Highlights
-
•
Basal ganglia segmentation appears reliable in children with perinatal stroke.
-
•
Alterations from perinatal stroke to basal ganglia development may be bihemispheric.
-
•
Stroke type may dictate nucleus-specific differences in basal ganglia development.
-
•
Putamen volume is associated with motor function in children with perinatal stroke.
Keywords: Basal ganglia, Perinatal Stroke, Volumetrics, Pediatric, Motor function, Cerebral palsy
Abstract
Introduction
Perinatal stroke affects millions of children and results in lifelong disability. Two forms prevail: arterial ischemic stroke (AIS), and periventricular venous infarction (PVI). With such focal damage early in life, neural structures may reorganize during development to determine clinical function, particularly in the contralesional hemisphere. Such processes are increasingly understood in the motor system, however, the role of the basal ganglia, a group of subcortical nuclei that are critical to movement, behaviour, and learning, remain relatively unexplored. Perinatal strokes that directly damage the basal ganglia have been associated with worse motor outcomes, but how developmental plasticity affects bilateral basal ganglia structure is unknown. We hypothesized that children with perinatal stroke have alterations in bilateral basal ganglia volumes, the degree of which correlates with clinical motor function.
Methods
Children with AIS or PVI, and controls, aged 6–19 years, were recruited from a population-based cohort. MRIs were acquired on a 3 T GE MR750w scanner. High-resolution T1-weighted images (166 slices, 1 mm isotropic voxels) underwent manual segmentations of bilateral caudate and putamen. Extracted volumes were corrected for total intracranial volume. A structure volume ratio quantified hemispheric asymmetry of caudate and putamen (non-dominant/dominant hemisphere structure volume) with ratios closer to 1 reflecting a greater degree of symmetry between structures. Participants were additionally dichotomized by volume ratios into two groups, those with values above the group mean (0.8) and those below. Motor function was assessed using the Assisting Hand Assessment (AHA) and the Box and Blocks test in affected (BBTA) and unaffected (BBTU) hands. Group differences in volumes were explored using Kruskal-Wallis tests, and interhemispheric differences using Wilcoxon. Partial Spearman correlations explored associations between volumes and motor function (factoring out age, and whole-brain white matter volume, a proxy for lesion extent).
Results
In the dominant (non-lesioned) hemisphere, volumes were larger in AIS compared to PVI for both the caudate (p < 0.05) and putamen (p < 0.01) but comparable between stroke groups and controls. Non-dominant (lesioned) hemisphere volumes were larger for controls than AIS for the putamen (p < 0.05), and for the caudate in PVI (p = 0.001). Interhemispheric differences showed greater dominant hemisphere volumes for the putamen in controls (p < 0.01), for both the caudate (p < 0.01) and putamen (p < 0.001) in AIS, and for the caudate (p = 0.01) in PVI. Motor scores did not differ between AIS and PVI thus groups were combined to increase statistical power. Better motor scores were associated with larger non-dominant putamen volumes (BBTA: r = 0.40, p = 0.011), and larger putamen volume ratios (BBTA: r = 0.52, p < 0.001, AHA: r = 0.43, p < 0.01). For those with relatively symmetrical putamen volume ratios (ratio > group mean of 0.8), age was positively correlated with BBTA (r = 0.54, p < 0.01) and BBTU (r = 0.69, p < 0.001). For those with more asymmetrical putamen volume ratios, associations with motor function and age were not seen (BBTA: r = 0.21, p = 0.40, BBTU: r = 0.37, p = 0.13).
Conclusion
Specific perinatal stroke lesions affect different elements of basal ganglia development. PVI primarily affected the caudate, while AIS primarily affected the putamen. Putamen volumes in the lesioned hemisphere are associated with clinical motor function. The basal ganglia should be included in evolving models of developmental plasticity after perinatal stroke.
1. Introduction
Perinatal stroke causes focal damage early in brain development, resulting in lifelong disability (Kirton and deVeber, 2013). With an estimated incidence of 1 in 1100 live births (Dunbar et al., 2020), perinatal stroke affects quality of life for millions of families worldwide. Most survivors suffer morbidities, with motor dysfunction (cerebral palsy) being most common but sensory, cognitive, behavioural, and language disorders, as well as epilepsy, may also occur (Kirton and deVeber, 2013). The term perinatal stroke captures multiple specific vascular diseases that can be defined based on timing and mechanism of injury (Dunbar and Kirton, 2019). Attention to the specific differences between each stroke disease state is advancing understanding of the developmental plasticity that occurs to determine long-term outcomes.
Perinatal arterial ischemic strokes (AIS) are the most common type, caused by an occlusion of a cerebral artery, most often the middle cerebral artery (MCA), usually near term. MCA AIS can additionally be classified by vascular territories (Kirton et al., 2008). While the size of AIS lesions may impact outcome, (Laredo et al., 2018, Rogers et al., 1997) lesion size per se is only modestly predictive of motor function (Carlson et al., 2020). The location of the lesion as defined by vascular territories has also been associated with functional outcomes (Kirton et al., 2008, López-Espejo et al., 2017, Boardman et al., 2005). Proximal MCA occlusion includes the lateral lenticulostriate arteries, which supply major basal ganglia structures including the putamen and caudate. In contrast, distal M1 occlusions beyond the lenticulostriate arteries, spare the basal ganglia and limit damage to regional white matter and cortex. More rarely, the lenticulostriate arteries alone may be injured in isolation. In contrast, periventricular venous infarction (PVI) results from a fetal germinal matrix hemorrhage leading to secondary venous infarction prior to 32 weeks gestation, damaging only the periventricular white matter while sparing both cortical and subcortical grey matter (Kirton et al., 2008). As the two most common perinatal stroke diseases and leading causes of hemiparetic cerebral palsy, the differences between AIS and PVI in regards to mechanism, location, and timing provide an optimal human model for the study of developmental plasticity.
How the young brain develops following the early, unilateral injury of perinatal stroke is increasingly understood (Kirton et al., 2021). The role of the contralesional (uninjured) hemisphere appears to be a particularly important element of this developmental plasticity. For example, ipsilateral fetal corticospinal tracts are often preserved from the contralesional hemisphere to the affected limbs in children with perinatal stroke and cerebral palsy (Staudt, 2007, Eyre, 2007, Zewdie and Kirton, 2016). Similarly, language outcomes are often remarkably normal, due in large part to the installation of language networks into the contralesional hemisphere (Carlson et al., 2019). Such bihemispheric changes, and the balance between them (Eng et al., 2018, Larsen et al., 2021), appear to be important determinants of outcome. Furthermore, changes in additional components of the motor system removed from the primary areas of injury in both hemispheres may dictate function. These include the thalamus, (Craig et al., 2019a) cerebellum (Craig et al., 2019b), and white matter connectome (Craig et al., 2020, Craig et al., 2022) where changes in the contralesional hemisphere have been associated with motor outcome. Strikingly absent from these increasingly informative models of motor system development after perinatal stroke are the basal ganglia.
The basal ganglia are a group of subcortical nuclei that facilitate movement, behaviour, and learning. Organized into a series of loops and subloops (Haber, 2016, Alexander et al., 1986, Frank et al., 2001), the basal ganglia integrate information from multiple sources subserving complex cognitive functions such as motor sequence learning and adaptability (Doyon et al., 2009), the selection of appropriate motor responses (Schroll and Hamker, 2013, Gurney et al., 2001, Schroll et al., 2012, Humphries et al., 2006), response initialization and termination (Schroll and Hamker, 2013, Nambu, 2004), learning of stimuli-response associations (Packard and Knowlton, 2002, Antzoulatos and Miller, 2011), developing automaticity (Ashby et al., 2007), and various other capabilities (Schroll and Hamker, 2013, Lanciego et al., 2012). The caudate receives significant projections from multimodal association cortices, while the putamen primarily receives inputs from the primary and secondary sensory cortices, as well as the premotor and motor cortices (Purves et al., 2001) suggesting that the putamen may be more closely linked to motor function than the caudate (Leisman et al., 2014). In adult stroke, quantifiable volumetry of the basal ganglia has explored associations with motor function (Boyd et al., 2009, Boyd and Winstein, 2004, Baudat et al., 2020). Similar methods have demonstrated utility in other disorders of the basal ganglia though most of these are neurodegenerative conditions of older adults, the exception being Tourette syndrome (Pitcher et al., 2012, Peterson et al., 2003, Aylward et al., 1996, Jurgens et al., 2008). One small study suggested that involvement of the basal ganglia was predictive of hemiparesis in children with arterial perinatal stroke (Boardman et al., 2005). In contrast, the diagnostic criteria for PVI (Kirton et al., 2008) deliberately include sparing of the caudal basal ganglia (specifically the putamen) which is not typically drained by the medullary venous system (Raets et al., 2015). Likewise, a recent study has shown that ipsilesional volume loss in the basal ganglia due to perinatal stroke was associated with poor hand function, however this relationship was highly dependent on the type of stroke (Ilves et al., 2022). Given the extensive functions of the basal ganglia, damage to these structures may result in complex disabilities following perinatal stroke but their role remains poorly defined.
