Abstract
Introduction
Obesity has been believed to be closely linked with many kinds of diseases including atherosclerosis, hypertension, cerebrovascular thrombosis, and diabetes. Ghrelin and Homeobox transcript antisense RNA (HOTAIR) were believed to be involved in the regulation of myocardial injury.
Methods
The obesity mice model was established through feeding mice (C57BL/6J, male, eight-week-old) with high-fat diet and palmitate (PA)-induced cardiomyocyte injury. RNA and protein levels were detected with Quantitative real-time PCR and Western blotting. The levels of TG, TCH, LDL, CK-MB, cTnl, and BNP in the serum or cell medium supernatant were measured using ELISA kits. The ROS level was detected with the DCFH-DA method. Binding sites between different targets were identified using detection of dual luciferase reporter assay. Cell apoptosis was analyzed by flow cytometry. RNA-binding protein immunoprecipitation and chromatin immunoprecipitation were used to detect the binding of DNMT3B with HOTAIR or miR-196b promoter.
Results
The expression of HOTAIR was downregulated, and miR-196b was upregulated in the obese myocardial injury. Ghrelin attenuated PA-induced cardiomyocyte injury by increasing HOTAIR. HOTAIR regulated the expression of miR-196b by recruiting DNMT3B to induce methylation of the miR-196b gene promoter. The binding site between miR-196b and IGF-1 was identified.
Discussion/Conclusion
We demonstrated that ghrelin attenuated PA-induced cardiomyocyte injury by regulating the HOTAIR/miR-196b /IGF-1 signaling pathway. Our findings might provide novel thought for the prevention and treatment of obesity-induced myocardial injury by targeting HOTAIR/miR-196b.
Keywords: Ghrelin, Obesity, miR-196b, Homeobox transcript antisense RNA
Introduction
Obesity is characterized by a body mass index equal or more than 30 kg/m2, and it is becoming common in the Western world. The incidence of obesity in China is increasing greatly in the past decades with the changes of diet habits, and over 1/3 American adults are obese [1]. The obesity condition is closely linked with many diseases including atherosclerosis, dyslipidemia, hypertension, cerebrovascular thrombosis, diabetes, and heart disease [2]. In addition, obesity exerts negative effect on the cardiac remodeling and function including myocardial fibrosis, hemodynamic load, and heart failure [3]. However, the specific regulatory mechanism is not clear. Therefore, more attention has been paid to the pathogenesis of obese myocardial injury and the exploration of new targets and related signaling molecular mechanisms.
Ghrelin, a 28-amino acid peptide, has been proved to be closely linked with the regulation of cardiovascular disease. Ghrelin exerts cardioprotective function through growth hormone secretagogue receptor. Ghrelin and its receptor GHS-R 1A have been reported to be constitutively expressed in cardiomyocytes and other myocardial cells [4, 5], suggesting an autocrine/paracrine role of ghrelin in the heart. Meanwhile, total ghrelin levels were proved to be decreased in several obesity-associated heart disorders, such as left ventricular hypertrophy [6], coronary heart disease [7], and myocardial infarction [8]. The inhibition of endothelial dysfunction, sympathetic nerve activation, apoptosis, and inflammation by ghrelin accounts for the cardiovascular protection [9]. We previously demonstrated that ghrelin reduces the cardiac injury induced by obesity by affecting the lncRNA H19/miR-29a/IGH1 signaling pathway [10]. However, whether ghrelin could protect against cardiac injury through other signaling pathway needs to be further explored.
Long noncoding RNAs have been proved to play an important role in many types of life activities [11]. Some reports indicated that long noncoding RNA acted a regulatory role in myocardial cell injury [12]. Homeobox transcript antisense RNA (HOTAIR) is located at 12q12.13, and it is known as a custom tilling array of HOXC locus. It is reported that HOTAIR protected cardiomyocytes from oxidative stress-induced injury [13, 14]. However, its role and regulatory mechanism in obese myocardial injury are still unclear.
It was reported that DNMT3B regulated the Rictor levels through miR-196b promoter methylation [15]. We speculate that ghrelin upregulates the expression of HOTAIR, which promotes hypermethylation of the miR-196b gene by recruiting DNMT3B, and then increase the expression of IGF-1, thus alleviating the progress of obesity-induced myocardial injury. This report might provide novel thought for the prevention and treatment of obesity-induced myocardial injury by targeting HOTAIR/miR-196b.
Materials and Methods
Establishment of the Obesity Mice Model
C57BL/6J mice (Male, 8-week-old, 20–24 g) were purchased from Charles River (Beijing, China). All experiments were approved by the Second Affiliated Hospital of Nanchang University Medical Research Ethics Committee (the Examination and Approval No. Review [2020] No. [A901]). The animals were raised with free access to water and food. Mice in the group high-fat diet (HFD) were fed with HFD (carbohydrate-protein-fat ratio: 20%-20%-60%, 25 kJ/g) for 8 weeks. The mice in the control group were fed with normal diet (carbohydrate-protein-fat ratio: 20%-70%-10%, 14 kJ/g) for 8 weeks. After sacrifice, the blood and heart samples were collected for further experiments.
