Abstract
Background and Objectives
Motor speech function, including speech timing, is a key domain for diagnosing nonfluent/agrammatic variant primary progressive aphasia (nfvPPA). Yet, standard assessments use subjective, specialist-dependent evaluations, undermining reliability and scalability. Moreover, few studies have examined relevant anatomo-clinical alterations in patients with pathologically confirmed diagnoses. This study overcomes such caveats using automated speech timing analyses in a unique cohort of autopsy-proven cases.
Methods
In a cross-sectional study, we administered an overt reading task and quantified articulation rate, mean syllable and pause duration, and syllable and pause duration variability. Neuroanatomical disruptions were assessed using cortical thickness and white matter (WM) atrophy analysis.
Results
We evaluated 22 persons with nfvPPA (mean age: 67.3 years; 13 female patients) and confirmed underlying 4-repeat tauopathy, 15 persons with semantic variant primary progressive aphasia (svPPA; mean age: 66.5 years; 8 female patients), and 10 healthy controls (HCs; 70 years; 5 female patients). All 5 speech timing measures revealed alterations in persons with nfvPPA relative to both the HC and svPPA groups, controlling for dementia severity. The articulation rate robustly discriminated individuals with nfvPPA from HCs (area under the ROC curve [AUC] = 0.95), outperforming specialist-dependent perceptual measures of dysarthria and apraxia of speech severity. Patients with nfvPPA exhibited structural abnormalities in left precentral and middle frontal as well as bilateral superior frontal regions, including their underlying WM. The articulation rate correlated with atrophy of the left pars opercularis and supplementary/presupplementary motor areas. Secondary analyses showed that, controlling for dementia severity, all measures yielded greater deficits in patients with nfvPPA and corticobasal degeneration (nfvPPA-CBD, n = 12) than in those with progressive supranuclear palsy pathology (nfvPPA-PSP, n = 10). The articulation rate robustly discriminated between individuals in each subgroup (AUC = 0.82). More widespread cortical thinning was observed for the nfvPPA-CBD than the nfvPPA-PSP group across frontal regions.
Discussion
Automated speech timing analyses can capture specific markers of nfvPPA while potentially discriminating between patients with different tauopathies. Thanks to its objectivity and scalability; this approach could support standard speech assessments.
Classification of Evidence
This study provides Class III evidence that automated speech analysis can accurately differentiate patients with nonfluent PPA from normal controls and patients with semantic variant PPA.
Nonfluent/agrammatic variant primary progressive aphasia (nfvPPA) is a clinical phenotype of frontotemporal dementia spectrum disorders, often caused by frontotemporal lobar degeneration (FTLD). Most cases present with corticobasal degeneration (CBD) or progressive supranuclear palsy (PSP) pathology,1-4 2 types of FTLD 4-repeat tauopathy (4Rtau).4-7 Both involve aberrant deposition of the microtubule-associated protein tau; varying patterns of frontal, insular, and/or striatal atrophy; underlying white matter (WM) abnormalities; and early motor speech deficits that may be accompanied by agrammatism.4-7 Although isolated agrammatism is rare, motor speech deficits are a defining clinical feature of nfvPPA and often appear on their own—a pattern sometimes termed primary progressive apraxia of speech (PPAOS).8
Prominent among these deficits is abnormal speech timing, a form of dysprosody affecting the rhythm, pace, and duration of oral production. Speech timing disruptions are variant-specific markers of nfvPPA, including path-confirmed cases.2,9-13 Most patients show slow articulation rate and/or prolonged syllables and extended pauses between syllables or words. Speech timing measures have thus been proposed as targets for diagnosis and follow-up.11 Indeed, these alterations might discriminate between CBD and PSP pathology, although the link remains unclear. Some studies report that abnormal speech timing and other prosodic disturbances are predominant in PPAOS caused by PSP,8 while others note their distinct presence in CBD.2,10
Yet, evidence from path-confirmed nfvPPA is restricted to perceptual evaluations, limiting its translational potential. Subjective impressions are prone to expertise and perceptual bias effects,14,15 thereby presenting low validity and reliability.16,17 In addition, they are typically formalized using short rating scales,18 bound to ceiling effects, and blind to pathologic differentiations. Furthermore, their administration requires trained experts, who may be unavailable across centers, especially in low-income countries.19
These limitations can be overcome with automated speech analysis, an objective approach which detects patterns that escape human raters20 and outperforms perceptual evaluations.20 In studies on clinically diagnosed nfvPPA, automated speech timing measures (e.g., articulation rate as well as sound and pause duration, rate, and variability) differentiate patients from healthy controls (HCs) and other PPA groups,12,15,21 capture disease progression,21 and correlate with phosphorylated tau level12 and atrophy of left motor and inferior frontal cortices.12,15,21 In particular, the articulation rate is positively associated with cortical thickness of the left primary motor cortex (PMC) and supplementary/presupplementary motor areas (SMA, preSMA).21 Validating these measures in autopsy-proven nfvPPA would be critical to expand current assessments and discover differential markers of CBD and PSP pathology.
This study aimed to capture speech timing signatures in a well-characterized, autopsy-proven FTLD-4Rtau nfvPPA cohort relative to HCs and patients with svPPA. We raised 3 primary research questions: First, can automated speech timing measures discriminate persons with autopsy-confirmed nfvPPA from both HCs and persons with svPPA? Second, do they capture information that escapes specialist-dependent evaluations of dysarthria and apraxia of speech severity? Third, do they capture distinct atrophy patterns across syndrome-sensitive regions? To address these questions, we administered an overt reading task,18 analyzed 5 relevant measures, assessed their correlations with specialist-dependent motor speech ratings, and examined underlying patterns of cortical thickness and WM atrophy. We hypothesized that automated speech timing measures would (a) differentiate persons with nfvPPA from both HCs and persons with svPPA, (b) robustly classify individuals with nfvPPA, and (c) reveal distinctions that are only partly captured by perceptual assessments. Moreover, we expected such deficits to correlate with atrophy of the left PMC, SMA, preSMA, and/or inferior frontal regions.21,22 As a secondary aim, we explored whether speech timing deficits can discriminate between individuals with nfvPPA-CBD and nfvPPA-PSP.
