Abstract
PURPOSE OF REVIEW:
Up to 80% of survivors of right brain stroke leave acute care without being diagnosed with a major invisible disability. Studies indicate that a generic cognitive neurologic evaluation does not reliably detect spatial neglect, nor does it identify unawareness of deficit after right brain stroke; this article reviews the symptoms, clinical presentation, and management of these two cognitive disorders occurring after right brain stroke.
RECENT FINDINGS:
Stroke and occupational therapy practice guidelines stress a quality standard for spatial neglect assessment and treatment to reduce adverse outcomes for patients, their families, and society. Neurologists may attribute poor outcomes associated with spatial neglect to stroke severity. However, people with spatial neglect are half as likely to return to home and community, have one-third the community mobility, and require 3 times as much caregiver supervision compared with similar stroke survivors. Multiple randomized trials support a feasible first-line rehabilitation approach for spatial neglect: prism adaptation therapy; more than 20 studies reported that this treatment improves daily life independence. Evidence-based treatment of anosognosia is not as developed; however, treatment for this problem is also available.
SUMMARY:
This article guides neurologists’ assessment of right brain cognitive disorders and describes how to efficiently assemble and direct a treatment team to address spatial neglect and unawareness of deficit.
INTRODUCTION
Up to 80% of survivors of right brain stroke are not diagnosed with cognitive disorders of spatial cognition or awareness1,2 during routine acute stroke care, and thus, they cannot receive a plan for personalized cognitive treatment. Worse, right brain stroke itself is underdiagnosed, so survivors of right brain stroke are at risk of undertreatment or incorrect treatment3–5 because of the difficulty in identifying the cognitive hallmarks of right brain disorders during routine care.
This article reviews the symptoms and clinical presentation of two of the cognitive syndromes occurring after right brain stroke. They are not rare; probably about half or even up to 60% to 80% of survivors of right brain stroke have these symptoms for the first weeks and months after stroke (TABLE 6-16–15).11,14,16
TABLE 6-1.
Prevalence of Spatial Neglecta
| Study | Total study, N | Left brain stroke, % | Right brain stroke, % | Setting | Country |
|---|---|---|---|---|---|
| Gainotti et al, 1972 7 | 222 | 31 | 42 | Outpatient clinic | Italy |
| Denes et al, 1982 8 | 48 | 21 | 33 | Geriatric hospital | Italy |
| Fullerton et al, 1986 9 | 205 | 25 | 49 | General hospital | Ireland |
| Stone et al, 1993 10 | 171 | 65 | 82 | General hospital | United Kingdom |
| McGlone et al, 1997 11 | 138 | 31 | 62 | General hospital | Canada |
| Kalra et al, 1997 12 | 145 | 21 | 43 | General hospital | United Kingdom |
| Ringman et al, 2004 13 | 750 | 20 | 43 | Acute care hospital | United States |
| Chen et al, 2015 14 | 121 | 47 | 76 | Inpatient rehabilitation | United States |
| Hammerbeck et al, 2019 15,b | ~90,000 | 37 | 41.5 | Acute care hospital | England, Wales, Northern Ireland |
Modified with permission from Chen P, et al, Top Stroke Rehabil.6 © 2012 Taylor & Francis Ltd.
Side of stroke assigned based on side of arm weakness on the National Institutes of Health Stroke Scale.
Cognitive symptoms after right brain stroke are important to neurologic care for two major reasons. First, they may be the only indication of a stroke event or right brain dysfunction (eg, seizure) requiring medical care. Thus, for example, detecting cognitive symptoms after right brain stroke may allow the clinician to give acute stroke treatment to more patients and improve community outcomes and quality of life. Second, by identifying cognitive symptoms of the spatial neglect syndrome, defined as asymmetric reporting, responding, or orienting to one side of space after a brain lesion, causing functional disability, one can provide management, education, and treatment.17 Assessment of spatial neglect and rehabilitation of this cognitive disorder, which can improve daily life function and independence, are also discussed. Anosognosia, or pathologic unawareness of deficit, is also covered in this article.18 Although both spatial neglect and anosognosia may occur after other forms of focal brain injury (eg, after brain tumor resection), they have only been studied in systematic ways in the context of right brain stroke. Thus, clinical practice guidelines for the assessment and treatment of spatial neglect are only available for stroke care.19 People with anosognosia may be pathologically unaware of several different kinds of deficits; however, anosognosia for left body paralysis is very strongly associated with right brain injury.20 Although rehabilitation approaches for anosognosia are not as well-researched as those for spatial neglect, evidence shows they may improve deficits and so this article describes how these treatments can be used. Neurologists need to advocate for patients with right brain syndromes to ensure quality care and good outcomes.
Right brain stroke is a major identified cause of spatial neglect.21 Spatial neglect symptoms may occur in other disorders associated with focal brain injury, such as traumatic brain injury and brain tumor, and spatial neglect symptoms may even occur in multiple sclerosis and Parkinson disease.22–25 However, in contrast to the high-quality evidence available supporting the use of spatial neglect assessment and treatment in quality stroke care,26 no prospective, systematic studies of the clinical presentation and treatment of spatial neglect in these disorders have been performed. Thus, the evidence is not yet appropriate to use as a basis for planning routine clinical care in these areas. This is an important area for future research.
SPATIAL NEGLECT
Clinically identifying spatial neglect is important; a challenge exists in diagnosing this problem, and routine care processes are insufficient to identify this disorder.
Clinical Presentation
Spatial neglect is a common disorder, occurring in about half of patients with stroke during the first months after their event (TABLE 6-1). The unsafe behaviors observed as symptoms of spatial neglect can be misattributed to intellectual, reasoning, personality, or generic cognitive problems, which can be devastating to patient dignity. Misattribution of spatial neglect symptoms can also delay diagnosis of right brain stroke past the window for acute stroke interventions; thus, clinicians should be alert to new spatial bias in patients with stroke risk factors.
SPATIAL NEGLECT SYMPTOMS.
Considerable inequity exists in management and outcomes between strokes based on which side of the brain is affected. Although cognitive symptoms caused by right brain stroke are characteristic and disabling, clinicians frequently miss them and, thus, fail to offer appropriate treatment.27 This is probably true in other disorders that cause focal brain damage and spatial neglect (eg, brain tumor); however, formal examination of underdiagnosis of spatial neglect and its impact on the management of right versus left brain issues have not been studied in other brain disorders.
The most common reason why the symptoms are missed is that they are not assessed. An acute stroke assessment tool, the National Institutes of Health Stroke Scale, which is a quality care standard in stroke centers,28 underestimates the severity of right brain stroke29 because, on this 47-point scale, only 2 points (for item 11: involves testing extinction to bilateral simultaneous stimulation, see below) are specific to right brain stroke and spatial neglect, compared with 7 points total (for items 1b, 1c, and 9: answering questions, obeying a command, and aphasia testing) that are sensitive to detect aphasia after left brain stroke.
How to Assess for Spatial Neglect
This section describes the principles and testing methods used to approach accurate evaluation for spatial neglect.
NOT A VISION PROBLEM.
One of the reasons that neurologists underestimate right brain stroke symptoms is that they have often been informed that spatial neglect is a higher-order visual problem.30 However, people with vision problems do not behave like people with spatial neglect. People with vision problems report that they cannot recognize objects or that they struggle with reading. Although people with spatial neglect may have trouble reading the left side of sentences or words (neglect dyslexia), which is disabling, they may not seem at all concerned about their partial reading. People with vision issues report they cannot see well. However, spatial neglect is characteristically accompanied by unawareness of deficit (anosognosia), and the overwhelming majority of patients with spatial neglect deny having visual issues. Unawareness of deficit (anosognosia) is reviewed later in this article.
EXTINCTION TO BILATERAL SIMULTANEOUS STIMULATION.
Frequently, spatial neglect occurs in patients who do not have hemianopia. Patients may be able to see single stimuli on the contralesional side of space; however, when a stimulus is presented in both visual fields (bilateral simultaneous stimulation), especially in the lower visual fields, patients with spatial neglect after right brain stroke may confidently respond that the stimulus is on the right only (ie, extinction to double simultaneous stimulation). Extinction can occur in the visual, tactile, and auditory modalities. Clinicians should bear in mind that extinction to double simultaneous stimulation is not always present in 100% of trials. In many patients, a deficit of “Where” perceptual attention causes unawareness of some stimuli on the left side, and yet not 100% of stimuli are extinguished in the presence of a right-sided stimulus: perhaps only 40%, 60%, or 80% of left-sided stimuli are extinguished. Because extinction of the left-sided stimulus may be present only some of the time, examiners need to do enough trials (at least six or eight simultaneous stimulation trials) to capture errors; of six or eight trials, even one or two errors in which only the ipsilesional side stimulus is reported raises suspicion for spatial neglect.