The objective of this study was to explore developmental alterations in bilateral basal ganglia structures and possible associations with motor function in children with perinatal stroke. We hypothesized that participants with AIS would have reduced lesioned hemisphere, and increased contralesional hemisphere volumes of the caudate and putamen volumes, the degree of which would correlate with clinical motor outcome.
2. Methods
2.1. Participants
Participants with perinatal stroke were recruited through the Alberta Perinatal Stroke Project (APSP), a population-based research cohort (Cole et al., 2017). Inclusion criteria were: (1) unilateral perinatal stroke (AIS or PVI) per established criteria (Kirton et al., 2008), (2) high-resolution T1-weighted MR images taken between 6 and 19 years of age, (3) term birth (>36 weeks), (4) no additional neurological disorders, and (5) completion of motor function assessments. Proximal M1 occlusions were classified by the inclusion of the lateral lenticulostriate arteries respectively, while distal M1 occlusions occurred beyond these arteries (Kirton et al., 2008). Typically developing controls (TDC) were recruited from a healthy controls database who had the same high-resolution T1-weighted anatomical MRI images acquired between the ages of 6–19 years (https://www.hiccupkids.ca). Controls were right-handed and had no self/parent-reported neurodevelopmental disorders or MRI contraindications. Written, informed parental consent and participant assent were obtained in accordance with the University of Calgary Research Ethics Board that approved this study.
2.2. Imaging
Magnetic resonance imaging was performed at the Alberta Children’s Hospital Diagnostic Imaging Suite using a 3.0 Tesla General Electric MR750w MRI scanner (GE Healthcare, Waukesha, WI) with an MR Instruments (Minnetonka, MN) 32-channel head coil. High-resolution anatomical T1-weighted fast spoiled gradient echo (FSPGR) images were acquired in the axial plane [166 slices, no skip; voxel size = 1.0 mm isotropic; repetition time = 8.5 ms; echo time = 3.2 ms; flip angle = 11°].
2.3. Putamen and caudate segmentation
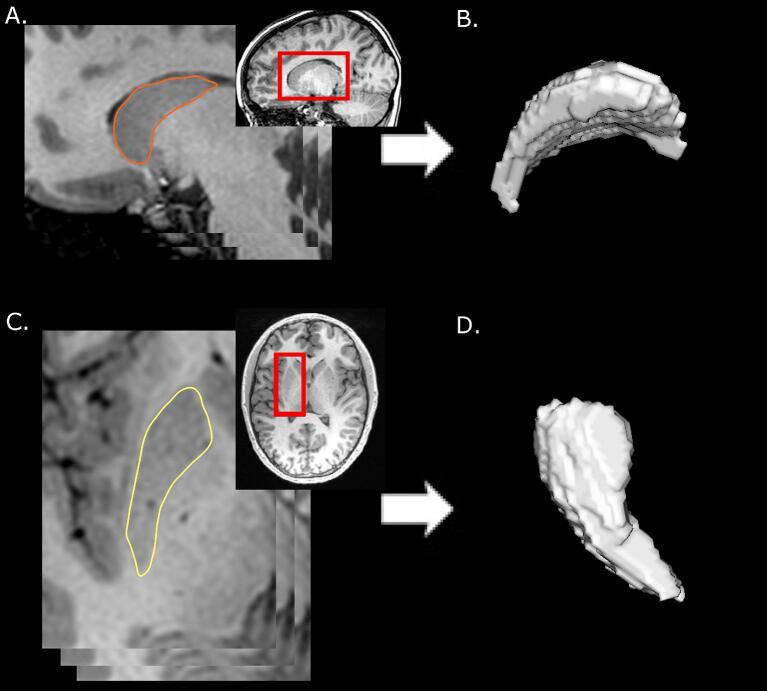
Bilateral putamen and caudate segmentations for each participant were performed manually using the Horos DICOM medical image viewer (Horosproject.org, Annapolis, MD USA). This software enables contrast adjustment to best visualize the structures of interest, and a precise region of interest selection function well-suited for anatomical segmentations. Segmentation involved carefully tracing the boundary of each structure based on image intensity, slice-by-slice, creating a 3D volume from the series, and extracting a volume measurement (in cm3) (Fig. 1). These were then used to calculate relative structure volumes as a percentage of total intracranial volume (TIV) using the equation below, to account for individual differences.
Fig. 1.
3D volume generation from ROI series. A) The caudate was traced slice by slice on the sagittal view, creating a series of ROI slices. B) This series was then used by Horos to compute a 3D volume. C & D) The putamen was segmented similarly but used axial slices for maximal visibility. Structures in each hemisphere were segmented and volumes recorded separately.
To maximize structure visibility, the putamen was traced in the axial view, superior-to-inferior, while the caudate was segmented using a sagittal view, medial-to-lateral. Image contrast was adjusted for each participant until boundaries were clearly visible.
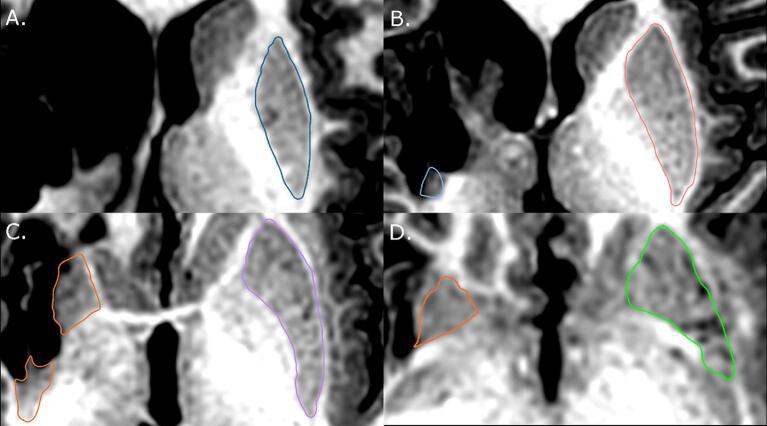
During segmentation, a number of difficult areas arose, especially so in severely damaged structures. To deal with this, a number of protocols were set in place. First, image contrast was adjusted a number of times for each slice when necessary, which clarified what was part of the structure as opposed to surrounding areas that appeared so due to similar contrast. Second, adjacent slices were a crucial guide to segmenting difficult areas. Slice-by-slice segmentation ensured that there were few drastic changes in the shape or location of a structure. Surrounding slices provided a template of how the segmentation of the difficult slice should appear. Third, white matter tracts bisecting the structure were extended through, when surrounding slices reflected similar patterns. This helped to keep the caudate and putamen separate from each other, and from other subcortical structures when boundaries were blurry. Last, for severely damaged structures, in the approximate region where the structure should be found, the areas surrounding the lesion were carefully examined for grey matter exhibiting clear demarcation from surrounding white matter, cerebral spinal fluid and other grey matter structures. Contrast was adjusted and surrounding slices were checked in order to decide whether this grey matter was part of the structure of interest. If not, the next slice was examined in a similar fashion. In many cases, no, or only a few slices were able to be segmented. See Fig. 2 for an example of these protocols in use.
Fig. 2.
Example of a severely damaged putamen segmentation (left side of the image). A) No apparent evidence of putamen tissue. B) Putamen tissue is successfully segmented. While not evident, navigating through surrounding slices reinforce the selection of this tissue as part of the putamen. C) Putamen tissue and shape clearly visible, although not intact. Dominant hemisphere structure is carefully kept separate from globus pallidus. D) White matter tracts inform the segmentation of both structures in this slice, and all subsequent slices.
All controls were right-handed, therefore the dominant hemisphere refers to the left hemisphere, and non-dominant the right. In stroke participants, the lesioned hemisphere is referred to as the non-dominant hemisphere, and the non-lesioned hemisphere was considered dominant. For simplicity, only dominant/non-dominant terminology will be used henceforth.