Methods
Participants
This study involved 47 English-speaking participants completing prospective longitudinal assessments at the Memory and Aging Center (University of California, San Francisco) between 2001 and 2018 (for demographics, see Table 1; for neurologic details, see eAppendix 1, eTable1, links.lww.com/WNL/C70). These comprised 22 persons with nfvPPA, 15 with svPPA, and 10 HCs. This study focused on persons with clinical diagnosis of nfvPPA caused by 4Rtau, with postmortem confirmation of CBD or PSP pathology.
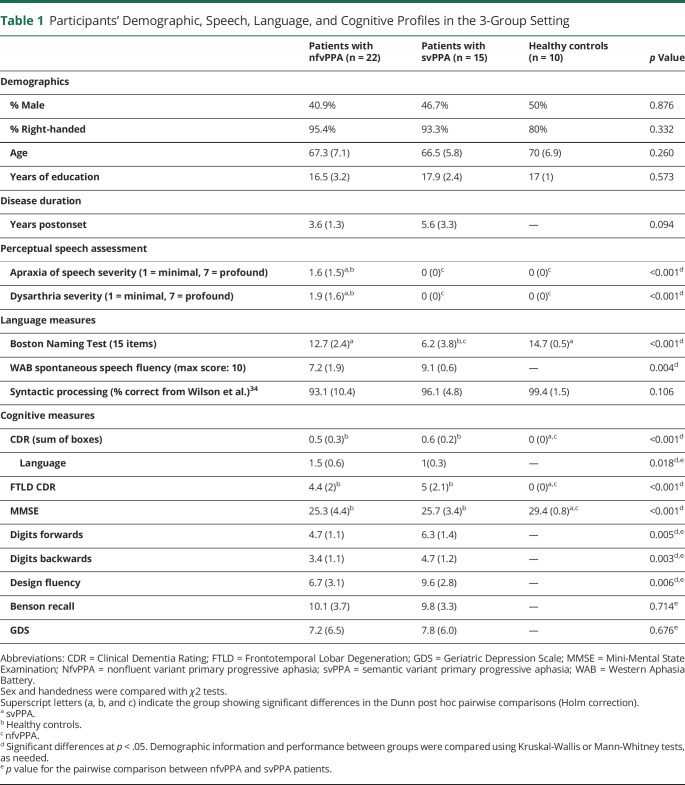
Table 1.
Participants' Demographic, Speech, Language, and Cognitive Profiles in the 3-Group Setting
Clinical nfvPPA diagnoses were made by a multidisciplinary team including neurologists, neuropsychologists, and speech-language pathologists, following validated criteria.23 In all cases, speech production challenges were the first and main complaint as well as the primary cause of functional impairment. Subtle grammatical deficits were noted in most patients. Postmortem, these individuals were autopsied at the University of California, San Francisco, the University of Pennsylvania, or the Vancouver General Hospital. Pathologic diagnoses were made following the consensus FTLD guidelines24 and standard procedures.25 FTLD-4Rtau analysis revealed that primary pathologic diagnosis was CBD for 12 individuals and PSP for the remaining 10. After tau immunohistochemistry, all CBD patients exhibited astrocytic plaques and thread-like inclusions,26 while those with PSP presented globose tangles and tufted astrocytes.27 These subgroups had partly distinct clinical profiles (eAppendix 2, links.lww.com/WNL/C70), and they were sociodemographically matched (eAppendix 3).
The remaining participants comprised 10 HCs (with normal neurologic, cognitive, speech, and language profiles) and 15 persons with svPPA (included to establish the syndromic specificity of predicted deficits). The study's goal did not include assessments of logopenic variant PPA. Persons with svPPA were diagnosed following the abovementioned consensus criteria. No pathologic information was required for these patients to enter this study, although TDP type C pathology is most common in our historical cohort. They exhibited naming and word comprehension deficits with preserved grammar and motor speech. These 2 groups were matched to the overall nfvPPA sample (Table 1) and its pathologically defined subgroups (eAppendix 3, eTable 2, links.lww.com/WNL/C70) for sex, age, years of education, and handedness. Our sample size reached a power of 0.93 for the 3-group analyses (nfvPPA, HCs, svPPA) and 0.90 for the 4-group analyses (nfvPPA-CBD, nfvPPA-PSP, HCs, svPPA)—see eAppendix 4.
Perceptual assessments of videotaped sessions by certified speech pathologists, based on the validated Motor Speech Evaluation,18 indicated that all patients with nfvPPA exhibited dysarthria (with mixed spastic, flaccid, and/or hypokinetic speech features)18 and/or apraxia of speech (with abnormal articulation characterized by slow speech rate, sound distortions, and sequencing errors).28 Clinical ratings of dysarthria or apraxia of speech severity did not differ significantly between the nfvPPA-CBD and nfvPPA-PSP subgroups. No signs of dysarthria or apraxia of speech were observed in HCs or patients with svPPA. The results of these assessments are presented alongside linguistic and cognitive results in Table 1 and in eAppendix 3 (eTable 2, links.lww.com/WNL/C70).
Standard Protocol Approvals, Registrations, and Patient Consents
All participants or their caregivers provided written informed consent before inclusion, pursuant to the Declaration of Helsinki. The 3 studies participants consented to (10-03946, 10-00619, 12-10512) were approved by the Institutional Review Board of the Human Research and Protection Program of the University of California, San Francisco (UCSF).
Automated Speech Assessment
All participants performed an overt reading task, using the Grandfather Passage (eAppendix 5, links.lww.com/WNL/C70). This 129-word text elicits all phonemes and main phoneme clusters in English.18 Unlike spontaneous or semispontaneous speech tasks, overt reading tasks keep verbal information constant across participants, circumventing potential confounds related to linguistic demands. Moreover, they prove sensitive to speech timing alterations in nfvPPA and other neurodegenerative conditions.15,29 Participants were asked to read at their own pace, with normal volume and cadence. Their speech was recorded, saved as .wav files (44.1 KHz, 16 bits), and analyzed to capture 5 timing measures: articulation rate (syllables per second of phonated time, after pause removal), syllable duration (mean across all syllables), pause duration (mean across all pauses, defined as silent intervals between speech sound offset and onset), syllable duration variability (SDs of syllabic durations), and pause duration variability (SD of pause durations).
