Abstract
West Nile virus (WNV) is an arthropod-borne virus (arbovirus). It circulates in an enzootic cycle between ornithophilic mosquitoes as vectors and reservoirs and avian host species for amplification, but humans can be infected as accidental hosts. In most individuals, WNV infection remains silent, while 20% develop mild symptoms of West Nile fever, and only 1% develop neuroinvasive disease (WNND). Human WNV cases have been identified in Southern and Eastern Europe for more than 20 years, but until 2018, Germany was considered to be a non-endemic country. This changed when in the exceptionally warm summer of 2018, conditions for viral replication in mosquitoes were ideal, and the first WNV cases among birds and horses were identified. The widespread domestic Culex mosquitoes are efficient vectors for WNV. Autochthonous mosquito-borne WNV infections in humans were reported in all following years, indicating a continuous circulation in the affected areas of Central-East Germany. So far, no clear expansion of the affected areas is discernible but may develop. WNV is a transfusion-transmissible-infection, and donor deferral or testing of donations after a stay in an affected area are effective means to ensure transfusion safety. WNV transmissions via blood products often result in WNND due to the predisposing underlying medical conditions of transfusion recipients. From 2020 onwards, roughly 80% of all blood establishments in Germany tested their donations for WNV using nucleic acid amplification techniques in the transmission season. Altogether, 19 confirmed WNV infections were identified from 2020–2021. As long as effective and affordable pathogen reduction is not available for all blood components, WNV testing or donor deferral will be essential. In order to timely identify affected areas, combined results of human and veterinary surveillance are needed. Partnerships between public health experts, transfusion medicine specialists, veterinarians, and entomologists should be strengthened to ensure a One Health approach.
Keywords: West Nile virus, Virus safety, Transfusion-associated infections, Transfusion risks, Epidemiology
Introduction: Virus Characteristics
West Nile virus (WNV) is an arthropod-borne virus (arbovirus) of the family Flaviviridae and is part of the Japanese encephalitis virus complex. Seven phylogenetic WNV lineages have been identified [1] with lineages 1 and 2 being relevant for human infections. The first detection of WNV in humans was made in Uganda in 1937 [2], and very few outbreaks of human disease were recorded before the 1990s.
WNV Ecology
WNV as an arbovirus circulates in an enzootic cycle between ornithophilic mosquitoes as vectors and reservoirs and avian host species for amplification [3]. Infection in birds is often subclinical, although highly susceptible species, such as birds of prey, owls, or various passerine birds, may develop fatal disease [4]. Via bridging vectors (mosquitoes feeding on both bird and mammalian species), transmission of WNV to other vertebrates is possible. These are dead-end hosts [5] because they develop only a low viraemia and are therefore unable to efficiently transmit the virus to uninfected mosquitoes [1]. Clinical signs are almost exclusively seen in humans and horses.
The principal vectors of WNV are ornithophilic Culex mosquitoes, especially Culex pipiens complex [6, 7]. German Culex pipiens have been shown to be highly susceptible for WNV, even at the relatively low temperature of 18°C [8, 9]. Overwintering of the virus in Culex mosquitoes and their eggs in Germany has been documented [10, 11]. Additionally, even small increases in temperature have been shown to result in significant increases in transmission potential for WNV in Culex pipiens mosquitoes [12]. Therefore, the likelihood for ongoing disease transmission to occur is thought to correlate more with sufficient summer temperatures rather than minimum winter temperatures [13]. Importantly, the Culex pipiens biotype molestus is present widespread throughout Germany and is noted for its propensity to feed on humans, autogeny or ability to lay eggs without first requiring a blood meal, as well as its ability to breed throughout the winter in man-made underground receptacles. Such adaptations likely support its role in the transmission of WNV to humans.
Clinical Signs
WNV was long thought to cause only mild and sporadic disease in humans. Only about 20% of the human vector-borne WNV infections are thought to become symptomatic. Typical mild and self-limited symptoms include headache, arthralgia, rash, and fever (West Nile fever). Of the symptomatic patients, approximately 1/20 develops neuroinvasive disease (WNND). WNND occurs predominately in the elderly and those with pre-existing conditions. WNND carries a case fatality ratio of about 10% [14, 15]. To date, no specific therapy for WNV is available. While a vaccine for equines is approved and recommended in areas with WNV transmission, for humans, there are yet no vaccination options.
Human-to-Human Transmission
Although the most common, transmission via mosquito bites is not the only route of infection. The relevance of WNV as a transfusion-transmissible-infection (TTI) was seen when it was first detected in the USA in 1999 [16]. It was then discovered that recipients of non-virus-inactivated blood components contracted the disease via transfusion: 23 TTIs were discovered between 1999 and 2003 [17]. In addition, transmission via organ and tissue transplantation was also recorded [18]. This led to the quick development of suitable WNV screening tests using nucleic acid amplification techniques (NATs) for potential blood donors which were implemented in the USA in 2003. Even after the introduction of universal NAT screening of blood donations, an additional 9 TTIs occurred [19]. Typical transfusion recipients are prone to more severe infections − irrespective of the mode of transmission − due to their underlying conditions, especially when immunocompromized [20]. Of the 32 reported transfusion-associated WNV infections in the USA, 19 (59%) developed WNND [17, 19]. The prevention of WNV transmission via blood products is therefore important in endemic regions, and WNV-NAT tests for screening of donations are commercially available.
WNV in Europe
Sporadic cases of WNV in humans and animals have been recorded in Europe since 1958 with evidence of sustained local transmission in Italy from as early as 1967 and Greece from 1970 [21, 22]. However, the first major outbreaks of symptomatic disease amongst humans in Europe were only recorded in Romania in 1996 [23] and in 1999 in the Volgograd region of Russia [24]. Despite this, the disease was still considered of limited relevance to human or animal health until its emergence and explosive spread in the USA from 1999 [25].
Compared to the rapid spread of WNV in North America, WNV occurrence in Southern Europe appears more stable. WNV-infected mosquitoes, birds, horses, or humans are mostly identified in areas characterized by the presence of wetlands. Since the introduction of WNV lineage 2 from Africa into Europe, this virus lineage has become widespread and dominant within a few years (described here [26]). In the early and mid-2010s, WNV had also affected parts of Austria, Hungary, and the Czech Republic [27], but no gradual northward spread was discernible. In 2018, there was very intense WNV circulation and a resulting strong increase in human case numbers in Southern Europe during a very long unusually warm summer [28, 29]. In the same summer, WNV was first identified in Germany. In a very recent development, in 2020, WNV was also identified in mosquitoes, birds, and humans in the Netherlands, in an area non-adjacent to the affected area in Germany [30]. However, in 2021, WNV may have been absent again from the Netherlands. The spread of WNV is well documented in the maps provided by the European Centre for Disease Prevention and Control which are available online [27].
