Abstract
Infectious bronchitis virus (IBV) has been frequently reported in chickens worldwide, including in the Eastern Region of Saudi Arabia (ERS). Several IBV outbreaks were recently reported in chickens despite the massive use of various vaccines. Based on partial sequencing of the S1 gene, at least three genotypes were reported (CK/CH/LDL/97I, IS/720/99, and IS/Variant2/98) in the ERS with no available homologous vaccines. Herein, we tried to evaluate the protection provided by some selected commercial-available vaccines against these three genotypes. We divided the experimental chickens into eight groups. Representative isolates from these genotypes were inoculated into three groups of broiler chickens vaccinated with the H-120 vaccine at the age of 1 day and boosted with the 4/91 vaccine at the age of 14 days (challenged groups). One group of chickens had received the same protocol of IBV vaccines but was kept without infection to serve as a vaccine control group. The three isolates were inoculated into three other similar but unvaccinated groups of broiler chickens (infected groups). Group eight chickens were neither vaccinated nor infected and used as a negative control group. Evaluation of the protection induced by the tested vaccination schedule was assessed by several criteria, including the ability to reduce the severe clinical signs caused by IBV infection, changes in the body temperature of various groups of chickens, the reduction in the magnitude of IBV-induced lesions, and the reduction in the viral loads in tracheas of a different group of chicken. Monitoring the immune status of chickens was also recorded based on the hemagglutination inhibition antibodies in sera of various groups of chickens. Our results show clinical and tracheal protection against IBV/IS/Variant2/98-like and IBV/IS/720/99-like strains. Moderate protection was observed in the IBV/CK/CH/LDL/97I-like pressure. The kidneys of the challenged groups of chickens showed minimal or no gross lesions compared with the infected groups, even in those chickens challenged with the IBV/CK/CH/LDL/97I-like strain. In conclusion, this is the first study to perform the protectotyping of some IBV strains from Saudi Arabia. It demonstrated the proficiency of the investigated vaccination schedule in control of infection of broiler chickens with IBV/IS/Variant2/98 and IBV/IS/720/99 strains. It is highly recommended to introduce the homologous IBV/CK/CH/LDL/97I-based vaccine to the vaccination protocols of chickens in the ERS to match the circulating strains and ensure better protection.
Keywords: IBV, Vaccine, Prime-boost, Antibody, Challenge
Introduction
Infectious bronchitis (IB) is an acute, highly contagious respiratory disease of chickens (Gallus gallus) that is present virtually in all regions with the intensive poultry industry. In addition to respiratory involvement, IBV may also affect the urogenitalandr alimentary tracts of chickens. It poses significant economic losses to the chicken industry by adversely affecting the body weight gain, the quality and quantity of the produced e and as acts as a predisposing factor for secondary infections [13]. IBV belongs to group III Coronaviruses of the family Coronaviridae and other viral diseases affecting several poultry spec,ies particularly turkey coronavirus (TCoV) [19–21]. It is an enveloped virus with a single-stranded positive-sense RNA genome 27.6 Kb in length. Four major structural proteins, including the Spike (S), the Envelope (E), the Membrane (M), and the Nucleocapsid (N) proteins, were encoded by the 3’ end of the viral genome [12]. Numerous IBV types were reported worldwide, and several methods were used for IBV typing. Serotyping assembles IBV strains according to their relatedness in the virus neutralization test. Genotyping groups IBV strains according to the similarity in their genome sequence. Protectotyping clusters of IBV strains according to their liableness against complete immune components induced in chickens by other IBV strains. Though not the general role, it has been reported that strains of a single genotype/serotype may gather in different protectotypes [6].
Vaccination is the most efficient approach to control IBV infection. The main factors that hampered the success of IBV vaccines and protection are many IBV types, the continuous emergence of new viral strains/variants, and the poor cross-protection among various strains of IBV [9, 10]. Theoretically, complete protection against IBV infection is usually dependent on the administration of the homologous vaccine; the protection conferred by heterologous vaccines ranges from very poor to moderate protection, depending on the assigned criteria of the protectotyping. Therefore, to avoid/minimize the IBV vaccination failure, a homologous vaccine to the circulating field strains in chickenparticularcertain region should be carefully selected [11]. Another apapplying the prime-boost vaccination regimen using two different types of IBV strains. For example, priming of chicken with Mass type vaccine and boosting with 4/91 vaccine was reported to broaden the acquired protection against heterologous IBV strains [15, 37].
IBV was repeatedly reported in poultry of Saudi Arabia and, in particular, poultry of the ERS where the circulation of several genotypes including CK/CH/LDL/97I, 4/91, Mass, IS/720/99, IS/Variant2/9,8 and QX were reported [1, 2, 4, 6, 17, 24, 25]. Our previous work showed that IBV was detected at flock level seenin 42 out of 115 (36.52%) broiler flocks with respiratory involvement [35].the At bird level, seroprevalence against IBV in unvaccinated chicken flocks ranged between 14 and 54% [30]. The IBV/Mass and 4/91 vaccine strains have been used for many years to control the IBV infections among the chicken population in the ERS. The present study was designated to evaluate the level of chicken protection granted by the consecutive administration of some commercial IBV vaccines (Mass-H120 and 4/91) in a prime-boost regimen against some heterologous strains that are currently circulating in the ERS (CK/CH/LDL/97I, IS/720/99 and IS/Variant2/98).
Materials and methods
IBV isoaltes
We used three field IBV isolates (SA/IH16/13, SA/IC8/13, and SA/IH1/12). Thesebelonged belonging to lineages/genotypes GI-16/CK/CH/LDL/97I, GI-23/IS/720/99, and GI-23/IS/Var,iant2/98 respectively. The GenBank accession numbers of these isolates are (MH648701, MH648724, and MH449644) respectively. These isolates were isolated from some broiler poultry farmsand showed respiratory and renal involvement in the ERS. Isolation and identification of these isolates wdescribed out as previously described [5].
Quantitative estimation of IBV inoculum
The IBV titration and quantification were carried out in 10-days old embryonated specific-pathogen-free (SPF) eggs (Nile SPF, Egypt). The virus titers were calculated according to the Reed and Muench (1938) formula (35). The virus titer was expressed as an embryo infectious dose of 50% (EID50) /100 μl. The purified titrated allantoic fluids per each strain were used as inoculum for the challenge stuand as to prepare the antigens for the haemagglutination test.