Acoustic features were extracted using onset detection functions and custom MATLAB scripts. Onset and offset detection was based on acoustic power and summed spectral energy measures across frequencies between 20 and 4,000 Hz, using validated methods adapted here30 and at The University of Melbourne. Time series were smoothed within 50-ms windows and normalized to yield values between 0 and 1. Time points above or below 0.1 were considered onsets or offsets. An 80-ms threshold between offset/onsets was used to limit inclusion of intrasyllabic pauses.
Analysis of Speech Features
Speech timing variables were compared among groups using factorial ANCOVAs, covarying for the Mini-Mental State Examination (MMSE) score as a measure of dementia severity—as in previous work.31 Widely used in PPA research,4,5,9,12,15,18,22,32-34 the MMSE was chosen over the Clinical Dementia Rating (CDR) and the FTLD-CDR because these 2 measures have a restricted score range, little variance across our (fairly mild) patients, and scores of 0 (null variability) across HCs, thus proving suboptimal as potential covariates. Analyses were conducted in a 3-group setting (nfvPPA, HCs, svPPA) and in a 4-group setting (nfvPPA-CBD, nfvPPA-PSP, HCs, svPPA). For each variable, participants with values ≥2.5 SDs from the group's mean were removed as outliers. Data were excluded from (1) a single HC for 3 variables (syllable duration variability, pause duration, pause duration variability) in both the primary and secondary analyses and (2) a single patient with nfvPPA-PSP for a single variable, only in the secondary analyses. No further participants had outlier values in any analysis. Missing data represented <5% in each group for primary analyses, and it was restricted to a single person with nfvPPA. Significant effects (p < 0.05) were inspected using Tukey honest significant difference post hoc tests. Effect sizes were calculated with ηp2 for ANCOVAs and Cohen d for pairwise comparisons.
Moreover, we ran binary logistic regressions to examine whether speech timing features could classify (1) individuals with nfvPPA from HCs and (2) persons with nfvPPA-CBD from those with nfvPPA-PSP. To overcome multicollinearity issues, the most sensitive automated measure across both groups (as seen in effect sizes) was used as a single predictor. For comparison, we also ran regression models including perceptual scores of apraxia of speech and dysarthria (Table 1) as separate predictors. Classification accuracy was evaluated considering the area under the receiving operating characteristic curve (AUC). We also used Pearson r to examine correlations between each automated measure and specialist-dependent perceptual ratings of apraxia of speech and dysarthria severity in the overall nfvPPA sample. Analyses were run on R (R Core Team, 2020) and JASP v.0.14.1 (JASP Team, 2020).
Neuroimaging Analyses
Data Acquisition and Preprocessing
All participants underwent whole-brain structural MRI on a 1.5T Siemens, 3T Siemens Trio, or 3T Siemens Prisma scanners, with a T1-weighted 3D magnetization-prepared rapid-acquisition gradient echo sequence. Standard parameters were used for the 1.5T scanner (164 coronal slices; voxel size = 1.0 × 1.5 × 1.0 mm3; FoV = 256 × 256 mm2; matrix size = 256 × 256; repetition time (TR) = 10 ms; echo time (TE) = 4 ms; T1 = 300 ms; flip angle = 15°), the 3T Trio scanner (160 sagittal slices; voxel size = 1.0 × 1.0 × 1.0 mm3; FoV = 256 × 256 mm; matrix size = 256 × 256; TR = 2,300 ms; TE = 2.98 ms; flip angle = 9°), and the 3T Prisma scanner (160 sagittal slices; voxel size = 1.0 × 1.0 × 1.0 mm3; FoV = 256 × 256 mm; matrix size = 256 × 256; TR = 2,300 ms; TE = 2.90 ms; flip angle = 9°). MRI data for patient groups were compared with those of a group of HCs (matched for sex, age, education, and scanner type) selected from the Hillblom Aging Network Project.
Cortical Thickness and WM Atrophy Analysis
T1-weighted images were visually inspected to ensure the absence of artifacts or excessive motion. Images were processed through the Computational Anatomy Toolbox (dbm.neuro.uni-jena.de/cat) in Statistical Parametric Mapping software (fil.ion.ucl.ac.uk/spm/software/spm12) running under MATLAB. Images were bias-field corrected with a spatial adaptive nonlocal means denoising filter35 and segmented into gray matter (GM), WM, and CSF. All these tissue classes underwent local intensity transformation before the final adaptive maximum a posteriori segmentation,36 refined by applying a partial volume.37 Segmented images were spatially normalized to the Montreal Neurologic Institute space using geodesic shooting registrations38 and modulated by scaling with the amount of volume changes because of spatial registration. WM images were smoothed for a voxel-based morphometry analysis with an 8-mm full-width at half-maximum Gaussian kernel.
Cortical thickness was estimated using surface-based morphometry, a measure that surpasses standard voxel-based analysis as brain surface meshes increase brain registration accuracy.39 The skull-stripped brain was parcellated into left and right hemisphere, subcortical regions, and cerebellum. Cortical thickness estimation and reconstruction of the central surface were performed using a projection-based thickness method.40 Final maps were resampled and smoothed using a 15-mm Gaussian heat kernel.41
Whole-brain analyses of between-group differences in cortical thickness and WM volume were performed using ANOVAs, including sex, age, handedness, and scanner type as nuisance variables. Total intracranial volume (TIV) was included for WM voxel-based morphometry but not for cortical thickness analysis, given that head size is associated only with the former measure.42 Alpha levels were set at p < 0.05. Family wise error (FWE) correction was used to detect areas of peak cortical thinning and of WM volume loss. For better visualization, between-group comparisons were also performed with a less stringent threshold (p < 0.001, uncorrected).
Brain-Behavior Analyses
Considering previous research,21 we assessed whether the articulation rate was associated with cortical atrophy in our target region of interest (ROIs). To this end, we performed linear regressions with sex, age, and TIV as covariates. We used a liberal uncorrected threshold (p < 0.05). Although this is suitable given our moderate sample size and hypothesis-driven analyses,21 we report adjusted R-squares values, controlling for the number of terms in the model. We targeted the following left hemisphere ROIs in specific Brodmann areas (BAs): PMC (BA4), SMA (BA6), and preSMA (BA8). We also included additional ROIs over the inferior frontal gyrus, namely pars opercularis (BA44) and pars triangularis (BA45). All ROIs were based on the Human Brainnetome Atlas.43
Data Availability
Public archiving of anonymized data is not contemplated by the study's institutional review board approval. Requests can be submitted through the resource request form of UCSF's Memory and Aging Center. After a UCSF-regulated procedure, access will be granted to designated individuals in line with the ethical guidelines on the reuse of sensitive data. This would require submission of a Material Transfer Agreement. Commercial use will not be approved.