Extinction to double simultaneous stimulation that is detected in two modalities is strongly suggestive of spatial neglect. To test tactile extinction, the examiner touches the patient on both hands, arms, knees, legs, or feet and asks where the stimulus was felt. If a single stimulus can be felt on the side of the body suspected to be contralesional, but the contralesional stimulus is not felt during double stimulation of both sides of the body, extinction is present. If a patient has extinction in two modalities (visual and tactile, for example), this helps clarify that the problem is spatial neglect and not a sensory issue because, clearly, a visual deficit should not, for example, cause errors in detecting a tactile stimulus.
EXTINCTION OR SPATIAL NEGLECT? OR ANOTHER TERM?
Clinicians will sometimes use three or four terms to describe spatial neglect symptoms. They may separate extinction from spatial neglect, and they may use other terms such as hemiinattention. Although this may be interesting when looking at laboratory-based analysis, this author discourages this approach in clinical practice. With the goal of increasing the clinical utility of spatial neglect diagnosis, this author and colleagues defined spatial neglect as pathologically asymmetric behavior caused by a brain lesion, resulting in functional disability.31 Thus, many symptoms can all be called part of the spatial neglect syndrome. Patients who do not have a visual field cut may yet collide with a door frame; thus, with extinction to double simultaneous stimulation, they can meet criteria for spatial neglect because it appears to be the basis of functional disability (a collision and safety risk) (CASE 6-1). Here, the extinction is a feature of, or sign within, the spatial neglect syndrome. The evidence of difficulty with safety while walking should, additionally, be enough to trigger physical therapy evaluation for fall risk, as well as gait and balance training.
CASE 6-1.
An 80-year-old woman who resided in an assisted living facility reported having heartburn one morning. The symptoms were gone a few hours later; however, she got out of bed and started moving around the facility, going into the rooms of three other residents and insisting they were in her space and needed to leave. The nurses could not redirect her; she kept breaking away and going into other resident rooms. The geriatrician on staff found her only mildly tachycardic, possibly dehydrated, and unable to tell him the day of the week or date, although she knew the month and year. She insisted she was in her room, although she was in the cafeteria lounge. About an hour after her symptoms started, the geriatrician diagnosed delirium, calling the patient’s behavior “impulsive,” but no medical condition could be identified on screening laboratory tests, ECG, and urine evaluation. Her past medical history was notable for hypertension, high cholesterol, and a possible past episode of atrial fibrillation.
Her family insisted on an assessment from a neurologist 2 days later. The neurologist found her irritable and saying “I’m fine. I want to go home.” Her gaze drifted to the right when not stimulated (right gaze preference), although she easily looked leftward when the examiner wiggled fingers on the left or told her to look leftward. She had no visual field cut to single stimuli; however, she made errors about half the time when tested with double simultaneous stimulation in the left and right lower visual fields, responding “right.” She also sometimes responded before the examiner showed a stimulus or when the examiner was not actually showing her anything, reporting she saw a stimulus on the left. The examiner gave her a pamphlet to read, and she started in the middle of the page, read the right side of several sentences in the first paragraph, then tossed the pamphlet aside, angrily saying “This is stupid.” She had no weakness; however, when drift was tested, her left palm rose, and she veered when walking. Sometimes she veered leftward, and she collided with the door frame as she left the examination room; however, within the examination room and when trying to find a chair, she moved in a tight, rightward circle, looking for the seat that was directly in front of her as she entered: she finally found it by nearly tripping over it. The neurologist ordered a brain MRI, which revealed a right parietal stroke; the geriatrician was much surprised, saying “she had no symptoms, just delirium.”
COMMENT
Spatial neglect symptoms can be misclassified or misattributed to problems with concentration or motivation. Losing orientation in space, in a patient who does not have an obvious hemiparesis, can be mistaken for a problem of continuous attention. A patient can have both problems; spatial neglect is a strong risk factor for developing delirium after stroke,32 either because cognitive problems increase delirium vulnerability or because right brain systems play a role in delirium development.33
Further, this patient not only failed to report a left-sided visual stimulus when one was simultaneously present on the right side, she also sometimes falsely reported a left-sided stimulus when none was there. Higher visual processing that occurs after stimuli is processed in the occipital lobes occurs in two parallel association cortex pathways in which dysfunctions can lead to agnosias. The dorsal pathway extends to the parietal lobe and processes location and motion and is therefore known as the Where pathway. The ventral pathway extends to the temporal lobe and processes shape, form, and color and is therefore known as the What pathway. This case is consistent with Where spatial neglect, in which distorted perceptual-attentional input lowers the resolution of sensory perception and thus reduces the accuracy of simple stimulus detection. Patients will make false-positive as well as false-negative (omission) errors.
“AIMING” SPATIAL NEGLECT: MOTOR-RELATED SYMPTOMS.
Another reason why clinicians can miss spatial neglect if they think of it as a higher-order visual problem is that it can manifest with only maladaptive spatial movements (eg, disinclination to move in one direction with the eyes, head, arms, or whole body [TABLE 6-2]). Patients may have head and eye deviation and spontaneous rotation rightward after a right brain lesion, as in CASE 6-2 (FIGURE 6-134). Even when lying in bed, they may rotate, positioning their bodies crookedly, lying unevenly and pushing their bodies farther to one side until one leg hangs off. These asymmetric movements are well-established as a primary manifestation of spatial neglect17 and may actually be associated with a better response to spatial retraining during rehabilitation.35 Maladaptive and spatially asymmetric movements may be limb-specific. The arm opposite the side of a stroke may not be particularly weak; however, it may not move well or may demonstrate poor persistence of movement.36
TABLE 6-2.
Key Components of Aiming Spatial Neglect
| Symptom | Abnormality (after right brain damage) | Finding (after right brain damage) |
|---|---|---|
| Motor extinction | Difficulty moving both sides of the body at the same time, with the left body failing to move properly when the right body is activated | Patient raises only the right arm when asked to raise both arms; however, strength tested to confrontation is good in both arms |
| Directional hypokinesia | Problems moving leftward with the eyes, head, limbs, or axial body; not accounted for by paralysis alone | Patient sits, stands, and moves with rightward rotation; veering while ambulating can cause collisions |
| Hemispatial hypokinesia | Smaller or weaker movements in left space as compared with right space | The patient’s grip with either hand is weaker to the left of the body than it is with the hand positioned in the right body space |
| Limb hypokinesia | Smaller or weaker movements by the left hand, arm, and even leg compared with the right limbs; not accounted for by paralysis alone | Similar to motor extinction, except that spontaneous left arm movements are weak or small even when that limb moves in isolation; however, strength tested to confrontation is good in both arms; patient may “forget” arm and leave it in an unsafe position |
| Defective motor response inhibition | Stimulus-evoked responses in a leftward direction or with the left body; cannot be inhibited by goal-oriented, conscious intention although the right body can be inhibited | Patient cannot inhibit leftward glances or cannot inhibit grasp or reach (leftward or with left hand or arm) while walking, during transfers, or during complex activities (eg, using power equipment); can interfere with safety |
CASE 6-2.
A 45-year-old man was brought to the emergency department by his employer because the employer noted that the patient suddenly “can’t turn left.” His employer found that the patient, a food service delivery driver, had made only four deliveries after a full day of driving and was behaving strangely. The global positioning system (GPS) records in his vehicle revealed he had been making deliveries by making exclusively right turns. The patient showed how he operated the steering wheel using only his right hand, and when asked why he was not using his left hand, he said it “is lazy” and “won’t work right.”
On examination, he had head and eye deviation to the right; when the examiner moved the patient’s head to the right, his eyes moved about 5 degrees conjugately leftward, supporting a cortical cause of his gaze deviation. He had left pronator drift and reduced left grip strength; however, during most of the examination, his left arm hung motionless, as if it were plegic. When asked to bisect a long line drawn on a piece of printer paper, he made a mark about 7.6 cm (3 in) to the right of the actual line center. While he was sitting on the gurney in the emergency department, he leaned to the right, and although when urged, he could sit straight again, his posture quickly returned to an asymmetrical lean; it looked like this postural bias could cause him to slip off the gurney. A nurse found him on the floor about 1 hour later. When the team reviewed the patient’s brain imaging, they found he had a right-sided putaminal ischemic stroke with some extension into subcortical white matter.
