Abstract
For most Gram-negative bacteria, pathogenicity largely depends on the type-III secretion system that delivers virulence effectors into eukaryotic host cells. The subcellular targets for the majority of these effectors remain unknown. Xanthomonas campestris, the causal agent of black rot disease of crucifers such as Brassica spp., radish, and turnip, delivers XopP, a highly conserved core-effector protein produced by X. campestris, which is essential for virulence. Here, we show that XopP inhibits the function of the host–plant exocyst complex by direct targeting of Exo70B, a subunit of the exocyst complex, which plays a significant role in plant immunity. XopP interferes with exocyst-dependent exocytosis and can do this without activating a plant NOD-like receptor that guards Exo70B in Arabidopsis. In this way, Xanthomonas efficiently inhibits the host’s pathogen-associated molecular pattern (PAMP)-triggered immunity by blocking exocytosis of pathogenesis-related protein-1A, callose deposition, and localization of the FLAGELLIN SENSITIVE2 (FLS2) immune receptor to the plasma membrane, thus promoting successful infection. Inhibition of exocyst function without activating the related defenses represents an effective virulence strategy, indicating the ability of pathogens to adapt to host defenses by avoiding host immunity responses.
XopP, a core bacterial effector of Xanthomonas campestris, manipulates the plant exocyst complex bypassing several host defense responses.
IN A NUTSHELL.
Background: Many phytopathogenic bacteria use type-III secretion systems to secrete protein effectors into the host plant cell cytoplasm, promoting their virulence. In defense, plants have evolved intracellular NOD-like receptors (NLRs) that recognize these effectors and activate host immunity. Some plant NLR receptors contain noncanonical protein domains known as integrated domains (IDs), which function as decoys for pathogen effectors. The origin of IDs as decoys may be from the duplication of the real targets of these bacterial effectors in host cells.
Questions: What host components are targeted by pathogen effectors? What are the mechanisms of virulence in these target/effector interactions? What essential physiological processes of the host plant do the effectors disrupt?
Findings: Building on our recent description of the NLR–ID model for effector interaction and defense activation, we screened a library of IDs with Xanthomonas core effectors and discovered several interesting interactions. We found that XopP, a Xanthomonas core-effector protein, interacted with a host EXO70-like ID without activating host immunity. Our in-depth investigations revealed that XopP interacts directly with EXO70B1, a component of the host exocyst complex. XopP blocked exocyst-dependent exocytosis of various immunity-promoting molecules in Nicotiana tabacum, N. benthamiana, and Arabidopsis cells, including pathogenesis-related protein-1A, the transmembrane immune receptor FLS2, and callose.
Next steps: We will conduct further screening to determine whether XopP targets other EXO70 paralogs. In this way, XopP will be a valuable tool to understand the role of effector–exocyst interactions during plant defense.
Introduction
Pathogens use common strategies to colonize their hosts. For the majority of the Gram-negative bacterial species, pathogenicity largely depends on the highly conserved Type-III protein Secretion System (T3SS) that delivers virulence proteins, known as effectors, into eukaryotic host cells. The type-III effector proteins (T3EPs) of pathogenic bacteria exert similar functions in eukaryotic host cells, often interfering with the host’s defense mechanisms (Staskawicz et al., 2001; Büttner and Bonas, 2003; Tampakaki et al., 2010; Duxbury et al., 2016; Dangl and Jones, 2019; Jo et al., 2019; Mermigka and Sarris, 2019). However, the role(s) and the particular subcellular targets of these T3EPs remain important open questions in molecular host–microbe interactions research field.
Bacterial species of the genus Xanthomonas are the causal agents of severe diseases on nearly 400 monocot and dicot plants, such as black rot of crucifers, bacterial blight of rice, black spot disease of mango, and bacterial spot disease of tomato and pepper. Thus, Xanthomonas has been used as an important model species for studies on bacterial pathogenicity and host adaptation (Niño-Liu et al., 2006; Ryan et al., 2011; An et al., 2020; Gluck-Thaler et al., 2020; Lu et al., 2020; Timilsina et al., 2020). Xanthomonas campestris pv. campestris (Xcc) infects the vascular system of members of the economically important plant family Brassicaceae, to which the model species Arabidopsis thaliana (Arabidopsis) belongs (Fargier et al., 2011). Besides the divergence of T3EPs secreted from different Xanthomonas, most of them are comprised by a core set of nine effectors (Ryan et al., 2011; Vicente and Holub, 2013), which in X. campestris is limited to only three T3EPs: XopP, XopF1, and XopAL1, and these are known as Xanthomonas outer proteins (Xops) (Roux et al., 2015).
The XopP effector was identified in X. campestris pv. vesicatoria using the AvrBs2/Bs2 reporter system (Roden et al., 2004). Though of unknown function, it was shown that XopP is essential for bacterial virulence in both monocot and dicot plant species. A phylogenetically distinct XopP homolog from Xanthomonas oryzae pv. oryzae (Xoo, XopPXoo) strongly suppresses pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) in Oryza sativa (rice) by targeting an ubiquitin-E3 ligase, PUB44, a positive regulator of plant immunity (Ishikawa et al., 2014). Recently, it was reported from the same research group that PUB44 interacts with rice PBI1, which is degraded during PTI. Either the presence of XopP or silencing of PUB44 effectively suppressed PBI1 degradation (Ichimaru et al., 2022). It is noteworthy that a XopP mutant generated by Tn5gusA5 insertion in the model strain X. campestris pv. campestris (Xcc 8004) results in significantly reduced infection of Raphanus sativus (radish) plants (Jiang et al., 2009).
The exocyst is a conserved eukaryotic protein complex, tethering post-Golgi secretory vesicles to the plasma membrane (PM). Besides its role to the conventional secretion, it also has a role in unconventional secretion and autophagy, plus a role in plant immunity (Elias et al., 2003; Saeed et al., 2019). In plants, as in animals and other eukaryotes, the exocyst complex consists of eight subunits: SEC3, SEC5, SEC6, SEC8, SEC10, SEC15, EXO70, and EXO84. The plant EXO70 subunit shows the largest paralog expansion (Elias et al., 2003; Zhang et al., 2010). The exocyst complex plays an important role in PTI and resistance against bacteria, oomycetes, and fungi in Arabidopsis (Pečenková et al., 2011; Stegmann et al., 2012, 2014). The Arabidopsis paralog EXO70B1 is guarded by the atypical NOD-like receptor (NLR) immune receptor TN2, whereas exo70B1 mutants activate TN2-mediated plant immunity leading to the hypersensitive response (HR) (Zhao et al., 2015), a type of suicidal cell death that constrains pathogen spread.
Here, we report that the core T3EP, XopP, associates with the Arabidopsis exocyst complex, resulting in inhibition of immune-associated exocytosis and the resulting suppression of the host PTI response. XopP inhibits both the secretion of pathogenesis-related protein-1A (PR1a) and the deposition of callose in stable transgenic Arabidopsis lines expressing XopP. Likewise, the interaction between XopP and two EXO70B paralogs affects the EXO70B-mediated trafficking of FLS2 to the PM (Wang et al., 2020). Interestingly, this occurs without activating TN2-dependent immunity. From our results, we hypothesize that the virulence effector XopP inhibits the proper assembly of the exocyst components, thus manipulating exocytosis without activating host NLR-dependent defenses.
Results
XopP and EXO70B1 interact in vitro and in vivo via their N-termini
Some host NLR immune receptors contain integrated domains (IDs) that act as decoys for pathogen effectors (Sarris et al., 2016). IDs fused to NLRs may be used as an effective tool to identify host proteins that are targeted by pathogens (Sarris et al., 2016; Landry et al., 2021). Based on this rationale, we performed yeast two-hybrid (Y2H) screenings between an EXO70-like ID, conserved in a wide range of NLRs, and potential interactors of the Xanthomonas core effectorome (the repertoire of effectors of a microorganism). The EXO70-like ID used in this study is integrated into the RGH2 NLR of Hordeum vulgare ssp. vulgare cv. Baronesse (Brabham et al., 2018). From binary Y2H screenings, eight potential interactions were identified, including an interaction with the XopP effector. Because the EXO70-like ID/XopP pair had not been found and studied so far, we decided to investigate this interaction further (Figure 1A; Supplemental Figure S1A). The impact and the important role of XopP (XC_2994) in the context of a physiological infection has been previously reported (Jiang et al., 2009). A significant reduction in virulence, bacterial population growth, and lesion length was reported for a XopP Tn5gusA5-insertion mutant of the model strain X. campestris pv. campestris 8004 (Jiang et al., 2009).
Figure 1.
XopP targets an EXO70-like ID and EXO70B1, and the interaction is mediated by their N-termini. A, Y2H assays showed that XopP targeted an EXO70-like ID. All the yeast combinations tested here grew in –LW minimal medium, but only the EXO70B1-like ID/XopP combination survived in –LWA minimal medium. Additional Y2H results are presented in Supplemental Figure S1A. B, Interaction of EXO70B1 with the XopP effector in a Y2H assay. All yeast combinations grew in –LW minimal medium, but only the EXO70B1/effector combination survived in –LWA minimal medium, in contrast to EXO70A1 (both splice forms). Additional Y2H results and negative controls are presented in Supplemental Figure S1, B–D. C, Schematic representations of the EXO70-like ID, EXO70B1, and XopP truncation constructs. The green color shows that the homology of the ID to EXO70B1 begins at residue 277 and ends at residue 699. The gray color represents the EXO70 domain (residues 265–614), as identified using the biological database SMART. Bar = 100 residues. The results of the Y2H assays for each construct are shown at the right of each construct. Only the EXO70B1-AB truncation, which corresponds to the first 388 residues, interacted with full-length XopP (upper), as it was observed in SC–LWA medium. D, Interaction of the XopP N-terminal truncation with EXO70B1-AB. All yeast combinations grew in –LW minimum medium and only the combination of EXO70B1-AB/XopP-N terminal truncations survived in the –LWA minimum medium. As above, Y2H assay results are shown to the right of the constructs. E, SEC elution profiles for XopP (yellow), EXO70B1 (blue), and XopP/EXO70B1 complex (purple). A shift to the left (earlier elution) of the XopP/EXO70B1 complex was observed. F, In planta validation of the EXO70B1/XopP interaction using a BiFC assay in N. benthamiana plants. EXO70B1 and XopP were fused at their C-termini with nVENUS and cCFP epitope tags, respectively, and the YFP signal was observed in confocal microscopy 3 dpi (left column). A maximum projection of the z-stack is shown at the right. As negative control, the A. thaliana EXO70A1 homolog was used with XopP, and no YFP signal was observed (middle column). Bars = 15 μm. Protein expression of negative interactions of BiFC and additional negative controls for BiFC are presented in Supplemental Figure 1, E and F, respectively. G, In planta validation of the EXO70B1/XopP interaction in N. benthamiana plants using a co-IP assay. EXO70B1 and XopP were fused at their C-termini with HF (6xHis and 3xFLAG) and Myc epitope tags, respectively, and an IP-flag assay was performed. XopP interacted with EXO70B1 as shown in the immunoblot when probed with an a-myc. XopP is 86 kDa and EXO70B1-HF is 77 kDa. As a negative control for XopP, the AvrRps4 effector (17 kDa) was used (red asterisk) and its expression is shown using the anti-Flag antibody. A red asterisk denotes where XopP was expected if it coprecipitated with AvrRps4. Additional negative control for EXO70B1 is presented in Supplemental Figure S1G. a-Myc, anti-Myc antibody; AD, activation domain of Gal4; BD, binding domain of Gal4; EV, empty vector; SMART, Simple Modular Architecture Research Tool.
To determine which EXO70 homolog is targeted by XopP, we performed genome mining in Arabidopsis using the EXO70-like ID as a bait. All hits in our analysis were EXO70B1-homologs with almost 73% identity, along with EXO70A1, with 64% identity. We cloned the EXO70B1 and EXO70A1 genes from Arabidopsis and performed Y2H assay using the XopP effector. EXO70A1 has two splice forms in Arabidopsis, and both forms were used in Y2H assays. Neither form interacted with XopP, whereas EXO70B1 interacted strongly with this effector (Figure 1B; Supplemental Figure S1B). The EXO70A1 expression of the splice forms was verified in Saccharomyces cerevisiae by immunoblot analysis (Supplemental Figure S1C). In our study, we also included EXO70B2, the closest EXO70B1 homolog, as well as EXO70F1, a phylogenetically distant homolog (Stegmann et al., 2012) and we found that both also interacted with XopP (Figure 1B).
We sought to further characterize the interaction between EXO70B1 and XopP and to determine the essential protein domains that are required for this association. We constructed three truncated versions of EXO70B1, naming them the AB, BC, and CD truncations according to the domains found in the close homolog EXO70B2 (Figure 1C) (Brillada et al., 2021). Our Y2H analyses showed that only the AB domain (1–388 amino acid [aa]) of EXO70B1 interacted with the XopP effector (Figure 1C). Expression of the EXO70B1 BC and CD constructs was verified via immunoblot (Supplemental Figure S1D). We next split the XopP into N- and C-terminal half truncation fragments (Figure 1D). For this, we used predictions based on the ClustalW algorithm to ensure that no structure motifs would be cleaved. Our Y2H results revealed that EXO70B1/XopP interaction occurred via their N-terminal half domains (Figure 1D).
To investigate whether the two proteins interact in vitro, we performed a size exclusion chromatography (SEC) experiment. In general, when two proteins form a complex, the molecular weight is increased and the complex is eluted earlier. When XopP and EXO70B1 were combined at a ratio of 1:1 in the SEC experiment, they were eluted earlier (Figure 1E, purple color) compared to the individual proteins (Figure 1E, yellow and blue colors).
Furthermore, we validated the EXO70B1/XopP interaction in planta by bimolecular fluorescence complementation (BiFC) and co-immunoprecipitation (co-IP) assays (Figure 1, F and G, respectively). For the BiFC assays, we transiently co-expressed the EXO70B1-nVENUS (the N terminus of YFP) and XopP-cCFP (the C-terminus of YFP) constructs in Nicotiana benthamiana leaves. As negative controls, we used the EXO70A1 homolog from Arabidopsis and the C-terminal truncation of XopP. In parallel, their in planta expression was verified via immunoblot (Supplemental Figure S1E). EXO70B1 interacted specifically with XopP (Figure 1F) but not with the XopP C-terminal truncation (Supplemental Figure S1F). Accordingly, XopP did not interact with EXO70A1 (Figure 1F).
For the co-IP assays, we transiently co-expressed EXO70B1-HF (6xHis-3Xflag C-terminal epitope tag) and XopP-myc in N. benthamiana leaves and performed a co-IP using anti-FLAG beads. XopP effector co-immunoprecipitated with EXO70B1 (Figure 1G). As a negative control, we used the AvrRps4 effector (Figure 1G; Supplemental Figure S1G) from a previous study (Sarris et al., 2015). All the above results suggest that XopP and EXO70B1 form a complex.
We also investigated the potential self-interaction of XopP and EXO70B1. Each protein appeared to interact with itself in both BiFC and Y2H assays (Supplemental Figure S2, A and B, respectively).
Additionally, in order to detect the exact subcellular localization site of the XopP/EXO70B1 interaction, we investigated whether EXO70B1, XopP, or a combination of the two co-localize with: (1) a PM marker (plasma membrane intrinsic protein 2A) (Nelson et al., 2007); (2) the early/late endosomal marker DsRed-FYVE [Fab 1 (yeast ortholog of PIKfyve), YOTB, Vac 1 (vesicle transport protein), and EEA1] (Voigt et al., 2005); and (3) the autophagosome marker ATG8a-mCherry after treatment with concanamycin A (Con-A) (Nelson et al., 2007). We decided to include the autophagosome marker because the EXO70B1 has been reported to be involved in autophagy-related transport to the vacuole (Kulich et al., 2013). Supplemental Figure S2, C–E showed likely co-localization between EXO70B1, XopP, or the EXO70B1/XopP complex with the PM marker (Supplemental Figure S2, C–E, respectively). A co-localization analysis was also performed for EXO70B1-YFP and the PM marker using Pearson’s and Spearman’s correlation (PSC) coefficients. The data from the analysis suggested that EXO70B1 may be localized to the PM (Supplemental Figure S2F). To further study the co-localization with the PM marker, we performed fractionation of EXO70B1-HF, infiltrated into N. benthamiana leaves along with GUS-myc (negative control) or XopP-myc. EXO70B1 accumulation was observed mainly in the PM and nuclear fractions, in both the absence and presence of the XopP effector (Supplemental Figure S2G).
