Abstract
Background and Objectives
Pompe disease is a rare, progressive neuromuscular disorder caused by deficiency of lysosomal acid α-glucosidase (GAA) and subsequent glycogen accumulation. Avalglucosidase alfa, a recombinant human GAA enzyme replacement therapy designed for increased cellular uptake and glycogen clearance, has been studied for long-term efficacy and safety in patients with late-onset Pompe disease (LOPD). Here, we report up to 6.5 years' experience with avalglucosidase alfa during the NEO1 and NEO-EXT studies.
Methods
NEO1 participants with LOPD, either treatment naive (Naive Group) or receiving alglucosidase alfa for ≥9 months (Switch Group), received avalglucosidase alfa (5, 10, or 20 mg/kg every other week [qow]) for 6 months before entering NEO-EXT and continued their NEO1 dose until all proceeded with 20 mg/kg qow. Safety and efficacy, a prespecified exploratory secondary outcome, were assessed; slopes of change for efficacy outcomes were calculated from a repeated mixed-measures model.
Results
Twenty-four participants enrolled in NEO1 (Naive Group, n = 10; Switch Group, n = 14); 21 completed and 19 entered NEO-EXT; in February 2020, 17 participants remained in NEO-EXT, with data up to 6.5 years. Avalglucosidase alfa was generally well tolerated during NEO-EXT, with a safety profile consistent with that in NEO1. No deaths or treatment-related life-threatening serious adverse events occurred. Eighteen participants developed antidrug antibodies without apparent effect on clinical outcomes. No participants who were tested developed immunoglobulin E antibodies. Upright forced vital capacity %predicted remained stable in most participants, with slope estimates (95% CIs) of −0.473 per year (−1.188 to 0.242) and −0.648 per year (−1.061 to −0.236) in the Naive and Switch Groups, respectively. Six-minute walk test (6MWT) %predicted was also stable for most participants, with slope estimates of −0.701 per year (−1.571 to 0.169) and −0.846 per year (−1.567 to −0.125) for the Naive and Switch Groups, respectively. Improvements in 6MWT distance were observed in most participants aged <45 years at NEO1 enrollment in both the Naive and Switch Groups.
Discussion
Avalglucosidase alfa was generally well tolerated for up to 6.5 years in adult participants with LOPD either naive to alglucosidase alfa or who had previously received alglucosidase alfa for ≥9 months.
Classification of Evidence
This study provides Class IV evidence of long-term tolerability and sustained efficacy of avalglucosidase alfa in patients with LOPD after up to 6.5 years.
Trial Registration Information
NCT01898364 (NEO1 first posted: July 12, 2013; clinicaltrials.gov/ct2/show/NCT01898364); NCT02032524 (NEO-EXT first posted: January 10, 2014; clinicaltrials.gov/ct2/show/NCT02032524). First participant enrollment: NEO1—August 19, 2013; NEO-EXT—February 27, 2014.
Pompe disease is a rare, progressive, autosomal recessive glycogen storage disorder caused by pathogenic variants in the gene encoding acid α-glucosidase (GAA) resulting in lysosomal GAA deficiency. Consequently, lysosomal and eventually cytoplasmic glycogen accumulation occurs in cardiac, skeletal, and smooth muscle causing progressive muscle damage.1 Late-onset Pompe disease (LOPD) is characterized by progressive weakness in skeletal muscles, including the diaphragm and other respiratory muscles, leading to progressive respiratory and motor disability.2 Respiratory failure remains the main cause of death in LOPD, and respiratory muscle strength may predict long-term outcomes.3 Diaphragm dysfunction impairs inspiration and leads to hypercapnia; diaphragm and thoracoabdominal expiratory muscle weakness impairs forced expiration and cough.4,5 Motor function is also impaired, and the motor decline trajectory may be influenced by a natural age-related functional decrease.6
Alglucosidase alfa has improved survival,3 quality of life, and participation in daily life7 in patients with LOPD, and long-term benefits have established it as the current standard of care. However, unmet needs for improvement in muscle and respiratory function remain for those receiving alglucosidase alfa,8,9 and disease progression is partly attributed to suboptimal enzyme replacement therapy (ERT) uptake into skeletal muscle. Avalglucosidase alfa, a recombinant human GAA, is designed with a ≈15-fold increase in mannose-6-phosphate (M6P) content compared with alglucosidase alfa to improve cation-independent M6P receptor–mediated uptake, glycogen clearance, and clinical efficacy. Avalglucosidase alfa-ngpt (Nexviazyme; Sanofi) has received marketing authorization in several countries for infantile-onset Pompe disease and/or LOPD. In the United States, it was approved in August 2021 for patients with LOPD aged ≥1 year.
Here, we evaluate long-term (up to 6.5 years) safety, efficacy, and pharmacokinetics (PK) of avalglucosidase alfa in adults with LOPD, who enrolled in the 6-month, phase 1, NEO1 study10 and subsequently entered NEO-EXT, a NEO1 extension. The primary objective was to assess the long-term tolerability of avalglucosidase alfa in participants with LOPD after up to 6.5 years.
Methods
Standard Protocol Approvals, Registrations, and Participant Consents
NEO1 (NCT01898364) and its extension, NEO-EXT (NCT02032524), were conducted according to the International Conference on Harmonisation guidelines for Good Clinical Practice, Declaration of Helsinki principles, and local/national regulations. Study protocols were approved by independent ethics committees/institutional review boards at participating centers; the names of these independent ethics committees/institutional review boards have been previously published.10 Written informed consent was obtained from participants.
Study Design
NEO-EXT is an open-label, multicenter, multinational, long-term extension study of NEO1, a safety and PK study of repeated every other week (qow) IV infusions of avalglucosidase alfa in participants with LOPD. NEO1 participants received 5, 10, or 20 mg/kg body weight of avalglucosidase alfa qow for 6 months. On entering NEO-EXT, participants continued their assigned NEO1 avalglucosidase alfa dose (5, 10, or 20 mg/kg qow) for 104–156 weeks until receiving 20 mg/kg qow avalglucosidase alfa. For each participant, the study duration is up to 8 years from NEO-EXT enrollment, depending on local circumstances and/or avalglucosidase alfa approval in the participant's country. Data cutoff for this article was February 27, 2020, reflecting a prespecified 6.5-year interim data set.
Participants
Full inclusion and exclusion criteria for NEO1 participation are published.10 In brief, participants, aged ≥18 years, had a confirmed Pompe disease diagnosis and were treatment naive (Naive Group) or had previously received alglucosidase alfa for ≥9 months (Switch Group). Participants had to be able to walk ≥50 m without stopping or using an assistive device and have an upright forced vital capacity (FVC) ≥50 %predicted according to spirometric reference values from a US population sample.11 Participants were excluded from NEO1 if they were wheelchair dependent, required invasive ventilation, were pregnant or had other extenuating conditions, or were at high risk for severe allergic reaction to alglucosidase alfa (i.e., anaphylaxis, immunoglobulin [Ig]E antibodies, or high IgG antibodies).