COMMENT
Accurate line bisection cannot rule out spatial neglect, but a survivor of stroke who errs more than 1 cm (0.4 in) rightward in bisecting a horizontal line longer than 22 cm (8.7 in) is highly likely to have spatial neglect.
FIGURE 6-1. Photo of a patient with a right brain stroke and left spatial neglect who maintained an abnormal, asymmetric posture. His rightward head and eye turning (apparent rotation of his torso) caused severe neck and back pain.

Image courtesy of Victor W. Mark, MD. Reprinted with permission from Mark VW, Front Biosci.34 © 2003 The Author.
Asymmetric gaze and movements (eg, in ambulation) are integral components of Aiming spatial neglect as it is observed during functional performance. As illustrated in CASE 6-2 and FIGURE 6-1, Aiming spatial neglect can cause an abnormal, persistent ipsilesional turning tendency that affects the eye, head, and body. The abnormally asymmetric posture caused by Aiming spatial neglect may explain the greatly increased risk of falls in people with spatial neglect, which is at least 50% higher and may be as much as 5 times higher than in survivors of stroke who do not have spatial neglect with attendant increased risk of accidental injury.37,38 Aiming spatial neglect also causes rightward bias in environmental movements; affected survivors of stroke with right brain stroke and Aiming spatial neglect can have a right-turning bias, as they move in the home, in the neighborhood, and even when driving (CASE 6-2).
Identifying Aiming Spatial Neglect
Unfortunately, spatial neglect has been traditionally regarded as a visual problem, and thus, no method of bedside screening has been established for neurologists to use to reliably identify Aiming spatial neglect. The abnormalities that can be caused by Aiming spatial neglect after right brain stroke do occur independently of perceptual-attentional Where spatial neglect; thus, survivors of stroke with Aiming spatial neglect may have intact awareness and ability to detect stimuli in both sides of space.
Aiming spatial neglect at the bedside can be identified when patients have marked leaning, veering, or postural rotation to the ipsilesional side (FIGURE 6-1), although contralesional leaning (“pushing”) can sometimes be observed. The “hanging eyeglasses” sign shown in FIGURE 6-2 may be seen; it is caused by hypometric leftward movement of the hand when placing the eyeglasses on the head. Key components of Aiming spatial neglect are also described in TABLE 6-2.
FIGURE 6-2. Photo of a man with spatial neglect after right brain stroke manifesting the hanging eyeglasses sign, which is evidence of asymmetric movement. The left temple of the eyeglasses rests on the side of the head, above the ear, because the leftward movement of the hand is too small to seat the eyeglasses on the ear on the left side.

Image courtesy of Victor W. Mark, MD. Reprinted with permission from Mark VW, Front Biosci.34 © 2003 The Author.
Visual extinction to double simultaneous stimulation may indicate the presence of Where spatial neglect because of dysfunctional perceptual-attentional spatial processing. However, paper-and-pencil tasks, such as bisecting a line (CASE 6-2) or marking all the lines scattered on a piece of paper (the Albert line cancellation task39), seem to require both Where and Aiming spatial skills. Therefore, the clinician can use these paper-and-pencil tests to help with screening anyone suspected of having spatial neglect.40
Screening for extinction to double simultaneous stimulation and for abnormal performance on paper-and-pencil tests is not sufficient to evaluate Aiming spatial neglect, however. Some survivors of stroke have only the body movement and postural abnormalities that are characteristic of Aiming spatial neglect (TABLE 6-2) without demonstrating any abnormalities on paper-and-pencil screening.41 Because these spatial movement abnormalities are not assessed in any of the available bedside tests such as the National Institutes of Health Stroke Scale or paper-and-pencil tests and are likely responsible for motor disability, fall risk, and other limitations that affect function and freedom in spatial neglect, an assessment is needed that examines movement performance. Thus, in any survivor of stroke suspected of having spatial neglect, this author recommends referral to an occupational or physical therapist who can screen for functional performance errors with a standardized measure.
The Catherine Bergego Scale,42 which has been validated in many medical and rehabilitation settings, is one measure used by those professionals that is strongly recommended to detect spatial movement abnormalities that affect daily life function and safety. The test captures asymmetric functional body movements and predicts daily life disability related to spatial neglect.6 Because of the time required to administer the scale and its ease in combining with a functional assessment in therapy care, a therapist rather than a neurologist is most likely to use this scale. If performed as a completely separate evaluation, the Catherine Bergego Scale takes about 15 to 20 minutes. If it is combined with a clinically standard therapy assessment of activities of daily living, the Catherine Bergego Scale takes only about 5 minutes of documentation time in addition to the therapy assessment. It also requires the examiner to be reliability-trained at observing and scoring spatial performance in patients while they actually perform daily life tasks.
“WHERE” REPRESENTATIONAL FUNCTION IN SPATIAL NEGLECT.
Spatial cognition entails an intermediate information processing stage between Where perceptual-attention and Aiming motor-intention in which explicit and implicit spatial representations (ie, knowledge in the form of imagery, maps, and internal descriptions) are required. These can be visual-spatial, auditory-spatial, and even spatial-somesthetic representations, and any of these may be impaired in spatial neglect. Although disordered spatial representations may sound like a theoretical problem, this issue has functional consequences. In the modern world, we need internal maps while we are working, completing our social activities, and even interacting with groups and navigating crowded environments. The survivors of stroke who have an isolated deficit of Where spatial neglect affecting representational function may have trouble drawing or interpreting maps or charts and may be perceived as confused, intellectually impaired, or perseverative when the problem is a loss of spatial knowledge.
At the bedside, one sign of representational spatial neglect is right-sided bias affecting the way we use a mental number line.43 Mathematical operations rely on our ability to spatially map numeric information in our minds; the left side of these operations may be distorted when people have Where representational spatial neglect. To test this ability, the examiner can ask the patient to “tell me what number is halfway between 11 and 55.” Giving two numbers that require little analysis to calculate a midpoint (eg, 10 and 30) should be avoided. When administering this test to a survivor of stroke whose educational level and general ability to perform intellectually demanding tasks are appropriate for this testing of mental mathematics (eg, a college professor), the examiner may be surprised by a “rightward” biased response (eg, 50).
Summary of Spatial Neglect Subtypes
Although the daily life performance errors made by people with spatial neglect are often interpreted as being exclusively related to perceptual or attentional problems, many functional impairments and limitations on daily life activities and participation also result from Where, representational problems, or Aiming spatial neglect. For example, because people with directional hypokinesia have difficulty initiating leftward movement, they may find themselves unable to dress on the left or may steer a wheelchair rightward just as the patient in CASE 6-2 steered his truck with exclusively right turns. TABLE 6-344–46 summarizes some of the potential relationships between spatial neglect subtypes and observed daily life limitations.
TABLE 6-3.
Spatial Neglect Subtypes and Potential Associated Deficitsa
| Spatial neglect subtype | Key behaviors demonstrating spatial neglect | Frequently observed functional impairmentsb |
|---|---|---|
| Where, perceptual-attentional awareness 45 | Extinction to double simultaneous stimulation, decreased vigilance in left body space | Accidents due to failure to respond to left-sided events; failure to respond in social interaction; difficulty in eating entire meal |
| Where, representational imagery 46 | Visual imagery incomplete on left, neglect dyslexia, right bias on mental number line | Poor environmental navigation; illusions, difficulty recognizing objects especially under unfamiliar conditions; difficulty reading or completing mathematical operations accurately for work, financial activities |
| Aiming, motor intention | Abnormalities listed in TABLE 6-2 | Postural imbalance, veering while ambulating while walking or in a wheelchair, augmented hemiparesis (weakness out of proportion to motor dysfunction) |
Modified with permission from Barrett AM, et al, Handb Clin Neurol.44 © 2019 Elsevier Ltd.
Proposed, further systematic research demonstration is needed.
Trends
This section describes treatment that is now established to be effective in reducing daily life disability for people with spatial neglect. These protocols have been demonstrated to be effective in stroke; further research in spatial neglect associated with other disorders is needed.
REHABILITATION OF SPATIAL NEGLECT.
Although the Centers for Disease Control and Prevention reported that 30% to 35% of survivors of stroke receive rehabilitation after leaving acute hospital care in many states,47 the National Institute of Neurological Disorders and Stroke estimates that about twice as many would benefit from rehabilitation.48 Neurologists frequently find that survivors of stroke who received meticulous attention to highest-quality standards of care in the first hours and days after their stroke received little or no rehabilitation once they left acute settings. This treatment gap needs to be closed for all patients with spatial neglect, and, in particular, for survivors of stroke with the spatial neglect syndrome.