Arabidopsis EXO70B1 interacts with X. campestris XopP but not with other homologs, and the E347 residue is essential for this interaction
To determine whether the interaction of EXO70B1 and the X. campestris (Xc) XopP homolog is specific, we performed a phylogenetic analysis of all XopP-homologs, using a maximum-likelihood method as described in “Materials and methods” (Supplemental Data Set 1). Our results identified five subgroups, including the Ralstonia group, the Acidovorax group, and three Xanthomonas groups indicated as XopP1, XopP2 (Michalopoulou et al., 2018), and the EXO70B interacting XopP of Xc, respectively (Figure 2A; Supplemental Data Set 2). We cloned a XopP-homolog from each subgroup: Hlk3 from the Ralstonia group (RsHlk3), XopP from the Acidovorax group (AcXopP), and XopP1 and XopP2 from the monocots pathogen X. oryzae pv. oryzicola (XocXopP, XocXopP2), whose genome was recently sequenced (Michalopoulou et al., 2018). In Y2H screenings, EXO70B1 did not interact with these XopP homologs, whereas the XocXopP1 interacted with EXO70F1 homolog (Figure 2B), indicating specificity regarding the XopP/EXO70 interactions. The expression of all XopP homologs was verified via immunoblot analysis (Supplemental Figure S3A).
Figure 2.
The EXO70B–XopP interaction is specific for EXO70B from dicots and Xcc and the E347 amino acid of EXO70B1 is essential for this interaction. A, Phylogenetic analysis of XopP homologs. XopP homologs were grouped in different clusters and share identity with XopP within a range from 99% to 25%. The homologs presented in bold were used for the interaction assays. B, EXO70B1 interacted specifically with XopP and not with other orthologs. In contrast, AtEXO70F1 interacted only with XocXopP1. Additional information regarding the expression of XopP homologs in S. cerevisiae is presented in Supplemental Figure S3A. C, Y2H assays using XopP and ObEXO70B_small and _long versions. Both versions of ObEXO70B failed to interact with the effector. Additional information regarding the expression of noninteractors in S. cerevisiae is presented in Supplemental Figure S3C. D, Y2H screenings of XopP with EXO70B1, EXO70B1E347K, ObEXO70B_small or ObEXO70B_smallK134E. The mutated ObEXO70B gains an interaction with the XopP effector, but AtEXO70B1E347K still interacted with the effector. The protein alignment for all the XopP interactors is presented in Supplemental Figure S3D.
Additionally, to investigate the interspecies specificity of XopP, we cloned an EXO70B homolog from the wild relative of common rice, Oryza brachyantha (Ob). Interestingly, during the cloning, we discovered a smaller, version of EXO70B lacking the first 234 aa that had not been annotated. We cloned and used both the small and the long versions of ObEXO70B as baits (hereafter ObEXO70B_small and ObEXO70B_long, respectively) (Supplemental Figure S3B). In Y2H assays, we did not observe an interaction of XopP with the monocot ObEXO70B (both small and long versions) (Figure 2C). In parallel, their expression was verified via immunoblot analysis (Supplemental Figure S3C).
To identify potential amino acid changes in the noninteracting proteins, we next produced an amino acid alignment using all XopP-interacting and noninteracting EΧΟ70s, including the EXO70-like ID. Our alignment portions on the XopP/EXO70B-interacting platform. Even though the N-terminal parts of these proteins seem to be quite divergent, we identified only 1 aa substitution in the ObEXO70B_small, a Lys (K134) instead of a Glu in the rest of the EXO70s we aligned (Supplemental Figure S3D).
To test whether Glu is an essential amino acid residue for the XopP/EXO70B interaction, we constructed mutated forms of ObEXO70B_small and EXO70B1, corresponding to K134E and E347K, respectively. Using Y2H, we observed a partial but significant interaction between XopP/ObEXO70B_smallK134E (Figure 2D). However, AtEXO70B1E347K still interacted with XopP. Thus, the Glu residue seems to be necessary but not sufficient for XopP/EXO70 interaction.
XopP associates with multiple components of the exocyst complex but reduces the levels of only EXO70B1 and EXO84B
To determine whether XopP has a wider range of targets among the components of the two exocyst subcomplexes (Heider et al., 2016; Mei et al., 2018; Synek et al., 2021), we cloned the seven exocyst components of Arabidopsis (including SEC15a, SEC15b, EXO84b, and the EXO84c paralogs) and used the encoded proteins as preys for Y2H analysis and investigated whether they interact with XopP and EXO70B1. As previously reported, we confirmed the interactions between EXO70B1 and SEC5a as well as between SEC15b and EXO84b (Kulich et al., 2013; Zhao et al., 2015) (Figure 3A; Supplemental Figure S4A). We also observed a relatively weak interaction of EXO70B1 with SEC3a (Figure 3A; Supplemental Figure S4A), as previously described (Zhao et al., 2015). These interactions were mediated by the first 388 aa residues of EXO70B1 (AB-truncation) (Supplemental Figure S4B). Using Y2H assays, we also investigated the interactions between the components of the exocyst subcomplex II. Our analysis revealed an interaction pattern similar to the one previously described for subcomplex II of other eukaryotes (Hála et al., 2008; Heider et al., 2016), similar to recent findings in Arabidopsis plants (Synek et al., 2021). More specifically, SEC15b interacted with both SEC10a and EXO84b (Figure 3A; Supplemental Figure S4D). We also validated in planta all of the above interactions via BiFC assays (Figure 3A; Supplemental Figure S4, C and E).
Figure 3.
XopP associates with three components of the exocyst sub-complex II and reduces the EXO70B1 and EXO84B protein levels. A, Diagram of interactions as determined by Y2H and BiFC assays. Shown are confirmed binary interactions of XopP and EXO70B1 with the other components of the exocyst complex of A. thaliana as well as the interactions between the components of SC-II, via Y2H (solid lines) and BiFC (dashed lines) interactions. First, EXO70B1 and XopP were cloned as baits and the other components of the exocyst complex were cloned as preys, and all possible interactions were tested. In addition, SEC15b was also cloned as bait and was transformed into S. cerevisiae in combination with SEC10a or EXO84b that were cloned as preys. All plasmids were transformed into either the PJ694a or the AH109 strain of S. cerevisiae. Solid lines represent Y2H interactions. Dashed lines represent the observed BiFC interactions. All possible interactions with EXO70B1 and XopP as baits were made and the absence of lines represents negative interactions. The only combinations that were not tested were XopP with SEC6, SEC8, and SEC15a. Additional verification of the interactions in planta via BiFC is presented and described in Supplemental Figure S4. B, EXO70B1 and XopP in planta localization and their co-localization in N. benthamiana leaves. EXO70B1 and XopP were fused C-terminally with YFP and mCherry fluorophore tags and each construct, as well as their combinations, were infiltrated into N. benthamiana leaves. EXO70B1 was localized not only at the PM but also into the nucleus. XopP had the same localization pattern as EXO70B1, but was found in the nuclear periphery instead of inside the nucleus. In co-localization of both proteins, their localization was not altered, but the EXO70B1–YFP fluorescence signal was significantly reduced. The confocal localizations were repeated three times with similar results. All parameters were the same for the two different conditions. Bars = 10 μm. Additional data are presented in Supplemental Figure S2F. C, Quantification of the protein levels of some representative components of the subcomplex I and subcomplex II of the exocyst, in the presence and absence of XopP. Nicotiana benthamiana leaves were infiltrated with A. tumefaciens GV3101 carrying any of SEC6-HF, SEC8-HF, SEC10a-HF, EXO70B1-HF, or EXO84b-HF in combination with XopP-myc or GUS-myc (as a negative control). Protein extractions were performed at 3 dpi, and immunoblotting with an anti-flag antibody showed reduced levels of EXO70B1 and EXO84b in the presence of XopP, but no reduced levels for the rest of the exocyst components. The quantifications were performed using Image J and the statistical analyses performed using multiple t tests. The P-value for EXO70B1 between mock and XopP was 9.4E-05 and P-value for EXO84B between mock and XopP was 0.00015. The experiment was repeated three times with similar results. Data in (C) show means 96.94 ± 3.76 for Sec6, 101.80 ± 2.27 for Sec8, 98.96 ± 1.04 for Sec10, 52.35 ± 2.01 for EXO70B1, and 55.29 ± 2.26 for EXO84B. The parameters of the statistical analysis are shown in Supplemental Data Set 3. A representative immunoblot is presented in the Supplemental Figure S5A. D and E, EXO70B1 localization in Arabidopsis leaves (D) and quantification of total protein levels (E). EXO70B1 tagged C-terminally with YFP for localization studies or tagged with HF for immunoblotting was co-infiltrated with XopP-myc in Arabidopsis leaves. The C-terminal region of XopP was used as a negative control. When the effector was present, EXO70B1-YFP fluorescence was reduced, as was the total level of EXO70B1-HF. A representative immunoblot is shown in Supplemental Figure S5B. The statistical analysis from three independent experiments was performed using paired parametric t test and P-value was 0.0022. Data in (E) show means 11.77 ± 7.21. The parameters of the statistical analysis are shown in Supplemental Data Set 3. Bars in (D) = 10 μm. SC-II, subcomplex II. **P < 0.005, ***P < 0.0005.
Furthermore, in Y2H assays, we noticed that XopP interacted with two other components of the exocyst complex, namely SEC10a and EXO84b (Figure 3A; Supplemental Figure S4F). These interactions were mediated by the N-terminal half of the effector (Supplemental Figure S4G). However, in BiFC analyses, we could only validate our Y2H results for the XopP/EXO84B pair (Supplemental Figure S4H). In the case of XopP and SEC10, we could not detect any YFP signal, although SEC10a-nVENUS was normally expressed in N. benthamiana leaves (Supplemental Figure S4, H and I, respectively).
We sought to further investigate the molecular function of the effector, regarding the integrity of the exocyst components. When EXO70B1-YFP co-localized with XopP-mCherry in transient expression studies in N. benthamiana, we observed a downregulation of YFP signal (Figure 3B). This reduction could mean that in the presence of XopP, EXO70B1 is degraded, a result consistent with what we had already observed in PM and nuclear fractions of EXO70B1 when co-expressed with XopP (Supplemental Figure S2F). To verify the above observations, we performed immunoblot analysis to examine the levels of EXO70B1 and the other XopP interactors, EXO84b and SEC10a, in the presence and absence of XopP. All the proteins for this immunoblot analysis were transiently expressed in N. benthamiana leaves. As a negative control, we used some exocyst components of the subcomplex I that do not associate with XopP. Levels of EXO70B1 and EXO84b were reduced in the presence of XopP (Figure 3C; Supplemental Figure S5A), whereas levels of SEC10a, SEC6, and SEC8 were unaffected (Figure 3C; Supplemental Figure S5A).To verify the above observations in Arabidopsis, we transiently co-expressed EXO70B1-YFP or EXO70B1-HF with XopP-myc in Arabidopsis leaves. As a negative control, we used the XopP C-terminal truncation construct. Confocal and immunoblot analysis verified that EXO70B1 protein levels are downregulated in the presence of XopP (Figure 3, D and E; Supplemental Figure S5B).
EXO70B1 downregulation caused by XopP is autophagy independent
EXO70B1 is known for its additional positive role in autophagy (Kulich et al., 2013; Pečenková et al., 2017). Recently, the EXO70B2 was also shown to be degraded via the same pathway (Brillada et al., 2021). Because XopP causes a reduction in the level of EXO70B1, we investigated whether this downregulation is mediated by the autophagy pathway. We already had observed that, when XopP was present, EXO70B1-YFP did not co-localize with the intravacuolar autophagic bodies in co-expression assays using ATG8a-mCherry (Supplemental Figure S2E; Figure 4A). Additionally, we transiently co-expressed the EXO70B1-HF/XopP-myc or GUS-myc proteins in N. benthamiana leaves, and 2-day post infiltration (dpi), we treated them with 1-μM ConA, a vacuolar H+-ATPase inhibitor. We observed that ConA did not attenuate XopP-induced EXO70B1 downregulation (Figure 4B; Supplemental Figure S6A), thus suggesting that the degradation pathway is autophagy independent.
Figure 4.
The downregulation of EXO70B1 levels caused by XopP effector is autophagy independent. A, Co-localization of EXO70B1 with the autophagosome marker Atg8a-mCherry in the presence and absence of XopP using the transient expression system in N. benthamiana leaves. Following treatment with 1-μM ConA, EXO70B1 tagged C-terminally with the YFP epitope tag did not co-localize with Atg8a-mCherry. The same results were obtained in the presence of XopP. Bars = 10 μm. Additional images are shown in Supplemental Figure S2, C and E. B, Quantification of EXO70B1 levels following treatment with the autophagy inhibitor, ConA. ConA treatment did not reveal any autophagy-dependent pathway of EXO70B1 downregulation caused by XopP. The quantification was performed using Image J software, and the samples were normalized by CBB staining of the PVDF membrane. The experiment was repeated four times with similar results. Data show means 43.92 ± 16.26 for XopP and 49.25 ± 14.98 for XopP+ConA. Statistical analysis was performed with paired parametric t test, and P-values were 0.0062 between GUS and XopP and 0.0076 between GUS and XopP+ConA. The parameters of the statistical analysis are shown in Supplemental Data Set 3. A representative immunoblot is shown in Supplemental Figure S6A. C, Localization of EXO70B1 in the presence or absence of XopP in transgenic atg9-3 Arabidopsis leaves stably expressing EXO70B1-YFP. The atg9-3+EXO70B1-YFP plants were infiltrated with XopP-myc or infiltration solution and images were taken 3 dpi via confocal microscopy. EXO70B1-YFP fluorescence decreased in the presence of the XopP effector. The experiment was repeated three times with similar results. Bars = 10 μm. A representative immunoblot is shown in Supplemental Figure S6B. The same experiment was also performed with transient expression of EXO70B1 in Arabidopsis and the results are shown in Supplemental Figure S6C. D, Quantification of the EXO70B1-YFP levels of atg9-3 Arabidopsis plants expressing EXO70B1-YFP in the absence (mock) and presence of XopP. The quantification was performed using Image J software, and the three experiments from Figure 4C are included in the analysis. Statistical analysis was performed with paired parametric t test and P-value was 0.0041. A representative immunoblot is shown in Supplemental Figure S6B. The parameters of the statistical analysis are shown in Supplemental Data Set 3. Data show means 34.27 ±7.30 in the case of XopP. A representative immunoblot from the transient expression of EXO70B1 in Arabidopsis is shown in Supplemental Figure S6D. **P < 0.005, ***P < 0.0005.
To verify the above observations also in Arabidopsis, we generated transgenic Arabidopsis lines expressing EXO70B1-YFP in the atg9-3 mutant background. The atg9-3 loss-of-function Arabidopsis mutants have compromised autophagosome progression from the ER membrane (Zhuang et al., 2017). In our transgenic plants, we transiently expressed XopP-myc and at 3 dpi used confocal microscopy to observe EXO70B1-YFP levels in comparison to the mock treatment (infiltration solution) (Figure 4C). Additionally, we performed an immunoblot analysis using the anti-C-terminal YFP antibody and found that downregulation of EXO70B1 caused by XopP was autophagy independent (Figure 4D; Supplemental Figure S6B). The result was similar when both EXO70B1-YFP and EXO70B1-HF were transiently co-expressed with XopP-myc in atg9-3 Arabidopsis leaves (Supplemental Figure S6, C and D).
