considered to be the major regulators of fruit size (de Jong et al., 2009; Fenn and Giovannoni, 2021). After successful fertilization, the rapidly growing young fruit experiences a rapid increase in cell number due to auxin-mediated cell divisions. Auxin and gibberellin also act together to influence cell expansion during the entire fruit growth cycle (Kumar et al., 2014). In contrast to auxin and gibberellin production, ethylene production decreases after successful fertilization (Shinozaki et al., 2015), and transcriptome studies have shown that the transcript levels of ethylene biosynthesis and signaling genes decrease in early-developing tomato fruits (Vriezen et al., 2008; Wang et al., 2009). Application of the ethylene precursor ACC to pollinated ovaries resulted in smaller tomatoes (Shinozaki et al., 2015), suggesting a negative role of ethylene in fruit growth. However, this growth inhibition required a relatively high ACC concentration (2 mM), while ovaries treated with 0.2 mM or 1 mM ACC showed no difference from the mock treatment (Shinozaki et al., 2015).
It was recently reported that the tomato SlACS2 knockdown mutant exhibited fewer flowers and a lower fruit set but larger fruits than the WT, whereas the gain-of-function of SlACS2 mutant showed the opposite effects (Sharma et al., 2021). Given the growing evidence of ethylene-independent functions for ACC (Mou et al., 2020), the role of ethylene in fruit growth is in question. Studies in cucumber (Cucumis sativus) showed that mutants with either higher or reduced ethylene production showed fewer cell divisions and shorter fruits than the WT (Xin et al., 2019), suggesting that ethylene exerts its regulatory function in a dosage-dependent manner during fruit growth. In this study, we explored whether and how endogenous ethylene functions during tomato fruit growth, particularly when ethylene signaling is largely or totally suppressed. To this end, we generated a series of loss-of-function tomato mutants in the SlEIN2, SlEIL1–4, and SlEBF1–3 genes. Unexpectedly, the smaller fruit size observed in slein21 and sleil1 sleil2 sleil3/SlEIL3 sleil4 plants provided strong evidence that ethylene plays a positive role in tomato fruit growth (Supplemental Figures S4, E and S6, A). Notably, the positive role of ethylene in controlling fruit size has not been observed before, including the Nr mutant in the Pearson cultivar background (Lanahan et al., 1994). Considering that ethylene-overproducing tomatoes also showed smaller fruit size (Liu et al., 2014; Sharma et al., 2021), we speculate that, similar to the situation in cucumber, ethylene confers bidirectional regulation of fruit size in tomato: an appropriate basal concentration of ethylene is optimal for fruit growth while the lack or excess of ethylene production is inhibitory. Moreover, we demonstrated that auxin biosynthesis is impaired in early-developing slein2-1 fruits and that the application of 2,4-D partially restored the fruit size of slein2-1 (Figure 2, H and I), implying that endogenous ethylene positively regulates fruit growth, at least partly by modulating auxin biosynthesis and the auxin response pathway. However, the elaborate mechanism by which ethylene and auxin signaling are integrated to control fruit size requires further investigation.
Whereas ethylene biosynthesis and signaling pathways are highly conserved in plants, mounting evidence suggests that ethylene exerts specific functions in different species. For instance, the ethylene-induced etiolated seedling response is quite different between dicotyledonous Arabidopsis and monocotyledonous rice (Oryza sativa; Yang et al., 2015). Ethylene has long been recognized as a growth inhibitor based on the larger rosettes and larger leaves of Arabidopsis mutants with impaired positive regulators of ethylene signaling (Dubois et al., 2018). However, in contrast to the ein2 null mutants in Arabidopsis, we observed comparable leaf size in tomato WT and slein2-1 (Supplemental Figure S4A), indicating that, at least in terms of leaf size control, the function of ethylene differs considerably in these two plant species.
Accumulating evidence suggests that ethylene can also promote growth, particularly at the level of cell division. In Arabidopsis, cell division stimulated by ethylene is required for apical hook development (Raz and Koornneef, 2001); ethylene and the downstream TFs ERF018 and ERF109 promote cell division during vasculature development in stems (Etchells et al., 2012). In poplar (Populus trichocarpa), ethylene is an endogenous stimulator of cell division of the cambial meristem (Lovea et al., 2009). In cucumber, transient exposure to ethylene stimulates the division of hypocotyl epidermal cells (Kazama et al., 2004). Given that auxin is a general regulator of plant cell division (Pierre-Jerome et al., 2018), the impaired auxin biosynthesis, the decreased number of cell layers, as well as the smaller size of young slein2-1 fruits observed in this study establish a regulatory axis involving ethylene–auxin crosstalk and fruit growth regulation. In fact, the crosstalk between ethylene and auxin in tomato has been well studied in recent years: SlERF.B3 mediates root growth via the direct regulation of Sl-Aux/IAA27 expression (Liu et al., 2018a, 2018b); SlARF2, SlSAUR69, and SlGH3.2 participate in the transition of system-1 to system-2 ethylene to initiate fruit ripening (Breitel et al., 2016; Sravankumar et al., 2018; Shin et al., 2019); and carotenoid accumulation during tomato fruit ripening is modulated by the auxin–ethylene balance (Su et al., 2015).
Compared to its role in fruit setting and fruit growth, the importance of ethylene in fruit ripening has been extensively investigated. The ripening program can be dramatically influenced by altering ethylene biosynthesis, perception, or signal transduction (Grierson, 2013). In tomato, antisense-mediated suppression of the ethylene biosynthetic gene ACS2 blocked ripening, which was alleviated by exogenous ethylene treatment (Oeller et al., 1991). Although not consistent in all tomato cultivars, the spontaneous mutant Nr shows severe ripening defect in the cultivar Pearson background (Lanahan et al., 1994). The suppression of four SlEIL genes simultaneously or the overexpression of a single SlEBF3 gene resulted in transgenic tomatoes with impaired ripening (Tieman et al., 2001; Yokotani et al., 2009; Deng et al., 2018).