Participants who completed 24 weeks' avalglucosidase alfa (13 doses) in NEO1 were eligible to enter NEO-EXT, unless they were unable to adhere to study requirements or they had clinically significant non-Pompe organic disease, an extenuating circumstance precluding study participation, or potentially decreased survival. Treatment compliance was monitored based on participants receiving an avalglucosidase alfa infusion qow within a ±7-day window from the previous infusion.
Study Objectives
The primary objective of NEO-EXT was to assess the long-term safety and PK of avalglucosidase alfa in participants with Pompe disease who previously completed NEO1. The secondary objective was to assess the long-term effects of avalglucosidase alfa on pharmacodynamic and prespecified exploratory efficacy variables to assess whether the avalglucosidase alfa benefits observed in NEO1 were maintained and to evaluate the time course of response.
Assessments
Safety, PK, pharmacodynamic, pharmacogenetic, and exploratory efficacy assessments were performed at scheduled visits. Adverse events (AEs) and concomitant medications were collected continuously.
Safety, the primary end point, was assessed via AEs/treatment-emergent AEs (TEAEs), including infusion-associated reactions (IARs) and deaths; physical examinations; clinical laboratory evaluations including hematology, biochemistry, and urinalysis; vital signs; body weight; 12-lead ECG; and immunogenicity assessments. An independent Data Monitoring Committee reviewed safety information semiannually and on an ad hoc basis.
Participants were tested for anti–avalglucosidase alfa antibodies monthly during the first 6 months and thereafter every 3 months. Every time a participant tested seropositive antidrug antibody (ADA), serum was also tested for neutralizing antibodies (NAbs) to avalglucosidase alfa including inhibition of enzyme activity and uptake. Samples collected from participants who previously received alglucosidase alfa were evaluated for anti–alglucosidase alfa IgG antibodies every 6 months for up to the first 6 years of NEO-EXT. Following moderate, severe, or recurrent mild IARs suggestive of hypersensitivity reactions, participants were tested for IgE, complement activation, and serum tryptase. If a participant had signs or symptoms suggestive of systemic immune-mediated reactions involving skin and other organs while receiving alglucosidase alfa, serum samples were collected for circulating immune complexes evaluation. Secondary end points included PK, pharmacodynamic, pharmacogenetic, and exploratory efficacy assessments.
PK sampling times were preinfusion, end of infusion, and 1, 4, 8, 12, and 24 hours postinfusion. Preinfusion blood samples and all samples immediately following the end of infusion through 8 hours postinfusion were collected within 15 minutes of the scheduled time. Analyses were performed at month 6 (week 26) and yearly thereafter (weeks 52, 104, 156, 208, 260, and 312). For participants who switched to 20 mg/kg after receiving the 5 or 10 mg/kg dose, assessments were performed after the first 20 mg/kg administration (reported here as rebaseline [20 mg/kg]), at month 6, and yearly thereafter. The following were calculated using noncompartmental methods: maximum plasma concentration observed, area under the plasma concentration–time curve from time zero to last measurable concentration, area under the plasma concentration–time curve, terminal elimination half-life (t1/2z), total body clearance from plasma at steady state (CLss), and steady state volume of distribution (Vss). Plasma samples were analyzed using validated, sensitive, and specific bioanalytical methods, namely, fluorometric assay using a 4-methylumbelliferyl-α-d-glucoside substrate to detect avalglucosidase alfa activity with a lower limit of quantitation (LLOQ) of 0.0125 or 0.0120 μg/mL.
Skeletal muscle MRI was performed every 2 years. In NEO1, glycogen content was measured in skeletal muscle biopsies at baseline and week 27 in all participants. In NEO-EXT, biopsies were sampled only in participants with muscle glycogen content ≥5% or significant clinical decline. MRI and biopsy data will be published separately. Morning-sampled fasting urinary hexose tetrasaccharide (Hex4) was assessed every 2 weeks in NEO1 and 6 monthly in NEO-EXT. Exploratory plasma biomarkers, creatine kinase (CK), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), were assessed every 2 weeks in NEO1 and monthly in NEO-EXT for 3 years and thereafter quarterly.
Exploratory efficacy end points included a 6-minute walk test (6MWT) and pulmonary function (FVC, maximum inspiratory pressure [MIP], and maximum expiratory pressure [MEP]). Testing was conducted every 6 months according to American Thoracic/European Respiratory Society guidelines.12,13
Statistical Analysis
Baseline was set at NEO1 enrollment, and the interim data cutoff was February 27, 2020. Because of sequential enrollment, participants had up to 6.5 years (range, 16–340 weeks) of data for avalglucosidase alfa treatment (mean ± SD, Naive: 221 ± 137 weeks; Switch: 242 ± 125 weeks).
For efficacy outcomes, observed measurements and changes from baseline were calculated and summarized using summary statistics. Upright FVC %predicted was calculated according to spirometric prediction equations.14 MIP %predicted was calculated as 100 × (actual MIP)/(120 − [0.41 × age]) for males and 100 × (actual MIP)/(108 − [0.61 × age]) for females. MEP %predicted was calculated as 100 × (actual MEP)/(174 − [0.83 × age]) for males and 100 × (actual MEP)/(131 − [0.86 × age]) for females. 6MWT %predicted was calculated using reference values.15
In a post hoc analysis, baseline functional status was compared in participants aged <45 and ≥45 years at baseline; these subgroups were selected for consistency with age group analyses from COMET (NCT02782741).16 In a post hoc analysis, plots of individual participant trajectories for FVC %predicted and 6MWT %predicted were overlaid with results from a repeated mixed-measures model showing a summary trend over time. This analysis was restricted to participants ever on 20 mg/kg dosing during the study and included time points on all dosing regimens (i.e., time points for participants who received 5 or 10 mg/kg avalglucosidase alfa at the start of the study, before starting 20 mg/kg, were also included). Importantly, the lower doses may have influenced the course of outcome measures. Analyses were performed separately for Naive (n = 7) and Switch (n = 12) participants. Biomarker data were summarized using summary statistics, with mean ± SD over time plotted for individual treatment groups within Naive and Switch Groups.
Data Availability
Qualified researchers may request access to participant-level data and study-related documents including the clinical study report, blank case report form, and data set specifications. Participant-level data are anonymized, and study documents redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at clinicalstudydatarequest.com/. The study protocol and statistical analysis plan are available in eSAP 1 and eSAP 2 (links.lww.com/WNL/C77), respectively.
Results
Participants
The first participant enrolled into NEO1 on August 19, 2013, and into NEO-EXT on February 27, 2014. Of the 24 participants enrolled into NEO1, 21 completed NEO1, and 19 entered NEO-EXT. Two discontinued NEO-EXT for personal reasons, and at data cutoff (February 27, 2020), 17 remained in NEO-EXT (Figure 1), with data for up to 6.5 years of avalglucosidase alfa treatment due to sequential enrollment.