The commercial IBV vaccines
Two live attenuated IBV vaccines, including the Izovac IB H120 vaccine (IZO, Spa Italy) and the NOBILIS®IB 4/91 (Intervet International B.V. Boxmeer, Holland) were used in this study. Vaccines were administered according to manufacturer instructions.
Embryonated chicken eggs and chickens
Fertile eggs from a commercial hatchery of a local chicken breed were obtained and incubated in the Virology Laboratory, College of Veterinary Medicine, King Faisal University. The hatched chicks were transferred to the isolation units in the Agriculture and Veterinary Training and Research Station (AVTRS), King Faisal University. Those chickens received the water and food ad libitum. Sex was not considered in the present study.
Experimental design
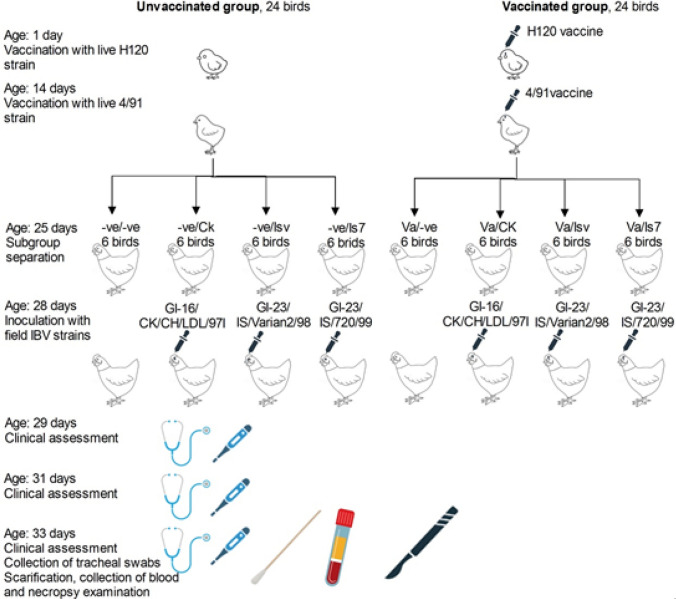
A total of 48 one-day-old chicks were used in this study. Chicks were divided into two equal divisions, vaccinated and unvaccinated, and further divided intoeight8 experimen,tal groups as shown in Fig. 1 and Table 1.
Fig. 1.
Experimental design, 48 one-day-old chicks were divided into two divisions (vaccinated and unvaccinated,) each containing 24 chicks. Vaccination was performed intraocularly with live attenuated H120 at the age of 1 day and lived attenuated 4/91 periodat the age of 14 periodthe age ofage of 25 days, each division was further divided into four groups (each containing six chickenperiodt the age ofe of 28 days, three vaccinated groups were inoculated with field strains while the fourth was kept as a vaccine control group. Similarly, three unvaccinated groups were infected with the same field st,rains while the fourth group was left as negative control (unvaccinated/uninfected) group. Clinical assessment was performed at 1, 3, and 5 days post-inoculation (DPI). At the 5 DPI, a tracheal swab was collected from each chicken, chickens were sacrificed, blood was collected from each chicken, and necropsy findings were assessed
Table 1.
Experimental design and inoculated virusesare givenn ages of 1-day, 14-days, and 28-days
| Group name | *Group abbreviationOne | Vaccine strain | Inoculated field strain (Lineage/Genotype) | |
|---|---|---|---|---|
| 1 day | 14 day | 28 day | ||
| Vaccinated groups | ||||
| Challenged groups | Va/Ck | H120 | 4/91 | SA/IH16/13 (GI-16/CK/CH/LDL/97I) |
| Va/Isv | H120 | 4/91 | SA/IH1/12 (GI-23/IS/Variant2/98) | |
| Va/Is7 | H120 | 4/91 | SA/IC8/13 (GI-23/IS/720/99) | |
| Vaccine control | Va/−ve | H120 | 4/91 | None |
| Unvaccinated groups | ||||
| Infected groups | −ve/Ck | None | None | SA/IH16/13 (GI-16/CK/CH/LDL/97I) |
| −ve/Isv | None | None | SA/IH1/12 (GI-23/IS/Variant2/98) | |
| −ve/Is7 | None | None | SA/IC8/13 (GI-23/IS/720/99) | |
| Negative control | −ve/−ve | None | None | None |
*Each groupcosixtainsn 6 birds
The vaccinated 24 chicks receivedthe IBV/H120 vaccine at 1 day of age and then boosted with the 4/91 vaccine at 14 daysAccording to the producer's recommended dilution, both vaccines were administered via the intraocular route ministered via the intraocular route according to the producer’s recommended dilution (103 and 103.6 EID50/dose for H120 and 4/91 vaccines, respectively). At 25 days of age, vaccinated chickens were randomly divided into four groups (6 chickens each). Chickens were housed in four isolated, separate units at the AVTRS. At 28 days of age, three groups of the vaccinated chickens were challenged with the field isolated IBV strains described above (herein referred to as challenged groups). The challenge dose was 103.0 EID50 per 100 μl per bird, as shown in Table 1. The inoculum was dispensed in both conjunctival sacs per chicken, approximately 50 µl per sac. The fourth group was not challenged and kept as a vaccine control (Va/−ve) group of chickens. Clinical observation and measuringof chicken’s body temperaturen per group was performed on the 1st, then 3rd, and the 5th Day Post-Inoculation (DPI). Chickens were examined individually for the appearance of clinical signs and scored a on scale of 5 degrees (normal, mild, moderate, severe, death) according to [8, 29, 32] with modifications. Scoring was performed as followsy healthy birds with no respiratory sound for one minute after provoked movement (average, score 0), birds with any respiratory sound during 1 min immediately after provoked activity (mild, score 1), and birds with any rales audible from about 50 cm apart during one minute without provoked movement (moderate, score 2), birds with signs of gasping, coughing or rales audible from about 1.5 m without provoked movement (severe, score 3), birds found dead (score four until the end of the experiment). The chicken’s body temperature was measured by inserting the thermometer probe (Hanna instrument, HI98511) about 2 cm in the cloaca. The remaining 24 one-day-old chicks in the non-vaccinated division of chickens were treated similarly, except for vaccination, as shown in Fig. 1 and includes 1. This division include three unvaccinated/infected groups which were used as positive control groups and one unvaccinated/uninfected group that served as a negative control group.
