Abstract
Variation in the mitochondrial tRNA Lys gene at position 8296 was previously found to be associated with maternally inherited diabetes mellitus and deafness, hypertrophic cardiomyopathy, myoclonic epilepsy with ragged-red fibers and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes. The pathogenicity of the m.8296A>G variation is unclear. In this study, we aimed to analyze the mitochondrial proteome in a patient with m.8296A>G variation to elucidate the effects of this mutation at the protein level. Whole-exome sequencing and mitochondrial genome analysis were performed in a patient with sensorineural hearing impairment, cognitive impairment, leukodystrophy, migraine-like headaches, and gastrointestinal dysmotility. Mitochondrial genome analysis identified a homoplasmic m.8296A>G variation in the mitochondrial tRNA Lys gene in the proband and unaffected mother. Global mitochondrial proteome analysis was carried out in the muscle mitochondria of the index patient and a control subject. Comparative muscle mitochondrial proteome analysis revealed a total of 13 nuclear-encoded mitochondrial proteins differently expressed with respect to the control. Ten of the 13 proteins were downregulated. Most of the proteins were involved in ATP synthesis and Krebs cycle and have strong interactions with each other. We considered the m.8296A>G variation to be pathogenic with variable penetrance for our patient's phenotype, and this variation led to different expressions of nuclear-encoded proteins involved in energy metabolism.
Keywords: A8296G mutation, Leukodystrophy, Mitochondrial proteome, Muscle mitochondria, Sensorineural hearing impairment
Introduction
Mitochondrial diseases are a group of heterogeneous disorders that arise as a result of dysfunction of the mitochondrial respiratory chain and affect various systems and organs in different combinations, predominantly high energy-demanding tissues such as the brain, skeletal muscle, and heart. They can be caused by mutations of nuclear or mitochondrial DNA (mtDNA). Human mtDNA is a double-stranded, circular molecule of 16,569 bp and contains 37 genes coding for 2 ribosomal RNAs (rRNA), 22 transfer RNAs (tRNA), and 13 polypeptides. The mtDNA-encoded polypeptides are all subunits of enzyme complexes of the oxidative phosphorylation system [Anderson et al., 1981]. The mitochondrial genome, especially mitochondrial tRNA genes, are sensitive to mutations. Although tRNA genes comprise about 10% of the mtDNA, because of their central role in protein synthesis, mutations in mt-tRNA genes contribute to more than half of the disorders caused by defects in oxidative phosphorylation [Pütz et al., 2007]. Diagnosis of a mitochondrial disease is usually challenging because of genetic and clinical heterogeneity and mitochondrial heteroplasmy in mitochondrial genome variants.
The human mitochondrial proteome consists of an estimated 1,100–1,400 distinct proteins, of which 13 are encoded by mtDNA. Approximately 1,100 of these proteins have been identified, mainly through large-scale proteomics, microscopy, and computation. In the last decade, mitochondrial proteomics has increasingly been applied to study diseases characterized by mitochondrial dysfunction. Mitochondrial proteomics covers not only pathways for energy metabolisms, such as oxidative phosphorylation (OXPHOS) and the citric acid cycle, but also mitochondrial proteins from several metabolic pathways, including fatty acid oxidation, heme biosynthesis, pyrimidine biosynthesis, calcium homeostasis, and apoptosis. Comparison of mitochondrial proteomics between healthy and diseased tissues provides better understanding of the pathogenesis associated with mitochondria [Calvo and Mootha, 2010].
In the current study, we present a girl with sensorineural hearing impairment, leukodystrophy, cognitive impairment, migraine-like headaches, and gastrointestinal dysmotility with a m.8296A>G variation in the mitochondrial tRNA Lys gene, which has been previously reported to have an association with diabetes mellitus, deafness, hypertrophic cardiomyopathy, mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS), and myoclonic epilepsy with ragged-red fibers (MERRF) [Kameoka et al., 1998a, b; Arenas et al., 1999; Fischel-Ghodsian, 1999; Akita et al., 2000; Sakuta et al., 2002].
To elucidate the effects of the m.8296A>G variation on the mitochondrial proteome, we carried out global mitochondrial proteome analysis using two-dimensional gel electrophoresis (2DE). This analysis allowed us to create a list of proteins that may be used to create a link between the mutation and the pertinent case.
Methods
Case Presentation
A 9-year-old girl was referred to our clinic because of a hearing impairment on the right side occurring in the 2 months before presentation. She was born to unrelated healthy Turkish parents after a normal pregnancy and delivery. Developmental milestones were within normal limits. She had a healthy sister. There is no family history of similar disorders.
She was vomiting nearly every day since birth, at most twice a day, usually in the morning, unrelated to feeding, but investigations for vomiting were normal. At the initial presentation, her weight was 20 kg (−0.74 standard deviation score [SDS]), height 130 cm (+1.68 SDS), body mass index 11.83 kg/m2 (−2.91 SDS), and her head circumference was 49.8 cm (−1.11 SDS). She was using a hearing aid for her right ear. The results of other physical examinations, including fundoscopic examination of her eyes, were normal.
Molecular Genetic Analysis
DNA was extracted from whole blood using the Puregene Blood Extraction Kit (Gentra Systems, Qiagen, Mississauga, ON, Canada). The proband's DNA was indexed and pooled using a Illumina TruSeq Rapid Capture Exome Library Prep Kit (Illumina, San Diego, CA, USA) and subjected to whole-exome sequencing on an Illumina NextSeq 500 system (Illumina). For all samples, the mean depth of the target region covered was 112×, and >97% of bases in the consensus coding sequences were covered by at least 20 reads. Variants were annotated using Alamut-HT software (Interactive Biosoftware, Rouen, France) and visualized on an Alamut Viewer 2.2 (Interactive Biosoftware). We excluded variants with minor allele frequency (MAF) >1% in the 1,000 Genomes Project, Exome Aggregation Consortium (ExAC v0.3), or ESP6500. A filtering pipeline was established to remove known and frequent single nuclear polymorphisms or benign polymorphisms. The remaining variants were cross-checked with the Human Gene Mutation Database (http://www.biobase-international.com/product/hgmd) and with the ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar/). Variant types leading to protein truncation, such as nonsense or frameshift, were given higher priority. The pathogenicity of missense variants was checked by 3 protein prediction algorithms: Sorting Intolerant from Tolerant (http://sift.jcvi.org/), Polymorphism Phenotyping (PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/), and Mutation Taster (http://mutationtaster.org/).
mtDNA was amplified in 2 overlapping PCR fragments (9,731 bp and 12,083 bp) using a Roche Expand Long Range PCR dNTPack (Roche Applied Science, Indianapolis, IN, USA).