Development-associated TFs have also been documented as key players in fruit ripening. RIN, a MADS-box TF, is considered to be the master regulator of tomato fruit ripening. RIN was identified by positional cloning of a spontaneously ripening-deficient mutant that exhibited complete ripening inhibition but normal fruit growth and mature seeds (Vrebalov et al., 2002). During the past decade, much attention has focused on uncovering how RIN participates in fruit ripening, including identifying its interaction partners and target genes. TAGL1, FUL1, and FUL2, three MADS-box family members that can interact with RIN, play positive roles in fruit ripening (Vrebalov et al., 2009; Bemer et al., 2012; Fujisawa et al., 2014). NOR, a NAC domain TF, is another master regulator of tomato fruit ripening. NOR was identified by positional cloning of another spontaneously ripening-deficient mutant with complete ripening inhibition but normal fruit growth and mature seeds (United States Patent, No. US6762347B1; Wang et al., 2020). Several additional NAC TFs, including NOR-like1, SlNAM1, and SlNAC4, positively regulate fruit ripening (Zhu et al., 2014; Gao et al., 2018, 2021). Due to the complete loss of system-2 ethylene biosynthesis in the natural rin and nor mutants, it has been widely accepted that ethylene acts downstream of these developmental TFs in ripening control. However, the accepted paradigm of the transcriptional regulation of ripening was recently challenged by the introduction of CRISPR/Cas9 mutagenesis in tomato. The observation of milder effects on ripening in CRISPR/Cas9-generated true null mutant alleles compared to the corresponding traditional mutants suggests that the current model of the role of these TFs and their relationships with the ripening phytohormone ethylene need to be revisited (reviewed by Li et al., 2019; Brumos, 2021). The rin and nor mutants were recently reported to carry dominant-negative mutations instead of loss-of-function mutations as initially thought (Wang et al., 2019; Gao et al., 2020), urging a reassessment of the roles of RIN and NOR in ripening control.
In this study, we sought to clarify the relationship between ethylene signaling and these developmental TFs in the ripening regulatory network. First and foremost, the slein2-1 mutant displayed a ripening cessation phenotype comparable to that of rin, indicating that tomato fruit ripening is largely controlled by ethylene (Figure 3A). This observation is essential to our understanding of ethylene during fruit ripening in tomato, as the inability to induce ripening of rin fruits by exogenous ethylene was thought to point to the existence of ethylene-independent pathways for ripening control (Giovannoni, 2007; Fujisawa et al., 2013). The comparable ripening inhibition in slein2-1, rin, and nor fruit supports the notion that ethylene and developmental TFs contribute equally to fruit ripening and that SlEIN2, RIN, and NOR are most likely in the same genetic pathway. Next, we corroborated the finding that rin fruits are, to some extent, responsive to ethylene (Giovannoni, 2007), as shown by their shorter color breaker time (Figure 4, A and B) and the inducible expression of ethylene signaling genes in rin fruits upon exogenous ethylene treatment (Figure 4, D and E). Furthermore, EMSA and Dual-LUC assays showed that the NOR, RIN, and FUL1 promoters are direct targets of tomato SlEIL1 (Figure 5, B–D). Most importantly, overexpression of either NOR, RIN, or FUL1 partially rescued the fruit ripening phenotype of the slein2-1 mutant, while the intermediate fruit ripening phenotype of the slein2 slebf1 double mutant was eliminated when combined with the rin or nor mutant (Figure 5E). These findings, together with the ethylene responsiveness of the rin-KO mutant (Li et al., 2020), and our observations that ethylene treatment neither induced ripening of rin fruits nor recovered the expression of system-2 ethylene biosynthesis genes in rin fruits (Figure 4, A and C), place rin downstream of ethylene signaling but upstream of other pivotal ripening activities, including autocatalytically associated system-2 ethylene biosynthesis. Altogether, we conclude that, during fruit ripening, ethylene mediates a largely linear pathway involving SlEIN2–SlEBF1–SlEILs that induces the expression of the TF genes NOR, RIN, and FUL1/2 (Supplemental Figure S14).
Once ripening has been initiated, tomato fruits undergo a series of metabolic changes in color, flavor, texture, and aroma to achieve full ripeness in a few days, suggesting that a highly organized and coordinated regulatory network drives ripening. The moderate ripening inhibition revealed by CRISPR/Cas9-mediated mutagenesis of RIN, FUL1/2, and NOR genes (Ito et al., 2017; Wang et al., 2019), and the partial rescue of ripening for slein2 35S:NOR, slein2 35S:FUL1, and slein2 35S:RIN fruits presented here (Figure 5E), suggest that a single TF is not sufficient to fulfill the entire ripening program. Supporting this notion, a growing number of TFs from different families, including SlMYB12, SlARF2, SlGRAS38, SlHY5, and SlWRKY32, have been described as positive regulators of ripening in tomato (Adato et al., 2009; Hao et al., 2015; Shinozaki et al., 2018; Wang et al., 2021; Zhao et al., 2021). Meanwhile, RIN and FUL1/2 might also possess ethylene-independent functions: compared to WT, fruits from the FUL1/2 RNAi lines and the gene-edited rin-KO mutants exhibit excess fleshy softening (Bemer et al., 2012; Ito et al., 2020; Li et al., 2020), which contrasts with the notable resistance of slein2-1 fruits to fleshy softening (Figure 6, G and H). Indeed, despite their greatly repressed expression compared to the WT, RIN, FUL1, and NOR transcript levels were elevated in slein2-1 fruits during ripening (Supplemental Figure S11, A and B), indicating that their transcriptional control is both dependent and independent of ethylene.
The reduced ethylene emissions in tomato acs2-2 and acs4-1 mutants delays the progression of ripening (Hoogstrate et al., 2014; Sharma et al., 2021). By analyzing mutants with a variation of ethylene signal intensity, we established a correlation between system-2 ethylene signal intensity and the efficiency of the ripening machinery, providing an explanation for why climacteric fruits usually exhibit an ethylene burst during fruit ripening. Although the positive correlation between ethylene production and ripening program has been documented extensively in the past decades (reviewed by Grierson, 2013; Liu et al., 2015; Li et al., 2019), our knowledge of the biological significance of the high volume of ethylene production (ethylene burst) remains scarce and is an emerging issue for ripening control, as recent studies suggested that a relatively low concentrations of system-2 ethylene are sufficient to initiate the ripening program in tomato (Ito et al., 2017; Li et al., 2020). Indeed, we observed that mutants with largely suppressed ethylene signaling could ultimately initiate ripening and achieve full ripeness (Supplemental Figure S13B). slein2-1 fruits, which are 100% ethylene insensitive, could never achieve red ripening. SlEILs CS fruits, which showed strong but not 100% ethylene insensitivity, could eventually achieve red ripening, but in a much longer period of time. The substantial difference between slein2-1 and SlEILs CS fruits suggests that, for climacteric fruits with an autocatalytic ethylene system, the low initial levels of ethylene can be sufficiently amplified to fulfill the ripening process. Thus, compared to Nr or SlEILs CS fruits, the 100% ethylene insensitivity of slein2 plants is essential to define ethylene function and, more importantly, to define the relationship between ethylene and other ripening determinants, for instance, ethylene and ripening-related TFs, as has been shown in this study.