Results
Automated Speech Analysis Results
ANCOVA Results
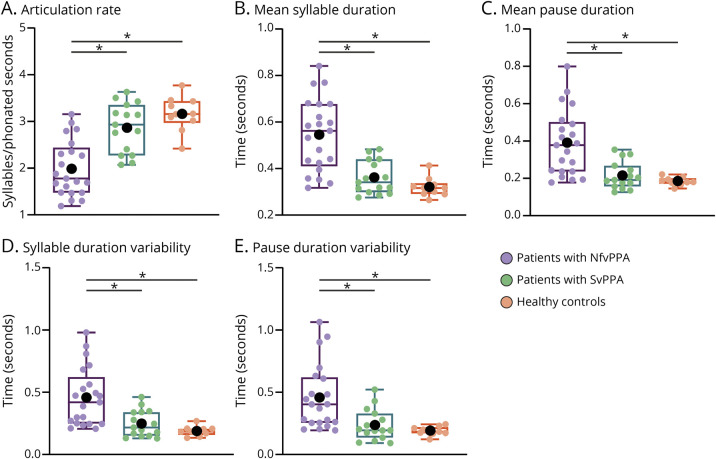
In the 3-group setting (collapsing all patients with nfvPPA), the 5 speech timing measures yielded significant effects of group, adjusting for MMSE scores. Post hoc analyses consistently revealed impaired motor speech in the nfvPPA group relative to both HCs and patients with svPPA, there being no significant differences between the latter 2 groups (Figure 1 and eAppendix 6, eTable 3, links.lww.com/WNL/C70).
Figure 1. Results for the 3-Group Setting (All Patients With nfvPPA Together).
All 5 speech timing measures revealed significant impairments in patients with nfvPPA relative to both healthy controls and patients with svPPA. No differences were observed between the latter 2 groups. In the box plot, middle horizontal lines show each group's median, with lower and upper lines representing the 25th and 75th percentiles, respectively. The whiskers represent the smallest and largest values in the distribution. Colored dots indicate each participant's individual value. Black dots represent the group's mean. *Denotes significant differences at p < 0.05. All statistics were calculated after outlier removal (namely, a single nfvPPA-PSP participant). NfvPPA = nonfluent variant primary progressive aphasia; svPPA = semantic variant primary progressive aphasia.
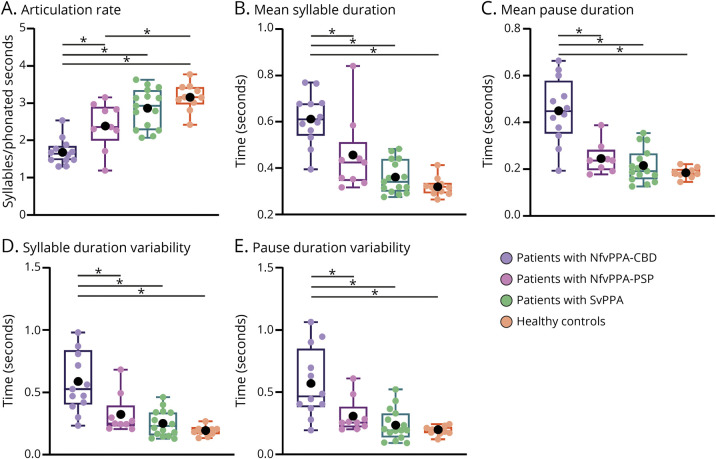
The results from the 4-group analysis revealed that abnormal speech timing in the nfvPPA group was driven by patients with nfvPPA-CBD, who differed from HCs and patients with svPPA across all 5 automated measures. Conversely, deficits in the nfvPPA-PSP group were confined to the articulation rate. Moreover, all 5 measures showed greater deficits in the nfvPPA-CBD than in the nfvPPA-PSP group, adjusting for MMSE scores (Figure 2 and eAppendix 6, eTable 4, links.lww.com/WNL/C70).
Figure 2. Results of the 4-Group Analysis (Separating Patients With nfvPPA-CBD From nfvPPA-PSP).
All 5 speech timing measures captured significant impairments in patients with nfvPPA-CBD compared with both healthy controls and patients with svPPA. By contrast, only the articulation rate was impaired in the patients with nfvPPA-PSP. Deficits in all measures were significantly greater for patients with nfvPPA-CBD than nfvPPA-PSP. In the box plot, middle horizontal lines show each group's median, with lower and upper lines representing the 25th and 75th percentiles, respectively. The whiskers represent the smallest and largest values in the distribution. Colored dots indicate each participant's individual value. Black dots represent the group's mean. *Denote significant differences at p < 0.05. All statistics were calculated after outlier removal (namely, a single participant with nfvPPA-PSP). NfvPPA-CBD = nonfluent variant primary progressive aphasia with corticobasal degeneration pathology; nfvPPA-PSP = nonfluent variant primary progressive aphasia with progressive supranuclear palsy pathology; svPPA = semantic variant primary progressive aphasia.