Muscle Sample Collection
Samples of the case and the control muscles were obtained by biopsy from the gastrocnemius muscle. For histopathologic examination, sections were stained with hematoxylin and eosin, modified Gomori trichrome, periodic acid-Schiff, succinate dehydrogenase (SDH), cytochrome c oxidase (COX), nicotinamide adenine dinucleotide-tetrazolium reductase, phosphorylase, acid phosphatase, and oil red O.
A biopsy of the control (a 9-year-old boy with asymptomatic CK elevation [CK levels between 622 and 2,274 U/L in a period of 2 months]) was performed with a clinical diagnosis of myopathy or muscular dystrophy.
Unfortunately, fibroblast samples for biochemical studies from patients and controls could not be obtained under the conditions in which the study was carried out.
Isolation of Crude Mitochondria and Mitochondrial Protein Extraction
The tissue biopsy specimens (150 mg) were cut into small pieces and washed twice with cold phosphate-buffered saline for the removal of excess blood contamination. Crude mitochondria were isolated from the tissue using Pierce's Mitochondrial Isolation kit (Pierce, cat no. 89801) and following the manufacturer's instructions. Homogenization was performed using a Dounce glass grinder (approximately 15 strokes) followed by sonication (2 × 10 s). The samples were centrifuged at 12,000 × g for 15 min at 4°C. After centrifugation, the supernatant was saved as the cytosolic fraction. Once the mitochondrial pellet was obtained, it was resuspended in 30 µL of 2DE rehydration buffer (8 M urea, 2 M thiourea, 4% CHAPS, 30 mM Tris [pH 8.5], and 1× protease inhibitor cocktail).
Two-Dimensional Gel Electrophoresis and Image Analysis
For 2DE analysis, the protein concentration was determined using a modified Lowry assay with a BSA standard (Bio-Rad, USA). Protein (300 μg) was loaded onto immobilized pH gradient strips (IPG) (17 cm, pH 3–10) (Bio-Rad) via passive rehydration. Separation based on isoelectric points was performed using a Protean isoelectric focusing cell under the recommended conditions (Bio-Rad). After isoelectric focusing, the strips were subjected to SDS-PAGE (12%). Gels were stained with colloidal Coomassie stain (KeraFast, USA) and visualized with VersaDoc4000 MP (Bio-Rad). PDQuest Advance (Bio-Rad) 2DE analysis software was used to compare the protein spot profiles. All experiments were repeated twice using the same mitochondrial protein extract. All matching protein spots were selected among the groups. The spots were cut using an automated spot cutting tool (ExQuest spot cutter; Bio-Rad) and placed into 96-well plates for protein identification.
Mass Spectrometry and Database Searching
Identification of selected protein spots was performed at Kocaeli University DEKART proteomics laboratory (http://kabiproteomics.kocaeli.edu.tr/) using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF/TOF) spectrometry on a 5800 system (Absciex, USA). In-gel tryptic digestion of the proteins was performed using an in-gel digestion kit following the manufacturer's recommended protocol (Pierce, USA). Before deposition onto a MALDI plate, all samples were desalted with ZipTips following the recommended protocol (Millipore, USA). The peptides were eluted using an α-cyano-4-hydroxycinnamic acid matrix and spotted onto the MALDI target plate. The TOF spectra were recorded in positive ion reflector mode with a mass range from 400 to 2,000 Da. Each spectrum was the cumulative average of 2,000 laser shots. The spectra were calibrated with trypsin autodigestion ion peaks (m/z 842.510 and 2211.1046) as internal standards. Ten of the strongest peaks of the TOF spectra per sample were chosen for tandem mass spectrometry (MS/MS) analysis.
The peak list was searched in MASCOT version 2.5 (Matrix Science) using streamlined software (ProteinPilot; Absciex, USA), with the following criteria: National Center for Biotechnology Information non-redundant (NCBInr); species restricted to Homo sapiens; enzyme of trypsin; at least 5 independent peptides matched; at most one missed cleavage site; MS tolerance set to ±50 ppm and MS/MS tolerance set to ±0.4 Da; carbamidomethyl (Cys) for fixed modification and oxidation (Met) for variable modification; peptide charge of 1+ and monoisotopic. Only significant hits, as defined by the MASCOT probability analysis (p < 0.05), were accepted.
Classification of the proteins into functional categories was based on the use of PANTHER (http://www.pantherdb.org/), a freely available classification system, as well as NCBI (http://www.ncbi.nlm.nih.gov/pubmed), Swiss-Prot/TrEMBL annotations (http://www.expasy.org/), and a literature search for each protein identified.
Western Blot Analysis
The samples were separated on 12% SDS gels and then transferred onto nitrocellulose membranes. To prevent non-specific binding, the membranes were blocked for 1 h at room temperature. After blocking, the membranes were incubated overnight at 4°C with appropriately diluted primary antibodies in tris-buffered saline with Tween 20 (TBS-T) against voltage-dependent anion channel 1 (VDAC1) (1:2,000; Santa Cruz Biotechnology, sc-8828, rabbit polyclonal), Hsp60 (1:5,000; Thermo Fisher Scientific, MA3-012, mouse monoclonal), β-actin (1:1,000; Santa Cruz Biotechnology, sc-47778, mouse monoclonal), and total OXPHOS antibody cocktail (1:250; Mito-Sciences, ab-110413, mouse monoclonal). Hsp60 and β-actin antibodies were used for the normalization of each protein sample to ensure equal protein loading. VDAC1 mitochondrial marker was used to determine the enrichment of mitochondrial proteins in mitochondria fractions isolated from the muscle biopsy samples. After 3 washes with TBS-T, the membranes were incubated with secondary anti-mouse IgG HRP antibody (Bio-Rad) or anti-rabbit IgG (Bio-Rad). Then, the proteins were visualized with an ECL Plus Western blotting detection system (GE Healthcare). The bands were quantitated using Quantity One 1D image analysis software (Bio-Rad).
Results
Laboratory and Radiologic Findings of the Study Case
The brainstem auditory evoked potential test revealed profound hearing loss (90 dB) in the right ear and normal hearing in the left ear. Cranial magnetic resonance imaging (MRI) revealed multiple T2 hyperintense lesions in the periventricular and subcortical white matter, more prominent in the parieto-occipital area but also extending through the frontoparietal region. There was no contrast enhancement (Fig. 1). Inborn errors of metabolic diseases were thought to be possibly peroxisomal, lysosomal, or mitochondrial disorders. The results of laboratory investigations, including complete blood count, extended biochemistry, blood lactate, ammonia, MS/MS, urinary organic acid evaluation, very-long-chain fatty acid, phytanic acid, pristanic acid, arylsulfatase A levels, were normal. There was no lactate peak on the mass spectrum. For systemic evaluation, her electroencephalogram, electrocardiogram, and echocardiogram were also performed and found to be normal.