At least 10% of people who have spatial neglect acutely continue to have chronic symptoms.49 As discussed earlier, ipsilesional bias can be observed in people with spatial neglect. It is not uncommon to observe that, years after stroke, a patient may have trouble eating from the left side of a plate (CASE 6-3). This is not only interesting to observe, it is pragmatically important. The Intercollegiate Stroke Working Party50 in the United Kingdom warns that people with stroke and spatial neglect should “be monitored to ensure that they do not eat too little through missing food on one side of the plate.” Patients with spatial neglect after right brain damage may fail to dress the left side of their body or may favor right space when navigating a wheelchair. This limits their independence, but it also has a devastating effect on their dignity.
CASE 6-3.
A 62-year-old man presented to a neurologist after his wife noticed that he ate only from the right side of his plate and was falling frequently. He had a right middle cerebral artery ischemic stroke 1 year before.
On examination, he had a mild left hemiparesis. Screening for extinction in the visual and tactile modalities and errors on line bisection and cancellation tasks revealed chronic spatial neglect. The patient had received acute stroke care at a comprehensive stroke center and had sought care from tertiary-care specialists at the local academic medical center for his cardiology and internal medicine needs. He had also received nursing and some kind of therapy (unspecified in his records; possibly physical therapy) through home health for the first few weeks after the stroke to address left-sided weakness; however, since the home health therapy ended, the patient and his wife reported that he received no specific outpatient therapy and no therapy targeted to improve spatial neglect.
COMMENT
The classic teaching to clinicians was that spatial neglect invariably resolves spontaneously. However, 10% or more of survivors of stroke have chronic spatial neglect years after a stroke.49
The Centers for Disease Control and Prevention reports that more than 60% of survivors of stroke receive absolutely no outpatient rehabilitation.47 Referring patients with spatial neglect for spatial retraining, as endorsed by professional organizations, helps them recover to greater independence and quality of life.19
The American Heart Association19 and American Occupational Therapy Association,51 as well as stroke organizations worldwide, recommend spatial neglect treatment as part of quality stroke care. Neurologists cannot usually administer spatial neglect treatment in their offices; however, the neurologists’ efforts are very important in assembling and directing a treatment team to administer rehabilitation. Evidence on which professional guidelines and recommendations are based is not yet available for other neurologic disorders associated with spatial neglect. Further research in this area is needed.
PRACTICE-BASED IMPROVEMENT.
Guideline-based early rehabilitation protocols for spatial neglect are available. Keeping this information for reference is an area for practice-based improvement.
Practice-based improvement for spatial neglect also requires the neurologist to digest information about personalized care (spatial neglect subtypes, noted earlier) because spatial neglect is undiagnosed in patients who receive no formal assessment or who have Aiming spatial neglect symptoms. However, a second reason, beyond the need to diagnose the presence of spatial neglect, is that personalized care is important. Just as different kinds of cardiac disease (eg, arrhythmia, heart failure, ischemia) should be prioritized and addressed differently in a treatment plan, patients with Aiming spatial neglect symptoms35 may be especially likely to improve after early spatial retraining (see TABLE 6-452–57 for treatment options).
TABLE 6-4.
Professional Guidelines for Spatial Neglect Treatmenta
| Professional organization endorsement | Spatial neglect treatment for any patient | Targeted treatment to improve Aiming spatial neglect and arousal/activation | How might this treatment work to improve outcomes?b |
|---|---|---|---|
| American Heart Association (AHA),19 American Occupational Therapy Association,51 Intercollegiate Stroke Working Party50 | Prism adaptation therapy17 | Prism adaptation therapy | May reduce Aiming spatial neglect, making leftward and left-body movements more adaptive |
| US Department of Veterans Affairs,52 Intercollegiate Stroke Working Party | Compensatory, self-mediated cuing strategies53 | Not applicable | Not a treatment, rather a management technique that may reduce the possibilities for error |
| AHA, Intercollegiate Stroke Working Party | Visual scanning training54 | Not applicable | Neuropsychological mechanisms uncertain |
| AHA, Intercollegiate Stroke Working Party | Limb activation55,56 | Limb activation | Proposed to stimulate body-based spatial systems, alter interhemispheric interaction; may also improve limb hypokinesia and Aiming spatial neglect |
| Canadian Partnership for Stroke Recovery 57 | Medications (acetylcholinergic, nicotine, noradrenergic) | Medications (dopaminergic, noradrenergic) | May improve attention, alertness, arousal, generative behavior, persistence, memory; paradoxical worsening can occur with dopaminergic treatment |
Modified with permission from Barrett AM, et al, Handb Clin Neurol.44 © 2019 Elsevier Ltd.
Proposed, further research is needed.
Aiming spatial neglect may predict an excellent response to treatment as compared with Where spatial neglect.58 The brain mechanisms explaining this observation are under research; however, this may be related to frontal lobe disconnection in spatial function. Different neuroanatomic regions might play different roles in the networks supporting Where versus Aiming spatial cognitive processing, with posterior, temporal-parietal cortical sensory association networks more critical in supporting information input (Where spatial neglect) or storage (Where, representational spatial neglect59) whereas anterior or motor-related subcortical networks are more critical to output cognition (Aiming spatial neglect17).
Treatment
Because it is supported by both the American Heart Association and American Occupational Therapy Association and because it is a low-risk, 10-day protocol that therapists can learn to use in a few days, prism adaptation therapy is appropriate as a first-line treatment. This author recommends that neurologists refer survivors of stroke with spatial neglect, especially if Aiming spatial neglect is suspected, for this treatment protocol. FIGURE 6-360 illustrates improvement in daily life function after prism adaptation therapy, in multiple studies; although the impact of treatment varied in different studies, better-designed studies showed stronger benefit, supporting the value of this treatment approach.
FIGURE 6-3. Evidence of prism adaptation treatment effects on daily living skills: reading/writing, activities of daily living (ADL) direct tests, ADL questionnaires, and environmental navigation tests.
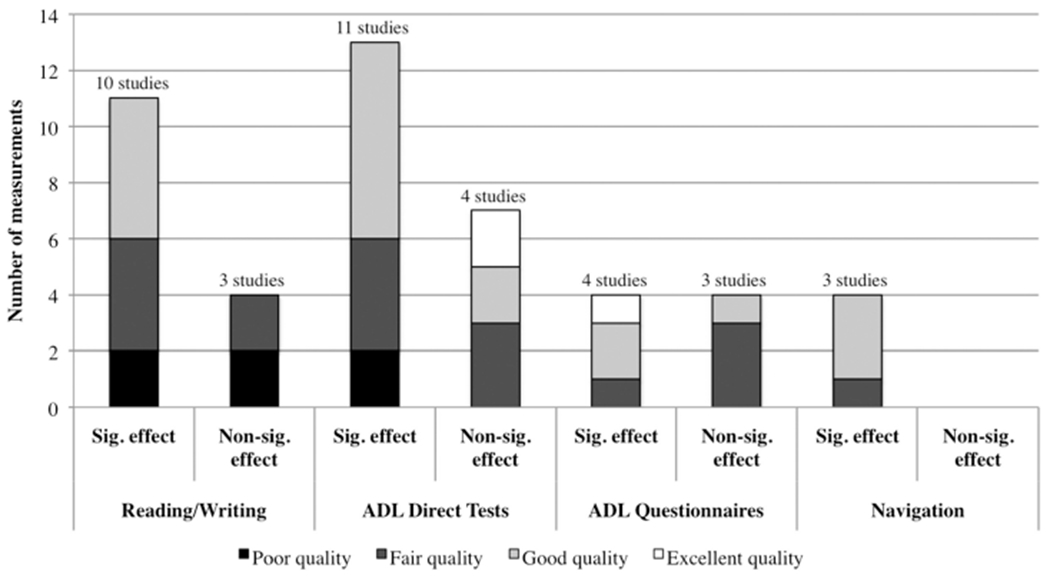
Sig. = significant; non-sig. = non-significant.
Reprinted with permission from Champod AS, et al, Neuropsychol Rehabil.60 © 2016 Taylor & Francis Ltd.
During prism adaptation therapy for left-sided spatial neglect,61 patients wear left-based, yoked optical prisms, which shift what they see rightward. They then make multiple goal-directed hand movements for a prescribed session of about 20 to 30 minutes. Although in randomized clinical trials of this approach some parameters of the treatment varied, in one standard regimen, patients wore 20-diopter wedge prisms and completed 10 sessions over 14 days.58 Patients wear prisms only during treatment sessions; at other times, they may engage as usual in other activities or rehabilitation.
FINDING A TREATMENT TEAM.