In summary, through a comprehensive genetic analysis in tomato, we defined the critical functions of ethylene during both fruit growth and fruit ripening (Supplemental Figure S14). System-1 ethylene is crucial for seed development. In developing fruits, seeds are a rich source for the biosynthesis of auxin, which diffuses to the surrounding tissues where it mediates cell division and fruit growth. During fruit ripening, system-2 ethylene controls ripening via a regulatory module comprising SlEIN2, SlEILs, and development-related TFs including NOR, RIN, and FUL1. These ripening-related developmental TFs are essential for the positive feedback regulation of ethylene biosynthesis and ethylene signal amplification, thus leading to the rapid upregulation of ripening-related genes and the achievement of full ripeness. The current results, combined with previous findings, strongly suggest that, in addition to the fundamental role of ethylene, the synergistic action of multiple ripening-related TFs may be crucial for the elaborate regulation of fruit ripening at multiple levels.
Materials and methods
Plant material and growth conditions
The tomato cultivar S.lycopersicum cv. MT is used as WT and parental strain of the ripening-impaired mutants Nr, rin, and nor. The genetic materials used in this study, including the MT cultivar WT, Nr, rin, and nor, were obtained from Professor Mondher Bouzayen’s laboratory (University of Toulouse, INPT, Laboratory of Genomics and Biotechnology of Fruit, Castanet-Tolosan, France). This group published a series of papers in the past few decades using the MT cultivar. Importantly, they claimed that the ripening phenotypes of Nr, rin, and nor in the cultivar MT background are strictly comparable to those described in other genotypes (Liu et al., 2016). For in vitro culture, seeds were surface sterilized by 3 min treatment with 70% ethanol, followed by 18 min with 4% hypochlorite solution. After a thorough rinse with sterile distilled water, the seeds were sown on sterile solidified medium based on Murashige and Skoog (MS) medium with pH adjusted to 5.7 using KOH. Five-day-old seedlings were transplanted to pots with soil and grown in controlled greenhouse conditions with 16-h light (5,000 Lux): 8-h dark cycles, 25°C day: 20°C night temperature, 60% relative humidity, and weekly irrigation with plant nutrient solution. Mature and ripening fruits were harvested as described by Shinozaki et al. (2018): MG stage (full-size green fruit, ∼35 dpa for both TM WT and slein2 fruit), Br stage (definite break in color from green to tannish-yellow on ˂10% of the surface, ∼40 and 52 dpa for WT and slein2, respectively). Fruit samples were harvested from six randomly selected individual plants at the same time each day.
Constructs and plant transformation
To KO the ethylene signaling genes by CRISPR/Cas9, sgRNAs were designed containing 20-bp target sequences specific to the 5ʹ-coding region of the corresponding genes followed by the NGG protospacer adjacent motif. To facilitate mutation efficiency, two or three target sequences were designed for each gene. The corresponding sgRNAs were amplified using specific primers (Supplemental Table S1), digested, and cloned into the binary vector pHNCas9 with two Golden Gate-compatible Esp3 I restriction sites placed after the plant codon-optimized version of Cas9 expressed under the control of the constitutive CaMV 35S promoter (Hu et al., 2019). To generate the overexpression and co-suppression plants, the coding sequences of the indicated genes (primers are listed in Supplemental Table S2) were cloned into modified plant binary vector K303 under the control of the CaMV 35S promoter (Huang et al., 2017).
The binary constructs were transformed into tomato by co-cultivation of cotyledons derived from 8-day-old seedlings using Agrobacterium tumefaciens-mediated transformation (strain GV3101), followed by regeneration on selective kanamycin-containing medium as described previously (Huang et al., 2017). Further validation was performed by PCR of genomic DNA with the primer pair Cas9-F and Cas9-R (Supplemental Table S1) to detect the 35S:Cas9 transgene.
Generation and identification of multigenic mutants
Homozygous alleles of each single mutant (sleil1, sleil2, sleil3, sleil4, sleil4, slebf1, slebf2, and slebf3) and the sleil1eil4 double mutant were identified by sequencing of PCR amplicons from their respective DNA extracts from T1 progenies. Homozygous double mutants (sleil1 sleil2, sleil1 sleil3, sleil2 sleil4, sleil3 sleil4, slebf1 slebf2, slebf1 slebf3, slebf2 slebf3, slein2 slebf1, slein2 slebf2, and slein2 slebf3) were generated by crosses between the homozygous single mutants and validated by sequencing of PCR amplicons from their respective DNA extracts from F2 progenies. The heterozygous double mutant sleil2−/+ sleil3 was generated by a cross between sleil2 and sleil3 and identified by sequencing of PCR amplicons from DNA extracts from more than 100 F2 progenies, with all sequencing results showing double peaks at the putative cleavage site. Homozygous triple mutants (sleil1 sleil2 sleli4, sleil1 sleil3 sleil4, slein2 slebf1 rin, and slein2 slebf1 nor) were generated by crosses between the homozygous double mutants and validated by sequencing PCR amplicons from their respective DNA extracts from F2 progenies. Heterozygous triple mutants (sleil1 sleil2−/+ sleil3, sleil2−/+ sleil3 sleil4, slebf1−/+slebf2 slebf3, slebf1 slebf2−/+slebf3, slebf1 slebf2 slebf3−/+) were generated by crosses between the homozygous/heterozygous double mutants and identified by sequencing of PCR amplicons from the respective DNA extracts of more than 100 F2 progenies, with all sequencing results showing double peaks at the putative cleavage site. Heterozygous quadruple mutants (sleil1 sleil2−/+sleil3 sleil4, sleil1 sleil2 sleil3−/+sleil4) were generated by crosses between the homozygous/heterozygous triple mutants and identified by sequencing PCR amplicons amplified from the respective DNA extracts of more than 100 F2 progenies, with all sequencing results showing double peaks at the putative cleavage site.