Collection and processing of samples
At the end of the 5th DPI, tracheal swabs were collected from each chicken and placed in 1 ml sterile phosphate-buffered saline (PBS) containingan antibiotic and antifungal cocktail. Chickens were then euthanized by cutting the jugular veins, and blood samples were collected for sera separation. All chickens were then examined individually for the presence of any gross tracheal, renal, splenic, or enteric lesions. Collected swabs and sera were stored at -80 °C until tested. Tracheal swabs in PBS were vigorously vortexed for one minute. The obtained fluid was used as a starting material for nucleic acid extraction. Collected sera were heat-inactivated at 56 °C for 30 min and tested for antibodies using the HI test.
Extraction of the total viral RNA and the Real-Time-RT-qPAccording to the manufacturer instructions, the total viral RNAs were extracted from the tracheal swab washes by IQeasy™ Plus Viral RNA Extraction Kit (Cat # 17153, iNtRON Biotmanufacturer’south Korea)onsThe Thermo Scientific Nanodrop 2000 Spectrophotometer measured the concentration of the extracted RNAsAccording to manufacturer instructions, the Reverse Transcription (RT) was performed using Reverse Transcription System (Cat # A3500, Promega, USA) using Reverse Transcription System (Cat # A3500, Promega, USA) according to manufacturer instructions. Quantitative PCR (qPCR) was performed using the primers and probe previously described by [21response2. Briefly, the reaction was performed using Quanti-Tect Virus Kit (Cat # 211011, Qiagen, Hilden, Germany) in a final volume of 20 µ. Each reaction includes 4 µl of QuantiTect Virus Master Mix, 1 µl of each primer (final concentration of 0.4 µM of each), 1 µl of the TaqMan probe (final concentration of 0.15 µM), 2 µl of the RT product, and 11 µl of RNase-free water. These reactions were peusing Agilent qPCR tubes using Agilent Stratagene Mx3000P instrumentCR tubes. The reaction parameters were as follows (95 °C/5 min, 40x (95 °C/15 s; 60 °C/45 s)). Data were analyzed using Agilent Mx3000P software. All samples were tested in two replicates to ensure the consistency and validation of the obtained results.
Preparation of the standard curve and quantification of the IBV viral load in tracheal swabs
To quantify the IBV viral load in tracheal swabs, a quantification curve was generated using a DNA amplicenclosed amplicon was a 403 base pair (bp) of the N gene of IBV that enclose the sequence targeted by, the qPCR as shown in Table 2.
Table 2.
PCR primers used for quantification of viral load in tracheas
| Name | Sequence 5′-3′ | Product | References |
|---|---|---|---|
| Primers used for PCR to generate DNA amplicon | |||
| N784 | AATTTTGGTGATGACAAGATGA |
403 bp (26,626–27,028)* |
[16] |
| N1145 | CATTGTTCCTCTCCTCATCTG | ||
| PB | |||
| AIBV-fr | ATGCTCAACCTTGTCCCTAGCA |
130 pb (26,683–26,813)* |
[30] |
| AIBV-as | TCAA-ACTGCGGATCA-TCACGT | ||
| AIBV-TM | FAM-TTGGAAGTAGAGTGACGCCCAAACTTCA-BHQ1 | ||
The qPCR targeted region is part of the DNA amplicon region used to generate DNAn
*Nucleotide position based on H120 genome, GB# FJ888351
The H120 vaccine was grown in embryonated SPF eggs (Nile SPF, Egypt), and allantoic fluid was used as starting material for RNA extraction. Extraction of viral nucleic acid, RT-PCR reaction, and purification of PCR product was performed as previously described [6]. The Thermo Scientific Nanodrop 2000 Spectrophotometer measured the concentration of the purified DNA. The online Sequence Manipulation Suite (SMS) was used to calculate the molecular weight of the amplicon double-stranded DNA [8]. The online DNA Copy Number and Dilution Calculator calculated copy number/ng and dilution factor to prepare a solution containing 108 copies/µl (www.thermofisher.com). Tenfold-serial dilutiondraftedn prepared in Quanti-Tect Nucleic Acid Dilution Buffer and used to establish a standard curve. Amplification efficiency was calculated using the formula: Efficiency = −1 + 10(− 1/SThe generated standard curve converted sample Ct valuesnverted to logarithmic values (Log10) of copy number using GraphPad Prism version 9 (GraphPad Software San Diego, CA, USA). Any Cts lower than the quantification limit were arbitrary set at 101 copies.
The hemagglutination inhibition (HI) test
Preparation and titration of the HA antigen from the allawereic fluids of the IBV field isolate infectarried out as previously described [29]. The HI test was performed as per the OIE guidelines [32]. Each sample was tested in duplicate, including the negative and positive controls. The test was repeated for samples that generatedinconsistente results. The HI titers were expressed as Log2 geometric mean of reciprocal of the highest serum dicausesn that cause inhibition of the hemagglutination. The HI titer of the vaccine control (Va/−ve) and negative control (−ve/−ve) groups was measured three times using each IBV field isolate.
Statistical analysis
Prism was used for statistical analysis (GraphPad software version 9, San Diego, CA, USA). The median, 25% percentile, and 75% prepresentto express the results. Kruskal–Wallis was used to compare groups over three with Duncan Test variance analysis for quantitative results with non-parametric distribution of Levene’s test. Significant was defined as a p-value of less than 0.05.
Results
Clinical observations on the IBV experimental chickens
The clinical signs were observed for 5 DPI and scored at 1st DPI, 3rd DPI, and 5th DPI. In challenged groups, symptoms were mild, observed after provoked movemmainlynd mostly commence at the 3rd DPI. In contrast, in the infected groups, signs erupted at the 1st DPI and were more severe as shown in Fig. 2A, with a noticeable decrease in feed consumption during the next couple of days. There was one death at the 5 DPI in CK/CH/LDL/97I-infected group. Neither clinical signs nor hostilere observed in the vaccine control and negative control groupssymptoms. Scores of clinical signs in infected groups were significantly higher than scores of the negative control and the vaccine control groups (Table 3).