Hematoxylin-Eosin Staining
Tissue was isolated and fixed using 4% formaldehyde for 24 h. The optimal cutting temperature compound (OCT, Sigma, Ronkonkoma, NY, USA) was used for tissue embedding, and a cryostat was used to make 10-micron thick sections. Hematoxylin was used to stain tissues for 5 min. Tap water was used to wash 3 min, and 1% acid alcohol was used for differentiation. After washing with water for 30 s, 0.1% ammonia water (20 s) was used for bluing. Tap water was used to wash 3 min, and tissues were rinsed using 95% alcohol. Then, the slides were stained using eosin for 15 s. Then, the slides were captured using Zeiss AxioVision (Jena).
Cell Culture and Treatment by Palmitate
Palmitate (PA) was purchased from Sigma (Ronkonkoma, NY, USA). PA (5 mM) was prepared using 5% BSA. Cardiac myocytes were cultured using DMEM medium (Gibco, Germany) containing 5% FBS on the condition of 5% CO2 at 37°C. After treatment with PA (5 mM) for 24 h, the cells were used for different experiments.
Cell Transfection
si HOTAIR, pcDNA-HOTAIR, and miR-196b mimics were purchased from RiboBio (Guangzhou, China). Cells (1 × 106 cells/well) were seeded into 6-well plates. Lipofectamine 2000 (Invitrogen, Waltham, MA, USA) was used to package the plasmids into cells according to the instructions of manufacturer.
Detection of the GSH/GSSG Ratio
The measurement of GSH/GSSG ratio was performed as described previously [16]. Cells were plated into 6-well plates and cultured on the condition of 5% CO2 and 37°C. After treatment with transfection, cells were digested. GSSG and GSH were detected in cell extracts using HPLC method. Then, the GSH/GSSG ratio was calculated.
Flow Cytometry
Cells were plated into 6-well plates and cultured on the condition of 5% CO2 and 37°C. After treatment with transfection, cells were digested. After removing supernatant, the pellet was resuspended using 300 μL PBS buffer containing PI (5 μL) and Annexin V-FITC (5 μL). After incubation for 20 min, apoptosis was measured using flow cytometry.
RNA Isolation and Quantitative Real-Time PCR
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was applied to extract RNA. Reverse transcription was performed with the TaqMan miRNA Reverse Transcript Kit (Applied Biosystems, Waltham, MA, USA). The Prism 7500 Fast sequence detection system of Applied Biosystems was used to perform PCR reaction. Primers were listed as follows: miR-196b (forward: 5′-TTTTATTTGTTGTGATTAGGTGGAG-3′, reverse: 5′-AACCTATAACTTCCCCTTCCTTAAC-3′), HOTAIR (forward: 5′-GGCGGATGCAAGTTAATAAAAC-3′, reverse: 5′-TACGCCTGAGTGTTCACGAG-3′), IGF-1 (forward: 5′-CACATCACATCCTCTTCG-3′, reverse: 5′-CTGGAGCCGTACCCTGTG-3′), U6 (forward: 5′-CTCGCTTCGGCAGCACA-3′, reverse: 5′-AACGCTTCAGAATTTGCCT-3′), GAPDH (forward: 5′-ACAACAGCCTCAAGATCATCAG-3′, reverse: 5′-GGTCCACCACTGACACGTTG-3′).
Western Blotting
Proteins were isolated with RIPA lysine buffer and measured using the BCA method. The proteins were electrophoresed on polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were cultivated with related primary antibodies (Abcam, UK) overnight at 4°C. After washing with PBS, membranes were cultivated with the secondary antibody (Abcam, UK) for 2 h at room temperature. Then, an ECL chemiluminescent kit (Advansta, San Jose, CA, USA) was applied to expose protein bands.
Measurement of TG, TCH, LDL, CK-MB, cTnI, and BNP
Blood samples were collected and centrifuged for 10 min at 1,000 g. Then, the concentrations of TG, TCH, LDL, CK-MB, cTnI, and BNP in the serums were measured through related commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Measurement of MDA and SOD
Blood samples were collected and centrifuged for 10 min at 1,000 g. The pyrogallol method (420 nm) was used to measure SOD activity. The SOD measurement kit was purchased from Shanghai Westang (Shanghai, China). The MDA level was measured using a commercial kit purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Measurement of ROS with the DCFH-DA Method
After treatments with transfection and PA, the cells were incubated with DCFH-DA (2 μM, Sigma, Ronkonkoma, NY, USA) for 30 min at 37°C. After washing 2 times with PBS, the ROS intensity was detected using a fluorescence microscope (488 nm).
Methylation-Specific PCR Assay
The miR-196b methylation was measured using methylation-specific PCR. The primer used for miR-196b was: forward: 5′-TTTTATTTGTTGTGATTAGGTGGAG-3′, reverse: 5′-AACCTATAACTTCCCCTTCCTTAAC-3′. The PCR reaction was set as follows: initial denaturation (95°C, 4 min), 35 cycles of denaturation (95°C, 30 s), annealing (55°C, 40 s), extension (72°C, 55 s), and final extension (72°C, 5 min). Finally, electrophoresis was performed to separate PCR products.