Figure 1. Participant Disposition.
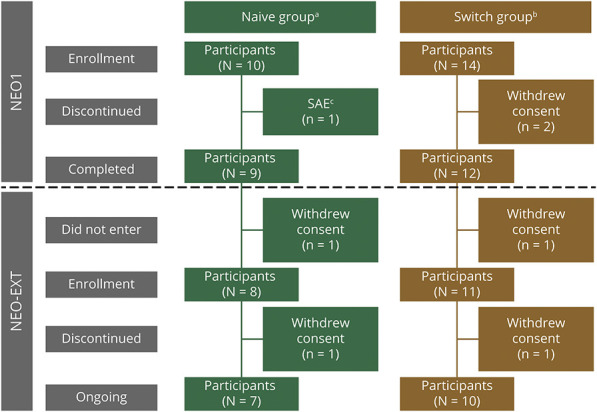
aNaive to alglucosidase alfa therapy. bPrior alglucosidase alfa therapy for ≥9 months. cSerious adverse events (SAEs) of respiratory distress and chest discomfort occurring during the ninth avalglucosidase alfa infusion; these SAEs were considered to be infusion-associated reactions. In NEO1, participants received 5, 10, or 20 mg/kg of avalglucosidase alfa every other week (qow) for 6 months. On entering NEO-EXT, they continued their current assigned NEO1 dose of avalglucosidase alfa for 104–156 weeks prior to all participants proceeding to receive 20 mg/kg qow of avalglucosidase alfa.
In the 10 mg/kg Naive Group, 2 participants became pregnant on study. One received treatment from baseline until week 27, continued follow-up without treatment, and discontinued at week 78. The other participant received treatment from baseline until week 182, stopped during pregnancy, and resumed at week 221; she continued follow-up throughout the study, including laboratory testing.
Participant's demographic and baseline characteristics were previously reported.10 In brief, age at study enrollment (mean ± SD) was 44.8 ± 20.3 years for Naive and 46.7 ± 14.1 years for Switch participants (overall range, 19.8–78.3 years). Of the 24 participants, 12 (50%) were male (Naive: 3 [30%]; Switch: 9 [64%]). The majority were White (Naive: 8 [80%]; Switch: 13 [93%]). The baseline body mass index (mean ± SD) was 22.3 ± 3.2 and 24.6 ± 3.7 for Naive and Switch participants, respectively (overall range, 17.0–31.0). Baseline mean ± SD (range) for 6MWT distance was 449 ± 118 (208–593) m for Naive and 440 ± 141 (201–657) m for Switch participants, and upright FVC %predicted was 69.2% ± 19.3% (51%–107%) for Naive and 77.3% ± 16.4% (51%–116%) for Switch participants.
Baseline functional status was better in participants aged <45 years (n = 13) compared with those aged ≥45 years (n = 11), regardless of the Naive or Switch Group. For Naive participants, aged <45 and ≥45 years, the baseline mean ± SD 6MWT distance was 511 ± 69 and 356 ± 122 m, respectively, and for Switch participants, it was 516 ± 113 and 364 ± 130 m, respectively. 6MWT %predicted was 71.6% ± 9.9% and 56.2% ± 19.3% for Naive and 70.3% ± 13.4% and 54.2% ± 18.5% for Switch participants aged <45 years and ≥45 years, respectively. Upright FVC %predicted was 74.8% ± 21.4% and 60.9% ± 14.1% for Naive participants aged <45 and ≥45 years, respectively, and 79.6% ± 19.9% and 75.0% ± 13.4% for Switch participants, aged <45 and ≥45 years, respectively.
Safety
Study Drug Exposure
After up to 6.5 years, participants had been exposed during NEO1 and NEO-EXT to 2,685 avalglucosidase alfa infusions (Naive: 1,043; Switch: 1,642). The median (range) duration of avalglucosidase alfa exposure was 293.3 (17–329) weeks (67.5 [3.9–75.7] months) for Naive and 304.4 (16–340) weeks (70.0 [3.7–78.2] months) for Switch participants. The median (range) number of infusions/participants was 137.5 (9–162) for Naive and 151.0 (8–164) for Switch participants. All participants had ≥80% drug compliance.
Adverse Events
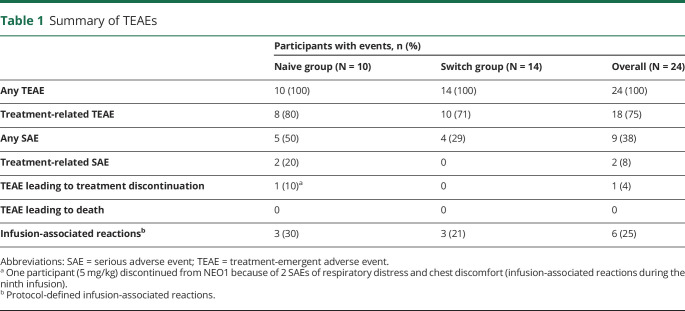
Overall, all 24 (100%) participants had ≥1 TEAE (Table 1). In total, 960 TEAEs were reported, with generally mild (563/960; 58.6%) TEAEs across all doses. Most TEAEs (91.6%) were considered unrelated to study drug. Overall, 18 participants experienced 81 TEAEs considered study drug related, with most events experienced by only 1 participant (eTable 1, links.lww.com/WNL/C77). Most frequently reported treatment-related TEAEs were fatigue, headache, nausea, and rash (3 participants each) and dizziness, dyspnea, erythema, hypertension, myalgia, muscle spasms, and pruritus (2 participants each). No deaths/life-threatening treatment-related serious adverse events (SAEs) occurred during avalglucosidase alfa treatment.
Table 1.
Summary of TEAEs
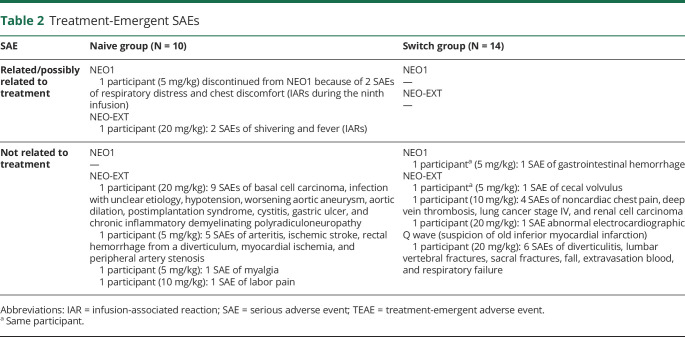
SAEs are shown in Table 2. During NEO1 and NEO-EXT, 2 participants had SAEs related or possibly treatment related. As previously reported,10 1 Naive participant receiving 5 mg/kg discontinued NEO1 for study drug-related SAEs of respiratory distress and chest discomfort; these were considered IARs, occurred during the ninth infusion (ADA titer: 1,600; peak titer: 3,200) and were not considered life threatening. In NEO-EXT, an 83-year-old participant, originally in the NEO1 20 mg/kg Naive Group who underwent surgery for worsening of aortic aneurysm, presented at week 169 with IARs of fever and chills during subsequent avalglucosidase alfa infusion that were characterized as SAEs (ADA titer at the time of SAE: 12,800; peak titer during study: 51,800, last available titer 3,200 [range, 100–51,800]). The same participant had 9 SAEs unrelated to treatment, including basal cell carcinoma, infection with unclear etiology, hypotension, worsening aortic aneurysm, aortic dilation, postimplantation syndrome, cystitis, gastric ulcer, and chronic inflammatory demyelinating polyradiculoneuropathy (because the participant was critically ill, FVC and 6MWT were not conducted between weeks 182 and 234).