Monitoring the ethylene triple response, leaf epinastic response, and ethylene production
For the ethylene triple response assay, germinated seeds of different genotypes were sown on 1/2 MS medium with or without 5-μM ACC and cultured at 25°C in the dark. Root and hypocotyl length were measured at 3-day postsowing. For each line, at least 30 seedlings were measured. The epinasitic response assay was performed using 4-week-old plants. Ethylene was injected into sealed chambers at the designed concentration (50 ppm), and the plants were incubated in growth chambers for 24 h in the light (5,000 Lux) at 25°C. Ethylene production was determined using an ETD-300 laser-based photo-acoustic spectrophotometer (Sensor Sense, Nijmegen, Netherlands) as previously described (Van de Poel and Van de Straeten, 2017).
Transient expression in N. benthamiana
Transient expression in N. benthamiana was performed as described previously (Li et al., 2015). Agrobacterium strain GV3101 expressing SlEIN2pro:SlEIN2-HA-YFP was cultured in liquid Luria–Bertani medium overnight. The dense cultures were inoculated into fresh medium at 1:100 dilution and incubated for 6–8 h. The bacteria were then pelleted at 4,000 g for 15 min and resuspended in infiltration buffer (5 g L−1 glucose, 10-mM MgCl2, 10-mM MES-KOH, pH 5.7; adding 150-µM acetosyringone and 100-µM ACC right before use) to an OD600 (Optical Density 600) of 0.4. The resuspended Agrobacteria were infiltrated into N. benthamiana leaves using a 1-mL needleless syringe. Nicotianabenthamiana plants were cultured in the dark for 48 h, and YFP fluorescence was visualized under a confocal microscope (Zeiss LSM710) with a 488-nm Argon laser line.
Pollen germination and pollen tube growth assays
Pollen from WT and slein2 plants at the anthesis stage was used for the pollen germination assay. A 30-μL drop of medium (20-mM MES buffer, pH 6.0, 2% sucrose, 15% PEG 4000, 1-mM KNO3, 3-mM Ca(NO3)2·4H2O, 0.8-mM MgSO4·7H2O, 1.6-mM H3BO3) was placed on a glass slide in a Petri dish. A flower undergoing anthesis was shaken to let the pollen fall onto the medium. The Petri dish was sealed and kept in the dark at 25°C for 6 h. Observations were made under a light microscope (ZEISS Axioscope 5). For the pollen tube growth assay, 1 dpa ovaries were soaked in buffer (95% ethanol-glacial acetic acid, v/v, 3:1) for 24 h, transferred to 8-M NaOH, and incubated for 2 days. The samples were washed 3 times in water and stained with 0.05% aniline blue for 4 h in the dark. Pollen tubes were observed under a microscope with ultraviolet light excitation (ZEISS Axioscope 5).
GUS staining and histological analysis
GUS activity was assayed by submerging fruit samples in 0.5-mg mL–1 X-Gluc solution (0.1-M sodium phosphate buffer pH 7.0, 10-mM EDTA, 0.1% Triton X-100, 0.5-mM potassium ferrocyanide, 0.5-mM potassium ferricyanide), infiltrating them under a vacuum, and incubating them at 37°C.
For histological analysis, 5-dpa fruits were embedded in FAA solution (50% [v/v] ethanol, 5% [v/v] acetic acid, and 3.7% [v/v] paraformaldehyde). The samples were placed under a vacuum for 10 min, incubated at room temperature for 24 h, dehydrated in an ethanol gradient, and embedded in paraffin. Observations were performed under a light microscope.
Total protein extraction and immunoblotting
Tomato seedlings were ground into a fine powder in liquid N2 and homogenized in the same volume of 2× SDS (Sodium dodecyl sulfate) loading buffer [100-mM Tris–HCl (pH 6.8), 4% (w/v) SDS, 10% (v/v) glycerol, 50-mM DTT, and 0.02% (w/v) bromophenol blue]. The extracts were thoroughly mixed, maintained on ice for 15 min, and heated at 95°C for 10 min. After centrifugation for 10 min at 13,000 g, the supernatants were fractionated by 7.5% SDS–PAGE (Polyacrylamide gel electrophoresis). Immunoblot analysis was performed with specific antibodies, including anti-EIN2 (1:5,000 dilution; Wen et al, 2012), anti-EIN3 (1:5,000 dilution; Guo and Ecker, 2003), and the corresponding anti-Mouse-HRP antibodies purchased from Promega (Madison, WI, USA).
Extraction and quantification of endogenous IAA
Pure IAA was purchased from Sigma Chemical Co. (St Louis, MO, USA). Isotopically labeled internal standards [2H5] IAA were purchased from ICON Isotopes (Summit, NJ). Plant hormones were extracted and quantified as previously described (Huang et al., 2017) with some modifications. Approximately 0.2 g of 3 dpa WT or slein2-1 fruits were frozen in liquid nitrogen and ground into a powder. Two milliliters of isopropanol–HCl buffer solution (2:0.002, v/v) was added to the powder and shaken for 30 min at 4°C. After adding dichloromethane (4 mL), the sample was shaken for 30 min and centrifuged at 13,000g for 5 min. After centrifugation, the lower organic phase was transferred to a 10-mL tube and evaporated in a constant stream of nitrogen. Each sample was kept in the dark and resolubilized in 150-μL methanol containing 0.1% formic acid, and the solution was filtered through a 0.45-μm microfilter for HPLC–MS/MS (High performance liquid chromatography-mass spectrum/mass spectrum) analysis. The samples were injected into a reversed-phase column (C18 ZORBAX 300SB 3 μm, 4.6 × 150 mm, Agilent, CA, USA) using a binary solvent system composed of methanol (solvent A) and water with 0.1% formic acid (solvent B) as the mobile phase. The column thermostat was set at 30°C. Separations were performed using a solvent gradient that started at 20% methanol for 2 min, increased linearly to 80% over 14 min and was maintained for 5 min, and returned to 20% methanol for 0.1 min, when it was allowed to equilibrate for 5 min. A hybrid triple quadrupole/linear ion trap mass spectrometer (SCIEX 6500 QTrap, Applied Biosystems, Foster City, CA, USA) was used with nebulizer gas pressure set at 75 psi, drying gas pressure at 65 psi, curtain gas pressure at 15 psi, source voltage at 4.5 kV, and source temperature at 500°C.