RNA-Binding Protein Immunoprecipitation Assay
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) was used to conduct RNA-binding protein immunoprecipitation. The HOTAIR antibody was used in this study. The coprecipitated RNA was measured using RT-PCR and quantitative PCR. The primers were listed in the part of “Quantitative real-time PCR”. Total RNA (input controls) and isotype controls were assayed simultaneously to demonstrate that the detected signals were the result of RNA specifically binding to HOTAIR.
Chromatin Immunoprecipitation Method
The chromatin immunoprecipitation was performed according to the previous report [17]. The tissue was incubated with formaldehyde (2%) for 10 min. Then glycine (2 mol/L) was added to incubate tissues for 15 min. After washing with PBS 3 times, the tissues were incubated with protease inhibitors and lysis buffer. After treatment with a homogenizer, 300–400 bp DNA fragments were achieved. After centrifugation (12,500 g, 5 min), chromatin was diluted using buffer and immunoprecipitated with protein A magnetic beads and nonspecific IgG or DNMT3B antibody. Then, the solutions were incubated for 12 h at 4°C. Immunoprecipitated complex-magnetic beads were collected using a magnetic separator. The primer used for miR-196b was: forward: 5′-TTTTATTTGTTGTGATTAGGTGGAG-3′, reverse: 5′-AACCTATAACTTCCCCTTCCTTAAC-3′.
Detection of Dual Luciferase Reporter Assay
Cells were plated into 24-well plates first. Igf1 -WT plasmid, Igf1 -MUT plasmid, NC mimics, and miR-196b mimics were mixed using medium. The mixed solution was incubated with a transfection reagent for 24 h. Signals were identified using a Dual Luciferase Reporter Assay Kit (Promega, Madison, WI, USA).
Statistical Analysis
Data are presented as means ± SD. Student's t test was used to analysis between two groups. ANOVA was used to analyze among more than two groups. p < 0.05 was considered to be statistical significance. Experiments were conducted at least for three times.
Results
The Expression of HOTAIR Was Downregulated, and miR-196b Was Upregulated in the Obese Myocardial Injury
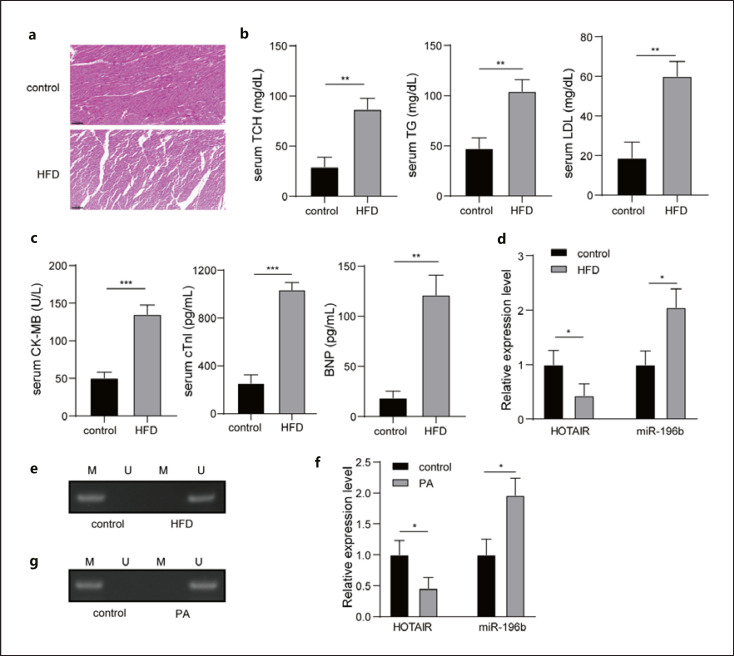
First, the HFD animal model was established. Compared with the group control, myocardial hypertrophy and fibrous disorder were observed in the HFD group through hematoxylin-eosin staining (Fig. 1a). In addition, remarkable higher levels of TG, TCH, LDL, CK-MB, cTnl, and BNP in the HFD group were found compared to the group control (Fig. 1b, c). Meanwhile, the expression of HOTAIR was significantly downregulated, but the level of miR-196b was upregulated in the HFD group (Fig. 1d). In the control group, the methylation of miR-196b gene was obvious, while in the HFD group, the methylation of miR-196b gene promoter was not detected (Fig. 1e). Compared with the control group, the expression of HOTAIR was significantly downregulated, and miR-196b was significantly upregulated in the PA group (Fig. 1f). In the control group, the methylation of miR-196b gene was obvious, while in the PA-induced HCM cells, no methylation of miR-196b gene promoter was observed (Fig. 1g). Therefore, obesity caused myocardial injury, and HOTAIR and miR-196b might be related to this process.
Fig. 1.
Expression of HOTAIR was downregulated, and miR-196b was upregulated in the obese myocardial injury. a Histological changes of myocardial tissues were measured using HE staining. b, c Levels of TG, TCH, LDL, CK-MB, cTnl, and BNP in the serum were detected by the ELISA kit. d, f Expression of HOTAIR and miR-196b was measured using qRT-PCR. e, g Methylation of the miR-196b gene was detected by MSP. HE, hematoxylin-eosin; qRT, quantitative real-time; MSP, methylation-specific PCR.
