Abstract
PCDH19-related epilepsy is a developmental and epileptic encephalopathy typically presenting with epilepsy and varying degrees of intellectual disability. Seizures typically present in clusters of focal or generalized seizures, sometimes in the setting of fever. We present the case of a 7-month-old girl presenting with new-onset refractory status epilepticus that followed routine vaccine administration and ensuing cytokine storm. She was diagnosed with a pathogenic variant in PCDH19. The patient required 5 antiseizure medications and pentobarbital-induced burst suppression for control of seizures. She was noted to have elevated serum cytokine levels (interleukin [IL]-2, IL-4, IL-10, IL-13, IL-17, IL-1, IL-1β, and IL-8) and CSF cytokine levels (IL-6 and IL-13). Anakinra was initiated and titrated based on serial cytokine levels, with doses ranging from 5 to 20 mg/kg/d resulting in reduction in cytokine levels and seizure reduction. By age 14 months, she was able to be maintained on 3 active antiseizure medications and ketogenic diet for seizure control.
PCDH19-related epilepsy, also called girls clustering epilepsy, is an X-linked, female-predominant, developmental epileptic encephalopathy that presents with multiple seizure semiologies and is associated with varying degrees of intellectual disability; patients initially present with difficult-to-control seizures that are often triggered by fever and occur in clusters of multiple seizures in a day.1,2 Although clusters of seizures are frequently reported with PCDH19-related epilepsy, initial presentation as a new-onset refractory status epilepticus (NORSE) in infancy has not been well reported, and the current evolving literature regarding NORSE related to the PCDH19 gene has been investigated but not confirmed.3,4 We present a clinically challenging case of NORSE with documented cytokine storm in a female infant found to have a pathogenic variant in PCDH19 and in whom we highlight successful treatment of seizures with anakinra.
Case Report
A 7-month-old girl with no previous medical history presented to the hospital for frequent clusters of seizures for 1 day. The day before seizure onset, the child had received the third dose of the pneumococcal vaccine and the second influenza booster, without documented fever. The patient was noted to have multiple episodes of seizures consisting of behavioral arrest, cyanosis, and generalized tonic-clonic and tonic activity. In the emergency department, seizures evolved into refractory status epilepticus (SE) despite use of multiple antiseizure medications (ASMs) (Figure), necessitating pentobarbital-induced coma to achieve burst suppression twice after seizures were refractory to midazolam and ketamine infusions. She was additionally started on a 3:1 ketogenic diet due to the refractory nature of her SE, which escalated to 4:1 ketogenic ratio at the peak of her seizure frequency. Extensive initial evaluation for infectious, metabolic, and structural causes of epilepsy was unrevealing.
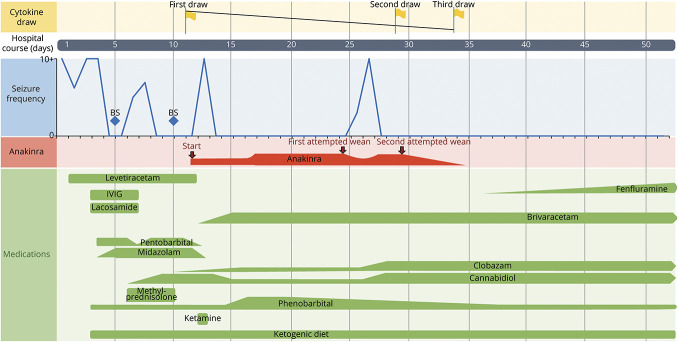
Figure. Relationship Between Immunomodulatory and Antiseizure Medications and Seizure Frequency.
Relationship of seizure frequency and medication interventions, most notably with the start and modulation of anakinra with respect to elevated cytokine levels and recurring seizures. We note the increase in seizures when anakinra was first decreased as well as downtrending cytokine levels correlating with successful reduction of seizure frequency in this patient. BS = burst suppression; IVIG = IV immunoglobulin.