WNV in Germany
There were continuous efforts to screen German mosquitoes (different national screening programmes) and hundreds of birds in the framework of the German wild-bird monitoring network for evidence of WNV RNA and specific WNV antibodies in non-migratory birds for decades [31]. Before 2018, there was however no evidence for autochthonous WNV infections in the tested mosquitoes and birds and no autochthonous human infections. Thus, Germany was not considered a WNV-endemic country.
This changed in 2018, when WNV lineage 2 was first detected in 12 resident, wild, and aviary birds and 2 horses in Germany [32]. The main focus was in East-Central Germany, where the long and warm summer provided excellent transmission conditions [33]. In 2019 and 2020, a considerable WNV epizootic among birds and horses was observed. Areas with WNV circulation were parts of the federal states of Berlin, Brandenburg, Saxony-Anhalt, Saxony, and Thuringia. A clear trend of expansion or shifting to the West of the area so far has not been seen. The number of detected infected animals increased from 2018 to 2019, remained at the same level in 2020, but decreased in 2021 [34, 35].
Usutu Virus
Another closely related flavivirus, Usutu virus (USUV), is circulating between mosquitoes and birds in the same enzootic cycle. It is pathogenic to various bird species (similar to WNV) and has caused die-offs, especially in blackbirds in the past 10 years in Germany. Originating from Africa, it was first discovered in mosquitoes in Germany in 2010 and has been circulating in the whole country since 2018 [31, 36]. WNV and USUV co-infections in birds [37] have been noted, and one co-infection in humans has been reported in Austria [28]. The pathogenic potential of USUV in humans is still unclear, but human infections clearly occur and were noted in Germany as early as 2016 in a blood donor from Western Germany [38]. Because both serological tests as well as some NAT tests are cross-reactive between WNV and USUV, diagnostics and screenings aimed at WNV often reveal human USUV infections upon confirmatory testing.
Diagnostics
One of the greatest challenges in identifying and confirming a WNV infection is the cross-reactivity with related flaviviruses such as USUV [39]. Most detections of WNV infection in a clinical setting are of more severe cases of West Nile fever and WNND, where the disease presents enough symptoms to trigger specific testing. Since the window to detect WNV RNA in plasma or cerebrospinal fluid samples is very short (2–8 days), diagnosis of clinical cases often rests on detection of WNV antibodies. IgM-capture ELISAs and IgG immunoassays such as immunofluorescence tests are highly sensitive but very specific and may show an unacceptably high rate of false positives [40]. WNV-specific serological tests have been developed [40] but are not commercially available [40]. IgG detection can be confirmed by a virus neutralization test to rule out serological cross-reactivity. Alternatively, confirmation of a WNV infection can be achieved by NAT, e.g., from the urine or whole blood, where higher concentrations of WNV RNA are present for longer periods of time (weeks) [40, 41, 42].
Only NAT is used for blood donor testing in order to identify WNV-infectious donations. WNV-NAT can be performed as a single donor or mini-pool NAT, depending on the desired limit of detection (LoD). Where both WNV and USUV are circulating and a non-discriminatory NAT is used for blood donor testing in Germany, confirmation of positive NAT results as WNV by nucleotide sequencing or specific NAT is highly recommended. The different diagnostic tests for specific situations are listed in Table 1.
Table 1.
Diagnostic tests for WNV infections
| Clinical case | Blood donor | |
|---|---|---|
| Initial test | NAT (plasma, whole blood, urine, CSF) WNV-IgM |
WNV-NAT (LoD: 250 copies/mL relating to the single donation) |
|
| ||
| Confirmation/follow up | WNV IgG titre increase (repeated testing) WNV IgM titre increase (repeated testing) Specific NAT for WNV and USUV, respectively Nucleotide sequencing |
Specific NAT for WNV and USUV, respectively Nucleotide sequencing |
CSF, cerebrospinal fluid.
Surveillance in Germany
Since birds play a key role in the transmission cycle of zoonotic arthropod-borne viruses, they are an ideal tool for monitoring. To evaluate the risks of WNV and USUV for human and veterinary health the Friedrich Loeffler Institute (FLI) has been conducting a wild-bird monitoring programme for many years. Together with external partners, blood samples and bird carcasses of wild and captive birds (e.g., zoo birds) are tested by molecular and/or serological methods for the occurrence of WNV and USUV annually. This nationwide, unique wild-bird monitoring programme is conducted as a research project supported by numerous sample collectors, including hobby ornithologists as well as members of the veterinary state investigation offices, avian clinics of the veterinary medicine faculties, the Bernhard Nocht Institute for Tropical Medicine (BNITM), the German Mosquito Control Association (KABS), the Nature and Biodiversity Conservation Union (NABU), and several avian clinics/practices and bird sanctuaries.
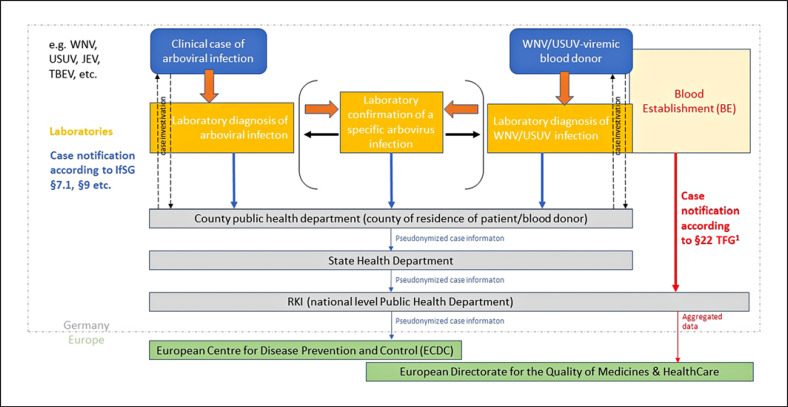
Human WNV and also USUV infections are notifiable according to the German “Protection against infection” law (IfSG) which since 2016 requires the notification of all arbovirus infections diagnosed in humans. The broad scope was chosen in response to a series of worldwide emerging arboviruses with at least travel-associated relevance to Germany, to be able to gather surveillance data from the start of such an emergence. Laboratory evidence of acute arbovirus infection has to be reported by laboratories to the patients' or blood donors' residential county public health departments. The latter then investigate exposure information including possible travel association and often also arrange for further laboratory confirmation of the infection (distinguishing WNV from other flavivirus infections). Pseudonymized case information is shared with state and national public health authorities. On the national level, the Robert Koch Institute (RKI) hosts the national database, differentiating between autochthonous and travel-associated infections. Cases are currently counted for the national statistic if WNV or USUV infection is specifically confirmed, and at least one typical symptom was present. RKI provides information on confirmed autochthonous WNV infections to ECDC on the European level according to the EU case definition [43] which also counts asymptomatic infections confirmed by nucleic acid detection. Figure 1 shows the case notification scheme for human arbovirus infections.