XopP inhibits the interaction between the components of the exocyst sub-complex II and the interaction between the two subcomplexes
Because XopP associates with more than one component of the exocyst complex, we hypothesized that it may act as an inhibitor of their interactions and the proper assembly of the exocyst subcomplex II (Ahmed et al., 2018; Mei et al., 2018; Synek et al., 2021). Using yeast-three hybrid (Y3H) analyses, we tested the potential interaction between EXO70B1 and SEC5a or SEC15b, as well as between SEC15b and SEC10a, in the presence of XopP. We observed inhibition of the interaction between the EXO70B1/SEC15b and EXO70B1/SEC5a pairs when XopP was expressed (Figure 5A). EXO70B1/EXO84b failed to interact, even in the absence of XopP (Supplemental Figure S7A). However, EXO84b interacted with SEC15b and in the presence of XopP this interaction was also inhibited (Supplemental Figure S7A). Additionally, in the presence of XopP, the SEC15b/SEC10a association was inhibited (Figure 5A).
Figure 5.
XopP inhibits the EXO70B1/SEC5a, EXO70B1/SEC15b, and SEC15b/SEC10a interactions in both Y3H and in planta assays. A, XopP interfered with EXO70B1/SEC5a, EXO70B1/SEC15b, and SEC15b/SEC10a associations in a Y3H analysis. The constructs were transformed into the Y3H-compatible yeast strain AH109. EXO70B1 interacted with SEC5a and SEC15b, and the latter associated with SEC10a, as shown by growth in medium lacking Leu, Trp, and Ade (SC–LWA). When Met was absent from the medium, XopP expression was induced by the MET25pro-inducible promoter and the above associations were inhibited, as shown in medium lacking Leu, Trp, Ade, and Met (SC–LWAM). B, Validation of XopP interference in exocyst components interactions, via FRET-SE. EXO70B1-YFP was co-infiltrated into N. benthamiana leaves with either SEC15b-mCherry or SEC5a-mCherry in the presence of the XopP-myc effector. As negative controls, AvrRps4-myc and/or GUS-myc were used. When XopP was present, the EXO70B1/SEC15b and EXO70B1/SEC5a interactions were inhibited. The images were taken 3 dpi via a Leica SP8 confocal laser microscopy. The box plots indicate the relative FRET efficiency (%). See the “Statistical analysis” section for a description of the symbols used. The significance test is Wilcoxon and the P-values are indicated. Data represent three independent experiments, each containing 70 measurements (n = 210 measurements). The parameters of the statistical analysis are shown in Supplemental Data Set 3. Visualization of representative images is presented in Supplemental Figure S7.
Furthermore, to support our Y3H results, we used Försters resonance energy transfer sensitized emission (FRET-SE). We transiently co-expressed EXO70B1-YFP with SEC15b-mCherry or SEC5a-mCherry in N. benthamiana leaves in either the presence or absence of XopP. As negative controls, the AvrRps4 and/or GUS were used. When XopP was expressed, EXO70B1 interactions with SEC15b and SEC5a were significantly reduced (Figure 5B; Supplemental Figure S7B). Expression in planta of either XopP-myc or AvrRps4-myc/GUS-myc was verified by immunoblot analysis (Supplemental Figure S7C). Via Y3H assay, we were also able to exclude any possible effect of AvrRps4 on EXO70B1/SEC5A and EXO70B1/SEC15B interactions (Supplemental Figure S7D).
XopP targets EXO70B without activating its guarding NLR immune receptor or inhibiting EXO70B1/RIN4 association
The EΧΟ70B1 is guarded by the atypical NLR immune receptor TN2 (Zhao et al., 2015). Moreover, the ubiquitination of EXO70B1 by the AvrPtoB effector of Pseudomonas syringae, leads to TN2-dependent HR induction in both Arabidopsis and tobacco (Nicotiana tabacum, Wang et al., 2019).
To investigate whether EXO70B1 targeting by XopP also activates TN2-dependent immunity, we cloned the TN2 resistance gene from Brassica napus (Bn; rapeseed). We tagged the TN2 protein with the YFP C-terminal tag (BnTN2-YFP). BnTN2 is a close homolog to A. thaliana TN2, sharing 82% similarity (Supplemental Figure S8A). We verified TN2 expression in planta using confocal microscopy (Figure 6A), and transiently expressed BnTN2-YFP with or without EXO70B1-HF in N. tabacum leaves. A TN2-dependent HR induction was observed (Figure 6, B and C), and this was abolished when TN2 was co-expressed with EXO70B1-HF in a 1:1 ratio (using the same culture density of Agrobacteria) (Figure 6, B and C), in agreement with previous results (Wang et al., 2019). Interestingly, and unlike AvrPtoB (Wang et al., 2019), XopP-myc did not re-activate the TN2-dependent HR when co-expressed with BnTN2/EXO70B1 complex (Figure 6, B and C). Our results led us to assume that XopP did not lead to proteolysis of EXO70B1, which is the case for AvrPtoB. Besides, we verified that XopP did not interact with TN2 in Y2H assays (Supplemental Figure S8B). The expression of TN2 in S. cerevisiae was confirmed via immunoblotting (Supplemental Figure S8C). Using Y2H assays, we also investigated which part of EXO70B1 interacted with TN2, and we found that EXO70B1 interacts via its N-terminus, as does XopP (Supplemental Figure S8D). The expression of all EXO70B1 truncations in S. cerevisiae was confirmed via immunoblot analysis (Supplemental Figure S8E).
Figure 6.
XopP targets EXO70B without activating its guarding NLR immune receptor, TN2. A, Expression in N. benthamiana leaves of BnTN2 fused C-terminally to the YFP epitope tag. Micrographs were obtained via confocal microscopy at 3 dpi. Bars = 10 μm. B and C, Transient co-expression of the NLR immune receptor BnTN2-YFP with EXO70B1-HF and XopP-myc in N. tabacum leaves (B) and quantification of the resulting HR (C). EXO70B1-HF suppressed the BnTN2-YFP-dependent HR induction when combined in a 1:1 ratio. In contrast, XopP-myc itself was not able to abolish the BnTN2-YFP-dependent HR induction at the 1:1 expression ratio. However, when combining all three constructs in a 1:1:1 ratio, no HR reaction was observed at 72 hpi. The experiment was repeated four times with identical results. The vertical axis shows the average number of plants from all four experiments that either produce or do not produce the HR. For the HR quantification, 10 plants were used for the statistical analysis (four independent experiments) and two-way ANOVA tests were performed. The P-value for interaction was <0.0001. Additional Y2H screenings and in planta experiments using TN2 are presented in Supplemental Figure S8. Bar = 1 cm.
RIN4, a known regulator of plant defense, has been reported to interact and recruit EXO70B1 to the PM in N. benthamiana. This interaction is shown to be inhibited by the AvrRpt2 effector protein of P. syringae pv. pisi (Sabol et al., 2017; Ray et al., 2019). We investigated whether the observed downregulation of EXO70B1 in the PM fraction is a consequence of XopP interference with the EXO70B1/RIN4 interaction. In Y3H assay, we observed an interaction between EXO70B1 and RIN4, and this interaction was not attenuated by XopP (Supplemental Figure S8F). Thus, the reduction of PM EXO70B1 protein levels could not be attributed simply to an effect of XopP on the EXO70B1/RIN4 association.
Membrane localization of the immune receptor FLS2 is inhibited by XopP
Both EXO70B1 and EXO70B2 regulate the trafficking of the transmembrane immunity pattern recognition receptor (PRR) FLAGELLIN SENSITIVE2 (FLS2) to the PM (Wang et al., 2020). The FLS2 receptor is responsible for both the recognition of a bacterial flagellin epitope (flg22) in plants and the activation of the flg22-dependent PTI (Wang et al., 2020). We investigated whether XopP acted as a negative regulator of EXO70B-dependent localization of FLS2 to the PM. To test this hypothesis, we analyzed the fluorescence intensity of the FLS2–GFP fusion itself or when transiently co-expressed with XopP-mCherry in N. benthamiana plants. When XopP was present, a significant reduction of approximately 50% of the FLS2–GFP signal at the PM was observed (Figure 7A; Supplemental Figure S9A).
Figure 7.
Localization of the transmembrane immune receptor FLS2 localization to the PM is downregulated in the presence of XopP. A, The box plot shows the intensity of FLS2–GFP transient expression at the PM of N. benthamiana leaves, in the presence (XopP column) or absence (control column) of XopP-mCherry. See the “Statistical analysis” section for a description of the symbols used. The significance test used was Wilcoxon and the P-values are indicated. Data represent three independent experiments, each containing 77 measurements (n = 232 measurements). The parameters of the statistical analysis are shown in Supplemental Data Set 3. Representative images are presented in Supplemental Figure S9A. B, The box plot shows the intensity of FLS2–GFP expression at the PM of transgenic FLS2–GFP Arabidopsis plants that were infected with Pf0-1 EtHAn carrying either the full-length XopP (XopP column) or the C-terminal truncation of XopP (control column). The significance test used was Wilcoxon and the P-values are indicated. Data represent two independent experiments, each containing 95 measurements (n = 190 measurements). The parameters of the statistical analysis are shown in Supplemental Data Set 3. Representative images and positive control for Pf0-1 infections are presented in Supplemental Figure S9, B and C, respectively. C, Quantification of FLS2 PM localization in WT and XopP-E transgenic Arabidopsis plants. Fractionation was performed using WT plants and a T3 Arabidopsis line (22-1-4) expressing XopP-mCherry. The levels of FLS2 were reduced in XopP-E plants. Quantification was performed using Image J software, and the samples were normalized with the H+-ATPase expression levels. The phenotypes of T1 and T3 XopP-E transgenic Arabidopsis plants are shown in Supplemental Figure S9D. A representative immunoblot is presented in Supplemental Figure S9E. AU, absorbance units.
To further validate our observations, we used Arabidopsis transgenic plants expressing FLS2–GFP (Beck et al., 2012). For this, we transformed Pseudomonas fluorescens (Pf01) strain EtHAn (Thomas et al., 2009) with the pEDV3 vector (Sohn et al., 2007), which contains the XopP effector. The pEDV3 vector contains the T3S signal of AvrRps4 and proteins cloned into this vector are fused to this secretion signal. We also cloned the XcvXopQ effector into the pEDV3 vector, and when we inoculated N. tabacum and Arabidopsis with Pf01-XcvXopQ, we observed HR at 36-h post-inoculation (hpi), thus confirming the translocation of Xanthomonas effectors via the pEDV3/Pf01 secretion system (Supplemental Figure S9C).
We then inoculated the FLS2–GFP transgenic Arabidopsis with our Pf01-XopP strain. A significant reduction of the FLS2–GFP signal at the PM was observed at 30 hpi (Figure 7B; Supplemental Figure S9B). As a negative control, we used the pEDV3 vector containing the XopP-C terminal truncation, and this did not interact with EXO70B1 (Supplemental Figure S9B).
Furthermore, we decided to investigate the reduced accumulation of the FLS2 receptor at the PM in Arabidopsis plants. For this, we generated stable transgenic Arabidopsis lines expressing an XopP–mCherry fusion under the transcription regulation of the Rubisco promoter. The XopP-expressing (XopP-E) Arabidopsis lines did not show any altered phenotype (Supplemental Figure S9D). Next, we performed a PM/cytosolic fractionation experiment in both Arabidopsis wild-type (WT) and XopP-E plants. In the XopP-E lines, we observed a reduction of the PM-localized FLS2 signal compared to WT plants, thus concluding that XopP downregulates accumulation of the FLS2 protein at the PM via EXO70Bs interaction (Figure 7C; Supplemental Figure S9E).
XopP inhibits the exocytosis of the PR1a
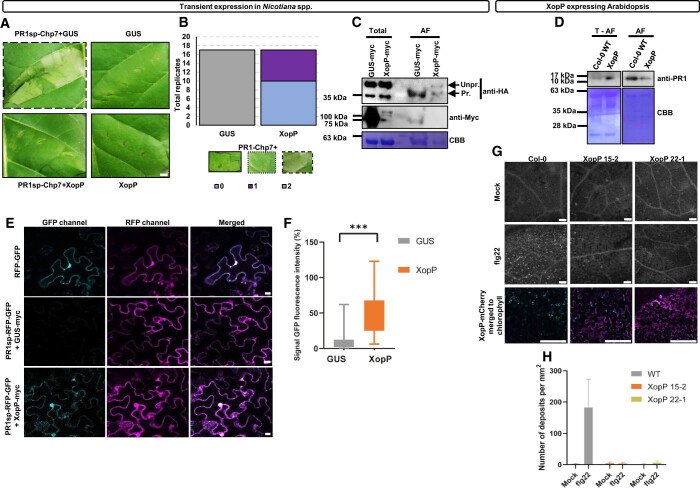
PR1a is produced in plants as part of the PTI response, and it depends on the exocyst complex for trafficking and secretion (Gu and Innes, 2012; Du et al., 2015). Recently, it was found that it contains a C-terminal motif that is essential for the transport from the ER to the apoplast (Pečenková et al., 2022). Our finding regarding the XopP targeting of the exocyst complex led us to speculate that this effector may inhibit exocytosis during PTI activation. To investigate this hypothesis, we initially checked whether PR1a secretion is compromised by XopP. For this, we used the PR1a secretion peptide (PR1sp) fused to the Ser protease effector Chp7 of Clavibacter michiganensis ssp. sepedonicus. Chp7 is an apoplastic effector that induces cell death in N. tabacum only when it is targeted to the apoplast but not when it occurs intracellularly (Lu et al., 2015). After co-expressing the chimeric PR1sp-Chp7 construct together with XopP or GUS (negative control) in the transient system of N. tabacum leaves, we observed induction of the HR at 3 dpi when the combination of PR1sp-Chp7/GUS was co-expressed, but not for the PR1sp-Chp7/XopP combination (Figure 8, A and B).
Figure 8.