Given the patient's fulminant onset of refractory SE, she was diagnosed with NORSE. Given presumed immunologic factors that lead to NORSE in general, she was treated symptomatically with IV methylprednisolone and IV immunoglobulin (IVIg). Initial serum cytokine panels revealed elevated serum interleukin (IL)-1, IL-1β, IL-2, IL-4, IL-8, IL-10, IL-13, and IL-17 levels (Table) and CSF IL-6 and IL-13 levels. Given the elevated cytokine levels, an evaluation for secondary hemophagocytic lymphohistiocytosis was performed and was negative. A rapid epilepsy gene panel revealed the pathogenic PCDH19 variant c.1211C>T, resulting in the p.T404I amino acid substitution.
Table.
Serum Cytokine Panel Results

At the peak, the patient presented with greater than 25 seizures in a day, requiring clobazam 20 mg nightly (2 mg/kg/d), brivaracetam 50 mg twice a day (10 mg/kg/d), phenobarbital 97 mg 3 times a day (30 mg/kg/d), and cannabidiol 100 mg nightly (10 mg/kg/d). Given the elevated cytokine levels, anakinra was started with dose escalation (5–20 mg/kg/d) and slowly weaned off, guided by repeat cytokine panels. She was successfully weaned off pentobarbital with subsequent cytokine panels showing continued improvement of the inflammatory markers. Following an attempt to reduce the anakinra dose, seizures recurred, and cytokine levels were rechecked, showing residual cytokine elevation notably in markers of adaptive immunity, such as IL-4, IL-5, and proinflammatory IL-17, which led to retitration of anakinra (Figure). Eventually, seizures abated, and anakinra was weaned off successfully with no recurrence of seizures. Given the similarities in presentation between our case and some patients with Dravet syndrome (DS), fenfluramine was included as part of the final antiseizure regimen in preparation for discharge using doses employed for patients with DS.5
After 52 days, the patient was discharged home on 5 ASMs: clobazam, cannabidiol, and fenfluramine and the ketogenic diet that were maintained and phenobarbital and brivaracetam that were actively being weaned. She remained seizure-free at 6-month follow-up. She has progressed from nasogastric tube feeding to oral intake of pureed feeds, has shown improved strength, and is achieving some developmental milestones, such as sitting up with support, with speech, physical, occupational, and feeding therapies in place.
Discussion
We report the case of an infant girl with PCDH19-related epilepsy who presented with NORSE 1 day after administration of routine vaccinations without concurrent febrile illness or vaccine-associated febrile response. Vaccination-induced seizures have been well documented in patients with genetically predisposed epilepsy, such as those with DS1,6 and anecdotally with PCDH19-related epilepsy.7 Although fever is reported as a trigger for seizures in girls with PCDH19-related epilepsy, as with DS, initial presentation of NORSE in a previously healthy child has not been previously reported in the context of PCDH19-related epilepsy.
Our case report demonstrates the presence of an immune-mediated process in the form of an unrelenting cytokine storm that we posit contributed to the sudden presentation and refractoriness of our patient's epilepsy presentation as NORSE. Serum cytokine panels demonstrated a robust cytokine reaction, and CSF findings demonstrated IL-6 elevation, a proinflammatory marker, that may have contributed to the severity of NORSE in this child. Given that fever and presumably associated inflammation is known to trigger seizures in patients with pathogenic variants in PCDH19, we posit that this patient's cytokine-mediated response to vaccination in the setting of PCDH19 dysfunction led to her severe NORSE presentation. Although the pathogenesis of PCDH19-related epilepsy remains unclear, case studies focused on courses of corticosteroids have demonstrated efficacy in prophylaxis and abortion of repetitive seizure clusters.8 This further underscores the possible involvement of an underlying neuroinflammatory mechanism for PCDH19 epilepsy.
