Abstract
In five vancomycin-resistant laboratory step mutants selected from the highly and homogeneously methicillin-resistant Staphylococcus aureus strain COL (MIC of methicillin, 800 μg/ml; MIC of vancomycin, 1.5 μg/ml), the gradually increasing levels of resistance to vancomycin were accompanied by parallel decreases in the levels of methicillin resistance and abnormalities in cell wall metabolism. The latter included a gradual reduction in the proportion of highly cross-linked muropeptide species in peptidoglycan, down-regulation of the production of penicillin-binding protein 2A (PBP2A) and PBP4, and hypersensitivity to β-lactam antibiotics each with a relatively selective affinity for the various staphylococcal PBPs; the PBP2-specific inhibitor ceftizoxime was particularly effective.
The mechanism of resistance in the recently described glycopeptide-resistant clinical isolates of Staphylococcus aureus is not well understood. A laboratory mutant, VM50 (formerly called VM), with high-level resistance to vancomycin (MIC, 100 μg/ml) and selected from the methicillin-resistant S. aureus strain COL through several steps of exposure to vancomycin, showed several unusual properties. The most striking was a massive reduction in the level of methicillin resistance, from an MIC of 800 μg/ml for the parental strain to an MIC of 1.5 μg/ml for mutant VM50. This decrease paralleled an increase in the vancomycin MIC from 1.5 μg/ml for the parental strain COL to 100 μg/ml for the mutant (9). Evidence was also obtained for the extensive perturbation of cell wall metabolism in mutant VM50, including a reduction of the highly oligomeric muropeptide components of the peptidoglycan and inactivation of penicillin-binding protein 2A (PBP2A) and PBP4 (11, 12).
The mechanism of resistance in this laboratory mutant most likely involves the accumulation of point mutations. In an effort to better understand the nature of these mutations, we undertook to characterize the four low- and intermediate-level-resistance step mutants VM3, VM6, VM12, and VM25, which were the precursors of the already characterized highly vancomycin-resistant mutant VM50.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are listed in Table 1. All strains were grown in tryptic soy broth (TSB) (Difco, Detroit, Mich.) at 37°C with aeration. Growth was monitored by measuring the optical density at 620 nm with an LKB spectrophotometer (Pharmacia LKB Biotechnology, Inc., Uppsala, Sweden). Viable titers and antibiotic resistance levels (population analysis) were determined by plating diluted cultures on tryptic soy agar (TSA) (Difco) as described before (13). The plates were incubated at 37°C for 96 h.
TABLE 1.
Strains used in this study
| Strain | Relevant characteristicsa | Source or reference |
|---|---|---|
| COL | Homogeneous Mcr Vms | The Rockefeller University collection |
| VM3b | Mcr Vmr | This study |
| VM6b | Mcr Vmr | This study |
| VM12b | Mcr Vmr | This study |
| VM25b | Mcr Vmr | This study |
| VM50b | Heterogeneous Mcr Vmr | 9 |
| RUSA4 | COL (mecA::Tn551) | 3 |
| RUSA130 | COL (pbp2::Tn551) | 7 |
| VM-RU4 | VM50 (mecA::Tn551) | This study |
| VM-RU130 | VM50 (pbp2::Tn551) | This study |
Abbreviations: Mc, methicillin; Vm, vancomycin.
The number in the strain designation represents the highest concentration of vancomycin in a twofold drug concentration series that does not prevent the majority of the strain population from growing.
Mutant isolation.
The lowest-level step mutant, VM3, was isolated from a synergy plate containing methicillin plus a sub-MIC concentration of vancomycin (9). Subsequent step mutants with increasing levels of vancomycin resistance were isolated by picking rare colonies that were capable of growth on agar containing vancomycin at concentrations above the MIC for the majority of the cells. Such colonies were then used as inocula of overnight cultures of TSB supplemented with the same concentration of the antibiotic as the agar plate from which the colony was picked.
Sequencing of mecA and pbp4 genes.
DNA fragments including the mecA and pbp4 genes were amplified by PCR from chromosomal DNA and sequenced as described before (11, 12).
Isolation of RNA and Northern blot hybridization.