Binary Logistic Regression Results
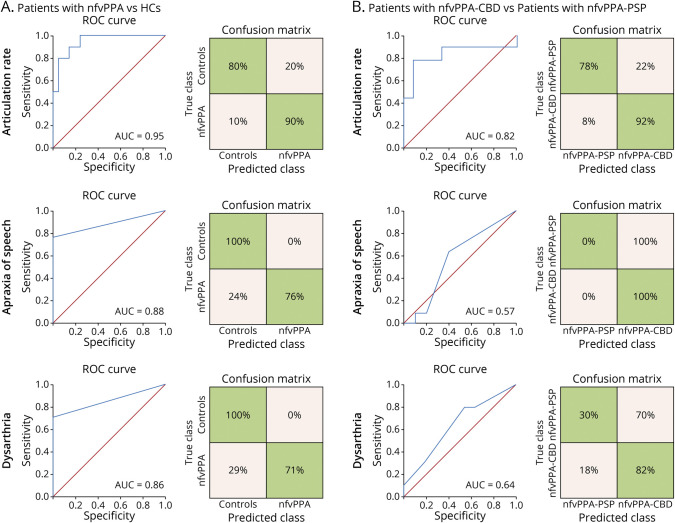
Binary logistic regressions were run based on the articulation rate, namely the measure yielding the largest effect size in the 3-group analyses and the only 1 revealing deficits in both nfvPPA subgroups. This predictor discriminated between patients with nfvPPA and HCs [Wald χ2 (1) = 6.53, p = 0.012] with high accuracy (AUC = 0.95). The model correctly classified 90% of persons with nfvPPA and 80% of HCs. Group membership was not predicted when based on independent models of apraxia of speech [Wald χ2 (1) = 1.60, p = 0.99] or dysarthria [Wald χ2 (1) = 0, p = 0.99] severity ratings, both yielding less accurate classification than the articulation rate model (AUC = 0.88 and 0.86, respectively)—Figure 3A.
Figure 3. Binary Logistic Regression Results.
(A) Classification between patients with nfvPPA and HCs was significant for the automated measure of the articulation rate (top inset) as well as the perceptual measures of apraxia of speech severity (middle inset) and dysarthria severity (bottom inset), with maximal AUC score for the former. (B) Classification between patients with nfvPPA-CBD and nfvPPA-PSP was significant for the automated measure of the articulation rate (top inset) but not for the perceptual measures of apraxia of speech severity (middle inset) and dysarthria severity (bottom inset), the former yielding a robust AUC value. AUC = area under the ROC curve; HCs = healthy controls; nfvPPA = nonfluent variant primary progressive aphasia; nfvPPA-CBD = nonfluent variant primary progressive aphasia with corticobasal degeneration pathology; nfvPPA-PSP = nonfluent variant primary progressive aphasia with progressive supranuclear palsy pathology; ROC = receiving operating characteristic; svPPA = semantic variant primary progressive aphasia.
In addition, the articulation rate discriminated between pathologically defined subgroups [Wald χ2 (1) = 5.51, p = 0.019]. This model yielded high classification accuracy (AUC = 0.82), correctly identifying 92% of persons with nfvPPA-CBD and 78% of persons with nfvPPA-PSP. Conversely, apraxia of speech severity did not predict group membership [Wald χ2 (1) = 0.003, p = 0.954], correctly identifying 100% of with nfvPPA-CBD but 0% of persons with nfvPPA-PSP (AUC = 0.57). Similarly, group membership was not predicted by dysarthria severity [Wald χ2 (1) = 1.20, p = 0.273], identifying 82% of persons with nfvPPA-CBD and only 30% of persons with nfvPPA-PSP (AUC = 0.64)—Figure 3B.
Correlations Between Automated and Perceptual Speech Measures
Across all patients with nfvPPA, perceptual ratings of apraxia of speech severity were correlated with articulation rate (p = 0.04, r = −0.45) and mean syllable duration (p = 0.04, r = 0.46). Nonsignificant results were observed for every other correlation between speech timing measures and perceptual ratings of apraxia of speech and dysarthria severity (all p values > 0.19). For details, see eAppendix 7, eTable 5 (links.lww.com/WNL/C70).
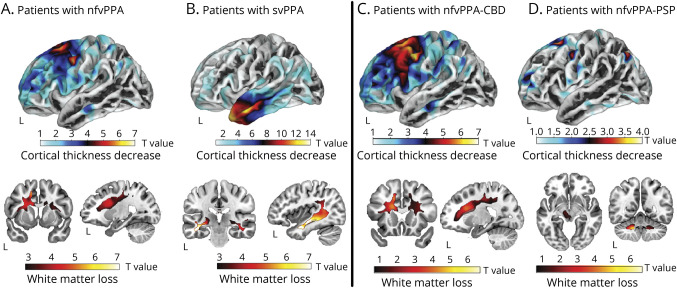
Cortical Thinning and WM Loss Across Patient Groups
The combined nfvPPA group exhibited cortical thinning in the left precentral and caudal/rostral middle frontal cortices as well as the bilateral superior frontal gyrus (including the supplementary motor area), together with atrophy in the underlying WM (p < 0.05, FWE-corrected)—Figure 4A. Patients with svPPA exhibited reduced cortical thickness in the left temporal pole (extending onto inferior, middle, and superior temporal gyri) and lower volume in the underlying WM (p < 0.05, FWE-corrected)—Figure 4B. Secondary analyses in the nfvPPA-CBD and nfvPPA-PSP subgroups revealed structural abnormalities in the same areas as the combined nfvPPA group, with the nfvPPA-CBD subgroup showing more involvement and additional thinning of the left pars opercularis (p < 0.05, FWE-corrected) relative to HCs and the nfvPPA-PSP subgroup exhibiting compromise of the angular gyrus as well as the bilateral dentate nucleus, the left thalamus, and the left midbrain (although not surviving FWE correction) relative to HCs. Structural abnormality was more widespread in patients with CBD than in those with PSP (Figure 4, C and D).
Figure 4. Brain Structural Abnormality Patterns of Each Patient Group.
The combined nfvPPA group (A) and the svPPA group (B), as well as subgroups with nfvPPA-CBD (C) and nfvPPA-PSP (D), were compared with HCs to estimate their patterns of cortical thickness (top insets) and white matter volume (bottom insets) using surface-based and voxel-based morphometry, respectively. For better visualization of differences and similarities across groups, t-map values are reported at an uncorrected p < 0.001 threshold. NfvPPA = nonfluent variant primary progressive aphasia; nfvPPA-CBD = nonfluent variant primary progressive aphasia with corticobasal degeneration pathology; nfvPPA-PSP = nonfluent variant primary progressive aphasia with progressive supranuclear palsy pathology; svPPA = semantic variant primary progressive aphasia.
Brain-Behavior Associations
Across the nfvPPA group, the articulation rate was associated with cortical atrophy in the left SMA (p = 0.03, adjusted R2 = 0.18), preSMA (p = 0.02, adjusted R2 = 0.34), and pars opercularis (p < 0.01, adjusted R2 = 0.47), but not in the PMC (p = 0.07, adjusted R2 = 0.14) or the pars triangularis (p = 0.65, adjusted R2 = −0.07).