Table 2.
Treatment-Emergent SAEs
Efficacy
Respiratory Function
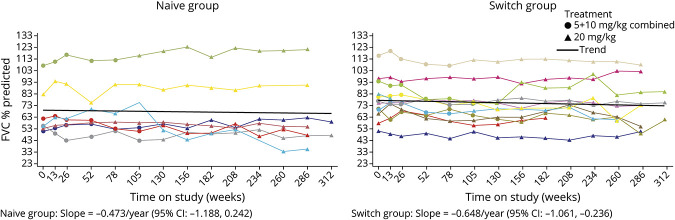
Changes in respiratory parameters from baseline up to 312 weeks' (6 years') avalglucosidase alfa treatment are shown in eTable 2 (links.lww.com/WNL/C77). Upright FVC %predicted remained stable in most Naive and Switch participants over up to 6.0 years' avalglucosidase alfa (Figure 2). Slope estimates (95% CI) for trends in upright FVC %predicted after up to 6 years of avalglucosidase alfa were −0.473 per year (−1.188 to 0.242) for Naive and −0.648 per year (−1.061 to −0.236) for Switch participants (Figure 2). MIP and MEP %predicted were more variable among participants, but overall remained stable, with slope estimates (95% CI) for MIP %predicted of 0.151 per year (−1.041 to 1.344) for Naive and −0.627 per year (−1.556 to 0.301) for Switch participants and for MEP %predicted of 0.726 per year (−0.494 to 1.946) for Naive and 0.949 per year (−0.273 to 2.170) for Switch participants.
Figure 2. Upright FVC %Predicted Over Up to 6 Years of Avalglucosidase Alfa Treatment (All Participants Ever Received 20 mg/kg Dose).
Black lines are trend lines for the slope, derived from a linear mixed-effects model. Individual participant trajectories are color coded to enable the FVC %predicted (Figure 2) and 6MWT distance (Figure 3) for individual participants to be compared. Naive group: 83-year-old participant (turquoise line): worsening aortic aneurysm at week 156, underwent surgery, and presented at week 169 with infusion-associated reaction of fever and shivering during subsequent avalglucosidase alfa infusion (ADA: 12,800; peak titer 51,800; NAb enzyme uptake positive); week 208: chronic inflammatory demyelinating polyneuropathy (ADA: 6,400; NAb enzyme uptake positive; last available sample ADA: 6,400; NAb negative). Naive group: 43-year-old participant (red line): week 208 exanthema and swelling at the infusion site (ADA: 100; peak titer 1,600; NAb enzyme uptake positive; last available sample ADA: 200; NAb negative). Switch group: 68-year-old participant (yellow line): medical history of left upper lobectomy for upper left lobe lung cancer and developed right upper lobe lung cancer, rib cage pain, and right renal cell carcinoma (ADA: negative; peak titer 200; NAb negative). Switch group: 39-year-old participant (olive green line): medical history of anxiety, car accident at week 168, events of sinus infection, influenza, and cold until last available visit (ADA: negative; peak titer <100; NAb negative). Switch group: 49-year-old participant (lime green line): medical history of depression, seasonal allergies, asthma, pain, fatigue, arthritis, and muscle soreness (ADA: negative; peak titer 1,600; NAb negative); recovery of FVC may be due to the change to 20 mg/kg dose from initial 5 mg/kg dose. 6MWT = 6-minute walk test; ADA = antidrug antibody; FVC = forced vital capacity; NAb = neutralizing antibody.
Motor Function
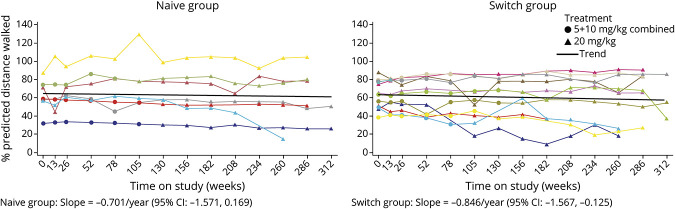
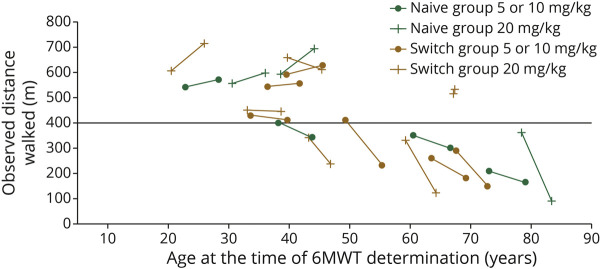
Change in 6MWT distance from baseline up to 312 weeks' (6 years') avalglucosidase alfa is shown in eTable 2 (links.lww.com/WNL/C77). 6MWT %predicted remained stable among most Naive and Switch participants over up to 6 years' avalglucosidase alfa (Figure 3). 6MWT %predicted slope estimates (95% CI) were −0.701 per year (−1.571 to 0.169) for Naive and −0.846 per year (−1.567 to −0.125) for Switch participants (Figure 3). In both Naive and Switch Groups, the 6MWT distance improved in most participants aged <45 years at the start of NEO1 and ever received 20 mg/kg avalglucosidase alfa (Figure 4).
Figure 3. 6MWT Distance %Predicted After Up to 6 Years of Avalglucosidase Alfa Treatment (All Participants Ever Received 20 mg/kg Dose).