Overnight cultures were inoculated into fresh TSB and grown to the mid-log phase (optical density, ≈0.7). RNA was extracted by use of a FastRNA Blue isolation kit (Bio 101, Inc., Vista, Calif.) according to the manufacturer's recommendations. After the concentration was adjusted with a Gene Quant spectrophotometer (Pharmacia), RNA (5 μg) was resolved by electrophoresis on 1.2% agarose–0.66 M formaldehyde gels in morpholinepropanesulfonic acid (MOPS) running buffer. Blotting of RNA onto Hybond N+ membranes (Amersham, Arlington Heights, Ill.) was performed with the Turbo Blotter Neutral Transfer System (Schleicher & Schuell, Inc., Keene, N.H.). For detection of specific transcripts, DNA probes were labeled with [α-32P]dCTP (Amersham) and hybridized under high-stringency conditions. The blots were subsequently washed and autoradiographed.
Membrane purification and analysis of PBPs.
Membranes were prepared from cells grown to the late exponential stage as described previously (11), and then proteins (80 μg per sample) were labeled with [3H]benzylpenicillin NEP salt (87.4 mCi per mg) (Merck & Co., Inc., Rahway, N.J.) for 10 min at 30°C. The reaction was stopped by the addition of an excess of nonlabelled benzylpenicillin. The labeled PBPs were resolved by the technique of Laemmli (2) and visualized by fluorography.
Preparation of peptidoglycan and analysis.
Cell wall peptidoglycan was prepared, and the muropeptide composition of peptidoglycan was analyzed by reversed-phase high-performance liquid chromatography (HPLC) as described before (1), except that the alkaline phosphatase step was omitted.
Transductional crosses and analysis of transductants.
Crosses were performed with phage 80α as described previously (6) with mutant VM50 as a recipient and RUSA4 and RUSA130 as donor strains. Transductants were screened as described before (10).
RESULTS
Gradual decrease in the methicillin MICs for vancomycin-resistant step mutants.
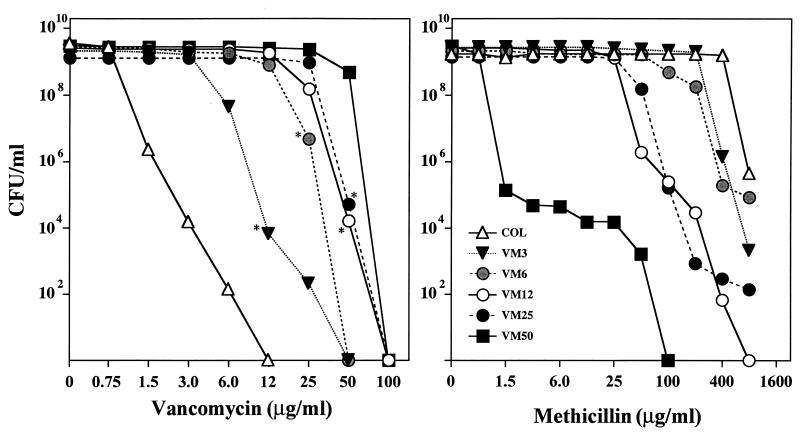
Figure 1 illustrates the antibiotic susceptibility profiles of the methicillin-resistant S. aureus parental strain COL and its five vancomycin-resistant mutant derivatives for two antimicrobial agents, vancomycin and methicillin. Overnight cultures of the bacteria were plated at various dilutions on TSA supplemented with increasing concentrations of either methicillin or vancomycin in the concentration ranges indicated in Fig. 1. The right panel of Fig. 1 shows the gradual decrease in the degree of methicillin resistance of mutant strains VM3, VM6, VM12, VM25, and VM50 (formerly called VM). For the most highly vancomycin-resistant strain, VM50, capable of growing in the presence of 50 μg of vancomycin per ml, the highest concentration of methicillin on which the majority of these bacteria was capable of forming colonies was about 0.75 μg/ml. Gradually increasing vancomycin MICs for mutants VM3 through VM50 are shown in the left panel of Fig. 1. The great majority of cells in cultures of VM50 were still capable of forming colonies on 50 μg of vancomycin per ml, but no survivors were detected (less than 10−8) on agar containing 100 μg of vancomycin per ml.
FIG. 1.
Phenotypic expression of resistance to vancomycin (left) and methicillin (right) in a series of vancomycin-resistant step mutants. Bacterial cultures were grown in TSB to stationary phase and plated at several dilutions on TSA containing various concentrations of vancomycin or methicillin, as described for the method of population analysis (13). Asterisks indicate colonies that served as sources for higher-level step mutants.
Gradual decrease in the growth rate of vancomycin-resistant step mutants.