A neurologist may be tempted to delegate all of the tasks of evaluation and treatment for rehabilitation to the therapist with whom care is being shared. However, as evidence-based protocols of treatment emerge, it is very helpful for a neurologist to share the responsibility with the therapy clinicians to ensure that patients receive quality care. When neurologists work with a partner organization to whom they send stroke therapy referrals, it is very helpful to meet with this partner, project the need for quality standard care, estimate the potential volume of referrals, and describe the benefit in improved outcomes, providing information about how therapists may need to be trained or what equipment may be needed. In the experience of this author, many therapists or therapy business administrators have busy day-to-day experiences that have prevented them from researching new treatment options. However, they are often willing to consider how their organization can offer a new treatment protocol, which can distinguish their therapists as experts, drive referrals, and help engage their staff and enhance staff retention.
DIRECTING SPATIAL NEGLECT TREATMENT PROTOCOLS.
In the past, allied health professionals received little support from neurologists about how to plan or execute rehabilitation care. Fortunately, most therapy clinicians are very resourceful and can make treatment decisions independently. However, focus group studies indicate, consistent with this author’s personal experience, that evidence-based treatments recommended by professional associations are not being selected by therapists in this kind of independent consultation partnership.62 Therapists may have learned about spatial neglect during training and may feel more comfortable with continuing to use other treatment approaches (eg, self-reminders to “look left,” or prism exposure, in which patients wear prisms without making visually guided movements).
To be successful in leading a spatial neglect treatment team, the neurologist should take a collaborative, decisive leadership role. Unlike the isolated practitioner of the past, the modern neurologist “leads and facilitates clinical teams, builds tools to implement new therapies, and communicates the vision and purpose of team-based care.”63 Because most neurologists do not directly employ or supervise speech-language pathologists or occupational or physical therapists, this means that the neurologist should use interpersonal leadership skills (persuasion and influence) to motivate and engage the treatment team and help the team examine and continuously improve performance. In a 2018 article, this author and colleagues in the American Academy of Neurology Transforming Leaders Program63 described this vision for the 21st century: neurologist-led team-care pathways are “essential to achieving the triple aim … improving functional outcomes, cost-effectiveness, and the patient experience. The care pathway from diagnosis to treatment for spatial neglect, using prism adaptation, traverses this new territory…”
UNAWARENESS OF DEFICIT (ANOSOGNOSIA) AFTER RIGHT BRAIN STROKE
Anosognosia means, literally, “without knowledge of disease.” People with neurologic disorders can demonstrate reduced awareness, or complete disavowal, of disabling consequences of brain dysfunction; notably, after right brain stroke, patients can appear unaware of left hemiparesis.64,65
Clinical Presentation
Anosognosia for hemiparesis has long been identified as a sign of right brain stroke,66 especially when it is moderate to severe.67 However, people with anosognosia after right brain stroke can also be unaware of visual and other sensory disturbance67 and other problems such as memory and cognitive deficits and gait disorder.18
Unawareness of deficit is a major barrier to receiving health care, although formal studies of this relationship are not available. Numerous anecdotal reports suggest that unawareness of deficits after right brain stroke prevents patients from presenting promptly for stroke evaluation. Many patients continue to drive under unsafe circumstances after having a stroke, unaware of their signs and symptoms. For example, a patient may have a stroke caused by the effect of recreational stimulant use on preexisting hypertension and cerebrovascular disease, lose control of the car while driving, and have an accident. Anosognosia after right brain stroke also interferes with reporting other medical symptoms that require immediate attention. In one report, a patient who was previously able to report angina reliably lost awareness of this left-body symptom after a stroke.68
The inability to recognize functional limitations poses a significant safety risk, is associated with falls,69 and is known to be a major barrier to effective rehabilitation.70 Anosognosia can take several forms, with different degrees of disavowal of the neurologic deficit (TABLE 6-571). It can be associated with a loss of the feeling of ownership of an impaired body part (asomatognosia) or a distorted experience of an impaired body part (somatoparaphrenia) and, at the far end of the continuum, a dislike or hatred of a dysfunctional body part (misoplegia).72 Although misoplegia is uncommon, it can cause dramatic behavioral problems, such as patients throwing themselves from their beds “in order to get rid of that awful leg.” Although classic studies support an association of right brain dysfunction with anosognosia for hemiparesis,65 awareness networks for motor and cognitive function are complex and still being investigated.73
TABLE 6-5.
Different Forms of Anosognosia That Can Manifest After Right Brain Strokea
| Term | Anosognosia type |
|---|---|
| Anosodiaphoria | Lack of emotional concern for deficits that are verbally acknowledged |
| Anosognosia with causal attribution abnormality | Acknowledgment of the deficit without linking the problem to a neurologic issue (eg, a patient saying, “I don’t feel like moving my left arm right now.”); inconsistent verbal acknowledgment (patient reports that others believe the deficit is present) |
| Anosognosia with implicit awareness | Conscious disavowal of deficits that are acknowledged through behavior (eg, patient claims the ability to walk but never actually tries to get out of bed) |
| Anosognosia with modality specificity | Acknowledgment of one deficit but not others (eg, patient is aware of a visual field cut but not aware of hemiplegia) |
| Anosognosia without implicit awareness | Combined explicit and implicit disavowal of deficits (both behavioral and verbal reports) without acknowledgment of deficits |
Data from Ofrei MD, et al.71
How to Assess for Anosognosia
Formal assessment of awareness of deficit is very important because of its potential impact on the outcomes of survivors of stroke and their caregivers.74 It is also easy to miss anosognosia for hemiparesis when patients have an amotivational state, are saying little, or have depression or decreased engagement. When a survivor of stroke is aware of other symptoms, the neurologist may incorrectly assume that the patient is aware of hemiparesis. Thus, this author recommends that neurologists always assess awareness of hemiparesis as part of acute care of stroke or right brain injury. People with right brain stroke and anosognosia for hemiparesis experience the same range of emotions as control subjects.75 In many of these people, awareness of the disabling consequences of their stroke and hemiparesis may be implicit (unconscious). This means they may not be able to articulate the awareness or their distress.76 Thus, they may even be at higher risk of depression than other patients with right brain stroke. It also means that we should not assume survivors of stroke are aware of deficits just because they have dysthymia or depression.
Formal screening for anosognosia for hemiparesis is described in CASE 6-4 and can be completed in just a few seconds. Although with this quick formal screening it is not possible to identify anosognosia that causes relatively small-magnitude overestimation of self-performance, large-magnitude inconsistencies between self-rating and performance are regularly identified by using this method.
CASE 6-4.
A 27-year-old man was seen in neurologic consultation on a brain injury unit at a rehabilitation hospital after admission subsequent to a car accident that had occurred while he was intoxicated with stimulants and cocaine. He reportedly experienced a “hemorrhagic brain injury,” a pelvic fracture, and multiple other injuries. Medical record review revealed elevated blood pressure since admission (systolic blood pressure approximately 150 mm Hg to 170 mm Hg).
On examination, his memory was normal for orientation and three-object recall and calculations were intact. He demonstrated psychomotor slowing; however, all of his reporting about his history and prior work experience was consistent with the chart and with normal reasoning ability. His apparent mood was euthymic. Spontaneously, he appeared to have left hemiparesis affecting his face, arm, and leg, with the greatest weakness in his arm and hand. Effort was decreased on confrontation strength testing; the examiner had to reposition the patient’s left arm several times when testing pronator drift because, although he was strong enough to elevate his hand, he kept dropping it, as if tired. At the end of the interview, the examiner drew a vertical line on a piece of paper, wrote “normal strength” at the top of the line and “cannot move” at the bottom of the line, and told the patient “I want you to rate the strength you showed on the testing just now for the left side of your body and your left arm. You might mark here [gesturing to the top of line] if your strength is normal; you might mark here [gesturing to the bottom of line] if you cannot move your left body at all. Or, you might mark your strength somewhere in between [gesturing to show the whole line].” The patient took the pen and, without hesitation, made a mark at the very top of the vertical line. The examiner asked, “Does this mean you don’t feel weak on the left side?” The patient looked at the examiner mildly and said, “Everyone says I am weak, but I don’t feel weak at all.” The examiner was subsequently able to view the patient’s brain image from the acute care hospital; it revealed a right putaminal hemorrhage.
COMMENT
A classic teaching about anosognosia is that only patients with global cognitive impairment have unawareness of deficit.77 However, many patients, like the one described in this case, have relatively normal memory and yet are unaware of their neurological deficits. Testing for unawareness of deficit (anosognosia) with a visual analog scale (the line the patient marked) can be very helpful and useful to show to staff or caregivers so that the neurologist can discuss anosognosia as a neurologic deficit independent of motivation, psychological denial, or willingness to recover.