Fig. 1.
Case notification scheme for human cases of arbovirus infection in Germany. Mandatory reporting for hemovigilance purposes according to the Medicines Act and the Transfusion Act remains unaffected. WNV, West Nile virus; USUV, Usutu virus; JEV, Japanese encephalitis virus; TBEV, tick-borne encephalitis virus; IfSG, German protection against infection law; TFG, transfusion act; RKI, Robert Koch Institute; ECDC, European Centre for Disease Prevention and Control.
Autochthonous WNV and USUV Infections from 2017 through 2021 Notified according to IfSG
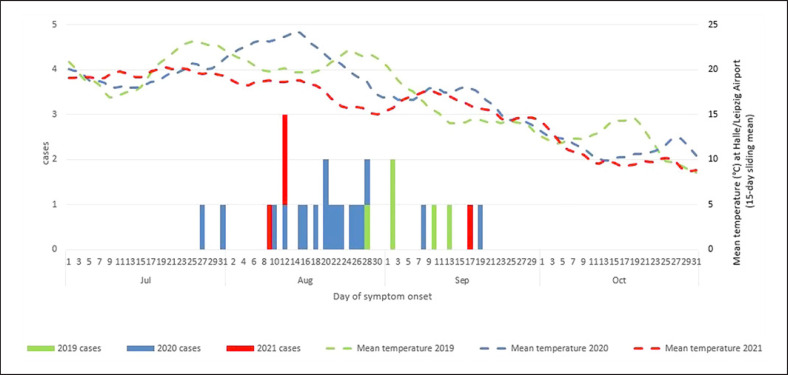
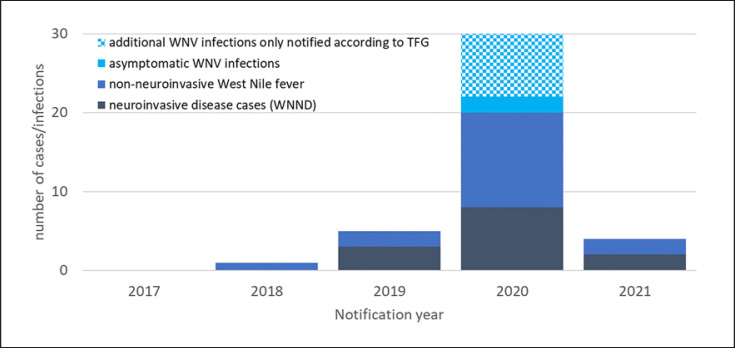
Notified as autochthonous symptomatic human WNV infections and confirmed by additional laboratory assays like specific NAT or nucleotide sequencing were 1 case in 2018, 5 cases in 2019, 20 cases in 2020, and 4 cases in 2021. In 2020, there were 2 additional asymptomatic infections also notified to ECDC. The case in 2018 pertains to a veterinarian who had close contact to a zoo bird (deceased of WNV) during the necropsy; all 31 later infections reported here are considered mosquito-borne. They affected 21 (67%) men and 10 women (33%) 24–85 years of age. Twelve (39%) of these cases had WNND, and one of these patients, a man in his 70s, died [44]. The 27 known dates of the symptom onset ranged from 27 July to 19 September, with a predominance in the second half of August. Figure 2 shows the distribution of onset dates in 2019–2021 and the timing in close association with the period of highest ambient air temperatures as measured at a weather station relatively central to the most affected areas (Leipzig/Halle Airport).
Fig. 2.
Symptom onset of human autochthonous WNV infections from 2019 to 2021, relative to ambient air temperature in the endemic region (weather station at Halle/Leipzig Airport).
The 31 infections from 2019–2021 occurred in the states of Berlin, Saxony, Saxony-Anhalt, and Brandenburg − all cases notified according to IfSG resided in counties with documented WNV infections in birds and horses at least 1 year since 2018 [34, 35]. Suspected cases were also notified from other regions, but none of those could be confirmed. In one particularly interesting non-confirmed case of a blood donor in 2021, the cross-reactivity was due to a vaccination (inactivated vaccine) against another closely related flavivirus, Japanese encephalitis virus, on the day before the positive blood donation.
In 2020 and 2021, approximately half of the human cases were discovered by blood donation screening. Autochthonous USUV infections notified include a geographic cluster of 3 infections in South-West Germany in 2018, an infection in Western Germany in 2020, and an infection in Western Germany in 2021. These USUV infections were diagnosed by blood donor screening. Symptoms were not notified for any of these cases. USUV to our knowledge has not been confirmed in any of the clinical cases investigated and notified as possible WNV infection in Germany.
Travel-Associated WNV and USUV Infections from 2017 through 2021 Notified according to IfSG
Travel-associated cases of WNV or USUV had been notified occasionally in years prior to the formal arbovirus notification requirement but not in every year. After notification became mandatory, ten, seven, one, and one symptomatic travel-associated WNV infections were notified in 2018, 2019, 2020, and 2021, respectively. Countries of infection for these 19 cases were: Greece (4×), Serbia (3×), Italy (2×), Montenegro (2×), Turkey (2×), Romania, Hungary, Bulgaria, the USA, and the Dominican Republic; one infection was diagnosed in a refugee who reached Germany from Africa via Malta, but the actual place of infection was undeterminable.
Travel-associated USUV cases were only detected in asymptomatic blood donors, 1 case each in 2018, 2019, and 2021. Countries of infection were Bulgaria, Croatia, and Italy. The case numbers 2020 and 2021 are influenced by changed travel patterns due to the SARS-CoV-2 pandemic.
Blood Donor Regulation in Germany
According to the Commission Directives 2004/33/EC and 2014/110/EC, potential blood donors of whole blood or blood components have to be deferred for 28 days after leaving an area with ongoing transmission of WNV to humans unless an individual NAT is negative. Prior to 2020, this rule applied to potential donors who had travelled to areas with an ongoing transmission of WNV abroad. After the first autochthonous WNV infections were reported in 2019, deferral or testing of potential donors was extended to areas in Germany in 2020. Affected regions are defined on the county-level and the competent authority (Paul Ehrlich Institute [PEI]) provides the “Data Base Emerging Infections for look-up” which has to be used by blood establishment (BE). Testing or deferral is limited to the transmission season from June 1 to November 30, and exposure defined as a stay of at least 2 consecutive days in an affected region. If testing is performed, the LoD is currently defined as 250 copies/mL based on the single donation [45]. From June 2023 onwards, the LoD will be defined as 120 IU/mL [46].