Classification of Evidence
This study provides Class III evidence that automated speech analysis can accurately differentiate patients with nonfluent PPA from normal controls and patients with semantic variant PPA.
Discussion
We used automated speech timing analysis to identify patients with autopsy-confirmed nfvPPA. All 5 measures discriminated these individuals from HCs and from patients with svPPA, and the articulation rate surpassed specialist-dependent perceptual evaluations in distinguishing individuals with nfvPPA. Abnormal speech timing was accompanied by GM and WM alterations in left frontal cortices, and significantly associated with motor region atrophy. In addition, relative to patients with nfvPPA-PSP, those with nfvPPA-CBD exhibited greater deficits in all automated metrics and more widespread frontal atrophy. Finally, the articulation rate robustly discriminated between patients in each subgroup. These findings suggest that automated speech timing measures are sensitive tools for nfvPPA assessments.
Relative to HCs and patients with svPPA, the nfvPPA group produced fewer syllables per phonated second as well as longer and less isochronous syllables and pauses. Although variant-specific alterations of speech timing and other prosodic dimensions have been reported in clinically diagnosed nfvPPA,11,12 our study successfully captured them automatically in autopsy-confirmed patients. This finding extends evidence from perceptual measures in persons with confirmed FTLD-tauopathy,5 indicating that speech timing markers are critically linked to such pathology.12 Notably, these distinctions proved significant on controlling for dementia severity, which is notable given that cognitive impairment can influence motor speech deficits.29
The articulation rate (our most sensitive automated measure) classified individuals with nfvPPA from HCs with an AUC of 0.95. This result surpassed the highest AUC scores in the nfvPPA literature, based on both single and combined motor speech measures.12 As observed in other diseases,20,44 the classifier based on the articulation rate outperformed those based on perceptual measures. Although useful when administered by specialists, listener-based assessments may yield inconsistent results and overlook fine-grained speech dimensions.16,17 The latter point may account for the better performance of our automated tools.
Both articulation rate and mean syllable duration were correlated with apraxia of speech (but not with dysarthria) severity ratings. This might reflect the prominence of phonated segment length (as opposed to pausing) in perceptual assessments because both automated measures lean heavily on such a factor. Still, no other correlation between automated and perceptual measures reached significance, suggesting that they capture partly different phenomena. In brief, regression results support the value of automated speech timing assessments for nfvPPA,11 showing that subtle dysfunctions can be objectively established at a probabilistic single-patient level.
The nfvPPA group exhibited cortical thinning across left precentral, left middle frontal, and bilateral superior frontal regions, including the SMA—alongside atrophy of the underlying WM. These regions (particularly, the precentral and supplementary motor cortices) are central hubs of the motor speech network,32 likely accounting for the patients' behavioral profile. Moreover, WM alterations beneath these and other substrates are typical of nfvPPA with FTLD-tau.33 In fact, damage to frontal regions, especially including the SMA, is typically observed in nonagrammatic patients with motor speech disorders.8 Interestingly, although prosodic alterations may also involve noncortical degeneration (e.g., in the superior cerebellar peduncle),45 our study suggests that at least some such disruptions (namely, speech timing alterations) may become significant without marked subcortical damage.
The articulation rate was associated with atrophy in the left SMA and preSMA, as observed in a previous study.21 However, unlike that work, we also observed an association with atrophy of the left pars opercularis, while failing to find associations with PMC or the pars triangularis. These differences may reflect the fact that brain-behavior correlations in the previously cited report.21 were conducted on collapsing patients from all 3 PPA variants, each presenting distinct atrophy patterns.
As seen in secondary analyses, all automated measures revealed greater deficits in nfvPPA-CBD than in nfvPPA-PSP; the latter group, indeed, was impaired only on the articulation rate. This measure classified persons in each subgroup with an AUC of 0.82—unlike perceptual speech measures, which failed to discriminate between groups. Interestingly, a previous study found that prosodic deficits were more strongly related to PSP than CBD pathology.8 Yet, that study targeted (semi)spontaneous speech while we used overt reading. These tasks' discrepant demands elicit distinct predominant motor speech disruptions, with prosodic alterations proving more salient during reading.29 In addition, no specific assessment of speech timing was conducted therein. Furthermore, dysprosodic signs have been highlighted in CBD,2,10 and slow syllabically segmented prosody is a diagnostic criterion46 for a speech-predominant disorder more likely caused by CBD than PSP.47 Thus, automated timing assessments could capture distinct signatures of nfvPPA-CBD that may be underestimated in listener-based studies.
Neuroimaging results inform these differentiations. Structural abnormality along premotor speech regions and underlying WM was present in both subgroups, as previously observed.8,45 Yet, such patterns proved markedly more pronounced and widespread in persons with nfvPPA-CBD, potentially accounting for their more severe speech timing impairments. This finding opens new avenus to investigate the role of neocortical motor-network hubs in speech timing.
This study underscores the usefulness of automated acoustic measures for nfvPPA assessments. Perceptual evaluations may not always be valid or reliable,14-17 they are not well suited to monitoring change,48 and their optimal administration may be unfeasible in low-income countries lacking specialized staff.19 Conversely, computerized systems offer an affordable and objective framework that can be applied remotely. In particular, unlike dimensions such as loudness or pitch, speech timing measures are robust to variability in noise and recording conditions, highlighting their scalability. By corroborating their sensitivity in autopsy-confirmed patients, this study addresses current calls to complement standard protocols with cutting-edge metrics.
Moreover, the potential to discriminate between CBD and PSP pathology holds clinical promise. Each of those tauopathies may be prodromal to either corticobasal syndrome (typified by asymmetric movement abnormalities, myoclonus, and dystonia) or PSP syndrome (with symmetric motor symptoms and vertical supranuclear palsy),2,10 whose differentiation in early clinical testing is very challenging. In addition, CBD and PSP pathology might entail distinct tau prion conformations,49 potentially requiring different therapies.5 Thus, these and other tools enabling automatic identification of nfvPPA-CBD and nfvPPA-PSP may optimize individualized treatment, prognosis, and monitoring. Note, however, that the reported measures may only discriminate between these subgroups if administered in early stages because both will likely become more pervasive, less distinguishable speech deficits as disease progresses.