Fig. 2.
Evaluation of the protection conferred by the investigated vaccination schedule A median, 25% percentile, and 75% percentithe observed clinical signs scores signs at 1st, 3rd, and 5th Days Post Inoculation (DPI). B Median, 25% percentile, and 75% percentile of the body temperature (°C) of chickens at 1st, 3rd, and 5th DPI. C Median, 25% percentile, and 75% percentile of Log 2 Haemagglutination Inhibition (HI) titers of sera collected from experimental chickens at ththree 5th DPI. HI, titers were measured using 3 hemagglutinins derived from the 3 field isolates. Negative control and vaccine control groups werethreetested 3 times uthreeing the 3 hemagglutinins. D Median, 25% ercentile, and 75% percentile of Log 10 viral load (genome copy number per reaction) measured using qPCR in tracheal swabs from experimental chickens at the 5th DPI. Related data are presented in supplementary Table 1.
Table 3.
Statistical significance of differences, expressed as P-values, between scores of clinical signs, rectal temperatures, hemagglutination inhibition titers, and viral loads in the trachea of the experimental groups
| Compared experimental groups | Group abbr | A score of clinical signs | Rectal temperature | HI titers | Viral load | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st DPI* | 3rd DPI | 5th DPI | 1st DPI | 3rd DPI | 5th DPI | Ck-Antigena | Isv-Antigen | Is7-Antigen | |||
| Negative control group vs. vaccine control group | |||||||||||
| Unvaccinated/uninfected vs. vaccinated/unchallenged | −ve/−ve vs. Va/−ve | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | 0.4157 | > 0.9999 |
| Negative control gr.oup vs infected groups | |||||||||||
| Unvaccinated/Uninfected vs. Unvaccinated/Ck-Infected | −ve/−ve vs. −ve/Ck | > 0.9999 | 0.0013 | 0.0024 | 0.0011 | 0.21 | 0.2538 | > 0.9999 | < 0.0001 | ||
| Unvaccinated/Uninfected vs. Unvaccinated/Is7-Infected | −ve/−ve vs. −ve/Is7 | 0.9757 | 0.0336 | 0.0034 | 0.0952 | 0.0066 | 0.0214 | > 0.9999 | < 0.0001 | ||
| Unvaccinated/Uninfected vs. Unvaccinated/Isv-Infected | −ve/−ve vs. −ve/Isv | > 0.9999 | 0.1009 | 0.0104 | 0.2961 | 0.1687 | 0.0568 | > 0.9999 | < 0.0001 | ||
| Negative control group vs. challenged groups | |||||||||||
| Unvaccinated/Uninfected vs. Vaccinated/CK-Challenged | −ve/−ve vs. Va/Ck | > 0.9999 | 0.266 | 0.1808 | 0.1552 | 0.0212 | 0.1118 | 0.0599 | < 0.0001 | ||
| Unvaccinated/Uninfected vs. Vaccinated/Is7-Challenged | −ve/−ve vs. Va/Is7 | > 0.9999 | > 0.9999 | 0.3975 | 0.8113 | 0.0768 | 0.2113 | 0.0032 | 0.3586 | ||
| Unvaccinated/Uninfected vs. Vaccinated/Isv-Challenged | −ve/−ve vs. Va/Isv | > 0.9999 | > 0.9999 | > 0.9999 | 0.1503 | > 0.9999 | > 0.9999 | 0.1313 | > 0.9999 | ||
| Infected vs. challenged groups | |||||||||||
| Unvaccinated/Ck-Infected vs. Vaccinated/CK-Challenged | −ve/Ck vs. Va/Ck | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | 0.0026 | > 0.9999 | ||
| Unvaccinated/Is7-Infected vs. Vaccinated/Is7-Challenged | −ve/Is7 vs. Va/Is7 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | > 0.9999 | 0.0028 | 0.037 | ||
| Unvaccinated/Isv-Infected vs. Vaccinated/Isv-Challenged | −ve/Isv vs. Va/Isv | > 0.9999 | > 0.9999 | 0.113 | > 0.9999 | > 0.9999 | > 0.9999 | 0.0441 | 0.0045 | ||
| Vaccine control group vs. challenged groups | |||||||||||
| Vaccinated/Unchallenged vs. Vaccinated/CK-Challenged | Va/−ve vs. Va/Ck | > 0.9999 | 0.266 | 0.1808 | 0.141 | 0.0169 | 0.0699 | > 0.9999 | 0.001 | ||
| Vaccinated/Unchallenged vs. Vaccinated/Is7-Challenged | Va/−ve vs. Va/Is7 | > 0.9999 | > 0.9999 | 0.3975 | 0.7496 | 0.0625 | 0.1359 | > 0.9999 | > 0.9999 | ||
| Vaccinated/Unchallenged vs. Vaccinated/Isv-Challenged | Va/−ve vs. Va/Isv | > 0.9999 | > 0.9999 | > 0.9999 | 0.1365 | 0.8711 | > 0.9999 | > 0.9999 | > 0.9999 | ||
| Vaccine control group vs. infected groups | |||||||||||
| Vaccinated/Unchallenged vs. Unvaccinated/Ck-Infected | Va/−ve vs. −ve/Ck | > 0.9999 | 0.0013 | 0.0024 | 0.001 | 0.1741 | 0.1646 | > 0.9999 | < 0.0001 | ||
| Vaccinated/Unchallenged vs. Unvaccinated/Is7-Infected | Va/−ve vs. −ve/Is7 | 0.9757 | 0.0336 | 0.0034 | 0.0861 | 0.0051 | 0.0125 | 0.3814 | < 0.0001 | ||
| Vaccinated/Unchallenged vs. Unvaccinated/Isv-Infected | Va/−ve vs. −ve/Isv | > 0.9999 | 0.1009 | 0.0104 | 0.2707 | 0.1393 | 0.0346 | > 0.9999 | 0.0006 | ||
*Time of measuring the parameter
aThe antigen used in the haemagglutination inhibition test
Vsucceededion succeed in minimizing the score of clinical signs in challenged groups to the level that showed no significabetweenifference from the negative control and the vaccine control groups.