XopP inhibits both the exocytosis of PR1a and the deposition of callose. A and B, HR inhibition assay (A) and quantification (B). XopP inhibited the apoplastic HR induction by the chimeric protein PR1sp–Chp7. Inhibition of the HR (left lower lane) appeared 3 dpi in N. tabacum leaves. GUS protein was used as an HR negative control. The expression of GUS or XopP alone did not induce HR (right lanes). Data represent three independent experiments, each containing approximately five measurements (n = 17 measurements). Additional negative controls are presented in Supplemental Figure S10, A–D. For the quantification, the data are from four independent experiments, each containing approximately five measurements (n = 17). Representative images are shown along with the score, where 0 = no HR, 1 = medium HR, 2 = HR in all the area of the infiltrated leaf. The statistical analysis was made with two-way RM ANOVA (Interaction P <0.0001, Row factor P = 0.1221, and Column factor P >0.9999). The parameters of the statistical analysis are shown in Supplemental Data Set 3. Bar = 1 cm. C, Inhibition of the chimeric protein PR1sp–Chp7 secretion by XopP. PR1sp–Chp7–HA was co-expressed with GUS-myc or XopP-myc in N. benthamiana leaves. AF separation and total protein extraction were performed at 3 dpi. Immunoblot analysis with an anti-HA antibody showed that, in the presence of XopP, a significant portion of the chimeric protein PR1sp–Chp7 was not processed (Unpr.) as it was observed by the shift in molecular weight of the protein before the cleavage of PR1sp of the secreted PR1sp–Chp7 (Pr.). PR1sp–Chp7–HA (C) is 43 kDa, whereas Chp7–HA (A) is 38 kDa. Expression of XopP-myc (86 kDa) and GUS-myc (74 kDa) was shown using an anti-myc antibody. Staining of the PVDF membrane with CBB was used as loading control. D, Inhibition of PR1 secretion in XopP overexpressing transgenic Arabidopsis plants. Arabidopsis WT and XopP transgenic plants were infiltrated with 1-μM flg22 and after 12 h, AF separation was performed. The proteins of the rest of the leaves (Total − AF fraction) were also extracted. Immunoblot analysis using anti-PR1 antibody showed reduced PR1 secretion in XopP transgenic plants relative to WT Col-0 Arabidopsis. Staining of the PVDF membrane with CBB was used as loading control. PR1 is approximately 15 kDa. E and F, Expression (E) and quantification (F) of the chimeric protein PR1sp–RFP–GFP in the absence or presence of XopP. In the upper, the chimeric protein RFP–GFP (without being fused with the secretion peptide) was expressed in the cytoplasm. Fusion of the PR1sp to the N-terminus of the RFP–GFP chimera (PR1sp–RFP–GFP) resulted in exocytosis to the apoplast of the RFP–GFP. Only RFP was visualized (magenta) as the GFP (cyan) was quenched in the acidic environment of the apoplast. When XopP was expressed along with PR1sp–RFP–GFP, exocytosis of RFP–GFP exocytosis was inhibited, and it relocalized to the cytoplasm and nuclei, as both fluorescent proteins were detectable. The images were taken via confocal microscopy at 3 dpi of N. benthamiana leaves. Bars in (E) = 10 μm. The experiment was repeated four times with similar results. Quantification of GFP fluorescence intensity of PR1sp–RFP–GFP infiltrated with GUS or XopP in N. benthamiana plants was performed. Quantification was performed using Image J software, and statistical analysis was made with unpaired t test. Data in (F) show means 8.93 for GUS and 47.05 for XopP and the difference between means (XopP – GUS) ± sem is 38.12 ± 2.90 (n =110 measurements each). The measurement has been done from three different biological replicates. The P <0.0001. The parameters of the statistical analysis are shown in Supplemental Data Set 3. G and H, Visualization (D) and quantification (E) of callose deposition after PTI elicitation with 20-μm flg22 (middle lane) or mock solution (upper lane), in T1 lines of transgenic A. thaliana lines (15-2 and 22-1 lines) expressing XopP-mCherry. In the lower, XopP-mCherry expression (magenta) together with chlorophyll (cyan) was imaged via confocal microscopy, and it was in accordance with the levels of callose secretion. Bars in (G) = 15 μm. Quantification has been made using Image J software, and statistical analysis was made with ordinary two-way ANOVA (Interaction P <0.0001, Row factor P = 0.0001, and Column factor P <0.0001). Data in (H) show means 3.00 for WT mock, 182.30 for WT flg-22 treated, 4.50 for XopP 15-2 mock, 3.67 for XopP 15-2 flg22 treated, 1.75 for XopP 22-1 mock, and 6.33 for XopP 22-1 flg22 treated. The measurements are from two biological replicates. ***P < 0.0005.
Furthermore, to validate the inhibition of apoplastic secretion of PR1sp-Chp7 induced by the XopP effector, we performed an apoplastic fluid (AF) extraction from N. benthamiana leaves that were transiently co-expressed with PR1sp-Chp7-HA and GUS-myc (negative control) or XopP-myc. We observed that in the absence of XopP (GUS), we could visualize only the secreted (processed) Chp7 (after the cleavage of PR1sp) (Figure 8C). However, in the presence of XopP, both processed and unprocessed forms of Chp7 were observed (Figure 8C).
In addition, we performed an apoplastic PR1 accumulation comparison between XopP-E Arabidopsis and WT plants. We treated the plants with 1,000-nM flg22 for 12 h to elicit PR1 expression and then performed an AF extraction. A total minus the AF (T − AF) protein extraction was performed for the treated Arabidopsis leaves. Immunoblotting with an anti-PR1 antibody showed reduced PR1 levels in the AF of XopP-E Arabidopsis plants compared to WT, whereas the protein levels were reversed in the T − AF fraction (Figure 8D). Finally, to rule out the possibility that XopP is a general cytoplasmic cell-death inhibitor, we co-expressed XopP with the XcvXopQ effector, which induces the HR (Adlung et al., 2016) in N. tabacum. XcvXopQ-dependent cell death was not inhibited by XopP (Supplemental Figure S10A). Similarly, we ruled out the potential interaction of XopP with Chp7 in a Y2H analysis (Supplemental Figure S9B). Chp7 expression in S. cerevisiae was verified via immunoblot analysis (Supplemental Figure S9C). In addition, we verified that XopP did not change the expression levels of the Chp7 effector (Supplemental Figure S9D).
We further validated whether XopP inhibited PR1a exocytosis by using confocal microscopy. For this, we constructed the chimeric RFP-GFP and the PR1sp–RFP–GFP proteins and co-expressed them using the transient system in N. benthamiana leaves in the presence of either XopP or GUS (negative control). RFP-GFP showed a nucleocytoplasmic localization, and both RFP and GFP signals were detected (Figure 8E). In contrast, when chimeric PR1sp–RFP–GFP was targeted to the apoplast, we predominantly observed an RFP signal, because the GFP signal was quenched in the acidic environment of the apoplast (Figure 8E) (Zheng et al., 2004; Dean et al., 2007). When XopP was co-expressed with PR1sp–RFP–GFP, both fluorescent signals were observed, indicating inhibited secretion of PR1sp–RFP–GFP (Figure 8E). Quantification of the fluorescence intensity of GFP of different samples is shown in Figure 8F. Overall, our results indicate that XopP intervenes in exocytosis through its association with the exocyst complex, preventing PR1a secretion.
XopP inhibits both callose deposition in the host cell wall and H2O2 production through exocyst targeting
Callose deposition in the cell wall is part of the PTI response in plants during pathogen attack, and it is secreted via the exocyst complex (Xu and Mendgen, 1994; Robatzek, 2007; Nielsen et al., 2012; Du et al., 2015; Redditt et al., 2019). We evaluated callose deposition in two of our transgenic Arabidopsis lines expressing XopP. The PTI was elicited using 20-μm flg22 (Figure 8, G and H). We compared our results with WT Arabidopsis plants and noticed that in XopP-E plants, almost no callose was secreted, in contrast to WT-treated plants (Figure 8, G and H). We measured XopP–mCherry expression levels using confocal microscopy, and we observed that callose deposition inversely correlated with levels of XopP–mCherry expression (Figure 8H). Taken together, these data indicate that XopP may either directly inhibit the exocyst-dependent exocytosis process that is responsible for the callose deposition in our transgenic Arabidopsis lines or indirectly inhibit the localization of FLS2 to the PM.
Upon PTI, reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) or superoxide accumulate as an early defense response (Yu et al., 2017). To examine whether XopP could also alter the ROS levels produced upon PTI, we investigated the H2O2 levels in transgenic XopP-E Arabidopsis plants by performing 3, 3′-diaminobenzidine (DAB)–HCl staining. We infiltrated leaves of WT and XopP-E plants with 100-nM flg22 for 8 and 16 min and used DAB staining to detect H2O2 levels. In XopP-E plants, very low levels of H2O2 were produced in 8 min (Supplemental Figure S10E). Treatment with the PTI elicitor for 16 min caused slightly elevated H2O2 levels that were, nonetheless, still reduced compared to levels in the WT plants (Supplemental Figure S10, E and F).
Finally, we examined whether our XopP-E transgenic Arabidopsis plants would be more susceptible to Pseudomonas infection in comparison to WT plants. For the infection assay, we sprayed the plants with the Pseudomonas cannabina pv. alisalensis strain ES4326 (former P. syringae pv. maculicola ES4326), which is one of the three Pseudomonas model species in plant-bacterial interactions research field (Pto DC3000; Pma ES4326; Pph 1448a) and one of the two species that infect Arabidopsis (Pto DC3000; Pma ES4326). After 3–6 dpi, we examined the phenotypes. We did not observe any difference in the appeared symptoms, but saw a quicker onset of the symptoms in XopP-E transgenic Arabidopsis plants (Supplemental Figure S11).
Discussion
NLR IDs function as decoys for pathogens’ effectors and seem to be conserved in the plant kingdom, and they have recently been described in mammals (Daskalov et al., 2015; Sandstrom et al., 2019; Zhang et al., 2019). It has been suggested that IDs could represent the authentic targets of the pathogen in the host cell, and thus they can be a suitable tool to identify host proteins targeted by pathogen effectors (Daskalov et al., 2015; Ellis, 2016; Sarris et al., 2016; Sandstrom et al., 2019; Zhang et al., 2019; Landry et al., 2021).
Based on this concept, we used an EXO70-like ID and we identified the exocyst component EXO70B1 as an authentic host virulence target of the highly conserved effector XopP of X. campestris pv. campestris (Xcc, XopPXcc). The fact that XopP, whose function is yet unknown, is one of the three core T3EPs in X. campestris species, indicates its importance in bacterial virulence. The importance of XopP (XC_2994) in host colonization has been extensively reported in the context of physiological infection (Jiang et al., 2009). Significant reduction in virulence, bacterial population growth, and lesion length were observed for an XccΔXopP insertion mutant (Jiang et al., 2009). However, the molecular mechanism(s) of its function in disease development remain(s) unknown.
XopP interacts with EXO70B homologs of Arabidopsis, and it is implicated in host defense. However, the effector did not interact with either an EXO70B homolog from monocots or the EXO70A1 homolog, which was also involved in biotic stress responses (Pečenková et al., 2020). Similarly, EXO70B1 revealed interaction specificity to XopP of X. campestris (Xc) but not to its homologs of other species. The specificity of Xc XopP to Arabidopsis EXO70B1 supports the view that the plants and phytopathogens co-evolve (Sacristán and García-Arenal, 2008; Burdon and Thrall, 2009; Lyu et al., 2021; Manriquez et al., 2021). Understanding the evolution of virulence has been a major goal in understanding molecular host–microbe interactions. Generalism and evolutionary dynamics leading to specialism, as well as the co-evolution of virulent microbes in host–pathogen interactions—including plant pathogenic bacteria—has resulted in an extensive debate for many years (Baumler and Fang, 2013). The potential to expand into new niches is implicated as an important driving force in specialism. Furthermore, specific genetic changes, including the adaptation and specialization of particular pathogenicity components such as effector proteins like XopP, could enhance adaptation to a particular niche and may reduce fitness for another niche. This so-called antagonistic pleiotropy can lead to evolutionary tradeoffs, which are not necessarily a requirement for specialization, but may reinforce tendencies toward specialization (Baumler and Fang, 2013). This could explain the specificity of XopPXcc regarding targeting of EXO70B proteins but no other homologs. Similarly, the XopP1 homolog of Xoc has evolved to interact with EXO70F1, and this needs to be investigated further in the future.
EXO70B1 and XopP interacted via their N-terminal regions. Likewise, EXO70B1 interacted with other exocyst components, as well as, with TN2 via its N-terminal region. Our overall results support the conclusion that the EXO70B1 N-terminal domain serves as the area for protein–protein interactions. It is noteworthy that the C-terminus of EXO70B1 binds to the cell PM (Guo et al., 1999; Pečenková et al., 2017), although we cannot exclude a conformational change of EXO70B1 when interacting with XopP.
In yeast and mammals, the exocyst complex forms two subcomplexes (SC-I and SC-II) consisting of the components Sec3, Sec5, Sec6, Sec8, and Sec10, Sec15, EXO70, EXO84, respectively (Heider et al., 2016; Mei et al., 2018). Recently, it has been proposed that the two subcomplexes are composed of the same components in plants (Synek et al., 2021). We showed that XopP interacts with EXO70B1 but also associates with SEC10a and EXO84b, all belonging to SC-II, enhancing our theory that XopP may inhibit the proper formation of SC-ΙI.
In our interaction screenings, we observed downregulation of EXO70B1 levels when XopP was present. Similarly, XopP also affected the protein levels of EXO84B but not those of SEC10, SEC6, or SEC8. This finding is consistent with the recent discovery that the Arabidopsis exocyst subcomplexes consist of the same exocyst components as those of mammals (Synek et al., 2021). Using Y3H and SE-FRET (sensitized emission FRET) analyses, we observed inhibition of the SEC10a/SEC15b association, as well as inhibition of the EXO70B1/SEC5a, EXO70B1/SEC15b, and EXO84b/SEC15b associations, by XopP. Additionally, the observed inhibition could be a double effect of both inhibition of the exocyst components association as well as the downregulation of EXO70B1 and EXO84B protein levels, caused by XopP. As it has been reported, the N. benthamiana sec10 VIGS mutant plants showed aberrant development (Du et al., 2018). Nonetheless, this was not the case for the sec10a and sec10b single-mutant Arabidopsis plants, which showed a phenotype similar to WT, due to gene redundancy; however, the double mutant had a dwarfism phenotype (Vukašinović et al., 2014). The observed inhibition of association of Arabidopsis SEC10a/SEC15b via Y3H assay needs to be further examined to determine whether this also occurs in planta. On the other hand, this inhibition of interaction could be specific for the EXO70B-participating exocyst complex, but not for the complexes that are formed by other EXO70 homologs, and this could also explain the absence of particular phenotypes. This additional data and theories that resulted from our studies of the XopP/exocyst association need to be investigated further, and the XopP could be a valuable tool for these studies. A complete molecular understanding of exocyst regulation in regard to plant defense remains challenging.
It is worth mentioning that we did not observe co-localization of either EXO70B1 or the EXO70B1/XopP complex with ATG8a after ConA treatment. However, in these assays, we did not use the elicitor that was reported to trigger EXO70B1 co-localization with Atg8f (Kulich et al., 2013). Likewise, treatment of agro-infiltrated N. benthamiana plants with ConA did not rescue the downregulated EXO70B1 levels by XopP. Additionally, in Arabidopsis atg9-3 mutant plants stably expressing EXO70B1-YFP, we also observed a reduction of the latter’s levels in the presence of XopP. Thus, we conclude that the EXO70B1 downregulation caused by XopP occurs in an autophagy-independent way.
The function of the exocyst in immunity has been reported mainly for EXO70 homologs in plants. The close homologs EXO70B1 and EXO70B2 are required for the full activation of signaling mediated by the PM-localized PRRs (Wang et al., 2020) and early- and late-PAMP responses (Stegmann et al., 2012). However, the EXO70B1 and EXO70B2 homologs do not functionally overlap, although both are required for PTI signaling (Stegmann et al., 2012; Wang et al., 2020). This rationale is supported by the fact that EXO70B2 is transferred to the vacuole for degradation via autophagy. It specifically interacts with the ATG8 via its two AIMs domains localized to its C-terminus (Brillada et al., 2021). However, in the absence of a PTI elicitor, the EXO70B2 protein is localized to the PM (Brillada et al., 2021) and, with EXO70B1, both are responsible for FLS2 accumulation to the PM (Wang et al., 2020). It is of note that in exo70B1-3/tn2-1 double Arabidopsis mutants, as well as in exo70B1-3/adr1s quadruple Arabidopsis mutants, the exo70B1-3-related cell death phenotypes are absent (Zhao et al., 2015; Wang et al., 2019). These reports may decrease the role of the reduced autophagy in exo70B1-3-related cell death. Future studies are needed in order to elucidate the potential upstream function of EXO70B1 in autophagy activation/function and to investigate the potential role of TN2 in activation of autophagy. To date, the NLR immune receptors have not directly been implicated in autophagy pathways (Leary et al., 2018, 2019), although the HR is linked to autophagy via restriction of the HR in distal uninfected tissue via autophagy genes (Liu et al., 2005).
Furthermore, only EXO70B1 is guarded by the atypical NLR immune receptor TN2 (Zhao et al., 2015; Wang et al., 2019). Before the discovery of TN2 and its role in guarding EXO70B1, it had been reported that the exo70b1 Arabidopsis mutants reveal a lesion mimic phenotype similar to the HR (Stegmann et al., 2014), and this was later attributed to TN2 (Zhao et al., 2015; Wang et al., 2019), as well as the compromised autophagy pathway (Kulich et al., 2013). The Activated Disease Resistance (ADR1s) NLRs in Arabidopsis have been reported to be essential for TN2-dependent autoimmunity (Wang et al., 2021). The ADR1s are RPW8-NLRs that are implicated in TIR-NLRs- and CC-NLRs-dependent immunity as well as some responses to autoimmune mutants (Collier et al., 2011; Bonardi et al., 2011). Nonetheless, whether other NLRs downstream of ADR1s are mediated in the activation of ETI remains to be investigated (Wang et al., 2021).