Analysis of carotenoids
Br + 10 stage fruits were harvested, weighed, immediately frozen in liquid nitrogen, and stored at −80°C until needed. Plant materials (100 mg fresh weight) were frozen in liquid nitrogen, ground into a powder, and extracted with n-hexane: acetone: ethanol (2:1:1, v/v/v). The extract was vortexed (30 s), subjected to ultrasound-assisted extraction for 20 min at room temperature, and centrifuged (12,000 g for 5 min). The supernatants were collected, and the above steps were repeated. The two supernatants were collected, evaporated to dryness under a nitrogen gas stream, and reconstituted in 75% methanol (v/v). The solution was centrifuged, and the supernatant was collected for LC–MS (Liquid chromatography-mass spectrum) analysis.
Dual-LUC reporter assay
A dual-LUC assay of the transcriptional activation of the RIN, NOR, and FUL1 promoters by SlEIL1 was performed in Arabidopsis protoplasts extracted from 10-day-old seedlings. The 1- to 2-kb promoter regions were cloned into the pGreenII 0800-LUC double-reporter vector, while the SlEIL1 coding sequence was cloned into the pEAQ vector as an effector. The effector plasmid was transformed into protoplasts together with the reporter plasmid at a 2:1 ratio. Cells were cultured at 22°C for 16 h. In the control group, empty pGreen II 62-SK vector replaced the effector plasmid at the same concentration. The ratio of firefly (Photinus pyralis) relative to REN (Renilla reniformis) LUC activity was used as an indicator of the transcriptional efficiency of SlEIL1 for each promoter. Primers for vector construct are listed in Supplemental Table S2.
EMSA
Plasmid construction for the expression of recombinant SlEIL1 protein in Escherichia coli (primers are listed in Supplemental Table S2) and protein purification were conducted as described (Li et al., 2013). A standard binding reaction was performed in a total volume of 20 mL by incubating an appropriate amount of purified SlEIL1 protein with 20 fmol of Digoxigeninor biotin-labeled probe DNA and 1 mg poly (dI-dC) in buffer (25-mM HEPES-potassium hydroxide, pH 7.5, 100-mM KCl, 0.1-mM EDTA, 10% [v/v] glycerol, 1-mM DTT) at room temperature for 30 min. The binding reaction products were resolved on a 6% polyacrylamide gel run in 0.53 Tris–borate–EDTA. The oligonucleotide probes containing the putative EIN3 bind site in the promoter regions of RIN, NOR, and FUL1 are listed in Supplemental Table S2.
Quantitive Real Time-PCR (qRT-PCR)
Total RNA was extracted from the samples using an RNeasy-Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. One microgram of total RNA (RNeasy-Plant Mini Kit, Qiagen) was used to synthesize first-strand cDNA (RevertAid First Strand cDNA Synthesis Kit, Fermentas, Burlington, Canada). Transcript levels were determined by qPCR as described in Huang et al. (2015) using specific primers. The primer sequences used for qRT-PCR are listed in Supplemental Table S3. mRNA levels in the samples were quantified using three biological replicates. Each replicate was collected from more than 12 pooled seedlings or from more than three pooled fruits at the same time each day.
Statistical analysis
Two-tailed Student’s t tests were used to determine significant differences between populations (Supplemental Data Set 1).
Accession numbers
Sequence data from this article can be found in Supplemental Table S4, and the genetic materials generated in this article are listed in Supplemental Table S5.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Triple response assay of slein2-2 etiolated seedlings and fruit phenotypes of slein2-1 and slein2-2.
Supplemental Figure S2. Generation of the transgenic line SlEIN2pro:SlEIN2-HA-YFP and analysis of its ability to complement slein2-1.
Supplemental Figure S3. Statistics of fruit number, fruit-set rate, fruit diameter, parthnocarpic fruit rate, seed number per fruit, and fruit yield of WT and slein2-1.
Supplemental Figure S4. Detailed phenotypes of WT and slein2-1 at different developmental stages.
Supplemental Figure S5. Schematic diagrams showing the mutations of SlEILs (SlEIL1–SlEIL4) and SlEBFs (SlEBF1–SlEBF3) genes created through CRISPR/Cas9-mediated mutagenesis.
Supplemental Figure S6. Phenotypes of fruits and etiolated seedlings of different genotypes.
Supplemental Figure S7. Expression data of four SlEILs and three SlEBF genes.
Supplemental Figure S8. Fertility and fruit growth characteristics of WT and slein2-1.
Supplemental Figure S9. Relative mRNA levels of auxin biosynthesis and auxin transporter genes in 3 dpa WT and slein2-1 fruits.
Supplemental Figure S10. Validation of fruit size control capacity of ethylene in the tomato cultivar AC.
Supplemental Figure S11. Relative mRNA levels of ripening-related TFs genes in WT and slein2-1 fruits at different ripening stages.
Supplemental Figure S12. Examination of DNA and mRNA levels of 35S:NOR, 35S:RIN, and 35S:FUL1 transgenic plants in different genetic backgrounds.
Supplemental Figure S13. Ripening phenotype of sleil1 sleil2/SlEIL2 sleil3 sleil4 and SlEILs co-suppression fruits.
Supplemental Figure S14. Proposed working model for ethylene-mediated fruit growth and fruit ripening control.
Supplemental Table S1. List of primers used for CRISPR/Cas9 vector construction and validation in this study.
Supplemental Table S2. List of primers used to construct other vectors in this study.
Supplemental Table S3. List of primers used for qRT-PCR in this study.
Supplemental Table S4. Solanum lycopersicum ID numbers of the genes mentioned in this study.
Supplemental Table S5. Genetic materials module.
Supplemental Data Set 1 . Summary of statistical analyses.
Supplementary Material
Acknowledgments
We thank Dr. Ming-Chun Liu (Sichuan University, China) for kindly providing Nr, rin, and nor mutant seeds. We thank Dr. Jinzhe Zhang and Dr. Sanwen Huang (Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, China) for assistance in the generation of slein2 mutants in tomato cultivar AC. We thank Dr. Xing Wen (Southern University of Science and Technology, China) for technical guidance of protein immunoblotting.