Monitoring of the body temperature of the IBV experimental chickens
IBV infection-induced increase in body temperature (Fig. 2B). Body temperatures of CK/CH/LDL/97I-infected and IS/720/99-infected groups were significantly higher than that of either the negative control or the vaccine control groups (Table 3). The body temperature of IS/Variant2/98-infected group was significantly higher than the body temperature of the vaccine control group. Vaccisucceedsucceededecreasing body temperature of IS/720/99-challenged and IS/Variant2/98-challenged groups to the level that showed no significant difference from hat of the negative control and the vaccine control groups. On the other hand, the body temperature of the CK/CH/LDL/97I-challenged group remained high and, like the CK/CH/LDL/97I-infected group, showed a significant difference from that of the negative control and the vaccine control groups.
Necropsy findings of the IBV experimental chickens
Necropsy examination of the infected groups of chickens revealed congestion and the presence of catarrhal exudates in the trachea. Congestion of the intestines and enlargement of the spleen were also reported. However, kidney lesions were the most prominent findings, especially in the case of the infected chickens with the CK/CH/LDL/97I-like strain. Kidneys were enlarged in size than usual, and pale-mottled color (Fig. 3). Necropsy examination of the challenged groups of chickens showed mild tracheal congestion and infiltration of exudates. Edema and congestion of kidneys in the challenged groups were also seen, especially in CK/CH/LDL/97I-challenged group. No apparent lesions were observed in the case of the vaccine control and the hostile control groups of chickens.
Fig. 3.
Kidney lesions revealed by necropsy of A negative control (unvaccinated/ uninfected (−ve/−ve)) chicken, B vaccinated-CK/CH/LDL/97I-challenged (Va/Ck) chicken, C unvaccinated-CK/CH/LDL/97I-infected (−ve/Ck) chicken and unvaccinated-IS/720/99-infected (−ve/Is7). The whiten. White arrow in B shows the swollen lobules of tand he kidney, Black arrows in C an show hows swollen mottled kidney
Monitoring the hemagglutination inhibition antibody titers
There was a significant increase in the titers of HI antibodies in all challenged grohen compared to the corresponding infected groups (Fig. 2C and Table 3). HI, titer in the IS/720/99-challenged group was also significantly higher than the HI titer of the negative control group. Minimal and insignificant differences in HI titers of the vaccine control group due to using hemagglutinin from the three field strains were also seen.
Quantification of the IBV load in tracheal swabs collected from the experimental chickens
Amplification of the ten-fold dilution series of the DNA amplicon presents linearity over the dilutions containing 102 to 107 copies/reaction (Supplementary Figures A and B). Higher dilutions of the DNA amplicon were detectable but out of linearity. Accordingly, viral load was quantified in the tracheas from chickens.
The IBV was detected within the quantification limit in samples from all experimental groups (Fig. 2D) except the IS/Variant2/98-challenged, the vaccine control, and the hostile control groups. The IBV was detected within the quantification limit in a sample from only one chicken and out of the quantification limit in samples from two- other chickens from the IS/Variant2/98-challenged group. Additionally, the IBV was detected but out of the quantification limit in samples from two chickens of the vaccine control group. The IBV was not seen in the negative control group of chickens.
Tracheal viral load in the infected groups was significantly higher that in the negative control and vaccine control groups (Table 3). Vaccination succeeds in minimizing tracheal viral load in IS/720/99-challenged and IS/Variant2/98-challenged groups to the levels that differ insignificantly from either the negative control or the vaccine control groups but are significantly different from that of the corresponding infected groups. On the other hand, tracheal viral load in CK/CH/LDL/97I-challenged group remained high and, like the CK/CH/LDL/97I-infected group, showed a significant difference from that of the negative control and the vaccine control groups.
Discussion
Active surveillance and protectotyping are essentialcontrollingcontrol of the continuously evolving IBV strains. In Saudi Arabia, several IBV genotypes were detected even in the presence of active vaccination programs. This highlights the importance of selecting the homologous IBV vaccines that match the field circulating strains [5, 6, 25]. Several IBV genotypes were recently detected and identified in t,his region including, but not limited to, CK/CH/LDL/97I, 4/91, Mass, IS/729/99, and IS/Variant2/98 [5, 6, 25]. The most commonly available commercial IBV vaccines belong to the Mass serotype (H120, Ma5) and 4/91 serotype. Unfortunately, there are no homologous commercial vaccines available in this region for the other three IBV genotypes. The main goal of this study was to evaluate the efficacy of some of the currently available commercial IBV vaccines in the protection against the three heterologous IBV types circulating in the ERS.
In the current study, we examined the efficacy of some commercial live attenuated IBV vaccines in a prime-post regimen (Mass and 4/91) in commercial broiler chickens. It was previously reported that the combination of some IBV antigenic-variable vaccines improved the protection rates provided against infection with IBV heterologous strains [37]. A previous study on the effects of variable sequential application of Mass (Ma5 or H120) and 4/91 vaccine,sands combining Ma5 and 4/91 vaccines was conducted [15]. This study showed that priming chickens with Mass type of vaccines followed by a booster with 4/91 vaccine provide superior chicken protection against IBV infection. This was compared to priming chickens with 4/91, followed by boosting with the Ma5 vaccine. It was also much more efficient in protecting chickens than combining both vaccinessimultaneouslye or booster with the same initial vaccine [15]. Similarly, administration of Mass vaccine as eonerly as 1 day of age and revaccination with 4/91 vaccine at 14 daysan excellentd a good rate of protection against nephrogenic Brazilian IBV strains [38]. Similarly, the IBV vaccine combination provided high protection rates against the heterologous QX strains [7, 34, 37], though better protection was achieved with homologous QX vaccine [23]. In the current study, we decided to use the H120 rather than the Ma5 because the immunity driven by its administration is well characterized, and it was also shown to be safe at vaccination of day-old chickens [4]. We used the commercial broiler chickens in this study, rather than SPF chickens, to mimic the field conditions. Logically, the immune response against the used IBV vaccines in the SPF chickens was much more superior to the commercial chickens. This difference may be attributed to the maternally derived immunity that may interfere with the replication of the vaccine in the case of commercial chickens [37].