Fig. 1.
Cranial MRI of the patient. a T2-weighted axial image. b T2-weighted sagittal image. c Fluid-attenuated inversion recovery (FLAIR) sequence. d T1-weighted image with gadolinium injection. There are symmetrical lesions (hyperintense in the T2-weighted and FLAIR sequence and hypointense in the T1-weighted image, without contrast enhancement) of the white matter with parieto-occipital predominance (arrows). Cerebellar white matter is spared.
Disease Course
At 14 years of age, the patient complained of headaches, easy fatigability, and decline in academic performance. Headaches were consistent with migraine headaches, including phonophobia, photophobia, and nausea. Although she was a successful student in the first 3 years of elementary school, her performance declined gradually over the last 4 years. She had impaired running and stair climbing compared with her peers. The physical examination revealed increased deep tendon reflexes (3+) in both upper and lower extremities. There was no clonus, and Babinski reflex was negative. Muscle strength at the distal upper extremities (elbow extension and hand extensor muscles) was 4+ according to the Medical Research Council scale. Palmar and plantar atrophy and bilateral pes cavus deformity were observed. The results of a sensory examination for pain, light touch, and vibration were normal. Nerve conduction velocities were at normal levels. She was evaluated with the Wechsler Intelligence Scale for Children–Revised Form (WISC-R) and her verbal intelligence quotient (IQ) was 46 (moderate impairment), her performance IQ was 50 (mild cognitive impairment), and her general IQ was 45 (moderate cognitive impairment).
At the last follow-up, she was 18 years old, her weight was 41 kg (−2.74 SDS), height 165 cm (+0.28 SDS), body mass index 15.06 kg/m2 (−3.7 SDS), and her head circumference was 52 cm (−3.32 SDS). The results of a neurologic examination were similar to the results of the previous examination. Daily vomiting was still present. Endoscopy of the upper gastrointestinal tract showed chronic active gastritis. Endoscopic biopsy revealed mild chronic inflammation and moderate Helicobacter pylori infection. H. pylori eradication treatment resulted in temporary partial improvement and weight gain, but daily vomiting persisted. Cranial MRI was repeated at 18 years of age, and the radiologic findings were similar to the findings at the initial presentation. The results of cranial MR angiography were normal, and there was no lactate peak in the MR spectroscopy. Nerve conduction studies were repeated because of pes cavus and palmoplantar atrophy, and the results were normal.
Mitochondrial disease was considered based on the findings, including sensorineural hearing loss, cognitive impairment, loss of acquired skills, exercise intolerance, leukodystrophy, gastrointestinal dysmotility, and migraine-like headaches. The mitochondrial disease criteria score was 5 (score 5–7, probable mitochondrial disorder) [Morava et al., 2006].
Coenzyme Q10 and riboflavin were given when the patient was 10 years old as a supportive treatment for mitochondrial disorders. Objective improvement was not observed after supplementary treatment, however, she did not complain about migraine-like headaches for the last 3 years, and no progression was seen in other symptoms during the 9-year follow-up.
Molecular Genetic Analysis
Mitochondrial genome analysis was performed on peripheral blood when she was 10 years old. An adenine to guanine point variation at nucleotide 8296 (m.8296A>G) in the MT-TK (tRNA Lys) gene was identified with 97.7% heteroplasmy. The mitochondrial genome from the peripheral blood of the control patient was normal.
Muscle DNA was extracted from our patient's muscle biopsy sample, and a homoplasmic m.8296A>G variant was also found in muscle DNA. The index patient's mother was tested for this variant, and she was found to be homoplasmic for this mutant sequence in peripheral blood. The results of a neurologic examination, a hearing test, and cranial MRI of the mother were normal. Mitochondrial genome analysis was not performed on the muscle sample of the healthy mother for ethical reasons.
Whole-exome sequencing was performed for nuclear-encoded mitochondrial diseases and revealed a heterozygous variant [c.35del/p.(Gly12valf s*2)] in the GJB2 gene (NM_004004.5). This heterozygous variant was also found in the healthy father's peripheral whole-blood sample.
Histopathology of the Muscle and Respiratory Chain Enzyme Activities
For the proband, histopathology of the muscle biopsy sample was normal at 10 years of age. The biopsy was repeated when she was 14 years old, and mild differences in the muscle fiber diameter were reported. Respiratory chain enzyme activities were studied in the biochemistry laboratory at Hacettepe University using spectrophotometric assays. The ratios of complex I/citrate synthase (0.11; n = 0.17–0.56) and complex IV/citrate synthase (0.84; n = 1.1–5) were found to be decreased in skeletal muscle.
The histopathology of the control patient's muscle biopsy sample was normal. He was 18 years old at last follow-up. He had no symptoms and CK level was normal (277 U/L).
Proteomic Data
We purified the mitochondria from the muscle biopsy samples of the index patient (from the muscle biopsy sample at 10 years of age) and the control. Western blot analysis was performed for a mitochondrial protein marker, VDAC1, to validate the purity of the mitochondrial fractions derived from the tissue biopsy samples. The results demonstrated the presence of enriched mitochondrial protein extracts (online suppl. Fig. 1; for online suppl. material, see www.karger.com/doi/10.1159/519526).
The enriched mitochondrial protein extracts were subjected to Western blotting with OXPHOS antibody so that any fluctuation in the mitochondrial protein complexes relative to the control was revealed. The patient contained 45, 46, and 39% less respiratory complexes I, II, and III, respectively (Fig. 2). OXPHOS signals were quantified and normalized relative to β-actin in the mitochondrial fractions from the control and the patient.
Fig. 2.
Levels of oxidative phosphorylation (OXPHOS) complexes. Fibroblast mitochondria were prepared from the control (C) and the patient (P). Mitochondria underwent Western blotting and were probed with an mAb against OXPHOS complexes I, II, III, IV, and V, which are components of the inner membrane, and Hsp60 and β-actin (as controls for equal loading). a Representative Western blot analysis. The levels of complex I (NDUFB8), complex II (SDHB), and complex IV (MTCO1) were reduced in the patient compared with the control (100%). b Relative amounts of the respiratory complexes in the mitochondrial fractions of the samples (the y axis shows the % of band intensities). The patient had 45, 46, and 39% less respiratory complexes I, II, and III, respectively. The results are normalized to control mitochondria.