Funding
This work was funded by the Shenzhen Science and Technology Program (KQTD20190929173906742 to H.G.), the National Key Research and Development Program of China (2019YFA0903904 to H.G.), the Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes (SUSTech) (2019KSYS006 to H.G.), the National Nature Science Foundation of China (32070322 to Y.Y.), and the research funding for Postdocs of Shenzhen (K20227505 to W.H.).
Conflict of interest statement. The authors declare that they have no conflict of interest.
Contributor Information
Wei Huang, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
Nan Hu, Key Laboratory of Plant Hormones and Development Regulation of Chongqing, School of Life Sciences, Chongqing University, Chongqing 401331, China.
Zhina Xiao, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
Yuping Qiu, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
Yan Yang, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
Jie Yang, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
Xin Mao, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
Yichuan Wang, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
Zhengguo Li, Key Laboratory of Plant Hormones and Development Regulation of Chongqing, School of Life Sciences, Chongqing University, Chongqing 401331, China.
Hongwei Guo, Department of Biology,Institute of Plant and Food Science, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China; Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China.
H.G. and W.H. conceived the program and designed the research plan. W.H. performed most experiments and data analysis. N.H. and Z.X. generated part of constructs and transgenic plants. Y.Q. participated in protein purification. J.Y. and X.M. participated in genotyping. Y.Y., Y.W., and Z.L. participated in research design and data analysis. W.H. and H.G. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Hongwei Guo (guohw@sustech.edu.cn).
References
- Adato A, Mandel T, Mintz-Oron S, Venger I, Levy D, Yativ M, Domínguez E, Wang Z, De Vos RCH, Jetter R, et al. (2009) Fruit-surface flavonoid accumulation in tomato is controlled by a SlMYB12-regulated transcriptional network. PLoS Genet 5: e1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- An F, Zhao Q, Ji Y, Li W, Jiang Z, Yu X, Zhang C, Han Y, He W, Liu Y, et al. (2010) Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22: 2384–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Giovannoni JJ (2006) Ripening in the tomato Green-ripe mutant is inhibited by ectopic expression of a protein that disrupts ethylene signaling. Proc Natl Acad Sci USA 103: 7923–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D (2000) The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemer M, Karlova R, Ballester AR, Tikunov YM, Bovy AG, Wolters-Arts M, Rossetto Pde B, Angenent GC, de Maagd RA (2012) The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell 24: 4437–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM (2020) Ethylene signaling in plants. J Biol Chem 295: 7710–7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD (2007) The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 19: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitel DA, Chappell-Maor L, Meir S, Panizel I, Puig CP, Hao Y, Yifhar T, Yasuor H, Zouine M, Bouzayen M, et al. (2016) AUXIN RESPONSE FACTOR 2 intersects hormonal signals in the regulation of tomato fruit ripening. PLoS Genet 12: e1005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumos J (2021) Gene regulation in climacteric fruit ripening. Curr Opin Plant Biol 63: 102042. [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- de Jong M, Mariani C, Vriezen WH (2009) The role of auxin and gibberellin in tomato fruit set. J Exp Bot 60: 1523–1532 [DOI] [PubMed] [Google Scholar]
- de Jong M, Wolters-Arts M, Schimmel BCH, Stultiens CLM, de Groot PFM, Powers SJ, Tikunov YM, Bovy AG, Mariani C, Vriezen WH, et al. (2015) Solanum lycopersicum AUXIN RESPONSE FACTOR 9 regulates cell division activity during early tomato fruit development. J Exp Bot 66: 3405–3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Pirrello J, Chen Y, Li N, Zhu S, Chirinos X, Bouzayen M, Liu Y, Liu MC (2018) A novel tomato F-box protein, SlEBF3, is involved in tuning ethylene signaling during plant development and climacteric fruit ripening. Plant J 95: 648–658 [DOI] [PubMed] [Google Scholar]
- Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58: 318–332 [DOI] [PubMed] [Google Scholar]
- Dubois M, Van den Broeck L, Inzé D (2018) The pivotal role of ethylene in plant growth. Trends Plant Sci 23: 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Turner SR (2012) Plant vascular cell division is maintained by an interaction between PXY and ethylene signaling. PLoS Genet 8: e1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA (2011) Gene structure and spatiotemporal expression profile of tomato genes encoding YUCCA-like flavin monooxygenases: the ToFZY gene family. Plant Physiol Biochem 49: 782–791 [DOI] [PubMed] [Google Scholar]
- Fenn MA, Giovannoni JJ (2021) Phytohormones in fruit development and maturation. Plant J 105: 446–458 [DOI] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Shima Y, Ito Y (2013) A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25: 371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Shima Y, Nakagawa H, Kitagawa M, Kimbara J, Nakano T, Kasumi T, Ito Y (2014) Transcriptional regulation of fruit ripening by tomato FRUITFULL homologs and associated MADS box proteins. Plant Cell 26: 89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Zhao WH, Qu HO, Wang QS, Zhao LX (2016) The yellow-fruited-tomato 1 (yft1) mutant has altered fruit carotenoid accumulation and reduced ethylene production as a result of a genetic lesion in ETHYLENE INSENSITIVE 2. Theor Appl Genet 129: 717–728 [DOI] [PubMed] [Google Scholar]
- Gao Y, Fan ZQ, Zhang Q, Li HL, Liu GS, Jing Y, Zhang YP, Zhu BZ, Zhu HL, Chen JY, et al. (2021) A tomato NAC transcription factor, SlNAM1, positively regulates ethylene biosynthesis and the onset of tomato fruit ripening. Plant J 108: 1317–1331 [DOI] [PubMed] [Google Scholar]
- Gao Y, Wei W, Fan Z, Zhao X, Zhang Y, Jing Y, Zhu B, Zhu H, Shan W, Chen J, et al. (2020) Re-evaluation of the nor mutation and the role of the NAC-NOR transcription factor in tomato fruit ripening. J Exp Bot 71: 3560–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Wei W, Zhao X, Tan X, Fan Z, Zhang Y, Jing Y, Meng L, Zhu B, Zhu H, et al. (2018) A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Hortic Res 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 14: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ (2007) Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol 10: 283–289 [DOI] [PubMed] [Google Scholar]