2.4. Reliability
To assess intra-rater reliability of manual segmentation volume measurements, the same rater repeated bilateral volume measurements for the putamen 8 weeks after the initial rating session on a subset of six randomly selected subjects (∼10 % of final sample). In addition, to assess inter-rater agreement, a second blinded researcher performed putamen and caudate volumetric measurements on a subset of nine randomly chosen participants (∼14 % of final sample).
2.5. Structure volume ratio
To quantify the relative degree of basal ganglia damage between the hemispheres, a structure volume ratio (similar to the commonly used laterality index) was calculated. Taken as the volume of the non-dominant hemisphere structure divided by its respective dominant hemisphere structure volume (non-dominant structure volume/dominant structure volume), this ratio uses each participant as their own reference to calculate a metric describing relative basal ganglia damage that is comparable across participants and has previously been validated in a large study of basal ganglia volumetrics (Wyciszkiewicz and Pawlak, 2014), as well as for other structures in children with perinatal stroke (Craig et al., 2019a, Craig et al., 2019b). A ratio close to 1.0 reflects similar volumes in each hemisphere. A higher structure volume ratio (approaching 1.0) reflects that basal ganglia volumes in the non-dominant hemisphere are relatively similar to the other hemisphere, while a lower value indicates that the non-dominant basal ganglia have relatively lower volumes.
2.6. Relative white matter volume
Relative white matter volume (RWM) was calculated for each participant, serving as a comparable metric for degree of stroke damage across stroke types. Measuring lesion size in a comparable way across stroke etiologies is challenging given that underlying injury mechanism and resulting anatomical changes vary. For PVI, dilatation of ventricles typically occurs with little damage to cortical grey matter (GM), whereas in AIS, lesions typically affect both GM and white matter (WM). We therefore used RWM volumes (corrected for TIV) to capture both injury patterns in a quantifiable way. First, the unified segmentation tool in SPM12 [Statistical Parametric Mapping (SPM12), Wellcome Centre for Human Neuroimaging, UCL, UK] running through Matlab (Mac i64 version R2020a, Mathworks, Natick, MA), was used to generate tissue probability maps for WM, GM, and cerebrospinal fluid (CSF). Total intracranial volume (TIV) was calculated as the sum of GM, WM and CSF volumes. RWM represents the proportion of WM in the brain in relation to TIV where lower numbers reflect less WM relative to brain volume. RWM was used as a covariate in subsequent statistical models.
2.7. Motor function
Motor function was evaluated using two validated assessments. The Assisting Hand Assessment (AHA) (Krumlinde-sundholm and Eliasson, 2003) measures the use of the affected hand in children with hemiparetic cerebral palsy using play-based bimanual activity with logit scores expressed from 0 to 100 scored by a qualified occupational therapist. The Box and Blocks Test (Mathiowetz et al., 1985) (BBT) measures unilateral hand function via a block moving task where participants move blocks from one box to another over a barrier as quickly as possible. BBT is performed for both the affected (BBTA) and unaffected (BBTU) hands. Scores reflect the number of blocks successfully moved in 60 s. Higher scores on both tasks indicate better motor function. These tests are explained in greater detail elsewhere (Wagner and Davids, 2012).
2.8. Statistical analyses
Distribution normality was assessed using Shapiro-Wilk revealing that putamen and caudate volumes in the non-dominant hemisphere, as well as age, deviated significantly from normality in stroke groups, while values in the TDC group were normally distributed. Group comparisons of demographic variables used Kruskal-Wallis (age), and chi-square (sex, side of stroke). Inter- and intra-rater reliability were assessed using an intraclass correlation coefficient (ICC). Group comparisons of relative structure volumes between the three participant groups (AIS, PVI, TDC) were conducted using a Kruskal-Wallis test followed by a Dunn’s post hoc test for pairwise comparisons, and a Bonferroni adjustment for multiple comparisons. Differences in relative volumes between hemispheres (dominant versus non-dominant) were explored using a Wilcoxon signed-rank test for stroke groups, and a paired-samples t-test for the TDC group. Correlations investigating associations between relative structure volume and motor function, as well as structure volume ratio and motor function, utilized partial Spearman’s tests, correcting for age, and RWM. To additionally explore how basal ganglia volumes were associated with motor function development over age, the population was separated by structure volume ratio as to whether the ratio was above the group mean or below. This created two subgroups based on the relative degree of basal ganglia damage (volume ratio < mean and volume ratio > mean). Correlations between age and motor function were performed for each group (relatively less or more damage), as well as for the entire sample. For correlations, a false discovery rate (FDR) correction was performed to correct for multiple comparisons (Benjamini and Hochberg, 1995). Uncorrected p-values were reported, with a denotation where FDR corrections affected statistical significance. All statistics were performed using SPSS (IBM SPSS statistics, version 26, USA).
3. Results
3.1. Population
The final population consisted of 64 participants (mean age (SD) = 12.24 (3.5) years, range 6.5–19.7 years), including 20 AIS, 24 PVI and 20 TDC participants. See Table 1 for complete demographics. Of the AIS participants, 12 had distal and 8 had proximal M1 occlusions (Table 2 & Fig. 3). One of the stroke participants was missing an AHA motor function assessment score and another was missing the BBTA score. There were no significant group differences for age (H = 3.10, p = 0.21), sex (χ2 = 0.54, p = 0.77), or side of stroke (χ2 = 0.01, p = 0.91). There were no sex differences in relative structure volumes or motor scores in the combined stroke group or TDC, however, relative dominant hemisphere caudate volumes were significantly larger in females for PVI (t = −2.415, p = 0.024), while relative dominant hemisphere putamen volumes were larger in males for AIS, nearing significance (t = 2.097, p = 0.05).
Table 1.
Patient demographics.
| Category | PVI | AIS | All stroke | TDC |
|---|---|---|---|---|
| Mean age (SD) [range] years | 11.50 (3.5) [6.7–19.7] | 13.11 (3.5) [8.2–19.0] | 12.24 (3.6) [6.7–19.7] | 12.46 (2.9) [6.5–17.3] |
| Sex (N) [%] | ||||
| Male | N = 13 [54 %] | N = 13 [65 %] | N = 26 [59 %] | N = 12 [60 %] |
| Female | N = 11 [46 %] | N = 7 [35 %] | N = 18 [41 %] | N = 8 [40 %] |
| Total | N = 24 | N = 20 | N = 44 | N = 20 |
| Side of stroke (N) [%] | ||||
| Right | N = 10 [32 %] | N = 7 [35 %] | N = 15 [34 %] | |
| Left | N = 21 [68 %] | N = 13 [65 %] | N = 29 [66 %] | |
| Vascular territory (N) [%] | ||||
| DM1 | – | N = 12 [60 %] | – | – |
| PM1 | – | N = 8 [40 %] | – | – |
| Mean motor score (SD) | ||||
| AHA (N = 43) | 66.04 (13.5) | 56.85 (19.4) | 61.77 (17.0) | – |
| BBTA (N = 42) | 30.26 (10.3) | 23.84 (15.4) | 27.36 (13.1) | – |
| BBTU (N = 43) | 52.92 (14.1) | 51.68 (9.7) | 52.37 (12.3) | – |
Table note: PVI - periventricular venous infarction, AIS - arterial ischemic stroke, TDC - typically developing control, DM1 - distal M1 occlusion, PM1 - proximal M1 occlusion, AHA - assisting hand assessment, BBTA - box-and-blocks test of the affected hand, BBTU - box-and-blocks test of the unaffected hand. Vascular territory for the AIS group was categorized using T1-weighed MRI scans.
Table 2.