Black lines are trend lines for the slope, derived from a linear mixed-effects model. Individual participant trajectories are color coded to enable the FVC %predicted (Figure 2) and 6MWT distance (Figure 3) for individual participants to be compared. Naive group: 83-year-old participant (turquoise line): worsening aortic aneurysm at week 156, underwent surgery, and presented at week 169 with infusion-associated reaction of fever and shivering during subsequent avalglucosidase alfa infusion (ADA: 12,800; peak titer 51,800; NAb enzyme uptake positive); week 208: chronic inflammatory demyelinating polyneuropathy (ADA: 6,400; NAb enzyme uptake positive; last available sample ADA: 6,400; NAb negative). Naive group: 43-year-old participant (red line): week 208 exanthema and swelling at the infusion site (ADA: 100; peak titer 1,600; NAb enzyme uptake positive; last available sample ADA: 200; NAb negative). Switch group: 68-year-old participant (yellow line): medical history of left upper lobectomy for upper left lobe lung cancer and developed right upper lobe lung cancer, rib cage pain, and right renal cell carcinoma (ADA: negative; peak titer 200; NAb negative). Switch group: 64-year-old participant (royal blue line): history of gout, Bell palsy, episodes of acute diverticulosis and Clostridium difficile, and chronic hip/back pain (ADA: negative; NAb negative). Switch group: 73-year-old participant (turquoise line): history of degenerative disc disease, back pain, and arthritis (ADA: 6,400; peak titer 12,800; NAb negative; last available sample ADA: 1,600; Nab negative). 6MWT = 6-minute walk test; ADA = antidrug antibody; FVC = forced vital capacity; NAb = neutralizing antibody.
Figure 4. 6MWT Distance by Age at First (Baseline) and Last Assessment (Up to Week 312) for Individual Participants Who Ever Received Avalglucosidase Alfa 20 mg/kg Dose.
6MWT = 6-minute walk test.
Immunogenicity
ADAs developed in 18 of 24 participants. The median peak titer was 1,600 (range, 100–51,200). Nine participants developed peak ADA titers ranging from 1,600 to 6,400, 7 ranging from 100 to 800, and 2 had peak titers at 12,800 and 51,200, respectively. ADA titers decreased in 6 participants, and 2 participants tolerized. Both participants with peak titers ≥12,800 had decreasing ADA titers over time and last available titers at 3,200.
Participants who seroconverted and subsequently had at least 2 consecutive samples testing negative were considered to have become immunologically nonresponsive and were classified as having tolerized. Five Naive and 2 Switch participants tested positive for NAb at intermittent time points without an apparent effect on clinical outcomes. Among the 5 Naive participants, ADA titers ranged between 100 and 3,200 in 4 participants and between 100 and 51,200 in 1 participant; last available titers were decreased (range, 200–3,200). The 2 Switch participants had low titer ranges (100–400) and last available titers. Six participants tested positive for enzyme uptake-inhibitory antibodies, among whom 3 also tested positive for enzyme activity inhibition. Three participants were positive for uptake inhibition alone, and 1 was positive for catalytic inhibition alone. In both groups, no participant among those tested developed IgE antibodies (IgE was tested only in participants who presented with IARs suggestive of hypersensitivity reactions). IARs and contemporaneous ADA titers after up to 6.5 years' avalglucosidase alfa are shown in eTable 3 (links.lww.com/WNL/C77).
Biomarkers
Pharmacodynamic Pompe disease biomarkers demonstrate avalglucosidase alfa's ability to reduce the burden of glycogen accumulation (Hex4) and muscle damage (CK), which is maintained in NEO-EXT participants up to 6 years. Mean ± SD Hex4 and CK from baseline up to 312 weeks' (6 years') avalglucosidase alfa is shown in eFigures 1 and 2 (links.lww.com/WNL/C77), respectively.
Mean ± SD ALT and AST from baseline up to 312 weeks' avalglucosidase alfa is shown in eFigures 3 and 4 (links.lww.com/WNL/C77), respectively. Overall, in both Naive and Switch Groups, ALT and AST decreased from baseline to last on-treatment measurement. Changes (mean ± SD) from baseline in ALT at last on-treatment measurement were −29.4 ± 30.8 and −14.8 ± 11.6 IU/L for the Naive (n = 10) and Switch (n = 14) Groups, respectively, and for AST, they were −36.0 ± 40.0 and −14.0 ± 11.0 IU/L, respectively.
Pharmacokinetics
The PK population included 18 participants. At week 26, rebaseline (20 mg/kg), and week 208, approximately 70% of participants had predose concentrations below the LLOQ of 0.0125 μg/mL, whereas the others had predose concentrations slightly above the LLOQ.
Mean ± SD concentrations of avalglucosidase alfa at week 208 for Naive and Switch Groups after 20 mg/kg are shown in eFigure 5 (links.lww.com/WNL/C77). At 20 mg/kg, participants previously treated with alglucosidase alfa (Switch Group) exhibited similar avalglucosidase alfa PK to that in the Naive Group, whatever the dose. Consequently, only PK parameters are presented at week 26, rebaseline (20 mg/kg), and week 208 on the pooled groups (eTable 4).
Avalglucosidase alfa exposures increased with no major deviation in dose proportionality between 5 and 20 mg/kg. No accumulation was observed following qow dosing and avalglucosidase alfa PK parameters appeared similar at week 26, rebaseline (20 mg/kg), and week 208, indicating time-independent PK. After avalglucosidase alfa 20 mg/kg qow, mean ranges were 1.25–1.47 hours for t1/2z, 840–1,140 mL/h for CLss, and 4.5–6.0 L for Vss.
Among the 19 participants receiving the 20 mg/kg dose, 15 had normal renal function, and 4 had mild impairment. Based on the estimated glomerular filtration rate (eGFR; Modification of Diet in Renal Disease study formula17), no participant had moderate or severe renal impairment as defined by eGFR <60 mL/min, and no meaningful difference in exposure was observed between participants with mild renal impairment and normal renal function.
Classification of Evidence
This study provides Class IV evidence of long-term tolerability and sustained efficacy of avalglucosidase alfa in patients with LOPD after up to 6.5 years.
Discussion
The availability of ERT has remarkably extended survival of patients with classical infantile-onset Pompe disease18,19 and improved survival,3 quality of life, and participation in daily life7 for patients with LOPD. Alglucosidase alfa's long-term benefits established it as the standard of care for Pompe disease. However, an unmet need for improvement in muscle and respiratory function still remains for patients receiving alglucosidase alfa.8,9 Disease progression while receiving alglucosidase alfa is partly attributed to suboptimal uptake of ERT into skeletal muscle.20,21 To maximize ERT benefit for patients with Pompe disease, avalglucosidase alfa was designed to target M6P-mediated uptake because it is anticipated that conjugating M6P moieties can enhance cellular uptake by skeletal muscle, resulting in improved glycogen clearance and outcomes.21
LOPD natural history is defined by progressive deterioration in the strength and function of skeletal muscles, including respiratory muscles.22 In a meta-analysis of untreated patients, FVC %predicted declined on average by 2.3% after 12 months and by 6.2% after 4 years' follow-up, whereas the 6MWT distance remained relatively stable or very gradually declined.23 After a few months' alglucosidase alfa treatment, patients' FVC and 6MWT distance improved rapidly and gradually stabilized or returned to baseline at ∼2–3 years; thereafter, both parameters gradually declined.23 In the recent multicenter STIG study,9 sitting FVC %predicted data were available for 57 participants at baseline who had received ERT for ≥3 years before study inclusion. At 1-year follow-up, participants remained stable with a 2% increase in FVC %predicted; however, this was followed by a secondary decline in the following years and a significant decline over 10 years. For most participants (83.5%), the authors attributed progressive decline while on ERT to baseline disease severity.9 In a longitudinal data analysis from patients with LOPD enrolled in the Pompe Registry, the effect of early treatment initiation and long-term ERT in real-world settings was associated with long-term preservation of FVC, with better respiratory function at the time of treatment initiation.24 Because Pompe disease is a progressive disorder and it is known from experience with alglucosidase alfa that functional ability declines over time, maintaining patients long term on a high functional level is meaningful because it delays the need for walking and ventilatory support.