Ghrelin Attenuated PA-Induced Cardiomyocyte Injury by Increasing HOTAIR
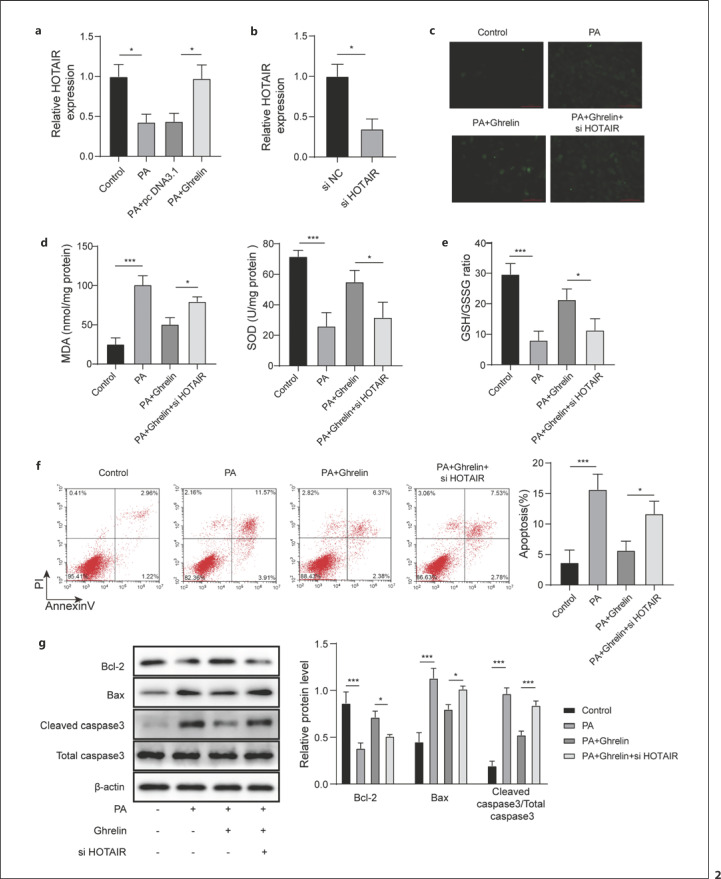
Compared with the control group, the expression of HOTAIR was significantly downregulated by PA, while the overexpression of ghrelin promoted the expression of HOTAIR (Fig. 2a). The expression of HOTAIR was significantly suppressed by si HOTAIR (Fig. 2b). The production of ROS induced by PA was significantly increased compared with the control group. Overexpression of ghrelin significantly inhibited the level of ROS induced by PA, but knockdown of HOTAIR significantly reversed the content of ROS (Fig. 2c). Meanwhile, PA induced a significant increase of MDA production and a remarkable decrease of SOD. However, overexpression of ghrelin significantly inhibited PA-induced MDA increase and promoted SOD levels. The changes of MDA and SOD by ghrelin induced were remarkably reversed by HOTAIR knockdown (Fig. 2d). Meanwhile, the PA-induced GSH/GSSG ratio was significantly reduced, and overexpression of ghrelin significantly reversed the inhibition of GSH/GSSG ratio by PA. While, HOTAIR knockdown significantly reversed the effect of ghrelin overexpression (Fig. 2e). In addition, compared with the control group, PA significantly increased the cell apoptosis rate, and ghrelin overexpression significantly inhibited PA-induced apoptosis. However, knockdown of HOTAIR reversed the influence of ghrelin on cell apoptosis (Fig. 2f). The expression level of Bcl-2 was suppressed, and the levels of Bax and cleaved caspase 3 were increased by PA. Overexpression of ghrelin significantly promoted the expression of Bcl-2 and inhibited the expression of Bax and cleaved caspase 3, while the expression of these apoptosis-related proteins was significantly reversed after knockdown of HOTAIR (Fig. 2g). Therefore, ghrelin alleviated PA-induced cardiomyocyte injury by upregulating HOTAIR.
Fig. 2.
Ghrelin attenuated PA-induced cardiomyocyte injury by increasing HOTAIR. a HOTAIR expression was measured using qRT-PCR. b Knockdown of HOTAIR was established using si HOTAIR transfection. c, d Levels of ROS, MDA and SOD were detected by using a commercial kit. e Ratio of GSH/GSSG was detected by using a commercial kit. f Cell apoptosis was analyzed by flow cytometry. g Apoptosis-related proteins were measured by Western blot. qRT, quantitative real-time.