Some patients “know what to say” when asked about their deficits after stroke; however, they do not seem concerned about this information (anosodiaphoria) (TABLE 6-5)71; this represents a form of anosognosia. They may report, for example, “Everyone tells me that my arm is weak.”
It is very helpful to communicate with other clinicians (nurses, therapists, social workers) and with caregivers about the anosognosia assessment because many people confuse this disorder with psychological denial or think that it is protective from the emotional impact of having a brain disorder. The vertical line rating is a convenient way of introducing the message that this problem is a reality distortion caused by the brain injury. Neurologists can, for example, show the vertical line as in CASE 6-4 and communicate that the patient actually experiences left arm strength as being normal. Researchers specifically examining whether anosognosia protects patients from depression found no evidence that it does.78 In the past, when members of the care team assumed that anosognosia was protective to the patient, efforts to provide cognitive rehabilitation were reduced, and this would be expected to increase disability.
Trends
This section reviews the need for rehabilitation of anosognosia after right brain stroke and approaches to this rehabilitation.
REHABILITATION OF ANOSOGNOSIA AFTER RIGHT BRAIN STROKE.
It is, first, extremely important for the neurologist to understand that studies very clearly demonstrate that patients with right brain disorders and anosognosia still benefit from intensive multidisciplinary rehabilitation, such as inpatient rehabilitation hospitalization. Second, specific approaches have been developed to improve anosognosia in stroke and traumatic brain injury. Even though randomized controlled trials of these treatments to improve anosognosia for hemiparesis after stroke are not yet available, several treatment approaches have been associated with improved rehabilitation outcome benefits in open-label studies, including self-awareness training,79 spatial neglect interventions such as vestibular stimulation,80 and self-observation via video feedback.81 As described earlier, many therapy clinicians delivering stand-alone care may not implement specific anosognosia treatment because they are familiar with using techniques based on expert wisdom, such as reminders, caregiver counseling, or management for safety. However, for best-quality care, neurologists may want to ensure that their patients receive specific treatment for anosognosia, which may often require an interprofessional program that administers particular behavioral protocols. This is yet another justification for considering inpatient rehabilitation if a survivor of stroke is otherwise eligible.
Such a therapy team includes neuropsychology, speech-language pathology, and occupational and physical therapy, and clinicians may have certification as “brain injury specialists.” Frequently, clinicians cross-apply approaches to remediate awareness, approaches that were developed in traumatic brain injury settings, to survivors of stroke or people with brain tumors or other focal brain disorders who are enrolled in therapy.
CONCLUSION
In this article, two major cognitive syndromes resulting from right brain injury, spatial neglect and anosognosia, were reviewed. As stroke centers begin to evaluate cognitive assessment with standardized measures during inpatient stroke care, it is likely that more and more people with these disorders after stroke will be diagnosed and that neurologists will play a greater role in leading a treatment team to address these important invisible disabilities. Unfortunately, not much evidence is available at present to guide assessment and treatment of spatial neglect due to other focal brain disorders, and this is an appropriate topic for future research.
The diagnosis and treatment of spatial neglect and the feasibility and utility of using prism adaptation treatment as a first-line therapy with a rehabilitation team were also reviewed in this article. Although randomized controlled trials and professional guideline recommendations strongly support specific identification and treatment of poststroke spatial neglect, neurologists can help this move forward.
For unawareness of deficit, neurologists can play a vital role in counseling caregivers and other clinicians about the crucial distinction between anosognosia and psychological denial and steering the patient to an interdisciplinary setting where specific protocols of care for anosognosia are used. Both stroke and traumatic brain injury are associated with anosognosia to left hemiparesis. In a broader sense, this author is concerned that anosognosia presents a public health barrier to allocation of resources to people with right brain dysfunction. People who have experienced a right brain injury may be unable to report their significant disability, leading to falsely inflated reports of good quality of life or well-being. It is also concerning how anosognosia affects access to health care and rehabilitation. In recent years, patients with unawareness of deficit have been systematically limited from admission to inpatient rehabilitation or other intensive treatments. However, if patient-reported outcomes become the main path of entry to rehabilitation care, it is up to the neurology community to assure access to inpatient rehabilitation and other forms of intensive therapy for our patients who cannot self-advocate.82
KEY POINTS.
The unsafe behaviors observed as symptoms of spatial neglect can be misattributed to intellectual, reasoning, personality, or generic cognitive problems, which can be devastating to patient dignity.
Misattribution of spatial neglect symptoms can delay diagnosis of right brain stroke past the window for acute stroke interventions; thus, clinicians should be alert to new spatial bias in patients with stroke risk factors.
Aiming spatial neglect causes several different movement-related problems. Spatial neglect can cause an ipsilesional turning tendency and disinclination to move in the direction opposite the brain lesion. This symptom is disabling beyond the effects of hemiparesis or awareness problems.
In-hospital fall riskcan be more than 5 times higher in people with spatial neglect than in those without it. Further, active movements that are posturally biased means that the best supervision and guarding by nursing assistants and other personnel may not be effective.
Line bisection is a simple spatial neglect screening test. Accurate line bisection cannot rule out spatial neglect, but more than 1 cm (0.4 in) of rightward error in bisecting a horizontal line longer than 22 cm (8.7 in) is highly likely to indicate that a patient has spatial neglect.
Many survivors of stroke benefit from continuing rehabilitation, but most receive no outpatient rehabilitation. Referring patients with spatial neglect for spatial retraining, as endorsed by professional organizations, helps them recover to greater independence and quality of life.
Ten percent or more of survivors of stroke have chronic spatial neglect years later.
The degree of unawareness of deficit can be varied; patients with anosognosia may “know what to say,” about their deficits and yet they may not believe they are disabled.
RELATIONSHIP DISCLOSURE:
Dr Barrett has received research/grant support from the Kessler Foundation, and her institution has received research/grant support from the National Institutes of Health/Veteran Health Association and the Wallerstein Foundation for Geriatric Improvement.
Footnotes
UNLABELED USE OF PRODUCTS/INVESTIGATIONAL USE DISCLOSURE:
Dr Barrett reports no disclosure.