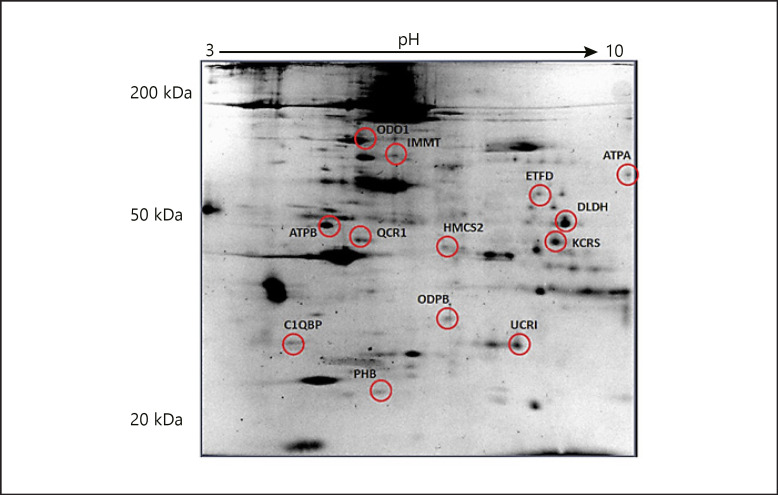
These observations along with the clinical symptoms allowed us to search for the molecular mechanisms that may be responsible for the observed phenotype. We used a 2DE-based approach to analyze the changes at the global mitochondrial proteome. Well-resolved and reproducible 2DE gel maps were produced. Using PDQuest Advance gel analysis software, changes in spot intensities that were up- or downregulated more than 2-fold were considered to be subjected to regulation. Among the matching spots that were compared (p < 0.05), we found that 13 protein spots were regulated (Fig. 3).
Fig. 3.
Comparative proteome analysis of the patient and the control. Muscle biopsy samples were subjected to mitochondrial protein isolation and loaded onto IPG strips at pH 3 to pH 10 for the first dimension and 12% SDS-PAGE gels for the separation of the second dimension and stained with colloidal Coomassie blue for 24 h after fixation for 24 h. The gel is representative for the selected protein spots and 13 were identified. The gel image was created using PDQuest Advance software to determine the number of spots that differentiated every member. The numbers are the numbers of the identified protein assigned to each spot by the researchers. These spots were cut from the gels and were subjected to MALDI-TOF/TOF analysis. Protein identification was performed by peptide mass fingerprinting by MASCOT.
The regulated protein spots were cut from a preparative gel with an automated spot cutting instrument and subjected to in-gel tryptic digestion followed by MALDI-TOF/TOF(MS/MS) analysis. Collectively, 13 protein spots were identified, 10 of which were downregulated (Table 1). The identified proteins were analyzed based on their molecular function and their involvement in biological processes (Table 2; Fig. 4). String analysis was performed to evaluate the protein-protein interactions of the 13 differentially expressed proteins (Fig. 5) [Szklarczyk et al., 2019].
Table 1.
Protein spots that were identified by MALDI-TOF/TOF (MS/MS) analysis
| Protein name/entry name (gene name) | SwissProt accession number | Best protein score | Sequence coverage, % | Up/down regulation | Mw, Da/pI | Unique peptides detected | Peptide sequence | |
|---|---|---|---|---|---|---|---|---|
| 1 | ATP synthase subunit beta, mitochondrial/ATPB (ATP5B) | P06576 | 540 | 23 | ↓ | 56,525/5.26 | 6(6) | K.VLDSGAPIKIPVGPETLGR.I |
|
| ||||||||
| 2 | Cytochrome b-c1 complex subunit 1, mitochondrial/QCR1 (UQCRC1) | P31930 | 125 | 15 | ↓ | 52,612/5.94 | 3(2) | R.ADLTEYLSTHYKAPR.M |
|
| ||||||||
| 3 | 2-Oxoglutarate dehydrogenase, mitochondrial/ODO1 (OGDH) | Q02218 | 145 | 7 | ↓ | 11,5861/6.4 | 4(1) | R.VIPEDGPAAQNPENVKR.L |
|
| ||||||||
| 4 | Dihydrolipoyl dehydrogenase, mitochondrial/DLDH (DLD) | P09622 | 90 | 8 | ↑ | 54,143/7.95 | 2(2) | R.VCHAHPTLSEAFR.E + Carbamidomethyl (C) |
|
| ||||||||
| 5 | Hydroxymethylglutaryl-CoA synthase, mitochondrial/HMCS2 (HMGCS2) | P54868 | 136 | 16 | ↓ | 56,599/8.4 | 4(1) | K.YNNVEAGKYTVGLGQTR.M |
|
| ||||||||
| 6 | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial/ODPB (PDHB) | P11177 | 216 | 20 | ↓ | 39,208/6.2 | 4(3) | R.IMEGPAFNFLDAPAVR.V + Oxidation (M) |
|
| ||||||||
| 7 | Cytochrome b-c1 complex subunit Rieske, mitochondrial/UCRI (UQCRFS1) | P47985 | 61 | 8 | ↓ | 29,649/8.55 | 3(1) | K.VPDFSEYRR.L |
|
| ||||||||
| 8 | ATP synthase subunit alpha, mitochondrial/ATPA (ATP5A1) | P25705 | 139 | 11 | ↓ | 59,714/9.16 | 3(3) | R.VGLKAPGIIPR.I |
|
| ||||||||
| 9 | Complement component 1 Q subcomponent-binding protein, mitochondrial/C1QBP (C1QBP) | Q07021 | 69 | 4 | ↑ | 31,343/4.74 | 1(1) | R.EVSFQSTGESEWK.D |
|
| ||||||||
| 10 | Prohibitin/PHB (PHB) | P35232 | 213 | 21 | ↓ | 29,786/5.57 | 4(3) | R.IFTSIGEDYDER.V |
|
| ||||||||
| 11 | Mitochondrial inner membrane protein/IMMT (IMMT) | Q16891 | 197 | 10 | ↑ | 83,626/6.08 | 5(3) | K.VVSQYHELVVQAR.D |
|
| ||||||||
| 12 | Electron transfer flavoprotein-ubiquinone oxidoreductase, mitochondrial/ETFD (ETFDH) | Q16134 | 78 | 3 | ↓ | 68,464/7.31 | 2(2) | R.ITTHYTIYPR.D |
|
| ||||||||
| 13 | Creatine kinase S-type, mitochondrial/KCRS (CKMT2) |
P17540 | 191 | 14 | ↓ | 47,474/8.46 | 3(3) | K.ITQGQFDEHYVLSSR.V |
|
| ||||||||
| Mw, molecular weight; pI, isoelectric point. | ||||||||
Table 2.