HOTAIR Regulated the Expression of miR-196b by Recruiting DNMT3B to Induce Methylation of the miR-196b Gene Promoter
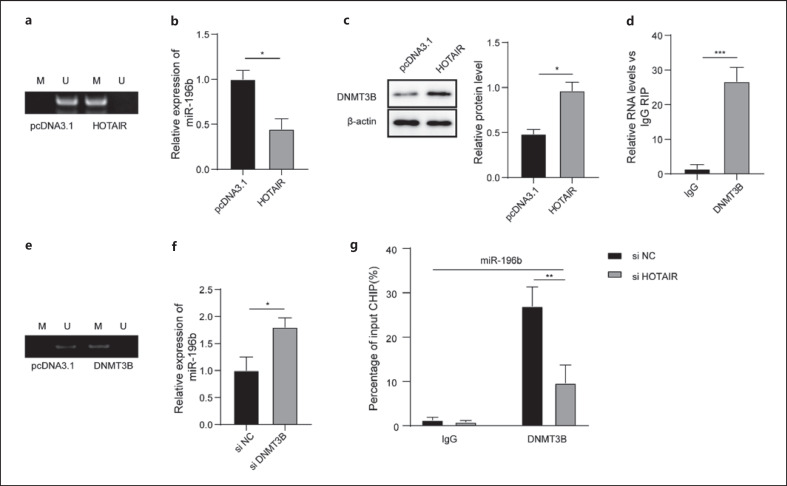
Overexpression of HOTAIR promoted the methylation of miR-196b gene promoter significantly compared with group pcDNA3.1 (Fig. 3a). Meanwhile, overexpression of HOTAIR remarkably decreased the expression of miR-196b (Fig. 3b) and induced the upregulation of DNMT3B (Fig. 3c). In addition, we demonstrated that HOTAIR could bind with DNMT3B (Fig. 3d). In addition, overexpression of DNMT3B increased the methylation of the miR-196b gene promoter remarkably (Fig. 3e), but knockdown of DNMT3B markedly increased the expression of miR-196b (Fig. 3f). However, the ability of DNMT3B to bind with the miR-196b promoter region was significantly decreased after HOTAIR knockdown (Fig. 3g).
Fig. 3.
HOTAIR regulated the expression of miR-196b by recruiting DNMT3B to induce methylation of the miR-196b gene promoter. a Methylation of the miR-196b gene promoter after overexpression of HOTAIR was measured by MSP. b Expression of miR-196b was detected by qRT-PCR. c Protein expression of DNMT3B was measured by Western blot. d Binding between HOTAIR and DNMT3B was measured by RIP. e Methylation of the miR-196b gene promoter after overexpression of DNMT3B was measured by MSP. f Expression of miR-196b was detected after DNMT3B knockdown by qRT-PCR. g Ability of DNMT3B to bind to the miR-196b promoter region was measured by ChIP. qRT, quantitative real-time; MSP, methylation-specific PCR; RIP, RNA-binding protein immunoprecipitation; ChIP, chromatin immunoprecipitation.
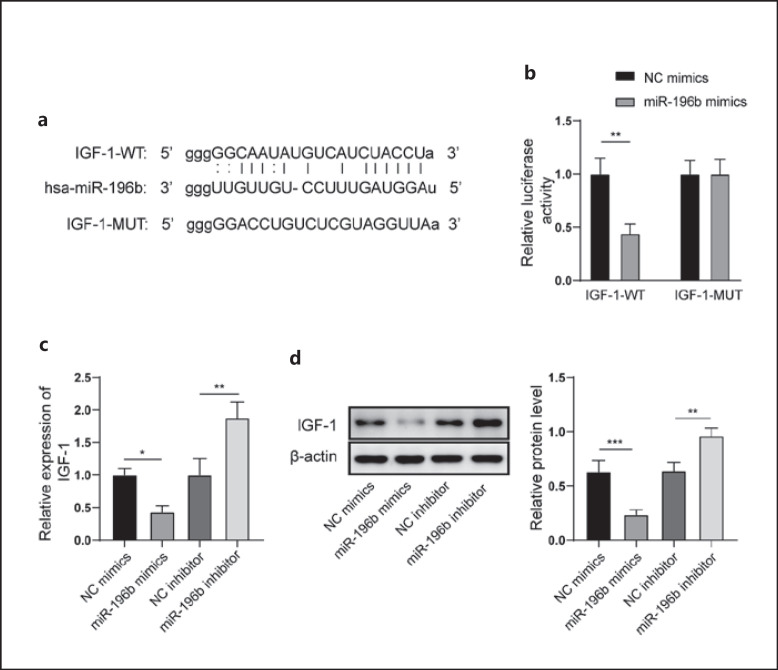
Igf1 Was a Target Gene of miR-196b
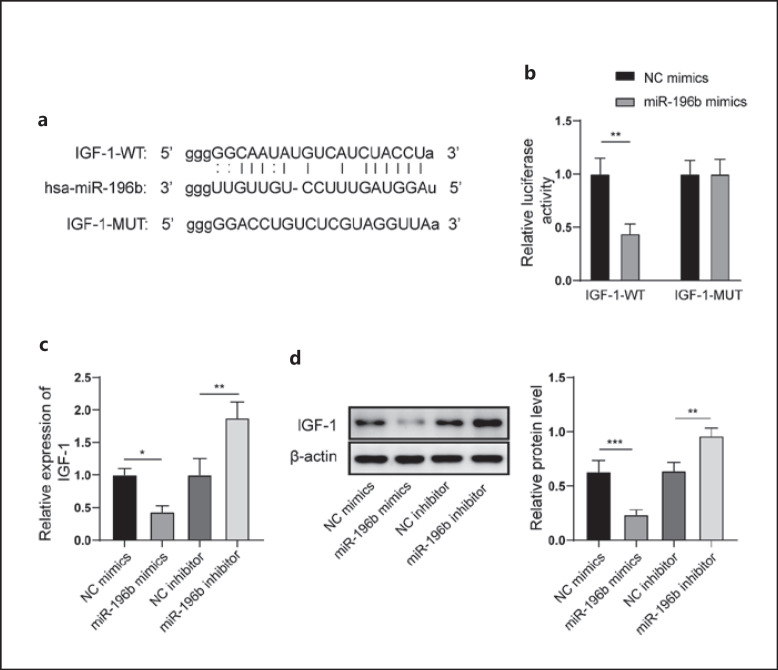
The relationship between miR-196b and Igf1 was predicted by StarBase (http://starbase.sysu.edu.cn/) in this study. The potential binding sites between miR-196b and Igf1 were found (Fig. 4a). Co-transfection of miR-196b mimics and the wild-type Igf1 vector significantly decreased the luciferase activity, but no significant change was found after treatment with the mutant-type Igf1 (Fig. 4b). miR-196b mimics significantly suppressed the mRNA and protein expression of IGF-1. Conversely, miR-196b inhibitor significantly promoted the mRNA and protein expression of IGF-1 (Fig. 4c, d).