- Grierson D (2013) Ethylene and the control of fruit ripening. The Molecular Biology and Biochemistry of Fruit Ripening. Blackwell Publishing Ltd., Ames, IA, pp 43–73 [Google Scholar]
- Guo H, Ecker JR (2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Hao Y, Hu G, Breitel D, Liu M, Mila I, Frasse P, Fu Y, Aharoni A, Bouzayen M, Zouine M (2015) Auxin response factor SlARF2 is an essential component of the regulatory mechanism controlling fruit ripening in tomato. PLoS Genet 11: e1005649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Xian Z, Li N, Liu Y, Huang W, Yan F, Su D, Chen J, Li Z (2019) Rapid and user-friendly open-source CRISPR/Cas9 system for single- or multi-site editing of tomato genome. Hortic Res 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Peng S, Xian Z, Lin D, Hu G, Yang L, Ren M, Li Z (2017) Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol J 15: 472–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Xian Z, Kang X, Tang N, Li Z (2015) Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol 15: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstrate SW, van Bussel LJ, Cristescu SM, Cator E, Mariani C, Vriezen WH, Rieu I (2014) Tomato ACS4 is necessary for timely start of and progression through the climacteric phase of fruit ripening. Front Plant Sci 5: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Nishizawa-Yokoi A, Endo M, Mikami M, Shima Y, Nakamura N, Kotake-Nara E, Kawasaki S, Toki S (2017) Re-evaluation of the rin mutation and the role of RIN in the induction of tomato ripening. Nat Plants 3: 866–874 [DOI] [PubMed] [Google Scholar]
- Ito Y, Sekiyama Y, Nakayama H, Nishizawa-Yokoi A, Endo M, Shima Y, Nakamura N, Kotake-Nara E, Kawasaki S, Hirose S, et al. (2020) Allelic mutations in the Ripening-Inhibitor locus generate extensive variation in tomato ripening. Plant Physiol 183: 80–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Zeng J, Li Z, Song Y, Yan H, He J, Jiang Y, Duan X (2020) Redox regulation of the NOR transcription factor is involved in the regulation of fruit ripening in tomato. Plant Physiol 183: 671–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, Garrett WM, Kessenbrock M, Growth G, Tucker ML, et al. (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA 109: 19486–19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama H, Dan H, Imaseki H, Wasteneys GO (2004) Transient exposure to ethylene stimulates cell division and alters the fate and polarity of hypocotyl epidermal cells. Plant Physiol 134: 1614–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S (2008) Ethylene signaling in Arabidopsis involves feedback regulation via the elaborate control of EBF2 expression by EIN3. Plant J 55: 821–831 [DOI] [PubMed] [Google Scholar]
- Kumar R, Khurana A, Sharma AK (2014) Role of plant hormones and their interplay in development and ripening of fleshy fruits. J Exp Bot 65: 4561–4575 [DOI] [PubMed] [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ (1994) The Never Ripe mutation blocks ethylene perception in tomato. Plant Cell 6: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen K, Grierson D (2019) A critical evaluation of the role of ethylene and MADS transcription factors in the network controlling fleshy fruit ripening. New Phytol 221: 1724–1741 [DOI] [PubMed] [Google Scholar]
- Li S, Zhu B, Pirrello J, Xu C, Zhang B, Bouzayen M, Chen K, Grierson D (2020) Roles of RIN and ethylene in tomato fruit ripening and ripening-associated traits. New Phytol 226: 460–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma M, Feng Y, Li H, Wang Y, Ma Y, Li M, An F, Guo H (2015) EIN2-directed translational regulation of ethylene signaling in Arabidopsis. Cell 163: 670–683 [DOI] [PubMed] [Google Scholar]
- Li Z, Peng J, Wen X, Guo H (2013) Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 25: 3311–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MC, Chen Y, Chen Y, Shin JH, Mila I, Audran C, Zouine M, Pirrello J, Bouzayen M (2018a) The tomato ethylene response factor Sl-ERF. B3 integrates ethylene and auxin signaling via direct regulation of Sl-Aux/IAA27. New Phytol 219: 631–640 [DOI] [PubMed] [Google Scholar]
- Liu MC, Diretto G, Pirrello J, Roustan JP, Li Z, Giuliano G, Regad F, Bouzayen M (2014) The chimeric repressor version of an Ethylene Response Factor (ERF) family member, Sl-ERF.B3, shows contrasting effects on tomato fruit ripening. New Phytol 203: 206–218 [DOI] [PubMed] [Google Scholar]
- Liu MC, Gomes BL, Mila I, Purgatto E, Peres LE, Frasse P, Maza E, Zouine M, Roustan ZP, Bouzayen M, et al. (2016) Comprehensive profiling of ethylene response factor expression identifies ripening-associated ERF genes and their link to key regulators of fruit ripening in tomato. Plant Physiol 170: 1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MC, Pirrello J, Chervin C, Roustan JP, Bouzayen M (2015) Ethylene control of fruit ripening: revisiting the complex network of transcriptional regulation. Plant Physiol 169: 2380–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang Y, Feng Q, Qin L, Pan C, Lamin-Samu AT, Lu G (2018b) Tomato AUXIN RESPONSE FACTOR 5 regulates fruit set and development via the mediation of auxin and gibberellin signaling. Sci Rep 8: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovea J, Bjorklunda S, Vahala J, Hertzberg M, Kangasjarvi J, Sundberg B (2009) Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc Natl Acad Sci USA 106: 5984–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü P, Yu S, Zhu N, Chen YR, Zhou B, Pan Y, Tzeng D, Fabi JP, Argyris J, Garcia-Mas J, et al. (2018) Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat Plants 4: 784–791 [DOI] [PubMed] [Google Scholar]
- Martí E, Gisbert C, Bishop GJ, Dixon MS, García-Martínez JL (2006) Genetic and physiological characterization of tomato cv. Micro-Tom. J Exp Bot 57: 2037–2047 [DOI] [PubMed] [Google Scholar]
- McAtee P, Karim S, Schaffer R, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci 4: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurchie EJ, McGlasson WB, Eaks IL (1972) Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature 237: 235–236 [DOI] [PubMed] [Google Scholar]
- Merchante C, Brumos J, Yun J, Hu Q, Spencer KR, Enríquez P, Binder BM, Heber S, Stepanova AN, Alonso JM (2015) Gene-specific translation regulation mediated by the hormone signaling molecule EIN2. Cell 163: 684–697 [DOI] [PubMed] [Google Scholar]