Classification of the proteins that were identified by MALDI-TOF/TOF analysis. The classifications were made based on PANTHER analysis and Swiss-Prot annotations
| SwissProt Accession number | Protein name | Biological process | Cellular component | |
|---|---|---|---|---|
| 1 | P06576 | ATP synthase subunit beta, mitochondrial | ATP synthesis; hydrogen ion transport; ion transport | Mitochondrion; mitochondrion inner membrane |
|
| ||||
| 2 | P31930 | Cytochrome b-c1 complex subunit 1, mitochondrial | Electron transport; respiratory chain; transport | Mitochondrion; mitochondrion inner membrane |
|
| ||||
| 3 | Q02218 | 2-Oxoglutarate dehydrogenase, mitochondrial | Glycolysis | Mitochondrion |
|
| ||||
| 4 | P09622 | Dihydrolipoyl dehydrogenase, mitochondrial | Mitochondrial electron transport, NADH to ubiquinone; regulation of acetyl-CoA biosynthetic process from pyruvate | Mitochondrial matrix; nucleus |
|
| ||||
| 5 | P54868 | Hydroxymethylglutaryl-CoA synthase, mitochondrial | Cholesterol, lipid, steroil and sterol biosynthesis/metabolism | Mitochondrion |
|
| ||||
| 6 | P11177 | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial | Carbohydrate metabolism; glucose metabolism; tricarboxylic acid cycle | Mitochondrion |
|
| ||||
| 7 | P47985 | Cytochrome b-c1 complex subunit Rieske, mitochondrial | Electron transport; respiratory chain | Mitochondrion; mitochondrion inner membrane |
|
| ||||
| 8 | P25705 | ATP synthase subunit alpha, mitochondrial | ATP synthesis; hydrogen ion transport | Mitochondrion; mitochondrion inner membrane |
|
| ||||
| 9 | Q07021 | Complement component 1 Q subcomponent-binding protein, mitochondrial | Adaptive immunity; apoptosis; mRNA processing; ribosome biogenesis; transcription regulation | Cell membrane; cytoplasm; membrane; mitochondrion |
|
| ||||
| 10 | P35232 | Prohibitin | DNA synthesis | Mitochondrion; mitochondrion inner membrane |
|
| ||||
| 11 | Q16891 | Mitochondrial inner membrane protein | Mitochondrial calcium ion homeostasis | Mitochondrion; mitochondrion inner membrane |
|
| ||||
| 12 | Q16134 | Electron transfer flavoprotein-ubiquinone oxidoreductase, mitochondrial | Electron transport | Mitochondrion; mitochondrion inner membrane |
|
| ||||
| 13 | P17540 | Creatine kinase S-type, mitochondrial | Creatine metabolic process; catalyzes the transfer of phosphate between ATP and various phosphogens | Mitochondrion; mitochondrion inner membrane |
Fig. 4.
Classification of the proteins that were identified by MALDI-TOF/TOF analysis. The pie charts present the distribution of the 13 proteins identified based on their molecular function, biological processes, cellular component, protein class, and pathway. Assignments were made based on information from PANTHER analysis (http://www.pantherdb.org/) as well as NCBI (http://www.ncbi.nlm.nih.gov/pubmed) and Swiss-Prot/TrEMBL annotations (http://www.expasy.org/).
Fig. 5.
String interaction analysis (http://string-db.org/; version 9.1) of the proteins identified in the MALDI-TOF/TOF analysis [Szklarczyk et al., 2019].
Discussion
We have presented a girl with sensorineural hearing impairment, leukodystrophy, cognitive impairment, migraine-like headaches, and gastrointestinal dysmotility. A heterozygous variant in the GJB2 gene was found in whole-exome sequencing. This variant was also found in her healthy father and was not considered to be pathogenic in our case. The inheritance pattern of non-syndromic hearing loss associated with the GJB2 gene is autosomal recessive. Rarely, an autosomal dominant inheritance pattern may be seen in the GJB2 gene, but it is related to skin diseases such as ichthyosis and palmoplantar keratoderma [Smith and Jones, 2016].
The mitochondrial genome analysis from peripheral blood revealed a nearly homoplasmic m.8296A>G variation in the mitochondrial tRNA Lys gene and a homoplasmic variant in skeletal muscle tissue of the proband. The variation site was located at the start point of the aminoacyl acceptor stem of tRNALys, and this nucleotide is highly conserved among species from humans to Drosophila [Kameoka et al., 1998a]. m.8296A>G might have an impact on the stability of the tRNA protein. After excluding possible lysosomal (metachromatic leukodystrophy, etc.) and peroxisomal (refsum disease, adrenoleukodystrophy, etc.) diseases that are characterized by deafness and leukodystrophy, the diagnosis of a mitochondrial disorder was suspected and the m.8296A>G variation was considered to be causative of our patient's findings based on the literature related to this variation [Kameoka et al., 1998a; Fischel-Ghodsian, 1999; Sakuta et al., 2002; Morava et al., 2006]. A homoplasmic m.8296A>G variant was found in the healthy mother's peripheral blood. Heteroplasmy/homoplasmy can modulate phenotypic expression, therefore extensive tissue-related heteroplasmy needed to be shown in the mother's tissues. However, we were unable to perform further mtDNA analysis of the mother's tissues other than blood because she does not have a clinical indication of diseases.
The pathogenicity of the m.8296A>G variation is unclear. This variation is reported in the MITOMAP database (https://www.mitomap.org). The Mito-TIP score, an in silico tool to predict the pathogenicity of mt-tRNA variants, was 72.3%, with a status of likely pathogenic [Sonney et al., 2017]. The Genome Aggregation Database (https://gnomad.broadinstitute.org/) v3.1 frequency is 0.044. HmtVar (https://www.hmtvar.uniba.it), a manually-curated database exploring pathogenicity information about mtDNA variants, pathogenicity prediction score of this variation is 0.85 (disease score ≥0.35 is rated as pathogenic by HmtVar). ClinVar classified this variant as pathogenic due to submission by Kameoka et al. [1998a]. (Accession: SCV000030424.2). In 2019, they updated this variant as benign, based on the modified ACMG guidelines by Wong et al. [2020], who developed criteria to interpret mitochondrial transfer RNA variants according to heteroplasmy correlations with phenotype, tissue distribution, family members, and unrelated families from the literature (Accession: SCV000993169.1).
This variation was first investigated by Kameoka et al. [1998a]. They screened 10 diabetic patients with clinical features suggesting mitochondrial DNA mutations and identified an adenine-to-guanine point variation in the MT-TK gene at position 8296. Then they screened 1,216 individuals with diabetes, 44 patients with sensorineural deafness, and 300 controls without diabetes for this variation. They identified the variation in 11 (0.90%) unrelated individuals with diabetes, 1 (2.3%) patient with deafness, and no controls without diabetes. Seven of the 12 patients showed maternal inheritance. Deafness was seen in 7 of 12 probands. Using MRI and xenon computed tomography cerebral blood flow, this group also studied the neuroimaging findings in 10 of 12 probands who had no neurologic symptoms or signs at the time of the study [Isotani et al., 1999]. They found abnormal high-intensity areas in 6 patients on T2-weighted images (60%) on MRI. On xenon computed tomography of cerebral blood flow, a decrease in local blood flow values in the parieto-occipital region was found in 4 patients. They concluded that the parieto-occipital region, a region of altered brain-glucose metabolic rates, is a common site of cerebral infarctions in patients with MELAS [De Volder et al., 1988; Suzuki et al., 1990]. The changes in white matter in our patient were diffuse but more prominent in the parieto-occipital region.