Fig. 4.
Igf1 was a target gene of miR-196b. a Binding sites between miR-196b and Igf1 were predicted by StarBase. b Binding between miR-196b and Igf1 was identified using the dual luciferase reporter method. c mRNA expression of IGF-1 was measured by qRT-PCR. d Protein expression of IGF-1 was measured by Western blot. qRT, quantitative real-time.
Ghrelin Attenuated PA-Induced Cardiomyocyte Injury by Regulating the HOTAIR/miR-196b /IGF-1 Signaling Pathway
Overexpression of ghrelin significantly inhibited the level of ROS induced by PA. miR-196b mimics significantly promoted the level of ROS. Meanwhile, overexpression of IGF-1 significantly reversed the increased level of ROS caused by miR-196b mimics (Fig. 5a). Overexpression of ghrelin significantly inhibited the level of MDA induced by PA and promoted the level of SOD. miR-196b mimics significantly promoted the level of MDA and inhibited the content of SOD. Overexpression of IGF-1 reversed the changes of MDA and SOD induced by miR-196b mimics (Fig. 5b). The decreased GSH/GSSG ratio induced by PA was significantly promoted by ghrelin overexpression. In addition, miR-196b mimics significantly inhibited the GSH/GSSG ratio, and overexpression of IGF-1 reversed the GSH/GSSG change caused by miR-196b mimics (Fig. 5c). The PA-induced cell apoptosis was inhibited by ghrelin overexpression, and miR-196b mimics remarkably promoted cell apoptosis. However, simultaneous overexpression of IGF-1 reversed the increase apoptosis rate induced by miR-196b mimics (Fig. 5d). In addition, the expression of Bcl-2 was increased and Bax and total caspase 3 were inhibited by overexpression of ghrelin. However, miR-196b mimics exerted the opposite effect on the levels of these apoptosis-related proteins. Overexpression of IGF-1 remarkably reversed the influence of miR-196b mimics on apoptosis-related proteins (Fig. 5e).
Fig. 5.
Ghrelin attenuated PA-induced cardiomyocyte injury by regulating the HOTAIR/miR-196b /IGF-1 signaling pathway. a, b Level of ROS MDA and SOD were measured by a commercial kit. c GSH/GSSG ratio was measured by a commercial kit. d Cell apoptosis was analyzed by flow cytometry. e Apoptosis-related proteins were measured by Western blot.
Discussion
It was reported that more than 650 million adults were obese in the world, and the obese condition is closely associated with several types of diseases including cancers, type 2 diabetes, stroke, ischemic heart disease, and high blood pressure. Previous reports indicated that obesity might lead to cardiac injury through inducing apoptosis, lipotoxicity, mitochondrial dysfunction, and oxidative stress. However, the further mechanism how obesity affects cardiac injury has not been fully clarified.
Previous report suggested that the expression of HOTAIR was promoted in the plasma and cardiac tissues of congenital heart diseases patients, and HOTAIR might be a potential marker for the congenital heart diseases patients [18]. HOTAIR could also act as the ceRNA through sponging miR-613 in regulating atrial fibrillation [19]. HOTAIR presented protective function against hypoxia-induced injury and myocardial infarction via sponging miR-519d [20]. It was reported that miR-196b was linked negatively with cardiomyocytes differentiation [21]. In this study, the expression of HOTAIR was significant lower, and miR-196b was increased in the myocardial injury induced by obesity. HOTAIR and miR-196b played an important role regulating the obesity-induced myocardial injury.
Ghrelin has been believed to be an important regulator for vasculature and heart diseases. The myocardial injury caused by obesity was relieved by ghrelin by targeting the H19/miR-29a/IGF-1 signaling pathway [10] and TLR4/NLRP3 inflammasome [22]. Ghrelin also suppressed the hypoxia injury of cardiomyocyte via Akt-mTOR [23]. In this study, we demonstrated that ghrelin attenuated PA-induced cardiomyocyte injury by increasing HOTAIR, which is in line with previous research.
DNA methylation in mammals acts an important role in many different kinds of life activities, and it mainly is established by de novo DNMT3B, DNMT3A, and DNMTs [15]. Telomerase reverse transcriptase affects DNA methylation in hepatocellular carcinoma through influencing DNMT3B expression [24]. In addition, increase of DNMT3B -mediated DNA methylation accelerates the leukemia development [25]. We demonstrated that HOTAIR regulated the expression of miR-196b by recruiting DNMT3B to induce methylation of the miR-196b gene promoter, which might be the potential mechanism how HOTAIR protects cardiomyocyte.