It has been proposed that pathogens, through adaptation, have developed strategies to manipulate the host exocyst complex and specifically its EXO70 component. This theory is based on the importance of the exocyst complex in the host PTI response, which involves tethering the post-Golgi secretory vesicles to the PM (Zhao et al., 2015; Saeed et al., 2019). The AVR-Pii effector of the rice pathogen Magnaporthe oryzae interacts with EXO70F2 and EXO70F3 homologs in co-IP assays in rice cells (Fujisaki et al., 2015). EXO70F3 is required for AVR-Pii to trigger defense mediated by the rice Pii gene that is present in some cultivars and confers resistance to M. oryzae (Fujisaki et al., 2015). Based on these findings, it was hypothesized that Pii might “guard” EXO70F. These results are consistent with the scenario that TN2 “guards” EXO70B1 (Zhao et al., 2015). Deletion of exo70B1 gene or the targeting of EXO70B1 protein by the P. syringae effector AvrPtoB (an E3 ubiquitin ligase) leads to activation of immune responses (Zhao et al., 2015; Wang et al., 2019). Both AVR-Pii and AvrPtoB effectors specifically interfere with host EXO70 homologs, and this is translated to a constitutive defense activation. In addition, the mammalian pathogen Shigella flexneri also hijacks the Rab8/Rab11 pathway to relocate the exocyst complex to promote the disassociation of its vacuole (bacteria-containing vacuole) and to be able to proliferate inside the host (Chang et al., 2020).
In contrast to these findings, we showed that XopP targets EXO70B1 without activating the TN2-dependent defense. This finding represents a novel pathogen adaptation strategy indicating that the evolution of pathogens is also driven by the host defenses. Our transient data are also supported by results using our stable transgenic Arabidopsis lines that constitutively express XopP. These lines do not show any TN2-dependent phenotype, unlike either the previously reported AvrPtoB transgenics, which were lethal due to TN2 activation (Wang et al., 2019), or the exo70B1 deletion mutant in Arabidopsis plants (Stegmann et al., 2014; Zhao et al., 2015). In the context of bacterial infection conditions, we observed a downregulation of FLS2 protein from the PM upon delivery of XopP through the T3SS. It has recently been reported that EXO70B2 protein is localized to the PM (Brillada et al., 2021), and with EXO70B1, both are responsible for the FLS2 immune receptor’s regulation of homeostasis at the PM (Wang et al., 2020). In our assays, XopP was translocated in Arabidopsis cells using the P. fluorescens (Pf01) EtHAn strain (Thomas et al., 2009) and the pEDV3 vector (containing the N-terminal secretion signal of AvrRps4 that is cleaved after the translocation of the cargo protein into the host cell) via the T3SS (Sohn et al., 2007). It is worth mentioning that T3SSs of Pseudomonas and Xanthomonas belong to different groups, revealing differential expression and translocation specificities. This means that the effectors’ secretion signals are not common in these species.
Our data using Pf0-1 EtHAn-XopP show that even if the XopP is translocated through a heterologous system, it can interfere with FLS2 localization to the host PM. Unfortunately, we could not reproduce the above conditions in Xanthomonas, as the available tools are limited for this species. There is no available Xanthomonas strain that lacks effectors containing both an intact and a functional T3SS, as already existing in Pseudomonas (P. fluorescens Pf01 harboring a functional T3SS). On the other hand, we could not use the XccΔXopP mutant for this assay, because this strain carries a large set of effectors (more than 35 distinct ones) besides XopP. The majority of these effectors are of unknown function, so any result would not be easily assigned to XopP, as other effectors may also have a similar or redundant function. The overlapping activities of bacterial effectors in phytopathogens by targeting the same host targets have been extensively reported (Badel et al., 2006; Kvitko et al., 2009; Munkvold et al., 2009; Cunnac et al., 2011; Worley et al., 2013; Laflamme et al., 2020). Furthermore, we verified EXO70Bs-dependent reduction of FLS2 levels from the PM fraction in XopP-E Arabidopsis plants.
In plants, both secretion of the PR1a and callose deposition require the exocyst complex (Xu and Mendgen, 1994; Robatzek, 2007; Gu and Innes, 2012; Nielsen et al., 2012; Du et al., 2015; Redditt et al., 2019). These are common responses during PTI induction in plants, and they are targeted by many pathogens, including several Xanthomonas species (Popov et al., 2016). We identified in planta that, in the presence of XopP, PR1a accumulation and callose deposition in the apoplast are downregulated in both transient expression assays and stable XopP-E Arabidopsis plants. Furthermore, in XopP-E Arabidopsis and in N. benthamiana plants transiently expressing XopP, unprocessed PR1sp–Chp7 was observed. Likewise, in the presence of XopP, no HR was induced in N. tabacum leaves agroinfiltrated to express PR1sp–Chp7.
The reduction of the apoplastic PR1 levels in XopP-E Arabidopsis plants, as well as the presence of unprocessed PR1sp–Chp7 when co-expressed with XopP, could be either through the direct inhibition of PR1 secretion or as a result of PR1sp–Chp7/PR1 failure to exit the ER, caused by the effector. The latter remains to be further investigated.
Moreover, the loss of HR induction in N. tabacum when PR1sp–Chp7 was co-expressed with XopP, could also be attributed to a double effect: either to the inhibition of PR1sp–Chp7 protein secretion, or/and to the inhibition of the PM localization of a yet unknown PRR that recognizes Chp7 in the plant cell apoplast (Lu et al., 2015).
Similarly, taking into account the observed FLS2 reduced levels from the PM and the highly reduced levels of callose deposition in XopP-E transgenic Arabidopsis plants, there could be a double effect that includes the downregulation of FLS2 protein from the PM as well as the inhibition of the consequent callose exocytosis process. Furthermore, we investigated the inhibition of downstream ROS production in XopP-E transgenic Arabidopsis plants upon treatment with the bacterial elicitor flg22. Reduced levels of H2O2 were produced in XopP-E plants compared to WT Arabidopsis.
In order to investigate whether the XopP-E Arabidopsis transgenic plants could have a deviation in phenotype, we infected them with P. cannabina pv. alisalensis str. ES4326. The XopP-E plants did not show any alteration in symptoms compared to WT Arabidopsis, but there was a minor quicker onset of symptom development in XopP-E plants. The lack of any great difference could be explained by the fact that Pcal ES4326 (like other P. syringae pathogens) has an arsenal of more than 30 effectors that successfully suppress PTI in Arabidopsis. Thus, the addition of an extra effector (XopP) would not make much difference during the infection procedure.
A distant XopP homolog from X. oryzae pv. oryzae (XopPXoo) has been shown previously to target the rice PUB44 (OsPUB44) via its U-box domain, inhibiting its E3 ubiquitin ligase activity (Ishikawa et al., 2014). OsPUB44 belongs to the class III PUB family, together with Arabidopsis PUB22, PUB23, and PUB24 (Azevedo et al., 2001; Ishikawa et al., 2014). Additionally, EXO70B1 and EXO70B2 are ubiquitinated via PUB18 and PUB22, respectively. This ubiquitination is dependent on the presence of the U-box N-terminal domain (Seo et al., 2016). In future studies, it is of high interest to identify whether XopPXcc interacts with PUB18 possibly interfering in EXO70B1 ubiquitination (Seo et al., 2016).
Here, we report the molecular mechanism by which a core Xanthomonas T3EP affects exocytosis probably through dissociation of proper formation of the exocyst (sub)complex. It is impressive that this pathogen has developed a strategy to inhibit exocyst-dependent exocytosis, avoiding host immunity. This virulence function clearly indicates the ability of pathogens to adapt to and overcome host defenses (Figure 9). It is important both to elucidate the molecular target(s) and role(s) of the bacterial T3EPs and to understand the nature of bacterial pathogenicity. Future structural studies will reveal whether XopP can mimic EXO70B1 structurally, enabling the effector to replace it with a nonfunctional component at the SC-II. Overall, our data represent a newly described strategy for pathogenicity in a Gram-negative Gamma proteobacterium. Our findings will also be of interest for researchers in the broader field of molecular host–pathogen interactions because the exocyst complex is a conserved defense mechanism found in of various eukaryotic hosts from different kingdoms.
Figure 9.

Schematic abstract. This model shows how XopP manipulates the exocytosis via associating with members of the subcomplex II of the exocyst, most likely by affecting its proper assembly into a functional complex. In physiological conditions (green color), the components of the exocyst complex are recruited to subcomplexes and then to a holocomplex. Upon X. campestris infection (brown color), XopP is delivered into the cytoplasm along with the other effectors that are secreted. XopP manipulates the interactions between the exocyst components of the subcomplex II and the interaction between EXO70B1 and SEC5a, which belongs to subcomplex I. As a consequence, this affects the exocytosis process of different cargoes, such as PR1, FLS2, and possibly other unknown PRR receptors and callose deposition. The schematical abstract was created in BioRender.
Material and methods
Plant materials and growth conditions
The N. benthamiana, N. tabacum cv. Petit Gerard, and N34-4 plants that we used in our study were grown in greenhouse conditions. A. thaliana ecotype Columbia-0 (Col-0) was used in our study as the WT. Arabidopsis seeds were stratified at 4°C in water for 3 days before sowing in soil and then were grown in greenhouse conditions. To generate constructs for stable expression of XopP effector from X. campestris pv. campestris in WT background, we used Golden Gate (GG) cloning (Engler et al., 2008, 2014) to insert, in series, the native Rubisco promoter, the gene, a C-terminal mCherry epitope tag, and the NOS terminator into pICSL86955OD vector (Engler et al., 2008). The plants were transformed using the floral dip method (Clough and Bent, 1999). The transformants were selected with BASTA (Bayer, 60-ppm Glufosinate, ammonium salt). In brief, plants were sprayed twice a week with BASTA and the survivors were screened via polymerase chain reaction (PCR). To generate constructs for stable expression of EXO70B1 in atg9-3 background, we transformed the Arabidopsis plants with ICH86988-35Spro:EXO70B1-YFP-NosT using the floral dip method and selected transformants having kanamycin resistance on MS plates (M0222, Duchefa), supplemented with 1% w/v sucrose.
Bacterial strains
Escherichia coli (Stellar or DH10b strains) were grown on LB (1% tryptone, 0.5% yeast extract, and 1% sodium chloride) plates with the appropriate concentrations of antibiotics at 37°C and kept at 4°C for up to 2 weeks (kanamycin sulfate 50 μg mL–1, ampicillin 100 μg mL–1, tetracycline 10 μg mL–1, spectinomycin 50 μg mL–1). For liquid cultures, bacteria were inoculated into liquid LB containing the appropriate antibiotic at the same concentration as for the solid cultures and grown at 37°C with shaking at approximately 200 rpm.
Agrobacterium tumefaciens (AGL1, C58C1, and GV3101 strains) were grown on LB plates with an appropriate concentration of antibiotics at 28°C and kept at 4°C for up to 2 weeks (empty AGL1: rifampicin 50 μg mL–1 and ampicillin 100 μg mL–1, empty C58C1: rifampicin 50 μg mL–1, empty GV3101: rifampicin 50 μg mL–1 and tetracycline 10 μg mL–1). For transient expression in Arabidopsis, the AGL1 strains were grown on YEB plates with the appropriate concentration of antibiotics, as mentioned above (Zhang et al., 2020). For liquid cultures, bacteria were inoculated into 5-mL LB containing the appropriate antibiotic at the same concentration as for the solid cultures and grown at 28°C with shaking at approximately 200 rpm.
P. fluorescens (Pf01) strain EtHAn was grown on LB plates with the appropriate concentration of antibiotics (chloramphenicol 34 μg mL–1) at 28°C and kept at 4°C for up to 5 days. For in planta assays, in transgenic Arabidopsis FLS2pro:FLS2–GFP plants, 2 mL of an overnight culture were centrifuged at 1,157g for 5 min, washed with 10-mM MgCl2, and resuspended with 10-mM MgCl2. Optical density (OD600) of 0.2 of bacteria was used.
P. cannabina pv. alisalensis strain ES4326 was used to infect WT and XopP-E transgenic Arabidopsis plants. The bacteria were grown on LB plates without any antibiotics at 28°C and kept at 4°C for up to 5 days. For in planta assays, a liquid culture was grown and bacteria were centrifuged at 2,000g for 2 min and diluted with 10-mM MgCl2. The plants were sprayed with bacteria at an OD600 of 0.002 with 0.025% Silwet (74056, Momentive).
Cloning and constructs
For all constructs used in the study, we used GG cloning and Phusion-HF polymerase (NEB). All constructs generated were verified by Sanger sequencing. All genes were first cloned into the pBluescript GG compatible vector unless stated otherwise, before cloning into the final destination vector. The BsaI restriction site of this vector (pBluescript SK(-)) has been domesticated in our lab. For Y2H analyses, all genes were amplified with a stop codon, because the Gal4 domain was N-terminal and cloned to the final destination vector pGBKT7-RFP (bait) or pGADT7-RFP (prey). These vectors were kindly given to us by Mr Mark Youles (TSL). For in planta analyses, all genes were amplified without a stop codon, because the tags were C-terminal in the final destination vector pICH86988 unless otherwise stated.
EXO70-like ID was amplified from pDONR221_Exo70_C_C119, kindly given by Prof. M. Moscou (TSL), using BsaI sites and cloned directly into pGBKT7-RFP GG for Y2H assays. The EXO70-like ID is a C-terminal fusion of the RGH2 NLR of H. vulgare ssp. vulgare cv. Baronnesse (LOC102707079). AtEXO70B1 and AtEXO70F1, as there are intronless genes, were amplified from Arabidopsis genomic DNA (gDNA) in three parts, domesticating (silencing) the internal BsaI sites. AtEXO70B2 was amplified with BsaI sites from pUG41308 vector, kindly given to us by Prof. Marco Trujillo (University of Freiburg). AtEXO70A1 (both splice forms) were amplified from Arabidopsis cDNA. ObEXO70B1_small was amplified from cDNA of O. brachyantha, in two parts, domesticating the internal BsaI site. ObEXO70B1_long was amplified from gDNA of O. brachyantha, first amplifying the whole open reading frame with the 5′-UTR and then using nested PCR with an internal FW primer. XopP was amplified from gDNA of X. campestris pv. campestris ATCC33913 in two parts, domesticating the internal BsaI site. Besides, Y2H and in planta vectors, XopP gene was also inserted into the pET26b (ColE1 plasmids) vector, carrying a C-terminal 6-His epitope tag, for expression in E. coli. XopP1 and XopP2 were amplified from gDNA of X. oryzae pv. oryzicola WHRI 5234 (NCPPB 1585), whose draft genome was recently sequenced (Michalopoulou et al., 2018). Hlk3 was amplified from gDNA of Ralstonia solanacearum GMI1000, a kind donation of Prof. Nemo Peeters (INRA-CNRS). AcXopP was amplified from gDNA of Acidovorax citrulli, a kind donation of Prof. Goumas (HMU).
Truncation forms of AtEXO70B1 were amplified using pGBKT7-AtEXO70B1 as a template. PCR fragments representing amino acid residues 1–388 (AB), 319–511 (BC), and 389–625 (CD) were generated, including start and stop codons. Truncation forms of XopP were generated using the CLUSTALW bioinformatics program. Using pGADT7-XopP as a template, PCR fragments from residues 1–420 and 421–733, including start and stop codons were amplified. AvrRps4 effector from P. syringae pv. pisi was used from a previous study (Sarris et al., 2015).