Finally, some researchers do not consider that isolated motor speech deficits constitute aphasia, and they use the term PPAOS instead. Still, current criteria indicate that clinical nfvPPA diagnosis is warranted even if patients exhibit core motor speech deficits without agrammatism and complex sentence comprehension deficits. Moreover, not all patients with predominant motor speech deficits are characterized by apraxia of speech (as the PPAOS label would suggest), but rather by dysarthria or some combination therefrom. In addition, subtle grammatical difficulties were observed in our cohort during clinical interviews, supporting the view that nfvPPA is a spectrum, as the weight of motor speech and grammatical deficits varies across patients and throughout time. Future studies should address these definitional questions in greater depth.
Our study is not without limitations. First, although we assembled the largest autopsy-confirmed cohort in the automated speech analysis literature, our sample size was modest. Future replications should involve more participants, especially for subgroup analyses. Second, inconsistent recording conditions (e.g., divergent volume and noise levels) over our 17-year data collection period precluded analysis of other acoustic dimensions, such as articulation and phonation. Relevant breakthroughs could be made, under standardized recording conditions, by comparing the predominance of different motor speech alterations in each nfvPPA subgroup, as performed elsewhere.8,45 Third, our approach could be more stringently tested through comparisons between nfvPPA and lvPPA, whose clinical differentiation proves particularly challenging when using standard speech measures.13,15 In addition, postmortem confirmation for participants with svPPA would be useful to refine pathology-related conclusions. Fourth, although the MMSE was statistically preferable to other severity measures for covariance analyses, it relies heavily on speech and language functions. In this sense, each group mainly missed different items (sentence comprehension, repetition, and writing for nfvPPA-CBD and nfvPPA-PSP; object naming and backward spelling for svPPA). Future studies could further explore how speech timing is affected by different language profiles and use relevant, nonverbal measures for covariance analyses. Fifth, because data were collected through various protocols over 17 years, we lacked a single, objective grammatical measure across patients. Although this precludes detailed characterizations of the patients' aphasic profile, future research could use morphosyntactic analyses of other recorded samples to elucidate the issue. Sixth, our cohort included too few persons with Pick disease, preventing their inclusion for statistical analysis and inviting new research on their particular speech timing profiles. Finally, our approach could be implemented on platforms offering remote audio recording. Free open-source applications already provide user-friendly capabilities for clinical research. These tools could foster continual remote testing, opening exciting opportunities for longitudinal research, and disease progression monitoring.
Automated speech timing measures can discriminate persons with autopsy-confirmed nfvPPA from HCs and patients with svPPA, identify persons with autopsy-confirmed nfvPPA at the probabilistic single-person level, capture distinct atrophy patterns across syndrome-sensitive regions, and potentially differentiate between patients with CBD and PSP pathology. Computerized speech tools could, thus, contribute to differential diagnosis and pathology prediction in this population. New avenues can be envisaged toward developing scalable, objective innovations in clinicopathologic FTLD assessments.
Acknowledgment
We thank all participants and their families for contributing to this research.
Glossary
- 4Rtau
FTLD-4-repeat tauopathy
- AUC
area under the ROC curve
- BA
Brodmann area
- CBD
corticobasal degeneration
- CDR
Clinical Dementia Rating
- FTLD
frontotemporal lobar degeneration
- FWE
family wise error
- HCs
healthy controls
- MMSE
Mini-Mental State Examination
- nfvPPA
nonfluent/agrammatic variant primary progressive aphasia
- nfvPPA-CBD
nonfluent/agrammatic primary progressive aphasia with underlying corticobasal degeneration pathology
- nfvPPA-PSP
nonfluent/agrammatic parimary progressive aphasia with underlying progressive supranuclear palsy pathology
- PMC
primary motor cortex
- PPA
primary progressive aphasia
- PPAOS
primary progressive apraxia of speech
- preSMA
presupplementary motor area
- PSP
progressive supranuclear palsy
- ROI
region of interest
- SMA
supplementary motor area
- svPPA
semantic variant primary progressive aphasia
- TIV
total intracranial volume
- WM
white matter
- GM
gray matter
- TE
echo time
- TR
repetition time
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Editorial, page 181
Study Funding
A.M. García is an Atlantic Fellow at the Global Brain Health Institute (GBHI) and is supported with funding from GBHI, Alzheimer's Association, and Alzheimer's Society (GBHI ALZ UK-22-865742); as well as CONICET; ANID (FONDECYT Regular 1210176); and Programa Interdisciplinario de Investigación Experimental en Comunicación y Cognición (PIIECC), Facultad de Humanidades, USACH. M.L. Henry is supported by the NIH (NIDCD R01DC016291). M.J. Torres-Prioris has been funded by a postdoctoral fellowship under the program Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI 2020) (DOC_00421). J. Deleon is supported by the NIH (NIDCD K23 DC018021). D.L. Lorca-Puls is supported by a postdoctoral fellowship from the National Agency for Research and Development (ANID BECAS-CHILE 74200073). The Memory and Aging Center is also supported by the Larry L. Hillblom Foundation; John Douglas French Alzheimer's Foundation; Koret Family Foundation; Consortium for Frontotemporal Dementia Research; and McBean Family Foundation. M.L. Gorno Tempini is supported by grants from the NIH (NINDS R01 NS050915, NIDCD K24 DC015544; NIA U01 AG052943).