IGF-1 is believed to be closely linked with the regulation process of myocardial injury. It was reported that miR-150 protected myocardial infarction by targeting TP53/IGF-1 [26]. IGF-1 protects against myocardial infarction through activating PI3K/Akt [27]. Meanwhile, IGF-1 also suppresses myocardial injury via strengthening the function of BMSC [28]. In this study, the direct binding site between IGF-1 and miR-196b was identified. Meanwhile, we proved that ghrelin inhibited PA-induced cardiomyocyte injury by regulating the HOTAIR/miR-196b /IGF-1 signaling pathway.
In this study, the low expression of HOTAIR and high expression of miR-196b in the obese myocardial injury tissues were observed. We demonstrated that ghrelin might suppress PA-induced cardiomyocyte injury by targeting the HOTAIR/miR-196b /IGF-1 signaling pathway. This study might provide a new thought for the treatment and prevention of cardiomyocyte injury induced by obesity.
Statement of Ethics
All experiments were approved by the Second Affiliated Hospital of Nanchang University Medical Research Ethics Committee; the Examination and Approval No. Review (2020) No. (A901).
Conflict of Interest Statement
The authors report no conflict of interest.
Funding Sources
This work was supported by the National Natural Science Foundation of China (82060162) and Natural Science Foundation of Jiang Xi (20202BABL206040).
Author Contributions
Guarantor of integrity of the entire study: Yang Liu; study concepts: Yuan-Yuan Lang; study design: Yang Liu; definition of intellectual content: Yan-Ling Liu; literature research: Chun-Feng Ye; clinical studies: Na Hu; experimental studies: Xin-Yue Xu; data acquisition: Qing Yao; data analysis: Wen-Shu Cheng; statistical analysis: Zu-Gen Cheng; manuscript preparation: Xin-Yue Xu; manuscript editing: Yuan-Yuan Lang; manuscript review: Yang Liu.
Data Availability Statement
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Yuan-Yuan Lang and Xin-Yue Xu are the first co-authors.
References
- 1.Tchang BG, Saunders KH, Igel LI. Best practices in the management of overweight and obesity. Med Clin North Am. 2021 Jan;105((1)):149–74. doi: 10.1016/j.mcna.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Chao AM, Wadden TA, Berkowitz RI, Quigley K, Silvestry F. The risk of cardiovascular complications with current obesity drugs. Expert Opin Drug Saf. 2020 Sep;19((9)):1095–104. doi: 10.1080/14740338.2020.1806234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam BT, Morais JA, Santosa S. Obesity and ageing: two sides of the same coin. Obes Rev. 2020 Apr;21((4)):e12991. doi: 10.1111/obr.12991. [DOI] [PubMed] [Google Scholar]
- 4.Iglesias MJ, Pineiro R, Blanco M, Gallego R, Dieguez C, Gualillo O, et al. Growth hormone releasing peptide (ghrelin) is synthesized and secreted by cardiomyocytes. Cardiovasc Res. 2004 Jun 1;62((3)):481–8. doi: 10.1016/j.cardiores.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Beiras-Fernandez A, Kreth S, Weis F, Ledderose C, Pottinger T, Dieguez C, et al. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides. 2010 Dec;31((12)):2222–8. doi: 10.1016/j.peptides.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez A, Gomez-Ambrosi J, Catalan V, Becerril S, Sainz N, Gil MJ, et al. Association of plasma acylated ghrelin with blood pressure and left ventricular mass in patients with metabolic syndrome. J Hypertens. 2010 Mar;28((3)):560–7. doi: 10.1097/HJH.0b013e328334327c. [DOI] [PubMed] [Google Scholar]
- 7.Yang D, Liu Z, Luo Q. Plasma ghrelin and pro-inflammatory markers in patients with obstructive sleep apnea and stable coronary heart disease. Med Sci Monit. 2013 Apr 8;19:251–6. doi: 10.12659/MSM.883874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voros K, Prohaszka Z, Kaszas E, Alliquander A, Terebesy A, Horvath F, et al. Serum ghrelin level and TNF-alpha/ghrelin ratio in patients with previous myocardial infarction. Arch Med Res. 2012 Oct;43((7)):548–54. doi: 10.1016/j.arcmed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Yuan MJ, Li W, Zhong P. Research progress of ghrelin on cardiovascular disease. Biosci Rep. 2021 Jan 29;41((1)):BSR2020338. doi: 10.1042/BSR20203387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Xu XY, Shen Y, Ye CF, Hu N, Yao Q, et al. Ghrelin protects against obesity-induced myocardial injury by regulating the lncRNA H19/miR-29a/IGF-1 signalling axis. Exp Mol Pathol. 2020 Jun;114:104405. doi: 10.1016/j.yexmp.2020.104405. [DOI] [PubMed] [Google Scholar]