Blood Donor Surveillance in Germany
Reporting of the number of WNV tested donations and any positive results is mandatory according to §22 of the Transfusion Act (TFG). For WNV-positive donations, additional information has to be provided, e.g., travel history and confirmatory testing. Apart from the donor epidemiology reporting, WNV-positive repeat donors involved in look-back investigations are reportable to the competent authority (PEI) and the supreme federal authorities according to the §63i Medicines Act and §19 TFG. Any look-back procedures in the context of a suspected WNV transmission also have to be notified to the PEI.
For logistical reasons, most BEs implemented general WNV screening in the transmission period rather than testing or deferring donors on an individual level. In 2020, 114 of the 141 BEs (81%) collecting whole-blood donations or platelets tested their donations for the presence of WNV genome. Altogether, 2,138,008 donations were screened, and 32 initially WNV-positive donations were identified: 17 were confirmed as WNV infections (0.8/100,000 donations; 95% CI 0.5–1.3). Fifteen initially reactive donations could not be confirmed as WNV; 11 turned out to be USUV infections and in 4, no specific viral RNA was identifiable. One of the 17 donors with confirmed WNV infection harboured an USUV co-infection. Methods used for confirmation were discriminatory NAT and nucleotide sequencing. All donors were eligible to donate at the time of donation, but for the 10 WNV-positive blood donors with available data on the course of infection, 8 reported mild symptoms postdonation consistent with WNV infection like headache, rash, or muscle pain when later questioned broadly by the local health department. The 17 WNV-infected blood donors had a median age of 38 years, and 9 were male − none of the infections were acquired abroad. All confirmed WNV-positive donors resided in areas where WNV had been detected in animals between 2018 and 2020. Six confirmed WNV-positive donors donated plasma, ten whole blood, and one platelets. Nine of the 17 WNV infections and 1 of the 12 USUV infections were also notified according to IfSG.
Data for 2021 are still preliminary. Until May 11, results of 2,213,744 WNV tested donations were available. Four initially WNV-positive donations were identified. Of these, two could be confirmed (0.1/100,000 donations; 95% CI 0.0–0.5), one turned out to be USUV, and in one case, no specific viral RNA was identified. Both WNV-positive donors reported mild symptoms postdonation consistent with WNV infection. The two female WNV-infected whole-blood donors were 53 and 57 years old. They resided in areas where WNV had been detected in animals between 2018 and 2021. Both confirmed WNV infections were also notified according to IfSG, but the USUV infection was not notified.
Testing results and donor characteristics are shown in Table 2. Blood donor surveillance revealed an additional 8 confirmed WNV infections in 2020 to the number notified according to IfSG. Figure 3 shows an epicurve of WNV infections notified according to IfSG and TFG.
Table 2.
Reported reactive WNV-NAT results in blood donors
| 2020 | 2021 ** | |
|---|---|---|
| Number of tested donations | 2,134,568 | 2,213,744 |
| Rate per 100,000 tested donations | 0.8 | 0.1 |
| Number of reported initially reactive WNV-NT tests | 32 | 4 |
| Confirmed WNV infection | 17* | 2 |
| Confirmed USUV infection | 12* | 1 |
| No specific viral RNA detected | 4 | 1 |
| Proportion confirmed WNV-positive male donors, % | 52 | 0 |
| Median age of confirmed WNV-positive donors, years | 38 | 55 |
One donor with a combined WNV and USUV infection.
Data not complete for 2021.
Fig. 3.
Autochthonous human WNV infections notified in Germany from 2017 to 2021 according to IfSG and TFG by the form of infection.
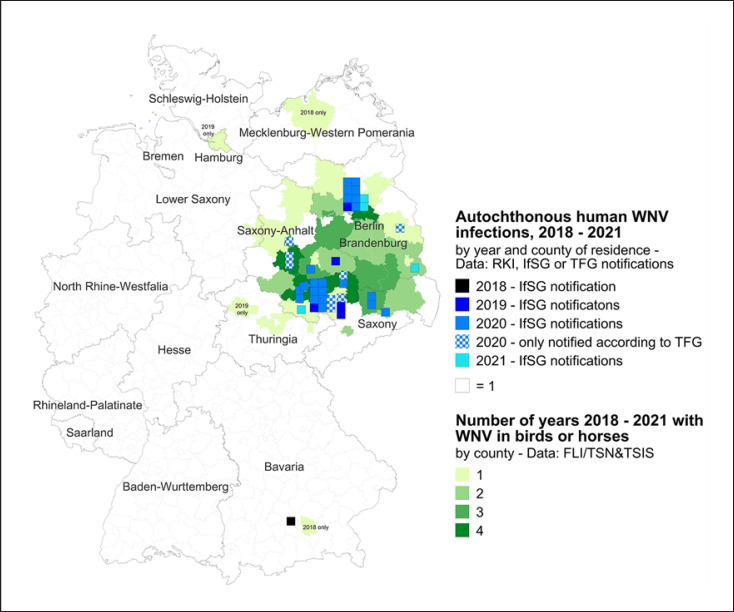
Figure 4 demonstrates the distribution of human WNV infections from 2018 to 2021 which were reported by either surveillance system (IfSG or TFG) and are marked by blue squares by county of residence. Green-shaded counties were affected by WNV-positive birds and/or horses − the darker the green, the more years. Data for 2021 are incomplete for notifications according to TFG.
Fig. 4.
Map of Germany. Human WNV infections from 2018–2021 are marked by blue squares by county of residence. Aside from cases notified according to IfSG for 2020, additional infections identified in the context of blood donations and reported through mandatory donor vigilance (TFG) are shown. Green-shaded counties were affected by WNV-positive birds and/or horses − the darker the green, the more years. Data for 2021 are incomplete for notifications according to TFG.
Discussion
WNV was not detected in Germany until 2018 when the first avian and equine cases were identified. Additionally, the first human case was observed, which was attributed to direct bird contact. In 2019, the first vector-borne infections occurred and affected regions needed to be identified quickly in order to ensure transfusion safety. Surveillance systems were therefore challenged.