The m.8296A>G variation has also been described independently in association with hypertrophic cardiomyopathy and MELAS syndrome [Akita et al., 2000; Sakuta et al., 2002]. In the report that associated the m.8296A>G variation with MELAS, a 14-year-old patient was described presenting with optic atrophy, hypertrophic cardiomyopathy, bilateral striatal necrosis, sensorineural hearing loss, short stature, epilepsy, myopathy with ragged-red fibers in skeletal muscle pathology, lactic acidosis, and stroke-like episodes. Our case shares some common features with MELAS syndrome, such as sensorineural hearing impairment, recurrent vomiting, migraine-like headaches, and non-specific white matter changes. However, our patient does not have stroke-like episodes or the typical MRI findings and high lactate levels that are typically seen in MELAS. During her 8-year follow-up, she did not develop diabetes, cardiomyopathy, or epilepsy. This can be explained by phenotypic variability. The clinical picture may also settle down in time as previously reported.
Sensorineural hearing impairment was the main symptom that brought our patient to medical care. Mitochondrial hearing loss may occur as non-syndromic, presenting only with hearing loss, or as one of several symptoms in syndromic diseases such as Kearns-Sayre syndrome, MELAS, MERRF, and maternally inherited diabetes mellitus and deafness [Guan, 2004]. Mitochondrial tRNA mutations are responsible for most cases of syndromic and non-syndromic mitochondrial deafness. It is suggested that inner ear hair cells and their connecting neuronal circuitry may be specifically affected by mt-tRNA mutations [Yan et al., 2011].
Arenas et al. [1999] documented a family with MERRF syndrome associated with a double variation: m.8296A>G and m.8363G>A in the MT-TK (tRNA Lys) gene. In this report, the authors concluded that the m.8363G>A variation is pathogenic; the co-occurrence of the 8296 variation is likely to be a rare polymorphism, based on functional studies and because the m.8296A>G variation was almost homoplasmic in muscle and blood from the index patient and his oligosymptomatic maternal relatives. In 2002, using a trans-mitochondrial cybrid approach, the same group reported that this variation does not cause mitochondrial dysfunction [Bornstein et al., 2002]. They showed that m.8347A>G variation severely impairs oxidative phosphorylation, suggesting that it is highly pathogenic. In contrast, the behavior of cybrids homoplasmic for the m.8296A>G mutation is similar to cybrids containing wild-type mtDNA.
Some mitochondrial transfer RNA variants, like Leber's hereditary optic neuropathy and mitochondrial non-syndromic sensorineural hearing loss, are reported as homoplasmic in all types of tissues of the proband and unaffected mothers. In these disorders, it is suggested that the nuclear background can affect the expression of mitochondrial transfer RNA variants, and mtDNA mutations are pathogenic in the presence of a nuclear modifier. The nuclear modifier could be a common polymorphism in a tissue-specific protein [Carelli et al., 2003; Wong et al., 2020]. Studies based on trans-mitochondrial cybrid assays may not detect the pathogenic effects occurring due to the nuclear-mitochondrial interaction. In our case, although the main symptom was hearing loss, multi-system involvement was present. Nevertheless, the unaffected mother with a homoplasmic m.8296A>G variation can be explained by variable penetrance and a possible nuclear modifier. The clinical features, especially hearing impairment, were associated with the m.8296A>G variation in the literature [Kameoka et al., 1998a; Pütz et al., 2007]. When the first muscle biopsy was performed, the proband was 10 years old; she had no muscle weakness, and the histopathology results were normal. But respiratory complexes I, II, and III were decreased less than 50% with respect to a control. Muscle biopsy was repeated when she was 14 years of age, although histopathology showed non-specific findings; mild decreases in complexes I and IV were found. The difference in decreased respiratory chain complexes in our patient may be attributed to the time of the biopsy and the dynamic process of mitochondrial respiratory chain activities, and/or the different methods used. The first skeletal muscle sample was studied using Western blot analysis on a research basis when the proband was 10 years of age. The second muscle sample was obtained when she was 14 years of age and studied in a laboratory where mitochondrial respiratory chain enzymes are examined routinely by phosphospectrometry methods. Our patient was taking coenzyme Q10 and riboflavin treatments, which may also affect respiratory chain complex activities. Given the previous reports of m.8296A>G associated with sensorineural deafness, MELAS, and our patient's phenotype with multi-system involvement, loss of acquired skills, decrease in combined respiratory chain complexes, we considered that the m.8296A>G variation was pathogenic.
The proteomic data revealed 13 proteins differently expressed with respect to the control, and all of them were nuclear encoded. The fact that all of the proteins that were differentially regulated are coded by the nuclear genome may raise the question of how a genetic variation in the mitochondrial genome could alter the level of proteins encoded by the nucleus. Perhaps one of the explanation that might be presented underlies in the evolution of the mitochondrial genome. The nucleus and mitochondrial genomes have evolved to work together to provide the proper organelle function and protein homeostasis. This collaborative arrangement is not the result of one-way signaling but the interaction of signals in both directions (anterogate and retrograde). Anterograde mechanisms regulate gene expression in organelles in response to endogenous and environmental stimuli sensed by the nucleus, whereas retrograde mechanisms transmit signals from mitochondria to regulate nuclear gene expression, which in turn can alter anterograde control [Liu and Butow, 2006; Woodson and Chory, 2008].
Rabilloud et al. [2001] studied 2 human mitochondrial tRNA disorders, MELAS and MERRF, by comparative proteomics. They showed decreased levels of 2 proteins that are nuclear-encoded subunits of cytochrome c oxidase and concluded that there is a link between the effects of mutations in mitochondrial tRNA genes and the steady-state level of nuclear-encoded proteins in mitochondria. In our case, there was no alteration in cytochrome c oxidase subunits; however, subunits of cytochrome reductase (cytochrome b-c1 complex subunit 1, cytochrome b-c1 complex subunit Rieske) and ATP synthase (ATP synthase subunit beta, ATP synthase subunit alpha) were altered.