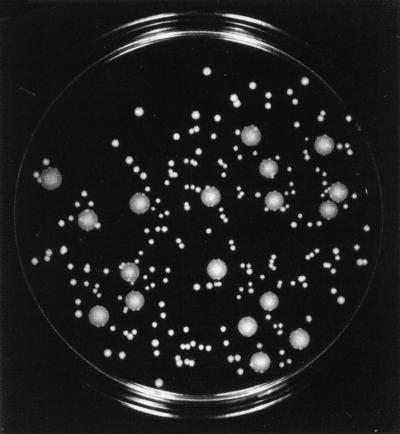
Cultures of the parental strain COL and the five step mutants VM3, VM6, VM12, VM25, and VM50 were grown in TSB at 37°C with aeration. Culture growth was monitored by the determination of optical density. The doubling times of the vancomycin-resistant step mutants increased gradually from 32 min for the parental strain COL to 50 min for VM3, 60 min for VM6, 72 min for VM12, and 78 min for VM25 and VM50. The slow growth rate and the general growth deficiency of vancomycin-resistant mutant VM50 were evident by its small colony size on solid medium (Fig. 2), which did not increase upon prolonged incubation.
FIG. 2.
Slow growth rate of mutant VM50. A mixture of mutant VM50 and parental strain COL was plated on TSA and incubated for 72 h at 37°C. The large colonies represent the parental strain; the small colonies represent the mutant.
Transcription of mecA.
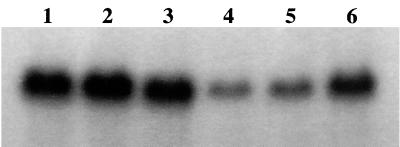
The drastic reduction of methicillin resistance in the most highly vancomycin-resistant mutant, VM50, was recently shown to be associated with the inactivation of the mecA gene (12). Sequencing of the mecA region from the low- or intermediate-level-resistance step mutants VM3 through VM25 showed no evidence for sequence alterations (data not shown). When total RNA from mutants VM3 through VM25 was hybridized with the mecA probe in Northern blot analysis, a radioactive band with a molecular size of 2 kb, corresponding to the size of the mecA transcript, was detected in each preparation (Fig. 3). However, the intensity of this band was significantly reduced only in mutants VM12 and VM25. Thus, the gradually declining methicillin resistance level in these mutants seems to be associated with the decreased transcription of mecA.
FIG. 3.
Levels of mecA transcription in the parental strain COL (lane 1) and the vancomycin-resistant step mutants VM3 through VM50 (lanes 2 to 6). RNA was extracted from mid-log-phase cultures and resolved by electrophoresis on agarose-formaldehyde gels, and mecA RNA was located after hybridization with a 32P-labeled mecA DNA probe as described in Materials and Methods.
Expression of PBP4 in step mutants.
Plasma membrane preparations isolated from the parental strain COL and the vancomycin-resistant step mutants were tested by use of a fluorographic assay with [3H]penicillin for the presence of staphylococcal PBPs. Figure 4 shows that there was a gradual decrease in the cellular amounts of PBP4 in mutants VM3, VM6, VM12, and VM25 and a complete disappearance of PBP4 in mutant VM50, in which the disruption of pbp4 was recently demonstrated (11). Sequencing of the pbp4 gene and its promoter region from step mutants VM3 through VM25 did not reveal any alterations (data not shown).
FIG. 4.
PBP patterns of parental strain COL (lane 1) and its vancomycin-resistant derivatives VM3 (lane 2), VM6 (lane 3), VM12 (lane 4), VM25 (lane 5), and VM50 (lane 6). Purified plasma membranes were incubated with [3H]benzylpenicillin and were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then fluorography as described in Materials and Methods.
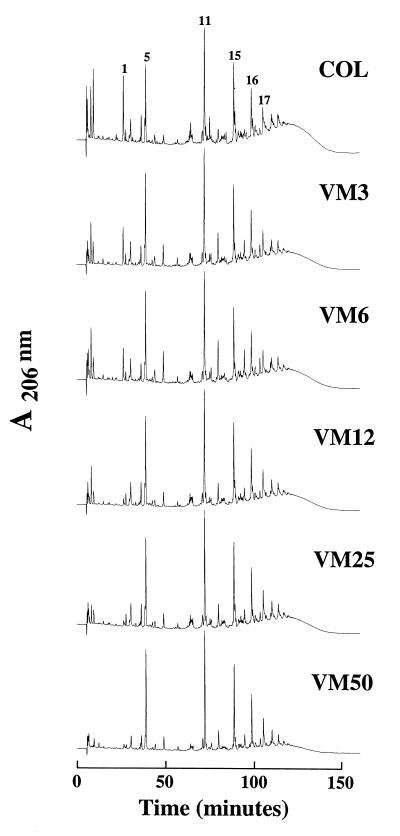
Changes in the muropeptide composition of peptidoglycan in vancomycin-resistant step mutants.