Cases of uncomplicated West Nile fever are often not subjected to virological diagnosis. Thus, as in other affected countries, surveillance of human disease relies on clinically more severe cases of WNND and infections discovered in blood donation screening. Given the novelty of the virus in Germany, it is possible that it may be erroneously missed in differential diagnoses of cases of meningoencephalitis, even in endemic areas. Massive underdiagnosis of WNV as a cause of meningoencephalitis in Germany however is unlikely − a centre in Berlin screened more than 600 cerebrospinal fluid samples of patients with meningitis or encephalitis from 2019 to 2020, finding only one WNV infection in 2020 [47]. Berlin has registered infected birds since 2018 and the first human case in 2019.
Presumptive WNV diagnosis in patients with neuroinvasive disease is often first achieved by serology. However, NAT from the urine or whole blood can confirm WNV infection later in the course of disease [41, 44]. Early inclusion of WNV in differential diagnosis of causes of meningoencephalitis may spare patients empirical treatment inappropriate for the infection [48]. In order to quickly identify WNV infections, clinicians in Germany should consider WNV in patients with neurological symptoms (especially meningoencephalitis) and mosquito exposures in areas affected by ongoing WNV transmission (or such transmission in previous years) in season (in Germany July–October, in North America and Southern Europe also later in the fall). WNV diagnostics should also be applied if unusual clusters of patients with mild disease matching West Nile fever, e.g., fever and rash, are observed, indicating intense local transmission. The National Reference Laboratory for tropical pathogens at the BNITM can aid in confirming the diagnosis. Human surveillance data are frequently published in the RKI's journal Epidemiologisches Bulletin (https://www.rki.de/DE/Content/Infekt/EpidBull/epid_bull_node.html).
Integrated surveillance of birds and mosquitoes and the cooperation between human and animal health are essential in detecting hot spots and circulation of WNV and USUV in Germany. Therefore, the German wild-bird monitoring network is a very useful tool to follow the geography of WNV and USUV circulation and can serve as an early warning system for a human exposure risk [31]. It would be very helpful if the structures of veterinary surveillance could be perpetuated to aid in timely defining affected areas. Real-time results of the WNV animal surveillance are publicly available in TSIS [34], and summaries are published regularly in the FLI's “LabLoeffler” publication (https://www.fli.de/de/publikationen/der-labloeffler/).
Currently, the EU Directive 2004/33/EC uses only human infections to define an area with ongoing transmission. Bearing in mind that the majority of WNV-infected individuals do not experience specific symptoms of a WNV infection and that donor screening only detects viraemia, which is very short, diagnosed WNV infections in humans only show the tip of the iceberg. In addition, the confirmation of a WNV infection is often lengthy, and thus, information about affected areas is delayed. Given the available information about WNV in Germany, the option of a broader definition of affected areas including data on virus circulation, e.g., in non-migratory birds, especially if repeatedly detected in consecutive years, should be discussed. Given the current stability of the German WNV-affected region, these areas can be considered endemic, with potential transmission to be expected in following seasons. This is supported by our findings that human WNV infections only occurred in areas with prior avian or equine cases.
The identification of affected areas could also be useful for targeted screening of organ and tissue donors as these are not routinely tested and WNV transmission via organ transplantation has occurred with poor outcomes [49]. Raising awareness of WNV occurrence in the general population is also important. Since no specific therapy or vaccination is available to date, personal protective measures like the use of mosquito repellents, wearing long-sleeved shirts and long trousers, and sleeping and resting in screened or air-conditioned rooms are recommended. This is especially important for vulnerable populations like the elderly or immunocompromized patients. While any property holder can aid in reducing mosquito breeding sites on their own properties, regional vector control measures fall into the responsibility of the local authorities.
Even though WNV only causes a short viraemia in humans, it is now accepted that it poses a considerable threat to transfusion safety if donors were exposed in endemic areas. Even donations from donors with recent infections and extremely low virus concentrations can efficiently transmit infections [29]. In Europe, prior to mandatory screening, one asymptomatic WNV-infected donor caused WNV infection in two recipients in Greece, one of whom developed WNND [50].
Blood donor screening with NAT is an effective means to reduce the possibility of transfusion transmitted WNV infections. It was successfully implemented in the USA in 2003 and in Europe in Italy, Greece, and Austria in 2008, 2012, and 2013, respectively. It is currently limited to the transmission season and to specific areas [51]. Additionally, the option to defer potential donors for non-virus-inactivated products who spent time in areas with ongoing transmission for 28 days is widely used in Europe. In Germany, both donor deferral and NAT testing are used. Some EU member states cancel blood collection altogether in affected regions, sometimes resulting in supply shortages [51]. The current pathogen reduction techniques targeting nucleic acids effectively reduce WNV in the product [52]. Yet, to date, these methods are not licenced for red blood cell concentrates in Germany and thus not an alternative to testing and donor deferral for all products. In the future, however, this approach could be useful in effectively eliminating the blood product-associated WNV transmission risk, especially if WNV became more widespread in Germany.
The prevalence of acute WNV infection in the German blood donor population of 0.8/100,000 tested donations in 2020 was lower than the reported prevalence in Austria (6.4/100,000 2014–2017) and Italy (4.9/100,000 donations 2009–2015) [53], possibly reflecting the climatic differences and the only recent introduction of the virus to Germany. In the first 4 years after its detection in Germany, WNV has only been discovered in a limited geographical area. Given that competent vectors and favourable climatic conditions are present in many parts of the country, effective vector, veterinary, and human surveillance is of utmost importance to timely identify affected regions, especially if blood safety measures are limited to exposures in such an area. The immediate and complete reporting of cases in the notification system according to IfSG and TFG is also crucial. The drop in reported cases in humans, bird, and horses in 2021 compared to 2019 and 2020 is probably a result of less suitable climatic conditions in the cooler summer of 2021, but the recurrence of WNV in the coming years is likely, although it remains unclear to what extent. A comprehensive multifaceted veterinary and human arbovirus surveillance − including the already established tools like vector (see also Mückenatlas.de) and veterinary surveillance and mandatory reporting − could also be helpful to identify other pathogens which could pose a risk for transfusion safety in the future, like chikungunya virus. This pathogen caused an outbreak in two regions in Italy in 2017 and disrupted the Italian blood supply in the densely populated metropolitan region of Rome [54].