Disclosure
A.P. Vogel is Chief Science Officer of Redenlab Inc. The remaining authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. 2008;21(6):688-692. [DOI] [PubMed] [Google Scholar]
- 2.Peterson KA, Patterson K, Rowe JB. Language impairment in progressive supranuclear palsy and corticobasal syndrome. J Neurol. 2021;268(3):796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol. 2017;81(3):430-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos-Santos MA, Mandelli ML, Binney RJ, et al. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol. 2016;73(6):733-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(pt 6):1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josephs KA, Duffy JR, Clark HM, et al. A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nat Commun. 2021;12(1):3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karnik NS, D'Apuzzo M, Greicius M. Non-fluent progressive aphasia, depression, and OCD in a woman with progressive supranuclear palsy: neuroanatomical and neuropathological correlations. Neurocase. 2006;12(6):332-338. [DOI] [PubMed] [Google Scholar]
- 10.Kertesz A, McMonagle P. Behavior and cognition in corticobasal degeneration and progressive supranuclear palsy. J Neurol Sci. 2010;289(1-2):138-143. [DOI] [PubMed] [Google Scholar]
- 11.Matias-Guiu JA, Suárez-Coalla P, Pytel V, et al. Reading prosody in the non-fluent and logopenic variants of primary progressive aphasia. Cortex. 2020;132:63-78. [DOI] [PubMed] [Google Scholar]
- 12.Nevler N, Ash S, Irwin DJ, Liberman M, Grossman M. Validated automatic speech biomarkers in primary progressive aphasia. Ann Clin translational Neurol. 2019;6(1):4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley KL, Jacks A, Jarrett J, et al. Speech metrics and samples that differentiate between nonfluent/agrammatic and logopenic variants of primary progressive aphasia. J Speech Lang Hear Res. 2021;64(3):754-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent RD. Hearing and believing. Am J Speech Lang Path. 1996;5(3):7-23. [Google Scholar]
- 15.Ballard KJ, Savage S, Leyton CE, Vogel AP, Hornberger M, Hodges JR. Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLoS One. 2014;9(2):e89864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cernak M, Orozco-Arroyave JR, Rudzicz F, Christensen H, Vásquez-Correa JC, Nöth E. Characterisation of voice quality of Parkinson's disease using differential phonological posterior features. Comp Speech Lang. 2017;46:196-208. [Google Scholar]
- 17.Aronson AE, Bless D. Clinical Voice Disorders. Thieme; 2011. [Google Scholar]
- 18.Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21(4):S23-S30. [DOI] [PubMed] [Google Scholar]
- 19.Parra MA, Baez S, Allegri R, et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology. 2018;90(5):222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huh YE, Park J, Suh MK, et al. Differences in early speech patterns between Parkinson variant of multiple system atrophy and Parkinson's disease. Brain Lang. 2015;147:14-20. [DOI] [PubMed] [Google Scholar]
- 21.Cordella C, Quimby M, Touroutoglou A, Brickhouse M, Dickerson BC, Green JR. Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology. 2019;92(17):e1992-e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandelli ML, Welch AE, Vilaplana E, et al. Altered topology of the functional speech production network in non-fluent/agrammatic variant of PPA. Cortex. 2018;108:252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995;146(6):1388-1396. [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada T, McGeer PL, McGeer EG. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992;135(1):99-102. [DOI] [PubMed] [Google Scholar]
- 28.Duffy J. Motor Speech Disorders. Mosby; 1995. [Google Scholar]
- 29.García AM, Arias-Vergara T, Vasquez-Correa JC, et al. Cognitive determinants of dysarthria in Parkinson's disease: an automated machine learning approach. Mov Disord. 2021;36(12):2862-2873. [DOI] [PubMed] [Google Scholar]
- 30.Schultz BG, O'Brien I, Phillips N, McFarland DH, Titone D, Palmer C. Speech rates converge in scripted turn-taking conversations. App Psycholing. 2016;37:1201-1220. [Google Scholar]
- 31.Marczinski CA, Kertesz A. Category and letter fluency in semantic dementia, primary progressive aphasia, and Alzheimer's disease. Brain Lang. 2006;97(3):258-265. [DOI] [PubMed] [Google Scholar]
- 32.Mandelli ML, Caverzasi E, Binney RJ, et al. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci. 2014;34(29):9754-9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caso F, Mandelli ML, Henry M, et al. In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology. 2014;82(3):239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson SM, Dronkers NF, Ogar JM, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J Neurosci. 2010;30(50):16845-16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manjón JV, Coupé P, Martí-Bonmatí L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31(1):192-203. [DOI] [PubMed] [Google Scholar]
- 36.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16(2):176-186. [DOI] [PubMed] [Google Scholar]
- 37.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. NeuroImage. 2004;23(1):84-97. [DOI] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. Diffeomorphic registration using geodesic shooting and Gauss-Newton optimisation. NeuroImage. 2011;55(3):954-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desai R, Liebenthal E, Possing ET, Waldron E, Binder JR. Volumetric vs. surface-based alignment for localization of auditory cortex activation. NeuroImage. 2005;26(4):1019-1029. [DOI] [PubMed] [Google Scholar]
- 40.Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. NeuroImage. 2013;65:336-348. [DOI] [PubMed] [Google Scholar]
- 41.Yotter RA, Nenadic I, Ziegler G, Thompson PM, Gaser C. Local cortical surface complexity maps from spherical harmonic reconstructions. NeuroImage. 2011;56(3):961-973. [DOI] [PubMed] [Google Scholar]
- 42.Barnes J, Ridgway GR, Bartlett J, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 2010;53(4):1244-1255. [DOI] [PubMed] [Google Scholar]
- 43.Fan L, Li H, Zhuo J, et al. The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliasova I, Mekyska J, Kostalova M, Marecek R, Smekal Z, Rektorova I. Acoustic evaluation of short-term effects of repetitive transcranial magnetic stimulation on motor aspects of speech in Parkinson's disease. J Neural Transm. 2013;120(4):597-605. [DOI] [PubMed] [Google Scholar]
- 45.Utianski RL, Duffy JR, Clark HM, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang. 2018;184:54-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Höglinger GU, Respondek G, Stamelou M, et al. ; Movement Disorder Society-endorsed PSP Study Group. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali F, Martin PR, Botha H, et al. Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord. 2019;34(8):1144-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel AP, Maruff P. Monitoring change requires a rethink of assessment practices in voice and speech. Logoped Phoniatr Vocol. 2014;39(2):56-61. [DOI] [PubMed] [Google Scholar]
- 49.Sanders DW, Kaufman SK, DeVos SL, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82(6):1271-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Public archiving of anonymized data is not contemplated by the study's institutional review board approval. Requests can be submitted through the resource request form of UCSF's Memory and Aging Center. After a UCSF-regulated procedure, access will be granted to designated individuals in line with the ethical guidelines on the reuse of sensitive data. This would require submission of a Material Transfer Agreement. Commercial use will not be approved.