REFERENCES
- 1.Chen P, McKenna C, Kutlik AM, Frisina PG. Interdisciplinary communication in inpatient rehabilitation facility: evidence of under-documentation of spatial neglect after stroke. Disabil Rehabil 2013;35(12):1033–1038. doi: 10.3109/09638288.2012.717585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards DF, Hahn MG, Baum CM, et al. Screening patients with stroke for rehabilitation needs: validation of the post-stroke rehabilitation guidelines. Neurorehabil Neural Repair 2006;20(1):42–48. doi: 10.1177/1545968305283038 [DOI] [PubMed] [Google Scholar]
- 3.Palmerini F, Bogousslavsky J. Right hemisphere syndromes. Front Neurol Neurosci 2012;30:61–64. doi: 10.1159/000333411 [DOI] [PubMed] [Google Scholar]
- 4.Losoi H, Kettunen JE, Laihosalo M, et al. Predictors of functional outcome after right hemisphere stroke in patients with or without thrombolytic treatment. Neurocase 2012;18(5):377–385. doi: 10.1080/13554794.2011.608369 [DOI] [PubMed] [Google Scholar]
- 5.McCluskey G, Wade C, McKee J, et al. Stroke laterality bias in the management of acute ischemic stroke. J Stroke Cerebrovasc Dis 2016;25(11):2701–2707. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 6.Chen P, Hreha K, Fortis P, et al. Functional assessment of spatial neglect: a review of the Catherine Bergego Scale and an introduction of the Kessler Foundation Neglect Assessment Process. Top Stroke Rehabil 2012;19(5):423–435. doi: 10.1310/tsr1905-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gainotti G, Messerli P, Tissot R. Qualitative analysis of unilateral spatial neglect in relation to laterality of cerebral lesions. J Neurol Neurosurg Psychiatry 1972;35(4):545–550. doi: 10.1136/jnnp.35.4.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denes G, Semenza C, Stoppa E, Lis A. Unilateral spatial neglect and recovery from hemiplegia: a follow-up study. Brain 1982;105(pt 3):543–552. doi: 10.1093/brain/105.3.543 [DOI] [PubMed] [Google Scholar]
- 9.Fullerton KJ, McSherry D, Stout RW. Albert’s test: a neglected test of perceptual neglect. Lancet 1986;1(8478):430–432. doi: 10.1016/s0140-6736(86)92381-0 [DOI] [PubMed] [Google Scholar]
- 10.Stone SP, Halligan PW, Greenwood RJ. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age Ageing 1993;22(1):46–52. doi: 10.1093/ageing/22.1.46 [DOI] [PubMed] [Google Scholar]
- 11.McGlone J, Losier BJ, Black SE. Are there sex differences in hemispatial visual neglect after unilateral stroke? Neuropsychiatry Neuropsychol Behav Neurol 1997;10(2):125–134. [PubMed] [Google Scholar]
- 12.Kalra L, Perez I, Gupta S, Wittink M. The influence of visual neglect on stroke rehabilitation. Stroke 1997;28(7):1386–1391. doi: 10.1161/01.str.28.7.1386 [DOI] [PubMed] [Google Scholar]
- 13.Ringman JM, Saver JL, Woolson RF, et al. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology 2004;63(3):468–474. doi: 10.1212/01.wnl.0000133011.10689.ce [DOI] [PubMed] [Google Scholar]
- 14.Chen P, Chen CC, Hreha K, et al. Kessler Foundation Neglect Assessment Process uniquely measures spatial neglect during activities of daily living. Arch Phys Med Rehabil 2015;96(5):869–876.e1. doi: 10.1016/j.apmr.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerbeck U, Gittins M, Vail A, et al. Spatial neglect in stroke: identification, disease process and association with outcome during inpatient rehabilitation. Brain Sci 2019;9(12):374. doi: 10.3390/brainsci9120374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone SP, Halligan PW, Marshall JC, Greenwood RJ. Unilateral neglect: a common but heterogeneous syndrome. Neurology 1998;50(6):1902–1905. doi: 10.1212/wnl.50.6.1902 [DOI] [PubMed] [Google Scholar]
- 17.Barrett AM, Houston KE. Update on the clinical approach to spatial neglect. Curr Neurol Neurosci Rep 2019;19(5):25. doi: 10.1007/s11910-019-0940-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adair JC, Barrett AM. Anosognosia. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York, NY: Oxford University Press, 2012:198–213. [Google Scholar]
- 19.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47(6):e98–e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 20.Thomas JO, Barrett AM. Right brain stroke syndromes. In: Wilson R, Raghavan P, editors. Stroke rehabilitation. St. Louis, MO: Elsevier, 2019:71–89. [Google Scholar]
- 21.Riestra AR, Barrett AM. Rehabilitation of spatial neglect. Handb Clin Neurol 2013;110:347–355. doi: 10.1016/B978-0-444-52901-5.00029-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P, Ward I, Khan U, et al. Spatial neglect hinders success of inpatient rehabilitation in individuals with traumatic brain injury: a retrospective study. Neurorehabil Neural Repair 2016;30(5):451–460. doi: 10.1177/1545968315604397 [DOI] [PubMed] [Google Scholar]
- 23.Villardita C, Smirni P, Zappala G. Visual neglect in Parkinson’s disease. Arch Neurol 1983;40(12):737–739. doi: 10.1001/archneur.1983.04050110055008 [DOI] [PubMed] [Google Scholar]
- 24.Gilad R, Sadeh M, Boaz M, Lampl Y. Visual spatial neglect in multiple sclerosis. Cortex 2006;42(8):1138–1142. doi: 10.1016/s0010-9452(08)70226-0 [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Lander V, Noce N, Hreha K. Prism adaptation treatment for spatial neglect post brain tumour removal: a case report. Hong Kong J Occup Ther 2020;33(1):25–29. doi: 10.1177/1569186120921472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillen G, Nilsen DM, Attridge J, et al. Effectiveness of interventions to improve occupational performance of people with cognitive impairments after stroke: an evidence-based review. Am J Occup Ther 2015;69(1):6901180040p1-9. doi: 10.5014/ajot.2015.012138 [DOI] [PubMed] [Google Scholar]
- 27.Foerch C, Misselwitz B, Sitzer M, et al. Difference in recognition of right and left hemispheric stroke. Lancet 2005;366(9483):392–393. doi: 10.1016/S0140-6736(05)67024-9 [DOI] [PubMed] [Google Scholar]
- 28.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864 [DOI] [PubMed] [Google Scholar]
- 29.Fink JN, Selim MH, Kumar S, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke 2002;33(4):954–958. doi: 10.1161/01.str.0000013069.24300.1d [DOI] [PubMed] [Google Scholar]
- 30.Zebhouser PT, Vernet M, Unterberger E, Brem A-K. Visuospatial neglect: a theory-informed overview of current and emerging strategies and a systematic review on the therapeutic use of non-invasive brain stimulation. Neuropsychol Rev 2019;29(4):397–420. doi: 10.1007/s11065-019-09417-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett AM, Burkholder S. Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. J Rehabil Res Dev 2006;43(3):337–345. doi: 10.1682/jrrd.2005.01.0015 [DOI] [PubMed] [Google Scholar]
- 32.Pasinska P, Kowalska K, Klimiec E, et al. Poststroke delirium clinical motor subtypes: the PRospective Observational POLIsh Study (PROPOLIS). J Neuropsychiatry Clin Neurosci 2019;31(2):104–111. doi: 10.1176/appi.neuropsych.18040073 [DOI] [PubMed] [Google Scholar]
- 33.Boukrina O, Barrett AM. Disruption of the ascending arousal system and cortical attention networks in post-stroke delirium and spatial neglect. Neurosci Biobehav Rev 2017;83:1–10. doi: 10.1016/j.neubiorev.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 34.Mark VW. Acute versus chronic functional aspects of unilateral spatial neglect. Front Biosci 2003;8:e172–e189. doi: 10.2741/973 [DOI] [PubMed] [Google Scholar]
- 35.Goedert KM, Chen P, Boston RC, et al. Presence of motor-intentional aiming deficit predicts functional improvement of spatial neglect with prism adaptation. Neurorehabil Neural Repair 2014;28(5):483–492. doi: 10.1177/1545968313516872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laplane D, Degos JD. Motor neglect. J Neurol Neurosurg Psychiatry 1983;46(2):152–158. doi: 10.1136/jnnp.46.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czernuszenko A, Czlonkowska A. Risk factors for falls in stroke patients during inpatient rehabilitation. Clin Rehabil 2009;23(2):176–188. doi: 10.1177/0269215508098894 [DOI] [PubMed] [Google Scholar]
- 38.Chen P, Hreha K, Kong Y, Barrett AM. Impact of spatial neglect on stroke rehabilitation: evidence from the setting of an inpatient rehabilitation facility. Arch Phys Med Rehabil 2015;96(8):1458–1466. doi: 10.1016/j.apmr.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albert ML. A simple test of visual neglect. Neurology 1973;23(6):658–664. doi: 10.1212/wnl.23.6.658 [DOI] [PubMed] [Google Scholar]
- 40.Daffner KR, Gale SA, Barrett AM, et al. Improving clinical cognitive testing: report of the AAN Behavioral Neurology Section Workgroup. Neurology 2015;85(10):910–918. doi: 10.1212/WNL.0000000000001763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goedert KM, Chen P, Botticello A, et al. Psychometric evaluation of neglect assessment reveals motor-exploratory predictor of functional disability in acute-stage spatial neglect. Arch Phys Med Rehabil 2012;93(1):137–142. doi: 10.1016/j.apmr.2011.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azouvi P, Marchal F, Samuel C, et al. Functional consequences and awareness of unilateral neglect: study of an evaluation scale. Neuropsychol Rehabil 1996;6(2):133–150. [Google Scholar]