The explicit extension of the 28-day deferral or testing requirement after exposure in an affected region of Germany by the PEI in 2020 [45] led to an immediate widespread screening of blood donations. Aside from assuring blood safety, the sheer number of tests performed becomes in itself a WNV surveillance system. The LoD of 250 copies/mL allows pool testing and seems sufficient under the current situation. From June 2023 onwards, an LoD of 120 IU WNV-RNA/mL is required for WNV testing in Germany which may require smaller pool sizes, depending on the results of the validation of the tests using the WHO standard [46]. If WNV community transmission increased, ID-NAT could be considered in order to identify low titre viraemic donations. The switch from pool to ID-NAT is practised in Italy and the USA, e.g., in regions with higher WNV incidence [19, 51]. This is important for donations made in the very early phase of the infection as these donors have not yet developed IgM antibodies. The presence of antibodies greatly reduces the risk of a TTI, and that is why decreasing WNV concentrations which might also be missed by pool-NAT testing will most likely not cause TTIs [55].
A larger percentage of USUV infections presumably are currently being missed by human surveillance. This is in part due to the almost exclusively asymptomatic presentation, but also because the virus − and the universal notification requirement for all arboviruses infecting humans − is less well known. USUV infections can be identified upon confirmatory testing of initially WNV screening positive blood donations due to NAT cross-reactivity [28, 38]. In fact, roughly one-third of the reported donations initially positive for WNV by NAT in 2020 turned out to be USUV instead. And this might even underestimate the number of USUV infections among blood donors as they have probably not been notified completely. Similarly, the proportion of USUV infections in initially WNV-positive donations was high in Austria in 2018, when 17 out of 23 positive donations contained USUV instead of WNV RNA [28], and all 5 WNV-NAT-reactive blood donations in 2017 and 2018 in the region of Lazio in Italy were identified as USUV and not WNV infections [56]. These findings are not surprising, given that USUV is currently circulating more widely than WNV in Europe [57].
The human pathogenic potential of USUV is still under debate. Few case reports have reported that neuroinvasive disease might occur in severely immunocompromized patients [58, 59]. Some USUV positive blood donors reported mild symptoms comparable to West Nile fever when questioned after diagnosis [28]. However, in Germany, none of the clinical cases initially suspected as WNV infections were found to have USUV infection instead. The German Advisory Board Blood (Arbeitskreis Blut) has reviewed the situation and in 2013, concluded that a reassessment of the pathogen in the context of blood safety might become necessary if new data on the pathogenicity of USUV infections became available [60]. The National Advisory Board “Blood” is regularly assessing the situation and has so far not changed its position that there is no need for specific USUV screening or measures for donation non-virus-inactivated blood products are necessary. To date, no transfusion-associated USUV infection has been reported.
Conclusion
WNV may have become permanently endemic in Germany. Therefore, partnerships between public health experts, transfusion medicine specialists, veterinarians, and entomologists should be strengthened to ensure a One Health approach. Combined results of human and veterinary surveillance aid in understanding the spread of the pathogen in Germany. This is necessary to assess the need for additional preventive measures. Clinicians and vulnerable individuals need to be informed about the relatively new threat so they can consider WNV infections in patients with typical symptoms or intensify personal protection, respectively. Testing of organ or tissue donors with recent stay in an affected area should be considered.
To ensure blood safety, the continuation of blood donor testing using NAT is necessary. It would be helpful to harmonize confirmatory testing also to speed up reporting and eventual identification of newly affected areas. As some questions remain with regard to the relevance of USUV infections, completeness of reporting of these infections as well is essential and should be added to the pathogens reportable in the donor vigilance. The development of safe, effective, and affordable pathogen reduction techniques for all blood components would ameliorate the problem of testing for WNV and other potentially emerging transfusion-relevant arboviruses.
Statement of Ethics
Data on human infections were derived from mandatory national reporting according to the Transfusion Act and the Infection Protection Act.
Conflict of Interest Statement
Christina Frank, Ute Ziegler, Raskit Lachmann, Karina Preußel, and Ruth Offergeld have no conflicts of interest to declare. Jonas Schmidt-Chanasit declares that he does not have conflicts of interest with respect to this article but received funding for other projects, lectures, and travel grants from the following organizations: Perkin Elmer, BASF, Roche, Sonic Healthcare, Wynn Macau, Bangkok Bank, Sanofi, Mahidol University, Vietnam Military Medical University, Takeda, Pfizer, INSTAND e.V., and QCMD.
Funding Sources
There were no funding sources.
Author Contributions
Writing the first draft of the manuscript: Christina Frank, Ruth Offergeld, Jonas Schmidt-Chanasit, and Ute Ziegler; analysing data from the general population: Christina Frank and Raskit Lachmann; analysing data from blood donors: Ruth Offergeld and Karina Preußel; analysing veterinary surveillance data: Ute Ziegler; performing confirmatory testing and data analysis: Jonas Schmidt-Chanasit; critically reviewing the paper and assistance in editing: Christina Frank, Ruth Offergeld, Jonas Schmidt-Chanasit, Ute Ziegler, Karina Preußel, and Raskit Lachmann.
Data Availability Statement
Surveillance data on human infections are published on the RKI website (www.rki.de), and data on animal infections are available at https://tsis.fli.de/. Further enquiries can be directed to the corresponding author.