Stabilization was observed in exploratory respiratory and motor function outcomes in most Naive and Switch participants, indicating a sustained benefit contrasting with Pompe disease's natural history. In a recent meta-analysis, longitudinal changes in FVC were positively associated with changes in LOPD measures and outcomes across multiple domains, including the 6MWT and 36-item Short-Form Survey–Physical Component Score.25 Change in therapeutic landscape of Pompe disease with ERT availability, as well as improved diagnostic awareness, is reflected in the enrolled participants with a median (range) age at diagnosis of 36.4 (15.8–78.2) and 34.2 (3.4–62.9) years for the Naive and Switch Groups, respectively, with 3 participants aged >60 years per group. Functional respiratory and motor status, measured by FVC %predicted and 6MWT distance, at study start was reflective of newly diagnosed participants in the Naive Group and participants who had received alglucosidase alfa for 0.9–7.9 years before switching to avalglucosidase alfa, including participants with normal FVC and 6MWT.10 Although room for short-term improvement of outcome measures in these otherwise clinically symptomatic patients is probably limited, the effect on long-term functional maintenance with treatment before impairment of function occurs is a desirable treatment goal because it allows patients to maintain a high functional level and delays the need for respiratory or walking support. Data from NEO-EXT, an open-label treatment extension study enrolling participants who completed NEO1,10 support evidence of the long-term effects of avalglucosidase alfa, with evaluable efficacy data available for up to 6.0 years' avalglucosidase alfa because of sequential enrollment.
FVC %predicted and 6MWT distance were maintained in participants during long-term treatment with avalglucosidase alfa 20 mg/kg qow for up to ∼6.0 years, as they were for participants randomized to avalglucosidase alfa 5 or 10 mg/kg qow in NEO1 and whose dosage increased to 20 mg/kg qow during NEO-EXT. This was more pronounced among Naive and less consistent among Switch participants. Because there was no placebo, an expectation for untreated participants would have been that their FVC would have declined more significantly.22 Participants aged <45 years at enrollment tended to show individual improvements in 6MWT distance from baseline to up to 6.0 years' avalglucosidase alfa; many aged ≥45 years showed not only lower baseline 6MWT values but also individual declines in 6MWT distance over this period. This effect likely reflects the interaction of age-related physiologic muscle wasting (sarcopenia) with LOPD and possibly other comorbidities affecting mobility or exercise tolerance in older patients.
NEO-EXT data show a tendency for improvement of elevated baseline Hex4 and CK over time in most participants, indicating stabilization of pathologic process in muscles. Although a dose response in Hex4 was not observed, likely because of heterogeneous baseline levels and small numbers of participants in each group, a continued response in Hex4 was observed throughout the study in the overall Naive and Switch Groups, with continued decreases observed following a dose increase to 20 mg/kg among participants initially receiving lower doses. The peak in Hex4 observed at ∼234 weeks for the only participant with data in the 10 mg/kg Naive Group at this time may be reflective of disease reoccurrence; the participant had temporarily discontinued treatment after week 182 while pregnant and resumed treatment at week 221.
Pharmacodynamic data show maintained long-term efficacy of avalglucosidase alfa in participants with Pompe disease and have been presented separately.26 In regard to safety and tolerability, the data reflect positive benefit-risk and no new safety signal after up to 6.5 years' avalglucosidase alfa. There was good tolerance to avalglucosidase alfa over time, with no deaths and only 1 treatment discontinuation due to a treatment-related SAE, which occurred early in NEO1, after the participant's ninth infusion. The majority of TEAEs and treatment-emergent SAEs were reflective of the underlying disease and other associated comorbidities due to participants' age. During the study, anti–avalglucosidase alfa IgG/IgM antibodies developed in the initial months of treatment with decreasing titers and tolerization over time. Most participants developed low or moderate ADA titers <12,800, and no clear effect of ADA development on safety or efficacy could be evidenced. In addition, the fact that in the transaminases, AST and ALT levels decreased over time is a positive signal for the absence of apparent drug-induced liver toxicity.
Participants previously treated with alglucosidase alfa showed similar avalglucosidase alfa PK compared with treatment-naive participants. Avalglucosidase alfa PK parameters appeared similar over time, indicating a time-independent PK and no apparent accumulative effect of qow dosing. PK findings in NEO-EXT confirm those in NEO1.10 No participant had moderate or severe renal impairment, and no meaningful difference in exposure was observed between the 4 participants with mild renal impairment and the 15 with normal renal function.
Safety data from the 49-week, primary analysis period of COMET support the NEO-EXT findings that avalglucosidase alfa was generally well tolerated in participants with LOPD.16 COMET also provided evidence of clinically meaningful improvement with avalglucosidase alfa vs alglucosidase alfa in respiratory function, ambulation, and functional endurance.
The study population was relatively small, with 24 participants enrolled into NEO1, 21 completing NEO1, 19 entering NEO-EXT, and at the interim data cut, 17 remained in NEO-EXT with data up to 6.5 years from treatment start. The study had no comparator arm because participants initially enrolled in the phase 1 NEO1 study and all received avalglucosidase alfa. During NEO1 and at NEO-EXT start, participants were treated with 5, 10, or 20 mg/kg qow avalglucosidase alfa, the use of the lower doses in NEO1 and at NEO-EXT start may have influenced outcomes. Participants' wide age range at NEO1 baseline (19.8–78.3 years) may have created some variability in observed outcomes. At NEO1 baseline, participant upright FVC %predicted ranged from 51% to 116%, indicating that they did not have severe respiratory impairment, and room for short-term improvement on outcome measures could be limited. However, because Pompe disease is a progressive disorder and it is known from experience with alglucosidase alfa that functional ability declines over time, maintaining patients long term at a high functional level is meaningful because it delays the need for respiratory and walking support.
NEO1 and NEO-EXT results provide evidence of long-term and overall maintained effect of avalglucosidase alfa on measures of respiratory function, endurance, and walking ability as well as pharmacodynamic data in participants with LOPD. Avalglucosidase alfa's safety profile during NEO-EXT is consistent with the first 6 months' treatment in NEO1.10 No deaths/treatment-related life-threatening SAEs were reported. Anti–avalglucosidase alfa IgG/IgM antibodies developed in the initial months of treatment with decreasing titers and tolerization over time.