- 43.Zorzi M, Priftis K, Umilta C. Brain damage: neglect disrupts the mental number line. Nature 2002;417(6885):138–139. doi: 10.1038/417138a [DOI] [PubMed] [Google Scholar]
- 44.Barrett AM, Abdou A, Caulfield MD. The cingulate cortex and spatial neglect. Handb Clin Neurol 2019;166:129–150. doi: 10.1016/B978-0-444-64196-0.00009-1 [DOI] [PubMed] [Google Scholar]
- 45.Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York, NY: Oxford University, 2012:296–348. [Google Scholar]
- 46.Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex 1978;14(1):129–133. doi: 10.1016/s0010-9452(78)80016-1 [DOI] [PubMed] [Google Scholar]
- 47.Ayala C, Fang J, Luncheon C, et al. Use of outpatient rehabilitation among adult stroke survivors–20 states and the District of Columbia, 2013, and four states, 2015. MMWR Morb Mortal Wkly Rep 2018;67(20):575–578. doi: 10.15585/mmwr.mm6720a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Office of Communications and Public Liaison, National Institute of Neurological Disorders and Stroke. “Post-stroke fact sheet.” NIH Publication 20-NS-4846. April 2020. Cited January 10, 2021. ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Post-Stroke-Rehabilitation-Fact-Sheet
- 49.Gerafi J, Samuelsson H, Viken JI, et al. The presence and prediction of lateralized inattention 7 years post-stroke. Acta Neurol Scand 2020;141(5):423–430. doi: 10.1111/ane.13221 [DOI] [PubMed] [Google Scholar]
- 50.Intercollegiate Stroke Working Party. National clinical guidelines for stroke. London, UK: Royal College of Physicians, 2016. [Google Scholar]
- 51.Wolf TJ, Nilsen DM. Occupational therapy practice guidelines for adults with stroke. Bethesda, MD: American Occupational Therapy Association, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Management of Stroke Rehabilitation Working Group, VA/DOD Clinical practice guideline for the management of stroke rehabilitation. Washington, DC: Office of Quality, Safety and Value, 2019:2–170. [Google Scholar]
- 53.Niemeier JP. The lighthouse strategy: use of a visual imagery technique to treat visual inattention in stroke patients. Brain Inj 1998;12(5):399–406. doi: 10.1080/026990598122511 [DOI] [PubMed] [Google Scholar]
- 54.Weinberg J, Diller L, Gordon WA, et al. Visual scanning training effect on reading-related tasks in acquired right brain damage. Arch Phys Med Rehabil 1977;58(11):479–486. [PubMed] [Google Scholar]
- 55.Robertson IH, North N. Active and passive activation of left limbs: influence on visual and sensory neglect. Neuropsychologia 1993;31(3):293–300. doi: 10.1016/0028-3932(93)90093-f [DOI] [PubMed] [Google Scholar]
- 56.Eskes GA, Butler B, McDonald A, et al. Limb activation effects in hemispatial neglect. Arch Phys Med Rehabil 2003;84(3):323–328. doi: 10.1053/apmr.2003.50012 [DOI] [PubMed] [Google Scholar]
- 57.Saikaley M, Iruthayarajah J, Salter K, et al. Chapter 13: rehabilitation of unilateral spatial neglect. In: Evidence-based review of stroke rehabilitation. London, Ontario: Heart and Stroke Foundation, Canadian Partnership for Stroke Recovery, 2018. [Google Scholar]
- 58.Goedert KM, Chen P, Foundas AL, Barrett AM. Frontal lesions predict response to prism adaptation treatment in spatial neglect: a randomised controlled study. Neuropsychol Rehabil 2020;30(1):32–53. doi: 10.1080/09602011.2018.1448287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Na DL, Adair JC, Williamson DJ, et al. Dissociation of sensory-attentional from motor-intentional neglect. J Neurol Neurosurg Psychiatry 1998;64(3):331–338. doi: 10.1136/jnnp.64.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Champod AS, Frank RC, Taylor K, Eskes GA. The effects of prism adaptation on daily life activities in patients with visuospatial neglect: a systematic review. Neuropsychol Rehabil 2016;28(4):491–514. doi: 10.1080/09602011.2016.1182032 [DOI] [PubMed] [Google Scholar]
- 61.Frassinetti F, Angeli V, Meneghello F, et al. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain 2002;125(pt 3):608–623. doi: 10.1093/brain/awf056 [DOI] [PubMed] [Google Scholar]
- 62.Menon-Nair A, Korner-Bitensky N, Ogourtsova T. Occupational therapists’ identification, assessment, and treatment of unilateral spatial neglect during stroke rehabilitation in Canada. Stroke 2007;38(9):2556–2562. doi: 10.1161/STROKEAHA.107.484857 [DOI] [PubMed] [Google Scholar]
- 63.Barrett AM, The AAN 2016-2017 Transforming Leaders. The speed of trust advances treatments. Pract Neurol 2018:32–35. [Google Scholar]
- 64.Fowler EA, Sala SD, Hart SR, McIntosh RD. Over- and underestimation of motor ability after a stroke: implications for anosognosia. Neuropsychologia 2018;119:191–196. doi: 10.1016/j.neuropsychologia.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 65.Heilman KM, Barrett AM, Adair JC. Possible mechanisms of anosognosia: a defect in self-awareness. Philos Trans R Soc Lond B Biol Sci 1998;353(1377):1903–1909. doi: 10.1098/rstb.1998.0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bisiach E, Vallar G, Perani D, et al. Unawareness of disease following lesions of the right hemisphere: anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia 1986;24(4):471–482. doi: 10.1016/0028-3932(86)90092-8 [DOI] [PubMed] [Google Scholar]
- 67.Baier B, Geber C, Müller-Forell W, et al. Anosognosia for obvious visual field defects in stroke patients. Brain Struct Funct 2015;220(3):1855–1860. doi: 10.1007/s00429-014-0753-5 [DOI] [PubMed] [Google Scholar]
- 68.Kasius KM, Lamfers EJP, Venderink DJ, Verhagen WIM. Silent cardiac ischemia after an ischemic stroke of the right hemisphere. Cardiology 2011;119(3):160–163. doi: 10.1159/000330931 [DOI] [PubMed] [Google Scholar]
- 69.McKechnie D, Fisher MJ, Pryor J. A case-control study examining the characteristics of patients who fall in an inpatient traumatic brain injury rehabilitation setting. J Head Trauma Rehabil 2016;31(2):E59–E70. doi: 10.1097/HTR.0000000000000146 [DOI] [PubMed] [Google Scholar]
- 70.Barker-Collo S, Feigin V. The impact of neuropsychological deficits on functional stroke outcomes. Neuropsychol Rev 2006;16(2):53–64. doi: 10.1007/s11065-006-9007-5 [DOI] [PubMed] [Google Scholar]
- 71.Orfei MD, Caltagirone C, Spalletta G. The evaluation of anosognosia in stroke patients. Cerebrovasc Dis 2009;27(3):280–289. doi: 10.1159/000199466 [DOI] [PubMed] [Google Scholar]
- 72.Giacino JT, Cicerone KD. Varieties of deficit unawareness after brain injury. J Head Trauma Rehabil 1998;13(5):1–15. doi: 10.1097/00001199-199810000-00003 [DOI] [PubMed] [Google Scholar]
- 73.Besharati S, Forkel SJ, Kopelman M, et al. Mentalizing the body: spatial and social cognition in anosognosia for hemiplegia. Brain 2016;139(pt 3):971–985. doi: 10.1093/brain/awv390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chesnel C, Jourdan C, Bayen E, et al. Self-awareness four years after severe traumatic brain injury: discordance between the patient’s and relative’s complaints. Results from the PariS-TBI study. Clin Rehabil 2018;32(5):692–704. doi: 10.1177/0269215517734294 [DOI] [PubMed] [Google Scholar]
- 75.Turnbull OH, Solms M. Awareness, desire, and false beliefs: Freud in the light of modern neuropsychology. Cortex 2007;43(8):1083–1090. doi: 10.1016/s0010-9452(08)70706-8 [DOI] [PubMed] [Google Scholar]
- 76.Nardone IB, Ward R, Fotopoulou A, Turnbull OH. Attention and emotion in anosognosia: evidence of implicit awareness and repression? Neurocase 2007;13(5):438–445. doi: 10.1080/13554790701881749 [DOI] [PubMed] [Google Scholar]
- 77.Levine DN, Calvania R, Rinn WE. The pathogenesis of anosognosia for hemiplegia. Neurology 1991;41(11):1770–1781. doi: 10.1212/wnl.41.11.1770 [DOI] [PubMed] [Google Scholar]
- 78.Starkstein SE, Fedoroff JP, Price TR, et al. Anosognosia in patients with cerebrovascular lesions. A study of causative factors. Stroke 1992;23(10):1446–1453. doi: 10.1161/01.str.23.10.1446 [DOI] [PubMed] [Google Scholar]
- 79.Toglia J, Kirk U. Understanding awareness deficits following brain injury. NeuroRehabilitation 2000;15(1):57–70. doi: 10.3233/NRE-2000-15104 [DOI] [PubMed] [Google Scholar]
- 80.Cappa S, Sterzi R, Vallar G, Bisiach E. Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia 1987;25(5):775–782. doi: 10.1016/0028-3932(87)90115-1 [DOI] [PubMed] [Google Scholar]
- 81.Besharati S, Kopelman M, Avesani R, et al. Another perspective on anosognosia: self-observation in video replay improves motor awareness. Neuropsychol Rehabil 2015;25(3):319–352. doi: 10.1080/09602011.2014.923319 [DOI] [PubMed] [Google Scholar]
- 82.Barrett AM. Rose-colored answers: neuropsychological deficits and patient-reported outcomes after stroke. Behav Neurol 2010;22(1-2):17–23. doi: 10.3233/BEN-2009-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]