- Mou W, Kao YT, Michard E, Simon AA, Li D, Wudick MM, Michael A, Lizzio M, Feijó JA, Chang C (2020) Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat Commun 11: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeller PW, Lu MW, Taylor LP, Pike DA, Theologis A (1991) Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254: 437–439 [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Yang SF (1995) The role of ethylene in fruit ripening. Acta Hortic 398: 167–178 [Google Scholar]
- Ozga JA, van Huizen R, Reinecke DM (2002) Hormone and seed specific regulation of pea fruit growth. Plant Physiol 128: 1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison RJ, Csukasi F, Zheng Y, Fei Z, van der Knaap E, Catalá C (2015) Comprehensive tissue-specific transcriptome analysis reveals distinct regulatory programs during early tomato fruit development. Plant Physiol 168: 1684–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D (1993) Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J 3: 469–481 [Google Scholar]
- Pierre-Jerome E, Drapek C, Benfey PN (2018) Regulation of division and differentiation of plant stem cells. Annu Rev Cell Dev Biol 34: 289–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR (. 2009) Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev 23: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, Ecker JR (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338: 390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V, Koornneef M (2001) Cell division activity during apical hook development. Plant Physiol 125: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Gupta S, Sarma S, Rai M, Sreelakshmi Y, Sharma R (2021) Mutations in tomato 1‐aminocyclopropane carboxylic acid synthase2 uncover its role in development beside fruit ripening. Plant J 106: 95–112 [DOI] [PubMed] [Google Scholar]
- Shin JH, Mila I, Liu M, Rodrigues MA, Vernoux T, Pirrello J, Bouzayen M (2019) The RIN-regulated small auxin-up RNA SAUR 69 is involved in the unripe-to-ripe phase transition of tomato fruit via enhancement of the sensitivity to ethylene. New Phytol 222: 820–836 [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Hao SH, Kojima M, Sakakibara H, Ozeki-Iida Y, Zheng Y, Fei ZJ, Zhong SL, Giovannoni JJ, Rose JKC, et al. (2015) Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J 83: 237–251 [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Nicolas P, Fernandez-Pozo N, Ma Q, Evanich DJ, Shi Y, Xu Y, Zheng Y, Snyder SI, Martin LBB, et al. (2018) High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat Commun 9: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sravankumar T, Naik N, Kumar R (2018) A ripening-induced SlGH3-2 gene regulates fruit ripening via adjusting auxin-ethylene levels in tomato (Solanum lycopersicum L.). Plant Mol Biol 98: 455–469 [DOI] [PubMed] [Google Scholar]
- Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z, Roustan JP, Bouzayen M, Giuliano G, Chervin C (2015) Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol 15: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ (2001) Members of the tomato LeEIL (EIN3‐like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26: 47–58 [DOI] [PubMed] [Google Scholar]
- van de Poel B, Bulens I, Markoula A, Hertog ML, Dreesen R, Wirtz M, Vandoninck S, Oppermann Y, Keulemans J, Hell R, et al. (2012) Targeted systems biology profiling of tomato fruit reveals coordination of the Yang cycle and a distinct regulation of ethylene biosynthesis during postclimacteric ripening. Plant Physiol 160: 1498–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poel B, van Der Straeten D (. 2017) Plant ethylene detection using laser-based photo-acoustic spectroscopy. Ethylene Signaling. Humana Press, New York, NY, pp 11–26 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Pan IL, Arroyo AJ, McQuinn R, Chung M, Poole M, Rose J, Seymour G, Grandillo S, Giovannoni JJ, et al. (2009) Fleshy fruit expansion and ripening are regulated by the tomato SHATTERPROOF gene TAGL1. Plant Cell 21: 3041–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni JJ (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177: 60–76 [DOI] [PubMed] [Google Scholar]
- Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latche A, Pech JC, Fernie AR, Bouzayen M (2009) Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21: 1428–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Angenent GC, Seymour G, de Maagd RA (2020) Revisiting the role of master regulators in tomato ripening. Trends Plant Sci 1: 291–301 [DOI] [PubMed] [Google Scholar]
- Wang R, da Rocha Tavano EC, Lammers M, Martinelli AP, Angenent GC, de Maagd RA (2019) Re-evaluation of transcription factor function in tomato fruit development and ripening with CRISPR/Cas9-mutagenesis. Sci Rep 9: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang P, Li X, Wang Y, Tian S, Qin G (2021) The transcription factor SlHY5 regulates the ripening of tomato fruit at both the transcriptional and translational levels. Hortic Res 8: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, Jiang L, Guo H (2012) Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res 22: 1613–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ (1995) An ethylene-inducible component of signal transduction encoded by Never-ripe. Science 270: 1807–1809 [DOI] [PubMed] [Google Scholar]
- Xin TX, Zhang Z, Li S, Zhang S, Li Q, Zhang ZH, Huang SW, Yang XY (2019) Genetic regulation of ethylene dosage for Cucumber fruit elongation. Plant Cell 31: 1063–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Lu X, Ma B, Chen SY, Zhang JS (2015) Ethylene signaling in rice and Arabidopsis: conserved and diverged aspects. Mol Plant 8: 495–505 [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu Y, Pirrello J, Regad F, Bouzayen M, Deng W, Li Z (2010) Silencing Sl-EBF1 and Sl-EBF2 expression causes constitutive ethylene response phenotype, accelerated plant senescence, and fruit ripening in tomato. J Exp Bot 61: 697–708 [DOI] [PubMed] [Google Scholar]
- Yokotani N, Nakano R, Imanishi S, Nagata M, Inaba A, Kubo Y (2009) Ripening-associated ethylene biosynthesis in tomato fruit is autocatalytically and developmentally regulated. J Exp Bot 60: 3433–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Li Y, Fan S, Wen T, Wang M, Zhang L, Zhao L (2021) The tomato WRKY32 transcription factor affects ripe fruit color by regulating YFT1, a core component of ethylene signal transduction. J Exp Bot 72: 4269–4282 [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen G, Zhou S, Tu Y, Wang Y, Dong T, Hu Z (2014) A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol 55: 119–135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.