Structure volumes (cm3) in proximal and distal M1 occlusions.
| Structure | Proximal | Distal |
|---|---|---|
| Dominant hemisphere caudate (Mean (SD) [Range]) | 4.49 (0.55) [3.95–5.53] | 4.24 (0.75) [2.75–5.34] |
| Dominant hemisphere putamen (Mean (SD) [Range]) | 4.68 (0.47) [4.10–5.28] | 4.32 (0.78) [3.03–5.50] |
| Non-dominant hemisphere caudate (Mean (SD) [Range]) | 2.87 (1.47) [0.85–4.20] | 3.73 (0.95) [2.03–5.03] |
| Non-dominant hemisphere putamen (Mean (SD) [Range]) | 1.83 (1.50) [0–3.48] | 3.62 (1.09) [1.71–5.22] |
| Caudate Ratio (Mean (SD) [Range]) | 0.65 (0.34) [0.15–0.97] | 0.88 (0.16) [0.54–1.04] |
| Putamen ratio (Mean (SD) [Range]) | 0.39 (0.33) [0–0.76] | 0.82 (0.15) [0.56–1.04] |
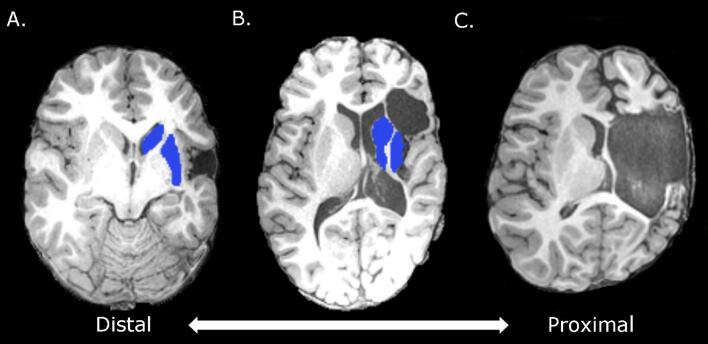
Fig. 3.
A) Distal middle cerebral artery occlusion: possible damage to primary motor cortex, but basal ganglia structures are spared. B) Occlusion closer to middle cerebral artery: basal ganglia are partially damaged. C) Proximal middle cerebral artery occlusion: M1 and basal ganglia are severely damaged.
3.2. Volume measurement reliability
Basal ganglia volume measurements had very high intra-rater reliability (dominant putamen: ICC = 0.99, p < 0.001; dominant caudate: ICC = 0.85, p < 0.05; non-dominant putamen: ICC = 1.0, p < 0.001; non-dominant caudate: ICC = 1.0, p < 0.001). There was also high reliability between raters for all structures (dominant putamen: ICC = 0.77, p < 0.01; dominant caudate: ICC = 0.72, p < 0.05; non-dominant putamen: ICC = 0.97, p < 0.001; non-dominant caudate: ICC = 0.97, p < 0.001).
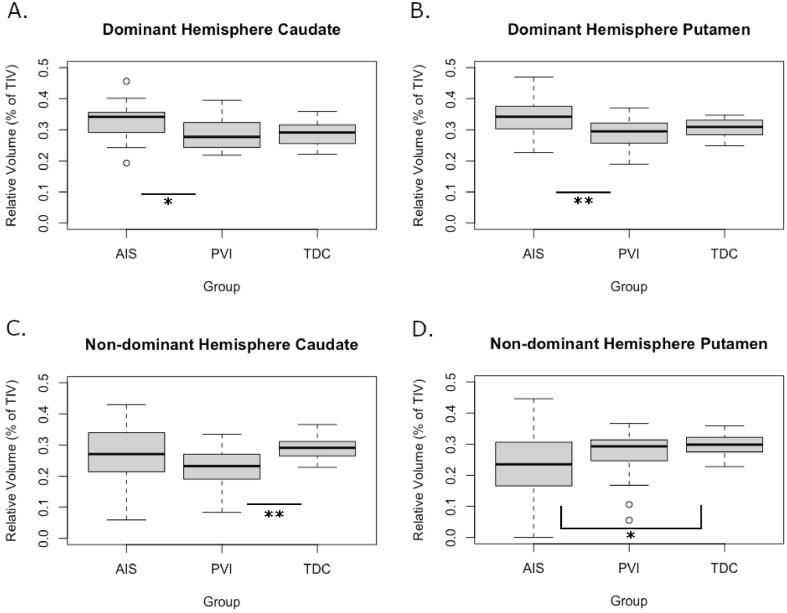
3.3. Group differences
Group comparisons were completed using relative volumes (structure volume/TIV) (Table 3 & Fig. 4). In the dominant hemisphere, relative volumes were higher in AIS than PVI for both the caudate and putamen (p = 0.024, and p = 0.008 respectively). Mean volumes did not differ between AIS and controls in the dominant hemisphere (caudate: AIS = 0.33, TDC = 0.29, p = 0.055; putamen: AIS = 0.34, TDC = 0.31, p = 0.14). In the non-dominant hemisphere, relative volumes compared to TDC were lower in the putamen for AIS (p = 0.039) and lower in caudate for PVI (p = 0.001).
Table 3.
Group differences in relative structure volumes.
| Structure | AIS and PVI | AIS and TDC | PVI and TDC |
|---|---|---|---|
| Dominant hemisphere caudate (Mean (SD)) | AIS: 0.33 (0.06) | AIS: 0.33 (0.06) | PVI: 0.29 (0.05) |
| PVI: 0.29 (0.05) | TDC: 0.29 (0.04) | TDC: 0.29 (0.04) | |
| H = 15.0, p = 0.024* | H = 13.9, p = 0.055 | H = 1.1, p = 1.00 | |
| Dominant hemisphere putamen (Mean (SD)) | AIS: 0.34 (0.06) | AIS: 0.34 (0.06) | PVI: 0.29 (0.04) |
| PVI: 0.29 (0.04) | TDC: 0.31 (0.03) | TDC: 0.31 (0.03) | |
| H = 16.9, p = 0.008** | H = 11.7, p = 0.141 | H = 5.2, p = 1.00 | |
| Non-dominant hemisphere caudate (Mean (SD)) | AIS: 0.26 (0.1) | AIS: 0.26 (0.1) | PVI: 0.23 (0.06) |
| PVI: 0.23 (0.06) | TDC: 0.29 (0.03) | TDC: 0.29 (0.03) | |
| H = 12.4, p = 0.082 | H = -7.4, p = 0.63 | H = 19.8, p = 0.001** | |
| Non-dominant hemisphere putamen (Mean (SD)) | AIS: 0.22 (0.1) | AIS: 0.22 (0.1) | PVI: 0.27 (0.08) |
| PVI: 0.27 (0.08) | TDC: 0.30 (0.03) | TDC: 0.30 (0.03) | |
| H = -8.2, p = 0.43 | H = -14.6, p = 0.039* | H = 6.4, p = 0.78 |
Table note: Relative structure volumes are reported as a percentage of TIV. PVI - periventricular venous infarction, AIS - arterial ischemic stroke, TDC - typically developing control, *p < 0.05, **p < 0.01.
Fig. 4.
Group differences in relative structure volume for the dominant hemisphere caudate (A) and putamen (B), as well as the non-dominant hemisphere caudate (C) and putamen (D). PVI - periventricular venous infarction, AIS - arterial ischemic stroke, TDC - typically developing control, *p < 0.05, **p < 0.01.
3.4. Hemispheric differences
For TDC, the volume of the putamen in the dominant hemisphere was greater than the non-dominant hemisphere (p = 0.023). Caudate volumes between hemispheres were comparable in TDC (p = 0.23). In AIS, the dominant hemisphere had larger volumes for both the caudate (p = 0.020) and the putamen (p < 0.001). The same relationship was only observed for the caudate in PVI (p = 0.010). Additional details are in Table 4.
Table 4.
Hemispheric differences in relative structure volumes by group.
| Group |
|||
|---|---|---|---|
| Structure | AIS | PVI | TDC |
| Caudate (Mean (SD)) | Dominant: 0.33 (0.06) | Dominant: 0.29 (0.05) | Dominant: 0.29 (0.04) |
| Non-dominant: 0.26 (0.1) | Non-dominant: 0.23 (0.06) | Non-dominant: 0.29 (0.03) | |
| Z = 1.2, p = 0.020* | Z = 1.17, p = 0.010* | t = -1.26, p = 0.23 | |
| Putamen (Mean (SD)) | Dominant: 0.34 (0.06) | Dominant: 0.29 (0.04) | Dominant: 0.31 (0.03) |
| Non-dominant: 0.22 (0.1) | Non-dominant: 0.27 (0.08) | Non-dominant: 0.30 (0.03) | |
| Z = 2.00, p < 0.001*** | Z = 0.42, p = 1.00 | t = 2.48, p = 0.023* | |
Table note: Relative structure volumes are reported as a percentage of TIV. PVI - periventricular venous infarction, AIS - arterial ischemic stroke, TDC - typically developing control, *p < 0.05, **p < 0.01, ***p < 0.001.