The Chp7 construct was kindly given by Prof. Jane Glazebrook (Lu et al., 2015). The pMDC-spC7HPB sequence was verified by restriction endonuclease digestion and transferred to AGL-1 Ag. tumefaciens strain for plant assays. RIN4 was amplified from Arabidopsis cDNA. XopQ was amplified from gDNA of X. campestris pv. vesicatoria, a kind donation of Prof. Goumas (HMU). For the generation of PR1sp–RFP–GFP, the PR1 secretion peptide was amplified from the pMDC:spC7HPB construct. The final construct was then cloned into pICSL86900_OD under the expression of the cauliflower mosaic virus 35S promoter and the NOS terminator. The pCambia-FLS2pro:FLS2–GFP construct used in this study was a kind donation of Dr. Katarzyna Rybak (LMU, Institute of Genetics), and had been already transformed into GV3101 strain of Ag. tumefaciens. Tn2 NLR gene was amplified from A. thaliana and B. napus zs11 gDNAs for in planta assays and from cDNAs of the above plant species for Y2H assays.
The Sec3a, Sec5a, Sec6, Sec8, Sec10a, Sec15a, Sec15b, Exo84b, and Exo84c exocyst components were amplified from Arabidopsis cDNA. The DsRed-FYVE confocal marker was kindly given to us in a C58C1 Agrobacterium strain by Prof. František Baluška (University of Bonn). PM-RFP and Ubi10pro:mCherry-Atg8A-Alli-mCherry confocal markers were kindly given to us and already transformed into AGL-1 and GV3101 Agrobacterium strains, respectively, from Dr. Yasin Dagdas (Gregor Mendel Institute of molecular plant biology).
Phylogenetic analysis of XopP
In KEGG (Kyoto Encyclopedia of Genes and Genomes) (Kanehisa et al., 2021), using X. campestris pv. campestris XopP (XCC_1247) as a search term, we identified the orthologs from various pathogens, including Xanthomonas species, Ralstonia, and Acidovorax. The annotated protein sequences of 73 orthologs were manually downloaded from KEGG, and two of them were recovered from sequence analysis of draft genome annotation of X. oryzae pv. oryzicola WHRI 5234 (NCPPB 1585) (Michalopoulou et al., 2018). The identity percentage of orthologs used did not drop below 24.4%. Moreover, to confirm that the resultant orthologs are indeed homologous to Xcc_1247, we did a reverse Blastp search in National Center for Biotechnology Information for each of the 73 protein sequences. From this search, we excluded nine protein sequences. All 67 proteins were aligned via MEGA5 (Tamura et al., 2011). MEGA5 was also used to construct the maximum likelihood phylogenetic tree using the Poisson correction model (Zuckerkandl and Pauling, 1965; Tamura et al., 2011). The tree with the highest log likelihood (–25,278.2594) is shown. Initial tree(s) for the heuristic search were obtained automatically as follows. When the number of common sites was less than 100 or less than one-fourth of the total number of sites, the maximum parsimony method was used; otherwise BIONJ (Biotech Innovation Organization (BIO) Neighbour-Joining) method with MCL (Markov Clustering) distance matrix was used (Gascuel, 1997). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 67 aa sequences. All positions with <0% site coverage were eliminated. Fewer than 100% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 532 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 (Tamura et al., 2011). See Supplemental File 1 for the alignment.
Electroporation of P. fluorescens
Pf01 strain EtHAn, used for in planta assays in Arabidopsis transgenic plants, was electroporated with the pEDV3 empty vector or pEDV3 carrying XopP C-terminal truncation and XopP full gene. pEDV3 was kindly given to us by Prof. M. Moscou (TSL). For electroporation, a large portion of streaked bacteria was taken and diluted in 300-mM sucrose. The cells were centrifuged at 1,157g for 5 min and washed twice with 300-mM sucrose. They were resuspended in 100 μL of 300-mM sucrose and moved to a fresh Eppendorf tube. Two micrograms of plasmid DNA was added to each sample and were electroporated at a BTX electroporator, using one pulse (5 ms) at 2.2 kV. The cells were collected and LB up to 1 mL was added for outgrowth with shaking at 28°C. Lastly, the cells were plated on a selective medium (chloramphenicol 34 μg mL–1 and gentamycin 10 μg mL–1) for 2 days at 28°C. Single colonies were selected for the in planta experiment.
Y2H and Y3H assays
All plant proteins were cloned as baits (in the pGBKT7-RFP vector) and bacterial effectors were cloned as preys (in the pGADT7-RFP vector), or as otherwise stated. Different pairs of constructs were cotransformed into PJ694a or AH109, and the cells were grown on SD–Leu–Trp medium (SD–LW). Two single clones were transferred from the SD–LW medium and were re-cultured on SD–LW (verification of growth) and SD–Leu–Trp–Ade (–LWA, auxotrophic selection) medium. For the dilutions, a single clone was transferred to a liquid medium and diluted as stated in the figures. Accordingly, the vectors used for Y3H assay were pBridge, kindly given to us by Prof. Subba Rao Gangi Setty (Indian Institute of Science Bangalore) and pGADT7-RFP. Either EXO70B1 or SEC15B were cloned into the MCS I site under the expression of the constitutive yADH1pro promoter and XopP was cloned into the MCS II site under the expression of the induced METpro promoter from the pBridge vector (Takara) and SEC5A, SEC15B, and SEC10 were cloned in pGADT7-RFP vector. The AH109 yeast strain was transformed with both vectors carrying different pairs of constructs, and the cells were grown on SD–LW. The two-hybrid analysis (for visualization of the interaction) was performed with a selection of the auxotrophic marker adenine with supplementation of 1-mM methionine in the medium, for repression of XopP expression. The Y3H analysis (for visualization of inhibition) was performed in medium lacking Leu, Trp, Ade, and Met (–LWAM). The yeasts were also grown in the control plate containing medium lacking Leu, Trp, and Met in order to check growth upon Met limitation. All yeast strains were left to grow at 30°C.
Subcellular co-/localizations, co-localization analyses, and BiFC
Nicotiana benthamiana leaves were infiltrated with Agrobacterium strains at OD600 = 0.4–0.5 expressing indicated constructs. For subcellular co-/localizations and BiFC, leaf discs were analyzed 3 dpi. All images were captured in a Leica SP8 laser scanning confocal microscope. The co-localization analyses were performed with PSC using the Coloc2 in Fiji (French et al., 2008; Moschou et al., 2013). The threshold regression used was Costes, PSF 100 and Costes randomizations 10 (Costes et al., 2004). A total of 15 regions of interest (ROIs) were chosen for the PSC analysis. For BiFC, all indicated constructs were fused to C-terminal nVENUS or cCFP epitope tags (plasmids: pCR8GW-TOPOnVENUS for C-terminal tagging and pCR8GW-TOPOcCFP for C-terminal tagging). All samples were imaged with a 40× water objective. The confocal images were processed with ImageJ. GFP was excited using a 488-nm argon laser and emission was collected at 496–520 nm. YFP was excited using a 514-nm argon laser and emission was collected at 520–550 nm. mCherry, DsRed, and RFP were excited using a 561-nm laser and emission was collected at 593–628, 573–616, or 573–620 nm, respectively. eCFP (enhanced Cyan Fluorescent Protein) was excited using the 405 nm and emission was collected at 463–487 nm. Chlorophyll was detected between 653 and 676 nm and excited at 561 nm. Quantification of GFP (Green Fluorescent Protein) signal from infiltrations with FLS2–GFP was processed with Image J software, as described in https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html.
FRET-SE analysis
Leaves of N. benthamiana were co-infiltrated with EXO70B1-YFP and SEC15B- or SEC5A-mCherry in the presence or absence of XopP-myc. As controls, AvrRps4-myc and/or GUS-myc were used. Leaf tissue was examined at 3 dpi using the FRET-SE module of SP8 confocal microscopy. For FRET-SE, we selected the photomultiplier tube (PMT) detectors for donor and acceptor and, in between, a Hybrid detector for the FRET. Details of this method are described in the Confocal Application Notes Pdf prepared by Myriam Gastard, Ph.D. Exton Support Group, Leica Microsystems, Inc. (Wouters et al., 2001; van Rheenen et al., 2004). For calculation of FRET efficiency, method 1 was used (Wouters et al., 2001). For quantification of FRET-SE signal, at least 200 counts were measured using FIJI v. 1.49 software (rsb.info.nih.gov/ij).
HR assay
For HR assays with PR1sp–Chp7 in the presence or absence of XopP, N. tabacum cv. Petit Gerard leaves were infiltrated with Agrobacterium C58C1 or AGL-1 strains at OD600 = 0.1 for PR1sp–Chp7 and OD600 = 0.4 for GUS and XopP. For HR assay with At- or BnTN2 constructs, N. tabacum cv. N34-4 and Petit Gerard were infiltrated with Agrobacterium C58C1 strain of TN2, EXO70B1, or XopP at OD600 = 0.5 (equimolar ratio).
Inhibition treatments
For the inhibition of autophagy assays, 1-μM Con-A was applied to N. benthamiana areas of leaves that were already agroinfiltrated with the constructs stated above. The treatment was performed for 16 h, and plants were imaged using confocal microscopy together with the Atg8a-mCherry marker. Total protein extraction was followed by immunoblot analysis.
Callose deposition assay
Leaves from 4-week-old Arabidopsis plants were infiltrated with 20 μM of flg22 in 10 mM of MgCl2 or mock solution and were detached 12 h after infiltration. Callose staining, image acquisition, and processing were carried out as described in Jin and Mackey (2017) with a change in destaining. In brief, clearing leaves of chlorophyll was carried out overnight (12 h) using acetic acid:ethanol in a 1:3 ratio. Callose visualization was done using a confocal SP8 microscope with a 10× dry objective with excitation at 405 nm and emission collected between 490 and 530 nm. Images were processed as described in Jin and Mackey (2017) with Image J software, and a graph was created depicting callose deposits per mm2 from six images per genotype and treatment. Some samples with extreme values were removed from the quantification in the WT sample of Arabidopsis treated with callose, as some areas had too many deposits because of tissue damaging during the process using the forceps.
ROS assay
H2O2 production was visually detected in leaves of WT and XopP expressing plants by staining with 1 mg mL–1-DAB solution. The leaves were infiltrated with 100-nM flg22 in 10-mM MgCl2 or mock solution (10-mM MgCl2) for 8–16 min. After the treatment, the leaves were collected and incubated in the DAB solution for 8 h at room temperature in darkness. The leaves were then decolorized in bleaching solution (1:3 acetic acid:ethanol) before collecting the images.
Protein extraction from plant leaves and co-immunoprecipitation
Nicotiana benthamiana was infiltrated with Agrobacterium strains at OD600 = 0.5 expressing indicated constructs. Agroinfiltrated leaves were ground at 72 hpi in liquid nitrogen, and proteins were extracted in GTEN buffer (10% glycerol, 25-mM Tris–Cl pH 7.5, 1-mM ethylenediaminetetraacetate, 150-mM NaCl) supplemented with fresh 5-mM DTT, protease inhibitor cocktail (Calbiochem), and 0.15% v/v NP-40. After 10-min centrifugation at 4°C at 14,000g, the supernatant was collected in a clean microcentrifuge tube and incubated for 2 h at 4°C with an anti-flag mouse antibody at 1:4,000 dilution (Sigma, F1804), followed by a 1-h incubation at 4°C with PureProteomeProtein A/G Magnetic Beads Mix (Millipore). Beads were washed with GTEN buffer, before adding 1× SDS loading dye and boiling the samples for 5 min.
AF extraction
AF was extracted from N. benthamiana and Arabidopsis plants. In brief, the leaves were excised and washed with distilled water before they syringe-infiltrated with vesicle isolation buffer (20-mM 2-[N-morpholino]ethanesulfonic acid, 2-mM CaCl2, and 0.01 M NaCl, pH 6.0). After wiping carefully the external buffer from the leaves, they were rolled inside a syringe or a 1.5-mL Eppendorf tube and centrifuged for 10 min at 800g (4°C). The apoplastic wash was collected and protease inhibitors and 1× SDS loading dye were added.
PM/Cell fractionation in Arabidopsis and N. benthamiana plants
PM proteins were extracted using a Minute Plasma Membrane Protein Isolation Kit for Plants (Invent Biotechnologies) as described previously (Shen et al., 2017). The proteins were extracted either from WT and XopP expressing lines or from N. benthamiana plants at 48 hpi with the Agrobacterium constructs. H+-ATPase was detected with an anti-H+-ATPase (AS07 260; Agrisera) antibody and the verification of cytosolic fraction was performed by staining of the polyvinylidene difluoride (PVDF) membrane with Coomassie Brilliant Blue (CBB) R-250 dye. Also, the first nuclear/chloroplastic (and larger debris) fraction was kept, as well the cytosolic and organelle membrane fraction and used in immunoblot analysis.
Protein extraction from yeast
Yeast, transformed with both bait and prey constructs, was harvested from a plate containing minimum medium lacking Leu and Trp and grown overnight in liquid culture in the same medium. Protein extraction was performed according to Horvath et al. (1994) and 40 μL of the protein extraction were separated at SDS–PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis).
SDS–PAGE and immunoblotting
Protein samples from plant leaves were separated via 8% or 12% SDS–PAGE (stated in images captions), electroblotted to a PVDF membrane (Millipore), then blocked for 1 h with 5% w/v milk in Tris-buffered saline (TBS; 150-mM NaCl, 50-mM Tris–HCl, pH 7.6) and incubated for 1 h with primary antibodies. The dilutions used were the following: anti-flag at 1:4,000 (Sigma, #F1804), anti-myc at 1:4,000 (Cell Signaling, #2276) anti-HA at 1:5,000 (Roche, #12CA5), anti-FLS2 at 1:1,000 (Agrisera, #AS12 1857), anti-PR1 at 1:1,000 (Agrisera, # AS10 687), anti-N-YFP at 1:2,000 (Agrisera, #AS11 1776), anti-C-YFP at 1:1,000 (Agrisera, # AS11 1775), and anti-H+-ATPase (Agrisera, #AS07 260) at 1:4,000. Membranes were then incubated for 1 h with a secondary antibody (anti-mouse HRP (horse radish peroxidase) conjugate at 1:10,000 dilution Cell Signaling) or an anti-rabbit HRP conjugate at 1:8,000 dilution (Cell Signaling) and chemiluminescent substrate ultra (Millipore) was applied for 5 min before camera detection in Sapphire Biomolecular Imager (Azure Biosystems). Total protein lysate from yeast transformations were separated in 8%, 10%, or 12% SDS–PAGE, electro-blotted at PVDF membrane (Millipore), then blocked for 90 min with 5% w/v milk in TBS supplemented with 0.05% Tween-20 and incubated for 1 h with the primary antibody anti-Gal4-DBD at a 1:500 dilution (Santa Cruz Biotechnology). The rest of the procedure was similar to proteins extracted from plant leaves.
Protein expression conditions in E. coli
The XopP gene fragment was cloned and inserted into the pET26b (ColE1 plasmids) vector, which encodes a C-terminal 6-His epitope tag and transformed into the E. coli strain BL21. A sufficient amount of soluble protein was obtained after induction using the following conditions: Cells were grown in LB medium containing 30-μg mL–1 kanamycin until an OD600 value of 0.6 was reached. The culture was induced with 0.4-mM isopropylthio-β-galactoside and grown at 20°C overnight. The cell paste was resuspended in 100-mL lysis buffer containing 25-mM Tris pH 8.0, 300-mM NaCl, 5-mM imidazole, and 15-mM β-mercaptoethanol and homogenized. After adding protease inhibitors (20-μg mL–1 leupeptin, 1-mM PMSF, and 150-μg mL–1 benzamidine, Sigma-Aldrich, P9599), the solution was sonicated on ice, 14 sonication cycles of 30 s each, with cooling intervals of 30 s. The precipitate was subsequently removed by centrifugation at 18.000g at 4°C for 45 min. Purification was carried out using His-tag affinity chromatography at 4°C using an 8-mL Ni-NTA Qiagen column that was pre-equilibrated in lysis buffer and initially washed stepwise with 10-, 20-, and 30-mM imidazole. With a subsequent increase in imidazole concentration, the protein started eluting at 100-mM imidazole. Fractions containing the protein were dialyzed against the storage buffer containing 25-mM Tris pH 8.0, 100-mM NaCl, and 10-mM β-mercaptoethanol and concentrated to approximately 2.8 mg mL–1. In the case of EXO70B1, the full-length gene was cloned and inserted into the LIC 1.10 vector carrying an N-terminal 6-His-SUMO3 tag, and transformed into E. coli strain Rosetta (DE3). The LIC (ligation independent cloning) 1.10 vector carrying the tag was kindly donated by NKI. The same protocol was followed and the fractions containing the protein were dialyzed against the storage buffer (25-mM Tris pH 8.0, 100-mM NaCl, 10-mM β-mercaptoethanol, and 0.5-nM SENP-2 protease, E-710). Subsequently, a reverse purification protocol was carried out using His-tag affinity chromatography, and the fractions containing the protein were concentrated to approximately 8.5 mg mL–1.