Figure 5 shows the HPLC elution profiles of enzymatic peptidoglycan hydrolysates prepared from the parental strain COL and its five vancomycin-resistant mutant derivatives. Inspection of elution profiles shows that the gradual decrease in the relative proportions of the highly oligomerized muropeptides (peak 17 [a pentamer] and larger oligomers with retention times longer than that of peak 17), seen on the HPLC profile as a “hump” of unresolved muropeptide species, parallels the increase in the vancomycin MICs for the mutants. The numbers in Fig. 5 indicate muropeptides with identified chemical structures (1).
FIG. 5.
HPLC elution profiles of muropeptides isolated from the parental strain COL and the vancomycin-resistant step mutants VM3 through VM50. Peptidoglycan was purified and digested with muramidase, and the muropeptides were separated by HPLC as described in Materials and Methods.
Hypersensitivity to β-lactams in strain VM50.
In order to further explore the relationship between susceptibility to β-lactams and that to vancomycin, the sensitivities of the parental strain COL and mutant VM50 to a number of inhibitory β-lactams were compared. The β-lactam compounds were chosen for their known preferential affinities for the various staphylococcal PBPs (5). For these experiments, it was necessary to construct a transductant derivative (VM-RU4) in which the mecA gene of VM50 was inactivated by a Tn551 insert so as to exclude the presence of the mecA gene product, PBP2A, which is known to provide blanket resistance against all β-lactam antibiotics, thus preventing the titration of normal PBP1 through PBP4 by appropriate β-lactam inhibitors. Table 2 clearly shows the hypersensitivity of vancomycin-resistant mutant VM-RU4 to most β-lactam inhibitors tested. Particularly striking was the reduction of the MIC of ceftizoxime, a β-lactam compound with a selective high affinity for PBP2 (5), for the vancomycin-resistant mutant from 3.0 μg/ml to 0.4 μg/ml. There was no reduction in the MIC of cefoxitin.
TABLE 2.
β-Lactam susceptibility profiles of VM-RU4, a derivative of vancomycin-resistant mutant VM50 with an interrupted mecA gene
| β-Lactam | MIC (μg/ml) for:
|
|
|---|---|---|
| RUSA4a | VM-RU4b | |
| Cloxacillin | 0.8 | 0.2 |
| Ceftizoxime | 3.0 | 0.4 |
| Cephalexin | 6.0 | 1.5 |
| Cefoxitin | 3.0 | 3.0 |
RUSA4 is a Tn551 mutant of COL with transposon-inactivated mecA.
VM-RU4 is a RUSA4 transductant with the VM50 background.
Effect of ceftizoxime on the expression of vancomycin resistance.
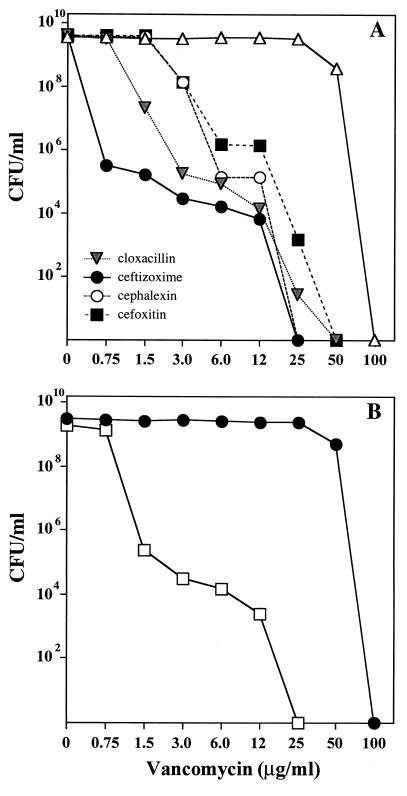
To further test the possible involvement of PBP2 with vancomycin resistance, the same selective inhibitors of the staphylococcal PBPs were also tested for their effect on the expression of vancomycin resistance. Figure 6 shows that of all the β-lactam inhibitors used at 0.25× their respective MICs, ceftizoxime was by far the most potent compound, causing a reduction in the vancomycin MIC for strain VM50 from 100 μg/ml to 0.75 μg/ml, i.e., close to the MIC for the parental strain COL. However, the population analysis profile generated by ceftizoxime was heterogeneous, and bacteria capable of expressing at least partial resistance to vancomycin in the presence of a sub-MIC concentration of ceftizoxime were also present at frequencies of about 10−6 (Fig. 6A).