Interhemispheric volume ratios varied between TDC and combined stroke group participants (caudate: U = 770, p < 0.001, putamen: U = 587, p = 0.033). Mean ratios were significantly lower than 1.0 in the combined stroke group (caudate mean(SD) = 0.8(0.23), t = −5.98, p < 0.001; putamen mean(SD) = 0.79(0.29), t = −4.69, p < 0.001), reflecting hemispheric differences. In TDC, only the mean ratio for the putamen differed from 1.0 (caudate mean(SD) = 1.02(0.06), t = 1.35, p = 0.19; putamen mean(SD) = 0.97(0.05), t = −2.44, p < 0.05) consistent with volume differences seen above. However, ratios for both structures were significantly larger in TDC, suggesting a greater degree of hemispheric symmetry relative to stroke. Specifically, ratios were higher in TDC than AIS for the caudate (U = 354, p < 0.001) and putamen (U = 351, p < 0.001), as well as the caudate for PVI (U = 416, p < 0.001) but not the putamen. Additionally, PVI had a higher ratio for the putamen than AIS (U = 386, p < 0.001).
3.5. Motor function correlations
AHA, BBTA and BBTU scores did not differ significantly between AIS and PVI (AHA: t = −1.82, p = 0.076, BBTA: t = −1.61, p = 0.115, BBTU: t = −0.32, p = 0.748). BBTU scores were higher than BBTA scores as expected (p < 0.001).
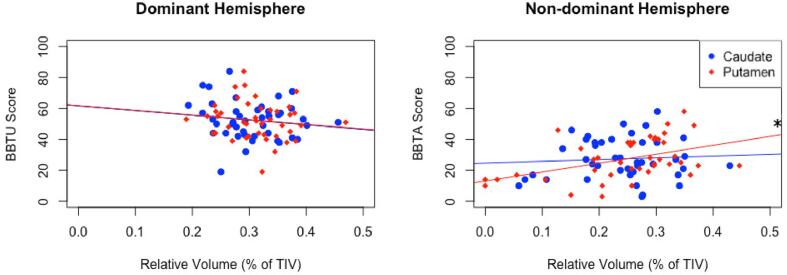
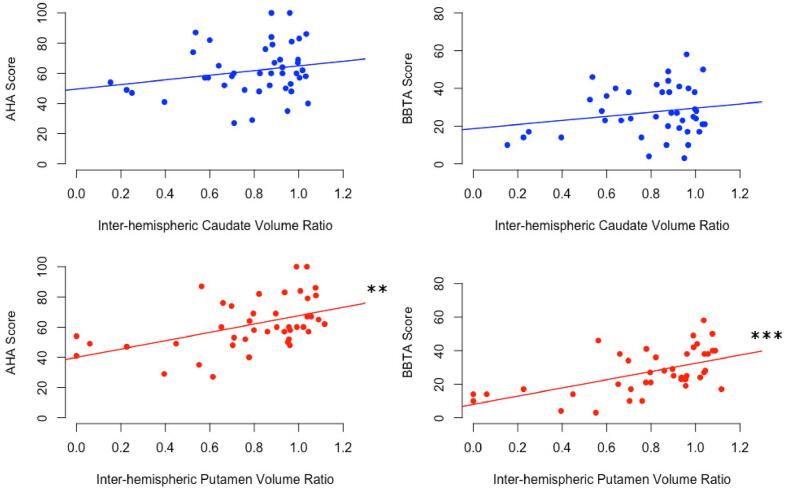
Putamen volumes in the non-dominant hemisphere demonstrated a positive correlation with BBTA values (rs = 0.396, p < 0.05) (Fig. 5, Table 5). Caudate volumes in the non-dominant hemisphere were negatively correlated with BBTU scores in PVI only (rs = −0.481, p < 0.05, not significant following FDR correction). Dominant hemisphere basal ganglia volumes were not correlated with motor function in stroke participants. However, putamen volume ratios were positively correlated with both AHA (rs = 0.428, p < 0.01) and BBTA (rs = 0.518, p < 0.001) for all stroke (Fig. 6). An association was also observed between BBTA and AIS specifically (r = 0.556, p < 0.05, not significant following FDR correction). Volume ratios for the caudate were not associated with motor function outcomes.
Fig. 5.
Box-and-blocks test scores with corresponding relative structure volume corrected for RWM and Age. BBTU scores are plotted against relative caudate and putamen volumes in the dominant hemisphere. BBTA scores are plotted against relative caudate and putamen volumes in the non-dominant hemisphere. RWM - Relative White Matter, BBTA - box-and-blocks test of the affected hand, BBTU - box-and-blocks test of the unaffected hand, *p < 0.05.
Table 5.
Correlations between relative basal ganglia volumes and motor function, correcting for age and RWM.
| Structure | AHA | BBTA | BBTU |
|---|---|---|---|
| Dominant hemisphere caudate | All-stroke: rs = -0.224, p = 0.17 | All-stroke: rs = -0.105, p = 0.52 | All-stroke: rs = -0.110, p = 0.50 |
| AIS: rs = 0.008, p = 0.98 | AIS: rs = 0.044, p = 0.87 | AIS: rs = 0.064, p = 0.81 | |
| PVI: rs = -0.165, p = 0.47 | PVI: rs = -0.117, p = 0.62 | PVI: rs = -0.171, p = 0.46 | |
| Non-dominant hemisphere caudate | All-stroke: rs = -0.020p = 0.90 | All-stroke: rs = -0.021, p = 0.90 | All-stroke: rs = -0.129, p = 0.43 |
| AIS: rs = -0.198, p = 0.45 | AIS: rs = 0.019, p = 0.94 | AIS: rs = 0.235, p = 0.36 | |
| PVI: rs = 0.323, p = 0.15 | PVI: rs = 0.028, p = 0.90 | PVI: rs = -0.481, p = 0.027*FD | |
| Dominant hemisphere putamen | All-stroke: rs = -0.212, p = 0.19 | All-stroke: rs = -0.137, p = 0.40 | All-stroke: rs = -0.225, p = 0.16 |
| AIS: rs = -0.389, p = 0.12 | AIS: rs = -0.207, p = 0.43 | AIS: rs = -0.172, p = 0.51 | |
| PVI: rs = 0.184, p = 0.43 | PVI: rs = 0.120, p = 0.60 | PVI: rs = -0.244, p = 0.29 | |
| Non-dominant hemisphere putamen | All-stroke: rs = 0.293, p = 0.066 | All-stroke: rs = 0.396, p = 0.011* | All-stroke: rs = 0.052, p = 0.75 |
| AIS: rs = 0.167, p = 0.52 | AIS: rs = 0.410, p = 0.10 | AIS: rs = 0.186, p = 0.47 | |
| PVI: rs = 0.344, p = 0.13 | PVI: rs = 0.271, p = 0.24 | PVI: rs = -0.263, p = 0.25 | |
| Caudate volume ratio | All-stroke: rs = 0.176, p = 0.28 | All-stroke: rs = 0.059, p = 0.72 | All-stroke: rs = -0.012, p = 0.94 |
| AIS: rs = -0.122, p = 0.64 | AIS: rs = 0.041, p = 0.88 | AIS: rs = 0.266, p = 0.30 | |
| PVI: rs = 0.383, p = 0.086 | PVI: rs = 0.050, p = 0.83 | PVI: rs = -0.310, p = 0.17 | |
| Putamen volume ratio | All-stroke: rs = 0.428, p = 0.006** | All-stroke: rs = 0.518, p < 0.001*** | All-stroke: rs = 0.211, p = 0.19 |
| AIS: rs = 0.382, p = 0.13 | AIS: rs = 0.556, p = 0.021*FD | AIS: rs = 0.247, p = 0.34 | |
| PVI: rs = 0.344, p = 0.13 | PVI: rs = 0.394, p = 0.077 | PVI: rs = 0.008, p = 0.97 |
Table note: AHA - Assisting Hand Assessment, BBTA - box-and-blocks test of the affected hand, BBTU - box-and-blocks test of the unaffected hand, rs - Spearman’s rho, *p < 0.05, **p < 0.01, ***p < 0.001, *FD - Not significant following FDR correction.
Fig. 6.
Motor assessments and inter-hemispheric structure volume ratio (non-dominant structure/ dominant structure) corrected for RWM and age. RWM - Relative White Matter, AHA - Assisting Hand Assessment, BBTA - box-and-blocks test of the affected hand, **p < 0.01, ***p < 0.001.