Our proposed vaccination schedule appears to provide high protection against the IS/Variant2/98-like strains. This was evident by the reduction in the severity of the developed clinical signs and necropsy lesions as well as a decrease in both the body temperature and the IBV-viral load in the trachea of the challenged group of chickens (Fig. 2D). This is in addition to the induction of production of circulating anti-IS/Variant 2/98 antibodies. These findings are in agreement with those reported previously [23]. The latter study vaccinated SPF chickens with the H120 and 1/96 strains at 1 and 14 days of age, respectively, then challenged them with the IS/1494/06 strain at age of 35 days. This vaccination/challenge protocol resulted in good protection manifested clinically with mild clinical and necropsy lesions, as well as resulted in a marked reduction in the IBV viral load in the tracheas of the infected chickens. This approach also resulted in a 69.2% protection score based on ciliostasis scoring [23]. Administration of the 4/91 vaccine to chickens at the 1 and 14 days old was reported to provide relatively lower protection compared to that provided by the administration of the Mass and 4/91 combination. This combination was not the only factor but also the sequential application of the vaccines, as priming chickens with the 4/91 type and boosting with the Mass type provide lower protection than that induced by either Mass prime and 4/91 boost or prime and boost with 4/91 vaccine [6]. In contrast, single or twice use of the homologous vaccine at the 1st or 1st and 14th day of age followed by the challenge at 2–3 weeks later was reported to provide complete protection from clinical signs, 73–85% protection scores based on ciliostasis and reduction in the virus shedding [4, 6].
Our vaccination protocol offered relatively good protection against the IS/720/99-like strain with regards to viral load, body temperature, induction of immune response, and protection from the severe clinical signs and lesions. This is in fine agreement with some previous studies [7] where both the H120 and CR88 vaccines were used to vaccinate some commercial broilers at 1 and 14 days of age respectively. Results of this experiment revealed a 60% ciliary protection rate, complete clinical protection, and variable protection from gross lesions [7]. On the other hand, it has been reported that similar vaccination/challenge programs of the SPF chickens provide complete clinical protection, 40% ciliary protection rate, and decrease in the rate of virus recovery from kidney up to 20%. This suggests the unsatisfactory protection against the challenged strain, EG/1212B, a IS/720/99-like strain [41]. Some non-consistent findings were reported after vaccination of SPF chicks with the H120 vaccine at 14 days old and then challenged with the IS/720/99 type. Based on virus re-isolation in embryonated SPF eggs, the tracheal protection rate of 36% was reported after the challenge with IS/720/99 strain [18] and 91%, together with 25% renal protection rate, was reported after challenge with IS/885 strain [31]. On the other hand, administration of the formalin-inactivated homologous vaccine in 14 days-old SPF chicks provided about 69% ciliary protection against challenge with EG/1212B, an IS/885 like strain at age of 42 days. The virus was also detected in tracheal and/or kidney tissues in 60% of the involved chickens [3, 31].
CK/CH/LDL/97I is a dominant IBV genotype in the ERS that was detected at a frequency of 28.6% of the total IBV detections. A mortalities of approximately 30% of the affected flocks as well as losses related to treatment, control programs and reduction of body weight was induced by this genotype [17, 36]. In the present work, based on the clinical signs protection scoring, the vaccinated-CK/CH/LDL/97I-challenged group remained at a good level of protection when compared with the corresponding infected group. Additionally, the vaccination scheme induces a significant rise in HI antibody titer. This contributed to the reduction in the severity of renal lesions as well as resulted in a marked reduction in the mortality rates. On the other hand, vaccination was not able to provide a significant reduction in tracheal viral load or body temperature of the challenged group of chickens. Based on these findings, it is reasonable to state that the used vaccination program provides some protection against CK/CH/LDL/97-like strain, however, this protection was incomplete and relatively lower than that achieved with other investigated genotypes. This is compatible with our observation about the prevalence of CK/CH/LDL/97I- genotype in chickens in this region despite the use of Mass and 4/91 vaccines [5].
Ideally, complete protection against IBV infection usually requires the use of the homologous vaccine, while vaccination with a heterologous vaccine provides questionable protection [26, 27, 33]. Our protection study on the IBV/CK/CH/LDL/97I-like strain support this hypothesis. Previous studies showed that variable protection against IBV infection was provided by the administration of several heterologous vaccines and one candidate-homologous vaccine against CK/CH/LDL/97I strain [27]. This study showed that administration of a single homologous IBV vaccine dose to the SPF chickens resulted in the complete protection against CK/CH/LDL/97I strain [27]. However, the IBV was recovered from the tracheas of 50–100% of the chickens vaccinated with heterologous CK/CH/LDL/97I strains and from tracheas of 70% of the chicks vaccinated with H120 strain [27]. On the other hand, high protection rate against CK/CH/LDL/97I-like strain was also reported to be induced by heterologous vaccine strains. Both H120 and CR88 live attenuated vaccines were used to vaccinate commercial broiler chicks in a prime-boost regimen at (0 and 14) days of age, respectively. Chickens were then challenged with the IBV-Q1 strain, a CK/CH/LDL/97I-like strain, at 28 days of age. This was based on several criteria such as protection from the severe clinical signs, high ciliary protection (90%), and reduction in the viral loads from about 104.4 to around 101 equivalent units [14]. However, this partial disagreement may result from the use of different strains of vaccine, though of the same type (CR88 of the 4/91 type). In this context, it has been reported that strains of a single genotype/serotype may behave differently in protectotyping [39]. Vaccination of the SPF chicken with 4/91 vaccine at day 1 of age provided variably low protection against challenges with the IBV/GI-16 strain, CK/CH/LDL/97I type, at 21 days of age [22]. Similarly, both 4/91 and CR88 vaccine strains were reported to provide variable degrees of protection against the QX strain [8].
The systemic humoral immune response was observed in all challenged groups in the present study. The circulating antibody plays important role in kidneys protection as reported earlier [28]. In that study, kidney protection may be directly related to the effect of the circulating specific IBV antibodies. This was in the light of earlier study demonstrated that the high level of the HI antibody titers induced by the heterologous IBV vaccine correlated well with the low recovery rate of IBV from the kidney or the genital tract. This was despite the lack of correlation with ciliary movement, reduction in the severity of the induced clinical signs, and virus recovery from the trachea [40]. Similar results were observed with nephropathy induced by CK/CH/LDL/97I-like strains [27].