References
- 1.Barrett ADT. West Nile in Europe: an increasing public health problem. J Travel Med. 2018 Jan 1;25((1)) doi: 10.1093/jtm/tay096. [DOI] [PubMed] [Google Scholar]
- 2.Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 1940;20((4)):471–92. [Google Scholar]
- 3.Pauli G. West Nile virus. Prevalence and significance as a zoonotic pathogen [in German] Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz. 2004 Jul;47((7)):653–60. doi: 10.1007/s00103-004-0864-x. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Ramírez E, Llorente F, Jiménez-Clavero M. Experimental infections of wild birds with West Nile virus. Viruses. 2014 Feb 13;6((2)):752–81. doi: 10.3390/v6020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossner CM, Marrama L, Carson M, Allerberger F, Calistri P, Dilaveris D, et al. West Nile virus surveillance in Europe: moving towards an integrated animal-human-vector approach. Euro Surveill. 2017 May 4;22((18)):30526. doi: 10.2807/1560-7917.ES.2017.22.18.30526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004 Jul 22;351((4)):370–8. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 7.Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, et al. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008 Jan;45((1)):125–8. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Leggewie M, Badusche M, Rudolf M, Jansen S, Börstler J, Krumkamp R, et al. Culex pipiens and Culex torrentium populations from Central Europe are susceptible to West Nile virus infection. One Health. 2016 Dec;2:88–94. doi: 10.1016/j.onehlt.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holicki CM, Ziegler U, Raileanu C, Kampen H, Werner D, Schulz J, et al. West Nile virus lineage 2 vector competence of indigenous culex and aedes mosquitoes from Germany at temperate climate conditions. Viruses. 2020 May 19;12((5)):561. doi: 10.3390/v12050561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kampen H, Holicki CM, Ziegler U, Groschup MH, Tews BA, Werner D. West Nile virus mosquito vectors (Diptera: Culicidae) in Germany. Viruses. 2020 Apr 28;12((5)):493. doi: 10.3390/v12050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kampen H, Tews BA, Werner D. First evidence of West Nile virus overwintering in mosquitoes in Germany. Viruses. 2021 Dec 9;13((12)):2463. doi: 10.3390/v13122463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008 Jun 27;4((6)):e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiter P. Climate change and mosquito-borne disease. Environ Health Perspect. 2001 Mar;109((Suppl 1)):141–61. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014 Feb 6;6((2)):606–23. doi: 10.3390/v6020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck C, Leparc Goffart I, Franke F, Gonzalez G, Dumarest M, Lowenski S, et al. Contrasted epidemiological patterns of West Nile virus lineages 1 and 2 infections in France from 2015 to 2019. Pathogens. 2020 Oct 30;9((11)):908. doi: 10.3390/pathogens9110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen LR, Hayes EB. Westward ho? — The spread of West Nile virus. N Engl J Med. 2004;351((22)):2257–59. doi: 10.1056/NEJMp048261. [DOI] [PubMed] [Google Scholar]
- 17.Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003 Sep 25;349((13)):1236–45. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) West Nile virus transmission via organ transplantation and blood transfusion: Louisiana, 2008. MMWR Morb Mortal Wkly Rep. 2009 Nov 20;58((45)):1263–7. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) West Nile virus transmission through blood transfusion--South Dakota, 2006. MMWR Morb Mortal Wkly Rep. 2007 Feb 2;56((4)):76–9. [PubMed] [Google Scholar]
- 20.Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation. 2004 Feb 15;77((3)):399–402. doi: 10.1097/01.TP.0000101435.91619.31. [DOI] [PubMed] [Google Scholar]
- 21.Joubert L, Oudar J, Hannoun C, Beytout D, Corniou B, Guillon JC, et al. Epidemiology of the West Nile virus: study of a focus in Camargue. IV. Meningo-encephalomyelitis of the horse. Annales de l'Institut Pasteur. 1970 Feb;118((2)):239–47. [PubMed] [Google Scholar]
- 22.Hubalek Z, Halouzka J. West Nile fever--a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999 Sep–Oct;5((5)):643–50. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998 Sep 5;352((9130)):767–71. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 24.Platonov AE, Shipulin GA, Shipulina OY, Tyutyunnik EN, Frolochkina TI, Lanciotti RS, et al. Outbreak of West Nile virus infection, Volgograd Region, Russia. Emerg Infect Dis. 2001 Jan–Feb;7((1)):128–32. doi: 10.3201/eid0701.010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007 Oct 15;45((8)):1039–46. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 26.Bakonyi T, Haussig JM. West Nile virus keeps on moving up in Europe. Euro Surveill. 2020 Nov;25((46)):2001938. doi: 10.2807/1560-7917.ES.2020.25.46.2001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control (ECDC) West Nile virus in Europe in 2021; human cases compared to previous seasons, updated 11 November 2021 [map] 2021. Available from https://www.ecdc.europa.eu/en/publications-data/west-nile-virus-europe-2021-human-cases-compared-previous-seasons-updated-11.
- 28.Aberle SW, Kolodziejek J, Jungbauer C, Stiasny K, Aberle JH, Zoufaly A, et al. Increase in human West Nile and Usutu virus infections, Austria, 2018. Euro Surveill. 2018 Oct;23((43)):1800545. doi: 10.2807/1560-7917.ES.2018.23.43.1800545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control (ECDC) Epidemiological update: West Nile virus transmission season in Europe, 2018. 2018. Available from http://ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2018.
- 30.Vlaskamp DR, Thijsen SF, Reimerink J, Hilkens P, Bouvy WH, Bantjes SE, et al. First autochthonous human West Nile virus infections in the Netherlands, July to August 2020. Euro Surveill. 2020 Nov;25((46)):2001904. doi: 10.2807/1560-7917.ES.2020.25.46.2001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michel F, Sieg M, Fischer D, Keller M, Eiden M, Reuschel M, et al. Evidence for West Nile virus and Usutu virus infections in wild and resident birds in Germany, 2017 1nd 2018. Viruses. 2019 Jul 23;11((7)):674. doi: 10.3390/v11070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler U, Lühken R, Keller M, Cadar D, van der Grinten E, Michel F, et al. West Nile virus epizootic in Germany, 2018. Antiviral Res. 2019 Feb;162:39–43. doi: 10.1016/j.antiviral.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler U, Santos PD, Groschup MH, Hattendorf C, Eiden M, Höper D, et al. West Nile virus epidemic in Germany triggered by epizootic emergence, 2019. Viruses. 020 Apr 15;12((4)):448. doi: 10.3390/v12040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedrich Loeffler Institute (FLI) Tierseuchen-Informationssystem (TSIS) 2021. Available from https://www.tsis.fli.de/Default.aspx.
- 35.Friedrich Loeffler Institute (FLI) Tierseuchen-Nachrichtensystem (TSN) 2021. Available from https://tsn.fli.de.
- 36.Sieg M, Schmidt V, Ziegler U, Keller M, Höper D, Heenemann K, et al. Outbreak and cocirculation of three different Usutu virus strains in Eastern Germany. Vector Borne Zoonotic Dis. 2017 Sep;17((9)):662–64. doi: 10.1089/vbz.2016.2096. [DOI] [PubMed] [Google Scholar]
- 37.Santos PD, Michel F, Wylezich C, Höper D, Keller M, Holicki CM, et al. Co-infections: simultaneous detections of West Nile virus and Usutu virus in birds from Germany. Transbound Emerg Dis. 2021 Mar;69((2)):776–92. doi: 10.1111/tbed.14050. [DOI] [PubMed] [Google Scholar]
- 38.Cadar D, Maier P, Müller S, Kress J, Chudy M, Bialonski A, et al. Blood donor screening for West Nile virus (WNV) revealed acute Usutu virus (USUV) infection, Germany, September 2016. Euro Surveill. 2017 Apr 6;22((14)):30501. doi: 10.2807/1560-7917.ES.2017.22.14.30501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzoli A, Jimenez-Clavero MA, Barzon L, Cordioli P, Figuerola J, Koraka P, et al. The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Euro Surveill. 2015 May 21;20((20)):21135. doi: 10.2807/1560-7917.es2015.20.20.21135. [DOI] [PubMed] [Google Scholar]
- 40.Berneck BS, Rockstroh A, Barzon L, Sinigaglia A, Vocale C, Landini MP, et al. Serological differentiation of West Nile virus- and Usutu virus-induced antibodies by envelope proteins with modified cross-reactive epitopes. Transbound Emerg Dis. 2021 Dec 16; doi: 10.1111/tbed.14429. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Barzon L, Pacenti M, Franchin E, Squarzon L, Sinigaglia A, Ulbert S, et al. Isolation of West Nile virus from urine samples of patients with acute infection. J Clin Microbiol. 2014 Sep;52((9)):3411–3. doi: 10.1128/JCM.01328-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietsch C, Trawinski H, Lübbert C, Liebert UG. Short communication: West Nile fever imported from Austria to Germany. Transbound Emerg Dis. 2019 Mar;66((2)):1033–6. doi: 10.1111/tbed.13079. [DOI] [PubMed] [Google Scholar]
- 43.European Union Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. 2018. Available from https://data.europa.eu/eli/dec_impl/2018/945/oj.