3.6. Developmental trends
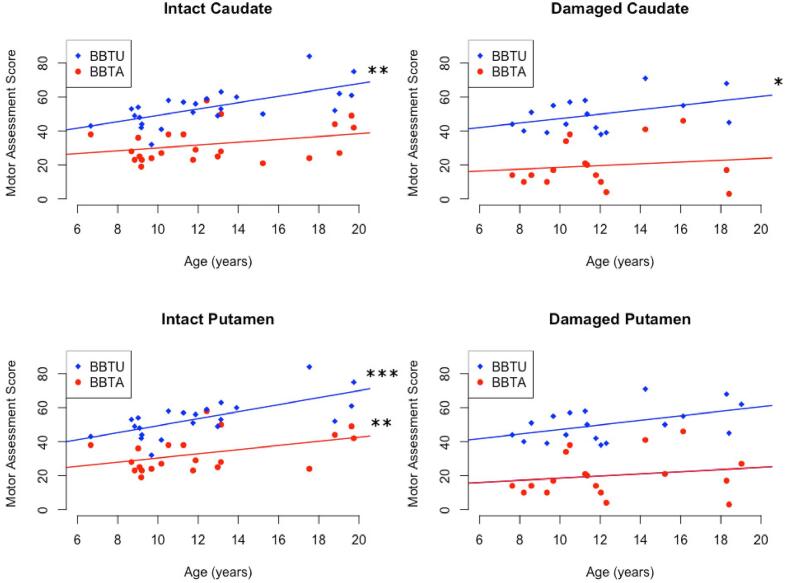
Age was associated with higher scores for both BBTU (r = 0.568, p < 0.001) and BBTA (r = 0.333, p = 0.031) across all stroke participants. When participant groups were dichotomized by structure volume ratios, differences in correlations between age and motor function were seen. For participants with caudate volume ratios greater than the group mean of 0.8 (relatively less damage to the caudate), there was a positive correlation between age and BBTU scores (r = 0.552, p < 0.01) but not for BBTA scores (r = 0.325, p = 0.098). For the group with caudate ratio values<0.8 (relatively more damage to the caudate), age was positively associated with BBTU scores (r = 0.525, p < 0.05), but not BBTA scores (r = 0.248, p = 0.37).
Participants with a putamen volume ratio greater than the group mean of 0.8 (relatively less damage to the putamen) exhibited positive correlations between age and both BBTA (r = 0.542, p < 0.01) and BBTU values (r = 0.694, p < 0.001). However, no associations were seen with age for participants with a putamen volume ratio<0.8 (BBTA: r = 0.210, p = 0.40, BBTU: r = 0.371, p = 0.13). Age did not correlate with AHA scores for any of the four individual groups. See Table 6 & Fig. 7.
Table 6.
Correlations between age and motor scores, by relative level of damage to basal ganglia.
| Correlation | Intact caudate | Damaged caudate | Intact putamen | Damaged putamen |
|---|---|---|---|---|
| Age & AHA scores | rs = 0.026, p = 0.90 | rs = 0.177, p = 0.51 | rs = 0.297, p = 0.16 | rs = 0.099, p = 0.69 |
| Age & BBTA scores | rs = 0.325, p = 0.098 | rs = 0.248p = 0.37 | rs = 0.542, p = 0.006** | rs = -0.210, p = 0.40 |
| Age & BBTU scores | rs = 0.552, p = 0.003** | rs = 0.525, p = 0.037* | rs = 0.694, p = 0.000*** | rs = 0.371, p = 0.13 |
Table note: Intact - participants with a structure volume ratio (non-dominant/dominant) greater than 0.8, Damaged - participants with a structure volume ratio<0.8, AHA - Assisting Hand Assessment, BBTA - box-and-blocks test of the affected hand, BBTU - box-and-blocks test of the unaffected hand, rs - Spearman’s rho, *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 7.
Motor assessment score plotted against age in subgroups with relatively intact caudate, damaged caudate, intact putamen, or damaged putamen. Groups were defined by structure volume ratio (non-dominant hemisphere structure/dominant hemisphere structure) such that a ratio greater than the mean (0.8) reflected a relatively intact structure, while a ratio less than the mean indicated damage to the structure in the non-dominant hemisphere. BBTA - box-and-blocks test of the affected hand, BBTU - box-and-blocks test of the unaffected hand, *p < 0.05, **p < 0.01, ***p < 0.001.
While development trajectories were originally tested with groups stratified by mean ratio (0.8), we additionally tested stratification points ranging from 0.75 to 0.85, which yielded the same pattern of results for all four groups.
4. Discussion
We have demonstrated both between-hemisphere and between-group differences in relative volumetric measurements of basal ganglia structures (caudate and putamen) in children with perinatal stroke. Specifically, compared to controls, non-dominant hemisphere caudate volumes were smaller for PVI while putamen volumes were smaller for AIS. In the dominant hemisphere, relative volumes in AIS were higher than that of PVI for both structures, though were not different from controls. We also found that relative putamen volumes in the non-dominant hemisphere (and inter-hemispheric ratios) were positively associated with functional motor outcomes. We conclude that basal ganglia structural development is altered after perinatal stroke, possibly in a lesion-specific manner, and correlations to functional outcome suggest clinical relevance.
Stroke-specific group differences in relative basal ganglia structure volumes were seen in the non-dominant, lesioned hemisphere. Specifically, relative caudate volumes were smaller in PVI while putaminal volumes were smaller in AIS. This finding is consistent with differing mechanisms of injury between the two stroke groups. Due to the position of the germinal matrix and subsequent infarction, PVI primarily affects periventricular tissue (i.e., the caudate). In contrast, PVI is unlikely to damage the more caudal putamen which is not typically drained by the medullary veins (Raets et al., 2015). In fact, the original imaging criteria for diagnosing PVI specifically include sparing of the lentiform nuclei (Kirton et al., 2008). By contrast, the AIS group showed smaller putamen volumes in the lesioned hemisphere. This may relate to the common involvement of the lateral lenticulostriate arteries that occurs in proximal M1 occlusions. However, AIS lesions also include much larger cortical injuries and indirect volume effects from damage to corticostriatal pathways (or diaschisis, see below) cannot be excluded. Finally, with PVI occurring mid-gestation while AIS injuries are typically close to term, different effects of injury timing on basal ganglia development cannot be excluded. More advanced imaging methods including structural and functional connectivity are feasible in the perinatal stroke population (Carlson et al., 2020, Craig et al., 2021) and could shed light on these different possibilities for the alterations we have observed in basal ganglia structure.
Inter-hemispheric differences in relative putamen volume within all groups favoured the dominant hemisphere, a result that was expected and also echoed in the volume ratios. It was interesting to find that the control group also showed interhemispheric asymmetry for putamen volumes in the absence of lesion-related damage. This could suggest some level of activity dependency whereby the dominant hemisphere, controlling the dominant hand, shows larger volumes perhaps underlying more extensive use and better dexterity of the dominant hand, though this was not directly measured. The same was true for both of the stroke groups; basal ganglia structures involved in the function of the less-affected limb were larger than that of the affected limb, likely due to a combination of direct lesion damage, subsequent degeneration of surrounding tissues, less use of the paretic limb, and possibly more extensive use of the non-paretic limb. Our results highlight both the importance and potential limitations of performing within-subject comparisons between hemispheres and we suggest both approaches should be considered.
Larger volumes of both caudate and putamen were observed in the dominant hemisphere of AIS participants compared to PVI. Alterations in the development of the non-lesioned hemisphere are well appreciated after perinatal stroke. Both animal and human studies have established the common, abnormal perseverance of fetal ipsilateral corticospinal projections from the non-lesioned hemisphere to the affected limbs (Martin et al., 2007, Staudt, 2007, Eyre, 2007, Zewdie and Kirton, 2016). Alterations in additional components of the sensorimotor system have been shown including increased volumes of key components of the motor system such as the thalamus (Craig et al., 2019a) and cerebellum (Craig et al., 2019b). Broader influence of early unilateral injury on the entire structure and connectivity of the non-lesioned hemisphere has been observed, including the white matter connectome (Craig et al., 2020), myelination (Yu et al., 2018) and cortical morphometry (Al Harrach et al., 2019). Considering the above, it may not be surprising that structural changes including increased volumes of the putamen and caudate may occur though, like the other components mentioned, parcellating these structures to understand the why and how of these changes requires further study.