Conclusions
In conclusion, our results demonstrate that vaccination of commercial broiler chickens in a prime-boost regimen using the Mass live attenuated vaccine at age of 1 day, and boosting with 4/91 live attenuated vaccine at 14 days of age provided a variable degree of protection against heterologous IBV strains. The protection rates were high against the IS/Variant2/98-like strain, good against IS/720/99-like strain, and moderate against CK/CH/LDL/97I-like strain. These data should help in the control of IBV in broiler chickens in a targeted area. Continuous monitoring of the circulating IBV strains in chickens in certain regions and preparation of some homologous vaccines to them is the main key role in the protection cycle against IBV natural infections.
Institutional review board statement
All handling and chicken experiments carried out in this study were conducted as per the instructions of the Animal Ethics protocols and the National Committee of Bio-Ethics, King Abdul-Aziz City of Science and Technology, Royal Decree No. M/59. The chicken experiments and protocols were reviewed and approved by the animal ethics committee of the deanship of scientific research, King Faisal University, Saudi Arabia (Approval No: KFU-REC/2020-12-35). All the necessary paperwork for sample collections was obtained.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
“Conceptualization, MGH and AM; methodology, MGH, AK, AM; software, MGH, AK, AM; validation, MGH, AK, AM; formal analysis, MGH, AK, AM; investigation, MGH, AM; resources, MGH, AM; data curation, MGH, AK, AM; writing—original draft preparation, MGH, AK, AM; writing—review and editing, MGH, AK, AM; visualization, MGH, AK, AM; supervision, MGH, AM; Project administration, AM; funding acquisition, AM. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to thank King Abdul-Aziz City for Science and Technology for their generous funding through the Strategic Technologies program, Grant Number 11-BIO1823-06.
Data availability
All the data will be available upon request from the corresponding authors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13337-022-00780-0.
References
- 1.Ababneh M, Dalab AE, Alsaad S, Al-Zghoul M. Presence of infectious bronchitis virus strain CK/CH/LDL/97I in the Middle East. ISRN Vet Sci. 2012;2012:201721. doi: 10.5402/2012/201721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ali A, El-sabagh IM, Mohamed MH, Alluaimi AM, Arif IA. Molecular characterization of common respiratory viral infections in broilers in Al-Hassa, Eastern Province, Saudi Arabia. Thai J Vet Med. 2018;48(2):235–245. [Google Scholar]
- 3.Al-Ebshahy E, Abdel-Sabour M, Abas O, Yanai T. Protection conferred by a vaccine derived from an inactivated Egyptian variant of infectious bronchitis virus: a challenge experiment. Trop Anim Health Prod. 2019;51(7):1997–2001. doi: 10.1007/s11250-019-01898-y. [DOI] [PubMed] [Google Scholar]
- 4.Ali A, Kilany WH, Zain El-Abideen MA, Sayed ME, Elkady M. Safety and efficacy of attenuated classic and variant 2 infectious bronchitis virus candidate vaccines. Poult Sci. 2018;97(12):4238–4244. doi: 10.3382/ps/pey312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Mubarak AIA, Al-Kubati AAG. Cocirculation of four infectious bronchitis virus lineages in broiler chickens in the Eastern Region of Saudi Arabia from 2012 to 2014. Vet Med Int. 2020;2020:6037893. doi: 10.1155/2020/6037893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsultan MA, Alhammadi MA, Hemida MG. Infectious bronchitis virus from chickens in Al-Hasa, Saudi Arabia 2015–2016. Vet World. 2019;12(3):424–433. doi: 10.14202/vetworld.2019.424-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awad F, Forrester A, Baylis M, Lemiere S, Ganapathy K. Protection conferred by live infectious bronchitis vaccine viruses against variant Middle East IS/885/00-like and IS/1494/06-like isolates in commercial broiler chicks. Vet Rec Open. 2015;2(2):e000111. doi: 10.1136/vetreco-2014-000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awad F, Hutton S, Forrester A, Baylis M, Ganapathy K. Heterologous live infectious bronchitis virus vaccination in day-old commercial broiler chicks: clinical signs, ciliary health, immune responses and protection against variant infectious bronchitis viruses. Avian Pathol. 2016;45(2):169–177. doi: 10.1080/03079457.2015.1137866. [DOI] [PubMed] [Google Scholar]
- 9.Bande F, Arshad SS, Bejo MH, Moeini H, Omar AR. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J Immunol Res. 2015;2015:424860. doi: 10.1155/2015/424860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butt SL, Erwood EC, Zhang J, Sellers HS, Young K, Lahmers KK, et al. Real-time, MinION-based, amplicon sequencing for lineage typing of infectious bronchitis virus from upper respiratory samples. J Vet Diagn Investig. 2021;33(2):179–190. doi: 10.1177/1040638720910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(6):567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 13.Cavanagh D, Gelb J. Infectious Bronchitis. In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. Diseases of Poultry. Diseases of Poultry. 12 ed. Ames, Iowa, USA: Blackwell Publishing; 2008. p. 117–35.