Acknowledgment
The authors thank the participants and their families for their participation in the NEO1 and NEO-EXT clinical trials. The authors acknowledge editorial assistance from Jane M. Gilbert, BSc, CMPP, of Elevate Medical Affairs, contracted by Sanofi for publication support services. The authors exerted sole scientific control, were responsible for all content and editorial decisions, and received no honoraria related to the development of this article.
Glossary
- 6MWT
6-minute walk test
- ADA
antidrug antibody
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CK
creatine kinase
- CLss
total body clearance from plasma at steady state
- CV
coefficient of variation
- eGFR
estimated glomerular filtration rate
- ERT
enzyme replacement therapy
- FVC
forced vital capacity
- GAA
acid α-glucosidase
- Hex4
hexose tetrasaccharide
- IAR
infusion-associated reaction
- Ig
immunoglobulin
- LLOQ
lower limit of quantification
- LOPD
late-onset Pompe disease
- MEP
maximum expiratory pressure
- M6P
mannose-6-phosphate
- MIP
maximum inspiratory pressure
- NAb
neutralizing antibody
- PK
pharmacokinetic
- qow
every 2 weeks
- SAE
serious adverse event
- t1/2z
terminal elimination half-life
- TEAE
treatment-emergent adverse event
- Vss
steady state volume of distribution
Appendix. Authors
Appendix 2. Coinvestigators

Footnotes
Class of Evidence: NPub.org/coe
Editorial, page 183
Study Funding
This study was funded by Sanofi.
Disclosure
M.M. Dimachkie serves or recently served as a consultant—Amazentis, argenx, Catalyst, Cello, Covance/Labcorp, CSL-Behring, EcoR1, Janssen, Kezar, Momenta, NuFactor, Octapharma, RaPharma/UCB, Roivant Sciences Inc., RMS Medical, Sanofi Genzyme, Shire Takeda, Scholar Rock, Spark Therapeutics, Third Rock, and UCB Biopharma—and received research grants or contracts or educational grants from Alexion, Alnylam Pharmaceuticals, Amicus, BioMarin, Bristol-Myers Squibb, Catalyst, Corbus, CSL-Behring, FDA/OOPD, GlaxoSmithKline, Genentech, Grifols, Kezar, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Octapharma, Orphazyme, Ra Pharma/UCB, Sanofi Genzyme, Sarepta Therapeutics, Shire Takeda, Spark Therapeutics, UCB Biopharma/RaPharma, Viromed/Helixmith, and TMA. R.J. Barohn: consulting fees—NuFactor and Momenta Pharmaceutical; contracted research—FDA Office of Orphan Products Development, NIH, Orphazyme, PCORI, PTC Therapeutics, Ra Pharma, and Sanofi Genzyme. B. Byrne: advisory board—Sanofi Genzyme. O. Goker-Alpan: advisory board—Amicus, BioMarin, Sanofi Genzyme, and Takeda; consulting fees—Amicus, BioMarin, Sanofi Genzyme, Shire HGT, and Takeda; contracted research—Amicus, Freeline, Genentech, Protalix, Sangamo, Sanofi Genzyme, and Takeda; and speaker's bureau—Sanofi Genzyme and Takeda. P.S. Kishnani: advisory board—Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme, Amicus Therapeutics, and Baebies; consulting fees—Sanofi Genzyme, Amicus Therapeutics, Maze Therapeutics, JCR Pharma, and Asklepios BioPharmaceutical, Inc. (AskBio); contracted research—Sanofi Genzyme, Valerion Therapeutics, and Amicus Therapeutics; honoraria—Sanofi Genzyme, Maze Therapeutics, Asklepios Biopharmaceutical, Inc. (AskBio), and Amicus Therapeutics; ownership interest less than 5% (stocks, stock options, or other ownership interest excluding diversified mutual funds)—Asklepios Biopharmaceutical, Inc. (AskBio) and Maze Therapeutics; and travel expenses—Sanofi Genzyme and Amicus Therapeutics. S. Ladha: advisory board—Sanofi Genzyme; consulting fees—Sanofi Genzyme; contracted research—Sanofi Genzyme; and speaker's bureau—Sanofi Genzyme. P. Laforêt: advisory board—Sanofi Genzyme and Spark Therapeutics; consulting fees—Sanofi Genzyme; contracted research—Sanofi Genzyme; honoraria—BioMarin, Sanofi Genzyme, and Spark Therapeutics; and travel expenses—Sanofi Genzyme, Amicus Therapeutics, and Spark Therapeutics. K.E. Mengel: advisory board—Actelion, Sanofi Genzyme, Takeda Shire, and Amicus; consulting fees—Orphazyme and Prevail; and contracted research—Orphazyme, Sanofi Genzyme, Shire, Idorsia, and Takeda Shire. L.D.M Peña: advisory board—AveXis Inc; consulting fees—AveXis Inc.; contracted research—Shire Takeda, Chiesi, and ModernaTx; Fees for Non-CME/CE Services Received Directly from a Commercial Interest or their Agents—Castle IRB; speaker's bureau—France Foundation; and travel expenses—France Foundation. S. Sacconi: advisory board—Alnylam Pharmaceuticals, Biogen, BioMarin, and Sanofi Genzyme; consulting fees—Alnylam Pharmaceuticals, Biogen, BioMarin, Sanofi Genzyme, AEC partners, BresMed, Sanofi-Aventis France, and argenx BV; contracted research—AFM; and travel expenses—Biogen, BioMarin, and Sanofi Genzyme. V. Straub: consulting fees—AveXis Inc, Exonics Therapeutics, Roche, Sanofi Genzyme, and Sarepta Therapeutics; contracted research—Sanofi Genzyme and Ultragenyx; and honoraria—Sanofi Genzyme. J. Trivedi: contracted research—Sanofi Genzyme. P. Van Damme: advisory board—Biogen, Alexion Pharmaceuticals, Ferrer, QurAlis, and argenx. A.T. van der Ploeg: advisory board—Amicus, BioMarin, Sanofi Genzyme, and Spark Therapeutics; consulting fees—Amicus, BioMarin, Sanofi Genzyme, and Spark Therapeutics; and contracted research—Amicus, BioMarin, Sanofi Genzyme, and Spark Therapeutics. J. Vissing: advisory board—argenx, Roche, Viela Bio, Biogen, Amicus Therapeutics, PTC Therapeutics, Sanofi Genzyme, Ultragenyx Pharmaceutical, Fulcrum Therapeutics, ML Bio Solutions, Sarepta Therapeutics, Novartis Pharma AG, Stealth Biotherapeutics, Zogenix, Regeneron, and Lupin Limited; consulting fees—argenx, Roche, Viela Bio, Biogen, Amicus Therapeutics, PTC Therapeutics, Sanofi Genzyme, Ultragenyx Pharmaceutical, Fulcrum Therapeutics, ML Bio Solutions, Sarepta Therapeutics, Novartis Pharma AG, Stealth Biotherapeutics, Zogenix, Regeneron, and Lupin Limited; contracted research—argenx, Amicus Therapeutics, PTC Therapeutics, Sanofi Genzyme, Novartis Pharma AG, Stealth Biotherapeutics, UCB Pharma, and Genethon; employee/salary—Edgewise; and travel expenses—argenx, Roche, Viela Bio, Biogen, Sanofi Genzyme, ML Bio Solutions, and Sarepta Therapeutics. P. Young: advisory board—Biogen, BioMarin, Medice, Sanofi Genzyme, and Vanda; honoraria—Biogen, BioMarin, Löwenstein Medical, Medice, Sanofi Genzyme, UCB, and Vanda; and speaker's bureau—Biogen, BioMarin, Löwenstein Medical, Medice, Sanofi Genzyme, UCB, and Vanda. K. An Haack: employee/salary—Sanofi Genzyme (spouse: employee/salary—Sanofi Genzyme). M. Foster: employee/salary—Sanofi Genzyme; ownership interest less than 5% (stocks, stock options, or other ownership interest excluding diversified mutual funds)—Sanofi Genzyme. J.M. Gilbert: employee/salary—Elevate Medical Affairs (contracted by Sanofi Genzyme for publication support services). P. Miossec: employee/salary—Sanofi Genzyme; ownership interest less than 5% (stocks, stock options, or other ownership interest excluding diversified mutual funds)—Sanofi Genzyme. O. Vitse: employee/salary—Sanofi Genzyme; ownership interest less than 5% (stocks, stock options, or other ownership interest excluding diversified mutual funds)—Sanofi Genzyme. T. Zhou: employee/salary—Sanofi Genzyme; ownership interest less than 5% (stocks, stock options, or other ownership interest excluding diversified mutual funds)—Sanofi Genzyme. B. Schoser: advisory board—Amicus, Alexion, Audentes, Dyne, Lupin, Sanofi Genzyme, and UCB; contracted research—Amicus and Sanofi Genzyme; honoraria—Kedrion; and travel expenses—Sanofi Genzyme. Go to Neurology.org/N for full disclosures.