- 44.Pietsch C, Michalski D, Münch J, Petros S, Bergs S, Trawinski H, et al. Autochthonous West Nile virus infection outbreak in humans, Leipzig, Germany. Euro Surveill. 2020 Nov;25((46)):2001786. doi: 10.2807/1560-7917.ES.2020.25.46.2001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul Ehrlich Institute (PEI) Notice of approval of medicinal products: Ordering of measures that can minimize the risk of transmission of a West Nile Virus (WNV) infection acquired in Germany through blood components for transfusion (cellular blood preparations and fresh therapeutic plasma) and through stem cell preparations for haematopoietic reconstitution [in German] Bundesanzeiger. 2020. Available from https://www.bundesanzeiger.de/pub/publication/C125NFKbnDG9Liyxe7T/content/C125NFKbnDG9Liyxe7T/BAnz%20AT%2004.06.2020%20B6.pdf?inline.
- 46.Paul Ehrlich Institute (PEI) Requirements for the validation and routine application of nucleic acid amplification methods (NATs) for the demonstration of viral nucleic acid in donated blood [in German] 2022. Available from https://www.pei.de/SharedDocs/Downloads/DE/regulation/blut/spendertestung/pei-anforderungen-validierung-nat.pdf?__blob=publicationFile&v=4.
- 47.Schneider J, Bachmann F, Choi M, Kurvits L, Schmidt ML, Bergfeld L, et al. Autochthonous West Nile virus infection in Germany: Increasing numbers and a rare encephalitis case in a kidney transplant recipient. Transbound Emerg Dis. 2021 Nov 30;69:221–226. doi: 10.1111/tbed.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karrasch M, Pein U, Fritz A, Lange D, Moritz S, Amann K, et al. West-Nile-virus infection acquired in Germany in a kidney transplant recipient. Dtsch Med Wochenschr. 2021 Apr;146((7)):482–6. doi: 10.1055/a-1218-9096. [DOI] [PubMed] [Google Scholar]
- 49.Soto RA, McDonald E, Annambhotla P, Velez JO, Laven J, Panella AJ, et al. West Nile virus transmission by solid organ transplantation and considerations for organ donor screening practices, United States. Emerg Infect Dis. 2022 Feb;28((2)):403–6. doi: 10.3201/eid2802.211697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pervanidou D, Detsis M, Danis K, Mellou K, Papanikolaou E, Terzaki I, et al. West Nile virus outbreak in humans, Greece, 2012: third consecutive year of local transmission. Eurosurveillance. 2014;19((13)):20758. doi: 10.2807/1560-7917.es2014.19.13.20758. [DOI] [PubMed] [Google Scholar]
- 51.Domanović D, Gossner CM, Lieshout-Krikke R, Mayr W, Baroti-Toth K, Dobrota AM, et al. West Nile and Usutu virus infections and challenges to blood safety in the European Union. Emerg Infect Dis. 2019 Jun;25((6)):1050–7. doi: 10.3201/eid2506.181755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.German Advisory Committee Blood Pathogen inactivation systems for platelet concentrates [in German] Bundesgesundheitsblatt Gesundheitsforsc Gesundheitsschutz. 2018 2018;61((7)):874–93. [Google Scholar]
- 53.Pisani G, Cristiano K, Pupella S, Liumbruno GM. West Nile virus in Europe and safety of blood transfusion. Transfus Med Hemother. 2016 May;43((3)):158–67. doi: 10.1159/000446219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marano G, Pupella S, Pati I, Masiello F, Franchini M, Vaglio S, et al. Ten years since the last Chikungunya virus outbreak in Italy: history repeats itself. Blood transfus. 2017 Oct;15((6)):489–90. doi: 10.2450/2017.0215-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busch MP, Caglioti S, Robertson EF, McAuley JD, Tobler LH, Kamel H, et al. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005 Aug 4;353((5)):460–7. doi: 10.1056/NEJMoa044029. [DOI] [PubMed] [Google Scholar]
- 56.Carletti F, Colavita F, Rovida F, Percivalle E, Baldanti F, Ricci I, et al. Expanding Usutu virus circulation in Italy: detection in the Lazio region, central Italy, 2017 to 2018. Eurosurveillance. 2019;24((3)):1800649. doi: 10.2807/1560-7917.ES.2019.24.3.1800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vilibic-Cavlek T, Petrovic T, Savic V, Barbic L, Tabain I, Stevanovic V, et al. Epidemiology of Usutu virus: the European scenario. Pathogens. 2020 Aug 26;9((9)):699. doi: 10.3390/pathogens9090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pecorari M, Longo G, Gennari W, Grottola A, Sabbatini A, Tagliazucchi S, et al. First human case of Usutu virus neuroinvasive infection, Italy. Euro Surveill. 2009 Dec 17;14((50)):19446. [PubMed] [Google Scholar]
- 59.Santini M, Vilibic-Cavlek T, Barsic B, Barbic L, Savic V, Stevanovic V, et al. First cases of human Usutu virus neuroinvasive infection in Croatia, August-September 2013: clinical and laboratory features. Journal of neurovirology. 2015 Feb;21((1)):92–7. doi: 10.1007/s13365-014-0300-4. [DOI] [PubMed] [Google Scholar]
- 60.Pauli G, Bauerfeind U, Blümel J, Burger R, Drosten C, Gröner A, et al. Usutu virus. Transfus Med Hemother. 2014 Feb;41((1)):73–82. doi: 10.1159/000357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Surveillance data on human infections are published on the RKI website (www.rki.de), and data on animal infections are available at https://tsis.fli.de/. Further enquiries can be directed to the corresponding author.