- 14.Chhabra R, Forrester A, Lemiere S, Awad F, Chantrey J, Ganapathy K. Mucosal, cellular, and humoral immune responses induced by different live infectious bronchitis virus vaccination regimes and protection conferred against infectious bronchitis virus Q1 strain. Clin Vaccine Immunol. 2015;22(9):1050–1059. doi: 10.1128/CVI.00368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook JK, Orbell SJ, Woods MA, Huggins MB. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999;28(5):477–485. doi: 10.1080/03079459994506. [DOI] [PubMed] [Google Scholar]
- 16.Farsang A, Ros C, Renstrom LH, Baule C, Soos T, Belak S. Molecular epizootiology of infectious bronchitis virus in Sweden indicating the involvement of a vaccine strain. Avian Pathol. 2002;31(3):229–236. doi: 10.1080/03079450220136530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganapathy K, Ball C, Forrester A. Genotypes of infectious bronchitis viruses circulating in the Middle East between 2009 and 2014. Virus Res. 2015;210:198–204. doi: 10.1016/j.virusres.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Gelb J, Jr, Weisman Y, Ladman BS, Meir R. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000) Avian Pathol. 2005;34(3):194–203. doi: 10.1080/03079450500096539. [DOI] [PubMed] [Google Scholar]
- 19.Gomaa MH, Barta JR, Ojkic D, Yoo D. Complete genomic sequence of turkey coronavirus. Virus Res. 2008;135(2):237–246. doi: 10.1016/j.virusres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomaa MH, Yoo D, Ojkic D, Barta JR. Seroprevalence of turkey coronavirus in North American turkeys determined by a newly developed enzyme-linked immunosorbent assay based on recombinant antigen. Clin Vaccine Immunol. 2008;15(12):1839–1844. doi: 10.1128/CVI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomaa MH, Yoo D, Ojkic D, Barta JR. Use of recombinant S1 spike polypeptide to develop a TCoV-specific antibody ELISA. Vet Microbiol. 2009;138(3–4):281–288. doi: 10.1016/j.vetmic.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzman M, Saenz L, Hidalgo H. Molecular and antigenic characterization of GI-13 and GI-16 avian infectious bronchitis virus isolated in Chile from 2009 to 2017 regarding 4/91 vaccine introduction. Animals (Basel) 2019;9(9):656. doi: 10.3390/ani9090656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habibi M, Karimi V, Langeroudi AG, Ghafouri SA, Hashemzadeh M, Farahani RK, et al. Combination of H120 and 1/96 avian infectious bronchitis virus vaccine strains protect chickens against challenge with IS/1494/06 (variant 2)-like infectious bronchitis virus. Acta Virol. 2017;61(2):150–160. doi: 10.4149/av_2017_02_04. [DOI] [PubMed] [Google Scholar]
- 24.Hemida MG, Al-Hammadi MA, Daleb AHS, Gonsalves CR. Molecular characterization and phylogenetic analyses of virulent infectious bronchitis viruses isolated from chickens in Eastern Saudi Arabia. Virusdisease. 2017;28(2):189–199. doi: 10.1007/s13337-017-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemida MG, Al-Hammadi M, Gonzalves C, Ismail MM. The experimental infection with a field isolate of the infectious bronchitis virus from eastern Saudi Arabia resulted in seroconversion of the challenged birds with no apparent clinical diseases. Virusdisease. 2021:1–7. [DOI] [PMC free article] [PubMed]
- 26.Lin KY, Wang HC, Wang CH. Protective effect of vaccination in chicks with local infectious bronchitis viruses against field virus challenge. J Microbiol Immunol Infect. 2005;38(1):25–30. [PubMed] [Google Scholar]
- 27.Liu S, Zhang X, Wang Y, Li C, Liu Q, Han Z, et al. Evaluation of the protection conferred by commercial vaccines and attenuated heterologous isolates in China against the CK/CH/LDL/97I strain of infectious bronchitis coronavirus. Vet J. 2009;179(1):130–136. doi: 10.1016/j.tvjl.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald JW, Randall CJ, McMartin DA, Dagless MD, Gazdzinski P. Active and passive immunisation against nephritis induced by an avian infectious bronchitis virus. Avian Pathol. 1981;10(2):121–129. doi: 10.1080/03079458108418466. [DOI] [PubMed] [Google Scholar]
- 29.Mahmood MS, Siddique M, Hussain I, Khan A. Trypsin-induced hemagglutination assay for the detection of infectious bronchitis virus. Pak Vet J. 2004;24(2):54–58. [Google Scholar]
- 30.Meir R, Maharat O, Farnushi Y, Simanov L. Development of a real-time TaqMan RT-PCR assay for the detection of infectious bronchitis virus in chickens, and comparison of RT-PCR and virus isolation. J Virol Methods. 2010;163(2):190–194. doi: 10.1016/j.jviromet.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meir R, Rosenblut E, Perl S, Kass N, Ayali G, Perk S, et al. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 2004;48(3):635–641. doi: 10.1637/7107. [DOI] [PubMed] [Google Scholar]
- 32.OIE. Avian Infectious Bronchitis OIE Terrestrial Manual Office international des épizooties. Chapter 232. 2013:1–15.
- 33.Pensaert M, Lambrechts C. Vaccination of chickens against a Belgian nephropathogenic strain of infectious bronchitis virus B1648 using attenuated homologous and heterologous strains. Avian Pathol. 1994;23(4):631–641. doi: 10.1080/03079459408419033. [DOI] [PubMed] [Google Scholar]
- 34.Pohuang T, Tanasatian S, Sasipreeyajan J. Efficacy of different vaccination programs of live 4/91 strain against Thai QX-like infectious bronchitis virus in broiler chickens. Thai J Vet Med. 2016;46(3):419. [Google Scholar]
- 35.Reed LJMH. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 36.Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques. 2000;28(6):1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- 37.Terregino C, Toffan A, Beato MS, De Nardi R, Vascellari M, Meini A, et al. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37(5):487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- 38.De Wit JJ, Brandao P, Torres CA, Koopman R, Villarreal LY. Increased level of protection of respiratory tract and kidney by combining different infectious bronchitis virus vaccines against challenge with nephropathogenic Brazilian genotype subcluster 4 strains. Avian Pathol. 2015;44(5):352–357. doi: 10.1080/03079457.2015.1058916. [DOI] [PubMed] [Google Scholar]
- 39.De Wit J, Dijkman R, Guerrero P, Calvo J, Gonzalez A, Hidalgo H. Variability in biological behaviour, pathogenicity, protectotype and induction of virus neutralizing antibodies by different vaccination programmes to infectious bronchitis virus genotype Q1 strains from Chile. Avian Pathol. 2017;46(6):666–675. doi: 10.1080/03079457.2017.1346782. [DOI] [PubMed] [Google Scholar]
- 40.Yachida S, Aoyama S, Sawaguchi K, Takahashi N, Iritani Y, Hayashi Y. Relationship between several criteria of challenge-immunity and humoral immunity in chickens vaccinated with avian infectious bronchitis vaccines. Avian Pathol. 1985;14(2):199–211. doi: 10.1080/03079458508436222. [DOI] [PubMed] [Google Scholar]
- 41.Zanaty A, Arafa A, Selim A, Hassan MK, El-Kady MF. Evaluation of the protection conferred by heterologous attenuated live infectious bronchitis viruses againest an Egyptian variant IBV [EG/1212B] J Am Sci. 2013;9(6):599–606. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data will be available upon request from the corresponding authors.