References
- 1.van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet. 2008;372(9646):1342-1353. doi: 10.1016/S0140-6736(08)61555-X. [DOI] [PubMed] [Google Scholar]
- 2.Mellies U, Lofaso F. Pompe disease: a neuromuscular disease with respiratory muscle involvement. Respir Med. 2009;103(4):477-484. doi: 10.1016/j.rmed.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Güngör D, de Vries JM, Hop WC, et al. Survival and associated factors in 268 adults with Pompe disease prior to treatment with enzyme replacement therapy. Orphanet J Rare Dis. 2011;6:34. doi: 10.1186/1750-1172-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiesshoefer J, Henke C, Kabitz HJ, et al. The nature of respiratory muscle weakness in patients with late-onset Pompe disease. Neuromuscul Disord. 2019;29(8):618-627. doi: 10.1016/j.nmd.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Gaeta M, Barca E, Ruggeri P, et al. Late-onset Pompe disease (LOPD): correlations between respiratory muscles CT and MRI features and pulmonary function. Mol Genet Metab. 2013;110(3):290-296. doi: 10.1016/j.ymgme.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Lachmann R, Schoser B. The clinical relevance of outcomes used in late-onset Pompe disease: can we do better? Orphanet J Rare Dis. 2013;8:160. doi: 10.1186/1750-1172-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Güngör D, Kruijshaar ME, Plug I, et al. Quality of life and participation in daily life of adults with Pompe disease receiving enzyme replacement therapy: 10 years of international follow-up. J Inherit Metab Dis. 2016;39(2):253-260. doi: 10.1007/s10545-015-9889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harlaar L, Hogrel JY, Perniconi B, et al. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology. 2019;93(19):e1756-e1767. doi: 10.1212/WNL.0000000000008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutschmidt K, Musumeci O, Diaz-Manera J, et al. STIG study: real-world data of long-term outcomes of adults with Pompe disease under enzyme replacement therapy with alglucosidase alfa. J Neurol. 2021;268(7):2482-2492. doi: 10.1007/s00415-021-10409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pena LDM, Barohn RJ, Byrne BJ, et al. Safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy of the novel enzyme replacement therapy avalglucosidase alfa (neoGAA) in treatment-naive and alglucosidase alfa-treated patients with late-onset Pompe disease: a phase 1, open-label, multicenter, multinational, ascending dose study. Neuromuscul Disord. 2019;29(3):167-186. doi: 10.1016/j.nmd.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 12.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518-624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 14.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21(2):87-93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Manera J, Kishnani PS, Kushlaf H, et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): a phase 3, randomised, multicentre trial. Lancet Neurol. 2021;20(12):1012-1026. doi: 10.1016/S1474-4422(21)00241-6. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Chien YH, van der Ploeg A, Jones S, et al. Survival and developmental milestones among Pompe Registry patients with classic infantile-onset Pompe disease with different timing of initiation of treatment with enzyme replacement therapy. J Neuromuscul Dis. 2015;2(s1):S61-S62. [PubMed] [Google Scholar]
- 19.Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11(3):210-219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- 20.Chien YH, Hwu WL, Lee NC. Pompe disease: early diagnosis and early treatment make a difference. Pediatr Neonatol. 2013;54(4):219-227. doi: 10.1016/j.pedneo.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Jiang JL, Gumlaw NK, et al. Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol Ther. 2009;17(6):954-963. doi: 10.1038/mt.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Beek NA, Hagemans ML, Reuser AJ, et al. Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul Disord. 2009;19(2):113-117. doi: 10.1016/j.nmd.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Schoser B, Stewart A, Kanters S, et al. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis. J Neurol. 2017;264(4):621-630. doi: 10.1007/s00415-016-8219-8. [DOI] [PubMed] [Google Scholar]
- 24.Stockton DW, Kishnani P, van der Ploeg A, et al. Respiratory function during enzyme replacement therapy in late-onset Pompe disease: longitudinal course, prognostic factors, and the impact of time from diagnosis to treatment start. J Neurol. 2020;267(10):3038-3053. doi: 10.1007/s00415-020-09936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger KI, Kanters S, Jansen JP, et al. Forced vital capacity and cross-domain late-onset Pompe disease outcomes: an individual patient-level data meta-analysis. J Neurol. 2019;266(9):2312-2321. doi: 10.1007/s00415-019-09401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Díaz-Manera J, Vissing J, Carlier P, et al. NEO1/NEO-EXT studies: muscle MRI results in patients with Pompe disease after long-term avalglucosidase alfa treatment. J Neuromuscul Dis. 2021;S8:S132-S133. doi: 10.3233/JND-219006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to participant-level data and study-related documents including the clinical study report, blank case report form, and data set specifications. Participant-level data are anonymized, and study documents redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at clinicalstudydatarequest.com/. The study protocol and statistical analysis plan are available in eSAP 1 and eSAP 2 (links.lww.com/WNL/C77), respectively.