Most of the proteins identified were involved in oxidative phosphorylation and the Krebs cycle and have strong interaction with each other. 2-Oxoglutarate dehydrogenase, dihydrolipoamide dehydrogenase, and pyruvate dehydrogenase E1 component subunit beta were found to be altered in our patient and function in the Krebs cycle. 2-Oxoglutarate dehydrogenase complex is the key regulatory enzyme of the Krebs cycle. The pyruvate dehydrogenase complex is the “gateway” enzyme for mitochondrial oxidative metabolism of carbohydrates and catalyzes the overall conversion of pyruvate to acetyl-CoA and CO2 and thereby links the glycolytic pathway to the Krebs cycle. Pyruvate dehydrogenase complex deficiency is a relatively common mitochondrial disorder, which presents primarily with neurologic manifestations and lactic acidosis [Patel and Korotchkina, 2006].
Another downregulated protein was electron transfer flavoprotein-ubiquinone oxidoreductase, mitochondrial (ETFD). Deficiencies in ETFD result in deficiency of riboflavin responsive multiple acyl-CoA dehydrogenase [Zhang et al., 2006]. The clinical efficacy of riboflavin treatment may be based on a chaperone effect that can compensate for inherited folding defects of ETFD [Cornelius et al., 2012]. A variety of vitamins and co-factors have been used in the treatment of mitochondrial diseases, although a Cochrane systematic review has shown that evidence supporting their use is lacking [Pfeffer et al., 2012]. Individuals with complex I and/or complex II deficiency may benefit from oral administration of riboflavin [Chinnery, 2014]. The modulation of ETFDH protein in our results may support this hypothesis.
Both ETFDH and DLD are proteins related to diseases with riboflavin responses [Zhang et al., 2006; Carrozzo et al., 2014]. In this perspective, we suggest that riboflavin may be a supplementary treatment with secondary involvement of these proteins as in our case.
Creatine kinase S-type is another differentially expressed protein localized in the inner membrane of mitochondria. It plays an important role in muscle energy homeostasis by catalyzing reversible phosphotransfer reaction between creatine and ATP in excitable tissues, including muscle. Elimination of the creatine kinase reaction should result in much faster oxygen consumption dynamics during transitions in the ATP turnover rate [Roman et al., 2002].
Numerous parameters can affect our proteomic data, including the technique, temporal changes in muscle mitochondrial protein, the environment, and the nuclear background. Changes in the levels of multiple proteins involved in energy metabolism, including glycolysis, the Krebs cycle, ATP synthesis, and electron transfer, can result in decreased ATP production, which may have a significant impact on disease symptoms.
The literature regarding proteomic studies in mitochondrial diseases usually involves in vitro studies and one control with a wild-type cell line [Tryoen-Tóth et al., 2003; Yan et al., 2011]. There may be some challenges with in vivo proteomic studies, because in vitro studies provide the same nuclear background to better understand proteomic changes in the disease state. However, a mtDNA variant can be pathogenic in a certain nuclear background, where a nuclear modifier contributes to the pathogenic effect. The main limitation of our study is that we have only one control patient, a patient with a medical indication for a muscle biopsy. Skeletal muscle studies are usually preferred for mitochondrial disease. In our case, although mild myopathic symptoms and findings were present, sensorineural hearing impairment and leukodystrophy were the main findings. But tissue-specific studies were not possible. Proteomic studies may be more applicable in target tissues. Further studies are required to evaluate changes in the mitochondrial proteome to understand the link between the clinical phenotype and genomic and proteomic data.
Conclusion
We presented a patient with sensorineural hearing impairment, cognitive impairment, leukodystrophy, migraine-like headaches, exercise intolerance, and gastrointestinal dysmotility. A homoplasmic m.8296A>G variation was found in the tRNA lys gene in the proband and unaffected mother. Common phenotypic presentations reported with the m.8296A>G variation include deafness and MELAS syndrome. The interpretation of mitochondrial tRNA variants is dependent on the heteroplasmy/homoplasmy level. Nuclear background may also affect the pathogenicity of this variation. Detection of m.8296A>G in additional individuals would facilitate its classification. Mitochondrial proteomic analysis was performed to explore the effect of this variation. Proteomic data revealed 13 differently expressed nuclear-encoded proteins, most of which are involved in energy metabolism. Although these proteins are usually regarded as housekeeping proteins that exist only to maintain mitochondrial function, it is important to reevaluate and recognize their roles in health and disease. These proteins might participate in or affect other cellular processes. Two of the proteins are related to riboflavin responsive diseases, which we also used as a supplementary treatment for our patient. However, our study is preliminary, and further genomic-proteomic data can contribute significant understanding of disease pathogenesis and treatment strategies. This study emphasizes the importance of genomic and proteomic data for a better understanding of challenging disorders such as mitochondrial diseases.
Statement of Ethics
The study procedures were performed according to the clinical guidelines of the Ethical Committee of our institution (KAEK, 2012/37). Written informed consent was obtained from the patient and the parents of the healthy control.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The study was financially supported by Kocaeli University Scientific Research Project unit (Project no. 2012/16).
Data Availability Statement
All data generated or analyzed during this study are included in this article and/or its supplementary material files. Further enquiries can be directed to the corresponding author.
Author Contributions
H.M.G., D.U., and B.K. designed the work; H.M.G., B.K., G.A., and M.K. applied for and received funding. H.M.G., E.U.Y., and B.K. performed the clinical investigations of the index and control patients. D.U. and A.D.A. conducted the genetic analyses (mitochondrial genome analysis and whole-genome analysis). G.A. and M.K. performed the proteomic analysis and interpreted the data. H.M.G., G.A., and B.K. prepared the manuscript. All authors were involved in revision of the manuscript and final approval.