Our findings of positive correlations between relative putamen volumes and motor function suggest possible clinical significance. Putamen volumes were positively correlated with BBTA scores, such that motor function of the paretic limb was associated with higher non-dominant putamen volumes, consistent with our initial hypothesis. Also, the putamen volume ratio, favouring the dominant hemisphere, was positively correlated with both AHA and BBTA scores. By contrast, neither relative caudate volumes, nor volume ratios, were significantly positively correlated with any of the motor tests. These findings taken together support the observation that motor control is more closely associated with the putamen compared to the caudate (Purves et al., 2001, Leisman et al., 2014).
Interestingly, there was a negative correlation between relative non-dominant caudate volume and BBTU scores in the PVI group that may suggest effects of developmental neuroplasticity in the basal ganglia following perinatal stroke. Though not statistically significant following an FDR correction, it is possible that a greater sample size would have allowed this correlation to hold. As such, we believe it is still worth consideration, also given that the correlation coefficient as a reflection of effect size is in the moderate range (rs = −0.48). That a relative non-dominant hemisphere volume was negatively linked to a dominant hand motor test, may suggest that broader, bihemispheric plasticity is taking place. For example, as noted above, it is possible that impaired motor function associated with the non-dominant hemisphere could result in larger structure volumes in the dominant hemisphere. How this affects functions normally assigned to the opposite hemisphere is not well understood though it is well established that the function of the so-called “unaffected” hand is often abnormal in children with perinatal stroke (Kuczynski et al., 2018). However, the lack of definitive group differences in the dominant hemisphere between the stroke groups and TDC, suggest that this relationship is more complicated (though we may have been underpowered to detect such subtle differences). Direct analogies exist for the effects of perinatal stroke-induced volumetric changes in “contralesional” structures including the thalamus (Craig et al., 2019a) and cerebellum (Craig et al., 2019b) where volumes were not only larger but the degree of which correlated with poorer motor outcome. It must be considered that these and the current study are only volumetric in nature and of limited spatial and functional resolution, suggesting that differences exist but with limited ability to understanding underlying mechanisms.
Our results also suggest that alterations to the basal ganglia after perinatal stroke may be associated with disruptions in developmental trajectories of motor function. After dichotomizing our population by their inter-hemispheric volume ratios, we observed that positive correlations between age and bilateral motor function scores for BBTA and BBTU, were only observed for those with less putaminal damage, while for those with relatively more damage, these associations with age were lost. How the developmental trajectory was affected cannot be determined using a cross-sectional study such as this one, however, a longitudinal study of motor development over time could shed additional light. While not definitive, these suggestions of possible differences in developmental trajectories are of clinical importance and relevant to the pursuit of personalized rehabilitation strategies. Our findings add to increasingly complex models of motor system development following perinatal stroke where complex, bihemispheric differences are increasingly understood (Kirton et al., 2021). Taken together, the current results suggest that the relationship between basal ganglia volumes and motor function is relatively complex. It’s possible that stroke-induced damage to the basal ganglia disrupts the mechanism responsible for building and maintaining the neural architecture (cortico-striatal loops) that enables the learning and execution of complex motor sequences. For example, in the dominant hemisphere, an undamaged putamen could have an intact, possibly adaptable, motor program more easily fine-tuned (Doyon et al., 2009), resulting in limited correlations between relative volume and motor function. In the non-dominant hemisphere, a damaged putamen may be unable to mediate the construction and execution of the motor networks required to perform well on the motor tests, but may also limit improvements that should occur with aging and/or motor learning interventions. This is consistent with the widely variable motor function seen in the perinatal stroke population, the associations between relative basal ganglia volume and motor function, and the lack of association we observed between age and motor function in participants with more extensive putamen damage. Given the central role of the basal ganglia in cortico-striatal loops (Haber, 2016, Alexander et al., 1986, Frank et al., 2001) and the degree to which basal ganglia damage results from perinatal stroke, these structures could be considered in future models to better inform understanding and neurorehabilitation targets.
This study has limitations that should be considered. First, the sample size, while large for perinatal stroke, was limited by the availability of motor assessment scores. Second, only the caudate and putamen could be segmented by our current methods. While these are primary structures associated with the motor circuit, exploring additional subcortical structures such as the globus pallidus may provide a more comprehensive picture. Detection of alterations in smaller basal ganglia structures such as the subthalamic nucleus, substantia nigra, and specific thalamic nuclei will likely require alternative methods in this population. Third, the segmentation process was manually performed slice by slice and while every effort was made to make this accurate and objective, subjectivity is inherent in such segmentations. Although automated basal ganglia segmentation programs do exist, pilot testing revealed that results were inaccurate in perinatal stroke participants, likely due to the deviation from normal architecture caused by perinatal stroke lesions, as well as most programs being designed for the adult brain. However, our interhemispheric ratios for TDC were very similar to those found in a large study that did employ an automated segmentation technique (Wyciszkiewicz and Pawlak, 2014), enhancing our confidence in the accuracy of our segmentations. Additionally, our very high inter- and intra-rater reliability values for basal ganglia volumes, combined with normalization of measurements within subjects, further mitigates concerns regarding manual segmentation accuracy. While ICC was much lower for the dominant hemisphere than the non-dominant hemisphere, this was not entirely surprising. Because dominant hemisphere structures are undamaged, we found that the variability in volume was relatively low between subjects, reflected as a smaller range. By contrast, in the non-dominant hemisphere, the volume of a severely damaged structure varied considerably and therefore had a wider range. As such, the ICCs reported for the dominant hemisphere are likely more sensitive to inter-rater variations due to the smaller range of volumes. By contrast, the higher range of volumes in the non-dominant hemisphere is likely less sensitive to small amounts of inter-rater disagreement. Lastly, RWMs were not direct measures of lesion size, but rather the extent of damage caused by the lesion. As such, lesions that don’t extensively affect WM may have been underestimated.
5. Conclusion
Specific perinatal stroke lesions affect different elements of basal ganglia development. PVI injuries primarily affected the caudate, while AIS injuries affected the putamen. Alterations in basal ganglia structural development likely occur in both hemispheres despite the isolated unilateral injury of perinatal stroke. Putamen volumes in the lesioned hemisphere are associated with clinical motor function. The basal ganglia should be included in evolving models of developmental plasticity after perinatal stroke.
CRediT authorship contribution statement
Jordan Hassett: Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Visualization. Helen Carlson: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision. Ali Babwani: Validation, Investigation, Writing – review & editing. Adam Kirton: Conceptualization, Methodology, Formal analysis, Resources, Data curation, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the children and families for participating, as well as the Canadian Institute of Health Research, and the Heart and Stroke Foundation.