Anakinra was added to this patient's regimen because of observed increased cytokine levels, resulting in elimination of seizures. Although tocilizumab has been reported as treatment for super-refractory status epilepticus (not specific to a genetic etiology), tocilizumab was not administered in the patient in this report due to its side effect profile, including immunosuppression, which would affect the vaccination schedule in this age group. A limitation of this case report is the difficulty in determining whether anakinra acutely helped to reduce seizures directly or helped slow progression of disease or whether the seizures reduced in frequency over time, as has been reported in the natural history of NORSE.9 Another potential confound is that the frequent adjustments of the patient's ASMs may also have contributed to seizure reduction and elimination. However, it appeared at one period during this patient's hospitalization that anakinra was required to be increased to a higher dose for seizure control after an attempt to taper the dose (Figure).
In summary, we add NORSE to the PCDH19 disease spectrum. Although the presence of SE per se in the setting of PCDH19 is not surprising and PCDH19 has been hypothesized as a possible cause of NORSE,4 PCDH19-related epilepsy presenting as NORSE has not been appreciated. Attention to cytokine levels in a child with NORSE led to treatment of our patient with an immunomodulatory medication that appeared to have a favorable acute response. Rapid genetic testing allowed for a precise explanation for the child's predisposition to epilepsy. We used fenfluramine, which is approved for use in patients with Dravet Syndrome, for our patient given that her seizures in the setting of a PCDH19 variant bear similarities to those seen in Dravet Syndrome.10 Future studies with more patients will be needed to determine whether the use of either of these strategies—anakinra or fenfluramine—can be generalized to all patients with PCDH19-related epilepsy.
Appendix. Authors

Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Kolc KL, Sadleir LG, Scheffer IE, et al. A systematic review and meta-analysis of 271 PCDH19-variant individuals identifies psychiatric comorbidities, and association of seizure onset and disease severity. Mol Psychiatry. 2019;24(2):241-251. doi: 10.1038/s41380-018-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith L, Singhal N, El Achkar CM, et al. PCDH19-related epilepsy is associated with a broad neurodevelopmental spectrum. Epilepsia. 2018;59(3):679-689. doi: 10.1111/epi.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Specchio N, Pietrafusa N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev Med Child Neurol. 2020;62(8):897-905. doi: 10.1111/dmcn.14553. [DOI] [PubMed] [Google Scholar]
- 4.Sculier C, Gaspard N. New onset refractory status epilepticus (NORSE). Seizure. 2019;68:72-78. doi: 10.1016/j.seizure.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Vavers E, Zvejniece L, Maurice T, Dambrova M. Allosteric modulators of sigma-1 receptor: a review. Front Pharmacol. 2019;10:223. doi: 10.3389/fphar.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntosh AM, McMahon J, Dibbens LM, et al. Effects of vaccination on onset and outcome of Dravet syndrome: a retrospective study. Lancet Neurol. 2010;9(6):592-598. doi: 10.1016/S1474-4422(10)70107-1. [DOI] [PubMed] [Google Scholar]
- 7.Verbeek NE, Jansen FE, Vermeer-de Bondt PE, et al. Etiologies for seizures around the time of vaccination. Pediatrics. 2014;134(4):658-666. doi: 10.1542/peds.2014-0690. [DOI] [PubMed] [Google Scholar]
- 8.Pracucci E, Pillai V, Lamers D, Parra R, Landi S. Neuroinflammation: a signature or a cause of epilepsy? Int J Mol Sci. 2021;22(13):6981. doi: 10.3390/ijms22136981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolc KL, Sadleir LG, Depienne C, et al. A standardized patient-centered characterization of the phenotypic spectrum of PCDH19 girls clustering epilepsy. Transl Psychiatry. 2020;10(1):127. doi: 10.1038/s41398-020-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoonjans AS, Lagae L, Ceulemans B. Low-dose fenfluramine in the treatment of neurologic disorders: experience in Dravet syndrome. Ther Adv Neurol Disord. 2015;8(6):328-338. doi: 10.1177/1756285615607726. [DOI] [PMC free article] [PubMed] [Google Scholar]