FIG. 6.
Inhibitory effect of β-lactams on the phenotypic expression of vancomycin resistance in strain VM50 (A) and massive reduction of vancomycin resistance in strain VM50 with inactivated PBP2 (B). (A) Cultures of VM50 grown in TSB overnight were plated at different cell concentrations on TSA containing various concentrations of vancomycin and one-fourth the MIC of β-lactam antibiotics with selective affinities for PBP1, PBP2, PBP3, and PBP4. (B) The pbp2 gene in strain VM50 was inactivated by allele replacement with strain RU130 (7) as described in Materials and Methods. Open squares represent strain VM50 with inactivated PBP2 (VM-RU130); solid circles represent VM50 with the intact pbp2 gene. Colonies were counted, and data were plotted to provide population analysis profiles as described before (13).
A similar, extensive inhibitory effect on the expression of vancomycin resistance was observed for a derivative of VM50 in which the pbp2 gene was inactivated by transduction with DNA from the auxiliary mutant RU130, in which this gene is interrupted by a Tn551 insertion (7). The vancomycin MIC for the resulting transductant, VM-RU130, was reduced from 100 μg/ml to 1.5 μg/ml (Fig. 6B).
DISCUSSION
Expanding the study of the mechanism of vancomycin resistance in S. aureus to the four low- to intermediate-level-resistance step mutants that are the precursors of the highly vancomycin-resistant mutant VM50 characterized earlier (9–11) provided new insights into one of the most striking aspects of this mechanism, namely, the apparent incompatibility between the optimal phenotypes of methicillin resistance and vancomycin resistance. The most striking feature observed in the four mutants, VM3, VM6, VM12, and VM25, was the gradual and unidirectional change in several properties involved with resistance to β-lactam antibiotics. These changes included a gradual increase in doubling time, a gradual decrease in resistance to methicillin, and a gradual reduction in the representation of the highly cross-linked muropeptides in the peptidoglycan of the vancomycin-resistant step mutants. Highly cross-linked muropeptides (i.e., oligomers including muropeptide 17 and components with higher retention times), which represent over 60% of all muropeptide components in the parental strain COL (1), were decreased to 40% in strain VM6, 35% in VM25, and below 30% in VM50. Membrane preparations obtained from the mutants and evaluated for the relative concentrations of various staphylococcal PBPs showed a similar gradual reduction in the quantity of PBP4, which was no longer detectable in the most highly resistant mutant, VM50. Interruption of pbp4 in this mutant was described recently (11). No structural alteration in pbp4 could be detected in mutants VM3 through VM25.
Still another alteration in cell wall metabolism that paralleled the increasing vancomycin resistance of the step mutants was detected when the transcription of the mecA gene (the genetic determinant of PBP2A) was evaluated by Northern analysis. Compared with that in the parental strain COL, the mecA mRNA was clearly down-regulated in strains VM12 and VM25. In a recent study, it was shown that in the most highly resistant mutant of this series, VM50, PBP2A could no longer be detected by a fluorographic assay, and the mecA determinant of VM50 was interrupted by a 19-bp duplication which generated a stop codon at position 286 (12). No alteration in the mecA sequence was detectable in mutants VM3 through VM25. Interestingly, Northern analysis revealed the production of mecA mRNA in mutant VM50 in amounts comparable to those seen in the parental strain COL (Fig. 3).
The unidirectional change in the properties of the vancomycin-resistant step mutants suggests a gradual “tuning down” of the transpeptidase system of cell wall synthesis, beginning with the gradual reduction in the amounts of PBP4 and decreased transcription of PBP2A and culminating in the complete inactivation of both of these genes in the most highly vancomycin-resistant mutant, VM50. The impact of the tuning down of these two transpeptidases is apparent from the reduction of highly cross-linked oligomeric components in the peptidoglycan.