SEC assays for in vitro protein–protein interactions
XopP and EXO70B1, which were previously purified by His-tag affinity chromatography, were used for gel filtration assay. To determine the interaction between the EXO70B1 and the entire XopP protein, SEC (Superdex 200 column, Pharmacia Biotech) was employed. The XopP and EXO70B1 were used individually but also were mixed in a final 1:1 concentration ratio. Buffer containing 25-mM Tris pH 8.0, 100-mM NaCl, and 10-mM β-mercaptoethanol was used for the assay with a flow rate of 0.5 mL min–1.
Statistical analysis
Image analyses and intensity measurements were done using FIJI v. 1.49 software (rsb.info.nih.gov/ij). The intensity of the fluorescence signal was measured as the integrated density in a ROI. Statistical analyses were done by Rstudio (R-project.org) or GraphPad Prism 8.0.2 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com. Each dataset was tested for normal distribution of measurements using the Shapiro normality test. To determine whether the differences observed between the datasets were statistically significant, the Wilcoxon test was used for Figures 5, B, 8, A and B. The significance threshold was set at P < 0.05 (significance claim) and the exact values are shown in the respective graphs. Graphs were generated by Microsoft Excel or Rstudio. In the box plots, upper and lower box boundaries represent the first and third quantiles, respectively, horizontal lines mark the median, and whiskers mark the highest and lowest values. For Figures 3, C, D, 4, B and D parametric t tests were performed and P-value was set at P < 0.05 and the exact values are shown in each figure legend. For Figures 6, C and 8, E ordinary two-way ANOVA was performed and Interaction, row and column P-values are shown in the figure legend.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EXO70A1: AT5G03540.3 and AT5G03540.1, EXO70B1: AT5G58430, EXO70B2: AT1G07000, EXO70F1: AT5G50380, ObEXO70B: LOC102707079 (NC_023163.2), EXO84B: AT5G49830.1, EXO84C: AT1G10180.1, Sec3a: AT1G47550, Sec5a: AT1G76850, Sec6: AT1G71820.2, Sec8: AT3G10380, Sec10a: AT5G12370, Sec15a: AT3G56640, Sec15b: AT4G02350, XopP: AE008922, PR1: AT2G14610, TN2: AT1G17615.1, BnTN2: HG994360.1
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The EXO70-like ID and EXO70B1 interact specifically with the XopP effector.
Supplemental Figure S2. XopP and EXO70B1 self-interact in Y2H and in planta assays; EXO70B1, XopP, as well as their combination, co-localize with the PM marker but not with the autophagosomal or early/late endosomal markers.
Supplemental Figure S3. Expression of the XopP homologs and the EXO70B homolog from Oryza brachyantha in S. cerevisiae; Schematic representation of long and small versions of ObEXO70B compared to EXO70B1 of Arabidopsis
Supplemental Figure S4. Interactions of the components of SC-II and of the XopP effector with the components of the exocyst complex in Y2H screenings and in planta assays.
Supplemental Figure S5. XopP reduces levels of EXO70B1 and EXO84b but not SEC10a, with which it interacts.
Supplemental Figure S6. EXO70B1 downregulation caused by XopP is autophagy independent.
Supplemental Figure S7. XopP inhibits the EXO70B1/SEC5a, EXO70B1/SEC15b, EXO84b/SEC15b, and SEC15b/SEC10a associations in Y3H and in planta assays.
Supplemental Figure S8. Protein alignment of AtTN2 with BnTN2; BnTN2 expression in S. cerevisiae; TN2 does not interact with XopP, but with the N-terminal domain of EXO70B1, in Y2H screenings; EXO70B1/RIN4 interaction was not inhibited by XopP, in a Y3H assay.
Supplemental Figure S9. FLS2-GFP localization in PM is significantly reduced in the presence of XopP in transient expression, as well as in XopP-expressing transgenic Arabidopsis lines.
Supplemental Figure S10. XopP is not a general cytoplasmic HR inhibitor nor does it interact with the Chp7 effector; levels of Chp7 are not affected by XopP; H2O2 is reduced in XopP-expressing Arabidopsis plants.
Supplemental Figure S11. Infection of XopP-expressing plants with P. cannabina pv. alisalensis ES4326 (formerly P.s. pv. maculicola ES4326) shows a quicker onset of symptoms compared to WT plants.
Supplemental Data Set 1. Sequence alignment of XopP homologs used to build the phylogenetic tree in Figure 2A, in FASTA format.
Supplemental Data Set 2. Phylogenetic tree file, as it was created in MEGA5, in Newick format.
Supplemental Data Set 3. Statistical analyses parameters for all statistical analyses performed in this study.
Supplemental Data Set 4. Primers used in this study.
Supplemental File 1. Sequence alignment used to produce the phylogenetic tree in Figure 2A.
Supplementary Material
Acknowledgements
We especially want to thank Prof. Marco Trujillo for his generous donation of the AtEXO70B2 construct as well as for his advice on construction of the truncations construction; Prof. M. Moscou for his kind donation of some of the IDs used in this research; Dr. Katarzyna Rybak for the kind donation of the FLS2–GFP construct; Prof. František Baluška for his kind donation of the DsRed-FYVE confocal marker; Dr. Yasin Dagdas for his generous donation of all the other confocal markers used in the research, and for fruitful discussion; Mr Marc Youles and the Synthetic Biology Group of TSL for the kind donation of Golden-Gate compatible Y2H vectors; Prof. Nemo Peeters for his kind donation of Rs GMI1000; Prof. Dimitris Goumas for his generous donation of Xcv; Prof. Subba Rao Gangi Setty for his kind donation of the pBridge vector; Dr. Jeff Chang for his kind donation of Pf01 EtHAn; Prof. D. Alexandraki for her kind donation of PJ69 yeast strain and her guidance with Y2H experiments; and lastly undergraduate student Aspa Papanikolaou for her kind contribution in some of the Y2H assays.
Funding
V.A.M. was supported by the Hellenic Foundation for Research and Innovation (HFRI) and the General Secretariat for Research and Technology (GSRT), under the HFRI PhD Fellowship grant (GA. no. 4776) and by the internal PhD supporting scheme of IMBB-FORTH. K.K. and P.F.S. were supported by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (“INNOVA-PROTECT” with project code: T1EDK-01878). P.N.M and A.M. were supported by the FORMAS Research Council Grant MOP-86675, and HFRI grants Always Strive for Excellence 1624 and Ph.D. scholarship. P.H.N.C.’s work benefited from access to the NKI Protein Facility and the Instruct-ERIC center, and was supported by iNEXT-Discovery, project number 871037, funded by the Horizon 2020 program of the European Commission
Conflict of interest statement. The authors declare no competing interests.
Contributor Information
Vassiliki A Michalopoulou, Department of Biology, University of Crete, Heraklion, Crete 714 09, Greece; Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, Heraklion, Crete 70013, Greece.
Glykeria Mermigka, Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, Heraklion, Crete 70013, Greece.
Konstantinos Kotsaridis, Department of Biology, University of Crete, Heraklion, Crete 714 09, Greece; Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, Heraklion, Crete 70013, Greece.
Andriani Mentzelopoulou, Department of Biology, University of Crete, Heraklion, Crete 714 09, Greece.
Patrick H N Celie, Division of Biochemistry, the Netherlands Cancer Institute, Amsterdam, The Netherlands.
Panagiotis N Moschou, Department of Biology, University of Crete, Heraklion, Crete 714 09, Greece; Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, Heraklion, Crete 70013, Greece; Department of Plant Biology, Swedish University of Agricultural Sciences, Uppsala BioCenter, Linnean Center for Plant Biology, Uppsala S-75007, Sweden.
Jonathan D G Jones, The Sainsbury Laboratory, Norwich Research Park, Norwich, UK.
Panagiotis F Sarris, Department of Biology, University of Crete, Heraklion, Crete 714 09, Greece; Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, Heraklion, Crete 70013, Greece; Biosciences, University of Exeter, Exeter, UK.
P.F.S. designed the research and supervised the project; V.A.M. carried out most of the experiments; K.K. and P.H.N.C. purified recombinant proteins and performed in vitro interaction assays; G.M. performed additional experiments and contributed to organizing some of the experiments in the lab. V.A.M. performed Arabidopsis transformation; P.N.M. and A.M. contributed to FRET analysis and some confocal microscopy experiments and A.M and V.A.M performed the statistical analysis. P.F.S. and V.A.M. initiated the project; P.F.S. and V.A.M. wrote the manuscript. P.N.M and J.D.G.J. edited the manuscript. All authors have read and approved the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Panagiotis F. Sarris (p.sarris@imbb.forth.gr; p.sarris@uoc.gr).
References
- Adlung N, Prochaska H, Thieme S, Banik A, Blüher D, John P, Nagel O, Schulze S, Gantner J, Delker C, et al. (2016) Non-host resistance induced by the Xanthomonas effector XopQ is widespread within the genus Nicotiana and functionally depends on EDS1. Front Plant Sci 7: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SM, Nishida-Fukuda H, Li Y, McDonald WH, Gradinaru CC, Macara IG (2018) Exocyst dynamics during vesicle tethering and fusion. Nat Commun 9: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SQ, Potnis N, Dow M, Vorhölter FJ, He YQ, Becker A, Teper D, Li Y, Wang N, Bleris L, et al. (2020) Mechanistic insights into host adaptation, virulence and epidemiology of the phytopathogen Xanthomonas. FEMS Microbiol Rev 44: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C, Santos-Rosa MJ, Shirasu K (2001) The U-box protein family in plants. Trends Plant Sci 6: 354–358 [DOI] [PubMed] [Google Scholar]
- Badel JL, Shimizu R, Oh HS, Collmer A (2006) A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol Plant-Microbe Interact 19: 99–111 [DOI] [PubMed] [Google Scholar]
- Baumler A, Fang FC (2013) Host specificity of bacterial pathogens. Cold Spring Harb Perspect Med 3: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Zhou J, Faulkner C, Mac DL, Robatzek S (2012) Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell 24: 4205–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi V, Tang S, Stallmann A, Roberts M, Cherkis K, Dangl JL (2011) Correction: expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc Natl Acad Sci USA 108: 16463–16468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabham HJ, Hernández-Pinzón I, Holden S, Lorang J, Moscou MJ (2018) An ancient integration in a plant NLR is maintained as a trans-species polymorphism. BioRxiv 239541. 10.1101/239541 [DOI] [Google Scholar]
- Brillada C, Teh O, Ditengou FA, Lee C, Klecker T, Furlan G, Zietz M, Hause G, Eschen-lippold L, Lee J, et al. (2021) Exocyst subunit Exo70B2 is linked to immune signalling and autophagy. Plant Cell 33(2): 404–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon JJ, Thrall PH (2009) Co-evolution of plants and their pathogens in natural habitats. Science 324: 755–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D, Bonas U (2003) Common infection strategies of plant and animal pathogenic bacteria. Curr Opin Plant Biol 6: 312–319 [DOI] [PubMed] [Google Scholar]
- Chang YY, Stévenin V, Duchateau M, Gianetto QG, Hourdel V, Rodrigues CD, Matondo M, Reiling N, Enninga J (2020) Shigella hijacks the exocyst to cluster macropinosomes for efficient vacuolar escape. PLoS Pathog 16: 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1999) Floral dip : a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Collier SM, Hamel LP, Moffett P (2011) Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol Plant-Microbe Interact 24: 918–931 [DOI] [PubMed] [Google Scholar]
- Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86: 3993–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnac S, Chakravarthy S, Kvitko BH, Russell AB, Martin GB, Collmer A (2011) Genetic disassembly and combinatorial reassembly identify a minimal functional repertoire of type III effectors in Pseudomonas syringae. Proc Natl Acad Sci USA 108: 2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Jones JDG (2019) A pentangular plant inflammasome. Science 364: 31–32 [DOI] [PubMed] [Google Scholar]
- Daskalov A, Habenstein B, Martinez D, Debets AJM, Sabaté R, Loquet A, Saupe SJ (2015) Signal transduction by a fungal NOD-like receptor based on propagation of a prion amyloid fold. PLOS Biol 13: e1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean GH, Zheng H, Tewari J, Huang J, Young DS, Yeen TH, Western TL, Carpita NC, McCann MC, Mansfield SD, et al. (2007) The Arabidopsis MUM2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 19: 4007–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Mpina MH, Birch PRJ, Bouwmeester K, Govers F (2015) Phytophthora infestans RXLR effector AVR1 interacts with exocyst component Sec5 to manipulate plant immunity. Plant Physiol 169: 1975–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Overdijk EJR, Berg JA, Govers F, Bouwmeester K (2018) Solanaceous exocyst subunits are involved in immunity to diverse plant pathogens. J Exp Bot 69: 655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury Z, Ma Y, Furzer OJ, Huh SU, Cevik V, Jones JDG, Sarris PF (2016) Pathogen perception by NLRs in plants and animals: parallel worlds. BioEssays 38: 769–781 [DOI] [PubMed] [Google Scholar]
- Elias M, Drdova E, Ziak D, Bavlnka B, Hala M, Cvrckova F, Soukupova H, Zarsky V (2003) The exocyst complex in plants. Cell Biol Int 27: 199–201 [DOI] [PubMed] [Google Scholar]
- Ellis JG (2016) Integrated decoys and effector traps: how to catch a plant pathogen. BMC Biol 14: 14–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Kandzia R, Marillonnet S (2008) A one pot, one step, precision cloning method with high throughput capability. PLoS One 3: e3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Youles M, Gruetzner R, Ehnert TM, Werner S, Jones JDG, Patron NJ, Marillonnet S (2014) A golden gate modular cloning toolbox for plants. ACS Synth Biol 3: 839–843 [DOI] [PubMed] [Google Scholar]
- Fargier E, Fischer-Le Saux M, Manceau C (2011) A multilocus sequence analysis of Xanthomonas campestris reveals a complex structure within crucifer-attacking pathovars of this species. Syst Appl Microbiol 34: 156–165 [DOI] [PubMed] [Google Scholar]
- French AP, Mills S, Swarup R, Bennett MJ, Pridmore TP (2008) Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat Protoc 3: 619–628 [DOI] [PubMed] [Google Scholar]
- Fujisaki K, Abe Y, Ito A, Saitoh H, Yoshida K, Kanzaki H, Kanzaki E, Utsushi H, Yamashita T, Kamoun S, et al. (2015) Rice Exo70 interacts with a fungal effector, AVR-Pii, and is required for AVR-Pii-triggered immunity. Plant J 83: 875–887 [DOI] [PubMed] [Google Scholar]
- Gascuel O (1997) BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14: 685–695 [DOI] [PubMed] [Google Scholar]
- Gluck-Thaler E, Cerutti A, Perez-Quintero AL, Butchacas J, Roman-Reyna V, Madhavan VN, Shantharaj D, Merfa MV, Pesce C, Jauneau A et al. (2020) Repeated gain and loss of a single gene modulates the evolution of vascular pathogen lifestyles. Sci Adv 6: eabc4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Innes RW (2012) The KEEP on going protein of Arabidopsis regulates intracellular protein trafficking and is degraded during fungal infectionC W OA. Plant Cell 24: 4717–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hála M, Cole R, Synek L, Drdová E, Pečenková T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, et al. (2008) An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20: 1330–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Gu M, Duffy CM, Mirza AM, Marcotte LL, Walls AC, Farrall N, Hakhverdyan Z, Field MC, Rout MP, et al. (2016) Subunit connectivity, assembly determinants and architecture of the yeast exocyst complex. Nat Struct Mol Biol 23: 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Horvath A, Riezman H, Riezman H (1994) Rapid protein extraction from Saccharomyces cerevisiae. Yeast 10: 1305–1310 [DOI] [PubMed] [Google Scholar]