References
- Al Harrach M., et al. Alterations in Cortical Morphology after Neonatal Stroke: Compensation in the Contralesional Hemisphere? Dev. Neurobiol. 2019;79:303–316. doi: 10.1002/dneu.22679. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Antzoulatos E.G., Miller E.K. Differences between neural activity in prefrontal cortex and striatum during learning of novel abstract categories. Neuron. 2011;71:243–249. doi: 10.1016/j.neuron.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby F.G., Ennis J.M., Spiering B.J. A neurobiological theory of automaticity in perceptual categorization. Psychol. Rev. 2007;114:632–656. doi: 10.1037/0033-295X.114.3.632. [DOI] [PubMed] [Google Scholar]
- Aylward E.H., et al. Basal Ganglia Volume and Proximity to Onset in Presymptomatic Huntington Disease. Arch. Neurol. 1996;53:1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- Baudat C., et al. Automated MRI-based volumetry of basal ganglia and thalamus at the chronic phase of cortical stroke. Neuroradiology. 2020;62:1371–1380. doi: 10.1007/s00234-020-02477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Boardman J.P., et al. Magnetic resonance image correlates of hemiparesis after neonatal and childhood middle cerebral artery stroke. Pediatrics. 2005;115:321–326. doi: 10.1542/peds.2004-0427. [DOI] [PubMed] [Google Scholar]
- Boyd L.A., et al. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol. Learn. Mem. 2009;92:35–44. doi: 10.1016/j.nlm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Boyd L.A., Winstein C.J. Providing Explicit Information Disrupts Implicit Motor Learning After Basal Ganglia Stroke. Learn. Mem. 2004;11:388–396. doi: 10.1101/lm.80104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson H.L., et al. Structural and functional connectivity of motor circuits after perinatal stroke: A machine learning study. NeuroImage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson H.L., Sugden C., Brooks B.L., Kirton A. Functional connectivity of language networks after perinatal stroke. NeuroImage Clin. 2019;23 doi: 10.1016/j.nicl.2019.101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L., et al. Clinical characteristics, risk factors, and outcomes associated with neonatal hemorrhagic stroke: A population-based case-control study. JAMA Pediatr. 2017 doi: 10.1001/jamapediatrics.2016.4151. [DOI] [PubMed] [Google Scholar]
- Craig B.T., et al. Crossed Cerebellar Atrophy in Perinatal Stroke. Stroke. 2019;50:175–177. doi: 10.1161/STROKEAHA.118.022423. [DOI] [PubMed] [Google Scholar]
- Craig B.T., et al. Developmental neuroplasticity of the white matter connectome in children with perinatal stroke. Neurology. 2020;95:e2476–e2486. doi: 10.1212/WNL.0000000000010669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig B.T., Carlson H.L., Kirton A. Thalamic diaschisis following perinatal stroke is associated with clinical disability. NeuroImage Clin. 2019;21 doi: 10.1016/j.nicl.2019.101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig B.T., Hilderley A., Kirton A., Carlson H.L. Imaging Developmental and Interventional Plasticity Following Perinatal Stroke. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2021;48:157–171. doi: 10.1017/cjn.2020.166. [DOI] [PubMed] [Google Scholar]
- Craig B.T., Kinney-Lang E., Hilderley A.J., Carlson H.L., Kirton A. Structural connectivity of the sensorimotor network within the non-lesioned hemisphere of children with perinatal stroke. Sci. Rep. 2022;12:3866. doi: 10.1038/s41598-022-07863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J., et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Dunbar M., et al. Population based birth prevalence of disease-specific perinatal stroke. Pediatrics. 2020;146 doi: 10.1542/peds.2020-013201. [DOI] [PubMed] [Google Scholar]
- Dunbar M., Kirton A. Perinatal stroke. Semin. Pediatr. Neurol. 2019;32 doi: 10.1016/j.spen.2019.08.003. [DOI] [PubMed] [Google Scholar]
- Eng D., Zewdie E., Ciechanski P., Damji O., Kirton A. Interhemispheric motor interactions in hemiparetic children with perinatal stroke: Clinical correlates and effects of neuromodulation therapy. Clin. Neurophysiol. 2018;129:397–405. doi: 10.1016/j.clinph.2017.11.016. [DOI] [PubMed] [Google Scholar]
- Eyre J.A. Corticospinal tract development and its plasticity after perinatal injury. Neurosci. Biobehav. Rev. 2007;31:1136–1149. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Loughry B., O’Reilly R.C. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cogn. Affect. Behav. Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Gurney K., Prescott T.J., Redgrave P. A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol. Cybern. 2001;84:401–410. doi: 10.1007/PL00007984. [DOI] [PubMed] [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M.D., Stewart R.D., Gurney K.N. A Physiologically Plausible Model of Action Selection and Oscillatory Activity in the Basal Ganglia. J. Neurosci. 2006;26:12921–12942. doi: 10.1523/JNEUROSCI.3486-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilves N., et al. Ipsilesional volume loss of basal ganglia and thalamus is associated with poor hand function after ischemic perinatal stroke. BMC Neurol. 2022;22:23. doi: 10.1186/s12883-022-02550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens C.K., et al. Basal ganglia volume and clinical correlates in ‘preclinical’ Huntington’s disease. J. Neurol. 2008;255:1785–1791. doi: 10.1007/s00415-008-0050-4. [DOI] [PubMed] [Google Scholar]
- Kirton A., et al. Perinatal stroke: mapping and modulating developmental plasticity. Nat. Rev. Neurol. 2021;17:415–432. doi: 10.1038/s41582-021-00503-x. [DOI] [PubMed] [Google Scholar]
- Kirton A., deVeber G. Life after perinatal stroke. Stroke. 2013;44:3265–3271. doi: 10.1161/STROKEAHA.113.000739. [DOI] [PubMed] [Google Scholar]
- Kirton A., Deveber G., Pontigon A.-M., Macgregor D., Shroff M. Presumed perinatal ischemic stroke: vascular classification predicts outcomes. Ann. Neurol. 2008;63:436–443. doi: 10.1002/ana.21334. [DOI] [PubMed] [Google Scholar]
- Krumlinde-sundholm L., Eliasson A.-C. Development of the assisting hand assessment: A Rasch-built measure intended for children with unilateral upper limb impairments. Scand. J. Occup. Ther. 2003;10:16–26. [Google Scholar]
- Kuczynski A.M., Kirton A., Semrau J.A., Dukelow S.P. Bilateral reaching deficits after unilateral perinatal ischemic stroke: a population-based case-control study. J. Neuroeng. Rehabil. 2018;15:77. doi: 10.1186/s12984-018-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego J.L., Luquin N., Obeso J.A. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo C., et al. Prognostic Significance of Infarct Size and Location: The Case of Insular Stroke. Sci. Rep. 2018;8:9498. doi: 10.1038/s41598-018-27883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N., et al. Frontal interhemispheric structural connectivity, attention, and executive function in children with perinatal stroke. Brain Behav. 2021;e2433 doi: 10.1002/brb3.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman G., Braun-Benjamin O., Melillo R. Cognitive-motor interactions of the basal ganglia in development. Front. Syst. Neurosci. 2014;8 doi: 10.3389/fnsys.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Espejo M., Hernández-Chávez M., López-Espejo M., Hernández-Chávez M. Could infarct location predict the long-term functional outcome in childhood arterial ischemic stroke? Arq. Neuropsiquiatr. 2017;75:692–696. doi: 10.1590/0004-282X20170124. [DOI] [PubMed] [Google Scholar]
- Martin J.H., Friel K.M., Salimi I., Chakrabarty S. Activity- and use-dependent plasticity of the developing corticospinal system. Neurosci. Biobehav. Rev. 2007;31:1125–1135. doi: 10.1016/j.neubiorev.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiowetz V., Federman S., Wiemer D. Box and block test of manual dexterity norms for 6–19 year olds. Can. J. Occup. Ther. 1985;52:241–245. [Google Scholar]
- Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog. Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- Packard M.G., Knowlton B.J. Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Peterson B.S., et al. Basal Ganglia Volumes in Patients With Gilles de la Tourette Syndrome. Arch. Gen. Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Pitcher T.L., et al. Reduced striatal volumes in Parkinson’s disease: a magnetic resonance imaging study. Transl. Neurodegener. 2012;1:17. doi: 10.1186/2047-9158-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves, D., et al., 2021. Projections to the Basal Ganglia. Neurosci. 2nd Ed. (2001).
- Raets M., et al. Brain vein disorders in newborn infants. Dev. Med. Child Neurol. 2015;57:229–240. doi: 10.1111/dmcn.12579. [DOI] [PubMed] [Google Scholar]
- Rogers Derek C., Campbell Colin A., Stretton Jennifer L., Mackay Kenneth B. Correlation Between Motor Impairment and Infarct Volume After Permanent and Transient Middle Cerebral Artery Occlusion in the Rat. Stroke. 1997;28:2060–2066. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Schroll H., Hamker F.H. Computational models of basal-ganglia pathway functions: focus on functional neuroanatomy. Front. Syst. Neurosci. 2013;7 doi: 10.3389/fnsys.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll H., Vitay J., Hamker F.H. Working memory and response selection: a computational account of interactions among cortico-basalganglio-thalamic loops. Neural Netw. Off. J. Int. Neural Netw. Soc. 2012;26:59–74. doi: 10.1016/j.neunet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Staudt M. Reorganization of the developing human brain after early lesions. Dev. Med. Child Neurol. 2007;49:564. doi: 10.1111/j.1469-8749.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Wagner L.V., Davids J.R. Assessment tools and classification systems used for the upper extremity in children with cerebral palsy. Clin. Orthop. 2012;470:1257–1271. doi: 10.1007/s11999-011-2065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyciszkiewicz, A. & Pawlak, M. A., 2014. Basal Ganglia Volumes: MR-Derived Reference Ranges and Lateralization Indices for Children and Young Adults. Neuroradiol. J. 27, 595–612. [DOI] [PMC free article] [PubMed]
- Yu S., et al. Bihemispheric alterations in myelination in children following unilateral perinatal stroke. NeuroImage Clin. 2018;20:7–15. doi: 10.1016/j.nicl.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zewdie E., Kirton A. In: Pediatric Brain Stimulation: Mapping and Modulating the Developing Brain. Kirton A., Gilbert D.L., editors. Elsevier; 2016. TMS Basics: Single and Paired Pulse Neurophysiology. [Google Scholar]