The gradual and extensive decrease in the peptide network cross-linking the staphylococcal peptidoglycan in the vancomycin-resistant mutants suggests that some compensatory changes in other aspects of the cell wall structure must occur to ensure the stability of the cell wall. Preliminary evidence suggesting that such a compensatory change involves the increased dependence of the vancomycin-resistant staphylococci on the glycan component of the cell wall comes from the observation that the cell wall of mutant VM50 acquired relative resistance to lysostaphin and hypersensitivity to the action of the M1 murein hydrolase (11). The reduction in the MIC for the VM50 mutant of ceftizoxime, a selective inhibitor of PBP2, and the drastic reduction in vancomycin resistance observed for cells exposed to sub-MIC concentrations of ceftizoxime or inactivation of pbp2 suggest that one of the PBPs that may acquire a major role in the assembly of the cell wall of vancomycin-resistant mutants may be PBP2, a postulated bifunctional enzyme with both transpeptidase and transglycosylase activities (4). Overexpression of PBP2 in glycopeptide-resistant mutants of S. aureus was described previously (8). Nevertheless, the results described in this communication indicate that not only ceftizoxime but also selective β-lactam inhibitors of PBP1 and PBP3 had significant inhibitory effects on strain VM50.
The tuning down of transpeptidases and the gradual reduction in the proportion of the highly cross-linked muropeptides should shift the composition of the cell wall of vancomycin-resistant staphylococci toward enrichment of monomeric muropeptide components carrying the intact d-ala-d-ala carboxy-terminal residues, which are the known attachment sites for glycopeptide antibiotics. In recent publications, we summarized evidence suggesting that the increased capacity of such monomer-rich cell walls for capturing and immobilizing glycopeptide molecules before they could reach cell wall synthetic sites on the plasma membrane may be part of the mechanism of glycopeptide resistance in S. aureus (10, 11).
ACKNOWLEDGMENT
This study received partial support from the Irene Diamond Fund.
REFERENCES
- 1.De Jonge B L M, Chang Y-S, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain: the role of penicillin binding protein 2A. J Biol Chem. 1992;267:11248–11254. [PubMed] [Google Scholar]
- 2.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 3.Matthews P, Tomasz A. Insertional inactivation of the mec gene in a transposon mutant of a methicillin-resistant clinical isolate of Staphylococcus aureus. Antimicrob Agents Chemother. 1990;34:1777–1779. doi: 10.1128/aac.34.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami K, Fujimura T, Doi M. Nucleotide sequence of the structural gene for the penicillin-binding protein 2 of Staphylococcus aureus and the presence of a homologous gene in other staphylococci. FEMS Microbiol Lett. 1994;117:131–136. doi: 10.1111/j.1574-6968.1994.tb06754.x. [DOI] [PubMed] [Google Scholar]
- 5.Okonogi K, Noji Y, Nakao M, Imada A. The possible physiological roles of penicillin-binding proteins of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. J Infect Chemother. 1995;1:50–58. [Google Scholar]
- 6.Oshida T, Tomasz A. Isolation and characterization of a Tn551-autolysis mutant of Staphylococcus aureus. J Bacteriol. 1992;174:4952–4959. doi: 10.1128/jb.174.15.4952-4959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho M, Ludovice A M, Wu S, de Lencastre H. Massive reduction in methicillin resistance by transposon inactivation of the normal PBP2 in a methicillin resistant strain of Staphylococcus aureus. Microb Drug Resist. 1997;3:409–413. doi: 10.1089/mdr.1997.3.409. [DOI] [PubMed] [Google Scholar]
- 8.Shlaes D M, Shlaes J H, Vincent S, Etter L, Fey P D, Goering R V. Teicoplanin-resistant Staphylococcus aureus expresses a novel membrane protein and increases expression of penicillin-binding protein 2 complex. Antimicrob Agents Chemother. 1993;37:2432–2437. doi: 10.1128/aac.37.11.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieradzki K, Tomasz A. Suppression of glycopeptide resistance in a highly teicoplanin resistant mutant of Staphylococcus aureus by transposon inactivation of genes involved in cell wall synthesis. Microb Drug Resist. 1998;4:159–168. doi: 10.1089/mdr.1998.4.159. [DOI] [PubMed] [Google Scholar]
- 11.Sieradzki K, Pinho M G, Tomasz A. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J Biol Chem. 1999;274:18942–18946. doi: 10.1074/jbc.274.27.18942. [DOI] [PubMed] [Google Scholar]
- 12.Sieradzki, K., S. W. Wu, and A. Tomasz. Inactivation of the methicillin resistance gene mecA in vancomycin-resistant Staphylococcus aureus. Microb. Drug Resist., in press. [DOI] [PubMed]
- 13.Tomasz A, Nachman S, Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991;35:124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]