- Ichimaru K, Yamaguchi K, Harada K, Nishio Y, Hori M, Ishikawa K, Inoue H, Shigeta S, Inoue K, Shimada K, et al. (2022). Cooperative regulation of PBI1 and MAPKs controls WRKY45 transcription factor in rice immunity. Nat Commun 13, 10.1038/s41467-022-30131-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Yamaguchi K, Sakamoto K, Yoshimura S, Inoue K, Tsuge S, Kojima C, Kawasaki T (2014) Bacterial effector modulation of host E3 ligase activity suppresses PAMP-triggered immunity in rice. Nat Commun 5: 1–11 [DOI] [PubMed] [Google Scholar]
- Jiang W et al. (2009) Identification of six type III effector genes with the pip box in Xanthomonas campestris pv. campestris and five of them contribute individually to full pathogenicity. Mol Plant-Microbe Interact 22: 1401–1411 [DOI] [PubMed] [Google Scholar]
- Jin L, Mackey DM (2017) Measuring callose deposition, an indicator of cell wall reinforcement, during bacterial infection in Arabidopsis. Methods Mol Biol 1578: 195–205 [DOI] [PubMed] [Google Scholar]
- Jo SH, Lee J, Park E, Kim DW, Lee DH, Ryu CM, Choi D, Park JM (2019) A human pathogenic bacterium Shigella proliferates in plants through adoption of type III effectors for shigellosis. Plant Cell Environ 42: 2962–2978 [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M (2021) KEGG: integrating viruses and cellular organisms. Nucleic Acids Res 49: D545–D551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich I, Pečenková T, Sekereš J, Smetana O, Fendrych M, Foissner I, Höftberger M, Žárský V (2013) Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14: 1155–1165 [DOI] [PubMed] [Google Scholar]
- Kvitko BH, Park DH, Velásquez AC, Wei CF, Russell AB, Martin GB, Schneider DJ, Collmer A (2009) Deletions in the repertoire of Pseudomonas syringae pv. tomato DC3000 type III secretion effector genes reveal functional overlap among effectors. PLoS Pathog 5: e1000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme B, Dillon MM, Martel A, Almeida RND, Desveaux D, Guttman DS (2020) The pan-genome effector-triggered immunity landscape of a host-pathogen interaction. Science 367: 763–768 [DOI] [PubMed] [Google Scholar]
- Landry D, Mila I, Sabbagh CRR, Zaffuto M, Pouzet C, Tremousaygue D, Dabos P, Deslandes L, Peeters N (2021) An NLR integrated domain toolkit to identify plant pathogen effector targets. BioRxiv [DOI] [PubMed]
- Leary AY, Sanguankiattichai N, Duggan C, Tumtas Y, Pandey P, Segretin ME, Salguero Linares J, Savage ZD, Yow RJ, Bozkurt TO (2018) Modulation of plant autophagy during pathogen attack. J Exp Bot 69: 1325–1333 [DOI] [PubMed] [Google Scholar]
- Leary AY, Savage Z, Tumtas Y, Bozkurt TO (2019) Contrasting and emerging roles of autophagy in plant immunity. Curr Opin Plant Biol 52: 46–53 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 121: 567–577 [DOI] [PubMed] [Google Scholar]
- Lu L, Yang D, Tang D, Li S, Chen Z (2020) Transcriptome analysis of different rice cultivars provides novel insights into the rice response to bacterial leaf streak infection. Funct Integr Genomics 20: 681–693 [DOI] [PubMed] [Google Scholar]
- Lu Y, Hatsugai N, Katagiri F, Ishimaru CA, Glazebrook J (2015) Putative serine protease effectors of Clavibacter michiganensis induce a hypersensitive response in the apoplast of Nicotiana species. Mol Plant-Microbe Interact 28: 1216–1226 [DOI] [PubMed] [Google Scholar]
- Lyu D, Msimbira LA, Nazari M, Antar M, Pagé A, Shah A, Monjezi N, Zajonc J, Tanney CAS, Backer R, et al. (2021) The coevolution of plants and microbes underpins sustainable agriculture. Microorganisms 9: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manriquez B, Muller D, Prigent-Combaret C (2021) Experimental evolution in plant-microbe systems: a tool for deciphering the functioning and evolution of plant-associated microbial communities. Front Microbiol 12:619122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei K et al. (2018) Cryo-EM structure of the exocyst complex. Nat Struct Mol Biol 25: 139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermigka G, Sarris PF (2019) The rise of plant resistosomes. Trends Immunol 40: 670–673 [DOI] [PubMed] [Google Scholar]
- Michalopoulou VA, Vicente JG, Studholme DJ, Sarris PF (2018) Draft genome sequences of pathotype strains for three pathovars belonging to three Xanthomonas species. Microbiol Resour Announc 7: 4–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou PN, Smertenko AP, Minina EA, Fukada K, Savenkov EI, Robert S, Hussey PJ, Bozhkov PV (2013) The caspase-related protease separase (EXTRA SPINDLE POLES) regulates cell polarity and cytokinesis in Arabidopsis. Plant Cell 25: 2171–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkvold KR, Russell AB, Kvitko BH, Collmer A (2009) Pseudomonas syringae pv. tomato DC3000 type III effector HopAA1-1 functions redundantly with chlorosis-promoting factor PSPTO4723 to produce bacterial speck lesions in host tomato. Mol Plant-Microbe Interact 22: 1341–1355 [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Nielsen ME, Feechan A, Böhlenius H, Ueda T, Thordal-Christensen H (2012) Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc Natl Acad Sci USA 109: 11443–11448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niño-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7: 303–324 [DOI] [PubMed] [Google Scholar]
- Pečenková T, Hála M, Kulich I, Kocourková D, Drdová E, Fendrych M, Toupalová H, Žárský V (2011) The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J Exp Bot 62: 2107–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pečenková T, Marković V, Sabol P, Kulich I, Zárský V (2017) Exocyst and autophagy-related membrane trafficking in plants. J Exp Bot 69: 47–57 [DOI] [PubMed] [Google Scholar]
- Pečenková T, Pejchar P, Moravec T, Drs M, Haluška S, Šantrůček J, Potocká A, Žárský V, Potocký M (2022) Immunity functions of Arabidopsis pathogenesis‐related 1 are coupled but not confined to its C‐terminus processing and trafficking. Mol Plant Pathol 23: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pečenková T, Potocká A, Potocký M, Ortmannová J, Drs M, Janková Drdová E, Pejchar P, Synek L, Soukupová H, Žárský V, et al. (2020) Redundant and diversified roles among selected Arabidopsis thaliana EXO70 paralogs during biotic stress responses. Front Plant Sci 11: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov G, Fraiture M, Brunner F, Sessa G (2016) Multiple Xanthomonas euvesicatoria type III effectors inhibit flg22-triggered immunity. Mol Plant-Microbe Interact 29: 651–660 [DOI] [PubMed] [Google Scholar]
- Ray SK, Macoy DM, Kim WY, Lee SY, Kim MG (2019) Role of RIN4 in regulating PAMP-triggered immunity and effector-triggered immunity: current status and future perspectives. Mol Cells 42: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redditt TJ, Chung EH, Karimi HZ, Rodibaugh N, Zhang Y, Trinidad JC, Kim JH, Zhou Q, Shen M, Dangl JL, et al. (2019) AvrRpm1 functions as an ADP-ribosyl transferase to modify NOI domain-containing proteins, including Arabidopsis and soybean RPM1-interacting protein4. Plant Cell 31: 2664–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S (2007) Vesicle trafficking in plant immune responses. Cell Microbiol 9: 1–8 [DOI] [PubMed] [Google Scholar]
- Roden JA, Belt B, Ross JB, Tachibana T, Vargas J, Mudgett MB (2004) A genetic screen to isolate type III effectors translocated into pepper cells during Xanthomonas infection. Proc Natl Acad Sci USA 101: 16624–16629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B et al. (2015) Genomics and transcriptomics of Xanthomonas campestris species challenge the concept of core type III effectome. BMC Genomics 16:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RP, Vorhölter FJ, Potnis N, Jones JB, Van Sluys MA, Bogdanove AJ, Dow JM (2011) Pathogenomics of Xanthomonas: understanding bacterium-plant interactions. Nat Rev Microbiol 9: 344–355 [DOI] [PubMed] [Google Scholar]
- Sabol P, Kulich I, Žárský V (2017) RIN4 recruits the exocyst subunit EXO70B1 to the plasma membrane. J Exp Bot 68: 3253–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacristán S, García-Arenal F (2008) The evolution of virulence and pathogenicity in plant pathogen populations. Mol Plant Pathol 9: 369–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed B, Brillada C, Trujillo M (2019) Dissecting the plant exocyst. Curr Opin Plant Biol 52: 69–76 [DOI] [PubMed] [Google Scholar]
- Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, Vance RE (2019) Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science 364: eaau1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris PF, Duxbury Z, Huh SU, Ma Y, Segonzac C, Sklenar J, Derbyshire P, Cevik V, Rallapalli G, Saucet SB et al. (2015) A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 161: 1089–1100 [DOI] [PubMed] [Google Scholar]
- Sarris PF, Cevik V, Dagdas G, Jones JDG, Krasileva KV (2016) Comparative analysis of plant immune receptor architectures uncovers host proteins likely targeted by pathogens. BMC Biol 14: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DH, Ahn MY, Park KY, Kim EY, Kim WT (2016) The N-terminal und motif of the Arabidopsis U-box E3 ligase pub18 is critical for the negative regulation of aba-mediated stomatal movement and determines its ubiquitination specificity for exocyst subunit Exo70B1. Plant Cell 28: 2952–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Bourdais G, Pan H, Robatzek S, Tang D (2017) Arabidopsis glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity. Proc Natl Acad Sci USA 114: 5749–5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn KH, Lei R, Nemri A, Jones JDG (2007) The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell 19: 4077–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz BJ, Mudgett MB, Dangl JL, Galan JE (2001) Common and contrasting themes of plant and animal diseases. Science 292: 2285–2289 [DOI] [PubMed] [Google Scholar]
- Stegmann M, Anderson RG, Ichimura K, Pecenkova T, Reuter P, Žárský V, McDowell JM, Shirasu K, Trujillo M (2012) The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 24: 4703–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M, Anderson RG, Westphal L, Rosahl S, McDowell JM, Trujillo M (2014) The exocyst subunit Exo70B1 is involved in the immune response of Arabidopsis thaliana to different pathogens and cell death. Plant Signal Behav 8: e27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Pleskot R, Sekereš J, Serrano N, Vukašinović N, Ortmannová J, Klejchová M, Pejchar P, Batystová K, Gutkowska M et al. (2021) Plasma membrane phospholipid signature recruits the plant exocyst complex via the EXO70A1 subunit. Proc Natl Acad Sci USA 118: e2105287118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampakaki AP, Skandalis N, Gazi AD, Bastaki MN, Sarris PF, Charova SN, Kokkinidis M, Panopoulos NJ (2010) Playing the “Harp”: evolution of our understanding of hrp/hrc genes. Annu Rev Phytopathol 48: 347–370 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WJ, Thireault CA, Kimbrel JA, Chang JH (2009) Recombineering and stable integration of the Pseudomonas syringae pv. syringae 61 hrp/hrc cluster into the genome of the soil bacterium Pseudomonas fluorescens Pf0-1. Plant J 60: 919–928 [DOI] [PubMed] [Google Scholar]
- Timilsina S, Potnis N, Newberry EA, Liyanapathiranage P, Iruegas-Bocardo F, White FF, Goss EM, Jones JB (2020) Xanthomonas diversity, virulence and plant–pathogen interactions. Nat Rev Microbiol 18: 415–427 [DOI] [PubMed] [Google Scholar]
- van Rheenen J, Langeslag M, Jalink K (2004) Correcting confocal acquisition to optimize imaging of fluorescence resonance energy transfer by sensitized emission. Biophys J 86: 2517–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente JG, Holub EB (2013) Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to Brassica crops. Mol Plant Pathol 14: 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt B, Timmers ACJ, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, et al. (2005) Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol 84: 609–621 [DOI] [PubMed] [Google Scholar]
- Vukašinović N, Cvrčková F, Eliáš M, Cole R, Fowler JE, Žárský V, Synek L (2014) Dissecting a hidden gene duplication: the Arabidopsis thaliana SEC10 locus. PLoS One 9: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Liu N, Gao C, Cai H, Romeis T, Tang D (2020) The Arabidopsis exocyst subunits EXO70B1 and EXO70B2 regulate FLS2 homeostasis at the plasma membrane. New Phytol 227: 529–544 [DOI] [PubMed] [Google Scholar]
- Wang W, Liu N, Gao C, Rui L, Jiang Q, Chen S, Zhang Q, Zhong G, Tang D (2021) The truncated TNL receptor TN2‐mediated immune responses require ADR1 function. Plant J 108: 672–689 [DOI] [PubMed] [Google Scholar]
- Wang W, Liu N, Gao C, Rui L, Tang D (2019) The Pseudomonas syringae effector AvrPtoB associates with and ubiquitinates Arabidopsis exocyst subunit EXO70B1. Front Plant Sci 10: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley JN, Russell AB, Wexler AG, Bronstein PA, Kvitko BH, Krasnoff SB, Munkvold KR, Swingle B, Gibson DM, Collmera A (2013) Pseudomonas syringae pv. tomato DC3000 CmaL (PSPTO4723), a DUF1330 family member, is needed to produce L-allo-isoleucine, a precursor for the phytotoxin coronatine. J Bacteriol 195: 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters FS, Verveer PJ, Bastiaens PIH (2001) Imaging biochemistry inside cells. Trends Cell Biol 11: 203–211 [DOI] [PubMed] [Google Scholar]
- Xu H, Mendgen K (1994) Endocytosis of 1,3-β-glucans by broad bean cells at the penetration site of the cowpea rust fungus (haploid stage). Planta 195: 282–290 [Google Scholar]
- Yu X, Feng B, He P, Shan L (2017) From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol 55: 109–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yao Y, Qiu X, Wang G, Hu Z, Chen S, Wu Z, Yuan N, Gao H, Wang J et al. (2019) Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat Immunol 20: 433–446 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen M, Siemiatkowska B, Toleco MR, Jing Y, Strotmann V, Zhang J, Stahl Y, Fernie AR (2020) A highly efficient Agrobacterium-mediated method for transient gene expression and functional studies in multiple plant species. Plant Commun 1: 100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu CM, Emons AMC, Ketelaar T (2010) The plant exocyst. J Integr Plant Biol 52: 138–146 [DOI] [PubMed] [Google Scholar]
- Zhao T, Rui L, Li J, Nishimura MT, Vogel JP, Liu N, Liu S, Zhao Y, Dangl JL, Tang D (2015) A truncated NLR protein, TIR-NBS2, is required for activated defense responses in the exo70B1 mutant. PLoS Genet 11: 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Kunst L, Hawes C, Moore I (2004) A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J 37: 398–414 [DOI] [PubMed] [Google Scholar]
- Zhuang X, Chung KP, Cui Y, Lin W, Gao C, Kang BH, Jiang L, Bassham DC (2017) ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis. Proc Natl Acad Sci USA 114: E426–E435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In Bryson V, Vogel HJ, eds, Evolving Genes and Proteins, the Netherlands, Academic Press, pp. 97–166 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.