Supplementary Material
Supplementary data
References
- 1.Akita Y, Koga Y, Iwanaga R, Wada N, Tsubone J, Fukuda S, et al. Fatal hypertrophic cardiomyopathy associated with an A8296G mutation in the mitochondrial tRNA(Lys) gene. Hum Mutat. 2000;15:382. doi: 10.1002/(SICI)1098-1004(200004)15:4<382::AID-HUMU15>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 3.Arenas J, Campos Y, Bornstein B, Ribacoba R, Martin MA, Rubio JC, et al. A double mutation (A8296G and G8363A) in the mitochondrial DNA tRNA (Lys) gene associated with myoclonus epilepsy with ragged-red fibers. Neurology. 1999;52:377–82. doi: 10.1212/wnl.52.2.377. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein B, Mas JA, Fernández-Moreno MA, Campos Y, Martín MA, del Hoyo P, et al. The A8296G mtDNA mutation associated with several mitochondrial diseases does not cause mitochondrial dysfunction in cybrid cell lines. Hum Mutat. 2002;19:234–9. doi: 10.1002/humu.10050. [DOI] [PubMed] [Google Scholar]
- 5.Calvo SE, Mootha VK. The mitochondrial proteome and human disease. Annu Rev Genomics Hum Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carelli V, Giordano C, d'Amati G. Pathogenic expression of homoplasmic mtDNA mutations needs a complex nuclear-mitochondrial interaction. Trends Genet. 2003;19:257–62. doi: 10.1016/S0168-9525(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 7.Carrozzo R, Torraco A, Fiermonte G, Martinelli D, Di Nottia M, Rizza T, et al. Riboflavin responsive mitochondrial myopathy is a new phenotype of dihydrolipoamide dehydrogenase deficiency. The chaperon-like effect of vitamin B2. Mitochondrion. 2014;18:49–57. doi: 10.1016/j.mito.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Chinnery PF. Mitochondrial disorders overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean JHL, editors. GeneReviews® [Internet] Seattle: University of Washington; 2014. pp. 1993–2021. [Google Scholar]
- 9.Cornelius N, Frerman FE, Corydon TJ, Palmfeldt J, Bross P, Gregersen N, et al. Molecular mechanisms of riboflavin responsiveness in patients with ETF-QO variations and multiple acyl-CoA dehydrogenation deficiency. Hum Mol Genet. 2012;21:3435–48. doi: 10.1093/hmg/dds175. [DOI] [PubMed] [Google Scholar]
- 10.De Volder A, Ghilain S, de Barsy T, Goffinet AM. Brain metabolism in mitochondrial encephalomyopathy: a PET study. J Comput Assist Tomogr. 1988;12:854–7. doi: 10.1097/00004728-198809010-00024. [DOI] [PubMed] [Google Scholar]
- 11.Fischel-Ghodsian N. Mitochondrial deafness mutations reviewed. Hum Mutat. 1999;13:261–70. doi: 10.1002/(SICI)1098-1004(1999)13:4<261::AID-HUMU1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Guan MX. Molecular pathogenetic mechanism of maternally inherited deafness. Ann N Y Acad Sci. 2004;1011:259–71. doi: 10.1007/978-3-662-41088-2_25. [DOI] [PubMed] [Google Scholar]
- 13.Isotani H, Kameoka K, Nagano Y, Kitaoka H, Ohsawa N. Characteristic neuroimaging findings in patients with diabetes and the 8296 mitochondrial tRNA(Lys) Diabetologia. 1999;42:1266–7. doi: 10.1007/s001250051304. [DOI] [PubMed] [Google Scholar]
- 14.Kameoka K, Isotani H, Tanaka K, Azukari K, Fujimura Y, Shiota Y, et al. Novel mitochondrial DNA mutation in tRNA(Lys) (8296A-->G) associated with diabetes. Biochem Biophys Res Commun. 1998a;245:523–7. doi: 10.1006/bbrc.1998.8437. [DOI] [PubMed] [Google Scholar]
- 15.Kameoka K, Isotani H, Tanaka K, Kitaoka H, Ohsawa N. Impaired insulin secretion in Japanese diabetic subjects with an A-to-G mutation at nucleotide 8296 of the mitochondrial DNA in tRNA(Lys) Diabetes Care. 1998b;21:2034–5. doi: 10.2337/diacare.21.11.2034. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu Rev Genet. 2006;40:159–85. doi: 10.1146/annurev.genet.40.110405.090613. [DOI] [PubMed] [Google Scholar]
- 17.Morava E, van den Heuvel L, Hol F, de Vries MC, Hogeveen M, Rodenburg RJ, et al. Mitochondrial disease criteria: diagnostic applications in children. Neurology. 2006;67:1823–6. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- 18.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–22. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment for mitochondrial disorders. Cochrane Database Syst Rev. 2012;2012(4):CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pütz J, Dupuis B, Sissler M, Florentz C. Mamit-tRNA, a database of mammalian mitochondrial tRNA primary and secondary structures. RNA. 2007;13:1184–90. doi: 10.1261/rna.588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabilloud T, Strub JM, Carte N, Luche S, Van Dorsselaer A, Lunardi J, et al. Comparative proteomics as a new tool for exploring human mitochondrial tRNA disorders. Biochemistry. 2001;41:144–50. doi: 10.1021/bi0114776. [DOI] [PubMed] [Google Scholar]
- 22.Roman BB, Meyer RA, Wiseman RW. Phosphocreatine kinetics at the onset of contractions in skeletal muscle of MM creatine kinase knockout mice. Am J Physiol Cell Physiol. 2002;283:C1776–83. doi: 10.1152/ajpcell.00210.2002. [DOI] [PubMed] [Google Scholar]
- 23.Sakuta R, Honzawa S, Murakami N, Goto Y, Nagai T. Atypical MELAS associated with mitochondrial tRNA(Lys) gene A8296G mutation. Pediatr Neurol. 2002;27:397–400. doi: 10.1016/s0887-8994(02)00456-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith RJH, Jones MKN. In: Nonsyndromic hearing loss and deafness, DFNB1. Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean JHL, Mirzaa G, et al., editors. Seattle: University of Washington; 2016. pp. 1993–2021. [Google Scholar]
- 25.Sonney S, Leipzig J, Lott MT, Zhang S, Procaccio V, Wallace DC, et al. Predicting the pathogenicity of novel variants in mitochondrial tRNA with MitoTIP. PLoS Comput Biol. 2017;13:e1005867. doi: 10.1371/journal.pcbi.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Koizumi J, Shiraishi H, Ishikawa N, Ofuku K, Sasaki M, et al. Mitochondrial encephalomyopathy (MELAS) with mental disorder: CT, MRI and SPECT findings. Neuroradiology. 1990;32:74–6. doi: 10.1007/BF00593949. [DOI] [PubMed] [Google Scholar]
- 27.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tryoen-Tóth P, Richert S, Sohm B, Mine M, Marsac C, Van Dorsselaer A, et al. Proteomic consequences of a human mitochondrial tRNA mutation beyond the frame of mitochondrial translation. J Biol Chem. 2003;278:24314–23. doi: 10.1074/jbc.M301530200. [DOI] [PubMed] [Google Scholar]
- 29.Wong LC, Chen T, Wang J, Tang S, Schmitt ES, Landsverk M, et al. Interpretation of mitochondrial tRNA variants. Genet Med. 2020;22((5)):917–26. doi: 10.1038/s41436-019-0746-0. [DOI] [PubMed] [Google Scholar]
- 30.Woodson JD, Chory J. Coordination of gene expression between organellar and nuclear genomes. Nat Rev Genet. 2008;9:383–95. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan X, Wang X, Wang Z, Sun S, Chen G, He Y, et al. Maternally transmitted late-onset non-syndromic deafness is associated with the novel heteroplasmic T12201C mutation in the mitochondrial tRNAHis gene. J Med Genet. 2011;48:682–90. doi: 10.1136/jmedgenet-2011-100219. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Frerman FE, Kim JJ. Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool. Proc Natl Acad Sci U S A. 2006;103:16212–7. doi: 10.1073/pnas.0604567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and/or its supplementary material files. Further enquiries can be directed to the corresponding author.