- 11.Choudhari R, Sedano MJ, Harrison AL, Subramani R, Lin KY, Ramos EI, et al. Long noncoding RNAs in cancer: from discovery to therapeutic targets. Adv Clin Chem. 2020;95:105–47. doi: 10.1016/bs.acc.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Wei JL, Wu CJ, Chen JJ, Shang FT, Guo SG, Zhang XC, et al. LncRNA NEAT1 promotes the progression of sepsis-induced myocardial cell injury by sponging miR-144-3p. Eur Rev Med Pharmacol Sci. 2020 Jan;24((2)):851–61. doi: 10.26355/eurrev_202001_20069. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Zhang M, Chen W, Wang R, Ye Z, Wang Y, et al. LncRNA-HOTAIR inhibition aggravates oxidative stress-induced H9c2 cells injury through suppression of MMP2 by miR-125. Acta Biochim Biophys Sin. 2018 Oct 1;50((10)):996–1006. doi: 10.1093/abbs/gmy102. [DOI] [PubMed] [Google Scholar]
- 14.Meng K, Jiao J, Zhu RR, Wang BY, Mao XB, Zhong YC, et al. The long noncoding RNA hotair regulates oxidative stress and cardiac myocyte apoptosis during ischemia-reperfusion injury. Oxid Med Cell Longev. 2020;2020:1645249. doi: 10.1155/2020/1645249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micevic G, Muthusamy V, Damsky W, Theodosakis N, Liu X, Meeth K, et al. DNMT3b modulates melanoma growth by controlling levels of mTORC2 component RICTOR. Cell Rep. 2016 Mar 8;14((9)):2180–92. doi: 10.1016/j.celrep.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romagnoli C, Marcucci G, Favilli F, Zonefrati R, Mavilia C, Galli G, et al. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013 Feb;280((3)):867–79. doi: 10.1111/febs.12075. [DOI] [PubMed] [Google Scholar]
- 17.Su X, Wang S, Zhang H, Yang G, Bai Y, Liu P, et al. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 through epigenetic modification. J Cell Mol Med. 2021 May;25((9)):4408–19. doi: 10.1111/jcmm.16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, Mo H, Luo J, Zhao S, Liang S, Zhang M, et al. HOTAIR is a potential novel biomarker in patients with congenital heart diseases. Biomed Res Int. 2018;2018:2850657. doi: 10.1155/2018/2850657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai W, Chao X, Li S, Zhou S, Zhong G, Jiang Z. Long noncoding RNA HOTAIR functions as a competitive endogenous RNA to regulate connexin43 remodeling in atrial fibrillation by sponging microRNA-613. Cardiovasc Ther. 2020;2020:5925342. doi: 10.1155/2020/5925342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D, Wang B, Ma M, Yu K, Zhang Q, Zhang X. lncRNA HOTAIR protects myocardial infarction rat by sponging miR-519d-3p. J Cardiovasc Transl Res. 2019 Jun;12((3)):171–83. doi: 10.1007/s12265-018-9839-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Chen ZY, Yang HT. [Expression profile of microRNAs in the cardiomyocytes derived from mouse embryonic stem cells] Sheng Li Xue Bao. 2014 Dec 25;66((6)):702–8. [PubMed] [Google Scholar]
- 22.Wang Q, Lin P, Li P, Feng L, Ren Q, Xie X, et al. Ghrelin protects the heart against ischemia/reperfusion injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci. 2017 Oct 1;186:50–8. doi: 10.1016/j.lfs.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Lu Y, Liu X, Wang X. Ghrelin protected neonatal rat cardiomyocyte against hypoxia/reoxygenation injury by inhibiting apoptosis through Akt-mTOR signal. Mol Biol Rep. 2017 Apr;44((2)):219–26. doi: 10.1007/s11033-017-4098-z. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Yuan X, Sjoholm L, Liu T, Kong F, Ekstrom TJ, et al. Telomerase reverse transcriptase regulates DNMT3B expression/aberrant DNA methylation phenotype and AKT activation in hepatocellular carcinoma. Cancer Lett. 2018 Oct 10;434:33–41. doi: 10.1016/j.canlet.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Schulze I, Rohde C, Scheller-Wendorff M, Baumer N, Krause A, Herbst F, et al. Increased DNA methylation of Dnmt3b targets impairs leukemogenesis. Blood. 2016 Mar 24;127((12)):1575–86. doi: 10.1182/blood-2015-07-655928. [DOI] [PubMed] [Google Scholar]
- 26.Zheng S, Gong M, Chen J. Extracellular vesicles enriched with miR-150 released by macrophages regulates the TP53-IGF-1 axis to alleviate myocardial infarction. Am J Physiol Heart Circ Physiol. 2021 Mar 1;320((3)):H969–79. doi: 10.1152/ajpheart.00304.2020. [DOI] [PubMed] [Google Scholar]
- 27.Liao Y, Li H, Pi Y, Li Z, Jin S. Cardioprotective effect of IGF-1 against myocardial ischemia/reperfusion injury through activation of PI3K/Akt pathway in rats in vivo. J Int Med Res. 2019 Aug;47((8)):3886–97. doi: 10.1177/0300060519857839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang GW, Gu TX, Guan XY, Sun XJ, Qi X, Li XY, et al. HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Prolif. 2015 Dec;48((6)):661–70. doi: 10.1111/cpr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.